Highlights
-
•
Grapes are a rich source of bioactive molecules which contribute to the health benefits.
-
•
Bioactive phytochemicals of grapes include phenolic compounds such as hydroxycinnamic acids, anthocyanins, proanthocyanidins and stilbenes.
-
•
Grape consumption is linked to reduced incidence of cardiovascular disease and its major risk factors including hypertension.
-
•
Grapes and its products can be considered as potential functional food in reducing hypertension.
Keywords: Grape berries, Phenolic compounds, Flavonoids, Anthocyanins, Stilbenes, Fatty acids, Hypertension
Abstract
Grapes are a rich source of bioactive molecules including phenolic acids, flavonoids, anthocyanins, stilbenes, and lipids. These are the compounds which contribute to the health benefits of grape and grape-derived products. They possess antioxidant, antimicrobial, anti-inflammatory, and anti-carcinogenic activities and have wide applications in food and nutraceutical industries. Use of grape extracts rich in these bioactive compounds are linked to reduced incidence of cardiovascular disease and its major risk factors including hypertension (high blood pressure); a clinical condition associated with high mortality worldwide. Therefore, considerable attention has been given to grape-based products to alleviate and treat hypertension. The aim of this review is to summarize the bioactive compounds of grapes, composition changes in different grape extracts and the potential benefits in reducing hypertension.
1. Introduction
Maintaining health and disease prevention are major goals driving today’s consumer food choices, and there is an ever increasing demand for “healthier” or “functional foods”. By definition, functional foods are “foods or part of foods” that provide medicinal or health benefits beyond basic nutritional requirements (Adefegha, 2018, Gul et al., 2016). In this regard, lifestyle changes, specifically improvement of dietary habits, have been suggested to favorably modify many disease risk factors and positively impact the development of disease. This notion is consistent with the modern concept of food which has expanded beyond its traditional role in basic nutrition and satiety, to include the prevention and treatment of disease (Adefegha, 2018, Gul et al., 2016). The continuing demand for “functional foods” is sending processors in search of edible crops with bioactive compounds that impart health benefits. For example, fruits that have significant amount of bioactive compounds possess antioxidant, antimicrobial, anti-inflammatory, and anti-tumorigenic activities (Ono et al., 2020). Indeed, fruits such as berries and grapes provide significant health benefits because of their high vitamin, mineral, lipid, fiber content, in addition to phenolic compounds which are the predominant phytochemicals present in these fruits (Gnanavinthan, 2013).
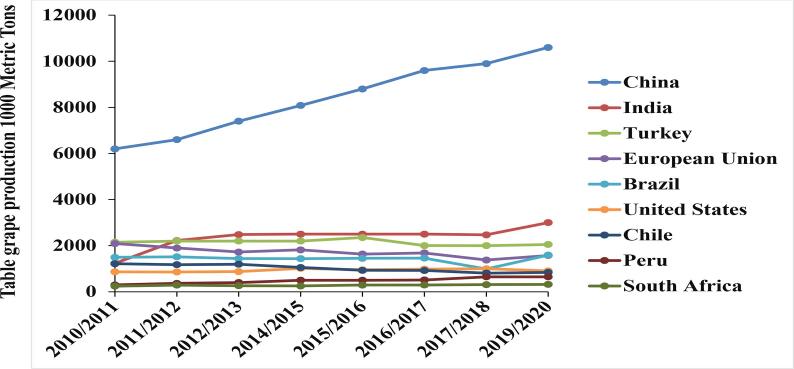
Use of grapes (Vitis species) is known to date back to Neolithic times (McGovern et al., 2017). Greek philosophers believed in the healing powers of whole grapes and products derived from grapes (Wang et al., 2014). The global grape production was about 23.38 million metric tons during the marketing year 2019/2020 (Shahbandeh, 2020). China leads the world table grape production followed by India and Turkey ranking 2nd and 3rd according to USDA Foreign Agricultural Service Report (Fig. 1). The cultivation of grapes all over the world vary based on cultivation suitability, disease resistance, color, taste, texture and having seeds or seedless (Santos et al., 2020). There are currently between 8000 and 10,000 cultivars of V. vinifera grapes in the world that are of commercial significance for wine, raisin, and table grape production (McGovern et al., 2017).
Fig. 1.
Production of top global table grape producers from 2010 to 2019 (source: USDA Foreign Agricultural Service Report).
Cardiovascular diseases have increased recently due to changes in life style, smoking, lack of physical activity and other factors (Caligiuri & Pierce, 2017). Hypertension is one of the major risk factors for developing cardiovascular disease and premature death in humans worldwide (Mills et al., 2020). The World Health Organization reported that in 2015 one in four men and one in five women were afflicted by hypertension globally (Haskell et al., 2017). More than a billion people (1.13 billion) are living with hypertension worldwide, and it is one of the major risk factors responsible for developing cardiovascular disease (https://www.who.int/news-room/fact-sheets/detail/hypertension). Despite the success of anti-hypertensive medications, hypertension and its complications remain as one of the major burdens both on the health and on the economy, thus, there is a critical need to discover novel strategies. A recent meta-analysis which reviewed the impact of different antihypertensive strategies in human clinical trials summarized that the use of functional foods and dietary supplements could be as effective as anti-hypertensive medications (Caligiuri & Pierce, 2017).
Grapes are a rich source of bioactive compounds, however the accumulation of these compounds in grapes is affected by different factors including the variety, maturity, post- harvest storage, environmental factors such as location, light conditions, temperature, nutrition, water, micro-organisms and viticulture practices (Chen et al., 2020, Colombo et al., 2020, Perestrelo et al., 2012, Yang et al., 2020). The main phenolic compounds in grape berries are hydroxycinnamic acids, stilbenes, flavonoids including anthocyanins and proanthocyanidins (Gouot et al., 2019). Quercetin which is a flavonoid and resveratrol which belongs to stilbene group are potent antioxidants, and have been suggested to have a role in protecting against cardiovascular diseases (Raj et al., 2015). Condensed tannins named as proanthocyanadins were also reported to have cholesterol-lowering propertiesand reducing blood pressure (Kumar et al., 2018). Grapes are also rich in phytosterols and fatty acids (Górnaś et al., 2019, Ruggiero et al., 2013) which may partially inhibit the intestinal absorption of both dietary and biliary endogenously produced cholesterol, lowering their circulating levels and exerting anti-atherogenic and cardio-protective effects (Garavaglia et al., 2016).
In this review, we will restrict the information included to the effects of grape bioactives in only one area of health – i.e. hypertension, commonly termed as high blood pressure. Given the value of the phytochemicals and fatty acids in grapes, we summarize the bioactive molecules in grapes, composition changes in different grape extracts and their associated potential benefits in hypertension.
2. Bioactive molecules of grapes
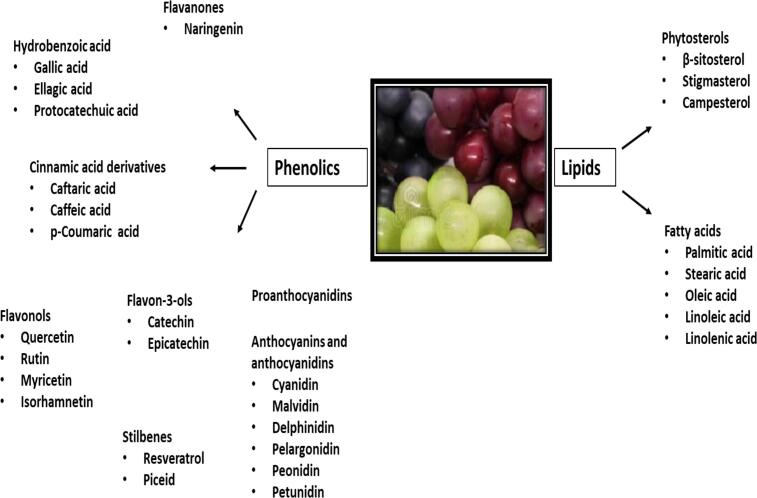
Phenolic acids, flavonoids, anthocyanins, proanthocyanadines and stilbenes are considered major bioactive compounds in grapes and their products contributing to the human health (Nassiri-Asl and Hosseinzadeh, 2016, Yang and Xiao, 2013). Various bioactive phenolic compound extracts in grapes are summarised in Table 1 and Fig. 2. Most of the phenolic compounds, such as anthocyanins, flavonols, hydroxycinnamic acid derivatives, and proanthocyanidins are concentrated in the skin of the berry with minimum amount of resveratrol in the seeds (Rebello et al., 2013). In addition, flavan-3-ols and proanthocyanidins contents are predominant in the seeds, while the flesh contains only trace amounts of anthocyanins and other phenolics.
Table 1.
Extraction and analysis of bioactives in grapes.
