Abstract
Management of kidney cancer has undergone a paradigm shift with the approval of new therapies over the last two decades. Although these drugs have improved clinical outcomes in patients with kidney cancer, there are still a large number of patients who do not show objective responses. A multitude of investigators, including those for The Cancer Genome Atlas have biologically characterized and sub-classified kidney cancer. However, we have not been able to identify molecular targets to effectively treat patients with kidney cancer. As we familiarize ourselves with newer drugs for patients with kidney cancer, it is important to understand that these drugs may not work in every patient and instead may expose patients to unnecessary toxic effects along with burdening society with the financial impact. As we head toward the era of “precision medicine”, validated biomarkers are being utilized to guide treatment choices and help identify pathways of resistance in other tumor types. The current review aims at evaluating the progress made so far in this realm for patients with kidney cancer.
Keywords: Kidney cancer, Clear cell, Non-clear cell, Immunotherapy, Biomarkers
1. Introduction
Per American Cancer Society's most recent statistical analysis, an estimated 73 750 new cases of kidney cancer will be diagnosed in the United States in 2020, of which about 14 830 people will die from this disease [1]. According to the International Agency for Research on Cancer, there has been a 22% increase in the number of people developing the disease worldwide since 2012 [2]. As these numbers have increased, we have most certainly expanded treatment options to treat patients with kidney cancer and are developing a better understanding of the molecular pathways and genomic aberrations associated with kidney cancer. With an expansion in treatment algorithms, we are now using immune checkpoint inhibitors (ICIs) alone or in combination with anti-vascular endothelial growth factor (VEGF) therapies in the upfront management of patients with metastatic renal cell cancer (mRCC). Novel combinations have led to response rates being increased by almost ten-fold and improvement in median overall survival (OS) as well [3]. Based on robust data from phase III clinical trials, regulatory agencies have approved ICIs for the management of patients with mRCC. We know however, from these trials, as well as from treating RCC patients in clinic, while some patients have a robust deep response to immunotherapy, there are many patients that do not respond at all. In CheckMate-214, a phase III study comparing programmed death-1 (PD-1) inhibitor, nivolumab plus cytotoxic T-lymphocyte-associated protein-4 (CTLA4) inhibitor, ipilimumab to sunitinib, the proportion of patients achieving an objective response was 41% vs. 34% respectively in the two arms [4]. Similarly in KEYNOTE-426, where the combination of PD-1 inhibitor, pembrolizumab plus axitinib was compared to sunitinib, we have seen an objective response rate (ORR) of 59.3% and 35.7% respectively [5]. Therefore, while the immunotherapeutic combinations whether with other ICIs or with VEGF inhibitors have led to responses in a higher proportion of patients on trials, there are many patients in whom an objective response is not achieved. The clinical conundrum is how to identify responders to each of these combinations and avoid unnecessary toxicity—both clinical and financial. It is also important to recognize prognostic biomarkers to guide treatment decisions in these patients, especially for patients such as those that undergo surgery for localized RCC and are in need of adjuvant therapies. In this review, we hope to shed light on work that has been done and is expected to be done in the near future in the realm of biomarker analysis in kidney cancer.
1.1. Overview of evolution of drugs for mRCC
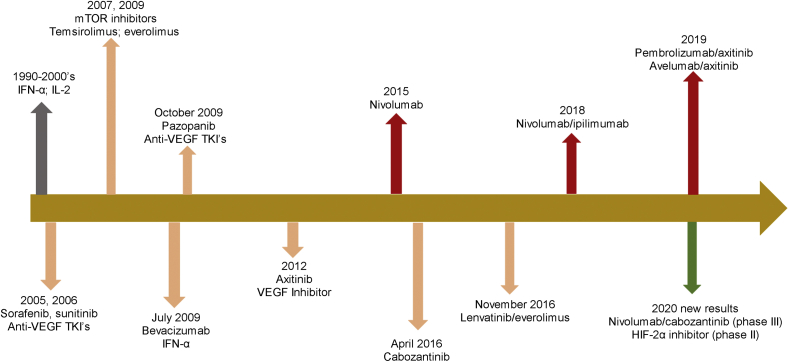
Studies during the 1980's with chemotherapy yielded extremely low ORR, on the order of 1%–5% [6]. Partially effective treatment with cytokine therapy (interferon-α [IFN-α] and interlukin-2 [IL-2]) was subsequently used for kidney cancer. These drugs, especially IL-2 led to some durable remissions but involved intense monitoring and required the patient to be in perfect health. The early two decades of the 2000's saw an evolution of treatment of mRCC with tyrosine kinase inhibitors (TKIs)/VEGF inhibitors and mammalian target of rapamycin (mTOR) inhibitors becoming available. Subsequently, in 2015, the first PD-1 inhibitor, nivolumab was approved by the Food and Drug Administration (FDA) based on OS benefit when compared to the mTOR inhibitor everolimus [7]. Nivolumab was then combined with CTLA-4 inhibitor ipilimumab in a phase III trial (CheckMate-214) and the combination received regulatory approval from the United States (US) FDA approval for patients with intermediate to poor risk disease [4]. Over the last few years, phase III trials combining ICIs (KEYNOTE-426, combining PD-1 inhibitor pembrolizumab and axitinib as well as JAVELIN- Renal-101 combining avelumab and axitinib) have evolved and these drugs are now part of guidelines to treat patients with mRCC [5,8]. Recently results from the phase III CheckMate 9 ER trial were presented where the combination of nivolumab and cabozantinib has been shown to be superior to sunitinib in terms of progression-free survival (PFS), OS, and ORR in the front-line treatment of patients with mRCC [9]. The combination of programmed death ligand-1 (PD-L1) inhibitor, atezolizumab with VEGF inhibitor, and bevacizumab was studied in the phase III IMmotion-151 trial but is not FDA approved due to lack of OS benefit [10]. These are some of the pivotal trials that have changed the way we manage mRCC patients and several phase III trials investigating ICI combinations are forthcoming in the near future (Fig. 1).
Figure 1.
Evolution of treatment landscape for metastatic clear-cell renal cell carcinoma. Drugs shown here are the ones approved by the FDA for use in this setting and recently presented phase III data. HIF-2α, hypoxia inducible factor-2 alpha; IFN, interferon; IL-2, Interleukin-2; TKI, tyrosine kinase inhibitor; VEGF, vascular endothelial growth factor.
1.2. Risk models for RCC treatment selection
Before delving into the available literature for molecular and genomic markers for mRCC, it is important to shed light on the risk models that are used in clinic to date to guide management decisions. These models are important to discuss as they are also used to risk stratify patients for participation in clinical trials.
In the phase III trial that compared mTOR inhibitor temsirolimus, temsirolimus plus interferon-α, or interferon-α monotherapy, 626 patients were randomized, however were required to meet three of six inclusion criteria (high lactate dehydrogenase [LDH], high serum calcium, low hemoglobin, less than 1 year gap between diagnosis to randomization, Karnofsky performance status of 60 or 70, and metastases in multiple organs) [11]. In this group of patients (considered to be “poor-risk”), temsirolimus led to an improvement in OS and was FDA approved. Even though the use of temsirolimus has declined with the advent of ICIs, this trial is important as it used a “risk-directed” approach to decide treatment modality.
International Metastatic RCC Database Consortium (IMDC) model was subsequently developed and it uses six characteristics—time to initiation of systemic treatment, performance status, hypercalcemia, anemia, thrombocytopenia, and neutrophilia for risk stratification [12]. Similarly, the Memorial Sloan-Kettering Cancer Center (MSKCC) model uses five features (time to initiation of systemic therapy, performance status, anemia, hypercalcemia, and elevated LDH) [13]. While there are other clinical models developed for RCC, the IMDC model is most commonly used. This model has been externally validated using a cohort of 1028 real-world patients from international cancer centers and against the Cleveland Clinic Foundation (CCF) model, the International Kidney Cancer Working Group (IKCWG) model, the French model, and the MSKCC model [14]. The model stratifies patients into three distinct prognostic groups: Favorable (zero risk factors), intermediate (one to two risk factors), and poor risk (more than two risk factors). The IMDC model has been used to stratify patients in clinical trials of nivolumab, ipilimumab/nivolumab, lenvatinib/everolimus and cabozantinib, and has been retrospectively validated for patients receiving pazopanib [15], as well as non-clear cell advanced renal cell carcinoma (nccRCC) [16] and thus makes a robust, practical and easy to use tool for risk stratification prior to deciding treatment options.
As IMDC model has been incorporated in practice, studies have emerged to describe association of risk groups with responsiveness to treatments. For example, in 2008 Heng et al. [12] described a retrospective cohort of 645 patients with mRCC, where patients with IMDC good risk disease were found to have a better OS when treated with VEGF inhibitors (sunitinib, sorafenib, or bevacizumab). In KEYNOTE-426 study (pembrolizumab/axitinib vs. sunitinib), the benefit of the ICI-VEGF combination was seen across all IMDC risk groups [5]. On the other hand, in CheckMate-214 trial (nivolumab/ipilimumab vs. sunitinib), the initial report had suggested that OS and ORR were significantly higher with the nivolumab plus ipilimumab combination in patients with intermediate- and poor-risk disease [4]. Interestingly, in patients with good risk disease, ORR and PFS favored the sunitinib arm: ORR 29% vs. 52% (p<0.001); PFS 15.3 months vs. 25.1 months (hazard ratio [HR] 2.18; p<0.001). On long-term follow-up, however, survival probabilities between favorable and intermediate/poor risk groups were similar when treated with the ICI combination of nivolumab and ipilimumab [17].
Because of these data and the durability of responses seen with the nivolumab and ipilimumab combination even in the favorable risk patients, several clinicians choose to use this combination upfront, while some clinicians prefer to use the VEGF inhibitor/ICI combination based on the data from KEYNOYE-426 discussed above. The clinical question of whether to choose an ICI combination or use a VEGF inhibitor upfront therefore remains somewhat unclear. The search for molecular and genomic markers is on and will be discussed in subsequent sections.
