Abstract
Transforming growth factor‐beta (TGFβ) is a multifunctional cytokine with a well‐established role in mammary gland development and both oncogenic and tumor‐suppressive functions. The extracellular matrix (ECM) indirectly regulates TGFβ activity by acting as a storage compartment of latent‐TGFβ, but how TGFβ is released from the ECM via proteolytic mechanisms remains largely unknown. In this study, we demonstrate that hepsin, a type II transmembrane protease overexpressed in 70% of breast tumors, promotes canonical TGFβ signaling through the release of latent‐TGFβ from the ECM storage compartment. Mammary glands in hepsin CRISPR knockout mice showed reduced TGFβ signaling and increased epithelial branching, accompanied by increased levels of fibronectin and latent‐TGFβ1, while overexpression of hepsin in mammary tumors increased TGFβ signaling. Cell‐free and cell‐based experiments showed that hepsin is capable of direct proteolytic cleavage of fibronectin but not latent‐TGFβ and, importantly, that the ability of hepsin to activate TGFβ signaling is dependent on fibronectin. Altogether, this study demonstrates a role for hepsin as a regulator of the TGFβ pathway in the mammary gland via a novel mechanism involving proteolytic downmodulation of fibronectin.
Keywords: breast cancer, fibronectin, hepsin, TGFβ, tumor microenvironment
Subject Categories: Cancer; Cell Adhesion, Polarity & Cytoskeleton
TGFβ is released from the ECM compartments of the mammary glands by hepsin mediated proteolytic cleavage of the ECM component fibronectin.
Introduction
The human genome encodes 566 proteases, and of these, 273 have been found in extracellular compartments or the lumen of secretory compartments, 277 in intracellular compartments, and a small fraction at the plasma membrane (Overall & Blobel, 2007). Extracellular proteolysis plays a key role in regulating the physical properties of the extracellular matrix (ECM) through turnover, which, for example, affects branching morphogenesis in the mammary gland (Wiseman et al, 2003; Green & Lund, 2005; Lu et al, 2011). Another critical role of extracellular proteolysis lies in the processing of growth factor pro‐forms (e.g., pro‐HGF; Herter et al, 2005, pro‐MSP Ganesan et al, 2011, and EGF Higashiyama et al, 2011). Therefore, extracellular proteolysis orchestrates the interaction between ECM remodeling and growth factor signaling during development and tissue regeneration (Mohammed & Khokha, 2005; Fukushima et al, 2018). Most studies on proteolytic regulation of the ECM have focused on the diverse class of matrix metalloproteinases (MMPs) (Chang & Werb, 2001). Interestingly, in addition to MMPs, serine proteases have been demonstrated to be involved in pericellular proteolysis of ECM components and plasma membrane proteins, allowing localized ECM remodeling and receptor signaling regulation (Del Rosso et al, 2002).
Hepsin is a protease belonging to the type II transmembrane serine protease (TTSP) family, a special group of membrane‐anchored proteases whose enzymatic activity is confined to the pericellular space (Hooper et al, 2001), making TTSPs accessible for function‐blocking antibodies and small molecule inhibitors (Antalis et al, 2010). Hepsin knockout mice display defects in inner ear development and have altered kidney function (Guipponi et al, 2007; Olinger et al, 2019). A recent study reported reduced liver size and browning of fat tissue in hepsin knockout mice, leading to a reduction of body fat in mice on a high‐fat diet (Li et al, 2020). Apart from a role for hepsin‐mediated cleavage of pro‐HGF in the liver (Hsu et al, 2012) and laminin‐332 during invasion (Klezovitch et al, 2004; Tripathi et al, 2008; Pant et al, 2018a), the molecular mechanisms underlying other physiological functions of hepsin remain poorly defined.
