Abstract
Objectives
To characterize the effect of ultra-short glucocorticoids followed by Tocilizumab monotherapy on the intima-media thickness (IMT) in GCA.
Methods
Eighteen GCA patients received 500 mg for 3 consecutive days (total of 1500mg) i.v. methylprednisolone on days 0–2, followed by i.v. Tocilizumab (8 mg/kg) on day 3 and thereafter weekly s.c. Tocilizumab injections (162 mg) over 52 weeks. US of temporal (TAs), axillary (AAs) and subclavian (SAs) arteries was performed at baseline, on days 2–3, and at weeks 4, 8, 12, 24 and 52. The largest IMT of all segments and IMT at landmarks of AA/SA were recorded. IMT was scaled by mean normal values and averaged. Each segment was classified according to diagnostic cut-offs.
Results
Of the 18 GCA patients, 16 patients had TA and 6 had extracranial large artery involvement. The IMT showed a sharp decline on day 2/3 in the TAs and AAs/SAs. In TAs, this was followed by an increase to baseline levels at week 4 and a subsequent slow decrease, which was paralleled by decreasing symptoms and achievement of clinical remission. The AAs/SAs showed a new signal of vasculitis at week 4 in three patients, with an IMT increase up to week 8.
Conclusion
Glucocorticoid pulse therapy induced a transient decrease of the IMT in TAs and AAs/SAs. Tocilizumab monotherapy resulted in a slow and steady decrease in IMT of the TAs and a smaller and delayed effect on the AAs/SAs. The data strongly support a remission-inducing effect of Tocilizumab and argue the case for US having an important role in monitoring disease activity in GCA.
Trial registration
ClinicalTrials.gov, www.clinicaltrials.gov, NCT03745586.
Keywords: giant cell arteritis, tocilizumab, glucocorticoids, ultrasound, vascular response, vasculitis
Rheumatology key messages
Three days of high-dose intravenous glucocorticoids lead to a profound but transient IMT reduction.
Tocilizumab induces a slow but steady IMT reduction in temporal arteries over 52 weeks.
Ultrasound remains a valid technique for diagnosing GCA, even after 3 days of high-dose glucocorticoids.
Introduction
GCA is the most frequent form of vasculitis in people above 50 years of age. The standard treatment still consists of glucocorticoids (GCs) [1]. Due to a high relapse rate, the duration of GCs therapy is often very long. Two randomized controlled trials (RCTs) showed that Tocilizumab (TCZ) may replace GCs in maintaining remission, resulting in a reduction of ∼50% of the cumulative GC dose [2, 3]. In an attempt to further reduce GCs side effects, the ‘GCA treatment with Ultra-Short glucocorticoids and TOcilizumab’ (GUSTO) trial was conducted (NCT03745586). In brief, patients with newly diagnosed GCA were treated with a GCs pulse over 3 days, followed by TCZ monotherapy over 52 weeks [4].
US is proposed as the first-line imaging modality for diagnosing GCA, according to the EULAR recommendations [5–7]. However, whether US is suitable for monitoring disease activity at the level of the vessel wall and thereby helps to define treatment intensity is unknown. Recent data show that none of the currently used imaging modalities can reliably differentiate between active vessel wall inflammation and residual local hyperaemia or wall remodelling [5, 8]. In PET/CT imaging and MRI, persistent tracer activity or arterial wall enhancement is present in the majority of patients, even if otherwise considered in clinical remission [5, 9, 10]. In a first approach to defining the value of US in monitoring disease activity, longitudinal US studies in GCA evaluated the halo sign and demonstrated its disappearance in temporal arteries (TAs) in the majority of patients. They also found reduced, but persistently elevated intima-media thickness (IMT) in the supra-aortic large arteries during therapy [11, 12]. More recently, diagnostic IMT cut-offs and mean normal values for the TAs, axillary (AAs) and subclavian arteries (SAs) were published, a scoring method for the quantitative IMT assessment has been proposed and first studies have evaluated quantitative IMT monitoring in GCA [13–20].
A tool for assessing disease activity in GCA at the level of the vessel wall is of particular importance if therapeutic agents are used that inhibit the IL-6 pathway. As the hepatic acute phase response is under the control of IL-6, drugs such as TCZ render the two inflammatory markers ESR and CRP unreliable. Therefore, in the case of IL-6 blocking therapies, the assessment of the treatment response relies entirely upon reported signs and symptoms.
