Abstract
Thrombosis and Thrombocytopenia Syndrome (TTS) has been associated with the AstraZencea (AZ) COVID-19 vaccine (Vaxzevria). Australia has reported low TTS incidence of < 3/100,000 after the first dose, with case fatality rate (CFR) of 5–6%. Risk-benefit analysis of vaccination has been challenging because of rapidly evolving data, changing levels of transmission, and variation in rates of TTS, COVID-19, and CFR between age groups. We aim to optimise risk–benefit analysis by developing a model that enables inputs to be updated rapidly as evidence evolves. A Bayesian network was used to integrate local and international data, government reports, published literature and expert opinion. The model estimates probabilities of outcomes under different scenarios of age, sex, low/medium/high transmission (0.05%/0.45%/5.76% of population infected over 6 months), SARS-CoV-2 variant, vaccine doses, and vaccine effectiveness. We used the model to compare estimated deaths from AZ vaccine-associated TTS with i) COVID-19 deaths prevented under different scenarios, and ii) deaths from COVID-19 related atypical severe blood clots (cerebral venous sinus thrombosis & portal vein thrombosis). For a million people aged ≥ 70 years where 70% received first dose and 35% received two doses, our model estimated < 1 death from TTS, 25 deaths prevented under low transmission, and > 3000 deaths prevented under high transmission. Risks versus benefits varied significantly between age groups and transmission levels. Under high transmission, deaths prevented by AZ vaccine far exceed deaths from TTS (by 8 to > 4500 times depending on age). Probability of dying from COVID-related atypical severe blood clots was 58–126 times higher (depending on age and sex) than dying from TTS. To our knowledge, this is the first example of the use of Bayesian networks for risk–benefit analysis for a COVID-19 vaccine. The model can be rapidly updated to incorporate new data, adapted for other countries, extended to other outcomes (e.g., severe disease), or used for other vaccines.
Keywords: SARS-CoV-2, Vaccination, Adverse events, Thrombosis/thrombocytopenia syndrome, Bayesian networks, Model
1. Introduction
The AstraZeneca ChAdOx1 (AZD1222) COVID-19 vaccine, Vaxzevria (AZ vaccine) has been widely used globally, with one billion doses released in over 170 countries by August 2021 [1]. The vaccine has been reported to be 40–90% effective against symptomatic infection, and 75–90% effective against death from COVID-19 [2], [3], [4], [5]. In March 2021, reports of AZ vaccine-associated Thrombosis with Thrombocytopenia Syndrome (TTS) began emerging from multiple countries, including Norway [6], Canada [7], the United Kingdom [8], and Australia [9], and appeared to be more common in younger age groups [9]. Although the incidence of TTS was very low (< 3/100,000 first doses in Australia), initial reports of high case fatality rates (CFR) (44% in Germany [10] and 18% in United Kingdom [8]) prompted some countries to recommend alternative COVID-19 vaccines in younger persons.
In Australia, a change in recommendation was made in April 2021 to preference the Pfizer/BioNTech (BNT162b2) COVID-19 vaccine (Comirnaty) over the AZ vaccine in those aged < 50 years [11]. In June 2021, after the death of a 52-year-old woman, the recommendation was revised to preference the Pfizer vaccine over the AZ vaccine in those aged < 60 years [12], [13]. AZ vaccine-associated TTS and the associated changes in government advice received significant media attention in Australia, which may have contributed to vaccine reluctance, and uncertainty amongst the public and clinicians about risks versus benefits of vaccination [14]. The only other COVID-19 vaccine available in Australia at the time (and up to early September 2021) was the Pfizer vaccine, but limited supplies meant that those choosing the Pfizer vaccine had to wait weeks or months to be vaccinated.
Initial risk assessments and decisions about age-based recommendations for the AZ vaccine in Australia were made at a time when there was almost no local community transmission of COVID-19. Unfortunately, since late June 2021, outbreaks have occurred in multiple states and territories, resulting in prolonged lockdowns of millions of people in New South Wales (NSW), Victoria (VIC), and the Australian Capital Territory (ACT) [15]. Therefore, the risks versus benefits of the AZ vaccine changed significantly over a few weeks, and those aged < 60 years were advised to see a doctor to help them make individual risk assessments.
On 29 June 2021, the Australian Government produced a document on ‘Weighing up the potential benefits against the risk of harm from COVID-19 Vaccine AstraZeneca’ for clinicians to help patients make informed decisions [16]. The document provides helpful information for each age group such as the risk of TTS versus the number of deaths, intensive care unit (ICU) admissions, and hospitalisations prevented by vaccination under low, medium, and high levels of transmission. However, calculations of risks versus benefits changed rapidly because of many factors, including evolving incidence and CFR of TTS (both in Australia and internationally), changing levels of community transmission, changing age distribution of cases associated with the arrival of the delta variant (the predominant variant in Australia since June 2021), and new data on vaccine effectiveness against the delta variant. These and other factors that influence risk–benefit analysis are likely to continue to evolve at a rapid pace.
To address the challenge of providing up-to-date risk–benefit analysis of COVID-19 vaccination, one of the authors (JL) suggested to the Immunisation Coalition (https://www.immunisationcoalition.org.au/) that they support the development of the COVID-19 Risk Calculator (CoRiCal), a set of tools designed to facilitate shared decision making between clinicians and patients. The Immunisation Coalition is a not-for-profit organization with representation from clinical and other professional groups interested in promoting vaccination best practice in Australia. CoRiCal aims to support informed risk assessment by providing relevant and accurate estimates of the risks and benefits of COVID-19 vaccines, taking into account locally relevant factors.
This paper describes the first CoRiCal model, focused on risk–benefit analysis of the AZ vaccine. The model was developed using a Bayesian network (BN), which allows the model’s inputs to be updated easily as the outbreak situation changes and as scientific evidence evolves. The model aims to provide more precise risk–benefit analysis of AZ vaccination by comparing the risk of death from vaccine-associated TTS versus benefits of vaccination through reduction in deaths from i) COVID-19 infection, and ii) atypical severe blood clots (cerebral venous sinus thrombosis [CVST] and portal vein thrombosis [PVT]) related to COVID-19 infection. The model enables scenario analysis based on age, sex, current transmission intensity, predominant SARS-CoV-2 variant, number of AZ vaccine doses received, and vaccine effectiveness against symptomatic infection and death. The model could also be extended for outcomes other than death (e.g., ICU admission, long COVID), other vaccines, other adverse events following immunization (AEFI), or adapted for use in other countries.
2. Methods
2.1. Bayesian networks
BNs are directed acyclic graphical models that explicitly represent relationships between variables in terms of conditional probabilities [17]. Variables are represented by nodes, each categorized into several possible states (e.g., yes/no, high/low, age groups). Links (or edges) represent the relationships between nodes and point in the direction from a parent node (independent variable) to a child node (dependent variable) (Fig. 1 ). Nodes are quantified using probability tables that define the probability of a node being in a given state, either based on some prior distribution (for nodes with no parent nodes) or conditional on the states of all parent nodes i.e., conditional probability tables (CPTs).
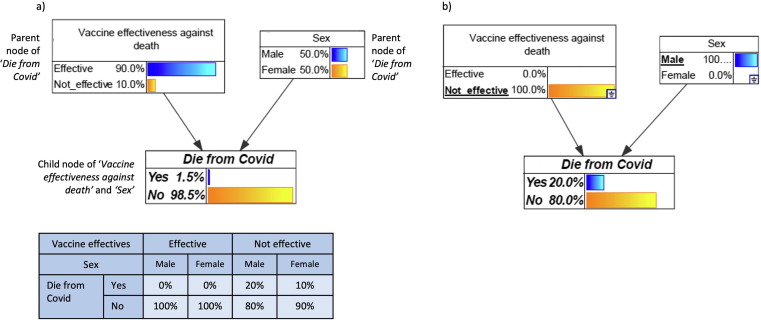
Fig. 1.
