Abstract
The capacity for neurogenesis in the adult mammalian brain is extremely limited and highly restricted to a few regions, which greatly hampers neuronal regeneration and functional restoration after neuronal loss caused by injury or disease. Meanwhile, transplantation of exogenous neuronal stem cells into the brain encounters several serious issues including immune rejection and the risk of tumorigenesis. Recent discoveries of direct reprogramming of endogenous glial cells into functional neurons have provided new opportunities for adult neuro-regeneration. Here, we extensively review the experimental findings of the direct conversion of glial cells to neurons in vitro and in vivo and discuss the remaining issues and challenges related to the glial subtypes and the specificity and efficiency of direct cell-reprograming, as well as the influence of the microenvironment. Although in situ glial cell reprogramming offers great potential for neuronal repair in the injured or diseased brain, it still needs a large amount of research to pave the way to therapeutic application.
Keywords: Direct cell-reprogramming, Glial cell-to-neuron conversion, Cross-differentiation neuronal regeneration, Brain repair
The capacity for tissue regeneration is inversely correlated with increasing levels in the evolutionary hierarchy in animals [1]. For instance, the retina of teleost fishes has a remarkable ability to regenerate new neurons after injury, whereas such an ability is severely limited in birds and completely absent in rodents [2–4]. In most mammals, the potential for organ repair is very low, and the central nervous system (CNS) in particular exhibits extremely little ability for neuronal self-regeneration after injury or disease. It is generally believed that mature mammalian brains are unable to generate many new neurons. However, many neurological diseases, in particular the neurodegenerative disorders such as Parkinson’s disease (PD), Huntington’s disease (HD), and amyotrophic lateral sclerosis are associated with a significant loss of specific subtypes of neuron [5]. Although neural stem cells (NSCs) have been found to persist in the subventricular zone, hippocampal dentate gyrus, and hypothalamus in the adult brain of a few mammalian species, where NSCs can proliferate and then differentiate into mature neurons mostly in local sites [6–9], whether the same NSCs exist in the human brain is still debated [10]. Furthermore, it has also been argued that the number of new neurons supplied by NSCs is too small to repair the neuronal loss caused by neurological diseases in the adult human or animal brain [11].
A long-favored theory postulates that regeneration of new neurons in the cell-lesion site in the CNS is a viable therapeutic approach for curing neural diseases [12]. In light of the limited regenerative capacity of endogenous NSCs, a great research effort has been made to develop other approaches to achieve neuro-regeneration in the CNS in the past several decades. Two new prevalent strategies, transplanting pluripotent NSCs and reprogramming endogenous glial cells, have emerged for enhancing neuro-regeneration in the mature mammalian CNS. However, it should be noted that the transplantation of NSCs or their derivatives has encountered a few challenges, such as the associated immuno-rejection and the risk of gene mutations and tumorigenesis, as well as ethical concerns and the shortage of donors. Moreover, transplantation also inevitably disrupts the internal environment at the graft location and often leads to severe inflammation or cerebral edema-induced obstructive hydrocephalus [13]. On the contrary, the glial cell-reprogramming approach not only avoids the above obstacles but also has other advantages, including much higher safety and efficiency in generating new neurons than NSC transplantation. Thus, it has gained increasing attention. In this review, we summarize the key findings on glial cell-reprogramming and its underlying molecular mechanisms and cellular factors, the latter of which include the origin of glial cells and the neural microenvironment. We also discuss current challenges in the potential for future therapeutic application.
Glial Cell-Reprogramming
Cell-reprogramming, also known as cell trans-differentiation, is a cell-fate conversion process bypassing an intermediate pluripotent state, induced by the forced expression of certain transcription factors (TFs) or the application of chemicals [14–19]. In the adult mammalian CNS, glial cells have an intrinsic proliferative capability and a ubiquitous distribution, which make them ideal candidates for conversion into neurons [20]. In 2002, Heins and colleagues first demonstrated that the forced expression of the TF Pax6 in cultured astrocytes directs these glial cells towards neurogenesis [21]. In a later study of mouse cerebral cortex, forced expression of a dominant-negative form of Olig2 in injury-induced reactive glial cells was found to be capable of reprogramming these reactive glial cells into neuronal cells expressing the immature neuron marker doublecortin [22]. These two pioneering studies uncovered a new method for neuronal regeneration from glial cells. Moreover, expression of another TF, Ngn2 or Ascl1 (also named Mash1), had similar effects on cultured glial cells [23]. In addition to expressing neuron-specific molecular markers, the glial cell reprogramming-induced neurons generated action potentials and formed postsynaptic compartments for receiving synaptic input from neighboring neurons. Furthermore, the expression of Ngn2 and Dlx2 in cultured astrocytes generated glutamatergic and GABAergic neurons, respectively [24], while the expression of Ascl1, Lmx1b, and Nurr1 was sufficient to reprogram astrocytes into dopaminergic neurons in culture [25].
Meanwhile, in vivo studies of reprogramming glial cells in the mammalian brain have also advanced rapidly since 2013. Shortly after the finding that co-expression of three TFs—Ascl1, Brn2, and Myt1l—is able to induce the direct conversion of mouse fibroblasts into functional neurons in culture [26], Torper et al. found that this combination of three TFs is sufficient to convert the resident glial cells into NeuN-expressing neurons in the mouse striatum in vivo [27]. Interestingly, ectopic expression of Sox2 alone also reprograms astrocytes into neuroblasts, which further develop into mature functional neurons in both the striatum and spinal cord in vivo, albeit with lower efficiency [28–30]. Moreover, over-expression of Sox2 is also sufficient to induce the conversion of another subtype of glial cell that specifically expresses the chondroitin sulfate proteoglycan NG2, known as NG2 glia, into mature neurons in a model of injured cerebral cortex and spinal cord, through an intermediate progenitor cell stage [31, 32]. Similarly, Ngn2 alone reprograms glial cells into NeuN-expressing neurons in adult mouse neocortex and striatum, and most of the new reprogramming-induced neurons are immunopositive for GABA in the striatum and glutamate in the cortex [33]. However, the efficiency of Sox2 or Ngn2-induced glial cell reprogramming is relatively low in vivo except in the model of spinal cord injury [28, 29, 32, 33]. Notably, it has also been reported that co-expression of the anti-apoptotic protein Bcl-2 or the TF Nurr1 with Ngn2 enhances the efficiency of Ngn2-driven astrocyte reprogramming [34, 35], Moreover, neural growth factors, including brain-derived neurotrophic factor, fibroblast growth factor 2, and epidermal growth factor also enhance the efficiency of Ngn2- or Sox2-drived glial cell reprogramming by promoting the transition from progenitor cells to functional neurons in the process of glial cell reprograming (Fig. 1) [28–33].
Fig. 1.
