Abstract
Objectives
To gather, synthesize, and meta-analyze data regarding the risk factors associated with a severe course of COVID-19 among patients with multiple sclerosis (pwMS).
Methods
MEDLINE, Embase, Scopus, and WoS were searched in May 2021. Briefly, the eligibility criteria included: 1) studies assessing COVID-19 severity among adult pwMS; 2) definitive diagnoses or high clinical suspicion of COVID-19; 3) a categorization of COVID-19 severity into at least two categories; 4) quantitative effect size and precision measurements; and 5) English language; and 6) clear effect size/precision measures. internal validity of studies was assessed using the NIH Quality Assessment Tools. A list of possible risk factors was created based on the search results and was later used in extraction, synthesis, and meta-analysis of the data.
Results
Thirteen studies were included in the syntheses. Outcome measures were either extracted from the papers, obtained from the primary researchers or calculated manually. The meta-analyses showed a significantly (P < 0.05) increased odds of a severe COVID-19 in pwMS with all of the assessed risk factors, except smoking and most DMTs.
Conclusion
This study facilitates evidence-based risk/benefit assessments in practice. Older men with progressive MS on anti-CD20 therapies are more at risk of an unfortunate COVID-19 outcome.
Keywords: Multiple sclerosis, COVID-19, Disease-modifying therapies, Meta-analysis
1. Introduction
Since the beginning of the COVID-19 pandemic, major concerns were raised among the people with Multiple Sclerosis (pwMS) and their physicians, considering their disabilities, their chronic states of immunosuppression, and their higher susceptibility to infections and their unfortunate outcomes [1]. Therefore, ever since, a lot of research has been aiming at investigating the possible relationships between COVID-19 and Multiple Sclerosis (MS). Identification of the risk factors, which render pwMS more susceptible to worse COVID-19 outcomes, has been among the aims of these studies. Results however, have been contrary, as expected from primary observational investigations. Managing pwMS during the pandemic, requires careful evidence-based assessments of individual risk/benefit profiles. Therefore, we aimed to gather and synthesize the published evidence concerning the risk factors reportedly associated with a more severe course of COVID-19, in a systematic review of literature, followed by a meta-analysis.
2. Methods
2.1. Framework, search strategy and eligibility criteria
The PRISMA guidelines were followed in the conduction of this study. Initially, a comprehensive search of MEDLINE, Embase, Scopus and Web of Science was conducted by two independent reviewers (SM and MB, the search team) in May 2021, using the terms: (coronavirus disease 2019 OR COVID-19 OR SARS-CoV-2 OR COVID) AND (Multiple Sclerosis). The search team used the Mendeley application to detect, scan, and remove duplicates. The same application was used to identify the reviews, case reports, and commentaries, which were excluded after confirmation. The remaining studies were scanned for relevant titles/abstracts. This process was conducted two separate times by two independent reviewers. The studies that at least one reviewer from the search team considered relevant, were sought for retrieval.
The inclusion criteria were pre-defined as: 1) observational studies assessing COVID-19 severity among adult (> 18 years old) pwMS ; 2) laboratory- or radiology-based diagnoses or high clinical suspicion of COVID-19; 3) a categorization of COVID-19 severity into at least two clearly defined categories (e.g., mild, severe, or similar terminologies); 4) estimation of the outcome of possible pre-defined risk factors, using proper quantitative effect size and precision measurements; and 5) studies published in the English language. Exclusion criteria included: 1) unclear effect size and/or precision measures. Full texts of the relevant papers were assessed for eligibility and quality independently by two reviewers (AAS and HN, the methodology team). In cases of reviewers not reaching a consensus regarding a study, a third reviewer's (NS) opinion was sought. For quality (internal validity) assessments, the NIH Quality Assessment Tools were used [2].
2.2. Risk factors and outcomes, eligibility for syntheses, and data extraction
The search team created an initial list of risk factors before data extraction, including all of the assessed risk factors in the relevant studies from their search. Later, if a specified risk factor was not assessed in at least two of the included studies, it was excluded from the final list of assessed risk factors (Table 1 ). The COVID-19 infection severity was defined as the outcome: mild or severe, with mild describing a patient who did not require hospitalization and severe a patient who did, regardless of ICU admission or final disease outcomes.
Table 1.
