Abstract
The global population is aging, and elderly people have a higher incidence of lower airway diseases owing to decline in swallowing function, airway ciliary motility, and overall immunity associated with aging. Furthermore, lower airway diseases in the elderly tend to have a high mortality rate. Their prevention is important for extending healthy life expectancy and improving the quality of life of each individual.
In recent years, the relationship between “chronic periodontitis and oral bacteria, especially the periodontopathic ones” and “respiratory diseases” (e.g., pneumonia, chronic obstructive pulmonary disease, and influenza) has become clear. In addition, the association of several periodontal pathogens with the onset and aggravation of coronavirus disease 2019 (COVID-19) is also being reported.
In support of these findings, oral health management has shown to reduce deaths from pneumonia and prevent influenza in nursing homes and inpatient wards. This has led to clinical and multidisciplinary cooperation between physicians and dentists, among others. However, to date, the mechanisms by which “chronic periodontitis and oral bacteria” contribute to lower airway diseases have not been well understood. Clarifying these mechanisms will lead to a theoretical basis for answering the question, “Why is oral health management effective in preventing lower airway diseases?”
Keywords: Oral bacteria, Chronic periodontitis, Pneumonia, COPD, Influenza, COVID-19
1. Introduction
The oral cavity is the entry point for bacteria and viruses to enter the body, and it is also the entrance to the lower airway, including the bronchi and lungs, in which inflammation occurs during lower airway diseases. Therefore, if aspiration of oral bacteria has an adverse effect on lower airway diseases, it is not difficult to imagine such an effect. In fact, a number of clinical studies have shown that “chronic periodontitis and oral bacteria” are associated with the onset, progression, and exacerbation of the following diseases: pneumonia, influenza, and chronic obstructive pulmonary disease (COPD) [[1], [2], [3], [4]]. It has also been reported that coronavirus disease 2019 (COVID-19) becomes more severe and results in higher mortality rates in SARS-CoV-2-infected individuals with underlying diseases such as diabetes, heart disease, and COPD [5,6], which are known to be closely related to “chronic periodontitis and oral bacteria” [4]. In fact, oral bacteria have been detected in the sputum and bronchial lavage fluid (BALF) of patients with COVID-19 [7,8], and reports stating that chronic periodontitis is associated with the severity of COVID-19 are accumulating [9].
Considering that the bacterial density in the normal salivary flora of healthy subjects is 108 CFUs/mL at maximum, people with poor oral hygiene are speculated to have more than 108 CFUs/mL of bacteria in their saliva [10]. Because humans swallow 1.0–1.5 L of saliva a day, it is calculated that they swallow more than 1011 CFUs of oral bacteria daily. When some of these bacteria enter the trachea through aspiration, they cause inflammation in the bronchi and lungs and are thought to be involved in the development of lower airway diseases [[11], [12], [13]]. The mechanism, however, is not well understood.
On the other hand, it has been reported that oral health management, including oral care, is effective in preventing the onset of lower airway diseases such as aspiration pneumonia and influenza [1,2].
Therefore, active oral health care is now being provided mainly to elderly people who need nursing care.
In this review, the effects of oral health management on the development of lower airway diseases will be examined, in addition to the relationship between “chronic periodontitis and oral bacteria” and lower airway diseases.
2. Relationship between oral bacterial flora and the development of pneumonia
As a result of global bacterial flora analysis using the BALF of patients with community-acquired pneumonia, many Streptococcus pneumoniae and Haemophilus influenzae, which are causative agents of pneumonia, have been detected, along with many oral bacteria.
In addition, many anaerobic bacteria, which are difficult to detect using conventional culture methods, have also been found. Among those anaerobes, the periodontopathic bacterial genera Prevotella and Fusobacterium show the possibility that oral bacteria are also involved in the development of community-acquired pneumonia. Furthermore, global bacterial flora analysis from the viewpoint of aspiration has shown that genera of oral Streptococcus are significantly more abundant in the BALF of pneumonia patients, and the ratio is even higher in patients with suspected aspiration pneumonia [14]. These results suggest that oral bacteria may be involved in the development of not only community-acquired pneumonia but also aspiration pneumonia.
The association between chronic periodontitis and pneumonia has also been reported by a number of studies. Awano et al. followed 697 subjects, aged 80 years, for 4 years and reported that people with 10 or more teeth with periodontal pockets (pathologically deepened grooves that form mainly between the roots of the teeth and the gums) of 4 mm or more had a 3.9 times higher risk of dying from pneumonia than those without periodontal pockets [15]. Furthermore, it has been reported that patients with moderate to severe chronic periodontitis have a 4.4-fold higher risk of developing community-acquired pneumonia [16] and a 2.9-fold higher risk of developing nosocomial pneumonia [17]. The risk of developing pneumonia is increased in patients with chronic periodontitis, not only in the elderly, suggesting that prevention of chronic periodontitis can lead to prevention of pneumonia.