| Compounds identified | Derivative | Matrix | Method of extraction | Method of analysis | Reference |
|---|---|---|---|---|---|
|
Hydroxybenzoic acids 1. Gallic acid |
Seed flours | NA | HPLC | Lutterodt et al. (2011) | |
| Methyl gallate | Seed extracts(V. vinifera) | Ultrafilteration clean-up procedure | RP-HPLC-PAD-MS | Prodanov et al. (2013) | |
| Whole fruit and Seed extracts(V. vinifera) | Ultrafilteration clean-up procedure | RP-HPLC-PAD-MS HPLC-DAD-MS |
Ivanova et al., 2011, Prodanov et al., 2013 | ||
| 2. Ellagic acid | Protocatechuic aldehyde | Seed extracts(V. vinifera) | Ultrafilteration clean-up procedure | RP-HPLC-PAD-MS | Prodanov et al. (2013) |
| 3. Protocatechuic acid | Not known | Solid-phase extraction (SPE) | HPLC-DAD-ESI-MS/MS) | Colombo et al. (2020) | |
| 4. Vanillic acid | Berry extract | Ultrafilteration clean-up | HPLC- DAD | Eyduran et al. (2015) | |
| 5. Syringic acid | Berry extract | Ultrafilteration clean-up | HPLC- DAD | Eyduran et al. (2015) | |
|
Cinnamic acid derivatives 1. Caftaric acid |
Juice and wine | NA | RP-HPLC/DAD | da Silva Padilha et al. (2017) | |
| Skin, seeds and flesh of hybrid cultivar | SPE | HPLC-DAD-ESI-MS/MS | Rebello et al. (2013) | ||
| 2. Caffeic acid | Esters of caffeic acid | Skin, seeds and flesh of hybrid cultivar | SPE | HPLC-DAD-ESI-MS/MS | Rebello et al. (2013) |
| Coumaryl-glucose | Skin and seeds (V. vinifera) | Solid phase extraction (SPE) | HPLC-DAD-ESI-MS/MS | Pérez-Navarro et al., 2019 | |
| 3. p-Coumaric acid | Esters of p-coumaric acid | Skin, seeds and flesh of hybrid cultivar | SPE | HPLC-DAD-ESI-MS/MS | Rebello et al. (2013) |
| Not known | Solid-phase extraction (SPE) | HPLC-DAD-ESI-MS/MS) | Colombo et al. (2020) | ||
| 4. Ferulic acid | Berry extract | Ultrafilteration clean-up | HPLC- DAD | Eyduran et al. (2015) | |
| Flavonols | Flavonols/flavonol glucosides | Grape pomace | EtOH | HPLC-DAD | Amico et al. (2008) |
| Quercetin-3-glucuronide | Skin and seeds (V. vinifera) | Solid phase extraction (SPE) | HPLC-DAD-ESI-MS/MS | Pérez-Navarro et al., 2019 | |
| 1. Quercetin | Seed flours | NA | HPLC | Lutterodt et al. (2011) | |
| Quercetin 3-glucoside | Skin, seeds and flesh of hybrid cultivar | SPE | HPLC-DAD-ESI-MS/MS | Rebello et al. (2013) | |
| 2. Rutin | Myricetin-3-glucoside | Skin and seeds (V. vinifera) | Solid phase extraction (SPE) | HPLC-DAD-ESI-MS/MS | Perez-Pérez-Navarro et al., 2019 |
| 3. Myricetin | Myricetin 3-glucuronide | Skin and seeds (V. vinifera) | Solid phase extraction (SPE) | HPLC-DAD-ESI-MS/MS | Perez-Pérez-Navarro et al., 2019 |
| Skin, seeds and flesh of hybrid cultivar | SPE | HPLC-DAD-ESI-MS/MS | Rebello et al. (2013) | ||
| 4. Isorhamnetin | 4 flavan-3-ols | Skin and seed extract | NA | HPLC-DAD | Muñoz et al. (2008) |
| Flavan-3-ols | Flavan-3-ol monoglucosides | Skin and seeds (V. vinifera) | Solid phase extraction (SPE) | HPLC-DAD-ESI-MS/MS | Pérez-Navarro et al., 2019 |
| Galloylated flavan-3-ol dimers | Skin and seeds (V. vinifera) | Solid phase extraction (SPE) | HPLC-DAD-ESI-MS/MS | Pérez-Navarro et al., 2019 | |
| Not known | Solid-phase extraction (SPE) | HPLC-DAD-ESI-MS/MS) | Colombo et al. (2020) | ||
| Galloylated and non-glalloylated flavan-3-ols | Grape pomace | NA | Capillary electrophoresis, HPLC-DAD-MSn, LC-ESI-FTICR-MS | Rockenbach et al. (2012) | |
| 1.Catechin | Seed flours | NA | HPLC | Lutterodt et al. (2011) | |
| Seeds | Infrared-assisted extraction (IRAE) | HPLC | Cai et al. (2011) | ||
| Skin, seeds and flesh of hybrid cultivar | SPE | HPLC-DAD-ESI-MS/MS | Rebello et al. (2013) | ||
| Wines and musts | NA | HPLC | Gürbüz et al. (2007) | ||
| Catechin gallate | Skin and seeds (V. vinifera) | Solid phase extraction (SPE) | HPLC-DAD-ESI-MS/MS | Pérez-Navarro et al., 2019 | |
| Epicatechin gallate | Skin and seeds (V. vinifera) | Solid phase extraction (SPE) | HPLC-DAD-ESI-MS/MS | Perez-Pérez-Navarro et al., 2019 | |
| 2. Epicatechin | Epicatechin gallate | Seed flours | NA | HPLC | Lutterodt et al. (2011) |
| Seeds | Infrared-assisted extraction (IRAE) | HPLC | Cai et al. (2011) | ||
| Wines and musts | NA | HPLC | Gürbüz et al. (2007) | ||
| Juice and wine | NA | RP-HPLC/DAD | da Silva Padilha et al. (2017) | ||
|
Flavanones 1. Naringenin |
Not known | Solid-phase extraction (SPE) | HPLC-DAD-ESI-MS/MS) | Colombo et al. (2020) | |
| Grape pomace | EtOH | HPLC-DAD | Amico et al. (2008) | ||
|
Anthocyanins and anthocyanidins 1. Cyanidin |
Anthocyanins | Skin and seed extract | NA | HPLC-DAD | Muñoz et al. (2008) |
| wine grape varieties (V. vinifera) and 1 hybrid variety | NA | HPLC-DAD-MS | Fraige et al. (2014) | ||
| Grape (V. vinifera) pomace | Ultrasonication with acidified MeOH | Semi-preparative HPLC, HPLC-DAD-MS/MS |
Zhao et al. (2020) | ||
| Skin and seeds (V. vinifera) | Solid phase extraction (SPE) | HPLC-DAD-ESI-MS/MS | Pérez-Navarro et al., 2019 | ||
| Cyanidin 3-(6″-Coumaroyl)-glucoside | Skin of Muscadine grapes | NA | HPLC-ESI-MS | Huang et al. (2009) | |
| Cyanidin 3,5-diglucoside | Skin and seeds (V. vinifera) | Solid phase extraction (SPE) | HPLC-DAD-ESI-MS/MS | Pérez-Navarro et al., 2019 | |
| 2. Malvidin | Malvidin-3-glucoside | Skin and seeds (V. vinifera) | Solid phase extraction (SPE) | HPLC-DAD-ESI-MS/MS | Pérez-Navarro et al., 2019 |
| Malvidin-3,5-diglucoside | Skin of Muscadine grapes | NA | HPLC-ESI-MS | Huang et al. (2009) | |
| Malvidin 3,5-diglucoside | Skin and seeds (V. vinifera) | Solid phase extraction (SPE) | HPLC-DAD-ESI-MS/MS | Pérez-Navarro et al., 2019 | |
| Delphinidin3-glucoside | Skin of Muscadine grapes | NA | HPLC-ESI-MS | Huang et al. (2009) | |
| 3. Delphinidin | Delphinidin 3,5-diglucoside | Skin and seeds (V. vinifera) | Solid phase extraction (SPE) | HPLC-DAD-ESI-MS/MS | Pérez-Navarro et al., 2019 |
| Delphinidin 3-(6″-acetyl)-glucoside | Skin and seeds (V. vinifera) | Solid phase extraction (SPE) | HPLC-DAD-ESI-MS/MS | Pérez-Navarro et al., 2019 | |
| Delphinidin 3-(6″-caffeoyl)-glucoside | Skin and seeds (V. vinifera) | Solid phase extraction (SPE) | HPLC-DAD-ESI-MS/MS | Pérez-Navarro et al., 2019 | |
| Delphinidin 3-(6″-coumaroyl)-glucoside | |||||
| Skin of Muscadine grapes | NA | HPLC-ESI-MS | Huang et al. (2009) | ||
| 4. Pelargonidin | Peonidin 3,5-diglucoside | Skin and seeds (V. vinifera) | Solid phase extraction (SPE) | HPLC-DAD-ESI-MS/MS | Pérez-Navarro et al., 2019 |
| 5. Peonidin | Petunidin 3-glucoside | Skin of Muscadine grapes | NA | HPLC-ESI-MS | Huang et al. (2009) |
| 6. Petunidin | Petunidin 3,5-diglucoside | Skin and seeds (V. vinifera) | Solid phase extraction (SPE) | HPLC-DAD-ESI-MS/MS | Perez-Pérez-Navarro et al., 2019 |
| Petunidin 3-(6″-acetyl)-glucoside | |||||
| Wine | Laser-induced electrochemical sensor | Zhang et al. (2020) | |||
|
Stilbenes 1. t-Resveratrol |
Trans and cis isomers of resveratrol dimers | Wine grapes | NA | HPLC/MSn, HPLC/DAD-UV | Kong et al. (2011) |
| Grape skin of V. vinifera | Supercritical fluid extraction (SFE) with ethanol | HPLC | Pascual-Martı et al. (2001) | ||
| Wines and musts | NA | HPLC | Gürbüz et al. (2007) | ||
| Skin and seeds (V. vinifera) | Solid phase extraction (SPE) | HPLC-DAD-ESI-MS/MS | Pérez-Navarro et al., 2019 | ||
| Seed flours | NA | HPLC | Lutterodt et al. (2011) | ||
| 2. t-Piceid | Procyanidin B1, B2 | Seeds | Infrared-assisted extraction (IRAE) | HPLC | Cai et al. (2011) |
| Proanthocyanidins | Procyanidin B1, B2 | Seed extracts(V. vinifera) | Ultrafilteration clean-up procedure | RP-HPLC-PAD-MS | Prodanov et al. (2013) |
| Procyanidin B2 | Seed extracts | NA | HPLC | Kuhnert et al. (2015) | |
| Procyanidin dimmers (B1, B2, B3, B4, B5 and B6), procyanidin trimer, procyanidin tetramer | Seed extracts(V. vinifera) | Ultrafilteration clean-up procedure | RP-HPLC-PAD-MS | Prodanov et al. (2013) | |
| Monomeric flavan-3-ols, dimeric, polymeric, pentameric proanthocyanidins | Seeds of Cabernet Sauvignon | NA | Centrifugal partition chromatography (CPC) and Q-TOF-MS | Ma et al. (2018) | |
| Flavan-3-ol monomers | Seed extracts(V. vinifera) | Ultrafilteration clean-up procedure | RP-HPLC-PAD-MS | Prodanov et al. (2013) |
Fig. 2.
Bioactive phenolic compounds and lipids in grapes that may have beneficial roles in hypertension.
2.1. Phenolic acids
Phenolic acids can be subdivided into hydroxybenzoic (C6-C1) and hydroxycinnamic acids (C6-C3) and their derivatives (Kumar et al., 2018; Table 1). These acids are found in plants and food as conjugates and rarely found in free forms. Caffeic acid, chlorogenic acid, coumaric acid, sinapic acid, and ferulic acid are the major hydroxycinnamic acids, while syringic acid, gallic acid, gentisic acid, ellagic acid, protocatechuic acid and vanillic acid are the most significant hydroxybenzoic acids found in grapes (Colombo et al., 2020, Eyduran et al., 2015, Lutterodt et al., 2011). These compounds can exist in skin, pulp or seeds with the highest abundance in the skins (Hornedo-Ortega et al., 2021). The quantities of total hydroxycinnamic or hydroxybenzoic acids in grape skins vary based on cultivar and origin (Hornedo-Ortega et al., 2021). These phenolic acids can be found in free or conjugated forms with sugars, anthocyanins or condensed tannins through methylation, glycosylation and acylation processes (Kumar & Goel, 2019). In white grape varieties, hydroxycinnamic acids are esterified with tartaric acid forming diverse derivatives (Hornedo-Ortega et al., 2021). Phenolic acids are used as co-pigment agents because they form stable pigments in red wine. In addition they contribute to the astringency and bitterness characteristics of wine (Hornedo-Ortega et al., 2021). It has been reported that concentration and reconstitution process of grapes resulted in significant losses of phenolic acids such as trans-caftaric acid, chlorogenic-acid, gallic-acid, caffeic-acid, p-coumaric-acid and syringic-acid compounds with unchanged antioxidant capacity (Dutra et al., 2021).
2.2. Flavonoids
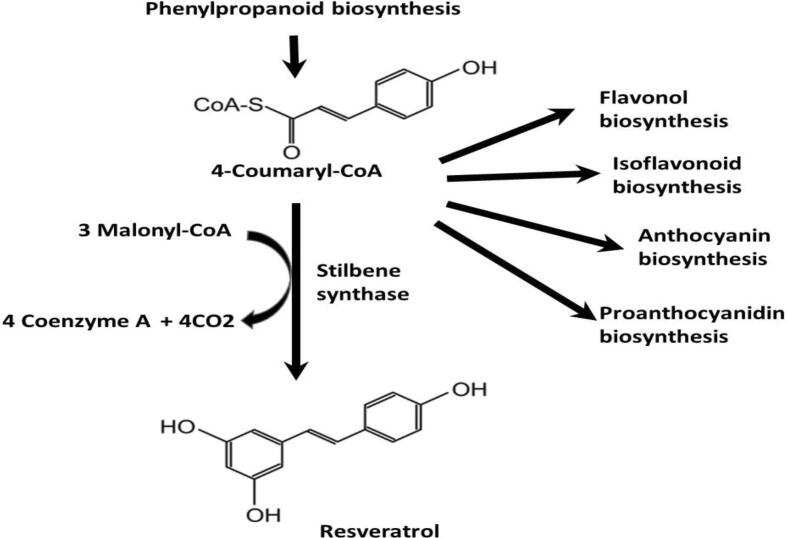
Flavonoids are generic secondary metabolites derived from the phenylpropanoid pathway (Fig. 3), which starts with the phenylalanine amino acid. The entry enzyme in this pathway is phenylalanine ammonia lyase (PAL), the first step in the phenylpropanoids pathway that converts phenylalanine into 4-coumaryl-CoA and subsequently to tetrahydroxychalcone, from which flavonols are synthesized (Flamini et al., 2013). Flavonoids have the common C6-C3-C6 backbone structure and are divided into subfamilies including flavonols, anthocyanins, flavan-ols and their derivative proanthocyanidins (Gouot et al., 2019). They are important secondary metabolites that have roles in protecting plants against biotic and abiotic stresses (Petrussa et al., 2013). Flavonoids are concentrated in the outer epidermal layer of the berry skin as they are involved in the protection against UV light. Their concentration differs with the developmental stages, where the highest concentration occurs after few weeks from veraison, then steady state concentration during early fruit development, followed by a decrease in their level with the increase in the berry size (Downey et al., 2003).
Fig. 3.