Studied risk factors and their main effect measures.
| Possible risk factors for COVID-19 severity in pwMS | Effect and precision measures |
|---|---|
| Age | OR per 10 years, 95% CI |
| Male sex | OR compared to female, 95% CI |
| Comorbidities | |
| Obesity | OR compared to non-obese pwMS, 95% CI |
| Diabetes mellitus | OR compared to non-diabetic pwMS, 95% CI |
| Cardiovascular comorbidities | OR compared to pwMS without CV comorbidities, 95% CI |
| Pulmonary comorbidities | OR compared to pwMS without pulmonary comorbidities, 95% CI |
| Hypertension | OR compared to pwMS without hypertension, 95% CI |
| Past/current Smoking | OR compared to non-smokers, 95%CI |
| Progressive MS | OR compared to non-progressive MS, 95% CI |
| 3 ≤ EDSS < 6 | OR compared to EDSS < 3, 95% CI |
| 6 ≤ EDSS | OR compared to EDSS < 3, 95% CI |
| Disease duration | Mean difference, 95% CI |
| Corticosteroids within 2 months | OR compared to no corticosteroid therapy within 2 months, 95%CI |
| Disease Modifying Therapies (DMTs) | OR compared to no DMT, 95% CI |
pwMS: people with multiple sclerosis; CI: confidence interval; OR: odds ratio; CV: cardiovascular; EDSS: expanded disability status scale.
As the studies used different variables to measure the effect size of each risk factor, the most abundant effect measure among all, for each risk factor, was considered the primary effect measure and was used in the syntheses. We tried to minimize the exclusion of data from studies that used different variables to measure the effect size of each risk factor and contacted the primary investigators of those studies. They were asked to calculate and share the required effect measures or share their raw data, enabling us to do so. If the primary investigators failed to share the required measures, the data extraction team (NS and MRM) aimed to manually calculate the effect measures from the descriptive data presented in their paper. Finally, if the required effect measures could not be obtained, either way, the study was excluded from synthesis regarding the particular risk factors. Studies also differed in the method of effect measure calculations (e.g., accounting for different confounding factors). This was regarded as a source of possible inter-study heterogeneity. In the case of a study estimating a specific effect size via different statistical methods, the result from the method accounting for the most confounding factors was extracted and used in the synthesis. The final list of risk factors and their extracted effect and precision measures can be interpreted from Table 1.
2.3. Syntheses and meta-analyses
Forest plots were used to display the results of syntheses visually. Based on the inter-study heterogeneity regarding each outcome, determined by Cochran's Q and I-squared tests, fixed- or random-effects models were used for meta-analysis of homo- and heterogenous results, respectively. More specifically, a random-effects model was used in the case of I2 > 50%. In case of a significant inter-study heterogeneity (I2 > 50%), a meta-regression analysis was utilized to assess the inter-study difference in analysis methods as a possible source of heterogeneity. Sensitivity analyses included performing meta-analysis only on studies with good quality based on NIH Quality Assessment Tools [2]. Egger's test and visual asymmetry of funnel plots were used to assess the risk of bias due to missing results (reporting/publication bias) in the syntheses. Statistical significance was defined as P-value < 0.05. The certainty of evidence was rated using the GRADE approach, by three independent reviewers (NS, ME, and MRM). The STATA14 software for macOS was used to gather, synthesize, and analyze the data.
2.4. Ethical considerations and registration
An approval from ethics committee was not required to initiate this study, as it was not a primary investigation on individuals. Before initiation, the protocol of the study was approved and registered by the internal research board of Isfahan University of Medical Sciences (registration number: IR.MUI.MED.REC.1400.244).
