Abstract
Objective: Delirium is common and highly distressing for the palliative care population. Until now, no study has systematically reviewed the risk factors of delirium in the palliative care population. Therefore, we performed a systematic review and meta-analysis to evaluate delirium risk factors among individuals receiving palliative care.
Methods: We systematically searched PubMed, Medline, Embase, and Cochrane database to identify relevant observational studies from database inception to June 2021. The methodological quality of the eligible studies was assessed by the Newcastle Ottawa Scale. We estimated the pooled adjusted odds ratio (aOR) for individual risk factors using the inverse variance method.
Results: Nine studies were included in the review (five prospective cohort studies, three retrospective case-control studies and one retrospective cross-section study). In pooled analyses, older age (aOR: 1.02, 95% CI: 1.01–1.04, I2 = 37%), male sex (aOR:1.80, 95% CI: 1.37–2.36, I2 = 7%), hypoxia (aOR: 0.87, 95% CI: 0.77–0.99, I2 = 0%), dehydration (aOR: 3.22, 95%CI: 1.75–5.94, I2 = 18%), cachexia (aOR:3.40, 95% CI: 1.69–6.85, I2 = 0%), opioid use (aOR: 2.49, 95%CI: 1.39–4.44, I2 = 0%), anticholinergic burden (aOR: 1.18, 95% CI: 1.07–1.30, I2 = 9%) and Eastern Cooperative Oncology Group Performance Status (aOR: 2.54, 95% CI: 1.56–4.14, I2 = 21%) were statistically significantly associated with delirium.
Conclusion: The risk factors identified in our review can help to highlight the palliative care population at high risk of delirium. Appropriate strategies should be implemented to prevent delirium and improve the quality of palliative care services.
Keywords: delirium, risk factors, palliative care, systematic review, meta-analysis
Introduction
Delirium is a complex neuropsychiatric syndrome characterized by acute onset and fluctuating disturbance in attention, awareness, and other cognitive abilities, including memory, orientation, language, and perception (1, 2). Delirium is commonly experienced during individuals receiving palliative care. The prevalence of delirium in palliative care settings has been reported at a range of 28–42% on admission, whereas it can rise to 88% before death (3, 4). Delirium is known to be associated with reduced quality of life, shortened survival expectancy, and increased health care costs (5, 6), which is highly distressing for the palliative care population, caregivers, as well as clinicians (7).
The etiology of delirium is multifactorial, and the pathophysiologic cause is not well-understood (8). It is important to recognize risk factors for delirium, which could promote the early identification, prevention, and treatment of delirium. Palliative care services are usually provided for patients with cancer or organ failure who are susceptible to delirium. However, despite the high occurrence of delirium in palliative care units, only very few studies investigate the risk factors in this vulnerable population, and the results from different studies are inconsistent (9, 10). Until now, the contributing factors of delirium in the palliative care population remain unclear, so it is necessary to perform a comprehensive evaluation of this area.
Until now, there has not been a systematic review to evaluate risk factors of delirium in the palliative care population. Therefore, the aims of our systematic review and meta-analysis were (a) to identify risk factors of delirium among individuals receiving palliative care; (b) to examine the methodology and quality of included studies.
Methods
We did a systematic review and meta-analysis following the recommendations of Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines (PRISMA) (11).
Search Strategy
We systematically searched PubMed, Medline, Embase, and Cochrane databases to identify relevant observational studies without language restrictions from database inception to June 2021. The words “delirium” and “palliative care” were used as key terms for searching. The detailed search strategy for MEDLINE was reported in the supplementary materials (Supplementary Table 1). We also hand-searched the reference lists of eligible studies and previous relevant reviews to identify additional studies.
Study Selection
Studies were independently selected by two reviewers (DG and TL) using a two-stage process. First, the titles and abstracts of studies were screened, and then the full text of screened articles was assessed for eligibility. Any discrepancy regarding the screening and selection of studies or the following data extraction was resolved through consensus with a third independent reviewer (JY).
The selection criteria were as follows: (a) observational studies (cross-sectional, cohort, and case-control studies); (b) primary research evaluating potential risk factors including predisposing factors and precipitating factors for prevalent delirium or incident delirium; (c) studies with validated instruments or criteria to identify delirium; (d) studies conducted within any palliative care settings: inpatient palliative care units, stand-alone inpatient hospices, hospital palliative care teams, and community palliative care services (12); (e) studies with two comparator groups: a delirium group and a non-delirium group; (f) risk factors analyzed in multivariable models. Studies were excluded if: (a) they were case reports, letters, comments, editorials, reviews, and protocols; (b) they had incomplete or unavailable data.
Data Extraction and Analysis
Data from all eligible studies were independently extracted by two reviewers (DG and YZ). Extracted information included the first author's name, publication year, country, number of patients, male proportion, age, study design, prevalence or incidence of delirium, criteria for delirium, and potential risk factors for delirium. A third reviewer (LG) reviewed the data extraction, and any disagreement was resolved by discussion. We contacted authors when the relevant information was not available in the publication. If the risk factors for delirium were assessed at admission and during admission simultaneously, the adjusted effect measures during admission were extracted.
We conducted the meta-analysis if two or more studies evaluated a risk factor using a multivariable approach and available data. We estimated the pooled adjusted odds ratios (aORs) using the inverse variance method in Review Manager (Version 5.4). We used the I2 test to assess the statistical heterogeneity. A random-effects model was chosen when statistical heterogeneity was present (I2 ≥ 50%). Otherwise, a fixed-effect model was applied. For heterogeneity, we performed subgroup analysis or sensitivity analysis to investigate possible causes of heterogeneity. If more than five studies were eligible for comparison, funnel plot and Egger's test were applied to assess publication bias (13).
