Abstract
A cDNA expression library prepared from Babesia caballi merozoite mRNA was screened with a monoclonal antibody BC11D against the rhoptry protein of B. caballi merozoite. A cDNA encoding a 48-kDa protein of B. caballi was cloned and designated BC48. The complete nucleotide sequence of the BC48 gene had 1,828 bp and was shown to contain no intron. Southern blotting analysis indicated that the BC48 gene contained more than two copies in the B. caballi genome. Computer analysis suggested that this sequence contained an open reading frame of 1,374 bp with a coding capacity of approximately 52 kDa. The recombinant protein expressed by the vaccinia virus vector in horse cells had an apparent molecular mass of 48 kDa, which was the same as that of the native B. caballi 48-kDa protein. Moreover, recombinant proteins expressed by the pGEX4T expression vector in Escherichia coli as glutathione S-transferase fusion proteins were used for antigen in an enzyme-linked immunosorbent assay (ELISA). The ELISA was able to differentiate very clearly between B. caballi-infected horse sera and B. equi-infected horse sera or noninfected normal horse sera. These results suggest that this simple and highly sensitive test might be applicable to the detection of B. caballi-infected horses in the field.
Babesia caballi is a tick-borne protozoan parasite which causes fever, anemia, jaundice, and edema in horses and, in some cases, the death of the infected animals (5, 9, 19, 22). The disease leads to great economic losses in the horse industry. The complement fixation test has been used as the standard test for the detection of antibodies against Babesia infection in horses since 1969 (9). However, it has been reported that, because of its low sensitivity and specificity, the complement fixation test fails to discriminate accurately between negative and carrier animals (27). Moreover, a large quantity of antigens are required to carry out this test. Since the parasitemia of B. caballi is usually very low in horses, it is very difficult to prepare the antigen from B. caballi-infected erythrocytes. By using the lysates of B. caballi-infected erythrocytes, the enzyme-linked immunosorbent assay (ELISA) causes extensive cross-reaction between B. caballi and B. equi (3, 28). Moreover, the serum from B. equi-infected horses sometimes shows a cross-reaction to B. caballi-infected erythrocytes by Western blotting (11). Therefore, the development of a high-quality system is required for the diagnosis of B. caballi infection.
Monoclonal antibody (MAb) BC11D was produced against a 48-kDa protein of B. caballi, and this MAb seemed to bind the rhoptry of merozoite observed by confocal laser microscopy (12). The 48-kDa protein has been shown to be an immunodominant protein (2–4, 11) and might be an important antigen for diagnosis (3, 4, 11, 12). Rhoptry proteins were reported to be involved in the invasion of erythrocytes (21) and might be suitable candidates for incorporation in vaccines against B. caballi merozoites. The aim of this study was to examine ultrastructural localization of the protein recognized by MAb BC11D, to sequence and express the 48-kDa rhoptry protein of B. caballi by using a pGEX4T expression vector in Escherichia coli, and to apply the expressed protein for the development of a highly specific and sensitive diagnostic ELISA. A possible use of the gene encoding the 48-kDa protein and its product for the development of a vaccine is also discussed.
MATERIALS AND METHODS
Parasite.
B. caballi (U.S. Department of Agriculture [USDA] strain) was grown in horse erythrocytes in continuous microaerophilous stationary-phase cultures as described by Avarzed et al. (1).
MAb and immunoelectron microscopy.
MAb BC11D against B. caballi merozoite was used because the confocal laser microscopic study has suggested that the location of protein recognized by MAb BC11D was within the rhoptry (12). Immunoelectron microscopy was done to examine the precise localization of epitope recognized by MAb BC11D as described before (26). Briefly, B. caballi-infected erythrocytes were collected when parasitemia was ca. 10% and were then washed three times in phosphate-buffered saline (PBS). Infected erythrocytes were then fixed with periodate-lysine-paraformaldehyde in phosphate buffer for 1 h. After three rinses with PBS, infected erythrocytes were dehydrated in a graded ethanol series (30 to 100%) and embedded in LR White resin (London Resin, Basingstoke, United Kingdom). Ultrathin sections mounted on nickel grids were blocked for 30 min in PBS containing 1% bovine serum albumin and then incubated with MAb BC11D for 12 h at room temperature. The ultrathin section were rinsed with PBS and then incubated with goat anti-mouse immunoglobulin G (IgG) + IgM conjugated with 10-nm gold particles (BioCell, Cardiff, United Kingdom) for 2 h at room temperature. After another rinse with PBS, the immunolabeled sections were fixed with 1% osmium tetroxide and then stained with a mixture of uranyl acetate and methyl cellulose. They were examined with a Hitachi-7500 (Hitachi, Tokyo, Japan) electron microscope at 80 kV. The specificity of immunohistochemical staining was confirmed by replacing MAb with normal mouse IgG.
Construction and immunoscreening of the cDNA expression library.
