Abstract
This report describes the clinical context and autopsy findings in the first reported fatal case of acute disseminated encephalomyelitis (ADEM), developed after being vaccinated using the Oxford/AstraZeneca COVID-19 vaccine. ADEM is a rare autoimmune disease, causing demyelination in the brain and spinal cord. A wide variety of precipitating factors can trigger ADEM, and it has long been known to be a rare adverse event following some types of vaccinations. Recently, ADEM has also been associated with COVID-19 infection and (very rarely) with COVID-19 vaccination. The reports of the latter however all pertain to living patients. Our case demonstrates that ADEM should be considered in patients developing neurological symptoms post COVID-19 vaccination, although that this adverse reaction is likely to remain extremely rare. Our report further emphasizes the added value of comprehensive post mortem investigation to confirm ante mortem diagnosis and to determine vaccination safety.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12024-021-00440-7.
Keywords: COVID-19, Vaccination, Adverse event, Forensic pathology, Acute disseminated encephalomyelitis, ADEM
Introduction
Vaccination is the backbone of the global response to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), also known as coronavirus disease 2019 (COVID-19). After the rapid development and registration of multiple vaccines, a global mass-vaccination effort is well underway. It is anticipated that billions of people will eventually be vaccinated, all having then received at least two doses of vaccine. Against this background, (potential) adverse events of COVID-19 vaccination receives much attention in scientific literature and public debate.
We report the first fatal case of a rare side-effect of vaccination: acute disseminated encephalomyelitis (ADEM), which developed after immunization with the Oxford/Astra-Zeneca COVID-19 vaccine (also referred to as AZD1222 or ChAdOx1).
Acute disseminated encephalomyelitis (ADEM)
ADEM is an autoimmune disease, causing demyelination in the brain and spinal cord. It is generally monophasic and characterized by acute-onset and rapidly progressive multifocal neurological deficits. Its exact incidence is unknown. Population-based studies estimate the prevalence at 3.3 per 100,000 individuals [1]. ADEM occurs more commonly in children and young adults under 20 years of age, and affects men more than women (ratio 1.3:1) [2, 3]. When diagnosed in a timely manner and treated appropriately, ADEM ordinarily has a favorable outcome [4]. Reports describing its autopsy pathology are therefore rare.
The pathogenic mechanisms of ADEM are postulated to be based on a T-cell mediated autoimmune response to myelin basic protein. The majority of cases are associated with preceding viral or bacterial infection. Infections with Mycoplasma pneumoniae and HIV, influenza, Epstein-Barr virus, herpes simplex virus, human herpes virus-6, smallpox, measles, rubella, mumps and varicella have all been implicated [4–8]. There is also an association with preceding immunization, with post-vaccination ADEM thought to historically account for 5% of ADEM cases [9]. Reports of “neuroparalytic accidents” following Jenner’s smallpox and Pasteur’s rabies vaccine have been documented since the mid-nineteenth century [3]. However, ADEM was first linked to vaccination in the 1950s after exposure to the now defunct rabies (Semple) vaccine [10]. A review of the largely case-report literature from 1976 to 2007 reports associations between ADEM and vaccination for rabies, diphtheria–tetanus–polio, smallpox, measles, mumps, rubella, Japanese B encephalitis, pertussis, influenza, hepatitis B, and the Hog vaccine [9]. The average time from non-neural vaccine exposure to symptom onset was generally 1 to 14 days. There is a suggestion that ADEM is more common after primary vaccination rather than revaccination [11].
The link between ADEM and COVID-19 is well-established [12–22], but only four cases of (suspected) ADEM have been reported in association with COVID-19 vaccination [23–26]. All these reports pertain to living patients. No fatal cases of ADEM following COVID-19 or COVID-19 vaccination have been published.
Case presentation
A 63-year-old man, with a medical history including insulin-dependent type II diabetes mellitus, ischemic heart disease and atrial fibrillation, electively received a first dose of the Oxford/AstraZeneca COVID-19 vaccine. Twelve days later he presented to hospital with non-specific symptoms including vertigo, abdominal pain and fatigue. On admission, preliminary tests prompted a working diagnosis of ketoacidosis and silent myocardial infarction. In the first days after admission he deteriorated neurologically, with declining cognition, emerging disorientation and impaired attention. On day four, he suddenly became poorly responsive. Clinical tests led to a presumed diagnosis of encephalitis, and empiric antibiotics and antivirals were started. He was intubated the following day.
