Abstract
Toxoplasma gondii infection is an important cause of chorioretinitis in the United States and Europe. Most cases of Toxoplasma chorioretinitis result from congenital infection. Patients are often asymptomatic during life, with a peak incidence of symptomatic illness in the second and third decades of life. Diagnosis is mainly supported by ophthalmological examination and a good response to installed therapy. However, establishment of a diagnosis by ophthalmological examination alone can be difficult in some cases. To determine the diagnostic value of PCR for the detection of T. gondii, 56 blood and 56 aqueous humor samples from 56 immunocompetent patients were examined. Fifteen patients with a diagnosis of ocular toxoplasmosis had increased serum anti-T. gondii immunoglobulin G levels but were negative for anti-T. gondii immunoglobulin M (group 1), and 41 patients were used as controls (group 2). Samples were taken before antiparasitic therapy was initiated, and only one blood sample and one aqueous humor sample were obtained for each patient. Single nested PCRs and Southern blot hybridization were performed with DNA extracted from these samples. The results obtained showed sensitivity and specificity values of 53.3 and 83%, respectively. Interestingly, among all patients with ocular toxoplasmosis, a positive PCR result with the aqueous humor sample was accompanied by a positive PCR result with the blood sample. This result suggests that ocular toxoplasmosis should not be considered a local event, as PCR testing of blood samples from patients with ocular toxoplasmosis yielded the same result as PCR testing of aqueous humor samples. PCR testing may be useful for discriminating between ocular toxoplasmosis and other ocular diseases, and also can avoid the problems associated with ocular puncture.
Toxoplasma gondii infection is an important cause of chorioretinitis in the United States and Europe. Most cases of Toxoplasma chorioretinitis result from congenital infection (16). Patients are often asymptomatic, with a peak incidence of symptomatic disease in the second and third decades of life. The characteristic lesion is a focal necrotizing retinitis that initially appears in the fundus as a yellowish white, elevated cotton patch with indistinct margins, usually on the posterior pole (15). The clinical ophthalmological findings together with positive anti-T. gondii serology provide sufficient information on which to base a diagnosis; the latter would be confirmed by a good response to installed therapy. However, in immunocompromised patients the clinical findings are sometimes not clear enough to make a definitive diagnosis, raising the question of which therapeutic strategy should be used. A wrong decision not only could cause a delay in adequate treatment and the preventable loss of a functioning retina for the infected patient but also could expose the uninfected patient to the toxic side effects of unnecessary medication. Regarding the laboratory diagnosis of ocular toxoplasmosis, cell culture of intraocular fluids is particularly insensitive when only a small amount of material is available; furthermore, it may take days to weeks to obtain a result. Detection of locally produced antibodies may be useful for immunocompetent patients but is probably not useful for immunocompromised patients. PCR detects the DNAs of microorganisms and is a rapid method which has been used to detect T. gondii DNA in different biological samples (4, 9, 12, 14, 19). Most of these samples can be obtained only by invasive procedures, and only blood can easily be obtained from the patients. This technique has also been used with immunocompetent and immunosuppressed patients with sight-threatening retinitis; so far, the results that have been obtained are inconsistent, and there are discrepancies in the sensitivity values (1, 2, 10, 18, 21). Therefore, we examined the diagnostic value of PCR for the detection of T. gondii in blood and aqueous humor samples from a large group of patients with and without ocular toxoplasmosis.
MATERIALS AND METHODS
Patients.