Due to the sequential administration of GCs (3-day pulse) and TCZ (subsequent monotherapy), the GUSTO trial offered a unique opportunity for the assessment and differentiation of the effects of the two drugs on the IMT of the TAs, AAs and SAs. In addition, the feasibility of IMT monitoring with US as a surrogate for monitoring disease activity could be assessed and a flexible scoring method evaluated.
Methods
GUSTO trial (NCT03745586)
GUSTO is a single-arm, single-centre, open-label proof of concept study. Eighteen patients with newly diagnosed GCA were enrolled and received 500 mg for 3 consecutive days (total of 1500mg) i.v. methylprednisolone on days 0–2. I.v. TCZ (8 mg/kg body weight) was administered on day 3, followed by weekly s.c. TCZ injections (162 mg) until week 52. The primary end point was the proportion of patients who achieved remission within 31 days and did not relapse until week 24. Secondary outcomes included remission rate until week 52. Remission was defined as complete absence of GCA symptoms; partial remission included the presence of mild symptoms (for details, see the study protocol in Supplementary Data S1, available at Rheumatology online). The therapeutic response of the IMT was an exploratory secondary outcome [4]. The study complies with the Declaration of Helsinki and the local ethics committee has approved the research protocol; informed consent was obtained from all patients.
Ultrasound
US scans were done at baseline, days 2 or 3 (after the third dose of methylprednisolone), and at weeks 4, 8, 12, 24 and 52. The examiner (L.S.), a physician with 6 years of US experience, performed all but two scans; he was not blinded to the clinical data. To evaluate inter-reader agreement, IMTs from 121 images (15 scans from 5 patients on GE-Logiq-E9) were remeasured on raw images with a GE-Logiq-E10 by an expert (W.A.S.) who was blinded to clinical data. Seven patients were examined using GE-Logiq-E9 and the remaining 11 with Canon-Aplio-i800. The B-Mode frequency was 18–22 MHz for the TA and 9–16.5 MHz for the AA/SA. The equipment details are listed in Supplementary Table S1, available at Rheumatology online. In the TAs, the IMT was measured after complete compression. For the AAs/SAs, the measurements were single-sided on the deep wall. Measurements were made strictly in B-Mode in longitudinal images for the AAs/SAs and transverse images after compression for the TAs. Colour Doppler was only used for faster identification of the arteries.
The maximum (not landmark-based) IMTs of bilateral TA segments [common superficial TAs (CSTAs), frontal branch, parietal branch] were registered. CSTAs, in which measurements were not possible due to incompressibility or due to a very proximal TA bifurcation, and biopsied segments were not followed up. Initially, follow-up scans were planned only for abnormal baseline scans. For IMT measurements of the AAs/SAs, landmarks were specified individually at baseline, usually the mid humeral head for the AAs and an arterial branch coming off the superficial wall for the SAs were used. In case the IMT at the landmark of the AAs/SAs was below the diagnostic cut-off, the whole of the AAs/SAs were screened for the largest IMT available, and the maximum IMT of the segment was registered. The IMT at the AA/SA landmarks was initially only registered in vasculitic segments, i.e. segments with an IMT above the diagnostic cut-off at baseline. While having normal IMT values in the AA/SA at baseline, patient number 7 showed an unexpected IMT increase in subsequent scans, which promoted adjustments of the protocol (see Supplementary Table S2, available at Rheumatology online): For subsequent patients, follow-up scans were always performed, and for every AA/SA (even if normal at baseline) the landmark-based IMT was registered. Again, the rest of the AA/SA segment was only screened for the additional maximum IMT if the landmark-based IMT was below the diagnostic cut-off. Based on the largest registered IMTs of the TAs and AAs/SAs, every segment was classified as being above or below the diagnostic cut-off values (i.e. as vasculitic or not-vasculitic) as defined by Schäfer et al. (TAs and AAs) or Ješe et al. (SA) [13, 18]. For the IMT as a quantitative measure, the maximum IMT of the TAs, and the landmark-based IMT of the AAs/SAs were used. To account for the different number of segments in individual patients, the IMT was scaled and averaged as follows. For each TA segment, the IMT was divided by 2 and again by the mean normal value [13]. (See Supplementary Table S3, available at Rheumatology online, for diagnostic cut-off and mean normal values.) The sum of these values was averaged by dividing it by the number of segments. For the AAs/SAs, the same calculation was used, omitting the factor 2, due to single-sided IMT measurements. (For the formula, see Supplementary Fig. S1, available at Rheumatology online.)
