Abstract
Gab1 is a member of the Gab/DOS (Daughter of Sevenless) family of adapter molecules, which contain a pleckstrin homology (PH) domain and potential binding sites for SH2 and SH3 domains. Gab1 is tyrosine phosphorylated upon stimulation of various cytokines, growth factors, and antigen receptors in cell lines and interacts with signaling molecules, such as SHP-2 and phosphatidylinositol 3-kinase, although its biological roles have not yet been established. To reveal the functions of Gab1 in vivo, we generated mice lacking Gab1 by gene targeting. Gab1-deficient embryos died in utero and displayed developmental defects in the heart, placenta, and skin, which were similar to phenotypes observed in mice lacking signals of the hepatocyte growth factor/scatter factor, platelet-derived growth factor, and epidermal growth factor pathways. Consistent with these observations, extracellular signal-regulated kinase mitogen-activated protein (ERK MAP) kinases were activated at much lower levels in cells from Gab1-deficient embryos in response to these growth factors or to stimulation of the cytokine receptor gp130. These results indicate that Gab1 is a common player in a broad range of growth factor and cytokine signaling pathways linking ERK MAP kinase activation.
Cytokine and growth factor receptors trigger multiple signaling cascades regulating cell growth and differentiation. Many growth factor receptors have a protein tyrosine kinase domain in their cytoplasmic domain (receptor tyrosine kinase). In contrast, cytokine receptors, such as those for interleukins, interferons, and colony-stimulating factors, do not have an intrinsic kinase domain but instead constitutively associate with Janus tyrosine kinases (JAKs). Binding of growth factors and cytokines to their cognate receptors induces the homo- and heterodimerization of the receptors, which position the kinase domains close to each other (reviewed in references 8 and 14). This leads to transphosphorylation and thereby activation of the receptor tyrosine kinase and the receptor-associated JAKs. The activated kinases further phosphorylate other tyrosine residues in the cytoplasmic domain, which recruit various signaling molecules containing Src homology 2 (SH2) or phosphotyrosine binding (PTB) domains and activate these molecules. In addition to the receptors, scaffolding adapter molecules are tyrosine phosphorylated by the receptor tyrosine kinases or the receptor-associated kinases and subsequently recruit SH2 or PTB domain-containing signaling molecules. Such scaffolding adapter molecules contribute to specification and amplification of signal transduction downstream of the receptors (26). These include IRS family adapter molecules, Shc, Dok, FRS2, and Gab family adapter molecules, all of which act downstream of tyrosine kinases (26).
Gab1 (Grb2-associated binder 1) is a member of the Gab/DOS (Daughter of Sevenless) family of adapter molecules. Gab1 contains a pleckstrin homology (PH) domain in the amino-terminal region, as well as tyrosine-based motifs and proline-rich sequences (PXXP), which are potential binding sites for various SH2 domains and the SH3 domains, respectively (12). Gab1 is tyrosine phosphorylated upon stimulation of receptors by growth factors such as epidermal growth factor (EGF), NGF, BDNF, platelet-derived growth factor (PDGF), insulin, hepatocyte growth factor (HGF), and stem cell factor (SCF), cytokines such as interleukin-6 (IL-6), IL-3, erythropoietin, and thrombopoietin, lysophosphatidic acid, and T- and B-cell-antigen receptors in cell lines (5, 12, 13, 22, 24, 33, 37, 38). Gab1 interacts with multiple signaling molecules, such as SHP-2, p85 phosphatidylinositol 3-kinase, phospholipase C-γ, and Grb2 (12, 24, 33, 37). Overexpression of Gab1 in cell lines enhances or mimics EGF-mediated cell growth and anchorage-independent transformation (12), HGF-mediated cell scattering, branching morphogenesis, extracellular signal-regulated kinase mitogen-activated protein kinase (ERK MAPK) activation (37), and gp130-mediated ERK MAPK activation (33). Gab2, another member of the Gab/DOS family, is tyrosine phosphorylated in response to IL-2, IL-3, Tpo, Epo, SCF, and the stimulation of gp130 and T- and B-cell-antigen receptors (7, 24, 38, 40). Overexpression of Gab2 enhances gp130- and IL-3-dependent ERK MAPK activation (7, 24), and the presence of Gab2 mutant proteins lacking the SHP-2 binding sites inhibits IL-3-dependent c-fos promoter activity (7). Consistent with these observations, genetic studies with Drosophila revealed that DOS acts downstream of receptor tyrosine kinases Sevenless and Torso (a homologue of the PDGF receptor) and EGF receptors, possibly in combination with Corkscrew, a Drosophila homologue of SHP-2 (9, 27). These reports suggest that Gab/DOS family adapter proteins function in signal transduction through a variety of cytokine and growth factor receptors and possibly other receptor systems. However, it has not yet been established whether Gab adapter molecules are required for cytokine- and growth factor-mediated signal transduction in vivo.
For the initial attempt to reveal the functions of Gab proteins in vivo, we generated mice lacking Gab1 by gene targeting. Gab1-deficient mice died in utero and displayed developmental defects in the heart, placenta, and skin. The phenotypes were similar to those observed in mice deficient for signaling of the HGF, PDGF, and EGF pathway. Furthermore, activation of ERK MAP kinase in response to EGF, PDGF, and HGF and to stimulation of the cytokine receptor gp130 was strongly reduced in Gab1-deficient fibroblasts. The data provide evidence that Gab1 plays a crucial role in a broad range of growth factor and cytokine signaling pathways in vivo.
MATERIALS AND METHODS
Generation of mutant mice.
Mouse genomic DNA containing an exon encoding the PH domain was isolated from a λFixII 129sv mouse strain genomic library. A targeting vector was constructed by replacing a 3.0-kb BamHI fragment containing a part of the PH domain with a cassette consisting of a splice acceptor site from mouse En-2, an internal ribosomal entry site (IRES) from picornavirus (21), and a fusion sequence consisting of the β-galactosidase-neomycin resistance gene (β-geo). Transfected embryonic stem cell colonies that survived after selection with G418 and ganciclovir were subcloned, and homologous recombination events were detected by PCR and Southern blotting with a probe located on the 5′ side of the exon. Targeted cells were injected into C57BL/6 mouse blastulas to create chimeric male founders. Chimeric offspring were mated to C57BL/6 mice to generate F1 heterozygous progeny. The F1 progeny were intercrossed to generate F2 progeny. The F1 heterozygous males were crossed with the F1 or F2 heterozygous females to generate Gab1+/+, Gab1+/−, and Gab1−/− embryos. The genotypes of the embryos were identified routinely by PCR, using a combination of three primers. Primer sequences and PCR conditions are available on request.
Embryonic fibroblasts.
