Abstract
The root endodermis forms a selective barrier that prevents the free diffusion of solutes into the vasculature; to make this barrier, endodermal cells deposit hydrophobic compounds in their cell walls, forming the Casparian strip. Here, we showed that, in contrast to vascular and epidermal root cells, endodermal root cells do not divide alongside the root apical meristem in Arabidopsis thaliana. Auxin treatment induced division of endodermal cells in wild-type plants, but not in the auxin signaling mutant auxin resistant3-1. Endodermis-specific activation of auxin responses by expression of truncated AUXIN-RESPONSIVE FACTOR5 (ΔARF5) in root endodermal cells under the control of the ENDODERMIS7 promoter (EN7::ΔARF5) also induced endodermal cell division. We used an auxin transport inhibitor to cause accumulation of auxin in endodermal cells, which induced endodermal cell division. In addition, knockout of P-GLYCOPROTEIN1 (PGP1) and PGP19, which mediate centripetal auxin flow, promoted the division of endodermal cells. Together, these findings reveal a tight link between the endodermal auxin response and endodermal cell division, suggesting that auxin is a key regulator controlling the division of root endodermal cells, and that PGP1 and PGP19 are involved in regulating endodermal cell division.
The endodermal auxin response, which is regulated by centripetal auxin flow, determines division of the endodermal cells.
Introduction
The root system determines the plant’s capacity to take up water and nutrients, anchors the plant, and supports its growth and productivity (Wang et al., 2006; Rogers and Benfey, 2015). The roots of vascular plants contain three specialized tissues: the epidermis, ground tissue (comprising the endodermis and cortex), and the vascular tissue. These tissues are derived from stem cell niches at the root apical meristem (RAM; Dolan et al., 1993; Pernas et al., 2010).
The endodermis has an essential physiological role as a selective barrier that restricts the free diffusion of nutrients to the central vasculature (Geldner, 2013; Lee et al., 2013). Nutrients absorbed in the root epidermis are moved into the central xylem across the concentric root layers of the cortex and endodermis through symplastic and apoplastic transport. Endodermal cells deposit a Casparian strip in their cell walls; the Casparian strip is composed of hydrophobic lignin polymers and blocks free diffusion of the absorbed nutrients through the apoplastic space, thereby facilitating plasmodesmata-mediated symplastic transport of nutrients (Naseer et al., 2012; Doblas et al., 2017). In Arabidopsis thaliana, the root endodermis is derived from the cortex/endodermis initial cell, the progenitor of the ground tissues (Scheres et al., 1994), and SHORT-ROOT (SHR) and SCARECROW (SCR) function in the asymmetric division of cortex/endodermis initials (Benfey et al., 1993; Scheres et al., 1995; Helariutta et al., 2000; Koizumi and Gallagher, 2013). However, our knowledge of the development of the root endodermis is still limited.
The cellular auxin response is essential for plant growth and development, and the auxin signaling pathway, including activation of AUXIN-RESPONSIVE FACTORs (ARFs), which are responsible for the transcription of auxin-responsive genes, is a key step controlling the cellular auxin response. ARFs typically consist of four domains: a DNA-binding domain in the N-terminal region, an activation domain in the central region, and domains III and IV in the C-terminal region (Ulmasov et al., 1999; Krogan et al., 2012; Boer et al., 2014; Nanao et al., 2014; Zhang et al., 2014). Domains III and IV mediate the interaction with AUXIN/INDOLE-3-ACETIC ACIDs (Aux/IAAs), and removal of domains III and IV in ARF5 increases the activity of ARF5 to promote the auxin response, indicating that activation of ARFs is sufficient for promoting the auxin response.
In the absence of auxin, ARFs are inactivated through interaction with auxin signaling repressor proteins, Aux/IAAs. When auxin accumulates in response to endogenous or environmental signals, cellular auxin induces proteolysis of the Aux/IAAs by promoting the interaction between Aux/IAAs and the SCFTIR1 E3 ubiquitin ligase complex, leading to release and activation of ARFs (Gray et al., 2001; Dharmasiri et al., 2005; Weijers et al., 2005; Mockaitis and Estelle, 2008).
Development of root cells largely depends on the auxin response, which provides the positional information required for determining root cell fate. In the RAM, the auxin response is position-dependent and the quiescent center/stem cell niche, vascular xylem, and epidermis display high auxin responses. The auxin response is tightly linked to the development of the quiescent center/stem cell niche, vascular xylem, and epidermis (Friml et al., 2003; Aida et al., 2004; Swarup et al., 2005; Galinha et al., 2007; Vanneste and Friml, 2009; Bishopp et al., 2011; Fabregas et al., 2015; Liu et al., 2017). Directional auxin flows, including acropetal, basipetal, and centripetal auxin flows, are deeply involved in the cell-specific auxin response in the RAM. The spatial arrangement of auxin carriers, such as PIN-FORMEDs (PINs) and P-GLYCOPROTEINs (PGPs), mediates this process, and it has been suggested that PIN1, 2, 3, 4, and 7 are involved in the acropetal (toward the root tip) and basipetal (toward the root base) flow of auxin, and PGP1 and PGP19 are involved in the centripetal flow toward the central xylem (Zamski and Wareing, 1974; Gälweiler et al., 1998; Noh et al., 2001; Friml et al., 2002; Blilou et al., 2005; Bouchard et al., 2006; Bandyopadhyay et al., 2007; Lewis et al., 2007; Kovrizshnykh et al., 2015; Ng et al., 2020). Mutant plants lacking the activity of auxin carriers have defects in the positional auxin response and root cell development, which suggests the essential role of directional auxin flows in determining root cell fate by controlling positional auxin accumulation and responses (Luschnig et al., 1998; Marchant et al., 1999; Sabatini et al., 1999; Geldner et al., 2001; Friml et al., 2002; Marchant et al., 2002; Friml, 2003; Grieneisen et al., 2007; Benjamins and Scheres, 2008; Kleine-Vehn and Friml, 2008; Laskowski et al., 2008; Jones et al., 2009).
In this study, we show that auxin is a key regulator determining the division of endodermal cells in Arabidopsis. Endodermis-specific activation of the auxin response by expressing truncated ARF5 (ΔARF5) in root endodermal cells under the control of the endodermis-specific ENDODERMIS7 (EN7) or SCR promoters (EN7::ΔARF5 or SCR::ΔARF5) revealed a tight link between auxin and endodermal cell division, and characterization of endodermal cell division in plants treated with N-1-naphthylphthalamic acid (NPA), an auxin transport inhibitor, or the double knockout mutant of PGP1 and PGP19, which are responsible for centripetal auxin movement, supported this finding. Collectively, these findings suggest that the endodermal auxin response regulates the division of endodermal cells, and PGP1 and PGP19 mediate the auxin-dependent division of endodermal cells by controlling centripetal auxin flow via the endodermis.
Results
Endodermal cells do not divide alongside the RAM
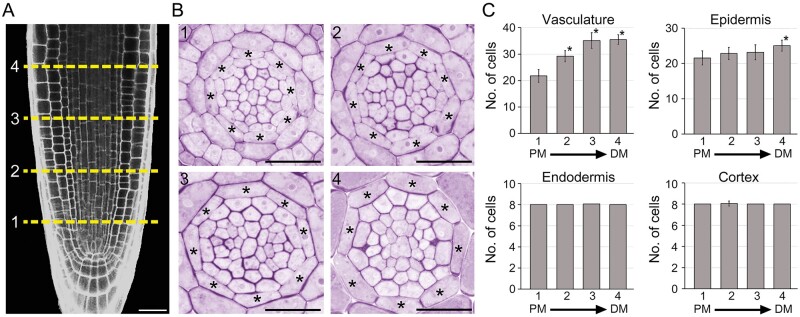
Cells in the RAM divide and produce daughter cells that give rise to differentiated cells with specialized functions. To investigate the development of the endodermis, we tracked changes in the number of vascular, epidermal, cortical, and endodermal cells alongside RAMs (n > 16; Figure 1). The number of cells in vascular tissues significantly increased with RAM growth, indicating that vascular procambial cells divide alongside the RAMs. The number of epidermal cells tended to increase with RAM growth, and the number of epidermal cells in the most distal region of the RAM was significantly increased compared with that in the proximal region. However, no change was seen in the number of ground tissue cells. The number of endodermal and cortical cells was exactly eight in the proximal regions of the RAM, and the number of endodermal cells was almost always identical to the number found at other longitudinal positions of the RAM, with no or very little variation. This indicates that ground tissue, including the endodermis, does not divide in the RAM.
