Abstract
Purpose
The purpose of this study was to investigate the effect of aging on bladder function and caveolin protein expression in rat urothelium.
Materials and Methods
Female Sprague-Dawley rats were divided into the following two groups: young age control group (12 weeks) and old-aged group of rats (80 weeks). Urodynamic measurements were taken to compare the contraction interval and the contraction pressure between the two groups. The expression and cellular localization of caveolin 1 and 2 in the urothelium of the rat urinary bladder were determined by Western blot and immunofluorescence microscopy.
Results
In cystometrograms, the contraction interval (min) was significantly shorter in the old-aged group (3.7±0.5 min) than in the young age control group (6.2±0.8 min). Also, the average contraction pressure (mmHg) was lower in the old-aged group (8.4±0.6 mmHg) than in the young age control group (13.2±1.3 mmHg). Caveolin 1 and 2 were expressed in the subepithelial area in the urothelium. The protein expression of both caveolin 1 and 2 was significantly lower in the old-aged group than in the young age control group.
Conclusions
Aging caused a significant change in the expression of caveolin 1 and 2 in the urothelium of the rat urinary bladder. These findings suggest that these molecules might have specific roles in the functional change of the urinary bladder that occurs in association with aging.
Keywords: Aging, Bladder, Caveolin, Rat
INTRODUCTION
The urothelium has been considered a simple passive membrane barrier between the urinary tract and urine. Recently, the urothelium has been found to be an actively involved responsive organ capable of mediating a variety of signals from the urinary bladder [1]. Many studies support the view that the urothelium exhibits specialized sensory and signaling properties that regulate normal bladder function [1,2]. The urothelium has an important role as it can release chemical mediators, express a number of sensor molecules, and respond to external stimuli, and alterations in urothelial features may in part contribute to abnormalities such as various bladder disorders [2].
Caveolae are 50 to 100 nm flask-shaped small invaginations in the plasma membrane that are found in most cell types, but are abundant in adipocytes, endothelial cells, and smooth and striated muscle cells [3]. Caveolins are the main protein component of the caveolae structure necessary for the formation of caveolae. In the mammalian cells, at least three different isoforms of the caveolin family have been identified (caveolin 1, 2, 3). Caveolae are shown to represent membrane compartments enriched in a large number of signaling molecules which are essential for many cell signaling processes that regulate various signal molecules and may facilitate and integrate the cellular response to a specific extracellular stimulus [4,5,6]. Caveolin 1 is an essential structural component of cell surface caveolae biogenesis and is regarded as an important molecule for trafficking and mobility of the caveolae. Caveolin 1 and 2 are usually co-expressed in many cells and tissues. Caveolin-null animals provide valuable insights regarding the roles of caveolin proteins in cell function. Functional roles of caveolin 1 in the urinary bladder are beginning to emerge with the study of caveolin 1 knockout animals. Lai et al. [7] reported that loss of caveolin 1 expression was associated with disruption of M3 muscarinic cholinergic activity, showing a 70% reduction in acetylcholine release in the bladder by deletion of caveolin 1. They suggested that caveolin 1 is involved in pre-junctional acetylcholine release from the neuromuscular junction and post-junctional M3 receptor-mediated signal transduction in bladder smooth muscle [7]. Caveolin 1 knockout mice exhibit several urological abnormalities showing decreased electrical neural and carbachol-evoked detrusor contraction power [8].
Functional changes of the urinary bladder, such as impaired detrusor smooth muscle contraction and detrusor overactivity, are a prevalent feature in the aging population [9,10]. Age-associated change of caveolins has been reported in various organs, resulting in functional alterations [11,12]. In the aging bladder, structural change of bladder smooth muscle including an apparent reduction in bladder smooth muscle caveolae has been described. Lowalekar et al. [13] investigated the effect of aging on caveolin in the smooth muscle of the aging bladder. They reported that biological aging significantly decreased caveolae number (50% fewer caveolae) and morphology combined with alteration of caveolin expression in the bladder smooth muscle. But the impact of aging on the urothelial tissue and related caveolin expression are unclear. Since the urothelium is highly associated with bladder function and is an emerging target for the treatment of bladder dysfunction, the relationship between aging and caveolin expression in the urothelium warrants investigation.