Example Bayesian network showing three nodes (white boxes), links (arrows), and conditional probability table (CPT) for the Die from Covi node (blue table). Data in figure are fictitious and for illustrative purposes only. The model assumes 90% vaccine effectiveness against death and even distribution of males and females in the population; these numbers are entered as priors because the nodes do not have parents. The model assumes case fatality rates of 20% in males and 10% in females. The CPT for Die from Covid shows that if the vaccine is effective, probability of dying is 0% for both sexes. If the vaccine is not effective, the probability of dying is 20% for males and 10% for females. Fig. 1a) shows the BN in its default state; if the vaccine was 90% effective, we expect an overall 1.5% chance of dying from COVID-19 (e.g., in a population of 1000 people, the vaccine was not effective in 100 people. Of these 100 people, we estimate 10 deaths out of 50 males, and 5 deaths out of 50 females, i.e., total of 15 deaths out of 1000 people, or 1.5%). Fig. 1b) shows an example scenario analysis of ‘what is the chance of a male dying if the vaccine was not effective?’ Selected states in each node are underlined, and the model updates the chance of dying from COVID-19 to 20% under this scenario.
BN modelling frameworks offer many advantages relevant to the problem at hand. Firstly, BNs provide visual and transparent representation of relationships between variables and the probabilistic assumptions between each variable. The graphical structure enables users to understand how the system has been modelled, clearly shows the underlying assumptions, how model outputs were derived, and enables interactive scenario analysis. Secondly, CPTs can be derived from a range of data formats, allowing relevant data to be integrated from diverse sources including studies, reports, and expert opinion. Thirdly, the probabilistic framework of BNs is well suited to risk analysis where the uncertainty surrounding estimates can be tested through sensitivity analysis for a range of plausible inputs. Finally, model assumptions and CPTs can be updated easily as more information become available or as the situation evolves. Rapid adaptability is particularly important for modelling rapidly evolving situations such as outbreaks.
BNs have been used for a variety of COVID-19 models, including modelling transmission and outbreak response [18], [19], decision making [20], risk analysis [21], [22], risk assessment and contact tracing [23], [24], interpretation of SARS-CoV-2 test results [25], and predictive diagnosis [26]. However, to our knowledge, BNs have not yet been applied for risk–benefit analysis of COVID-19 vaccines.
2.2. Model design
Our BN model structure was based on the best available scientific evidence from multiple sources. Evidence was integrated using facilitated expert elicitation, with subject matter experts (JL, AB, KRS, AKE, CLL, Thrombosis & Haemostasis Society of Australia and New Zealand [THANZ]) working closely with experienced BN modellers (HJM, KM, CLL). The four subject matter experts included clinicians and researchers with expertise in infectious disease epidemiology, virology, thrombosis, general practice, and public health. The experts defined the scope of the model, key outcome variables of interest, and major predictor variables (inputs) that influenced the outcomes. Experts reviewed published literature and reports to determine whether there was sufficient evidence to include each predictor variable in the model. Predictors were linked to outcomes if there were sufficient evidence and data to quantify the conditional probabilities between them.
Based on the discussions between the experts and modellers, and evidence collected by the experts, a draft BN model structure was derived by the modellers, then reviewed and refined with the team over several iterations of facilitated discussions. To parameterize the model, questionnaires were developed to help experts structure the systematically collected data into CPTs. Variables were not linked if there were only weak quantitative relationships between them because the links would have little impact on model predictions. Data were updated as new evidence became available during the model development process from July-September 2021. External consistency was considered during model design so that predictions were consistent with other commonly used information sources such as the Australian Technical Advisory Group on Immunisation (ATAGI) and the Therapeutic Goods Administration (TGA), e.g., using the same age groups and definitions of low/medium/high community transmission intensity (equivalent to 0.05%/0.45%/5.76% of population infected over 6 months) [16].
2.3. Atypical severe blood clots
TTS is a rare and atypical form of blood clotting associated with paradoxical thrombosis and low platelets. TTS is immunologically mediated, and can be trigged by medications (e.g., heparin) or vaccines (e.g., AZ vaccine) [27]. TTS is diagnosed on the basis of clinical criteria and includes atypical severe blood clots such as CVST and splanchnic vein thrombosis (SVT) [28], [29], [30], which have also been reported in COVID-19 patients [31], [32]. CVST and SVT are distinct from the common types of venous thromboembolism such as deep vein thrombosis or pulmonary embolus, or other types of thrombocytopenia and have the highest risk of mortality in this group. SVT includes portal vein thrombosis (PVT), Budd Chiari Syndrome and mesenteric vein thrombosis. The data included in the model focused on PVT as it is the most common type of SVT, and has been specifically reported post-COVID-19, enabling a direct comparison. Our model therefore focused on comparing the risk of AZ vaccine-associated TTS with the risk of CVST and PVT in COVID-19 patients.
In Australia, the TGA used the same definition of vaccine-induced TTS as the US Centers for Disease Control and Prevention [33]. In addition, every patient with TTS that was counted by TGA was discussed at both state based and central national committees to ensure that all clinical and laboratory criteria had been evaluated, and a consensus on TSS diagnosis was reached.
2.4. Data sources
While the assumptions and model structure were defined by experts, the CPTs were based on empirical data. Experts compiled evidence from peer-reviewed literature, government websites and reports, and through discussion with external clinical experts (e.g., haematologists regarding the evidence for AZ vaccine- associated TTS, and background rates of CVST and PVT). Official data from Australian authorities were used wherever possible (e.g., local data on AZ vaccine-associated TTS). Otherwise, data were obtained from other robust and publicly available sources (e.g., background rates of CVST and PVT). Data from different sources were consolidated for the CPTs, e.g., summarising data to match the age groups used in the BN. Data analyses were conducted for some variables to convert them into probabilities for the CPTs, e.g., converting incidence of COVID-19 into probability of infection over 6 months for the Intensity of local transmission node. Table 1 [5], [15], [16], [28], [29], [32], [34], [35], [36], [37], [38] and Appendix A provide a summary of data sources, model assumptions, and rationale.
Table 1.
Summary of data sources, assumptions, and prior distributions for a Bayesian network to assess risks versus benefits of the AstraZeneca COVID-19 vaccine.