Quality Assessment
The quality of the included studies was evaluated independently by two reviewers (DG and TL) using the Newcastle–Ottawa Scale (NOS) (14), which assessed the quality of observational studies in three aspects (selection, comparability, and exposure/outcomes). The total score was classified as high quality (≥7 points), moderate quality (5–6 points), or low quality (≤4 points). Any disagreement about the quality of the studies was resolved by a third reviewer (JY) through discussion.
Results
Study Selection
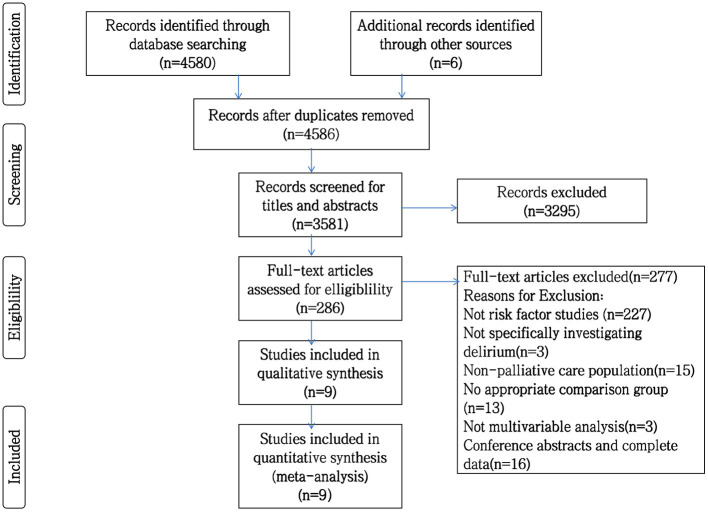
A total of 4,586 studies were identified through the initial electronic search and manual review of references. After removing duplicates, 3,581 studies remained. Of these, 3,295 studies were excluded by screening the title and abstract. The remaining 286 studies were assessed by full-text review, and nine were finally included in our synthesis. Figure 1 shows the flowchart and details of the study selection process.
Figure 1.
PRISMA flowchart of studies included in systematic review.
Study Characteristics
The characteristics of the nine included studies were presented in Table 1 (9, 10, 15–21). Among these studies, three were case-control studies (9, 19, 20), and five were cohort studies (10, 15–18). The studies involved 4,939 subjects (899 delirium cases and 4,040 non-delirious controls) from five countries. Four studies were conducted in Japan (9, 10, 15, 20), two studies in Italy (18, 21), and one each from United States (19), Switzerland (17), and Korea (16). Three multicenter prospective studies enrolled patients receiving palliative care services (10, 15, 18). A retrospective cross-sectional study was conducted in end-of-life patients in a hospice or cared for at home by palliative care physicians (21). Another study retrospective reviewed palliative care inpatients admitted to the Veterans Affairs Boston Healthcare System (19). The remaining four single-center studies were conducted in hospital-based palliative care units (9, 16, 17) or hospice-based palliative care units (20).
Table 1.
Characteristics of the included studies examining risk factors for delirium in palliative population.
| References | Country | Study design | Total number | Age(mean ± SD)unless otherwise stated | No. of male | Delirium prevalence or incidence(%) | Criteria for delirium | Delirium subtype | NOS |
|---|---|---|---|---|---|---|---|---|---|
| Hamano et al. (15) | Japan | Prospective cohort | 2,829 | 72.4 ± 12.2 | 1,492 | 6.9 | DSM-V, MDAS | Hyperactive type | 6 |
| Kang et al. (16) | Korea | Prospective cohort | 102 | Delirium group: 71.83 ± 9.82 Non-delirium group: 62.04 ± 14.35 |
52 | 23.52 | CAM, DSM-IV | No mention | 6 |
| Seiler et al. (17) | Switzerland | Prospective cohort | 410 | Delirium group: 66.4 ± 14.1 Non-delirium group: 64.5 ± 12.4 |
244 | 55.9 | DOS, DSM-V | No mention | 6 |
| Matsuo et al. (10) | Japan | Prospective cohort | 207 | Median:73 | 98 | 17 | CAM | No mention | 6 |
| Mercadante et al. (18) | Italy | Prospective cohort | 263 | 72.1 ± 13.7 | 146 | 41.8 | MDAS | No mention | 8 |
| Matsuoka et al. (9) | Japan | Case Control | 166 | 68.4 ± 11.6 | 97 | 35 | DSM-IV | Hyperactive and mixed type | 5 |
| Zimmerman et al. (19) | United state | Case control | 217 | 72.9 ± 12.8 | 210 | 31 | A chart review instrument | No mention | 7 |
| Morita et al. (20) | Japan | Case Control | 284 | 64 ± 14 | 153 | 20.4 | MDAS, Agitation Distress Scale | Hyperactive type | 6 |
| Pasina et al. (21) | Italy | Cross-section | 461 | Median of delirium group:82.6 median of non-delirium group:78.1 |
226 | 26.9 | 4AT | No mention | 5 |
NOS, Newcastle-Ottawa Scale; DSM, Diagnostic and Statistical Manual of Mental Disorders; CAM, Confusion Assessment Method; MDAS, Memorial Delirium Assessment Scale; DOS, Delirium Observation Screening; 4AT, 4 “A”s Test.