Previously, we investigated the effect of functional abnormality of the urinary bladder showing detrusor overactivity on the expression of caveolins in the urinary bladders using animal models [14,15]. In those studies, we reported that alterations of detrusor function cause a significant change in the expression of caveolins in the urinary bladder, which suggests that caveolin might have a functional role in the detrusor overactivity.
Most studies to date have demonstrated caveolin expression in the detrusor muscle of the urinary bladder. However, there have been no studies to investigate the changes in the expression of caveolin 1, 2 in the urothelium and functional activity of this protein in response to the clinically important aging condition. The purpose of this study was to investigate the effect of aging on bladder function and protein expression of caveolin in rat urothelium. Understanding the effect of caveolin 1 and 2 on the urothelium induced by the aging process would provide a valuable insight into the mechanism regulating bladder function and could identify caveolin protein as a promising target for the treatment of dysfunctional change of the urinary bladder or bladder overactivity that occurs in association with the aging bladder.
MATERIALS AND METHODS
Female Sprague-Dawley rats (12 weeks old, 230–240 g, n=30) were divided into the following two groups: young age control group (12 weeks, n=15) and old-aged group (80 weeks, n=15).
All experimental animals were fed a standard diet until the day before the experiment. Animals having an estrous cycle confirmed via a vaginal smear were anesthetized with an intramuscular injection of ketamine (15 mg/kg) for tissue dissection after the cystometry. The study protocol was reviewed and approved by the Institutional Animal Care and Use Committee of the Chonnam National University Hospital (approval number: CNU IACUC-H-2015-10). In addition, the study was performed in accordance with the Declaration of Helsinki and the Ethical Guidelines for Clinical Studies.
1. Cystometrogram
Five rats in each group were anesthetized with 1.2 g/kg urethane injected subcutaneously. A suprapubic midline incision was performed to expose the bladder, a transvesical catheter with a fire-flared tip (polyethylene catheter-50) was inserted into the dome of the bladder and secured with a ligature, and then the abdomen was closed. The catheter was connected to a pressure transducer and syringe pump via a 3-way stopcock to record the intravesical pressure and to infuse saline into the bladder (PowerLab/400 system, ADInstruments, Sydney, Australia). After 30 minutes equilibrium the bladder was emptied and cystometry was performed with saline infused at a rate of 0.04 mL/min using the infusion pump (CMA 100; Microject, Solna, Sweden). The contraction pressure and the contraction interval were calculated.
2. Immunofluorescence staining
Samples were frozen in liquid nitrogen mixed with optimal cutting temperature compound in the cryomold and subsequently stored in liquid nitrogen. Each tissue was cut by 6 micrometer thick. The tissue sections (n=5 for each group, eight sections of each tissue) were rinsed in phosphate-buffered saline (PBS), and then treated with 3% hydrogen peroxide in 60% methanol for 30 minutes to quench endogenous peroxidase activity. After washing in PBS, tissue sections were treated with normal chicken serum for 30 minutes to block nonspecific binding. After being washed in PBS, the sections were incubated with a 1:100 dilution in PBS of antibody for caveolin 1 or caveolin 2 (BD Biosciences, San Jose, CA, USA) for 12 to 14 hours at 4℃. Immunoreactivity for caveolin 1 and caveolin 2 was detected with the use of Alexa-Fluor 594 chicken anti-mouse IgG (1;100, H+L; Invitrogen, Carlsbad, CA, USA). Tissues were mounted with the use of a mounting solution containing 4-6-diamidino-2-phenylindole. For a negative control, tissues were prepared in a similar manner, except that caveolin 1 and caveolin 2 antibody were omitted from the incubation solution. Tissues were examined with a model LSM 510 confocal microscope (Carl Zeiss, Jena, Germany) with an excitation wavelength appropriate for Alexa-Fluor (594 nm). Final images were constructed with the use of LSM Image Examiner software. Densitometric analysis was performed with Studio Star Scanner using the NIH Image V1-57 software.