Hepsin is one of the most frequently overexpressed proteins in prostate cancer (Dhanasekaran et al, 2001; Luo et al, 2001; Magee et al, 2001; Stamey et al, 2001; Welsh et al, 2001; Ernst et al, 2002; Chen et al, 2003; Stephan et al, 2004). Hepsin is also very frequently overexpressed in breast cancer, in up to 70% of breast tumors (Tervonen et al, 2016). Hepsin overexpression is suggested to promote cancer progression and metastasis (Tanimoto et al, 1997; Klezovitch et al, 2004; Tervonen et al, 2016), and several hepsin‐regulated oncogenic pathways have been described. For example, overactive hepsin damages epithelial integrity and promotes degradation of the basement membrane (Klezovitch et al, 2004; Tripathi et al, 2008; Partanen et al, 2012; Tervonen et al, 2016), and hepsin directly cleaves oncogenic growth factors pro‐HGF and pro‐MSP (Herter et al, 2005; Ganesan et al, 2011). However, given that most proteases have a broad substrate range, additional signaling pathways downstream of hepsin probably exist that contribute to its oncogenic properties.
Here, we demonstrate that hepsin controls TGFβ signaling via proteolytic regulation of fibronectin. The findings are corroborated in vivo by using a new hepsin CRISPR knockout mouse model, alongside an established model of hepsin overexpression in breast cancer.
Results
CRISPR/Cas9‐generated Hpn knockout mice manifest diminished liver size and partial loss of hearing
We generated hepsin knockout mice with CRISPR/Cas9‐mediated gene editing using a guide RNA targeting exon 4 of the Hpn gene. Genetic analysis revealed a 50 bp frameshift deletion in the coding region of the Hpn gene (Fig 1A), which resulted in the loss of the hepsin protein in mice homozygous for the frameshift allele (Figs 1A and EV1A). Hpn knockout mice did not display any obvious histopathological changes, gross morphological, or tissue architecture deviations, nor did they show any differences in external appearance, lean mass, free fluid, or body fat (Fig EV1B–D). Consistent with Hpn knockout mice created via traditional homologous recombination methods (Wu et al, 1998; Li et al, 2020), our CRISPR hepsin knockout (Hpn −/−) mice displayed diminished liver size and reduced hearing (Figs 1B and C, and EV1E).
Figure 1. The knockout of hepsin with CRISPR/Cas9 inhibits TGFβ signaling in the mouse mammary gland.
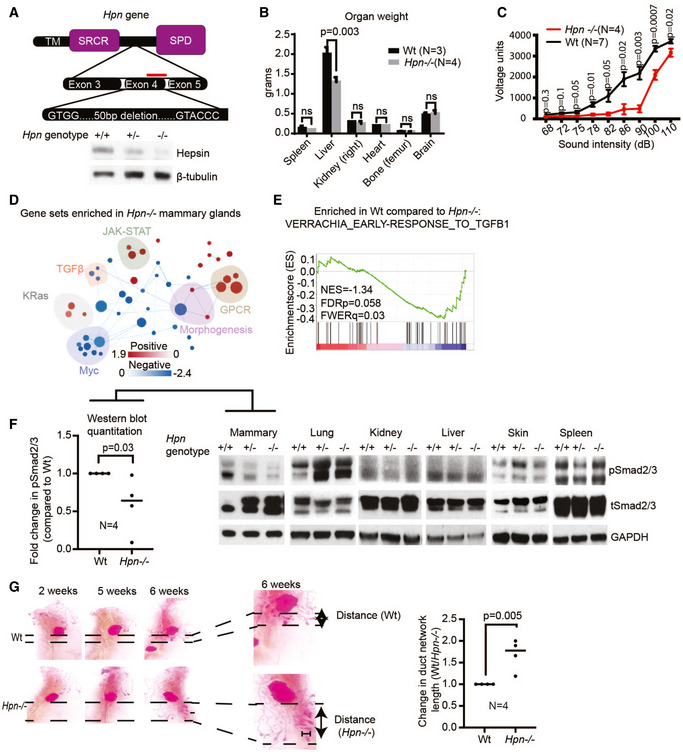
- Hepsin knockout mice harbor a 50 bp frameshift deletion in the 4th exon of the Hpn gene (TM = transmembrane domain, SRCR = scavenger receptor cysteine‐rich domain, SPD = serine protease domain; red bar indicates gRNA‐binding site). Immunoblot from whole mammary lysates against hepsin protein (Hpn +/+ = Wt, Hpn +/− = heterozygous deletion, Hpn −/− = homozygous deletion) (representative of 3 mice per group).
