Abstract
Objective:
To examine associations of changes in leptin and adiponectin concentrations from birth to age 12 years with adolescent adiposity and cardiometabolic risk in the HOME Study, a prospective birth cohort (Cincinnati, OH, N=166).
Methods:
Adiposity and cardiometabolic risk factors were assessed at age 12 years using anthropometry, dual-energy X-ray absorptiometry, and fasting serum biomarkers. Cardiometabolic risk scores were calculated by summing age- and sex- standardized z-scores for individual cardiometabolic risk factors.
Results:
Most serum adipocytokine concentrations at birth were not associated with adiposity or cardiometabolic risk outcomes. Leptin and adiponectin concentrations at age 12 years were associated with all outcomes in the expected direction. Adolescents with increasing (β:4.2; 95%CI:3.2, 5.2) and stable (β:2.2; 95%CI:1.2, 3.2) leptin concentrations from birth to age 12 had higher cardiometabolic risk scores than adolescents with decreasing concentrations (reference group). Adolescents with increasing (e.g., fat mass index: β:−1.04; 95%CI:−1.27, −0.80) and stable (β:−0.66; 95%CI:−0.92, −0.40) adiponectin/leptin ratios had more favorable adiposity outcomes than adolescents with decreasing ratios.
Conclusions:
In this cohort, changes in leptin concentrations and adiponectin/leptin ratios over childhood were associated with adiposity and cardiometabolic risk scores, indicating that adipocytokine concentrations are potential biomarkers for predicting excess adiposity and cardiometabolic risk in adolescence.
Keywords: Leptin, Adiponectin, Adolescent, Adiposity, Cardiometabolic risk
Introduction
The metabolic syndrome is a cluster of risk factors associated with a heightened risk of cardiovascular disease in adults.(1) Although definitions of metabolic syndrome vary, they generally require that a person presents with obesity, glucose dysregulation, dyslipidemia, and hypertension (at least 3) in order to have the syndrome. Alternatively, others have used continuous scores of metabolic syndrome components, as they may better predict later life cardiovascular disease risk and detect more subtle or earlier manifestations of cardiometabolic disease in children and adolescents.(2, 3) Strong evidence supports the hypothesis that metabolic risk, such as the abnormalities associated with metabolic syndrome, stem from perturbations of normal developmental programing in utero and in early infancy.(4)
Leptin and adiponectin are energy metabolism hormones that are released from both adipose and placental tissue.(5) Leptin and adiponectin are both positively associated with gestational age and birthweight, and in the newborn period, are markers of fetal metabolism.(6, 7) In animal models, perinatal leptin and adiponectin exposure independently modify offspring diet-induced weight gain and fat deposition.(8, 9) Additionally, the development of insulin resistance and hyperlipidemia in rodent offspring exposed to a high fat maternal diet is mediated by alterations in methylation patterns of adiponectin and leptin genes.(10) In children, we and others have shown that concentrations of leptin and adiponectin at birth are associated with adiposity gains from birth through mid-childhood and adolescence.(11, 12)
There is compelling evidence that increased leptin and decreased adiponectin concentrations in adolescents and adults are correlated with cardiometabolic disease. (13-15) However, few studies have examined the impact of fetal adipocytokine concentrations on cardiometabolic risk factors during adolescence. One study examined the association of leptin at birth with cardiometabolic outcomes during adolescence, finding that increasing leptin concentrations from birth to mid-childhood were associated with higher metabolic risk scores in early adolescence compared with children with low-stable leptin concentrations.(16) A second study found that increasing leptin concentrations from birth to mid childhood, but not adiponectin concentrations, were associated with adiposity at age 9 years.(17)
Identification of early biochemical phenotypes of adiposity and metabolic abnormalities may influence risk stratification and prevention strategies to ameliorate the morbidities associated with the obesities.(18) To better understand the role of leptin and adiponectin in the development of cardiometabolic abnormalities, we examined the associations of leptin and adiponectin concentrations at birth and in early adolescence with adiposity and cardiometabolic risk assessments in early adolescence in a prospective cohort. We hypothesized that higher cord blood leptin and lower cord blood adiponectin concentrations would be predictive of higher adiposity and cardiometabolic risk at age 12 years, and that increases in leptin concentrations and decreases in adiponectin concentrations over time would be predictive of greater cardiometabolic risk.
Materials/Subjects and Methods
Study Participants
We used data from the Health Outcomes and Measures of Environment (HOME) Study, an ongoing, longitudinal birth cohort study of women and their children. Pregnant women were recruited at approximately 16±3 weeks gestational age in Cincinnati, Ohio between 2003 and 2006, and follow up visits have been conducted of their children through age 12 years.(19) Women were included if they were ≥ 18 years of age, living in the Cincinnati area in a home built before 1978, without the diagnosis of diabetes, schizophrenia, bipolar disorder, cancer, or HIV infection, and not taking any medications for thyroid or seizure disorders. The institutional review boards (IRBs) at Cincinnati Children’s Hospital Medical Center (CCHMC) and participating delivery hospitals approved the study. The Brown University IRB deferred to the CCHMC IRB. Additionally, all mothers provided informed consent for themselves and their children at all visits, and children assented to the study procedures at the age 12-year visit.
Details of our most recent study visit at age 12 years were previously published.(20) In brief, among 441 eligible participants, we conducted follow-up on 256 adolescents at an average of 12.4 years of age. At this visit, we conducted anthropometry, body composition, and cardiometabolic health assessments. A total of 166 mother - singleton child pairs with cord blood leptin and adiponectin concentrations, at least one individual adiposity or cardiometabolic risk outcome, and relevant covariate data were included in the analysis for this study.
Leptin and Adiponectin Measurement
We measured concentrations of leptin and total adiponectin in previously frozen umbilical venous cord blood and adolescent overnight-fasting venous blood samples, using valid and reliable ELISA sandwich assays: adiponectin (Millipore/Linco, Linco Research, St Charles MO, Catalog #EZHADP-61K) and leptin (Millipore/EMD, St. Charles MO, Catalog #EZHL-805K). All assays were performed by trained technicians at the CCHMC NIH-funded Clinical Translational Research Center Core Laboratory. The levels of detection were 0.8 ng/mL for leptin and <2 μg/mL for adiponectin. Quality control samples and reagent blanks were included in each analytic batch, with inter- and intra-assay coefficients of variation of <11% for leptin and <17% for adiponectin. All adipocytokine concentrations were log2-transformed for analyses. Additionally, we calculated the adiponectin-to-leptin ratio for both the umbilical cord and adolescent adipocytokine measurements. Adiponectin-to-leptin ratio has been associated with metabolic abnormalities in children and adolescents.(21)
Adolescent Adiposity and Cardiometabolic Risk Assessment
We measured adolescent adiposity and cardiometabolic risk outcomes at the 12-year follow up study visit. Trained research staff measured weight and height using a digital calibrated Scaletronix 5002 scale (Hill-Rom Inc., Chicago IL) and wall-mounted Harpenden stadiometer (Holtain Ltd., Crymych UK), respectively. They measured waist circumference at the level of the iliac crest and hip circumference at the maximum protuberance of the buttocks using a Gulick II fiberglass no-stretch retractable measuring tape (Country Technology, Inc., Gays Mills WI). We calculated body mass index (BMI, kg/m2) and waist-to-hip ratio. We calculated BMI z-scores using the World Health Organization (WHO) age- and sex-specific standard data. Overweight and obese were defined as BMI z-score >1 and >2 standard deviations above the WHO growth reference median, respectively.(22) We obtained measures of whole-body adipose tissue mass (kg) and visceral fat area (cm2; defined as cross-sectional area of fat inside the abdominal cavity) using dual x-ray absorptiometry (DXA, Hologic Horizon densitometer, Hologic Inc., Bedford MA). Whole body DXA scans were analyzed using the National Health and Nutrition Examination Survey (NHANES) body composition analysis option. We divided the total fat mass (kg) by the square of height (m) to calculate the whole-body fat mass index (kg/m2). Sex- and age- standardized z-scores of fat mass index were calculated according to the NHANES 1999-2004 child and adolescent participants.(23)
We obtained an overnight-fasting blood sample via venipuncture and measured serum glucose, insulin, hemoglobin A1C, triglycerides, LDL, HDL, and total cholesterol using immunoassays. All assays were performed in the above-mentioned laboratory, using the following assays from Roche Diagnostics (Indianapolis, IN): cholesterol (catalog #03039773), HDL (catalog #04399803), LDL (catalog #07005717), triglycerides (catalog #20767107). Coefficients of variation for glucose, insulin, triglycerides, LDL, HDL, and total cholesterol assays were 2.0%, 9.5%, 6.5%, 4.2%, 2.9% and 2.6%, respectively. We calculated homeostatic model assessment for insulin resistance (HOMA-IR) using a standard formula (HOMA-IR = fasting insulin (mIU/L) x fasting glucose (mg/dL)/405).(24) Higher values of HOMA-IR indicate increased insulin resistance. Additionally, we calculated the triglyceride to HDL ratio. We took 3 sitting blood pressure measurements, each one minute apart, using a Dinamap Pro100 automated monitor (Critikon, Tampa FL) using previously described methods.(25) For statistical analyses, we excluded the first measure and used the average of the second and third blood pressure measures.(26)
Next, we calculated z-scores for the individual cardiometabolic risk outcomes. We calculated sex-, age-, and height- standardized blood pressure z-scores and percentiles according to a national childhood blood pressure database.(27) We additionally standardized these blood pressure z-scores for our study participants. For outcomes in which standards from the U.S. population were unavailable, we calculated standardized z-scores according to our own study participants. To do this, we ran linear regression models of each individual cardiometabolic risk factor as the dependent variable with age and sex as predictors. We then standardized the residuals from those models to derive the age- and sex-specific z-scores.(28) Insulin, HOMA-IR, triglycerides, triglycerides to HDL ratio, waist circumference, total fat mass, and visceral fat area were not normally distributed, and thus were log2-transformed prior to calculating standardized z-scores.