Figure 1.
Development of root endodermal tissue. Longitudinal (A) and transverse sections (B) of the RAM showing development of the endodermal tissue in roots grown in 1/2 MS solid media for 7 d. The numbers 1, 2, 3, and 4 show the longitudinal positions where physical sectioning was performed and asterisks indicate endodermal cells. Scale bars = 20 μm. C, Quantification of the number of vascular, epidermal, endodermal, and cortical cells in the roots (n > 16). PM, proximal region of the RAM; DM, distal region of the RAM. Error bars represent sd. Asterisks indicate statistically significant differences between the corresponding samples and their control (PM1; P < 0.01, two-tailed t test).
Auxin regulates division of endodermal cells
To address whether development of the root endodermis is affected by phytohormones, we analyzed the number of endodermal cells in the wild-type (WT) roots supplemented with auxin, cytokinin, gibberellic acid, or brassinosteroid (Figure 2A;Supplemental Figure S1). Auxin promoted the division of endodermal cells, but the other phytohormones, including cytokinin, had no effect. Auxin-treated roots (n = 23) contained approximately nine endodermal cells, while roots treated with the other hormones (n > 18) contained eight endodermal cells. Unlike the development of endodermal cells, the development of cortical cells was not affected by these hormones. These findings suggested that auxin positively regulates endodermal cell division in roots. Examination of endodermal cell development in the auxin signaling mutant auxin resistant3-1 (axr3-1) supported the involvement of auxin in endodermal cell division (Figure 2B). The auxin response is severely compromised in the axr3-1 mutant (Leyser et al., 1996; Bishopp et al., 2011). Unlike WT plants, in the axr3-1 mutant, auxin treatment did not affect division of endodermal cells in the roots, and the number of endodermal cells was identical between auxin-treated and untreated axr3-1 plants (n > 20). This indicated that auxin regulates the division of root endodermal cells.
Figure 2.
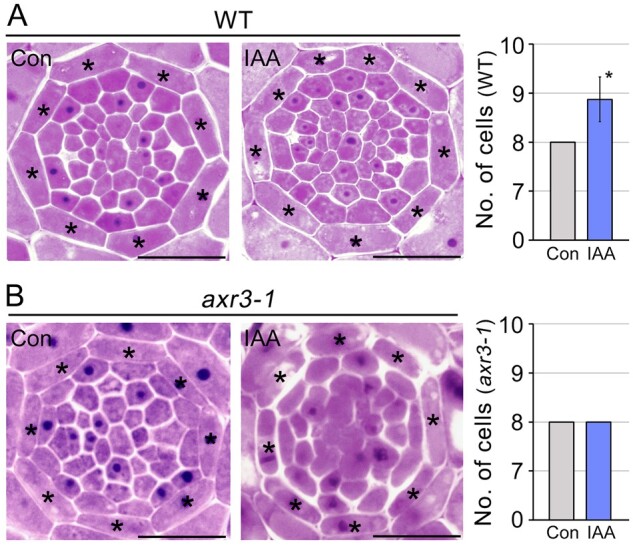
Auxin promotes division of the endodermal cells. Transverse sectioning images of WT (A) and auxin signaling mutant axr3-1 (B) roots grown in auxin-treated (IAA, 200 nM indole-3-acetic acid) and untreated (Con) conditions for 7 d, and quantification of the number of endodermal cells in the roots (n > 20). Asterisks on the images indicate endodermal cells and the asterisk in the graph indicates a significant difference from the control (P < 0.01, two-tailed t test). Error bars represent sd. Scale bars = 20 μm.
Activation of the endodermis-specific auxin response promotes endodermal cell division
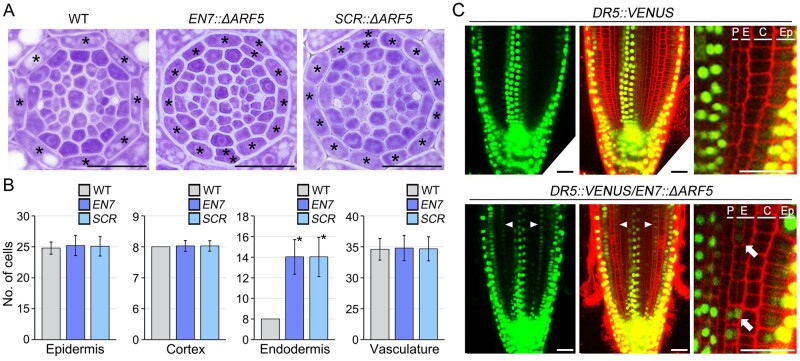
If auxin is indeed a positive regulator of endodermal cell division, then activation of the auxin response in endodermal cells may be sufficient to promote endodermal cell division. To test this, we generated transgenic plants that specifically expressed a truncated ARF5/MONOPTEROS (ΔARF5) in root endodermal cells under the control of the EN7 promoter (EN7::ΔARF5 transgenic lines; Heidstra et al., 2004; Wachsman et al., 2011; Koizumi and Gallagher, 2013). The ΔARF5 protein lacks domains III and IV, which are responsible for interacting with Aux/IAA repressors (Supplemental Figure S2), and thus possesses a greater ability than intact ARF5 to provoke an auxin response (Krogan et al., 2012; Zhang et al., 2014). We visualized the development of root endodermal tissues in the distal region of the RAM by sectioning, and noted that endodermis-specific expression of ΔARF5 strongly induced endodermal cell division: EN7::ΔARF5 plants (n = 34) produced approximately 14 root endodermal cells, a marked increase compared with the WT (Figure 3, A and B). Furthermore, this increase was caused by anticlinal and periclinal division of endodermal cells in contrast to the auxin-treated roots, where the endodermal cells only undergo anticlinal division.
Figure 3.
The endodermis-specific auxin response promotes division of endodermal cells. Transverse section images of the distal regions of RAMs of WT, EN7::ΔARF5 (EN7), and SCR::ΔARF5 (SCR) plants grown in normal MS media conditions for 7 d (A), and quantification of the number of epidermal, cortical, endodermal, and vascular cells in these plants (n > 30; B). The asterisks on the images indicate endodermal cells and asterisks on the graphs indicate statistically significant differences between the corresponding samples and their control (P < 0.01, two-tailed t test). Error bars represent sd. C, Visualization of the auxin response domain using the DR5::VENUS system in the roots of WT (DR5::VENUS, top) and EN7::ΔARF5 (DR5::VENUS/EN7::ΔARF5, bottom) plants. Green and red fluorescence correspond to VENUS and PI, respectively. White arrowheads indicate VENUS signals in endodermal cells in the roots of DR5::VENUS/EN7::ΔARF5 transgenic lines. White arrows point to VENUS signals in the endodermal cells and division of endodermal cells. P, pericycle; E, endodermis; C, cortex; Ep, epidermis. Scale bars = 20 μm.