- Weight of indicated organs isolated from Wt (N = 3) and Hpn −/− (N = 4) mice.
- Acoustic startle reflex test to compare hearing ability between Wt (N = 7) and Hpn −/− (N = 4) mice.
- Cytoscape enrichment map of pathways affected in Hpn −/− whole mammary glands compared with Wt controls (N = 3 per group). Node size correlates with the number of genes in the signature; node color correlates with either gene set enrichment (red) or reduction (blue) in Hpn −/− mammary glands. A full list of gene signatures affected in Hpn −/− mammary glands is shown in Table EV1.
- Gene Set Enrichment Analysis (GSEA) graphs showing enrichment of indicated TGFβ1 signaling gene sets in Hpn −/− mammary glands compared with Wt mammary glands (FDRp—P‐value; FWERq—false discovery rate; NES—normalized enrichment score).
- Immunoblot analysis of phospho‐Smad2/3 (TGFβ pathway signaling marker) and total Smad2/3 in lysates from indicated tissues isolated from Wt, Hpn +/−, and Hpn −/− mice. GAPDH was used as the loading control. The histogram depicts quantification of pSmad2/3 compared to Wt, normalized to total Smad2/3.
- Representative Carmine alum stained mammary gland whole mounts from Wt and Hpn −/− mice. The histogram depicts quantification of duct length normalized to duct length in Wt mammary glands. The scale bar represents 1 mm. Data in (B, C, F, G) are represented as mean ± SD, and Student’s t‐test was used for statistical analyses.
Source data are available online for this figure.
Figure EV1. Description of gross morphology and histology of Hpn −/− mice.

- Immunoblot analysis of hepsin in Wt and Hpn −/− tissues.
- Photographs of representative 6‐week‐old Wt and Hpn −/− mice. Scale bar is equal to 3 cm.
- Graph showing body composition and body weight analysis of 6‐week‐male and female Wt and Hpn −/− mice using the Bruker minispec LF50 NMR Body Composition Analyzer (N = 5 mice each).
- Representative H&E stainings of paraffin tissue sections from the mammary gland, lung, skin, kidney, spleen, and liver from female Wt and Hpn −/− mice (6‐week‐old littermates). Scale bar equals to 100 μm.
- Liver mass (% of whole‐body weight) in 6‐week‐old virgin female Wt (N = 3) and Hpn −/− (N = 4) mice. Data in (C, E) are represented as mean ± SD, and Student’s t‐test was used for statistical analyses (n.s. = not significant).
Hpn −/− mice are deficient in TGFβ signaling in the mammary gland and show increased ductal branching
Hepsin is expressed in human and mouse epithelial tissues, where it typically localizes to desmosomal and hemidesmosomal junctions (Miao et al, 2008). Moreover, in the mammary gland, oncogenic deregulation of hepsin has been coupled to loss of epithelial integrity (Partanen et al, 2012; Tervonen et al, 2016). These findings prompted us to examine whether the loss of hepsin might affect signaling pathways relevant to cohesiveness or morphogenesis of the mammary epithelial tissue. Whole mammary glands were isolated from wild‐type and Hpn −/− mice and analyzed in parallel by RNAseq and Reverse Phase Protein Array (RPPA). The RPPA analysis revealed Hpn knockout‐specific changes in the levels of 12 proteins, including osteopontin, periostin, and endostatin (Fig EV2A and B). RNAseq analysis demonstrated that while the expression levels of mRNAs encoding 2 of these proteins were also altered, expression changes in other 10 proteins were not accompanied by corresponding changes in mRNA expression, suggesting that hepsin regulates proteomes via both transcription‐associated and post‐translational/proteolytic mechanisms (Fig EV2A–D).
Figure EV2. Molecular profiling of Hpn −/− mammary glands.

- Proteome profiling of whole mammary tissue lysates prepared from 6‐week‐old virgin female Wt and Hpn −/− littermates. Small rectangles indicate proteins with differential expression (see (B)).
- Graph showing quantification of signal intensity from proteome profiling depicted in (A). Proteins were considered differently expressed starting from a 20% change in expression (indicated with red x‐axis labels).