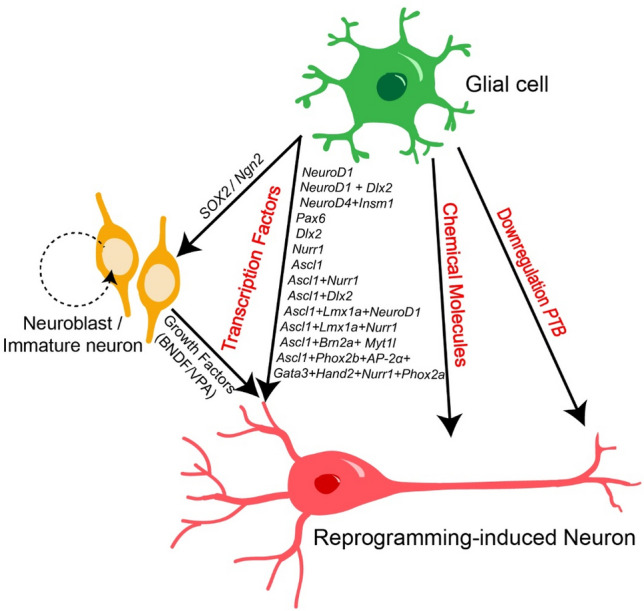
Summary of known approaches for reprogramming glial cells into neurons. The diagram illustrates the three approaches that have been used to induce the reprogramming of glial cells into neurons: (1) the over-expression of a single TF or TF cocktail including direct and indirect approaches (e.g., via neuroblasts or immature neurons by SOX2 or Ngn2 signaling); (2) the use of small chemical molecules; and (3) suppression of the PTB gene.
In contrast, several other TFs have been found to directly reprogram glial cells into neurons (Fig. 1). For example, virus-mediated expression of NeuroD1 in astrocytes induces their direct conversion into glutamatergic neurons in mouse cortex [36, 37]. Moreover, co-expression of NeuroD1 and Dlx2 specifically reprograms astrocytes into GABAergic neurons in the mouse striatum in vivo [38]. A TF cocktail of Ascl1, Lmx1a, and Nurr1 is needed to convert adult striatal NG2 glial cells into GABAergic neurons [39], and most of these reprogrammed striatal GABAergic neurons are parvalbumin (PV)-expressing fast-spiking cells [40]. However, a different TF combination (NeuroD1, Ascl1, and Lmx1a) with addition of the microRNA miR218 is required to generate reprogrammed dopaminergic neurons in the mouse mid-brain [41]. Another TF Ascl1 is also effective in inducing direct glia-to-neuron conversion in the striatum, neocortex, and retina [42, 43]. Remarkably, in some instances, a combination of multiple TFs increases the conversion efficiency, in comparison with that of a single TF, indicating synergistic action among TFs [35, 38].
Differing from the TF over-expression approach, Fu and colleagues revealed that repression of polypyrimidine tract binding protein (PTBP1), an RNA-binding protein, is sufficient to directly convert fibroblasts into functional neurons in culture [44]. On the basis of this original finding, three groups recently demonstrated that the down-regulation of PTBP1 is also successful for reprogramming oligodendrocytes, astrocytes, or Müller cells into neurons in multiple mouse brain regions in vivo [45–47]. It is worth noting that these PTBP1 repression-reprogrammed neurons possess the same identity or nature as endogenous local neurons in a specific brain circuit, e.g. GABAergic neurons from oligodendrocytes in the striatum [46], dopaminergic neurons from astrocytes in the substantia nigra [45], and ganglion cells from Müller cells in the retina [47] (except for the odd result of dopaminergic neurons from astrocytes in the striatum) [47]. These findings also suggest a unique advantage of the PTBP1-repression approach over TF over-expression, that is, manipulating a single factor, PTBP1, reprograms various types of glial cell into local neurons ubiquitously in different circuits. In contrast, the TF over-expression approach often requires combinations of different TFs with or without extra molecules to generate a certain type of induced neuron by the glia-to-neuron reprogramming process.
Moreover, recent studies have established another reprogramming approach with the use of small chemical molecules (Fig. 1). In 2015, Zhang and colleagues first identified a chemical cocktail containing nine small molecules that successfully reprogrammed human astrocytes into functional neurons in culture [14], and a follow-up further narrowed it down to 3–4 essential molecules that were sufficient for efficient reprogramming [48]. However, in the latter studies, the chemical cocktails were not effective in converting mouse astrocytes into neurons either in vitro or the mouse brain in vivo. On the contrary, a very recent study using a different chemical cocktail reported the successful reprogramming of mouse astrocytes into neurons in vitro and in vivo. In the mouse brain, local infusion of this chemical cocktail into the striatum and cortex converted astrocytes into GABAergic neurons and glutamatergic neurons, respectively, but with a low reprogramming efficiency (<10%) [49]. These studies have demonstrated the feasibility of using small chemical compounds to induce astrocyte–to–neuron conversion, and selecting better combinations of chemical compounds seems to be key for future studies and applications.
Molecular Mechanisms
Although multiple cell-reprogramming strategies have been established, their underlying molecular mechanisms are still largely unclear. Nevertheless, recent progress in in vivo time-lapse cell imaging [23], real-time qRT-PCR [50, 51], and RNA sequencing (RNA-seq) [51, 52] has made it possible to shed light on the molecular mechanism.
As generally understood, the programming process consists of at least two steps: suppressing glial-specific gene expression, and eliciting the expression of critical genes for the control of neuronal fate and differentiation [20, 41, 49, 51]. Our recent study using the high-throughput RNA-seq and chromatin immunoprecipitation sequencing (ChIP-seq) methods identified approximately 107 downstream target genes of Ascl1 in its induction of astrocyte–to–neuron conversion [51]. Among these target genes, the TFs Klf10 as well as Myt1 and Myt1l regulate the early neuritogenesis and the late electrophysiological maturation of Ascl1-reprogrammed neurons, respectively, while the TF Neurod4 and the chromatin remodeling factor Chd7 are required for efficient cell-fate conversion [51].
Moreover, transcriptional regulation and epigenetic modification are also involved in cell-reprogramming by increasing the chromatin accessibility for the TFs, which allows large TF overexpression-induced changes in gene expression [52–54]. For example, Pollak et al. reported that Ascl1 directly binds the promoter or proximal enhancer sites of the progenitor genes Hes5, Dll1, Hes6, and Dll3, and then remodels the chromatin from a repressive state to an active expression state in the process of reprogramming mouse Müller glia into neurogenic retinal progenitors [55]. When the process of Ngn2-binding to regulatory elements of its downstream targets NeuroD1, NeuroD4, and Trnp1 is inhibited, Ngn2 over-expression in glial cells fails to induce direct conversion to neurons [56].
Ectopic expression of Ascl1 in mouse Müller glia also decreases the level of histone-3 lysine-27 trimethylation (H3K27me3) but increases the H3K27 acetylation (H3K27Ac) level in the progenitor genes Hes5, Hes 6, dll1, and dll3 [55]. Because H3K27me3 and H3K27Ac are generally correlated with the repression and activation states of gene expression, respectively, these histone modifications elicited by Ascl1 overexpression in Müller glia result in significant increases in the expression of progenitor genes [55]. Thus, these findings suggest that epigenetic regulation or histone modification is a key step in the glia-to-neuron conversion induced by TF overexpression. Similarly, substantially increased methylation at the glia-specific GFAP gene and reduced methylation in the neuronal gene NEFM have been found in the chemically-induced reprogramming of astrocytes to neurons [14].
Taken together, these molecular findings have revealed that during cell-reprograming, certain TFs or small molecule cocktails trigger complex regulation of transcription that switches glia-specific gene expression profiles to progenitor- or neuronal-specific profiles and further up-regulates the neurogenic ability in late differentiation and maturation. Epigenetic modification and chromatin remodeling actively reinforce the latter process [49, 56]. Recently, a transcriptional dynamic model illustrated the temporal hierarchies of molecular regulation for neuronal fate acquisition during the glia-to-neuron reprogramming induced by Ascl1 or Ngn2 [56].