3. Western blot
The whole bladder tissues were harvested for the protein extraction. Proteins (n=5 for each group, 30 µg) were separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes (Amersham Pharmacia Biotech, UK). The blots were then washed with Tris-Buffered Saline Tween (10 mM Tris-HCl, pH 7.6, 150 mM NaCl, 0.05% Tween-20). Each membrane was blocked with 5% skimmed milk for 1 hour and incubated with the appropriate primary antibody. Monoclonal mouse antibodies for caveolin 1 and caveolin 2 (1:1,000; BD Biosciences) and polyclonal rabbit antibody against glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (1:4,000; Cell Signaling Technology, Danvers, MA, USA) were used. The membrane was then washed, and caveolin 1, caveolin 2, and GAPDH were detected with goat anti-mouse-IgG and goat anti-rabbit-IgG conjugated to horseradish peroxidase, respectively. Antibody incubations were performed in a 4℃ incubator. The bands were visualized with LAS-3000 (Fujifilm Life-science, Tokyo, Japan). GAPDH was used as an internal control. Densitometric scanning was conducted by the Multi gauge V3.0 (Fujifilm Life-science) analysis software and chemiluminescence system.
4. Statistical analysis
The results are expressed as mean±standard deviation, except for the data for the cystometric parameters, which are expressed as mean±standard error of the mean. Analysis of variance was used to test the null hypothesis that there would be no differences in the mean expression levels between the two groups. Differences were considered statistically significant at p<0.05.
RESULTS
1. Effect of aging on the cystometric parameters
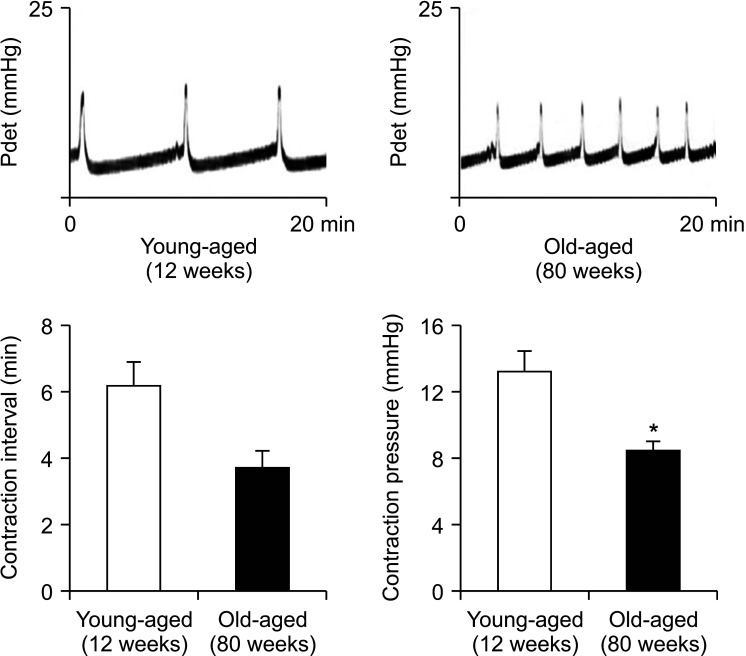
In cystometrograms, the contraction interval (min) was significantly shorter in the old-aged group (3.7±0.5 min) than in the young age control group (6.2±0.8 min). Also, the average contraction pressure (mmHg) was lower in the old-aged group (8.4±0.6 mmHg) than in the young age control group (13.2±1.3 mmHg) (Fig. 1).
Fig. 1. Representative cystometric profiles of the following two groups of rats: the young age control group and the old-aged group. The values denote the mean±standard error of the mean of 5 experiments for each condition determined by cystometrogram. *p<0.05.
2. Effect of aging on the immunofluorescent expression of caveolin 1 and caveolin 2
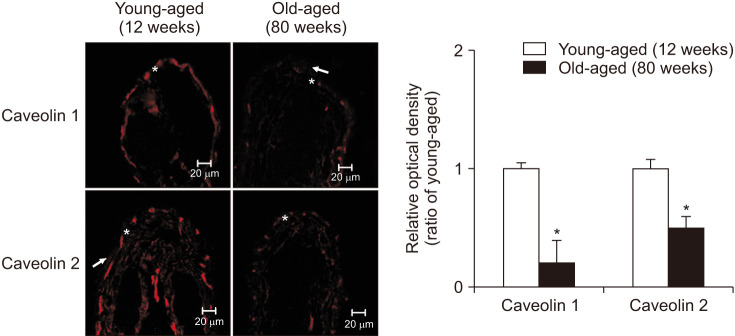
Confocal microscopy was performed and immunofluorescence of caveolin 1 and caveolin 2 in each group was compared. Distinct staining of caveolin 1 and 2 was apparent subepithelial area in the urothelium. Immunofluorescence staining showed that the old-aged group had decreased expression of caveolin 1 (p=0.01) and caveolin 2 (p=0.02) (Fig. 2).