2. Biomarkers in clear cell renal cell carcinoma
2.1. Tumor histology
Sarcomatoid differentiation is not a distinct entity of RCC per World Health Organization (WHO) classification, but is high-grade differentiation that can occur in any RCC type and it is estimated that approximately 10%–15% of all RCC tumors could contain sarcomatoid elements [17]. This distinction is important given that tumors with sarcomatoid differentiation tend to be associated with poorer outcomes [17]. Lately, this recognition has become important, because of difference in response to various treatment modalities seen in patients with sarcomatoid features on post-hoc analysis done on pivotal clinical trials as described here.
KEYNOTE-426 (phase III trial comparing axitinib plus pembrolizumab vs. sunitinib) included 105 patients with sarcomatoid features. The pembrolizumab plus axitinib arm was shown to have improved OS (HR 0.58, 95% confidence interval [CI] 0.21–1.59), PFS (HR 0.54, 95% CI 0.29–1.00; median not reached vs. 8.4 months), and ORR (58.8% vs. 31.5%) for patients with sarcomatoid features [18]. In the phase III CheckMate-214 study (ipilimumab plus nivolumab vs. sunitinib), 112 patients had sarcomatoid features [19]. OS and PFS in this group of patients were better in the ICI arm with similar hazard ratios as KEYNOTE-426; ORR was 56.7% versus 19.2% in favor of the ipilimumab plus nivolumab arm. A pooled analysis from CheckMate-025 (phase III trial comparing nivolumab vs. everolimus), real world patient data from Harvard, and data obtained from IMDC confirmed better treatment outcomes of patients with sarcomatoid features when treated with ICI based therapies [20].
These data point to the role of tumor histology, specifically sarcomatoid, in preferentially choosing ICI based therapies for these patients.
2.2. PD-L1 status
PD-L1 status was first reported to be a marker of poor prognosis in a study of 200 RCC specimens in 2004 with an almost 4.5 times higher risk of dying reported in the PD-L1+ patients [21]. A meta-analysis that included 1323 cases showed that a higher expression of PD-L1 by immunohistochemistry (IHC) led to an increase in mortality of patients with clear cell RCC (ccRCC) by >50% [22]. A post-hoc analysis of the phase III COMPARZ trial (comparing pazopanib and sunitinib), found that patients treated with either TKI had significantly worse OS and PFS if they were PD-L1+ compared to the PD-L1− patients [23]. From these studies, tumor PD-L1 expression was proposed as a negative prognostic factor in kidney cancer and as a biomarker that predicts poor response to anti-VEGF agents.
In the era of immunotherapy, the role of PD-L1 as a biomarker of response has been descried here and is summarized in Table 1. In CheckMate-025 trial (investigating nivolumab vs. everolimus in patients who had progressed on prior VEGF targeted therapy), nivolumab was shown to have better efficacy than everolimus in both PD-L1+ and negative patients [7]. The median OS was numerically higher in PD-L1− patients (median OS: 27.4 months [in those with PD-L1<1%] vs. 21.8 months median [in those with ≥1%]), thus indicating that the PD-L1 status could represent a prognostic biomarker but was not seen to be predictive of response to ICI based therapy.
Table 1.
Phase III clinical trial data from immunotherapy trials to show clinical outcomes by PD-L1 expression status.
| Study | Regimen | mOS PD-L1+ | mOS ITT population | mPFS PD-L1+ | mPFS ITT population |
|---|---|---|---|---|---|
| CheckMate025 [7] | Nivo vs. everolimus | -21.8 mo for Nivo vs. 18.8 mo for everolimus (HR 0.78) |
-25.0 vs. 19.6 mo (95% CI 17.6–23.1) | NR | -4.6 vs. 4.4 mo (HR 0.88; 95% CI, 0.75–1.03; p=0.11) |
| IMmotion151 [10] | Atezolizumab+bevacizumab vs. sunitinib | -HR 0.84; 95% CI 0.62–1.15; p=0.2857 | -HR 0.93; 95% CI 0.76–1.14; p=0.4751 | -11.2 mo vs. 7.7 mo (HR 0.74; 95% CI 0.57–0·96; p=0.0217) |
-11.2 vs. 8.4 mo (HR 0.83; 95% CI 0.70–0.97) |
| CheckMate 214 [4] | Nivo+Ipi vs. sunitinib |
-NR for Ipi/Nivo vs. 19.6 mo (HR 0.45; 95% CI 0.29–0.71) | -NR vs. 26.0 mo (HR 0.63) |
-22.8 mo for Ipi/Nivo vs. 5.9 mo (HR 0.46; 95% CI, 0.31–0.67) | -11.6 vs. 8.4 mo (HR 0.82; 99.1% CI 0.64–1.05; p=0.03) |
| KEYNOTE-426 [5,19] | Pembro+Axi vs. sunitinib |
-HR 0.54; 95% CI 0.34–1.03 (12-mo OS) | -Pembro+Axi improved OS (HR, 0.68; 95% CI 0.55–0.85; p<0.001) | -15.3 mo for Pembro/Axi vs. 8.9 mo (HR 0.62; 95% CI 0.47–0.80) | -Pembro+Axi improved PFS (HR 0.71; 95% CI 0.60–0.84; p<0.001) |
| JAVELIN Renal 101 [26,27] | Avelumab+Axi vs. sunitinib |
-NR in avelumab/Axi group vs. 25.8 mo (HR 0.83; 95% CI 0.596–1.151; p=0.1301) | -NR vs. NR (HR 0.80; 95% CI 0.616–1.027; p=0.03920) | -13.8 mo for avelumab/Axi vs. 7 mo (HR 0.62; 95% CI 0.490–0.777; p<0.0001) | -13.3 mo in avelumab/Axi arm vs. 8 mo in sunitinib arm (HR 0.69; 95% CI 0.574–0.825; p<0.0001) |
CI, confidence interval; mo, months; HR, hazard ratio; NS, non-significant; NR, not reached; Nivo, Nivolumab; Ipi, ipilimumab; Pembro, pembrolizumab; Axi, axitinib; mOS, median overall survival; OS, overall survival; mPFS, median progression free survival; ITT, intention to treat.
Similarly, in CheckMate-214 (comparing nivolumab in combination with ipilimumab vs. sunitinib) multivariate analysis was presented in a 32-month follow-up study [24]. PD-L1 expression was measured using the Dako PD-L1 IHC 28-8 pharmDx test and considered positive if >1% expression was seen on tumor cells. Here the investigators showed that PD-L1+ patients had worse survival only in sunitinib treated patients (HR 0.70; 95% CI 0.52–0.93) and PD-L1 positivity was not associated with survival in the nivolumab plus ipilimumab treated patients. This could suggest that combination immunotherapy was able to negate the negative prognostic effects associated with PD-L1 expression. Exploratory analysis was done in the intermediate- and poor-risk patient population according to PD-L1 expression. Median PFS for PD-L1+ patients was higher with nivolumab plus ipilimumab compared to sunitinib (22.8 months vs. 5.9 months [HR 0.46; 95% CI, 0.31–0.67]) while the median PFS was not significantly different among PD-L1− patients (HR 1.00; 95% CI, 0.80–1.26). OS was significantly longer with nivolumab plus ipilimumab in both the PD-L1 positive and negative groups. The ORR with nivolumab plus ipilimumab was higher in the PD-L1+ group and was statistically significant (58% with nivolumab plus ipilimumab vs. 22% with sunitinib [p<0.001]), compared to the PD-L1 negative (37% with nivolumab plus ipilimumab vs. 28% with sunitinib [p=0.03]). More frequent complete responses (CRs) were also seen in the PD-L1+ group (nivolumab plus ipilimumab with 16% CR sunitinib with 1%) as compared to the PD-L1 group (7% with nivolumab plus ipilimumab vs. 1% with sunitinib). These data thus also reiterated the fact that while PD-L1 status could be used as a prognostic biomarker, it is not entirely predictive of treatment response.
On the other hand, in IMmotion-150 (phase II trial comparing atezolizumab with or without bevacizumab vs. sunitinib in metastatic ccRCC in the front-line setting), PD-L1 expression was correlated with response to ICI based treatment [25]. In this study, PD-L1 status was measured using the Ventana SP142 IHC assay and considered positive if >1% expression was seen in tumor infiltrating cells. Median PFS was numerically higher in patients with PD-L1 positive tumors receiving the ICI based combination (11.2 months with atezolizumab plus bevacizumab vs. 7.7 months with sunitinib [HR 0.74; 95% CI, 0.38–1.08]), while the median PFS was 11.7 months in the intention to treat population with atezolizumab/bevacizumab versus 8.4 months with sunitinib (HR 1.00; 95% CI, 0.69–1.45).
Similarly, IMmotion-151 (phase III trial comparing atezolizumab plus bevacizumab vs. sunitinib in both clear cell as well as sarcomatoid histologies) analyzed this relationship further [10]. PD-L1 was measured using the same technique as described in IMmotion-150 study. Similar to IMmotion-150, median PFS was 11.2 months in the atezolizumab plus bevacizumab arm vs. 7.7 months in the sunitinib arm (HR 0.74; 95 CI 0.57–0.96; p=0.0217) in the PD-L1 positive patients. OS difference in the ITT population was not found to be significant. ORR in the PD-L1 positive patients was 43% in the atezolizumab plus bevacizumab arm vs. 35% in the sunitinib arm. In the PD-L1 negative arm, the ORR was similar in both groups (33% for atezolizumab plus bevacizumab vs. 32% for sunitinib).
KEYNOTE-426 (phase III trial comparing pembrolizumab plus axitinib versus sunitinib) provided data from exploratory analysis regarding PD-L1 status [5]. Here PD-L1 was tested using the IHC 22C3 pharmDx assay (Agilent Technologies, Santa Clara, CA, United States), calculated by combined positive score (CPS) (PD-L1+ cells [tumor, lymphocytes, macrophages] divided by total tumor cells and multiplied by 100). In the PD-L1+ patients, 12-month OS rates were 90.1% with pembrolizumab plus axitinib versus 78.4% with sunitinib (HR 0.54, 95% CI 0.34–0.84). In the PD-L1− group, the 12-month OS rates were 91.5% in pembrolizumab plus axitinib versus 78.3% in the sunitinib group (HR 0.59, 95% CI 0.34–1.02). Similarly, median PFS in PD-L1+ patients was 15.3 months with pembrolizumab plus axitinib versus 8.9 months with sunitinib (HR 0.62, 95% CI 0.47–0.80), and in the PD-L1– group 15.0 months versus 12.5 months (HR 0.87, 95% CI 0.62–1.23). Thus, there was a benefit of treating patients in both the PD-L1+ and PD-L1− of treating with the ICI based regimen and hence PD-L1 expression was not seen to be a predictive biomarker.