With regard to the molecular mechanism underlying PTBP1 down-regulation-induced glia-to-neuron conversion, a key event is the sequential suppression of gene-regulation loops as follows. First, PTB (polypyrimidine tract binding protein 1) repression in the glial cell relieves the suppression of neuronal induction by the microRNA miR-124/REST (RE1-silencing transcription factor) signaling in the early neurogenesis stage [44, 45, 57]. Second, further down-regulation of the neuronal analogue nPTB elevates the levels of the transcription activator BRN2 and miR-9, both of which are required for neuronal maturation [45, 57].
Glial Cell Origin
Glial cells are highly heterogeneous and abundant in the mammalian brain, and the major types are the astrocyte, the NG2 cell, and the microglia [37, 58, 59]. To date, studies have elucidated that all three types can be reprogrammed into neurons in vivo [38–42, 46, 60] (see Table 1). In the retina, Müller glia are converted into retinal bipolar neurons, amacrine cells, and ganglion projection neurons by Ascl1 expression or PTB downregulation [43, 47, 55, 61]. Interestingly, accumulating evidence has suggested that the different origins of glial cells could be a factor determining the identity or type of newly-reprogrammed neurons. For example, NeuroD1 reprograms astrocytes into glutamatergic neurons, but converts NG2 cells into both glutamatergic and GABAergic neurons in cultured mouse glia cells, whereas NeuroD1 is effective for human astrocyte reprogramming but not for human microglia [37]. However, it is not clear what intrinsic factors among the different glial subtypes influence the fate determination of reprogramming-induced neurons.
Table 1.
Summary of recent approaches to glia-to-neuron conversion in vitro and in vivo.
| Glial source | Region | Targeted TFs | Small molecules/micro RNA/Others | Neuronal type | Conversion efficiency | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| Mouse | Astrocyte | in vitro (cultured cell) | Forskolin, ISX9, CHIR99021, I-BET151 | Glut+/GABA+ | High | [49] | ||
| Cortex | NeuroD1 | Glut+ neuron | High | [37] | ||||
| Cortex | Ngn2 | GEND1 | GABA+/TH+ cell | High | [50] | |||
| Cerebral cortex | Ngn2 | Glut+ neuron | High | [23, 24, 56] | ||||
| Cerebral cortex | Pax6 | Undefined | ? | [21, 23] | ||||
| Cerebral cortex | NeuroD4+ Insm1 | Glut+ neuron | Low | [56] | ||||
| Cerebral cortex | Dlx2 | GABA+ neuron | Low | [24] | ||||
| Cerebral cortex | Ascl1+ Dlx2 | Interneurons | High | |||||
| Cerebral cortex | Ascl1 | Undefined | Low | [23, 56] | ||||
| Ascl1+Lmx1b+Nurr1 | Dopaminergic neuron | Low | [25] | |||||
| Midbrain | Ascl1+Phox2b+AP−2α+Gata3+Hand2+Nurr1+Phox2a | Noradrenergic neuron | High | [82] | ||||
| Dorsal midbrain | Ascl1 | Glut+/GABA+ | High | [42] | ||||
| in vivo | Midbrain | Ascl1 | Glut+/GABA+ | High | [42] | |||
| Striatum | Ascl1+Lmx1a+ Nurr1 | Glut+ (16%)/GABA+ (68%) | ? | [39] | ||||
| Striatum | Ascl1+Lmx1a+NeuroD1 | miR218 | Dopaminergic neuron | Low | [41] | |||
| Striatum | Ascl1+Brn2a+Myt1l | Un-defined | Low | [27] | ||||
| Striatum | Forskolin+ CHIR99021+ IBET151+ISX9+Y-27632 | GABA+ (87.1%) | low | [49] | ||||
| Striatum | NeuroD1 + Dlx2 | GABA+ neuron | High | [38] | ||||
| Striatum | Ngn2 | FGF2+EGF | GABA+ neuron | Low | [33] | |||
| Striatum | Sox2 | DCX+ cell | ? | [28, 29] | ||||
| Spinal cord | Sox2 | VPA | GABA+ neuron | Low | [30] | |||
| Substantia nigra | PTB/sh RNA | Dopaminergic (30 %)/Others | high | [45] | ||||
| Cortex | Forskolin+ CHIR99021+ IBET151+ISX9+Y−27632 | Glut+ (72.8%) | Low | [49] | ||||
| Cortex | NeuroD1 | Undefined | High | [37] | ||||
| Cortex | Ngn2 | FGF2+EGF | Glut+ neuron | Low | [33] | |||
| Cerebral cortex | Ngn2+Nurr1 | Pyramidal neuron | High | [35] | ||||
| Cerebral cortex | Ascl1+Nurr1 | Undefined | High | |||||
| Motor cortex | NeuroD1 | Pyramidal neuron(90%)/GABA+ (10%) | High | [36] | ||||
| NG 2 cell | in vitro | Cortex | NeuroD1 | Glut+ (90%)/GABA+ (10%) neuron | High | [37] | ||
| in vivo | Cortex | NeuroD1 | Undefined | High | [37] | |||
| Spinal cord | Sox2 | Glut+/GABA+ | High | [32] | ||||
| Cerebral cortex | Sox2 | DCX+ cell | Low | [31] | ||||
| Cerebral cortex | Ascl1+ Lmx1a + Nurr1 | Glut+ (16%)/GABA+ (68%) neuron | High | [39, 40] | ||||
| Striatum | siRNAs 3/4 | GABA+ neuron | High | [46] | ||||
| Microglia | in vitro | NeuroD1 | Glut+ (74.2%) / GABA+ (25.7%) | Low | [56] | |||
| in vivo | Striatum | NeuroD1 | Striatal neuron | High | [56] | |||
| Müller glia | in vitro | Ascl1 | Bipolar cell | Low | [55] | |||
| Ascl1 | Multipolar/uni/bipolar cells | High | [80] | |||||
| Ngn2 | Unipolar/bipolar cell | High | ||||||
| in vivo | Injured retina | Ascl1 | Bipolar/amacrine cell | ? | [43] | |||
| Injured retina | Ascl1 | TSA | Bipolar cell | ? | [61] | |||
| Human | Astrocytes | in vitro | Cortex | LDN193189+ SB431542+ TTNPB+Tzv+ CHIR99021+ VPA+DAPT+ SAG+Purmo | Glut+ (88%)/GABA+ (8.2%) neuron | High | [14] | |
| Cortex | SB431542+ LDN193189+ CHIR9902+ DAPT | Glut+ (78%)/GABA+ (2%)/Dopaminergic (1%) | High | [48] | ||||
| Cortex | NeuroD1 | Glut+ neuron | High | [37] | ||||
| NeuroD1+Ascl1+ Lmx1a | miR218 | Dopaminergic neuron | Low | [41] | ||||
| NG2 cell | in vitro | Sox2+Dlx5+Lhx6+ Ascl1+Foxg1 | sh REST | GABA+ neuron | high | [84] | ||
| Ascl1+Lmx1a+Nurr1 | sh REST | Dopaminergic neuron | ? | [85] | ||||
TFs transcription factors, GABAergic (GABA+) or glutamatergic (Glut+) neurons; high efficiency: reprogramming ratio >35%.
