Highlights
-
•
Mandarin peel extracts inhibited the growth of A. flavus by up to 52% over 7 days.
-
•
The MIC of mandarin extracts was 300-400 mg mL−1 depending on the extraction solvent.
-
•
Phenolic-rich SPE fractions showed 40% higher antifungal activity than crude extracts.
-
•
Narirutin and hesperidin were most abundant phenolic compounds in mandarin extracts.
Abbreviations: SPE, Solid phase extraction; PDB, Potato dextrose broth; PDA, Potato dextrose agar; CE, Crude extract; WF, Washing fraction; EF, Elution fraction; TPC, Total phenolic content; TFC, Total flavonoids content; MIC, Minimum inhibitory concentration; PMF, Polymethoxylated flavones
Keywords: Antifungal activity, Citrus sp., Aspergillus flavus, Polyphenols, Solid Phase Extraction
Abstract
Aspergillus flavus is a pathogenic fungus associated with food safety issues worldwide. This study investigated the antifungal activity of citrus peel extracts prepared using food-grade solvents (hot water or ethanol). Mandarin (Citrus reticulata) peel ethanol extracts inhibited the mycelial growth of A. flavus (39.60%) more effectively than those of orange (32.31%) and lemon (13.51%) after 7 days of incubation. The growth of A. flavus could be completely inhibited by mandarin extracts at 300–400 mg mL−1, depending on the extraction solvent. Solid-phase extraction (SPE) separated the polyphenol-rich fractions, which showed up to 40% higher antifungal activity than crude extracts. Twelve polyphenols (2 phenolic acids and 10 flavonoids) were identified by HPLC-DAD, narirutin and hesperidin were the most abundant. In conclusion, citrus peels are promising bioresources of antifungal agents with potential applications in food and other industries.
1. Introduction
Mycotoxigenic fungi are considered as major threats to food safety worldwide. The fungi cause food spoilage leading to loss and waste, but can additionally produce toxic mycotoxins that pose serious health problems to both human and livestock (Jing et al., 2014). Aflatoxins are mycotoxins produced by Aspergillus flavus which are of particular concern because of their hepatotoxicity and carcinogenicity (Abdel-Kareem, Rasmey, & Zohri, 2019). A good approach to prevent aflatoxins in food is by inactivating A. flavus and preventing its growth (Liu, Galani Yamdeu, Gong, & Orfila, 2020) The usage of biological metabolites to prevent fungal growth is regarded as a safe, effective and environmental-friendly preventative method (Rasheed et al., 2020).
Citrus (genus Citrus L.) is one of the most important fruit crops, growing widely in tropical and subtropical regions. According to the FAO, >140 million tons of citrus were produced in 2019 (FAOSTATS, 2021). Apart from fresh produce, citrus fruits are processed into juice, canned or dehydrated products, marmalades, jams, and flavouring agents. Around 50–60% of the fruit weight, including peels, seeds and segment membranes, are generated as by-products after processing (Mahato, Sinha, Sharma, Koteswararao, & Cho, 2019). These citrus by-products contain bioactive compounds such as vitamins, minerals, phenolic compounds, terpenoids and dietary fibre (Mahato et al., 2019). Polyphenols are bioactive molecules widely found in plant species, affecting their morphology, growth, reproduction and resistance to pathogens and environmental stresses (Bahorun, Luximon-Ramma, Crozier, & Aruoma, 2004). Flavonoids are the most common group of polyphenols in plants, playing important roles in plant responses (Xi, Fang, Zhao, Jiao, & Zhou, 2014) and also showing antifungal activities (Al Aboody & Mickymaray, 2020). The most abundant flavonoids in citrus have been identified as naringin, hesperidin, narirutin, and neohesperidin (Xu, Liu, Chen, Ye, & Shi, 2009).
Studies have shown antibacterial activity of citrus metabolites towards pathogenic gram positive and gram negative bacteria that cause human diseases (Lemes et al., 2018), animal diseases (El-Desoukey, Saleh, & Alhowamil, 2018), and food spoilage (Ben Hsouna, Ben Halima, Smaoui, & Hamdi, 2017). Regarding antifungal properties, distilled citrus essential oils (mainly containing terpenes) have shown to inhibit Aspergillus sp., Penicillium sp. Fusarium sp., Candida sp., Cladosporium sp., Eurotium sp., and Rhizopus sp. (Jing et al., 2014). Studies on the antifungal activity of citrus polyphenols are limited. One study showed that purified flavanones (naringin at concentration of 68 mg mL−1, hesperidin at 153 mg mL−1 and neohesperidin at 153 mg mL−1) could inhibit growth of A. flavus by 33–41% (Salas, Céliz, Geronazzo, Daz, & Resnik, 2011). These polyphenols are proposed to inhibit the fungus by altering the ultrastructure of the fungal cell walls and endomembrane system (Pok et al., 2020). In addition, these three flavanones were found to reduce the accumulation of aflatoxin B1 produced by Aspergillus sp. by 80% to 100% (Pok et al., 2020). However, there is limited knowledge on the activity of food-grade extracts, which may contain mixtures of polyphenols, as well as other bioactives. Most studies that investigated extraction of polyphenols from plant materials used organic solvents (Karim et al., 2016, Mohotti et al., 2020, Olakunle et al., 2019). In the food industry, the safety and sustainability of these solvents, as well as policy restrictions, calls for green extraction solvents to be considered. Therefore, this study aimed to investigate the antifungal properties of citrus peel extracts, comparing the efficacy of water and ethanol as food-grade extraction solvents. Solid phase extraction (SPE) was then applied to fractionate the crude extracts. The extracts were analysed for total polyphenol, total flavonoid content, as well for polyphenol composition by HPLC. The hypothesis is that citrus fruit peel extracts prepared with food-grade solvents show antifungal bioactivity and can be considered as sustainable antifungal agents for a range of industry applications.
2. Material and methods
2.1. Plant material and chemicals
Orange (Citrus sinensis) and lemon (C. limon) peels were obtained from Biopower Technologies Limited (BioPower, UK). Mandarin (C. reticulata) peel was obtained from Xiangshan Huayu Foodstuffs Co. Ltd (China). The peels were diced, dried at 65 °C and then micronized to particle size of < 150 µm. All the samples were packed in polythene bags and stored at −20 °C until needed.
Amberlite (R) XAD-7HP resin (20–60 mesh), Folin-Ciocalteu, Na2CO3, gallic acid, and sodium acetate were purchased from Sigma (UK). Ethanol was procured from VWR (USA). AlCl3 was purchased from Honeywell (USA). tert-Butylhydroquinone (TBHQ) was from Aldrich. Dimethyl sulfoxide (DMSO) was from Fluorochem (UK). Milli-Q water was used for extraction. Reagents used in HPLC analysis were of analytical grade. Formic acid, acetonitrile was purchased from Merck Life (UK). HPLC grade standards (purity > 97%) were used. Naringenin narirutin, naringin, hesperetin, hesperidin, neohesperidin, quercitrin, rutin, isorhoifolin, rhoifolin, luteolin, cymaroside, didymin, poncirin, eriodictyol, eriocitrin, neoeriocitrin, isorhamnetin, diosmetin, neodiosmin, sinensetin, tangeretin, taxifolin, nobiletin, and protocatechuic acid were purchased from Extrasynthese (France). Quercetin, p-coumaric acid, chlorogenic acid, ferulic acid and gallic acid were obtained from Sigma (UK). Apigenin was purchased from PhytoLab (Germany), p-hydroxybenzoic acid from SΛFC (USA), vanillic acid from Alfa Aesar (USA), and caffeic acid from Cayman Chemical (USA).
