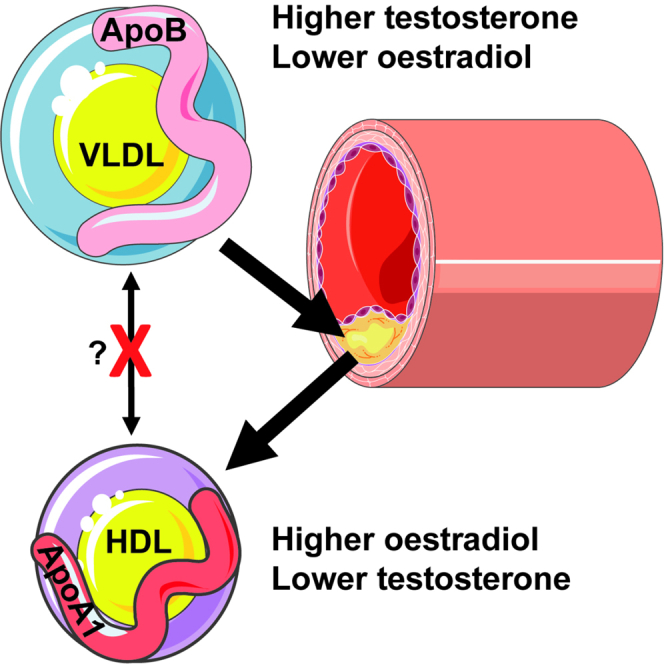
Summary
Women have a reduced cardiovascular disease (CVD) risk compared with men, which could be partially driven by sex hormones influencing lipid levels post puberty. The interrelationship between sex hormones and lipids was explored in pre-pubertal children, young post-pubertal cis-men/women, and transgender individuals on cross-sex-hormone treatment (trans-men/women) using serum metabolomics assessing 149 lipids. High-density lipoproteins (HDL, typically atheroprotective) were significantly increased and very-low- and low-density lipoproteins (typically atherogenic) were significantly decreased in post-pubertal cis-women compared with cis-men. These differences were not observed pre-puberty and were induced appropriately by cross-sex-hormone treatment in transgender individuals, supporting that sex hormones regulate lipid metabolism in vivo. Only atheroprotective apolipoprotein (Apo)A1 expressing lipoproteins (HDL) were differentially expressed between all hormonally unique comparisons. Thus, estradiol drives a typically atheroprotective lipid profile through upregulation of HDL/ApoA1, which could contribute to the sexual dimorphism observed in CVD risk post puberty. Together, this could inform sex-specific therapeutic strategies for CVD management.
Subject areas: Biological sciences, Biochemistry, Metabolomics
Graphical abstract
Highlights
-
•
Estradiol increases typically atheroprotective HDL/ApoA1 in cis- and trans-women
-
•
Differences in HDL/ApoA1 are induced in a dose-dependent manner by estradiol
-
•
Sex differences are not identified pre-puberty and are disrupted in autoimmunity
-
•
Serum lipid metabolites could inform sex-tailored strategies for CVD risk management
Biological sciences; Biochemistry; Metabolomics
Introduction
Prior to menopause, it is known that women have a lower risk of cardiovascular disease (CVD) and coronary heart disease compared with age-matched men; it is reported that women have around half the CVD risk and almost a 10-year delay in the first myocardial infarction event compared with men (Lloyd, 2011; Roger et al., 2011; Wilmot et al., 2015). Various traditional risk factors have been shown to confer differential CVD susceptibilities for men compared with women (Yusuf et al., 2004).
Atherosclerosis, a chronic inflammation of the medium-sized to large arteries, secondary to lipid deposition within the sub-endothelial intimal layer by oxidized apolipoprotein (Apo)B expressing lipoproteins, is a major cause of CVD and mortality. Sex differences in CVD risk could be due to reduced low and very-low-density lipoproteins (LDL and VLDL, typically atherogenic) and increased high-density lipoproteins (HDL, typically atheroprotective) in women, as shown in the Framingham Offspring Study (Freedman et al., 2004). Sex hormones have been proposed to drive these differences mechanistically (Arnold et al., 2017), and there is evidence that early versus late menarche may result in differential long-term cardiovascular traits, including altered lipoprotein levels, in women (Bell et al., 2018). In support of this observation, following menopause a reduction in circulating estrogen levels increases susceptibility to developing metabolic diseases including metabolic syndrome, non-alcoholic fatty liver disease, and diabetes in women (Della Torre et al., 2014). In addition, LDL is increased post menopause in women to the same levels as observed in age-matched men, thus reducing CVD protection, although HDL remains higher in women compared with men at all ages (Matthews et al., 1989; Abbey et al., 1999; Kannel, 1983).
Despite previous studies reporting differences in lipoproteins between men and women, less is known about the sex-biased cardiovascular risk in younger individuals and the specific role of sex hormones in driving these differences post puberty. It is of particular importance to investigate changes in lipoproteins and CVD risk in individuals with gender dysphoria undergoing cross-sex-hormone therapy. In this case, young individuals are issued with gonadotrophin-releasing hormone agonists (“puberty blockers”), followed by testosterone (in those born phenotypically female, trans-men), or estradiol (in those born phenotypically male, trans-women) (Butler et al., 2018). Another group of particular interest are individuals with autoimmune diseases characterized by a significant sex bias such as juvenile systemic lupus erythematosus (JSLE) (with a common onset around puberty and significant female bias), juvenile idiopathic arthritis (JIA) overall (with onset before 16 years of age, with a slight female bias overall, but with clear sex differences in incidence based on the disease phenotype), systemic sclerosis, autoimmune thyroid disease, multiple sclerosis, and rheumatoid arthritis (with strong female predominance and onset in adulthood), suggesting a sex hormone influence in disease etiopathogenesis (Watson et al., 2012; Cattalini et al., 2017; Chiaroni-Clarke et al., 2016), as well as dyslipidemia and increased CVD risk through accelerated atherosclerosis (Barsalou et al., 2013; Schanberg et al., 2009; Coulson et al., 2013). Therefore, both young individuals with gender dysphoria undergoing sex hormone replacement therapy and patients with autoimmunity could be significantly affected by changes in lipoprotein metabolism mediated by sex hormones, which could influence long-term cardiovascular health outcomes; this represents an unmet research need.
Investigating the relationship between sex hormones and lipid metabolism is therefore important for understanding the etiology of atherosclerosis in different physiological and disease settings. Using an unbiased analysis of lipid metabolomics in a cohort of young post-pubertal cis-gendered-men and women, as well as age matched transgender individuals on cross-sex-hormone treatment, we found a direct association between estradiol (in young cis-woman and trans-women) and increased typically atheroprotective HDL subsets and ApoA1, the dominant apolipoprotein associated with HDL. Conversely, testosterone (in young cis-men and trans-men) was associated with an increase in typically atherogenic VLDL subsets and ApoB:ApoA1 ratio. Strikingly, these sex differences in lipids were lost or altered between young post-pubertal men and women with JSLE or JIA, respectively, and were not observed in healthy pre-pubertal children. Together, this indicates that sex hormones are likely to play a crucial role in lipoprotein metabolism, contributing to the sexual dimorphism observed in atherosclerotic and CVD risk post puberty. This detailed knowledge of sex differences in lipoprotein taxonomy post puberty could help inform sex-tailored strategies for cardiovascular risk management from a younger age, potentially leading to a decrease in CVD-related morbidity and mortality overall.