Reactive glial cells following neurodegeneration or traumatic injury can proliferate and then form a glial scar to prevent further damage to neuronal tissue. Reactive glial cells are composed of multiple types of glia including microglia, astrocytes, and NG2 cells, which play different roles at different stages. For example, reactive microglia provide an immediate immuno-like defensive response, while reactive astrocytes form protective barriers (or scars) against the further spread of damage. However, the latter process has also been thought to greatly decrease the possibility of further neural repair of certain circuits [20, 62, 63]. Recruiting reactive glial cells for reprogramming alleviates the glial barrier that inhibits neuronal repair to some extent [17]. Considering such complex progressive processes involving various reactive glial cells, it would be ideal if glia-to-neuron conversion can be well controlled to affect specific glial types at certain time points after damage, e.g. controlling the induction of astrocyte reprogramming at the glial scar-formation stage. Precise control of the reprogramming of different types of reactive glial cell would have maximal benefits in therapeutic application. Certainly this demands sophisticated reprogramming routes specifically for reactive glial cells. Nevertheless, proliferating reactive glial cells provide an additional glia resource for reprogramming. Guo et al. used the retrovirus-based approach, which transduces exogenous gene expression specific for proliferating cells, to achieve NeuroD1 expression in reactive astrocytes and NG2 cells and found reprogrammed functional neurons in a mouse model of cortical injury [37]. Similar results of reprogramming reactive glial cells into functional neurons were obtained by retrovirus-mediated Ngn2 over-expression in the mouse striatum after local injury [33]. Lentivirus-mediated TF expression has also been used to target the reprogramming of reactive NG2 cells in the injured mouse spinal cord [30].
Regional Specificity and the Microenvironment
A few in vivo studies have suggested that brain regions or neural microenvironments can exert an important influence on the efficiency of glial cell-reprogramming and the fate control of reprogrammed neurons in the mouse CNS [31, 33, 35, 42, 45, 47, 49]. For an example, forced expression of Ngn2 reprograms non-neuronal cells into GABAergic neurons in the striatum, while it generates glutamatergic neurons in the neocortex [33]. We also reported that similar AAV-mediated expression of Ascl1 in the astrocytes of the mouse striatum and cortex have different efficiencies in the direct conversion to functional neurons [42]. Similarly, the same TFs (Nurr1+Ngn2 or Nurr1+Ascl1) induces glial-to-neuron conversion with high efficiency in the grey matter in the mouse cortex, but are not effective in the white matter, although comparable numbers of cortical astrocytes were infected by AAV vectors in both regions [35, 64]. The regional specificity is more evident in the direct glia-to-neuron conversion induced by PBT down-regulation, in which local neuronal constituents seem to determine the identity of programming-induced neurons [45, 47]. Several studies have suggested that variations in the glial transcriptome among different brain regions may account for the differences in the outcome of reprogramming [45, 49, 65, 66].
Changes in the microenvironment after injury or the onset of neurological disease, can affect the outcome of reprogramming, especially the indirect reprogramming mediated via neuroblasts. Brain injury or disease often substantially alters the levels of cytokines, growth factors, and morphogens in the microenvironment, and extracellular signaling mediated by these molecules is known to play differential roles in neuroblast survival and neuronal differentiation [67, 68], which may facilitate glial cell reprogramming [68]. Grande et al. reported that ischemic injury augments the production and subsequent maturation of Ngn2-induced new neurons [33]. In the mouse cortex, the altered cortical microenvironment at the stab wound injury site reprograms the astrocytes with a deletion of Rbpj-k, a key regulator of Notch signaling, into GABAergic interneurons through a process mediated via the amplified neuroblast population. More interestingly, this neurogenic potential can spread widely across different sensory cortices [69]. Although it is not clear how the glial Notch-signaling blockade and local injury alter the transcriptome profiles of astrocytes and the microenvironmental conditions, the extensive spread of neurogenic induction to uninjured cortical areas implies the importance of microenvironmental signaling in the process of glia-to-neuron reprogramming.
Function of Reprogramming-Derived New Neurons
It remains a fundamental question as to whether the functions of the newly generated neurons resemble the endogenously existing neurons regarding the formation of synapses, the ability to communicate electrically, and the ability to form correct afferent/efferent connections with host neurons. Elucidating these functional aspects of reprogramming-induced new neurons is demanding in the field for the potential application to neural repair [70]. Berninger et al. provided the first evidence that astroglia-derived neurons in culture exhibit several electrophysiological hallmarks of functional neurons, including the formation of characteristic neuronal morphology and the ability to fire action potentials and receive phasic synaptic inputs [23]. In a previous study, we reported similar processes of functional maturation of Ascl1 induced neurons approximately 5 weeks after induction, plus demonstrating their efferent synaptic transmission to pre-existing neurons, in the mouse mid-brain and neocortex in vivo [42]. Analogous functional maturation processes have also been reported in many other studies [35, 36, 38–41, 45, 47]. All these morphological and electrophysiological results from culture and brain slice studies directly suggest that reprogramming of glial cells can generate neurons with the same functional properties as neighboring endogenous neurons. Moreover, further studies have shown that these new glia-derived neurons can integrate into the existing neural circuits by forming correct afferent and efferent connections in various brain regions in vivo. By using retrograde trans-synaptic tracing with modified rabies virus, Torper et al. showed that individual striatal NG2-derived new neurons connect with 3–4 local pre-existing neurons on average [39]. In the mouse spinal cord, NG2 glia-derived neurons receive not only local connections from dorsal root ganglia but also long-rang projection synapses from the brain stem [32]. Further studies in the neocortex [35], nigrostriatum [45], striatum [38], and retina [47], have consistently reported that the in situ glia reprogramming-induced neurons send long-range axonal projections to the same target areas as the pre-existing neurons [35, 38, 45, 47]. These anatomical studies have demonstrated that newly reprogrammed neurons are able to form largely correct synaptic connections with local neurons and distant brain nuclei, endorsing their potential for neural network reconstruction after injury or in neurodegenerative diseases [32, 35, 38, 45, 47]. In this direction, however, a fundamental question remains as to whether and how these new glial reprogramming-induced neurons perform the correct neuronal functions in sensory perception, motor control, learning/memory, and other brain functions.
Prospectives and Challenges
Over the past decade, several different strategies have been established for successfully reprogramming various types of glia into defined neurons in the CNS in animal studies (Table 1). These newly-induced neurons have been reported to integrate into the existing neuronal circuits and form correct connections in vivo [32, 35, 36, 38, 45–47, 61]. All these studies have raised the potential of glial-reprogramming for providing an alternative cell source for neuro-regeneration in the injured or diseased brain, besides the transplantation of NSCs [70, 71]. In this direction, several pioneering studies have tested the feasibility of the glial cell reprogramming-based approach for neuro-regeneration of defined types of neuron in mouse models of some prominent neurological diseases, including PD, AD, and HD. Rivetti di Val Cervo et al. reported that in an adult PD mouse model, in situ reprogramming of striatal astrocytes into functional dopaminergic neurons dramatically alleviates the symptoms in spontaneous motor behavior, such as increasing the coordination of limbs during voluntary locomotion, axial symmetry, and gait [41]. Two recent studies have also reported that similar motor deficits are largely reversed after regeneration of dopaminergic neurons by PTBP1 repression-induced astrocyte reprogramming in the substantia nigra [45] and in the striatum [47]. In a mouse model of ischemic injury, partial restoration of motor function and fear memory function have been reported 3–9 weeks after the induction of reactive astrocyte-reprogramming in the mouse motor cortex and amygdala, respectively [36]. Similarly, in situ reprogramming of astrocytes into GABAergic neurons in the striatum reduces striatal atrophy, improves motor function, and extends the life span of HD-model mice [38]. Thus, these animal model findings strongly endorse the potential application of in vivo glia-to-neuron reprogramming for repairing certain circuits in the injured or degenerating brain. However, a causal link between the re-supply of reprogrammed neurons and the restoration of certain brain functions or behaviors in these disease models is not yet fully clarified. Recently, chemo- or opto-genetic tools have been used to suppress the activity of transplanted stem cells to confirm their contribution to rescuing motor functions in a PD mouse model [45, 72, 73]. In the PD model, Fu and colleagues were the first group to use a similar approach to clarify the causal link between glia-derived dopaminergic neurons and the rescue of PD-like symptoms [41]. Further future works are still needed to elucidate the exact contributions of glia-derived new neurons in the normal and diseased brain.