Unlike endodermal cells, the number of cells in the epidermis, cortex, and vasculature at the distal region of the RAM was similar between EN7::ΔARF5 and WT plants. To explore this, we also analyzed the number of epidermal, cortical, endodermal, and vascular cells at the proximal and middle regions of RAMs (Supplemental Figure S3). Similar to the result at the distal region, the number of endodermal cells in EN7::ΔARF5 transgenic plants strongly increased compared to that of WT plants, whereas there was no obvious difference in the number of epidermal, cortical, and vascular cells between EN7::ΔARF5 and WT plants both at the proximal and middle regions of RAMs.
To verify this, we generated another transgenic line, SCR::ΔARF5, in which ΔARF5 was expressed under the control of the endodermis-specific promoter SCR (Malamy and Benfey, 1997; Sabatini et al., 1999; Helariutta et al., 2000). Like EN7::ΔARF5 plants, the number of endodermal cells markedly increased in SCR::ΔARF5 plants at the distal region of the RAM, while the number of cells in the epidermis, cortex, and vasculature was similar between SCR::ΔARF5 and WT plants (n > 30). The developmental pattern observed in the distal regions of RAMs was similarly observed in the proximal and middle regions (Figure 3, A and B; Supplemental Figure S3). These results indicate that an endodermal auxin response is sufficient to promote the division of endodermal cells, suggesting that auxin is a key regulator in the division of endodermal root cells.
We also visualized the auxin response in EN7::ΔARF5 roots using the DR5::VENUS reporter (Figure 3C). Consistent with previous reports (De Smet et al., 2007; Brunoud et al., 2012), in WT plants expressing DR5::VENUS, VENUS fluorescent signals were specifically observed in epidermal cells and xylem cells in the middle of the vasculature, but not in the endodermal cells. This indicated that in the endodermis, the auxin response is either absent or too weak to be detected using DR5::VENUS. However, in the EN7::ΔARF5 plants expressing DR5::VENUS (DR5::VENUS/EN7::ΔARF5), the VENUS signals were detected in endodermal cells as well as in epidermal and xylem cells. Furthermore, the visualization revealed a double layer of endodermis, indicating the periclinal division of endodermal cells. Collectively, these findings suggested that the endodermis-specific auxin response is sufficient to promote the division of endodermal cells, and auxin is a key regulator in endodermal root cell division.
Polar auxin transport regulates the development of endodermal cells
Polar auxin transport is key to the control of auxin-related root development by generating tissue-specific auxin accumulation. Since endodermal cells do not display an auxin response, but activation of the endodermis-specific auxin response strongly promotes the division of endodermal cells, we surmised that polar auxin transport may be involved in the regulation of the endodermal auxin response and endodermal cell division. To explore this, we analyzed changes in the auxin response in DR5::VENUS roots treated with NPA, an inhibitor of auxin transport, by monitoring VENUS fluorescence (Figure 4A). The DR5::VENUS roots grown in media supplemented with 1 μM NPA exhibited reduced VENUS signal intensity in xylem cells compared with untreated DR5::VENUS roots. Instead, the NPA treatment appeared to induce the accumulation of VENUS signal in the endodermis. This suggested that auxin accumulation in xylem cells is mediated by centripetal auxin movement from the endodermis to the vasculature. The effect of NPA was dosage-dependent, and a 5 μM NPA treatment induced dramatic changes in the auxin response domains in roots. In response to 5 μM NPA, xylem-specific VENUS signals completely disappeared while endodermal cells predominantly exhibited VENUS signals. This indicated that the induction of the endodermis-specific auxin response in the NPA-treated roots is tightly associated with the reduction of the xylem-specific auxin response, and suggested that the endodermis plays a key role in centripetal auxin movement to the vasculature. These findings were also supported by examination of the expression of ROOT HAIR DEFECTIVE SIX-LIKE4 (RSL4) and IAA2 (Figure 4B). RSL4 and IAA2 are specifically expressed in the epidermis and xylem, and their expression is positively regulated by auxin (Rusak et al., 2010; Yi et al., 2010; Bishopp et al., 2011; Vijayakumar et al., 2016). Reverse transcription quantitative PCR (RT-qPCR) assays showed that NPA reduced the expression of IAA2, but did not affect the expression of RSL4. In addition, the effect of NPA on IAA2 expression was more evident at high concentrations than at low concentrations.
Figure 4.
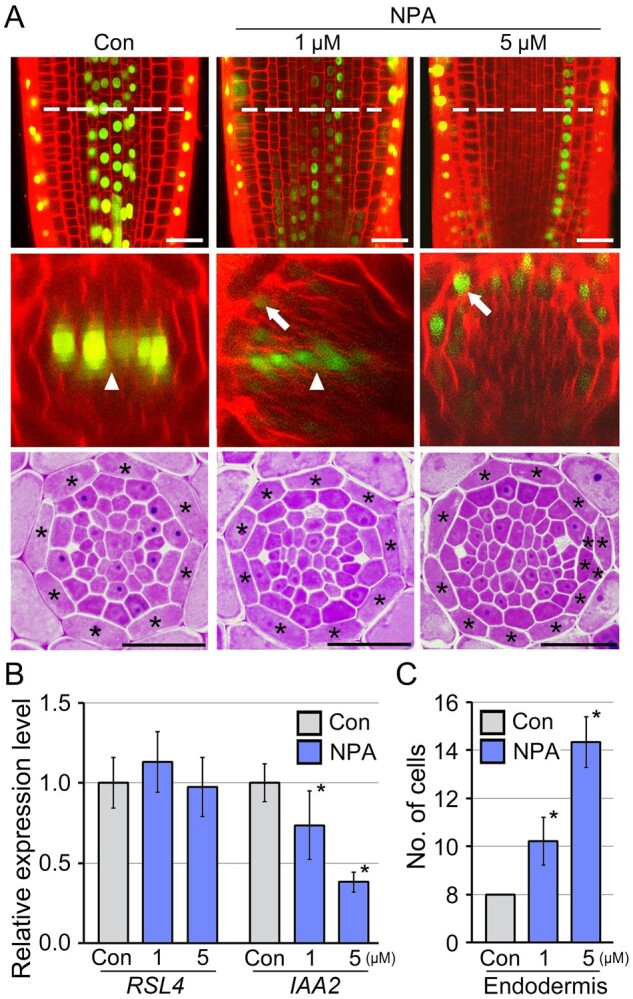
Polar auxin transport regulates division of endodermal cells. A, Fluorescence (longitudinal-optical sections, top; cross-optical sections, middle; and physical sections, bottom) images of DR5::VENUS roots grown with and without NPA (1 and 5 μM NPA) for 7 d. Dotted lines indicate the longitudinal position of confocal optical cross-sectioning. Arrowheads and arrows indicate fluorescent signals in the xylem and endodermis, respectively. Expression levels of RSL4 and IAA2 by RT-qPCR (B), and quantification of the number of endodermal cells in the NPA-treated samples (n > 20; C). Error bars represent sd. Asterisks on the graphs indicate statistically significant differences between the corresponding samples and their control (P < 0.01, two-tailed t test). Scale bars = 20 μm.
To understand whether endodermis-specific auxin accumulation affects the division of endodermal cells, we physically sectioned and visualized the morphology of the endodermis in the NPA-treated roots (Figure 4, A and C). As expected, NPA increased the number of endodermal cells, and the increase was dependent on the concentration of NPA. In response to 1 and 5 μM NPA, roots developed ∼10 and 14 endodermal cells, respectively (n > 20). In addition, the increase of endodermal cells in 5 μM NPA-treated roots was caused not only by the anticlinal division but also by the periclinal division of endodermal cells, as shown in EN7::ΔARF5 and SCR::ΔARF5 transgenic plants, and the periclinal division was also observed through confocal microscope-based optical sectioning (Supplemental Figure S4). These data indicate that the endodermal auxin response is tightly linked to endodermal cell division, supporting the idea that the endodermal auxin response, which is determined by polar auxin transport, controls the division of endodermal cells.