JAVELIN Renal-101 trial (phase III study evaluating avelumab plus axitinib versus sunitinib) recently reported results where PD-L1 has been incorporated as part of the primary end-points [8,26]. Assay used in this study was the Ventana PD-L1 SP263 assay similar to the IMmotion trials above. No difference in PFS was seen in the avelumab plus axitinib arm in PD-L1 positive or negative tumors (HR 0.89; 95% CI 0.652–1.220; p=0.4734). In the sunitinib arm, PFS was seen to be shorter in the PD-L1+ tumors compared to the PD-L1− tumors (HR 1.57; 95% CI 1.156–2.142; p=0.0037). Thus PD-L1 expression was not seen to be predictive or response to ICI based regimen and similar trends were maintained even when immune cell PD-L1– expression thresholds were increased to 5%, 10%, and 25%.
In conclusion, unlike other tumors, such as urothelial cancer, lung cancer, head and neck cancer, where PD-L1 expression is incorporated into treatment algorithms to decide role of ICIs in management decisions upfront, its role is less clearly defined in patients with mRCC. Even PD-L1 negative patients seem to benefit from ICI based regimens (Table 1). This is further complicated by differences between PD-L1 assays for each drug (e.g. 28–8 Dako assay for nivolumab and the SP142 Ventana assay for atezolizumab), where each assay has a different threshold to define PD-L1 positivity [27]. Moreover, discordance has been reported in PD-L1 staining between primary tumors and metastatic sites (up to 20.8%, according to one study) [28]. All these factors have contributed to PD-L1 not being used in mRCC patients as a predictive biomarker for responsiveness to immune checkpoint inhibitor therapy.
2.3. Tumor mutational burden (TMB)
TMB is a reflection of the volume of neoantigens in the tumor and has the potential to predict response to checkpoint inhibitors in several cancer types including bladder cancer, melanoma and lung cancer [29]. RCC, however, stands out distinctly from these cancers in that these tumors have been reported to have a low TMB, which has not been shown to be predictive of response to ICI based regimens [29]. For example, in a study that included over 1600 solid tumor samples that were genomically profiled using the MSK-IMPACT assay, TMB and response to immunotherapy were analyzed and a pre-specified cutoff percentage for TMB in each histology type was used [29,30]. Using 20% as a cut-off, a significant improvement in OS was observed across the entire cohort (HR 0.061; p=1.3×10−7). The cohort of patients with RCC (151 patients) on the other hand behaved differently in that no significant difference was found in OS between the patients in the top 20% of TMB and those below (cutoff here was 5.9 muts/Mb), thus implying that TMB did not correlate with response to ICI based therapy in RCC. Several retrospective studies have further explored the role of TMB in predicting response to ICI and have uniformly found no such association. Labriola et al. [31] evaluated patients with mRCC treated with ICI based regimens. TMB score was not seen to be different in patients with progressive disease (PD) versus those with stable disease (SD) or partial response (PR) (mean TMB of 3.01 muts/Mb among the PD group versus 2.63 muts/Mb in those with SD/PR). Wood et al. [32] reported data from 431 patients, of which 58 tumors samples were from RCC patients. They also concluded that TMB was not predictive of response to immunotherapy in patients with RCC (p=0.894). Dizman et al. [33] evaluated 91 patients with mRCC who had undergone genomic profiling. Patients included in the analysis had undergone genomic profiling prior to starting systemic treatment (32 started on immunotherapy and 43 started on VEGF-TKI therapy). As reported in previous studies, median TMB was low at 1.2 muts/Mb (range 0.03–4.0 muts/Mb) and no significant difference was seen in the TMB between patients who responded to immunotherapy cohort versus who did not (p=0.82).
Similar studies were performed on tumor samples collected from clinical trials. Data from CheckMate-025 trial and CheckMate-010 (comparing nivolumab vs. everolimus) [34] were combined with existing genomic data from CheckMate-009 [35]. Of the total 592 tumor samples, data were available for 261 patients that had been treated with nivolumab. TMB was calculated by adding all non-synonymous mutations in each tumor sample. Response to nivolumab was not correlated with TMB (p=0.81).
Analyses from both CheckMate-214 (nivolumab plus ipilimumab vs. sunitinib) and JAVELIN Renal-101 (avelumab plus axitinib vs. sunitinib) have not shown a difference in efficacy with ICI based regimens between patients with low and high TMB [36,26]. Exploratory analysis from IMmotion-150 study showed no relationship between TMB in RCC and responsiveness to ICI based therapy (atezolizumab alone as well as in combination with bevacizumab) [25].
Recently, a checkpoint inhibitor, pembrolizumab was FDA approved for the treatment of patients with unresectable or metastatic cancers with a high tumor mutational burden (TMB) (≥10 mut/Mb), if they have progressed following prior standard of care treatment options [37]. However, given the paucity of evidence connecting high TMB to responsiveness to immunotherapy-based regimens, this tumor agnostic approval likely does not hold true for patients with RCC.
2.4. Single gene mutations for prognosis and prediction of response to treatment
Loss of heterozygosity at chromosome 3p (between 3p25 and 3p21 segments) is seen in more than 90% ccRCC tumor samples. As a result, there are mutations in various genes—von Hippel-Lindau (VHL) being the most common, followed by Polybromo 1 (PBRM1), BRCA1 associated protein-1(BAP1) and SET domain containing protein 2 (SETD2), which are amongst the most commonly mutated genes in ccRCC [38]. While VHL gene is inactivated by either mutation or methylation, other genes listed here are mutated for the most part. PBRM1, SETD2, and BAP1 are chromatin remodeling genes, which can be mutated in non-clear cell RCC as well. The role of identification of single gene alterations as predictive of response or prognosis is discussed in this section.
2.4.1. VHL
The 2016 WHO classification characterizes genomic profile of ccRCC as biallelic loss of function of the VHL gene (mapped to chromosome 3p) [38,39]. Loss of heterozygosity on chromosome 3p is one genetic hit that is seen in 80%–90% of ccRCC cases while a second hit (mutation [in about 50%] or promoter methylation [in about 10%]), makes the VHL protein inactive, eliminates regulation of hypoxia-inducible factor α subunits (HIF1α and HIF2α) and ultimately leads to constitutive activation of HIF and transcription of genes [38,40]. This further results in various disease characteristics, including enhanced angiogenesis, tumor growth, and metastasis. Development of targeted therapies such as VEGF inhibitors for mRCC was based on reports that had shown early loss of VHL and overexpression of HIF and HIF target genes (e.g. VEGF) [41]. More recently direct inhibitors of HIF2α are being tested in clinical trials for advanced ccRCC [42]. The HIF2α inhibitor, MK-6482 was granted accelerated approval to treat patients with VHL disease associated RCC [43].
Mixed results have been reported, with some studies showing that VHL alterations portended a poor prognosis, while some showing that VHL alterations were linked to positive outcomes [38,44,45]. Further, investigators have not been able to confirm an association between response to VEGF inhibitors and VHL inactivation either [46,47]. A meta-analysis on 663 patients with ccRCC, of which 410 (61.8%) had an alteration in VHL confirmed this finding and found no association between alterations in VHL and ORR or PFS when treated with VEGF-targeted agents (sorafenib, sunitinib, pazopanib, and bevacizumab) [48]. Therefore, despite VHL gene driving the use of certain targeted drugs in mRCC, their association with prognosis and response to treatment has not been established.
2.4.2. PBRM1
Protein polybromo-1 (PB1), also known as BRG1-associated factor 180 (BAF180) is a protein that is encoded by the PBRM1 gene, which is a tumor suppressor gene that belongs to the SWItch/Sucrose Non-Fermentable (SWI/SNF) chromatin remodeling complex [49]. PBRM1 is mutated in about 40%–50% of ccRCC patients and makes for the second most common mutation overall [50,51]. The role of PBRM1 as a predictive or prognostic biomarker has been variable across different studies.
A retrospective study conducted by The Cancer Genome Atlas (TCGA) Research Network that included a total of 488 ccRCC samples, concluded that PBRMI1 mutations did not correlate with survival in ccRCC. Another retrospective study that included 132 patients with ccRCC, concluded that PBRM1 mutated tumors had a non-significant trend toward a longer relapse-free survival (RFS) [50].
Further, for utility of PBRM1 as a predictor of response to treatment, data emerged from studies correlating PBRM1 mutations with responsiveness to treatments. A retrospective study conducted on 31 patients with ccRCC showed that patients with PBRM1 mutations had a longer duration of response when treated with VEGF inhibitors [51]. Subsequently data on genomics pertaining to correlation with response to targeted treatments were obtained from RECORD-3 (a phase III trial comparing first-line mTOR inhibitor everolimus and sunitinib) that included 220 samples from patients with clear cell histology. Unlike the previous study, PFS was prolonged in patients with PBRM1 mutation who received the mTOR inhibitor but not for those that received the VEGF inhibitor [52]. Both these scenarios present the discrepancy and variation in conclusions related to PBRM1 mutation across studies. In the IMmotion150 trial, 305 patients with metastatic RCC were included. For PBRM1 mutated tumors, PFS was significantly longer in arms containing VEGF inhibitors (both atezolizumab/bevacizumab and the sunitinib arms) as compared to the ICI arm [25]. A subsequent retrospective study looked at 143 ccRCC patients treated with ICIs in the front line/second line or in combination with VEGF inhibitors [55]. This study showed that PBRM1 mutations were not associated with an impact on survival in ICI treated patients. Contrasting results were presented from analysis of pre-treatment samples of metastatic ccRCC patients on a clinical trial, where patients who had truncating mutations in PBRM1 were seen to experience clinical benefit from ICI therapy [35].