Statistics
Statistical analysis was performed using Stata version 16 (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC.) plots using R version 4.0.3 [R Core Team (2020)] [21]. The scaled IMT were analysed separately for TAs and AAs/SAs using a linear mixed-effects model, with the time point as a categorical covariate and random intercepts and slopes for patient and branch. Models were fitted with restricted maximum likelihood. The fitted values at each time point and the change from baseline were calculated with 95% CIs using Satterthwaite’s approximation for the degrees of freedom and the t-distribution. Inter-reader agreement was assessed using the intra-class correlations (ICCs) and Krippendorff’s alpha. The ICC represents the fraction of the total variance that is within-reader; values close to 1 indicate a high correlation between the two readers. It was calculated from linear mixed models of the scaled IMT determined by the two readers, including random intercepts for patient, branch (if applicable) and time point. Separate models were fitted for each branch and overall. For Krippendorff’s alpha, 0 represents no agreement and 1 perfect agreement. It was calculated using the difference function for ratio data and accompanied by bootstrap 95% CIs based on 1000 repetitions.
Results
The main characteristics of the 18 patients are shown in Table 1. Sixteen patients had involvement of TA only [cranial GCA (cGCA)], 6 patients had cGCA and involvement of large extracranial arteries and 2 patients had no vasculitis on US examination. Five patients dropped out before week 24, 3 due to non-response and 2 due to adverse events. Of the 18 patients, 3 achieved remission within 31 days and 14 within 24 weeks after a mean of 11.1 weeks (95% CI 8.3–13.9). Partial remission was achieved by 14 patients within 24 weeks after a mean of 6.3 weeks (95% CI 3.7–8.7) [4].
Table 1.
Patients’ characteristics (n = 18)
| Age (years) | 72 (67, 75) |
|---|---|
| Female | 12 (67%) |
| Ethnicity: Caucasian | 18 (100%) |
| BMI (kg/m2) | 24 (23, 26) |
| Prior GCs treatmenta | 11 (61%) |
| Days since GCA symptom onset | 28 (21, 59) |
| CRP at screening (mg/l) | 61 (50, 78) |
| CRP at baseline (mg/l) | 44 (18, 62) |
| ESR at screening (mm/h) | 83 (61, 89) |
| ESR at baseline (mm/h) | 71 (44, 79) |
| Cranial symptoms | 15 (83%) |
| Headache | 12 (67%) |
| Jaw claudication | 10 (56%) |
| Visual symptoms | 6 (33%) |
| PMR symptoms | 10 (56%) |
| Weight loss >2 kg/4 weeks | 6 (33%) |
| Positive TA US | 16 (89%) |
| Aortitis on MRI | 14 (78%) |
| Vasculitis on cranial MRI | 14 (78%) |
| Positive histology (inflammatory infiltrate) | 13 (72%) |
for a median of 1 (min 1, max 7) days. Values are n (%, referring to 18) or median (lower quartile, upper quartile). GCs: glucocorticoids; TA: temporal artery.
In total, 96 US scans were performed. For the TAs, of the potential 108 segments (18 × 6), 92 segments could be classified into vasculitic/not-vasculitic segments at baseline: 14 CSTA were unavailable due to proximal bifurcation and/or incompressibility and one anatomic variant (singular frontal branch) with loss of one parietal branch and CSTA. Moreover, due to 16 unilateral and 2 bilateral TA biopsies, only 72/92 segments could be scaled for IMT follow-up. For the AAs/SAs, 70/72 segments were available for classification into vasculitic/not-vasculitic segments at baseline, and 66/72 for scaled IMT follow-up. Omitting biopsied segments and dropouts, 45 TA and 50 AA/SA segments were available for scaled IMT follow-up at week 52.