Results
Young post-pubertal men and women have unique serum lipid profiles-associated atherosclerotic risk that is partially reversed by cross-sex-hormone treatment
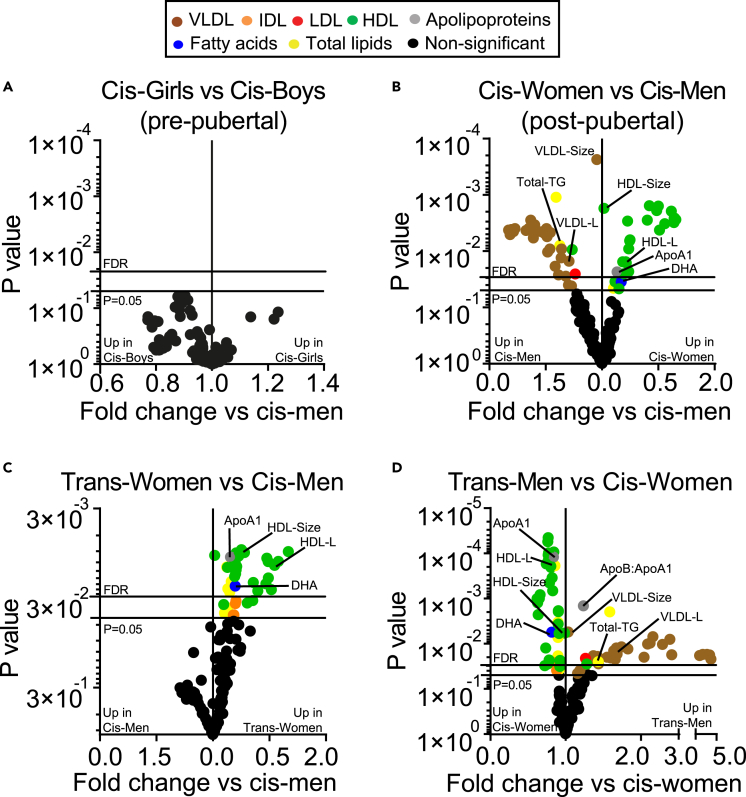
The onset of puberty is known to alter lipid profiles (Freedman et al., 2001, 2004; Fonseca et al., 2019; Mascarenhas et al., 2015; Eissa et al., 2016). In-depth nuclear magnetic resonance (NMR) spectroscopy analysis of 149 serum lipid metabolites was performed on pre-pubertal children and young post-pubertal cis-men and cis-women (Figure 1 for study plan and detailed explanation of study groups, Table S1 for list of metabolites). This analysis accounts for >20 times the number of lipid measures included in a routine serum lipid profile panel. No sex-associated differences were observed in lipid metabolites from age-matched pre-pubertal children (Figure 2A, Table S2 for demographics). By contrast, following false discovery rate (FDR) correction for multiple t-tests, 31 lipid metabolites were significantly increased in young post-pubertal cis-men compared with 22 lipid metabolites increased in age-matched post-pubertal cis-women (Figures 2B and Table 1 for demographics, Table SD1 for full metabolite analysis), strongly supporting a role for sex hormones in altering lipid metabolism. Of note, a significant increase in VLDL particle subsets (VLDL particle concentration and size and VLDL lipid composition including cholesterol, cholesterol esters, free cholesterol, phospholipids, and triglycerides [TG]) and serum TGs was identified in young post-pubertal cis-men. Conversely, in young post-pubertal cis-women a significant increase in HDL particle subsets (HDL particle concentration and size and HDL lipid composition including cholesterol, cholesterol esters, and free cholesterol), HDL-associated ApoA1, polyunsaturated fatty acid (PUFA), and docosahexaenoic acid (DHA) was seen post puberty.
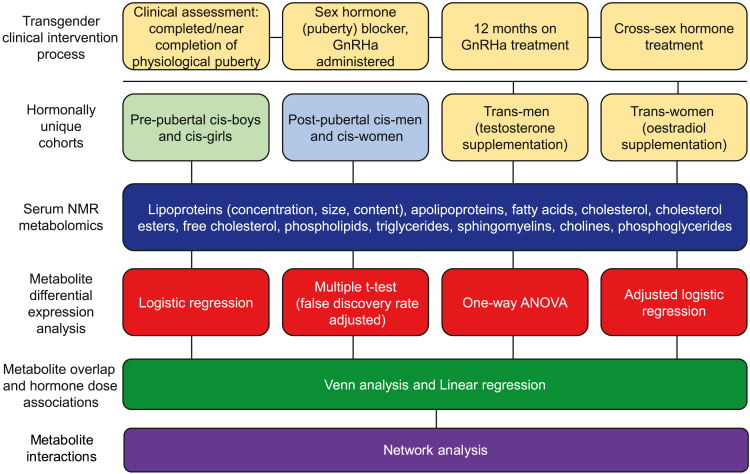
Figure 1.
Study design and analysis flow diagram
Terminology: cis-woman: a person who identifies as female and was also assigned female at birth. This may have been based on genitals and/or having two X chromosomes (XX); cis-man: a person who identifies as male and was also assigned male at birth. This may have been based on genitals and/or having one X and one Y chromosome (XY); trans-woman: a person who identifies as female but was assigned male at birth, on the basis of their genitals and/or having one X and one Y chromosome (XY). Sometimes known as MTF, male-to-female; trans-man: a person who identifies as male but was assigned female at birth, on the basis of their genitals and/or having two X chromosomes (XX). Sometimes known as FTM, female-to-male; puberty blocker: medication that blocks the production of the hormones estrogen and testosterone, which cause female or male puberty, respectively. These are given to young people before they begin taking cross-sex hormones to begin their transition. In this case, GnRHa (gonadotrophin-releasing hormone agonists).
Figure 2.
Young post-pubertal men and women have an altered serum lipid profile driven by hormones. Metabolites were measured in serum by Nightingale Metabolomics (Table S1)
(A–D) Volcano plots displaying fold change of all metabolites and Log10 p values from multiple unpaired t tests comparing (A) pre-pubertal boys and girls (n = 10 and 10), (B) young post-pubertal cis-men and cis-women (n = 15 and 17) (Table SD1), (C) young transgender individuals undergoing cross-sex-hormone treatment with estradiol, trans-woman (n = 25) and young post-pubertal cis-men (assigned male at birth, n = 15) (Table SD2) (D) young transgender individuals undergoing cross-sex-hormone treatment with testosterone, trans-men (n = 26) (Table SD3), and post-pubertal cis-women (assigned female at birth, n = 17). Bottom y axis line, p = 0.05; top y axis line, adjusted p value threshold following 6% false discovery rate (FDR) adjustment for multiple comparisons (Benjamini, Krieger, and Yekutieli approach). Colored dots represent different metabolite groups.
Abbreviations: VL/I/L/HDL, very low/intermediate/low/high-density lipoproteins; Apo, apolipoprotein.
Table 1.
Demographic and clinical comparisons between young post-pubertal cis-men and cis-women and transgender cohorts
| Demographic: | Cis-men | Cis-women | Trans-men | Trans-woman | p Valuea |
|---|---|---|---|---|---|
| Number | 15 | 17 | 26 | 25 | – |
| Age, mean (SD) | 19 (2.73) | 18.94 (3.07) | 18.38 (0.57) | 18.44 (0.87) | 0.6316“ |
| BMI, mean (SD) | 23.99 (2.18) | 22.28 (3.59) | 24.75 (4.38) | 24.03 (4.92) | 0.4757“ |
| Ethnicity, number (%): | |||||
| White | 7 (47) | 8 (47) | 23 (88) | 22 (88) | 0.0008a |
| Asian | 5 (33) | 3 (18) | 0 (0) | 1 (4) | 0.0046a |
| Black | 1 (7) | 1 (6) | 1 (4) | 0 (0) | 0.6608a |
| Other | 2 (13) | 5 (29) | 2 (8) | 2 (8) | 0.1604a |
| Tanner stage at time of sample, n (%): | |||||
| Tanner stage 2-3 | 0 (0) | 0 (0) | – | – | >0.9999a |
| Tanner stage 4-5 | 15 (100) | 17 (100) | – | – | >0.9999a |
| Tanner stage at time of puberty block, n (%): | |||||
| Tanner stage 2-3 | – | – | 1 (4) | 11 (44) | 0.0008a |
| Tanner stage 4-5 | – | – | 25 (96) | 14 (56) | 0.0008a |
| Cross-sex-hormone treatment: | |||||
| Time on treatment (months), mean (SD) | – | – | 11.83 (7.40) | 12.04 (8.34) | 0.9234+ |
| Estradiol valerate (2-6 mg oral/day), n (%) | – | – | – | 19 (76) | – |
| Estradiol/Evorel patch (1.6–6.4 mg/patch), n(%) | – | – | – | 6 (24) | – |
| Estrogen serum level (pmol/L), median (IQR) | – | – | 75.50 (45.5–111.5) | 126.0 (44.0–256.0) | 0.0470+ |
| Sustanon (100–250 mg IM/4 weeks), n (%) | – | – | 23 (88) | – | – |
| Nebido (1,000 mg IM/12 weeks), n (%) | – | – | 2 (8) | – | – |
| Testogel (25 mg/day), n (%) | – | – | 1 (4) | – | – |
| Testosterone serum level (nmol/L), median (IQR) | – | – | 15.5 (6.1–25.8) | 0.4 (0.4–1.1) | <0.0001+ |
| Other medication, number (%): | |||||
| Contraception (combined pill) | – | 2 (12) | – | – | – |
| Anti-depressants | 0 (0) | 0 (0) | 4 (15) | 1 (4) | 0.0862a |
IM, intramuscular.
Fisher's exact (two groups) or chi-square (four groups) test, “one-way ANOVA, or +unpaired t test was used. For transgender individuals, the Tanner stage was their most recent Tanner stage prior to puberty blocking therapy.
To investigate the extent to which sex hormones can influence lipid metabolism, we explored the serum lipid profile of young transgender individuals on puberty blockers and early cross-sex-hormone treatment: trans-men (on puberty blockers and exogenous testosterone) and trans-women (on puberty blockers and exogenous estradiol) (Table 1 for demographics and clinical data). Strikingly, young trans-women had an increase in HDL subsets, ApoA1, and DHA, compared with age-matched cis-men (Figure 2C and Table SD2 for full metabolite analysis), resembling the profile of young post-pubertal cis-women. In contrast, young trans-men had decreased HDL subsets, ApoA1, and DHA; an increased ApoB:ApoA1 ratio; and an up to 4-fold significant increase in VLDL subset expression compared with age matched cis-women (Figure 2D and Table SD3 for full metabolite analysis), resembling the profile of young post-pubertal cis-men. These statistically significant differences were validated by logistic regression analysis (Figure S1). No significant differences in lipid measures were observed between young cis-men and trans-men or between young cis-women and trans-women following FDR correction (Table SD4 for full metabolite analysis). There was no significant difference in body mass index (BMI) between each gender group; however, ethnicity did vary between cis- and trans-gender populations, with trans-men and trans-women being predominately white (Table 1 for demographics and clinical data).