2.2. Fungal strain and culture conditions
A. flavus 9643 (non-toxigenic strain that does not produce aflatoxins) was purchased from ATCC (UK). The fungus was cultivated in 24 g L-1potato dextrose broth (PDB, Sigma, UK), for 2 days. Culture medium (0.5 mL) containing conidia was spread on 15 mL of 39 g/L potato dextrose agar (PDA, Sigma, UK) in 9 mm petri dish, the dish was incubated at 30 °C under dark conditions for 7 days, and the diameter of the fungal colony was measured to 0.1 mm with a ruler every day.
2.3. Citrus peel extraction
Ten grams of dried citrus peel powder were extracted in 200 mL of absolute ethanol or water at 60 °C in shaking water bath for 2 h, followed by centrifugation at 3220 g for 10 min. The supernatant was filtered through Whatman #1 filter paper to separate any remaining solid residue. The extraction process was repeated twice. The supernatants were finally collected and combined. For antifungal experiments, the extraction solution was concentrated by evaporation under vacuum using a Genevac (Fisher Scientific, UK) at room temperature, and then freeze-dried (Labconco, UK). The extracts were stored at −20 °C.
2.4. Solid phase extraction (SPE) of crude mandarin extracts
Mandarin peel polyphenols were fractionated by SPE. Pre-conditioned amberlite (R) XAD7HP resin (4.8 g) was added to 8 mL of 5-fold pre-concentrated extracts. The mixture was then allowed to stand for 24 h at room temperature. Later, the resin was washed 3 times with 10 mL distilled water. The unbound extract and washing solutions were collected as the washing fraction (WF). After washing, the resin was eluted 4 times with 10 mL of absolute ethanol. The elution solutions were collected and combined as the elution fraction (EF). Both WF and EF were concentrated in a rotary evaporator (Heidolph, Germany) at 60 °C, and then freeze-dried. All samples were stored at −20 °C until use.
2.5. Determination of the effect of crude and SPE citrus extracts on A. flavus mycelia growth
The antifungal activity of citrus extracts against A. flavus mycelia growth was based on agar dilution test using PDA medium (Prakash et al., 2011). Citrus extracts (150 mg) were dissolved 1 mL of 10% DMSO and added to 15 mL molten PDA at around 50 °C to achieve a final concentration of 10 mg mL−1. PDA medium containing 1 mL of DMSO solution was used as negative control, and 10 mg/mL of tert-Butylhydroquinone (TBHQ) as positive control. To avoid microbial contamination, the PDA medium with extracts was autoclaved at 121 °C for 15 min before pouring into petri dishes. Autoclaving was found not to affect antifungal activity significantly (Supplementary Data S1) Next, a 4 mm medium disc with fungi cut from 2-day-old A. flavus solid culture was placed at the centre of each petri dish. The dishes were placed in an incubator at 30 °C for 7 days. The diameter of the fungal colony was measured every day.
The antifungal activity was calculated as:
Where, Dt: the diameter of fungal colony in the treated dishes; Dc: the diameter of fungal colony in the negative control dishes.
2.6. Effect of concentration on A. flavus mycelia growth of crude mandarin extracts
Requisite amounts of water and ethanol mandarin extracts were dissolved in 15 mL molten PDA to get the final concentration of 5, 10, 50, 100, 200, 300, 400 mg mL−1 followed by autoclaving, plating, inoculation and incubation, as previously described.
2.7. Identification and quantification of polyphenols
2.7.1. Determination of total phenolic content (TPC)
TPC of extracts was determined by the Folin-Ciocalteu reagent method, based on Singleton, Orthofer, and Lamuela-Raventós (1999) with some modifications. Briefly, 10 μL of sample was mixed with 40 μL of Folin-Ciocalteu (12.5%) and 150 μL of Na2CO3 solution (4%). The mixed sample was incubated for 30 min at room temperature in the dark. The absorbance was then measured at 765 nm with plate reader (Tecan, Switzerland). Ten μL of 50% ethanol solution was used as negative control. The same process was applied to the standard solution of gallic acid (7.81–500 mg mL−1) and the obtained standard curve was used to calculate the TPC of samples, expressed as μg gallic acid equivalent (µg GAE).
2.7.2. Determination of total flavonoids content (TFC)
TFC of each extract was determined by aluminium chloride colorimetric assay following the method described by Sembiring, Elya, and Sauriasari (2017) with a slight modification. Briefly, 50 μL of each sample was orderly mixed with 10 μL of 10% AlCl3, 150 μL of 96% ethanol and 10 μL of 1 M sodium acetate. After mixing, the reaction solution was incubated for 45 min at room temperature in the dark. The absorbance was measured at 415 nm with plate reader. Fifty μL of 50% ethanol solution was used as negative control. Serially diluted rutin (6.25–200 mg mL−1) was used to make the standard curve and determine the content of flavonoids, which was expressed as μg rutin equivalents (µg RE).
2.7.3. HPLC analysis of phenolics compounds
The phenolic compounds were determined by HPLC method as previous reported by Huang et al. (2018) with some modifications. The chromatography was carried out with Agilent HPLC 1200 Series comprising an autosampler set at 4 °C, a UV detector (DAD) set at 254, 280, 330 and 540 nm, and a column oven (Agilent Co., UK). Separation of compounds was done on an Agilent Eclipse XDB-C18 4.6 × 250 mm, 5 µm reverse phase column, maintained at 40 °C. The mobile phase consisted of solvent A (0.5% formic acid) and solvent B (acetonitrile) with the flow rate of 0.5 mL/min. Gradient elution was performed as follows: 0–30 min, 10–25% B; 30–40 min, 25–70% B; 40–50 min, 70–90% B; 50–52 min, 90% B; 52–54 min, 10% B. The injection volume was 10 μL. Thirty-four phenolic compounds were identified using their retention time at indicated wavelength (Fig. 2S).
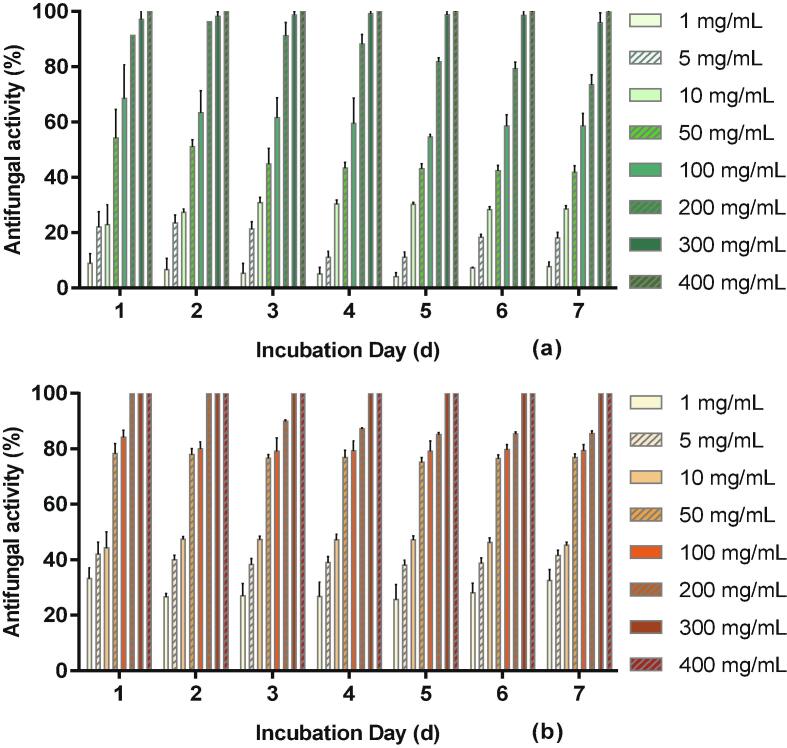
Fig. 2.