Two studies explored the risk factors for hyperactive delirium only (15, 20), while another study investigated risk factors for hyperactive and mixed-type delirium (9). The remaining six studies did not mention the subtype of delirium (10, 17–19, 21). Six studies reported the mean age of study participants from 64 to 72.9 years (9, 10, 15, 18–20). All studies reported gender: 2,718 (55.03%) males, and 2,221 (44.97%) were female. Tools used to identify delirium were the Diagnostic and Statistical Manual of Mental Disorders (DSM) fourth or fifth edition (9, 15–17), Confusion Assessment Method (CAM) (10, 16), Memorial Delirium Assessment Scale (MDAS) (15, 18, 20), Delirium Observation Screening (DOS) (17), Agitation Distress Scale (20), 4 'A's Test (4AT) (21) and a chart review instrument (19). The prevalence of delirium ranged between 6.9 to 55.9%.
Quality Assessment
The overall quality scores of the NOS scale for the included studies ranged from 5 to 8 (Table 1). Only a cohort study and a case-control study were graded as high quality (9, 10), the remaining seven studies as moderate quality (15–21). Only one cohort study achieved the maximum of four scores in the 'selection' criteria (10). The other four cohort studies failed to demonstrate that the outcome of interest was not present at the start of studies (15–18). All the three case-control studies and a cross-sectional study did not select community as controls (9, 19–21), and two of these studies did not report independent validation of delirium (19, 21). Only two studies scored maximum points in the 'comparability' criteria (9, 10). No study met all three quality criteria in the 'outcome/exposure' criteria due to lack of independent blind assessment of delirium in cohort studies and no respondents described in case-control studies. The detailed methodological quality of included studies according to the NOS scale was presented in the supplementary materials (Supplementary Table 2).
Risk Factors
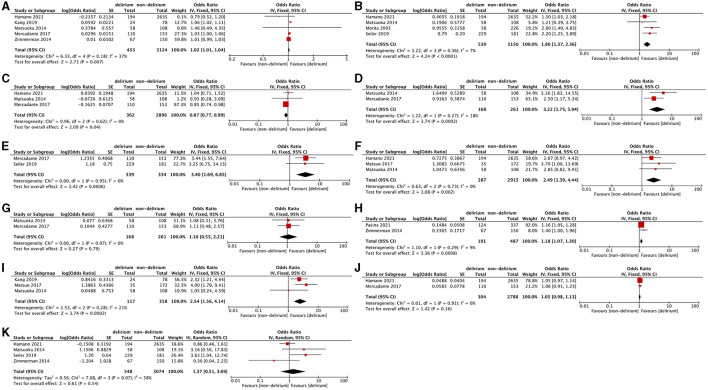
All nine included studies explored 41 risk factors in multivariable analysis. Of these, 18 were reported as independent risk factors (Table 2). Meta-analysis was performed for 11 risk factors described in two or more studies (Figure 2).
Table 2.
Multivariable analyses of risk factors for delirium in palliative care population.
| Risk factors | aOR(95% CI) | Reference |
|---|---|---|
| Age | 1.061 (1.016–1.108) | Kang et al. (16) |
| 1.03 (1–1.07) | Mercadante et al. (18) | |
| Gender(male) | 1.5 (1.03–2.17) | Hamano et al. (15) |
| 2.19 (1.251–3.841) | Seiler et al. (17) | |
| 2.6 (1.4–5.0) | Morita et al. (20) | |
| Brain tumor | 3.63 (1.033–12.776) | Seiler et al. (17) |
| Hearing impairment | 3.52 (1.721–7.210) | Seiler et al. (17) |
| Visual impairment | 3.15 (1.765–5.607) | Seiler et al. (17) |
| Frailty | 15.28 (5.885–39.665) | Seiler et al. (17) |
| 2.39 (1.52–3.74) | Hamano et al. (15) | |
| Acute renal failure | 16.79 (1.062–43.405) | Seiler et al. (17) |
| Dehydration | 2.50 (1.17–5.34) | Mercadante et al. (18) |
| 5.16 (1.83–14.59) | Matsuoka et al. (9) | |
| Metabolic abnormalities | 4.3 (1.43–12.96) | Matsuoka et al. (9) |
| Hypoxia | 0.85 (0.74–0.96) | Mercadante et al. (18) |
| Icterus | 2.4 (1.3–4.4) | Morita et al. (20) |
| Cachexia | 3.44 (1.55–7.63) | Mercadante et al. (18) |
| ECOG PS | 2.320 (1.212–4.44) | Kang et al. (16) |
| 4 (1.7–9.3) | Matsuo et al. (10) | |
| Karnofsky score | 0.93 (0.87–0.98) | Mercadante (18) |
| Pressure sores | 3.66 (1.102–12.149) | Seiler et al. (17) |
| Opioid use | 3.7 (1.0–13) | Matsuo et al. (10) |
| Chemotherapeutic drugs penetrating the blood-brain barrier | 18.92 (1.08–333.04) | Matsuoka et al. (9) |
| Anticholinergic burden | 1.4 (1–1.9) | Zimmerman et al. (19) |
| 1.16 (1.05–1.28) | Pasina et al. (21) |
aOR, adjusted Odds Ratio; ECOG PS, Eastern Cooperative Oncology Group Performance Status.
Figure 2.
