Abstract
Obesity can lead to cardiovascular disease, diabetes, and erectile dysfunction (ED) which decreases overall quality of life. Mechanisms responsible for obesity induced ED are unknown. Current mouse models of high fat diet (HFD) induced obesity yield conflicting results. Genetic variants among common “wild type” strains may explain contradictory data. Adult male C57BL/6N and 6J mice were fed a 45% HFD for 12 weeks. Weekly food intake, weight gain, and body fat percentage were measured. After 12 weeks, ex vivo vascular reactivity was measured in aortas, internal pudendal arteries, and penises. We assessed smooth muscle contractility, endothelial-dependent and -independent relaxation, and penile neurotransmitter mediated relaxation. C57BL/6N mice developed greater obesity and glucose sensitivity compared to C57BL/6J mice. Aortas from both strains fed a HFD had decreased contraction, yet contraction was unchanged in HFD pudendal arteries and penises. Interestingly, endothelial-dependent and -independent relaxation was unchanged in both systemic and penile vasculature. Likewise, HFD did not impair penile neurotransmitter mediated relaxation. Both strains fed 12 weeks of HFD developed obese phenotypes. However, HFD did not impair pre-penile or penile smooth muscle vasoreactivity as demonstrated in previous studies, suggesting this preclinical model does not accurately represent the clinical phenotype of obesity induced ED.
Introduction
Obesity is a growing epidemic in the United States as more than two-thirds of Americans are either overweight or obese (1). Although obesity is a well-known risk factor for cardiovascular diseases, diabetes and atherosclerosis, the negative impact of obesity on quality of life is often overlooked. For instance, obese men have lower sexual satisfaction (2). In fact, nearly 79% of men living with erectile dysfunction (ED) are obese (3,4). Obesity induced ED is considered a vasculogenic etiology. A central mechanism linking obesity to vasculogenic ED is endothelial damage and impaired nitric oxide (NO) release, which prevents penile vasodilation and tumescence (5,6). Obesity induced endothelial damage is linked to inflammation and atherosclerosis, but the exact mechanisms of obesity induced ED are unclear. Thus, animal models are needed to better understand the progression of obesity induced ED.
A commonly used obese animal model is the C57BL/6 mouse fed a high fat diet (HFD) (7). After a 10- to 12-week HFD, these mice have been shown to exhibit penile endothelial damage, decreased nitrergic relaxation and increased smooth muscle contractility (8–10). Interestingly, these studies did not specify which specific C57BL/6 strain was used. Physiological variants in C57BL/6 strains have been distinguished. Within Jackson Laboratory, the current C57BL/6J (6J) mouse possesses 236 genetic variations from the original strain (11,12). One significant mutation is the nicotinamide nucleotide transhydrogenase (NNT) gene – a mutation that alters mitochondrial function and fat metabolism (13). A mutation free C57BL/6N (6N) strain which mirrors the original C57BL/6 mouse is available. These strains have distinct metabolic responses to HFD. The 6J strain gains less body fat, is less glucose tolerant and secretes less insulin in response to glucose (13). To date, no study has identified how the progression of HFD induced ED differs between these strains.
This study determines how a 12-week HFD impacts the metabolic phenotype and vascular reactivity of both 6J and 6N mice. To mimic human fat consumption, we used a 45% HFD. Both systemic and penile vascular beds are examined. Aortas are commonly used to assess systemic vascular function and allow us to distinguish between any systemic HFD effects and those specific to the pelvic vasculature. Within the pelvis, the internal pudendal arteries (IPA) bifurcate into the penile arteries supplying the cavernosum and damage to these small sensitive vessels directly contributes to ED (14). By examining multiple vascular beds in two different strains of mice, this study broadly focuses on the overall physiological HFD effects on systemic and penile vasoreactivity.
Methods
Animal Model and Handling
Eight week-old male C57BL/6J (n=12) and C57BL/6N (n=12) mice purchased from Jackson Laboratory (Bar Harbor, ME) were single housed (22 ± 2°C, 12 hour light/dark cycle) and provided water and standard rodent chow ad libitum for the first two weeks. At 10 weeks of age, animals were randomized into groups and began either a 10% fat control diet (CON: n=6 per strain; D12450K, Research Diets, New Brunswick, NJ) or 45% high fat diet (HFD: n=6 per strain; D12451, Research Diets). Mice remained on CON or HFD for 12 weeks with body weight and food intake measured weekly. Body composition was assessed every 3 weeks using EchoMRI (Houston, TX). After respective 12 week diets, mice were fasted from 8am-1pm and underwent a glucose tolerance test (GTT). Mice were given an intraperitoneal injection of glucose (2 mg/kg lean body weight; Sigma Aldrich, St. Louis, MO), blood was collected from the tail at baseline, 30, 60, 90 and 120 minutes and blood glucose levels were measured with the Blood Glucose Alpha Track Monitoring System. All mice were euthanized via isoflurane overdose. Animal experiments were authorized by the Brody School of Medicine Institutional Animal Care and Use Committee under guidelines set forth by the National Institutes of Health (Guide for the Care and Use of Laboratory Animals, 8th edition) and the American Veterinary Medical Association. The ARRIVE guidelines were taken into consideration when planning, performing, and analyzing our data.
