Abstract
Environmental light that animal receives (i.e., photoperiod and light intensity) has recently been shown that it affects avian central nervous system for the physiological responses to the environment by up or downregulation of dopamine and serotonin activities, and this, in turn, affects the reproductive function and stress-related behavior of birds. In this study, the author speculated on the intriguing possibility that one of the proposed avian deep-brain photoreceptors (DBPs), i.e., melanopsin (Opn4), may play roles in the dual sensory-neurosecretory cells in the hypothalamus, midbrain, and brain stem for the behavior and physiological responses of birds by light. Specifically, the author has shown that the direct light perception of premammillary nucleus dopamine-melatonin (PMM DA-Mel) neurons is associated with the reproductive activation in birds. Although further research is required to establish the functional role of Opn4 in the ventral tegmental area (VTA), dorsal raphe nucleus, and caudal raphe nucleus in the light perception and physiological responses of birds, it is an exciting prospect because the previous results in birds support this hypothesis that Opn4 in the midbrain DA and serotonin neurons may play significant roles on the light-induced welfare of birds.
Keywords: light, melanopsin (Opn4), premammillary nucleus, ventral tegmental area, raphe nucleus, dopamine, serotonin, welfare
Introduction
Light perception and integration of photic information in the diurnal animals are critical for their proper adaptation to the environment, and therefore, animals can respond to daily and annual environmental change (Chmura et al., 2019; Hussein et al., 2021). Light plays a central role in modulating animal behavior and is a critical environmental factor that can affect the physiological processes, performance, and welfare of many animals and birds (Wilson and Cunningham, 1980; Manser, 1996; Deep et al., 2010; Fernandes et al., 2013; Aulsebrook et al., 2021). The physiological roles and effects of light include facilitating sight, regulating reproductive hormone release, and affecting social behavior. The most visible physiological effects of light on birds are the effect of photoperiod and light intensity on the seasonal reproduction, health, and behavior of birds (Deep et al., 2010; Olanrewaju et al., 2018; ViviD and Bentley, 2018).
Several studies provide evidence that light can affect the central physiology of animals independent of retinal function (Chiu et al., 1975; Routtenberg et al., 1978; Underwood et al., 1984; Wade et al., 1988; Fernandes et al., 2013). In avian species, photoperiodic synchronization is achieved independently of the pineal melatonin through direct light perception by avian deep-brain photoreceptors (DBPs), which project directly to the median eminence near the pars tuberalis (PT) in the anterior pituitary (Kang et al., 2010; Nakane et al., 2010; Chmura et al., 2019). However, evidence is not available regarding the pathway used by the photoperiodic message to reach the PT independently of pineal melatonin in mammals. The melatonin-independent photoperiodic entrainment of the annual thyroid-stimulating hormone (TSH) rhythm was reported in the European hamster, suggesting the presence of the non-visual DBPs in mammals (Saenz De Miera et al., 2018). Interestingly, encephalopsin (Opn3) was found to be expressed in different areas of the rodent brain, indicating a potential role of Opn3 in the non-visual photic process due to the changes in light (Blackshaw and Snyder, 1999; Nissila et al., 2012).
The initiation of light-induced physiological change is particularly important for diurnal animals such as mammals and birds. However, those within the avian brain have not been studied extensively. In this study, the author explored and derived how non-visual photoreceptive cells in the avian brain may connect to circuits controlling the aspects of feeding and emotional behaviors, which will provide an intriguing perspective on how environmental light can be a critical cue for the welfare of birds.
Effect of Light on the Behavior and Physiology of Birds
Light information characterizing the particular day length (i.e., photoperiod) and intensity can be stored within the organism and subsequently used to provide time signals for the adjustments of the physiological behavior of animals (Farner and Wingfield, 1980; Gwinner, 1989; Brandstatter et al., 2000). Animals must be able to discriminate between short and long days to perform photoperiodic time measurement. The differences of circadian changes related to the reproductive activation between mammals and avian species were well-reviewed by recent reports (Ikegami and Yoshimura, 2013; Kuenzel et al., 2015; ViviD and Bentley, 2018). In comparison with mammals, the avian circadian pacemaking system seems to be more complicated, being composed of at least three major components containing autonomous circadian oscillators as follows: the pineal gland, the retina, and a central nervous hypothalamic component possibly equivalent to the mammalian suprachiasmatic nucleus (SCN). The avian pineal organ contains photoreceptors with different photopigments including melanopsin (Opn4, an opsin-based photopigment), and synthesizes and secretes melatonin which is regulated by light (Sato, 2001; Kang et al., 2007, 2010).
The effects of artificial light on wild birds are critical for their various biological responses. Especially, artificial light at night alters natural light/dark cycles to be problematic for many avian species, suggesting that disrupting circadian rhythms causes multiple direct and indirect physiological consequences of birds because the unnatural sleep deprivation is associated with cardiovascular disease and endocrine disruption and has a profound effect on the circadian expression of genes associated with the immune and stress response (Dominoni et al., 2016).
Light intensity has a significant effect on the behavior, diurnal activity, and immune function of chickens (Blatchford et al., 2009). When birds are in the higher light intensity, they show a more dramatic circadian rhythm, spending more time active, eating and drinking, walking, foraging, and preening during the photophase (light), and resting more time during the scotophase (dark) compared with birds kept at lower light intensities (Alvino et al., 2009; Blatchford et al., 2009; Rault et al., 2017). The rhythms of the multiunit neuronal activity in the premammillary nucleus (PMM) of the caudal hypothalamus of temperate zone bird were demonstrated to show the photoperiod-dependent durations of high activity (Kang et al., 2007; El Halawani et al., 2009). Moreover, in the follow-up confirmation study, low light intensity (10 lux) could not activate PMM in the turkey hypothalamus even in long-day photoperiod (Moore et al., 2018), indicating that light intensity is also a key stimulant of initiation of avian reproductive function as well as photoperiod in avian species. Melanopsin (Opn4) is one of the DBPs which was characterized in the PMM of female turkey (Kang et al., 2007, 2009, 2010; El Halawani et al., 2009; Leclerc et al., 2010).