Forest plots of meta-analysis of risk factors for delirium in the palliative care population. (A) Age. (B) Male sex. (C) Hypoxia. (D) Dehydration. (E) Cachexia. (F) Opioid use. (G) Steroid use. (H) Anticholinergic burden. (I) Eastern Cooperative Oncology Group Performance Status. (J) Comorbidity. (K) Tumor of central nervous system.
Age and Gender
Age was the most frequent risk factor discussed by five studies using multivariable analyses (9, 15, 16, 18, 19). A meta-analysis of these studies presented that older age was statistically associated with increased delirium risk (aOR: 1.02, 95%CI: 1.01–1.04, P = 0.007; I2 = 37%, P = 0.18). Regarding gender, the pooled analysis of four studies showed that male sex was significantly associated with delirium (aOR: 1.80, 95%CI: 1.37–2.36, P < 0.0001; I2 = 7%, P = 0.36).
Hypoxia
Hypoxia was discussed in three studies (9, 15, 18). One of these studies found that hypoxia is significantly associated with delirium in multivariate analysis (18).This association remained statistically significant in pooled analysis (aOR: 0.87, 95%CI: 0.77–0.99, P = 0.04; I2 = 0%, P = 0.62).
Dehydration
Two studies reported dehydration as an independent risk factor for delirium (9, 18). The meta-analysis of the two studies also confirmed the association between dehydration and delirium (aOR: 3.22, 95%CI: 1.75–5.94, P = 0.002; I2 = 18%, P = 0.27).
Cachexia
Cachexia was explored in two studies (17, 18), both of which did not mention the diagnostic criteria of cachexia. A statistical association between cachexia and delirium was found in one multivariable analysis (18) and pooled analysis (aOR: 3.40, 95% CI: 1.69–6.85, P = 0.0006; I2 = 0%, P = 0.95).
Medication
Three studies explored the association between opioid use and delirium (9, 10, 15).Use of opioid was significantly associated with delirium in one multivariable (10) and in pooled analysis (aOR: 2.49, 95%CI: 1.39–4.44, P =0.16; I2 = 0%, P = 0.73).The relationship between steroid use and delirium was investigated in two studies (9, 18). However, no significant association was found in multivariable analyses and the pooled analysis (aOR: 1.1, 95% CI: 0.55–2.21, P = 0.79; I2 = 0%, P = 0.97). The anticholinergic burden was discussed in two studies using the Anticholinergic Risk Scale and Anticholinergic Cognitive Burden scale, respectively (19, 21). The anticholinergic burden was identified as an independent risk factor for delirium in the two studies, which was confirmed in the pooled analysis (aOR: 1.18, 95% CI: 1.07–1.30, P = 0.0008; I2 = 9%, P = 0.29).
Performance Status
The performance status, a patient's ability to perform everyday activities, was routinely evaluated for survival prediction of cancer patients in palliative care settings (22, 23).Three studies (9, 10, 16) used Eastern Cooperative Oncology Group Performance Status Scale (ECOG PS) and one study (18) used Karnofsky score to rate patients' performance status. The association between performance status assessed by ECOG PS and delirium was statistically significant in two multivariable analyses and confirmed in pooled analysis (aOR: 2.54, 95%CI: 1.56–4.14, p = 0.0002; I2 = 21%, P = 0.28). Since only one study reported impaired performance status evaluated by Karnofsky score as an independent risk factor for delirium (18), we did not perform a meta-analysis.
Comorbidity
Comorbidity was investigated in two studies (15, 18). One study used the Charlson Comorbidity Index to evaluate comorbidity (15), while the other study did not report the tool to assess comorbidity. The association between comorbidity and delirium was not statistically significant in multivariate analysis and pooled analysis of the two studies (aOR: 1.05, 95%CI: 0.98–1.13, P = 0.16; I2 = 0%, P = 0.91).
Tumor of the Central Nervous System
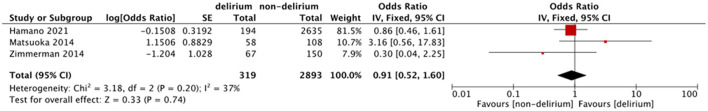
Three studies explored the association between central nervous system metastasis and delirium (9, 15, 19). Another study reported brain tumors as an independent risk factor for delirium (17). However, no significant association was found in the meta-analysis of these four studies (aOR: 1.37, 95% CI: 0.51–3.69, P = 0.54), while a significant heterogeneity was found (I2 = 58%, P = 0.07). As brain tumors included primary brain tumor and brain metastasis, we conducted a sensitivity analysis in which we excluded the study investigating brain tumors and restricted studies to central nervous system metastasis (17). Although the heterogeneity improved (I2 = 37%, P = 0.2), the combined odds ratio was still no significance (aOR: 0.91, 95% CI: 0.52–.6, P = 0.74). The result of sensitive analysis was shown in Figure 3.
Figure 3.
Sensitive analysis of tumor of central nervous system.
Others
The remaining eight independent risk factors were reported only in one study: visual impairment (17), hearing impairment (17), frailty (17), acute renal failure (17), metabolic abnormalities (9), icterus (20), pressure sores (17), and chemotherapeutic drugs penetrating the blood-brain barrier (9) (Table 2). Therefore, we did not perform any pooled analyses among these risk factors.
Discussion
Our systematic review and meta-analysis revealed that older age, male sex, dehydration, hypoxia, cachexia, using opioids, anticholinergic burden, and poor performance status were risk factors for delirium in the palliative care population, while comorbidity, tumor of the central nervous system, and use of steroid were not. The remaining eight independent risk factors including visual impairment, hearing impairment, frailty, acute renal failure, metabolic abnormalities, icterus, pressure sores and chemotherapeutic drugs penetrating the blood-brain barrier reported only in one study were not involved in pooled analyses.