3. Results
3.1. Study selection
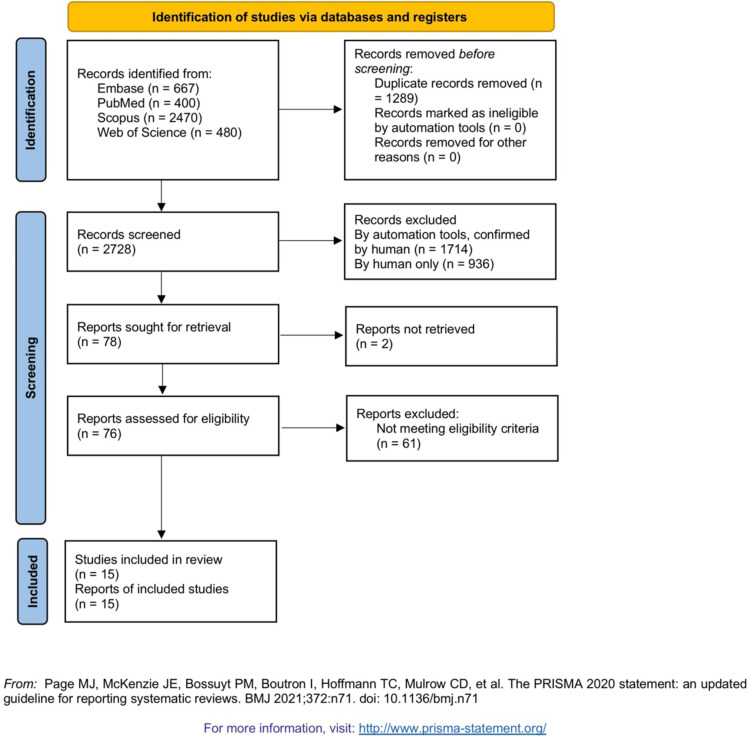
The PRISMA flow diagram, describing the study selection processes, from the search to the final inclusion in syntheses, can be interpreted from Fig. 1 . Thirteen studies were included in the final syntheses based on the eligibility criteria. Two studies [3], [4] were excluded despite meeting the eligibility criteria, because no data could be extracted from them in any way. General information pertaining to each included study and the extracted results from each can be interpreted from Table 2 . The reviewers did not detect any sign of duplicate data among the included studies, although its possibility should be acknowledged, considering the large extents of global and regional shared data registries and databases.
Fig. 1.
PRISMA flow diagram.
Table 2.
Included studies and the extracted results used in the syntheses.
| Study | Country/region | Number of participants (female/male ratio) | Study qualitya | Extracted results regarding risk factors (X: yes) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Male sex | Obesity | DM | CV | Pulm | HTN | Smoking | PMS | EDSS | DD | CS | DMTc | ||||
| [5] | North America | 1626 | Good | X | X | X | X | X | X | X | X | X | X | X | X | X |
| [6] | Italy | 844 | Good | X | X | X | X | X | X | X | X | X | X | X | X | |
| [7] | International | 715 | Fair | X | X | X | X | X | X | X | X | |||||
| [8] | France | 347 | Good | X | X | X | X | X | X | X | X | X | ||||
| [9] | International | 344 | Fair | X | X | X | X | X | X | X | ||||||
| [10] | Turkey | 309 | Fair | X | X | X | X | X | X | |||||||
| [11] | Latin America | 129 | Fair | X | X | X | X | X | X | |||||||
| [12] | Spain | 93 | Fair | X | X | X | X | X | X | X | X | X | X | X | X | |
| [13] | The Netherlands | 86 | Fair | X | X | X | ||||||||||
| [14] | United States | 76 | Fair | X | X | X | X | X | X | X | X | |||||
| [15] | Iran | 68 | Fair | X | X | X | ||||||||||
| [16] | Spain | 51 | Fair | X | X | X | X | X | X | X | X | X | X | |||
| [17] | United States | 40 | Fair | X | X | X | X | X | X | Xb | X | X | ||||
DM: diabetes mellitus; CV: cardiovascular comorbidities; Pulm: pulmonary comorbidities; HTN: hypertension; PMS: progressive multiple sclerosis; EDSS: expanded disability status scale; DD: disease duration; CS: corticosteroid within 2 months; DMT: disease-modifying therapy.
Based on a consensus reached by two independent reviewers (AAS and HN), using NIH quality assessment tools.
Only extracted data for EDSS > 6.
Data pertaining to some DMTs could not be extracted from some mentioned studies.
3.2. Results of individual studies and syntheses
Regarding each risk factor, the extracted outcomes from each study and the results of their synthesis along with Cochran's Q and I-squared results and methods of meta-analyses are presented via forest plots and can be found in the supplementary material. As mentioned, each study accounted for a different set of confounding variables in its analyses, which was regarded as a source of bias/heterogeneity. Studies differed in their methodology, some being registry-based, while others being questionnaire- and follow-up-based. Some outcomes required for syntheses were not presented in most of primary studies, forcing us to contact the primary researchers, of which the majority could not share the measures apart from the ones presented in their papers. Therefore, some of the required outcomes were calculated manually using univariate analysis and others were excluded from syntheses. This should be regarded as a source of possible bias, which was investigated in the sensitivity analyses, by performing no manual calculations and extracting all the outcome measures from the studies with good quality based on NIH Quality Assessment Tools [2]. Furthermore, most studies were conducted with the primary goal of investigating the contraction of COVID-19 rather than investigating its different courses among pwMS. This led to limited numbers of COVID-19-contracted pwMS being a subgroup in the study samples and therefore, may have presented possible biases due to limited sample sizes and analytic power. This problem might have been amplified by using the random-effects model regarding the risk factors that showed inter-study heterogenous results. This was also investigated via the sensitivity analyses, in which the meta-analyses were performed on the studies with sufficient sample sizes and analytic powers. The results of bias assessments in each individual study, using NIH Quality Assessment Tools [2], can be retrieved from the supplementary material.
