Abstract
RNA cleavages by many ribonucleases generate RNA molecules that contain a 2′,3′-cyclic phosphate (cP) at their 3′-termini, and many cP-containing RNAs (cP-RNAs) are expressed as functional molecules in cells and tissues. 5′-tRNA half molecules are representative examples of functional cP-RNAs, playing important roles in various biological processes. We here show in vitro production of cP-containing 5′-tRNA half molecules that is able to prepare abundant synthetic cP-RNAs enough for functional analyses. Furthermore, we report a multiplex TaqMan RT-qPCR method which can simultaneously quantify multiple cP-containing 5′-tRNA half species. The method enabled us to efficiently quantify 5′-tRNA halves using samples with limited amounts, such as human plasma samples, revealing drastic enhancement of 5′-tRNA half levels at approximately 1000-fold in patients infected with Mycobacterium tuberculosis. These in vitro production and multiplex quantification methods can be applied to any cP-RNAs, and they provide cost-effective, in-house techniques to accelerate expressional and functional characterizations of 5′-tRNA halves and other cP-RNAs.
1. Introduction
In biogenesis of non-coding RNA (ncRNA) molecules, newly transcribed RNAs usually undergo multiple maturation steps in which enzymatic cleavages of the RNAs play crucial roles. RNA cleavages by many ribonucleases generate RNA molecules that contain a 2′,3′-cyclic phosphate (cP) at their 3′-termini [1]. The 3′-terminal cP end is also formed by ribozyme cleavages or is generated de novo by the enzymes, such as RtcA and MthRnl, that convert a 3′-terminal phosphate (P) to a cP [1]. Many cP-containing RNAs (cP-RNAs) have been demonstrated to be expressed as functional molecules. Representative examples of functional cP-RNAs include the 5′-tRNA half molecules produced from tRNA anticodon cleavage by angiogenin (ANG), a member of the RNase A super family [1]. In mammalian cells, ANG cleavage of tRNAs is induced by various biological phenomenon, such as stress stimuli, the sex hormone signaling pathway, and mycobacterial infection [2–6]. The resultant cP-containing 5′-tRNA half molecules play important roles in a variety of biological processes. Stress-induced 5′-tRNA halves promote stress granule formation, regulate translation, and trigger cellular stress responses and apoptosis in neurodevelopmental disorders [2, 7–10]. Sex hormone-dependent 5′-tRNA halves promote cell proliferation in hormone-dependent breast and prostate cancers [4, 11]. Infection-induced 5′-tRNA halves promote immune response by activating Toll-like receptor 7 (TLR7) [5]. 5′-tRNA halves further serve as direct precursors of Piwi-interacting RNAs (piRNAs) in germ cells [12]. Because cP-RNAs cannot be ligated to a 3’-adaptor (AD) in standard RNA-seq procedure, cP-RNAs are not captured by standard RNA-seq, forming a hidden component in the transcriptome [1]. We developed cP-RNA-seq that is able to specifically sequence cP-RNAs [4, 13] and recently performed genome-wide identification of short cP-RNA transcriptome, revealing abundant expression of numerous cP-RNAs derived not only from tRNAs but also from rRNAs and mRNAs [6, 14].
To further expand cP-RNA research, it is imperative to continuously unravel expressional regulations and biological roles of cP-RNAs. Many previous functional characterizations of 5′-tRNA halves relied on the transfection of cP-lacking synthetic RNAs into cultured cells. However, cP formation is not just the consequence of specific ribonuclease digestions—cP formation itself could have a functional significance: cP formation regulates RNA-protein interaction and RNA stability [15, 16] and it is required for specific ligation reaction [17–19]. Recent discovery of cP-specific phosphatase further suggests the importance of cP-formation [20]. Thus, even though cP-RNA syntheses are relatively costly and not many companies offer them, it is ideal to utilize synthetic cP-RNAs, instead of cP-lacking RNAs, for functional analyses of cP-RNAs. Utilization of synthetic cP-RNAs would also greatly contribute to unraveling fundamental roles of a cP. In this light, we here show in vitro production of cP-containing 5′-tRNA half molecules that is able to prepare abundant synthetic cP-RNAs enough for functional analyses. Furthermore, given that the expression levels of 5′-tRNA half molecules are drastically changed in various biological processes and diseases [2, 4–6, 21], a specific cP-RNA quantification method would be an indispensable technique in cP-RNA research. We here report a multiplex TaqMan RT-qPCR method that efficiently, specifically, and simultaneously quantifies multiple cP-containing 5′-tRNA half species. These methods provide cost-effective, in-house techniques to accelerate characterizations of 5′-tRNA halves and other cP-RNAs.