In summary, above evidence suggests that PBRM1 altered tumors have a better prognosis and these mutations may be able to predict responsiveness to checkpoint inhibitor therapy. However, large studies are required to validate these findings and at this time, are not being used in clinic to decide on treatment options.
2.4.3. BAP1
BRCA1 associated protein-1 (BAP1) gene is also located on chromosome 3. It is a tumor suppressor gene which is a ubiquitin carboxy-terminal hydrolase and like PBRM1, has chromatin remodeling properties [56]. BAP1 mutations were seen in about 10%–15% patients with ccRCC and were reported to be mutually exclusive from the PBRM1 mutations [56,57]. The role of BAP1 as a predictive and prognostic biomarker is discussed here. Per the TCGA analysis, BAP1 mutations were associated with reduced survival in those with ccRCC [51], which was also seen in a retrospective analysis of 145 patients [58]. Another large retrospective study conducted on nephrectomy specimens of 1439 patients with localized ccRCC showed that BAP1 protein loss (which correlates with BAP1 gene mutation) was associated with an increased risk of death in patients with ccRCC [59].
From a predictive standpoint, a combined model including mutation status of six genes of interest (BAP1, PBRM1, TP53, TERT, KDM5C, and SETD2) were added to the MSKCC risk model to create a genomically annotated model in patients enrolled on the COMPARZ trial (sunitinib and pazopanib, used as the training cohort) and RECORD-3 trial (everolimus and sunitinib, used as the validation cohort) [60]. Mutation status of BAP1, PBRM1, and TP53 were shown to have independent prognostic value in metastatic RCC patients treated with first-line TKIs and recommended further investigation in prospective trials [60].
2.4.4. SETD2
SET domain containing protein 2 (SETD2) gene is also located as the previous genes on chromosome 3. This H3 lysine 36 histone methyltransferase gene mediates its function through effector proteins which bind trimethylated H3K36, which in turn causes multiple chromatin-regulated processes (RNA splicing, DNA damage repair, and DNA methylation) [61,62]. The frequency of SETD2 gene alteration has been reported at around 13% in the TCGA dataset [51], and in metastatic tumors, this frequency is higher at around 30% [57,63]. Prognostically, in 421 patients in the TCGA cohort, those with the SETD2 mutations had worse cancer specific survival [63]. In analysis from COMPARZ and RECORD-3 clinical trials that included patients with metastatic disease, no correlation was demonstrated between SETD2 mutations and survival [60]. Further studies on SETD2 are needed to better elucidate this role.
2.4.5. DNA damage repair (DDR) genes
These are being explored as prognostic or predictive markers in metastatic RCC as well as potential targets for drugs in clinical trials. Especially interesting are results from studies looking at DDR mutations and prognosis and responsiveness to treatment. In other tumors, like lung cancer, presence of DDR mutations has been associated with high tumor mutation burden and potential responsiveness to ICIs as well as other agents such as PARP inhibitors [64,65]. In RCC, however, variable results are seen across studies. For example, in one study that retrospectively analyzed data on 229 patients, 19% had deleterious DDR gene alterations. While DDR deleterious status was associated with better OS for patients treated with ICIs, no such association was seen with tyrosine kinase inhibitors [66]. Another study that analyzed 34 RCC samples, showed that DNA damage response gene mutations were correlated with a high tumor mutational burden, and samples from patients with progressive disease had a significantly higher mutations in genes involved in this pathway [31]. Also, even though restrictive due to smaller sample size, these data showed that DNA damage response gene mutations were associated with responsiveness to ICI based therapy [31]. These smaller studies have proposed DDR gene mutations as biomarkers of responsiveness to ICI based therapy that need to be validated in larger cohorts. The role of DDR mutations will also become important in RCC if some of the ongoing trials with PARP inhibitors are positive in RCC (e.g. the phase II study for olaparib in metastatic RCC patients with DDR gene mutations and the phase II trial studying the combination of talazoparib and avelumab in patients with metastatic RCC), which are currently enrolling patients [67].
2.5. Genomic signatures to predict response to treatment
As alluded to in the earlier sections, there is a paucity of data to confirm the association between single gene mutations and outcomes in ccRCC. Transcriptomic gene signatures are now being studied in this setting to predict response to various treatment modalities and to predict prognosis.
2.5.1. Gene expression signatures in localized ccRCC
Patients with high risk localized ccRCC have ≥40% risk of recurrence after surgery [68]. Presently, the only drug approved for patients with high-risk localized ccRCC who have undergone nephrectomy is the tyrosine kinase inhibitor, sunitinib. Approval of sunitinib is based on disease free survival benefit seen in the S-TRAC phase III trial but no overall survival benefit was determined. Also, administration of this drug comes at the cost of a high risk of adverse events [69]. Multiple other trials that evaluated targeted agents in this setting (sorafenib/sunitinib in ASSURE, axitinib in ATLAS, and pazopanib in PROTECT study) showed no clinical benefit [[70], [71], [72]]. Several ongoing trials are investigating checkpoint inhibitors in high risk localized ccRCC (atezolizumab in IMMOTION-010, pembrolizumab in KEYNOTE-564, nivolumab in PROSPER, nivolumab and ipilimumab in CHECKMATE-914, and durvalumab in RAMPART clinical trial) [73]. As suitability of multiple drugs is being evaluated in the peri-operative setting, there is a great need for identifying biomarkers to prognosticate and predict outcomes of high risk localized ccRCC patients.
2.5.1.1. 16-gene recurrence score
Using a large cohort of 1568 patients, 732 genes were identified, of which 11 genes related to cancer and five house-keeping genes were selected for correlation with recurrence free survival [74]. The signature was validated in the phase III S-TRAC study (sunitinib adjuvant treatment for patients at high risk of recurrence of renal cell carcinoma following nephrectomy). The recurrence score was able to risk-stratify patients in both placebo and sunitinib groups, however the assay was not predictive of treatment benefit with sunitinib [74].
2.5.1.2. ClearCode34 gene signature
Investigators utilized data from 48 localized RCC samples, which identified two independent clusters (ccA and ccB) and further developed a 34-gene classifier (ClearCode34), which was then applied to RNA-sequencing data from TCGA for 380 non-metastatic ccRCC samples and to 157 formalin-fixed clinical samples from University of North Carolina [75]. In both cohorts, ccB was seen to be associated with poor prognosis.
2.5.1.3. Cell-cycle progression (CCP) score
The Myriad cell cycle score was first developed to predict outcomes in localized prostate cancer [76]. Later it was adapted for bladder, lung cancers [77] and subsequently for localized RCC as well [78]. While Morgan et al. [78] found that a higher CCP score correlated with a higher 5-year mortality, subsequently Ueno et al. [79] found that in the TCGA cohort, CCP score was not able to be correlate with prognosis.
2.5.2. Gene expression signatures in metastatic RCC
The armamentarium for the management of metastatic renal cell cancer has hugely expanded in the last decade with immune checkpoint inhibitors being used in the frontline setting. Within the last decade, PD-1 inhibitors have become available alone or in combination with CTLA-4 inhibitors and TKIs. These drugs have improved outcomes in patients with RCC. As the treatment landscape expands, several gene signatures are being proposed in this realm to help predict response to treatment regimens for better patient selection. While several gene signatures are being reported from around the globe [[80], [81], [82]], the more important ones are described here.
2.5.2.1. IMmotion150
This study was a phase II clinical trial that compared sunitinib with either single-agent atezolizumab or an atezolizumab-bevacizumab combination [10]. The study investigators analyzed the molecular characteristics of tumors from patients enrolled on this study in a subsequent publication [25]. Three distinct gene signatures were identified—the Angiohigh gene signature (associated with high vascular density with CD31 IHC), immune signature (Teffhigh gene signature with T-effector presence and function, IFN-γ response, response to checkpoint inhibitors; high expression of PD-L1 and CD8+T cell infiltration) and the myeloidhigh gene signature [25]. Patients with the Angiohigh signature had the best response when treated with anti-angiogenic drug sunitinib, while those with the myeloidhigh did poorly when treated with atezolizumab alone or in combination with bevacizumab but treatment with sunitinib did not seem to be affected. Finally the patients with Teffhigh gene signature were reported to have better outcomes when treated with the atezolizumab and bevacizumab [25].
2.5.2.2. COMPARZ
COMPARZ is a phase III clinical trial that compared sunitinib with pazopanib and showed equal efficacy between the two drugs [83]. Archival tissue samples were analyzed and investigators found four distinct molecular subtypes and similar to findings from the IMmotion150, suggested that a higher angiogenesis signature was able to predict better outcomes when treated with a TKI [84].
2.5.2.3. JAVELIN renal 101
JAVELIN Renal 101 is a phase III trial comparing avelumab plus axitinib and sunitinib which lead to the approval of the former combination arm [8]. Translational work from this study showed that an elevated immune cluster was associated with better PFS when treated with avelumab combination rather than the TKI alone [26]. This group identified a 26-gene signature (Renal-101 Immuno signature), which predicted longer PFS when treated with the immunotherapy-based treatment but showed limited overlap with the IMmotion 150 effector T cell signature described above.
As these signatures are being developed further and validated in other cohorts, it seems such signatures are paving the path to precision medicine in RCC. While none of these signatures are currently approved for use, this certainly remains an active area of research.
3. Brief description of biomarkers in non-clear cell renal cell carcinoma
NC-RCC cases account for almost 25%–30% of all [85]. Amongst these papillary RCC accounts for most cases (about 15%), followed by chromophobe type (about 5%) and the remaining are accounted for by the much rarer subtypes (hereditary leiomyomatosis and RCC, collecting duct carcinoma, renal medullary carcinoma, MiT family translocation RCC, succinate dehydrogenase deficient renal carcinoma, mucinous tubular/spindle cell carcinoma, tubulocystic RCC, and unclassified types); often designated as “rare kidney cancers” [82].