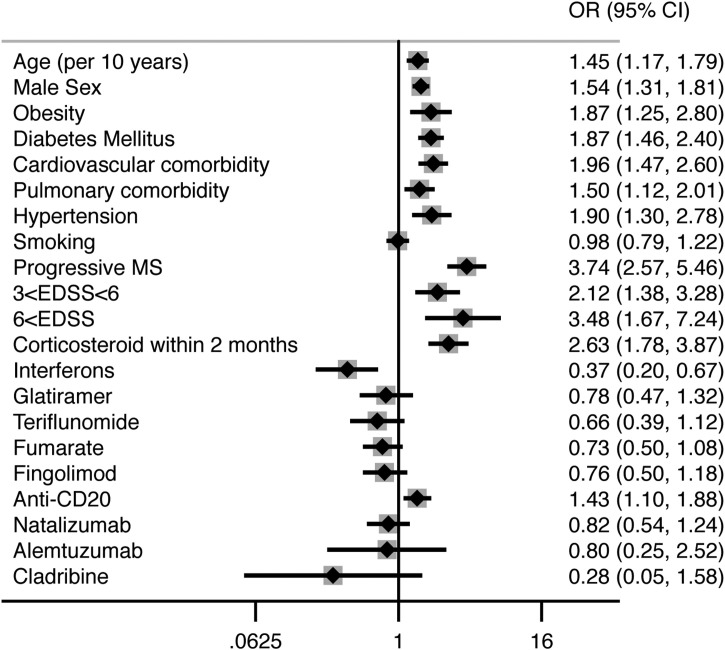
The final results of syntheses and meta-analyses are summarized in Table 3 and Fig. 2 . Egger's test showed statistically significant results in case of male sex (P = 0.039), interferons (P = 0.029), and glatiramer (P = 0.012) (Online material). Funnel plots for each risk factor can be found in the supplementary material. Sensitivity analyses confirmed the effect measure estimation for each risk factor, except for four risk factors, namely obesity, hypertension, EDSS and corticosteroid administration within two months (Online material). This may indicate an overestimation of the effects of obesity, hypertension, and EDSS, and an underestimation of the effects of corticosteroid administration within two months, in the overall analyses.
Table 3.
Summary of findings.
| Risk factor | Meta-analysis of all studies |
Effect size measure | Assessment of certainty (GRADE)a | |
|---|---|---|---|---|
| Effect size (95% CI) | P-value | |||
| Overall demographics | ||||
| Age | 1.45 (1.17, 1.79) | 0.001 | OR per 10 years | Moderateb |
| Male sex | 1.54 (1.31, 1.81) | < 0.001 | OR vs. female | High |
| Comorbidities | ||||
| Obesity | 1.87 (1.25, 2.80) | 0.002 | OR vs. non-obese) | Moderateb |
| Diabetes | 1.87 (1.46, 2.40) | < 0.001 | OR vs. non-diabetic | High |
| Pulmonary comorbidities | 1.50 (1.12, 2.01) | 0.007 | OR vs. no pulmonary comorbidities | High |
| Cardiovascular comorbidities | 1.96 (1.47, 2.60) | < 0.001 | OR vs. no CV comorbidities | High |
| Hypertension | 1.90 (1.30, 2.78) | 0.001 | OR vs. no HTN | Moderateb |
| Current/past Smoking | 0.98 (0.79, 1.22) | 0.845 | OR vs. never-smokers | High |
| MS characteristics | ||||
| Progressive MS | 3.74 (2.57, 5.46) | < 0.001 | OR vs. non-progressive MS | High |
| 3 < EDSS < 6 | 2.12 (1.38, 3.28) | 0.001 | OR vs. EDSS < 3 | Moderateb |
| EDSS > 6 | 3.48 (1.67, 7.24) | 0.001 | ||
| Disease duration | 3.93 (3.07, 4.80) | < 0.001 | Mean difference | Moderatec |
| Corticosteroid in past 2 months | 2.63 (1.78, 3.87) | < 0.001 | OR vs. no corticosteroid in past 2 months | Moderatec |
| DMTs | ||||
| Interferons | 0.37 (0.20, 0.67) | 0.001 | OR vs. no DMTs | High |
| Glatiramer | 0.78 (0.47, 1.32) | 0.358 | Moderated | |
| Teriflunomide | 0.66 (0.39, 1.12) | 0.125 | Moderatee | |
| Fumarate | 0.73 (0.50, 1.08) | 0.116 | Moderatee | |
| Fingolimod | 0.76 (0.50, 1.18) | 0.222 | Moderatee | |
| Anti-CD20 | 1.43 (1.10, 1.88) | 0.008 | High | |
| Natalizumab | 0.82 (0.54, 1.24) | 0.342 | Moderatee | |
| Alemtuzumab | 0.80 (0.25, 2.52) | 0.698 | Lowf | |
| Cladribine | 0.28 (0.05, 1.58) | 0.148 | Lowf | |
CI: confidence interval; OR: odds ratio; CV: cardiovascular; HTN: hypertension; MS: multiple sclerosis; EDSS: expanded disability status scale; DMT: disease-modifying therapy.