2. Materials and Methods
2.1. In vitro production of cP-containing 5′-tRNA halves
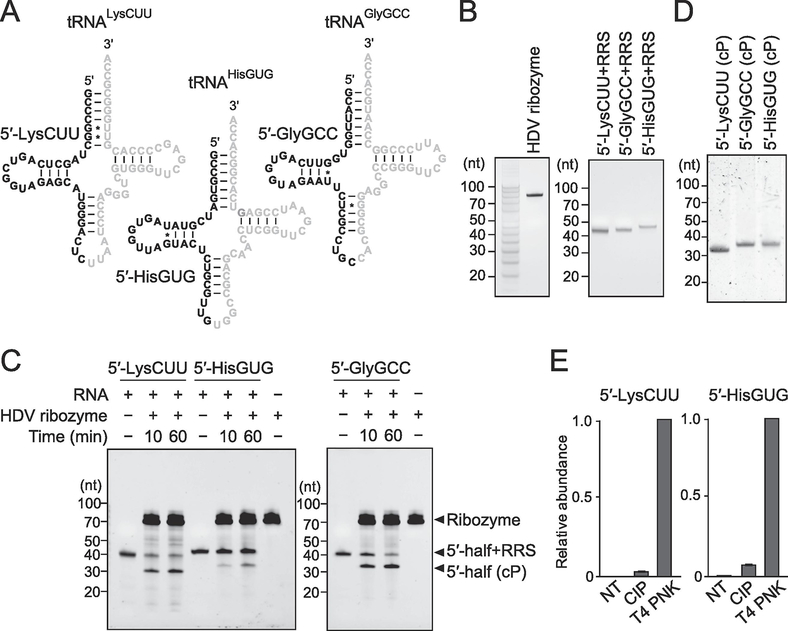
The sequences of three cP-containing 5′-tRNA halves (5′-tRNALysCUU half, 5′-tRNAHisGUG half, and 5′-tRNAGlyGCC half) synthesized in this study are shown in Fig. 1A. We produced the three cP-RNAs according to the Ciesiołka’s method [22] with modifications. The method consists of the two steps: 1) in vitro synthesis of RNAs containing a 3′-terminal hepatitis delta virus (HDV) ribozyme recognition site (RRS: 5′-GGGUCGG-3′); and 2) 3′-end cleavage of the synthesized RNAs by trans-acting HDV ribozyme to form a cP end. In vitro RNA synthesis was performed as described previously [5, 23]. dsDNA templates were synthesized using the primers shown in Table S1. The templates were then subjected to an in vitro transcription reaction with T7 RNA polymerase (New England Biolabs) at 37°C for 4 h. The synthesized RNAs were then gel-purified using denaturing PAGE with single-nucleotide resolution. The in vitro synthesized RRS-containing 5′-tRNA half (3 μM) and HDV ribozyme (6 μM) were subjected to annealing procedure by incubating in 18 μl mixture containing 2 μl of 10× reaction buffer [500 mM Tris-HCl (pH 7.5) and 1 mM EDTA] at 90°C for 2 min, followed by incubation at 0°C for 10 min and then at 37°C for 10 min. Subsequently, RNA cleavage by HDV ribozyme was started by adding 2 μl of 100 mM MgCl2 solution and incubating at 37°C for 60 min. The cleaved RNAs were then gel-purified using denaturing PAGE.
Figure 1. In vitro production of cP-containing 5′-tRNA halves.
(A) The targeted 5′-tRNA half’s regions are shown in black in the cloverleaf secondary structure of respective cytoplasmic tRNAs.
(B) In vitro synthesized HDV ribozyme and RRS-containing 5′-tRNA halves [5′-tRNALysCUU half (5′-LysCUU), 5′-tRNAHisGUG half (5′-HisGUG), and 5′-tRNAGlyGCC half (5′-GlyGCC)] were gel-purified and developed in denaturing PAGE.
(C) For trans-acting HDV ribozyme cleavage, the in vitro synthesized RNAs were incubated with HDV ribozyme for 10 min or 60 min. The bands of resultant cP-containing 5′-tRNA halves were observed after the cleavage reactions.
(D) The produced cP-containing 5′-tRNA halves were gel-purified and developed in denaturing PAGE.
(E) The 3′-terminal phosphate states of the produced 5′-tRNA halves were analyzed enzymatically. The RNAs were treated with CIP or T4 PNK (NT: nontreated samples used as negative controls) and subjected to 3′-AD ligation. The ligation efficiency was estimated by quantifying the AD-ligated RNAs using TaqMan RT-qPCR. The amounts from T4 PNK-treated RNA were set as 1, and relative amounts are indicated. Averages of three technical replicates with SD values are shown.
2.2. Confirmation of a cP end in the produced 5′-tRNA halves
The 3′-terminal phosphate states of the produced 5′-tRNA halves were analyzed as described previously [6, 12, 14]. The RNAs were treated with calf intestinal phosphatase (CIP; New England Biolabs) or T4 polynucleotide kinase (T4 PNK; New England Biolabs). The RNAs were then subjected to specific quantification of 5′-tRNA halves using 3′-AD ligation and TaqMan RT-qPCR as described previously [4–6, 12, 14].