According to a TCGA analysis of 161 patients with papillary RCC tumors, almost 80% of the type-1 tumors were found to have an alteration of the MET gene (amplification, mutation, or duplication) or a gain of chromosome-7 (which harbors the MET gene) [86]. This leads to clinical trials for papillary RCC using MET inhibiting drugs (foretinib and crizotinib, which are multi-kinase inhibitors, as well as savolitinib, which is highly MET specific). Savolitinib is of special interest because even though there were preclinical, phase I and phase II data to support its use in MET-driven papillary RCC, the phase III trial comparing savolitinib versus sunitinib did not meet pre-specified primary endpoint of PFS (7 months for savolitinib vs. 5.6 months with sunitinib [HR 0.71; p-value was not significant]) [87]. Even though the PFS, OS and ORR were numerically higher in the savolitinib arm, these were not statistically significant differences and the study has been terminated and will not be accruing further. Meanwhile, the clinical trial, PAPMET (NCT02761057), a recently completed Southwest Oncology Group (SWOG) phase II randomized control trial initially designed to compare cabozantinib, crizotinib, savolitinib, and sunitinib in patients with papillary NC-RCC is ongoing. While the crizotinib and savolitinib arms have been removed for futility, the trial is expected to report the efficacy of multi-kinase inhibitor, cabozantinib as compared to sunitinib for MET mutated as well as MET expressing tumors [88]. CALYPSO is a phase I/II trial where savolitinib is being used in combination with the PD-L1 inhibitor, durvalumab, in patients [89]. Therefore, even though we know from literature that papillary RCCs are MET driven tumors, use of MET alterations as a biomarker of efficacy is not validated yet because of varying results from the trials utilizing these drugs as well as different methods used across trials to detect these mutations. Future studies analyzing this relationship further as well as other biomarkers such as gene signatures are required to be incorporated in the non-clear cell RCC clinical trials as well.
4. Novel biomarkers in kidney cancer
4.1. Role of biomarkers based on metabolic derangements in kidney cancer
Metabolic reprogramming is a cardinal feature of ccRCC, characterized by induction of glycolysis, nucleotides and lipids biosynthetic pathways as well as downregulation of multiple metabolic genes [[90], [91], [92]]. Recently, a distinct metabolic subtype of clear cell renal cell carcinoma in tobacco smokers which was independent of genomic alterations was described using an integrated transcriptomic, metabolomic, and metallomic approach [93]. The metabolic subtype was characterized by activation of oxidative phosphorylation coupled with reprogramming of the malate-aspartate shuttle. Further metallomic analysis showed redistribution of copper among intracellular pools, including that into the cytochrome c oxidase complex. A gene expression signature (MG-154), developed from the tumors analyzed was able to prognosticate localized ccRCC tumors in The Cancer Genome Atlas (TCGA).
Circulating metabolic pathway substrates have also been identified. Analysis of patients on CheckMate-025 (phase III study comparing nivolumab vs. everolimus) showed that there was an enhanced tryptophan to kynurenine conversion in response to ICI (nivolumab), and a decrease in the kynurenine/tryptophan ratio over time while on ICI was associated with improved OS [94]. The same group has previous data on the metabolite, adenosine, where they showed that low level of adenosine in patients treated with nivolumab was associated with a better response when treated with the checkpoint inhibitor [95].
Metabolic pathways need to be further explored in ccRCC, which is a highly metabolically driven tumor and clearly current evidence suggests a paucity of standard biomarkers that have been established for other tumor types.
4.2. Other exploratory biomarkers
4.2.1. Neutrophil/lymphocyte ratio (NLR)
In several malignant tumors, including RCC, the ratio of pretreatment NLR has been explored as a predictor of response to therapy [96,97]. Analysis of 1199 patients from the IMDC cohort of VEGF targeted treated patients, showed that patients with a higher NLR at baseline had worse OS and if patients experienced a decline in NLR by Week 6 while on treatment, they had better outcomes [98]. In ICI based therapies studies, including a meta-analysis on >6000 patients have corroborated that higher pretreatment NLR predicted worse PFS and OS and a decrease in NLR compared to baseline while on treatment was associated with improved outcomes [[99], [100], [101]]. Since evaluation of neutrophils and lymphocytes involves a simple blood test, this can become an easy to obtain biomarker, however, needs further validation in larger prospective clinical trials for incorporation into clinical practice.
4.2.2. Circulating DNA (ctDNA) in RCC
Unlike other genitourinary malignancies (especially prostate cancer), the role of ctDNA is less clear in RCC. While liquid biopsies are an easy, non-invasive method of performing next generation sequencing (NGS), the level of tumor derived DNA in circulation seems to be low even in studies that included metastatic RCC [102]. When comparing genomic alterations between tumor tissue and ctDNA, a study reported similar median rate of mutations in both, but the concordance rate was only 8.6% [103]. Another study revealed significant differences in genomic alterations observed at the time of first-line treatment versus at subsequent lines [104]. These findings suggest the role of ctDNA needs further evaluation and at this point cannot be recommended as a stand-alone diagnostic or prognostic test.
4.2.3. Role of the microbiome in predicting response to treatments in RCC
Analysis of the human microbiome is done by amplification and sequencing of bacterial nucleic acids obtained from human stool, urine and saliva. In RCC, a correlation between response to ICIs and the relative abundance of Akkermansia muciniphila in stool samples of patients with metastatic RCC has been reported [105,106]. Further, supplementation with Akkermansia muciniphila, Alistipes indistinctus, or Enterococcus hirae was shown to help recover the efficacy of ICIs in germ-free mice [105]. In RCC patients, the role of stool microbiome has been recognized in a previous study on 20 patients with mRCC receiving VEGF inhibitors. The study reported high Bacteroides spp. and low Prevotella spp. in patients who developed diarrhea; less relative abundance of Bifidobacterium spp. in VEGF-TKI treated patients as compared to previously reported healthy subjects [107]. As more studies continue to evolve, the role of the human microbiome as a predictor of outcomes will continue to evolve.
5. Conclusion
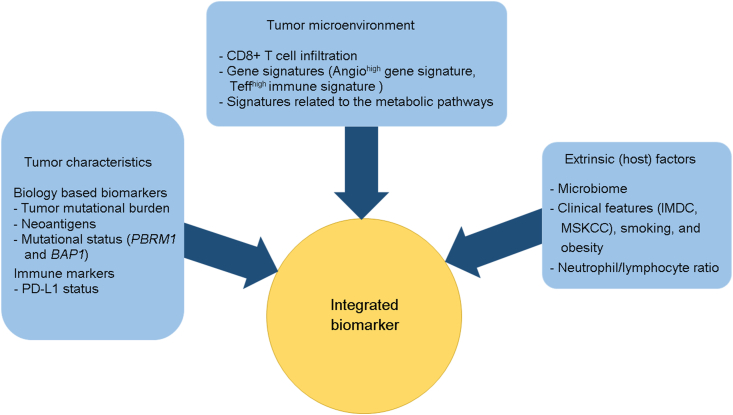
In summary, management of advanced RCC has undergone a paradigm shift over the last decade. As a number of highly effective therapies have become available and more trials are ongoing, it is imperative to develop means to develop a biomarker-based approach for treatment selection. This has been difficult to achieve as there are no actionable or driver mutations in RCC. Moreover, tumor heterogeneity, variability of assays between clinical trials and lack of validation across studies have made it difficult to develop predictive biomarkers. It seems from the information we have available from recent studies that a single biomarker such as a single gene mutation or a single gene expression signature will likely not be helpful in kidney cancer. Instead it will be important for investigators to think of an integrated biomarker approach, wherein a composite biomarker incorporating tumor characteristic, tumor microenvironment related changes and host factors (microbiome) is incorporated (Fig. 2). In our review we have attempted to comprehensively summarize studies done so far for the discovery of predictive as well as prognostic biomarkers for kidney cancer. We also point out some future directions as this field continues to evolve.
Figure 2.
Proposed approach to develop an “integrated biomarker” for patients with kidney cancer. IMDC, International Metastatic Renal Cell Carcinoma Database Consortium; PDL1, programmed death ligand-1; MSLCC, the Memorial Sloan-Kettering Cancer Center.
Author contributions
Study concept and design: Shuchi Gulati, Nicholas J. Vogelzang.
Data acquisition: Shuchi Gulati, Nicholas J. Vogelzang.
Data analysis: Shuchi Gulati, Nicholas J. Vogelzang.
Drafting of manuscript: Shuchi Gulati, Nicholas J. Vogelzang.
Critical revision of the manuscript: Shuchi Gulati, Nicholas J. Vogelzang.
Conflicts of interest
Shuchi Gulati declares no conflict of interest; Nicholas J. Vogelzang reports conflicts with Bayer, Janssen, Pfizer, Astrazeneca, Astellas, Eisai, Exelexis, Genetech, Merck, Myovant, Novartis, Pfizer, Sanofi-Genzyme, and Tolero.
Acknowledgement
We thank Dr. Primo Nery Lara, Jr., MD for critical review of the manuscript and for providing feedback. The project described was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health, under Award Number 2KL2TR001426-05A1. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
Footnotes
Peer review under responsibility of Second Military Medical University.