Effect of concentrations (mg/mL) of water (a) and ethanol (b) extracts on A. flavus colony growth at 25 °C during 7 days.
2.8. Statistical analysis
Compound analyses and antifungal experiments were carried out in triplicate. Data were analysed using GraphPad 7 and presented as means ± standard deviation. For comparison of antifungal activity of different citrus peel extracts, a two-way ANOVA followed by a Tukey-test (p < 0.05) was used to compare the difference of two factors (treatments × incubation day). For phenolic composition analysis, one-way ANOVA with a posthoc Tukey-test (p < 0.05) was applied to compare the difference between the extraction methods.
3. Results and discussion
3.1. The effect of citrus extracts on A. flavus mycelia growth
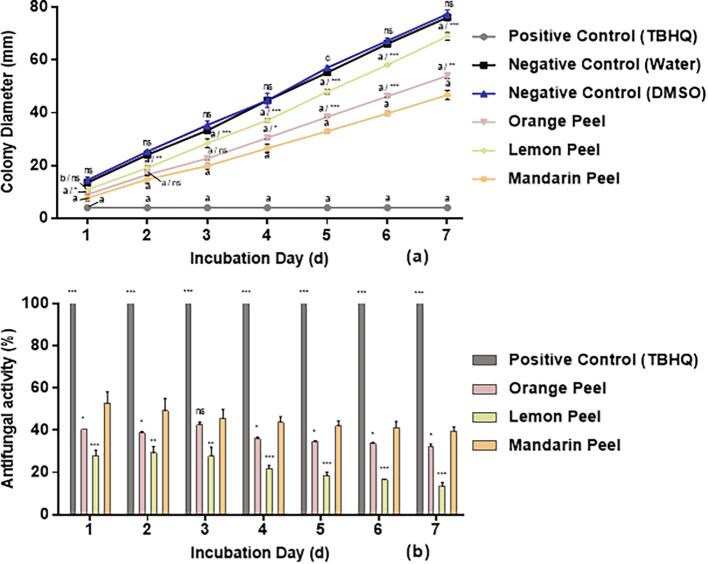
The effect of orange, lemon and mandarin peel ethanol crude extracts at a concentration of 10 mg mL−1 on A. flavus mycelia growth is shown in Fig. 1. Two-way ANOVA analysis showed that there were significant effects (p < 0.0001) of both treatments (positive control and citrus peel extracts) and incubation time on the antifungal activity. Then the pairwise comparison demonstrated that three crude extracts showed significant inhibitory activity (p < 0.05) compared to control (Fig. 1a). Among the extracts (Fig. 1b), mandarin peel extract displayed the highest inhibition activity (ranging from 53 to 40% over 7 days), followed by the orange extract (40–32%). Over 7 days, all extracts showed a decrease in antifungal effect. The activity of mandarin and lemon extracts decreased by 13% on day 7 compared to day 1, while the reduction was relatively lower for orange extract (8%). Our results are similar to the report of Okwu, Awurum, and Okoronkwo (2007) who compared the inhibitory effect of five citrus peel extracts, including C. reticulata, C. aurantifolia, C. limonum, C. sinensis and C. vitis, against F. oxysporum. Among these citrus peel extracts, C. sinensis showed the highest (83.55%) inhibitory effect compared to other extracts (42.15–71.10%). The higher activities observed by these authors suggests that F. oxysporum may be more susceptible to citrus extracts than A. flavus. This could also be due to the differences in origin and varieties of the citrus, which influence their content in antimicrobial compounds. In the present study, the highest antifungal activity was observed for mandarin peel extract, and therefore mandarin was selected for further work.
Fig. 1.
Antifungal activity (%) of ethanol extracts (10 mg/mL) from orange, lemon and mandarin citrus peels, assayed at 25 °C for 7 days. (a) Colony diameter (mm) including disc diameter of 4 mm and (b) antifungal activity (%) compared to TBQH positive control. ‘a, b, c’ represent significant differences comparing each peel extracted samples to negative control (DMSO) for colony diameter; * represents significant differences comparing orange and lemon extracts to mandarin extracts. a/***, p ≤ 0.0001; b/**, p ≤ 0.001; c/*, p ≤ 0.05 and ns, p > 0.05. Results are means of three replicated dishes.
3.2. Dose-dependent inhibitory activity of crude mandarin extracts on mycelia growth of A. flavus
To evaluate the effect of extract concentrations on fungal mycelia growth, water and ethanol crude extracts were added to the PDA medium to a final concentration of 1, 5, 10, 50, 100, 200, 300 and 400 mg mL−1. The results show that the fungal inhibition increased with the concentration of extracts (Fig. 2). A. flavus was 100% inhibited in first two days with 200 mg mL-1of ethanol extract, while antifungal activity decreased about 15% after 5 days. The minimum inhibitory concentration (MIC) of A. flavus for water and ethanol mandarin extracts over 7 days were 400 and 300 mg/mL respectively. The difference might be due to the variation in the composition of samples prepared with different extraction solvents. The preliminary research of the non-aflatoxigenic strain and aflatoxigenic strain (A. flavus NNRL 3375, Cranfield University) suggested that both strains performed similarly at 10 mg mL−1 of mandarin extracts (data not shown). Compared to a report by Oikeh, Omoregie, Oviasogie, and Oriakhi (2016) which investigated the effect of citrus juice concentrates, the MIC in the present study was higher. Three citrus juice concentrates were found to completely suppress A. niger and Penicillum sp. at concentrations ranging 50 to 100 µg mL−1. The differences may be attributed to the concentration and pH differences between juice and peel. Some studies also investigated the minimum fungicidal concentration (MFC) which is the lowest concentration required to prevent the formation of any colony forming units (CFUs), usually by killing 99.98% of the inoculum (Prakash et al., 2011, Prakash et al., 2012). The MFC is normally higher than the MIC. MFCs were no considered in this study, as the MICs were found to be already quite high.
It is also worth noting that the inhibitory effect of ethanol extract was higher than that of water extract at each concentration (Fig. 2). For instance, at the concentration of 10 mg/mL, ethanol extract inhibited the growth of A. flavus by 47% in 7 days, while the inhibition by water extract was about 28%, significantly lower (p < 0.05). This result is in agreement with the findings of Ndonkeu Mangoumou, Nguefack, Galani Yamdeu, Petchayo Tigang, and Amvam Zollo (2013) showing that the activity of ethanol extracts from Cameroonian plants was up to 53% higher than cold water extracts at same concentration. However, water extracts still possessed good levels of antifungal activity and water extraction may be a viable, greener option for industry to produce antifungal agents from citrus peel at scale.