Fig. 2. Immunofluorescence labeling for caveolin 1 and caveolin 2. Distinct staining of caveolin 1 and 2 was apparent subepithelial area in the urothelium (→ urothelium, * suburothelium). Immunofluorescence staining showed that the old-aged group had decreased expression of caveolin 1 (p=0.01) and caveolin 2 (p=0.02). The horizontal scale bar at the bottom left of each figure indicates the magnification power. The right panels denote the mean±standard deviation of 5 experiments for each condition determined by relative densitometry. *p<0.05.
3. Effect of aging on the protein expression of caveolin 1 and caveolin 2
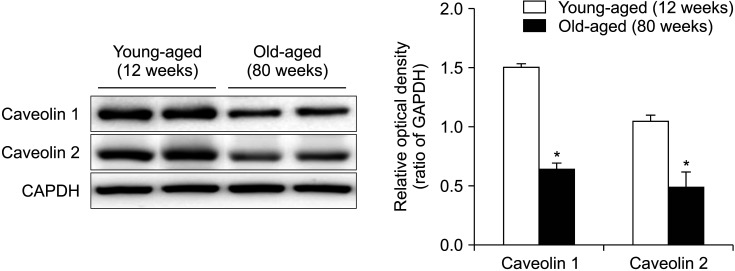
Western blot analysis revealed bands at 20 kDa corresponding to caveolin 1 and caveolin 2 proteins. Anti-GAPDH antibody recognized the 42 kDa band (Fig. 3). The protein expression of both caveolin 1 and 2 was significantly decreased in the old-aged group than in the young age control group (p<0.05) (Fig. 3).
Fig. 3. Immunoblotting of caveolin 1 and caveolin 2. Western blot analysis revealed bands at 20 kDa corresponding to caveolin 1 and caveolin 2 proteins. Anti-GAPDH antibody recognized the 42 kDa band. The protein expression of both caveolin 1 and 2 was significantly decreased in the old-aged group than in the young age control group. The lower panels denote the mean±standard deviation of 5 experiments for each condition determined by densitometry relative to GAPDH. *p<0.05.
DISCUSSION
Aging is commonly associated with various bothersome symptoms like frequency, urgency, nocturia weak stream, hesitancy and incomplete bladder emptying that affect the elderly population and these symptoms increase dramatically with age, becoming a major health problem in daily life. Despite its prevalence, the exact pathophysiology of the aging bladder is poorly understood. Many disease entities that increase with the aging process, such as Alzheimer’s disease, atherosclerosis, and diabetes mellitus, have been shown to be associated with structural and functional abnormalities of caveolin [16].
In the present study, the expression of caveolin 1 and caveolin 2 was significantly decreased in the old-aged group of rats. Cystometric results showed that bladders in the old-aged group exhibited a decreased voiding interval and voiding pressure compared to those in the young age control group. The immunohistochemical study showed that co-localization of caveolin 1 and caveolin 2 in the urinary bladder was detected in the subepithelial layer in the urothelium. These results suggest that the aging process may induce a change in the expression of urothelial caveolins in the rat urinary bladder and that caveolins may play a role in bladder dysfunction induced by aging. To the best of our knowledge, this is the first study to show the possible occurrence of signaling alteration in the urothelium via caveolins resulting in bladder dysfunction by using an aging rat model.
More than 50 years ago, caveolae are small subcellular structures that were originally described in the capillary endothelial cell [17]. Caveolae are plasma membrane invaginations that play a role in the cell signaling processes such as endocytosis and transcytosis in the endothelial and epithelial cells [18]. Signaling molecules such as G-proteins, eNOS, EGF receptors and some protein kinase-C isoenzymes that bind directly to the domain of caveolins are accumulated preferentially in these membrane invaginations [19].
There are a number of studies indicating that caveolae may have an important role in bladder function in an animal study. Several studies have shown that caveolae regulate bladder smooth muscle and modulate bladder contraction, and have documented pronounced alteration in caveolae under specific conditions [7,20,21]. Polyák et al. [20] reported decreased numbers of caveolae in hypertrophied detrusor smooth muscle, which might contribute to alterations in signal transduction pathways that regulate the downstream effects of agonist induced bladder contraction. Cristofaro et al. [21] examined the expression of caveolin 1, 2, and 3 in rat urinary bladder tissue and found reduced agonist induced bladder contraction after cholesterol depletion that was restored following cholesterol replacement. The authors concluded that caveolins have a central role in the regulation of receptor signaling pathways in bladder smooth muscle.