2.3. Multiplex TaqMan RT-qPCR for quantification of synthetic 5′-tRNA halves
The produced cP-containing 5′-tRNA halves (100 fmol each), mixed with 500 ng of E. coli total RNA, were treated with 10 units of T4 PNK in 10 μl reaction mixture at 37°C for 1 h. One μl of the mixture was then added to a 3′-AD ligation mixture (total 10 μl) containing 0.1 mg/ml BSA, 1 mM ATP, 5% PEG8000, 2 μM of 3′-AD [4], 20 units of RNasin® Ribonuclease Inhibitor (Promega), 5 units of T4 RNA ligase (T4 Rnl: Thermo Scientific), and 1× T4 Rnl reaction buffer. For 3′-AD ligation, the mixture was incubated at 37°C for 1 h, followed by overnight incubation at 4°C. The ligated RNAs were diluted 20-fold, and 2 μl of the diluted mixture was subjected to TaqMan RT-qPCR (reaction mixture volume 10 μl) using One Step PrimeScript RT-PCR Kit (Takara) and StepOne Plus Real-time PCR machine (Applied Biosystems). Two groups of multiplex quantification were established: Group 1 for 5′-tRNAHisGUG half (hexachlorofluorescein, HEX), 5′-tRNAGlyGCC half (carboxytetramethylrhodamine, TAMRA NHS Ester), and spike-in control RNA (6-carboxyfluorescein, FAM; 5′-GGGAGGCAAGCCCGACGUCGUCCAGAUUGUCCGC-3′), and Group 2 for 5′-tRNALysCUU half (HEX) and spike-in control RNA (FAM), respectively. The sequences and concentrations of primers and TaqMan probes are shown in Table S2. The cycle conditions of PCR were: 42°C for 5 min and 95°C for 10 s, followed by up to 40 cycles of 95°C for 10 s and 63°C for 34 s.
2.4. Cell culture, induction of oxidative stress, and RNA isolation
HeLa cells were cultured in DMEM (Life Technologies) containing 10% FBS. The cells were treated with 500 μM of sodium arsenite (SA; Sigma) to induce oxidative stress and expression of stress-induced tRNA halves as described previously [6]. Total RNA from the cells was isolated using TRIsure (Bioline).
2.5. Multiplex quantification of 5′-tRNA halves for cellular RNAs
To remove a cP from cP-RNAs, total RNA (500 ng) was first treated with T4 PNK as described previously [4]. Then, 50 ng of the treated RNAs were subjected to 3′-AD ligation reaction, followed by multiplex TaqMan RT-qPCR as described in 2.3.
2.6. Ethical approval, human plasma samples, and RNA isolation
The Office of Human Research (OHR) of Thomas Jefferson University (TJU) approved our use of human plasma samples from patients infected with Mycobacterium tuberculosis (Mtb) without private information in accordance with all federal, institutional, and ethical guidelines. We obtained the plasma samples from a biological specimen company, BioIVT, without receiving patients’ information. Human plasma samples were derived from healthy or Mtb-infected males aged 30–35 years. RNA isolation from the plasma samples was performed as described previously [5]. First, 500 μl of plasma was centrifuged at 16,060 g for 5 min, and then 400 μl of supernatant was mixed with 1 fmol of the spike-in control RNA and subjected to RNA extraction using TRIzol LS (Invitrogen). The extracted RNAs were further subjected to purification using the miRNeasy Mini Kit (Qiagen).
2.7. Multiplex quantification of 5′-tRNA halves for plasma RNAs
We adapted the above-described multiplex 5′-tRNA half quantification method to plasma RNAs by combining T4 PNK treatment and 3′-AD ligation into one-step reaction. Plasma RNA sample (2 μl) was added to the reaction mixture (total volume 20 μl) containing 0.1 mg/ml BSA, 2 mM ATP, 5% PEG8000, 1 μM 3′-AD, 40 units of RNasin® Ribonuclease Inhibitor, 10 units of T4 Rnl, 10 units of T4 PNK, 1 mM DTT, and 1× T4 RNA ligase reaction buffer. The reaction mixture was first incubated at 37°C for 1 h, followed by incubation at 4°C for 1 h. The reacted mixture was diluted 3-fold, and 2 μl of the diluted mixture was subjected to the multiplex TaqMan RT-qPCR (reaction mixture volume 10 μl) as described in 2.3.
3. Results and Discussion
3.1. Selection of 5′-tRNA half species for production and quantification
In this study, we selected the three 5′-tRNA halves (5′-tRNALysCUU half, 5′-tRNAHisGUG half, and 5′-tRNAGlyGCC half, Fig. 1A) because they are abundantly and differentially expressed in various cells and tissues. 5′-tRNALysCUU half and 5′-tRNAGlyGCC half were the two most abundant tRNA-derived cP-RNAs in oxidative stress-induced HeLa and U2OS cells [6]. Together, the 5′-tRNALysCUU half and 5′-tRNAHisGUG half occupied >87% of sex hormone-dependent tRNA-derived cP-RNAs in BT-474 breast cancer cells [4]. 5′-tRNAHisGUG half and 5′-tRNAGlyGCC half were among the top 4 abundant 5′-tRNA halves identified in extracellular vehicles (EVs) secreted from human monocyte-derived macrophages [5]. The EV-5′-tRNAHisGUG half, which plays an important role in promoting the immune response by activating TLR7, were greatly upregulated in plasma samples from Mtb-infected patients [5]. Regarding the 5′-tRNALysCUU half and 5′-tRNAHisGUG half, the molecules ranging from the 5′-end to the anticodon 1st nucleotide [nucleotide position (np) 1–34 according to the nucleotide numbering system of tRNAs [24]] (Fig. 1A) were commonly the most abundant molecules in the above cP-RNA-seq studies, so we targeted these molecules in this study. Regarding the 5′-tRNAGlyGCC half, its 3′-end position showed variations in cP-RNA-seq data from various cells and tissues. In this study, we targeted the 5′-tRNAGlyGCC half of np 1–35 (Fig. 1A), which accumulated the most prominently as stress-induced molecules [6] and which was the most abundant in mouse tissues [14].