Embryonic day 14.5 (E14.5) embryos were isolated by caesarean section, and their heads and blood were removed. The remainder was cut into small pieces and trypsinized. Cells were separated by centrifugation and plated on culture dishes. Embryonic fibroblasts were cultured in Dulbecco's modified Eagle's medium containing 10% fetal calf serum.
ERK assay and transfection.
Embryonic fibroblasts (2 × 106) were starved with 0.1% fetal calf serum for 5 h and stimulated with 100 ng of human IL-6/ml and 100 ng of recombinant soluble IL-6 receptor α (sIL-6Rα)/ml or 50 ng of EGF/ml and 100 ng of PDGF-BB/ml. Cells were lysed with 1 ml of a lysis buffer (1% Triton X-100, 20 mM Tris HCl, 150 mM NaCl, sodium vanadate, phenylmethylsulfonyl fluoride, pepstatin, leupeptin). An amount of lysate equivalent to 4 × 104 cells was fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotted with anti-active ERK antibodies (Promega) or anti-ERK2 antibody (C-14, which also cross-reacts with ERK1; Santa Cruz). To detect ERK kinase activities, cell lysates were immunoprecipitated with the anti-ERK2 antibody and subjected to an in vitro kinase reaction using myelin basic protein (MBP) (33). Immunoprecipitated ERK2 was incubated with MBP in the presence of [γ-32P]ATP. Phosphorylated MBP was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and incorporated 32P was measured with a BAS2000 image analyzer (Fuji Film Co.). For reexpression of Gab1 (see Fig. 6B), 2 × 106 embryonic fibroblasts were transfected with 5 μg of pcDNA3-Flag ERK2 and/or 5 μg of pcDNA3-HA Gab1. For the analysis of HGF signaling (see Fig. 7C), the expression vector for human c-Met (pRS2) was used (10). Embryonic fibroblasts were transfected with 5 μg of pRS2 and 5 μg of pcDNA3-Flag ERK2. Transfectants were stimulated with 30 ng of human HGF/ml for 10 min. Flag-tagged ERK2 was isolated from the stimulated cells with anti-Flag antibody and subjected to the in vitro kinase assay (see Fig. 6B and 7C).
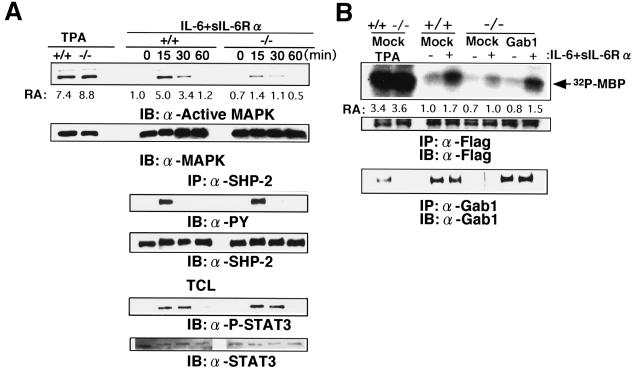
FIG. 6.
Reduction in gp130-dependent ERK MAP kinase activation in Gab1−/− cells. (A) gp130-mediated ERK activation. Gab1+/+ and Gab1−/− embryonic fibroblasts were stimulated with IL-6 and sIL-6Rα for the indicated periods or with tetradecanoyl phorbol acetate (TPA) for 30 min. Lysates were immunoblotted with anti-diphospho-ERKs or anti-ERK2 antibodies. Kinase activities were determined by an immunoprecipitation (IP) kinase assay using the anti-ERK2 antibody and MBP. The amount of incorporated 32P in MBP was determined and is indicated as relative activity (versus activity in unstimulated Gab1+/+ cells) (RA). Tyrosine phosphorylation and expression of SHP-2 and STAT3 are also indicated. TCL, total cell lysate. (B) Transfection of Gab1 rescued the ERK activation in Gab1−/− cells. Gab1+/+ or Gab1−/− fibroblasts were transfected with an expression vector for human Gab1 and Flag-tagged ERK2 and stimulated with IL-6 and sIL-6Rα for 15 min. Expression of Flag-tagged ERK2 and Gab1 is indicated. Flag-tagged ERK2 was immunoprecipitated with anti-Flag antibody and was subjected to an in vitro kinase assay. The results are shown by autoradiography and indicated as relative activities. IB, immunoblot.
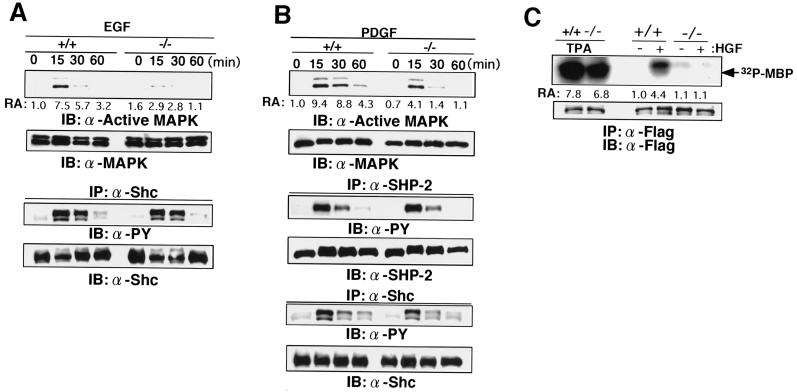
FIG. 7.
Reduced activation of ERK MAP kinase in response to EGF, PDGF, and HGF. (A) EGF-induced ERK activation and tyrosine phosphorylation of Shc. Gab1+/+ and Gab−/− embryonic fibroblasts were stimulated with EGF for the indicated periods. The indicated isoform of Shc is p52. PY, phosphotyrosine. (B) PDGF-induced ERK activation and tyrosine phosphorylation of SHP-2 and Shc. (C) c-Met-induced ERK activation. Embryonic fibroblasts were transfected with expression vectors for Flag-tagged ERK2 and c-Met. At 20 h after transfection, cells were stimulated with HGF for 10 min (+) or left unstimulated (−). ERK activities were determined by the in vitro kinase assay, using anti-Flag immunoprecipitates (IP). Similar amounts of c-Met were expressed in Gab1+/+ and Gab1−/− cells (data not shown). IB, immunoblot.
Immunoblotting.
The anti-Gab1 antibody was raised by immunizing rabbits with mouse Gab1 protein containing amino acids 452 to 695 fused to glutathione S-transferase and was purified with a glutathione S-transferase–Gab1 column. Antibodies used for immunoblotting were anti-SHP-2 (C-18; Santa Cruz), anti-tyrosine-phosphorylated STAT3 (New England Biolabs), anti-STAT3 (23), anti-Shc (Upstate Biotechnology Inc.), and antiphosphotyrosine antibodies (4G10; Upstate Biotechnology Inc.). Immune complexes were visualized with a Renaissance chemiluminescence system (Dupont, NEN Research Products).