Fifty-six blood and 56 aqueous humor samples from 56 patients were examined. Serum anti-T. gondii immunoglobulin G (IgG) and IgM were detected by enzyme-linked immunosorbent assay. Patients were divided into two groups. (i) Group 1 consisted of 15 patients with a clinical diagnosis of ocular toxoplasmosis reactivation. The mean age of the patients was 35.5 years (age range, 18 to 59 years). Anterior chamber taps were taken after topical application of oxybuprocaine chloride (0.4%) and tetracaine chloride (0.1%). The eye was gently grasped with a fixating forceps at the nasal side, and the anterior chamber was entered just anterior of the limbus from the temporal side with a 30-gauge needle on a 1-ml syringe. After the collection of 0.2 to 0.3 ml of aqueous humor, the needle was removed. Patients were examined after 1 h. No complications were seen. Samples were taken from these patients before specific antiparasitic therapy was started. No problems were associated with the puncture. All these patients had ocular symptoms, and indirect ophthalmoscopy of a dilated eye showed the typical active lesions of T. gondii retinitis adjacent to pigmented scars (the typical appearance of congenital ocular toxoplasmosis reactivation). Antitoxoplasmic drugs (oral pyrimethamine, 50 mg daily for 2 days and then 25 mg daily, plus oral folinic acid, 10 to 20 mg daily, and either 1 or 1.5 g of sulfadiazine four times daily) were administered for 6 to 8 weeks to patients with clinically diagnosed or suspected T. gondii retinitis. Ophthalmoscopic examination and the response to antitoxoplasmic therapy (resolution of the retinal lesion) suggested ocular toxoplasmosis. After antitoxoplasmic therapy, symptoms improved and ocular lesions resolved, leaving pigmented scars. (ii) Group 2 consisted of 41 patients with no clinical evidence of ocular toxoplasmosis. The mean age of the patients was 58.1 years (age range, 23 to 80 years). These patients had other ocular diseases, and aqueous humor and blood samples were obtained before vitreoretinal surgery. For all patients, only one blood sample and one aqueous humor sample were examined.
Serology.
Serologic determinations were performed with the kit VIDAS Toxo IgG and IgM (Bio Mérieux, Paris, France), and the manufacturer's directions were followed.
DNA preparation.
DNA was prepared by using a kit (DNA III Extraction; Real, Valencia, Spain) in accordance with the manufacturer's directions. For blood and aqueous humor samples, 5 ml of anticoagulated blood and a 200- to 300-μl portion of aqueous humor, respectively, were used for DNA extraction.
Amplification procedures.
The amplification reactions were performed with 50-μl reaction mixtures containing the following: 100 pmol of each primer, 20 mM Tris-HCl (pH 8.3), 50 mM KCl, 2.5 mM MgCl2, 200 μM concentrations of each deoxynucleoside triphosphate, and 2.5 U of Taq DNA polymerase (Perkin-Elmer, Norwalk, Conn.). A total of 2.0 μg of genomic DNA was used for blood samples, and an undetectable amount of DNA was used when PCR was done with aqueous humor samples. Samples were overlaid with 60 μl of paraffin oil, and the reactions were run in a Perkin-Elmer thermocycler. After initial denaturation of the DNA at 94°C for 4 min, 55 cycles were run, as follows: 94°C for 1 min, 42°C for 30 s, and 72°C for 2 min. The final extension step continued for an additional 10 min at 72°C. For the nested PCR, the amplification reactions were performed in 50-μl reaction mixtures containing the following: 30 pmol of each primer, 100 μM concentrations of each deoxynucleoside triphosphate, 20 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 0.02% gelatin, and 2.5 U of Taq DNA polymerase (Perkin-Elmer). A total of 20 μl of a 1:200 dilution of the first PCR product was used for the second amplification. Samples were overlaid with 60 μl of paraffin oil, and reactions were run in a Perkin-Elmer thermocycler. After initial denaturation of the DNA at 94°C for 4 min, 17 cycles were run, as follows: 94°C for 1 min 20 s, 53°C for 2 min, and 72°C for 2 min and 30 s. The final extension step was continued for an additional 5 min at 72°C.
Primer pair P1 (5′-TGCATAGGTTGCAGTCACTG-3′) and P3 (5′-TCTTTAAAGCGTTCGTGGTC-3′) (Cruachen, Progenetic, S.L., Madrid, Spain), which amplifies a 133-bp DNA fragment of the repetitive B1 gene of T. gondii (3), was used for the first PCR. Primer pair P1 and P2 (5′-GGCGACCAATCTGCGAATACAC-3′), which amplifies an internal 97-bp fragment of the first amplicon, was used for the nested PCR. After amplification, an aliquot of 25 μl from each reaction mixture was run on a 3.0% electrophoresis-grade agarose gel in 1× TBE buffer (0.09 M Tris-borate, 0.002 M EDTA), and DNA was stained with ethidium bromide (50 μg/ml). Bands were visualized under UV illumination.