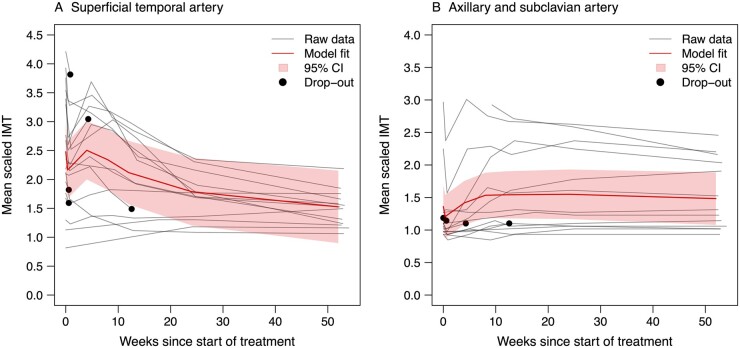
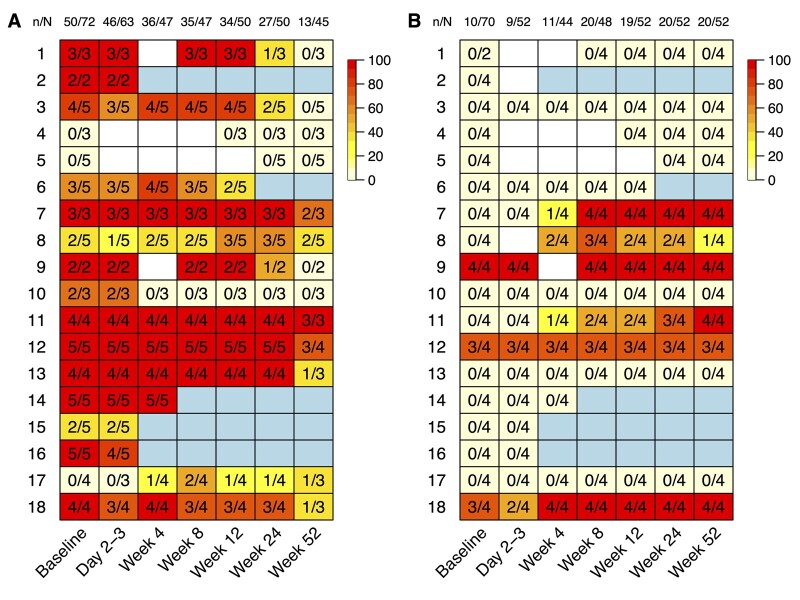
The development of the IMT over 52 weeks is displayed in Figs 1 and 2. Fig. 1 shows the fitted mean IMT values; the model coefficients are shown in Supplementary Table S4, available at Rheumatology online. The individual mean scaled IMT for the TAs and AAs/SAs are displayed in Fig. 2. Supplementary Fig. S2, available at Rheumatology online, shows the box-plots of scaled IMT, split up by segment and week. Fig. 3 displays the proportion of vasculitic segments for individual patients. Supplementary Table S5, available at Rheumatology online, shows more detailed information about the proportion of vasculitic segments on a patient and segment level.
Fig . 1.
Development of the overall mean scaled IMT from baseline to week 52
For all 18 patients, the raw data is shown in grey and the development overall (model fit) with the 95% CI is shown in red. IMT: intima-media thickness.
Fig . 2.
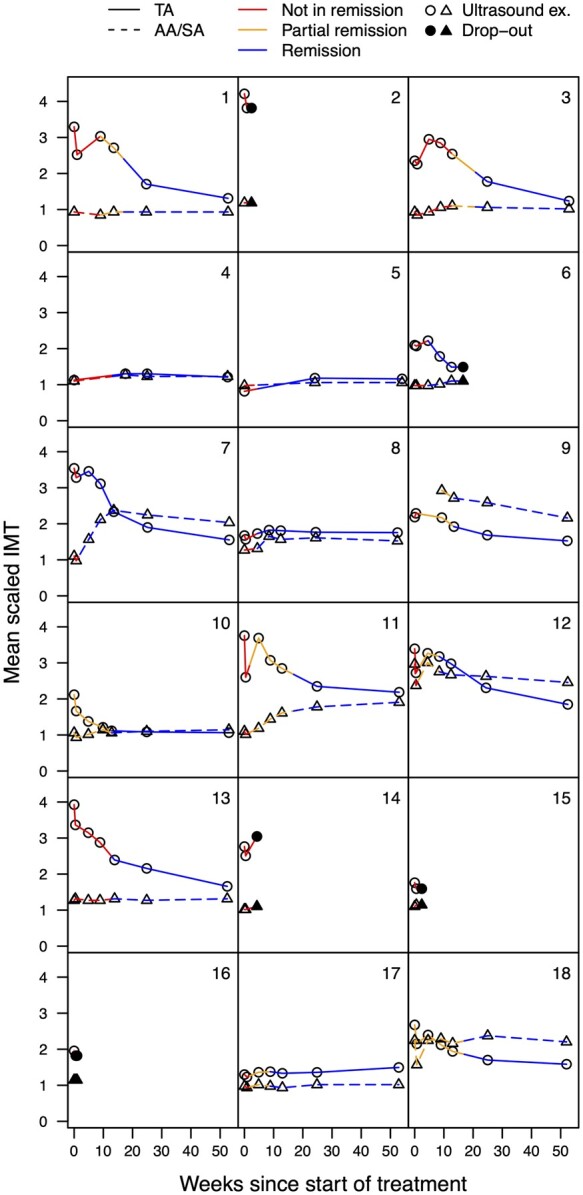
Development of the individual mean scaled IMT from baseline to week 52
For all 18 individual patients, the mean scaled IMT is shown for the TAs and for the AAs/SAs combined. Only patients 4 and 5 did not show any pathological IMT throughout the study. The colour coding (not in remission, partial remission and remission) shows the relationship of the IMT to the clinical status. The circles and triangles represent the time points of the US examinations or drop-outs (circles for TAs, triangles for SAs/AAs). TA: temporal artery; AA: axillary artery; SA: subclavian artery; IMT: intima-media thickness.
Fig. 3.
Proportion of vasculitic segments for individual patients (TA (A) and AA/SA (B))
In (A), the biopsied TA segments were already omitted at baseline and day 2–3. (A heat map of the TAs, including the biopsied segments, is shown in Supplementary Fig. S3, available at Rheumatology online.) At week 52, the denominator drops by one in five patients due to an additional biopsy after week 24. n = segments above diagnostic cut-offs; N = available segments; colour coding = proportion of vasculitic segments (0–100%); white square = missing value; light-blue square = drop out; n/N = column total.