Biosynthetic pathways of Vitis vinifera in producing major bioactives as a result of phenylpropanoid biosynthesis. Resveratrol is produced directly from the substrate 4-Coumaryl-CoA by stilbene synthase enzyme. The pathway metabolites such as flavonols, isoflavonoids, anthocyanins and proanthocyanidins are produced by the substrate 4-Coumaryl-CoA after several enzymatic reactions (Bonghi et al., 2012; https://pmn.plantcyc.org).
2.2.1. Proanthocyanidins
Proanthocyanidins are composed of oligomers and polymers of flavan-3-ol subunits singly linked through C4 → C6 or C4 → C8 bonds (Prior et al., 2001). They differ in the composition and length, but the nature of the polymerization process whether enzymatic or non-enzymatic or the number of units still exactly unknown (Gouot et al., 2019). Proanthocyanidins in grapes are mainly composed of catechin and epicatechin subunits and their composition and length differs based on the type of tissues (Chen & Yu, 2017). Berry skin contains longer polymers with higher mean degree of polymerization (mDP) and are located mainly in the hypodermal layers of the skin, while the seeds have shorter polymers with lower mDP and are located in the paranchyma cells of the seed coat (Gouot et al., 2019).
The total amount of flavonoid compounds in grape berries ranges from 1 to 80 mg/Kg of fresh berry with higher concentration in the red varieties than the white ones (Castillo-Munoz et al., 2010). Some wild grapes, such as Vitis riparia can contain higher concentration of flavonoids (111 mg/Kg) than the content in cultivars derived from Vitis vinifera (Flamini et al., 2013). In red grapes, quercetin, myricetin, and kaempferol constitute 90% of the total flavonols, while laricitin, isorhamnetin, and syringetin represent 10% of the flavonols (Mattivi et al., 2006).
2.2.2. Anthocyanins
Anthocyanins are a ubiquitous class of flavonoids that are synthesized from the flavonoid pathway through the condensation of anthocyanidins and sugars (Gouot et al., 2019). These compounds are responsible for all the orange, red, blue, pink, and purple color of grape berries and their products, such as wines and juices (Mattioli et al., 2020). Plants accumulate these types of compounds for their biological and ecological roles including the protection from solar exposure and the ultraviolet radiation, antioxidant activities, coloration of plants for attracting pollinators, and defense against pathogen attack and predators (Wen et al., 2020). In addition, anthocyanins are considered natural pigments for coloring foods due to their vibrant color, high water solubility and safety (Mattioli et al., 2020).
Anthocyanins are glycosides and acylglycosides of anthocyanidins, which contains the aglycones flavyliums that differ in the number of the hydroxyl and methoxyl substitutions in their structures (He et al., 2010). More than 60% of the anthocyanins in grapes are found as monoglycosylated form, while the remaining are acylated with non-flavonoid phenolics, such as caffeic and p-coumaric acids (Gouot et al., 2019). The major anthocyanidins found in grapes are pelargondin, cyanidin, delphinidin, petunidin, peonidin, and malvidin, which is the predominant anthocyanidins in most red grapes (Samoticha et al., 2017).
Generally, individual anthocyanins are not chemically stable and are susceptible to oxidation in grape-derived products (Tan et al., 2021). Anthocyanins in grapes undergo chemical reactions, such as O-glycosylation and C-glycosylation in which the sugar moiety is linked to anthocyanidins at the C3 positions through glycosidic bonds to form 3-O-monoglycoside anthocyanins particularly in V. vinifera species, while in other Vitis species, 3,5-O-diglycoside anthocyanins are the dominant type (He et al., 2010). For example, in V. vinifera varieties, 3-O-monoglucosides of cyanidin, delphinidin, petunidin, peonidin, and malvidin are the major types of anthocyanins, while in other varieties pertaining to V. labrusca, V. rotunfolia, V. rupestris and their hybrids, anthocyanins with both 3-O-monoglucosides and 3,5-O-diglucosides derivatives are present.
2.3. Stilbenes
Stilbenes are phytoalexins that naturally found in some edible plants including grapes (Yang & Xiao, 2013). Various types of stilbenes have been identified in grapes including cis-and trans-resveratrol (3,5,4′-trihydroxystilbene), resveratrol-3-O-β-d-glucopyranoside (piceid), resveratrol dimers (viniferins), piceatannol (3,4,3′,5′-tetrahydroxy-trans-stilbene) (Flamini et al., 2013). Glycosylation process of stilbenes influence the antioxidative and antimicrobial properties and the storage and translocation of these compounds and trace amounts of isomeric and glycosylated forms of resveratrol and piceatannol have found in grapes (Flamini et al., 2013). The active form of resveratrol is synthesized through the phenylpropanoid pathway from the substrate 4-coumaryl-CoA by stilbene synthase enzyme (Fig. 3).
2.4. Lipids
The Lipids are mainly found in seeds of grapes including fatty acids, tocopherols, tocotrienols, and phytosterols (Górnaś et al., 2019). Comparing the profile of these compounds in different interspecific crosses of Vitis Species (V. Vinifera, V. riparia, V. amurensis, V. rupestris, and V. labruska), it was found that the oil content in grape seeds ranged from 7 to 160 g/Kg DW and the major fatty acids identified were linoleic acid, oleic acid, and palmatic acid, which constituted more 92–97% from the total fatty acids content (Górnaś et al., 2019). On the other side, tocopherol and tocotrienol contents ranged between 0.78 and 9.03 g/Kg oil and the sterols content ranged from 2.91 to 105.9 g/Kg oil (Table 2). Another study has shown that different grape varieties have different concentration and profile from fatty acid and tocopherol constituents in the seed oils where the oil concentration ranged from 7.3 to 22.4% with linoleic acid being the major component (53.6–69.6%) followed by oleic acid, palmitic acid and stearic acid (Sabir et al., 2012). The seeds oil extracted from all varieties contained α-tocopherol as the major tocopherol type (153 to 260 mg/Kg oil extract), while β-tocopherol, γ-tocopherol and δ-tocopherol existed in greater less amounts (Sabir et al., 2012).
Table 2.
Content of major lipids in grape seed oil.
| Lipid | Amount | Reference |
|---|---|---|
|
Fatty acid 1. C16:0 |
(FAMEs %) 5.4–13.2 |
Özcan et al., 2012, Górnaś et al., 2019, Sabir et al., 2012 |
| 2. C18:0 | 1.44–4.69 | |
| 3. C18:1 cis-9 | 6.2–31.2 | |
| 4. C18:2 | 53.6–83.1 | |
| Phytosterols | (mg/kg) | Garavaglia et al., 2016, Martin et al., 2020 |
| 1. Stigmasterol | 10.2–10.8 | |
| 2. β-sitosterol | 66.4–67.4 | |
| 3. Sitostanol | 3.92–4.7 | |
| 4. Avenasterol 5. Estigmastenol |
2.96–3.2 1.99–2.3 |
|
| Tocols | (mg/kg) | |
1. Tocopherol
|
14–244 1.1–1.3 24.8–26.2 |
Sabir et al., 2012, Garavaglia et al., 2016 |
2. Tocotrienol
|
60–352 4–125 482–1575 |
Martin et al., 2020 |
3. Bioactive composition analysis in different grape extracts
Different extraction methods have been used to study the bioactives of grapes in order to identify their potential bioactive molecule composition. The quantification may be different based on the extraction method and the used equipment. Extraction and analysis procedures for phenolic compounds are helpful in determining their effects on bioactivity, bio-accessibility and bioavailability in determination of health benefits (Rodríguez-Pérez et al., 2019). For example, bioactive molecules such as resveratrol and quercetin in grape extracts have been an effect on hypertension and heart damage (Finotti and Di Majo, 2003, Raj et al., 2015, Theodotou et al., 2017).
Bioactive molecule extraction from grapes and its products is necessary prior to their utilization in food or nutraceutical preparations. Some conventional techniques, such as maceration and Soxhlet extraction are used in extraction but they have some disadvantages including use of large quantity of solvents, long extraction time, and low extraction efficiency (Dai & Mumper, 2010). Pressurized liquid extraction (PLE) is an efficient extraction technique for extracting bioactives from different matrices and has numerous advantages over the conventional methods of extraction, such as reduced solvent usage and high recovery percentage due to the use of high temperature and pressure (dos Santos Freitas et al., 2008). Among the various factors (solvent type, extraction time, flush time, sample size, number of cycles, temperature) that affect the extraction efficiency in PLE, it was found that solvent type had a significant effect on the yield obtained from grape seeds using PLE followed by temperature and number of extraction cycles (dos Santos Freitas et al., 2013). On the other hand, recent techniques, such as accelerated solvent extraction (ASE), supercritical fluid extraction (SFE), ultrasound-assisted extraction (UAE), microwave-assisted extraction (MAE) have numerous advantages including higher extraction efficiency, high throughput, reduced solvent and time, and more environmentally- friendly. Table 1 highlights some of these techniques that were used for extraction of phenolic compounds from the skin, flesh, seed, wine, juice, etc. of grape varieties.
3.1. Phenolic fraction
Grape seed extract is a rich and inexpensive source of bioactives particularly proanthocyanidins; however, its use in food industry is quite challenging due to its adverse effects on food quality. Phenolics in grape seed extract cause astringent and bitter taste. The chromatographic techniques that are used to identify and quantify phenolic compounds in grapes and grape products are summarized in Table 1. High performance liquid chromatography-diode array detection-electrospray ionization tandem mass spectrometry (HPLC-DAS-ESI-MS/MS) was used to identify more than 50 phenolic compounds including hydroxycinnamic acid derivatives, anthocyanins, flavonols, stilbenes and flavan-3-ols in the skins and seeds of two Vitis vinifera red genotypes (Pérez-Navarro et al., 2019). The distribution pattern of these compounds in grapes enabled the differentiation between the grape varieties (Huang et al., 2009). Infrared-assisted extraction (IRAE) coupled with HPLC was developed to determine the phenolic fraction in grape seeds and the major compounds identified were catechin, epicatechin, and procyanidin B2 (Cai et al., 2011). A method utilizing HPLC-DAD-ESI-MS enabled the identification of more than 40 phenolic compounds in the skin of some grape varieties including novel compounds such as protocatechuic acid-glucoside, p-hydroxybenzoyl glucoside, p-coumaric acid-erythroside, caftaric acid vanilloyl pentoside, malylated kaempferol-glucoside and resveratrol dimer (Perestrelo et al., 2012). Monomeric flav-3-ols and polymeric proanthocyanidins were quantified in grape seed extracts using HPLC method on a diol stationary phase (Kuhnert et al., 2015).
The phenolic compounds in different berry parts (skin, seeds, and flesh) of a hybrid grape cultivar BRS were characterized employing SPE and HPLC-DAD-ESI-MS/MS (Rebello et al., 2013). The fingerprint of anthocyanins of Brazil-grown wine grapes was determined by HPLC-DAD-MS and grape varieties showed different profiles, however, the growing region did not affect the anthocyanin composition (Fraige et al., 2014). HPLC method employing both DAD and fluorescent detectors enabled the analysis of wide array of phenolic compounds in skin, seed and wines from V. vinifera cultivar Cencibel showing 48 compounds pertaining to benzoic acids, hydroxycinnamic acid, flavan-3-ols, flavonols, anthocyanins, and stilbens (Gómez-Alonso et al., 2007).
3.2. Lipophilic fraction
Although most of the studies attribute the health benefits of grapes to the phenolics fraction, the lipophilic components in the seeds can be considered a source of healthy fatty acids, phytoserols and tocopherols, which can be used in a wide variety of food, cosmetic and pharmaceutical applications (Garavaglia et al., 2016, Ruggiero et al., 2013). Cold- pressed oils obtained from different grape varieties showed variation in the percentage of fatty acids with linoleic acid being the most abundant fatty acid (Lutterodt et al., 2011). Its content was 66.0 g/100 g of total fatty acids in the seed oil of ruby red variety, while its content was 75.3 g/100 g in the seed oil of concord variety. Other lipophilic compounds, such as lutein, zeaxanthin, cryptoxanthin, β-carotene and α-tocopherol were also detected in the oil (Lutterodt et al., 2011).