Age-associated change of the membrane microdomain and expression of caveolin protein have been exhibited in various organs such as aorta, penile smooth muscle and human prostate showing functional alterations [22,23,24]. In the rat heart, age-dependent dissociation of caveolin expression from the muscle sarcolemma that alters cell signaling in cardiomyocytes was detected [25]. Many studies have described a number of changes in lower urinary tract function that occur in the aging population as well as in animal models. Pfisterer et al. [26] assessed voiding function in a group aged between 20 and 90 years using bladder diary, uroflowmetry and detailed videourodynamics. They showed that bladder sensation, detrusor contraction power, maximal flow rate and maximum urethral closure pressure were negatively associated with age. They suggested a progressive decrease in detrusor contraction strength. Animal models allow detailed investigation of structural and functional aspects of the micturition pathways and changes occurring with aging. In what concerns the bladder structure, it seems undoubtedly that aging severely alters its properties. Elbadawi et al. [27] extensively studied the aged human bladder under the electron microscope. The membrane of detrusor muscle cells from healthy aged individuals becomes dominated by dense bands with depleted caveolae, slightly widening the spaces between muscle cells. The caveolin 1 knockout mouse model may support the importance of this structure in bladder aging. When comparing 3-month-old and 1-year-old caveolin 1 knockout mice vs. age-equivalent healthy mice, the knockout group showed a diminished contractile response to electric stimulation and carbachol [8]. Daly et al. [28] found significant changes on comparing 24-month-old mice with 3-month-old controls. They reported an elevated voiding frequency accompanied by an increased release of ATP and a decreased release of Ach, a rise in the number of spontaneous detrusor contractions, an increment in the contractile detrusor response to muscarinic and purinergic agonists and an increase in the frequency of afferent nerve impulses. Lowalekar et al. [13] established age-dependent reduction of caveolae and alteration in caveolin protein expression in bladder smooth muscle cells on comparing young (10 weeks old), adult (6 months old), and aged (12 months old) rat urinary bladders.
Significant advances have been made in recent years in the understanding of bladder sensory function in terms of crosstalk between the urothelium and afferent nerves. However, there have been no studies to date investigating the expression of caveolins in the urothelial tissue of the urinary bladder or changes in the functional activity of these proteins in response to aging. In the present study, the expression of caveolins in the urinary bladder was significantly affected by aging in association with the change of cystometric parameters. One of the possible reasons behind this influence on the expression of caveolins is believed to be the significance of the location of caveolins: the microvasculature is highly dependent on aging and altered blood supply of the urinary bladder may also be an important step in regulating bladder function. Our results suggest that aging may lead to significant down-regulation of caveolin expression in urinary bladder, providing presumptive evidence that caveolins are involved in the affected lower urinary tract symptoms induced by aging, probably via modification of specific cellular transmission and signaling pathway.
A limitation of our study is that an assessment of the precise morphological change of caveolins via electron microscopy was not conducted, although we showed the change in the expression of urothelial caveolins in the aging rat urinary bladder and suggested the possible relationship between bladder function and urothelial caveolins. Further studies are needed to investigate the expression and localization of all caveolin family members in the urinary bladder and to unveil the functional role of these molecules in the underlying mechanisms of bladder pathophysiology. Future translational studies should be aimed at unraveling the pathology of the aging bladder and developing new modalities targeting the pathological processes for the treatment of age-related dysfunction of the urinary bladder.
CONCLUSIONS
Aging caused a significant change in the expression of caveolin 1 and 2 in the urothelium of the rat urinary bladder. These findings suggest that these molecules might have functional roles in the functional change of the urinary bladder that occurs in association with aging.
ACKNOWLEDGMENTS
This study was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012-2139, 2013-2109), and by a grant from Chonnam National University Hospital Research Institute of Clinical Medicine (CRI15004-1).
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
- Research conception and design: Sun-Ouck Kim and Seong Hyeon Yu.
- Data acquisition: Sun-Ouck Kim and Seong Hyeon Yu.