Ante mortem imaging
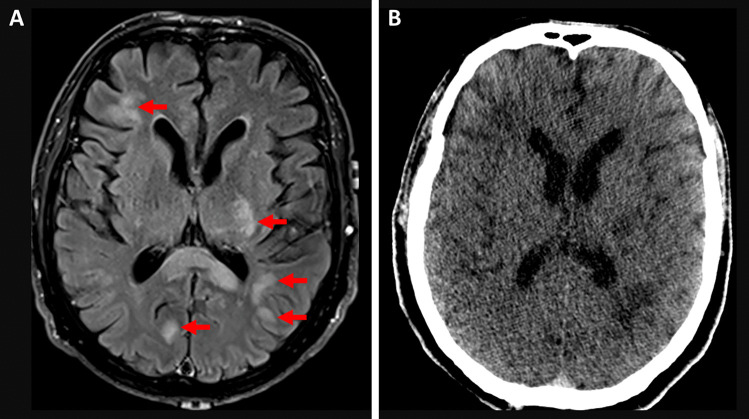
Non-contrast MRI brain and cervical spine were performed on day six, showing numerous bilateral foci (> 20) of high T2 and FLAIR signal in the cerebral white matter, with both periventricular and juxtacortical involvement (Fig. 1). The radiological differential diagnoses included a demyelinating condition (such as ADEM) and CNS lymphoma. A repeat MRI brain on day eight demonstrated some stable lesions, but also merging of previously separate lesions. Based on the rapid clinical and radiological progression, a diagnosis of ADEM was favored over CNS lymphoma.
Fig. 1.
Ante mortem and post mortem radiology. (a) Image
taken from MRI Brain FLAIR sequence, showing juxtacortical and periventricular white matter (circles). (b) Image taken from the post mortem CT scan, at the same level as (a)
Ante mortem neuropathology
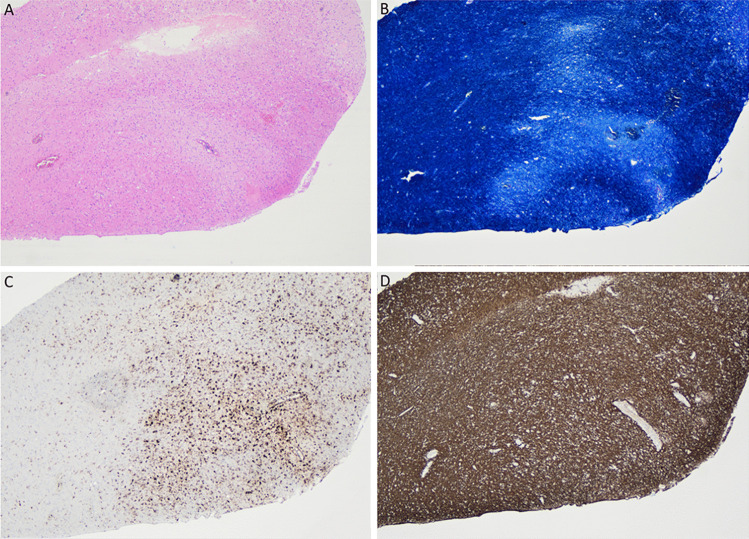
Stereotactic right frontal craniotomy and open biopsy of a right frontal lesion was performed on day nine. Histologic examination demonstrated white matter edema with areas of pallor in a perivascular distribution (Fig. 2). There were small focal perivascular hemorrhages, with no destructive vasculitis or vascular necrosis. Luxol fast blue stain showed perivascular demyelination with axonal preservation (demonstrated by neurofilament stain). CD68 immunohistochemistry highlighted a perivascular distribution of macrophages. CD3 stained a small number of perivascular T lymphocytes. Immunohistochemistry for infective causes including CMV, HSV1/2, EBER-ISH, toxoplasma and SV40 were negative. There was no evidence of malignancy.
Fig. 2.