HDL-associated metabolites were significantly influenced by all sex hormone changes
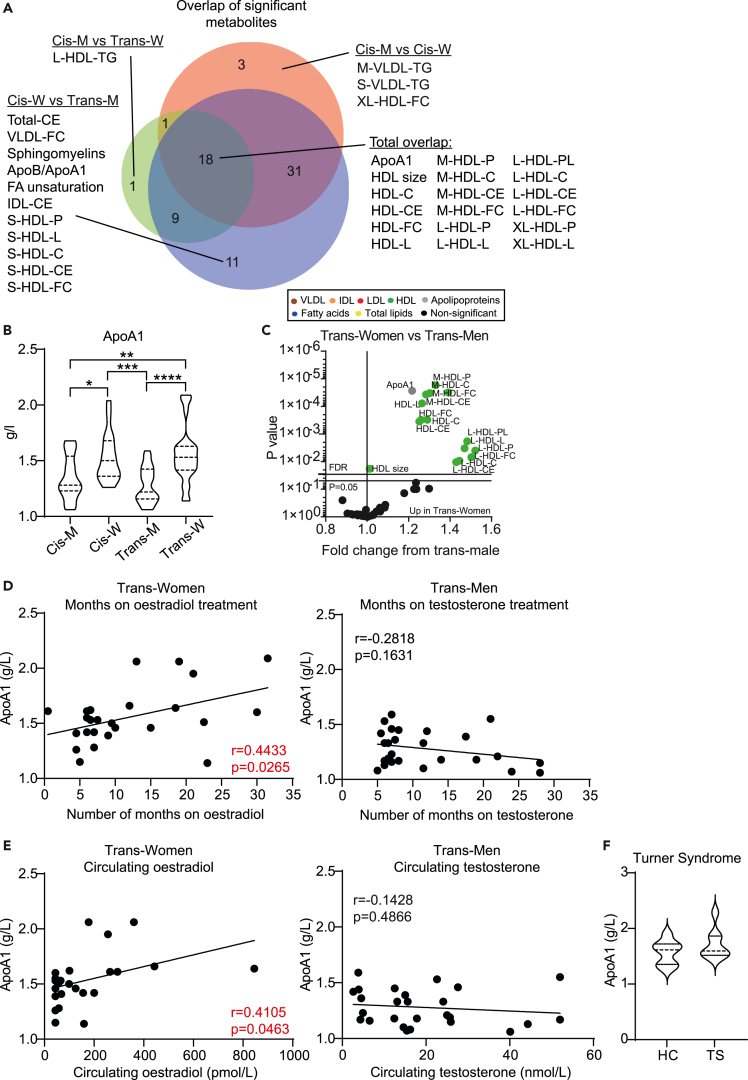
A total of 18 metabolites were significantly altered in all comparisons between hormonally unique adolescent groups using FDR-corrected multiple t tests (Figure 3A) and validation by logistic regression analysis (Figure S1). These metabolites were all HDL subsets (total, medium, large, and extra-large particles) and ApoA1 (Figures 3A and B, Table S3 for full metabolite overlap list, Figure S2 for raw data plots of 18 overlapping metabolites). Furthermore, HDL subsets and ApoA1, but not VLDL subsets, were significantly altered between young trans-women and trans-men (Figure 3C). ApoA1 remained statistically significant in all comparisons when adjusted for ethnicity by logistic regression, despite ethnic differences observed between cohorts (Table 1 for demographics, Table S4 for adjusted logistic regression data). In addition, circulating ApoA1 levels correlated positively with the duration of cross-sex-hormone (estradiol) treatment and with serum estradiol concentration in young trans-women (Figures 3D and E). However, ApoA1 did not correlate with testosterone levels in young trans-men (Figures 3D and E), suggesting that only estradiol may drive changes in HDL expression irrespective of the background sex chromosomes. In support, ApoA1 was not significantly altered in young post-pubertal individuals with Turner syndrome (females with only one functional X sex chromosome, rather than the usual two) compared with age, ethnicity, and puberty-matched young healthy controls (Figure 3F and Table S5 for clinical information).
Figure 3.
HDL metabolites are associated with the presence of circulating estradiol
(A) Venn diagram (http://www.biovenn.nl/) displaying the proportional overlap of statistically significantly altered metabolites (Table S3) that overlap between different gender group comparisons from Figure 2.
(B) Violin plot comparing levels of ApoA1 in young post-pubertal cis-men and cis-women (n = 15 and 17) and in young transgender individuals undergoing cross-sex-hormone treatment with estradiol, trans-women (n = 25), or testosterone, trans-men (n = 26). One-way ANOVA, ∗ = p < 0.05, ∗∗ = p < 0.01, ∗∗∗ = p < 0.001, ∗∗∗∗ = p < 0.0001.
(C) Volcano plot displaying fold change of all lipid metabolites (Table S1) and Log10 p values from multiple unpaired t tests comparing young trans-women (n = 25) with age-matched trans-men (n = 26). Bottom y axis line, p = 0.05; top y axis line, adjusted p value threshold following 6% false discovery rate adjustment for multiple comparisons (Benjamini, Krieger, and Yekutieli approach).
Colored dots represent different metabolite groups.
(D and E) Pearson's correlation between serum ApoA1 levels and (D) number of months on cross-sex-hormone treatment or (E) circulating serum levels of hormones following cross-sex-hormone treatment matched to the time of metabolomic analysis in either young trans-women (n = 25, estradiol treatment) or trans-men (n = 26, testosterone treatment).
(F) Violin plot comparing the level of ApoA1 between young post-pubertal individuals with Turner syndrome (females with only one functional X sex chromosome, rather than the usual two, n = 8) compared with age, ethnicity, and puberty-matched young healthy controls (n = 8). One-way ANOVA.
Abbreviations: M, men; W, women; VL/I/ HDL, very low/intermediate/ high-density lipoproteins; Apo, apolipoprotein; C, cholesterol; CE, cholesterol ester; FC, free cholesterol; L, total lipids; P, particles; PL, phospholipids; HC, healthy control; TS, Turner syndrome.
Lipoprotein interaction networks are altered under different in vivo hormone conditions
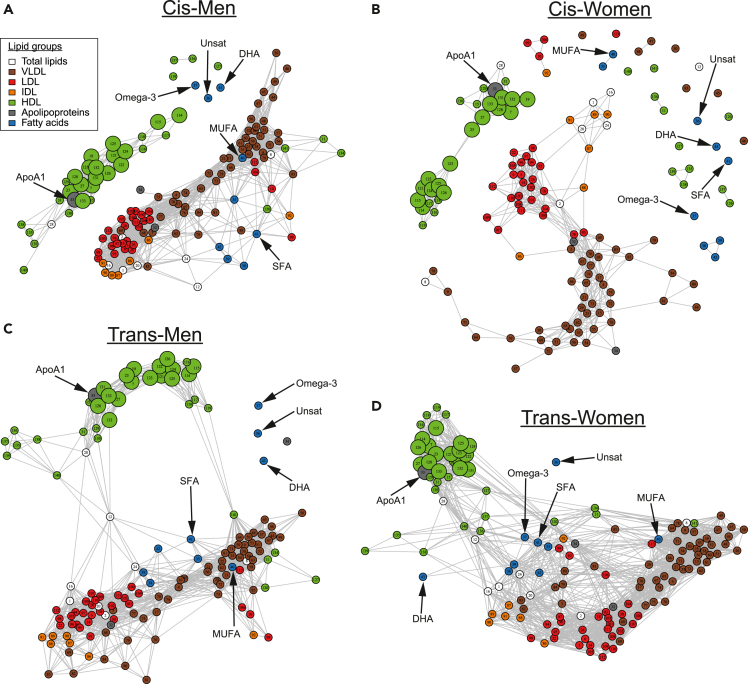
Lipoprotein metabolism is a tightly regulated network, largely co-ordinated by the liver, to control the concentration of circulating lipids and subsequent peripheral tissue lipid uptake and efflux (Zhang et al., 2014). Therefore, we next investigated associative networks between both lipoprotein subsets and other lipid metabolites to explore possible mechanistic metabolite interactions across the hormonally unique groups (Figure 4, Table S6 for key with node numbers matched to metabolites). This could help to explain how sex hormones systemically alter the lipoprotein metabolic network and change metabolites serum concentrations as identified previously. As expected, typically atheroprotective HDL subsets (including the 18 identified as overlapping in Figure 3A, represented as enlarged nodes in Figure 4) and ApoA1, were tightly and separately clustered away from typically atherogenic lipoproteins (LDL, VLDL, and intermediate density lipoprotein, IDL) and ApoB in all of the cohort networks (Figures 4A–4D). The typically atherogenic metabolite group appeared more tightly clustered and incorporated more lipoprotein subsets and fatty acids in young post-pubertal cis-men compared with cis-women (Figures 4A and B). In fact, the network for cis-women excluded multiple small-HDL subsets, extra-large-VLDL subsets, and the majority of high-TG-containing lipoproteins. In the young transgender groups on cross-sex-hormone treatment (Figures 4C and D), the typically atherogenic and atheroprotective metabolite clusters were cross-linked together through correlative relationships. This was more evident in the young trans-women group where many more connections were observed, suggesting that exogeneous estradiol may induce more tightly regulated lipoprotein networks.