PGP1 and PGP19 regulate the development of endodermal cells
To further explore the above findings, we attempted to identify genetic components that regulate endodermal auxin movement to the vasculature, and predicted that some auxin carriers expressed in the endodermis might mediate this process. To address this, we analyzed spatial expression patterns of the PIN auxin efflux carriers by visualizing green fluorescent protein (GFP) signals in PIN1::PIN1-GFP, PIN3::PIN3-GFP, PIN4::PIN4-GFP, and PIN7::PIN7-GFP transgenic plants (Supplemental Figure S5A). GFP fluorescence corresponding to PIN1, 3, 4, and 7 revealed that these PINs were not expressed in root endodermal cells. PIN3 and PIN7 are responsible for the centripetal auxin flow from procambial cells to xylem cells in vascular tissues (Bishopp et al., 2011; Miyashima et al., 2013), and we found that the pin3 pin7 double mutant did not have an increased number of endodermal cells (Supplemental Figure S5, B and C). These results suggested that these PINs, including PIN3 and PIN7, might not be involved in centripetal auxin movement from the endodermis to the vasculature.
Previous studies suggested that PGP19 is involved in the centripetal auxin flow from the epidermis to the vasculature, and PGP1 and PGP19 are functionally redundant in regulating auxin transport (Noh et al., 2001; Bouchard et al., 2006; Lewis et al., 2007). This suggested that PGP1 and PGP19 are involved in endodermal auxin movement. To address this, we analyzed the spatial expression patterns of PGP1 and PGP19 in the roots by monitoring GFP fluorescence signals in PGP1::PGP1-GFP and PGP19::PGP19-GFP transgenic plants (Figure 5A). In PGP1::PGP1-GFP and PGP19::PGP19-GFP plants, GFP signals corresponding to PGP1 and PGP19 were observed in root endodermal cells, and PGP19 was more specific to the endodermal cells than PGP1. These findings suggested that PGP1 and PGP19 are involved in endodermal cell development by controlling auxin movement from the endodermis to the vasculature.
Figure 5.
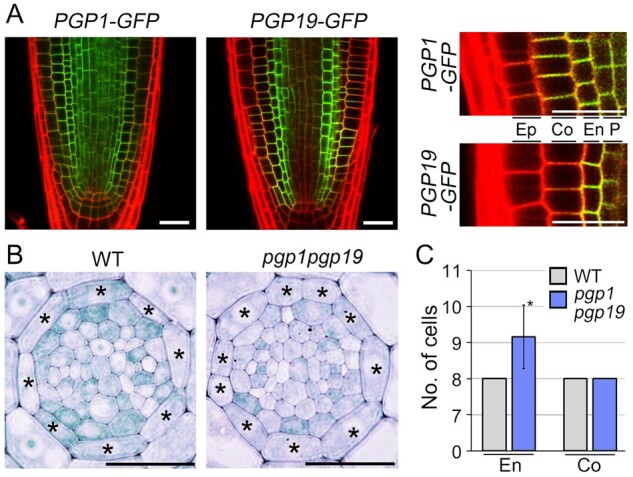
PGP1 and PGP19 regulate the division of endodermal cells. A, Visualization of fluorescent signals in PGP1::PGP1-GFP (PGP1-GFP) and PGP19::PGP19-GFP (PGP19-GFP) plants, and their high resolution images (right). Ep, epidermis; Co, cortex; En, endodermis; P, pericycle. B, Transverse sections of WT and pgp1pgp19 double mutant roots grown in 1/2 MS solid media for 7 d. Asterisks on the images indicate endodermal cells. C, Quantification of the number of endodermal cells in these plants (n > 22). En and Co indicate the endodermis and cortex, respectively. Error bars represent sd, and the asterisk indicates a statistically significant difference from the control (P < 0.01, two-tailed t test). Scale bars = 20 μm.
To explore this, we analyzed the development of endodermal cells in pgp1 and pgp19 single mutants by quantifying the number of endodermal cells, but there was no significant difference between the WT and the single mutants (n > 22; Supplemental Figure S6). However, the pgp1pgp19 double mutant had an increased number of endodermal cells, approximately nine (n = 26; Figure 5, B and C). In the double mutant, the number of cortical cells was identical to that in WT plants. When we analyzed the endodermal auxin response in WT and pgp1pgp19 plants using SCR::DII-VENUS, we found that endodermal DII-VENUS signals in the pgp1pgp19 mutant were weaker than those in WT plants (Supplemental Figure S7). Because DII-VENUS proteins are degraded in response to auxin (Brunoud et al., 2012), this finding suggested that the endodermal auxin level in the pgp1pgp19 mutant is higher than that in WT plants, explaining why the division of endodermal cells is activated in pgp1pgp19 mutant plants. These findings propose that PGP1 and PGP19 regulate the division of endodermal cells by controlling endodermal auxin movement, and these findings were partially supported by the result that the number of endodermal cells was similar among EN7::ΔARF5, SCR::ΔARF5, pgp1pgp19 and WT roots grown in MS media supplemented with NPA (n > 15; Supplemental Figure S8).
Lateral root formation in EN7::ΔARF5, SCR::ΔARF5, and pgp1pgp19 plants
To understand the relationship between endodermal cell division and root growth, we investigated root growth in EN7::ΔARF5, SCR::ΔARF5, and pgp1pgp19 plants (Figure 6). When root length was quantified and compared, there was no obvious difference between the WT and these plants. However, lateral root formation in EN7::ΔARF5, SCR::ΔARF5, and pgp1pgp19 plants differed from that in WT plants. EN7::ΔARF5, SCR::ΔARF5, and pgp1pgp19 plants formed more lateral roots compared to WT plants grown in the same conditions for 10 d (n > 30). Unlike the WT plants that formed approximately one lateral root, EN7::ΔARF5 and SCR::ΔARF5 transgenic plants developed approximately five lateral roots, and pgp1pgp19 mutant plants formed approximately two lateral roots. Comparing the number of lateral roots with the number of endodermal cells formed in EN7::ΔARF5, SCR::ΔARF5, and pgp1pgp19 plants suggested a close relationship between endodermal cell division and lateral root formation.
Figure 6.
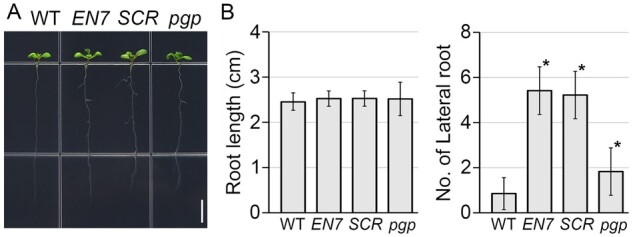
Lateral root formation in EN7::ΔARF5, SCR::ΔARF5, and pgp1pgp19 plants. A, Root development of WT, EN7::ΔARF5 (EN7), SCR::ΔARF5 (SCR), and pgp1pgp19 (pgp) plants grown in 1/2 MS solid media for 10 d. Scale bar = 0.5 cm. B, Quantification of root length and the number of lateral roots in these plants (n > 30). Error bars represent sd and asterisks indicate statistically significant differences between the corresponding samples and their control (P < 0.01, two-tailed t test).