- Quantification of mRNA levels (read counts) corresponding to proteins quantified in the RPPA (A–B), normalized to the housekeeping gene Pum1 mRNA levels. Data were derived from bulk RNA sequencing data from whole mammary tissue lysates prepared from 6‐week‐old virgin female Wt and Hpn −/− mice (see Fig 1D and E). Data are presented as mean ± SD, and Student’s t‐test was used for statistical analyses (n.s. = not significant, N = 3 indicates the number of biological replicates).
- Venn diagram showing differential expression of mRNAs (bulk RNA seq; Fig EV2C and D) and proteins (RPPA; Fig EV2A and B) in mammary tissue of 6‐week‐old virgin female Wt and Hpn −/− littermates. Resistin and IGFBP‐5 are differentially expressed both on mRNA and protein levels.
Gene Set Enrichment Analysis (GSEA) was performed on the RNAseq data. Functional clustering of gene signatures affected by the absence of hepsin suggests the downregulation of TGFβ1 signaling in Hpn −/− mammary glands (Fig 1D and E, Table EV1). TGFβ signaling regulation occurs via two discrete activation steps: the processing of latent‐TGFβ in the extracellular compartment and TGFβ receptor‐induced activation of downstream signaling, which includes phosphorylation of receptor‐associated Smads (R‐Smads), such as Smad2 and Smad3 (Derynck & Budi, 2019). To confirm the downmodulation of TGFβ signaling in Hpn −/− mice, we determined the levels of phospho‐Smad2/3 in tissue extracts from mammary, lung, kidney, liver, skin, and spleen. The level of phosphorylated Smad2/3 was significantly decreased in Hpn −/− mammary tissue (Fig 1F), indicating deficient canonical TGFβ signaling in the mammary gland upon loss of hepsin. Reduced phosphorylated Smad2/3 levels were not observed in the other tissues examined (Fig 1F), suggesting that this phenomenon is specific to the mammary gland.
TGFβ is a well‐established suppressor of mammary duct branching and proliferation (Ewan et al, 2002; Ingman & Robertson, 2008; Moses & Barcellos‐Hoff, 2011). Analysis of mammary gland whole mounts from 6‐week‐old wild‐type and Hpn −/− littermates showed that loss of hepsin increased duct branching into the fat pad (Fig 1G). This phenotype resembles the one observed in TGFβ1+/− mice (Ewan et al, 2002; Ingman & Robertson, 2008), thus showing that loss of hepsin affects mammary morphogenesis in a manner consistent with reduced TGFβ signaling.
Hepsin overexpression activates TGFβ signaling in a Wap‐Myc model of breast cancer
The TGFβ pathway is commonly dysregulated in human cancer but its impact on tumorigenesis is highly contextual—while TGFβ has tumor‐suppressive effects, it also exerts pro‐tumorigenic effects by modulating processes such as cell invasion, production of ECM, and inflammatory immune responses (Yeung et al, 2013; Bellomo et al, 2016; Mariathasan et al, 2018; Tauriello et al, 2018). To test whether ectopic overexpression of hepsin induces TGFβ signaling in the context of tumorigenesis, we made use of a previously published tumor syngraft model of Myc‐driven breast cancer (Partanen et al, 2012; Utz et al, 2020). Wap‐Myc mammary tumor cells, which express high Myc levels, were isolated from donor mice, transduced with the pIND21‐HPN lentiviral construct that allows doxycycline (DOX)‐induced hepsin overexpression (Tervonen et al, 2016), and subsequently transplanted into recipient mice (Fig 2A). Consistent with the notion that hepsin promotes TGFβ signaling, Western blot analysis revealed increased phospho‐Smad2/3 and upregulation of the TGFβ signaling downstream target SNAIL in Wap‐Myc tumors with DOX‐induced hepsin overexpression compared with control (DOX−) tumors (Fig 2B). RNAseq analysis of these tumors provided additional evidence for TGFβ pathway upregulation by hepsin as the most significantly upregulated gene signatures corresponded to the TGFβ signaling pathway (Figs 2C, D, and E, and EV3A and B). The largest cluster of gene signatures affected by hepsin overexpression, however, was related to the ECM and integrins (Fig 2C).
Figure 2. Overexpression of Hpn in Wap‐Myc‐driven mammary tumors induces TGFβ signaling.