Based on a consensus reached by three independent reviewers (NS, ME, and MRM). The baseline certainty was considered as moderate, as the meta-analysis was performed on observational non-randomized studies, however, if the results from an adequate number of studies showed no sign of inconsistency, imprecision, and risk of missing results, the certainty was upgraded to high.
Due to inconsistency.
Due to limited number of studies.
Due to imprecision and risk of missing results.
Due to imprecision.
Due to imprecision and limited number of studies.
Fig. 2.
Summary forest plot of the pooled results.
Overall, as it can be interpreted from Table 3, older age, male sex, obesity, diabetes mellitus, cardiovascular and pulmonary comorbidities, hypertension, progressive phenotypes of MS, higher EDSS, longer disease duration, administration of corticosteroids within two months, and anti-CD20 therapies were significantly associated with a severe course of COVID-19. On the other hand, interferons significantly reduced the odds of a severe COVID-19 (P = 0.001). Other DMTs and smoking did not show any significant correlation with a severe course of COVID-19.
Meta-regression did not explain the inter-study heterogeneities and its source remains unclear. Detailed results of meta-regression can be retrieved from the supplementary material.
4. Discussion
This study provided robust evidence regarding the risk factors of a severe COVID-19 among pwMS, guiding clinicians to better assess individualized risk profiles and better manage each patient in clinical practice. Among the studied risk factors, a progressive phenotype of MS seems to multiply the odds of a severe course of COVID-19, by roughly four times, which is an alarming number. Therefore, close observations of these pwMS and raising awareness among themselves and their relatives to take the protective measures seriously, may be of more importance than the general population or other pwMS. Also, prioritizing their vaccination against COVID-19, advising them of receiving booster doses and the continuation of protective measures, even after being vaccinated, may be reasonable. To a lesser extent, this also accounts for pwMS with other risk factors as well.
Most DMTs did not show to be associated with a severe COVID-19, and therefore, they can be continued with caution throughout the pandemic; with anti-CD20 drugs, however, the issue is slightly different. Anti-CD20 therapies were significantly associated with a severe course of COVID-19 in our meta-analysis, but whether or not anti-CD20 treatments should be halted or replaced by other therapies is controversial, and more practical approaches may be more appreciated [18]. Overall, when it comes to recovery from the infection, some suggest that B cells may not be the sole requirement of the host's immune system, with innate and cellular immune components exerting their antiviral effects as planned [19]. Currently, high-quality data on long-term immunity against SARS-CoV-2 in pwMS treated with anti-CD20 drugs is lacking. The matter gains more importance considering the controversial data on post-vaccination cellular responses [20], [21] and blunted humoral responses [21], [22], [23], [24] in these pwMS. As supported by theory, the reports of Sormani et al. [22] and Stefanski et al. [23] showed delaying doses of anti-CD20 therapies to be associated with more favorable post-vaccination humoral responses – a key point to keep in mind when developing vaccination strategies for pwMS on anti-CD20 therapies, who were shown to be at higher risk of unfavorable COVID-19 outcomes.