3.2. In vitro production of cP-containing 5′-tRNA halves
In vitro transcription by bacteriophage T7 RNA polymerase has been a standard, widely used method to synthesize RNAs of desired sequences [25], but the synthesized RNAs possess a hydroxyl group (OH) at their 3′-end. To enable rapid, abundant, and cost-effective synthesis of cP-containing 5′-tRNA halves, we employed the in vitro RNA synthesis, followed by 3′-terminal cleavage of the RNAs using a trans-acting HDV ribozyme based on the Ciesiołka’s method [22, 26]. As in the RNA cleavages catalyzed by all other self-cleaving ribozymes, HDV ribozyme cleavage forms a cP at the 3′-end of 5′-cleavage products [27]. To be cleaved by the HDV ribozyme, RNAs need to contain 7-nt RRS sequences; these sequences are recognized and hybridized by the ribozyme [22, 26]. In vitro transcription by T7 RNA polymerase successfully synthesized the three 5′-tRNA halves containing 3′-terminal RRS sequences, as well as the HDV ribozyme (Fig. 1B). After gel-purification, the RRS-containing 5′-tRNA halves were subjected to HDV ribozyme-catalyzed cleavage. Incubation of the RNAs with the HDV ribozyme produced RRS-lacking 5′-tRNA halves (Fig. 1C), which were expected to contain a cP. Compared to a 10-min incubation, an incubation of 60 min provided better yields of the cleavage products (Fig. 1C), prompting us to set 60 min as the default incubation time for future 5′-tRNA half synthesis by this method. After gel-purification, we confirmed the quality of the produced cP-containing 5′-tRNA halves as a single band in denaturing PAGE (Fig. 1D). To confirm the presence of a cP in the synthesized 5′-tRNA halves, we treated the RNAs with CIP (removes 3′-P) or T4 PNK (removes both 3′-P and cP). When we subsequently examined the efficiency of the ligations between each RNA and a 3′-AD by TaqMan RT-qPCR, as described in our previous studies [4–6, 12, 14], the produced 5′-tRNA halves exhibited the highest amplification signals upon T4 PNK treatment, while non-treated RNAs and CIP-treated RNAs did not yield significant amplification signals (Fig. 1E). These results indicate that the majority of the produced 5′-tRNA halves contain a cP at their 3′-ends and provide evidence of the successful production of cP-containing 5′-tRNA halves. Approximately 16–74 μg (~1.4–6.7 nmol) of cP-containing 5′-tRNA halves were produced from 1 ml of T7 RNA polymerase reaction. This method is easy to be scaled-up and is applicable to abundantly produce any short cP-RNAs. The produced cP-RNAs will be useful for various experiments in biochemical and functional characterization of cP-RNAs (e.g., transfection, pull-down assay, and electrophoretic mobility shift assay).
3.3. Multiplex TaqMan RT-qPCR for quantification of cellular 5′-tRNA halves
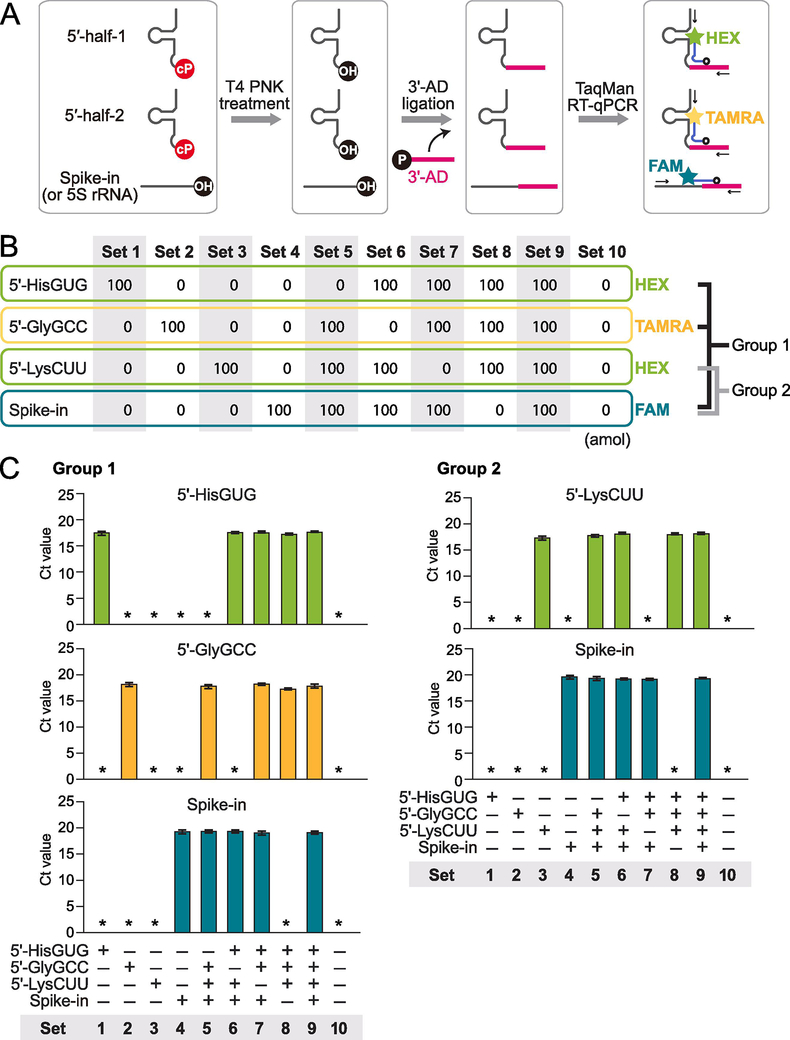
The majority of cP-RNAs are produced from abundant substrate RNAs [14]. As a result, cP-RNAs and their substrate RNAs usually coexist in the cells and in total RNA. For example, 5′-tRNA halves coexist with corresponding mature tRNAs and their precursors, which are usually much more abundant than 5′-tRNA halves. Standard RT-qPCR amplification of the interior sequences of 5′-tRNA halves cannot distinguish signals of 5′-tRNA halves and their substrate RNAs. Instead, we previously developed a specific TaqMan RT-qPCR method that is able to exclusively quantify 5′-tRNA halves [4] and utilized this method for quantification of 5′-tRNA halves in human cultured cells, Bombyx cells, and mouse tissues [5, 6, 12, 14]. While the previous