- Schematic representation of the mouse experiment.
- Immunoblot analysis of Wap‐Myc mammary tumor lysates for the indicated TGFβ signaling markers and hepsin (T# denotes individual tumors). GAPDH was used as the loading control. Lysates were derived from Wap‐Myc mammary tumors from six mice with and six mice without DOX‐induced hepsin overexpression (see (A)).
- Gene Set Enrichment Analysis (GSEA) enrichment map of pathways upregulated in hepsin overexpressing Wap‐Myc tumors (DOX+) compared with control tumors (DOX−) (N = 5 tumors in each group). Node size correlates with the number of genes in the signature; node color red correlates with enrichment in hepsin overexpressing Wap‐Myc tumors.
- GSEA graph comparing the expression of the HALLMARK_TGF_BETA_SIGNALING gene set in hepsin overexpressing (DOX+) to control (DOX−) Wap‐Myc tumors (N = 5 tumors per group; FDRp—P‐value; FWERq—false discovery rate; NES—normalized enrichment score).
- Heatmap showing changes in expression of all genes in the HALLMARK_TGF_BETA_SIGNALING gene set in hepsin overexpressing (DOX+) compared with control (DOX−) Wap‐Myc tumors. Red color indicates upregulation, and blue color indicates the downregulation of the indicated genes.
Figure EV3. Regulation of TGFβ signaling and levels, fibronectin protein, and mRNA.
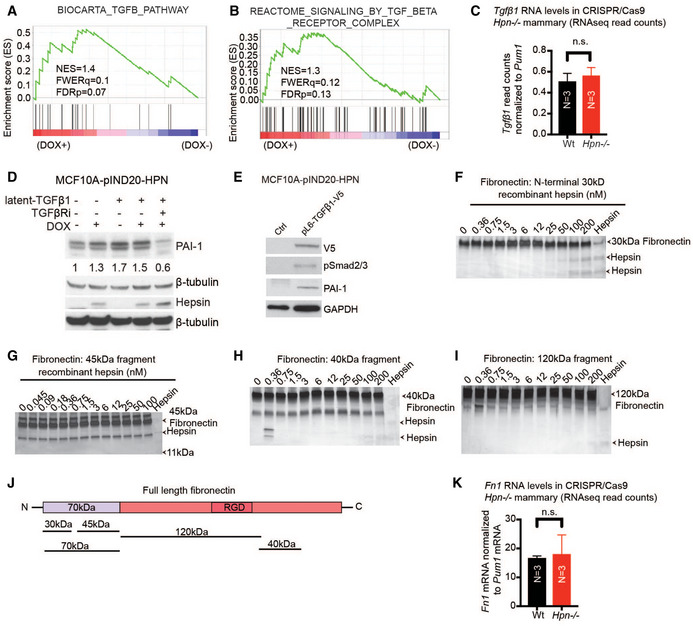
-
A, BGSEA enrichment maps from tumors overexpressing hepsin (DOX+) and control tumors (DOX−).
-
CGraph depicting quantification of Tgfβ1 mRNA levels (mean ± SD mRNA read counts), normalized to the housekeeping gene Pum1, in mammary tissue of 6‐week‐old virgin female Wt and Hpn −/− mice. Student’s t‐test was used for statistical analyses (n.s. = not significant, N = 3 indicates the number of biological replicates).
-
DImmunoblot analysis of PAI‐1, hepsin, and β‐tubulin (loading control) in MCF10A‐pIND20‐HPN cells treated with doxycycline, galunisertib, and latent‐TGFβ (small latent complex) as indicated. Numbers below the PAI‐1 blot indicate PAI‐1 levels, normalized to β‐tubulin.
-
EImmunoblot analysis of TGFβ‐V5 (antibody detecting V5), pSmad2/3, PAI‐1, and GAPDH (loading control) in cell lysates from MCF10A‐pIND20‐HPN cells with or without TGFβ‐V5 overexpression.
-
F–ISilver‐stained protein gels with samples from in vitro protease activity assays with recombinant hepsin and fragments of fibronectin. The respective fragments (30 kDa (F), 45 kDa (G), 40 kDa (H), and 120 kDa (I); marked by arrowheads) were incubated (1 μg/reaction) with increasing concentrations of recombinant hepsin (nM).