Vascular Reactivity Studies
Following euthanasia, thoracic aortas, IPA, and penises were excised, cleaned of connective tissue and maintained in cold Krebs solution (130mM NaCl, 4.7mM KCl, 1.18mM KH2PO4, 1.18mM MgSO4, 14.9mM NaHCO3, 5.6mM dextrose, and 1.56mM CaCl2 in distilled water). Aorta and IPA were cut into 2 mm rings and mounted in wire myographs (620M Danish Myograph Technology, Aarhus, DK) using 200 mm pins or 25 μm tungsten wires, respectively. Individual corpus cavernosum (approximately 2 mm x 8 mm) were mounted in muscle strip myographs (820M, Danish Myograph Technology). Each myograph chamber contained aerated (95% oxygen/5% carbon dioxide) Krebs solution maintained at 37°C. A 9.8 mN resting tension was applied to aorta and penis samples; IPA resting tension was determined by LabChart8 normalization software (AD Instruments, Colorado Springs, CO). After a 30 minute (aorta and IPA) or 1 hour (penis) equilibration period, contractile responses to a high potassium chloride (KCl, 120 mM) solution verified tissue viability. Contractile responses to increasing concentrations of phenylephrine (PE, 10−9 - 10−5 M) and endothelin-1 (ET-1, 10−10 - 10−7 M: Phoenix Pharmaceuticals, Burlingame, CA) were assessed. To evaluate vascular relaxation, tissues were precontracted with PE (IPA and penis: 10−5 M, aorta: 10−6 M) and concentration dependent relaxation responses to acetylcholine (ACh, 10−9 −10−5M) and diethylamine NONOate (DEA NONOate, 10−9 −10−5M: Cayman Chemicals, Ann Arbor, MI) were evaluated. Stock solutions of DEA NONOate were dissolved in 100% ethanol and further diluted in distilled water. Unless otherwise stated, all myograph chemicals and drugs were purchased from Sigma Aldrich (St. Louis, MO) and dissolved in distilled water. Electric field stimulation (EFS) was applied to PE contracted penises to induce relaxation responses as previously described (15). EFS parameters were 0.62 – 10 Hz, 2 msec duration, 10 volts, 45 sec stimulation, and a 5 minute recovery period between stimulations (S88: Grass Technologies, W. Warwick, RI).