Immunohistochemistry and β-galactosidase staining.
Embryos were isolated at different stages of gestation and fixed in buffered formalin, followed by paraffin embedding and sectioning. Sections were stained with hematoxylin and eosin. For immunohistochemistry, embryos or placentas were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) and frozen in ornithine carbamoyltransferase compound in liquid nitrogen. Immunohistochemistry was performed using the Vectastain Elite ABC kit (Vector Laboratories) according to the manufacturer's protocol. Affinity-purified anti-Gab1 antibody was used for the first antibody (1/200 dilution). Immune complexes were visualized with diaminobenzidine tetrahydrochloride precipitates. For determination of β-galactosidase activity, embryos were fixed for 15 min in 2% formaldehyde and 0.2% glutaraldehyde in PBS containing 0.1% NP-40 and were stained with 0.1% 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) in solution [2 mM MgCl2, 5 mM K3Fe(CN)6, 5 mM K4Fe(CN)6 in PBS] at room temperature.
RESULTS
Disruption of Gab1 leads to embryonic lethality.
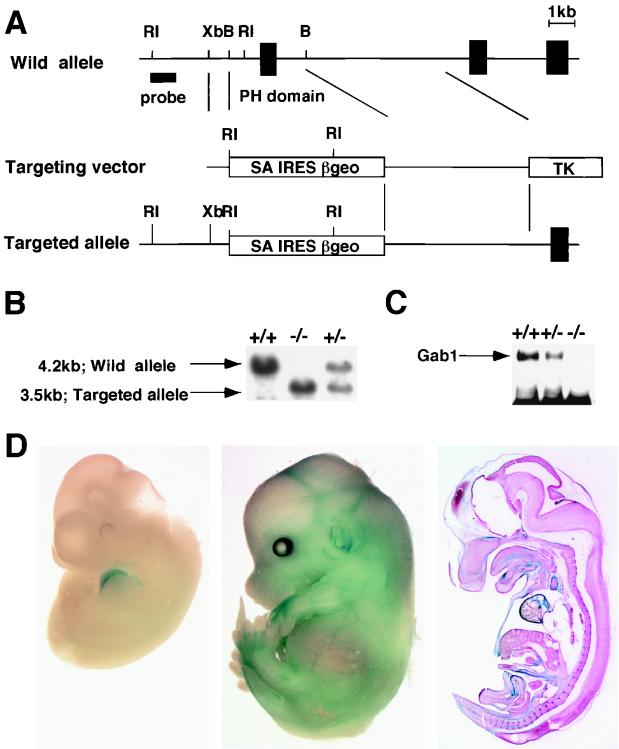
We disrupted the mouse Gab1 gene by replacing, in embryonic stem cells, an exon encoding part of the PH domain (amino acids 25 to 123) with a promoterless IRES β-geo cassette (Fig. 1A), which enabled us to determine the expression pattern of Gab1. Heterozygous mice appeared to be normal. However, no live homozygous offspring were born. To determine when the homozygotes died, we genotyped embryos at different stages of development (Table 1). The proportion of viable homozygotes versus wild-type embryos and heterozygotes decreased between E12.5 and E17.5, indicating that the homozygous embryos died at mid-to-late gestation. Immunoblot analysis confirmed that embryonic fibroblasts from the homozygous embryos lacked functional Gab1 protein (Fig. 1C). To visualize Gab1 expression during embryonic development, heterozygous and homozygous embryos at various developmental stages were stained for β-galactosidase activity. Gab1 was expressed ubiquitously at a low level, and high expression was detected in the heart from E10.5 to E13.5 and in the surface of the limbs, ears, some blood vessels, and the choroid plexus at E13.5 (Fig. 1D).
FIG. 1.
Targeting disruption of the Gab1 locus. (A) Restriction map of the Gab1 locus and targeting vector. The deletion region contains an exon encoding a part of the PH domain. This region was replaced by an en-2 splice acceptor-IRES–β-geo pA cassette. RI, EcoRI; Xb, XbaI; B, BamHI. (B) Southern blot analysis for genotyping embryos. EcoRI-digested DNA from wild-type (+/+), heterozygous (+/−), and homozygous (−/−) embryos was hybridized with the 5′ probe shown in panel A. TK, thymidine kinase. (C) Immunoblotting analysis of Gab1. Gab1 immunoprecipitates from Gab1−/−, Gab1+/−, and Gab1+/+ embryonic fibroblasts were analyzed by immunoblotting with the anti-Gab1 antibody. (D) β-Galactosidase staining. Heterozygous embryos were fixed at E11.5 (left) and E13.5 (middle) and stained with X-Gal. Sagittal sections of stained E13.5 embryos are also shown (right).
TABLE 1.
Genotypes of offspring obtained by intercrossing Gab1+/− mice
| Age | No. of live embryos with genotype
|
||
|---|---|---|---|
| +/+ | +/− | −/− | |
| E10.5–11.5 | 29 | 93 | 48 |
| E12.5–13.5 | 54 | 116 | 24 |
| E14.5–15.5 | 27 | 67 | 10 |
| E16.5–17.5 | 41 | 58 | 9 |
| E18.5 | 9 | 19 | 0 |
| 3 wk | 152 | 195 | 0 |
Developmental defects in the heart, placenta, and skin.