Figure 4.
Lipoproteins have altered interaction networks depending on hormone presence
Network analysis displaying correlative relationships between lipid metabolites in (A) young post-pubertal cis-men (n = 15), (B) young post-pubertal cis-women (n = 17), (C) young trans-men (n = 26) undergoing cross-sex-hormone treatment with testosterone, or (D) young trans-women (n = 25) undergoing cross-sex-hormone treatment with estradiol. The high-dimensional undirected graphs were produced using the R package “high dimensional undirected graph estimation” (HUGE). Each of the colored nodes represents a single lipid metabolite variable: a key showing metabolite names corresponding to numbers on the nodes is in Table S6. Edges between nodes show likely correlations between two lipid metabolites, where a dense cluster of nodes implies a stronger relationship between the metabolites than the sparser clustered nodes. Nodes (or small groups of nodes) with no linking edge implies that they are conditionally independent compared with the other metabolites. Enlarged nodes represent lipid metabolites that overlap between all hormone comparisons identified from Figure 3A. Relevant fatty acid metabolites and ApoA1 are labeled with arrows.
Abbreviations: VL/I/L/HDL, very low/intermediate/low/high density lipoproteins; Unsat, unsaturated; SFA, saturated fatty acids; MUFA, monounsaturated fatty acids; ApoA1, Apolipoprotein-A1; DHA, docosahexaenoic acid.
Saturated and monounsaturated fatty acids (SFA and MUFA) were incorporated into the typically atherogenic lipoprotein cluster, whilst total unsaturated fatty acids, omega-3, and DHA were excluded from the network entirely in young cis- and trans-men (Figures 4A and C). In contrast, all fatty acids were excluded from the network in young cis-women and only total unsaturated fatty acids were excluded from the network in young trans-women, where other fatty acid subtypes bridged connections between the clusters (Figures 4B and D).
Together, these data support that sex hormones may play a pivotal but mechanistically complex role in modifying serum lipid metabolism in post-pubertal young individuals, which may contribute to the sex-biased atherosclerotic risk.
Finally, lipoproteins were assessed in post-pubertal adolescents with JSLE and JIA, autoimmune diseases characterized by sex bias and onset at young age, as well as dyslipidemia and increased CVD risk through accelerated atherosclerosis linked to chronic inflammation/medication. This makes them of particular interest for exploring the role of sex hormones and lipoproteins on CVD risk in younger populations with autoimmune conditions. Strikingly, all previously identified post-pubertal sex differences in lipids (Figure 2B) were lost between young age-matched men and women with JSLE (Figure S3A, Table S7 for demographics and clinical data). In JIA, young women maintained the more typically atheroprotective HDL/ApoA1 profile compared with men, whilst men lost the VLDL profile observed in healthy individuals compared with women (Figure S3B, Table S8 for demographics and clinical data). Despite no statistical significance, HDL subsets were reduced, whereas VLDL subsets and TGs were increased in young women with JSLE compared with age-matched healthy women (Figure S3C); interestingly, the opposite was observed for young men with JSLE compared with age-matched healthy men, suggesting possible differential metabolic mechanisms by pathology and cardiovascular implications for patients with JSLE depending on their sex. Similar directional patterns were observed by sex in JIA for lipoproteins compared with age-matched healthy controls (Figure S3D).
These results suggest that sex hormone-driven changes in lipid metabolism as well as impact of low-grade chronic inflammation/stable medication associated with disease could influence the atherosclerotic risk associated with sex-biased chronic autoimmune inflammatory conditions such as JSLE and JIA, even when the autoimmune disease is well controlled (e.g., the majority of patients with low disease activity and none of the patients with JIA were treated with steroids or biologics, whereas patients with JSLE have been treated only with the equivalent of less than 10 mg Prednisolone daily).
Discussion
In this study we identified for the first time that cross-sex-hormone treatment induced early sex-specific lipoprotein and apolipoprotein changes in a dose-dependent manner, supporting a role for sex hormones in lipid metabolism; our data suggest that this may not be significantly affected by sex chromosome dosage and could explain the observation that, following puberty, young men develop a more typically atherogenic lipoprotein profile compared with women, who develop a more typically atheroprotective profile. We also identified that lipoprotein metabolic interaction networks may differ depending on the circulating sex hormone environment and that sex differences in lipid profiles were lost or altered in patients with JSLE or JIA, respectively, suggesting that sex hormone-induced lipoprotein metabolism may be disrupted in autoimmunity. The information reported here may help us to understand why men and women are differentially susceptible to CVD from a young age (Lloyd, 2011; Roger et al., 2011; Wilmot et al., 2015).
It is established that clinical measures of lipoproteins differ between healthy men and pre-menopausal women, where men are at an increased risk of developing CVD (Matthews et al., 1989; Abbey et al., 1999; Kannel, 1983). Despite this, the role of sex hormones in driving these differences in humans has not been explored using detailed metabolomic analysis. In this study, assessing in depth lipid fraction characterization, far greater than available in a clinical setting, we found that VLDL subsets and TGs were significantly increased in young post-pubertal cis-men, whereas HDL subsets, ApoA1, and DHA were increased in cis-women; no differences were observed pre-puberty. This provides evidence that, from the onset of puberty, young cis-men and cis-women are already segregated by unique metabolic signatures that could contribute to a differential CVD risk. In support, a large cohort study of cardiometabolic outcomes post puberty, including lipoprotein measures, showed trends toward increasing CVD traits, including increased VLDL and reduced HDL, in women with delayed menarche (Bell et al., 2018); the study, however, speculated that pre-pubertal BMI and adiposity were significant confounders. Measurement of adiposity was beyond the scope of this study; however, we found no difference in the mean BMI between the cohorts. The INTERHEART study, a standardized case-control study of acute myocardial infarction in 52 countries, showed that abnormal lipids had the highest population attributable risk to CVD in both men (49.5%) and women (47.1%), highlighting the importance of lipids when studying CVD risk factors (Yusuf et al., 2004).
Our study suggests that post-pubertal cis-men may need to consider specific dietary interventions from a younger age to counteract their more typically atherogenic lipid profile. A diet intervention trial where healthy individuals followed a diet rich in PUFAs from childhood to adulthood resulted in a decrease in circulating levels of saturated fat and LDL in both men and women, whereas monounsaturated fat levels decreased in men only (Lehtovirta et al., 2018). We identified lower levels of circulating DHA (a PUFA) in young cis- and trans-men compared with cis-women as well as altered sex-specific network interactions between fatty acids and lipoproteins. This supports a sex-specific differential role for dietary PUFAs in normalizing serum lipid profile; sex differences in DHA have also been reported in a systematic review of 51 publications, thus supporting these findings (Lohner et al., 2013). The Cardiovascular Risk in Young Finns Study, a prospective study following the dietary intake of children through to adulthood, showed that young women had a more favorable diet than young men in terms of cardiovascular health, whereas the men’s diet contained relatively more total, saturated, and monounsaturated fat (Mikkila et al., 2004). The study also showed that the children's diets were a significant determinant of their adult diet, even after the age of 21 years, supporting that improved sex-specific dietary public health advice given to young individuals could improve long-term cardiovascular outcomes, particularly for young cis- and trans-men who have more typically atherogenic lipid profiles as shown by our study.
Speculation that sex hormones may drive changes in lipid metabolism and CVD risk is not new. Studies have shown that the combined oral contraceptive pill (COCP) (combination of estradiol and progesterone) increased circulating TGs and HDL-cholesterol, whereas the progestin-only oral contraception had little effect on the serum lipid profiles (Wang et al., 2016). In the same study, COCP also decreased PUFAs, suggesting a differential role of sex hormones in regulating lipid metabolism. These observations support the association between exogeneous estradiol administration and an atheroprotective lipid serum profile. We found that HDL subsets were increased in young trans-women treated with estradiol (on puberty blockers) and were lost in young trans-men treated with testosterone (on puberty blockers) where an increase in VLDL and LDL was detected. It is well reported that TG-rich VLDL particles are responsible for residual cardiovascular risk (Duran and Pradhan, 2021). Of interest, no difference in VLDL or LDL subsets were seen in young trans-women, suggesting HDL levels and cellular lipid efflux to HDL may be more sensitive to estradiol fluctuations. Negative feedback interactions between HDL and the levels of typically atherogenic lipoproteins may explain why an increase in VLDL was observed following blockade of physiological estradiol production in young trans-men with a gonadotropin-releasing hormone agonist, as shown by our network analysis. Clinical studies of lipids in large transgender cohorts are rare; however, a few small studies have shown contradictory observations regarding routine serum lipid measurements. A study showed that adolescent and adult trans-men develop increased TGs, total cholesterol, and LDL-cholesterol as well as decreased HDL-cholesterol following puberty blockers and cross-sex-hormone treatment with testosterone. Adolescent and adult trans-women in the same study showed decreased total cholesterol and LDL-cholesterol (Fisher, 2016). Another report also identified a decrease in HDL levels in young trans-men but found no difference in the lipid profile of young trans-women compared with matched cis-gendered individuals (Jarin et al., 2017). Discrepancies between studies may be due to differences in circulating exogenous sex hormone concentrations following cross-sex-hormone treatment, length of time on therapy, and conventional and other CVD risk factors, such as BMI, hypertension, smoking, and associated medical conditions (e.g., diabetes or chronic inflammatory conditions). Longitudinal studies will help us to identify the long-term cardio-pathogenic effects of these sex hormone-driven lipid changes in individuals with gender dysphoria (Martinez et al., 2020). Together, these observations could explain why cis-women lose their cardio-protective advantage over cis-men following the cessation of ovarian functions post menopause (Della Torre et al., 2014); in addition, the atheroprotective benefits of estradiol are not age dependent, as there is also evidence that oral estradiol therapy can increase HDL post menopause (Walsh et al., 1999; Shufelt and Manson, 2021).