Discussion
The endodermis is derived from the cortex/endodermis initial cell, the progenitor of the ground tissues (Scheres et al., 1994). The endodermis with its Casparian strip has an essential physiological role as a selective barrier that restricts the free diffusion of solutes to vascular tissues (Geldner, 2013; Lee et al., 2013). In this study, we revealed that auxin is a key to regulating the division of root endodermal cells. We found that endodermal cells of WT plants do not divide in the RAM and that exogenous auxin induces endodermal cell division. Furthermore, expression of ΔARF5 under the control of the EN7 or SCR promoters activated the auxin response in endodermal cells, and promoted their division. Together with the result that endodermal cells of the auxin signaling mutant axr3-1 did not divide in response to auxin, these findings indicate that auxin is a key regulator of endodermal cell division.
In this study, our observations suggested that activation of endodermal-specific auxin responses by ΔARF5 expression or NPA treatment induces anticlinal and periclinal division of endodermal cells, and these periclinal divisions result in the formation of extra endodermal cells ectopically positioned outside of the endodermal cell file. The periclinal division of endodermal cells is partially supported by the examination of SHR::SHR-GFP and SCR::ΔARF5-GFP transgenic plants (Supplemental Figure S9). Unlike SHR::SHR-GFP plants grown in normal growth conditions, in which SHR-GFP fluorescent signals were detected in a single layer of the endodermis, NPA treatment induced a double endodermal layer phenotype, and the cells in the layers exhibited SHR-GFP fluorescent signals (Supplemental Figure S9A). In addition, SCR::ΔARF5-GFP plants showed the periclinal division of endodermal cells, and the cells displayed GFP signals (Supplemental Figure S9B). These findings support that the ectopic cells formed in EN7::ΔARF5, SCR::ΔARF5, and NPA-treated WT plants originate from periclinal division of endodermal cells.
The position-dependent auxin response plays an essential role in providing the cell or tissue-specific developmental information required for cell division and differentiation. Polar auxin transport is largely responsible for the position-dependent auxin response by controlling directional auxin flows, and the spatial expression and arrangement of auxin carriers mediate this process (Friml, 2003). Many studies suggest that auxin is transported acropetally toward the root tip through the central vasculature, and basipetally toward the root base through the epidermis; centripetal transport from the epidermis to the central vasculature links the acropetal and basipetal flow and establishes an auxin reflux loop in the RAM (Rashotte et al., 2000; Blilou et al., 2005; Kepinski and Leyser, 2005; Lewis et al., 2007). In this study, we found that division of endodermal cells is regulated by centripetal auxin flow. NPA treatment demonstrated the existence of the centripetal auxin flow by showing that the auxin response in the central xylem decreased in response to NPA, and the decrease was dependent on the NPA concentration. In contrast to the vascular auxin response, NPA promoted an endodermal auxin response, which was not observed in untreated control plants, and promoted division of endodermal cells. These results indicate a tight link between the endodermal auxin response and cell division, and that the endodermal auxin response is regulated by centripetal auxin movement via the endodermis. In addition, endodermal cell division was promoted in the double knockout mutant of PGP1 and PGP19, which are involved in centripetal auxin movement (Noh et al., 2001; Bouchard et al., 2006; Lewis et al., 2007), and the enodermal DII-VENUS signals in pgp1pgp19 mutant plants were weaker than those in WT plants. Collectively, these findings suggest that PGP1 and PGP19 regulate the division of endodermal cells by controlling centripetal auxin flow via the endodermis. However, it is likely that other auxin carriers besides PGP1 and PGP19 might be involved in endodermal auxin flow to the vasculature, because the pgp1pgp19 double mutant roots had fewer endodermal cells than the NPA-treated WT roots, or the EN7::ΔARF5 and SCR::ΔARF5 roots. Auxin efflux carriers regulate auxin movement in a coordinated manner (Noh et al., 2003; Blakeslee et al., 2007; Mravec et al., 2008; Petricka et al., 2012). Therefore, this finding suggests that PGP1 and PGP19 coordinate with other auxin efflux carriers to modulate endodermal auxin movement, and the result that single mutation of PGP1 or PGP19 did not affect endodermal cell division supports this idea.
The relationship between endodermal cell division and root growth has remained largely elusive. Previous studies show that endodermal auxin response is essential for the formation of lateral roots. Swarup et al. (2008) showed that an IAA3 loss-of-function mutant short hypocotyl2-24 (shy2-24) had an enhanced endodermal auxin response, and more lateral roots, whereas an IAA3 gain-of-function mutant short hypocotyl2-2 (shy2-2) had a reduced endodermal auxin response and fewer lateral roots. This indicates that the endodermal auxin response plays an essential role in lateral root formation (Swarup et al., 2008). The essential role of endodermal auxin was further demonstrated by Vermeer et al. (2014), who showed that endodermal-specific expression of shy2-2 under the control of the CASPARIAN STRIP MEMBRANE DOMAIN PROTEIN 1 promoter strongly inhibited lateral root formation (Vermeer et al., 2014). In addition, they showed that the endodermal auxin response mediates developmental changes of endodermal cells, such as endodermal shrinkage, resulting in the release of the mechanical constraints that suppress spatial expansion of lateral root primordia through the endodermis. This suggests that development of endodermal cells is deeply involved in lateral root formation. In this study, we showed that EN7::ΔARF5, SCR::ΔARF5, and pgp1pgp19 plants, which show increased endodermal auxin responses, formed more lateral roots than WT plants, supporting the previous results that the endodermal auxin response is essential for lateral root formation. When we compared the number of lateral roots with the number of endodermal cells formed in EN7::ΔARF5, SCR::ΔARF5, and pgp1pgp19 plants, we found a relationship between endodermal cell division and lateral root formation. This suggests that endodermal cell division might be involved in lateral root formation. However, our results are not sufficient to confirm the effect of the endodermal auxin response and endodermal cell division on lateral root formation, and we cannot exclude the possibility of organ autonomous effects of ΔARF5 expression driven by the EN7 or SCR promoters on lateral root formation. Therefore, further molecular and genetic approaches will expand our understanding of the mechanisms underlying this process.
Materials and methods
Plant materials, growth conditions, and treatments
The Arabidopsis thaliana PGP1::PGP1-GFP, PGP19::PGP19-GFP, PIN1::PIN1-GFP, PIN3::PIN3-GFP, PIN4::PIN4-GFP, PIN7::PIN7-GFP, DR5::VENUS, pgp1pgp19, pin3pin7, and SHR::SHR-GFP/shr-2 lines have been described previously (Nakajima et al., 2001; Noh et al., 2001; Friml et al., 2003; Noh et al., 2003; Blilou et al., 2005; Vieten et al., 2005; Mravec et al., 2008; Bishopp et al., 2011; Brunoud et al., 2012). Seeds were obtained from the Nottingham Arabidopsis Stock Centre or by kind donation from Dr Spalding, Dr Friml, and Dr Helariutta. Seeds were surface-sterilized and plated on half-strength Murashige and Skoog (1/2 MS) solid media. After 3 d of vernalization at 4°C in darkness, plants were grown vertically with a light regime of 16/8 h (light/dark) at 23°C. For the hormone treatments, plants were grown in 1/2 MS media supplemented with 200 nM indole-3-acetic acid, benzylaminopurine, gibberellic acid, or epibrassinolide.
Plasmid construction and transformation
To generate the EN7::ΔARF5 and SCR::ΔARF5 constructs, DNA fragments containing the SCR (2,164 bp) or EN7 (1,226 bp) promoters (Malamy and Benfey, 1997; Heidstra et al., 2004; Koizumi and Gallagher, 2013) were inserted into a HindIII/KpnI-digested pMDC plant binary vector using the Gibson Assembly Cloning system (New England BioLabs). The cDNA fragment of ΔARF5 was then introduced into the vector using the GATEWAY system (Invitrogen). To generate the SCR::ΔARF5-GFP construct, DNA fragments containing the SCR promoter, ΔARF5, and GFP sequences were amplified by PCR, and then introduced into P4-P1, P1-P2, and P2-P3 entry vectors by BP reaction, respectively. These DNA fragments were inserted into the dpGreen plant binary vector by MULTISITE GATEWAY system (Invitrogen).