Overall mean scaled IMT development
In the TAs, a sharp decline in IMT on day 2/3 was observed, followed by an increase to approximately baseline levels at week 4 (Fig. 1). This was followed by a steady decrease until week 52. For the AAs/SAs, a comparable decline in IMT was observed at day 2/3, with a subsequent slow increase up to week 8, a plateau until week 24, and a subtle decline thereafter until week 52. Because only 6 patients had involvement of extracranial large arteries, the individual IMT courses need to be considered.
Individual mean scaled IMT development and proportion of vasculitic segments
At week 4, 8 patients showed a rebound to larger IMT values compared with day 2/3 in the TAs, and 4 of them had larger IMT values compared with baseline (Figs 2 and 3). In the AA/SA at week 4, 3 patients had segments above the diagnostic cut-off for the first time, and 2 patients with baseline AA/SA involvement showed a rebound of the IMT. Of the 6 patients with AA/SA vasculitis, 5 had landmark-based IMT at baseline. The largest IMT of the AAs/SAs was reached after 4, 8, 12, 24 and 52 weeks, respectively, for one patient each. An example with normal baseline IMT and new onset vasculitis at week 12 in the AAs is shown in Supplementary Fig. S4, available at Rheumatology online.
Regarding the effects of i.v. GCs on the IMT, the following was observed: of 16 patients with TA involvement at baseline, 15 still showed at least one segment above the diagnostic cut-off at day 2/3. The single patient without any segment above the cut-off value at day 2/3 had the only pathological segment biopsied at baseline. Of the 3 patients with AA/SA vasculitis at baseline, all had multiple segments above the diagnostic cut-off at day 2/3. For the TAs, the IMT of only 4/50 vasculitic segments, and for the AAs/SAs, the IMT of only 1/10 vasculitic segments, dropped below the diagnostic cut-offs in response to GCs pulse therapy.
Inter-reader agreement
The inter-reader agreement of the IMT measurements was excellent, with an overall ICC of 0.98 (95% CI 0.97–0.99) and overall Krippendorff’s alpha of 0.97 (95% CI 0.95–0.98), being close to 1 (see Supplementary Table S6, available at Rheumatology online, for details).