Avian DBP Opn4 For Light Perception
The primary system to detect avian photoperiodic information has been thought to be non-retinal, non-pineal DBPs (Benoit and Assenmacher, 1953; Menaker et al., 1970; Yokoyama et al., 1978). Three DBPs (i.e., Opn4, Opsin 5, and Vertebrate ancient opsin) were proposed in the avian brain that responds to photoperiodic information affecting the onset and development of the reproductive function, and all three types of DBPs appear to be involved in priming the neuroendocrine system to activate the reproductive functions of birds (Halford et al., 2009; Kang et al., 2010; Nakane et al., 2010; Kang and Kuenzel, 2015). In this study, the author focused only on Opn4.
Melanopsin (Opn4) was first discovered by Provencio et al. (1998) in the photosensitive melanophores of Xenopus skin. In situ hybridization studies demonstrated that Opn4 mRNA is also expressed in other photosensitive tissues, such as the retina, the magnocellular preoptic nucleus, and the SCN in the brain (Brown and Robinson, 2004). Later, several studies make Opn4 an attractive candidate for circadian photopigment and non-visual photic responses (Gooley et al., 2003; Hannibal et al., 2013). In non-mammalian vertebrates, Opn4 has two isoforms, namely, mammal-like Opn4m and Xenopus-like Opn4x (Bellingham et al., 2006).
Avian Opn4 expression and functional role in the photoperiodic activation of reproductive function were reported in several avian species (Bailey and Cassone, 2005; Kang et al., 2010; Potter et al., 2018; Nakane et al., 2019). A recent study on Japanese quail showed the possible functional role of Opn4 in the mediobasal hypothalamus by evaluating an action spectrum for the expression of photoperiodically controlled beta subunit of TSH in the PT of the pituitary gland (Nakane et al., 2019). Interestingly, it has been suggested that Opn4 may have additional physiological roles beyond the reproductive system in the Pekin duck (Van Wyk and Frakey, 2021).
In mammals, specific populations within PMM were genetically defined as dopaminergic (DAergic) neurons and activated in specific social contexts and functions via glutamate release to regulate social interactions; moreover, mammalian PMM has a projection of the catecholaminergic input from locus coeruleus (LoC) (Sobrinho and Canteras, 2011; Soden et al., 2016).
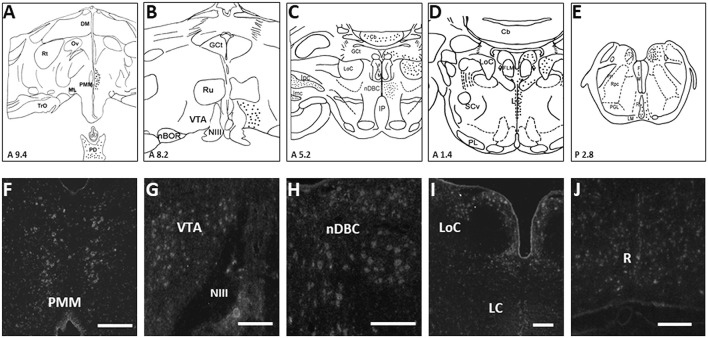
Avian PMM neurons co-express both dopamine and melatonin (DA-MEL, Kang et al., 2007) and are activated by light provided during the photosensitive phase for reproductive stimulation (Thayananuphat et al., 2007b). The regulation of rhythmic DAergic/melatoninergic (MELergic) activity may involve clock genes, which localize and cycle rhythmically within DA/MEL neurons (Leclerc et al., 2010). Moreover, light pulses that are provided during the photosensitive phase for reproductive stimulation activate these neurons, as indicated by the induction of c-fos (Thayananuphat et al., 2007a) and the upregulation of Cry1 and Per3 genes (Leclerc et al., 2010). Dopamine and MEL expressing neurons of avian PMM have been shown to have dual functionality, which consists of sensory of light information by Opn4 and neurosecretory functions by the diurnal activities of DA and MEL (Kang et al., 2007, 2009, 2010; Figures 1A,F), suggesting that PMM may be a conserved dual sensory-neurosecretory unit in avian species as suggested in the lower vertebrates (Tessmar-Raible et al., 2007; Conzelmann et al., 2013).
Figure 1.
Schematic drawings of rostral-to-caudal (A–E) coronal sections, showing the distribution of turkey Xenopus-like melanopsin (tOpn4x) expression labeled neurons (filled circles) of the turkey hen. Coronal illustrations were drawn from an unpublished turkey brain atlas with nomenclature taken from a chicken atlas (Kuenzel and Masson, 1988) and the revised nomenclature for avian brains (Reiner et al., 2004). Representative photomicrographs (F–J) showing the distribution of tOpn4x mRNA labeled neurons in the PMM, VTA, nDBC, LoC, LC, and R (refer to the abbreviations given below). A specific tOpn4x cRNA probe was used for in situ hybridization histochemistry (ISH). Darkfield photomicrographs of turkey brain sections processed for ISH with 33P-labeled tOpn4 antisense cRNA probes. Scale bar: 100 μm (G,H,J), 200 μm (F,I). The following abbreviations are used in the figure: Cb, cerebellum; DM, dorsomedial hypothalamic nucleus; MLF, medial longitudinal fasciculus; GCt, mesencephalic central gray; Ipc, parvocellular nucleus Isthmi; Imc, magnocellular nucleus isthmi; IP, interpeduncular nucleus; LC, caudal linear nucleus; LM, medial lemniscus; LoC, locus coeruleus; ML, lateral mammillary nucleus; nBOR, nucleus of the basal optic root; nDBC, nucleus decussationis brachiorum conjunctivorum; NIII, third cranial nerve; nTS, nucleus of the solitary tract; Ov, nucleus ovoidalis; PD, pars distalis; PH, plexus of Horsley; PL, lateral pontine nuclei; PMM, premammillary nucleus; R, raphe nucleus; Rpc, parvocellular reticular nucleus; Rt, nucleus rotundus; Ru, nucleus ruber; SCv, nucleus subcoeruleus ventralis; TrO, tractus opticus; VTA, ventral tegmental area (Modified from Kang et al., 2010).