| Model inputs | Data sources, assumptions, rationale | Reference |
|---|---|---|
| Age distribution of infections from delta variant | NSW COVID-19 case data from 1/6/2021 to 13/8/2021 were used to provide estimates of age distribution of infections from delta variant. Case data published daily by NSW Health, for the following age categories: 0–19, 5-year age groups from 20 to 69 years, and 70 + . For cases in the 0–19 age group, assumed that 40% were aged 0–9, and 60% aged 10–19 (based on age distribution of cases reported by NNDSS). Date range used was selected to reflect the first 6 weeks of delta outbreak, when vaccination coverage was relatively low. No significant change in age distribution of cases to 29/8/2021. See Appendix A, Table A1. | [15], [34] |
| Age distribution of infections from alpha/wild variants | COVID-19 cases reported in Australia from January to December 2020 were used to provide estimates of age distribution from alpha/wild variants. Data sourced from National Notifiable Diseases Surveillance System. See Appendix A, Table A2. | [15], [35] |
| Case fatality rates of COVID-19 cases | COVID-19 cases reported in Australia from January 2020 to 13/8/2021 were used to provide estimates of age-specific case fatality. Data sourced from National Notifiable Diseases Surveillance System. See Appendix A, Table A3. | [15], [35] |
| Community transmission levels | Chance of infection over 6 months calculated for different levels of community transmission. See Appendix A, Table A4. Definitions of low, medium, and high transmission as defined by ATAGI document ‘Weighing up the potential benefits and risk of harm from COVID-19 Vaccine AstraZeneca’. Low – similar to first wave in Australia (equivalent to 0.05% of population infected over 6 months). Medium – similar to second wave in VIC in 2020 (equivalent to 0.045% of population infected over 6 months). High – similar to Europe in January 2021 (equivalent to 5.76% of population infected over 6 months). Also included transmission scenarios equivalent to: zero transmission; 1% and 2% chance of infection over 6 months; 200 cases/day and 1000 cases/day in NSW; 1000 cases/day in VIC; 1000 cases/day in QLD. Other transmission levels can be added to model. |
[16] |
| Chance of infection by age group | Chance of infection differed between age groups and by variants. Calculated chance of infection by age group if overall community transmission of 1%. Calculations based on age distribution of infections from delta and alpha/wild variants, and age distribution of Australian population. See Appendix A, Table A5. | |
| Vaccine effectiveness against symptomatic infection | Delta variant (ATAGI recommended data used in Doherty transmission model):
Alpha variant (from Vaccine effectiveness Expert Panel, Public Health England):
|
[5], [36] |
| Vaccine effectiveness against death | Delta variant (ATAGI recommended data used in Doherty transmission model):
Alpha variant (from Vaccine effectiveness Expert Panel, Public Health England):
|
[5], [36] |
| Thrombosis and Thromobcytopenia Syndrome (TTS) after AZ vaccine | Model uses data reported by ATAGI update following weekly COVID-19 meeting on 25/8/2021.Estimated rate per 100,000 1st dose of AZ vaccinations:
Case fatality rate in Australia ∼ 5% (noting that higher rates reported in UK ∼ 18%). For sensitivity analysis, data from ATAGI reports on 1/9/2021, 8/9/2021, and 15/9/2021 were used. |
[37] |
| Background rates of atypical venous thrombotic disorders | Background rates (in population not infected with and not vaccinated for COVID-19) of atypical venous thrombotic disorder (CVST and PVT) over 6 weeks were calculated for each age group to provide a comparison with chance of TTS after AZ vaccine. CVST data from Kristoffersen et al.
|
[28], [29], [30] |
| Atypical venous thrombotic disorders associated with COVID-19 infection | Rates of CVST and PVT in COVID-19 cases from a retrospective cohort study using data primarily from the USA.CVT:
|
[32] |
| Prior distributions* | Assumptions | Reference |
| Age distribution of Australian population | Australian Bureau of Statistics. National population estimates, December 2020. See Appendix A, Table A6. | [38] |
| Sex distribution of Australian population | 50% male, 50% female | |
| Variants | 90% delta, 10% alpha/wild | |
| Vaccine coverage | 70% received 1st dose, 35% received 2nd dose |
ATAGI = Australian Technical Advisory Group on Immunisation; CVST = Cerebral venous sinus thrombosis; PVT = Portal vein thrombosis.
Note that prior distributions do not affect results of scenario analysis but enables the model to provide population-level estimates. Assumptions can be changed as the situation evolves.
The model includes default prior distributions for age group (using age distribution of Australia), sex (50% male, 50% female), SARS-CoV-2 variants (90% delta, 10% alpha/ancestral virus), and vaccine coverage (70% of population received first dose, 35% received two doses). The prior distributions do not affect the results of scenario analyses, e.g., if delta was selected in the variants node, the model outputs relate to delta only regardless of the prior distribution of variants entered into the model. Prior distributions can be changed to model other scenarios, e.g., different distributions of variants, and different levels of vaccine coverage.
2.5. Model validation
The final model was assessed by subject matter experts and the modelling team together, to determine if the structure, variables, and assumptions reflected current knowledge and evidence. The model’s predictive ability was not validated against a dataset because model parameters were not learned from data. Rather, we validated each section of the model by defining a range of scenarios and manually assessing whether model outputs were consistent with our assumptions and external evidence. Manual quantitative validations were conducted by a subject matter expert/BN modeller (CLL), a mathematician/statistician/BN modeller (KM) and a biostatistician (MW). Scenarios used for manual validations are provided in Appendix B. All authors evaluated the biological plausibility of estimates, e.g., for COVID-19 patients, the probability of dying from COVID-19 related atypical blood clots should be lower than the total probability of dying from COVID-19. Further details about the model development process are provided in a linked paper on modelling methods [49].
2.6. Risk-benefit analysis
We assessed the risks versus benefits of the AZ vaccine if 70% of the population received the first dose, and 35% had two doses. For this paper, the priors were chosen to represent vaccination coverage at the time of model development, but other priors can be used. We compared the following risks (vaccine-associated TTS) and benefits (potential deaths prevented) assuming the above vaccination coverage:
-
i)
Estimated deaths per million under low, medium, and high transmission scenarios (equivalent to 0.05%/0.45%/5.76% of population infected over 6 months), and potential deaths prevented by vaccination;
-
ii)
Estimated deaths per million from AZ vaccine-associated TTS;
-
iii)
Estimated deaths per million from CVST and PVT in COVID-19 patients (i.e., those with symptomatic infection), and potential deaths prevented by vaccination.
2.7. Sensitivity analysis
During the development of the model, data and evidence for many of the inputs continually evolved. To determine how often model assumptions need to be updated, sensitivity analysis was conducted for three predictor variables that were considered most likely to change over time. We examined actual changes in the reported incidence of AZ vaccine-associated TTS cases and deaths in Australia in August-September 2021. We also examined i) plausible differences in age-specific CFR for COVID-19 in Australia (e.g., if higher CFR with new variants or because of an overwhelmed health system), and ii) plausible reductions in vaccine effectiveness against symptomatic COVID-19 infection and death.
2.7.1. Incidence and CFR of vaccine-associated TTS
From July-September 2021, reported incidence of AZ vaccine-associated TTS and related deaths in Australia fluctuated by week because of low numbers. We examined ATAGI reports from 25/8/2021, 1/9/2021, 8/9/2021, and 15/9/2021 [37] to determine how fluctuations in data affected our model predictions of age-specific TTS-related deaths.
2.7.2. Age-specific CFR for COVID-19 in Australia
By 31/8/2021, COVID-19 CFRs in Australia were very low for younger age groups, with less than five deaths during the entire pandemic in each male/female subgroups in those aged 0–9, 10–19, 20–29, 30–39, and 40–49 years. We examined changes in CFR if (theoretically) there was one extra death or five extra deaths in each age-sex subgroup, and the potential impact of these changes on model predictions on COVID-19 related deaths.
2.7.3. Vaccine effectiveness against symptomatic infection and death
We examined the sensitivity of model outputs to reduced vaccine effectiveness against the delta variant using theoretical assumptions of 5% and 10% reduction in effectiveness against both symptomatic infection and death.
3. Results
3.1. Model description
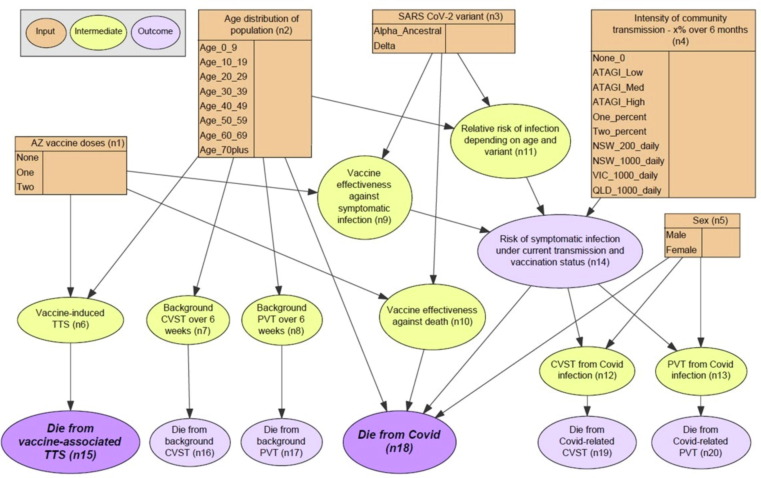
The final BN structure (Fig. 2 ) synthesises the assumptions regarding probabilistic relationships between variables and outcomes. Table 2 provides a summary of all nodes (n1 to n20) and their parent/child relationships. The model was designed to predict five outcomes:
-
i.