References
- 1.Key statistics about kidney cancer. https://www.cancer.org/cancer/kidney-cancer/about/key-statistics.html [Internet]
- 2.Kidney cancer. World Cancer Research Fund; 2018. https://www.wcrf.org/dietandcancer/kidney-cancer [Internet] [Google Scholar]
- 3.Gulati S., Vaishampayan U. Current state of systemic therapies for advanced renal cell carcinoma. Curr Oncol Rep. 2020;22:26. doi: 10.1007/s11912-020-0892-1. [DOI] [PubMed] [Google Scholar]
- 4.Motzer R.J., Tannir N.M., McDermott D.F., Frontera O.A., Melichar B., Choueiri T.K. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378:1277–1290. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rini B.I., Plimack E.R., Stus V., Gafanov R., Hawkins R., Nosov D. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380:1116–1127. doi: 10.1056/NEJMoa1816714. [DOI] [PubMed] [Google Scholar]
- 6.Yagoda A., Abi-Rached B., Petrylak D. Chemotherapy for advanced renal-cell carcinoma: 1983–1993. Semin Oncol. 1995;22:42–60. [PubMed] [Google Scholar]
- 7.Motzer R.J., Escudier B., McDermott D.F., George S., Hammers H.J., Srinivas S. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015 5;373:1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Motzer R.J., Penkov K., Haanen J., Rini B., Albiges L., Campbell M.T. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019 21;380:1103–1115. doi: 10.1056/NEJMoa1816047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choueiri T.K., Powles T., Burotto M., Bourlon M.T., Zurawski B., Juárez V.M.O. 696O_PR Nivolumab+cabozantinib vs. sunitinib in first-line treatment for advanced renal cell carcinoma: First results from the randomized phase III CheckMate 9ER trial. Ann Oncol. 2020;31:S1159. doi: 10.1016/j.annonc.2020.08.2257. [DOI] [Google Scholar]
- 10.Rini B.I., Powles T., Atkins M.B., Escudier B., McDermott D.F., Suarez C. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): A multicentre, open-label, phase 3, randomised controlled trial. Lancet. 2019;393:2404–2415. doi: 10.1016/S0140-6736(19)30723-8. [DOI] [PubMed] [Google Scholar]
- 11.Hudes G., Carducci M., Tomczak P., Dutcher J., Figlin R., Kapoor A. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 12.Heng D.Y.C., Xie W., Regan M.M., Warren M.A., Golshayan A.R., Sahi C. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: Results from a large, multicenter Study. J Clin Oncol. 2009;27:5794–5799. doi: 10.1200/JCO.2008.21.4809. [DOI] [PubMed] [Google Scholar]
- 13.Motzer R.J., Mazumdar M., Bacik J., Berg W., Amsterdam A., Ferrara J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. 1999;17:2530–2540. doi: 10.1200/JCO.1999.17.8.2530. [DOI] [PubMed] [Google Scholar]
- 14.Heng D.Y., Xie W., Regan M.M., Harshman L.C., Bjarnason G.A., Vaishampayan U.N. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: A population-based study. Lancet Oncol. 2013;14:141–148. doi: 10.1016/S1470-2045(12)70559-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pérez-Valderrama B., Arranz Arija J.A., Rodríguez Sánchez A., Pinto Marín A., Borrega García P., Castellano Gaunas D.E. Validation of the international metastatic renal-cell carcinoma Database consortium (IMDC) prognostic model for first-line pazopanib in metastatic renal carcinoma: The Spanish oncologic genitourinary group (SOGUG) SPAZO study. Ann Oncol. 2016;27:706–711. doi: 10.1093/annonc/mdv601. [DOI] [PubMed] [Google Scholar]
- 16.Kroeger N., Xie W., Lee J.-L., Bjarnason G.A., Knox J.J., MacKenzie M.J. Metastatic non-clear cell renal cell carcinoma (nccRCC) treated with targeted therapy agents: Characterization of survival outcome and application of the International mRCC Database Consortium (IMDC) Criteria. Cancer. 2013;119:2999–3006. doi: 10.1002/cncr.28151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Motzer R.J., Rini B.I., McDermott D.F., Arén Frontera O., Hammers H.J., Carducci M.A. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: Extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol. 2019;20:1370–1385. doi: 10.1016/S1470-2045(19)30413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Peralta-Venturina M., Moch H., Amin M., Tamboli P., Hailemariam S., Mihatsch M. Sarcomatoid differentiation in renal cell carcinoma: A study of 101 cases. Am J Surg Pathol. 2001;25:275–284. doi: 10.1097/00000478-200103000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Rini B.I., Plimack E.R., Stus V., Gafanov R., Hawkins R., Nosov D. Pembrolizumab (pembro) plus axitinib (axi) versus sunitinib as first-line therapy for metastatic renal cell carcinoma (mRCC): Outcomes in the combined IMDC intermediate/poor risk and sarcomatoid subgroups of the phase 3 KEYNOTE-426 study. J Clin Oncol. 2019;37:4500. doi: 10.1200/JCO.2019.37.15_suppl.4500. [DOI] [Google Scholar]
- 20.McDermott D.F., Choueiri T.K., Motzer R.J., Aren O.R., George S., Powles T. CheckMate 214 post-hoc analyses of nivolumab plus ipilimumab or sunitinib in IMDC intermediate/poor-risk patients with previously untreated advanced renal cell carcinoma with sarcomatoid features. J Clin Orthod. 2019 May 20;37:4513. doi: 10.1200/JCO.2019.37.15_suppl.4513. [DOI] [Google Scholar]
- 21.Bakouny Z., Braun D.A., Shukla S.A., Pan W., Gao X., Hou Y. Integrative molecular characterization of sarcomatoid and rhabdoid renal cell carcinoma. Nat Commun. 2021;12:808. doi: 10.1038/s41467-021-21068-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thompson R.H., Gillett M.D., Cheville J.C., Lohse C.M., Dong H., Webster W.S. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A. 2004;101:17174–17179. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iacovelli R., Nolè F., Verri E., Renne G., Paglino C., Santoni M. Prognostic role of PD-L1 expression in renal cell carcinoma. A systematic review and meta-analysis. Target Oncol. 2016;11:143–148. doi: 10.1007/s11523-015-0392-7. [DOI] [PubMed] [Google Scholar]
- 24.Choueiri T.K., Figueroa D.J., Fay A.P., Signoretti S., Liu Y., Gagnon R. Correlation of PD-L1 tumor expression and treatment outcomes in patients with renal cell carcinoma receiving sunitinib or pazopanib: Results from COMPARZ, a randomized controlled trial. Clin Canc Res. 2015;21:1071–1077. doi: 10.1158/1078-0432.CCR-14-1993. [DOI] [PubMed] [Google Scholar]
- 25.McDermott D.F., Huseni M.A., Atkins M.B., Motzer R.J., Rini B.I., Escudier B. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med. 2018;24:749–757. doi: 10.1038/s41591-018-0053-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Motzer R.J., Robbins P.B., Powles T., Albiges L., Haanen J.B., Larkin J. Avelumab plus axitinib versus sunitinib in advanced renal cell carcinoma: Biomarker analysis of the phase 3 JAVELIN Renal 101 trial. Nat Med. 2020;26:1733–1741. doi: 10.1038/s41591-020-1044-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu J., Armstrong A.J., Friedlander T.W., Kim W., Pal S.K., George D.J. Biomarkers of immunotherapy in urothelial and renal cell carcinoma: PD-L1, tumor mutational burden, and beyond. J Immunother Cancer. 2018;6:4. doi: 10.1186/s40425-018-0314-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Callea M., Albiges L., Gupta M., Cheng S.-C., Genega E.M., Fay A.P. Differential expression of PD-L1 between primary and metastatic sites in clear-cell renal cell carcinoma. Cancer Immunol Res. 2015;3:1158–1164. doi: 10.1158/2326-6066.CIR-15-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Samstein R.M., Lee C.-H., Shoushtari A.N., Hellmann M.D., Shen R., Janjigian Y.Y. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. 2019;51:202–206. doi: 10.1038/s41588-018-0312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng D.T., Mitchell T.N., Zehir A., Shah R.H., Benayed R., Syed A. Memorial sloan kettering-integrated mutation profiling of actionable cancer targets (MSK-IMPACT): A hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular Oncology. J Mol Diagn. 2015;17:251–264. doi: 10.1016/j.jmoldx.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Labriola M.K., Zhu J., Gupta R., McCall S., Jackson J., Kong E.F. Characterization of tumor mutation burden, PD-L1 and DNA repair genes to assess relationship to immune checkpoint inhibitors response in metastatic renal cell carcinoma. J Immunother Cancer. 2020;8 doi: 10.1136/jitc-2019-000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wood M.A., Weeder B.R., David J.K., Nellore A., Thompson R.F. Burden of tumor mutations, neoepitopes, and other variants are weak predictors of cancer immunotherapy response and overall survival. Genome Med. 2020;12:33. doi: 10.1186/s13073-020-00729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dizman N., Lyou Y., Salgia N., Bergerot P.G., Hsu J., Enriquez D. Correlates of clinical benefit from immunotherapy and targeted therapy in metastatic renal cell carcinoma: Comprehensive genomic and transcriptomic analysis. J Immunother Cancer. 2020;8 doi: 10.1136/jitc-2020-000953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Braun D.A., Hou Y., Bakouny Z., Ficial M., Sant’ Angelo M., Forman J. Interplay of somatic alterations and immune infiltration modulates response to PD-1 blockade in advanced clear cell renal cell carcinoma. Nat Med. 2020;26:909–918. doi: 10.1038/s41591-020-0839-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Motzer R.J., Choueiri T.K., McDermott D.F., Powles T., Yao J., Ammar R. Biomarker analyses from the phase III CheckMate 214 trial of nivolumab plus ipilimumab (N+I) or sunitinib (S) in advanced renal cell carcinoma (aRCC) J Clin Oncol. 2020;38:5009. doi: 10.1200/JCO.2020.38.15_suppl.5009. [DOI] [Google Scholar]