The aroma in grape and wine is determined by the lipophilc terpenoids which affect the quality and organoliptic properties due to its low olfactory threshold (Sun et al., 2020). During alcoholic fermentation, the level of unsaturated fatty acids in grape-must have been regulated by the biosynthesis of aroma compounds (Yan et al., 2019). The supplementation of fatty acids to grape juice modulated the growth and metabolism of Saccharomyces cervisiae suggesting that the fatty acids composition of the juice contribute to quality of finish products (Pinu et al., 2019). It was found that unsaturated fatty acids (UFAs) types and levels influence the aroma production in the final wine obtained from Cabernet Sauvignon grape (Liu et al., 2019). In their study, the addition of oleic acid enhanced the generation of acetate esters in wine, while linoleic acid facilitated more production of octanoic acid and decanoic acid. Recently, approximately 102 compounds were identified recently including monoterpene-triol, monoterpene-tetraol, and sesquiterpenol glycosides (Caffrey et al., 2020). Fifty eight volatile organic compounds were identified including monoterpene alcohols (e.g. myrtenol, p-cymen-7-ol, and p-mentha-1,8-dien-7-ol) using headspace solid-phase microextraction coupled to GC/MS in grape plants inoculated with Arbuscular mycorrhizal fungi and rhizobacteria (Velásquez et al., 2020).
3.3. Analysis of juices, wine, seed oil and pomace
The chemical composition of the grape juice differs according to the grape cultivar. In a study aimed at comparing the chemical composition of juices in white and red grape cultivars, it was found that red grape juices were predominated by anthocyanins and tannins and lower concentration of flavonols, while in white grape juice, phenolic acids and tannins existed at higher levels (Natividade et al., 2013). Bio-accessibility study suggested that greater fraction of skin and seed phenolics was extracted through juicing while 100% grape juice and whole fruit both deliver similar phenolic contents to consumers (Mohamedshah et al., 2020).
The phenolic compounds in the juices and wines of Brazilian grape varieties were determined using a RP-HPLC/DAD method (da Silva Padilha et al., 2017). They identified epigallocatechin and trans-caftaric acid as the major compounds while resveratrol concentration was affected by various conditions including the variety of grape vine and also by processing conditions, such as duration of maceration. It was found that the concentrations of total phenolics, flavonoids, anthocyanins as well as resveratrol reached its maximum with extended maceration time up to 250 days (Ulrih et al., 2020). Phenolic compounds possess a critical role due to their contribution to organoleptic wine quality as color, astringency, and bitterness (Hornedo-Ortega et al., 2021). In-addition, flavonoids are considered the best co-pigmentation cofactor that contribute to the color of young red wines, and the strength of co-pigmentation correlates with the quercetin 3-O-glucoside content (Rustioni et al., 2012). Despite wine is recognized as valuable source of phenolic compounds, certain restrictions occur due to its alcohol content where that cannot be classified as functional food product.
The chemical analysis of the grape seed oil showed that linoleic acid is the major compound with a percentage of more than 50% and less quantities of palmitic, stearic, and oleic acids (Pérez-Navarro et al., 2019, Prado et al., 2012, Santos et al., 2011). In addition, Lutterodt et al. (2011) found that linoleic acid, the most abundant fatty acid in the cold-pressed grape seed oil, differed by the variety. Other minor constituents, such as lutein, zeaxanthin, cryptoxanthin, β-carotene, and α-tocopherol were also identified in the oil. The GC and GC-MS analysis of fatty acids in grape seed and grape seed oil showed the presence of high levels of unsaturated fatty acids and a favourable ratio of omega-6: omega-3 fatty acids, which indicate their potential health benefits (Khan et al., 2020). The composition of the oils extracted from three white winemaking varieties pertaining to V. vinifera was studied and all the three varieties contained high levels of linoleic acid and eicosenoic acid. β-sitosterol, γ-and α-tocotrienol existed at elevated levels in the oil (Boso et al., 2018). In non-Vitis vinifera grape species, such as V. coignetiae and V. ficifolia that are native to Japan, linoleic acid was also the major unsaturated fatty acid in the seeds (Shiozaki & Murakami, 2016).
Grape pomace was shown to be used as a natural source of antioxidants where the existence of several galloylated and non-galloylated flavan-3-ols as well as the condensed derivatives of catechin and acetaldehyde were detected using HPLC-DAD-MS in the pomace of Cabernet Sauvignon (Rockenbach et al., 2012). More than 250 flavan-3-ol compounds including several isomers were characterized by Fourier-transform ion cyclotron resonance mass spectrometry (LC-ESI-FTICR-MS), which proved to be an efficient tool for analyzing complex phenolics matrix (Rockenbach et al., 2012). A single-step semi –preparative HPLC method was developed for the separation of high-purity anthocyanin monomers from grape pomace, the residue after red winemaking process (Zhao et al., 2020). After separation, they purified anthocyanin fractions using column chromatography and analysed by HPLC-DAD-MS/MS, which yielded 14 anthocyanins with high (90%) purity. It was found that chemical composition of grape varieties can predict the sensory attributes of the corresponding wines (Niimi et al., 2020). The musts and wines produced from white and red grape cultivars grown in Turkey were analyzed by the HPLC for their chemical composition. Red wines and musts contained higher amounts of phenolics, however products of the white grape cultivar had higher concentration of catechin and epicatechin while resveratrol only existed in low concentration (Gürbüz et al., 2007).
4. Role of grapes and its products in reducing hypertension
4.1. Potential bioactive molecules in grapes reducing hypertension
While the potential of antihypertensive medication in lowering blood pressure in human subjects with hypertension is well established, lifestyle practices such as regular exercise and eating a healthy diet have also been reported to have a positive influence on blood pressure control (Carey et al., 2018). In this regard, studies have reported that food sources rich in a wide variety of bioactive compounds including polyphenols, phenolics, peptides and polyunsaturated fatty acids have demonstrated blood pressure lowering potential (Ghaffari & Roshanravan, 2020). It has been suggested that fruit-derived phenolic compounds favorably affect four risk factors of cardiovascular diseases: platelet aggregation, elevated blood pressure, vascular dysfunction and hyperlipidemia (Chong et al., 2010). The bioactive compounds in grapes include phenolic compounds such as phenolic acids, flavonoids, anthocyanins, stilbenes, and lipids as described earlier in section 2. Studies reveal that fruit phenolic compounds affect four risk factors of cardiovascular diseases: platelet aggregation, elevated blood pressure, vascular dysfunction and hyperlipidemia (Chong et al., 2010). Phenolic acids have broader role in human health benefits including anti-inflammatory, therapeutic usage, antidiabetic, anti-cancer, antiapoptotic, antiageing, hepatoprotective, neuroprotective, radioprotective, pulmonary protective, hypotensive effect, and antiatherogenic effects (Kumar et al., 2018). In addition, anthocyanins have health benefits to humans related to cancer, hepatic and cardiovascular diseases (Bitsch et al., 2004). Proanthocyanidins from grapes especially from seeds have shown a broad pharmacological and therapeutic health effects against cardiovascular disease, diabetes mellitus, obesity or cancer that are related with oxidative stress and inflammatory processes (Rodríguez-Pérez et al., 2019). The antioxidant, anticancer, anti-inflammatory, and cardio-protective properties of resveratrol have been demonstrated through several in vitro studies (Hung et al., 2000, Tamura et al., 1994). Indeed quercetin and resveratrol are potent antioxidants, and have been suggested to have a role in protecting against cardiovascular diseases (Finotti and Di Majo, 2003, Raj et al., 2015). proanthocyanadins were reported to have cholesterol-lowering propertiesand reducing blood pressure (Kumar et al., 2018). Many lipid groups in berries (unsaturated fatty acids, sterols, terpenoids and others) also have demonstrated high biological activity and may be important in cardio-metabolic health. For example, berry seed oils contain high amounts of polyunsaturated fatty acids and phytosterols that may be important in cardio-metabolic health (Szakiel et al., 2012). Linoleic acid is the principal unsaturated fatty acid in grape oil, which is linked to promoting the cardiovascular health in several studies (Martin et al., 2020). In addition, pinoresinol, ethyl caffeate and ethyl gallate were identified in the cold-pressed grape seed oil and showed inhibitory effect against protein tyrosine phosphatise 1B enzyme (PTP-1B) in type-two diabetes (Cecchi et al., 2019).
It has been reported that berry sterols (phytosterols) have the ability to reduce cholesterol levels in humans (Dulf et al., 2012). Anti-inflammatory, antiviral, wound-, cardio-protective and anti-carcinogenic properties have been indicated for sterols and triterpenoids present in berry seed oils (Szakiel et al., 2012). For example, phytosterols mainly β-sitosterol, stigmasterol and campesterol in grape oil are minor lipophilic constituents that have health benefits due to their antioxidant properties and effect on cholesterol metabolism (Martin et al., 2020). Berry consumption also contributes to a healthy gut microbiome, and have been suggested to improve lipid profile of human plasma and thereby reduce the risk of cardiovascular diseases (Yang & Kortesniemi, 2015).
4.2. Bio-accesibility and bioavailability of grape bioactive molecules
It is generally recognized that to exert a biological effect, grape bioactives must be present in sufficient amounts in raw or prepared foods and should be both bio-accessible and bioavailable. Bio-accessibility is termed as the fraction released from the food matrix during gastrointestinal digestion and the bioavailability is termed as the fraction that reaches systemic circulation in amount high enough to elicit a response (Mattioli et al., 2020). Bioavailability could be affected by the food matrix and the food processing. For example, phenolics are prone to oxidation during food process, have limited solubility in water and low bioavailability (Sagis, 2015). To overcome these drawbacks, microencapsulation using various techniques, such as freeze drying and spray drying are commonly used to incorporate the grape seed extract in food and pharmaceutical industries. These techniques regulate the release of the these bioactives, prevent the contents from oxidation and increase their stability, and overall enhance the food quality characters (Sagis, 2015). The final bioactivity of phenolic compounds is dependent on their release from the grape product during digestion and their capacity to cross the intestinal barrier as discussed above.
It has been reported that the bio-accessibility of polyphenols from berry fruits such as grapes is higher compared to other fruits, due to their low content of non-digestible carbohydrates and protein associated with an efficient gastrointestinal delivery enhancing their bioavailability (Olivas-Aguirre et al., 2020). A bio-accessibility study of grapes and products showed that a greater fraction of skin and seed phenolics extracted through juicing were highly bio-accessible (Mohamedshah et al., 2020). It also stated that 100% grape juice and whole fruit consumptions showed a similar trend in phenolic delivery to consumers. The differences in bioaccessibility of anthocyanins were compared in grape juice (86–135%) to whole grape (14–39%) as well as for flavan-3-ols and phenolic acids from grape juice (48–101; 39–85%) to whole grape (0–3; 9–67%). Therefore, consumption of grapes/ its products may have beneficial bio-accessibilty and bioavailability to initiate a biological response. However, molecules such as resveratrol and anthocyanins, which are common bioactives present in grapes playing important role in hypertension exhibit low bioavailability (Mattioli et al., 2020, Rasines-Perea et al., 2018).