-
JSchematic mapping of the fibronectin fragments used in (G–J) onto full‐length fibronectin. RGD indicates the RGD‐binding domain in fibronectin.
-
KGraph depicting quantification of Fn1 mRNA levels (mean ± SD mRNA read counts), normalized to the housekeeping gene Pum1, in mammary tissue of 6‐week‐old virgin female Wt and Hpn −/− mice. Student’s t‐test was used for statistical analyses (n.s. = not significant, N = 3 indicates the number of biological replicates).
Hepsin regulates the extracellular activation of TGFβ signaling
Pro‐TGFβ protein, which consists of both the mature TGFβ and latency‐associated peptide (LAP), is released from cells in a latent form. This so‐called TGFβ small latent complex (SLC) consists of the receptor‐binding TGFβ growth factor dimer (12 kDa Western blot band under reducing conditions) and a dimer of the latency‐associated peptide (LAP) (40 kDa Western blot band under reducing conditions) (Miyazono et al, 1988), where LAP inhibits the TGFβ growth factor dimer from binding and activating TGFβ receptors. The latent‐TGFβ SLC is stored in the ECM through a covalent bond between LAP and latent‐TGFβ‐binding protein (LTBP), forming the large latent complex (LLC), which binds to fibronectin and fibrillins (Fig 3A) schematically depicts the TGFβ‐LAP‐LTBP complex). TGFβ can be released from LAP‐LTBP, and thus from ECM storage, and activated by multiple types of stimuli, such as reactive oxygen species, proteases, or an acidic environment (Lyons et al, 1988, 1990; Sato & Rifkin, 1989; Yu & Stamenkovic, 2000; Jenkins, 2008; Sounni et al, 2010; Liu & Desai, 2015). Active TGFβ then mediates the tetramerization of type II TGFβ receptor (TGFβR2) and type I TGFβ receptor (TGFβR1), leading to phosphorylation of downstream receptor‐associated R‐Smads. Subsequently, phosphorylated R‐Smads form oligomeric complexes with other Smads and translocate to the nucleus to regulate the transcription of target genes (Derynck & Budi, 2019).
Figure 3. Hepsin is a regulator of TGFβ storage.

- Schematic illustration of proteins involved in TGFβ storage in the extracellular matrix. The small latent complex (SLC) is a non‐covalently linked tetramer of 2 LAP (latency‐associated peptide) (40 kDa) and 2 TGFβ1 (12 kDa). LTBP1 + SLC together form the large latent complex (LLC) through covalent bonds between LAP and LTBP1. LLC interacts with the ECM through non‐covalent interactions of LTBP with fibrillins and fibronectin.
- Immunoblot analysis of LAP in mammary whole tissue lysates from Wt and Hpn −/− mouse. The 90 kDa band represents unprocessed pro‐TGFβ, and the 40 kDa band corresponds to mature TGFβ LAP peptide.
- Schematic representation of the mouse experiment, showing orthotopic transplantation of Wt Wap‐Myc tumor cells into syngeneic Wt and Hpn −/− recipients.
- Immunoblot analysis of phospho‐Smad2/3, total Smad2/3, and GAPDH (loading control) expression in Wt tumors transplanted into either Wt or Hpn −/− recipients (N = 10 tumors each).
- Western blot analysis of phospho‐Smad2/3, total Smad2/3, hepsin, and β‐tubulin (loading control) in MCF10A‐pIND20‐HPN cells treated with 1 µg/ml doxycycline for 48 hours (DOX; overexpression of hepsin) and the TGFβR1 inhibitor galunisertib (TGFβR1i, 10 µM) as indicated.
- Immunoblot analysis of conditioned medium (LAP peptide) and cell extracts (hepsin and vinculin) from control (DOX) or hepsin overexpressing (DOX+) MCF10A‐pIND20‐HPN supplemented with 100 ng/ml latent‐TGFβ (small latent complex). Ponceau was used as a loading control for the cell culture medium.