- 36.FDA approves pembrolizumab for adults and children with TMB-H solid tumors. FDA. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-pembrolizumab-adults-and-children-tmb-h-solid-tumors [Internet] Available from:
- 37.Czyzyk-Krzeska M.F., Landero Figueroa J.A., Gulati S., Cunningham J.T., Meller J., ShamsaeI B. Molecular and metabolic subtypes in sporadic and inherited clear cell renal cell carcinoma. Genes (Basel) 2021;12:388. doi: 10.3390/genes12030388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sato Y., Yoshizato T., Shiraishi Y., Maekawa S., Okuno Y., Kamura T. Integrated molecular analysis of clear-cell renal cell carcinoma. Nat Genet. 2013;45:860–867. doi: 10.1038/ng.2699. [DOI] [PubMed] [Google Scholar]
- 39.Moch H., Cubilla A.L., Humphrey P.A., Reuter V.E., Ulbright T.M. The 2016 WHO Classification of tumours of the urinary system and male genital organs—Part A: Renal, penile, and testicular tumours. Eur Urol. 2016;70:93–105. doi: 10.1016/j.eururo.2016.02.029. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y.J., Houldsworth J., Emmadi R., Dyer L., Wolff D.J. Assessing genomic copy number alterations as best practice for renal cell neoplasia: An evidence-based review from the cancer genomics consortium workgroup. Cancer Genet. 2020;244:40–54. doi: 10.1016/j.cancergen.2020.04.004. [DOI] [PubMed] [Google Scholar]
- 41.Kim W.Y., Kaelin W.G. Role of VHL gene mutation in human cancer. J Clin Oncol. 2004;22:4991–5004. doi: 10.1200/JCO.2004.05.061. [DOI] [PubMed] [Google Scholar]
- 42.Choueiri T.K., Kaelin W.G. Targeting the HIF2–VEGF axis in renal cell carcinoma. Nat Med. 2020;26:1519–1530. doi: 10.1038/s41591-020-1093-z. [DOI] [PubMed] [Google Scholar]
- 43.Jonasch E., Donskov F., Iliopoulos O., Rathmell W.K., Narayan V., Maughan B.L. Phase II study of the oral HIF2α inhibitor MK-6482 for Von Hippel-Lindau disease-associated renal cell carcinoma. J Clin Oncol. 2020;38(Suppl. 15):5003. doi: 10.1200/JCO.2020.38.15_suppl.5003. [DOI] [Google Scholar]
- 44.Cowey C.L., Rathmell W.K. VHL gene mutations in renal cell carcinoma: Role as a biomarker of disease outcome and drug efficacy. Curr Oncol Rep. 2009;11:94–101. doi: 10.1007/s11912-009-0015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Büscheck F., Fraune C., Simon R., Kluth M., Hube-Magg C., Möller-Koop C. Prevalence and clinical significance of VHL mutations and 3p25 deletions in renal tumor subtypes. Oncotarget. 2020;11:237–249. doi: 10.18632/oncotarget.27428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choueiri T.K., Vaziri S.A.J., Jaeger E., Elson P., Wood L., Bhalla I.P. von Hippel-Lindau gene status and response to vascular endothelial growth factor targeted therapy for metastatic clear cell renal cell carcinoma. J Urol. 2008;180:860–866. doi: 10.1016/j.juro.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 47.Choueiri T.K., Fay A.P., Gagnon R., Lin Y., Bahamon B., Brown V. The role of aberrant VHL/HIF pathway elements in predicting clinical outcome to pazopanib therapy in patients with metastatic clear-cell renal cell carcinoma. Clin Canc Res. 2013;19:5218–5226. doi: 10.1158/1078-0432.CCR-13-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim B.J., Kim J.H., Kim H.S., Zang D.Y. Prognostic and predictive value of VHL gene alteration in renal cell carcinoma: a meta-analysis and review. Oncotarget. 2017;8:13979–13985. doi: 10.18632/oncotarget.14704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shain A.H., Pollack J.R. The spectrum of SWI/SNF mutations, ubiquitous in human cancers. PloS One. 2013;8 doi: 10.1371/journal.pone.0055119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Varela I., Tarpey P., Raine K., Huang D., Ong C.K., Stephens P. Exome sequencing identifies frequent mutation of the SWI/SNF complex gene PBRM1 in renal carcinoma. Nature. 2011;469:539–542. doi: 10.1038/nature09639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ricketts C.J., De Cubas A.A., Fan H., Smith C.C., Lang M., Reznik E. The cancer genome atlas comprehensive molecular characterization of renal cell carcinoma. Cell Rep. 2018;23:313–326. doi: 10.1016/j.celrep.2018.03.075. https://10.1016/j.celrep.2018.03.075 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ho T.H., Choueiri T.K., Wang K., Karam J.A., Chalmers Z., Frampton G. Correlation between molecular subclassifications of clear cell renal cell carcinoma and targeted therapy response. Eur Urol Focus. 2016;2:204–209. doi: 10.1016/j.euf.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 55.Hakimi A.A., Ged Y., Flynn J., Hoen D.R., Di Natale R.G., Blum K.A. The impact of PBRM1 mutations on overall survival in greater than 2100 patients treated with immune checkpoint blockade (ICB) J Clin Oncol. 2019;37(Suppl 7):666. doi: 10.1200/JCO.2019.37.7_suppl.666. [DOI] [Google Scholar]
- 56.Carbone M., Harbour J.W., Brugarolas J., Bononi A., Pagano I., Dey A. Biological mechanisms and clinical significance of BAP1 mutations in human cancer. Canc Discov. 2020;10:1103–1120. doi: 10.1158/2159-8290.CD-19-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peña-Llopis S., Vega-Rubín-de-Celis S., Liao A., Leng N., Pavía-Jiménez A., Wang S. BAP1 loss defines a new class of renal cell carcinoma. Nat Genet. 2012;44:751–759. doi: 10.1038/ng.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kapur P., Peña-Llopis S., Christie A., Zhrebker L., Pavía-Jiménez A., Rathmell W.K. Effects on survival of BAP1 and PBRM1 mutations in sporadic clear-cell renal-cell carcinoma: A retrospective analysis with independent validation. Lancet Oncol. 2013;14:159–167. doi: 10.1016/S1470-2045(12)70584-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Joseph R.W., Kapur P., Serie D.J., Eckel-Passow J.E., Parasramka M., Ho T. Loss of BAP1 protein expression is an independent marker of poor prognosis in patients with low risk clear cell renal cell carcinoma. Cancer. 2014;120:1059–1067. doi: 10.1002/cncr.28521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Voss M.H., Reising A., Cheng Y., Patel P., Marker M., Kuo F. Genomically annotated risk model for advanced renal-cell carcinoma: A retrospective cohort study. Lancet Oncol. 2018;19:1688–1698. doi: 10.1016/S1470-2045(18)30648-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fahey C.C., Davis I.J. SETting the stage for cancer development: SETD2 and the consequences of lost methylation. Cold Spring Harb Perspect Med. 2017;7:a026468. doi: 10.1101/cshperspect.a026468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.González-Rodríguez P., Engskog-Vlachos P., Zhang H., Murgoci A.-N., Zerdes I., Joseph B. SETD2 mutation in renal clear cell carcinoma suppress autophagy via regulation of ATG12. Cell Death Dis. 2020;11:69. doi: 10.1038/s41419-020-2266-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hakimi A.A., Ostrovnaya I., Reva B., Schultz N., Chen Y.-B., Gonen M. Adverse outcomes in clear cell renal cell carcinoma with mutations of 3p21 epigenetic regulators BAP1 and SETD2: A report by MSKCC and the KIRC TCGA research network. Clin Canc Res. 2013;19:3259–3267. doi: 10.1158/1078-0432.CCR-12-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mamdani H., Chen J., Kim S., Ibrahim Y., Asad M.F.B., Nieva J.J. DNA damage response and repair (DDR) gene mutations and correlation with tumor mutation burden (TMB) in non-small cell lung cancer (NSCLC) J Clin Oncol. 2019;37:9100. doi: 10.1200/JCO.2019.37.15_suppl.9100. [DOI] [Google Scholar]
- 65.Ricciuti B., Recondo G., Spurr L.F., Li Y.Y., Lamberti G., Venkatraman D. Impact of DNA damage response and repair (DDR) gene mutations on efficacy of PD-(L)1 immune checkpoint inhibition in non-small cell lung cancer. Clin Canc Res. 2020;26:4135–4142. doi: 10.1158/1078-0432.CCR-19-3529. [DOI] [PubMed] [Google Scholar]
- 66.Ged Y., Chaim J.L., DiNatale R.G., Knezevic A., Kotecha R.R., Carlo M.I. DNA damage repair pathway alterations in metastatic clear cell renal cell carcinoma and implications on systemic therapy. J Immunother Cancer. 2020;8 doi: 10.1136/jitc-2019-000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Home-ClinicalTrials.gov. https://clinicaltrials.gov/ [Internet]
- 68.Lam J.S., Shvarts O., Leppert J.T., Pantuck A.J., Figlin R.A., Belldegrun A.S. Postoperative surveillance protocol for patients with localized and locally advanced renal cell carcinoma based on a validated prognostic nomogram and risk group stratification system. J Urol. 2005;174:466–472. doi: 10.1097/01.ju.0000165572.38887.da. [DOI] [PubMed] [Google Scholar]
- 69.Ravaud A., Motzer R.J., Pandha H.S., George D.J., Pantuck A.J., Patel A. Adjuvant sunitinib in high-risk renal-cell carcinoma after nephrectomy. N Engl J Med. 2016;375:2246–2254. doi: 10.1056/NEJMoa1611406. [DOI] [PubMed] [Google Scholar]
- 70.Haas N.B., Manola J., Dutcher J.P., Flaherty K.T., Uzzo R.G., Atkins M.B. Adjuvant treatment for high-risk clear cell renal cancer: Updated results of a high-risk subset of the ASSURE Randomized Trial. JAMA Oncol. 2017;3:1249–1252. doi: 10.1001/jamaoncol.2017.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Motzer R.J., Haas N.B., Donskov F., Gross-Goupil M., Varlamov S., Kopyltsov E. Randomized phase III trial of adjuvant pazopanib versus placebo after nephrectomy in patients with localized or locally advanced renal cell carcinoma. J Clin Oncol. 2017;35:3916–3923. doi: 10.1200/JCO.2017.73.5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gross-Goupil M., Kwon T.G., Eto M., Ye D., Miyake H., Seo S.I. Axitinib versus placebo as an adjuvant treatment of renal cell carcinoma: Results from the phase III, randomized ATLAS trial. Ann Oncol. 2018;29:2371–2378. doi: 10.1093/annonc/mdy454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wood E., Donin N., Shuch B. Adjuvant therapy for localized high-risk renal cell carcinoma. Urol Clin North Am. 2020;47:345–358. doi: 10.1016/j.ucl.2020.04.007. [DOI] [PubMed] [Google Scholar]