Data Analysis
The number of animals used was based on previous experiments and the minimum number required to provide adequate statistical power by log rank test (power=80%, alpha =.05). Myograph data was collected using LabChart8 software, and all data are represented as mean ± standard error of the mean (SEM). Vascular relaxation is reported as a percent decrease from the PE precontraction. Statistical significance was determined by two-way ANOVAs with Bonferroni post hoc t-tests and noted for all p-values ≤0.05 (Prism 5, GraphPad, San Diego, CA).
Results
Different Metabolic Phenotypes
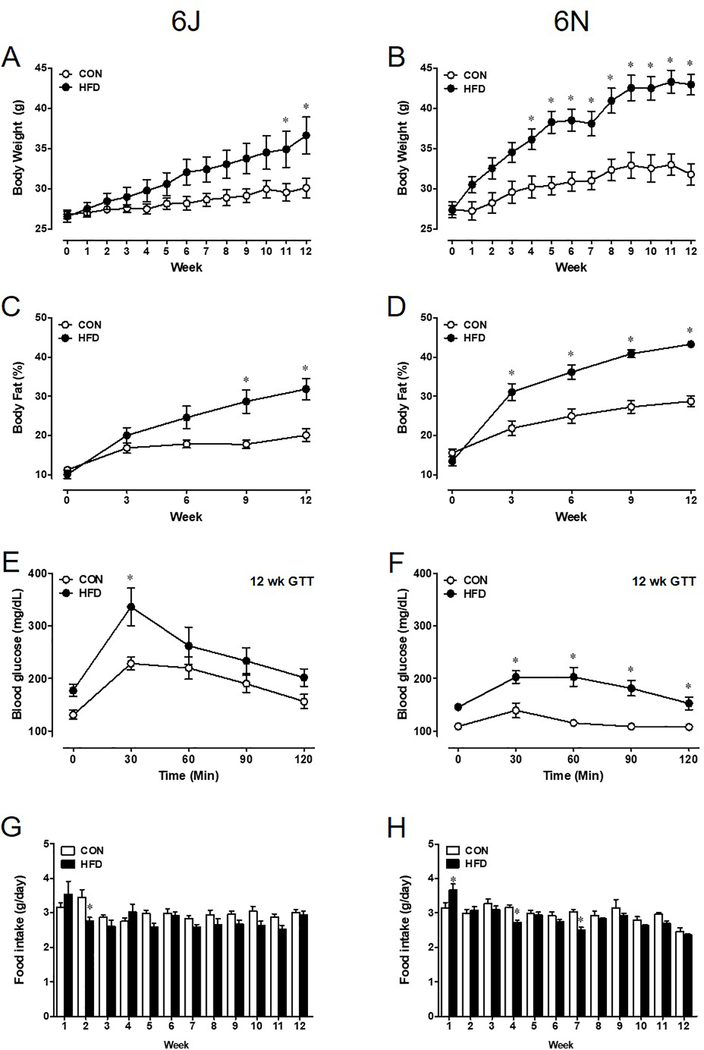
Both 6J and 6N mouse strains subjected to a 12-week HFD develop increased adiposity. Interestingly, a marked increase in 6J body weight is evident by week 11 (Figure 1A), yet 6N mice begin to exhibit this increase much earlier by 4 weeks (Figure 1B). In parallel to body weight, body fat composition increases with the duration of the diet. Significant accumulation of body fat in the 6J HFD group is noted at 9 weeks, but changes in body fat are present at 3 weeks in 6N HFD mice (Figure 1C,D). During the GTT, blood glucose 30 minutes post injection is significantly higher in both HFD groups; however, blood glucose remains elevated in the 6N strain (Figure 1E,F). Despite elevated glucose after 30 minutes, total area under the curve was not significantly different in CON and HFD 6J mice (data not shown). However, in 6N HFD mice, the area under the curve was significantly higher than CON (HFD: 18,225±1,495 vs. CON: 12,975±611 cm. p<0.05). Despite strain dependent changes in metabolic parameters, food intake was not different (Figure 1G,H).
Figure 1: Adult 6J and 6N mice fed a 12 week HFD exhibit unique metabolic phenotypes.
Obesity onset and severity differs between 6J (A,C) and 6N (B,D) strains. This results in variable glucose insensitivity in glucose tolerance test after 12 weeks of HFD (E,F) despite comparable daily food intake (G,H). n=6 per group. *P<0.05. Data are mean±SEM.