On the other hand, in one study, when professional oral care was provided in elderly care facilities, the incidence of pneumonia was successfully reduced by approximately 40% [1]. The importance of oral care in the prevention of pneumonia was highlighted in the “Guidelines for the Treatment of Pneumonia in Adults 2017,” published by the Japanese Respiratory Society, and oral care for the prevention of pneumonia is now being actively provided in elderly care facilities in Japan. Furthermore, from a life-saving perspective, it is clinically important to note that oral care can significantly reduce the incidence of ventilator-associated pneumonia in patients whose lives cannot be prolonged without ICU care [18].
3. Relationship between oral microbiota and the development of COPD
COPD is a general term for diseases that were previously known as chronic bronchitis and emphysema. The number of patients with COPD continues to increase, with the disease having become the third leading cause of death worldwide in 2020, and global countermeasures are required. COPD is mainly caused by prolonged inhalation of tobacco smoke and presents as airflow obstruction. As inflammation progresses, the disease destroys lung tissue and the ability to inhale oxygen and expel carbon dioxide; thus, external respiratory function is reduced, which interferes with daily life. Nowadays, COPD is regarded as chronic, systemic inflammation rather than local inflammation confined to the lungs, and it is associated with a high incidence of comorbidities such as diabetes, ischemic heart disease, and osteoporosis.
In recent years, there have been many reports on the association between COPD and chronic periodontitis as well as the association with pneumonia. Among smokers, those with severe chronic periodontitis had higher odds (ratio of 3.71) of developing COPD than those without chronic periodontitis [19]. A cross-sectional study of 13,792 patients in the U.S. showed that the more severe the chronic periodontitis, the more likely the patient was to develop COPD, even after taking into account the patient's age and smoking history [20]. In Japan, Takeuchi et al. conducted a follow-up study of 900 adults aged 60 years and older and reported that the rate of developing COPD within 5 years was 3.5 times higher in those with severe chronic periodontitis than in healthy individuals or those with mild chronic periodontitis [21]. In addition, a meta-analysis of observational studies showed a significant correlation between chronic periodontitis and COPD [22]. Interestingly, as inflammation improved as a result of chronic periodontitis treatment, the frequency of COPD exacerbations decreased [23,24].
These studies suggest that chronic periodontitis is a risk factor for COPD exacerbation and that oral bacteria also influence the pathogenesis of COPD. However, at this time, the causal relationship between oral microbiota and the pathogenesis of COPD, or the mechanism of COPD exacerbation, is not clear.
It is well known that COPD is exacerbated by lower airway infections such as pneumonia and bronchitis, which are caused by bacteria and viruses. The frequency of acute exacerbations of COPD has a significant impact on the progression and prognosis of COPD, and the process of exacerbation often leads to death in COPD patients. In a similar vein, the possibility that oral bacteria may be involved in the development and progression of COPD is investigated. From the perspective described above, if researchers can elucidate how oral bacteria, including the causative agents of chronic periodontitis, are involved in the onset and progression of pneumonia and the exacerbation of COPD, a theoretical basis for the effectiveness of oral health management can be provided.
4. Mechanisms of oral bacterial pathogenicity for the development and progression of pneumonia as well as the development and exacerbation of COPD
4.1. Aspiration of oral bacteria may promote infection with pneumonia-causing bacteria
Bacterial and viral infections require the bacteria/virus to bind to specific receptors expressed on the cell membranes of target cells, and this phenomenon is known as “bacterial attachment” or “viral adsorption.” Pneumonia-causing bacteria such as S. pneumoniae and H. influenzae infect lower airway epithelial cells and cause pneumonia by binding to the PAF receptor (PAFR), which is a receptor for platelet-activating factor (PAF). In addition to this, PAFR-mediated infection of lower airway epithelium with pneumonia-causing bacteria is also an important trigger for COPD exacerbations.
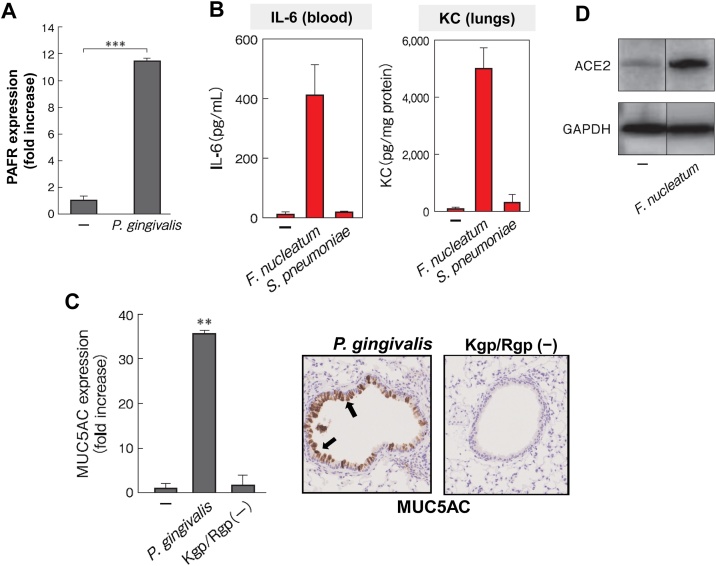
In one study, when alveolar epithelial cells were stimulated with culture supernatants of P. gingivalis, a major periodontopathic bacterium, the authors found that the expression of PAFR was upregulated, which was consequently accompanied by increased adhesion of S. pneumoniae to alveolar epithelial cells (Fig. 1A) [25]. Interestingly, among the virulence factors of P. gingivalis released into the culture supernatant, LPS and fimbriae were not involved in the expression of PAFR, but experiments using a gingipain-deficient mutant strain revealed that gingipain, a trypsin-like protease, was the stimulant that had promoted the observed increase in PAFR [25]. It has also been reported that another periodontopathic bacterium, Prevotella intermedia, promotes the expression of PAFR [26].