Opn4 Expression in the Dopaminergic and Serotonergic Nuclei and its Possible Roles in the Welfare of Birds
Photoreceptor Opn4 was observed in the brain areas that are associated with DA and serotonin [5-hydroxytryptamine (5-HT)] in birds (Kang et al., 2010), which were not appreciated hitherto (Figures 1B–E,G–J). It may be of interest to speculate that direct light perception may be involved in the physiological function of DA and 5-HT neurons in the avian brain. Light-induced feed intake in birds may be directly stimulated by central Opn4 because tryptophan hydroxylase 2 (TPH2: rate-limiting enzyme of serotonin biosynthesis) in the dorsal raphe nucleus (DRN) is also associated with food intake and energy balance (Flores et al., 2018; Liu et al., 2021).
Dopamine is predominantly synthesized in the ventral tegmental area (VTA) and substantia nigra (SN) of the midbrain. Dopaminergic neurons in the VTA integrate complex inputs to convert multiple signals that influence motivated behaviors via various neural projections underlying the different functions of these neurons in psychological processes and brain diseases (Beier et al., 2015; Bouarab et al., 2019). In mammals, the important roles of DA neurons were discovered in numerous behavioral or psychological processes other than rewards, such as aversion, depression, fear, social behavior, stress, and movement coordination (Pani et al., 2000; Bromberg-Martin et al., 2010; Zweifel et al., 2011; Lammel et al., 2012; Chaudhury et al., 2013; Matsumoto and Takada, 2013; Friedman et al., 2014; Walsh et al., 2014; Grace, 2016; Holly and Miczek, 2016). The major brain structures associated with positive emotion are the amygdala complex and nucleus accumbens (Janak and Tye, 2015). Importantly, the nucleus accumbens is the terminal site of the DAergic mesolimbic axis originating in the VTA (Ikemoto, 2007; Holly and Miczek, 2016). Ventral tegmental area neurons have long been implicated in feeding behaviors, and major neurons are DAergic neurons (about 60% of VTA neurons) (Ungless and Grace, 2012; Meye and Adan, 2014). In addition to DAergic neurons, VTA also contains gamma-aminobutyric acid (GABA) and glutamate neurons that account for about 35 and 2–3% of VTA neurons, respectively (Nair-Roberts et al., 2008; Taylor et al., 2014; Miranda-Barrientos et al., 2021). Besides DA, GABA, and glutamate neurons, several studies reported serotonergic (5-HTergic) neurons in the VTA of mammalian and avian brains (Kang et al., 2009; Carkaci-Salli et al., 2011; Morales and Margolis, 2017; Smith et al., 2019). Interestingly, the optogenetic activation of VTA GABAergic neurons stimulates food intake and anxiety-like behavior in mice (Chen et al., 2020).
The avian VTA contains cell bodies that label positively for tyrosine hydroxylase (TH; the rate-limiting enzyme in catecholamine biosynthesis) but not DA-β-hydroxylase (which is involved in converting DA to norepinephrine), indicating that the major population of avian VTA is DAergic neurons (Kang et al., 2009, Figure 2). The electrophysiological and pharmacological properties of VTA neurons have been studied using whole-cell recordings in the brain slices of birds (zebra finch) (Gale and Perkel, 2006), showing that zebra finch VTA DAergic neurons possess physiological properties very similar to those of mammalian DAergic neurons and also contain non-DAergic neurons similar to GABAergic neurons in the mammalian VTA. In addition, avian VTA DAergic neurons densely innervate the striatal areas of the basal ganglia and project more moderately to several other regions of the telencephalon, and the pharmacological agents and lesions targeting the DAergic system have many similar behavioral effects in birds and mammals (Durstewitz et al., 1999). Therefore, these results provide strong evidence for anatomical, physiological, and functional similarities between the VTA DAergic systems of mammals and birds (Gale and Perkel, 2006).
Figure 2.
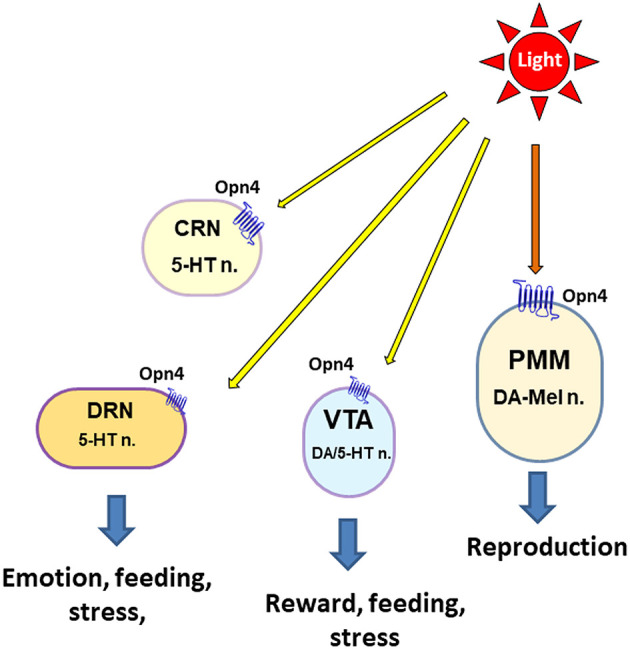
Schematic overview of extraocular light perception in the midbrain and brain stem of avian species for the physiological response. The following abbreviations are used in the figure: 5-HT, serotonin; DA, dopamine; CRN, caudal raphe nucleus; DRN, dorsal raphe nucleus; n, neuron; PMM, premammillary nucleus; VTA, ventral tegmental area.