In the same line, natalizumab is used as a second-line therapy, and treated pwMS are likely to have a higher EDSS when treatment is started. In fact, natalizumab therapy associates with a small increased risk of upper respiratory tract infections [25], and theoretically, due to its mechanism of action, natalizumab could be effective in reducing lymphocyte trafficking to the lung and mucosa [26]. However, natalizumab did not show to be associated with a severe COVID-19 course in this study.
Regarding corticosteroid therapy, a recent report in a cohort of 1289 patients with cancer and COVID-19 showed no significant effect of this therapy (long term or transient < 4 weeks) in a multivariate analysis of factors associated with all-cause mortality by COVID-19 [27]. Moreover, in MS, corticosteroids are reserved for treating relapses, a scenario that could be associated with an increase in neurological disability and, therefore, probable complications of COVID-19.
The significant results of the Egger's test regarding male sex, interferons, and glatiramer most probably stem from the use of multivariable analyses in the larger studies, rather than publication/reporting bias, especially regarding male sex, which is not an industry-involved factor. Smaller studies with limited sample sizes are obligated to using univariate analyses, as running multivariable analyses with insufficient data results in inaccurate and inconclusive measures. Also, it is worth mentioning that regarding DMT's, results from univariate analysis are probably far less accurate, as univariate analysis does not account for the fact that the majority of pwMS receiving no DMT's are among the older ones with more comorbidities, progressive MS phenotypes and higher EDSS or in other words, it does not account for age, comorbidities, progressive MS, and EDSS as major confounders. This is most probably the reason that the smaller studies, which ran univariate analyses, falsely show protective effects for DMTs.
The heterogeneity seen in the results from different studies, may be because of different COVID-19 situations across the globe. For instance, the COVID-19 mortality among general population in USA is roughly two times higher than in Italy [28]. This may raise serious concerns regarding the synthesis of data, as the pooling does not account for the different situations in different regions as a confounder. Also, as mentioned, the heterogeneity may stem in the use of different models among studies (e.g., univariate and multivariable), which makes some statisticians to argue that the pooling of the results from different models may not be reasonable. On the other hand, exclusion of heterogenous results solely because of their heterogeneity, and pooling the homogenous results because of their homogeneity, may introduce a significant amount of selection bias to the studies. The answer to this problem may lie in gathering of data as much as possible from all over the globe, and utilizing statistical tools (e.g., meta-regression) to account for different confounders and sources of inter-study heterogeneity. Nevertheless, this problem has been an issue over the years and still remains to be discussed. In this study, aiming to reduce selection bias, we approached the issue by including as much studies as we could regardless of their locations, methods of analysis, and overall heterogeneity compared to other studies, including in the syntheses the results from all over the globe and from both univariate and multivariable analyses, and thereafter, utilized meta-regression to investigate the possible source of inter-study heterogeneity. An alternative approach (which a lot of statisticians would not approve) is to scan the different studies, exclude the heterogenous ones, and synthesize/meta-analyze the most homogenous.
Nevertheless, there is still a need for more evidence to estimate the effects of mentioned risk factors more precisely on the course of COVID-19. Future studies can also focus on the mechanistic processes which render pwMS with risk factors more susceptible to COVID-19. Unfortunately, discussions in this regard do not fit the present paper.
4.1. Limitations
Most of the limitations in each step of this study, and the methods used to tackle them were mentioned in the methods and results section. One of the unmentioned limitations of this study, might be the two-levels outcome measurement. The two-level outcome measurement based on hospitalization was considered in order to facilitate the data extraction processes, although it might have presented uncertainty to the results. For instance, different countries have followed different guidelines for hospitalizing patients and it could not be determined if the hospitalized pwMS all experienced similar disease courses, a problem that was not investigated in this study and might have led to over- or underrepresentation of the results. Also, a two-member search team and limited searched databases still leaves a chance of missing relevant studies and more sources of inter-study heterogeneity (e.g., differences of COVID-19 situations and managements in different regions) could have been investigated in meta-regression. Nevertheless, this was a meta-analysis of observational studies and therefore, bears their limitations.
Funding
This study did not receive any funding.
Disclosure of interest
Dr Maria Sepúlveda received speaking honoraria from Roche and UCB Pharma, and travel reimbursement from Biogen, Sanofi and Zambon for national and international meetings. Dr Yolanda Blanco has received speaker honoraria from Novartis, Roche, Genzyme-Sanofi and Biogen. Dr Ana Zabalza has received travel expenses for scientific meetings from Biogen-Idec and Novartis, speaking honoraria from Eisai and a study grant from Novartis.