Embedding, sectioning, and staining
Technovit embedding and sectioning was performed as described with slight modifications (Jang et al., 2011). Arabidopsis roots were fixed in 4% paraformaldehyde solution (w/v) for 1.5 h and then washed in double-distilled H2O (ddH2O) four times for 1 h each time. The fixed samples were dehydrated in an ethanol series (25%, 50%, 75%, and 100% [v/v] in ddH2O). The dehydrated samples were sequentially incubated in a series of Technovit 7100 cold-polymerizing resin solutions (25%, 50%, 75%, and 100% [v/v] in ethyl alcohol) for 6 h each. Samples were further incubated in 100% Technovit resin for 1 d, and solidified with a 15:1 (v/v) mixture of Technovit and hardener solution II at room temperature in a mold for 1 d. Sections (3–4 μm) were taken from solidified samples with an RM 2145 microtome (Leica). Dehydrated sections were stained with 0.05% toluidine blue solution (w/v, pH 4.5).
Microscopy
To visualize fluorescent signals in the PGP1::PGP1-GFP, PGP19::PGP19-GFP, PIN3::PIN3-GFP, PIN7::PIN7-GFP, and DR5::VENUS lines, whole roots of the indicated plants grown in 1/2 MS media for 7 d were dipped in propidium iodide (PI) solution (10 µg/mL) for 3 min. After staining, the roots were mounted on glass slides in ddH2O. PI fluorescence was excited with 561 nm light, and GFP and VENUS fluorescence were excited with 488 nm light. Fluorescence was visualized at wavelengths of 591–635 nm for PI, and wavelengths of 505–530 nm for GFP and VENUS, using LSM700 laser scanning confocal microscope (Zeiss). The laser intensity was ∼70%, and the gain was below 800. Photographs of physical sections were taken with an Olympus BX41 light microscope.
RT-qPCR analysis
RT-qPCR analyses were performed using total RNA extracted from the indicated roots. Total RNA was extracted using the RNeasy plant mini-prep kit (Qiagen) according to the manufacturer’s instructions. To synthesize the first-strand cDNA, reverse transcription was carried out using 1 µg of total RNA and Superscript III reverse transcriptase (Invitrogen). For qPCR, a master mix was prepared using a LightCycler 480 SYBR GREEN I Master (Roche). PCR reactions and fluorescence detection were performed using a LightCycler NANO Real-Time PCR machine (Roche). PCR conditions were programmed according to the manufacturer’s instructions (initial denaturation at 95°C for 5 min, denaturation at 95°C for 10 s, annealing at 60°C for 10 s, and extension at 72°C for 10 s for 45 cycles). Three technical replicates of the RT-qPCR were performed using two biological replicates. ACTIN2 was used as an internal control. Primer sequence information is available in Supplemental Table S1.
Statistical analyses
Data are presented as mean values, and the number of samples is indicated in the figure legends. The statistical difference between the samples and their control was determined using two-tailed Student’s t test with a P < 0.01.
Accession numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: PGP1 (At2G36910), PGP19 (At3G28860), PIN1 (At1G73590), PIN3 (At1G70940), PIN4 (At2G01420), PIN7 (At1G23080), EN7 (At4G28100), SCR (At3G54220), ARF5 (At1G19850), RSL4 (At1G27740), IAA2 (At3G23030), and ACT2 (At3G18780).
Supplemental materials
The following materials are available in the online version of this article.
Supplemental Figure S1 . Regulation of endodermal cell division by auxin.
Supplemental Figure S2 . Amino acid sequence of ΔARF5.
Supplemental Figure S3 . Division of endodermal cells by endodermal expression of ΔARF5.
Supplemental Figure S4 . NPA induces the endodermal auxin responses and endodermal cell division.
Supplemental Figure S5 . Spatial expression pattern of PINs in roots.
Supplemental Figure S6 . Endodermal cell development in pgp1 and pgp19 single mutant roots.
Supplemental Figure S7 . Endodermal auxin response in the pgp1pgp19 mutant.
Supplemental Figure S8 . Development of endodermal cells in NPA-treated EN7::ΔARF5, SCR::ΔARF5, and pgp1pgp19 plants.
Supplemental Figure S9 . Periclinal division of endodermal cells.
Supplemental Table S1. Primers used in this study.
Supplementary Material
Acknowledgments
We deeply thank Dr Spalding at University of Wisconsin, Dr Friml at Institute of Science and Technology, and Dr Helariutta at University of Cambridge for donating mutant and transgenic plant seeds.
Funding
This work was carried out with the support of the BioGreen21 Agri-Tech Innovation Program (Project No. PJ01567301), Rural Development Administration, Republic of Korea, and the National Research Foundation of Korea Grant funded by the Korean Government (MOE) [NRF-2019R1A2C1007103 to G.J.].
Conflict of interest statement. The authors declare no conflict of interest.
Senior author.
G.J. conceived the original screening and research plans. D.S., H.J., and G.J. performed the experiments and analyzed the data. G.J. and Y.C. wrote the article with contributions of all the authors. G.J. agrees to serve as the author responsible for contact and ensures communication.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plphys/pages/general-instructions) is Geupil Jang (yk3@jnu.ac.kr).
References
- Aida M, Beis D, Heidstra R, Willemsen V, Blilou I, Galinha C, Nussaume L, Noh Y-S, Amasino R, Scheres B (2004) The PLETHORA genes mediate patterning of the Arabidopsis root stem cell niche. Cell 119: 109–120 [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay A, Blakeslee J, Lee OR, Mravec J, Sauer M, Titapiwatanakun B, Makam SN, Bouchard R, Geisler M, Martinoia E (2007) Interactions of PIN and PGP auxin transport mechanisms. Biochem Soc Trans 35: 137–141 [DOI] [PubMed] [Google Scholar]
- Benfey PN, Linstead PJ, Roberts K, Schiefelbein JW, Hauser M-T, Aeschbacher RA (1993) Root development in Arabidopsis: four mutants with dramatically altered root morphogenesis. Development 119: 57–70 [DOI] [PubMed] [Google Scholar]
- Benjamins R, Scheres B (2008) Auxin: the looping star in plant development. Annu Rev Plant Biol 59: 443–465 [DOI] [PubMed] [Google Scholar]
- Bishopp A, Help H, El-Showk S, Weijers D, Scheres B, Friml J, Benková E, Mähönen AP, Helariutta Y (2011) A mutually inhibitory interaction between auxin and cytokinin specifies vascular pattern in roots. Curr Biol 21: 917–926 [DOI] [PubMed] [Google Scholar]
- Blakeslee JJ, Bandyopadhyay A, Lee OR, Mravec J, Titapiwatanakun B, Sauer M, Makam SN, Cheng Y, Bouchard R, Adamec J (2007) Interactions among PIN-FORMED and P-glycoprotein auxin transporters in Arabidopsis. Plant Cell 19: 131–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, Friml J, Heidstra R, Aida M, Palme K, Scheres B (2005) The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 433: 39–44 [DOI] [PubMed] [Google Scholar]