Total RNA was prepared from an in vitro culture of B. caballi-infected horse erythrocytes (erythrocyte volume, 10 ml; parasitemia, ca. 10%) by acid guanidinium thiocyanate-phenol-chloroform extraction, and then polyadenylated RNA was purified by Oligotex-dT 30 (Takara, Tokyo, Japan). The cDNA was synthesized by using a Zap-cDNA synthesis kit, ligated to a λ Zap II phage expression vector, and packaged by using the Gigapack III packaging system (Stratagene, La Jolla, Calif.). The cDNA library (2.5 × 105 PFU) was screened with MAb BC11D recognizing 48-kDa antigen. MAb BC11D was incubated 1 h at room temperature with nitrocellulose sheets (Schleicher & Schuell, Keene N.H.) containing the phage plaques. Positive plaques were visualized by using alkaline phosphate-conjugated goat anti-mouse IgG (Stratagene) with nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate as substrates (Stratagene). Positive plaques were rescreened until 100% plaque purification was achieved. The cloned insert in the plaque-purified λ phage was subcloned into pBluescript SK(+) by using the in vivo excision capabilities of λ ZAP II (23).
cDNA sequencing.
Restriction enzyme-generated fragments for sequencing were subcloned into pBluescript SK(+) vectors (Stratagene). An insert cDNA designated BC48 was sequenced by the dideoxy chain-termination method by using M13 reverse and universal primers (Perkin-Elmer, Foster, Calif.). Sequence analysis was performed with the computer program GeneWorks (IntelliGenetics, Mountain View, Calif.).
Isolation of the BC48 genomic clone.
As shown in Table 1, three sets of oligonucleotide primers derived from BC48 were used. The nucleotide sequences of each primer, including an EcoRI restriction enzyme site and their corresponding positions on cDNA, are indicated in Table 1. The amplified products were inserted into the EcoRI site of pBluescript SK(+) and sequenced with M13 reverse and universal primers as described above.
TABLE 1.
Primers used for gene sequencing
| Primer | Sequence (5′-3′)a | Corresponding position on cDNA |
|---|---|---|
| Group I | ||
| F1 | ACGAATTC CCACAACAGCCGTGTT | 17–32 |
| R3 | AGAATTC GTAAAGCGTGGCCATG | 533–548 |
| Group II | ||
| F2 | ACGAATTC AAGAACGTGACTCGCG | 471–486 |
| R4 | ACGAATTC TGGTGTCGGTTGACAC | 960–975 |
| Group III | ||
| F3 | ACGAATTC GGTGAACAGGGTGTTC | 881–896 |
| R2 | ACGAATTC CTAGAGTGCAACCGAG | 1757–1772 |
BC48 sequences representing restriction enzyme sites are shown in italics.
Northern and Southern blotting analyses.
Formaldehyde-denatured total RNA (10 μg) was fractionated on 1.2% formaldehyde-agarose gel, transferred to a nylon membrane (Hybond-N; Amersham-Buchler, Braunschweig, Germany), and hybridized with a 32P-labeled probe derived from the BC48 cDNA by using the random primer DNA synthesis method in the presence of [32P]dCTP (Amersham-Buchler) (8). Prehybridization and hybridization were performed overnight at 42°C. Membranes were washed three times with 0.1× SSC (0.3 M NaCl plus 0.03 M trisodium citrate; pH 7.0) containing 0.1% sodium dodecyl sulfate (SDS) at 42°C for 15 min. Bands hybridizing to the probe were detected by standard techniques. B. equi merozoite RNA and horse leukocyte RNA were used as controls. For Southern blotting analysis, total DNA was extracted from B. caballi merozoites by the standard method (20). Restriction enzyme-digested B. caballi genomic DNA was run on a 0.7% agarose gel, and the DNA was transferred onto a nylon membrane as described earlier (13). Horse leukocyte DNA was used as a control. The membrane was processed and probed exactly as for Northern blotting analysis.
Expression of the BC48 gene in E. coli.
The insert BC48 gene in pBluescript SK(+) vectors was subcloned into the pGEX4T plasmid (Pharmarcia, Uppsala, Sweden) of E. coli expression vector after digestion with EcoRI and XhoI. The resulting plasmid pGEX/BC48 was checked for accurate insertion by restriction enzyme analyses. The pGEX/BC48 was used to transform E. coli (BL21 strain; Stratagene) by standard techniques (20). The recombinant protein was expressed as glutathione S-transferase (GST) fusion protein, designated GST-BC48 protein. The GST-BC48 protein was purified with glutathione-Sepharose 4B beads (Pharmarcia) (24) after lysis of the collected bacteria by sonication in PBS containing 1% Triton X-100.
Production of anti-GST-BC48 serum.