The distribution of 5-HT immunoreactivity and TPH2 mRNA expression was reported in the avian brain such as VTA, DRN, and caudal raphe nucleus (CRN) (Cozzi et al., 1991; Challet et al., 1996; Kang et al., 2009). The presence of TPH2-positive neurons in the VTA may provide an area of further investigation involving interactions between 5-HTergic and DAergic systems within the VTA (Carkaci-Salli et al., 2011). Serotonin is one of the main neurotransmitters to regulate the parasympathetic nervous system (PNS) and is involved in emotional states caused by stress, pain, or the availability of food (Chamas et al., 1999; Mosienko et al., 2012), while DA acts on the sympathetic nervous system (SNS). Serotonergic neurons can be identified based on the presence of TPH2 mRNA expression, and thereby the TPH2 expression levels can be used as a specific marker for 5-HT generation (Chamas et al., 1999; Kang et al., 2009, 2020; Carkaci-Salli et al., 2011; Liu et al., 2021). The DRN is a heterogeneous brain stem nucleus located in the midbrain and pons, which is involved in the control of various physiological functions, such as learning and memory (Michelsen et al., 2008). The most abundant neurotransmitter in the DRN is serotonin, and the TPH2 mRNA expression was observed in the avian DRN such as nucleus decussationis brachiorum conjunctivorum (nDBC), LoC, and caudal linear nucleus (LC) (Kang et al., 2009, Figure 2).
The presence of both DA and 5-HT systems in the VTA indicates that avian VTA is the critical area of the midbrain involved in the welfare of avian species (Kang et al., 2009, 2020; Carkaci-Salli et al., 2011). Several studies have proposed that DA and 5-HT could serve as positive indicators of animal welfare (Algers et al., 2007; Boissy et al., 2007; Polter and Kauer, 2014). Stress and negative experience alter the 5-HT metabolism in the brain by stimulating 5-HT turnover in the areas innervated by 5-HTergic neurons (Clement et al., 1993; Inoue et al., 1994; Amat et al., 1998). In mammals, repeated immobilization stress increased the TPH2 gene expression levels in the raphe nuclei of the brain stem (Chamas et al., 1999; Walther et al., 2003), indicating the elevation of 5-HT metabolism. In the recent study of DA and 5-HT activity, 5-HTergic and DAergic activities respond differently to light intensity and light intensity preference, and these results suggest the beneficial effects of dual intensity lighting program on the protection of the central nervous system of birds (Kang et al., 2020).
Perspective
Animals explore their surroundings to secure resources such as food, water, and shelter, and the regulation of their reproductive system for producing offspring depends on the environment day-and-night light condition.
The data discussed in this study and the previous light intensity study (Kang et al., 2020) suggest the possible roles of Opn4 in the VTA and DRN/CRN on the direct light perception for the physiological responses of birds such as feeding behavior and welfare. Although this observation makes the hypothesis that Opn4 is a positive candidate photoreceptor associated with direct light perception in the ancient brain (i.e., hypothalamus and brain stem) of birds, the functional role of Opn4 should be tested in the future study.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author/s.
Author Contributions
SK contributed to the conception, drafted the manuscript, edited and revised the manuscript, and approved the final version of the manuscript.
Funding
This study was financially supported by the US Poultry and Egg Association Research Grant.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The author thanks his current and past collaborators Dr. Karen Christensen, Dr. Wayne J. Kuenzel, and Dr. Mohamed El Halawani for their valuable contributions to the research underpinning this study.
References
- Algers B., Lundeheim N., Boyle L. A., Broom D. M., Eliasson-Selling L., Holmgren N., et al. (2007). Thoughts on farm animal welfare. J. Am. Vet. Med. Assoc. 230, 185−186; author reply 186–187. [PubMed] [Google Scholar]
- Alvino G. M., Blatchford R. A., Archer G. S., Mench J. A. (2009). Light intensity during rearing affects the behavioural synchrony and resting patterns of broiler chickens. Br. Poult. Sci. 50, 275–283. 10.1080/00071660902942775 [DOI] [PubMed] [Google Scholar]
- Amat J., Matus-Amat P., Watkins L. R., Maier S. F. (1998). Escapable and inescapable stress differentially alter extracellular levels of 5-HT in the basolateral amygdala of the rat. Brain Res. 812, 113–120. 10.1016/s0006-8993(98)00960-3 [DOI] [PubMed] [Google Scholar]
- Aulsebrook A. E., Johnsson R. D., Lesku J. A. (2021). Light, sleep and performance in diurnal birds. Clocks Sleep 3, 115–131. 10.3390/clockssleep3010008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey M. J., Cassone V. M. (2005). Melanopsin expression in the chick retina and pineal gland. Brain Res. Mol. Brain Res. 134, 345–348. 10.1016/j.molbrainres.2004.11.003 [DOI] [PubMed] [Google Scholar]
- Beier K. T., Steinberg E. E., Deloach K. E., Xie S., Miyamichi K., Schwarz L., et al. (2015). Circuit architecture of VTA dopamine neurons revealed by systematic input-output mapping. Cell 162, 622–634. 10.1016/j.cell.2015.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellingham J., Chaurasia S. S., Melyan Z., Liu C., Cameron M. A., Tarttelin E. E., et al. (2006). Evolution of melanopsin photoreceptors: discovery and characterization of a new melanopsin in nonmammalian vertebrates. PLoS Biol. 4:e254. 10.1371/journal.pbio.0040254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit J., Assenmacher I. (1953). [Role of superficial and deep photoreceptors in photostimulation of gonads in birds]. J. Physiol. (Paris) 45, 34–37. [PubMed] [Google Scholar]