Given there is currently no consensus on which individual components should be included in the children’s/adolescents’ cardiometabolic risk score, we constructed two continuous cardiometabolic risk summary scores, one with traditional cardiometabolic risk factors that were components of the metabolic syndrome definition and the other with novel cardiometabolic risk factors that were recently shown to have great reliability and predictability. Traditional cardiometabolic risk scores have utilized assessments of waist circumference, blood pressure, HDL, and triglyceride z-scores.(28) However, new data suggest that inclusion of factors such as visceral fat, HOMA-IR, and the TG to HDL ratio may be more predictive of cardiometabolic risk than traditional measures.(29, 30) The traditional cardiometabolic risk score was calculated by summing the standardized z-scores for glucose, insulin, triglycerides, HDL (multiplied by −1), the mean of systolic blood pressure and diastolic blood pressure, and waist circumference.(31) The novel cardiometabolic risk score was calculated by summing standardized z-scores for HOMA-IR, triglyceride to HDL ratio, systolic blood pressure, and visceral fat area. Higher cardiometabolic risk scores indicate higher cardiometabolic risk.
Covariates
We used published studies and direct acyclic graphs to identify potential confounders of the associations between birth adipocytokine concentrations and adolescent adiposity and cardiometabolic outcomes (Figs. S1, S2). Trained research assistants collected baseline data at enrollment using computer assisted questionnaires and medical chart abstractions. Maternal sociodemographic factors included age at delivery and education. Perinatal and infant factors included child sex, race, birth weight percentile, and maternal parity. Birth weight percentiles were calculated according to sex and gestational age using a U.S. national reference.(32) We used maternal self-reported height and weight (or imputed weight if missing) to calculate pre-pregnancy BMI.(33) To assess gestational weight gain, we used the difference between maternal weight at the last visit prior to delivery and maternal self-reported pre-pregnancy weight, and calculated weight gain for gestational age z-scores.(34) The average of the log10-transformed serum cotinine concentrations measured at 16- and 26-weeks’ gestation were used to assess tobacco smoke exposure (active smoking: >3 ng/mL; second hand exposure: 0.015-3; unexposed: <0.015). Additionally, length of breastfeeding was assessed through standardized interviewer-administered questionnaires, and categorized as a continuous variable in the statistical models.
At the 12-year study visit, we assessed adolescents’ physical activity levels with a validated Physical Activity Questionnaire for Older Children and calculated an activity summary score.(35) Trained research staff collected three 24-hour food recalls (2 weekdays and 1 weekend day) from adolescents, and we calculated Healthy Eating Index 2010 scores. The Healthy Eating Index is a measure of diet quality in terms of conformance with federal dietary guidance using the Nutrition Data Systems for Research software and foods database (University of Minnesota, MN).(36) We also provided adolescents with standardized instructions to self-evaluate their pubertal stage (stages I-V) based on pubic hair growth in a private room.(37) The exposure, outcome, and covariate variables for mothers and children are listed in Table S1.
Statistical Analysis
We started by comparing the covariate distribution between children included in this analysis and all children in the HOME Study cohort. We calculated univariate statistics of all exposure variables (serum concentrations of leptin, adiponectin, and adiponectin-to-leptin ratio at birth and age 12 years), outcome variables (adiposity and cardiometabolic risk outcomes), and by strata of covariates for whole body fat index, the novel cardiometabolic risk score, and all exposure variables. We also calculated Pearson correlation coefficients for serum adipocytokine concentrations between birth and age 12 years.
In the main analyses of cardiometabolic risk outcomes, we focused on the novel cardiometabolic risk score and its individual components. We used multivariable linear regression to estimate covariate-adjusted associations of serum concentrations of adipocytokines at birth and age 12 years with adolescents’ adiposity and cardiometabolic risk outcomes. We estimated the difference in each adiposity and cardiometabolic risk outcome (expressed as standardized z-scores) per unit increase in log2-transformed serum concentrations of leptin, adiponectin, and adiponectin-to-leptin ratio separately at birth and age 12 years.
To evaluate whether changes in adipocytokine concentrations between birth and adolescence were associated with adolescent adiposity and cardiometabolic risk outcomes, we subtracted the sex-standardized z-scores of serum adipocytokine concentrations at birth from the age- and sex- standardized z-scores of serum adipocytokine concentrations at age 12 years to calculate the change in the adipocytokine concentrations over time. We categorized these changes into three categories according to terciles of change over time. We used spaghetti plots to visualize the changes of adipocytokine z-scores over time for each child by tercile groups for leptin, adiponectin, and adiponectin-to-leptin ratio separately. We then estimated differences in adolescents’ adiposity and cardiometabolic risk outcomes across terciles using Tercile 1, the decreasing group, as the reference. Linear trends were tested by modeling the changes of adipocytokine z-scores as continuous variables, with p-values for this term <0.05 considered as evidence of linear trends across the three terciles.
In all models, we adjusted for mother's age, education, pre-pregnancy BMI, gestational serum cotinine concentrations, parity, child’s age and sex, child’s race, and length of breastmilk feeding. We also adjusted for visceral fat area in the models examining associations of the age 12 adipocytokine concentrations and cardiometabolic risk outcomes because central adiposity may be associated with both the exposure and the outcome. We also adjusted for cord serum concentrations of leptin, adiponectin, and adiponectin-to-leptin ratio in the models examining associations of the changes in the standardized z-scores of leptin, adiponectin, and adiponectin-to-leptin ratio over time with adiposity and cardiometabolic risk outcomes at age 12, respectively, as a baseline adjustment.
Secondary and Sensitivity Analyses
First, we examined the associations of adipocytokines (at birth, age 12 years, and changes over time) with the traditional cardiometabolic risk score and its individual components. Second, we included an adipocytokine x sex interaction term in the multivariable linear regression models to determine if the exposure-outcome associations differed by sex. We considered an adipocytokine x sex interaction term p-value <0.05 to be a significant interaction. This analysis was conducted for both the prospective and cross-sectional associations of adipocytokine concentrations with adiposity and cardiometabolic risk outcomes. Third, we adjusted for serum concentrations of leptin, adiponectin, and adiponectin-to-leptin ratio at age 12 years (instead of the levels at birth) in the models examining the changes in the leptin, adiponectin, and adiponectin-to-leptin ratio with associations of adiposity and cardiometabolic risk, respectively. We also adjusted for birth weight percentile, pubertal stage, and gestational weight gain z-score in both the prospective and cross-sectional associations. Finally, we adjusted for Healthy Eating Index 2010 total scores and physical activity summary scores in the cross-sectional associations since the presence and/or directionality of the associations of these covariates with the exposure and outcome is unclear. We performed all analyses using SAS version 9.4 (SAS Institute Inc., USA).