Probability of dying from AZ vaccine-associated TTS (n6) – depending on age (n2), 1st or 2nd dose of AZ vaccine (n1);
-
ii.
Background probability of deaths from CVST and PVT (in those who have not had AZ vaccine or COVID-19 infection) (n7, n8). Estimates were converted to probability of events over 6 weeks to enable comparison with the probability of vaccine-associated TTS (generally occurs within 6 weeks of vaccination);
-
iii.
Probability of symptomatic COVID-19 infection – depending on age (n2), sex (n5), variant (n3), intensity of community transmission (n4), vaccine effectiveness against symptomatic infection (n9);
-
iv.
Probability of dying from COVID-19 (n18) – depending on age (n2), sex (n5), variant (n3), intensity of community transmission (n4), vaccine effectiveness against symptomatic infection (n9), vaccine effectiveness against death (n10); and
-
v.
Probability of CVST and PVT deaths (n19, n20) related to COVID-19 infection – depending on sex (n5).
Fig. 2.
Bayesian network for assessing risks versus benefits of the AstraZeneca COVID-19 vaccine.
Table 2.
Summary of nodes and relationships between nodes in a Bayesian network for assessing risks versus benefits of the AstraZeneca COVID-19 vaccine.
| Node name (number) | Description | Potential values | Node type | Parent nodes | Child nodes |
|---|---|---|---|---|---|
| AZ vaccine doses (n1) | Version 1: Vaccine dose number Version 2: Vaccine coverage in population |
Version 1: None, 1st dose, 2nd dose Version 2: None, one dose only, two doses |
Input | N/A – Default priors: 30% unvaccinated, 35% had one dose only, 35% had two doses. | n6, n10, n9 |
| Age group (n2) | Age group | 0–9,10–19, 20–29,30–39, 40–49, 50–59, 60–69, 70+ | Input | N/A – Default priors: population distribution of Australia | n6, n7, n8, n11, n18 |
| SARS CoV-2 variant (n3) | Dominant SARS CoV-2 variant(s) currently circulating | Alpha/wild, Delta | Input | N/A – Default priors: 5% Alpha/wild and 95% Delta | n9, n10, n11 |
| Intensity of community transmission - x% over 6 months (n4) | Probability of infection over 6-months based on different levels of community transmission | None, ATAGI definitions of low, med, high, 1%, 2%, NSW 200 cases/day, NSW 1000 cases/day, Vic 1000 cases/day, QLD 1000 cases/day |
Input | N/A – Defaults to uniform distribution | n14 |
| Sex (n5) | Sex | Male, female | Input | N/A – Defaults to uniform distribution | n12, n13, n18 |
| Vaccine-associated TTS (n6) | Probability of AZ vaccine-associated TTS | Yes, no | Intermediate | AZ vaccine doses (n1), Age group (n2) | n15 |
| CVST over 6 weeks (n7) | Probability of developing CVST over 6 weeks | Yes, no | Intermediate | Age group (n2) | n16 |
| PVT over 6 weeks (n8) | Probability of developing PVT over 6 weeks | Yes, no | Intermediate | Age group (n2) | n17 |
| Vaccine effectiveness against symptomatic infection (n9) | Effectiveness of the vaccine at preventing symptomatic SARS CoV-2 infection | Yes, no | Intermediate | AZ vaccine doses (n1), SARS CoV-2 variant (n3) | n14 |
| Vaccine effectiveness against death (n10) | Effectiveness of the vaccine at preventing deaths from symptomatic SARS CoV-2 infection | Yes, no | Intermediate | AZ vaccine doses (n1), SARS CoV-2 variant (n3) | n18 |
| Relative risk of infection depending on age and variant (n11) | Relative risk of COVID-19 infection depending on age and variant | Yes, no | Intermediate | Age group (n2), SARS CoV-2 variant (n3) | n14 |
| CVST from Covid infection (n12) | Probability of developing CVST if develops symptomatic COVID-19 | Yes, no | Intermediate | Sex (n5), Risk of symptomatic infection under current transmission and vaccination status (n12) | n20 |
| PVT from Covid infection (n13) | Probability of developing PVT if develops symptomatic COVID-19 | Yes, no | Intermediate | Sex (n5), Risk of symptomatic infection under current transmission and vaccination status (n12) | N/A |
| Risk of symptomatic infection under current transmission and vaccination status (n14) | Probability of symptomatic COVID-19 | Yes, no | Intermediate | Intensity of community transmission - x% over 6 months (n4), Vaccine effectiveness against symptomatic infection (n9), Relative risk of infection depending on age and variant (n11) | n19 |
| Die from vaccine-associated TTS (n15) | Proportion of the population that will die from vaccine-associated TTS | Yes, no | Outcome | Vaccine-associated TTS (n6) | N/A |
| Die from CVST (n16) | Probability of dying from CVST (background rate in those who have not had vaccine or infection) | Yes, no | Outcome | CVST over 6 weeks (n7) | N/A |
| Die from PVT (n17) | Probability of dying from PVT (background rate in those who have not had vaccine or infection) | Yes, no | Outcome | PVT over 6 weeks (n8) | N/A |
| Die from Covid (n18) | Probability of dying from COVID-19 | Yes, no | Outcome | Age (n2), Sex (n5), Vaccine effectiveness against death (n10), Risk of symptomatic infection under current transmission and vaccination status (n12) | N/A |
| Die from Covid-related CVST (n19) | Probability of dying from COVID-19 related CVST | Yes, no | Outcome | CVST from SARS COV-2 infection (n13) | N/A |
| Die from Covid-related PVT (n20) | Probability of dying from COVID-19 related PVT | Yes, no | Outcome | PVT from SARS COV-2 infection (n14) | N/A |
The BN includes five input nodes (orange) that could be used for scenario analysis based on AZ vaccine doses (n1), age (n2), sex (n5), variant (n3) and intensity of community transmission (n4). Community transmission scenarios were converted to probability of infection over six months to more readily compare the risks versus benefits of vaccination, which is expected to be effective for at least six months. Scenarios were selected based on ATAGI definitions of low/medium/high risk [16], other scenarios that users could relate to (e.g., 1000 cases/day in NSW), and general scenarios that could be adapted to any setting (e.g., 1% chance of infection over 6 months). The model includes nine intermediate nodes (yellow) where modellers can readily update evidence on incidence of TTS (n6), background incidence of CVST and PVT (n7, n8), relative risk of infection based on age and variant (n11), vaccine effectiveness (n9, n10), and incidence and CFR of COVID-19 related CVST and PVT (n12, n13).
Two versions of the model were built using different definitions of the ‘AZ vaccine doses (n1)’ node:
-
•
Version 1: AZ vaccine doses defined as no doses, 1st dose, and 2nd dose. This version was used to estimate the probability of vaccine-associated TTS with each dose of vaccine.
-
•
Version 2: AZ vaccine doses defined as no doses, received only one dose, and received both doses. This version was used to estimate the probability of deaths in the population based on vaccine coverage rates.
3.2. Model validation
A model walk-through indicated that subject matter experts agreed the model structure matched their understanding of the problem space in terms of the relevant predictors and relationships drawn from the external data sources. Independent manual calculations by three authors (CLL, MW, KM) of probabilities of selected outcomes were consistent with model predictions (Appendix B).
A minor anomaly was detected in the model through assessment of biological plausibility. For COVID-19 cases in younger age groups (females < 30 years and males < 10 years), the estimated probability of dying from COVID-19 was lower than the probability of dying from COVID-19 related atypical severe blood clots. The reason for this anomaly is that data for COVID-19 CFR in Australia were used for the CPTs, and there have been no deaths to date in these age-sex subgroups (Table A3), while data on COVID-19 related atypical blood clots were extracted from a study where data were predominantly sourced from the USA and Europe, where high CFRs could have resulted from an overwhelmed health system during the outbreak. Although the discrepancies in probabilities were extremely low (probability of dying from COVID-19 related atypical blood clots < 0.0002% higher than dying from COVID-19 itself), the anomaly highlights that the model predictions should be interpreted as broad estimates rather than exact risks.