Antiserum against the GST-BC48 protein was produced in mice. Recombinant bacteria were washed three times in PBS, lysed in PBS by sonication, and used as crude antigen. Seven-week-old female BALB/c mice were injected both intraperitoneally and subcutaneously with crude antigen suspended in 0.2 ml of PBS (8 mg/ml) emulsified with 0.2 ml of complete Freund's adjuvant (Difco, Detroit Mich.). At 2-week intervals, three additional stimulations with the same amount of crude antigen emulsified with 0.2 ml of incomplete Freund's adjuvant (Difco) were given. Sera from immunized mice were collected 10 days after the last immunization.
Construction of the recombinant vaccinia virus.
Insert BC48 in pBluescript SK(+) vectors was subcloned after digestion with EcoRI and XhoI, blunt ended with a Klenow fragment of DNA polymerase, and then ligated into the SalI site of pAK8 plasmid (30) of the vaccinia virus transfer vector. The accurate insertion of the resulting plasmid pAK8/BC48 was checked by restriction enzyme analyses. RK13 cells infected with vaccinia virus LC16mO (mO) strain were transfected with the recombinant transfer vector pAK8/BC48 by using a Lipofectin reagent (Gibco, Grand Island, N.Y.). Thymidine kinase negative (TK−) viruses were isolated by plaque assay on 143TK− cells in the presence of 5-bromo-2′-deoxyuridine at a concentration of 50 μg/ml. TK− virus plaques were picked up and purified three times, and the recombinant vaccinia virus (rVV) carrying the BC48 gene (vvBC48) was obtained. E. Derm cells from the dermis of horses were infected with vvBC48 at 10 PFU per cell. After incubation for 48 h, cell lysates obtained by sonication in PBS containing 1% Triton X-100 were collected as recombinant protein and designated ED-BC48 protein.
Western blotting analysis.
ED-BC48 and GST-BC48 proteins were analyzed by Western blotting as described previously (12).
ELISA.
Ninety-six-well microtitration plates (Nunc-Immuno Plate; Nunc, Roskilde, Denmark) were coated overnight at 4°C with 50 μl (0.1 μg/μl) of purified GST-BC48 protein or GST protein as the control. These proteins were diluted in a 0.05 M carbonate-bicarbonate buffer (pH 9.6). To reduce the nonspecific binding, plates were blocked for 1 h at 37°C with PBS containing 3% skim milk. The microtiter plates were then incubated with individual horse serum diluted 1:80 in PBS containing 3% skim milk for 1 h at 37°C. After six washes times with PBS containing 0.05% Tween 20, the peroxidase-conjugated goat anti-horse IgG (Cappel, Durham, N.C.) antibody diluted 1:4,000 in PBS containing 3% skim milk was added to each well in 50 μl and incubated for 1 h at 37°C. The plates were washed as described above, and then substrate solution (0.1 M citric acid, 0.2 M sodium phosphate, 0.003% H2O2, and 0.3 mg/ml 2,2′-azide-bis[3-ethylbenzthiazoline-6-sulfonic acid]; Sigma, St. Louis, Mo.) was added to each well in 100-μl aliquots. The absorbance at 415 nm was read after 1 h of incubation at room temperature by using an ELISA reader (Corona Microplate Reader MTP-120; Corona, Tokyo, Japan).
Sera.
The following horse sera were used for ELISA: (i) 14 sera from noninfected horses; (ii) 4 sera from horses experimentally infected with B. equi; and (iii) 6 sera from horses experimentally infected with B. caballi. All sera were obtained from the Equine Research Institute, The Japan Racing Association, Tochigi, Japan. They were kept at −80°C until use.
Nucleotide sequence accession number.
The sequence of the BC48 gene of B. caballi (USDA strain) has been submitted to the GenBank database under accession no. AB017700.
RESULTS
Ultrastructural localization of the 48-kDa protein.
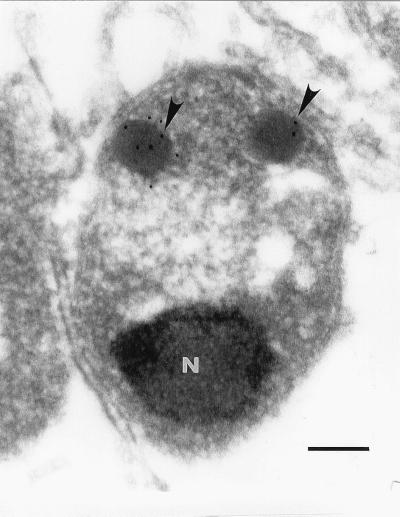
Immunomicroscopic studies were undertaken to determine the intracellular localization of the 48-kDa protein in B. caballi merozoites. Immunogold labeling showed specific binding of MAb BC11D to the rhoptries in B. caballi merozoites (Fig. 1). Gold particles were observed only in rhoptries of B. caballi merozoites, but not in the nucleus, spherical bodies, micronemes, or merozoite cytoplasm. Infected erythrocytes incubated with control mouse IgG did not have any particles bound to them (data not shown). These results have confirmed previous results with confocal laser microscopic observations, suggesting that MAb BC11D specifically bound to rhoptries of B. caballi merozoites (12).