- Statistical analysis: Sun-Ouck Kim and Seong Hyeon Yu.
- Data analysis and interpretation: Sun-Ouck Kim and Jae Hyeon Kim.
- Drafting of the manuscript: Jae Hyeon Kim.
- Critical revision of the manuscript: Sun-Ouck Kim.
- Obtaining funding: Sun-Ouck Kim.
- Supervision: Sun-Ouck Kim.
- Approval of the final manuscript: Sun-Ouck Kim.
References
- 1.de Groat WC. The urothelium in overactive bladder: passive bystander or active participant? Urology. 2004;64(6 Suppl 1):7–11. doi: 10.1016/j.urology.2004.08.063. [DOI] [PubMed] [Google Scholar]
- 2.Birder LA, de Groat WC. Mechanisms of disease: involvement of the urothelium in bladder dysfunction. Nat Clin Pract Urol. 2007;4:46–54. doi: 10.1038/ncpuro0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin AD, Levin R, Kogan B, Whitbeck C, Chichester P, Sokol R, et al. Estrogen induced functional hypertrophy and increased force generation of the female rabbit bladder. Neurourol Urodyn. 2006;25:473–479. doi: 10.1002/nau.20258. [DOI] [PubMed] [Google Scholar]
- 4.Smart EJ, Ying Y, Donzell WC, Anderson RG. A role for caveolin in transport of cholesterol from endoplasmic reticulum to plasma membrane. J Biol Chem. 1996;271:29427–29435. doi: 10.1074/jbc.271.46.29427. [DOI] [PubMed] [Google Scholar]
- 5.Galbiati F, Razani B, Lisanti MP. Emerging themes in lipid rafts and caveolae. Cell. 2001;106:403–411. doi: 10.1016/s0092-8674(01)00472-x. [DOI] [PubMed] [Google Scholar]
- 6.Hnasko R, Lisanti MP. The biology of caveolae: lessons from caveolin knockout mice and implications for human disease. Mol Interv. 2003;3:445–464. doi: 10.1124/mi.3.8.445. [DOI] [PubMed] [Google Scholar]
- 7.Lai HH, Boone TB, Yang G, Smith CP, Kiss S, Thompson TC, et al. Loss of caveolin-1 expression is associated with disruption of muscarinic cholinergic activities in the urinary bladder. Neurochem Int. 2004;45:1185–1193. doi: 10.1016/j.neuint.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 8.Lai HH, Boone TB, Thompson TC, Smith CP, Somogyi GT. Using caveolin-1 knockout mouse to study impaired detrusor contractility and disrupted muscarinic activity in the aging bladder. Urology. 2007;69:407–411. doi: 10.1016/j.urology.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 9.Resnick NM, Yalla SV. Detrusor hyperactivity with impaired contractile function. An unrecognized but common cause of incontinence in elderly patients. JAMA. 1987;257:3076–3081. doi: 10.1001/jama.257.22.3076. [DOI] [PubMed] [Google Scholar]
- 10.Thomas AW, Cannon A, Bartlett E, Ellis-Jones J, Abrams P. The natural history of lower urinary tract dysfunction in men: minimum 10-year urodynamic followup of transurethral resection of prostate for bladder outlet obstruction. J Urol. 2005;174:1887–1891. doi: 10.1097/01.ju.0000176740.76061.24. [DOI] [PubMed] [Google Scholar]
- 11.Finkbeiner AE. Is bethanechol chloride clinically effective in promoting bladder emptying? A literature review. J Urol. 1985;134:443–449. doi: 10.1016/s0022-5347(17)47234-x. [DOI] [PubMed] [Google Scholar]
- 12.Goodwin RJ, Swinn MJ, Fowler CJ. The neurophysiology of urinary retention in young women and its treatment by neuromodulation. World J Urol. 1998;16:305–307. doi: 10.1007/s003450050072. [DOI] [PubMed] [Google Scholar]