We think that there are three critical challenges in the potential application of glia-to-neuron reprogramming for future therapeutic application to the injured or diseased brain.
First, a major hurdle for therapeutic application is the risk of the adventitious virus associated with the virus-based gene delivery system. Currently, the lentiviruses, retroviruses, and adeno-associated viruses (AAVs) are commonly used to deliver recombinant TF DNA vectors [74, 75]. The use of lentivirus or retrovirus is known to increase the risk of genetic mutations and tumorigenicity, due to integration into the host-cell genome [13]. However, AAVs do not have the latter problem but have a comparable efficiency in expressing TFs for relatively long periods in glial cells [76]. However, AAV expression vectors normally have limited room for recombining exogenous genes, and thus they are not suitable for delivering several genes simultaneously [74]. In addition, a high dose of AAVs often leads to toxic effects after injection into animal tissues [76, 77], and is also accompanied by a significant leakage of expression of target genes to neural cells other than glial cells. The latter issue may produce false-positive results in research. For example, recently there has been a strong debate on the efficiency of the TF NeuroD1 in reprogramming astrocytes into neurons in vivo with AAV delivery, so a series of AAV dosage experiments are needed for different CNS sites in various animal species to avoid the AAV leakage problem [78]. Furthermore, cell lineage-tracing analysis is also needed to confirm the glia-to-neuron conversion following the AAV delivery of TF genes. To fully address the cell lineage, a combined time-lapse approach to characterizing the switch of morphology and transcriptomic profiles at different reprogramming stages should be applied to follow the fate of the reprogrammed progeny of glial cells and capture axon-dendritic growth in induced new neurons, either in the intact brain or 3-D organoids [79].
With regard to the possible application of chemically-induced glia-to-neuron reprogramming, this has several unique advantages over the genetic approach, such as easy drug application or delivery, and convenient combinations for inducing defined neurons. However, its outcome efficiency is substantially lower than that of the genetic approach. Moreover, the off-target action and potential toxicity to other neural cells have not yet been extensively addressed.
Second, the regeneration of different defined types of neuron that have been lost in various brain diseases requires varied combinations of specific TFs or chemicals. A quest to identify these strategic combinations demands numerous research efforts. To date, glial cells have been successfully reprogrammed into glutamatergic neurons [35–37], GABAergic neurons [30, 38, 40, 46], dopaminergic neurons [41, 45], retinal ganglion cells [80], and noradrenergic neurons [81]. However, attempts to generate other neuronal subtypes such as cholinergic and serotonergic neurons have not yet been successful. Considering that generating diverse neuronal subtypes is a crucial step towards the restoration of damaged neuronal networks, a newly developed approach using CRISPR/Cas9 technology may expedite the discovery process [82, 83]. Moreover, more understanding of the molecular mechanisms that gate cell fate switches will also greatly promote the identification of these critical molecular factors for designing new routes for the in vivo reprogramming of glial cells into specific neurons more efficiently in the CNS. Other factors related to glial cell origins and local microenvironments, especially in injured or diseased circuits, are also indispensable. Future research is required to explicitly elucidate how these cellular factors influence glia-to-neuron conversion.
Finally, there is still a lack of systematic research on the reprogramming of human glial cells. Almost all glia-to-neuron conversions in vivo have been done in animals (Table 1). Even in cultured cells, there are only eight studies exploring the feasibility of human glia-to-neuron reprogramming, and they are mainly focused on astrocytes and NG2 cells. For example, the TFs NeuroD1 and NeuroD4, or a combination of NeruoD1, Ascl1, Lmx1a, and miR218, have been found to convert human cortical astrocytes into functional neurons [37, 41, 56] but failed to reprogram human microglia into neurons [37]. The PTB repression approach is also effective in converting cultured human astrocytes into dopaminergic neurons [45]. In addition, two recent studies reported successful reprogramming of human NG2 cells into GABAergic neurons and dopaminergic neurons by the TF cocktails Ascl1, Dlx5, Lhx6, Sox2, and Foxg1 or Ascl1, Lmx1a, and Nurr1 with a short hairpin (sh)RNA against the RE1-silencing transcription factor (sh REST), respectively [84, 85]. On the contrary, reprogramming of human microglia has still not been established. Considering the different natures of transcription and epigenetic regulation among species, the reprogramming approaches developed from animal studies cannot be simply applied to human glia cells. Thus, future studies are needed to test these established approaches in cultured human glial cells in vitro or in human brain organoids ex vitro.
Meanwhile, due to ethical constraints, glia-to-neuron conversion cannot be directly tested in the human brain. A recent study by transplanting human astrocytes engineered to express the inducible forms of TF genes (Ascl1, Brn2, and Myt1l) into the rat brain further found that after doxycyline induction, some surviving human astrocytes were reprogrammed into neurons that expressed the human-specific neural cell adhesion molecule (hNCAM) [27]. This study provides an alternative system for testing or identifying molecular approaches to reprogramming human glia cells in vivo. It is unknown whether the mouse brain environment is similar to that in the human brain and how it affects the reprograming process. Thus, it is mandatory to test these glia-to-neuron reprogramming genes or chemicals in the CNS of non-human primates before any translation to cell therapy in human patients, following protocols similar to the pharmaceutical development of regular small molecular drugs.
Concluding Remarks
Over the past decade, rapid advances have been achieved in the field of glial cell reprogramming, which opens a future avenue for recruiting endogenous glial cells for the potential regeneration of defined functional neurons to treat traumatic injury and neurodegenerative diseases. This new strategy for neuron replacement can avoid some of the major obstacles of the ongoing stem cell-based therapies. To date, growing numbers of studies have established various TF or chemical cocktail approaches to efficiently reprogram various types of glial cells into glutamatergic, GABAergic, or dopaminergic, neurons in the mouse CNS in vivo. These reprogramming-induced neurons have also been shown to integrate into the existing neuronal circuits and restore some of the defective brain functions in mouse models of neurodegenerative diseases, including PD and HD. These findings imply that a re-supply of new induced neurons is able to reconstruct injured or diseased neural circuits. However, it is still completely unknown what factors instruct these induced neurons to properly build their functions and circuit connections, especially long-distance connections, similar to the pre-existing endogenous neurons. Finally, although several approaches established in animal studies have been verified in cultured human astrocytes or NG2 cells, little advance has been made in glia-to-neuron reprogramming in the brain of non-human primates or humans, primarily due to ethical issues and limited experimental resources. Moreover, other issues associated with toxicity, adventitious virus risk, and glia-specific gene-delivery methods have yet to be investigated extensively. Thus, from these perspectives, it is still an odyssey to translate glial reprogramming as a cell replacement for therapeutic application.