Although the role of sex hormones in modifying lipid metabolism is more difficult to explore in humans, studies of circulating estradiol in mice have begun to identify potential mechanisms. Della-Torre et al. identified that the reproductive cycle in mice determines the size and efficiency of HDL particles produced by the liver (Della Torre et al., 2016). Smaller, more lipid-efficient HDL are produced during high-estradiol phases of the menstrual cycle, and this results in greater cholesterol efflux from cells. In our study, we observed an increase in medium to large-sized HDL subsets in the presence of estradiol as well as an exclusion of small-HDL subsets and high-TG-containing HDL subsets from the lipid network in young women, suggesting complex mechanistic differences between human and mouse lipoprotein metabolism. It has been shown recently that cholesterol in larger versus smaller HDL particles infer a respective higher versus lower CVD protection humans, whereas TGs in all HDL particle sizes infer a greater CVD risk (Holmes et al., 2018); this supports an increased CVD protection in cis- and trans-women with respect to our analysis and lipid networks. This effect on HDL efflux in mice was due to increased DNA binding of estrogen receptor alpha when estradiol levels were high, speculated to promote the binding and transcriptional activity of Liver-X-Receptors, master regulators of cholesterol metabolism and HDL efflux (Della Torre et al., 2016). This suggests a key mechanism of action for estradiol and HDL metabolism. High plasma levels of estrogen in murine models have also shown to increase de novo lipid clearance of VLDL and LDL, increase synthesis of HDL and alter the expression of lipoprotein modifiers (Villa et al., 2012; Della Torre et al., 2016). In contrast, a study in macrophages taken from trans-women undergoing sex hormone therapy showed that the total HDL efflux decreased by 10.8% and ATP-binding cassette transporter A1 (ABCA1)-mediated HDL efflux by 23.8%; this hormone-driven effect may therefore be tissue specific (van Velzen et al., 2021). In addition, a study has reported that male patients with primary and secondary hypogonadism (low testosterone) presented with lower ABCA1/G1, suggesting an association with reduced cholesterol efflux (Adorni et al., 2019). The liver may therefore be a sexually dimorphic organ providing tight control of systemic lipid metabolism. As for mouse models (Della Torre and Maggi, 2017; Della Torre et al., 2018; Palmisano et al., 2017), investigating the mechanisms by which sex hormones regulate the hepatic transcriptome and function will be a key focus for research going forward in humans. Estradiol levels may also contribute to decreased CVD risk through direct effects on non-hepatic cell types including anti-inflammatory, apoptotic, and oxidative implications (Nofer, 2012). As supported by our study, the sex bias observed in JSLE and JIA and the increased autoimmune-associated CVD risk of patients with JSLE and JIA could therefore be due to a breakdown in estradiol signaling, influencing both atherogenic lipid metabolism and inflammation (Klein and Flanagan, 2016). With this respect, it has been shown in different species and cell culture systems that low levels of estradiol promote Th1-type and cell-mediated immunity, whereas high levels of estradiol promote Th2-type and humoral immune responses, thus adding to the complexity of sex-hormone signaling and inflammation (Straub, 2007). In addition, castration of male versus female mouse models increases versus decreases the respective incidence of autoimmune diseases (Voskuhl, 2011). Although chronic inflammation represents a CVD risk factor alone, changes in lipoprotein metabolism in autoimmunity may also be driven by inflammation of the liver, a master regulator of systemic lipid metabolism, where altered liver function is common in systemic lupus erythematosus (Gibson and Myers, 1975; Matsumoto et al., 2000; Runyon et al., 1980), as well as influenced by medications (such as corticosteroids or other targeted disease-modifying therapies). It is also plausible that testosterone drives atherogenic lipoprotein production and/or inhibits HDL production in men; this remains to be explored mechanistically, although the effects of testosterone replacement therapy on serum lipoproteins has been investigated by several studies with no clear conclusions (Gencer et al., 2021). One study showed that testosterone administration in men increased the activity of hepatic lipase and decreased the levels of HDL and size of LDL (although VLDL was not measured). This could explain the increase in VLDL seen in young trans-men through increased hydrolysis of TGs in chylomicrons to produce VLDL (Herbst et al., 2003). Despite this, treatment with testosterone has also been associated with improved CVD symptoms such as angina and claudication (Gencer et al., 2021). Alternatively, a study has shown that young trans-women on puberty blockers alone (prior to estradiol treatment) experienced a statistically significant decrease in lean mass over 12 months, whereas no difference was seen for young trans-men on puberty blockers alone (Ghelani et al., 2020). This suggests that testosterone may play a greater role in the glycolytic control of muscle development over lipid metabolism.
Limitations of the study
In our study, we had the unique opportunity to investigate the in vivo effects of sex hormones in young donors; this study, however, had some limitations. The cross-sectional design and low sample size may limit the statistical power of some of the metabolite analyses. Thus, it would be important to validate our findings in external cohorts; this will also account for genetic, demographic, and lifestyle differences between populations. In our analysis, a statistically significant difference in ethnicity was identified between cohorts. However, we observed a sustained statistically significant difference in ApoA1 concentration, the dominant apolipoprotein associated with HDL, when ethnicity was adjusted for in the logistic regression analysis. Furthermore, longitudinal data were not available, and analysis was performed at one time point per individual. This was specifically an early time point following initiation of cross-sex-hormone treatment to investigate the initial influences of sex hormones on lipids; this meant that hormone levels rarely reached physiological levels; however, this is common in young individuals where low doses are administered initially and tapered gradually to avoid adverse effects (Meyer et al., 2020). In addition, the stage of menstrual cycle was not recorded for young cis-women and it was beyond the scope of the study to quantify hormone levels in the cis-gendered cohorts. Based on our observations in transgender individuals, differing circulating hormone concentrations per individual may have influenced the lipid levels to some extent, something that is difficult to control in human studies. Heterogeneity in cross-hormone treatments was also difficult to control for as transgender individuals were treated differently based on individual factors, such as tolerability and absorption efficiency of different sex hormone formulations and route of administration, BMI, and rate of development of secondary sex characteristics upon initiation of cross-sex hormone therapy. Here we opted to perform metabolite analysis compared with circulating levels of hormones to account for differences in dose and absorption (and therefore bioavailability). It was beyond the scope of this study to karyotype the cis/transgender individuals; however, both of these cohorts had no clinical features suggestive of genetic disorders. In support, a recent study evaluating the same transgender cohort reported that all 44 transgender individuals evaluated had “normal” karyotypes for their birth genders (Carmichael et al., 2021). It is typical that individuals with Turner syndrome are treated with hormone therapy owing to the reduced/dysfunctional X chromosome; this confounder was therefore difficult to control for in our analysis. Prospective studies for the quantification of the risks and benefits of hormonal treatment are of high demand, particularly regarding the stability of metabolic changes and cardiovascular outcomes. Finally, the data reported here are mostly associative; further functional studies are required to investigate the mechanism of sex hormone regulation of lipid metabolism post puberty.
In conclusion, we report unique changes in lipoprotein metabolism induced by sex hormones following puberty in young healthy donors and in an age-matched cohort of young transgender individuals in the initial stages of cross-sex-hormone treatment. The presence of estradiol (and puberty blockers) was associated with a typically atheroprotective lipoprotein profile, whereas the presence of testosterone (and puberty blockers) was associated with a typically atherogenic lipoprotein profile. Together, this highlights the importance of considering sex determinants in all biological research studies and interventional clinical trials, and the results reported here will help to inform future sex-specific therapeutic considerations for CVD.
STAR★Methods
Key resources table
| REAGENTS or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological samples | ||
| Peripheral blood (serum) from healthy individuals | University College London Hospital and University College London | NA |
| Peripheral blood (serum) from trans-gender individuals with gender dysphoria | University College London Hospital | NA |
| Peripheral blood (serum) from patients with turner syndrome | University College London Hospital | NA |
| Peripheral blood (serum) from patients with JSLE | University College London Hospital or Great Ormond Street Hospital (GOSH) | NA |
| Peripheral blood (serum) from patients with JIA | University College London Hospital or Great Ormond Street Hospital (GOSH) | NA |
| Deposited data | ||
| Metabolomics data from healthy post-pubertal cis-men/women and individuals with gender dysphoria (trans-men/women). | Nightingale Health | Robinson et al. (2021), “Metabolomics data on serum from cis-gender and trans-gender individuals”, Mendeley Data, https://doi.org/10.17632/3nxny453ch.1 |
| Software and algorithms | ||
| Prism v9 | GraphPad | https://www.graphpad.com/ |
| RStudio | R | https://www.R-project.org/ |
| Venn analysis | BioVenn | https://www.biovenn.nl/ |
| High dimensional undirected graph estimation’ (HUGE) | R | https://rdrr.io/cran/huge/ |
| Graphical lasso (glasso) with the stability approach to regularization selection (StARs) | R | (Liu et al., 2010) |
Resource availability
Lead contact
Further information and requests for resources should be directed to and will be fulfilled by the lead contact, Dr Coziana Ciurtin (c.ciurtin@ucl.ac.uk).