Previous systematic reviews of risk factors for delirium were usually developed from studies of population recruited from surgical, intensive care and general medical settings. No systematic review has specifically evaluated risk factors for delirium in palliative care units. It is noteworthy that palliative care population with poor physical condition and multiple dimensions of distress may have unique risk factors for delirium. The European Society for Medical Oncology (ESMO) Clinical Practice Guideline (CPG) summarized direct risk factors (e.g., tumors of central nervous system, radiation to brain, chemotherapy) and indirect risk factors including physical complications, predisposing comorbidities and medications for delirium in adult cancer patients. However, the ESMO guidance did not use meta-analysis to identify key risk factors (24). A recent systematic review limited to older cancer adults receiving chemotherapy revealed six independent risk factors for delirium, but all the risk factors were not included in pooled analyses (25).Our study was the first systematic review focusing on palliative care population and use meta-analysis to evaluate risk factors for delirium, which filled a research gap on delirium.
Risk factors such as age, hypoxia, visual impairment, hearing impairment, frailty, renal failure, and metabolic abnormalities are well-recognized risk factors for delirium and demonstrated in many studies including our review (26–29). In addition, we found that certain risk factors such as dehydration and cachexia were more consistently associated with delirium in the palliative care population. Dehydration and cachexia are highly prevalent in palliative care units and associated with reduced quality of life and increased mortality (30, 31). Although the underlying mechanisms by which dehydration and cachexia cause delirium are not clear, metabolic insufficiency may be a possible explanation and could be changed by hydration and nutrition (30, 32). Hydration and nutrition have been considered effective non-pharmacologic approaches in preventing delirium in older persons (33). However, there is little sufficient or consistent evidence in palliative care population (34–36). So more clinical trials are needed to provide insight on the roles of hydration and nutrition for delirium in the future.
Symptom management is a key element of palliative care, but many drugs used to control symptoms (e.g., opioids, steroids and anticholinergic drugs) are considered to correlate with delirium (37). Opioids, as the mainstay of cancer pain management, could precipitate delirium by increase dopamine release in the nucleus accumbens through opioid receptors (38, 39), which has been confirmed in palliative care population (40–42). However, the impact of opioid dose on delirium is still controversial. Some studies considered that the risk of delirium was increased with higher doses of opioids (43, 44), but others found that receiving no opioids or very low doses of opioids could increase risk of delirium in patients with severe pain (45–47). So, pain may act as a confounding factor in the relationship between opioids and delirium. Our meta-analysis revealed that using opioids was a risk factor for delirium, but we did not evaluate the correlation between opioid dose and pain due to lack of data. As such, we should consider the possibility that the increased delirium risk may be related to refractory pain following a rapid increment in opioids consumption within a short time, irrespective of basal opioid dose. Therefore, further research is required to clarify the relationships among opioid dose, pain, and delirium. To reduce delirium occurrence, we should take some strategies, such as routine measurements for delirium, regular opioid dose titration, and adequate pain control (44). Furthermore, the opioid rotation could also be beneficial for reducing delirium since the risk of delirium may differ in various opioids due to their specific pharmacokinetic and pharmacodynamic properties (48, 49).
Anticholinergic drugs, such as scopolamine and loperamide are often used in palliative care for the anti-secretory, anti-emetic and anti-diarrheal effects. However, use of anticholinergic drugs may induce cholinergic deficiency and thus affect attention, sleep, and memory (50). The relationship between anticholinergic drugs and delirium has been investigated, but results were conflicting. This might partly attribute to different anticholinergic drug scales used in previous studies. A recent systematic review recommended Anticholinergic Risk Scale as a useful tool to identify patients at an increased risk for delirium (51). We also found that the cumulative anticholinergic burden could increase the risk of delirium. Further studies are needed to confirm it using uniform tool to measure anticholinergic drug burden, such as Anticholinergic Risk Scale. In addition, proper use of anticholinergic drugs and regular medication review may be beneficial for reducing delirium. Concerning steroids, only two studies explored the association with delirium and no correlation was found. Given the small number of included studies without mentioning drug dose and treatment course, the actual relationship between steroids and delirium in the palliative care population still needs further study.
There were still some risk factors such as male sex and poor performance status identified in our review were not exactly same as previous studies (52, 53). This could be partially explained by palliative care population we studied. More evidence is needed to draw definitive conclusions. Regarding comorbidity and tumor of the central nervous system, we did not find any significant association with delirium, which was inconsistent with previous findings (24, 26, 28). The possible reasons may be due to different appraisal of comorbidity in varied studies. Future studies should use standard measurement to evaluate comorbidity, such as Charlson Comorbidity Index. As for tumor of the central nervous system, there was substantial heterogeneity across the included studies. The different impacts of primary brain tumor and metastatic brain tumors on delirium could be responsible for this heterogeneity. Therefore, future studies are necessary to verify respective effect of primary brain tumor and metastatic brain tumor on delirium.
Despite existing uncertain risk factors, our study has important implications for clinical practice. Some risk factors identified in our meta-analysis are modifiable, for example, hypoxia, dehydration, use of opioids, and anticholinergic burden. Management of these risk factors could help prevent and improve delirium. The related strategies include oxygen therapy, hydration, nutrition, and prudent use of opioids and anticholinergic drugs. Besides this, the non-modifiable risk factors, such as age, gender, performance status are still useful for prediction of delirium in palliative care population. Overall, our findings highlight the palliative care population at high risk of delirium and suggest several directions for possible intervention.