- Blackshaw S., Snyder S. H. (1999). Encephalopsin: a novel mammalian extraretinal opsin discretely localized in the brain. J. Neurosci. 19, 3681–3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatchford R. A., Klasing K. C., Shivaprasad H. L., Wakenell P. S., Archer G. S., Mench J. A. (2009). The effect of light intensity on the behavior, eye and leg health, and immune function of broiler chickens. Poult. Sci. 88, 20–28. 10.3382/ps.2008-00177 [DOI] [PubMed] [Google Scholar]
- Boissy A., Manteuffel G., Jensen M. B., Moe R. O., Spruijt B., Keeling L. J., et al. (2007). Assessment of positive emotions in animals to improve their welfare. Physiol. Behav. 92, 375–397. 10.1016/j.physbeh.2007.02.003 [DOI] [PubMed] [Google Scholar]
- Bouarab C., Thompson B., Polter A. M. (2019). VTA GABA neurons at the interface of stress and reward. Front. Neural Circuits 13, 78. 10.3389/fncir.2019.00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandstatter R., Kumar V., Abraham U., Gwinner E. (2000). Photoperiodic information acquired and stored in vivo is retained in vitro by a circadian oscillator, the avian pineal gland. Proc. Natl. Acad. Sci. U.S.A. 97, 12324–12328. 10.1073/pnas.200354997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg-Martin E. S., Matsumoto M., Hikosaka O. (2010). Dopamine in motivational control: rewarding, aversive, and alerting. Neuron 68, 815–834. 10.1016/j.neuron.2010.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. L., Robinson P. R. (2004). Melanopsin–shedding light on the elusive circadian photopigment. Chronobiol. Int. 21, 189–204. 10.1081/cbi-120037816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carkaci-Salli N., Salli U., Kuntz-Melcavage K. L., Pennock M. M., Ozgen H., Tekin I., et al. (2011). TPH2 in the ventral tegmental area of the male rat brain. Brain Res. Bull. 84, 376–380. 10.1016/j.brainresbull.2011.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challet E., Miceli D., Pierre J., Reperant J., Masicotte G., Herbin M., et al. (1996). Distribution of serotonin-immunoreactivity in the brain of the pigeon (Columba livia). Anat. Embryol. (Berl.) 193, 209–227. 10.1007/BF00198325 [DOI] [PubMed] [Google Scholar]
- Chamas F., Serova L., Sabban E. L. (1999). Tryptophan hydroxylase mRNA levels are elevated by repeated immobilization stress in rat raphe nuclei but not in pineal gland. Neurosci. Lett. 267, 157–160. 10.1016/s0304-3940(99)00340-7 [DOI] [PubMed] [Google Scholar]
- Chaudhury D., Walsh J. J., Friedman A. K., Juarez B., Ku S. M., Koo J. W., et al. (2013). Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature 493, 532–536. 10.1038/nature11713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Lu Y. P., Chen H. Y., Huang S. N., Guo Y. R., Zhang J. Y., et al. (2020). Ventral tegmental area GABAergic neurons induce anxiety-like behaviors and promote palatable food intake. Neuropharmacology 173, 108114. 10.1016/j.neuropharm.2020.108114 [DOI] [PubMed] [Google Scholar]
- Chiu P. S., Lauber J. K., Kinnear A. (1975). Dimensional and physiological lesions in the chick eye as influenced by the light environment. Proc. Soc. Exp. Biol. Med. 148, 1223–1228. 10.3181/00379727-148-38721 [DOI] [PubMed] [Google Scholar]
- Chmura H. E., Wingfield J. C., Hahn T. P. (2019). Non-photic environmental cues and avian reproduction in an era of global change. J. Avian Biol. 51. 10.1111/jav.02243 [DOI] [Google Scholar]
- Clement H. W., Schafer F., Ruwe C., Gemsa D., Wesemann W. (1993). Stress-induced changes of extracellular 5-hydroxyindoleacetic acid concentrations followed in the nucleus raphe dorsalis and the frontal cortex of the rat. Brain Res. 614, 117–124. 10.1016/0006-8993(93)91024-m [DOI] [PubMed] [Google Scholar]
- Conzelmann M., Williams E. A., Tunaru S., Randel N., Shahidi R., Asadulina A., et al. (2013). Conserved MIP receptor-ligand pair regulates Platynereis larval settlement. Proc. Natl. Acad. Sci. U.S.A. 110, 8224–8229. 10.1073/pnas.1220285110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzi B., Viglietti-Panzica C., Aste N., Panzica G. C. (1991). The serotoninergic system in the brain of the Japanese quail. An immunohistochemical study. Cell Tissue Res. 263, 271–284. 10.1007/BF00318769 [DOI] [PubMed] [Google Scholar]
- Deep A., Schwean-Lardner K., Crowe T. G., Fancher B. I., Classen H. L. (2010). Effect of light intensity on broiler production, processing characteristics, and welfare. Poult. Sci. 89, 2326–2333. 10.3382/ps.2010-00964 [DOI] [PubMed] [Google Scholar]
- Dominoni D. M., Borniger J. C., Nelson R. J. (2016). Light at night, clocks and health: from humans to wild organisms. Biol. Lett. 12, 20160015. 10.1098/rsbl.2016.0015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durstewitz D., Kroner S., Gunturkun O. (1999). The dopaminergic innervation of the avian telencephalon. Prog. Neurobiol. 59, 161–195. 10.1016/s0301-0082(98)00100-2 [DOI] [PubMed] [Google Scholar]
- El Halawani M. E., Kang S. W., Leclerc B., Kosonsiriluk S., Chaiseha Y. (2009). Dopamine-melatonin neurons in the avian hypothalamus and their role as photoperiodic clocks. Gen. Comp. Endocrinol. 163, 123–127. 10.1016/j.ygcen.2008.11.030 [DOI] [PubMed] [Google Scholar]
- Farner D. S., Wingfield J. C. (1980). Reproductive endocrinology of birds. Annu. Rev. Physiol. 42, 457–472. 10.1146/annurev.ph.42.030180.002325 [DOI] [PubMed] [Google Scholar]