Fig. 1.
The presence of oral bacteria-induced PAFR, ACE2, and MUC5AC expression in alveolar epithelial cells and proinflammatory cytokine secretion and MUC5AC expression in murine lungs.
(A) When alveolar epithelial cells were incubated with the periodontal pathogen P. gingivalis, PAFR expression was induced in the cells at the gene level after 24 h [25].
(B) When F. nucleatum was introduced into the trachea of mice, a much larger amount of IL-6 and KC (IL-8 isoform in mice) was induced in the lungs and blood than when Streptococcus pneumoniae was introduced [27].
(C) When the culture supernatant of the P. gingivalis parent strain was introduced into mouse bronchi (Top), MUC5AC was induced in airway epithelia in the mouse lungs (brown), but no such phenomenon was observed with P. gingivalis gingipain-deficient (Kgp/Rgp(-)) mutant strains [30].
(D) When alveolar epithelial cells were incubated with the periodontal pathogen F. nucleatum, ACE2 expression was induced in the cells at the protein level [35].
Aspiration of periodontopathic bacteria may increase PAFR expression on the alveolar epithelium, which in turn may promote infection with pneumonia-causing bacteria.
4.2. Aspiration of oral bacteria may promote secretion of proinflammatory cytokines in the lower airway epithelium and exacerbate lung inflammation
Proinflammatory cytokines play a central role in lung inflammation during pneumonia and COPD exacerbations. Killed periodontopathic bacteria, such as P. gingivalis and Fusobacterium nucleatum, were reported to cause massive secretion of proinflammatory cytokines, such as IL-6 and IL-8, from human epithelial cell lines originating from the bronchi, alveoli, and pharynx (Fig. 1B) [27,28]. This result was observed not only in the above cell lines but also in primary human respiratory epithelial cells [29]. In addition, intratracheal inoculation of the above periodontal pathogens to mice strongly induced secretion of IL-6 and KC (the mouse isoform of IL-8) in the lungs and bronchi [27]. Surprisingly, high concentrations of these cytokines were also induced in the blood of the mice, and the concentrations were several times higher than those induced by S. pneumoniae (Fig. 1B).
Since periodontopathic bacteria are obligate anaerobes, they are unlikely to grow in the respiratory system, even when aspirated alive. Therefore, it is hypothesized that the mere contact of periodontal pathogens with respiratory epithelial cells would induce the production of proinflammatory cytokines.
The fact that large amounts of IL-6 and IL-8 secretion were induced from respiratory epithelial cells, even by dead bacterial cells, suggests that periodontal pathogens may induce inflammation simply by touching epithelial cells of the pharynx, bronchi, and alveoli, without causing infection. It is also possible that proinflammatory cytokines secreted by pharyngeal epithelial cells that are in contact with these periodontopathic bacteria before they are aspirated, combined with stimulation by aspirated bacteria, may induce inflammation of bronchi and alveoli in an additive or synergistic manner. Experiments hitherto suggest that P. gingivalis may utilize Toll-like receptor-2 (TLR2) of respiratory epithelial cells as a receptor for the bacterial stimulus [28].
Aspiration of periodontal bacteria, including P. gingivalis, induces the secretion of large amounts of inflammatory cytokines from respiratory epithelial cells below the pharynx, which may be involved in the development of pneumonia and exacerbation of COPD.
Furthermore, PAF itself, a ligand for PAFR, induces secretion of proinflammatory cytokines from respiratory epithelial cells. In other words, since it is known that patients with chronic periodontitis have high oral PAF levels, it is possible that aspirated PAF may also act on respiratory PAFR, whose expression has been elevated by bacterial aspiration. Thus, the inflammation of chronic periodontitis itself and the periodontopathogenic bacteria released from periodontitis lesions may promote inflammation of the lower airway, including the lungs.
4.3. Oral bacteria may promote bronchiole lumen obstruction and respiratory epithelial barrier breakdown
Excessive production of mucin in patients with pneumonia and COPD leads not only to excessive sputum production but also to bronchial lumen obstruction, thus reducing the respiratory function of patients. Using primary human bronchial epithelial cells, P. gingivalis was found to strongly induce the gene expression of MUC5AC, a core protein of mucin [30]. The gingipains of P. gingivalis, mentioned earlier, was revealed to play a central role in this effect. Furthermore, MUC5AC expression and mucin production were strongly induced by P. gingivalis also in the murine lung, while, as expected, no such effect was observed with the gingipain-deficient strains (Fig. 1C) [30]. It was suggested that gingipains from P. gingivalis may reduce respiratory function by inducing excessive mucin production in the lungs and bronchi, resulting in bronchial lumen narrowing.