Discussion
The GUSTO trial is the first study allowing separate and comparative assessment of the effect of GCs and of TCZ on signs of vessel wall inflammation in GCA using IMT measurements with US. Furthermore, the 52-week duration of the study made long-term monitoring possible. GCs-pulse therapy led to a profound yet transient decrease in IMT. Thereafter TCZ monotherapy resulted in a slow but steady decrease of the IMT in the TAs, with less pronounced effect on the IMT of the AAs/SAs. The presented US findings for the cranial arteries support the clinical results of a remission-inducing effect of TCZ, and they show the potential of US for monitoring disease activity.
While older studies examined the qualitative halo sign in GCA treated with GCs standard therapy [11, 12], prior data on quantitative IMT measurements with TCZ treatment without long-term GCs therapy is not available. Also, no prior study examined the IMT quantitatively before and after i.v. GCs. Although the IMT was reduced in our study, pathological segments in all assessable patients were still present on day 2–3. Thus, based on the proposed diagnostic IMT cut-offs [13, 15, 18], the diagnosis of GCA could still be made after 3 consecutive days of 500 mg i.v. methylprednisolone in all 15 patients. This indicates that the IMT could serve as a diagnostic tool for GCA, even after high-dose i.v. GCs. This contrasts with MRI data reporting a loss of diagnostic accuracy after as little as 2 days of prednisone with standard oral dosing [22].
The short-lasting decrease in IMT in response to GCs may be best explained by a strong anti-oedematous effect. It contrasts with the slow decrease in IMT induced by TCZ. TCZ not only suppresses the acute-phase response but also acts on maturation of B-lymphocytes and recruitment of Th17 cells [23]. The kinetics of the IMT decline in response to TCZ may suggest that indirect cellular effects come into play and gradually reduce inflammation. Of note, the slow and sometimes delayed control of vessel wall inflammation in the TAs by TCZ parallels the observed slow clinical response. Patients showed gradual improvement of signs and symptoms and achieved complete remission after a mean of only 3 months [4]. Collectively, the US data suggest synergic effects of GCs and TCZ in inducing clinical remission, which may explain the success of a rapid GCs reduction.
There appears to be a different effect of TCZ on TAs and AAs/SAs in some patients. Based on earlier studies [12] and our own experience, we did not expect the IMT of AAs/SAs to normalize within 1 year. However, the development of new vasculitic segments of AAs/SAs in three patients was surprising; it was even more surprising that two patients simultaneously showed an excellent response in the TAs. The rebound of the IMT in the TAs at week 4, the development of new-onset vasculitis of the AAs/SAs in three patients, and the increasing IMT of AAs/SAs in two patients until week 24 or later might be due to a slower than expected onset of action of TCZ, in spite of the rapid achievement of therapeutic blood levels with the chosen TCZ treatment regimen [4]. In some patients, TCZ cannot stabilize the IMT reduction after the effect of the i.v. GCs wears off. Also, TCZ shows less efficacy in IMT reduction in the larger arteries compared with in the TAs. This finding is consistent with previous studies with conventional therapy [12]. The observed unequal response of large vs medium-sized arteries may suggest the need to check the IMT of large arteries during the first weeks of treatment with IL-6 inhibitors.
Although some variations in timing and amplitude of the IMT changes in individual patients are notable, nearly all vasculitic arterial segments of the TAs followed a similar pattern, and a substantial number of segments ultimately normalized over 52 weeks (see Fig. 3A). Taken together, IMT appears to serve as a tool for monitoring remission in cGCA, more so than in extracranial large arteries. However, the very slow changes argue against a plausible role for IMT in the early recognition of relapse and/or non-responders. To fully answer these important questions, multicentre efforts will have to be deployed.
IMT monitoring was feasible, but we frequently observed thrombosis of adjacent segments after biopsy, had one anatomical variant of the TAs and either due to a proximal bifurcation or incompressibility, many CSTA could not be rated with our equipment and method. Thus, we believe an IMT scoring system should be flexible to account for such limitations. The presented scaled IMT would meet these demands. Due to the potentially divergent responses of large vs medium sized arteries, both territories should be scored individually, or their individual developments should remain comprehensible. Also, only using a dichotomous rating like in Fig. 3 results in loss of relevant information (i.e. multiple segments dropping below the diagnostic cut-off only after week 24) and should be accompanied by non-binary, quantitative IMT follow-up like in Figs 1 and 2. Incorporating IMT surveillance into GCA trials would be especially helpful with anti-IL-6 therapy and could facilitate inclusion of patients with rare clinical presentations, e.g. inflammation or fever of unknown origin, who are consistently excluded from trials. If US monitoring of IMT is used in trials, we expect patients in clinical remission but with increasing IMT to be reclassified as active disease.