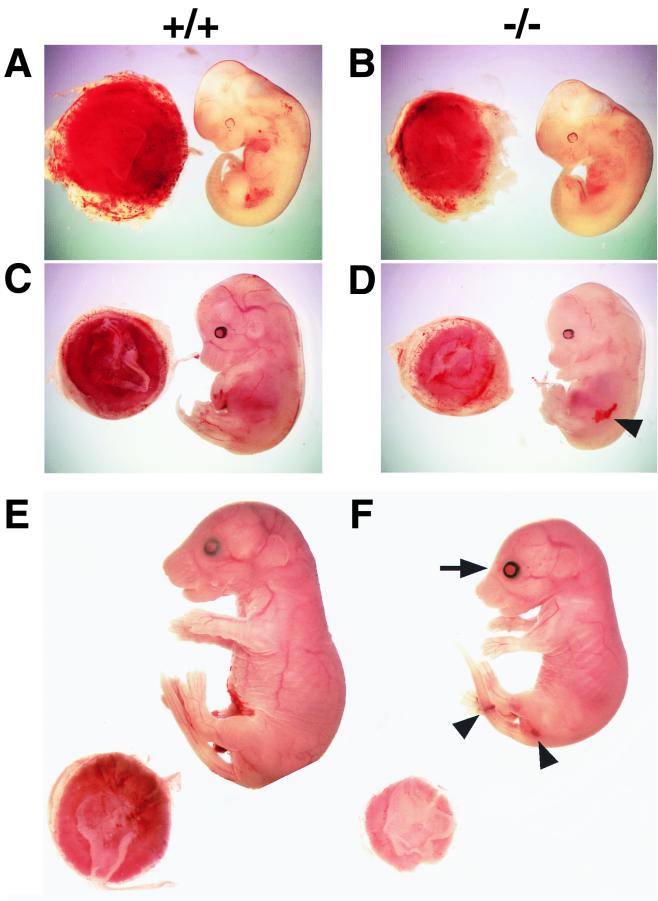
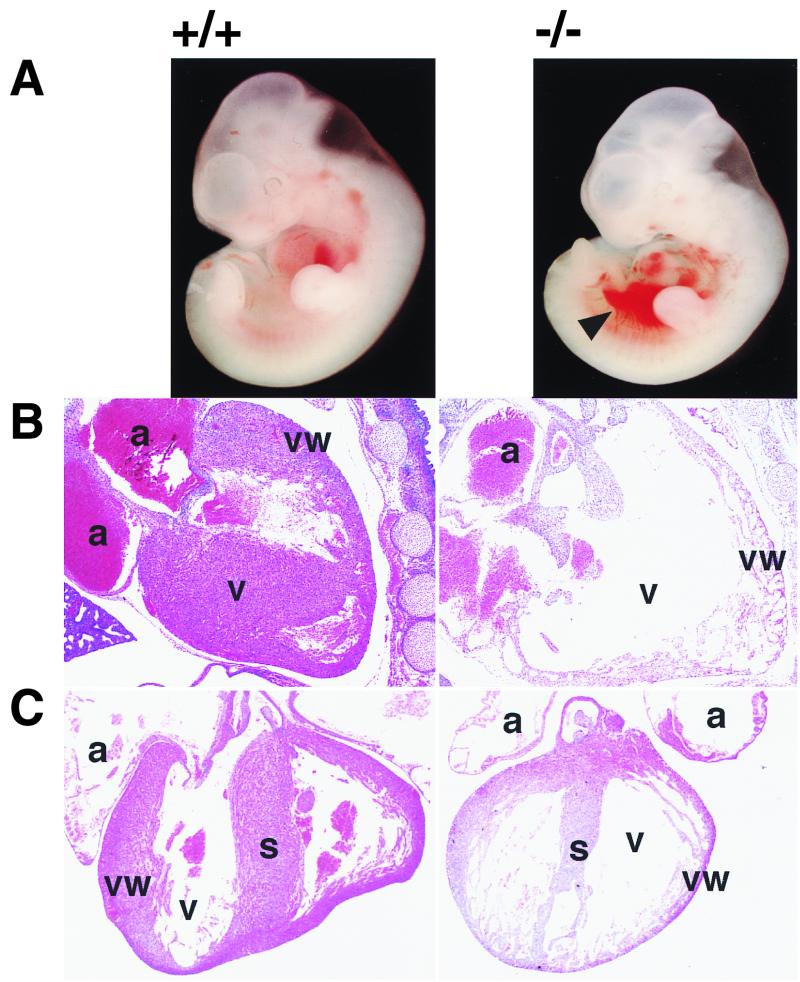
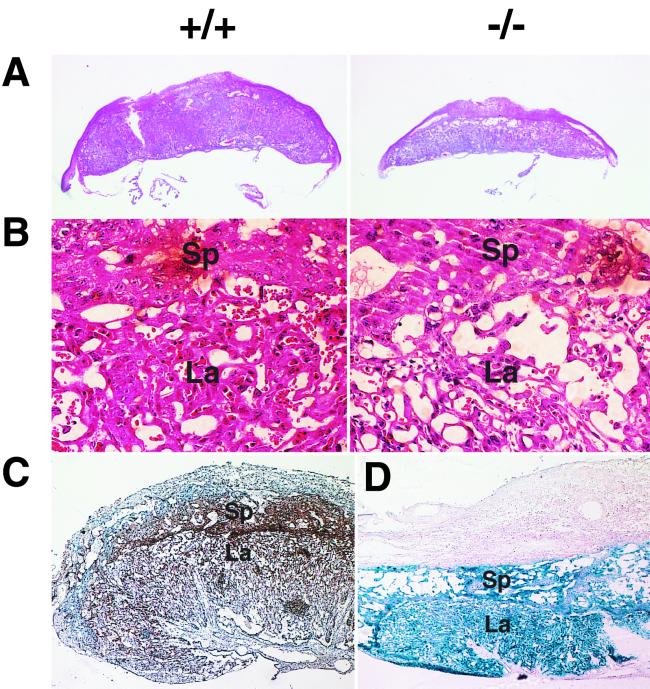
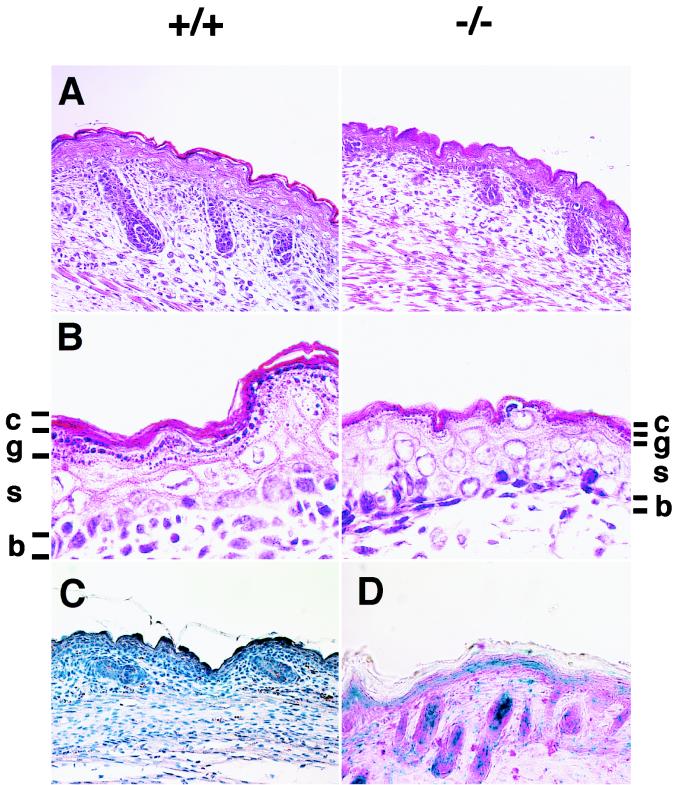
After E13.5, Gab1−/− embryos were smaller than their littermates (Fig. 2); some had hemorrhage (Fig. 2D and F) and petechiae (data not shown). Gab1−/− embryos that survived over E16.5 had open eyes and no eyelids (Fig. 2F). Heart development was impaired in the Gab1−/− mice but not in heterozygotes. About 40% (6 of 15) of the E10.5-to-E11.5 Gab1−/− embryos contained blood in the pericardial cavity (Fig. 3A). Later the ventricular chamber displayed hypoplasia and dilation and the ventricular wall was extremely thin in all the Gab1−/− embryos that survived past E13.5 (Fig. 3B and C). The placentas of Gab1−/− embryos were generally paler and smaller than those of their Gab1+/+ littermates (Fig. 2B, D, and F and 4A). Histological sections revealed that the number of trophoblast cells in the labyrinth region was severely reduced, resulting in hypoplasia of the labyrinth region (Fig. 4B). In contrast, the spongiotrophoblast cells of the junctional zone were not affected. Disorganization of the labyrinth region might affect the transport of nutrients and oxygen from the maternal side of the placenta and could lead to the death of the Gab1−/− embryos. Gab1 expression in the placenta was analyzed by immunostaining with an anti-Gab1 antibody and by X-Gal staining with β-galactosidase activity. Gab1 was expressed in both the labyrinth trophoblast cells and the spongiotrophoblast cells, suggesting that Gab1 acts cell autonomously in the labyrinth (Fig. 4C and D). Gab1 may not be required for the formation of the spongiotrophoblast, or there may be redundant mechanisms. For instance, Gab2 or other unidentified Gab/DOS-related molecules might compensate for the loss of Gab1 function. The epidermis of the Gab1−/− embryos was thinner than that of wild-type littermates, and the hair follicles were underdeveloped (Fig. 5). The basal layer of the epidermis of the Gab1−/− mice appeared to be normal, but the stratum granulosum of the Gab1−/− mice was thinner than that of the control littermates. Immunostaining and X-Gal staining showed the expression of Gab1 in the basal layer and hair follicles (Fig. 5C and D), suggesting a cell-autonomous role for Gab1 in skin development.