3.3. Risk-benefit analysis
3.3.1. Comparison between estimated deaths prevented from COVID-19 under different scenarios of community transmission intensity and vaccine coverage, and estimated deaths from AZ vaccine-associated TTS
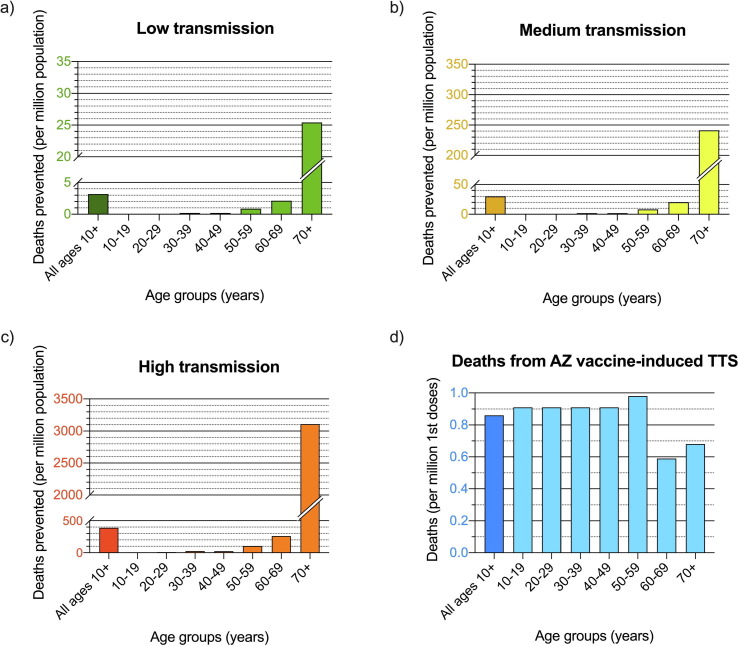
Model version 2 was used to estimate COVID-19 deaths prevented over 6 months per million population if 70% had a first dose, and 35% had two doses. Fig. 3 a, b, and c show the estimated deaths prevented for each age group under different levels of community transmission:
-
•
Low transmission (Fig. 3a), similar to 1st wave in Australia in 2020, equivalent to 0.05% of population infected over 6 months;
-
•
Medium transmission (Fig. 3b), similar to 2nd wave in VIC in 2020, equivalent to 0.45% of population infected over 6 months; and
-
•
High transmission (Fig. 3c), similar to Europe in January 2021, equivalent to 5.76% of population infected over 6 months.
Fig. 3.
Estimated COVID-19 deaths prevented over 6 months per million population of each age group if 70% had first dose, and 35% had two doses of AZ vaccine under a) low, b) medium, and c) high levels of community transmission; and d) estimated deaths from AZ vaccine-associated TTS if 70% of the population had first dose, and 35% had two doses. (Note the large variations in scale in y-axes between each graph).
The model shows that for a million people aged ≥ 70 years where 70% have had first dose and 35% two doses, an estimated 25 deaths would be prevented under low transmission (Fig. 3a) versus > 3000 deaths prevented under high transmission (Fig. 3c), with < 1 expected death from TTS (Fig. 3d). In a million people aged 60–69 years with the same vaccine coverage, the model estimates two deaths prevented under low transmission, and 260 deaths prevented under high transmission, with < 1 expected death from TTS. In contrast, for a million 20–29 year-olds with the same vaccine coverage, <0.1 deaths would be prevented under low transmission, ∼9 deaths prevented under high transmission, with < 1 expected death from TTS. Details on calculations are provided in Appendix C.
3.3.2. Comparison of risk of AZ vaccine-associated TTS with risk of atypical blood clots (CVST and PVT) in COVID-19 infected patients
Up to 25/8/2021 in Australia, age-specific incidence of AZ vaccine-associated TTS cases ranged from 16 to 27 per million first doses (Table 1), with an overall CFR of ∼ 5% (6 deaths out of 115 cases). Based on annual background rates of CVST and PVT reported by Kristoffersen et al. [28], Ageno et al. [29], and Søgaard et al. [30] our model estimated 6-week incidence of 0.38 (age < 20 years) to 2.69 (age ≥ 70 years) cases per million, and overall CFR ranging from 7.0% to 21.6% from youngest to oldest age groups (Table A7). The background CFR for CVST and PVT (combined) range from 0.03 (age < 20 years) to 0.58 (age ≥ 70 years) per million over 6 weeks, compared to CFR of 0.83 to 1.40 per million first doses of AZ vaccine depending on age (Table 3 ).
Table 3.
Evolving evidence on incidence and case-fatality rate (CFR) of vaccine-associated Thrombosis with Thrombocytopenia Syndrome (TTS) in Australia in August-September 2021, and influence on estimated TTS-related deaths by age group.
| Age group (years) |
Estimated incidence of TTS per million 1st doses of AZ vaccinationsa |
Casesb, deathsb, and CFRcfrom 1st doses of AZ vaccinations |
Estimated deaths per million 1st doses of AZ vaccinations based on observed CFR to date |
Difference in estimated deaths per million compared to 25/8/2021 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 25/8/2021 | 1/9/2021 | 8/9/2021 | 15/9/21 | 25/8/2021 | 1/9/2021 | 8/9/2021 | 15/9/21 | 25/8/2021 | 1/9/2021 | 8/9/2021 | 15/9/21 | 1/9/2021 | 8/9/2021 | 15/9/2021 | |
| <50 | 25 | 22 | 20 | 18 | Cases = 115 Deaths = 6 CFR = 5.2% (95% CI 1.9–11.0%) |
Cases = 125 Deaths = 8 CFR = 6.4% (95% CI 2.8–12.2%) |
Cases = 132 Deaths = 8 CFR = 6.1% (95% CI 2.7–11.6%) |
Cases = 134 Deaths = 8 CFR = 6.0% (95% CI 2.6–11.4%) |
1.30 | 1.41 | 1.22 | 1.08 | 0.11 | −0.08 | −0.22 |
| 50–59 | 27 | 26 | 29 | 28 | 1.40 | 1.66 | 1.77 | 1.68 | 0.26 | 0.37 | 0.28 | ||||
| 60–69 | 16 | 17 | 16 | 16 | 0.83 | 1.09 | 0.98 | 0.96 | 0.26 | 0.14 | 0.13 | ||||
| 70–79 | 21 | 21 | 20 | 20 | 1.09 | 1.34 | 1.22 | 1.20 | 0.25 | 0.13 | 0.11 | ||||
| ≥80 | 16 | 16 | 17 | 19 | 0.83 | 1.02 | 1.04 | 1.14 | 0.19 | 0.21 | 0.31 | ||||
Incidence of TTS in Australia reported by ATAGI.
Cumulative cases and deaths to date in Australia, all ages.
CFR = Case fatality rate for all ages combined, and binomial exact 95% confidence interval.
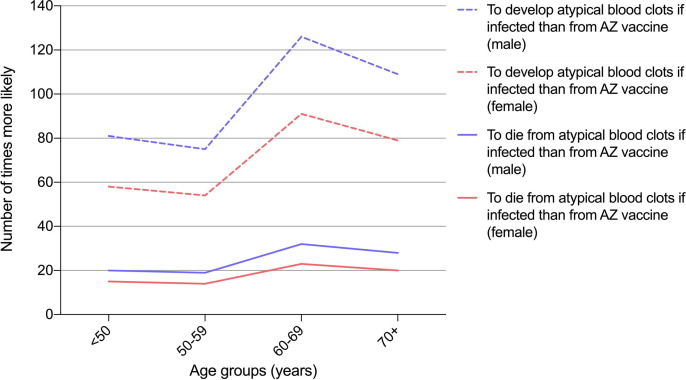
Model assumptions on the incidence and CFR of CVST and PVT in COVID-19 patients were obtained from a retrospective cohort study using linked electronic health records predominantly from the USA and Europe [32] because there were insufficient data from Australia. Model version 1 estimated that overall fatality from atypical severe blood clots (CVST and PVT combined) in COVID-19 patients were 51.1 and 37.3 per 100,000 in males and females, respectively. Fig. 4 shows that the probability of developing atypical blood clots if infected (in those with symptomatic infection) was 14–28 times higher than developing TTS after the first dose of the AZ vaccine, depending on age group and sex (Fig. 4, dashed lines). The probability of dying from atypical blood clots if infected (in those with symptomatic infection) was 58–126 times higher than dying from TTS after the first dose of AZ vaccine, again depending on age group and sex (Fig. 4, solid lines).