Materials availability
This study did not generate unique reagents.
Experimental model and subject details
Terminology describing human cohorts included in the study
Cis-woman
A person who identifies as female and was also assigned female at birth. This may have been based on genitals and/or having two X chromosomes (XX).
Cis-man
A person who identifies as male and was also assigned male at birth. This may have been based on genitals and/or having one X and one Y chromosome (XY).
Trans-woman
A person who identifies as female but was assigned male at birth, on the basis of their genitals and/or having one X and one Y chromosome (XY). Sometimes known as MTF; Male-to-female.
Trans-man
a person who identifies as male but was assigned female at birth, on the basis of their genitals and/or having two X chromosomes (XX). Sometimes known as FTM; Female-to-male.
Peripheral blood was collected from young healthy volunteers either children pre-puberty, recruited if blood was being taken for an unrelated clinical purpose (e.g. surgery for routine, non-inflammatory procedures, Table S2 for demographics and clinical information), or young post-puberty (Tanner stage 4-5), recruited from the local community at science outreach events (Table 1 for demographics and clinical information). Blood was also collected from age matched young transgender individuals undergoing cross-sex-hormone treatment (testosterone in those born phenotypically female; trans-men, or oestradiol in those born phenotypically male; trans-women), recruited from UCLH gender dysphoria endocrine clinics (Table 1 for demographics, clinical and treatment information, Figure 1 for study summary). Under current guidelines for NHS treatment of under 18 s with gender incongruence, transgender individuals were issued with gonadotrophin-releasing hormone agonists (GnRHa, "puberty blockers", either Gonapeptyl or Decapeptyl) for a minimum of 12 months prior to commencement of cross sex hormone treatment. These medications block the production of hormones estrogen and testosterone, which induce female or male puberty, respectively. These are given to young people before they begin taking cross-sex hormones to begin their transition. All transgender individuals had completed or were nearing completion of physiological puberty of their birth assigned sex prior to starting blocker treatment. All individuals in receipt of a puberty blocker and gender-affirming hormones are encouraged to take calcium and vitamin D supplementation. From the same endocrine clinics, 8 age-matched young post-pubertal women with Turner syndrome (only one functional X sex chromosome, rather than the usual two; all displayed the characteristic phenotype associated with a single X chromosome, although they had varying genotypes) were also recruited for blood collection (Table S5 for treatment and genotype information). Finally, peripheral blood was collected from 35 age matched young juvenile systemic lupus erythematosus (JSLE) patients (fulfilling The American College of Rheumatology (ACR) classification criteria for lupus (1997) (Hochberg, 1997) or the Systemic Lupus International Collaborating Clinics (SLICC) criteria (2012) (Petri et al., 2012) and 121 young juvenile idiopathic arthritis (JIA) patients (fulfilling the International League of Associations for Rheumatology criteria (Petty et al., 2004)) (puberty Tanner stage 4-5) attending a young adult or adolescent rheumatology clinic at University College London Hospital (UCLH) or Great Ormond Street Hospital (GOSH) respectively (Table S7 and 8 for demographics and clinical data). Patients on biologic therapy were not included in the cohorts due to their known effects on lipids (Hoffman et al., 2018; Daien et al., 2012; Fernandez-Nebro et al., 2014). Informed written consent was acquired from all donors under the ethical approval reference: REC11/LO/0330. Questionnaires provided the Tanner puberty stage of all donors as well as their current use of contraception and any other relevant medication. All information was stored as anonymised data. Detailed demographic and any clinical characteristics, including hormone levels and treatment details, were recorded from NHS databases and questionnaires.
Method details
Metabolomics
Measures of over 140 serum lipid biomarkers were acquired with an established NMR-spectroscopy platform (Nightingale Health) (Robinson et al., 2019) in the serum of study participants. These included both absolute concentrations (mmol/L) and ratios of lipids and apolipoproteins (g/L). Serum lipids measured included fatty acids and very low, low, intermediate density lipoprotein (VLDL, LDL, IDL), and high-density lipoprotein (HDL) particles of different sizes ranging from extremely large (XXL), very large, large (L), medium (M), small (S), and very small (XS). Lipids within each lipoprotein subclass included total lipid (L), phospholipids (PL), total cholesterol (C), cholesterol esters (CE), free cholesterol (FC), and triglycerides (TGs) (Table S1 for list of biomarkers). BioVenn (http://www.biovenn.nl/) was used to produce proportional Venn diagrams of overlapping metabolites between comparisons.
Quantification and statistical analysis
Statistical analysis was performed using GraphPad Prism-9. Data was tested for normal distribution using Kolmogorov-Smirnov test and parametric/non-parametric tests were used accordingly. Unpaired t-tests and One-way ANOVA (Turkey's post-hoc test) were used as appropriate. In some figures, p values are represented by ∗ = p < 0.05, ∗∗ = p < 0.01, ∗∗∗ = p < 0.001, and ∗∗∗∗ = p < 0.0001. Multiple testing was accounted for using a false discovery rate (FDR) approach (Benjamini, Krieger and Yekutieli) to p values and volcano plots were plotted accordingly. Linear regression was performed using a 95% confidence interval to calculate significance (Pearson correlation). Logistic regression was performed in RStudio (R Core Team, 2018. R: A language and environment for statistical computing. Available online at https://www.R-project.org/) on individual metabolomic biomarkers. For metabolomic data networks, high-dimensional undirected graphs were produced using the R package ‘high dimensional undirected graph estimation’ (HUGE) (Jiang et al., 2019). Data pre-processing, neighborhood screening, graph estimation, and model selection techniques were implanted in the ‘huge’ package pipeline. In the graph estimation stage, graphical lasso (glasso) was used to estimate the sparse inverse covariance matrix, with the stability approach to regularization selection (StARs) (Liu et al., 2010).
Acknowledgments
G.A.R. was supported by a PhD studentship from Lupus UK and The Rosetrees Trust (M409) and by a NIHR UCLH Biomedical Research Centre grant reference BRC773/III/CC/101350. J.P. is supported by Versus Arthritis grant reference 21226. This work was also supported by NIHR UCLH Biomedical Research Center grant reference BRC531/III/IPT/101350 and was performed within the Center for Adolescent Rheumatology Versus Arthritis at UCL UCLH and GOSH supported by grants from Versus Arthritis (21593 and 20164), GOSCC, and the NIHR-Biomedical Research Centres at both GOSH and UCLH. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
Author contributions
Design of research study, E.C.J., I.P.-T., C.C. Acquiring data, G.A.R., H.P., A.R. Recruiting patients, G.B., C.C., H.P., A.R. Analyzing data, G.A.R., J.P., E.C.J., I.P.-T.: Writing the manuscript, G.A.R., E.C.J.: Review of the manuscript, C.C., I.P.-T., G.B. All authors approved the final version.
Declaration of interests
The authors declare no competing interests.
Published: November 19, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.103257.
Contributor Information
George A. Robinson, Email: george.robinson.15@ucl.ac.uk.
Ines Pineda-Torra, Email: i.torra@ucl.ac.uk.
Elizabeth C. Jury, Email: e.jury@ucl.ac.uk.
Coziana Ciurtin, Email: c.ciurtin@ucl.ac.uk.
Supplemental information
Data and code availability
-
•
Metabolomic data can be found at Mendeley Data (https://data.mendeley.com/), with source and identifier detailed in the ‘Key resources table’.
-
•
This paper does not report original code. The source and identifier of analysis code used in the paper can be found in the ‘Key resources table’ and/or ‘Quantification and statistical analysis’ section.
-
•
Any additional information required to reanalyse the data reported in this paper is available from the lead contact upon request.