Histology of the ante mortem biopsy specimen,
taken from the right frontal lesion on day nine of patient admission. (a) Perivascular pallor on hematoxylin and eosin stain. (b) Perivascular loss of positivity in Luxol fast blue stain, indicating demyelination. (c) Perivascular distribution of macrophages on CD68 immunohistochemistry. (d) Preservation of perivascular axons on neurofilament stain. All images at 40 × magnification
Based on the radiological and histological findings, ADEM was diagnosed. Despite treatment with corticosteroids and plasmapheresis the patient failed to improve. MRI brain was repeated on day 19 and demonstrated no changes. A decision was made to move to a palliative model of care. He died on day 20 of admission. A more detailed description of the clinical presentation and ante mortem diagnostic workup is provided online (Supplementary Information).
Post mortem investigation
To confirm the ante mortem diagnosis, and to provide additional information to establish the suggested relation between vaccination and death, the case was referred to the presiding Coroner for further investigation. A comprehensive post mortem investigation was ordered.
Prior to autopsy, a full body CT scan showed the presence of multifocal hypodensities in the brain, in keeping with the findings at ante mortem imaging (Fig. 1). A full three-cavity autopsy was performed, during which the brain, dura and spinal cord were retained and fixed for 4 days in 8% buffered formalin for neuropathological examination.
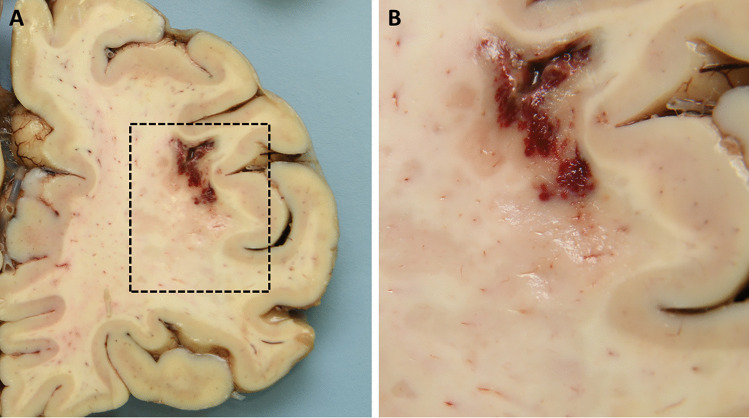
Macroscopic examination of the convexity and base of the brain was unremarkable, apart from a small cortical disruption consistent with ante mortem biopsy. Serial coronal sections of the brain showed multiple areas of ill-defined demyelination in the white matter of the left superior frontal gyrus; the right cingulate gyrus extending into the corpus callosum; and in the left and right parietal regions (Fig. 3). Less well-defined foci were present within the occipital white matter. In addition, the region of left anterior internal capsule and the mid-portion of the thoracic cord appeared softened. The brain stem and cerebellum were macroscopically unremarkable. Examination of the cord demonstrated a softened area at the mid-thoracic level.
Fig. 3.
Post mortem macroscopic neuropathology. (a) Right frontal cortex, with hemorrhage and disruption in the white matter due to ante mortem biopsy and demyelination of the adjacent white matter, visible as ill-defined geographical brown discoloration. (b) Higher power field of the demarcated area in (a)
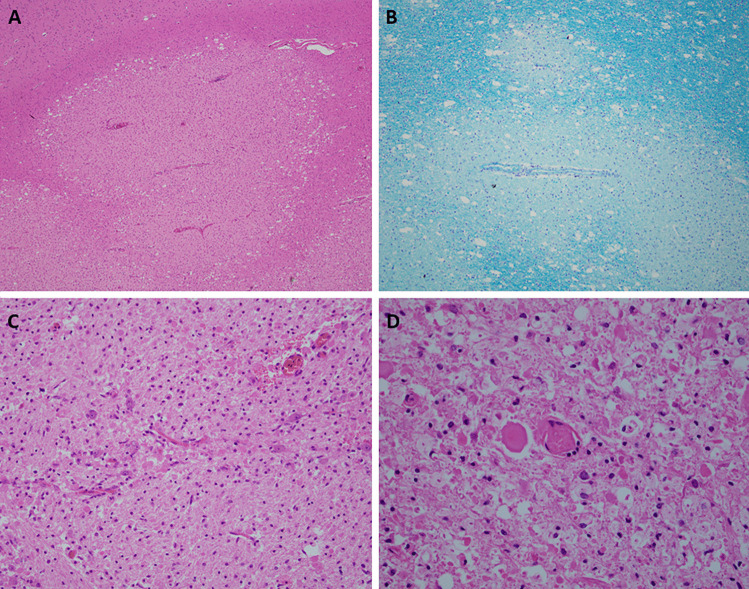
Extensive histological examination confirmed the presence of large geographic areas of acute demyelination, focally in a perivenular distribution (Fig. 4). The foci were characterized by loss of myelin, as shown in Luxol-Pas stain, with white matter vacuolization and necrosis. The lesions showed reactive astrocytes, microglia and foamy macrophages. Occasional perivenular lymphocytosis was seen, but lymphocytes were overall sparse, indicating advanced age of the lesions. Although predominantly located in the white matter, the cerebral lesions extended focally past the grey and white junction. There was no evidence of meningitis, vasculitis or encephalitis. No nuclear atypia was noted. There was extensive demyelination in the midbrain and pons, the medulla oblongata was unremarkable. The spinal cord demonstrated foci of demyelination at the level of the lower cervical cord, the mid-thoracic region and lumbosacral region.