Results
The covariate distribution was similar between children included in this analysis and all children in the HOME Study cohort (Table S2). The median (25th, 75th) serum concentrations of leptin, adiponectin, and adiponectin-to-leptin ratio were 10 (6, 16) ng/mL, 42 (30, 51) μg/mL, and 4.3 (2.4, 7.1) at birth and 10 (4, 22) ng/mL, 12 (9, 20) μg/mL, and 1.3 (0.6, 3.8) at age 12 years, respectively (Table 1, Table S3). The correlation between cord and age 12-year serum adipocytokine concentrations were moderate for leptin (Pearson’s r=0.42) and adiponectin-to-leptin ratio (Pearson r=0.36), and null for adiponectin (Pearson r=0.01).
Table 1.
Cord Blood Adipocytokine Concentrations, Whole Body Fat Mass Index, and Cardiometabolic Risk Scores at Age 12 Years According to Covariates among Children from the Health Outcomes and Measures of the Environment (HOME) Study (N=166).
| N | Leptin (ng/mL) | N | Adiponectin (μg/mL) |
N | Adiponectin- to-Leptin Ratio |
N | Whole Body Fat Mass Index |
N | Novel CM Risk Score |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Overall | 159 | 10 (6, 16) | 166 | 42 (30, 51) | 159 | 4.3 (2.4, 7.1) | 158 | 6.3 (4.7, 8.1) | 135 | 0.1 (2.7) |
| Child Sex | ||||||||||
| Female | 87 | 12 (8, 20) | 93 | 42 (33, 51) | 87 | 3.2 (2.1, 5.6) | 89 | 6.5 (5.3, 8.6) | 74 | 0.0 (2.8) |
| Male | 72 | 7 (3, 12) | 73 | 41 (27, 51) | 72 | 5.5 (3.2, 9.5) | 69 | 5.4 (4.4, 7.3) | 61 | 0.2 (2.6) |
| Child Race | ||||||||||
| Non-Hispanic White | 99 | 11 (6, 17) | 104 | 44 (34, 53) | 99 | 4.5 (2.3, 6.9) | 99 | 6.3 (5.0, 7.7) | 84 | −0.1 (2.8) |
| Non-Hispanic Black | 50 | 9 (7, 15) | 51 | 36 (26, 50) | 50 | 3.4 (2.4, 6.5) | 49 | 6.3 (4.7, 8.8) | 41 | 0.6 (2.7) |
| Other | 10 | 5 (1, 8) | 11 | 39 (20, 49) | 10 | 7.6 (3.3,14) | 10 | 5.2 (3.8, 8.0) | 10 | −0.4 (1.8) |
| Breastfeeding Duration | ||||||||||
| 0 month | 27 | 9 (4, 15) | 29 | 34 (22, 47) | 27 | 4.3 (2.4, 8.3) | 28 | 6.6 (4.7,11) | 22 | 1.4 (2.8) |
| >0-6 months | 67 | 10 (6, 16) | 68 | 45 (35, 55) | 67 | 4.9 (2.5, 8.2) | 64 | 5.9 (4.6, 7.3) | 58 | −0.4 (2.4) |
| >6 months | 65 | 10 (6, 17) | 69 | 41 (30, 50) | 65 | 4.0 (2.1, 6.5) | 66 | 6.5 (5.0, 8.4) | 55 | 0.1 (2.9) |
| Maternal Age | ||||||||||
| 18-25 Years | 35 | 7 (4, 12) | 35 | 39 (26, 47) | 35 | 5.4 (3.3, 8.6) | 33 | 5.4 (4.5, 8.3) | 28 | −0.2 (2.2) |
| >25-35 Years | 103 | 12 (7, 17) | 108 | 43 (33, 53) | 103 | 3.6 (2.3, 6.8) | 103 | 6.4 (5.0, 8.1) | 87 | 0.1 (2.8) |
| >35 Years | 21 | 8 (7, 12) | 23 | 45 (22, 53) | 21 | 5.4 (2.6, 7.1) | 22 | 5.4 (4.7, 8.0) | 20 | 0.4 (3.3) |
| Maternal Education | ||||||||||
| High School or Less | 29 | 8 (5, 15) | 30 | 38 (22, 55) | 29 | 5.4 (2.4, 7.4) | 28 | 7.1 (5.0, 9.7) | 22 | 1.1 (3.0) |
| Tech School or Some College | 45 | 10 (7, 17) | 48 | 41 (30, 48) | 45 | 3.3 (2.1, 6.8) | 46 | 6.0 (4.5, 7.8) | 42 | −0.3 (2.6) |
| Bachelor's or More | 85 | 11 (6, 15) | 88 | 44 (33, 53) | 85 | 4.9 (2.9, 6.9) | 84 | 6.3 (5.0, 7.8) | 71 | 0.0 (2.7) |
| Pre-Pregnancy BMI | ||||||||||
| <25 | 86 | 8 (4, 13) | 90 | 42 (33, 50) | 86 | 5.4 (3.2, 8.9) | 84 | 5.4 (4.6, 7.2) | 69 | −0.2 (2.6) |
| ≥25-30 | 39 | 12 (7, 17) | 39 | 44 (35, 55) | 39 | 3.4 (2.0, 6.8) | 39 | 6.5 (5.2, 8.6) | 37 | 0.0 (2.7) |
| ≥30 | 34 | 11 (7, 21) | 37 | 41 (24, 53) | 34 | 2.9 (2.1, 4.3) | 35 | 7.4 (5.8,10) | 29 | 1.0 (2.9) |
| Gestational Serum Cotinine Concentrations (ng/ml) | ||||||||||
| <0.015 (Unexposed) | 53 | 11 (4, 15) | 56 | 42 (35, 52) | 53 | 4.9 (2.5, 8.3) | 52 | 6.4 (5.3, 8.2) | 43 | −0.1 (2.6) |
| 0.015-3 (Secondhand) | 91 | 10 (7, 16) | 93 | 43 (30, 51) | 91 | 4.3 (2.5, 6.5) | 89 | 6.0 (4.7, 8.0) | 80 | 0.1 (2.8) |
| >3 (Active Smoker) | 15 | 7 (4, 17) | 17 | 33 (22, 59) | 15 | 3.0 (2.2, 12) | 17 | 6.3 (4.4, 8.6) | 12 | 1.0 (2.5) |
| Parity | ||||||||||
| 0 | 66 | 10 (6, 15) | 70 | 44 (34, 51) | 66 | 4.9 (2.5, 6.9) | 67 | 6.3 (5.1, 8.1) | 56 | −0.3 (2.6) |
| 1 | 55 | 8 (5, 15) | 58 | 43 (33, 52) | 55 | 5.1 (3.1, 8.0) | 54 | 5.8 (4.6, 8.1) | 44 | 0.5 (2.9) |
| ≥2 | 38 | 11 (7, 20) | 38 | 34 (20, 50) | 38 | 2.7 (1.4, 7.1) | 37 | 6.3 (4.9, 8.3) | 35 | 0.2 (2.6) |
All values expressed as Median (25th, 75th); except the CM Risk score, which is expressed as mean (SD)
BMI: body mass index; CM: cardiometabolic; SD: standard deviation; Whole body fat mass index (kg/m2) was calculated as whole body fat mass/ (height*height); Novel CM risk score was the sum of the standardized z-scores for Homeostatic Model Assessment of Insulin Resistance, triglyceride to HDL ratio, systolic blood pressure, and visceral fat area.
The median (25th, 75th) whole body fat mass index was 6.3 (4.7, 8.1) kg/m2, and the mean (SD) of the novel cardiometabolic risk score was 0.1 (2.7) (Table 1). On average, adolescents’ mean novel cardiometabolic risk scores were higher among children who were non-Hispanic black, not breastfed, or born to mothers who were >35 years at delivery, less educated (≤ high school education), actively smoked during pregnancy, or had higher pre-pregnancy BMI (≥30 kg/m2). The distribution of the adiposity and cardiometabolic risk outcomes are presented in Table S4. At age 12 years, 62% of the children were normal-weight, 25% were overweight, and 13% were obese. The distribution of pubertal stage at age 12 years were stage 1 (13%), stage 2 (25%), stage 3 (32%), stage 4 (18%) and stage 5 (12%).