3.3. Antifungal activity of mandarin extracts prepared by SPE
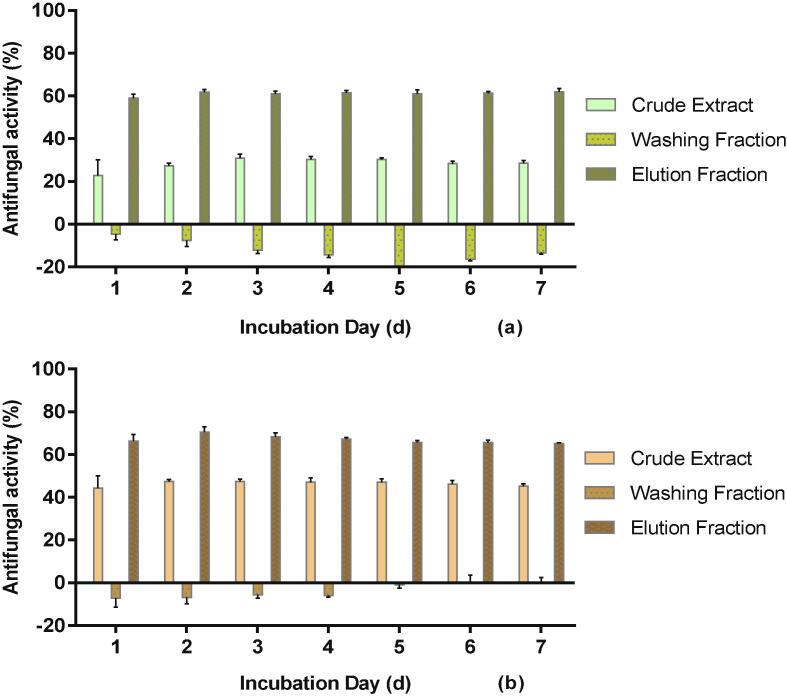
As shown in Fig. 3, the elution fraction (EF) of water extract (10 mg mL−1) inhibited the fungus by 60%; whereas the washing fraction (WF, 10 mg mL−1) promoted fungal growth by 4.61–19.74% after 7 days. Similarly, the EF of ethanol extract inhibited A. flavus growth by 65.13–70.47%, and WF increased fungal growth up to 7.15%. Therefore, SPE technique significantly (p ≤ 0.0001) increased antifungal ability for both water extract (by about 30% compared to the crude) and ethanol extract (by about 20%), presumably by concentrating antifungal components in the EF and removing the ingredients that did not help fungal inactivation (or actually promoted fungal growth) into the WF. In particular sugars, which could promote fungal growth, were washed into the WF (data not shown).
Fig. 3.
Antifungal activity (%) of a) water and b) ethanol mandarin peel extracts and their SPE fractions (10 mg/mL) at 25 °C during 7 days.
3.4. Identification and quantification of polyphenols in mandarin peel extracts
3.4.1. Total phenolic/flavonoid content of crude and SPE extracts
The content of total polyphenols and flavonoids was significantly different between water and ethanol crude extracts (p < 0.05) (Table 1). The results show that water could extract more phenolic compounds than absolute ethanol at 60 °C. In contrast, in the study by Lapornik, Prošek, and Golc Wondra (2005), TPC extracted by organic solvents (70% methanol and ethanol) was higher compared to water extracts. The TPC of crude water and ethanol extracts were 6242.80 and 4244.80 µg GAE, while the TFC were 3149.78 and 2080.72 µg RE respectively. The TPC and TFC measured in the present study were lower compared to those of methanol extracts of Citrus reticulata fruits peel (Zhang et al., 2014).
Table 1.
The total phenolic and total flavonoid content in mandarin peel crude and SPE extracts.
| Solvent |
Crude Extracts |
Washing Fractions |
Elution Fractions |
|||||
|---|---|---|---|---|---|---|---|---|
| Yield (%) |
Water |
42.24 |
33.16 |
5.68 |
||||
| Ethanol |
31.88 |
24.36 |
4.72 |
|||||
| Recovery (%)b | Recovery (%) | Recovery (%) | ||||||
| Phenolic Content | Content in 1 g Peel (µg GAEa) | Water | 6242.80 ± 198.56 | 100 | 2822.00 ± 103.52 | 45.20 | 3253.87 ± 153.60 | 52.12 |
| Content in 1 g Peel (µg GAE) | Ethanol | 4244.80 ± 222.33** | 100 | 1752.53 ± 24.49 | 41.29 | 2457.33 ± 127.27 | 57.89 | |
| Flavonoid Content | Content in 1 g Peel (µg REa) | Water | 3149.78 ± 1.54 | 100 | 243.17 ± 39.98 | 7.72 | 2470.22 ± 36.59 | 78.43 |
| Content in 1 g Peel (µg RE) | Ethanol | 2080.72 ± 35.18*** | 100 | 183.04 ± 30.16 | 8.80 | 1749.42 ± 21.34 | 84.08 | |
Data are presented as means ± SD of triplicate samples. * represents significant differences compare ethanol crude extract to water crude extract. ***, p ≤ 0.0001; **, p ≤ 0.001; *, p ≤ 0.05; ns, p > 0.05.
a) GAE, gallic acid equivalent; RE, rutin equivalent.
b) Recovery (%), the percentage of each fraction recovered from crude extracts.
The moderate-polar resin amberlite (R) XAD7HP used in this study has been previously utilized for polyphenol purification from plant crude extracts, such as olive leaf (Karakaya, 2011) and blackcurrant (Rose et al., 2018). The yield (dry weight as a proportion of crude extract) of the eluting (EF) and washing fractions (WF) after SPE of mandarin extracts are presented in Table. 1. The TFC of water and ethanol in EF were 2470.22 or 1749.42 µg GAE, which was about 78.43% or 84.08% of what is found in crude extracts respectively. However, significant amounts of phenolic compounds were washed into the WF, which were 2822.00 µg GAE for water extracts and 1752.53 µg GAE for ethanol extracts.
The Folin-Ciocalteu method is one of the most extensively used methods for the quantification of phenolic compounds content. However, TPC assay has some limitations. On the one hand, some compounds (e.g. sugars) are able to react with Folin-Ciocalteu reagent (Farooque, Rose, Benohoud, Blackburn, & Rayner, 2018). On the other hand, methoxylated polyphenols do not react with the reagent (Margraf, Karnopp, Rosso, & Granato, 2015). Thus, TPC would overestimate the ‘phenolic content’ when the sample contains high content of sugars, while would underestimate the total phenolic content when the sample has methoxylated polyphenols. Similarly, in TFC reaction, there are two types of complexes formed: acid stable complexes with flavones and flavonols having a C-4 carbonyl group and a C-3/5 hydroxyl group, and acid unstable complexes with catechol hydroxyl groups in the A-ring or B-ring. If the hydroxyl groups are unavailable due to glycosylation or methoxylation, this would prevent chelation with AlCl3 and consequently reduce the bathochromic effect. Thus, while flavones and flavonols are able to be detected with AlCl3 method, polymethoxylated flavones (PMFs) can be hardly measured and flavanones give low response to AlCl3 reagent, which might cause underestimation of the amount of TFC in samples (Huang et al., 2018). Therefore, to accurately determine the content of each phenolic compound in the extracts, HPLC analysis of the various extracts was performed.