Fig. 4.
Number of times more likely to develop and die from atypical blood clots (CVST and PVT) in COVID-19 patients (i.e., those with symptomatic infection) than from AZ-vaccine-induced TTS, by age group and sex.
3.4. Sensitivity analysis
3.4.1. Incidence and CFR of AZ vaccine-associated TTS
ATAGI reports from 25/8/2021 to 15/9/2021 [37] showed minor changes in the incidence of vaccine-associated TTS in Australia, and CFR ranging from 5.2% to 6.4% (Table 3). The model outputs showed that estimated deaths from TTS per million first doses did not change significantly over this time period, and ranged from differences of −0.08 to 0.37 deaths per million (depending on age group) when comparing data from 25/8/2021 with subsequent reports. Therefore, minor fluctuations in CFR from TTS did not have any significant influence on the point estimates of the number of deaths at a population level. Because of the small number of TTS cases and deaths in Australia so far, the 95% confidence intervals for CFR were wide, ranging from 1.9% to 12.2% over the four reports (Table 3). Our model currently assumes a CFR of 5%; it is plausible that true CFR is twice as high (10%), and if so, model estimates of TTS related deaths would be doubled. Changes in reported CFR for TTS should be monitored, and model assumptions updated if needed.
3.4.2. Age-specific CFR for COVID-19 in Australia
By August 2021, CFR for COVID-19 in Australia was 1.84% in males and 1.94% in females. In those aged under 50 years, CFR was < 0.1% in both sexes (Appendix A). If there was one extra death in each age-sex subgroup during this time, CFR would have increased the most in 60–69 year-olds and the least in 20–29 year-olds, but by less than 0.06% in any subgroup (Appendix D). The model was most sensitive to changes in the 60–69 year-old age group because of the small case numbers (small denominator). If there were five extra deaths in each age-sex subgroup, CFR would have increased by<0.3% in any subgroup. Therefore, our model was not very sensitive to minor changes in number of reported deaths, and model estimates of deaths per million would have differed by<0.06% or 0.3% if there were one or five extra deaths in any age-sex subgroup, respectively. Therefore, it is not necessary to update our model assumptions of age-specific CFR if changes are minor.
3.4.3. Vaccine effectiveness against symptomatic infection and death
Our model shows that for a population where 70% has received the first dose and 35% has received two doses, a theoretical 5% or 10% reduction in vaccine effectiveness against the delta variant results in a 7.1% or 15.1% increase in estimated deaths, respectively (Table 4 ). Therefore, sensitivity analyses show that model predictions of deaths are much more sensitive to changes in vaccine effectiveness than to changes in incidence and CFR of TTS, or changes in CFR from COVID-19 infection. Data on vaccine effectiveness should therefore be regularly reviewed and updated in the model.
Table 4.
Impact of theoretical reduction in vaccine effectiveness against delta variant on estimated deaths, assuming 70% of population had first dose, and 35% had two doses.
| Delta variant | Current model assumptions |
If 5% less effective | If 10% less effective |
|---|---|---|---|
Vaccine effectiveness against symptomatic infection after
|
33% 61% |
28% 56% |
23% 51% |
Vaccine effectiveness against death after
|
69% 90% |
64% 85% |
59% 80% |
| % Increase in estimated deaths compared to current model assumptions of vaccine effectiveness | N/A | 7.1% | 15.1% |
4. Discussion
Our study demonstrates that risk–benefit analysis of the AZ vaccine is complex, and depends on multiple factors including age, sex, vaccine effectiveness, and locally specific factors such as the intensity of community transmission, local incidence and CFR of TTS, and CFR from COVID-19. The model outputs and scenario analysis could facilitate more informed decision making between clinicians and individuals, e.g., by considering age, sex, community transmission, and local data on TTS. The model could also provide decision support at a public health level; if the priors were set to represent the population in age and sex distribution, vaccination coverage, circulating variants and level of community transmission, the model outputs could be used to estimate deaths or deaths prevented. To our knowledge, this is the first example of the use of Bayesian networks for risk–benefit analysis for a COVID-19 vaccine.
Our model quantifies the risks (deaths from TTS) versus benefits from vaccination (deaths prevented) for different age groups under different levels of community transmission (Fig. 3). Under any level of community transmission, the benefits of vaccination are greater in older age groups, particularly those aged over 70 years. While the benefits are significantly greater during higher transmission (especially for older age groups), decisions about vaccination should be based not only on the current level of transmission, but also potential future scenarios. For example, higher transmission will be almost inevitable with lifting of public health restrictions and opening of borders. We presented results for scenarios where 70% of the population have had first dose and 35% have had two doses. Other vaccination coverage scenarios can be easily defined in the model priors to assess the current vaccination coverage rates, or future vaccination targets.
To date, the CFR of TTS in Australia has been lower than those reported in other countries [8], [10]. Although numbers have been small, CFR have remained around 5% throughout August-September 2021 (Table 3). Possible reasons for the lower CFR in Australia include early shift to Pfizer as the preferred vaccine for those aged < 60 years; detailed expert review of each case of serious and fatal AEFI (reducing classification error) by state-based committees, ATAGI and TGA; high clinical vigilance of TTS based on lessons learnt from earlier reports from other countries; clinical guidelines for vaccine providers on early diagnosis and referral of possible TTS cases; and recommendations for early use of intravenous immunoglobulin [39], [40], [41]. Our sensitivity analysis showed that fluctuations in observed CFR from TTS had only minor impact on model outputs. Model assumptions can be readily updated if CFR changes significantly in the future. While vaccine-associated TTS can be serious and rarely fatal, mortality rates for atypical severe blood clots (CVST and PVT) in COVID-19 patients are estimated to be 58–126 times more likely than after the first dose of the AZ vaccine (Fig. 4).
Sensitivity analyses showed that the estimates of COVID-19 related deaths were very sensitive to changes in vaccine effectiveness. Therefore, model assumptions need to be updated to reflect evolving evidence on vaccine effectiveness against current and future variants of the virus, waning immunity over time, and effectiveness of vaccine boosters. From a public health perspective, our findings suggest that a decrease in vaccine effectiveness will likely have important implications on disease burden. In contrast, model outputs were not very sensitive to changes in age-specific CFR for COVID-19, and updates to model assumptions are unlikely to be required unless changes are dramatic.
We used an innovative and flexible modelling framework which allows model assumptions to be easily changed, either to reflect new data (e.g., vaccine effectiveness), ort the evolving situation (e.g., intensity of community transmission, changing CFR from TTS and COVID-19). While the current model is designed to estimate deaths from vaccine versus disease, it could be readily adapted for other outcomes (e.g., ICU admission, long COVID), other adverse events (e.g., immune thrombocytopenia), risk–benefit assessment of other vaccines (e.g., myocarditis/pericarditis from mRNA vaccines), and other types of scenario analysis (e.g., different combinations of vaccine doses and boosters). Our modelling approach also enables the use of multiple sources of data. We have used Australian data wherever possible and international sources if needed (e.g., low number of COVID-19 cases and vaccinations in Australia means that international data on vaccine effectiveness were likely to be more accurate). Our modelling approach therefore provides the potential for countries to develop a locally relevant risk–benefit assessment tool for COVID-19 vaccination even if there are limited local data. Although the directional links in a BN generally show association rather than explicit causal relationship, BNs also enable reasoning and causal inference by association. For example, what is the probability that COVID-19 related death in an unvaccinated person could have been prevented by vaccination?