Acknowledgements
This review was supported by grants from the National Natural Science Foundation of China (32071025), the Beijing Municipal Science & Technology Commission (Z181100001518001), and the Interdisciplinary Research Fund of Beijing Normal University, and the Science and Technology Program of Guangxi (AD21075052), the National Natural Science Foundation of China (31871037 and 32070976), and the Guangxi First-class Discipline Project for Basic Medicine Sciences (GXFCDP-BMS-2018).
Conflict of interests
All authors claim that there are no conflicts of interest.
Contributor Information
Leping Cheng, Email: lpcheng@gxmu.edu.cn.
Xiaohui Zhang, Email: xhzhang@bnu.edu.cn.
References
- 1.Pesaresi M, Sebastian-Perez R, Cosma MP. Dedifferentiation, transdifferentiation and cell fusion: In vivo reprogramming strategies for regenerative medicine. FEBS J. 2019;286:1074–1093. doi: 10.1111/febs.14633. [DOI] [PubMed] [Google Scholar]
- 2.Lenkowski JR, Raymond PA. Müller glia: Stem cells for generation and regeneration of retinal neurons in teleost fish. Prog Retin Eye Res. 2014;40:94–123. doi: 10.1016/j.preteyeres.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fischer AJ, Reh TA. Müller glia are a potential source of neural regeneration in the postnatal chicken retina. Nat Neurosci. 2001;4:247–252. doi: 10.1038/85090. [DOI] [PubMed] [Google Scholar]
- 4.Karl MO, Reh TA. Studying the generation of regenerated retinal neuron from Müller glia in the mouse eye. Methods Mol Biol, 2012, 884: 213–227. [DOI] [PubMed]
- 5.Lindvall O, Kokaia Z, Martinez-Serrano A. Stem cell therapy for human neurodegenerative disorders–how to make it work. Nat Med. 2004;10:S42–S50. doi: 10.1038/nm1064. [DOI] [PubMed] [Google Scholar]
- 6.Gonçalves JT, Schafer ST, Gage FH. Adult neurogenesis in the hippocampus: From stem cells to behavior. Cell. 2016;167:897–914. doi: 10.1016/j.cell.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 7.Goodman T, Hajihosseini MK. Hypothalamic tanycytes-masters and servants of metabolic, neuroendocrine, and neurogenic functions. Front Neurosci. 2015;9:387. doi: 10.3389/fnins.2015.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lim DA, Alvarez-Buylla A. The adult ventricular–subventricular zone (V-SVZ) and olfactory bulb (OB) neurogenesis. Cold Spring Harb Perspect Biol. 2016;8:a018820. doi: 10.1101/cshperspect.a018820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ming GL, Song H. Adult neurogenesis in the mammalian brain: Significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gage FH. Adult neurogenesis in mammals. Science. 2019;364:827–828. doi: 10.1126/science.aav6885. [DOI] [PubMed] [Google Scholar]
- 11.Goldman SA. Directed mobilization of endogenous neural progenitor cells: The intersection of stem cell biology and gene therapy. Curr Opin Mol Ther. 2004;6:466–472. [PubMed] [Google Scholar]
- 12.Steinbeck JA, Studer L. Moving stem cells to the clinic: Potential and limitations for brain repair. Neuron. 2015;86:187–206. doi: 10.1016/j.neuron.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldman SA. Stem and progenitor cell-based therapy of the central nervous system: Hopes, hype, and wishful thinking. Cell Stem Cell. 2016;18:174–188. doi: 10.1016/j.stem.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang L, Yin JC, Yeh H, Ma NX, Lee G, Chen XA, et al. Small molecules efficiently reprogram human astroglial cells into functional neurons. Cell Stem Cell. 2015;17:735–747. doi: 10.1016/j.stem.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to β-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barker RA, Götz M, Parmar M. New approaches for brain repair-from rescue to reprogramming. Nature. 2018;557:329–334. doi: 10.1038/s41586-018-0087-1. [DOI] [PubMed] [Google Scholar]
- 17.Torper O, Götz M. Brain repair from intrinsic cell sources: Turning reactive glia into neurons. Prog Brain Res. 2017;230:69–97. doi: 10.1016/bs.pbr.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 18.Wang LL, Zhang CL. Engineering new neurons: In vivo reprogramming in mammalian brain and spinal cord. Cell Tissue Res. 2018;371:201–212. doi: 10.1007/s00441-017-2729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lei WL, Li W, Ge LJ, Chen G. Non-engineered and engineered adult neurogenesis in mammalian brains. Front Neurosci. 2019;13:131. doi: 10.3389/fnins.2019.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heins N, Malatesta P, Cecconi F, Nakafuku M, Tucker KL, Hack MA, et al. Glial cells generate neurons: The role of the transcription factor Pax6. Nat Neurosci. 2002;5:308–315. doi: 10.1038/nn828. [DOI] [PubMed] [Google Scholar]
- 22.Buffo A, Vosko MR, Ertürk D, Hamann GF, Jucker M, Rowitch D, et al. Expression pattern of the transcription factor Olig2 in response to brain injuries: Implications for neuronal repair. Proc Natl Acad Sci U S A. 2005;102:18183–18188. doi: 10.1073/pnas.0506535102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berninger B, Costa MR, Koch U, Schroeder T, Sutor B, Grothe B, et al. Functional properties of neurons derived from in vitro reprogrammed postnatal astroglia. J Neurosci. 2007;27:8654–8664. doi: 10.1523/JNEUROSCI.1615-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heinrich C, Blum R, Gascón S, Masserdotti G, Tripathi P, Sánchez R, et al. Directing astroglia from the cerebral cortex into subtype specific functional neurons. PLoS Biol. 2010;8:e1000373. doi: 10.1371/journal.pbio.1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Addis RC, Hsu FC, Wright RL, Dichter MA, Coulter DA, Gearhart JD. Efficient conversion of astrocytes to functional midbrain dopaminergic neurons using a single polycistronic vector. PLoS One. 2011;6:e28719. doi: 10.1371/journal.pone.0028719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Südhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Torper O, Pfisterer U, Wolf DA, Pereira M, Lau S, Jakobsson J, et al. Generation of induced neurons via direct conversion in vivo. Proc Natl Acad Sci U S A. 2013;110:7038–7043. doi: 10.1073/pnas.1303829110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niu W, Zang T, Smith DK, Vue TY, Zou Y, Bachoo R, et al. SOX2 reprograms resident astrocytes into neural progenitors in the adult brain. Stem Cell Reports. 2015;4:780–794. doi: 10.1016/j.stemcr.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niu W, Zang T, Zou Y, Fang S, Smith DK, Bachoo R, et al. In vivo reprogramming of astrocytes to neuroblasts in the adult brain. Nat Cell Biol. 2013;15:1164–1175. doi: 10.1038/ncb2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Su Z, Niu W, Liu ML, Zou Y, Zhang CL. In vivo conversion of astrocytes to neurons in the injured adult spinal cord. Nat Commun. 2014;5:3338. doi: 10.1038/ncomms4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heinrich C, Bergami M, Gascón S, Lepier A, Viganò F, Dimou L, et al. Sox2-mediated conversion of NG2 glia into induced neurons in the injured adult cerebral cortex. Stem Cell Reports. 2014;3:1000–1014. doi: 10.1016/j.stemcr.