Our study has several strengths. It was the first systematic review and meta-analysis to quantitatively summarized risk factors for delirium in the palliative care population. We performed a comprehensive literature search following rigorous selection criteria. All included studies used multivariable techniques to analyze risk factors, which ensured the reliability of the results in our meta-analysis. However, there are some potential limitations of this review. First, relatively few original studies were included by limitations in multivariable analysis. Thus, we could not perform publication bias analysis and subgroup meta-analysis. Second, few studies explicitly stated the variables that were adjusted for in multivariable analysis. Therefore, the combined results should be interpreted with caution. Third, the independent risk factors reported only in one study could not be definitively confirmed without more information.
Conclusion
We found that older age, male sex, dehydration, hypoxia, cachexia, using opioids, anticholinergic burden, and poor performance status were risk factors for delirium in the palliative care population. These risk factors should be considered when developing strategies to prevent delirium in palliative care settings. The interaction among diverse risk factors for delirium need to be explored further.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
DG and JY contributed to conception and design of the study. DG and TL were responsible for the study selection and quality assessment. DG and YZ contributed to data extraction. JY and LG provided overall supervision to the project. DG and CD drafted the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This study was supported by Grants from National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University (Z20191003, Z20192009); 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYJC21005); Sichuan Science and Technology Program (2021YFS0139); and West China Nursing Discipline Development Special Fund Project, Sichuan University (HXHL20014).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2021.772387/full#supplementary-material
References
- 1.Sachdev PS, Blacker D, Blazer DG, Ganguli M, Jeste DV, Paulsen JS, et al. Classifying neurocognitive disorders: the DSM-5 approach. Nat Rev Neurol. (2014) 10:634–42. 10.1038/nrneurol.2014.181 [DOI] [PubMed] [Google Scholar]
- 2.Finucane AM, Jones L, Leurent B, Sampson EL, Stone P, Tookman A, et al. Drug therapy for delirium in terminally ill adults. Cochrane Database Syst Rev. (2020) 1:CD004770. 10.1002/14651858.CD004770.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hosie A, Davidson PM, Agar M, Sanderson CR, Phillips J. Delirium prevalence, incidence, and implications for screening in specialist palliative care inpatient settings: a systematic review. Palliat Med. (2013) 27:486–98. 10.1177/0269216312457214 [DOI] [PubMed] [Google Scholar]
- 4.Watt CL, Momoli F, Ansari MT, Sikora L, Bush SH, Hosie A, et al. The incidence and prevalence of delirium across palliative care settings: a systematic review. Palliat Med. (2019) 33:865–77. 10.1177/0269216319854944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fang C-K, Chen H-W, Liu S-I, Lin C-J, Tsai L-Y, Lai Y-L. Prevalence, detection and treatment of delirium in terminal cancer inpatients: a prospective survey. Jpn J Clin Oncol. (2008) 38:56–63. 10.1093/jjco/hym155 [DOI] [PubMed] [Google Scholar]
- 6.de la Cruz M, Yennu S, Liu D, Wu J, Reddy A, Bruera E. Increased symptom expression among patients with delirium admitted to an acute palliative care unit. J Palliat Med. (2017) 20:638–41. 10.1089/jpm.2016.0315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finucane AM, Lugton J, Kennedy C, Spiller JA. The experiences of caregivers of patients with delirium, and their role in its management in palliative care settings: an integrative literature review. Psychooncology. (2017) 26:291–300. 10.1002/pon.4140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grassi L, Caraceni A, Mitchell AJ, Nanni MG, Berardi MA, Caruso R, et al. Management of delirium in palliative care: a review. Curr Psychiatry Rep. (2015) 17:550. 10.1007/s11920-015-0550-8 [DOI] [PubMed] [Google Scholar]
- 9.Matsuoka H, Yoshiuchi K, Koyama A, Otsuka M, Nakagawa K. Chemotherapeutic drugs that penetrate the blood-brain barrier affect the development of hyperactive delirium in cancer patients. Palliat Support Care. (2014) 13:859–64. 10.1017/S1478951514000765 [DOI] [PubMed] [Google Scholar]
- 10.Matsuo N, Morita T, Matsuda Y, Okamoto K, Matsumoto Y, Kaneishi K, et al. Predictors of delirium in corticosteroid-treated patients with advanced cancer: an exploratory, multicenter, prospective, observational study. J Palliat Med. (2017) 20:352–9. 10.1089/jpm.2016.0323 [DOI] [PubMed] [Google Scholar]