- TGFβ ELISA assay to detect active and total TGFβ levels in conditioned medium from MCF10A‐pIND20‐HPN pL6‐TGFβ1 cells with (DOX+) or without (DOX−) hepsin overexpressing. The TGFβ ELISA assay detects active TGFβ levels (first 2 columns), but heat treatment of the conditioned medium activates all TGFβ, thus effectively measuring total TGFβ (right two columns). Data are presented as scatter plot (mean denoted by the black line), and paired Student’s t‐test was used for statistical analyses.
- Silver‐stained protein gel with samples from an in vitro protease activity assay with recombinant SLC and hepsin. SLC was incubated with increasing concentrations of recombinant hepsin (numbers indicate the concentration of hepsin in nM). Western blots in (B, E, F) are representative of at least three repeats.
Interestingly, further comparison of protein lysates from Wt and Hpn −/− mammary glands exposed markedly increased levels of LAP in Hpn −/− mammary glands, indicating accumulation of latent‐TGFβ (Fig 3B). Tgfb1 mRNA levels were not altered by the loss of hepsin (Fig EV3C), suggesting that these effects are due to post‐transcriptional events. To find further evidence for accumulation of latent‐TGFβ, we syngrafted Wt Wap‐Myc tumor cells into the fat pads of either Wt or Hpn −/− syngeneic hosts (Fig 3C) and measured phospho‐Smad2/3 levels in tumor lysates. In this experiment, we observed increased phospho‐Smad2/3 levels in tumors grown in Hpn −/− mice (Fig 3D), consistent with a model where latent‐TGFβ1 accumulates in ECM stores in mammary glands in the absence of hepsin and processed TGFβ1 is liberated by tumor cells, leading to activation of the TGFβ1 pathway. While these results leave room for several other interpretations, at this stage they prompted us to investigate two possible lines of inquiry: (i) Hepsin or one or more proteases regulated by hepsin could directly cleave LAP, which releases active TGFβ from SLC, or (ii) hepsin could be needed for the release of latent‐TGFβ from ECM stores, after which hepsin‐independent processes convert latent‐TGFβ into its active form.
To address whether hepsin directly or indirectly contributes to proteolytic processing of latent‐TGFβ1 LAP in cells, we performed several assays using the MCF10A‐pIND20‐HPN cell line (Tervonen et al, 2016). We established that DOX‐induced ectopic hepsin expression enhances Smad2/3 phosphorylation in this cell line (Fig 3E), thus demonstrating its suitability to model hepsin‐mediated regulation of TGFβ1 signaling. Importantly, hepsin‐induced phosphorylation of Smad2/3 was rescued by the TGFβR1 inhibitor galunisertib, confirming that the hepsin‐induced changes in pSmad2/3 levels were dependent on TGFβ receptor signaling (Fig 3E). First, we tested whether hepsin overexpression increases proteolytic processing of the inhibitory LAP peptide by incubating MCF10A‐pIND20‐HPN cells, with (DOX+) and without (DOX−) hepsin overexpression, with purified latent‐TGFβ1 (LAP‐TGFβ; SLC) for 24 h before harvesting conditioned medium and cell extracts for Western blotting (Fig 3F). No cleavage of the full‐length LAP peptide was observed upon hepsin overexpression, indicating that hepsin does not affect proteolytic processing of the LAP peptide. Secondly, we examined whether adding exogenous latent‐TGFβ1 further potentiates hepsin‐induced PAI‐1 induction in MCF10A‐pIND20‐HPN cells (Fig EV3D and E). While hepsin overexpression and treatment with exogenous latent‐TGFβ each enhanced TGFβ receptor signaling (PAI‐1 expression was used as a readout (Cichon et al, 2016)), the combination of hepsin overexpression and latent‐TGFβ did not have an additive effect (Fig EV3D), further suggesting that hepsin does not stimulate extracellular cleavage of TGFβ1 LAP. Thirdly, using a TGFβ ELISA assay (Brown et al, 1990), we measured active and total TGFβ levels in conditioned medium from TGFβ1‐overexpressing MCF10A‐pIND20‐HPN cells with (DOX+) or without (DOX−) hepsin overexpression (Fig 3G). While this TGFβ ELISA assay only detects active TGFβ1 (Brown et al, 1990), we utilized heat treatment of the conditioned medium to activate all TGFβ1 and thus to effectively measure total TGFβ1 levels. If hepsin would directly or indirectly promote cleavage of the LAP peptide, we expect hepsin overexpression to increase active TGFβ1 levels in the conditioned medium, i.e., released from the insoluble ECM. We did not observe such an increase in active TGFβ1 levels (Fig 3G; first 2 columns), however, providing additional evidence that hepsin does not affect cleavage of TGFβ1 LAP. We did, however, observe a modest but significant increase in total TGFβ (after heat activation of TGFβ) in the conditioned medium of hepsin overexpressing cells (Fig 3G; last 2 columns), which is consistent with the second hypothesis mentioned above, i.e., that hepsin could be needed for the release of latent‐TGFβ from ECM stores. Lastly, we performed an in vitro protease assay, which showed that recombinant hepsin is unable to proteolytically process recombinant latent‐TGF‐β1 LAP (Fig 3H), suggesting that hepsin does not directly cleave LAP.