A Southern blot hybridization (20) was performed with all the samples after the first PCR. After gel electrophoresis the amplified DNA samples were denatured (0.5 N NaOH, 1.5 M NaCl) for 30 min, renatured (1.0 M Tris-HCl [pH 7.5], 1.5 M NaCl) for 30 min, and then transferred to a positively charged nylon membrane (Boehringer Mannheim, Mannheim, Germany) by capillary transfer with 20× SSC buffer (3 M NaCl, 0.3 M sodium citrate [pH 7.0]). DNA was coupled to the membrane by using a UV chamber (Bio-Rad, Madrid, Spain) at 150 mJ. The membrane was washed with 2× SSC and prehybridized at 68°C for 1 h with hybridization solution (Boehringer Mannheim), and afterward, 5 pmol of the 133-bp amplicon that was 3′ labeled with digoxigenin-dUTP with terminal transferase was added to the mixture solution and the mixture was hybridized at 68°C overnight. After a washing step (with 2× SSC–0.1% sodium dodecyl sulfate and 0.1× SSC–0.1% sodium dodecyl sulfate for 1 h each) at 68°C, the probe was detected by a chemiluminescence method (Boehringer Mannheim).
A clinical sample was considered to be T. gondii DNA positive (PCR positive) if the PCR test (single and/or nested PCR) was positive and the 133-bp amplified fragment of the first PCR hybridized with the probe in the Southern blotting experiment. A patient was considered to be T. gondii DNA positive (PCR positive) if a positive amplification of one of the samples (blood or aqueous humor) was obtained.
The DNA extraction, amplification, and detection steps were physically separated and were performed in three different rooms. Positive controls and negative controls (reaction mixtures without DNA) were used in all experiments. The amplification experiment was considered to be invalid when a failure in any of the controls occurred.
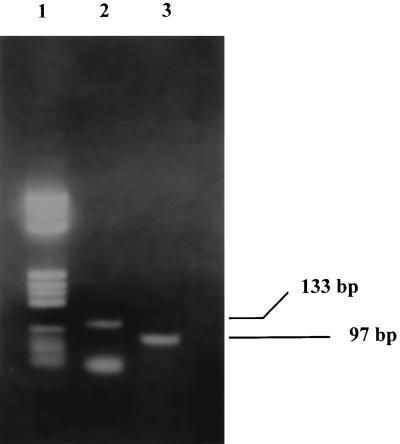
Virulent strain T. gondii RH was grown in monolayers of MRC-5 cells and was used as a positive control. When a cytopathic effect was observed in the culture (flask of 7.5 by 3.5 cm), cells and tachyzoites were collected and DNA was obtained and resuspended in 100 μl of distilled water. After the amplification reaction with primers P1 and P3 and further nested PCR under the conditions described above, the fragment of the correct size was detected up to a dilution of 1:106 with water. DNA extracted at a dilution of 1:105 was used as a positive control in all amplification experiments. A correct DNA amplification yielded a band of 133 bp in the first amplification and a band of 97 bp in the nested PCR (Fig. 1).
FIG. 1.
T. gondii PCR products resolved on a 3% agarose gel with 1× TBE buffer. Lane 1, DNA molecular size marker (λV; Boehringer Mannheim); lane 2, product of the first amplification; lane 3, product of the nested PCR.
RESULTS
The PCR was positive for 8 of the 15 blood samples and 7 of the 15 aqueous humor samples from patients in group 1. No amplification was observed with blood and aqueous humor samples from the other seven patients in group 1. Thus, a correspondence of positive results by PCR between blood and aqueous humor samples was observed for seven patients; for one patient with ocular toxoplasmosis, only the blood sample was positive. In addition, all these patients had higher serum anti-T. gondii IgG levels but were negative for anti-T. gondii IgM. With DNA from blood and aqueous humor samples from patients in group 2, no amplification of DNA fragments from blood was observed for 35 patients, whereas a positive PCR result was obtained for 7 patients. For these seven patients, we also observed a correspondence of positive PCR results between blood and aqueous humor samples for all except one patient, for whom only the aqueous humor sample was positive by DNA amplification. Twenty-five and 16 of the 41 patients in group 2 were positive and negative for anti-T. gondii IgG, respectively. Among the seven PCR-positive patients in group 2, five were positive for anti-T. gondii IgG, while the other two were negative for anti-T. gondii IgG. A summary of the results is shown in Table 1.
TABLE 1.