method was singleplex and targeted only one 5′-tRNA half per reaction, here we established an upgraded version of TaqMan RT-qPCR by employing a multiplex approach that involves three steps (Fig. 2A). First, we treated total RNA extracted from cells or tissues with T4 PNK to remove a cP from 5′-tRNA halves. Then we ligated 3′-AD to the 3′-dephosphorylated 5′-tRNA halves, followed by quantification of the ligation products by TaqMan RT-qPCR. Because TaqMan probe is designed to target the boundary of the targeted 5′-tRNA halves and 3′-AD, this method quantified only the 3′-AD-ligated 5′-tRNA half but not the corresponding mature tRNA and its precursor. To perform the TaqMan RT-qPCR, we conducted a one-step reaction of reverse transcription and qPCR quantification in the presence of multiple target primers and TaqMan probes (Fig. 2A). Two target 5′-tRNA halves were simultaneously quantified by TaqMan probes either with HEX or TAMRA, and control spike-in RNA was also detected by FAM-containing probe at the same time. This multiplex method has two advantages over the singleplex method previously used. First, more than one target 5′-tRNA half can be simultaneously quantified, which saves time, effort, cost, and RNA sample for 5′-tRNA half quantification. Saving the amounts of starting RNA sample is especially important when using precious and limited samples such as those from disease patients. Second, this method allows us to quantify 5′-tRNA halves and control RNA at the same time. Because the amounts of control RNA are used for normalization, the simultaneous quantification would enhance accuracy of the 5′-tRNA half quantification.
Figure 2. Establishment of multiplex TaqMan RT-qPCR method for 5′-tRNA halves.
(A) Schematic representation of multiplex quantification of 5′-tRNA halves.
(B) We used 10 sets of synthetic RNA mixtures to confirm the specificity of the multiplex TaqMan RT-qPCR method. The 5′-tRNAHisGUG half (HEX), 5′-tRNAGlyGCC half (TAMRA), and spike-in RNA (FAM) are simultaneously quantified in Group 1, and the 5′-tRNALysCUU half (HEX) and spike-in RNA (FAM) are quantified in Group 2.
(C) The multiplex method was applied to the 10 sets of synthetic RNAs. The results showed specific detection of targeted RNAs by each TaqMan probe without cross-reaction. Asterisk indicates that the amplification signal was not detected.
To establish our multiplex scheme and to confirm its specificity, we first used synthetic cP-containing 5′-tRNA halves produced by our in vitro method (Fig. 1D), as well as synthetic control spike-in RNA. Not all multiplex combinations of targeted RNAs resulted in specific quantification of each target RNA without cross-reaction. We eventually established two groups of multiplex quantifications: Group 1 for the quantification of the 5′-tRNAHisGUG half (HEX), 5′-tRNAGlyGCC half (TAMRA), and spike-in RNA (FAM); and Group 2 for the quantification of the 5′-tRNALysCUU half (HEX) and spike-in RNA (FAM) (Fig. 2B). To confirm their specificities, 10 sets of synthetic RNA mixtures consisting of various combinations of the three 5′-tRNA halves and spike-in RNA (Fig. 2B) were subjected to the multiplex TaqMan RT-qPCR for Groups 1 and 2. As shown in Fig. 2C, in both Groups 1 and 2, each targeted RNA was specifically detected without being influenced by the presence of other RNAs in the mixture. The mixtures lacking the targeted RNAs did not yield amplification signals at all. These results confirmed that our multiplex method is able to specifically quantify the targeted 5′-tRNA halves.