Furthermore, periodontopathic bacteria have been confirmed to disrupt the barrier function of the bronchial epithelium, including the alveolar epithelium, in experiments using cultured cell systems and COPD model mice. In the lungs of mice with intratracheal inoculation of periodontopathic bacteria, the mean interalveolar distance was significantly increased, indicating that the alveolar walls were destroyed. In the mice, MMP-12 expression, which is involved in the destruction of alveolar walls, was increased. By contrast, the gene expressions of claudin1 and JAM-A, which are involved in epithelial barrier formation, were significantly suppressed. In addition, Benedyk et al. have also reported that gingipain destroys mouse alveoli [31].
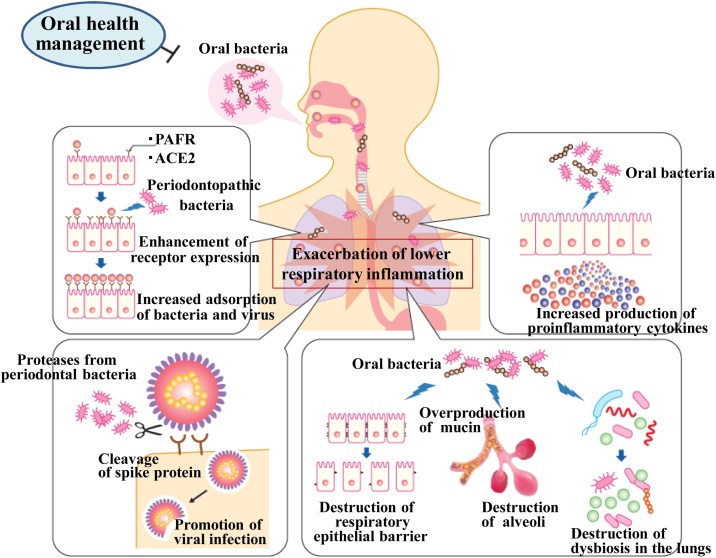
Aspiration of periodontopathic bacteria may promote overproduction of mucin in the alveoli and bronchial lumen, thereby impairing respiratory function, and disrupt tight junctions in the respiratory epithelia, facilitating tissue invasion of viruses and pneumonia-causing bacteria (Fig. 2).
Fig. 2.
Enzymes secreted by oral bacteria can spread influenza infection.
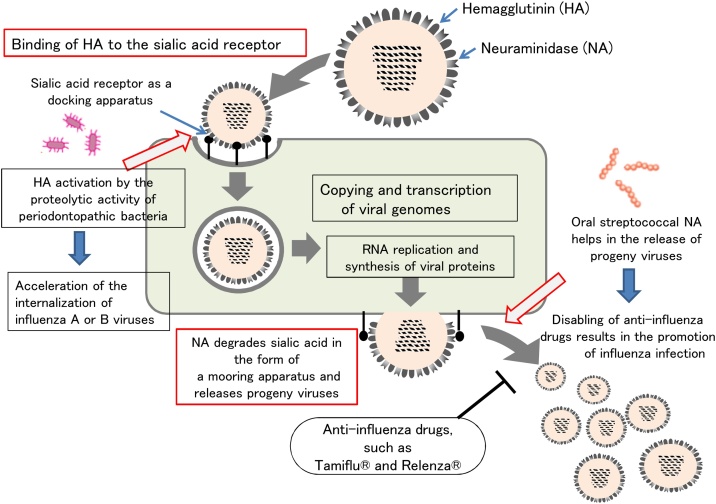
In the life cycle of influenza viruses, adsorption to target cells by hemagglutinin (HA) and release from target cells by neuraminidase (NA) are the most important processes for viral replication and spread.
Gingipains secreted by the periodontal pathogen Porphyromonas gingivalis can facilitate viral infection by cleaving influenza virus HA into HA1 and HA2. In addition, NA secreted by oral streptococci may act as a substitute for viral NA, whose activity is inhibited by anti-NA drugs, resulting in a weakened effect of anti-NA drugs and accelerated viral release.
5. Relationship between the composition of oral commensal flora and infectivity of influenza viruses in the host
Seasonal influenza caused by influenza A and B viruses often causes severe illness and consequently leads to death in those with underlying diseases and in the elderly. Hemagglutinin (HA) and neuraminidase (NA) are expressed as spikes on the surface of influenza A and B viruses, and they play important roles in adsorption and release during viral proliferation (Fig. 3). During adsorption, HA on the surface of the virus binds to sialic acid on the viral receptor expressed on the host's upper respiratory mucosa, allowing the virus to adsorb to the target cell and then be taken up into the target cell for entry. Viral nucleic acids and viral proteins are synthesized in the target cell, and these components are aggregated to form progeny viral particles, which exit the target cell by budding and are moored by sialic acids on the target cell surface. It should be noted that release of the progeny virus does not occur in this state. When the NA on the virus itself degrades sialic acid, which holds the progeny virus in place, the progeny virus then are released from the infected cell and spread to other target cells in the vicinity (Fig. 3).
Fig. 3.
Mechanism of lower airway inflammation exacerbation by oral bacteria.