Another advantage of BNs is the visual and interactive interface that allows intuitive scenario analysis for users and decision makers. While this paper presents results of specific scenarios, interactive versions of model outputs are also be freely available on the Immunisation Coalition website (https://corical.immunisationcoalition.org.au/). Interactive risk assessment tools for COVID-19 have been developed for estimating the risk of hospitalization and death (e.g., QCOVID tool in United Kingdom [42], COVID-19 risk tools in USA [43] and France [44]), predicting clinical outcomes [45], and assessing risk of infection from different activities [46], [47], [48]. Some tools were based on analysis of millions (e.g., QCOVID [42]) or hundreds of thousands (e.g., ISARIC4C [45]) of medical records. Our modelling approach provides an alternative option of developing risk assessment tools if large datasets are not available, and model inputs need to be obtained from alternative sources. We have not identified any web-based interactive tools that use Bayesian networks for risk–benefit analysis of COVID-19 vaccines for use in clinical or public health settings.
Our results should be interpreted considering the model’s limitations. There are uncertainties associated with some of our model inputs, either because of limited data, or the use of data from other countries. As illustrated with the anomaly regarding the risk of dying in younger persons, model outputs should be considered as broad estimates rather than exact risks, but estimates can be improved over time as more data become available. Our model provides population level estimates and does not consider individual risks such as behaviour and comorbidities. We plan to develop future models that include the individual’s comorbidities, similar to the QCOVID tool [42] but specific for the Australian context. In the results provided, we have assumed that 100% of infections were from the delta variant. Assumptions of age distribution of delta cases (if unvaccinated) were obtained from data during the early stages of the delta outbreak in NSW from June 2021. While vaccination rates were relatively low then, older age groups had higher vaccine coverage so infection rates for delta may have been underestimated in these groups. Data on CVST and PVT were obtained from studies outside Australia and may not reflect the local experience. While data used in the model were based on evidence rather than expert elicitation, we accept that biases, including information cascades, could still influence what evidence was gathered. We minimised these risks by encouraging experts to search the literature broadly and having regular group discussions (including with professional advisory groups) about where evidence could be challenged. The current model focuses on fatalities from COVID-19, TTS, and atypical blood clots, but does not consider other risks (e.g., adverse events) or other benefits (e.g., cases of severe COVID-19 prevented, or broader societal benefits). It is important to note that the model uses a 6-month time frame for COVID-19 related outcomes and 6-weeks for background rates of CVST and PVT; these time horizons could be updated as evidence evolves. Our model was not parameterised from any specific datasets, so model outputs could not be directly validated by data. Nevertheless, the model provides a powerful mechanism for complex synthesis of multiple sources of data, and the outputs reflect the latest available knowledge.
In conclusion, we developed a novel approach to risk–benefit analysis for the AZ vaccine by using an adaptable BN modelling framework. Our model enables more precise risk analysis based on demographics, the outbreak situation, local data on vaccine-associated TTS, and the best available international evidence on vaccine effectiveness and atypical blood clots. Although use of the AZ vaccine is expected to gradually decrease in Australia over coming months, the model can be readily adapted for use in other countries or risk–benefit assessment of other vaccines.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We thank Kim Sampson from Immunisation Coalition for facilitating the collaboration between authors, and A/Prof Hassan Valley (La Trobe University, Melbourne, Australia) for contributions to discussions about risk communication and data visualisation. We thank Aapeli Vuorinen (Columbia University) for building the interactive risk assessment tool. Our Bayesian network model was built using GeNIe Modeler (BayesFusion 2019), available free of charge for academic research and teaching use from https://www.bayesfusion.com/. We thank the THANZ Vaccine-induced Immune Thrombotic Thrombocytopenia (VITT) advisory group (in particular A/Prof Vivien Chen, Prof Huyen Tran, A/Prof Jennifer Curnow, Dr Ibrahim Tohidi and Dr Danny Hsu) for their feedback about TTS related data.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The CoRiCal project was supported by the Immunisation Coalition through educational grants received from pharmaceutical companies that manufacture vaccines. However, the CoRiCal project did not receive any direct funding from AstraZeneca, Pfizer, or any other companies that produce COVID-19 vaccines. All co-authors of this paper provided in-kind contribution of their time and expertise to develop the CoRiCal interactive tool, and the research and modelling that underpin the risk calculations provided by the tool. CLL was supported by an Australian National Health and Medical Research Council (NHMRC) Fellowship (APP1193826).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2021.10.079.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.University of Oxford. Oxford vaccine reaches one billion doses released. 202https://www.ox.ac.uk/news/2021-07-29-oxford-vaccine-reaches-one-billion-doses-released.
- 2.Voysey M., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voysey M., et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397(10277):881–891. doi: 10.1016/S0140-6736(21)00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sheikh A., et al. SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet. 2021;397(10293):2461–2462. doi: 10.1016/S0140-6736(21)01358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaccine Effectiveness Expert Panel (VEEP) VEEP: Vaccine effectiveness table, 16 July 2021. 2021. https://www.gov.uk/government/publications/veep-vaccine-effectiveness-table-16-july-2021.
- 6.Norwegian Institute of Public Health Norwegian Institute of Public Health's recommendation about AstraZeneca vaccine. 2021. https://www.fhi.no/en/news/2021/astrazeneca-vaccine-removed-from-coronavirus-immunisation-programme-in-norw/.
- 7.Michael Franklin; Timm Bruch Alberta doctors find 2nd case of blood clot linked to AstraZeneca vaccine in Canada. 2021. https://calgary.ctvnews.ca/alberta-doctors-find-2nd-case-of-blood-clot-linked-to-astrazeneca-vaccine-in-canada-1.5391531.
- 8.Medicines & Healthcare products Regulatory Agency, Coronavirus vaccine - weekly summary of Yellow Card reporting. 2021, GOV.UK: United Kingdom.
- 9.Australian Government Department of Health, COVID-19 vaccine weekly safety report - 12-08-2021, in COVID-19 vaccine weekly safety report. 2021: Australia.
- 10.Greinacher A., et al. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N Engl J Med. 2021;384(22):2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Australian Government Department of Health Joint statement from ATAGI and THANZ on Thrombosis with Thrombocytopenia Syndrome (TTS) and the use of COVID-19 Vaccine AstraZeneca. 2021. https://www.health.gov.au/news/joint-statement-from-atagi-and-thanz-on-thrombosis-with-thrombocytopenia-syndrome-tts-and-the-use-of-covid-19-vaccine-astrazeneca.
- 12.Australian Government Department of Health ATAGI statement on revised recommendations on the use of COVID-19 Vaccine AstraZeneca, 17 June 2021. 2021. https://www.health.gov.au/news/atagi-statement-on-revised-recommendations-on-the-use-of-covid-19-vaccine-astrazeneca-17-june-2021.
- 13.MacIntyre C.R., et al. Thrombosis with Thrombocytopenia Syndrome (TTS) following AstraZeneca ChAdOx1 nCoV-19 (AZD1222) COVID-19 vaccination – A risk–benefit analysis for people < 60 years in Australia. Vaccine. 2021;39(34):4784–4787. doi: 10.1016/j.vaccine.2021.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicholas Biddle; Ben Edwards; Matthew Gray; Kate Sollis, Vaccine willingness and concerns in Australia: August 2020 to April 2021. 2021, Australian National University.
- 15.Australian Government Department of Health COVID-19 summary statistics. Coronavirus (COVID-19) case numbers and statistics, 2021. https://www.health.gov.au/news/health-alerts/novel-coronavirus-2019-ncov-health-alert/coronavirus-covid-19-case-numbers-and-statistics#covid19-summary-statistics.