- 11.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. (2009) 339:b2700. 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawlor PG, Rutkowski NA, MacDonald AR, Ansari MT, Sikora L, Momoli F, et al. A scoping review to map empirical evidence regarding key domains and questions in the clinical pathway of delirium in palliative care. J Pain Symptom Manage. (2019) 57:661–81.e12. 10.1016/j.jpainsymman.2018.12.002 [DOI] [PubMed] [Google Scholar]
- 13.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 15.Hamano J, Mori M, Ozawa T, Sasaki J, Kawahara M, Nakamura A, et al. Comparison of the prevalence and associated factors of hyperactive delirium in advanced cancer patients between inpatient palliative care and palliative home care. Cancer Med. (2020) 10:1166–79. 10.1002/cam4.3661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang B, Kim YJ, Suh SW, Son K-L, Ahn GS, Park HY. Delirium and its consequences in the specialized palliative care unit: validation of the Korean version of memorial delirium assessment scale. Psychooncology. (2019) 28:160–6. 10.1002/pon.4926 [DOI] [PubMed] [Google Scholar]
- 17.Seiler A, Schubert M, Hertler C, Schettle M, Blum D, Guckenberger M, et al. Predisposing and precipitating risk factors for delirium in palliative care patients. Palliat Support Care. (2019) 18:437–46. 10.1017/S1478951519000919 [DOI] [PubMed] [Google Scholar]
- 18.Mercadante S, Masedu F, Balzani I, De Giovanni D, Montanari L, Pittureri C, et al. Prevalence of delirium in advanced cancer patients in home care and hospice and outcomes after 1 week of palliative care. Support Care Cancer. (2017) 26:913–9. 10.1007/s00520-017-3910-6 [DOI] [PubMed] [Google Scholar]
- 19.Zimmerman KM, Salow M, Skarf LM, Kostas T, Paquin A, Simone MJ, et al. Increasing anticholinergic burden and delirium in palliative care inpatients. Palliat Med. (2014) 28:335–41. 10.1177/0269216314522105 [DOI] [PubMed] [Google Scholar]
- 20.Morita T, Tei Y, Inoue S. Impaired communication capacity and agitated delirium in the final week of terminally ill cancer patients: prevalence and identification of research focus. J Pain Symptom Manage. (2003) 26:827–34. 10.1016/S0885-3924(03)00287-2 [DOI] [PubMed] [Google Scholar]
- 21.Pasina L, Rizzi B, Nobili A, Recchia A. Anticholinergic load and delirium in end-of-life patients. Eur J Clin Pharmacol. (2021) 77:1419–24. 10.1007/s00228-021-03125-w [DOI] [PubMed] [Google Scholar]
- 22.Dzierzanowski T, Gradalski T, Kozlowski M. Palliative performance scale: cross cultural adaptation and psychometric validation for polish hospice setting. BMC Palliat Care. (2020) 19:1–6. 10.1186/s12904-020-00563-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verweij NM, Schiphorst AHW, Pronk A, van den Bos F, Hamaker ME. Physical performance measures for predicting outcome in cancer patients: a systematic review. Acta Oncol. (2016) 55:1386–91. 10.1080/0284186X.2016.1219047 [DOI] [PubMed] [Google Scholar]
- 24.Bush SH, Lawlor PG, Ryan K, Centeno C, Lucchesi M, Kanji S, et al. Delirium in adult cancer patients: ESMO clinical practice guidelines. Ann Oncol. (2018) 29:iv143–65. 10.1093/annonc/mdy147 [DOI] [PubMed] [Google Scholar]
- 25.Jung P, Puts M, Frankel N, Syed AT, Alam Z, Yeung L, et al. Delirium incidence, risk factors, and treatments in older adults receiving chemotherapy: a systematic review and meta-analysis. J Geriatr Oncol. (2021) 12:352–60. 10.1016/j.jgo.2020.08.011 [DOI] [PubMed] [Google Scholar]
- 26.Wilson JE, Mart MF, Cunningham C, Shehabi Y, Girard TD, MacLullich AMJ, et al. Delirium. Nat Rev Dis Prim. (2020) 6:90. 10.1038/s41572-020-00223-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Persico I, Cesari M, Morandi A, Haas J, Mazzola P, Zambon A, et al. Frailty and delirium in older adults: a systematic review and meta-analysis of the literature. J Am Geriatr Soc. (2018) 66:2022–30. 10.1111/jgs.15503 [DOI] [PubMed] [Google Scholar]
- 28.Mattison MLP. Delirium. Ann Intern Med. (2020) 173:ITC49–64. 10.7326/AITC202010060 [DOI] [PubMed] [Google Scholar]
- 29.Engel GL, Romano J. Delirium, a syndrome of cerebral insufficiency. 1959. J Neuropsychiatry Clin Neurosci. (2004) 16:526–38. 10.1176/jnp.16.4.526 [DOI] [PubMed] [Google Scholar]
- 30.Lacey J, Corbett J, Forni L, Hooper L, Hughes F, Minto G, et al. A multidisciplinary consensus on dehydration: definitions, diagnostic methods and clinical implications. Ann Med. (2019) 51:232–51. 10.1080/07853890.2019.1628352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roeland EJ, Bohlke K, Baracos VE, Bruera E, Del Fabbro E, Dixon S, et al. Management of cancer cachexia: ASCO guideline. J Clin Oncol. (2020) 38:2438–53. 10.1200/JCO.20.00611 [DOI] [PubMed] [Google Scholar]