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tai WJ, Wu W, Wang LL, Ni HQ, Chen CH, Yang JJ, et al. In vivo reprogramming of NG2 glia enables adult neurogenesis and functional recovery following spinal cord injury. Cell Stem Cell. 2021;28:923–937. doi: 10.1016/j.stem.2021.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grande A, Sumiyoshi K, López-Juárez A, Howard J, Sakthivel B, Aronow B, et al. Environmental impact on direct neuronal reprogramming in vivo in the adult brain. Nat Commun. 2013;4:2373. doi: 10.1038/ncomms3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gascón S, Murenu E, Masserdotti G, Ortega F, Russo GL, Petrik D, et al. Identification and successful negotiation of a metabolic checkpoint in direct neuronal reprogramming. Cell Stem Cell. 2016;18:396–409. doi: 10.1016/j.stem.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 35.Mattugini N, Bocchi R, Scheuss V, Russo GL, Torper O, Lao CL, et al. Inducing different neuronal subtypes from astrocytes in the injured mouse cerebral cortex. Neuron. 2019;103:1086–1095.e5. doi: 10.1016/j.neuron.2019.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen YC, Ma NX, Pei ZF, Wu Z, Do-Monte FH, Keefe S, et al. A NeuroD1 AAV-based gene therapy for functional brain repair after ischemic injury through in vivo astrocyte-to-neuron conversion. Mol Ther. 2020;28:217–234. doi: 10.1016/j.ymthe.2019.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo Z, Zhang L, Wu Z, Chen Y, Wang F, Chen G. In vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer's disease model. Cell Stem Cell. 2014;14:188–202. doi: 10.1016/j.stem.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu Z, Parry M, Hou XY, Liu MH, Wang H, Cain R, et al. Gene therapy conversion of striatal astrocytes into GABAergic neurons in mouse models of Huntington's disease. Nat Commun. 2020;11:1105. doi: 10.1038/s41467-020-14855-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Torper O, Ottosson DR, Pereira M, Lau S, Cardoso T, Grealish S, et al. In vivo reprogramming of striatal NG2 glia into functional neurons that integrate into local host circuitry. Cell Rep. 2015;12:474–481. doi: 10.1016/j.celrep.2015.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pereira M, Birtele M, Shrigley S, Benitez JA, Hedlund E, Parmar M, et al. Direct reprogramming of resident NG2 glia into neurons with properties of fast-spiking parvalbumin-containing interneurons. Stem Cell Reports. 2017;9:742–751. doi: 10.1016/j.stemcr.2017.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rivetti di Val Cervo P, Romanov RA, Spigolon G, Masini D, Martín-Montañez E, Toledo EM, et al. Induction of functional dopamine neurons from human astrocytes in vitro and mouse astrocytes in a Parkinson's disease model. Nat Biotechnol 2017, 35: 444–452. [DOI] [PubMed]
- 42.Liu YG, Miao QL, Yuan JC, Han SE, Zhang PP, Li SL, et al. Ascl1 converts dorsal midbrain astrocytes into functional neurons in vivo. J Neurosci. 2015;35:9336–9355. doi: 10.1523/JNEUROSCI.3975-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ueki Y, Wilken MS, Cox KE, Chipman L, Jorstad N, Sternhagen K, et al. Transgenic expression of the proneural transcription factor Ascl1 in Müller glia stimulates retinal regeneration in young mice. Proc Natl Acad Sci U S A. 2015;112:13717–13722. doi: 10.1073/pnas.1510595112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xue Y, Ouyang K, Huang J, Zhou Y, Ouyang H, Li H, et al. Direct conversion of fibroblasts to neurons by reprogramming PTB-regulated microRNA circuits. Cell. 2013;152:82–96. doi: 10.1016/j.cell.2012.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qian H, Kang X, Hu J, Zhang D, Liang Z, Meng F, et al. Reversing a model of Parkinson's disease with in situ converted nigral neurons. Nature. 2020;582:550–556. doi: 10.1038/s41586-020-2388-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weinberg MS, Criswell HE, Powell SK, Bhatt AP, McCown TJ. Viral vector reprogramming of adult resident striatal oligodendrocytes into functional neurons. Mol Ther. 2017;25:928–934. doi: 10.1016/j.ymthe.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou H, Su J, Hu X, Zhou C, Li H, Chen Z, et al. Glia-to-neuron conversion by CRISPR-CasRx alleviates symptoms of neurological disease in mice. Cell. 2020;181:590–603.e16. doi: 10.1016/j.cell.2020.03.024. [DOI] [PubMed] [Google Scholar]
- 48.Yin JC, Zhang L, Ma NX, Wang Y, Lee G, Hou XY, et al. Chemical conversion of human fetal astrocytes into neurons through modulation of multiple signaling pathways. Stem Cell Rep. 2019;12:488–501. doi: 10.1016/j.stemcr.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ma Y, Xie H, Du X, Wang L, Jin X, Zhang Q, et al. In vivo chemical reprogramming of astrocytes into neurons. Cell Discov. 2021;7:12. doi: 10.1038/s41421-021-00243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aravantinou-Fatorou K, Ortega F, Chroni-Tzartou D, Antoniou N, Poulopoulou C, Politis PK, et al. CEND1 and NEUROGENIN2 reprogram mouse astrocytes and embryonic fibroblasts to induced neural precursors and differentiated neurons. Stem Cell Reports. 2015;5:405–418. doi: 10.1016/j.stemcr.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rao Z, Wang R, Li S, Shi Y, Mo L, Han S, et al. Molecular mechanisms underlying Ascl1-mediated astrocyte-to-neuron conversion. Stem Cell Reports. 2021;16:534–547. doi: 10.1016/j.stemcr.2021.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ma NX, Yin JC, Chen G. Transcriptome analysis of small molecule-mediated astrocyte-to-neuron reprogramming. Front Cell Dev Biol. 2019;7:82. doi: 10.3389/fcell.2019.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raposo AASF, Vasconcelos FF, Drechsel D, Marie C, Johnston C, Dolle D, et al. Ascl1 coordinately regulates gene expression and the chromatin landscape during neurogenesis. Cell Rep. 2015;10:1544–1556. doi: 10.1016/j.celrep.2015.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zaret KS, Carroll JS. Pioneer transcription factors: Establishing competence for gene expression. Genes Dev. 2011;25:2227–2241. doi: 10.1101/gad.176826.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pollak J, Wilken MS, Ueki Y, Cox KE, Sullivan JM, Taylor RJ, et al. ASCL1 reprograms mouse Müller glia into neurogenic retinal progenitors. Development. 2013;140:2619–2631. doi: 10.1242/dev.091355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Masserdotti G, Gillotin S, Sutor B, Drechsel D, Irmler M, Jørgensen HF, et al. Transcriptional mechanisms of proneural factors and REST in regulating neuronal reprogramming of astrocytes. Cell Stem Cell. 2015;17:74–88. doi: 10.1016/j.stem.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xue Y, Qian H, Hu J, Zhou B, Zhou Y, Hu X, et al. Sequential regulatory loops as key gatekeepers for neuronal reprogramming in human cells. Nat Neurosci. 2016;19:807–815. doi: 10.1038/nn.4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Janowska J, Gargas J, Ziemka-Nalecz M, Zalewska T, Buzanska L, Sypecka J. Directed glial differentiation and transdifferentiation for neural tissue regeneration. Exp Neurol. 2019;319:112813. doi: 10.1016/j.expneurol.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 59.Yavarpour-Bali H, Ghasemi-Kasman M, Shojaei A. Direct reprogramming of terminally differentiated cells into neurons: A novel and promising strategy for Alzheimer's disease treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2020;98:109820. doi: 10.1016/j.pnpbp.2019.109820. [DOI] [PubMed] [Google Scholar]