Decreased Systemic Vascular Contractility
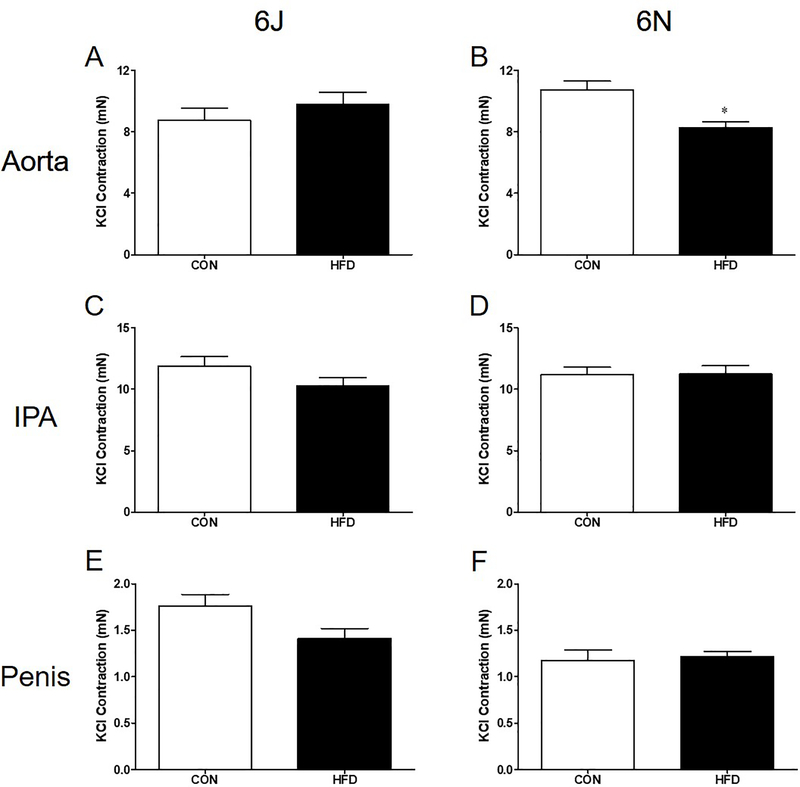
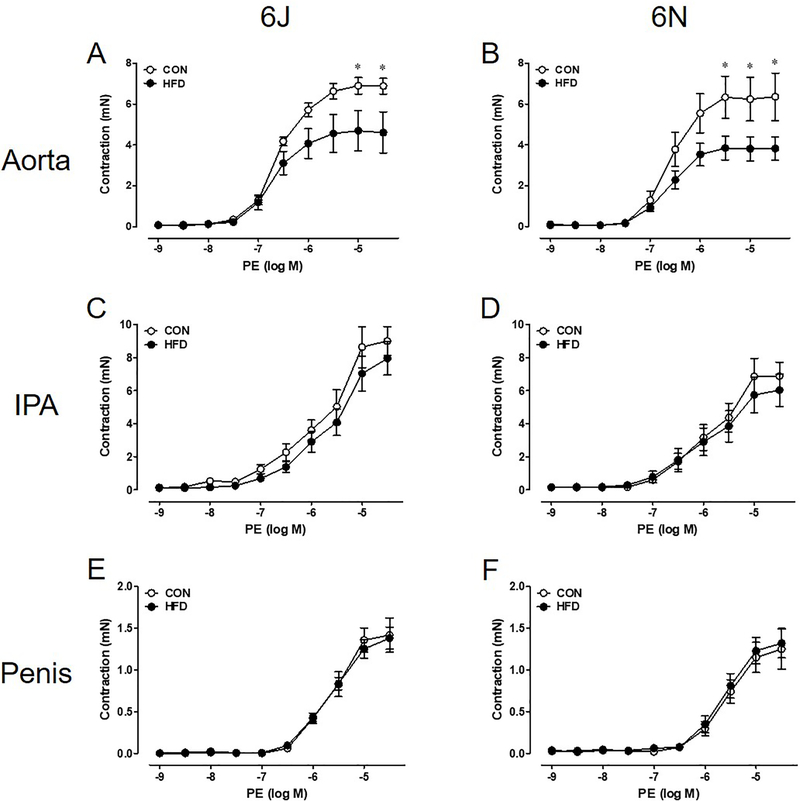
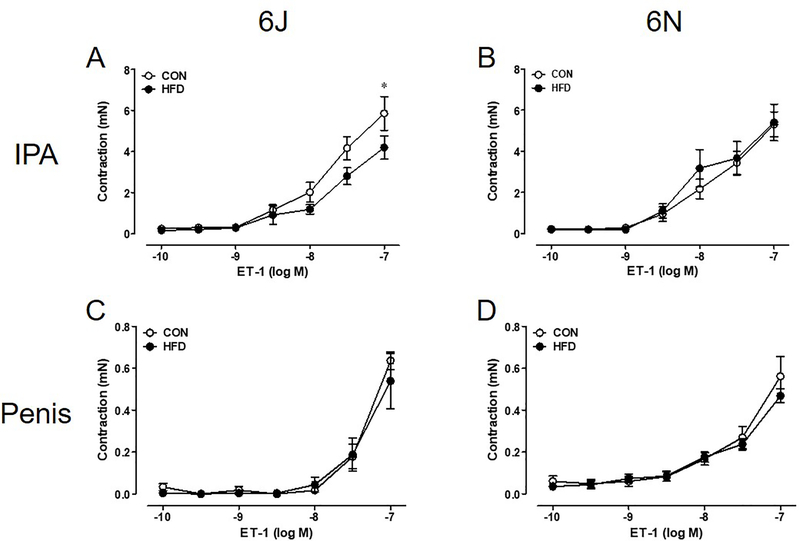
Aortas of 6N HFD mice exhibit decreases in non-receptor dependent KCl contractility; however, no difference is noted in KCl contractility between CON and HFD 6J aortas (Figure 2A,B). Surprisingly, KCl contractions in the IPA and penis remain unchanged between all groups (Figure 2C–F). Aortas from both HFD strains have lower PE (α1 agonist) contractions than CON aortas (Figure 3A,B). Again, HFD did not alter PE induced contractions in the IPA and penis (Figure 3C–F). Due to scarce receptor populations, ET-1 does not commonly cause contraction in mouse thoracic aorta and no contractions were evident in aortas from either strain [16]. ET-1 mediated contractions in IPA from 6J HFD mice are lower than 6J CON IPA (Figure 4A). In contrast, ET-1 contractions are unchanged in the IPAs from 6N HFD mice and in the HFD penises from both strains (Figure 4B–D).
Figure 2: HFD reduces KCl contractions exclusively in 6N aortas.
Contractions to 120 mM KCl solution were assessed in 6J and 6N tissue excised following a 12 week HFD. Aortic KCl contractions are reduced in 6N (B) but not 6J mice (A). No HFD induced changes in IPA or penis KCl contractility are noted (C-F). n=6 per group. *P<0.05. Data are mean±SEM.
Figure 3: Aortic adrenergic contractility decreases following a 12 week HFD.
In both 6J and 6N HFD aortas, lower PE contractions are evident (A,B). HFD does not affect IPA and penis PE contractility (C-F). n=6 per group. *P<0.05. Data are mean±SEM.
Figure 4: A modest HFD decrease in contraction to ET-1 in 6J IPA is noted.
ET-1 contractions are lower in 6J HFD IPA (A) but not 6N (B). Penis ET-1 contractions remain unchanged by a 12 week HFD (C-D). n=6 per group. *P<0.05. Data are mean±SEM.