- 16.Australian Government Department of Health COVID-19 vaccination – Weighing up the potential benefits against risk of harm from COVID-19 Vaccine AstraZenec. 2021. https://www.health.gov.au/resources/publications/covid-19-vaccination-weighing-up-the-potential-benefits-against-risk-of-harm-from-covid-19-vaccine-astrazeneca.
- 17.Norman Fenton; Martin Neil, Risk Assessment and Decision Analysis with Bayesian Networks. 1 ed. 2012, USA: CBC Press. 524.
- 18.Vincenzina Vitale;D’Urso, Pierpaolo, and De Giovanni, Livia, Spatio-temporal Object-Oriented Bayesian Network modelling of the COVID-19 Italian outbreak data. Spatial Statistics, 2021: p. 100529. [DOI] [PMC free article] [PubMed]
- 19.Bertone Edoardo, et al. Effectiveness of the Early Response to COVID-19: Data Analysis and Modelling. Systems. 2020;8(2) [Google Scholar]
- 20.Junyi Wang;Zhai, Xueting, and Luo, Qiuju. How COVID-19 Impacts Chinese Travelers’ Mobility Decision-Making Processes: A Bayesian Network Model. In: Information and Communication Technologies in Tourism 2021. 2021. Cham: Springer International Publishing.
- 21.Georgina Prodhan, Fenton, Norman, Extending the range of COVID-19 risk factors in a Bayesian network model for personalised risk assessment. medRxiv, 2020: p. 2020.10.20.20215814.
- 22.Lai K, Yanushkevich S. Machine Reasoning to Assess Pandemics Risks: Case of USS Theodore Roosevelt. ArXiv, 2020. abs/2008.11040.
- 23.Norman E. Fenton, et al., A Bayesian network model for personalised COVID19 risk assessment and contact tracing. medRxiv, 2021: p. 2020.07.15.20154286.
- 24.Scott McLachlan, et al., The fundamental limitations of COVID-19 contact tracing methods and how to resolve them with a Bayesian network approach. 2020.
- 25.Yue Wu., et al. Bridging the gaps in test interpretation of SARS-CoV-2 through Bayesian network modelling. Epidemiol Infect. 2021;149 doi: 10.1017/S0950268821001357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rachel Butcher and Fenton, Norman, Extending the range of symptoms in a Bayesian Network for the Predictive Diagnosis of COVID-19. medRxiv, 2020: p. 2020.10.22.20217554.
- 27.Edward Burn, et al., Background rates of five thrombosis with thrombocytopenia syndromes of special interest for COVID-19 vaccine safety surveillance: incidence between 2017 and 2019 and patient profiles from 25.4 million people in six European countries. medRxiv, 2021: p. 2021.05.12.21257083. [DOI] [PMC free article] [PubMed]
- 28.Kristoffersen Espen Saxhaug, et al. Incidence and Mortality of Cerebral Venous Thrombosis in a Norwegian Population. Stroke. 2020;51(10):3023–3029. doi: 10.1161/STROKEAHA.120.030800. [DOI] [PubMed] [Google Scholar]
- 29.Ageno W., et al. Incidence rates and case fatality rates of portal vein thrombosis and Budd-Chiari Syndrome. Thromb Haemost. 2017;117(4):794–800. doi: 10.1160/TH16-10-0781. [DOI] [PubMed] [Google Scholar]
- 30.Søgaard Kirstine Kobberøe, et al. Survival after splanchnic vein thrombosis: a 20-year nationwide cohort study. Thromb Res. 2016;141:1–7. doi: 10.1016/j.thromres.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 31.James B. Bussel; Jean M. Connors; Douglas B. Cines; Cynthia E. Dunbar; Laura C. Michaelis; Lisa Baumann Kreuziger; Agnes Y.Y. Lee; Ingrid Pabinger-Fasching Thrombosis with Thrombocytopenia Syndrome (also termed Vaccine-induced Thrombotic Thrombocytopenia). 2021. https://www.hematology.org/covid-19/vaccine-induced-immune-thrombotic-thrombocytopenia.
- 32.Taquet Maxime, et al. Cerebral venous thrombosis and portal vein thrombosis: A retrospective cohort study of 537,913 COVID-19 cases. EClinicalMedicine. 2021;39 doi: 10.1016/j.eclinm.2021.101061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tom Shimabukuro; National Center for Immunization & Respiratory Diseases Update: Thrombosis with thrombocytopenia syndrome (TTS) following COVID-19 vaccination. 2021. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-05-12/07-COVID-Shimabukuro-508.pdf.
- 34.NSW Government NSW COVID-19 cases data. 2021. https://data.nsw.gov.au/nsw-covid-19-data/cases.
- 35.Australian Government Department of Health National Notifiable Diseases Surveillance System public datasets. 2021. https://www1.health.gov.au/internet/main/publishing.nsf/Content/ohp-pub-datasets.htm.
- 36.Doherty Institute, Doherty modelling report revised 10th August 2021. 2021, Doherty Institute. p. Table S2.5.
- 37.Australian Government Department of Health News and Media. 2021. https://www.health.gov.au/news?f%5B0%5D=field_date_updated%3A2021.
- 38.Australian Bureau of Statistics Data downloads - time series spreadsheets. National, state and territory population, 2021. https://www.abs.gov.au/statistics/people/population/national-state-and-territory-population/dec-2020#data-download.
- 39.Australian Government Department of Health COVID-19 vaccination – Primary care approach to thrombosis with thrombocytopenia syndrome after COVID-19 AstraZeneca vaccine. 2021. https://www.health.gov.au/resources/publications/covid-19-vaccination-primary-care-approach-to-thrombosis-with-thrombocytopenia-syndrome-after-covid-19-astrazeneca-vaccine.
- 40.Vivien Chen; Jennifer L. Curnow; Huyen Tran; Philip Young-Ill Choi, Australian New Zealand approach to diagnosis and management of vaccine induced immune thrombosis and thrombocytopenia. Medical Journal of Australia, 2021. [DOI] [PMC free article] [PubMed]
- 41.VITT Communication Committee THANZ Multidisciplinary VITT Guideline for Doctors. 2021. https://www.thanz.org.au/news/vitt-multidisciplinary-guideline-for-doctors.
- 42.Clift Ash K., et al. Living risk prediction algorithm (QCOVID) for risk of hospital admission and mortality from coronavirus 19 in adults: national derivation and validation cohort study. BMJ. 2020;371 doi: 10.1136/bmj.m3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johns Hopkins. COVID-19 Risk Tools. COVID-19 Risk Tooks 2021 [cited 25 September 2021]; Available from: https://covid19risktools.com:8443/riskcalculator#mainbody.
- 44.Tran Viet-Thi, et al. Impact of an interactive web tool on patients’ intention to receive COVID-19 vaccination: a before-and-after impact study among patients with chronic conditions in France. BMC Med Inf Decis Making. 2021;21(1):228. doi: 10.1186/s12911-021-01594-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.ISARIC4C (Coronavirus Clinical Characterisation Consortium). ISARIC4C Mortality Score. ISARIC4C 2021; Available from: https://isaric4c.net.
- 46.National Institute on Ageing. My COVID-19 Visit Risk Calculator. 2021 [cited 25 September 2021]; Available from: https://covidvisitrisk.com/riskscore-english.html.
- 47.The microCOVID Project. microCOVID Project. 2021 [cited 25 September 2021]; Available from: https://www.microcovid.org/about.
- 48.Lifespan; Warren Alpert Medical School of Brown University. MyCOVIDRisk. 2021 [cited 25 September 2021]; Available from: https://mycovidrisk.app.
- 49.Mayfield Helen J, Lau Colleen L, Sinclair Jane E, Brown Samuel J, Baird Andrew, Litt John, et al. Designing an evidence-based Bayesian network for estimating the risk versus benefits of AstraZeneca COVID-19 vaccine. medRxiv. 2021 doi: 10.1101/2021.10.28.21265588. In this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.