- Fernandes A. M., Fero K., Driever W., Burgess H. A. (2013). Enlightening the brain: linking deep brain photoreception with behavior and physiology. Bioessays 35, 775–779. 10.1002/bies.201300034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores R. A., Da Silva E. S., Ribas A. S., Taschetto A. P. D., Zampieri T. T., Donato J., Jr., et al. (2018). Evaluation of food intake and Fos expression in serotonergic neurons of raphe nuclei after intracerebroventricular injection of adrenaline in free-feeding rats. Brain Res. 1678, 153–163. 10.1016/j.brainres.2017.10.021 [DOI] [PubMed] [Google Scholar]
- Friedman A. K., Walsh J. J., Juarez B., Ku S. M., Chaudhury D., Wang J., et al. (2014). Enhancing depression mechanisms in midbrain dopamine neurons achieves homeostatic resilience. Science 344, 313–319. 10.1126/science.1249240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale S. D., Perkel D. J. (2006). Physiological properties of zebra finch ventral tegmental area and substantia nigra pars compacta neurons. J. Neurophysiol. 96, 2295–2306. 10.1152/jn.01040.2005 [DOI] [PubMed] [Google Scholar]
- Gooley J. J., Lu J., Fischer D., Saper C. B. (2003). A broad role for melanopsin in nonvisual photoreception. J. Neurosci. 23, 7093–7106. 10.1523/JNEUROSCI.23-18-07093.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace A. A. (2016). Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat. Rev. Neurosci. 17, 524–532. 10.1038/nrn.2016.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwinner E. (1989). Photoperiod as a modifying and limiting factor in the expression of avian circannual rhythms. J. Biol. Rhythms 4, 237–250. 10.1177/074873048900400210 [DOI] [PubMed] [Google Scholar]
- Halford S., Pires S. S., Turton M., Zheng L., Gonzalez-Menendez I., Davies W. L., et al. (2009). VA opsin-based photoreceptors in the hypothalamus of birds. Curr. Biol. 19, 1396–1402. 10.1016/j.cub.2009.06.066 [DOI] [PubMed] [Google Scholar]
- Hannibal J., Georg B., Fahrenkrug J. (2013). Differential expression of melanopsin mRNA and protein in Brown Norwegian rats. Exp. Eye Res. 106, 55–63. 10.1016/j.exer.2012.11.006 [DOI] [PubMed] [Google Scholar]
- Holly E. N., Miczek K. A. (2016). Ventral tegmental area dopamine revisited: effects of acute and repeated stress. Psychopharmacology (Berl). 233, 163–186. 10.1007/s00213-015-4151-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein A. a. A., Bloem E., Fodor I., Baz E. S., Tadros M. M., et al. (2021). Slowly seeing the light: an integrative review on ecological light pollution as a potential threat for mollusks. Environ. Sci. Pollut. Res. Int. 28, 5036–5048. 10.1007/s11356-020-11824-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami K., Yoshimura T. (2013). Seasonal time measurement during reproduction. J. Reprod. Dev. 59, 327–333. 10.1262/jrd.2013-035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S. (2007). Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res. Rev. 56, 27–78. 10.1016/j.brainresrev.2007.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T., Tsuchiya K., Koyama T. (1994). Regional changes in dopamine and serotonin activation with various intensity of physical and psychological stress in the rat brain. Pharmacol. Biochem. Behav. 49, 911–920. 10.1016/0091-3057(94)90243-7 [DOI] [PubMed] [Google Scholar]
- Janak P. H., Tye K. M. (2015). From circuits to behaviour in the amygdala. Nature 517, 284–292. 10.1038/nature14188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S. W., Christensen K. D., Aldridge D., Kuenzel W. J. (2020). Effects of light intensity and dual light intensity choice on plasma corticosterone, central serotonergic and dopaminergic activities in birds, Gallus gallus. Gen. Comp. Endocrinol. 285, 113289. 10.1016/j.ygcen.2019.113289 [DOI] [PubMed] [Google Scholar]
- Kang S. W., Kuenzel W. J. (2015). Deep-brain photoreceptors (DBPs) involved in the photoperiodic gonadal response in an avian species, Gallus gallus. Gen. Comp. Endocrinol. 211, 106–113. 10.1016/j.ygcen.2014.11.020 [DOI] [PubMed] [Google Scholar]
- Kang S. W., Leclerc B., Kosonsiriluk S., Mauro L. J., Iwasawa A., El Halawani M. E. (2010). Melanopsin expression in dopamine-melatonin neurons of the premammillary nucleus of the hypothalamus and seasonal reproduction in birds. Neuroscience 170, 200–213. [DOI] [PubMed] [Google Scholar]
- Kang S. W., Leclerc B., Mauro L. J., El Halawani M. E. (2009). Serotonergic and catecholaminergic interactions with co-localised dopamine-melatonin neurones in the hypothalamus of the female turkey. J. Neuroendocrinol. 21, 10–19. 10.1111/j.1365-2826.2008.01804.x [DOI] [PubMed] [Google Scholar]
- Kang S. W., Thayananuphat A., Bakken T., El Halawani M. E. (2007). Dopamine-melatonin neurons in the avian hypothalamus controlling seasonal reproduction. Neuroscience 150, 223–233. 10.1016/j.neuroscience.2007.08.031 [DOI] [PubMed] [Google Scholar]
- Kuenzel W. J., Kang S. W., Zhou Z. J. (2015). Exploring avian deep-brain photoreceptors and their role in activating the neuroendocrine regulation of gonadal development. Poult. Sci. 94, 786–798. 10.3382/ps.2014-4370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuenzel W. J., Masson M. (1988). A Stereotaxic Atlas of the Brain of Chick (Gallus domesticus). Baltimore, MD: Johns Hopkins University Press. [Google Scholar]