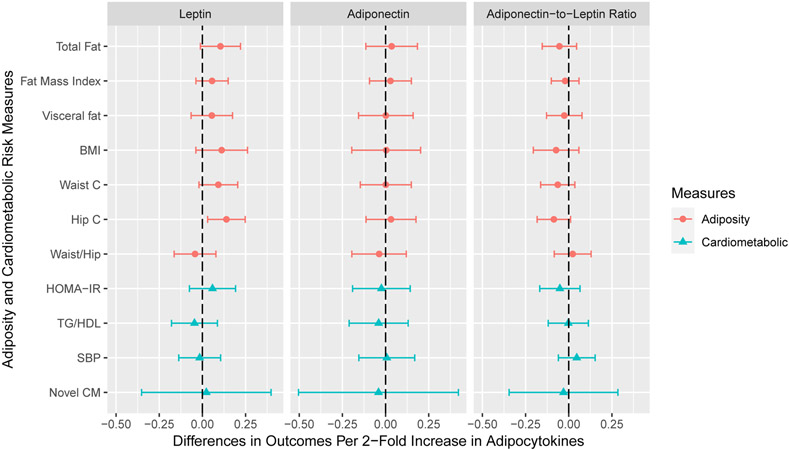
After adjusting for covariates, cord serum concentrations of leptin, adiponectin, and adiponectin-to-leptin ratio were not associated with adiposity or cardiometabolic risk outcomes, except that cord leptin was associated with increased hip circumference (β: 0.14; 95% confidence interval [CI]: 0.03, 0.25) (Figure 1). Cord leptin was marginally associated with increased total fat mass (β: 0.10; 95% CI: −0.01, 0.22) and waist circumference (β: 0.09; 95% CI: −0.02, 0.20) (Table S5).
Figure 1. Adjusted Differences in Adiposity and Cardiometabolic Risk Outcome Standardized Z-Scores with 1-Unit Increase in Log2-Transformed Cord Serum Adipocytokine Concentrations: The HOME Study.
BMI: body mass index; waist c: waist circumference; hip c: hip circumference; waist/hip: waist to hip ratio; HOMA-IR: Homeostatic Model Assessment of Insulin Resistance; TG/HDL: triglycerides to high density lipoprotein ratio; SBP: systolic blood pressure; novel CM: novel cardiometabolic risk score.
All models adjusted for maternal age, maternal education, maternal pre-pregnancy BMI, gestational smoking, parity; child age, sex, race, and length of breastfeeding.
All outcomes were sex- and age-standardized z-scores. Whole body fat mass, visceral fat area, waist circumference, HOMA-IR, and triglycerides to HDL ratio were not normally distributed, and thus were log2-transformed before standardization.
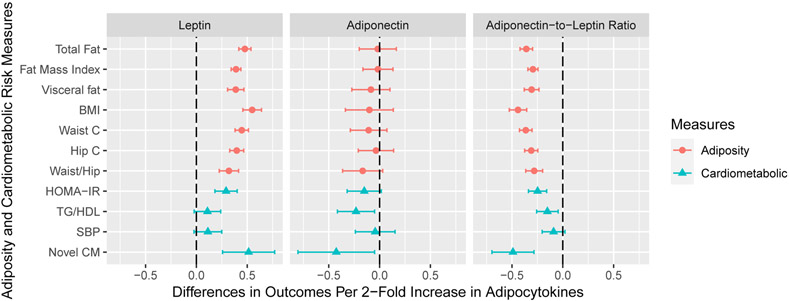
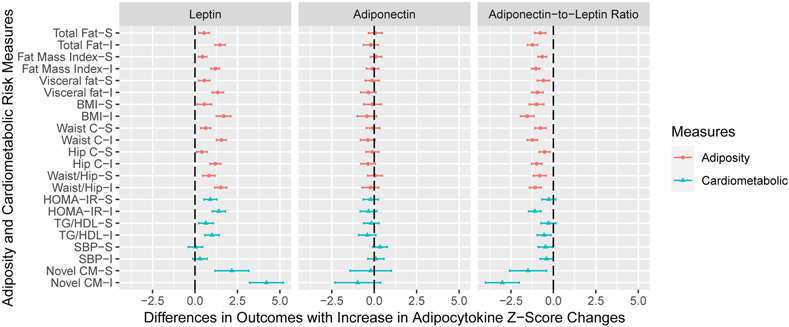
Serum leptin concentrations at age 12 years were positively associated with all adiposity outcomes (Figure 2). Adiponectin-to-leptin ratio at age 12 years was negatively associated with all adiposity outcomes, but adiponectin was not associated with the adiposity outcomes at age 12 years. Serum leptin (β 0.51; 95% CI: 0.26, 0.77) and adiponectin (β −0.43; 95% CI: −0.80, −0.05) at age 12 years had opposite associations with the novel cardiometabolic risk score (Figure 2). Adiponectin-to-leptin ratio was negatively associated with the novel cardiometabolic risk score (β −0.49; 95% CI: −0.70, −0.28) (Table S6).
Figure 2. Adjusted Differences in Adiposity and Cardiometabolic Risk Outcome Standardized Z-Scores with 1-Unit Increase in Log2-Transformed Adipocytokine Concentrations at Age 12 Years: The HOME Study.
BMI: body mass index; waist c: waist circumference; hip c: hip circumference; waist/hip: waist to hip ratio; HOMA-IR: Homeostatic Model Assessment of Insulin Resistance; TG/HDL: triglycerides to high density lipoprotein ratio; SBP: systolic blood pressure; Novel CM: novel cardiometabolic risk score.
All models adjusted for maternal age, maternal education, maternal pre-pregnancy BMI, gestational smoking, parity; child age, sex, race, and length of breastfeeding. Visceral fat area was adjusted in cardiometabolic risk models. All outcomes were sex- and age-standardized z-scores. Whole body fat mass, visceral fat area, waist circumference, HOMA-IR, and triglycerides to HDL ratio were not normally distributed, and thus were log2-transformed before standardization.