FIG. 2.
External appearance and placentas of wild-type and Gab1−/− embryos. (A and B) E11.5; (C and D) E13.5; (E and F) E17.5. Homozygous embryos were retarded in growth compared with wild-type littermates after E13.5. Mutant placentas were pale and smaller than the wild type. The arrowheads and arrow indicate hemorrhage and an open eye with no lid, respectively.
FIG. 3.
Developmental defects in heart of Gab1−/− embryos. (A) External appearance of E11.5 wild-type (+/+) and homozygous (−/−) embryos. Gab1−/− embryos contained blood in the pericardial cavity, indicated by the arrowhead. (B and C) Heart sections from wild-type (left) and Gab1−/− (right) embryos at E13.5 (B) E15.5 (C). Note the hypoblastic ventricle in the heart of Gab1−/− embryos (B and C). a, atrium; v, ventricle; s, septum; vw, ventricular wall.
FIG. 4.
Underdeveloped placentas in Gab1−/− embryos. Shown are sections of wild-type (+/+) and Gab1−/− placentas of E13.5 embryos at low (A) and high (B) magnifications. There were fewer trophoblast cells in the labyrinth region of Gab1−/− placentas. Sp, spongiotrophoblast; La, labyrinthine trophoblast. Gab1 expression was detected by immunohistochemistry with anti-Gab1 antibody (C) and by LacZ expression (D), with counterstaining with hematoxylin (C) and eosin (D). Expression of Gab1 was detected in the spongiotrophoblast and labyrinthine trophoblast.
FIG. 5.
Developmental defects in skin of Gab1−/− embryos. Shown are sections of epidermis in Gab1+/+ and Gab1−/− embryos at low (A) and high (B) magnifications. The epidermal layer was thinner and hair follicles were underdeveloped. c, stratum corneum; g, stratum granulosum; s, stratum spinosum; b, basal layer. Gab1 expression was detected by immunohistochemistry with anti-Gab1 antibody (C) and by LacZ expression (D), with counterstaining with hematoxylin (C) and eosin (D). Expression of Gab1 was detected in the epidermis and hair follicles.
Reduced ERK MAP kinase activation in Gab1-deficient cells.
To examine the roles of Gab1 in signal transduction of growth factors and cytokines, embryonic fibroblasts were isolated from Gab1−/− and Gab1+/+ E14.5 mice and stimulated with various growth factors and cytokines. When cells from the wild-type littermates were stimulated with IL-6 and sIL-6Rα to activate the cytokine receptor gp130, which is shared by the IL-6 family cytokine receptors for CNTF, LIF, OSM, IL-11, and CT-1 (11), the activity of ERK2 MAP kinase was increased within 15 min (Fig. 6A). Activated forms of ERK1 and ERK2 were recognized by an antibody that reacts with the diphospho-ERKs. When the Gab1−/− embryonic fibroblasts were stimulated with IL-6 and sIL-6Rα, induction of ERK activity was markedly reduced. However, the Gab1−/− embryonic fibroblasts could respond to tetradecanoyl phorbol acetate and activated ERK. Furthermore, the expression level of ERK was not altered in the Gab1−/− embryonic fibroblasts. The tyrosine phosphorylation of SHP-2 and STAT3 was not altered in response to IL-6 and IL-6Rα in the Gab1−/− embryonic fibroblasts, showing that the mutation specifically diminished the ERK activation in this pathway. Transfection of a Gab1 expression vector rescued the gp130-mediated ERK2 activation, confirming that the reduction in ERK activities was due to the lack of Gab1 expression (Fig. 6B). The activation of ERK in response to EGF and PDGF was also strongly reduced in the Gab1−/− embryonic fibroblasts, but tyrosine phosphorylation of SHP-2 and Shc was not altered in the EGF- and PDGF-stimulated Gab1−/− embryonic fibroblasts, respectively (Fig. 7A and B). Furthermore, c-Met-mediated ERK activation in the Gab1−/− embryonic fibroblasts was also reduced (Fig. 7C). Expression of gp130, the PDGF receptor, and the EGF receptor was not affected in the Gab1−/− embryonic fibroblasts (data not shown). These data indicate that the disruption of Gab1 specifically affected signaling between these receptors and the ERK MAP kinases.