FIG. 1.
Localization of the 48-kDa protein to rhoptries of B. caballi. Sections of periodate–lysine–paraformaldehyde-fixed parasites were reacted with MAb BC11D and then reacted with a 10-nm-gold-conjugated second antibody and examined by electron microscopy. Gold particles (arrowheads) were observed in two rhoptries located in the anterior area of merozoites. N, nucleus. Bar, 0.2 μm.
Cloning and sequencing of the BC48 cDNA clone.
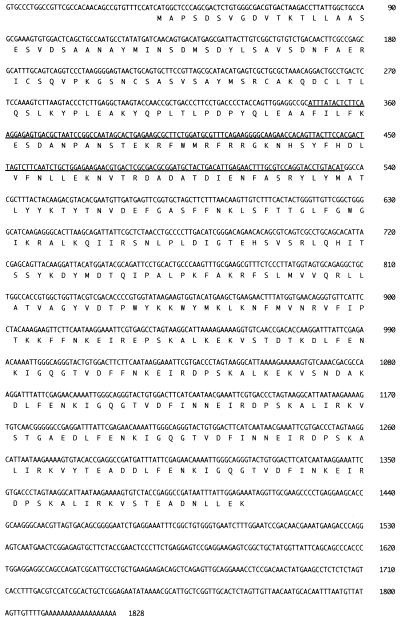
A cDNA clone containing a 1,828-bp insert was isolated after a screening with MAb BC11D. The cDNA sequence of the insert is shown in Fig. 2. Starting with methionine at position 45, a single open reading frame (ORF) of 1,374 nucleotides was present. Computer-aided searching of the GenBank database revealed that this ORF contained 189 bp of the B. caballi rhoptry protein gene previously reported by Dalrymple et al. (7) (GenBank accession number U46551) (Fig. 2).
FIG. 2.
Complete sequence, including the 5′- and 3′-untranslated regions, of BC48. The amino acid sequence translated from the long ORF is depicted. The sequence of 189 bp of the B. caballi rhoptry protein previously reported by Dalrymple et al. (GenBank accession number U46551) is underlined.
The ORF encodes a polypeptide of 458 amino acid residues, with a size of 52 kDa as calculated by computer. Two conserved sequences with tandemly repeated 27- and 8-residue periodicities, starting as F(X)N(Y)EIR, occur five and four times from residues 292 to 458, respectively (Fig. 3). The 27-residue sequence was almost identical, and the 8-residue sequence KIGQGTVD was exactly preserved.
FIG. 3.
Deduced amino acid sequence of the BC48 coding region from residues 292 to 458. Highly conserved residues are denoted by underlining.
Characterization of the BC48 gene.
A cDNA clone BC48 was hybridized to the total RNA isolated from B. caballi merozoites but not to horse leukocyte RNA or B. equi RNA by Northern blotting. Two mRNA species of 2.6 and 2.0 kb were identified (Fig. 4).
FIG. 4.
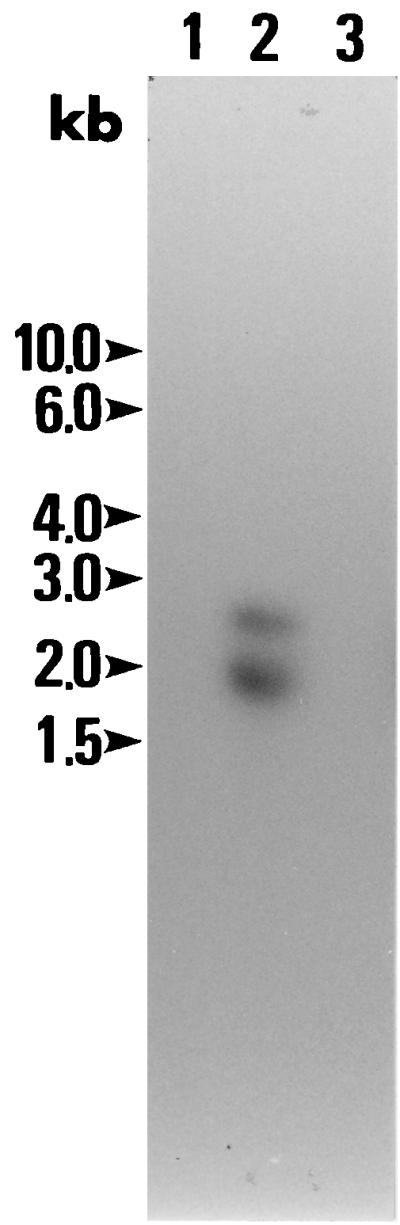
Northern blotting hybridization of the BC48 of B. caballi. Tracks contained 10 μg of total RNA prepared from B. equi derived from infected blood (lane 1), B. caballi-infected blood (lane 2), and horse leukocytes (lane 3) and were hybridized with the 32P-labeled BC48 cDNA. Molecular size markers are shown on the left (in kilobases).