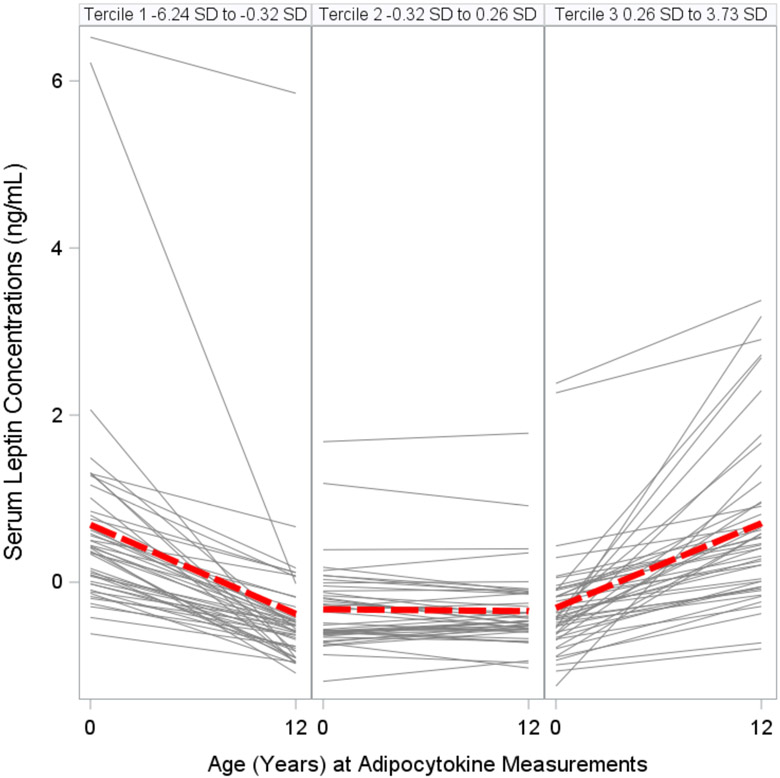
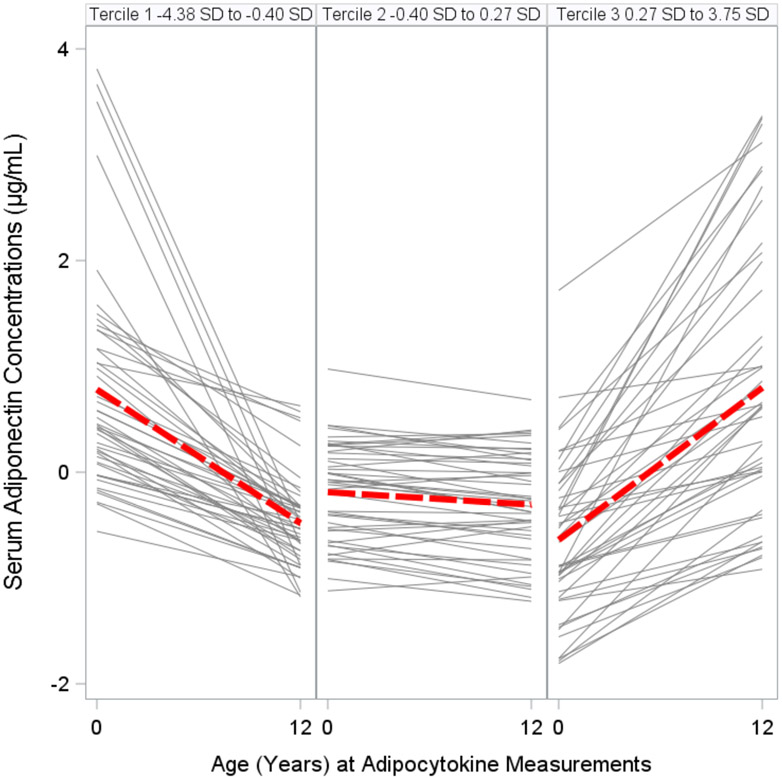
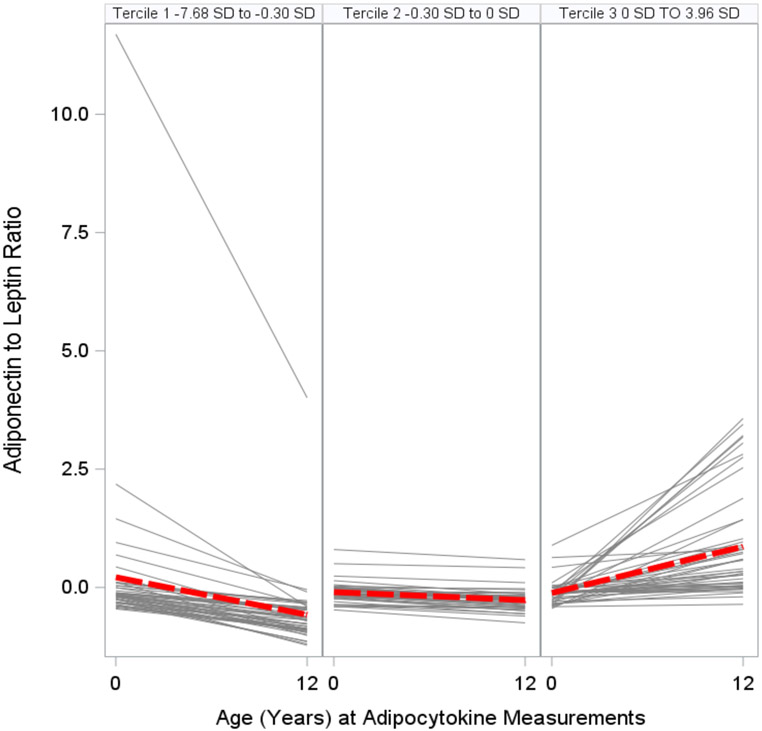
We categorized changes in adipocytokine standardized z-scores from birth to age 12 years into terciles: Tercile 1, decreasing; Tercile 2, stable; and Tercile 3, increasing (Figs. 3A, 3B, 3C, Table S7). Adolescents with increasing (Tercile 3: 0.26 SD to 3.73 SD range) or stable (Tercile 2: −0.32 SD to 0.26 SD range) leptin concentration z-scores from birth to age 12 years had higher adiposity and worse cardiometabolic risk scores than adolescents with decreasing (Tercile 1: −6.24 SD to −0.32 SD range) leptin concentration z-scores (all p-values for linear trend <0.01) (Figure 4). Similarly, adolescents with increasing (Tercile 3: 0 SD to 3.96 SD) or stable (Tercile 2: −0.30 SD to 0 SD) adiponectin-to-leptin ratio z-scores had lower adiposity and more favorable cardiometabolic risk scores than adolescents with decreasing (Tercile 1: −7.68 SD to −0.30 SD) adiponectin-to-leptin ratio z-scores (all p-values for linear trend <0.01). Changes in adiponectin concentration z-scores (Tercile 1: −4.38 D to −0.40 SD; Tercile 2: −0.40 SD to 0.27 SD; Tercile 3: 0.27 SD to 3.75 SD) were not associated with the adiposity outcomes or cardiometabolic risk scores (Table S8).
Figure 3A. Changes (by Terciles) in Serum Leptin Concentrations Standardized Z-Scores Between Birth and Age 12 Years among HOME Study Children.
The gray lines show the changes from birth to age 12 years for each individual. The red lines show the average trend in that tercile.
Figure 3B. Changes (by Terciles) in Serum Adiponectin Concentrations Standardized Z-Scores Between Birth and Age 12 Years among HOME Study Children.
The gray lines show the changes from birth to age 12 years for each individual. The red lines show the average trend in that tercile.
Figure 3C. Changes (by Terciles) in Serum Adiponectin-to-Leptin Ratio Standardized Z-Scores Between Birth and Age 12 Years among HOME Study Children.
The gray lines show the changes from birth to age 12 years for each individual. The red lines show the average trend in that tercile.
Figure 4. Adjusted Differences in Adiposity and Cardiometabolic Risk Outcome Standardized Z-Scores with 1- or 2- Tercile Increase in Adipocytokine Standardized Z-Score Changes (Age 12 Year – Cord), additionally Adjusted for Adipocytokine Concentrations at Birth.
Reference group is the decreasing group (Tercile 1). -S: stable group; -I: increasing group.
Leptin: Tercile 1 (Decreasing) −6.24 SD to −0.32 SD; Tercile 2 (Stable) −0.32 SD to 0.26 SD; Tercile 3 (Increasing) 0.26 SD to 3.73 SD.
Adiponectin: Tercile 1 (Decreasing) −4.38 SD to −0.40 SD; Tercile 2 (Stable) −0.40 SD to 0.27 SD; Tercile 3 (Increasing) 0.27 SD to 3.75 SD.
Adiponectin-to-leptin ratio: Tercile 1 (Decreasing) −7.68 SD to −0.30 SD; Tercile 2 (Stable) −0.30 SD to 0 SD; Tercile 3 (Increasing) 0 SD to 3.96 SD.
Fat: whole body fat mass; FMI: whole body fat mass index; Visc: visceral fat area; BMI: body mass index; W: waist circumference; H: hip circumference; W/H: waist to hip ratio; HOMA-IR: Homeostatic Model Assessment of Insulin Resistance; TG/HDL: triglycerides to high density lipoprotein ratio; SBP: systolic blood pressure; Novel: novel cardiometabolic risk score.
All models adjusted for maternal age, maternal education, maternal pre-pregnancy BMI, gestational smoking, parity; child age, sex, race, and length of breastfeeding.
All outcomes were sex- and age-standardized z-scores. Whole body fat mass, visceral fat area, waist circumference, HOMA-IR, and triglycerides to HDL ratio were not normally distributed, and thus were log2-transformed before standardization.