4. Preclinical evidence related to the consumption of grapes and their products
The results from preclinical studies suggest that grapes and their products may have antihypertensive properties. One study (Thandapilly et al., 2012) reported that treatment with whole grape powder for a period of 10 weeks was able to lower severely high blood pressure in 20-week-old spontaneously hypertensive rat (SHR). SHR is the most widely used animal model because it reflects closely the clinical condition of primary hypertension in humans; more than 90% of hypertensive subjects suffer from this form of hypertension (Carey et al., 2018). The blood pressure lowering effect of grape powder was associated with an improvement in blood vessel structure and function in 20-week-old SHR (Thandapilly et al., 2012). The results of a study using the extract from grape skins also showed that twelve week treatment (with the extract) prevented the elevation in blood pressure in 3 week old SHR (da Costa et al., 2020). The antihypertensive effects of the grape skin extract was associated with an improvement of superoxide dismutase activity and lowering of oxidative stress, as well as, lowering of plasma tryglcerides and cholesterol in grape skin treated SHR (da Costa et al., 2020). Another study reported that treatment with grape pomace obtained after red wine processing for a period of 4 weeks lowered high blood pressure in 12-week-old SHR (Del Pino-García et al., 2017). They reported that the blood pressure lowering effect of pomace was associated with an improvement in the expression of antioxidants such as hemoxygenase-1 and superoxide dismutase 2 in the aorta of SHR. The antihypertensive effect of pomace was also associated with a reduction in expression of angiotensin converting enzyme (ACE), as well as, an improvement in the expression of endothelial nitric oxide (eNOS) in the aorta of SHR. ACE is a critical enzyme responsible for the catalysis of angiotensin II, a major vasoconstrictor molecule involved in the genesis of hypertension, while eNOS is an enzyme responsible for the catalysis of the critical vasodilator molecule nitric oxide. In another study, Rasines-Perea et al. (2018) showed significant lowering of very high blood pressure in SHR upon of grape pomace supplementation. In this study the authors also identified metabolites in the urine, blood and tissues of SHR which showed a showed the presence of 5-(Hydroxyphenyl)-γ-valerolactone-O-glucuronide, (Epi)catechin-O-glucuronide 1, (Epi)catechin-O-glucuronide 2, O-Methyl-(epi)catechin-O-glucuronide 1, 5-(Hydroxyphenyl)-γ-valerolactone-O-glucuronide 2, Methyl-(epi)catechin-O-glucuronide 2, Methyl-(epi)catechin-O-glucuronide 3, Di-methyl-(epi)catechin-O-glucuronide 1, 5-(Hydroxyphenyl)-4-hydroxyvaleric acid-O-sulphate 2 Nd Nd Nd Di-methyl-(epi)catechin-O-glucuronide 2, and 5-(Hydroxyphenyl)-γ-valerolactone sulphate 1 in the plasma of SHR, and 5-(Hydroxyphenyl)-γ-valerolactone-O-glucuronide 2.50 ± 0.25 * 2 and 5-(Hydroxyphenyl)-γ-valerolactone-O-sulphate in the heart tissue. The authors suggest that these compounds may have contributed to the antihypertensive effects of grape pomace observed in the SHR.
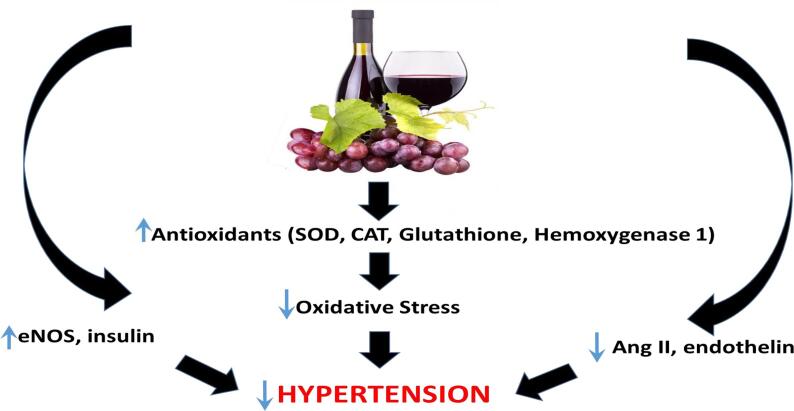
The effects of grape seed extract has also been studied in the SHR. One study reported a significant lowering of very high blood pressure in SHR supplemented with grape seed extract treatment (Margalef et al., 2017). In this study the authors identified flavanol metabolites in plasma and aorta of the SHR and its healthy control supplemented with grape seed extract (Margalef et al., 2017). The total amount of flavanol metabolites and total phenolic content observed were not significantly different in the plasma of healthy and SHR supplemented groups, however there were differences observed in the composition. There was a higher concentration of unconjugated flavanols in the supplemented SHR, in particular the levels of gallic acid which may be associated with an overactivation of microbial metabolism observed in the supplemented SHR. This study (Margalef et al., 2017) also reports that aortic tissue showed a significant reduction in flavanol metabolites in the supplemented SHR in comparison to healthy supplemented rats, however, the amounts of (-) epicatechin and its metabolites to catechin was significantly higher in the supplemented SHR group. The authors suggest that the increase in gallic acid and (-) epicatechin and its metabolites in plasma and aorta respectively, may have contributed to the reduction in blood pressure observed in SHR supplemented with grape seed extract. However, grape seed extract treatment for a longer duration (22 weeks) did not reduce severely high blood pressure in 20 week SHR (Liang et al., 2017). The variance in results observed on effects of in SHR could be due to a variety of reasons, including differences in type of extracts and the dose of treatment. The studies done by Margalef et al., 2017. used a an extract of grape seed which was enriched in phenolic compounds whereas the study carried out by Liang et al., 2017 used a grape seed extract which was enriched in proanthocyanidin. Furthermore Margalef et al used a higher dose (375 mg/kg/day) in their study, while Liang et al., 2017 used a lower dose (250 mg/kg/day) in their study. Fig. 4 shows the potential mechanisms by which grapes and grape-products may alleviate hypertension.
Fig. 4.
Potential mechanisms underlying the antihypertensive effects of grapes and grape-products. SOD – superoxide dismutase; CAT – catalase; eNOS – endothelial nitric oxide; Ang II – angiotensin II.
Two studies also showed the effects of supplementation with grape seed proanthocyanidin extract or grape seed polyphenol rich extract in rats fed with a cafeteria diet for 12 weeks (Pons et al., 2017) or 3 weeks (Mas-Capdevila et al., 2020), respectively. The results from both studies show that grape seed extract supplementation reduced moderately high blood pressure in the cafeteria-diet fed animals. The mechanisms underlying the antihypertensive affects include reduction of the vasoconstrictor molecule endothelin 1, and increase in the antioxidant glutathione (Pons et al., 2017). Supplementation of a proanthocyanidin rich grape seed extract was also reported to lower very high blood pressure in salt sensitive rats (Sato et al., 2020).
4.4. Clinical studies related to the consumption of grapes and their products
There is evidence from human studies to suggest that whole grapes and grape extract may have blood pressure lowering potential in individuals with hypertension. Vaisman and Niv (2015) reported that 12-week consumption of grape powder was effective in lowering blood pressure in individuals with prehypertension and mild hypertension; the antihypertensive effect of grape powder in these subjects was associated with improvement in vascular function and lowering of oxidative stress (Vaisman & Niv, 2015). Similarly, another study reported that administration of a wine grape extract for a period of 4 weeks was effective in reducing blood pressure in mildly hypertensive subjects (Draijer et al., 2015); the blood pressure lowering effects of the wine extract in these subjects was associated with the lowering of a potent vasco-constrictor molecule, endothelin However, clinical studies with grape seed extract have not showed clear outcomes. One study reported that supplementation of grape seed extract for 6 weeks lowered blood pressure in mildly hypertensive subjects; this benefit was associated with improvement in fasting insulin and insulin sensitivity (Park et al., 2016). Another study also reported a reduction in blood pressure in pre-hypertensive subjects who received grape seed proanthocyanidin extract for a period of 12 weeks (Odai et al., 2019) However, one study showed that 8-week supplementation with grape seed extract did not significantly affect blood pressure in individuals with mild and modest hypertension (Ras et al., 2013). The differences in results observed on effects of grape seed extracts could in part be due to the degree of hypertension in the study subjects. In the studies conducted by Park et al., 2016, Odai et al., 2019, the study subjects had a systolic blood pressure up to 139 mm Hg, whereas the study done by Ras et al included subjects that had systolic blood pressure up to 159 mm Hg. Other reasons for the variance observed could be different patient populations. While the Odai et al., 2019 study included Japanese subjects, the Park et al. study included subjects of mixed descent 2016, this information is not reported in the study done by Ras et al., 2013). Furthermore, the compositions of the phenolic compounds in grape seed extract used in the above mentioned studies are different (Park et al., 2016, Odai et al., 2019, Ras et al., 2013), and this may have also in part contributed to observed differences. As is the case with grape seed extract, there is no clear evidence for grape juice. An early study reported that grape juice supplementation for a period of 8 weeks lowered blood pressure in hypertensive subjects (Park et al., 2004). Another study also showed lowering of blood pressure in pre-hypertensive subjects with grape juice consumption for 8 weeks (Park et al., 2009). Furthermore, 4-week grape juice supplementation was reported to lower blood pressure at rest in mildly hypertensive patients (Neto et al., 2017). However, a later study showed that grape juice administration for 8 weeks was not effective in reducing blood pressure in individuals with pre-hypertension and mild hypertension (Dohadwala et al., 2010). Similarly, the results of the above-mentioned clinical study by Draijer et al. (2015) also showed that 4-week grape juice supplementation did not lower blood pressure in mildly hypertensive subjects (Draijer et al., 2015). The differences in results observed on effects of grape seed extract and grape juice could be due to the differences in length of treatment, severity of the disease, extracts from different grape cultivars as well as response of treatment in different populations. The reasons for the variance observed in these studies may in part be due to the different patient populations. While the studies of Park et al., 2004, Park et al., 2009 included Japanese subjects, the study by Dohadwala et al., 2010 included subjects of mixed descent; this information is not reported in the study done by Draijer et al. (2015). Furthermore, difference observed could also be attributed to the grape varieties used. The studies by Park et al., 2004, Park et al., 2009, Dohadwala et al., 2010 used juice from Concord grapes, whereas the study by Draijer et al. (2015) used a juice extract from Rubired grapes. The potential mechanisms underlying the antihypertensive effects of grapes and grape-products are shown in Fig. 4.
Recently, Asbaghi et al. (2021) revealed that grape products consumption compared to controls could significantly reduce systolic blood pressure and diastolic blood pressure. Authors used human clinical trials data which reported the effect of grape products supplementation on systolic blood pressure and diastolic blood pressure based on twenty eight studies comprising a total of 1344 subjects. Whether the benefits of grape in hypertension are attributed to certain compound(s) or group of compounds work synergistically in exerting their benefits is not known, but advancement in the pharmacokinetics could reveal some of these answers in the future.
5. Conclusion
Grapes and its derived-products can be rich sources of natural bioactive compounds, such as flavonoids, stilbenes, anthocyanins, and anthocyanidins, which can be identified and quantified in different matrices utilizing the advancement in the chromatographically analytical techniques. In addition, grapes contain a considerable amount of lipophilic constituents, such as essential fatty acids, phytosterols and tocols, which could also contribute to the health benefits associated with the consumption of grapes and products.
The potential mechanisms underlying the antihypertensive effects include a lowering of vasoconstrictory molecules such as angiotensin II and endothelin 1, an improvement of the vasodilatory molecule nitric oxide, and an attenuation of oxidative stress and inflammation. Pre-clinical and clinical evidence suggest blood pressure lowering potential for whole grapes and its derivatives, however, it must be noted that overall few studies have been carried out to make a strong case for the antihypertensive effects. Accordingly, it is important to conduct further preclinical studies using the most relevant animal models of human hypertension and clinical trials in humans [(at different stages of hypertension (mild and severe), examine the impact of sex difference, and different study populations (Caucasian, Asian, Black)], and build upon the limited evidence available to establish the antihypertensive potential of this amazing fruit.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
This project was funded by Agriculture and Agri-Food Canada (AAFC) start-up new scientist funding to Champa Wijekoon.
Author contributions
Champa Wijekoon and Thomas Netticadan planed and designed the manuscript. Ali Sabra, Thomas Netticadan and Champa Wijekoon contributed to literature review and writing. All authors read and approved the final manuscript.