Fig. 4.
Post mortem microscopic neuropathology. (a) Perivascular palor of the white matter of the right frontal cortex. Compare with adjacent normal white matter and the cortex in the upper left corner. Hematoxylin and eosin, 4 × magnification. (b) Perivascular demyelination in Luxol fast blue stain, 10 × magnification. (c) Necrosis of white matter and abundant foamy macrophages in a pontine lesion. Hematoxylin and eosin, 20 × magnification. (d) Sparse perivascular lymphocytes in a lesion in the lumbar spinal cord. Hematoxylin and eosin, 40 × magnification
Autopsy did not reveal other relevant findings. Given the protracted clinical presentation, no toxicological or biochemical analysis was performed. Post mortem virology of the brain was negative.
Based on the ante mortem medical records and the results of the post mortem investigation, the cause of death was registered as “acute disseminated encephalomyelitis (ADEM) in the setting of recent Astra Zeneca COVID-19 vaccination”.
Discussion
Four reports link COVID-19 vaccination with ADEM or ADEM-like presentations. The first case pertains to a 24-year-old woman from China, who presented with neurological symptoms two weeks after the first dose of the ‘SinoVac’ COVID-19 vaccine [23]. Based on cerebrospinal fluid analysis and imaging, ADEM was diagnosed. The patient was treated with intravenous immunoglobulin and clinically improved to a deficit-free baseline one month after discharge. An MRI brain showed total resolution of the lesions.
Kenangil et al. reported case of a 46-year old female who had the first tonic–clonic seizure in her life, one month after her second immunization with the “SinoVac” vaccine. When presenting at hospital, four days later, neurological tests were completely normal, but imaging was suggestive of ADEM. In absence of encephalopathy, the case was registered as “ADEM-like presentation after an inactivated coronavirus vaccination”.
Vogrig et al. reported a case of a 56-year old female who developed progressive neurological symptoms two weeks after vaccination with the “Pfizer” COVID-19 vaccine [25]. After an extensive diagnostic workup, a post-vaccine neuroinflammatory disorder (such as ADEM) was deemed most probable. At the time of publication, the patient had finished her corticosteroid treatment and showed a good recovery with overall progressive neurological improvement.
The fourth case was reported in the Korea Herald in March 2021 [26]. According to that source, a 45-year-old nursing assistant developed a headache soon after receiving the Oxford/AstraZeneca COVID-19 vaccine. One and a half weeks later she reportedly developed blurred vision and quadriparesis and was eventually diagnosed with ADEM. Further details were unavailable. The case has not been published in the mainstream scientific literature.
COVID-19 vaccine safety data to date has not described an increased risk of ADEM in study populations. Although the initial safety trial for the Oxford/Astra-Zeneca COVID-19 vaccine was temporarily paused after two cases of transverse myelitis, it was determined that vaccination was not clearly attributable to these cases [27]. Similarly, the initial efficacy and safety trials of the “Janssen” Ad26.COV2.S vaccine, “Moderna” mRNA-1273 SARS-CoV-2 vaccine, “Pfizer” BNT162b2 mRNA COVID-19 vaccine and “Sputnik V” Gam-COVID-vaccine have not identified ADEM as an adverse effect [28–31].