- 60.Matsuda T, Irie T, Katsurabayashi S, Hayashi Y, Nagai T, Hamazaki N, et al. Pioneer factor NeuroD1 rearranges transcriptional and epigenetic profiles to execute microglia-neuron conversion. Neuron. 2019;101:472–485.e7. doi: 10.1016/j.neuron.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 61.Jorstad NL, Wilken MS, Grimes WN, Wohl SG, VandenBosch LS, Yoshimatsu T, et al. Stimulation of functional neuronal regeneration from Müller glia in adult mice. Nature. 2017;548:103–107. doi: 10.1038/nature23283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Karimi-Abdolrezaee S, Billakanti R. Reactive astrogliosis after spinal cord injury-beneficial and detrimental effects. Mol Neurobiol. 2012;46:251–264. doi: 10.1007/s12035-012-8287-4. [DOI] [PubMed] [Google Scholar]
- 63.Qin C, Zhou LQ, Ma XT, Hu ZW, Yang S, Chen M, et al. Dual functions of microglia in ischemic stroke. Neurosci Bull. 2019;35:921–933. doi: 10.1007/s12264-019-00388-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu MH, Li W, Zheng JJ, Xu YG, He Q, Chen G. Differential neuronal reprogramming induced by NeuroD1 from astrocytes in grey matter versus white matter. Neural Regen Res. 2020;15:342–351. doi: 10.4103/1673-5374.265185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bayraktar OA, Bartels T, Holmqvist S, Kleshchevnikov V, Martirosyan A, Polioudakis D, et al. Astrocyte layers in the mammalian cerebral cortex revealed by a single-cell in situ transcriptomic map. Nat Neurosci. 2020;23:500–509. doi: 10.1038/s41593-020-0602-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boisvert MM, Erikson GA, Shokhirev MN, Allen NJ. The aging astrocyte transcriptome from multiple regions of the mouse brain. Cell Rep. 2018;22:269–285. doi: 10.1016/j.celrep.2017.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Russo I, Barlati S, Bosetti F. Effects of neuroinflammation on the regenerative capacity of brain stem cells. J Neurochem. 2011;116:947–956. doi: 10.1111/j.1471-4159.2010.07168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wan J, Zhao XF, Vojtek A, Goldman D. Retinal injury, growth factors, and cytokines converge on β-catenin and pStat3 signaling to stimulate retina regeneration. Cell Rep. 2014;9:285–297. doi: 10.1016/j.celrep.2014.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zamboni M, Llorens-Bobadilla E, Magnusson JP, Frisén J. A widespread neurogenic potential of neocortical astrocytes is induced by injury. Cell Stem Cell. 2020;27:605–617.e5. doi: 10.1016/j.stem.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grade S, Götz M. Neuronal replacement therapy: Previous achievements and challenges ahead. NPJ Regen Med. 2017;2:29. doi: 10.1038/s41536-017-0033-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vignoles R, Lentini C, d'Orange M, Heinrich C. Direct lineage reprogramming for brain repair: Breakthroughs and challenges. Trends Mol Med. 2019;25:897–914. doi: 10.1016/j.molmed.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 72.Chen Y, Xiong M, Dong Y, Haberman A, Cao J, Liu H, et al. Chemical control of grafted human PSC-derived neurons in a mouse model of Parkinson's disease. Cell Stem Cell. 2016;18:817–826. doi: 10.1016/j.stem.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Steinbeck JA, Choi SJ, Mrejeru A, Ganat Y, Deisseroth K, Sulzer D, et al. Optogenetics enables functional analysis of human embryonic stem cell-derived grafts in a Parkinson's disease model. Nat Biotechnol. 2015;33:204–209. doi: 10.1038/nbt.3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fang L, El Wazan L, Tan C, Nguyen T, Hung SSC, Hewitt AW, et al. Potentials of cellular reprogramming as a novel strategy for neuroregeneration. Front Cell Neurosci. 2018;12:460. doi: 10.3389/fncel.2018.00460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gascón S, Masserdotti G, Russo GL, Götz M. Direct neuronal reprogramming: achievements, hurdles, and new roads to success. Cell Stem Cell. 2017;21:18–34. doi: 10.1016/j.stem.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 76.Xiong WJ, Wu DM, Xue YL, Wang SK, Chung MJ, Ji XK, et al. AAV cis-regulatory sequences are correlated with ocular toxicity. Proc Natl Acad Sci U S A. 2019;116:5785–5794. doi: 10.1073/pnas.1821000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Khabou H, Cordeau C, Pacot L, Fisson S, Dalkara D. Dosage thresholds and influence of transgene cassette in adeno-associated virus-related toxicity. Hum Gene Ther. 2018;29:1235–1241. doi: 10.1089/hum.2018.144. [DOI] [PubMed] [Google Scholar]
- 78.Xiang Z, Xu L, Liu M, Wang Q, Li W, Lei W, et al. Lineage tracing of direct astrocyte-to-neuron conversion in the mouse cortex. Neural Regen Res. 2021;16:750–756. doi: 10.4103/1673-5374.295925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Qian C, Dong B, Wang XY, Zhou FQ. In vivo glial trans-differentiation for neuronal replacement and functional recovery in central nervous system. FEBS J. 2020 doi: 10.1111/febs.15681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.de Melo Guimarães RP, Landeira BS, Coelho DM, Golbert DCF, Silveira MS, Linden R, et al. Evidence of Müller glia conversion into retina ganglion cells using neurogenin2. Front Cell Neurosci. 2018;12:410. doi: 10.3389/fncel.2018.00410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li S, Shi Y, Yao X, Wang X, Shen L, Rao Z, et al. Conversion of astrocytes and fibroblasts into functional noradrenergic neurons. Cell Rep. 2019;28:682–697.e7. doi: 10.1016/j.celrep.2019.06.042. [DOI] [PubMed] [Google Scholar]
- 82.Chakraborty S, Ji H, Kabadi AM, Gersbach CA, Christoforou N, Leong KW. A CRISPR/Cas9-based system for reprogramming cell lineage specification. Stem Cell Reports. 2014;3:940–947. doi: 10.1016/j.stemcr.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu Y, Yu C, Daley TP, Wang F, Cao WS, Bhate S, et al. CRISPR activation screens systematically identify factors that drive neuronal fate and reprogramming. Cell Stem Cell. 2018;23:758–771.e8. doi: 10.1016/j.stem.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Giacomoni J, Bruzelius A, Stamouli CA, Rylander Ottosson D. Direct conversion of human stem cell-derived glial progenitor cells into GABAergic interneurons. Cells. 2020;9:2451. doi: 10.3390/cells9112451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nolbrant S, Giacomoni J, Hoban DB, Bruzelius A, Birtele M, Chandler-Militello D, et al. Direct reprogramming of human fetal- and stem cell-derived glial progenitor cells into midbrain dopaminergic neurons. Stem Cell Reports. 2020;15:869–882. doi: 10.1016/j.stemcr.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]