- Lammel S., Lim B. K., Ran C., Huang K. W., Betley M. J., Tye K. M., et al. (2012). Input-specific control of reward and aversion in the ventral tegmental area. Nature 491, 212–217. 10.1038/nature11527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc B., Kang S. W., Mauro L. J., Kosonsiriluk S., Chaiseha Y., El Halawani M. E. (2010). Photoperiodic modulation of clock gene expression in the avian premammillary nucleus. J. Neuroendocrinol. 22, 119–128. 10.1111/j.1365-2826.2009.01942.x [DOI] [PubMed] [Google Scholar]
- Liu H., Wang C., Yu M., Yang Y., He Y., Liu H., et al. (2021). TPH2 in the dorsal raphe nuclei regulates energy balance in a sex-dependent manner. Endocrinology 162, bqaa183. 10.1210/endocr/bqaa183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manser C. E. (1996). Effects of lighting on the welfare of domestic poultry: a review. Anim. Welf. 5, 341–360. 10.1258/002367796780744974 [DOI] [PubMed] [Google Scholar]
- Matsumoto M., Takada M. (2013). Distinct representations of cognitive and motivational signals in midbrain dopamine neurons. Neuron 79, 1011–1024. 10.1016/j.neuron.2013.07.002 [DOI] [PubMed] [Google Scholar]
- Menaker M., Roberts R., Elliott J., Underwood H. (1970). Extraretinal light perception in the sparrow. 3. The eyes do not participate in photoperiodic photoreception. Proc. Natl. Acad. Sci. U.S.A. 67, 320-325. 10.1073/pnas.67.1.320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meye F. J., Adan R. A. (2014). Feelings about food: the ventral tegmental area in food reward and emotional eating. Trends Pharmacol. Sci. 35, 31–40. 10.1016/j.tips.2013.11.003 [DOI] [PubMed] [Google Scholar]
- Michelsen K. A., Prickaerts J., Steinbusch H. W. (2008). The dorsal raphe nucleus and serotonin: implications for neuroplasticity linked to major depression and Alzheimer's disease. Prog. Brain Res. 172, 233–264. 10.1016/S0079-6123(08)00912-6 [DOI] [PubMed] [Google Scholar]
- Miranda-Barrientos J., Chambers I., Mongia S., Liu B., Wang H. L., Mateo-Semidey G. E., et al. (2021). Ventral tegmental area GABA, glutamate, and glutamate-GABA neurons are heterogeneous in their electrophysiological and pharmacological properties. Eur. J. Neurosci. 54, 4061–4084. 10.1111/ejn.15156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore A. F., Cassone V. M., Alloway K. D., Bartell P. A. (2018). The premammillary nucleus of the hypothalamus is not necessary for photoperiodic timekeeping in female turkeys (Meleagris gallopavo). PLoS ONE 13, e0190274. 10.1371/journal.pone.0190274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M., Margolis E. B. (2017). Ventral tegmental area: cellular heterogeneity, connectivity and behaviour. Nat. Rev. Neurosci. 18, 73–85. 10.1038/nrn.2016.165 [DOI] [PubMed] [Google Scholar]
- Mosienko V., Bert B., Beis D., Matthes S., Fink H., Bader M., et al. (2012). Exaggerated aggression and decreased anxiety in mice deficient in brain serotonin. Transl. Psychiatry 2, e122. 10.1038/tp.2012.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair-Roberts R. G., Chatelain-Badie S. D., Benson E., White-Cooper H., Bolam J. P., Ungless M. A. (2008). Stereological estimates of dopaminergic, GABAergic and glutamatergic neurons in the ventral tegmental area, substantia nigra and retrorubral field in the rat. Neuroscience 152, 1024–1031. 10.1016/j.neuroscience.2008.01.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane Y., Ikegami K., Ono H., Yamamoto N., Yoshida S., Hirunagi K., et al. (2010). A mammalian neural tissue opsin (Opsin 5) is a deep brain photoreceptor in birds. Proc. Natl. Acad. Sci. U.S.A. 107, 15264–15268. 10.1073/pnas.1006393107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane Y., Shinomiya A., Ota W., Ikegami K., Shimmura T., Higashi S.-I., et al. (2019). Action spectrum for photoperiodic control of thyroid-stimulating hormone in Japanese quail (Coturnix japonica). PLoS ONE 14:e0222106. 10.1371/journal.pone.0222106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissila J., Manttari S., Sarkioja T., Tuominen H., Takala T., Timonen M., et al. (2012). Encephalopsin (OPN3) protein abundance in the adult mouse brain. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 198, 833–839. 10.1007/s00359-012-0754-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olanrewaju H. A., Miller W. W., Maslin W. R., Collier S. D., Purswell J. L., Branton S. L. (2018). Influence of light sources and photoperiod on growth performance, carcass characteristics, and health indices of broilers grown to heavy weights. Poult. Sci. 97, 1109–1116. 10.3382/ps/pex426 [DOI] [PubMed] [Google Scholar]
- Pani L., Porcella A., Gessa G. L. (2000). The role of stress in the pathophysiology of the dopaminergic system. Mol. Psychiatry 5, 14–21. 10.1038/sj.mp.4000589 [DOI] [PubMed] [Google Scholar]
- Polter A. M., Kauer J. A. (2014). Stress and VTA synapses: implications for addiction and depression. Eur. J. Neurosci. 39, 1179–1188. 10.1111/ejn.12490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter H., Alenciks E., Frazier K., Porter A., Fraley G. S. (2018). Immunolesion of melanopsin neurons causes gonadal regression in Pekin drakes (Anas platyrhynchos domesticus). Gen. Comp. Endocrinol. 256, 16–22. 10.1016/j.ygcen.2017.08.006 [DOI] [PubMed] [Google Scholar]
- Provencio I., Jiang G., De Grip W. J., Hayes W. P., Rollag M. D. (1998). Melanopsin: an opsin in melanophores, brain, and eye. Proc. Natl. Acad. Sci. U.S.A. 95, 340–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rault J. L., Clark K., Groves P. J., Cronin G. M. (2017). Light intensity of 5 or 20 lux on broiler behavior, welfare and productivity. Poult. Sci. 96, 779–787. 10.3382/ps/pew423 [DOI] [PubMed] [Google Scholar]