- Boer DR, Freire-Rios A, van den Berg WA, Saaki T, Manfield IW, Kepinski S, López-Vidrieo I, Franco-Zorrilla JM, de Vries SC, Solano R (2014) Structural basis for DNA binding specificity by the auxin-dependent ARF transcription factors. Cell 156: 577–589 [DOI] [PubMed] [Google Scholar]
- Bouchard R, Bailly A, Blakeslee JJ, Oehring SC, Vincenzetti V, Lee OR, Paponov I, Palme K, Mancuso S, Murphy AS (2006) Immunophilin-like TWISTED DWARF1 modulates auxin efflux activities of Arabidopsis P-glycoproteins. J Biol Chem 281: 30603–30612 [DOI] [PubMed] [Google Scholar]
- Brunoud G, Wells DM, Oliva M, Larrieu A, Mirabet V, Burrow AH, Beeckman T, Kepinski S, Traas J, Bennett MJ (2012) A novel sensor to map auxin response and distribution at high spatio-temporal resolution. Nature 482: 103–106 [DOI] [PubMed] [Google Scholar]
- De Smet I, Tetsumura T, De Rybel B, dit Frey NF, Laplaze L, Casimiro I, Swarup R, Naudts M, Vanneste S, Audenaert D (2007) Auxin-dependent regulation of lateral root positioning in the basal meristem of Arabidopsis. Development 134: 681–690 [DOI] [PubMed] [Google Scholar]
- Dharmasiri N, Dharmasiri S, Estelle M (2005) The F-box protein TIR1 is an auxin receptor. Nature 435: 441–445 [DOI] [PubMed] [Google Scholar]
- Doblas VG, Geldner N, Barberon M (2017) The endodermis, a tightly controlled barrier for nutrients. Curr Opin Plant Biol 39: 136–143 [DOI] [PubMed] [Google Scholar]
- Dolan L, Janmaat K, Willemsen V, Linstead P, Poethig S, Roberts K, Scheres B (1993) Cellular organisation of the Arabidopsis thaliana root. Development 119: 71–84 [DOI] [PubMed] [Google Scholar]
- Fabregas N, Formosa-Jordan P, Confraria A, Siligato R, Alonso JM, Swarup R, Bennett MJ, Mähönen AP, Cano-Delgado AI, Ibanes M (2015) Auxin influx carriers control vascular patterning and xylem differentiation in Arabidopsis thaliana. PLoS Genet 11: e1005183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friml J (2003) Auxin transport—shaping the plant. Curr Opin Plant Biol 6: 7–12 [DOI] [PubMed] [Google Scholar]
- Friml J, Benková E, Blilou I, Wisniewska J, Hamann T, Ljung K, Woody S, Sandberg G, Scheres B, Jürgens G (2002) AtPIN4 mediates sink-driven auxin gradients and root patterning in Arabidopsis. Cell 108: 661–673 [DOI] [PubMed] [Google Scholar]
- Friml J, Vieten A, Sauer M, Weijers D, Schwarz H, Hamann T, Offringa R, Jürgens G (2003) Efflux-dependent auxin gradients establish the apical–basal axis of Arabidopsis. Nature 426: 147–153 [DOI] [PubMed] [Google Scholar]
- Gälweiler L, Guan C, Müller A, Wisman E, Mendgen K, Yephremov A, Palme K (1998) Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 282: 2226–2230 [DOI] [PubMed] [Google Scholar]
- Galinha C, Hofhuis H, Luijten M, Willemsen V, Blilou I, Heidstra R, Scheres B (2007) PLETHORA proteins as dose-dependent master regulators of Arabidopsis root development. Nature 449: 1053–1057 [DOI] [PubMed] [Google Scholar]
- Geldner N (2013) The endodermis. Annu Rev Plant Biol 64: 531–558 [DOI] [PubMed] [Google Scholar]
- Geldner N, Friml J, Stierhof Y-D, Jürgens G, Palme K (2001) Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature 413: 425–428 [DOI] [PubMed] [Google Scholar]
- Gray WM, Kepinski S, Rouse D, Leyser O, Estelle M (2001) Auxin regulates SCFTIR1-dependent degradation of AUX/IAA proteins. Nature 414: 271–276 [DOI] [PubMed] [Google Scholar]
- Grieneisen VA, Xu J, Marée AF, Hogeweg P, Scheres B (2007) Auxin transport is sufficient to generate a maximum and gradient guiding root growth. Nature 449: 1008–1013 [DOI] [PubMed] [Google Scholar]
- Heidstra R, Welch D, Scheres B (2004) Mosaic analyses using marked activation and deletion clones dissect Arabidopsis SCARECROW action in asymmetric cell division. Genes Dev 18: 1964–1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helariutta Y, Fukaki H, Wysocka-Diller J, Nakajima K, Jung J, Sena G, Hauser M-T, Benfey PN (2000) The SHORT-ROOT gene controls radial patterning of the Arabidopsis root through radial signaling. Cell 101: 555–567 [DOI] [PubMed] [Google Scholar]
- Jang G, Yi K, Pires ND, Menand B, Dolan L (2011) RSL genes are sufficient for rhizoid system development in early diverging land plants. Development 138: 2273–2281 [DOI] [PubMed] [Google Scholar]
- Jones AR, Kramer EM, Knox K, Swarup R, Bennett MJ, Lazarus CM, Leyser HO, Grierson CS (2009) Auxin transport through non-hair cells sustains root-hair development. Nat Cell Biol 11: 78–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepinski S, Leyser O (2005) Plant development: auxin in loops. Curr Biol 15: R208–R210 [DOI] [PubMed] [Google Scholar]
- Kleine-Vehn J, Friml J (2008) Polar targeting and endocytic recycling in auxin-dependent plant development. Annu Rev Cell Dev Biol 24: 447–473 [DOI] [PubMed] [Google Scholar]
- Koizumi K, Gallagher KL (2013) Identification of SHRUBBY, a SHORT-ROOT and SCARECROW interacting protein that controls root growth and radial patterning. Development 140: 1292–1300 [DOI] [PubMed] [Google Scholar]
- Kovrizshnykh V, Omelyanchuk N, Pasternak T, Mironova V (2015) The key role of PIN proteins in auxin transport in Arabidopsis thaliana Roots. Russ J Genet Appl Res 5: 279–285 [Google Scholar]
- Krogan NT, Ckurshumova W, Marcos D, Caragea AE, Berleth T (2012) Deletion of MP/ARF5 domains III and IV reveals a requirement for Aux/IAA regulation in Arabidopsis leaf vascular patterning. New Phytol 194: 391–401 [DOI] [PubMed] [Google Scholar]
- Laskowski M, Grieneisen VA, Hofhuis H, Colette A, Hogeweg P, Marée AF, Scheres B (2008) Root system architecture from coupling cell shape to auxin transport. PLoS Biol 6: e307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Rubio MC, Alassimone J, Geldner N (2013) A mechanism for localized lignin deposition in the endodermis. Cell 153: 402–412 [DOI] [PubMed] [Google Scholar]
- Lewis DR, Miller ND, Splitt BL, Wu G, Spalding EP (2007) Separating the roles of acropetal and basipetal auxin transport on gravitropism with mutations in two Arabidopsis multidrug resistance-like ABC transporter genes. Plant Cell 19: 1838–1850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyser HO, Pickett FB, Dharmasiri S, Estelle M (1996) Mutations in the AXR3 gene of Arabidopsis result in altered auxin response including ectopic expression from the SAUR‐AC1 promoter. Plant J 10: 403–413 [DOI] [PubMed] [Google Scholar]
- Liu Y, Xu M, Liang N, Zheng Y, Yu Q, Wu S (2017) Symplastic communication spatially directs local auxin biosynthesis to maintain root stem cell niche in Arabidopsis. Proc Natl Acad Sci 114: 4005–4010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luschnig C, Gaxiola RA, Grisafi P, Fink GR (1998) EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev 12: 2175–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy JE, Benfey PN (1997) Analysis of SCARECROW expression using a rapid system for assessing transgene expression in Arabidopsis roots. Plant J 12: 957–963 [DOI] [PubMed] [Google Scholar]