This study has the following limitations: the sample size, particularly the low number of patients with extracranial large artery involvement, precludes generalization of our findings. Furthermore, the very low number of relapsing patients did not allow for identification of US findings predicting relapse. The examiner (L.S.) was not blinded to signs and symptoms, and the examinations were not repeated in a blinded fashion by a second expert. Finally, the IMT measurement protocol of the AA/SA had to be changed while the trial was ongoing; however, this did not affect the results for the TA.
In summary, this study indicates a clinically important role for IMT measurements in diagnosing GCA despite commenced GCs treatment, and in monitoring gradual achievement of remission. This latter finding is of particular importance if IL-6 blocking strategies are used, as these strategies render the acute-phase proteins unreliable in the quantification of disease activity. The data furthermore support the notion of different reaction profiles of cranial arteries and large arterial vessels to TCZ.
Supplementary Material
Acknowledgements
P.V. initiated the study and was responsible for obtaining funding. L.S., L.C., L.B., S.R. and P.V. were involved in the study conception, design and implementation, and data interpretation. L.S. and F.L. performed US scans and marked biopsy sites. L.C., G.S., F.K. and A.S. performed study visits. L.B. undertook the statistical analysis. W.A.S. performed the independent reading on a subset of IMT measurements. L.S., F.L. and P.V. wrote the manuscript. All authors read and approved the manuscript.
Funding: This work was supported by the Research Funds of the Department of Rheumatology, Immunology and Allergology, University Hospital (Inselspital) and University of Bern, Switzerland (50%) and by F. Hoffmann-La Roche (50%). F. Hoffmann-La Roche had no role in study design, data collection, data analysis, data interpretation, or writing of the manuscript.
Disclosure statement: L.C. reports grant/research support from Gilead, Roche and Pfizer. F.K. is a shareholder of Roche, consultant of Actelion, BMS, Boehringer-Ingelheim, and Pfizer, has grant/research support from Gilead, Pfizer and Roche, is employed by Roche and was previously employed by Novartis. W.A.S. reports personal fees and other support from Abbvie, GlaxoSmithKline, Novartis, Roche and Sanofi. P.V. reports speaker fees, advisory fees and research support from Roche, MSD, Abbvie, Pfizer, Novartis, Celgene, Sanofi, Chugai and BMS. The other authors have declared no conflicts of interest.
Data availability statement
Research data will be available upon reasonable request. All requests should be submitted to the corresponding author for consideration.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Hellmich B, Agueda A, Monti S. et al. 2018 Update of the EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis 2020;79:19–30. [DOI] [PubMed] [Google Scholar]
- 2. Villiger PM, Adler S, Kuchen S. et al. Tocilizumab for induction and maintenance of remission in giant cell arteritis: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet 2016;387:1921–7. [DOI] [PubMed] [Google Scholar]
- 3. Stone JH, Tuckwell K, Dimonaco S. et al. Trial of tocilizumab in giant-cell arteritis. N Engl J Med 2017;377:317–28. [DOI] [PubMed] [Google Scholar]
- 4. Christ L, Seitz L, Scholz G. et al. Tocilizumab Mono- therapy after Ultra-Short Glucocorticoid Administration in Giant Cell Arteritis: a proof-of-concept trial. Lancet Rheumatol 2021;doi:10.1016/S2665-9913(21)00152-1. [DOI] [PubMed] [Google Scholar]
- 5. Dejaco C, Ramiro S, Duftner C. et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheum Dis 2018;77:636–43. [DOI] [PubMed] [Google Scholar]
- 6. Chrysidis S, Duftner C, Dejaco C. et al. Definitions and reliability assessment of elementary ultrasound lesions in giant cell arteritis: a study from the OMERACT Large Vessel Vasculitis Ultrasound Working Group. RMD Open 2018;4:e000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schäfer VS, Chrysidis S, Dejaco C. et al. Assessing vasculitis in giant cell arteritis by ultrasound: results of OMERACT patient-based reliability exercises. J Rheumatol 2018;45:1289–95. doi:10.3899/jrheum.171428. [DOI] [PubMed] [Google Scholar]