- Reiner A., Perkel D. J., Bruce L. L., Butler A. B., Csillag A., Kuenzel W., et al. (2004). Revised nomenclature for avian telencephalon and some related brainstem nuclei. J. Comp. Neurol. 473, 377–414. 10.1002/cne.20118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routtenberg A., Strop M., Jerdan J. (1978). Response of the infant rat to light prior to eyelid opening: mediation by the superior colliculus. Dev. Psychobiol. 11, 469–478. 10.1002/dev.420110510 [DOI] [PubMed] [Google Scholar]
- Saenz De Miera C., Sage-Ciocca D., Simonneaux V., Pevet P., Monecke S. (2018). Melatonin-independent photoperiodic entrainment of the circannual TSH rhythm in the pars tuberalis of the European Hamster. J. Biol. Rhythms 33, 302–317. 10.1177/0748730418766601 [DOI] [PubMed] [Google Scholar]
- Sato T. (2001). Sensory and endocrine characteristics of the avian pineal organ. Microsc. Res. Tech. 53, 2–11. 10.1002/jemt.1063 [DOI] [PubMed] [Google Scholar]
- Smith R. J., Vento P. J., Chao Y. S., Good C. H., Jhou T. C. (2019). Gene expression and neurochemical characterization of the rostromedical tegmental nucleus (RMTg) in rats and mice. Brain Struct. Funct. 224, 219–238. 10.1007/s00429-018-1761-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobrinho C. R., Canteras N. S. (2011). A study of the catecholaminergic inputs to the dorsal premammillary nucleus. Neurosci. Lett. 501, 157–162. 10.1016/j.neulet.2011.07.006 [DOI] [PubMed] [Google Scholar]
- Soden M. E., Miller S. M., Burgeno L. M., Phillips P. E. M., Hnasko T. S., Zweifel L. S. (2016). Genetic isolation of hypothalamic neurons that regulate context-specific male social behavior. Cell Rep. 16, 304–313. 10.1016/j.celrep.2016.05.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S. R., Badurek S., Dileone R. J., Nashmi R., Minichiello L., Picciotto M. R. (2014). GABAergic and glutamatergic efferents of the mouse ventral tegmental area. J. Comp. Neurol. 522, 3308–3334. 10.1002/cne.23603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessmar-Raible K., Raible F., Christodoulou F., Guy K., Rembold M., Hausen H., et al. (2007). Conserved sensory-neurosecretory cell types in annelid and fish forebrain: insights into hypothalamus evolution. Cell 129, 1389–1400. 10.1016/j.cell.2007.04.041 [DOI] [PubMed] [Google Scholar]
- Thayananuphat A., Kang S. W., Bakken T., Millam J. R., El Halawani M. E. (2007a). Rhythm-dependent light induction of the c-fos gene in the turkey hypothalamus. J. Neuroendocrinol. 19, 407–417. 10.1111/j.1365-2826.2007.01544.x [DOI] [PubMed] [Google Scholar]
- Thayananuphat A., Kang S. W., Bakken T., Millam J. R., El Halawani M. E. (2007b). Rhythmic dependent light induction of gonadotrophin-releasing hormone-I expression and activation of dopaminergic neurones within the premammillary nucleus of the turkey hypothalamus. J. Neuroendocrinol. 19, 399–406. 10.1111/j.1365-2826.2007.01545.x [DOI] [PubMed] [Google Scholar]
- Underwood H., Binkley S., Siopes T., Mosher K. (1984). Melatonin rhythms in the eyes, pineal bodies, and blood of Japanese quail (Coturnix coturnix japonica). Gen. Comp. Endocrinol. 56, 70–81. 10.1016/0016-6480(84)90063-7 [DOI] [PubMed] [Google Scholar]
- Ungless M. A., Grace A. A. (2012). Are you or aren't you? Challenges associated with physiologically identifying dopamine neurons. Trends Neurosci 35, 422–430. 10.1016/j.tins.2012.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wyk B., Frakey G. (2021). Ontogeny of OPN4, OPN5, GnRH and GnIH mRNA expression in the posthatch male and female Pekin duck (Anas platyrhynchos domesticus) suggests OPN4 may have additional functions beyond reproduction. Animals 11, 1121. 10.3390/ani11041121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ViviD D., Bentley G. E. (2018). Seasonal reproduction in vertebrates: melatonin synthesis, binding, and functionality using Tinbergen's four questions. Molecules 23, 652. 10.3390/molecules23030652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade P. D., Taylor J., Siekevitz P. (1988). Mammalian cerebral cortical tissue responds to low-intensity visible light. Proc. Natl. Acad. Sci. U.S.A. 85, 9322–9326. 10.1073/pnas.85.23.9322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh J. J., Friedman A. K., Sun H., Heller E. A., Ku S. M., Juarez B., et al. (2014). Stress and CRF gate neural activation of BDNF in the mesolimbic reward pathway. Nat. Neurosci. 17, 27–29. 10.1038/nn.3591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther D. J., Peter J. U., Bashammakh S., Hortnagl H., Voits M., Fink H., et al. (2003). Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 299, 76. 10.1126/science.1078197 [DOI] [PubMed] [Google Scholar]
- Wilson S. C., Cunningham F. J. (1980). Effect of increasing day length and intermittent lighting schedules in the domestic hen on plasma concentrations of luteinizing hormone (LH) and the LH response to exogenous progesterone. Gen. Comp. Endocrinol. 41, 546–553. [DOI] [PubMed] [Google Scholar]
- Yokoyama K., Oksche A., Darden T. R., Farner D. S. (1978). The sites of encephalic photoreception in phosoperiodic induction of the growth of the testes in the white-crowned sparrow, Zonotrichia leucophrys gambelii. Cell Tissue Res. 189, 441–467. 10.1007/BF00209132 [DOI] [PubMed] [Google Scholar]
- Zweifel L. S., Fadok J. P., Argilli E., Garelick M. G., Jones G. L., Dickerson T. M., et al. (2011). Activation of dopamine neurons is critical for aversive conditioning and prevention of generalized anxiety. Nat. Neurosci. 14, 620–626. 10.1038/nn.2808 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author/s.