- Marchant A, Bhalerao R, Casimiro I, Eklöf J, Casero PJ, Bennett M, Sandberg G (2002) AUX1 promotes lateral root formation by facilitating indole-3-acetic acid distribution between sink and source tissues in the Arabidopsis seedling. Plant Cell 14: 589–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant A, Kargul J, May ST, Muller P, Delbarre A, Perrot‐Rechenmann C, Bennett MJ (1999) AUX1 regulates root gravitropism in Arabidopsis by facilitating auxin uptake within root apical tissues. EMBO J 18: 2066–2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashima S, Sebastian J, Lee JY, Helariutta Y (2013) Stem cell function during plant vascular development. EMBO J 32: 178–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mockaitis K, Estelle M (2008) Auxin receptors and plant development: a new signaling paradigm. Annu Rev Cell Dev Biol 24: 55–80 [DOI] [PubMed] [Google Scholar]
- Mravec J, Kubeš M, Bielach A, Gaykova V, Petrášek J, Skůpa P, Chand S, Benková E, Zažímalová E, Friml J (2008) Interaction of PIN and PGP transport mechanisms in auxin distribution-dependent development. Development 135: 3345–3354 [DOI] [PubMed] [Google Scholar]
- Nakajima K, Sena G, Nawy T, Benfey PN (2001) Intercellular movement of the putative transcription factor SHR in root patterning. Nature 413: 307–311 [DOI] [PubMed] [Google Scholar]
- Nanao MH, Vinos-Poyo T, Brunoud G, Thévenon E, Mazzoleni M, Mast D, Lainé S, Wang S, Hagen G, Li H (2014) Structural basis for oligomerization of auxin transcriptional regulators. Nat Commun 5: 1–8 [DOI] [PubMed] [Google Scholar]
- Naseer S, Lee Y, Lapierre C, Franke R, Nawrath C, Geldner N (2012) Casparian strip diffusion barrier in Arabidopsis is made of a lignin polymer without suberin. Proc Natl Acad Sci 109: 10101–10106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng JL, Welvaert A, Wen J, Chen R, Mathesius U (2020) The Medicago truncatula PIN2 auxin transporter mediates basipetal auxin transport but is not necessary for nodulation. J Exp Bot 71: 1562–1573 [DOI] [PubMed] [Google Scholar]
- Noh B, Bandyopadhyay A, Peer WA, Spalding EP, Murphy AS (2003) Enhanced gravi-and phototropism in plant mdr mutants mislocalizing the auxin efflux protein PIN1. Nature 423: 999–1002 [DOI] [PubMed] [Google Scholar]
- Noh B, Murphy AS, Spalding EP (2001) Multidrug resistance–like genes of Arabidopsis required for auxin transport and auxin-mediated development. Plant Cell 13: 2441–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernas M, Ryan E, Dolan L (2010) SCHIZORIZA controls tissue system complexity in plants. Curr Biol 20: 818–823 [DOI] [PubMed] [Google Scholar]
- Petricka JJ, Winter CM, Benfey PN (2012) Control of Arabidopsis root development. Annu Rev Plant Biol 63: 563–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashotte AM, Brady SR, Reed RC, Ante SJ, Muday GK (2000) Basipetal auxin transport is required for gravitropism in roots of Arabidopsis. Plant Physiol 122: 481–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers ED, Benfey PN (2015) Regulation of plant root system architecture: implications for crop advancement. Curr Opin Biotechnol 32: 93–98 [DOI] [PubMed] [Google Scholar]
- Rusak G, Cerni S, Polancec DS, Ludwig-Müller J (2010) The responsiveness of the IAA2 promoter to IAA and IBA is differentially affected in Arabidopsis roots and shoots by flavonoids. Biol Plant 54: 403–414 [Google Scholar]
- Sabatini S, Beis D, Wolkenfelt H, Murfett J, Guilfoyle T, Malamy J, Benfey P, Leyser O, Bechtold N, Weisbeek P (1999) An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell 99: 463–472 [DOI] [PubMed] [Google Scholar]
- Scheres B, Di Laurenzio L, Willemsen V, Hauser M-T, Janmaat K, Weisbeek P, Benfey PN (1995) Mutations affecting the radial organisation of the Arabidopsis root display specific defects throughout the embryonic axis. Development 121: 53–62 [Google Scholar]
- Scheres B, Wolkenfelt H, Willemsen V, Terlouw M, Lawson E, Dean C, Weisbeek P (1994) Embryonic origin of the Arabidopsis primary root and root meristem initials. Development 120: 2475–2487 [Google Scholar]
- Swarup K, Benková E, Swarup R, Casimiro I, Péret B, Yang Y, Parry G, Nielsen E, De Smet I, Vanneste S (2008) The auxin influx carrier LAX3 promotes lateral root emergence. Nat Cell Biol 10: 946–954 [DOI] [PubMed] [Google Scholar]
- Swarup R, Kramer EM, Perry P, Knox K, Leyser HO, Haseloff J, Beemster GT, Bhalerao R, Bennett MJ (2005) Root gravitropism requires lateral root cap and epidermal cells for transport and response to a mobile auxin signal. Nat Cell Biol 7: 1057–1065 [DOI] [PubMed] [Google Scholar]
- Ulmasov T, Hagen G, Guilfoyle TJ (1999) Dimerization and DNA binding of auxin response factors. Plant J 19: 309–319 [DOI] [PubMed] [Google Scholar]
- Vanneste S, Friml J (2009) Auxin: a trigger for change in plant development. Cell 136: 1005–1016 [DOI] [PubMed] [Google Scholar]
- Vermeer JE, von Wangenheim D, Barberon M, Lee Y, Stelzer EH, Maizel A, Geldner N (2014) A spatial accommodation by neighboring cells is required for organ initiation in Arabidopsis. Science 343: 178–183 [DOI] [PubMed] [Google Scholar]
- Vieten A, Vanneste S, Wiśniewska J, Benková E, Benjamins R, Beeckman T, Luschnig C, Friml J (2005) Functional redundancy of PIN proteins is accompanied by auxin-dependent cross-regulation of PIN expression. Development 132: 4521–4531 [DOI] [PubMed] [Google Scholar]
- Vijayakumar P, Datta S, Dolan L (2016) ROOT HAIR DEFECTIVE SIX‐LIKE 4 (RSL 4) promotes root hair elongation by transcriptionally regulating the expression of genes required for cell growth. New Phytol 212: 944–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachsman G, Heidstra R, Scheres B (2011) Distinct cell-autonomous functions of RETINOBLASTOMA-RELATED in Arabidopsis stem cells revealed by the Brother of Brainbow clonal analysis system. Plant Cell 23: 2581–2591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Inukai Y, Yamauchi A (2006) Root development and nutrient uptake. Crit Rev Plant Sci 25: 279–301 [Google Scholar]
- Weijers D, Benkova E, Jäger KE, Schlereth A, Hamann T, Kientz M, Wilmoth JC, Reed JW, Jürgens G (2005) Developmental specificity of auxin response by pairs of ARF and Aux/IAA transcriptional regulators. EMBO J 24: 1874–1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi K, Menand B, Bell E, Dolan L (2010) A basic helix-loop-helix transcription factor controls cell growth and size in root hairs. Nat Genet 42: 264. [DOI] [PubMed] [Google Scholar]
- Zamski E, Wareing P (1974) Vertical and radial movement of auxin in young sycamore plants. New Phytol 73: 61–69 [Google Scholar]
- Zhang J-Y, He S-B, Li L, Yang H-Q (2014) Auxin inhibits stomatal development through MONOPTEROS repression of a mobile peptide gene STOMAGEN in mesophyll. Proc Natl Acad Sci U S A 111: E3015–E3023 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.