Summary of results of diagnosis of toxoplasmosis by PCR with 56 blood and 56 aqueous humor samples from 56 patients with and without ocular toxoplasmosis
| Group | Ocular toxoplasmosis | No. of patients
|
|||||
|---|---|---|---|---|---|---|---|
| PCR
|
Serologya
|
||||||
| Positive
|
Negative
|
Positive | Negative | ||||
| Blood | AHb | Blood | AH | ||||
| 1 (n = 15) | Yes | 8 | 7 | 7 | 8 | 15 | 0 |
| 2 (n = 41) | No | 6 | 7 | 35 | 34 | 25 | 16 |
Corresponds to IgG determination.
AH, aqueous humor sample.
DNAs from all PCR-positive samples hybridized with the B1 probe. None of the DNA samples that were PCR negative hybridized with the B1 probe. With these results we conclude that a very good correlation exists between the results of the nested PCR and those of the Southern blot assay.
DISCUSSION
We report here the results of our experience with the use of PCR for the identification of T. gondii in blood and aqueous humor samples from patients with and without ocular toxoplasmosis. Currently, the clinical diagnosis of ocular toxoplasmosis is based on the observation of a necrotizing lesion on the fundus, response to treatment, and serologic determination. However, in cases of atypical retinitis or when the fundus is hidden by vitreal inflammation, establishment of a diagnosis by ophthalmological examination alone can be difficult. In such cases the aqueous humor analysis may be used as a diagnostic tool for confirmation of ocular toxoplasmosis. Determination of intraocular production of antitoxoplasmic antibodies has been performed previously, but the results obtained had erratic values (2, 21). PCR has mostly been used to detect T. gondii in different biological samples (4, 9, 12, 14, 19). Aqueous humor samples from patients with ocular toxoplasmosis have also been used with the PCR technique (1, 2, 10, 18, 21). All these studies were carried out by examining aqueous humor samples exclusively. Sensitivities ranged from 2.3 to 75%, but specificities ranged from 75 to 100% (2, 10, 18, 21). In our opinion, these studies had several important limitations; first, most of them were retrospective studies; second, the final diagnosis was made on the basis of treatment response but was not microbiologically confirmed; and third, very small numbers of samples were tested in the studies. We report here sensitivity and specificity values of 53 and 83%, respectively, when we consider the results for both blood and aqueous humor samples and a sensitivity value of 46.6% when we consider only the results for aqueous humor samples among a collection of 112 samples from 56 patients. The low sensitivity values in this work are similar to those reported previously (2, 10, 18, 21). The distance between the anterior chamber and the initial chorioretinal site of inflammation must be considered in the interpretation of sensitivity values when a PCR is performed with an ocular sample (2). On the other hand, the likelihood that the necrotizing lesions are caused by an immunological mechanism and not by the presence of the parasite cannot be ruled out (17). Other investigators have reported that sample storage conditions before the amplification may have an important influence on the PCR sensitivity values (13). In our study, some of the DNA samples were kept at −20°C before the amplification was performed because immediate processing was impossible. This can account for some reduction in the sensitivity values. On the other hand, despite the very good DNA extraction method, the presence of inhibitors in the aqueous humor samples cannot be ruled out. The specificity values obtained in our assay are similar to those obtained in other reports (1, 18, 21). When the sequences of the P1 and P3 primers are compared with sequences in the GenBank database, only matches with the T. gondii B1 gene were observed, thus revealing a theoretical high degree of specificity of the amplification reactions.
An interesting point to be considered is that blood from patients with ocular toxoplasmosis yielded positive amplifications with the B1 primers with even higher band intensities than those with the DNAs from the aqueous humors from the same patients. This result strongly suggests that ocular toxoplasmosis should not be considered a local event. Thus, in our study a positive PCR result was obtained with the blood from 8 of 15 (53.3%) patients with a diagnosis of ocular toxoplasmosis. Similar findings have been reported by Dupouy-Camet et al. (6) in the case of cerebral toxoplasmosis in human immunodeficiency virus-positive patients. Those investigators detected T. gondii DNA by PCR in the blood of 9 of 13 patients (69%) with confirmed cerebral toxoplasmosis (6). Moreover, other studies (5, 7, 8, 11) showed sensitivity values of tests with blood of between 10 and 35%. An important question is this: Why was the T. gondii PCR positive with blood from 53% of the patients with reactivated congenital corioretinitis in our study? There are two hypotheses: (i) the source of parasitemia or T. gondii DNAemia was the ocular lesion or (ii) ocular toxoplasmosis reactivation occurs during immunosuppressive states (such as pregnancy) and is associated with reactivations in other body tissues (muscle, lung, brain, etc.). Tachyzoites or T. gondii DNA released from the cysts in these tissues may be the source of parasitemia or T. gondii DNA in blood. In any case, preventive measures should be taken for pregnant women after they have had ocular toxoplasmosis.