References
- Adefegha S.A. Functional foods and nutraceuticals as dietary intervention in chronic diseases; Novel perspectives for health promotion and disease prevention. Journal of Dietary Supplements. 2018;15:977–1009. doi: 10.1080/19390211.2017.1401573. [DOI] [PubMed] [Google Scholar]
- Amico V., Chillemi R., Mangiafico S., Spatafora C., Tringali C. Polyphenol-enriched fractions from Sicilian grape pomace: HPLC–DAD analysis and antioxidant activity. Bioresource Technology. 2008;99(13):5960–5966. doi: 10.1016/j.biortech.2007.10.037. [DOI] [PubMed] [Google Scholar]
- Asbaghi O., Naeini F., Moodi V., Najafi M., Shirinbakhshmasoleh M., Rezaei Kelishadi M.…Fadel A. Effect of grape products on blood pressure: A systematic review and meta-analysis of randomized controlled trials. International Journal of Food Properties. 2021;24(1):627–645. [Google Scholar]
- Bitsch R., Netzel M., Frank T., Strass G., Bitsch I. Bioavailability and biokinetics of anthocyanins from red grape juice and red wine. Journal of Biomedicine and Biotechnology. 2004;2004(5):293–298. doi: 10.1155/S1110724304403106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonghi C., Rizzini F.M., Gambuti A., Moio L., Chkaiban L., Tonutti P. Phenol compound metabolism and gene expression in the skin of wine grape (Vitis vinifera L.) berries subjected to partial postharvest dehydration. Postharvest Biology and Technology. 2012;67:102–109. [Google Scholar]
- Boso S., Gago P., Santiago J.-L., Rodríguez-Canas E., Martínez M.-C. New monovarietal grape seed oils derived from white grape bagasse generated on an industrial scale at a winemaking plant. LWT. 2018;92:388–394. [Google Scholar]
- Caffrey A.J., Lerno L.A., Zweigenbaum J., Ebeler S.E. Direct Analysis of Glycosidic Aroma Precursors Containing Multiple Aglycone Classes in Vitis vinifera Berries. Journal of Agricultural and Food Chemistry. 2020;68(12):3817–3833. doi: 10.1021/acs.jafc.9b08323. [DOI] [PubMed] [Google Scholar]
- Cai Y., Yu Y., Duan G., Li Y. Study on infrared-assisted extraction coupled with high performance liquid chromatography (HPLC) for determination of catechin, epicatechin, and procyanidin B2 in grape seeds. Food Chemistry. 2011;127:1872–1877. [Google Scholar]
- Caligiuri S.P.B., Pierce G.N. A review of the relative efficacy of dietary, nutritional supplements, lifestyle, and drug therapies in the management of hypertension. Critical Reviews in Food Science and Nutrition. 2017;57(16):3508–3527. doi: 10.1080/10408398.2016.1142420. [DOI] [PubMed] [Google Scholar]
- Carey R.M., Muntner P., Bosworth H.B., Whelton P.K. Prevention and control of hypertension: JACC health promotion series. Journal of the American College of Cardiology. 2018;72(11):1278–1293. doi: 10.1016/j.jacc.2018.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo-Muñoz N., Gómez-Alonso S., García-Romero E., Hermosín-Gutiérrez I. Flavonol profiles of Vitis vinifera white grape cultivars. Journal of Food Composition and Analysis. 2010;23:699–705. [Google Scholar]
- Cecchi L., Innocenti M., Urciuoli S., Arlorio M., Paoli P., Mulinacci N. In depth study of phenolic profile and PTP-1B inhibitory power of cold-pressed grape seed oils of different varieties. Food Chemistry. 2019;271:380–387. doi: 10.1016/j.foodchem.2018.07.140. [DOI] [PubMed] [Google Scholar]
- Chen H., Yang J., Deng X., Lei Y., Xie S., Guo S.…Xu T. Foliar-sprayed manganese sulfate improves flavonoid content in grape berry skin of Cabernet Sauvignon (Vitis vinifera L.) growing on alkaline soil and wine chromatic characteristics. Food Chemistry. 2020;314:126182. doi: 10.1016/j.foodchem.2020.126182. [DOI] [PubMed] [Google Scholar]
- Chen M., Yu S. Lipophilized grape seed proanthocyanidin derivatives as novel antioxidants. Journal of Agricultural and Food Chemistry. 2017;65(8):1598–1605. doi: 10.1021/acs.jafc.6b05609. [DOI] [PubMed] [Google Scholar]
- Chong M.-F., Macdonald R., Lovegrove J.A. Fruit polyphenols and CVD risk: A review of human intervention studies. British Journal of Nutrition. 2010;104(S3):S28–S39. doi: 10.1017/S0007114510003922. [DOI] [PubMed] [Google Scholar]
- Colombo R.C., Roberto S.R., Nixdorf S.L., Pérez-Navarro J., Gómez-Alonso S., Mena-Morales A.…Hermosín-Gutiérrez I. Analysis of the phenolic composition and yield of ‘BRS Vitoria’seedless table grape under different bunch densities using HPLC–DAD–ESI-MS/MS. Food Research International. 2020;130:108955. doi: 10.1016/j.foodres.2019.108955. [DOI] [PubMed] [Google Scholar]
- da Costa G.F., Ognibene D.T., da Costa C.A., Teixeira M.T., Cordeiro V.D.S.C., de Bem G.F.…de Moura R.S. Vitis vinifera L. grape skin extract prevents development of hypertension and altered lipid profile in spontaneously hypertensive rats: Role of oxidative stress. Preventive Nutrition and Food Science. 2020;25:25–31. doi: 10.3746/pnf.2020.25.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J., Mumper R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010;15(10):7313–7352. doi: 10.3390/molecules15107313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilha C.V.d.S., Miskinis G.A., de Souza M.E.A.O., Pereira G.E., de Oliveira D., Bordignon-Luiz M.T., Lima M.D.S. Rapid determination of flavonoids and phenolic acids in grape juices and wines by RP-HPLC/DAD: Method validation and characterization of commercial products of the new Brazilian varieties of grape. Food Chemistry. 2017;228:106–115. doi: 10.1016/j.foodchem.2017.01.137. [DOI] [PubMed] [Google Scholar]
- Del Pino-García R., Rivero-Pérez M.D., González-SanJosé M.L., Croft K.D., Muñiz P. Antihypertensive and antioxidant effects of supplementation with red wine pomace in spontaneously hypertensive rats. Food & Function. 2017;8(7):2444–2454. doi: 10.1039/c7fo00390k. [DOI] [PubMed] [Google Scholar]
- Dohadwala, M.M., Hamburg, N.M., Holbrook, M., Kim, B.H., Duess, M.A., Levit, A., … Frame, A.A. (2010). Effects of Concord grape juice on ambulatory blood pressure in prehypertension and stage 1 hypertension. The American Journal of Clinical Nutrition, 92, 1052–1059. [DOI] [PMC free article] [PubMed]
- Freitas L.D.S., Dariva C., Jacques R.A., Caramão E.B. Effect of experimental parameters in the pressurized liquid extraction of Brazilian grape seed oil. Separation and Purification Technology. 2013;116:313–318. [Google Scholar]
- dos Santos Freitas L., Jacques R.A., Richter M.F., Silva A.L.d., Caramão E.B. Pressurized liquid extraction of vitamin E from Brazilian grape seed oil. Journal of Chromatography A. 2008;1200(1):80–83. doi: 10.1016/j.chroma.2008.02.067. [DOI] [PubMed] [Google Scholar]
- Downey Mark O., Harvey John S., Robinson Simon P. Synthesis of flavonols and expression of flavonol synthase genes in the developing grape berries of Shiraz and Chardonnay (Vitis vinifera L.) Australian Journal of Grape and Wine Research. 2003;9(2):110–121. [Google Scholar]
- Draijer R., de Graaf Y., Slettenaar M., de Groot E., Wright C. Consumption of a polyphenol-rich grape-wine extract lowers ambulatory blood pressure in mildly hypertensive subjects. Nutrients. 2015;7(5):3138–3153. doi: 10.3390/nu7053138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulf F.V., Andrei S., Bunea A., Socaciu C. Fatty acids and phytosterol contents of seed oils. Botanical Studies. 2012;55:1–6. [Google Scholar]
- Dutra M.d.C.P., Viana A.C., Pereira G.E., Nassur R.d.C.M.R., Lima M.D.S. Whole, concentrated and reconstituted grape juice: Impact of processes on phenolic composition, “foxy” aromas, organic acids, sugars and antioxidant capacity. Food Chemistry. 2021;343:128399. doi: 10.1016/j.foodchem.2020.128399. [DOI] [PubMed] [Google Scholar]
- Eyduran S.P., Akin M., Ercisli S., Eyduran E., Maghradze D. Sugars, organic acids, and phenolic compounds of ancient grape cultivars (Vitis vinifera L.) from Igdir province of Eastern Turkey. Biological Research. 2015;48(1):2. doi: 10.1186/0717-6287-48-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finotti E., Di Majo D. Influence of solvents on the antioxidant property of flavonoids. Food/Nahrung. 2003;47(3):186–187. doi: 10.1002/food.200390043. [DOI] [PubMed] [Google Scholar]
- Flamini R., Mattivi F., Rosso M., Arapitsas P., Bavaresco L. Advanced knowledge of three important classes of grape phenolics: Anthocyanins, stilbenes and flavonols. International Journal of Molecular Sciences. 2013;14(10):19651–19669. doi: 10.3390/ijms141019651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraige K., Pereira-Filho E.R., Carrilho E. Fingerprinting of anthocyanins from grapes produced in Brazil using HPLC–DAD–MS and exploratory analysis by principal component analysis. Food Chemistry. 2014;145:395–403. doi: 10.1016/j.foodchem.2013.08.066. [DOI] [PubMed] [Google Scholar]
- Garavaglia J., Markoski M.M., Oliveira A., Marcadenti A. Grape seed oil compounds: Biological and chemical actions for health. Nutrition and Metabolic Insights. 2016;9:NMI.S32910. doi: 10.4137/NMI.S32910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaffari S., Roshanravan N. The role of nutraceuticals in prevention and treatment of hypertension: An updated review of the literature. Food Research International. 2020;128:108749. doi: 10.1016/j.foodres.2019.108749. [DOI] [PubMed] [Google Scholar]
- Gnanavinthan A. Bioactives in Fruit: Health Benefits and Functional Foods. John Wiley & Sons, Ltd; Oxford, UK: 2013. pp. 1–18. [DOI] [Google Scholar]
- Gómez-Alonso S., García-Romero E., Hermosín-Gutiérrez I. HPLC analysis of diverse grape and wine phenolics using direct injection and multidetection by DAD and fluorescence. Journal of Food Composition and Analysis. 2007;20(7):618–626. [Google Scholar]
- Górnaś P., Rudzińska M., Grygier A., Lācis G. Diversity of oil yield, fatty acids, tocopherols, tocotrienols, and sterols in the seeds of 19 interspecific grapes crosses. Journal of the Science of Food and Agriculture. 2019;99(5):2078–2087. doi: 10.1002/jsfa.9400. [DOI] [PubMed] [Google Scholar]
- Gouot J.C., Smith J.P., Holzapfel B.P., Walker A.R., Barril C. Grape berry flavonoids: A review of their biochemical responses to high and extreme high temperatures. Journal of Experimental Botany. 2019;70(2):397–423. doi: 10.1093/jxb/ery392. [DOI] [PubMed] [Google Scholar]
- Gul K., Singh A.K., Jabeen R. Nutraceuticals and Functional Foods: The Foods for the Future World. Critical Reviews in Food Science and Nutrition. 2016;56(16):2617–2627. doi: 10.1080/10408398.2014.903384. [DOI] [PubMed] [Google Scholar]
- Gürbüz O., Göçmen D., Dagˇdelen F., Gürsoy M., Aydin S., Şahin İ.…Usta M. Determination of flavan-3-ols and trans-resveratrol in grapes and wine using HPLC with fluorescence detection. Food Chemistry. 2007;100(2):518–525. [Google Scholar]
- Haskell S.G., Brandt C., Burg M., Bastian L., Driscoll M., Goulet J.…Dziura J. Incident Cardiovascular Risk Factors Among Men and Women Veterans After Return From Deployment. Medical Care. 2017;55(11):948–955. doi: 10.1097/MLR.0000000000000801. [DOI] [PubMed] [Google Scholar]