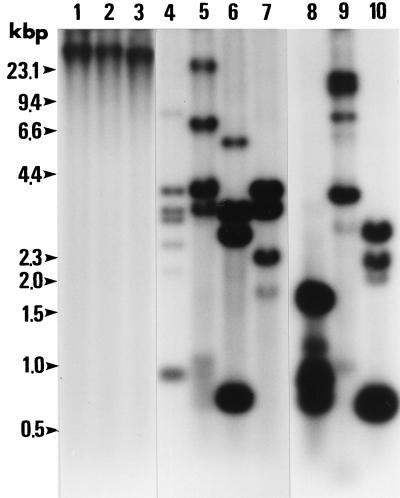
A probe derived from cDNA clone BC48 was strongly hybridized to B. caballi DNA fragments but not to horse leukocyte DNA in Southern blotting. As shown in Fig. 5, genomic DNA was digested by restriction enzymes HindIII, SacI, EcoRV, SalI, KpnI, DraII, PvuII, AccI, StyI, and PstI. The cDNA sequence, which did not contain any HindIII, SacI, and EcoRV sites, contained only a single SalI, KpnI, DraII, and PvuII site and two AccI, StyI, and PstI sites. However, the results showed the size of the fragments and the presence of more than two bands in lanes 4, 5, 6, and 7 and more than three bands in lanes 8, 9, and 10. These results indicate that BC48 contained more than two copies in the B. caballi genome.
FIG. 5.
Southern blotting hybridization of the BC48 of B. caballi. Genomic DNA (10 μg per lane) from B. caballi merozoites was digested with the indicated enzymes (lane 1, HindIII; lane 2, SacI; lane 3, EcoRV; lane 4, SalI; lane 5, KpnI; lane 6, DraII; lane 7, PvuII; lane 8, AccI; lane 9, StyI; lane 10, PstI) and hybridized with the 32P-labeled BC48 cDNA. Molecular size markers are shown on the left (in kilobase pairs).
B. caballi genomic DNA was amplified by PCR techniques by using three sets of primers, namely, groups I, II, and III (Table 1). The resulting DNA fragments were ca. 500, 500, and 900 bp, respectively. These amplified DNAs were molecularly cloned into each plasmid vector. The plasmids containing the gene from each representative group were isolated and subjected to DNA sequence analysis. The DNA sequences were found to coincide with BC48 (data not shown), which demonstrates that the BC48 gene contains no introns.
Western blotting analysis of the recombinant protein.
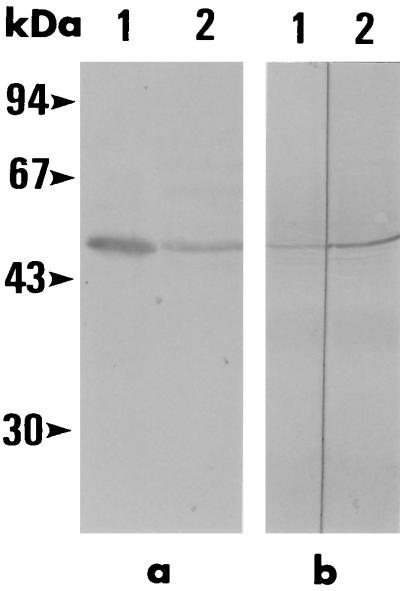
E. Derm cells infected with vvBC48 were analyzed by SDS-polyacrylamide gel electrophoresis and Western blotting to determine whether the ED-BC48 protein was expressed. A single band of ED-BC48 protein was observed in the cell lysate, and the molecular mass of the ED-BC48 protein was demonstrated to be the same as that of the native B. caballi 48-kDa protein by Western blotting (Fig. 6a), which proves that the ORF in the BC48 gene was the complete length. Moreover, the antibodies against GST-BC48 protein from mice recognized only the 48-kDa native protein as MAb BC11D (Fig. 6b). These results indicate that this 48-kDa protein did not contain the epitope of other constituent proteins in B. caballi and was unique among these proteins.
FIG. 6.
Western blotting analysis. (a) Native B. caballi and ED-BC48 protein with MAb BC11D. The molecular mass of the ED-BC48 protein (lane 1) was the same molecular mass as that of the native B. caballi protein (lane 2). (b) Native B. caballi protein with antibodies against GST-BC48 protein from mice (lane 1) and MAb BC11D (lane 2). The molecular mass of the reacted pattern was recognized in the same position. The positions of molecular mass standards are indicated on the left (in kilodaltons).
Detection of anti-GST-BC48 antibodies in B. caballi-infected horses by ELISA.