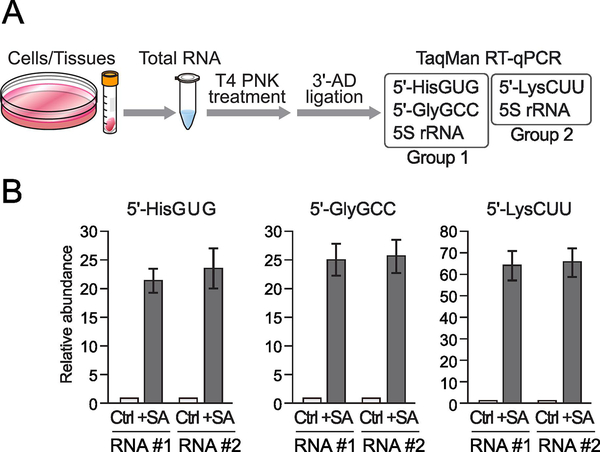
We next examined applicability of our multiplex TaqMan RT-qPCR method to cellular total RNA. When cellular RNAs are used as materials, instead of control spike-in RNA, the 5S rRNA is detected as a control for normalization. As a representative example of cellular samples which show differential expression of 5′-tRNA halves, we utilized total RNA from SA-treated HeLa cells where the induction of oxidative stress accumulated stress-induced, cP-containing 5′-tRNA halves [6]. Total RNAs from non-treated (control) or SA-treated HeLa cells were subjected to T4 PNK treatment, followed by 3′-AD ligation and multiplex TaqMan RT-qPCR for Groups 1 and 2 (Fig. 3A). Relative abundances of the 5′-tRNA halves between non-treated and SA-treated samples were normalized by the abundance of simultaneously quantified 5S rRNA. We observed upregulation of all of the quantified 5′-tRNA halves (Fig. 3B), which is consistent with our previous study [6]. Upon SA treatment, the expression of the 5′-tRNAHisGUG half, 5′-tRNAGlyGCC half, and 5′-tRNALysCUU half increased 22.6-, 25.3-, and 65.2-fold (average of two biological replicates), respectively.
Figure 3. Multiplex quantification of 5′-tRNA halves using cellular total RNA.
(A) Schematic representation of multiplex quantification of 5′-tRNA halves using cellular total RNA.
(B) The total RNA from HeLa cells treated with SA or water (control: Ctrl) for 2 h were subjected to multiplex TaqMan RT-qPCR for the indicated 5′-tRNA halves. The quantified 5′-tRNA half levels were normalized to the levels of 5S rRNA. The amounts from control cells were set as 1, and relative amounts are indicated. Averages of three experiments with SD values are shown.
3.4. Multiplex TaqMan RT-qPCR for quantification of plasma 5′-tRNA halves
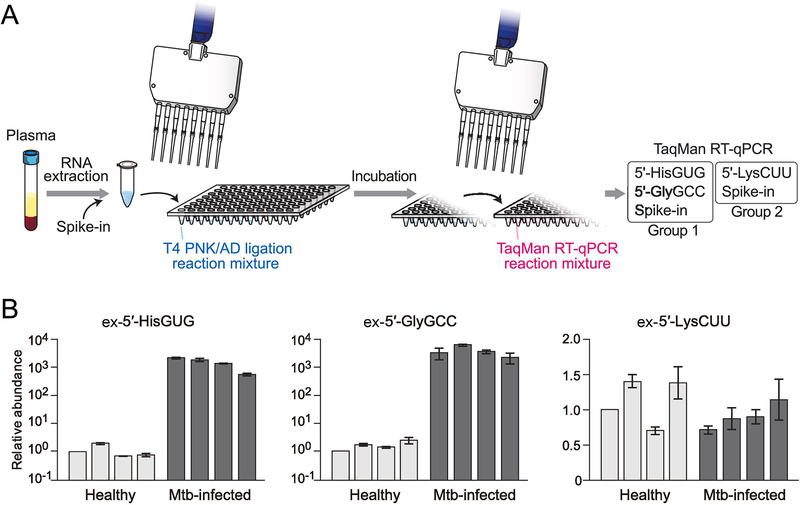
Dynamic secretion of RNA molecules and transportation of the secreted extracellular RNAs (exRNAs) into recipient cells can modulate their phenotypes, showing that exRNAs act as key transducers of intercellular communications [28, 29]. These exRNAs circulate in biofluids, and given their accessibility, abundance, and stability, coupled with their cellular roles, they are regarded as a powerful source of biomarkers for diseases [30]. Our recent study revealed upregulation of extracellular 5′-tRNA halves (ex-5′-tRNA halves) in plasma samples from Mtb-infected patients [5], prompting us to establish multiplex quantification method of ex-5′-tRNA halves for plasma samples. Because human tissue samples are precious and generally limited in their quantity, we established a more efficient and convenient version of the method for multiplex 5′-tRNA half quantification (Fig. 4A) by making the following modifications. First, we simultaneously performed the T4 PNK treatment and 3′-AD ligation as one step in one tube, which can reduce starting amounts of RNA samples. Second, we shortened the incubation time for the T4 PNK treatment/3′-AD ligation to 2 h (1 h at 37°C, followed by 1 h at 4°C), which increases detection efficiency of ex-5′-tRNA halves due to less degradation during the reactions. Both modifications are important, especially when starting RNA amounts are limited. At the same time, this method requires only two sample transfers between tubes or plates, enabling us to conveniently quantify ex-5′-tRNA halves using large numbers of samples (Fig. 4A).