DISCUSSION
Biochemical analyses of Gab1 suggest that Gab1 is involved in signaling of growth factor and cytokine receptors. Most of the analyses have focused on the tyrosine phosphorylation of Gab1, the interaction of Gab1 and signaling molecules, and the effects of overexpression of Gab1 and its mutant in cell lines. The roles of Gab1 in vivo and in a more physiological environment have not been investigated yet. Here we demonstrate that Gab1 plays a crucial role in the signal transduction of EGF, PDGF, HGF, and the cytokine receptor gp130 in vivo. First, Gab1-deficient mice displayed phenotypes similar to those observed in mice lacking signaling of the HGF, PDGF, and EGF family growth factors and leukemia inhibitory factor (Fig. 2, 3, 4, and 5). Open eyes lacking eyelids and underdeveloped skin phenotypes are observed in transforming growth factor α-, IGF receptor-, and EGF receptor-deficient mice (1, 18, 20, 34). Abnormal development of hair follicles is also observed in PDGF-A-deficient mice (15). Hemorrhage and cardiac hypoplasia are observed in mice lacking PDGF-B and PDGF receptor β (16, 32). Underdeveloped placenta, particularly in the labyrinth region, is observed in HGF/scatter factor, PDGF-B-, c-Met-, EGF receptor-, and leukemia inhibitory factor receptor-deficient mice (3, 19, 25, 30, 34–36). Second, ERK activation in response to EGF, PDGF, and HGF or to stimulation of gp130 was reduced in embryonic fibroblasts established from Gab1−/− embryos (Fig. 6 and 7). Collectively, the data suggest that Gab1 is a common player in a broad range of growth factor and cytokine signaling pathways in vivo and links the growth factor and cytokine receptors to ERK MAP kinases.
In addition to knockout mice lacking the ligands and the receptors described above, targeted disruption of genes known to play a role in Ras-ERK MAP kinase signaling has been reported. Grb2-deficient embryos die soon after implantation and Grb2 is required for development of the visceral endoderm and the epiblast, but its roles in later development remain to be elucidated (4). Mek-1-deficient embryos display abnormality in development of the labyrinthine region of the placenta, which is similar to the phenotype observed in the Gab1-deficient mice (Fig. 4). Targeted deletion of a part of the amino-terminal SH2 domain of SHP-2 leads to abnormal gastrulation and defects in mesodermal tissue (29). The ERK activation in response to EGF, fibroblast growth factor, PDGF, and IGF-1 is reduced in the embryonic fibroblasts containing the SHP-2 mutant alleles homozygously (29, 31). Gab1-deficient mice exhibit certain similarities with respect to the phenotypes and/or the ERK activation to Mek-1-deficient and SHP-2 mutant mice, suggesting that Gab1, SHP-2, and Mek-1 act in a line in a signal transduction cascade. In contrast, Mek-1-deficient and SHP-2 mutant mice, as well as Grb2-deficient mice, display more severe phenotypes than Gab1-deficient mice, suggesting that Gab1 may participate in a part but not all of Ras-ERK MAP kinase signaling. Genetic interaction studies using Gab1-deficient mice and targeted mice lacking the ligands, receptors, and signaling molecules may clarify the relationship between Gab1 and these signaling cascades.
Although ERK activation in response to these growth factors and to stimulation of gp130 was strongly reduced in Gab1−/− embryonic fibroblasts, ERK was weakly activated (Fig. 6 and 7). Immunoblotting analysis and reverse transcription-PCR revealed that embryonic fibroblasts expressed Gab1 but not Gab2 (data not shown). Thus, the remaining ERK activity should depend on pathways other than Gab1- and Gab2-mediated pathways. Tyrosine-phosphorylated SHP-2 interacts with Grb2 through the YXNX motifs in the carboxy-terminal region, and Grb2 constitutively associates with SOS, the GDP-GTP exchanging factor for Ras (2, 6, 17, 39). Shc also acts as an adapter molecule that links the receptor to Grb2-SOS (28). In Gab1−/− embryonic fibroblasts, SHP-2 was tyrosine phosphorylated in response to IL-6 and sIL-6Rα and to PDGF (Fig. 6 and 7), and Shc was also tyrosine phosphorylated in response to EGF and PDGF (Fig. 7). The Gab1-mediated pathway may collaborate or function in parallel with SHP-2- or Shc-mediated Grb2-SOS pathways to activate signals to ERK MAP kinases.
SHP-2 is one of the predominant Gab1-associated molecules. But the absence of Gab1 did not affect tyrosine phosphorylation of SHP-2 in response to the stimulation of gp130 and the PDGF receptor (Fig. 6 and 7). The data indicate that Gab1 is not required for tyrosine phosphorylation of SHP-2 in these signaling cascades. Members of our group previously demonstrated that SHP-2 is first recruited to gp130 and phosphorylated by the gp130-associated JAKs, and then tyrosine-phosphorylated SHP-2 interacts with tyrosine-phosphorylated Gab1 (33). In that scenario, Gab1 does not mediate the tyrosine phosphorylation of SHP-2 and other substrates by the JAKs and the PDGF receptor. Consistent with this, total tyrosine-phosphorylated protein profiles were not altered between Gab1+/+ and Gab1−/− embryonic fibroblasts stimulated by PDGF, EGF, or IL-6 and sIL-6Rα (data not shown).
The expression pattern of Gab1 overlaps with that of Gab2 in various tissues, and similar sets of stimuli induce tyrosine phosphorylation of Gab1 and Gab2 (7, 24). Gab1 and Gab2 also interact with a similar set of signaling molecules upon being stimulated (7, 24). These reports suggest redundant roles for Gab1 and Gab2 (7, 24). The disruption of the Gab1 gene, however, affected the development of various tissues whose normal development depends on growth factor and cytokine signaling, indicating a nonredundant role for Gab1 in these signal transduction pathways.
Like Gab1, DOS acts downstream of the receptor tyrosine kinases (9, 27). In the case of Sevenless signaling, both DOS- and SOS-mediated signals are necessary for the differentiation of R7 photoreceptor cells, which also requires the activity of the Drosophila ERK, Rolled. However, it is not yet known whether DOS acts upstream of ERK or affects other pathways for R7 differentiation. Furthermore, the roles of DOS in other signaling pathways have not been elucidated. Our data indicate that Gab1 mediates signals for a broad range of receptor tyrosine kinases and cytokine receptors. Furthermore, we first established that Gab1-mediated signals are involved in the activation of ERK. Since Gab1 binds a number of signaling molecules, such as SHP-2, phosphatidylinositol 3-kinase, phospholipase C-γ, Grb2, and Crk, in response to various stimuli, the Gab1-deficient mice may provide an avenue for determination of the link between receptor activation and biological responses.
ACKNOWLEDGMENTS
We thank K. Yamauchi-Takihara, K. Kunisada, S. I. Nishikawa, H. Yoshida, A. Miyajima, T. Hara, and Y. Mukouyama for their suggestions. We thank T. Nakamura for the c-Met expression vector. We also thank J. Ishikawa and K. Nishikawa for technical assistance and R. Masuda and A. Kubota for excellent secretarial assistance.
This work was supported by grants and a Grant-in-Aid for COE Research from the Ministry of Education, Science, Sports, and Culture in Japan, the Searle Scientific Research Fellowship, and the Osaka Foundation for the Promotion of Clinical Immunology.