The associations of adipocytokines at birth, at age 12 years, and change over time with the traditional cardiometabolic risk score and its individual components were similar to that of the novel cardiometabolic risk score (Tables S5, S6, S8). The associations of adipocytokine concentrations at birth and age 12 years with adolescents’ adiposity and cardiometabolic risk outcomes generally did not differ by sex (Tables S9, S10). For some associations of the age 12-year adipocytokine concentrations, the interaction p-values were <0.05; however, the directions of the sex-specific associations were the same (e.g., for the association between leptin and waist circumference, the βs [95%CIs] were 0.56 [0.46, 0.66] for girls and 0.36 [0.28, 0.44] for boys). When we adjusted for serum concentrations of leptin, adiponectin, and adiponectin-to-leptin ratio at age 12 years (instead of levels at birth) in the models examining the changes in adipocytokine concentrations, the associations were slightly attenuated. However, most of the significant associations of leptin and adiponectin-to-leptin ratio with adiposity and cardiometabolic risk outcomes remained (Figure S3). The associations of cord serum adipocytokine concentrations with adiposity and cardiometabolic risk outcomes were not substantially altered after additionally adjusting for birth weight percentile or pubertal stage. Similarly, the cross-sectional associations of age 12-year adipocytokine concentrations with adiposity and cardiometabolic risk outcomes were not substantially different from our main analysis after additionally adjusting for birth weight percentile, pubertal stage, gestational weight gain z-score, Healthy Eating Index 2010 total scores, or physical activity summary scores (data not shown).
Discussion
In this prospective cohort, adipocytokine concentrations at age 12 years, but not at birth, were associated with adolescents’ adiposity and cardiometabolic risk assessments. Changes in both leptin and adiponectin-to-leptin ratio from birth to age 12 years predicted both adolescent adiposity outcomes and cardiometabolic risk scores, independent of birth or 12-year levels. In our cohort, increasing and stable leptin concentrations were associated with higher adiposity and worse cardiometabolic risk scores, while increasing and stable adiponectin-to-leptin ratios were associated with lower adiposity and more favorable cardiometabolic risk scores.
Our observation that rises in leptin over time were associated with adiposity and cardiometabolic risk outcomes are consistent with findings from the Project Viva cohort, in which adolescents with increasing leptin from birth to mid-childhood had higher metabolic risk scores.(16) Both results support the notion that changing leptin concentrations over time may represent the development of leptin resistance in children, which is associated with insulin resistance, central, and visceral adiposity.(38) Another study examining trajectories of adipocytokine change over time have also found similar results. In that study, Volberg et al. examined correlations of leptin and adiponectin at 4 time points between birth and age 9 years, and found positive associations of rising leptin, but not adiponectin, with waist circumference and BMI in mid-childhood.(17)
Similar to our findings, several other studies have also found that higher leptin and lower adiponectin concentrations in adolescence were associated with adverse adiposity and cardiometabolic risk outcomes, including central adiposity, insulin resistance, blood pressure percentiles, and lipid profiles. (13, 15) Most of these studies, however, have not examined comprehensive indicators of cardiometabolic risk, including detailed assessments of fat mass and central adiposity by methods other than BMI or body circumferences. Additionally, factors such as puberty or measures of visceral or abdominal fat were not consistently adjusted for in the analyses in prior studies, and the proportion of subjects with overweight or obese status varied significantly in prior studies. These factors may explain some of the variation in the magnitude of results across studies. In our study, the associations of adipocytokine concentrations at birth and age 12 years with adolescents’ adiposity and cardiometabolic risk outcomes generally did not differ by sex. Consistent with this, no interaction by child sex was observed in the Project Viva cohort when examining age 3-year leptin in associations with age 7-year adiposity.(38) Other studies have not examined interaction by sex.
Our study is the first, to our knowledge, to assess the relationships of birth adiponectin and adiponectin-to-leptin ratio with adolescent cardiometabolic risk outcomes. Adiponectin-to-leptin ratio has been associated with adiposity and insulin resistance in children and adolescents.(21) Overall, we did not identify any significant associations between adiponectin concentrations at birth or change in adiponectin concentrations over time and the age 12-year outcomes. Our finding of adiponectin-to-leptin ratio predicting both traditional and novel cardiometabolic risk scores was driven mostly by leptin concentrations. Our results suggest that the association between changes in leptin and adiponectin-to-leptin ratio over time with adiposity and cardiometabolic risk may also be driven by the cross-sectional associations of adipocytokines and adiposity outcomes. Alternatively, adolescent adiposity and cardiometabolic dysfunction could cause changes in adipocytokines (i.e., reverse causation). Although, when we adjusted for age 12-year adipocytokine concentrations in our models, the associations remained.
In our prior work from the HOME Study, birth adiponectin and leptin were both associated with childhood body mass index and adiposity development over time.(11) Increased BMI and rate of weight gain in early childhood are important predictors of obesity and cardiometabolic disease in adolescence.(39) The exact mechanism of how adiposity development and cardiometabolic risk is programmed, and the role of early concentrations of adipocytokines in this process is largely unknown. In regards to cardiometabolic disease, animal models have shown that insulin resistance and hyperlipidemia among offspring exposed to a maternal high fat diet in pregnancy are due to methylation patterns in both adiponectin and leptin genes.(10) Recognizing the complex influence of the perinatal environment in the programming of both adiposity and cardiometabolic risk, there is a need to further identify these mechanisms to inform strategies to decrease adverse cardiometabolic consequences in vulnerable populations.
This study has several strengths. Our prospective and longitudinal design have allowed for examining adipocytokines at birth, age 12 years, and change over time in association with the adiposity and cardiometabolic risk outcomes. Additionally, we assessed a novel risk score of adiposity and cardiometabolic risk outcomes, creating standardized scores of insulin resistance, visceral fat area, triglycerides, and HDL. Inclusion of HOMA-IR, a robust surrogate for the gold standard euglycemic clamp diagnosis of insulin resistance;(24) directly measured visceral adipose tissue;(40) and the triglyceride to HDL ratio, which is a known predictor of cardiovascular disease,(29, 30) may better represent cardiometabolic risk in adolescents than other less refined measures (2, 41). While we used a novel cardiometabolic risk score, it is important to note that there are several metabolic syndrome definitions (at least 8) and ongoing debate regarding the clinical significance and predictive power of these definitions for adolescents.(42)
Limitations of the present study include our moderate sample size, loss to follow up, and self-reported assessment of adolescent puberty status. Additional limitations include the inability to include covariates such as detailed maternal nutrition and placental function in our analysis. Weight and adiposity at birth, which are influenced by maternal nutrition and other health conditions, are important predictors of cardiometabolic outcomes in childhood and adolescence.(43) However, adjustment for birth weight percentile did not substantially change our associations. We were also unable to measure maternal or early childhood adipocytokine concentrations, which may have further informed our trajectories of adipocytokine change over time or establish temporality of the association of adipocytokine concentrations with adolescent adiposity and cardiometabolic risk measures.(44) In light of this, our categories of adipocytokine change were consistent with other studies that examined concentrations at 3 or more time points during childhood and/or adolescence.(16, 17) There is limited information regarding changes in adipocytokines over time, particularly over the first two years of life, which represent critical developmental windows for adipose tissue development.(45) How such changes may influence adolescent adiposity and cardiometabolic risk outcomes is an important avenue for future study. Finally, while multiple comparisons may be a concern in this study, leptin, adiponectin, and adiponectin-to-leptin ratio were all consistently associated with different adiposity and cardiometabolic risk measures, which suggests that these results may not be solely due to chance. The potential concern of false positive results necessitates further replication of our findings.
Conclusion
In this prospective cohort study of pregnant women and their children, adolescents with increasing and stable leptin concentrations over time had higher adiposity and worse cardiometabolic risk scores than those with decreasing concentrations. Adipocytokines are potential biomarkers for predicting excess adiposity and cardiometabolic risk in adolescence. Further study on the mechanism linking adipocytokines to metabolic programming and the development of adipocytokine resistance may shed light on future risk stratification and prevention strategies for reducing cardiometabolic disease.
Supplementary Material
What is already known about this subject?
Evidence shows that metabolic abnormalities stem from perturbations of normal developmental programing in utero and in early infancy.
Adipocytokines, such as leptin and adiponectin are biomarkers of adipose tissue metabolism.
What are the new findings in this manuscript?
Change in leptin concentrations from birth to adolescence were associated with higher cardiometabolic risk scores among early adolescents.
Change in adiponectin-to-leptin ratios from birth to adolescence were associated with more favorable adiposity outcomes among early adolescents.