Figure 4. Multiplex quantification of 5′-tRNA halves using plasma RNA.
(A) Schematic representation of the convenient version of multiplex 5′-tRNA half quantification using plasma RNA.
(B) RNAs isolated from plasma samples of healthy individuals or Mtb-infected patients were subjected to the convenient version of multiplex TaqMan RT-qPCR for the indicated 5′-tRNA halves. The quantified 5′-tRNA half levels were normalized to the levels of spike-in RNA. The amounts from one of the healthy individuals were set as 1, and relative amounts are indicated. Averages of three experiments with SD values are shown.
To test the applicability of our method, we quantified the three ex-5′-tRNA halves in plasma samples from healthy individuals or Mtb-infected patients. Because the expression of tRNA halves can be affected by sex hormones [4] and aging [14], we limited the examined individuals to males aged 30 to 35 years as in our previous study [5]. A synthetic spike-in RNA was added during plasma RNA extraction, and its abundance was later used for normalization. We successfully quantified the relative abundance of the three examined ex-5′-tRNA halves by using the convenient version of multiplex TaqMan RT-qPCR (Fig. 4B). The expression levels of the ex-5′-tRNAHisGUG half and ex-5′-tRNAGlyGCC half were drastically enhanced approximately 1000-fold in Mtb-infected patients compared to healthy individuals, while the levels of the ex-5′-tRNALysCUU half showed similar abundance between the healthy individuals and the patients. These results proved the applicability of the convenient version of our method to the samples with limited RNA quantity, such as plasma samples.
3.5. Conclusion
In conclusion, we showed an in vitro method using HDV ribozyme cleavage for production of cP-containing 5′-tRNA halves, and we further established an efficient and convenient multiplex TaqMan RT-qPCR method for 5′-tRNA half quantification. Molecular function of 5′-tRNA halves/cP-RNAs can vary depending on their sequences and species, such that the 5′-tRNAHisGUG half activates TLR7 whereas the 5′-tRNAGluCUC half does not [5]. As cP-RNA research is being expanded, it will become more important to capture individual cP-RNA species and perform expressional and functional characterizations for each cP-RNA. Our cP-RNA production and quantification methods are widely applicable to any cP-RNAs, including recently-identified mRNA- and rRNA-derived cP-RNAs [6, 14]. Further, our multiplex method can be useful to screen various biological materials and disease samples to characterize differential cP-RNA expressions. As cP-RNA research is being expanded, our methods will provide much-needed techniques to produce and quantify the 5′-tRNA halves and other cP-RNAs.
Supplementary Material
Acknowledgments
We are grateful to Dr. Kamlesh Pawar for helpful discussion. This study was supported in part by the National Institutes of Health Grant (GM106047, HL150560, and AI151641, to YK) and American Cancer Society Research Scholar Grant (RSG-17-059-01-RMC, to YK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Shigematsu M, Kawamura T, Kirino Y, Generation of 2’,3’-Cyclic Phosphate-Containing RNAs as a Hidden Layer of the Transcriptome, Front Genet 9 (2018) 562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Yamasaki S, Ivanov P, Hu GF, Anderson P, Angiogenin cleaves tRNA and promotes stress-induced translational repression, J Cell Biol 185(1) (2009) 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Fu H, Feng J, Liu Q, Sun F, Tie Y, Zhu J, Xing R, Sun Z, Zheng X, Stress induces tRNA cleavage by angiogenin in mammalian cells, FEBS Lett 583(2) (2009) 437–42. [DOI] [PubMed] [Google Scholar]
- [4].Honda S, Loher P, Shigematsu M, Palazzo JP, Suzuki R, Imoto I, Rigoutsos I, Kirino Y, Sex hormone-dependent tRNA halves enhance cell proliferation in breast and prostate cancers, Proc Natl Acad Sci U S A 112(29) (2015) E3816–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pawar K, Shigematsu M, Sharbati S, Kirino Y, Infection-induced 5′-half molecules of tRNAHisGUG activate Toll-like receptor 7, PLoS Biol 18(12) (2020) e3000982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Shigematsu M, Kirino Y, Oxidative stress enhances the expression of 2’,3’-cyclic phosphate-containing RNAs, RNA Biol 17(8) (2020) 1060–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ivanov P, Emara MM, Villen J, Gygi SP, Anderson P, Angiogenin-induced tRNA fragments inhibit translation initiation, Molecular cell 43(4) (2011) 613–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Blanco S, Dietmann S, Flores JV, Hussain S, Kutter C, Humphreys P, Lukk M, Lombard P, Treps L, Popis M, Kellner S, Holter SM, Garrett L, Wurst W, Becker L, Klopstock T, Fuchs H, Gailus-Durner V, Hrabe de Angelis M, Karadottir RT, Helm M, Ule J, Gleeson JG, Odom DT, Frye M, Aberrant methylation of tRNAs links cellular stress to neuro-developmental disorders, The EMBO journal 33(18) (2014) 2020–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ivanov P, O’Day E, Emara MM, Wagner G, Lieberman J, Anderson P, G-quadruplex structures contribute to the neuroprotective effects of angiogenin-induced tRNA