No Change in Vascular Relaxation
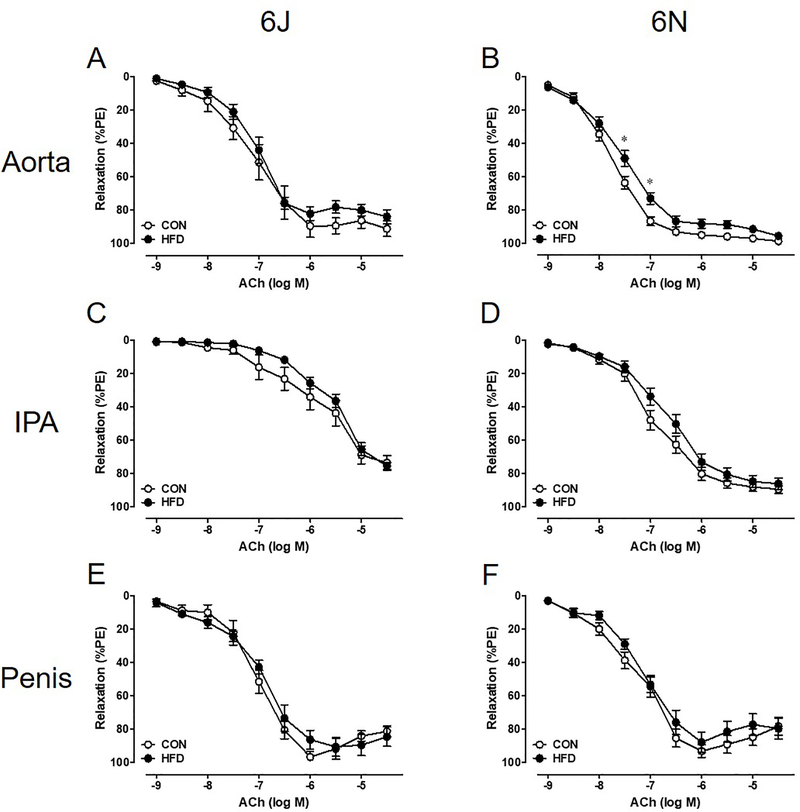
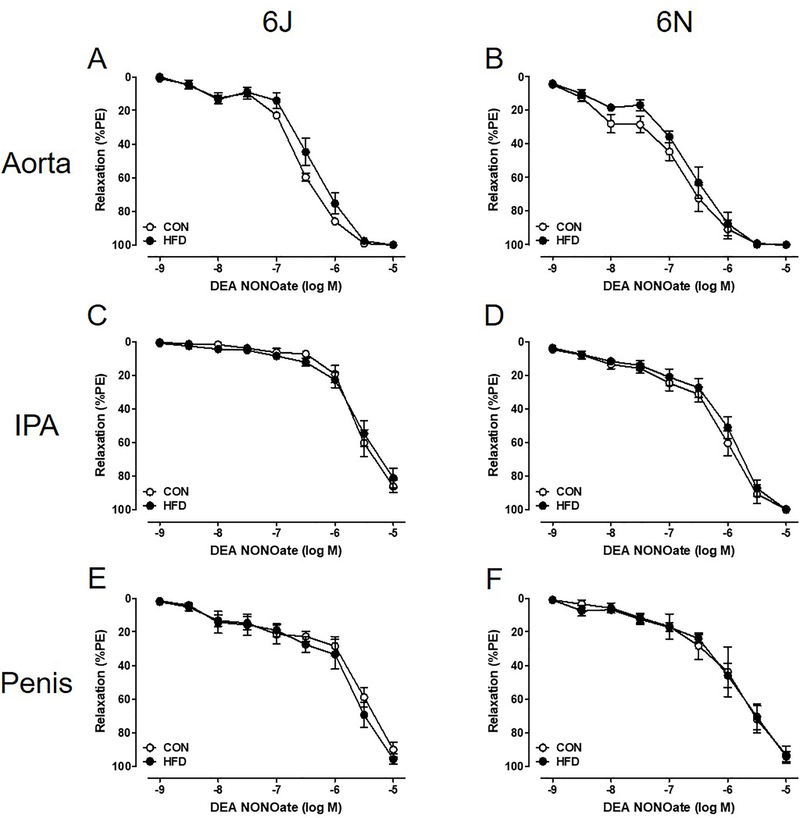
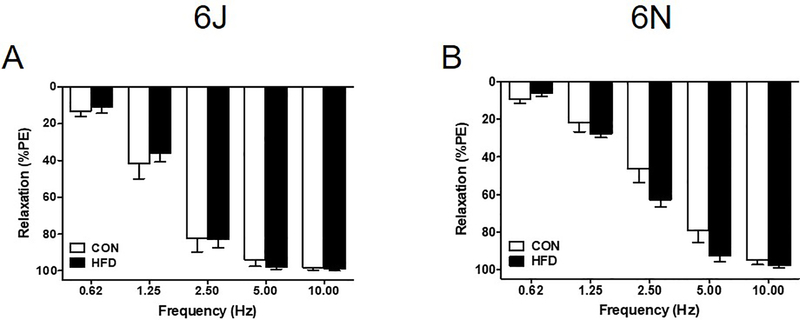
Unexpectedly, there is no evidence of physiologically relevant endothelial dysfunction in the systemic or penile vasculature. Vascular relaxation to ACh (endothelial-dependent, muscarinic receptor agonist) is unchanged following a chronic HFD, and both systemic and penile vasculature relaxes completely (Figure 5). Additionally, DEA NONOate (endothelial-independent, nitric oxide donor) fully relaxes all vascular beds independent of diet (Figure 6). EFS induced nerve-mediated relaxation in the penis is also unchanged in both HFD 6J and 6N mice (Figure 7).