How might the results change the direction of research or the focus of clinical practice?
Changes in adipocytokine concentration over time may predict excess adiposity and cardiometabolic risk in adolescence.
Funding:
This work was supported by National Institute of Environmental Health Sciences grants P01 ES011261, R01 ES014575, R01 ES025214, and R01 ES027224.
Footnotes
Disclosure: JMB received an honorarium from Quest Diagnostic for serving on an expert panel related to endocrine disrupting chemicals. JMB’s institution was financially compensated for his services as an expert witness for plaintiffs in litigation related to PFAS-contaminated drinking water; these funds were not paid to JMB directly. KTK is a founder and advisor for Cellintec, which played no role in this work. The other authors declare they have no actual or potential competing financial interests.
References
- 1.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–5. Epub 2009/10/07. doi: 10.1161/CIRCULATIONAHA.109.192644. PubMed PMID: 19805654. [DOI] [PubMed] [Google Scholar]
- 2.Kelly AS, Steinberger J, Jacobs DR, Hong CP, Moran A, Sinaiko AR. Predicting cardiovascular risk in young adulthood from the metabolic syndrome, its component risk factors, and a cluster score in childhood. Int J Pediatr Obes. 2011;6(2-2):e283–9. Epub 2010/11/13. doi: 10.3109/17477166.2010.528765. PubMed PMID: 21070100; PubMed Central PMCID: PMCPMC3684392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Expert Panel on Integrated Guidelines for Cardiovascular H, Risk Reduction in C, Adolescents, National Heart L, Blood I. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128 Suppl 5:S213–56. Epub 2011/11/16. doi: 10.1542/peds.2009-2107C. PubMed PMID: 22084329; PubMed Central PMCID: PMCPMC4536582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffman DJ, Reynolds RM, Hardy DB. Developmental origins of health and disease: current knowledge and potential mechanisms. Nutr Rev. 2017;75(12):951–70. Epub 2017/12/01. doi: 10.1093/nutrit/nux053. PubMed PMID: 29186623. [DOI] [PubMed] [Google Scholar]
- 5.D'Ippolito S, Tersigni C, Scambia G, Di Simone N. Adipokines, an adipose tissue and placental product with biological functions during pregnancy. Biofactors. 2012;38(1):14–23. doi: 10.1002/biof.201. PubMed PMID: 22287297. [DOI] [PubMed] [Google Scholar]
- 6.Karakosta P, Chatzi L, Plana E, Margioris A, Castanas E, Kogevinas M. Leptin levels in cord blood and anthropometric measures at birth: a systematic review and meta-analysis. Paediatric and Perinatal Epidemiology. 2011;25(2):150–63. doi: 10.1111/j.1365-3016.2010.01163.x. PubMed PMID: WOS:000286837200008. [DOI] [PubMed] [Google Scholar]
- 7.Sivan E, Mazaki-Tovi S, Pariente C, Efraty Y, Schiff E, Hemi R, et al. Adiponectin in human cord blood: relation to fetal birth weight and gender. J Clin Endocrinol Metab. 2003;88(12):5656–60. doi: 10.1210/jc.2003-031174. PubMed PMID: 14671149. [DOI] [PubMed] [Google Scholar]
- 8.Qiao L, Yoo HS, Madon A, Kinney B, Hay WW Jr., Shao J Adiponectin enhances mouse fetal fat deposition. Diabetes. 2012;61(12):3199–207. doi: 10.2337/db12-0055. PubMed PMID: 22872236; PubMed Central PMCID: PMCPMC3501876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeltser LM. Developmental influences on circuits programming susceptibility to obesity. Front Neuroendocrinol. 2015;39:17–27. doi: 10.1016/j.yfrne.2015.07.002. PubMed PMID: 26206662; PubMed Central PMCID: PMCPMC4681591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khalyfa A, Carreras A, Hakim F, Cunningham JM, Wang Y, Gozal D. Effects of late gestational high-fat diet on body weight, metabolic regulation and adipokine expression in offspring. Int J Obes (Lond). 2013;37(11):1481–9. Epub 2013/02/13. doi: 10.1038/ijo.2013.12. PubMed PMID: 23399773; PubMed Central PMCID: PMCPMC3701742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buck CO, Eliot MN, Kelsey KT, Chen A, Kalkwarf H, Lanphear BP, et al. Neonatal Adipocytokines and Longitudinal Patterns of Childhood Growth. Obesity (Silver Spring). 2019;27(8):1323–30. Epub 2019/06/15. doi: 10.1002/oby.22519. PubMed PMID: 31199076; PubMed Central PMCID: PMCPMC6656611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li LJ, Rifas-Shiman SL, Aris IM, Young JG, Mantzoros C, Hivert MF, et al. Associations of maternal and cord blood adipokines with offspring adiposity in project viva: Is there an interaction with child age? Int J Obes (Lond). 2017. doi: 10.1038/ijo.2017.256. PubMed PMID: 29026216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaibi GQ, Cruz ML, Weigensberg MJ, Toledo-Corral CM, Lane CJ, Kelly LA, et al. Adiponectin independently predicts metabolic syndrome in overweight Latino youth. J Clin Endocrinol Metab. 2007;92(5):1809–13. Epub 2007/02/22. doi: 10.1210/jc.2006-2294. PubMed PMID: 17311859. [DOI] [PubMed] [Google Scholar]
- 14.Nappo A, Gonzalez-Gil EM, Ahrens W, Bammann K, Michels N, Moreno LA, et al. Analysis of the association of leptin and adiponectin concentrations with metabolic syndrome in children: Results from the IDEFICS study. Nutr Metab Cardiovasc Dis. 2017;27(6):543–51. Epub 2017/05/18. doi: 10.1016/j.numecd.2017.04.003. PubMed PMID: 28511904. [DOI] [PubMed] [Google Scholar]
- 15.Stakos DA, Papaioannou HI, Angelidou I, Mantadakis E, Paraskakis E, Tsigalou C, et al. Plasma leptin and adiponectin concentrations correlate with cardiometabolic risk and systemic inflammation in healthy, non-obese children. J Pediatr Endocrinol Metab. 2014;27(3-4):221–8. Epub 2013/10/24. doi: 10.1515/jpem-2013-0195. PubMed PMID: 24150199. [DOI] [PubMed] [Google Scholar]
- 16.Li LJ, Rifas-Shiman SL, Aris IM, Mantzoros C, Hivert MF, Oken E. Leptin trajectories from birth to mid-childhood and cardio-metabolic health in early adolescence. Metabolism. 2019;91:30–8. Epub 2018/11/10. doi: 10.1016/j.metabol.2018.11.003. PubMed PMID: 30412696; PubMed Central PMCID: PMCPMC6366620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Volberg V, Heggeseth B, Harley K, Huen K, Yousefi P, Dave V, et al. Adiponectin and Leptin Trajectories in Mexican-American Children from Birth to 9 Years of Age. Plos One. 2013;8(10). doi: 10.1371/journal.pone.0077964. PubMed PMID: WOS:000326334500083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blundell JE, Dulloo AG, Salvador J, Fruhbeck G, BMI ESWGo. Beyond BMI--phenotyping the obesities. Obes Facts. 2014;7(5):322–8. Epub 2014/12/09. doi: 10.1159/000368783. PubMed PMID: 25485991; PubMed Central PMCID: PMCPMC5644899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Braun JM, Kalloo G, Chen A, Dietrich KN, Liddy-Hicks S, Morgan S, et al. Cohort Profile: The Health Outcomes and Measures of the Environment (HOME) study. Int J Epidemiol. 2017;46(1):24. Epub 2016/03/24. doi: 10.1093/ije/dyw006. PubMed PMID: 27006352; PubMed Central PMCID: PMCPMC5837495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braun JM, Buckley JP, Cecil KM, Chen A, Kalkwarf HJ, Lanphear BP, et al. Adolescent follow-up in the Health Outcomes and Measures of the Environment (HOME) Study: cohort profile. BMJ Open. 2020;10(5):e034838. Epub 2020/05/10. doi: 10.1136/bmjopen-2019-034838. PubMed PMID: 32385062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mihalopoulos NL, Urban BM, Metos JM, Balch AH, Young PC, Jordan KC. Breast-feeding, Leptin:Adiponectin Ratio, and Metabolic Dysfunction in Adolescents with Obesity. Southern Medical Journal. 2017;110(5):347–52. doi: 10.14423/smj.0000000000000653. PubMed PMID: WOS:000400588300006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WHO Child Growth Standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: Methods and development. Geneva: World Health Organization; [updated 2006]. Available from: http://www.who.int/childgrowth/standards/en/. [Google Scholar]