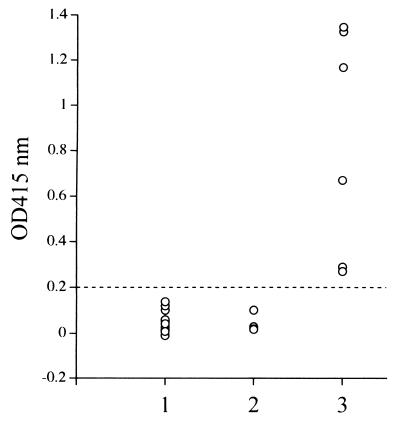
The recombinant protein was expressed as GST-BC48 protein with an apparent molecular mass of approximately 75 kDa (data not shown). Ninety-six-well plates coated with the GST-BC48 protein or the GST control protein were used for ELISA. Although the sera of B. caballi-infected horses and those of B. equi- or noninfected horses were confirmed to be nonreactive to the GST control protein (data not shown), the test was able to distinguish (optical density = 0.2) between the sera of B. caballi-infected horses and those of B. equi- or noninfected horses (Fig. 7). The GST-BC48 protein did not show any cross-reaction with anti-B. equi horse sera or normal horse sera in ELISA.
FIG. 7.
ELISA analysis of GST-BC48 protein with experimentally infected horse sera. Lane 1, noninfected horse sera; lane 2, B. equi-infected horse sera; lane 3, B. caballi-infected horse sera.
DISCUSSION
In the present study, the cDNA gene encoding a 48-kDa rhoptry protein of B. caballi merozoite was cloned, and its complete nucleotide sequence was determined. The genes encoding the rhoptry protein have been cloned and sequenced from a number of Babesia spp. (6, 7, 16, 17, 25). Those Bv60/p58 family genes encoding the rhoptry protein were found to be conserved in all isolates of B. bovis (18) and B. bigemina (14, 15). In our previous study, we developed a MAb BC11D that seemed to bind a 48-kDa rhoptry protein of B. caballi (12). The ultrastructural localization of a 48-kDa protein in the rhoptries of B. caballi merozoites was demonstrated by immunoelectron microscopic studies with MAb BC11D (Fig. 1). A cDNA expression library prepared from B. caballi merozoites was screened with MAb BC11D, and it was found that the complete DNA sequence encoding the rhoptry protein of B. caballi consisted of 1,828 bp. This DNA sequence contained 189 bp in B. caballi, as previously reported (7) with regions of highly conserved amino acid sequences in the Bv60/p58 in members of the genus Babesia. Therefore, the DNA sequence of B. caballi in the present study confirmed that the BC48 gene is a member of the Bv60/p58 family of the rhoptry protein in the genus Babesia. The 48-kDa protein was reported to be common to all strains of B. caballi obtained from different European countries and Brazil (2, 4), although it has not yet shown to be identical to the 48-kDa rhoptry protein. Taken together, these results may suggest that the BC48 gene encoding the rhoptry protein of B. caballi is an important gene for the conservation of the genus.
Two conserved sequences in the amino acids of the BC48 gene encoding the rhoptry protein of B. caballi with tandemly repeated 27- and 8-residue periodicities occurred five and four times from residues 292 to 458, respectively. The 27-residue sequence was almost identical, and the 8-residue sequence KIGQGTVD was exactly preserved. The polypeptide also contained a large region of two kinds of repeated amino acid sequences accounting for 36% of the total number of amino acids. Computer analysis indicated that the pattern present in this region has many T-cell epitopes. Suarez et al. (25) hypothesized that if the repeat regions of the amino acids were surface-exposed merozoite epitopes, the antibodies against the conserved and surface-exposed epitopes could block infectivity for host erythrocytes. Analyses of the mRNA from erythrocyte-stage parasites confirmed the transcription of mRNAs of two different sizes (2.6 and 2.0 kb). These results indicated that the BC48 gene is differentially expressed in the erythrocyte stage of the parasite. This BC48 gene was shown to be the product of more than two copies in the B. caballi genome by Southern blotting analysis, suggesting a multicopy gene. However, this protein expressed by the BC48 gene in B. caballi did not contain the epitope of other constituent proteins in B. caballi. Moreover, Western blotting indicated that it was unique among these proteins with antibodies against GST-BC48 protein from mice. The mechanisms for transcription and translation remain unclear at present because the origin of the rhoptries and the mechanism of their formation are still poorly understood. In addition, it is unclear whether proteins identified within the organelle are structural components of the organelle or whether they are inserted into the organelle posttranslationally.
Proteins with molecular masses of 48- and 50-kDa were detected by Western blotting with anti-B. caballi antibody at an early stage of infection and in a wide range of horses in different countries (2, 4). Sera from all experimentally infected horses were still positive against 48- and 50-kDa proteins more than 1 year after infection. The 48- and 50-kDa proteins were also confirmed to be major antigens of B. caballi merozoites (4, 11). Therefore, these proteins were considered to be suitable antigens for use in immunodiagnostic tests for B. caballi infection. GST-BC48 protein expressed by E. coli was used for ELISA in the present study. The ELISA was able to differentiate very clearly between B. caballi-infected horse sera and B. equi-infected horse sera or noninfected normal horse sera. However, the number of horse sera was small, and further studies on ELISA with this recombinant protein are thus necessary with a large number of horse sera to ascertain whether this simple and highly sensitive test is applicable to the detection of B. caballi-infected horses in the field.