3.4.2. Polyphenol composition analysis of crude and SPE extracts
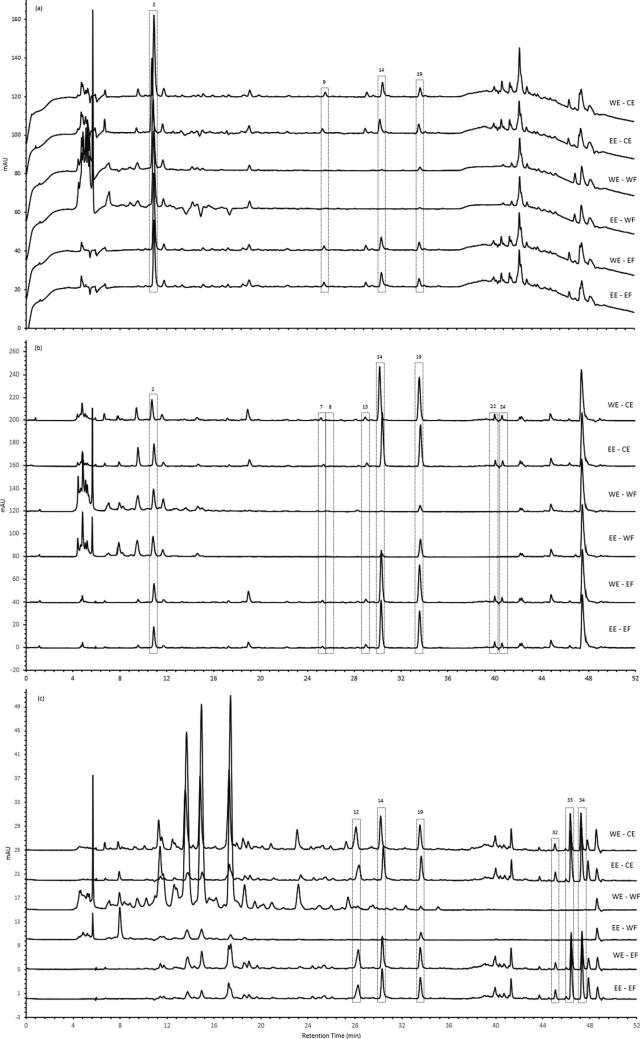
The polyphenol composition of extracts was analysed by HPLC with characteristic chromatograms for mixed standards (Fig. 2S) and mandarin peel extracts (Fig. 4). The compounds identified in each extract are listed in Table 2. They were identified based on their retention time based on major citrus phenolic contents mentioned in the literature (Table 2S). The chemical structure of these compounds is shown in Fig. 5. Mandarin peel extracts contained 12 out of 34 phenolic compounds analysed, including 5 flavanones, 5 PMFs and 2 phenolic acids (Fig. 5). These were identified and determined quantitatively.
Fig. 4.
HPLC chromatogram of mandarin peel extracts at (a) 254 nm, (b) 280 nm and (c) 330 nm. WE, water extract; EE, ethanol extracts; CE, crude extracts; WF, washing fraction; EF, elution fraction. Peaks: 2, Protocatechuic acid (internal standard); 7, ρ-coumaric acid; 8, Eriocitrin; 9, Rutin; 12, Ferulic acid; 13, Taxifolin; 14, Narirutin; 19, Hesperidin; 22, Didymin; 24, Eriodictyol; 32, Sinensetin; 33, Nobiletin; 34, Tangeretin.
Table 2.
Phenolic composition (µg/g of mandarin peel) of mandarin peel crude and SPE extracts with their theoretical partition coefficients (Log10P).
| Peak no |
Compounds | Log10P b) | Water Extract |
Ethanol Extract |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Crude Extracts | Washing Fractions | Elution Fractions | Recovery (%)a) | Crude Extracts | Washing Fractions | Elution Fractions | Recovery (%) | |||
| 7 | p-coumaric acid | 1.43 | 34.00 ± 0.01*** | – | 40.30 ± 11.03 ns | 118.53 | 40.77 ± 0.09 | – | 36.26 ± 0.00 | 88.94 |
| 8 | Eriocitrin | −0.86 | 87.24 ± 0.45*** | – | 82.76 ± 2.01** | 107.15 | 82.60 ± 0.10 | – | 64.78 ± 3.34 | 78.43 |
| 9 | Rutin | −1.06 | 221.90 ± 1.28 ns | – | 211.54 ± 8.38* | 95.33 | 214.50 ± 9.28 | – | 171.06 ± 4.46 | 79.75 |
| 12 | Ferulic acid | 1.25 | 67.05 ± 2.23 ns | – | 55.02 ± 4.78 ns | 82.06 | 65.47 ± 4.56 | – | 60.34 ± 5.57 | 92.16 |
| 13 | Taxifolin | 0.71 | 139.17 ± 6.67 ns | – | 115.66 ± 1.17 ns | 83.11 | 134.36 ± 3.71 | – | 108.93 ± 6.03 | 81.07 |
| 14 | Narirutin | −0.37 | 2155.57 ± 74.03 ns | 6.76 ± 0.71*** | 1962.55 ± 30.90 ns | 91.05 | 2044.46 ± 55.48 | 22.45 ± 1.28 | 1794.81 ± 68.02 | 87.79 |
| 19 | Hesperidin | −0.55 | 1643.60 ± 66.11* | 38.27 ± 1.18*** | 1304.77 ± 9.46 ns | 79.38 | 1346.44 ± 67.78 | 100.12 ± 2.29 | 1231.19 ± 32.72 | 91.44 |
| 22 | Didymin | 0.17 | 52.51 ± 4.40 ns | – | 52.63 ± 2.15 ns | 100.23 | 51.95 ± 44.42 | – | 57.51 ± 4.53 | 110.70 |
| 24 | Eriodictyol | 1.63 | 31.24 ± 0.79 ns | – | 30.25 ± 1.44 ns | 96.83 | 31.10 ± 4.11 | – | 26.72 ± 3.16 | 85.92 |
| 32 | Sinensetin | 3.19 | 82.58 ± 3.38** | – | 76.90 ± 1.18*** | 93.12 | 113.82 ± 4.73 | – | 105.30 ± 1.95 | 92.51 |
| 33 | Nobiletin | 3.37 | 121.09 ± 0.07*** | – | 121.07 ± 0.11*** | 99.98 | 218.02 ± 7.29 | – | 203.81 ± 4.76 | 93.48 |
| 34 | Tangeretin | 3.78 | 40.06 ± 1.93*** | – | 37.66 ± 0.53*** | 94.01 | 74.83 ± 3.01 | – | 70.90 ± 2.12 | 94.75 |
| Total | 4676.02 | 45.03 | 4091.11 | 4418.32 | 122.57 | 3931.62 | ||||
(-) represents the component was not detected in extracts.
Data are presented as means ± SD of triplicate samples. * represents significant differences comparing ethanol extracts to water extracts. ***, p ≤ 0.0001; **, p ≤ 0.001; *, p ≤ 0.05; ns, p > 0.05.
Recovery (%), the percentage of EF recovered from crude extracts.
Theoretical partition coefficients (Log10P), Theoretical log10P theoretical values were obtained via online logP calculator ‘molinspiration’, on Web site: https://molinspiration.com/services/logp.html (accessed in May 2021). The Canonical SMILES used in logP calculation was obtain from ‘PubChem’, on Web site: https://pubchem.ncbi.nlm.nih.gov/ (accessed in May 2021).