- 23.Weber DR, Moore RH, Leonard MB, Zemel BS. Fat and lean BMI reference curves in children and adolescents and their utility in identifying excess adiposity compared with BMI and percentage body fat. Am J Clin Nutr. 2013;98(1):49–56. Epub 2013/05/24. doi: 10.3945/ajcn.112.053611. PubMed PMID: 23697708; PubMed Central PMCID: PMCPMC3683820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. Epub 1985/07/01. doi: 10.1007/bf00280883. PubMed PMID: 3899825. [DOI] [PubMed] [Google Scholar]
- 25.Gillman MW, Cook NR. Blood pressure measurement in childhood epidemiological studies. Circulation. 1995;92:1049–57. [DOI] [PubMed] [Google Scholar]
- 26.Lacruz ME, Kluttig A, Kuss O, Tiller D, Medenwald D, Nuding S, et al. Short-term blood pressure variability - variation between arm side, body position and successive measurements: a population-based cohort study. BMC Cardiovasc Disord. 2017;17(1):31. Epub 2017/01/18. doi: 10.1186/s12872-017-0468-7. PubMed PMID: 28100183; PubMed Central PMCID: PMCPMC5241970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.U.S. Department of Health and Human Services. National Institutes of Health National Heart, Lung, and Blood Institute. The Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents. Revised May 2005. [Google Scholar]
- 28.Eisenmann JC. On the use of a continuous metabolic syndrome score in pediatric research. Cardiovasc Diabetol. 2008;7:17. doi: 10.1186/1475-2840-7-17. PubMed PMID: 18534019; PubMed Central PMCID: PMCPMC2430947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manco M, Grugni G, Di Pietro M, Balsamo A, Di Candia S, Morino GS, et al. Triglycerides-to-HDL cholesterol ratio as screening tool for impaired glucose tolerance in obese children and adolescents. Acta Diabetol. 2016;53(3):493–8. Epub 2015/12/22. doi: 10.1007/s00592-015-0824-y. PubMed PMID: 26687197. [DOI] [PubMed] [Google Scholar]
- 30.Krawczyk M, Ruminska M, Witkowska-Sedek E, Majcher A, Pyrzak B. Usefulness of the Triglycerides to High-Density Lipoprotein Cholesterol ratio (TG/HDL-C) in prediction of metabolic syndrome in Polish obese children and adolescents. Acta Biochim Pol. 2018;65(4):605–11. Epub 2018/11/20. doi: 10.18388/abp.2018_2649. PubMed PMID: 30451245. [DOI] [PubMed] [Google Scholar]
- 31.Väistö J, Eloranta AM, Viitasalo A, Tompuri T, Lintu N, Karjalainen P, et al. Physical activity and sedentary behaviour in relation to cardiometabolic risk in children: cross-sectional findings from the Physical Activity and Nutrition in Children (PANIC) Study. Int J Behav Nutr Phys Act. 2014;11:55. Epub 2014/04/26. doi: 10.1186/1479-5868-11-55. PubMed PMID: 24766669; PubMed Central PMCID: PMCPMC4008488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. doi: 10.1186/1471-2431-3-6. PubMed PMID: 12848901; PubMed Central PMCID: PMCPMC169185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Laan MJ, Polley EC, Hubbard AE. Super learner. Statistical applications in genetics and molecular biology. 2007;6:Article 25. doi: 10.2202/1544-6115.1309. PubMed PMID: 17910531. [DOI] [PubMed] [Google Scholar]
- 34.Hutcheon JA, Platt RW, Abrams B, Himes KP, Simhan HN, Bodnar LM. A weight-gain-for-gestational-age z score chart for the assessment of maternal weight gain in pregnancy. Am J Clin Nutr. 2013;97(5):1062–7. doi: 10.3945/ajcn.112.051706. PubMed PMID: 23466397; PubMed Central PMCID: PMCPMC3625243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kowalski K, Crocker P, Faulkner R. Validation of the Physical Activity Questionnaire for Older Children. Pediatric Exercise Science. 1997;9(2):174–86. [Google Scholar]
- 36.Guenther PM, Casavale KO, Reedy J, Kirkpatrick SI, Hiza HA, Kuczynski KJ, et al. Update of the Healthy Eating Index: HEI-2010. J Acad Nutr Diet. 2013;113(4):569–80. Epub 2013/02/13. doi: 10.1016/j.jand.2012.12.016. PubMed PMID: 23415502; PubMed Central PMCID: PMCPMC3810369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rasmussen AR, Wohlfahrt-Veje C, Tefre de Renzy-Martin K, Hagen CP, Tinggaard J, Mouritsen A, et al. Validity of self-assessment of pubertal maturation. Pediatrics. 2015;135(1):86–93. Epub 2014/12/24. doi: 10.1542/peds.2014-0793. PubMed PMID: 25535262. [DOI] [PubMed] [Google Scholar]
- 38.Boeke CE, Mantzoros CS, Hughes MD, Rifas-Shiman SL, Villamor E, Zera CA, et al. Differential Associations of Leptin with Adiposity Across Early Childhood. Obesity. 2013;21(7):1430–7. doi: 10.1002/oby.20314. PubMed PMID: WOS:000323351400017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sacco MR, de Castro NP, Euclydes VLV, Souza JM, Rondo PHC. Birth weight, rapid weight gain in infancy and markers of overweight and obesity in childhood. European Journal of Clinical Nutrition. 2013;67(11):1147–53. doi: 10.1038/ejcn.2013.183. PubMed PMID: WOS:000326666300007. [DOI] [PubMed] [Google Scholar]
- 40.Zhang MZ, Hu T, Zhang SY, Zhou L. Associations of Different Adipose Tissue Depots with Insulin Resistance: A Systematic Review and Meta-analysis of Observational Studies. Sci Rep-Uk. 2015;5. doi: ARTN 18495 10.1038/srep18495. PubMed PMID: WOS:000367080200001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kamel M, Smith BT, Wahi G, Carsley S, Birken CS, Anderson LN. Continuous cardiometabolic risk score definitions in early childhood: a scoping review. Obes Rev. 2018;19(12):1688–99. Epub 2018/09/18. doi: 10.1111/obr.12748. PubMed PMID: 30223304. [DOI] [PubMed] [Google Scholar]
- 42.Serbis A, Giapros V, Galli-Tsinopoulou A, Siomou E. Metabolic Syndrome in Children and Adolescents: Is There a Universally Accepted Definition? Does it Matter? Metab Syndr Relat Disord. 2020;18(10):462–70. Epub 2020/08/17. doi: 10.1089/met.2020.0076. PubMed PMID: 32795106. [DOI] [PubMed] [Google Scholar]
- 43.Lowe WL Jr., Scholtens DM, Kuang A, Linder B, Lawrence JM, Lebenthal Y, et al. Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study (HAPO FUS): Maternal Gestational Diabetes Mellitus and Childhood Glucose Metabolism. Diabetes Care. 2019;42(3):372–80. Epub 2019/01/19. doi: 10.2337/dc18-1646. PubMed PMID: 30655380; PubMed Central PMCID: PMCPMC6385693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aye IL, Powell TL, Jansson T. Review: Adiponectin--the missing link between maternal adiposity, placental transport and fetal growth? Placenta. 2013;34 Suppl:S40–5. doi: 10.1016/j.placenta.2012.11.024. PubMed PMID: 23245987; PubMed Central PMCID: PMCPMC3650089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woo Baidal JA, Locks LM, Cheng ER, Blake-Lamb TL, Perkins ME, Taveras EM. Risk Factors for Childhood Obesity in the First 1,000 Days: A Systematic Review. Am J Prev Med. 2016;50(6):761–79. Epub 2016/02/27. doi: 10.1016/j.amepre.2015.11.012. PubMed PMID: 26916261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.