Figure 5: A 12 week HFD does not induce endothelial dysfunction.
ACh relaxation was assessed in PE precontracted tissue. Maximum ACh relaxation does not change in 6J or 6N aorta (A,B), IPA (C,D), or penis (E,F). Data are presented as a percentage decrease from PE precontraction. n=6 per group. *P<0.05. Data are mean±SEM.
Figure 6: Endothelial independent smooth muscle relaxation is not affected by HFD.
Complete relaxation to DEA NONOate occurs in all PE precontracted tissue regardless of diet or strain (A-F). Data are presented as a percentage decrease from PE precontraction. n=6 per group. Data are mean±SEM.
Figure 7: HFD does not impair penile nerve mediated relaxation.
Electric field stimulation (EFS) was applied to PE precontracted penises to elicit neurotransmitter release and net relaxation response. Both 6J and 6N penises exhibit complete EFS relaxation despite a 12 week HFD (A,B). Data are presented as a percentage decrease from PE precontraction. n=6 per group. Data are mean±SEM.
Discussion
To investigate how strain dependent variations impact HFD induced obesity and vascular pathology responsible for ED, commonly used 6J and 6N mice were given a 12 week HFD under identical experimental conditions. The 12-week HFD causes 6J and 6N mice to develop distinct obese metabolic phenotypes yet neither strain developed penile vascular dysfunction commonly associated with ED. Both strains fed a HFD demonstrate significant increases in both body weight and percent body fat while consuming similar quantities of food. Following a bolus of glucose for GTT, both HFD strains show a significant increase in blood glucose after 30 minutes. Despite these metabolic impairments, HFD reduces systemic vascular contractility in both strains of mice, whereas neither strain exhibits impaired pre-penile or penile endothelial-dependent or -independent relaxation. The surprising lack of pre-penile and penile vascular pathology following a HFD questions the clinical applications for preclinical HFD induced obese mouse models of ED.
Our mouse models aim to replicate adult onset diet induced obesity. To avoid pubertal developmental complications associated with juvenile obesity (17), mice did not start respective CON or HFD diets until they were 10 weeks old and sexually mature. Initiating a HFD between 8–10 weeks of age is common; however, it’s not unusual for mice to begin a HFD before reaching sexual maturity – as early as 4 weeks of age (10,18–21). Starting the diet at 4 weeks of age will result in a more severe obese phenotype; however, this represents initiation of obesity in childhood into adulthood which can alter hormonal development. Our goal was to determine how obesity in adulthood impacts metabolic phenotype and vascular function. The composition of the HFD also plays a key role in the development of metabolic and vascular disorders. Western diets high in saturated fat and sucrose are considered responsible for the American obesity epidemic. Mouse models commonly use a 45% or 60% fat diet. The HFD used in this study was made up of a ratio of 177.5 g lard to 25 g soybean oil. It was important to minimize the soybean oil fat contribution. Soybean oil is rich in isoflavones, which are phytoestrogens thought to have anti-inflammatory and endocrine disrupting properties. When chronically administered to juvenile rats, isoflavones cause dose dependent ED in adulthood (22). In the current study, a 12 week 45% kcal HFD in adult mice is sufficient for both 6N and 6J strains to develop HFD induced obesity but not ED.
Though both strains become obese, the metabolic variations between the 6J and 6N obese phenotypes exist and studies are conflicting. Male 11-week old 6J and 6N mice fed a 60% HFD for 12 weeks had no difference between strains in weight gain (23). Another study fed 6 week old mice a 60% HFD for 14 weeks and report 6J mice gain more weight than 6N (24). In our study using 10-week old mice with 45% HFD, the 6N strain gained more body weight and greater fat composition. Differences in 6J and 6N glucose sensitivity are evident within 24 hours of starting a 60% HFD in 8-week old mice (13). Consistent with studies using similarly aged adult mice and 45–60% HFD; we find higher 6J blood glucose peaks higher during GTT than 6N (13,23). This is not surprising, since 6J mice have been shown to secrete less insulin in response to glucose (13). Mutations of the Nnt gene, which is involved in glucose-mediated insulin secretion, found in 6J mice may be the reason for impaired insulin response compared to 6N sub strains (25). We did not measure cholesterol levels or insulin sensitivity, which are study limitations. Moving forward, all studies should specify the strain of C57BL/6 mice used for rigor and reproducibility. Overall, the different metabolic strain outcomes in these studies could be due to diet sources, environment, or age the diet are initiated.