- 8. Schäfer VS, Jin L, Schmidt WA.. Imaging for diagnosis, monitoring, and outcome prediction of large vessel vasculitides. Curr Rheumatol Rep 2020;22:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reichenbach S, Adler S, Bonel H. et al. Magnetic resonance angiography in giant cell arteritis: results of a randomized controlled trial of tocilizumab in giant cell arteritis. Rheumatology (Oxford) 2018;57:982–6. [DOI] [PubMed] [Google Scholar]
- 10. Grayson PC, Alehashemi S, Bagheri AA. et al. 18F-fluorodeoxyglucose-positron emission tomography as an imaging biomarker in a prospective, longitudinal cohort of patients with large vessel vasculitis. Arthritis Rheumatol 2018;70:439–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. De Miguel E, Roxo A, Castillo C. et al. The utility and sensitivity of colour Doppler ultrasound in monitoring changes in giant cell arteritis. Clin Exp Rheumatol 2012;30:S34–8. [PubMed] [Google Scholar]
- 12. Aschwanden M, Schegk E, Imfeld S. et al. Vessel wall plasticity in large vessel giant cell arteritis: an ultrasound follow-up study. Rheumatology (Oxford) 2019;58:792–7. doi:10.1093/rheumatology/key383. [DOI] [PubMed] [Google Scholar]
- 13. Schäfer VS, Juche A, Ramiro S, Krause A, Schmidt WA.. Ultrasound cut-off values for intima-media thickness of temporal, facial and axillary arteries in giant cell arteritis. Rheumatology (Oxford) 2017;56:1479–83. Erratum in: Rheumatology (Oxford) 2017;56: 1632. [DOI] [PubMed] [Google Scholar]
- 14. Czihal M, Schröttle A, Baustel K. et al. B-mode sonography wall thickness assessment of the temporal and axillary arteries for the diagnosis of giant cell arteritis: a cohort study. Clin Exp Rheumatol 2017;35(Suppl 103):128–33. [PubMed] [Google Scholar]
- 15. De Miguel E, Beltran LM, Monjo I. et al. Atherosclerosis as a potential pitfall in the diagnosis of giant cell arteritis. Rheumatology (Oxford) 2018;57:318–21. [DOI] [PubMed] [Google Scholar]
- 16. Van der Geest KSM, Borg F, Kayani A. et al. Novel ultrasonographic Halo Score for giant cell arteritis: assessment of diagnostic accuracy and association with ocular ischaemia. Ann Rheum Dis 2020;79:393–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sebastian A, van der Geest KSM, Coath F. et al. Halo score (temporal artery, its branches and axillary artery) as a diagnostic, prognostic and disease monitoring tool for giant cell arteritis (GCA). BMC Rheumatol 2020;4:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ješe R, Rotar Ž, Tomšič M, Hočevar A.. The cut-off values for the intima-media complex thickness assessed by colour Doppler sonography in seven cranial and aortic arch arteries. Rheumatology (Oxford) 2020;60:1346–52. [DOI] [PubMed] [Google Scholar]
- 19. Sebastian A, Kayani A, Prieto-Pena D. et al. Efficacy and safety of tocilizumab in giant cell arteritis: a single centre NHS experience using imaging (ultrasound and PET-CT) as a diagnostic and monitoring tool. RMD Open 2020;6:e001417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ponte C, Serafim AS, Monti S. et al. Early variation of ultrasound halo sign with treatment and relation with clinical features in patients with giant cell arteritis. Rheumatology (Oxford) 2020;59:3717–26. [DOI] [PubMed] [Google Scholar]
- 21.R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. 2020. https://www.r-project.org.
- 22. Hauenstein C, Reinhard M, Geiger J. et al. Effects of early corticosteroid treatment on magnetic resonance imaging and ultrasonography findings in giant cell arteritis. Rheumatology (Oxford) 2012;51:1999–2003. [DOI] [PubMed] [Google Scholar]
- 23. Calabrese LH, Rose-John S.. IL-6 biology: implications for clinical targeting in rheumatic disease. Nat Rev Rheumatol 2014. Dec;10:720–7. Erratum in: Nat Rev Rheumatol 2014;10: i. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Research data will be available upon reasonable request. All requests should be submitted to the corresponding author for consideration.