References
- Abbey M., Owen A., Suzakawa M., Roach P., Nestel P.J. Effects of menopause and hormone replacement therapy on plasma lipids, lipoproteins and LDL-receptor activity. Maturitas. 1999;33:259–269. doi: 10.1016/s0378-5122(99)00054-7. [DOI] [PubMed] [Google Scholar]
- Adorni M.P., Zimetti F., Cangiano B., Vezzoli V., Bernini F., Caruso D., Corsini A., Sirtori C.R., Cariboni A., Bonomi M., Ruscica M. High-density lipoprotein function is reduced in patients affected by genetic or idiopathic hypogonadism. J. Clin. Endocrinol. Metab. 2019;104:3097–3107. doi: 10.1210/jc.2018-02027. [DOI] [PubMed] [Google Scholar]
- Arnold A.P., Cassis L.A., Eghbali M., Reue K., Sandberg K. Sex hormones and sex chromosomes cause sex differences in the development of cardiovascular diseases. Arterioscler. Thromb. Vasc. Biol. 2017;37:746–756. doi: 10.1161/ATVBAHA.116.307301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsalou J., Bradley T.J., Silverman E.D. Cardiovascular risk in pediatric-onset rheumatological diseases. Arthritis Res. Ther. 2013;15:12. doi: 10.1186/ar4212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J.A., Carslake D., Wade K.H., Richmond R.C., Langdon R.J., Vincent E.E., Holmes M.V., Timpson N.J., Smith G.D. Influence of puberty timing on adiposity and cardiometabolic traits: a Mendelian randomisation study. PLoS Med. 2018;15:25. doi: 10.1371/journal.pmed.1002641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler G., De Graaf N., Wren B., Carmichael P. Assessment and support of children and adolescents with gender dysphoria. Arch. Dis. Child. 2018;103:631. doi: 10.1136/archdischild-2018-314992. [DOI] [PubMed] [Google Scholar]
- Carmichael P., Butler G., Masic U., Cole T.J., De Stavola B.L., Davidson S., Skageberg E.M., Khadr S., Viner R.M. Short-term outcomes of pubertal suppression in a selected cohort of 12 to 15 year old young people with persistent gender dysphoria in the UK. PLoS One. 2021;16:e0243894. doi: 10.1371/journal.pone.0243894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattalini M., Soliani M., Caparello M.C., Cimaz R. Sex differences in pediatric rheumatology. Clin. Rev. Allergy Immunol. 2017;56:293–307. doi: 10.1007/s12016-017-8642-3. [DOI] [PubMed] [Google Scholar]
- Chiaroni-Clarke R.C., Munro J.E., Ellis J.A. Sex bias in paediatric autoimmune disease - not just about sex hormones? J. Autoimmun. 2016;69:12–23. doi: 10.1016/j.jaut.2016.02.011. [DOI] [PubMed] [Google Scholar]
- Coulson E.J., Ng W.F., Goff I., Foster H.E. Cardiovascular risk in juvenile idiopathic arthritis. Rheumatology. 2013;52:1163–1171. doi: 10.1093/rheumatology/ket106. [DOI] [PubMed] [Google Scholar]
- Daien C.I., Duny Y., Barnetche T., Daures J.P., Combe B., Morel J. Effect of TNF inhibitors on lipid profile in rheumatoid arthritis: a systematic review with meta-analysis. Ann. Rheum. Dis. 2012;71:862–868. doi: 10.1136/annrheumdis-2011-201148. [DOI] [PubMed] [Google Scholar]
- Della Torre S., Benedusi V., Fontana R., Maggi A. Energy metabolism and fertility-a balance preserved for female health. Nat. Rev. Endocrinol. 2014;10:13–23. doi: 10.1038/nrendo.2013.203. [DOI] [PubMed] [Google Scholar]
- Della Torre S., Maggi A. Sex differences: a resultant of an evolutionary pressure? Cell Metab. 2017;25:499–505. doi: 10.1016/j.cmet.2017.01.006. [DOI] [PubMed] [Google Scholar]
- Della Torre S., Mitro N., Meda C., Lolli F., Pedretti S., Barcella M., Ottobrini L., Metzger D., Caruso D., Maggi A. Short-term fasting reveals amino acid metabolism as a major sex-discriminating factor in the liver. Cell Metab. 2018;28:256–+. doi: 10.1016/j.cmet.2018.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Torre S., Mitro N., Fontana R., Gomaraschi M., Favari E., Recordati C., Lolli F., Quagliarini F., Meda C., Ohlsson C. An essential role for liver ERα in coupling hepatic metabolism to the reproductive cycle. Cell Rep. 2016;15:360–371. doi: 10.1016/j.celrep.2016.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran E.K., Pradhan A.D. Triglyceride-rich lipoprotein remnants and cardiovascular disease. Clin. Chem. 2021;67:183–196. doi: 10.1093/clinchem/hvaa296. [DOI] [PubMed] [Google Scholar]
- Eissa M.A., Mihalopoulos N.L., Holubkov R., Dai S.F., Labarthe D.R. Changes in fasting lipids during puberty. J. Pediatr. 2016;170:199–205. doi: 10.1016/j.jpeds.2015.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Nebro A., Marenco J.L., Lopez-Longo F., Galindo M., Hernandez-Cruz B.E., Narvaez J., Rua-Figueroa I., Raya-Alvarez E., Zea A., Freire M. The effects of rituximab on the lipid profile of patients with active systemic lupus erythematosus: results from a nationwide cohort in Spain (LESIMAB) Lupus. 2014;23:1014–1022. doi: 10.1177/0961203314534909. [DOI] [PubMed] [Google Scholar]
- Fisher A.D. Cross-sex hormone therapy in trans persons is safe and effective at short-time follow-up: results from the European Network for the Investigation of Gender Incongruence (vol 11, pg 1999, 2014) J. Sex. Med. 2016;13:732. doi: 10.1111/jsm.12571. [DOI] [PubMed] [Google Scholar]
- Fonseca M.J., Oliveira A., Azevedo I., Nunes J., Santos A.C. Association of pubertal development with adiposity and cardiometabolic health in girls and boys-findings from the generation XXI birth cohort. J. Adolesc. Health. 2019;65:558–563. doi: 10.1016/j.jadohealth.2019.05.014. [DOI] [PubMed] [Google Scholar]
- Freedman D.S., Bowman B.A., Srinivasan S.R., Berenson G.S., Otvos J.D. Distribution and correlates of high-density lipoprotein subclasses among children and adolescents. Metabolism. 2001;50:370–376. doi: 10.1053/meta.2001.21027. [DOI] [PubMed] [Google Scholar]
- Freedman D.S., Otvos J.D., Jeyarajah E.J., Shalaurova I., Cupples L.A., Parise H., D'Agostino R.B., Wilson P.W.F., Schaefer E.J. Sex and age differences in lipoprotein subclasses measured by nuclear magnetic resonance spectroscopy: the Framingham study. Clin. Chem. 2004;50:1189–1200. doi: 10.1373/clinchem.2004.032763. [DOI] [PubMed] [Google Scholar]
- Gencer B., Bonomi M., Adorni M.P., Sirtori C.R., Mach F., Ruscica M. Cardiovascular risk and testosterone - from subclinical atherosclerosis to lipoprotein function to heart failure. Rev. Endocr. Metab. Disord. 2021;22:257–274. doi: 10.1007/s11154-021-09628-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghelani R., Lim C., Brain C., Fewtrell M., Butler G. Sudden sex hormone withdrawal and the effects on body composition in late pubertal adolescents with gender dysphoria. J. Pediatr. Endocrinol. Metab. 2020;33:107–112. doi: 10.1515/jpem-2019-0045. [DOI] [PubMed] [Google Scholar]
- Gibson T., Myers A.R. Subclinical liver-disease IN systemic lupus-erythematosus. Scand. J. Rheumatol. 1975;4:112. [PubMed] [Google Scholar]
- Herbst K.L., Amory J.K., Brunzell J.D., Chansky H.A., Bremner W.J. Testosterone administration to men increases hepatic lipase activity and decreases HDL and LDL size in 3 wk. Am. J. Physiol. Endocrinol. Metab. 2003;284:E1112–E1118. doi: 10.1152/ajpendo.00524.2002. [DOI] [PubMed] [Google Scholar]
- Hochberg M.C. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- Hoffman E., Rahat M.A., Feld J., Elias M., Rosner I., Kaly L., Lavi I., Zisman D. Effects of tocilizumab, an anti-interleukin-6 receptor antibody, on serum lipid and adipokine levels IN patients with rheumatoid arthritis. Ann. Rheum. Dis. 2018;77:318. doi: 10.3390/ijms20184633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes M.V., Millwood I.Y., Kartsonaki C., Hill M.R., Bennett D.A., Boxall R., Guo Y., Xu X., Bian Z., Hu R.Y. Lipids, lipoproteins, and metabolites and risk of myocardial infarction and stroke. J. Am. Coll. Cardiol. 2018;71:620–632. doi: 10.1016/j.jacc.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarin J., Pine-Twaddell E., Trotman G., Stevens J., Conard L.A., Tefera E., Gomez-Lobo V. Cross-sex hormones and metabolic parameters in adolescents with gender dysphoria. Pediatrics. 2017;139:e20163173. doi: 10.1542/peds.2016-3173. [DOI] [PubMed] [Google Scholar]
- Jiang H., Fei X., Liu H., Roeder K., Lafferty J., Wasserman L. Huge: high- dimensional undirected graph estimation version 1.3.4 from CRAN. 2019. https://rdrr.io/cran/huge/2020
- Kannel W.B. Citation classic - serum-cholesterol, lipoproteins, and the risk OF coronary heart-disease - the framingham-study. Curr. Cont. Life Sci. 1983;29:18. doi: 10.7326/0003-4819-74-1-1. [DOI] [PubMed] [Google Scholar]
- Klein S.L., Flanagan K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016;16:626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- Lehtovirta M., Pahkala K., Niinikoski H., Kangas A.J., Soininen P., Lagstrom H., Viikari J.S.A., Ronnemaa T., Jula A., Ala-Korpela M. Effect of dietary counseling on a comprehensive metabolic profile from childhood to adulthood. J. Pediatr. 2018;195:190–+. doi: 10.1016/j.jpeds.2017.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Roeder K., Wasserman L. Stability approach to regularization selection (StARS) for high dimensional graphical models. Adv. Neural Inf. Process. Syst. 2010;24:1432–1440. [PMC free article] [PubMed] [Google Scholar]