The other authors declare that they have no competing interest.
Acknowledgments
We would like to thank Dr. Narges Motamedi, Dr. Maria Pia Sormani, and Dr. Irene Schiavetti for their valuable consultations regarding statistical methodologies.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.neurol.2021.10.003.
Online material. Supplementary data
References
- 1.Luna G., Alping P., Burman J., Fink K., Fogdell-Hahn A., Gunnarsson M., et al. Infection risks among patients with multiple sclerosis treated with fingolimod, natalizumab, rituximab, and injectable therapies. JAMA Neurol. 2020;77(2):184–191. doi: 10.1001/jamaneurol.2019.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Heart, Lung, and Blood Institute . National Institutes of Health, and US Department of Health and Human Services.; 2019. Study quality assessment tools. https://www.nhlbi.nih.gov/health‐topics/study‐quality‐assessment‐tools. [Google Scholar]
- 3.Kovvuru S., Nalleballe K., Onteddu S.R., Sharma R., Jasti M., Kapoor N., et al. Immunosuppression in chronic autoimmune neurological disorders during the COVID-19 pandemic. J Neurol Sci. 2021;420:117230. doi: 10.1016/j.jns.2020.117230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brum DG, REDONE.br – Neuroimmunology Brazilian Study Group Focused on COVID-19 and MS Incidence and clinical outcome of Coronavirus disease 2019 in a cohort of 11,560 Brazilian patients with multiple sclerosis. Mult Scler. 2021;1(5) doi: 10.1177/1352458520978354. [1352458520978354] [DOI] [PubMed] [Google Scholar]
- 5.Salter A., Fox R.J., Newsome S.D., Halper J., Li D.K.B., Kanellis P., et al. Outcomes and risk factors associated with SARS-CoV-2 infection in a North American registry of patients with multiple sclerosis. JAMA Neurol. 2021;78(6):699–708. doi: 10.1001/jamaneurol.2021.0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sormani M.P., De Rossi N., Schiavetti I., Carmisciano L., Cordioli C., Moiola L., et al. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann Neurol. 2021;89(4):780–789. doi: 10.1002/ana.26028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hughes R., Whitley L., Fitovski K., Schneble H.-M., Muros E., Sauter A., et al. COVID-19 in ocrelizumab-treated people with multiple sclerosis. Mult Scler Relat Disord. 2021;49:102725. doi: 10.1016/j.msard.2020.102725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Louapre C., Collongues N., Stankoff B., Giannesini C., Papeix C., Bensa C., et al. Clinical characteristics and outcomes in patients with coronavirus disease 2019 and multiple sclerosis. JAMA Neurol. 2020;77(9):1079–1088. doi: 10.1001/jamaneurol.2020.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reder A.T., Centonze D., Naylor M.L., Nagpal A., Rajbhandari R., Altincatal A., et al. COVID-19 in patients with multiple sclerosis: associations with disease-modifying therapies. CNS Drugs. 2021;35(3):317–330. doi: 10.1007/s40263-021-00804-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sen S., Karabudak R., Schiavetti I., Demir S., Ozakbas S., Tutuncu M., et al. The outcome of a national MS-COVID-19 study: what the Turkish MS cohort reveals? Mult Scler Relat Disord. 2021;52:102968. doi: 10.1016/j.msard.2021.102968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alonso R., Silva B., Garcea O., Diaz P.E.C., Dos Passos G.R., Navarro D.A.R., et al. COVID-19 in multiple sclerosis and neuromyelitis optica spectrum disorder patients in Latin America: COVID-19 in MS and NMOSD patients in LATAM. Mult Scler Relat Disord. 2021;51:102886. doi: 10.1016/j.msard.2021.102886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zabalza A., Cárdenas-Robledo S., Tagliani P., Arrambide G., Otero-Romero S., Carbonell-Mirabent P., et al. COVID-19 in multiple sclerosis patients: susceptibility, severity risk factors and serological response. Eur J Neurol. 2020;28(10):3384–3395. doi: 10.1111/ene.14690. [DOI] [PubMed] [Google Scholar]