- He F., Mu L., Yan G.-L., Liang N.-N., Pan Q.-H., Wang J.…Duan C.-Q. Biosynthesis of anthocyanins and their regulation in colored grapes. Molecules. 2010;15(12):9057–9091. doi: 10.3390/molecules15129057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornedo-Ortega R., Reyes González-Centeno M., Chira K., Jourdes M., Teissedre P.L. In Chemistry and Biochemistry of Winemaking. Wine Stabilization and Aging (IntechOpen) 2021 doi: 10.5772/intechopen.93127. [DOI] [Google Scholar]
- Huang Z., Wang B., Williams P., Pace R.D. Identification of anthocyanins in muscadine grapes with HPLC-ESI-MS. LWT-Food Science and Technology. 2009;42(4):819–824. [Google Scholar]
- Hung L.M., Chen J.K., Huang S.S., Lee R.S., Su M.J. Cardioprotective effect of resveratrol, a natural antioxidant derived from grapes. Cardiovascular Research. 2000;47:549–555. doi: 10.1016/s0008-6363(00)00102-4. [DOI] [PubMed] [Google Scholar]
- Ivanova V., Stefova M., Vojnoski B., Dörnyei Á., Márk L., Dimovska V.…Kilár F. Identification of polyphenolic compounds in red and white grape varieties grown in R. Macedonia and changes of their content during ripening. Food Research International. 2011;44(9):2851–2860. [Google Scholar]
- Khan Z.S., Mandal A., Maske S., Ahammed Shabeer T.P., Gaikwad N., Shaikh S., Banerjee K. Evaluation of fatty acid profile in seed and oil of Manjari Medika, a novel Indian grape cultivar and its comparison with Cabernet Sauvignon and Sauvignon Blanc. Sustainable Chemistry and Pharmacy. 2020;16:100253. doi: 10.1016/j.scp.2020.100253. [DOI] [Google Scholar]
- Kong Q.J., Ren X.Y., Hu N., Sun C.R., Pan Y.J. Identification of isomers of resveratrol dimer and their analogues from wine grapes by HPLC/MSn and HPLC/DAD-UV. Food Chemistry. 2011;127(2):727–734. doi: 10.1016/j.foodchem.2010.12.133. [DOI] [PubMed] [Google Scholar]
- Kuhnert S., Lehmann L., Winterhalter P. Rapid characterisation of grape seed extracts by a novel HPLC method on a diol stationary phase. Journal of Functional Foods. 2015;15:225–232. [Google Scholar]
- Kumar N., Goel N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnology Reports. 2019;1(24):e00370. doi: 10.1016/j.btre.2019.e00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Mosa K.A., Ji L., Kage U., Dhokane D., Karre S.…Pathania N. Metabolomics-assisted biotechnological interventions for developing plant-based functional foods and nutraceuticals. Critical Reviews in Food Science and Nutrition. 2018;58(11):1791–1807. doi: 10.1080/10408398.2017.1285752. [DOI] [PubMed] [Google Scholar]
- Liang Y., Gao H., Wang J., Wang Q., Zhao S., Zhang J., Qiu J. Alleviative effect of grape seed proanthocyanidin extract on small artery vascular remodeling in spontaneous hypertensive rats via inhibition of collagen hyperplasia. Molecular Medicine Reports. 2017;15:2643–2652. doi: 10.3892/mmr.2017.6292. [DOI] [PubMed] [Google Scholar]
- Liu P.-T., Duan C.-Q., Yan G.-L. Comparing the effects of different unsaturated fatty acids on fermentation performance of Saccharomyces cerevisiae and aroma compounds during red wine fermentation. Molecules. 2019;24(3):538. doi: 10.3390/molecules24030538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutterodt H., Slavin M., Whent M., Turner E., Yu L.L. Fatty acid composition, oxidative stability, antioxidant and antiproliferative properties of selected cold-pressed grape seed oils and flours. Food Chemistry. 2011;128(2):391–399. doi: 10.1016/j.foodchem.2011.03.040. [DOI] [PubMed] [Google Scholar]
- Ma W., Waffo-Téguo P., Alessandra Paissoni M., Jourdes M., Teissedre P.-L. New insight into the unresolved HPLC broad peak of Cabernet Sauvignon grape seed polymeric tannins by combining CPC and Q-ToF approaches. Food Chemistry. 2018;249:168–175. doi: 10.1016/j.foodchem.2018.01.005. [DOI] [PubMed] [Google Scholar]
- Margalef M., Pons Z., Iglesias-Carres L., Quiñones M., Bravo F.I., Arola-Arnal A., Muguerza B. Rat health status affects bioavailability, target tissue levels, and bioactivity of grape seed flavanols. Molecular Nutrition & Food Research. 2017;61(2):1600342. doi: 10.1002/mnfr.v61.210.1002/mnfr.201600342. [DOI] [PubMed] [Google Scholar]
- Martin M.E., Grao-Cruces E., Millan-Linares M.C., Montserrat-de la Paz S. Grape (Vitis vinifera L.) Seed oil: A functional food from the winemaking industry. Foods. 2020;9:1360. doi: 10.3390/foods9101360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mas-Capdevila A., Iglesias-Carres L., Arola-Arnal A., Suárez M., Bravo F.I., Muguerza B. Changes in arterial blood pressure caused by long-term administration of grape seed proanthocyanidins in rats with established hypertension. Food & Function. 2020;11(10):8735–8742. doi: 10.1039/d0fo00981d. [DOI] [PubMed] [Google Scholar]
- Mattioli R., Francioso A., Mosca L., Silva P. Anthocyanins: A Comprehensive review of their chemical properties and health effects on cardiovascular and neurodegenerative diseases. Molecules. 2020;25(17):3809. doi: 10.3390/molecules25173809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattivi F., Guzzon R., Vrhovsek U., Stefanini M., Velasco R. Metabolite profiling of grape: Flavonols and anthocyanins. Journal of Agricultural and Food Chemistry. 2006;54(20):7692–7702. doi: 10.1021/jf061538c. [DOI] [PubMed] [Google Scholar]
- McGovern Patrick, Jalabadze Mindia, Batiuk Stephen, Callahan Michael P., Smith Karen E., Hall Gretchen R.…Lordkipanidze David. Early neolithic wine of Georgia in the South Caucasus. Proceedings of the National Academy of Sciences. 2017;114(48):E10309–E10318. doi: 10.1073/pnas.1714728114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills Katherine T., Stefanescu Andrei, He Jiang. The global epidemiology of hypertension. Nature Reviews Nephrology. 2020;16(4):223–237. doi: 10.1038/s41581-019-0244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamedshah Zulfiqar, Chadwick-Corbin Sydney, Wightman JoLynne D., Ferruzzi Mario G. Comparative assessment of phenolic bioaccessibility from 100% grape juice and whole grapes. Food & Function. 2020;11(7):6433–6445. doi: 10.1039/d0fo00792g. [DOI] [PubMed] [Google Scholar]
- Muñoz Susanna, Mestres Montserrat, Busto Olga, Guasch Josep. Determination of some flavan-3-ols and anthocyanins in red grape seed and skin extracts by HPLC-DAD: Validation study and response comparison of different standards. Analytica Chimica Acta. 2008;628(1):104–110. [Google Scholar]
- Nassiri-Asl Marjan, Hosseinzadeh Hossein. Review of the Pharmacological Effects of Vitis vinifera (Grape) and its Bioactive Constituents: An Update. Phytotherapy Research. 2016;30(9):1392–1403. doi: 10.1002/ptr.5644. [DOI] [PubMed] [Google Scholar]
- Natividade Mariana Mirelle Pereira, Corrêa Luiz Claudio, Souza Scheilla Vitorino Carvalho de, Pereira Giuliano Elias, Lima Luiz Carlos de Oliveira. Simultaneous analysis of 25 phenolic compounds in grape juice for HPLC: Method validation and characterization of São Francisco Valley samples. Microchemical Journal. 2013;110:665–674. [Google Scholar]
- Neto M.M., da Silva T.F., de Lima F.F., Siqueira T.M.Q., Toscano L.T., de Moura S.K.M.S.F., Silva A.S.J. Whole Red Grape Juice Reduces Blood Pressure at Rest and Increases Post-exercise Hypotension. The Journal of the American College of Nutrition. 2017;36(7):533–540. doi: 10.1080/07315724.2017.1331385. [DOI] [PubMed] [Google Scholar]
- Niimi Jun, Tomic Oliver, Næs Tormod, Bastian Susan E.P., Jeffery David W., Nicholson Emily L.…Boss Paul K. Objective measures of grape quality: From Cabernet Sauvignon grape composition to wine sensory characteristics. LWT. 2020;123:109105. doi: 10.1016/j.lwt.2020.109105. [DOI] [Google Scholar]
- Odai T., Terauchi M., Kato K., Hirose A., Miyasaka N. Effects of grape seed proanthocyanidin extract on vascular endothelial function in participants with prehypertension: A randomized, double-blind, placebo-controlled study. Nutrients. 2019;11(12):2844. doi: 10.3390/nu11122844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono Kenjiro, Zhao Daisy, Wu Qingli, Simon James, Wang Jun, Radu Aurelian, Pasinetti Giulio Maria. Pine bark polyphenolic extract attenuates amyloid-β and tau misfolding in a model system of alzheimer’s disease neuropathology. Journal of Alzheimer's Disease. 2020;77(1):457. doi: 10.3233/JAD-209007. [DOI] [PubMed] [Google Scholar]
- Özcan Mehmet Musa, Ünver Ahmet, Gümüş Tuncay, Akın Aydın. Characteristics of grape seed and oil from nine Turkish cultivars. Natural Product Research. 2012;26(21):2024–2029. doi: 10.1080/14786419.2011.631133. [DOI] [PubMed] [Google Scholar]
- Park Eunyoung, Edirisinghe Indika, Choy Ying Yng, Waterhouse Andrew, Burton-Freeman Britt. Effects of grape seed extract beverage on blood pressure and metabolic indices in individuals with pre-hypertension: A randomised, double-blinded, two-arm, parallel, placebo-controlled trial. British Journal of Nutrition. 2016;115(2):226–238. doi: 10.1017/S0007114515004328. [DOI] [PubMed] [Google Scholar]
- Park Yoo Kyoung, Kim Jung-Shin, Kang Myung-Hee. Concord grape juice supplementation reduces blood pressure in Korean hypertensive men: Double-blind, placebo controlled intervention trial. BioFactors. 2004;22(1-4):145–147. doi: 10.1002/biof.5520220128. [DOI] [PubMed] [Google Scholar]
- Park Y.K., Lee S.H., Park E., Kim J.S., Kang M.H. Changes in antioxidant status, blood pressure, and lymphocyte DNA damage from grape juice supplementation. Annals of the New York Academy of Sciences. 2009;1171:385–390. doi: 10.1111/j.1749-6632.2009.04907.x. [DOI] [PubMed] [Google Scholar]
- Pascual-Martı M.C., Salvador A., Chafer A., Berna A. Supercritical fluid extraction of resveratrol from grape skin of Vitis vinifera and determination by HPLC. Talanta. 2001;54:735–740. doi: 10.1016/s0039-9140(01)00319-8. [DOI] [PubMed] [Google Scholar]
- Petrussa E., Braidot E., Zancani M., Peresson C., Bertolini A., Patui S., Vianello A. Plant flavonoids—biosynthesis, transport and involvement in stress responses. International Journal of Molecular Sciences. 2013;14(7):14950–14973. doi: 10.3390/ijms140714950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perestrelo Rosa, Lu Ying, Santos Sónia A.O., Silvestre Armando J.D., Neto Carlos P., Câmara José S., Rocha Sílvia M. Phenolic profile of Sercial and Tinta Negra Vitis vinifera L. grape skins by HPLC–DAD–ESI-MSn: Novel phenolic compounds in Vitis vinifera L. grape. Food Chemistry. 2012;135(1):94–104. [Google Scholar]
- Pérez-Navarro José, Izquierdo-Cañas Pedro Miguel, Mena-Morales Adela, Martínez-Gascueña Jesús, Chacón-Vozmediano Juan Luis, García-Romero Esteban.…Gómez-Alonso Sergio. Phenolic compounds profile of different berry parts from novel Vitis vinifera L. red grape genotypes and Tempranillo using HPLC-DAD-ESI-MS/MS: A varietal differentiation tool. Food Chemistry. 2019;295:350–360. doi: 10.1016/j.foodchem.2019.05.137. [DOI] [PubMed] [Google Scholar]
- Pinu Farhana R., Villas-Boas Silas G., Martin Damian. Pre-fermentative supplementation of fatty acids alters the metabolic activity of wine yeasts. Food Research International. 2019;121:835–844. doi: 10.1016/j.foodres.2019.01.005. [DOI] [PubMed] [Google Scholar]