- Lloyd J. Heart disease and stroke statistics-2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee (vol 119, pg e21, 2009) Circulation. 2011;124:E424. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- Lohner S., Fekete K., Marosvolgyi T., Decsi T. Gender differences in the long-chain polyunsaturated fatty acid status: systematic review of 51 publications. Ann. Nutr. Metab. 2013;62:98–112. doi: 10.1159/000345599. [DOI] [PubMed] [Google Scholar]
- Martinez C., Rikhi R., Haque T., Fazal A., Kolber M., Hurwitz B.E., Schneiderman N., Brown T.T. Gender identity, hormone therapy, and cardiovascular disease risk. Curr. Probl. Cardiol. 2020;45:1–24. doi: 10.1016/j.cpcardiol.2018.09.003. [DOI] [PubMed] [Google Scholar]
- Mascarenhas L.P.G., Leite N., Titski A., Brito L.M.S., Boguszewski M.C.S. Variability of lipid and lipoprotein concentrations during puberty in Brazilian boys. J. Pediatr. Endocrinol. Metab. 2015;28:125–131. doi: 10.1515/jpem-2013-0450. [DOI] [PubMed] [Google Scholar]
- Matsumoto T., Kobayashi S., Shimizu H., Nakajima M., Watanabe S., Kitami N., Sato N., Abe H., Aoki Y., Hoshi T., Hashimoto H. The liver in collagen diseases: pathologic study of 160 cases with particular reference to hepatic arteritis, primary biliary cirrhosis, autoimmune hepatitis and nodular regenerative hyperplasia of the liver. Liver. 2000;20:366–373. doi: 10.1034/j.1600-0676.2000.020005366.x. [DOI] [PubMed] [Google Scholar]
- Matthews K.A., Meilahn E., Kuller L.H., Kelsey S.F., Caggiula A.W., Wing R.R. Menopause and risk-factors for coronary heart-disease. N. Engl. J. Med. 1989;321:641–646. doi: 10.1056/NEJM198909073211004. [DOI] [PubMed] [Google Scholar]
- Meyer G., Boczek U., Bojunga J. Hormonal gender reassignment treatment for gender dysphoria. Dtsch. Arztebl. Int. 2020;117:725–+. doi: 10.3238/arztebl.2020.0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkila V., Rasanen L., Raitakari O.T., Pietinen P., Viikari J. Longitudinal changes in diet from childhood into adulthood with respect to risk of cardiovascular diseases: the Cardiovascular Risk in Young Finns Study. Eur. J. Clin. Nutr. 2004;58:1038–1045. doi: 10.1038/sj.ejcn.1601929. [DOI] [PubMed] [Google Scholar]
- Nofer J.R. Estrogens and atherosclerosis: insights from animal models and cell systems. J. Mol. Endocrinol. 2012;48:R13–R29. doi: 10.1530/JME-11-0145. [DOI] [PubMed] [Google Scholar]
- Palmisano B.T., Zhu L., Stafford J.M. Role of estrogens in the regulation of liver lipid metabolism. Adv. Exp. Med. Biol. 2017;1043:227–256. doi: 10.1007/978-3-319-70178-3_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri M., Orbai A.M., Alarcon G.S., Gordon C., Merrill J.T., Fortin P.R., Bruce I.N., Isenberg D., Wallace D.J., Nived O. Derivation and validation of the systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677–2686. doi: 10.1002/art.34473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petty R.E., Southwood T.R., Manners P., Baum J., Glass D.N., Goldenberg J., He X.H., Maldonado-Cocco J., Orozco-Alcala J., Prieur A.M. International league of associations for rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J. Rheumatol. 2004;31:390–392. [PubMed] [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; 2018. R: A Language and Environment for Statistical Computing.https://www.R-project.org/ [Google Scholar]
- Robinson G.A., Coelewij L., Radziszewska A., Wincup C., Peckham H., Waddington K., Isenberg D.A., Ioannou Y., Ciurtin C., Pineda-Torra I., Jury E.C. Metabolomics in juvenile-onset SLE: identifying new biomarkers to predict cardiovascular risk. medRxiv. 2019:19000356. [Google Scholar]
- Robinson G.A., Waddington K.E., Coelewij L., Peng J., Naja M., Wincup C., Radziszewska A., Peckham H., Isenberg D.A., Ioannou Y. Increased apolipoprotein-B:A1 ratio predicts cardiometabolic risk in patients with juvenile onset SLE. EBioMedicine. 2021;65:103243. doi: 10.1016/j.ebiom.2021.103243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger V.L., Go A.S., Lloyd-Jones D.M., Adams R.J., Berry J.D., Brown T.M., Carnethon M.R., Dai S.F., de Simone G., Ford E.S. Executive summary: heart disease and stroke statistics-2011 update a report from the American heart association. Circulation. 2011;123:459–463. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runyon B.A., Labrecque D.R., Anuras S. The spectrum of liver-disease in systemic lupus-erythematosus - report of 33 histologically-proved cases and review of the literature. Am. J. Med. 1980;69:187–194. doi: 10.1016/0002-9343(80)90378-2. [DOI] [PubMed] [Google Scholar]
- Schanberg L.E., Sandborg C., Barnhart H.X., Ardoin S.P., Yow E., Evans G.W., Mieszkalski K.L., Ilowite N.T., Eberhard A., Levy D.M. Premature atherosclerosis in pediatric systemic lupus erythematosus: risk factors for increased carotid intima-media thickness in the atherosclerosis prevention in pediatric lupus erythematosus cohort. Arthritis Rheum. 2009;60:1496–1507. doi: 10.1002/art.24469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shufelt C.L., Manson J.E. Menopausal hormone therapy and cardiovascular disease: the role of formulation, dose, and route of delivery. J. Clin. Endocrinol. Metab. 2021;106:1245–1254. doi: 10.1210/clinem/dgab042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub R.H. The complex role of estrogens in inflammation. Endocr. Rev. 2007;28:521–574. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- van Velzen D.M., Adorni M.P., Zimetti F., Strazzella A., Simsek S., Sirtori C.R., den Heijer M., Ruscica M. The effect of transgender hormonal treatment on high density lipoprotein cholesterol efflux capacity. Atherosclerosis. 2021;323:44–53. doi: 10.1016/j.atherosclerosis.2021.03.008. [DOI] [PubMed] [Google Scholar]
- Villa A., Torre S., Stell A., Cook J., Brown M., Maggi A. Tetradian oscillation of estrogen receptor alpha is necessary to prevent liver lipid deposition. Proc. Natl. Acad. Sci. U S A. 2012;109:11806–11811. doi: 10.1073/pnas.1205797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voskuhl R. Sex differences in autoimmune diseases. Biol. Sex Differ. 2011;2:1. doi: 10.1186/2042-6410-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh B.W., Spiegelman D., Morrissey M., Sacks F.M. Relationship between serum estradiol levels and the increases in high-density lipoprotein levels in postmenopausal women treated with oral estradiol. J. Clin. Endocrinol. Metab. 1999;84:985–989. doi: 10.1210/jcem.84.3.5571. [DOI] [PubMed] [Google Scholar]
- Wang Q., Wurtz P., Auro K., Morin-Papunen L., Kangas A.J., Soininen P., Tiainen M., Tynkkynen T., Joensuu A., Havulinna A.S. Effects of hormonal contraception on systemic metabolism: cross-sectional and longitudinal evidence. Int. J. Epidemiol. 2016;45:1445–1457. doi: 10.1093/ije/dyw147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson L., Leone V., Pilkington C., Tullus K., Rangaraj S., McDonagh J.E., Gardner-Medwin J., Wilkinson N., Riley P., Tizard J. Disease activity, severity, and damage in the UK juvenile-onset systemic lupus erythematosus cohort. Arthritis Rheum. 2012;64:2356–2365. doi: 10.1002/art.34410. [DOI] [PubMed] [Google Scholar]
- Wilmot K.A., O'Flaherty M., Capewell S., Ford E.S., Vaccarino V. Coronary heart disease mortality declines in the United States from 1979 through 2011 evidence for stagnation in young adults, especially women. Circulation. 2015;132:997–1002. doi: 10.1161/CIRCULATIONAHA.115.015293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuf S., Hawken S., Ounpuu S. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study (vol 364, pg 937m 2004) Lancet. 2004;364:2020. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- Zhang H., Temel R.E., Martel C. Cholesterol and lipoprotein metabolism: early career committee contribution. Arterioscler. Thromb. Vasc. Biol. 2014;34:1791–1794. doi: 10.1161/ATVBAHA.114.304267. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
Metabolomic data can be found at Mendeley Data (https://data.mendeley.com/), with source and identifier detailed in the ‘Key resources table’.
-
•
This paper does not report original code. The source and identifier of analysis code used in the paper can be found in the ‘Key resources table’ and/or ‘Quantification and statistical analysis’ section.
-
•
Any additional information required to reanalyse the data reported in this paper is available from the lead contact upon request.