Native and recombinant rhoptry proteins have been demonstrated to induce protective immune responses in cattle infection (15, 29). The ED-BC48 protein expressed by rVV has the same molecular mass as the native protein in the present study. Honda et al. (10) reported that the sporozoite surface antigen (p67) of Theileria parva expressed by rVV with rVV interleukin-2 of cattle enhanced protection against East Coast fever disease caused by T. parva, and that rVV expressing the p67 of T. parva with rVV interleukin-4 also enhanced anti-p67 antibody production. These results indicate the potential use of rVV as a delivery system in vaccination against protozoan infection. Further examination of the potency of vvBC48 as a potential subunit vaccine might provide interesting insights into how to control B. caballi infection in horses.
ACKNOWLEDGMENTS
This work was supported by grants-in-aid for Scientific Research from the Ministry of Education, Science, Culture, and Sports in Japan.
REFERENCES
- 1.Avarzed A, Igarashi I, Kanemaru T, Hirumi K, Omata Y, Saito A, Oyamada T, Nagasawa H, Toyoda Y, Suzuki N. Improved in vitro cultivation of Babesia caballi. J Vet Med Sci. 1997;59:479–481. doi: 10.1292/jvms.59.479. [DOI] [PubMed] [Google Scholar]
- 2.Böse R, Deaman K. Demonstration of the humoral immune response of horses to Babesia caballi by Western blotting. Int J Parasitol. 1992;22:627–630. doi: 10.1016/0020-7519(92)90011-9. [DOI] [PubMed] [Google Scholar]
- 3.Böse R, Peymann B. Diagnosis of Babesia caballi infections in horses by enzyme-linked immunosorbent assay (ELISA) and Western blot. Int J Parasitol. 1994;24:341–346. [PubMed] [Google Scholar]
- 4.Böse R, Peymann B, Barbosa P. Identification of diagnostic antigens for South American Babesia caballi infections. Int J Parasitol. 1994;24:255–258. doi: 10.1016/0020-7519(94)90034-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brüning A, Phipps P, Posnett E, Canning E U. Monoclonal antibodies against Babesia caballi and Babesia equi and their application in serodiagnosis. Vet Parasitol. 1997;58:11–26. doi: 10.1016/s0304-4017(96)01074-6. [DOI] [PubMed] [Google Scholar]
- 6.Dalrymple B P, Casu R E, Peters J M, Dimmock C M, Gale K R, Boese R, Wright I G. Characterization of a family of multi-copy genes encoding rhoptry protein homologues in Babesia bovis, Babesia ovis, and Babesia canis. Mol Biochem Parasitol. 1993;57:181–192. doi: 10.1016/0166-6851(93)90194-3. [DOI] [PubMed] [Google Scholar]
- 7.Dalrymple B P, Peters J M, Böse R, Wright I G. A polymerase chain-reaction method for the identification of genes encoding members of the Bv60/p58 family of rhoptry protein homologues in the genus Babesia. Exp Parasitol. 1996;84:96–100. doi: 10.1006/expr.1996.0094. [DOI] [PubMed] [Google Scholar]
- 8.Feinberg A P, Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983;132:6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- 9.Friedhoff K T. The piroplasms of equidae. Significance for the international equine trade. Berl Muench Tieraertzl Wochenschr. 1982;95:368–374. [PubMed] [Google Scholar]
- 10.Honda Y, Waithaka M, Taracha E L N, Duchateau L, Musoke A J, McKeever D J. Delivery of the Theileria parva p67 antigen to cattle using recombinant vaccinia virus: IL-2 enhances protection. Vaccine. 1998;16:1276–1282. doi: 10.1016/s0264-410x(98)00041-3. [DOI] [PubMed] [Google Scholar]
- 11.Ikadai, H., S. Kabamoto, X. Xuan, I. Igarashi, H. Nagasawa, K. Fujisaki, N. Suzuki, and T. Mikami. Protein analysis of Babesia caballi merozoites from in vitro culture by two-dimensional polyacrylamide gel electrophoresis and Western blotting. Submitted for publication. [DOI] [PubMed]
- 12.Ikadai, H., Y. Tamaki, X. Xuan, I. Igarashi, S. Kawai, H. Nagasawa, K. Fujisaki, Y. Toyoda, N. Suzuki, and T. Mikami. Inhibitory effect of monoclonal antibodies on the growth of Babesia caballi. Int. J. Parasitol., in press. [DOI] [PubMed]
- 13.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 14.McElwain T F, Perryman L E, Davis W C, McGuire T C. Antibodies define multiple proteins with epitopes exposed on the surface of live Babesia bigemina merozoites. J Immunol. 1987;138:2298–2304. [PubMed] [Google Scholar]