- Pons Zara, Margalef Maria, Bravo Francisca I., Arola-Arnal Anna, Muguerza Begoña. Chronic administration of grape-seed polyphenols attenuates the development of hypertension and improves other cardiometabolic risk factors associated with the metabolic syndrome in cafeteria diet-fed rats. British Journal of Nutrition. 2017;117(2):200–208. doi: 10.1017/S0007114516004426. [DOI] [PubMed] [Google Scholar]
- Prado Juliana M., Dalmolin Irede, Carareto Natália D.D., Basso Rodrigo C., Meirelles Antonio J.A., Vladimir Oliveira J.…Meireles M. Angela A. Supercritical fluid extraction of grape seed: Process scale-up, extract chemical composition and economic evaluation. Journal of Food Engineering. 2012;109(2):249–257. [Google Scholar]
- Prior Ronald L., Lazarus Sheryl A., Cao Guohua, Muccitelli Helen, Hammerstone John F. Identification of Procyanidins and Anthocyanins in Blueberries and Cranberries (Vaccinium spp.) Using High-Performance Liquid Chromatography/Mass Spectrometry. Journal of Agricultural and Food Chemistry. 2001;49(3):1270–1276. doi: 10.1021/jf001211q. [DOI] [PubMed] [Google Scholar]
- Prodanov Marin, Vacas Visitación, Hernández Teresa, Estrella Isabel, Amador Beatriz, Winterhalter Peter. Chemical characterisation of Malvar grape seeds (Vitis vinifera L.) by ultrafiltration and RP-HPLC-PAD-MS. Journal of Food Composition and Analysis. 2013;31(2):284–292. [Google Scholar]
- Olivas-Aguirre F.J., Mendoza S., Alvarez-Parrilla E., Gonzalez-Aguilar G.A., Villegas-Ochoa M.A., Quintero-Vargas J.T.J. Wall-Medrano A. First-Pass Metabolism of Polyphenols from Selected Berries: A High-Throughput Bioanalytical Approach. Antioxidants (Basel) 2020;9(4):311. doi: 10.3390/antiox9040311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj P., Zieroth S., Netticadan T. An overview of the efficacy of resveratrol in the management of ischemic heart disease. Annals of the New York Academy of Sciences. 2015;1348:55–67. doi: 10.1111/nyas.12828. [DOI] [PubMed] [Google Scholar]
- Ras Rouyanne T., Zock Peter L., Zebregs Yvonne E.M.P., Johnston Neil R., Webb David J., Draijer Richard. Effect of polyphenol-rich grape seed extract on ambulatory blood pressure in subjects with pre-and stage I hypertension. British Journal of Nutrition. 2013;110(12):2234–2241. doi: 10.1017/S000711451300161X. [DOI] [PubMed] [Google Scholar]
- Rasines-Perea Z., Ky I., Cros G., Crozier A., Teissedre P.L. Grape pomace: Antioxidant activity, potential effect against hypertension and metabolites characterization after intake. Diseases. 2018;6(3):60. doi: 10.3390/diseases6030060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebello Ligia Portugal Gomes, Lago-Vanzela Ellen Silva, Barcia Milene Teixeira, Ramos Afonso Mota, Stringheta Paulo César, Da-Silva Roberto.…Hermosín-Gutiérrez Isidro. Phenolic composition of the berry parts of hybrid grape cultivar BRS Violeta (BRS Rubea× IAC 1398–21) using HPLC–DAD–ESI-MS/MS. Food Research International. 2013;54(1):354–366. [Google Scholar]
- Rockenbach Ismael Ivan, Jungfer Elvira, Ritter Christina, Santiago-Schübel Beatrix, Thiele Björn, Fett Roseane, Galensa Rudolf. Characterization of flavan-3-ols in seeds of grape pomace by CE, HPLC-DAD-MSn and LC-ESI-FTICR-MS. Food Research International. 2012;48(2):848–855. [Google Scholar]
- Rodríguez-Pérez C., García-Villanova B., Guerra-Hernández E., Verardo V. Grape seeds proanthocyanidins: An overview of in vivo bioactivity in animal models. Nutrients. 2019;11(10):2435. doi: 10.3390/nu11102435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggiero Antonietta, Vitalini Sara, Burlini Nedda, Bernasconi Silvana, Iriti Marcello. Phytosterols in grapes and wine, and effects of agrochemicals on their levels. Food Chemistry. 2013;141(4):3473–3479. doi: 10.1016/j.foodchem.2013.05.153. [DOI] [PubMed] [Google Scholar]
- Rustioni Laura, Bedgood Danny R., Failla Osvaldo, Prenzler Paul D., Robards Kevin. Copigmentation and anti-copigmentation in grape extracts studied by spectrophotometry and post-column-reaction HPLC. Food Chemistry. 2012;132(4):2194–2201. [Google Scholar]
- Sabir Ali, Unver Ahmet, Kara Zeki. The fatty acid and tocopherol constituents of the seed oil extracted from 21 grape varieties (Vitis spp.) Journal of the Science of Food and Agriculture. 2012;92(9):1982–1987. doi: 10.1002/jsfa.5571. [DOI] [PubMed] [Google Scholar]
- Sagis L.M. Academic Press; 2015. editor. Microencapsulation and microspheres for food applications; pp. P235–P251. [Google Scholar]
- Samoticha J., Wojdyło A., Golis T. Phenolic composition, physicochemical properties and antioxidant activity of interspecific hybrids of grapes growing in Poland. Food Chemistry. 2017;15(215):263–273. doi: 10.1016/j.foodchem.2016.07.147. [DOI] [PubMed] [Google Scholar]
- Santos J.A., Fraga H., Malheiro A.C., Moutinho-Pereira J., Dinis L.-T., Correia C.…Schultz H.R. A review of the potential climate change impacts and adaptation options for European viticulture. Applied Sciences. 2020;10:3092. [Google Scholar]
- Santos Leandra Pereira, Morais Damila Rodrigues, Souza Nilson Evelázio, Cottica Solange Maria, Boroski Marcela, Visentainer Jesuí Vergílio. Phenolic compounds and fatty acids in different parts of Vitis labrusca and V. vinifera grapes. Food Research International. 2011;44(5):1414–1418. [Google Scholar]
- Sato A., Nishioka S., Kiuchi M., Imada Y., Makino K., Nakagawa K.…Ohkita M. Grape Extract from Chardonnay Seeds Restores Deoxycorticosterone Acetate-Salt-Induced Endothelial Dysfunction and Hypertension in Rats. Biological &/and Pharmaceutical Bulletin. 2020;43(1):59–67. doi: 10.1248/bpb.b19-00540. [DOI] [PubMed] [Google Scholar]
- Shahbandeh M. Global grape production 2012/13-2019/20. 2020. https://www.statista.com/statistics/237600/world-grape-production-in-2007-by-region/ Accessed 14th Jan 2021.
- Shiozaki Shuji, Murakami Kazunori. Lipids in the seeds of wild grapes native to Japan: Vitis coignetiae and Vitis ficifolia var. ganebu. Scientia Horticulturae. 2016;201:124–129. [Google Scholar]
- Sun Lei, Zhu Baoqing, Zhang Xuanyin, Wang Huiling, Yan Ailing, Zhang Guojun.…Xu Haiying. The accumulation profiles of terpene metabolites in three Muscat table grape cultivars through HS-SPME-GCMS. Scientific Data. 2020;7(1) doi: 10.1038/s41597-019-0321-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szakiel Anna, Pączkowski Cezary, Koivuniemi Heini, Huttunen Satu. Comparison of the triterpenoid content of berries and leaves of lingonberry Vaccinium vitis-idaea from Finlandand Poland. Journal of Agricultural and Food Chemistry. 2012;60(19):4994–5002. doi: 10.1021/jf300375b. [DOI] [PubMed] [Google Scholar]
- Tamura Hirotoshi, Hayashi Yoshiki, Sugisawa Hiroshi, Kondo Tadao. Structure determination of acylated anthocyanins in Muscat Bailey a grapes by homonuclear Hartmann-Hahn (HOHAHA) spectroscopy and liquid chromatography-mass spectrometry. Phytochemical Analysis. 1994;5(4):190–196. [Google Scholar]
- Tan C., Dadmohammadi Y., Lee M.C., Abbaspourrad A. Combination of copigmentation and encapsulation strategies for the synergistic stabilization of anthocyanins. Comprehensive Reviews in Food Science and Food Safety. 2021;20:3164–3191. doi: 10.1111/1541-4337.12772. [DOI] [PubMed] [Google Scholar]
- Thandapilly Sijo J., LeMaistre Jillian L., Louis Xavier L., Anderson Christopher M., Netticadan Thomas, Anderson Hope D. Vascular and cardiac effects of grape powder in the spontaneously hypertensive rat. American Journal of Hypertension. 2012;25(10):1070–1076. doi: 10.1038/ajh.2012.98. [DOI] [PubMed] [Google Scholar]
- Theodotou M., Fokianos K., Mouzouridou A., Konstantinou C., Aristotelous A., Prodromou D., Chrysikou A. The effect of resveratrol on hypertension: A clinical trial. Experimental and Therapeutic Medicine. 2017;13:295–301. doi: 10.3892/etm.2016.3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrih N.P., Skrt M., Košmerl T., Wondra M., Abram V. Part I. Polyphenols composition and antioxidant potential during ‘Blaufränkisch’grape maceration and red wine maturation, and the effects of trans-resveratrol addition. Food and Chemical Toxicology. 2020;137:111122. doi: 10.1016/j.fct.2020.111122. [DOI] [PubMed] [Google Scholar]
- USDA foreign agricultural service report. https://usda.library.cornell.edu/concern/publications/1z40ks800?locale=en (Accessed 16th Jan 2021).
- Vaisman Nachum, Niv Eva. Daily consumption of red grape cell powder in a dietary dose improves cardiovascular parameters: A double blind, placebo-controlled, randomized study. International Journal of Food Sciences and Nutrition. 2015;66(3):342–349. doi: 10.3109/09637486.2014.1000840. [DOI] [PubMed] [Google Scholar]
- Velásquez A., Vega-Celedón P., Fiaschi G., Agnolucci M., Avio L., Giovannetti M., D’Onofrio C., Seeger M. Responses of Vitis vinifera cv. Cabernet Sauvignon roots to the arbuscular mycorrhizal fungus Funneliformis mosseae and the plant growth-promoting rhizobacterium Ensifer meliloti include changes in volatile organic compounds. Mycorrhiza. 2020;30:161–170. doi: 10.1007/s00572-020-00933-3. [DOI] [PubMed] [Google Scholar]
- Wang Limei, Waltenberger Birgit, Pferschy-Wenzig Eva-Maria, Blunder Martina, Liu Xin, Malainer Clemens.…Atanasov Atanas G. Natural product agonists of peroxisome proliferator-activated receptor gamma (PPARγ): A review. Biochemical Pharmacology. 2014;92(1):73–89. doi: 10.1016/j.bcp.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Weiwei, Alseekh Saleh, Fernie Alisdair R. Conservation and diversification of flavonoid metabolism in the plant kingdom. Current Opinion in Plant Biology. 2020;55:100–108. doi: 10.1016/j.pbi.2020.04.004. [DOI] [PubMed] [Google Scholar]
- Yan G., Duan L.L., Liu P.T., Duan C.Q. Transcriptional comparison investigating the influence of the addition of unsaturated fatty acids on aroma compounds during alcoholic fermentation. Frontiers in Microbiology. 2019;10:1115. doi: 10.3389/fmicb.2019.01115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Bohan, He Shuang, Liu Yuan, Liu Buchun, Ju Yanlun, Kang Dengzhao.…Fang Yulin. Transcriptomics integrated with metabolomics reveals the effect of regulated deficit irrigation on anthocyanin biosynthesis in Cabernet Sauvignon grape berries. Food Chemistry. 2020;314:126170. doi: 10.1016/j.foodchem.2020.126170. [DOI] [PubMed] [Google Scholar]
- Yang B., Kortesniemi M. Clinical evidence on potential benefits of berries. Current Opinions Food Science. 2015;2:36–42. [Google Scholar]
- Yang Jun, Xiao Yang-Yu. Grape phytochemicals and associated health benefits. Critical Reviews in Food Science and Nutrition. 2013;53(11):1202–1225. doi: 10.1080/10408398.2012.692408. [DOI] [PubMed] [Google Scholar]
- Zhang Chao, Ping Jianfeng, Ying Yibin. Evaluation of trans-resveratrol level in grape wine using laser-induced porous graphene-based electrochemical sensor. Science of the Total Environment. 2020;714:136687. doi: 10.1016/j.scitotenv.2020.136687. [DOI] [PubMed] [Google Scholar]
- Zhao Xu, Zhang Shan-Shan, Zhang Xin-Ke, He Fei, Duan Chang-Qing. An effective method for the semi-preparative isolation of high-purity anthocyanin monomers from grape pomace. Food Chemistry. 2020;310:125830. doi: 10.1016/j.foodchem.2019.125830. [DOI] [PubMed] [Google Scholar]