- 13.Loonstra F.C., Hoitsma E., van Kempen Z.L., Killestein J., Mostert J.P. COVID-19 in multiple sclerosis: the Dutch experience. Mult Scler. 2020;26(10):1256–1260. doi: 10.1177/1352458520942198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parrotta E., Kister I., Charvet L., Sammarco C., Saha V., Charlson R.E., et al. COVID-19 outcomes in MS: observational study of early experience from NYU Multiple Sclerosis Comprehensive Care Center. Neurol Neuroimmunol Neuroinflamm. 2020;7(5):e835. doi: 10.1212/NXI.0000000000000835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sahraian M.A., Azimi A., Navardi S., Ala S., Naser Moghadasi A. Evaluation of the rate of COVID-19 infection, hospitalization and death among Iranian patients with multiple sclerosis. Mult Scler Relat Disord. 2020;46:102472. doi: 10.1016/j.msard.2020.102472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sepúlveda M., Llufriu S., Martínez-Hernández E., Català M., Artola M., Hernando A., et al. Incidence and impact of COVID-19 in MS: a survey from a Barcelona MS Unit. Neurol Neuroimmunol Neuroinflamm. 2021;8(2):e954. doi: 10.1212/NXI.0000000000000954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaudhry F., Bulka H., Rathnam A.S., Said O.M., Lin J., Lorigan H., et al. COVID-19 in multiple sclerosis patients and risk factors for severe infection. J Neurol Sci. 2020;418:117147. doi: 10.1016/j.jns.2020.117147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baker D., Roberts C.A.K., Pryce G., Kang A.S., Marta M., Reyes S., et al. COVID-19 vaccine-readiness for anti-CD20-depleting therapy in autoimmune diseases. Clin Exp Immunol. 2020;202(2):149–161. doi: 10.1111/cei.13495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baker D., Amor S., Kang A.S., Schmierer K., Giovannoni G. The underpinning biology relating to multiple sclerosis disease modifying treatments during the COVID-19 pandemic. Mult Scler Relat Disord. 2020;43:102174. doi: 10.1016/j.msard.2020.102174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madelon N., Lauper K., Breville G., Royo I.S., Goldstein R., Andrey D.O., et al. Patients treated with anti-CD20 therapy can mount robust T cell responses to mRNA-based COVID-19 vaccines. medRxiv. 2021 [2021.07.21.21260928] [Google Scholar]
- 21.Moor M.B., Suter-Riniker F., Horn M.P., Aeberli D., Amsler J., Möller B., et al. Humoral and cellular responses to mRNA vaccines against SARS-CoV-2 in patients with a history of CD20 B-cell-depleting therapy (RituxiVac): an investigator-initiated, single-centre, open-label study. The Lancet Rheumatology. 2021;3(11):e789–e797. doi: 10.1016/S2665-9913(21)00251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sormani M.P., Inglese M., Schiavetti I., Carmisciano L., Laroni A., Lapucci C., et al. Vol. 72. EBioMedicine; 2021. Effect of SARS-CoV-2 mRNA vaccination in MS patients treated with disease modifying therapies; p. 103581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stefanski A.-L., Rincon-Arevalo H., Schrezenmeier E., Karberg K., Szelinski F., Ritter J., et al. B cell numbers predict humoral and cellular response upon SARS-CoV-2 vaccination among patients treated with rituximab. medRxiv. 2021 doi: 10.1002/art.42060. doi:2021.07.19.21260803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tallantyre E.C., Vickaryous N., Anderson V., Asardag A.N., Baker D., Bestwick J., et al. COVID-19 vaccine response in people with multiple sclerosis. Ann Neurol. 2021 doi: 10.1002/ana.26251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kapoor R., Ho P.-R., Campbell N., Chang I., Deykin A., Forrestal F., et al. Effect of natalizumab on disease progression in secondary progressive multiple sclerosis (ASCEND): a phase 3, randomised, double-blind, placebo-controlled trial with an open-label extension. Lancet Neurol. 2018;17(5):405–415. doi: 10.1016/S1474-4422(18)30069-3. [DOI] [PubMed] [Google Scholar]
- 26.Woodside D.G., Vanderslice P. Cell adhesion antagonists. Bio Drugs. 2008;22(2):85–100. doi: 10.2165/00063030-200822020-00002. [DOI] [PubMed] [Google Scholar]
- 27.Lièvre A., Turpin A., Ray-Coquard I., Le Malicot K., Thariat J., Ahle G., et al. Risk factors for coronavirus disease 2019 (COVID-19) severity and mortality among solid cancer patients and impact of the disease on anticancer treatment: a French nationwide cohort study (GCO-002 CACOVID-19) Eur J Cancer. 2020;141:62–81. doi: 10.1016/j.ejca.2020.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.