- 15.McElwain T F, Perryman L E, Musoke A J, McGuire T C. Molecular characterization and immunogenicity of neutralization-sensitive Babesia bigemina merozoite surface proteins. Mol Biochem Parasitol. 1991;47:213–222. doi: 10.1016/0166-6851(91)90181-5. [DOI] [PubMed] [Google Scholar]
- 16.Mishra V S, McElwain T F, Dame J B, Stephens E B. Isolation, sequence, and differential expression of the p58 gene family of Babesia bigemina. Mol Biochem Parasitol. 1992;53:149–158. doi: 10.1016/0166-6851(92)90017-e. [DOI] [PubMed] [Google Scholar]
- 17.Mishra V S, Stephens E B, Dame J B, Perryman L E, McGuire T C, McElwain T F. Immunogenicity and sequence analysis of recombinant p58: A neutralization-sensitive, antigenically conserved Babesia bigemina merozoite surface protein. Mol Biochem Parasitol. 1991;47:207–212. doi: 10.1016/0166-6851(91)90180-e. [DOI] [PubMed] [Google Scholar]
- 18.Palmer G H, McElwain T F, Perryman L E, Davis W C, Reduker D R, Jasmer D P, Shkap V, Pipano E, Goff W L, McGuire T C. Strain variation of Babesia bovis merozoite surface-exposed epitopes. Infect Immun. 1991;59:3340–3342. doi: 10.1128/iai.59.9.3340-3342.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Purnell R E. Babesiosis in various hosts. In: Ristic M, Krier J P, editors. Babesiosis. New York, N.Y: Academic Press, Inc.; 1981. pp. 25–64. [Google Scholar]
- 20.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 21.Sam-Yellowe T Y. Rhoptry organelles of the apicomplexa: their role in host cell invasion and intracellular survival. Parasitol Today. 1996;12:308–316. doi: 10.1016/0169-4758(96)10030-2. [DOI] [PubMed] [Google Scholar]
- 22.Schein E. Equine babesiosis. In: Ristic M, editor. Babesiosis of domestic animals and man. Boca Raton, Fla: CRC Press, Inc.; 1988. pp. 197–208. [Google Scholar]
- 23.Short J M, Sorge J A, Huse W D. λZAP: a bacteriophage with in vivo excision properties. Nucleic Acids Res. 1988;16:7583–7600. doi: 10.1093/nar/16.15.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith D B, Johnson K S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 25.Suarez C E, Palmer G H, Jasmer D P, Hines S A, Perryman L E, McElwain T F. Characterization of the gene encoding a 60-kilodalton Babesia bovis merozoite protein with conserved and surface-exposed epitopes. Mol Biochem Parasitol. 1991;46:45–52. doi: 10.1016/0166-6851(91)90197-e. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka S, Yora T, Nakayama K, Inoue K, Kurosumi K. Proteolytic processing of pro-opiomelanocortin occurs in acidifying secretory granules of AtT-20 cells. J Histochem Cytochem. 1997;45:425–436. doi: 10.1177/002215549704500310. [DOI] [PubMed] [Google Scholar]
- 27.Tenter A M, Friedhoff K T. Serodiagnosis of experimental and natural Babesia equi and B. caballi infections. Vet Parasitol. 1986;20:49–61. doi: 10.1016/0304-4017(86)90092-0. [DOI] [PubMed] [Google Scholar]
- 28.Weiland G. Species-specific serodiagnosis of equine piroplasma infections by means of complement fixation test, immunofluorescence, and enzyme-linked immunosorbent assay. Vet Parasitol. 1986;20:43–48. doi: 10.1016/0304-4017(86)90091-9. [DOI] [PubMed] [Google Scholar]
- 29.Wright I G, Casu R, Commins M A, Dalrymple B P, Gale K R, Goodger B V, Riddles P W, Waltisbuhl D J, Abetz I, Berrie D A, Bowles Y, Dimmock C, Hayes T, Kalnins H, Leatch G, McCrae R, Montague P E, Nisbet I T, Parrodi F, Peters J M, Scheiwe P C, Smith W, Rode-Bramanis K, White M A. The development of a recombinant Babesia vaccine. Vet Parasitol. 1992;44:3–13. doi: 10.1016/0304-4017(92)90138-y. [DOI] [PubMed] [Google Scholar]
- 30.Yasuda A, Kimura-Kuroda J, Ogimoto M, Sata T, Sato T, Takamura C, Kurata T, Kojima A, Yasui K. Induction of protective immunity in animals vaccinated with recombinant vaccinia viruses that express pre M and E glycoproteins of Japanese encephalitis virus. J Virol. 1990;64:2788–2795. doi: 10.1128/jvi.64.6.2788-2795.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]