Both strains of HFD fed mice have similar vascular contractility changes. One noteworthy exception is the decrease in KCl induced contraction in aortas from 6N HFD mice. KCl contractions are rarely reported and often used to normalize contractions to other agonists. To avoid masking differences in contractile activity due to routine normalization, all contractile responses in this study are reported as units of force (mN). Interestingly, other HFD studies have reported similar reductions in adrenergic aortic contractions. Male C57BL/6 mice (unspecified strain) subjected to 24 weeks of HFD have reduced aortic PE contractions and increased heart rate, which the authors attributed to HFD induced autonomic dysfunction (21). Our study is the first to investigate how HFD impacts mouse pre-penile vasoreactivity. Two previous chronic HFD studies have assessed mouse penile contractility (8,10). Mice were fed a 55% HFD beginning at 4 weeks old and showed increased PE and EFS-mediated adrenergic cavernosal contraction. In contrast to our study, using a higher fat content and initiating the HFD at an earlier age causes greater penile adrenergic contractility.
Endothelial dysfunction is a hallmark characteristic of vasculogenic ED, yet mice in the current study exhibit no impaired vascular relaxation. We did not expect HFD to decrease aortic relaxation as other studies have shown that 24-week HFD mice experience no changes in ACh relaxation (20,21). In contrast, as other studies have demonstrated, we did expect impaired IPA and penile endothelial relaxation (8–10). For example, mice 10–13 weeks old (unknown strain) fed a 45% HFD for 12, 16 or 22 weeks show a significant reduction in the penile ACh ED50; however, they do not indicate if maximal ACh relaxation is decreased (9). Without showing maximal relaxation, we cannot conclude if the ACh results of this study differ from our current findings. It seems the age at which mice begin a HFD is a significant factor for ED development. Juvenile mice fed a HFD experience severe reduction in ACh relaxation and impaired neurotransmitter-mediated penile relaxation (8,10). In contrast, adult mice fed a HFD are not as susceptible at developing penile vascular dysfunction.
A major limitation to this study is we did not assess erectile function in vivo. However, this is also a limitation to most of the other mouse HFD studies reported in the discussion as they have also neglected to assess in vivo erectile function. If intracavernosal pressure is measured, it is in the absence of concurrent measurement of mean arterial pressure. Our mice were individually housed for duration of the HFD, whereas other studies housed their mice in pairs, or it was not indicated in the methods. The housing and the general institutional environments can lead to different stressors and may also play a role in disease development. The fat content, diet composition and duration of the diet should be considered. As researchers, we also need to consider the validity and translational relevance of manipulating juvenile animals to attain a disease phenotype developed in adulthood. Future studies should consider using even older animals (middle aged, retired breeders, or old) to induce obesity with HFD. Older HFD-fed animals are more likely to consistently develop obesity, severe vascular dysfunction, and ED. Additionally, older animals better model the aging male obese clinical population with a high incidence of ED.
Conclusions
Pre-clinical mouse models of diet induced obesity are commonly utilized for ED research. These HFD models vary widely in the age of HFD initiation, duration of HFD, development and severity of obesity, and genetic variations among commonly used mouse strains. We investigated strain dependent genetic variations as a causative factor for conflicting pre-clinical obesity induced ED data. A 12-week 45% HFD failed to develop impaired pre-penile or penile vascular or endothelial dysfunction in both 6J and 6N adult mouse strains. The conflicting results between our studies and others suggests mouse models of HFD induced adult onset obesity need to be critically assessed for their relevance as pre-clinical ED models. To fully understand the pathological progression of disease and develop efficacious ED therapies, clinically relevant HFD induced obesity and ED animal models are needed.
Acknowledgments
Funding
Funding was provided to MRO by a research grant from the Sexual Medicine Society of North America and by NIDDK K12-DK100024 grant, and to JLH by NIDDK Diabetic Complications Consortium (DiaComp, www.diacomp.org), grant DK076169 and DK115255.
Footnotes
Conflict of Interest
None.