REFERENCES
- 1.Baker J, Liu J P, Robertson E J, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73–82. [PubMed] [Google Scholar]
- 2.Bennett A M, Tang T L, Sugimoto S, Walsh C T, Neel B G. Protein-tyrosine-phosphatase SHPTP2 couples platelet-derived growth factor receptor beta to Ras. Proc Natl Acad Sci USA. 1994;91:7335–7339. doi: 10.1073/pnas.91.15.7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bladt F, Riethmacher D, Isenmann S, Aguzzi A, Birchmeier C. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature. 1995;376:768–771. doi: 10.1038/376768a0. [DOI] [PubMed] [Google Scholar]
- 4.Cheng A M, Saxton T M, Sakai R, Kulkarni S, Mbamalu G, Vogel W, Tortorice C G, Cardiff R D, Cross J C, Muller W J, Pawson T. Mammalian Grb2 regulates multiple steps in embryonic development and malignant transformation. Cell. 1998;95:793–803. doi: 10.1016/s0092-8674(00)81702-x. [DOI] [PubMed] [Google Scholar]
- 5.Daub H, Wallasch C, Lankenau A, Herrlich A, Ullrich A. Signal characteristics of G protein-transactivated EGF receptor. EMBO J. 1997;16:7032–7044. doi: 10.1093/emboj/16.23.7032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukada T, Hibi M, Yamanaka Y, Takahashi-Tezuka M, Fujitani Y, Yamaguchi T, Nakajima K, Hirano T. Two signals are necessary for cell proliferation induced by a cytokine receptor gp130: involvement of STAT3 in anti-apoptosis. Immunity. 1996;5:449–460. doi: 10.1016/s1074-7613(00)80501-4. [DOI] [PubMed] [Google Scholar]
- 7.Gu H, Pratt J C, Burakoff S J, Neel B G. Cloning of p97/Gab2, the major SHP2-binding protein in hematopoietic cells, reveals a novel pathway for cytokine-induced gene activation. Mol Cell. 1998;2:729–740. doi: 10.1016/s1097-2765(00)80288-9. [DOI] [PubMed] [Google Scholar]
- 8.Heldin C H. Dimerization of cell surface receptors in signal transduction. Cell. 1995;80:213–223. doi: 10.1016/0092-8674(95)90404-2. [DOI] [PubMed] [Google Scholar]
- 9.Herbst R, Carroll P M, Allard J D, Schilling J, Raabe T, Simon M A. Daughter of sevenless is a substrate of the phosphotyrosine phosphatase Corkscrew and functions during sevenless signaling. Cell. 1996;85:899–909. doi: 10.1016/s0092-8674(00)81273-8. [DOI] [PubMed] [Google Scholar]
- 10.Higuchi O, Mizuno K, Vande Woude G F, Nakamura T. Expression of c-met proto-oncogene in COS cells induces the signal transducing high-affinity receptor for hepatocyte growth factor. FEBS Lett. 1992;301:282–286. doi: 10.1016/0014-5793(92)80257-h. [DOI] [PubMed] [Google Scholar]
- 11.Hirano T, Nakajima K, Hibi M. Signaling mechanisms through gp130: a model of the cytokine system. Cytokine Growth Factor Rev. 1997;8:241–252. doi: 10.1016/s1359-6101(98)80005-1. [DOI] [PubMed] [Google Scholar]
- 12.Holgado-Madruga M, Emlet D R, Moscatello D K, Godwin A K, Wong A J. A Grb2-associated docking protein in EGF- and insulin-receptor signalling. Nature. 1996;379:560–564. doi: 10.1038/379560a0. [DOI] [PubMed] [Google Scholar]
- 13.Holgado-Madruga M, Moscatello D K, Emlet D R, Dieterich R, Wong A J. Grb2-associated binder-1 mediates phosphatidylinositol 3-kinase activation and the promotion of cell survival by nerve growth factor. Proc Natl Acad Sci USA. 1997;94:12419–12424. doi: 10.1073/pnas.94.23.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ihle J N. STATs: signal transducers and activators of transcription. Cell. 1996;84:331–334. doi: 10.1016/s0092-8674(00)81277-5. [DOI] [PubMed] [Google Scholar]
- 15.Karlsson L, Bondjers C, Betsholtz C. Roles for PDGF-A and sonic hedgehog in development of mesenchymal components of the hair follicle. Development. 1999;126:2611–2621. doi: 10.1242/dev.126.12.2611. [DOI] [PubMed] [Google Scholar]
- 16.Leveen P, Pekny M, Gebre-Medhin S, Swolin B, Larsson E, Betsholtz C. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev. 1994;8:1875–1887. doi: 10.1101/gad.8.16.1875. [DOI] [PubMed] [Google Scholar]
- 17.Li W, Nishimura R, Kashishian A, Batzer A G, Kim W J H, Cooper J A, Schlessinger J. A new function for a phosphotyrosine phosphatase: linking GRB2-Sos to a receptor tyrosine kinase. Mol Cell Biol. 1994;14:509–517. doi: 10.1128/mcb.14.1.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luetteke N C, Qiu T H, Peiffer R L, Oliver P, Smithies O, Lee D C. TGF alpha deficiency results in hair follicle and eye abnormalities in targeted and waved-1 mice. Cell. 1993;73:263–278. doi: 10.1016/0092-8674(93)90228-i. [DOI] [PubMed] [Google Scholar]
- 19.Maina F, Casagranda F, Audero E, Simeone A, Comoglio P M, Klein R, Ponzetto C. Uncoupling of Grb2 from the Met receptor in vivo reveals complex roles in muscle development. Cell. 1996;87:531–542. doi: 10.1016/s0092-8674(00)81372-0. [DOI] [PubMed] [Google Scholar]
- 20.Mann G B, Fowler K J, Gabriel A, Nice E C, Williams R L, Dunn A R. Mice with a null mutation of the TGF alpha gene have abnormal skin architecture, wavy hair, and curly whiskers and often develop corneal inflammation. Cell. 1993;73:249–261. doi: 10.1016/0092-8674(93)90227-h. [DOI] [PubMed] [Google Scholar]
- 21.Mountford P, Zevnik B, Duwel A, Nichols J, Li M, Dani C, Robertson M, Chambers I, Smith A. Dicistronic targeting constructs: reporters and modifiers of mammalian gene expression. Proc Natl Acad Sci USA. 1994;91:4303–4307. doi: 10.1073/pnas.91.10.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murakami H, Iwashita T, Asai N, Shimono Y, Iwata Y, Kawai K, Takahashi M. Enhanced phosphatidylinositol 3-kinase activity and high phosphorylation state of its downstream signalling molecules mediated by Ret with the MEN 2B mutation. Biochem Biophys Res Commun. 1999;262:68–75. doi: 10.1006/bbrc.1999.1186. [DOI] [PubMed] [Google Scholar]