When oral bacteria, including periodontal pathogens, are aspirated due to poor oral hygiene, lower airway epithelial cells express more PAFR and ACE2, which are receptors for pneumonia-causing bacteria and new coronaviruses, and lower airway epithelial cells also secrete inflammatory cytokines. In addition, aspiration of oral bacteria may induce excessive production of mucin from bronchial glands, leading to a decrease in external respiratory function, and disrupt alveolar and bronchial epithelial barriers, facilitating the entry of viruses and other microorganisms into the subepithelium. Furthermore, inflammatory substances produced by oral tissues may affect systemic organs, including the lungs, via the bloodstream (the effect of chronic periodontitis on systemic chronic inflammation), and aspiration of oral bacteria may cause lung dysbiosis (an imbalance in the symbiosis of the indigenous flora of the lungs). Through these mechanisms, “chronic periodontitis and oral bacteria” may be involved in the development and progression of lower airway diseases.
From this point of view, oral health management, including oral hygiene, is important in the New Normal, and it is thought that by suppressing the harmful effects of chronic periodontitis and oral flora described above, the onset and development of lower airway diseases can be prevented.
Okuda's group has reported that after weekly professional oral care intervention among elderly people, the number of people with influenza in the control group was 9 out of 92 (9.8%), while in the oral care intervention group it was 1 out of 92 (1.0%) [2]. It is thought that professional oral care reduces the number of oral bacteria that secrete NA and, thus, suppresses the number of influenza cases. However, what kind of effects do oral bacteria have on influenza infection? Okuda's group has also hypothesized that proteolitic enzymes produced by periodontal pathogens may disrupt the mucus layer of the airway, which in turn exposes receptors for the virus, thereby promoting infection. Another group has reported that oral bacteria induce apoptosis of respiratory epithelial cells [32], but the relationship between oral bacteria and influenza infectivity is not well understood.
Using an experimental system with cultured cells, NA from oral bacteria (Streptococcus mitis and Streptococcus oralis) helped the NA of influenza viruses to function, resulting in a 20-fold increase in virus replication [33]. The inhibitory effect of zanamivir, an NA inhibitor, on virus release has also been found to be attenuated in the presence of NA-producing oral bacteria. In other words, NA inhibitors that inhibit viral NA activity do not inhibit bacterial NA activity. Therefore, even if NA inhibitors are used for the treatment of influenza, in influenza-infected patients with poor oral hygiene, oral bacterial NA may act as a substitute for viral NA, thereby promoting the release of influenza viruses and helping the spread of influenza (Fig. 3).
In addition, gingipains from P. gingivalis have been found to cleave the HA of influenza viruses and make them infectious (manuscript under review). Immediately after release from infected cells, progeny viruses from infected cells are essentially not infectious because they are not capable of fusing with the membrane of new target cells. However, some proteases, such as trypsin, cleave specific sites on HA, resulting in the cleavage of HA into HA1 and HA2 subunits, and this in turn gives the membrane fusion ability to the newly released progeny virus. These proteases are thought to be mainly derived from the neighboring host tissues surrounding the target cells that have released progeny viruses, but proteases derived from Staphylococcus aureus and Pseudomonas aeruginosa also have this cleavage ability, and those are known to promote viral infection and cause severe influenza. Therefore, oral bacteria may contribute to the spread of influenza infection and the aggravation of infectious disease by facilitating both the adsorption and release of influenza viruses.
6. Relationship between oral commensal flora composition and severity of SARS-CoV-2 infection
The oral mucosae, especially the tongue mucosa, gingival mucosa, and salivary glands, express ACE2, which is the receptor for SARS-CoV-2. Since SARS-CoV-2 infections actually occur in the gingiva and salivary glands [34], and saliva is as useful as nasopharyngeal swabs for detecting COVID-19, the oral cavity is once again attracting attention [13]. Recently, it has been reported that the degree of chronic periodontitis is associated with the severity of COVID-19 and mortality [9]. In fact, oral bacteria, including periodontal pathogens, are detected in the sputum and BALF of COVID-19 patients [7,8]. Since COVID-19 is an inflammation of the lungs, aspiration of oral bacteria may have a negative impact on the development of COVID-19 as well as on that of pneumonia and COPD.
Periodontal pathogens have been found to induce the expression of ACE2 in alveolar epithelial cells at the gene and protein levels (Fig. 1D) [35]. Although it has been reported that the expression of ACE2 in alveolar epithelial cells is increased by stimuli such as tobacco smoke and PM2.5, this report is probably the first to show that the expression is increased by microorganisms. In addition, severe respiratory distress, such as acute respiratory distress syndrome (ARDS), is a major cause of death in COVID-19 patients, and cytokine storm has been implicated as a major factor in causing ARDS. In particular, elevated levels of proinflammatory cytokines and chemokines, such as IL-6, IL-8, and TNF-γ, have been correlated with elevated mortality by ARDS, suggesting that the lungs in critically ill patients are in a state of hyperinflammation. In a previous study, periodontopathogenic bacteria were found to induce large amounts of proinflammatory cytokines at the protein level in respiratory epithelial cells and in mice inoculated with the bacteria intratracheally [[27], [28], [29]]. Therefore, aspiration of periodontal pathogens in COVID-19 patients may cause severe lung inflammation in SARS-CoV-2-infected individuals through two phenomena: 1) increased expression of ACE2 in the lower airway epithelia, which may promote infection of these epithelia with SARS-CoV-2, and 2) induction of large amounts of proinflammatory cytokines in the lower airway epithelia, which, together with pneumonia caused by viral infection, leads to severe lung inflammation in SARS-CoV-2-infected individuals [13,36].