We have also detected T. gondii DNA in the blood and aqueous humor of patients without ocular manifestations of toxoplasmosis (6 of 41 blood samples and 7 of 41 aqueous humor samples from control patients in group 2, and among these patients five were positive for anti-T. gondii IgG). In order to explain this result, we consider it possible that a small number of parasites might be released from tissues into the blood at a subclinical level, and their presence can be detected by PCR in IgG-positive patients and under some circumstances (such as low-level immunosuppressive states). We cannot explain why the aqueous humors from these seven patients had positive PCR results because the patients had ocular diseases with no relationship to ocular toxoplasmosis. Moreover, by the PCR technique, results must be interpreted with caution; although no failures were observed among the controls, the appearance of a false-positive result cannot be ruled out.
In summary, although the diagnosis of the ocular reactivation of toxoplasmosis requires the presence of typical lesions, a positive result for anti-T. gondii IgG, a negative result for anti-T. gondii IgM, and a response to installed therapy, the results reported here suggest that the T. gondii PCR is a useful technique that can be used as a laboratory tool for the diagnosis of ocular toxoplasmosis. In addition, PCR testing of blood samples from patients with ocular toxoplasmosis yielded the same results as PCR testing of aqueous humor samples, thus avoiding all the problems associated with the ocular puncture. Considering the sensitivity and specificity values, PCR may be useful for discriminating between ocular toxoplasmosis and other ocular diseases.
ACKNOWLEDGMENTS
We thank the National Institute of Health (INSALUD) for economic support.
We thank L. de Rafael for critical reading of the manuscript.
REFERENCES
- 1.Aouizerate F, Cazenave J, Poirier L, Verin P, Cheyrou A, Begueret J, Lagoutte F. Detection of Toxoplasma gondii in aqueous humor by the polymerase chain reaction. Br J Ophthalmol. 1993;77:107–109. doi: 10.1136/bjo.77.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brezin A P, Eqwuagu C E, Silveria C, Thulliez P, Martins M, Mahdi R, Belfort R, Nusseblatt R B. Analysis of aqueous humor in ocular toxoplasmosis. N Engl J Med. 1991;10:699. [PubMed] [Google Scholar]
- 3.Burg J L, Grover C M, Pouletty P, Boothroyd J C. Direct and sensitive detection of a pathogenic protozoan, Toxoplasma gondii, by polymerase chain reaction. J Clin Microbiol. 1989;27:1787–1792. doi: 10.1128/jcm.27.8.1787-1792.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cingolani A, De Luca A, Ammassari A, Murri R, Linzalone A, Grillo R, Antinori A. PCR detection of Toxoplasma gondii DNA in CSF for the differential diagnosis of AIDS-related focal brain lesions. J Med Microbiol. 1996;45:472–476. doi: 10.1099/00222615-45-6-472. [DOI] [PubMed] [Google Scholar]
- 5.Dupon M, Cazenave J, Pellegrin J L, Ragnaud J M, Cheyrou A, Fischer I, Leng B, Lacut J Y. Detection of Toxoplasma gondii by PCR and tissue culture in cerebrospinal fluid and blood of human immunodeficiency virus-seropositive patients. J Clin Microbiol. 1995;33:2421–2426. doi: 10.1128/jcm.33.9.2421-2426.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dupouy-Camet J, de Souza S L, Maslo C, Paugam A, Saimot A, Benarous R, Tourte-Schaefer C, Derouin F. Detection of Toxoplasma gondii in venous blood from AIDS patients by polymerase chain reaction. J Clin Microbiol. 1993;31:1866–1869. doi: 10.1128/jcm.31.7.1866-1869.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Filice G A, Hitt J A, Mitchell C D, Blackstad M, Sorensen S W. Diagnosis of toxoplasma parasitemia in patients with AIDS by gene detection after amplification with polymerase chain reaction. J Clin Microbiol. 