Sudden death due to ADEM is extremely uncommon [32], and autopsy pathologists are unlikely to be confronted with previously unknown or unsuspected cases. Still, post mortem diagnosis can add substantially in various ways. First and foremost, it can provide a definitive histopathological diagnosis. Besides, determining the extent of disease can help to understand the ante mortem symptoms and clinical course. Autopsy can furthermore help to advance the knowledge on this still poorly understood disease by detailed neuropathological documentation. Post mortem neuropathological examination furthermore presents a unique opportunity for tissue sampling.
Determining a causal relationship between a fatal adverse event and vaccination is outside the remit of a forensic pathologist. In most countries, the evaluation of an ‘adverse events following immunization’ (AEFI) is delegated to governmental agencies. However, such evaluations often rely heavily on a detailed and independent investigation into the cause, mechanism and circumstances of death. As such, our case also emphasizes the important added value forensic pathology can have in determining vaccination safety.
Key points
The safety of COVID-19 vaccinations receives a lot of attention in medical literature and public debate.
Most reported adverse events of vaccination are mild, and potentially life-threatening complications are considered extremely rare.
We present the first fatal case of acute disseminated encephalomyelitis, following immunization with the Oxford/AstraZeneca COVID-19 vaccine.
The clinical presentation, ante mortem diagnostic workup and autopsy pathology are described in detail.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank Dr Jonathan Clark, Dr Nicholas Crump, Dr Greg Fitt and Dr Richard Macdonell (Austin Health), and Dr Linda Iles (Victorian Institute of Forensic Medicine) for their expertise and valuable suggestions.
Funding
No funds, grants, or other support was received.
Declarations
Informed consent
The deceased had no known relatives, therefore no informed consent could be obtained. Consent for publication was obtained from the State Coroner of the Coroner’s Court of Victoria.
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Fiona Permezel and Branko Borojevic these authors share first authorship
References
- 1.Dubey D, Pittock SJ, Kelly CR, et al. Autoimmune encephalitis epidemiology and a comparison to infectious encephalitis. Ann Neurol. 2018 doi: 10.1002/ana.25131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anilkumar AC, Foris LA, Tadi P. Acute disseminated encephalomyelitis. In: StatPearls. 2021; https://www.ncbi.nlm.nih.gov/books/NBK430934. Accessed 2 September 2021. [PubMed]
- 3.Bennetto L, Scolding N. Inflammatory/post-infectious encephalomyelitis. J Neurol Neurosurg Psychiatry. 2004 doi: 10.1136/jnnp.2003.034256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garg RK. Acute disseminated encephalomyelitis. Postgrad Med J. 2003 doi: 10.1136/pmj.79.927.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaji M, Kusuhara T, Ayabe M, Hino H, Shoji H, Nagao T. Survey of herpes simplex virus infections of the central nervous system, including acute disseminated encephalomyelitis, in the Kyushu and Okinawa regions of Japan. Mult Scler. 1996 doi: 10.1177/135245859600200204. [DOI] [PubMed] [Google Scholar]
- 6.Marchioni E, Ravaglia S, Piccolo G, et al. Postinfectious inflammatory disorders: subgroups based on prospective follow-up. Neurology. 2005 doi: 10.1212/01.wnl.0000179302.93960.ad. [DOI] [PubMed] [Google Scholar]
- 7.Silver B, McAvoy K, Mikesell S, Smith TW. Fulminating encephalopathy with perivenular demyelination and vacuolar myelopathy as the initial presentation of human immunodeficiency virus infection. Arch Neurol. 1997 doi: 10.1001/archneur.1997.00550170115023. [DOI] [PubMed] [Google Scholar]
- 8.Tsiodras S, Kelesidis T, Kelesidis I, Voumbourakis K, Giamarellou H. Mycoplasma pneumoniae-associated myelitis: a comprehensive review. Eur J Neurol. 2006 doi: 10.1111/j.1468-1331.2006.01174.x. [DOI] [PubMed] [Google Scholar]
- 9.Huynh W, Cordato DJ, Kehdi E, Masters LT, Dedousis C. Post-vaccination encephalomyelitis: literature review and illustrative case. J Clin Neurosci. 2008 doi: 10.1016/j.jocn.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Appelbaum E, Greenberg M, Nelson J. Neurological complications following antirabies vaccination. JAMA. 1953;151:188–191. [PubMed] [Google Scholar]