Cleavage of the S-protein by proteases such as TMSRPP2 and furin during SARS-CoV-2 infection is critical for the virus to adsorb to target cells and fuse with membranes, both of which are essential for the virus to enter target cells. Similar to the susceptibility of influenza virus HA to the proteases secreted by periodontal pathogens, the S-protein of SARS-CoV-2 may also be cleaved by the proteases produced by periodontal pathogens (Fig. 2), which is currently under active investigation.
7. Conclusion
In the future, as society ages, the number of people with oral infections, such as chronic periodontitis, and lower respiratory diseases, such as aspiration pneumonia and COPD, is expected to increase. When people live in evacuation centers for a long time after a major earthquake or flood, oral care tends to be inadequate, and the number of people dying from aspiration pneumonia is seen as a social problem. Poor oral hygiene is likely to lead to the inflammation of the lower airway caused by the aspiration of oral bacteria through the mechanisms described previously (Fig. 4). On the other hand, multidisciplinary cooperation, such as medical and dental collaboration, is being promoted to prevent pneumonia and influenza. From the perspective of saving medical costs and deterring the development of drug-resistant bacteria and viruses, infection prevention through oral health care may lead to a decrease in the number of antimicrobial and antiviral drugs administered and, ultimately, to a dramatic reduction in medical costs.
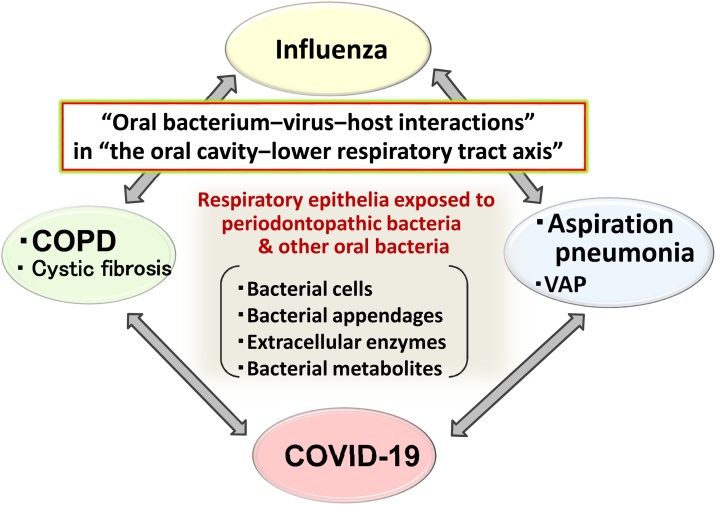
Fig. 4.
Relationship between oral cavity and respiratory diseases: “Oral bacterium–virus–host interactions” in the oral cavity–lower airway axis.
Pneumonia, COPD, influenza, and COVID-19, all of which have been shown to be associated with oral flora, are diseases in which inflammation of the lungs is the main symptom, and there is also an association between diseases. In addition to the mechanisms between chronic periodontitis and systemic chronic inflammation, those of lower airway inflammation caused by oral bacteria, their appendages (e.g., fimbriae and LPS), and their extracellular enzymes should be elucidated, and measures should be developed for their control. For this purpose, it is necessary to examine the etiology of lower airway inflammation from the viewpoint of microbial interactions, such as oral bacteria–pneumonia-causing bacteria, oral bacteria–influenza virus, and oral bacteria–SARS-CoV-2, rather than focusing only on the pathogenicity of a single microorganism.
It is hoped that further accumulation of evidence showing the relationship between oral commensal flora and lower airway diseases will lead to a wider public understanding of the usefulness of oral health management, which in turn will lead to extension of a healthy life expectancy for people.
Funding
This work was supported by JSPS KAKENHI, Uemura Fund, Dental Research Center, Nihon University School of Dentistry, and the Nihon University Multidisciplinary Research Grant for 2021.
Conflicts of interest
None.