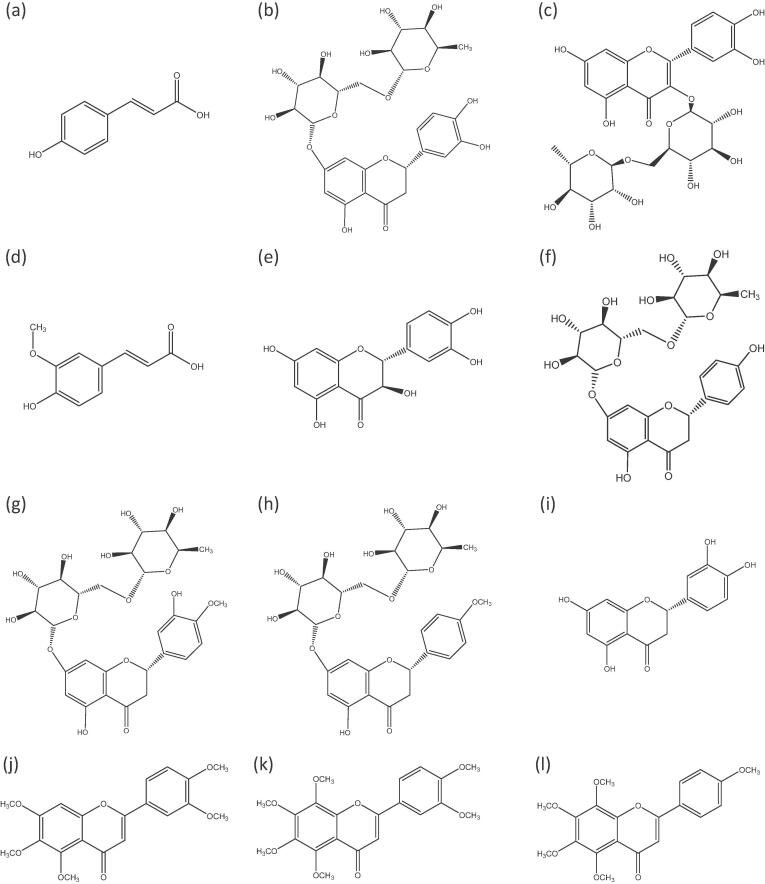
Fig. 5.
Chemical structures of (a) p-Coumaric acid, (b) Eriocitrin, (c) Rutin, (d) Ferulic acid, (e) Taxifolin, (f) Narirutin, (g) Hesperidin, (h) Didymin, (i) Eriodictyol, (j) Sinensetin, (k) Nobiletin and (l) Tangeretin.
Water and ethanol crude extracts were predominantly composed of hesperidin (1643.60 µg g−1 and 1304.77 µg g−1) and narirutin (2155.57 µg g−1 and 2044.46 µg g−1). This was partly in agreement with Zhang et al., 2014, Nogata et al., 2006 which showed high hesperidin content in mandarin fruits, while in this study, narirutin was the most abundant flavonoid in peel extracts. Both crude extracts also contained a non-negligible quantity of rutin (221.90 µg g−1 and 211.54 µg g−1 for water and ethanol extracts, respectively), taxifolin (139.17 µg g−1 and 134.36 µg g−1), sinensetin (82.58 µg g−1 and 113.82 µg g−1), nobiletin (121.09 µg g−1 and 218.02 µg g−1), and low amount of eriocitrin (87.24 µg g−1 and 82.60 µg g−1), didymin (52.51 µg g−1 and 51.95 µg g−1), eriodictyol (31.24 µg g−1 and 31.10 µg g−1), tangeretin (40.06 µg g−1 and 74.83 µg g−1). Flavanones are the typical polyphenols usually accounting for a great proportion in citrus polyphenols. In addition to hesperidin, narirutin, eriocitrin and didymin, there are other flavanones including naringin, neohesperdin, poncirin, naringenin identified in different citrus sp. (Table 4S), but were not found in our mandarin extract. Moreover, PMFs rutin, sinensetin, nobiletin and tangeretin, detected in the present study, were also found in other studies. Other PMFs including isorhoifolin, diosmin, neodiosmin, rhoifolin, luteolin, diosmetin (Table 4S) were previously found in other species, but not in our mandarin extract.
For phenolic acids, only 2 hydroxycinnamic acids were identified as ferulic acid (67.05 µg/g and 65.47 µg/g) and p-coumaric acid (34.00 µg/g and 40.77 µg/g) in both water and ethanol crude extracts. In most of the studies on mandarin phenolic acids, the hydroxycinnamic acid contents were much higher than benzoic acids, which is in agreement with the present study (Xi et al., 2014). Furthermore, there were other hydroxycinnamic acids, benzoic acids and p-hydroxybenzoic acid that have been previously identified in various citrus peels (Table 4S) that have not been found in the mandarin extracts.
These differences in the composition of citrus polyphenols may be caused by the genetic background and/or tissues of fruits, environment factors, and extraction solutions and methods (Nogata et al., 2006, Salas et al., 2011). Therefore, in order to improve the utilization of citrus peel, the extraction technology should be optimized depending on different citrus materials and the target compounds.
There was no big difference in the composition of phenolic compounds extracted by water or ethanol as quantified using HPLC (Table. 2). Phenolic compounds are described as amphiphilic molecules because they usually contain hydroxyl groups that can contribute to the hydrophilic nature of the molecules, as well as ring structures that can lead to their hydrophobicity (Chen et al., 2020). Therefore, aqueous and organic solvent in this study showed similar behaviour for phenolic extraction. When it comes to individual compounds, the difference in water and ethanol extracts could be explained by their Log10P values. Hence, amongst the 12 identified polyphenols, p-coumaric acid (1.43), sinensetin (3.19), nobiletin (3.37) and tangeretin (3.78) have a relatively higher theoretical Log10P value, which correlated with significantly higher (p < 0.05) solubility in ethanol than water. On the contrary, the Log10P of hesperidin and eriocitrin was −0.55 and −1.06, so that significantly more of these compounds were found in water extract (1643.60 and 87.24 µg/g respectively) than ethanol extract (1346.44 and 82.60 µg/g respectively). The rest of the 6 phenolics were extracted by two extraction solutions in similar quantities (p > 0.05), and this corresponded to their Log10P values which are closer to zero compared to above compounds.
XAD-7HP resin is an aliphatic non-ionic acrylic ester polymer with moderate polarity, showing both hydrophobic and hydrophilic behaviours, thus it is a good choice for isolating the components with both characteristics (Farooque et al., 2018, Karakaya, 2011). Polar solvent (water) was used in washing steps to remove polar molecules (e.g. sugars), while nonpolar solvent (ethanol) disrupts the interaction between hydrophobic components and the resin that elutes the adsorbate. According to the theoretical Log10P of narirutin and hesperidin, they have stronger hydrophilic characteristic than other phenolics, so a small amount of them were eluted during washing steps but not others. From present results, XAD-7HP showed a ‘good’ isolation ability of phenolics from crude extracts, as the recovery of each component was between 80 and 120% (hesperidin in water extract was 79.38%, eriocitrin and rutin in ethanol extract was 78.43% and 79.75% respectively). The lost phenolics might be retained in the resin. To improve the elution efficiency, SPE steps could be optimized with gradient elution solutions (Karakaya, 2011).
Compared to Folin-Ciocalteu and AlCl3 method, HPLC analysis more completely and accurately reflected the phenolic compounds in citrus extracts. However, the Folin-Ciocalteu and AlCl3 assays are still among the most common and easy-operated methods for comparing TPC and TFC between samples, and allow monitoring the distribution of phenolic compounds during extraction and SPE processing. Therefore, these two methods still could be applied as quick assays for total phenolic and flavonoid detection in research and industry.
3.4.3. Proposed mechanism of mandarin polyphenols for their fungal inhibition effect
The antifungal properties of the polyphenols maybe attributed to their functionality such as molecule size and functional groups (e.g. number and position of hydroxyl groups, their substitutions, with/without glycosylation and its position (Makarewicz et al., 2021, Sanver et al., 2016). For example, the aglycone hesperitin displayed a higher antifungal activity than the glycoside hesperidin. Meanwhile, neohesperidin has a higher activity than hesperidin, suggesting the configuration of the glycoside also influences activity. Furthermore, polyphenols can be chemically modified. For example, Salas et al. (2011) showed that flavonoids esterified with butyrate and decanoate had higher fungal inhibition ability than unsubstituted molecules, while esterification with stearate impeded the antifungal ability.