- 11.Booss J, Davis LE. Smallpox and smallpox vaccination: neurological implications. Neurology. 2003 doi: 10.1212/01.WNL.0000063319.64515.6B. [DOI] [PubMed] [Google Scholar]
- 12.de Miranda Henriques-Souza AM, de Melo ACMG, Madeiro BdACS, Freitas LF, Rocha-Filho PAS, Gonçalves FG. Acute disseminated encephalomyelitis in a COVID-19 pediatric patient. Neuroradiology. 2021; 10.1007/s00234-020-02571-0. [DOI] [PMC free article] [PubMed]
- 13.Delamarre L, Gollion C, Grouteau G, et al. COVID-19–associated acute necrotising encephalopathy successfully treated with steroids and polyvalent immunoglobulin with unusual IgG targeting the cerebral fibre network. J Neurol Neurosurg Psychiatry. 2020 doi: 10.1136/jnnp-2020-323678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langley L, Zeicu C, Whitton L, Pauls M. Acute disseminated encephalomyelitis (ADEM) associated with COVID-19. BMJ Case Rep. 2020 doi: 10.1136/bcr-2020-239597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCuddy M, Kelkar P, Zhao Y, Wicklund D. Acute demyelinating encephalomyelitis (ADEM) in COVID-19 Infection: A Case Series. Neurol India. 2020 doi: 10.4103/0028-3886.299174. [DOI] [PubMed] [Google Scholar]
- 16.Parsons T, Banks S, Bae C, Gelber J, Alahmadi H, Tichauer M. COVID-19-associated acute disseminated encephalomyelitis (ADEM) J Neurol. 2020 doi: 10.1007/s00415-020-09951-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paterson RW, Brown RL, Benjamin L, et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020 doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reichard RR, Kashani KB, Boire NA, Constantopoulos E, Guo Y, Lucchinetti CF. Neuropathology of COVID-19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol. 2020 doi: 10.1007/s00401-020-02166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shahmirzaei S, Moghadasi AN. Association of COVID-19 and acute disseminated encephalomyelitis (ADEM) in the absence of pulmonary involvement. Autoimmun Rev. 2021 doi: 10.1016/j.autrev.2021.102753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varadan B, Shankar A, Rajakumar A, et al. Acute hemorrhagic leukoencephalitis in a COVID-19 patient—a case report with literature review. Neuroradiology. 2021 doi: 10.1007/s00234-021-02667-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zanin L, Saraceno G, Panciani PP, et al. SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochir. 2020 doi: 10.1007/s00701-020-04374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zoghi A, Ramezani M, Roozbeh M, Darazam IA, Sahraian MA. A case of possible atypical demyelinating event of the central nervous system following COVID-19. Mult Scler Relat Disord. 2020 doi: 10.1016/j.msard.2020.102324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao L, Ren L. Acute disseminated encephalomyelitis after severe acute respiratory syndrome coronavirus 2 vaccination: a case report. Acta Neurol Belg. 2021 doi: 10.1007/s13760-021-01608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kenangil GO, Ari BC, Guler C, Demir MK. Acute disseminated encephalomyelitis-like presentation after an inactivated coronavirus vaccine. Acta Neurol Belg. 2021 doi: 10.1007/s13760-021-01699-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vogrig A, Janes F, Gigli GL, et al. Acute disseminated encephalomyelitis after SARS-CoV-2 vaccination. Clin Neurol Neurosurg. 2021 doi: 10.1016/j.clineuro.2021.106839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arin K. Korea keeps serious vaccine safety issues quiet. Korea Herald. 19 April 2021. http://www.koreaherald.com/view.php?ud=20210419000900. Accessed DD MMM YYYY.
- 27.Knoll MD, Wonodi C. Oxford–AstraZeneca COVID-19 vaccine efficacy. Lancet. 2021 doi: 10.1016/S0140-6736(20)32623-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021. 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed]
- 29.Logunov DY, Dolzhikova IV, Shcheblyakov DV, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021 doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020. 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed]
- 31.Sadoff J, Gray G, Vandebosch A, et al. Safety and efficacy of single-dose Ad26. COV2. S vaccine against Covid-19. N Engl J Med. 2021; 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed]
- 32.Schwarz S, Mohr A, Knauth M, Wildemann B, Storch-Hagenlocher B. Acute disseminated encephalomyelitis: a follow-up study of 40 adult patients. Neurology. 2001 doi: 10.1212/WNL.56.10.1313. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.