- 74.Rini B., Goddard A., Knezevic D., Maddala T., Zhou M., Aydin H. A 16-gene assay to predict recurrence after surgery in localised renal cell carcinoma: Development and validation studies. Lancet Oncol. 2015;16:676–685. doi: 10.1016/S1470-2045(15)70167-1. [DOI] [PubMed] [Google Scholar]
- 75.Brooks S.A., Brannon A.R., Parker J.S., Fisher J.C., Sen O., Kattan M.W. ClearCode34: A prognostic risk predictor for localized clear cell renal cell carcinoma. Eur Urol. 2014;66:77–84. doi: 10.1016/j.eururo.2014.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cuzick J., Swanson G.P., Fisher G., Brothman A.R., Berney D.M., Reid J.E. Prognostic value of an RNA expression signature derived from cell cycle proliferation genes in patients with prostate cancer: A retrospective study. Lancet Oncol. 2011;12:245–255. doi: 10.1016/S1470-2045(10)70295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dancik G.M., Theodorescu D. Robust prognostic gene expression signatures in bladder cancer and lung adenocarcinoma depend on cell cycle related genes. PloS One. 2014;9 doi: 10.1371/journal.pone.0085249. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Morgan T.M., Mehra R., Tiemeny P., Wolf J.S., Wu S., Sangale Z. A multigene signature based on cell cycle proliferation improves prediction of mortality within 5 yr of radical nephrectomy for renal cell carcinoma. Eur Urol. 2018;73:763–769. doi: 10.1016/j.eururo.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 79.Ueno D., Dancik G.M., Shuch B. The cell cycle progression score: Unclear role in renal cell carcinoma. Eur Urol. 2018;74:128–129. doi: 10.1016/j.eururo.2018.03.033. [DOI] [PubMed] [Google Scholar]
- 80.Zhan Y., Guo W., Zhang Y., Wang Q., Xu X., Zhu L. A five-gene signature predicts prognosis in patients with kidney renal clear cell carcinoma. Comput Math Methods Med. 2015;2015:842784. doi: 10.1155/2015/842784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yao M., Huang Y., Shioi K., Hattori K., Murakami T., Sano F. A three-gene expression signature model to predict clinical outcome of clear cell renal carcinoma. Int J Canc. 2008;123:1126–1132. doi: 10.1002/ijc.23641. [DOI] [PubMed] [Google Scholar]
- 82.Dai J., Lu Y., Wang J., Yang L., Han Y., Wang Y. A four-gene signature predicts survival in clear-cell renal-cell carcinoma. Oncotarget. 2016;7:82712–82726. doi: 10.18632/oncotarget.12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Motzer R.J., Hutson T.E., Cella D., Reeves J., Hawkins R., Guo J. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369:722–731. doi: 10.1056/NEJMoa1303989. [DOI] [PubMed] [Google Scholar]
- 84.Hakimi A.A., Voss M.H., Kuo F., Sanchez A., Liu M., Nixon B.G. Transcriptomic profiling of the tumor microenvironment reveals distinct subgroups of clear cell renal cell cancer: Data from a randomized phase III trial. Canc Discov. 2019;9:510–525. doi: 10.1158/2159-8290.CD-18-0957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gulati S., Philip E., Salgia S., Pal S.K. Evolving treatment paradigm in metastatic non clear cell renal cell carcinoma. Cancer Treat Res Commun. 2020;23:100172. doi: 10.1016/j.ctarc.2020.100172. [DOI] [PubMed] [Google Scholar]
- 86.Cancer Genome Atlas Research Network. Linehan W.M., Spellman P.T., Ricketts C.J., Creighton C.J., Fei S.S. Comprehensive molecular characterization of papillary renal-cell carcinoma. N Engl J Med. 2016;374:135–145. doi: 10.1056/NEJMoa1505917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Choueiri T.K., Heng D.Y.C., Lee J.L., Cancel M., Verheijen R.B., Mellemgaard A. Efficacy of savolitinib vs. sunitinib in patients with MET-driven papillary renal cell carcinoma. JAMA Oncol. 2020;6:1247–1255. doi: 10.1001/jamaoncol.2020.2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pal S.K., Tangen C., Thompson I.M., Balzer-Haas N., George D.J., Heng D.Y.C. A comparison of sunitinib with cabozantinib, crizotinib, and savolitinib for treatment of advanced papillary renal cell carcinoma: A randomised, open-label, phase 2 trial. Lancet. 2021;397:695–703. doi: 10.1016/S0140-6736(21)00152-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Powles T., Larkin J.M.G., Patel P., Pérez-Valderrama B., Rodriguez-Vida A., Glen H. A phase II study investigating the safety and efficacy of savolitinib and durvalumab in metastatic papillary renal cancer (CALYPSO) J Clin Oncol. 2019;37(Suppl. 7):545. doi: 10.1200/JCO.2019.37.7_suppl.545. [DOI] [PubMed] [Google Scholar]
- 90.Cancer Genome Atlas Research Network Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature. 2013;499:43–49. doi: 10.1038/nature12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Semenza G.L. HIF-1 mediates the Warburg effect in clear cell renal carcinoma. J Bioenerg Biomembr. 2007;39:231–234. doi: 10.1007/s10863-007-9081-2. [DOI] [PubMed] [Google Scholar]
- 92.Gatto F., Nookaew I., Nielsen J. Chromosome 3p loss of heterozygosity is associated with a unique metabolic network in clear cell renal carcinoma. Proc Natl Acad Sci U S A. 2014;111:e866–e875. doi: 10.1073/pnas.1319196111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Reigle J., Secic D., Biesiada J., Wetzel C., Shamsaei B., Chu J. Tobacco smoking induces metabolic reprogramming of renal cell carcinoma. J Clin Invest. 2021;131 doi: 10.1172/JCI140522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li H., Bullock K., Gurjao C., Braun D., Shukla S.A., Bossé D. Metabolomic adaptations and correlates of survival to immune checkpoint blockade. Nat Commun. 2019;10:4346. doi: 10.1038/s41467-019-12361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Giannakis M., Li H., Jin C., Gopal S., Desai K., Horak C. Metabolomic correlates of response in nivolumab-treated renal cell carcinoma and melanoma patients. J Clin Oncol. 2017;35(Suppl. 15):3036. doi: 10.1200/JCO.2017.35.15_suppl.3036. [DOI] [Google Scholar]
- 96.Bagley S.J., Kothari S., Aggarwal C., Bauml J.M., Alley E.W., Evans T.L. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer. 2017;106:1–7. doi: 10.1016/j.lungcan.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 97.Nunno V.D., Mollica V., Gatto L., Santoni M., Cosmai L., Porta C. Prognostic impact of neutrophil-to-lymphocyte ratio in renal cell carcinoma: A systematic review and meta-analysis. Immunotherapy. 2019;11:631–643. doi: 10.2217/imt-2018-0175. [DOI] [PubMed] [Google Scholar]
- 98.Templeton A.J., Knox J.J., Lin X., Simantov R., Xie W., Lawrence N. Change in neutrophil-to-lymphocyte ratio in response to targeted therapy for metastatic renal cell carcinoma as a prognosticator and biomarker of efficacy. Eur Urol. 2016;70:358–364. doi: 10.1016/j.eururo.2016.02.033. [DOI] [PubMed] [Google Scholar]
- 99.Jeyakumar G., Kim S., Bumma N., Landry C., Silski C., Suisham S. Neutrophil lymphocyte ratio and duration of prior anti-angiogenic therapy as biomarkers in metastatic RCC receiving immune checkpoint inhibitor therapy. J Immunother Cancer. 2017;5:82. doi: 10.1186/s40425-017-0287-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lalani A.-K.A., Xie W., Martini D.J., Steinharter J.A., Norton C.K., Krajewski K.M. Change in neutrophil-to-lymphocyte ratio (NLR) in response to immune checkpoint blockade for metastatic renal cell carcinoma. J Immunother Cancer. 2018;6:5. doi: 10.1186/s40425-018-0315-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shao Y., Wu B., Jia W., Zhang Z., Chen Q., Wang D. Prognostic value of pretreatment neutrophil-to-lymphocyte ratio in renal cell carcinoma: A systematic review and meta-analysis. BMC Urol. 2020;20:90. doi: 10.1186/s12894-020-00665-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Smith C.G., Moser T., Burge J., Eldridge M., Riediger A.L., Mouliere F. Comprehensive characterisation of cell-free tumour DNA in plasma and urine of patients with renal tumours. Genome Med. 2020;12:23. doi: 10.1186/s13073-020-00723-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hahn A.W., Gill D.M., Maughan B., Agarwal A., Arjyal L., Gupta S. Correlation of genomic alterations assessed by next-generation sequencing (NGS) of tumor tissue DNA and circulating tumor DNA (ctDNA) in metastatic renal cell carcinoma (mRCC): Potential clinical implications. Oncotarget. 2017;8:33614–33620. doi: 10.18632/oncotarget.16833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pal S.K., Sonpavde G., Agarwal N., Vogelzang N.J., Srinivas S., Haas N.B. Evolution of circulating tumor DNA profile from first-line to subsequent therapy in metastatic renal cell carcinoma. Eur Urol. 2017;72:557–564. doi: 10.1016/j.eururo.2017.03.046. [DOI] [PubMed] [Google Scholar]
- 105.Routy B., Le Chatelier E., Derosa L., Duong C.P.M., Alou M.T., Daillère R. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 106.Salgia N.J., Bergerot P.G., Maia M.C., Dizman N., Hsu J., Gillece J.D. Stool microbiome profiling of patients with metastatic renal cell carcinoma receiving anti-PD-1 immune checkpoint inhibitors. Eur Urol. 2020;78:498–502. doi: 10.1016/j.eururo.2020.07.011. [DOI] [PubMed] [Google Scholar]
- 107.Pal S.K., Li S.M., Wu X., Qin H., Kortylewski M., Hsu J. Stool bacteriomic profiling in patients with metastatic renal cell carcinoma receiving vascular endothelial growth factor—tyrosine kinase inhibitors. Clin Canc Res. 2015;21:5286–5293. doi: 10.1158/1078-0432.CCR-15-0724. [DOI] [PubMed] [Google Scholar]