Multiple mechanisms underpinning antifungal actions of polyphenolic compounds have been suggested including: 1) inhibition of glycans and chitin biosynthesis resulting in deformation of fungal cell wall, 2) disruption of plasma membrane and its biosynthesis leading to leakage of intracellular components, 3) suppression of fungal nucleic acid metabolism through inhibition of mitochondrial processes, 4) inhibition of metabolic enzymes (Al Aboody and Mickymaray, 2020, Makarewicz et al., 2021). The extract produced in the present study contained a mixture of polyphenols which may act through above through a combination of effects.
In the mandarin extracts, narirutin and hesperidin were the most abundant polyphenols. It was demonstrated that the C2 − C3 double bond in heterocyclic C ring, rendering a planar structure, has stronger interaction with biological membrane components than nonplanar chemical structure, while the attached glycoside groups sterically hinder the interaction (Salas et al., 2011, Sanver et al., 2016). Both narirutin (Fig. 5f) and hesperidin (Fig. 5g) lack of C2 − C3 double bond and have a glycoside group, they may have weaker interaction with cell membrane than rutin (third abundant compound, Fig. 5c). In addition, the aromatic and planar structure of the C ring with a -C O group at position of C4 in C ring allows the formation of a pseudo ring with a –OH group at position of C5 in A ring, leading to the disruption of enzyme binding and activity (Makarewicz et al., 2021). This kind of configuration is present in eriocitrin (Fig. 5b), rutin (Fig. 5c), taxifolin (Fig. 5e), narirutin (Fig. 5f) and eriodictyol (Fig. 5i). Therefore, the most abundant compounds in mandarin extracts may not be the most active, but other less abundant compounds may play the more the important role in antifungal properties. We can also not rule out synergistic effects between the compounds (Pok et al., 2020).
4. Conclusion
The present study provided evidence that citrus peel extracts inhibit the growth of A. flavus, and therefore are good candidates for further investigation as antifungal ingredients. Amongst the citrus extracts investigated, mandarin was the most effective. The antifungal activity of water extracts was less effective than ethanol extracts but still showed considerable activity. The MIC of both extracts was higher than other studies on plant extracts inhibiting A. flavus. The MFC was not determined in this study. SPE was used to fractionate antifungal components from crude extracts and significantly improved antifungal capability. Ten flavonoids and two phenolic acids were identified and quantified in the extracts. Among these phenolic compounds, narirutin and hesperidin was the most dominant in both extracts.
This study provides novel information on antifungal bioactivity of citrus fruit peel extracts prepared with food-grade solvents and potential usage of citrus peel by-products to improve food safety and extend shelf-life. These extracts could developed as antifungal agents for food raw material storage (e.g. cereals, spices), or considered as natural antifungal ingredients added directly to food or packaging material. For practical applications, the experiments need to be scaled up and wider considerations taken (e.g. toxicity, sensory aspects). Future work should elucidate mechanisms of action, and the effect of these extracts on aflatoxin production.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The authors acknowledge the technical support from Sara Viney, Dr Joanne Sier, Miles Ratcliffe, Dr Jin Chu, and Dr Lei Xia. We thank Phil Metcalfe at Biopower Ltd (Milton Keynes, UK) for providing the micronised citrus peels, and Xiangshan Huayu Foodstuffs Co. Ltd (China) for providing dried mandarin peels.
Funding
This project received financial support from the BBSRC and Innovate UK (BB/S020950/1, Citrusafe project) to MB and CO and the BBSRC (BB/P027784/1, AFRICAP project) to JHGY, YYG and CO.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2021.100144.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Abdel-Kareem M.M., Rasmey A.M., Zohri A.A. The action mechanism and biocontrol potentiality of novel isolates of Saccharomyces cerevisiae against the aflatoxigenic Aspergillus flavus. Letters in Applied Microbiology. 2019;68(2):104–111. doi: 10.1111/lam.13105. [DOI] [PubMed] [Google Scholar]
- Al Aboody M.S., Mickymaray S. Anti-Fungal efficacy and mechanisms of flavonoids. Antibiotics (Basel) 2020;9(2):1–42. doi: 10.3390/antibiotics9020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahorun T., Luximon-Ramma A., Crozier A., Aruoma O.I. Total phenol, flavonoid, proanthocyanidin and vitamin C levels and antioxidant activities of Mauritian vegetables. Journal of the Science of Food and Agriculture. 2004;84(12):1553–1561. doi: 10.1002/(ISSN)1097-001010.1002/jsfa.v84:1210.1002/jsfa.1820. [DOI] [Google Scholar]
- Ben Hsouna A., Ben Halima N., Smaoui S., Hamdi N. Citrus lemon essential oil: Chemical composition, antioxidant and antimicrobial activities with its preservative effect against Listeria monocytogenes inoculated in minced beef meat. Lipids in Health and Disease. 2017;16(1):146. doi: 10.1186/s12944-017-0487-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.X., Yang J., Ma L.L., Li J., Shahzad N., Kim C.K. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Science Reports. 2020;10(1):1–9. doi: 10.1038/s41598-020-59451-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Desoukey R.M., Saleh A.S., Alhowamil H.F. The phytochemical and antimicrobial effect of Citrus sinensis (Orange) peel powder extracts on some animal pathogens as eco-friendly. EC Microbiology. 2018:312–318. [Google Scholar]
- FAOSTATS. (2021). Food and Agriculture Organization of the United Nations. http://www.fao.org/faostat/en/#home.
- Farooque S., Rose P.M., Benohoud M., Blackburn R.S., Rayner C.M. Enhancing the potential exploitation of food waste: Extraction, purification, and characterization of renewable specialty chemicals from blackcurrants (Ribes nigrum L.) Journal of Agricultural and Food Chemistry. 2018;66(46):12265–12273. doi: 10.1021/acs.jafc.8b04373. [DOI] [PubMed] [Google Scholar]
- Huang R., Wu W.Y., Shen S.Y., Fan J.W., Chang Y., Chen S.G., Ye X.Q. Evaluation of colorimetric methods for quantification of citrus flavonoids to avoid misuse. Royal Society of Chemistry. 2018;10(22):2575–2587. doi: 10.1039/c8ay00661j. [DOI] [Google Scholar]
- Jing L.i., Lei Z., Li L., Xie R., Xi W., Guan Y.u.…Zhou Z. Antifungal activity of citrus essential oils. Journal of Agricultural and Food Chemistry. 2014;62(14):3011–3033. doi: 10.1021/jf5006148. [DOI] [PubMed] [Google Scholar]
- Karakaya, A. (2011). Purification of Polyphenolic Compounds from Crude Olive Leaf Extract. (Master of Science Master's thesis), İzmir Institute of Technology, Turkey. Retrieved from http://hdl.handle.net/11147/3085.