References
- 1.Yoneyama T., Yoshida M., Matsui T., Sasaki H. Oral care and pneumonia. Oral Care Working Group. Lancet. 1999;354(9177):515. doi: 10.1016/s0140-6736(05)75550-1. [DOI] [PubMed] [Google Scholar]
- 2.Abe S., Ishihara K., Adachi M., Sasaki H., Tanaka K., Okuda K. Professional oral care reduces influenza infection in elderly. Arch Gerontol Geriatr. 2006;43(2):157–164. doi: 10.1016/j.archger.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Scannapieco F.A., Bush R.B., Paju S. Associations between periodontal disease and risk for nosocomial bacterial pneumonia and chronic obstructive pulmonary disease. A systematic review. Ann Periodontol. 2003;8(1):54–69. doi: 10.1902/annals.2003.8.1.54. [DOI] [PubMed] [Google Scholar]
- 4.Scannapieco F.A., Cantos A. Oral inflammation and infection, and chronic medical diseases: implications for the elderly. Periodontol 2000. 2016;72(1):153–175. doi: 10.1111/prd.12129. [DOI] [PubMed] [Google Scholar]
- 5.Gao Y.D., Ding M., Dong X., Zhang J.J., Azkur A.K., Azkur D. Risk factors for severe and critically ill COVID-19 patients: a review. Allergy. 2021;76(2):428–455. doi: 10.1111/all.14657. [DOI] [PubMed] [Google Scholar]
- 6.Alsaied T., Aboulhosn J.A., Cotts T.B., Daniels C.J., Etheridge S.P., Feltes T.F. Coronavirus disease 2019 (COVID-19) pandemic implications in pediatric and adult congenital heart disease. J Am Heart Assoc. 2020;9(12) doi: 10.1161/JAHA.120.017224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen Z., Xiao Y., Kang L., Ma W., Shi L., Zhang L. Genomic diversity of severe acute respiratory syndrome-coronavirus 2 in patients with coronavirus disease 2019. Clin Infect Dis. 2020;71(15):713–720. doi: 10.1093/cid/ciaa203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ren L.L., Wang Y.M., Wu Z.Q., Xiang Z.C., Guo L., Xu T. Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study. Chin Med J (Engl) 2020;133(9):1015–1024. doi: 10.1097/CM9.0000000000000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marouf N., Cai W., Said K.N., Daas H., Diab H., Chinta V.R. Association between periodontitis and severity of COVID-19 infection: a case-control study. J Clin Periodontol. 2021;48(4):483–491. doi: 10.1111/jcpe.13435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nolte W.E. In: Oral microbiology with basic microbiology and immunology. 3rd ed. Nolte W.E., editor. Mosby; St. Louis: 1977. The oral flora; pp. 197–233. [Google Scholar]
- 11.Hasegawa A., Sato T., Hoshikawa Y., Ishida N., Tanda N., Kawamura Y. Detection and identification of oral anaerobes in intraoperative bronchial fluids of patients with pulmonary carcinoma. Microbiol Immunol. 2014;58(7):375–381. doi: 10.1111/1348-0421.12157. [DOI] [PubMed] [Google Scholar]
- 12.Ishida N., Sato T., Hoshikawa Y., Tanda N., Sasaki K., Kondo T. Microbiota profiling of bronchial fluids of elderly patients with pulmonary carcinoma. J Oral Biosci. 2015;57(2):110–117. [Google Scholar]
- 13.Imai K., Tanaka H. SARS-CoV-2 infection and significance of oral health management in the era of “the New Normal with COVID-19”. Int J Mol Sci. 2021;22(12):6527. doi: 10.3390/ijms22126527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akata K., Yatera K., Yamasaki K., Kawanami T., Naito K., Noguchi S. The significance of oral streptococci in patients with pneumonia with risk factors for aspiration: the bacterial floral analysis of 16S ribosomal RNA gene using bronchoalveolar lavage fluid. BMC Pulm Med. 2016;16(1):79. doi: 10.1186/s12890-016-0235-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Awano S., Ansai T., Takata Y., Soh I., Akifusa S., Hamasaki T. Oral health and mortality risk from pneumonia in the elderly. J Dent Res. 2008;87(4):334–339. doi: 10.1177/154405910808700418. [DOI] [PubMed] [Google Scholar]
- 16.de Melo Neto J.P., Melo M.S., dos Santos-Pereira S.A., Martinez E.F., Okajima L.S., Saba-Chujfi E. Periodontal infections and community-acquired pneumonia: a case-control study. Eur J Clin Microbiol Infect Dis. 2013;32(1):27–32. doi: 10.1007/s10096-012-1710-y. [DOI] [PubMed] [Google Scholar]
- 17.Gomes-Filho I.S., de Oliveira T.F., da Cruz S.S., Passos-Soares Jde S., Trindade S.C., Oliveira M.T. Influence of periodontitis in the development of nosocomial pneumonia: a case control study. J Periodontol. 2014;85(5):e82–e90. doi: 10.1902/jop.2013.130369. [DOI] [PubMed] [Google Scholar]
- 18.Mori H., Hirasawa H., Oda S., Shiga H., Matsuda K., Nakamura M. Oral care reduces incidence of ventilator-associated pneumonia in ICU populations. Intensive Care Med. 2006;32(2):230–236. doi: 10.1007/s00134-005-0014-4. [DOI] [PubMed] [Google Scholar]
- 19.Hyman J.J., Reid B.C. Cigarette smoking, periodontal disease: and chronic obstructive pulmonary disease. J Periodontol. 2004;75(1):9–15. doi: 10.1902/jop.2004.75.1.9. [DOI] [PubMed] [Google Scholar]
- 20.Scannapieco F.A., Ho A.W. Potential associations between chronic respiratory disease and periodontal disease: analysis of National Health and Nutrition Examination Survey III. J Periodontol. 2001;72(1):50–56. doi: 10.1902/jop.2001.72.1.50. [DOI] [PubMed] [Google Scholar]