- 23.Nakajima K, Yamanaka Y, Nakae K, Kojima H, Ichiba M, Kiuchi N, Kitaoka T, Fukada T, Hibi M, Hirano T. A central role for Stat3 in IL-6-induced regulation of growth and differentiation in M1 leukemia cells. EMBO J. 1996;15:3651–3658. [PMC free article] [PubMed] [Google Scholar]
- 24.Nishida K, Yoshida Y, Itoh M, Fukada T, Ohtani T, Shirogane T, Atsumi T, Takahashi-Tezuka M, Ishihara K, Hibi M, Hirano T. Gab-family adapter proteins act downstream of cytokine and growth factor receptors and T- and B-cell antigen receptors. Blood. 1999;93:1809–1816. [PubMed] [Google Scholar]
- 25.Ohlsson R, Falck P, Hellstrom M, Lindahl P, Bostrom H, Franklin G, Ahrlund-Richter L, Pollard J, Soriano P, Betsholtz C. PDGFB regulates the development of the labyrinthine layer of the mouse fetal placenta. Dev Biol. 1999;212:124–136. doi: 10.1006/dbio.1999.9306. [DOI] [PubMed] [Google Scholar]
- 26.Pawson T, Scott J D. Signaling through scaffold, anchoring, and adaptor proteins. Science. 1997;278:2075–2080. doi: 10.1126/science.278.5346.2075. [DOI] [PubMed] [Google Scholar]
- 27.Raabe T, Riesgo-Escovar J, Liu X, Bausenwein B S, Deak P, Maroy P, Hafen E. DOS, a novel pleckstrin homology domain-containing protein required for signal transduction between sevenless and Ras1 in Drosophila. Cell. 1996;85:911–920. doi: 10.1016/s0092-8674(00)81274-x. [DOI] [PubMed] [Google Scholar]
- 28.Rozakis-Adcock M, McGlade J, Mbamalu G, Pelicci G, Daly R, Li W, Batzer A, Thomas S, Brugge J, Pelicci P G, Schlessinger J, Pawson T. Association of the Shc and Grb2/Sem5 SH2-containing proteins is implicated in activation of the Ras pathway by tyrosine kinases. Nature. 1992;360:689–692. doi: 10.1038/360689a0. [DOI] [PubMed] [Google Scholar]
- 29.Saxton T M, Henkemeyer M, Gasca S, Shen R, Rossi D J, Shalaby F, Feng G S, Pawson T. Abnormal mesoderm patterning in mouse embryos mutant for the SH2 tyrosine phosphatase Shp-2. EMBO J. 1997;16:2352–2364. doi: 10.1093/emboj/16.9.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidt C, Bladt F, Goedecke S, Brinkmann V, Zschiesche W, Sharpe M, Gherardi E, Birchmeier C. Scatter factor/hepatocyte growth factor is essential for liver development. Nature. 1995;373:699–702. doi: 10.1038/373699a0. [DOI] [PubMed] [Google Scholar]
- 31.Shi Z Q, Lu W, Feng G S. The Shp-2 tyrosine phosphatase has opposite effects in mediating the activation of extracellular signal-regulated and c-Jun NH2-terminal mitogen-activated protein kinases. J Biol Chem. 1998;273:4904–4908. doi: 10.1074/jbc.273.9.4904. [DOI] [PubMed] [Google Scholar]
- 32.Soriano P. Abnormal kidney development and hematological disorders in PDGF beta-receptor mutant mice. Genes Dev. 1994;8:1888–1896. doi: 10.1101/gad.8.16.1888. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi-Tezuka M, Yoshida Y, Fukada T, Ohtani T, Yamanaka Y, Nishida K, Nakajima K, Hibi M, Hirano T. Gab1 acts as an adapter molecule linking the cytokine receptor gp130 to ERK mitogen-activated protein kinase. Mol Cell Biol. 1998;18:4109–4117. doi: 10.1128/mcb.18.7.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Threadgill D W, Dlugosz A A, Hansen L A, Tennenbaum T, Lichti U, Yee D, LaMantia C, Mourton T, Herrup K, Harris R C, Barnard J A, Yuspa S H, Coffey R J, Magnuson T. Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science. 1995;269:230–234. doi: 10.1126/science.7618084. [DOI] [PubMed] [Google Scholar]
- 35.Uehara Y, Minowa O, Mori C, Shiota K, Kuno J, Noda T, Kitamura N. Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature. 1995;373:702–705. doi: 10.1038/373702a0. [DOI] [PubMed] [Google Scholar]
- 36.Ware C B, Horowitz M C, Renshaw B R, Hunt J S, Liggitt D, Koblar S A, Gliniak B C, McKenna H J, Papayannopoulou T, Thoma B, Cheng L, Donovan P J, Peschon J J, Bartlett P F, Willis C R, Wright B D, Carpenter M K, Davison B L, Gearing D P. Targeted disruption of the low-affinity leukemia inhibitory factor receptor gene causes placental, skeletal, neural and metabolic defects and results in perinatal death. Development. 1995;121:1283–1299. doi: 10.1242/dev.121.5.1283. [DOI] [PubMed] [Google Scholar]
- 37.Weidner K M, Di Cesare S, Sachs M, Brinkmann V, Behrens J, Birchmeier W. Interaction between Gab1 and the c-Met receptor tyrosine kinase is responsible for epithelial morphogenesis. Nature. 1996;384:173–176. doi: 10.1038/384173a0. [DOI] [PubMed] [Google Scholar]
- 38.Wickrema A, Uddin S, Sharma A, Chen F, Alsayed Y, Ahmad S, Sawyer S T, Krystal G, Yi T, Nishada K, Hibi M, Hirano T, Platanias L C. Engagement of Gab1 and Gab2 in erythropoietin signaling. J Biol Chem. 1999;274:24469–24474. doi: 10.1074/jbc.274.35.24469. [DOI] [PubMed] [Google Scholar]
- 39.Wong L, Johnson G R. Epidermal growth factor induces coupling of protein-tyrosine phosphatase 1D to GRB2 via the COOH-terminal SH3 domain of GRB2. J Biol Chem. 1996;271:20981–20984. doi: 10.1074/jbc.271.35.20981. [DOI] [PubMed] [Google Scholar]
- 40.Zhao C, Yu D H, Shen R, Feng G S. Gab2, a new pleckstrin homology domain-containing adapter protein, acts to uncouple signaling from ERK kinase to Elk-1. J Biol Chem. 1999;274:19649–19654. doi: 10.1074/jbc.274.28.19649. [DOI] [PubMed] [Google Scholar]