- 32.da Silva SP, Santos JMO, Costa e Silva MP, Dil da Costa RM, Medeiros R. Cancer cachexia and its pathophysiology: links with sarcopenia, anorexia and asthenia. J Cachexia Sarcopenia Muscle. (2020) 11:619–35. 10.1002/jcsm.12528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oh ES, Fong TG, Hshieh TT, Inouye SK. Delirium in older persons: advances in diagnosis and treatment. JAMA. (2017) 318:1161–74. 10.1001/jama.2017.12067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hui D, Dev R, Bruera E. The last days of life: symptom burden and impact on nutrition and hydration in cancer patients. Curr Opin Support Palliat Care. (2015) 9:346–54. 10.1097/SPC.0000000000000171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galanakis C, Mayo NE, Gagnon B. Assessing the role of hydration in delirium at the end of life. Curr Opin Support Palliat Care. (2011) 5:169–73. 10.1097/SPC.0b013e3283462fdc [DOI] [PubMed] [Google Scholar]
- 36.Lokker ME, van der Heide A, Oldenmenger WH, van der Rijt CCD, van Zuylen L. Hydration and symptoms in the last days of life. BMJ Support Palliat Care. (2019) 11:335–43. 10.1136/bmjspcare-2018-001729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caraceni A. Drug-associated delirium in cancer patients. Eur J Cancer Suppl. (2013) 11:233–40. 10.1016/j.ejcsup.2013.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomkins DM, Sellers EM. Addiction and the brain: the role of neurotransmitters in the cause and treatment of drug dependence. C Can Med Assoc J. (2001) 164:817−21. [PMC free article] [PubMed] [Google Scholar]
- 39.Cowen MS, Lawrence AJ. The role of opioid-dopamine interactions in the induction and maintenance of ethanol consumption. Prog Neuropsychopharmacol Biol Psychiatry. (1999) 23:1171–212. 10.1016/S0278-5846(99)00060-3 [DOI] [PubMed] [Google Scholar]
- 40.Senel G, Uysal N, Oguz G, Kaya M, Kadioullari N, Koçak N, et al. Delirium frequency and risk factors among patients with cancer in palliative care unit. Am J Hosp Palliat Med. (2017) 34:282–6. 10.1177/1049909115624703 [DOI] [PubMed] [Google Scholar]
- 41.Lim KH, Nguyen NN, Qian Y, Williams JL, Lui DD, Bruera E, et al. Frequency, outcomes, and associated factors for opioid-induced neurotoxicity in patients with advanced cancer receiving opioids in inpatient palliative care. J Palliat Med. (2018) 21:1698–704. 10.1089/jpm.2018.0169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thwaites D, McCann S, Broderick P. Hydromorphone neuroexcitation. J Palliat Med. (2004) 7:545–50. 10.1089/jpm.2004.7.545 [DOI] [PubMed] [Google Scholar]
- 43.Gaudreau JD, Gagnon P, Harel F, Roy MA, Tremblay A. Psychoactive medications and risk of delirium in hospitalized cancer patients. J Clin Oncol. (2005) 23:6712–8. 10.1200/JCO.2005.05.140 [DOI] [PubMed] [Google Scholar]
- 44.Duprey MS, Dijkstra-Kersten SMA, Zaal IJ, Briesacher BA, Saczynski JS, Griffith JL, et al. Opioid use increases the risk of delirium in critically ill adults independently of pain. Am J Respir Crit Care Med. (2021) 204):566–72. 10.1164/rccm.202010-3794OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morrison RS, Magaziner J, Gilbert M, Koval KJ, McLaughlin MA, Orosz G, et al. Relationship between pain and opioid analgesics on the development of delirium following hip fracture. J Gerontol A Biol Sci Med Sci. (2003) 58:76–81. 10.1093/gerona/58.1.M76 [DOI] [PubMed] [Google Scholar]
- 46.Denny DL, Lindseth GN. Pain, opioid intake, and delirium symptoms in adults following joint replacement surgery. West J Nurs Res. (2020) 42:165–76. 10.1177/0193945919849096 [DOI] [PubMed] [Google Scholar]
- 47.Daoust R, Paquet J, Boucher V, Pelletier M, Gouin É, Émond M. Relationship between pain, opioid treatment, and delirium in older emergency department patients. Acad Emerg Med. (2020) 27:708–16. 10.1111/acem.14033 [DOI] [PubMed] [Google Scholar]
- 48.Bruera E, Franco JJ, Maltoni M, Watanabe S, Suarez-Almazor M. Changing pattern of agitated impaired mental status in patients with advanced cancer: association with cognitive monitoring, hydration, and opioid rotation. J Pain Symptom Manage. (1995) 10:287–91. 10.1016/0885-3924(95)00005-J [DOI] [PubMed] [Google Scholar]
- 49.Swart LM, van der Zanden V, Spies PE, de Rooij SE, van Munster BC. The comparative risk of delirium with different opioids: a systematic review. Drugs Aging. (2017) 34:437–43. 10.1007/s40266-017-0455-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hshieh TT, Fong TG, Marcantonio ER, Inouye SK. Cholinergic deficiency hypothesis in delirium: a synthesis of current evidence. J Gerontol A Biol Sci Med Sci. (2008) 63:764–72. 10.1093/gerona/63.7.764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Egberts A, Moreno-Gonzalez R, Alan H, Ziere G, Mattace-Raso FUS. Anticholinergic drug burden and delirium: a systematic review. J Am Med Dir Assoc. (2021) 22:65–73.e4. 10.1016/j.jamda.2020.04.019 [DOI] [PubMed] [Google Scholar]
- 52.Zhu C, Wang B, Yin J, Xue Q, Gao S, Xing L, et al. Risk factors for postoperative delirium after spinal surgery: a systematic review and meta-analysis. Aging Clin Exp Res. (2020) 32:1417–34. 10.1007/s40520-019-01319-y [DOI] [PubMed] [Google Scholar]
- 53.Ahmed S, Leurent B, Sampson EL. Risk factors for incident delirium among older people in acute hospital medical units: a systematic review and meta-analysis. Age Ageing. (2014) 43:326–33. 10.1093/ageing/afu022 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.