- 13.Lowalekar SK, Cristofaro V, Radisavljevic ZM, Yalla SV, Sullivan MP. Loss of bladder smooth muscle caveolae in the aging bladder. Neurourol Urodyn. 2012;31:586–592. doi: 10.1002/nau.21217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim SO, Song SH, Lee SC, Cho KA, Park JS, Kwon D, et al. Altered expression of caveolin 2 and 3 in smooth muscle of rat urinary bladder by 17β-estradiol. BMC Urol. 2013;13:44. doi: 10.1186/1471-2490-13-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim SO, Song SH, Lee SC, Cho KA, Yu HS, Hwang IS, et al. Expression of caveolin-1 in rat urinary bladder with cyclophosphamide-induced cystitis. Int Neurourol J. 2012;16:169–174. doi: 10.5213/inj.2012.16.4.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohno-Iwashita Y, Shimada Y, Hayashi M, Inomata M. Plasma membrane microdomains in aging and disease. Geriatr Gerontol Int. 2010;10 Suppl 1:S41–S52. doi: 10.1111/j.1447-0594.2010.00600.x. [DOI] [PubMed] [Google Scholar]
- 17.Palade GE. An electron microscope study of the mitochondrial structure. J Histochem Cytochem. 1953;1:188–211. doi: 10.1177/1.4.188. [DOI] [PubMed] [Google Scholar]
- 18.Cohen AW, Hnasko R, Schubert W, Lisanti MP. Role of caveolae and caveolins in health and disease. Physiol Rev. 2004;84:1341–1379. doi: 10.1152/physrev.00046.2003. [DOI] [PubMed] [Google Scholar]
- 19.Anderson RG. The caveolae membrane system. Annu Rev Biochem. 1998;67:199–225. doi: 10.1146/annurev.biochem.67.1.199. [DOI] [PubMed] [Google Scholar]
- 20.Polyák E, Boopathi E, Mohanan S, Deng M, Zderic SA, Wein AJ, et al. Alterations in caveolin expression and ultrastructure after bladder smooth muscle hypertrophy. J Urol. 2009;182:2497–2503. doi: 10.1016/j.juro.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 21.Cristofaro V, Peters CA, Yalla SV, Sullivan MP. Smooth muscle caveolae differentially regulate specific agonist induced bladder contractions. Neurourol Urodyn. 2007;26:71–80. doi: 10.1002/nau.20361. [DOI] [PubMed] [Google Scholar]
- 22.Schutzer WE, Reed JF, Mader SL. Decline in caveolin-1 expression and scaffolding of G protein receptor kinase-2 with age in Fischer 344 aortic vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2005;288:H2457–H2464. doi: 10.1152/ajpheart.01090.2004. [DOI] [PubMed] [Google Scholar]
- 23.Bakircioglu ME, Sievert KD, Nunes L, Lau A, Lin CS, Lue TF. Decreased trabecular smooth muscle and caveolin-1 expression in the penile tissue of aged rats. J Urol. 2001;166:734–738. [PubMed] [Google Scholar]
- 24.Herbert Z, Bötticher G, Aschoff A, Sendemir E, Zermann DH, Arnold R, et al. Changing caveolin-1 and oxytocin receptor distribution in the ageing human prostate. Anat Histol Embryol. 2007;36:361–365. doi: 10.1111/j.1439-0264.2007.00775.x. [DOI] [PubMed] [Google Scholar]
- 25.Ratajczak P, Damy T, Heymes C, Oliviéro P, Marotte F, Robidel E, et al. Caveolin-1 and -3 dissociations from caveolae to cytosol in the heart during aging and after myocardial infarction in rat. Cardiovasc Res. 2003;57:358–369. doi: 10.1016/s0008-6363(02)00660-0. [DOI] [PubMed] [Google Scholar]
- 26.Pfisterer MH, Griffiths DJ, Schaefer W, Resnick NM. The effect of age on lower urinary tract function: a study in women. J Am Geriatr Soc. 2006;54:405–412. doi: 10.1111/j.1532-5415.2005.00613.x. [DOI] [PubMed] [Google Scholar]
- 27.Elbadawi A, Yalla SV, Resnick NM. Structural basis of geriatric voiding dysfunction. III. Detrusor overactivity. J Urol. 1993;150(5 Pt 2):1668–1680. doi: 10.1016/s0022-5347(17)35868-8. [DOI] [PubMed] [Google Scholar]
- 28.Daly DM, Nocchi L, Liaskos M, McKay NG, Chapple C, Grundy D. Age-related changes in afferent pathways and urothelial function in the male mouse bladder. J Physiol. 2014;592:537–549. doi: 10.1113/jphysiol.2013.262634. [DOI] [PMC free article] [PubMed] [Google Scholar]