- 21.Takeuchi K., Matsumoto K., Furuta M., Fukuyama S., Takeshita T., Ogata H. Periodontitis is associated with chronic obstructive pulmonary disease. J Dent Res. 2019;98(5):534–540. doi: 10.1177/0022034519833630. [DOI] [PubMed] [Google Scholar]
- 22.Gomes-Filho I.S., Cruz S.S.D., Trindade S.C., Passos-Soares J.S., Carvalho-Filho P.C., Figueiredo A.C.M.G. Periodontitis and respiratory diseases: a systematic review with meta-analysis. Oral Dis. 2020;26(2):439–446. doi: 10.1111/odi.13228. [DOI] [PubMed] [Google Scholar]
- 23.Kucukcoskun M., Baser U., Oztekin G., Kiyan E., Yalcin F. Initial periodontal treatment for prevention of chronic obstructive pulmonary disease exacerbations. J Periodontol. 2013;84(7):863–870. doi: 10.1902/jop.2012.120399. [DOI] [PubMed] [Google Scholar]
- 24.Zhou X., Han J., Liu Z., Song Y., Wang Z., Sun Z. Effects of periodontal treatment on lung function and exacerbation frequency in patients with chronic obstructive pulmonary disease and chronic periodontitis: a 2-year pilot randomized controlled trial. J Clin Periodontol. 2014;41(6):564–572. doi: 10.1111/jcpe.12247. [DOI] [PubMed] [Google Scholar]
- 25.Kamio N., Hayata M., Tamura M., Tanaka H., Imai K. Porphyromonas gingivalis enhances pneumococcal adhesion to human alveolar epithelial cells by increasing expression of host platelet-activating factor receptor. FEBS Lett. 2021;595(11):1604–1612. doi: 10.1002/1873-3468.14084. [DOI] [PubMed] [Google Scholar]
- 26.Nagaoka K., Yanagihara K., Morinaga Y., Nakamura S., Harada T., Hasegawa H. Prevotella intermedia induces severe bacteremic pneumococcal pneumonia in mice with upregulated platelet-activating factor receptor expression. Infect Immun. 2014;82(2):587–593. doi: 10.1128/IAI.00943-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayata M., Watanabe N., Tamura M., Kamio N., Tanaka H., Nodomi K. The periodontopathic bacterium Fusobacterium nucleatum induced proinflammatory cytokine production by human respiratory epithelial cell lines and in the lower respiratory organs in mice. Cell Physiol Biochem. 2019;53(1):49–61. doi: 10.33594/000000120. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe N., Yokoe S., Ogata Y., Sato S., Imai K. Exposure to Porphyromonas gingivalis induces production of proinflammatory cytokine via TLR2 from human respiratory epithelial cells. J Clin Med. 2020;9(11):3433. doi: 10.3390/jcm9113433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koike R., Cueno M.E., Nodomi K., Tamura M., Kamio N., Tanaka H. Heat-killed Fusobacterium nucleatum triggers varying heme-related inflammatory and stress responses depending on primary human respiratory epithelial cell type. Molecules. 2020;25(17):3839. doi: 10.3390/molecules25173839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miya C., Cueno M.E., Suzuki R., Maruoka S., Gon Y., Kaneko T. Porphyromonas gingivalis gingipains potentially affect MUC5AC gene expression and protein levels in respiratory epithelial cells. FEBS Open Bio. 2021;11(2):446–455. doi: 10.1002/2211-5463.13066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benedyk M., Mydel P.M., Delaleu N., Płaza K., Gawron K., Milewska A. Gingipains: critical factors in the development of aspiration pneumonia caused by Porphyromonas gingivalis. J Innate Immun. 2016;8(2):185–198. doi: 10.1159/000441724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y., Zhou R., Yi Z., Li Y., Fu Y., Zhang Y. Porphyromonas gingivalis induced inflammatory responses and promoted apoptosis in lung epithelial cells infected with H1N1 via the Bcl-2/Bax/caspase-3 signaling pathway. Mol Med Rep. 2018;18(1):97–104. doi: 10.3892/mmr.2018.8983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kamio N., Imai K., Shimizu K., Cueno M.E., Tamura M., Saito Y. Neuraminidase-producing oral mitis group streptococci potentially contribute to influenza viral infection and reduction in antiviral efficacy of zanamivir. Cell Mol Life Sci. 2015;72(2):357–366. doi: 10.1007/s00018-014-1669-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang N., Pérez P., Kato T., Mikami Y., Okuda K., Gilmore R.C. SARS-CoV-2 infection of the oral cavity and saliva. Nat Med. 2021;27(5):892–903. doi: 10.1038/s41591-021-01296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takahashi Y., Watanabe N., Kamio N., Yokoe S., Suzuki R., Sato S. Expression of the SARS-CoV-2 receptor ACE2 and proinflammatory cytokines induced by the periodontopathic bacterium Fusobacterium nucleatum in human respiratory epithelial cells. Int J Mol Sci. 2021;22(3):1352. doi: 10.3390/ijms22031352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takahashi Y., Watanabe N., Kamio N., Kobayashi R., Iinuma T., Imai K. Aspiration of periodontopathic bacteria due to poor oral hygiene potentially contributes to the aggravation of COVID-19. J Oral Sci. 2020;63(1):1–3. doi: 10.2334/josnusd.20-0388. [DOI] [PubMed] [Google Scholar]