1993;31:2327–2331. doi: 10.1128/jcm.31.9.2327-2331.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franzen C, Altfeld M, Hegener P, Hartmann P, Arendt G, Jablonowski H, Rockstroh J, Diehl V, Salzberger B, Fatkenheuer G. Limited value of PCR for detection of Toxoplasma gondii in blood from human immunodeficiency virus-infected patients. J Clin Microbiol. 1997;35:2639–2641. doi: 10.1128/jcm.35.10.2639-2641.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuentes I, Rodríguez M, Domingo C J, Del Castillo F, Juncosa T, Alvar J. Urine sample used for congenital toxoplasmosis diagnosis by PCR. J Clin Microbiol. 1996;34:2368–2371. doi: 10.1128/jcm.34.10.2368-2371.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garweg J, Boehnke M, Koerner F. Restricted applicability of the polymerase chain reaction for the diagnosis of ocular toxoplasmosis. Ger J Ophthalmol. 1996;5:104–108. [PubMed] [Google Scholar]
- 11.Guy E C, Joynson D H. Potential of the polymerase chain reaction in the diagnosis of active Toxoplasma infection by detection of parasite in blood. J Infect Dis. 1995;172:319–322. doi: 10.1093/infdis/172.1.319. [DOI] [PubMed] [Google Scholar]
- 12.Ho-Yen D O, Joss A W L, Balfour A H, Smyth E T, Baird D, Chatterton J M. Use of the polymerase chain reaction to detect Toxoplasma gondii in human blood samples. J Clin Pathol. 1992;45:910–913. doi: 10.1136/jcp.45.10.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.James G S, Sintchenko V G, Dickenson D J, Gilbert G L. Comparison of cell culture, mouse inoculation, and PCR for detection of Toxoplasma gondii: effects of storage conditions on sensitivity. J Clin Microbiol. 1996;34:1572–1575. doi: 10.1128/jcm.34.6.1572-1575.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joss A W L, Chatterton J, Evans R, Yen D O. Toxoplasma polymerase chain reaction on experimental blood samples. J Med Microbiol. 1993;38:38–43. doi: 10.1099/00222615-38-1-38. [DOI] [PubMed] [Google Scholar]
- 15.Mets M B, Holfels E, Boyer K M, Swisher C N, Roizen N, Stein L, Stein M, Hopkins J, Withers S, Mack D, Luciano R, Patel D, Remington J S, Meier P, McLeod R. Eye manifestations of congenital toxoplasmosis. Am J Ophthalmol. 1996;122:309–324. doi: 10.1016/s0002-9394(14)72057-4. [DOI] [PubMed] [Google Scholar]
- 16.Montoya J G, Remington J S. Toxoplasmic chorioretinitis in the setting of acute acquired toxoplasmosis. Clin Infect Dis. 1996;23:277–282. doi: 10.1093/clinids/23.2.277. [DOI] [PubMed] [Google Scholar]
- 17.Nusseblatt R B, Mittal K K, Furhman S, Sharma S D, Palestine A G. Lymphocyte proliferative responses of patients with ocular toxoplasmosis to parasite and retinal antigens. Am J Ophthalmol. 1989;107:632–641. doi: 10.1016/0002-9394(89)90260-2. [DOI] [PubMed] [Google Scholar]
- 18.Robert F, Ouatas T, Blanche P, Tourte-Schaefer C, Sicard D, Dupouy-Camet J. Evaluation rétrospective de la détection de Toxoplasma gondii par réaction de polymérisation en chaîne chez des patients sidéens. Presse Med. 1996;25:541–545. [PubMed] [Google Scholar]
- 19.Rodrîguez J C, Martinez M M, Martínez A R, Royo G. Evaluation of different techniques in the diagnosis of Toxoplasma encephalitis. J Med Microbiol. 1997;46:597–601. doi: 10.1099/00222615-46-7-597. [DOI] [PubMed] [Google Scholar]
- 20.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 21.Verbraak F D, Galema M, Van den Horn G H, Bruinenberg M, Luyendijk L, Danner S A, Kijlstra A. Serological and polymerase chain reaction-based analysis of aqueous humor samples in patients with AIDS and necrotizing retinitis. AIDS. 1996;10:1091–1099. [PubMed] [Google Scholar]