- Karim H., Boubaker H., Askarne L., Talibi I., Msanda F., Boudyach E.H.…Ait Ben Aoumar A. Antifungal properties of organic extracts of eight Cistus L. species against postharvest citrus sour rot. Letters in Applied Microbiology. 2016;62(1):16–22. doi: 10.1111/lam.12507. [DOI] [PubMed] [Google Scholar]
- Lapornik B., Prošek M., Golc Wondra A. Comparison of extracts prepared from plant by-products using different solvents and extraction time. Journal of Food Engineering. 2005;71(2):214–222. doi: 10.1016/j.jfoodeng.2004.10.036. [DOI] [Google Scholar]
- Lemes R.S., Alves C.C.F., Estevam E.B.B., Santiago M.B., Martins C.H.G., Santos T.…Miranda M. Chemical composition and antibacterial activity of essential oils from Citrus aurantifolia leaves and fruit peel against oral pathogenic bacteria. An Acad Bras Cienc. 2018;90(2):1285–1292. doi: 10.1590/0001-3765201820170847. [DOI] [PubMed] [Google Scholar]
- Liu Yue, Galani Yamdeu Joseph Hubert, Gong Yun Yun, Orfila Caroline. A review of postharvest approaches to reduce fungal and mycotoxin contamination of foods. Comprehensive Reviews in Food Science and Food Safety. 2020:1–40. doi: 10.1111/1541-4337.12562. [DOI] [PubMed] [Google Scholar]
- Mahato N., Sinha M., Sharma K., Koteswararao R., Cho M.H. Modern extraction and purification techniques for obtaining high purity food-grade bioactive compounds and value-added co-products from citrus wastes. Foods. 2019;8(11) doi: 10.3390/foods8110523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarewicz M., Drozdz I., Tarko T., Duda-Chodak A. The interactions between polyphenols and microorganisms, especially gut microbiota. Antioxidants (Basel) 2021;10(2):1–70. doi: 10.3390/antiox10020188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margraf T., Karnopp A.R., Rosso N.D., Granato D. Comparison between Folin-Ciocalteu and prussian blue assays to estimate the total phenolic content of juices and teas using 96-well microplates. Journal of Food Science. 2015;80(11):C2397–C2403. doi: 10.1111/1750-3841.13077. [DOI] [PubMed] [Google Scholar]
- Mohotti S., Rajendran S., Muhammad T., Strömstedt A.A., Adhikari A., Burman R.…Gunasekera S. Screening for bioactive secondary metabolites in Sri Lankan medicinal plants by microfractionation and targeted isolation of antimicrobial flavonoids from Derris scandens. Journal of Ethnopharmacology. 2020;246:112158. doi: 10.1016/j.jep.2019.112158. [DOI] [PubMed] [Google Scholar]
- Ndonkeu Mangoumou G., Nguefack J., Galani Yamdeu J.H., Petchayo Tigang S., Amvam Zollo P.H. Antifungal potential of extracts from three plants against two major pathogens of celery (Apium graveolens L.) in Cameroon. International Journal of Current Research. 2013;5(12):4091–4096. [Google Scholar]
- Nogata Y., Sakamoto K., Shiratsuchi H., Ishii T., Yano M., Ohta H. Flavonoid composition of Friut tissues of citrus species. Bioscience, Biotechnology, and Biochemistry. 2006;70(1):178–192. doi: 10.1271/bbb.70.178. [DOI] [PubMed] [Google Scholar]
- Oikeh E.I., Omoregie E.S., Oviasogie F.E., Oriakhi K. Phytochemical, antimicrobial, and antioxidant activities of different citrus juice concentrates. Food Science & Nutrition. 2016;4(1):103–109. doi: 10.1002/fsn3.2016.4.issue-110.1002/fsn3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okwu D.E., Awurum A.N., Okoronkwo J.I. Phytochemical compositon and in vitro antifungal activity screening of extracts from citrus plants against Fusarium Oxysporum of okra plant (Hibiscus esculentus) Global Science Books. 2007;1(2):145–148. [Google Scholar]
- Olakunle O.O., Deborah J.B., Irene O.J. Antifungal activity and phytochemical analysis of selected fruit peels. Journal of Biology and Medicine. 2019;3(1):040–043. doi: 10.17352/jbm10.17352/jbm.v3i110.17352/jbm.000013. [DOI] [Google Scholar]
- Pok P.S., García Londoño V.A., Vicente S., Romero S.M., Pacín A., Tolaba M.…Resnik S.L. Evaluation of citrus flavonoids against Aspergillus parasiticus in maize: Aflatoxins reduction and ultrastructure alterations. Food Chemisty. 2020;318:126414. doi: 10.1016/j.foodchem.2020.126414. [DOI] [PubMed] [Google Scholar]
- Prakash B., Shukla R., Singh P., Mishra P.K., Dubey N.K., Kharwar R.N. Efficacy of chemically characterized Ocimum gratissimum L. essential oil as an antioxidant and a safe plant based antimicrobial against fungal and aflatoxin B1 contamination of spices. Food Research International. 2011;44(1):385–390. doi: 10.1016/j.foodres.2010.10.002. [DOI] [Google Scholar]
- Prakash B., Singh P., Kedia A., Dubey N.K. Assessment of some essential oils as food preservatives based on antifungal, antiaflatoxin, antioxidant activities and in vivo efficacy in food system. Food Research International. 2012;49(1):201–208. doi: 10.1016/j.foodres.2012.08.020. [DOI] [Google Scholar]
- Rasheed U., Ain Q.U., Yaseen M., Santra S., Yao X., Liu B. Assessing the aflatoxins mitigation efficacy of blueberry pomace biosorbent in buffer. Gastrointestinal Fluids and Model Wine. Toxins (Basel) 2020;12(7):1–21. doi: 10.3390/toxins12070466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose P.M., Cantrill V., Benohoud M., Tidder A., Rayner C.M., Blackburn R.S. Application of Anthocyanins from blackcurrant (Ribes nigrum L.) Fruit waste as renewable hair dyes. Journal of Agricultural and Food Chemistry. 2018;66(26):6790–6798. doi: 10.1021/acs.jafc.8b01044. [DOI] [PubMed] [Google Scholar]
- Salas M.P., Céliz G., Geronazzo H., Daz M., Resnik S.L. Antifungal activity of natural and enzymatically-modified flavonoids isolated from citrus species. Food Chemistry. 2011;124(4):1411–1415. doi: 10.1016/j.foodchem.2010.07.100. [DOI] [Google Scholar]
- Sanver D., Murray B.S., Sadeghpour A., Rappolt M., Nelson A.L. Experimental modeling of flavonoid-biomembrane interactions. Langmuir. 2016;32(49):13234–13243. doi: 10.1021/acs.langmuir.6b02219. [DOI] [PubMed] [Google Scholar]
- Sembiring E.N., Elya B., Sauriasari R. Phytochemical screening, total flavonoid and total phenolic content and antioxidant activity of different parts of Caesalpinia bonduc (L.) roxb. Pharmacognosy Journal. 2017;10(1):123–127. doi: 10.5530/pj10.5530/pj.2018.110.5530/pj.2018.1.22. [DOI] [Google Scholar]
- Singleton V.L., Orthofer R., Lamuela-Raventós R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods in Enzymology. 1999;299:152–178. [Google Scholar]
- Xi W.P., Fang B., Zhao Q.Y., Jiao B.N., Zhou Z.Q. Flavonoid composition and antioxidant activities of Chinese local pummelo (Citrus grandis Osbeck.) varieties. Food Chemistry. 2014;161:230–238. doi: 10.1016/j.foodchem.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Xu G.H., Liu D.H., Chen J.C., Ye X.Q., Shi J. Composition of major flavanone glycosides and antioxidant capacity of three citrus varieties. Journal of Food Biochemistry. 2009;33(4):453–469. doi: 10.1111/j.1745-4514.2009.00230.x. [DOI] [Google Scholar]
- Zhang Y., Sun Y., Xi W., Shen Y., Qiao L., Zhong L.…Zhou Z. Phenolic compositions and antioxidant capacities of Chinese wild mandarin (Citrus reticulata Blanco) fruits. Food Chemistry. 2014;145:674–680. doi: 10.1016/j.foodchem.2013.08.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.