References
- [1].Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA - J Am Med Assoc 2016;315:2284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Carr D, Murphy LF, Batson HD, Springer KW. Bigger Is Not Always Better: The Effect of Obesity on Sexual Satisfaction and Behavior of Adult Men in the United States. Men Masc 2013;16:452–77. [Google Scholar]
- [3].Walczak MK, Lokhandwala N, Hodge MB, Guay AT. Prevalence of cardiovascular risk factors in erectile dysfunction. J Gend Specif Med 2002;5:19–24. [PubMed] [Google Scholar]
- [4].Fillo J, Levcikova M, Ondrusova M, Breza J, Labas P. Importance of Different Grades of Abdominal Obesity on Testosterone Level, Erectile Dysfunction, and Clinical Coincidence. Am J Mens Health 2017;11:240–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Esposito K, Giugliano D. Obesity, the metabolic syndrome, and sexual dysfunction. Int J Impot Res 2005;17:391–8. [DOI] [PubMed] [Google Scholar]
- [6].Mobley DF, Khera M, Baum N. Recent advances in the treatment of erectile dysfunction. Postgrad Med J 2017;93:679–85. [DOI] [PubMed] [Google Scholar]
- [7].Heydemann A. An Overview of Murine High Fat Diet as a Model for Type 2 Diabetes Mellitus. J Diabetes Res 2016;2016:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Silva FH, Alexandre EC, Calmasini FB, Calixto MC, Antunes E. Treatment with Metformin Improves Erectile Dysfunction in a Murine Model of Obesity Associated with Insulin Resistance. Urology 2015;86:423.e1–423.e6. [DOI] [PubMed] [Google Scholar]
- [9].Xie D, Odronic SI, Wu F, Pippen A, Donatucci CF, Annex BH. Mouse Model of Erectile Dysfunction Due to Diet-Induced Diabetes Mellitus. Urology 2007;70:196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Toque HA, Da Silva FH, Calixto MC, Lintomen L, Schenka AA, Saad MJ, et al. High-fat diet associated with obesity induces impairment of mouse corpus cavernosum responses. BJU Int 2011;107:1628–34. [DOI] [PubMed] [Google Scholar]
- [11].Simon MM, Greenaway S, White JK, Fuchs H, Gailus-durner V, Wells S, et al. A comparative phenotypic and genomic analysis of C57BL / 6J and C57BL / 6N mouse strains. Genome Biology 2013:14:R82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Keane TM, Goodstadt L, Danecek P, White MA, Wong K, Yalcin B, et al. Mouse genomic variation and its effect on phenotypes and gene regulation. Nature 2011;477:289–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Fisher-Wellman KH, Ryan TE, Smith CD, Gilliam LAA, Lin C Te, Reese LR, et al. A direct comparison of metabolic responses to high-fat diet in c57bl/6j and c57bl/6nj mice. Diabetes 2016;65:3249–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hannan JL, Blaser MC, Oldfield L, Pang JJ, Adams SM, Pang SC, et al. Morphological and functional evidence for the contribution of the pudendal artery in aging-induced erectile dysfunction. J Sex Med 2010;7:3373–84. [DOI] [PubMed] [Google Scholar]
- [15].Luttrell IP, Swee M, Starcher B, Parks WC, Chitaley K. Erectile dysfunction in the type II diabetic db/db mouse: impaired venoocclusion with altered cavernosal vasoreactivity and matrix. Am J Physiol Heart Circ Physiol 2008;294:H22204–11. [DOI] [PubMed] [Google Scholar]
- [16].Zhou Y, Dirksen WP, Zweier JL, Periasamy M. Endothelin-1-induced responses in isolated mouse vessels: the expression and function of receptor types. Am J Physiol Hear Circ Physiol 2004;287:H573–8. [DOI] [PubMed] [Google Scholar]
- [17].Burt Solorzano CM, McCartney CR. Obesity and the pubertal transition in girls and boys. Reproduction 2010;140:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Montgomery MK, Hallahan NL, Brown SH, Liu M, Mitchell TW, Cooney GJ, et al. Mouse strain-dependent variation in obesity and glucose homeostasis in response to high-fat feeding. Diabetologia 2013;56:1129–39. [DOI] [PubMed] [Google Scholar]
- [19].Dou J, Li H, Ma X, Zhang M, Fang Q, Nie M, et al. Osteocalcin attenuates high fat diet-induced impairment of endothelium-dependent relaxation through Akt/eNOS-dependent pathway. Cardiovasc Diabetol 2014;13:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Xia N, Weisenburger S, Koch E, Burkart M, Reifenberg G, Förstermann U, et al. Restoration of perivascular adipose tissue function in diet-induced obese mice without changing bodyweight. Br J Pharmacol 2017;174:3443–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bruder-Nascimento T, Ekeledo OJ, Anderson R, Le HB, Belin de Chantemèle EJ. Long term high fat diet treatment: An appropriate approach to study the sex-specificity of the autonomic and cardiovascular responses to obesity in mice. Front Physiol 2017;8:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Pan L, Xia X, Feng Y, Jiang C, Cui Y, Huang Y. Exposure of juvenile rats to the phytoestrogen daidzein impairs erectile function in a dose-related manner in adulthood. J Androl 2008;29:55–62. [DOI] [PubMed] [Google Scholar]
- [23].Attané C, Peyot ML, Lussier R, Zhang D, Joly E, Madiraju SRM, et al. Differential insulin secretion of high-fat diet-fed C57BL/6NN and C57BL/6NJ mice: Implications of mixed genetic background in metabolic studies. PLoS One 2016;11:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Nicholson A, Reifsnyder PC, Malcolm RD, Lucas CA, MacGregor GR, Zhang W, et al. Diet-induced obesity in two C57BL/6 substrains with intact or mutant nicotinamide nucleotide transhydrogenase (Nnt) gene. Obesity 2010;18:1902–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Freeman HC, Hugill H, Dear NT, Ashcroft FM, Cox RD. Deletion of nicotinamide nucleotide transhydrogenase: a new quantitive trait locus accounting for glucose intolerance in C57BL/6J mice. Diabetes 2006;55(7):2153–6. [DOI] [PubMed] [Google Scholar]