fragments, Proceedings of the National Academy of Sciences of the United States of America 111(51) (2014) 18201–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Emara MM, Ivanov P, Hickman T, Dawra N, Tisdale S, Kedersha N, Hu GF, Anderson P, Angiogenin-induced tRNA-derived stress-induced RNAs promote stress-induced stress granule assembly, J Biol Chem 285(14) (2010) 10959–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Honda S, Kirino Y, SHOT-RNAs: A novel class of tRNA-derived functional RNAs expressed in hormone-dependent cancers, Mol Cell Oncol 3(2) (2016) e1079672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Honda S, Kawamura T, Loher P, Morichika K, Rigoutsos I, Kirino Y, The biogenesis pathway of tRNA-derived piRNAs in Bombyx germ cells, Nucleic Acids Res 45(15) (2017) 9108–9120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Honda S, Morichika K, Kirino Y, Selective amplification and sequencing of cyclic phosphate-containing RNAs by the cP-RNA-seq method, Nat Protoc 11(3) (2016) 476–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Shigematsu M, Morichika K, Kawamura T, Honda S, Kirino Y, Genome-wide identification of short 2’,3’-cyclic phosphate-containing RNAs and their regulation in aging, PLoS Genet 15(11) (2019) e1008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Licht K, Medenbach J, Luhrmann R, Kambach C, Bindereif A, 3’-cyclic phosphorylation of U6 snRNA leads to recruitment of recycling factor p110 through LSm proteins, RNA 14(8) (2008) 1532–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sporn MB, Lazarus HM, Smith JM, Henderson WR, Studies on nuclear exoribonucleases. 3. Isolation and properties of the enzyme from normal and malignant tissues of the mouse, Biochemistry 8(4) (1969) 1698–706. [DOI] [PubMed] [Google Scholar]
- [17].Popow J, Schleiffer A, Martinez J, Diversity and roles of (t)RNA ligases, Cell Mol Life Sci 69(16) (2012) 2657–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Moldovan JB, Wang Y, Shuman S, Mills RE, Moran JV, RNA ligation precedes the retrotransposition of U6/LINE-1 chimeric RNA, Proceedings of the National Academy of Sciences of the United States of America 116(41) (2019) 20612–20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Englert M, Xia S, Okada C, Nakamura A, Tanavde V, Yao M, Eom SH, Konigsberg WH, Soll D, Wang J, Structural and mechanistic insights into guanylylation of RNA-splicing ligase RtcB joining RNA between 3’-terminal phosphate and 5′-OH, Proceedings of the National Academy of Sciences of the United States of America 109(38) (2012) 15235–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Pinto PH, Kroupova A, Schleiffer A, Mechtler K, Jinek M, Weitzer S, Martinez J, ANGEL2 is a member of the CCR4 family of deadenylases with 2’,3’-cyclic phosphatase activity, Science 369(6503) (2020) 524–530. [DOI] [PubMed] [Google Scholar]
- [21].Anderson P, Ivanov P, tRNA fragments in human health and disease, FEBS Lett 588(23) (2014) 4297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wichlacz A, Legiewicz M, Ciesiolka J, Generating in vitro transcripts with homogenous 3’ ends using trans-acting antigenomic delta ribozyme, Nucleic acids research 32(3) (2004) e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Shigematsu M, Kirino Y, 5′-Terminal nucleotide variations in human cytoplasmic tRNAHisGUG and its 5′-halves, RNA 23(2) (2017) 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sprinzl M, Horn C, Brown M, Ioudovitch A, Steinberg S, Compilation of tRNA sequences and sequences of tRNA genes, Nucleic Acids Res 26(1) (1998) 148–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Milligan JF, Groebe DR, Witherell GW, Uhlenbeck OC, Oligoribonucleotide synthesis using T7 RNA polymerase and synthetic DNA templates, Nucleic acids research 15(21) (1987) 8783–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wrzesinski J, Legiewicz M, Smolska B, Ciesiolka J, Catalytic cleavage of cis- and trans-acting antigenomic delta ribozymes in the presence of various divalent metal ions, Nucleic acids research 29(21) (2001) 4482–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Schurer H, Lang K, Schuster J, Morl M, A universal method to produce in vitro transcripts with homogeneous 3’ ends, Nucleic acids research 30(12) (2002) e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Tsatsaronis JA, Franch-Arroyo S, Resch U, Charpentier E, Extracellular Vesicle RNA: A Universal Mediator of Microbial Communication?, Trends Microbiol 26(5) (2018) 401–410. [DOI] [PubMed] [Google Scholar]
- [29].O’Brien K, Breyne K, Ughetto S, Laurent LC, Breakefield XO, RNA delivery by extracellular vesicles in mammalian cells and its applications, Nat Rev Mol Cell Biol 21(10) (2020) 585–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Max KEA, Bertram K, Akat KM, Bogardus KA, Li J, Morozov P, Ben-Dov IZ, Li X, Weiss ZR, Azizian A, Sopeyin A, Diacovo TG, Adamidi C, Williams Z, Tuschl T, Human plasma and serum extracellular small RNA reference profiles and their clinical utility, Proceedings of the National Academy of Sciences of the United States of America 115(23) (2018) E5334–E5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.