Together, the experiments in Figs 3B, E–H, and EV3D strongly suggest that hepsin causes accumulation of latent‐TGFβ1 and promotes TGFβ signaling via mechanisms other than direct proteolytic cleavage of latent‐TGFβ.
Hepsin downregulates the levels of ECM protein fibronectin
To identify hepsin‐regulated ECM proteins, we examined both lysates and culture supernatants collected from MCF10A‐pIND20‐HPN cells with or without hepsin overexpression. The lysates and supernatants were separated on SDS–PAGE under reducing or non‐reducing conditions followed by Coomassie staining, which revealed eight protein bands visibly affected by hepsin overexpression (Fig 4A). These proteins were analyzed by mass spectrometry, identifying fibronectin (FN1) as one of the proteins showing reduced expression in culture supernatant upon hepsin overexpression (Fig 4A). Hepsin‐dependent downmodulation of fibronectin was of great interest, given the well‐established role for fibronectin in the storage of TGFβ family growth factors, and thus in TGFβ signaling (Dallas et al, 2005; Robertson et al, 2015). Immunoblotting of the cell culture supernatant from the same cells showed accumulation of lower MW fibronectin fragments upon hepsin overexpression, which could indicate that reduced fibronectin levels in the cell extracts are due to fibronectin degradation (Fig 4B). Immunoblot for fibronectin in lysates of MCF10A‐pIND20‐HPN cells with or without hepsin overexpression confirmed the reduction in full‐length fibronectin levels upon hepsin overexpression (Fig 4C).
Figure 4. Hepsin promotes the proteolytic processing of fibronectin.

- Coomassie‐stained protein gels with cell lysates and concentrated culture supernatants of MCF10A‐pIND20‐HPN cells, with (DOX+) or without (DOX−) hepsin overexpression. M indicates the media only control. R and NR above the gels indicate reducing and non‐reducing conditions, respectively. Numbered arrowheads indicate areas of the gel that were analyzed by mass spectrometry; corresponding proteins differently expressed in hepsin overexpressing cells are listed on the left. The image with two lanes on the right is a copy of the indicated area with supernatant under reducing conditions, highlighting the part from which fibronectin was identified. Red boxes indicate the lanes that were analyzed by mass spectrometry.
- Immunoblot analyses of fibronectin expression in concentrated culture supernatant from MCF10A‐pIND20‐HPN cells with (DOX+) or without (DOX−) hepsin overexpression. Ponceau staining of the Western blot is shown as the loading control.
- Immunoblot analysis of fibronectin, hepsin, and β‐tubulin (loading control) in cell lysates from MCF10A‐pIND20‐HPN cells with (DOX+) or without (DOX−) hepsin overexpression. The graph shows the quantification of fibronectin levels. Student’s t‐test was used for statistical analyses. Data are represented as mean ± SD.
- Silver‐stained protein gel with samples from an in vitro protease activity assay with recombinant hepsin and either full length purified plasma fibronectin (upper panel) or the 70 kDa most N‐terminal fragment of fibronectin (lower panel). Full‐length fibronectin (1 μg) or the 70 kDa fibronectin fragment (1 μg) was incubated with increasing concentrations of recombinant hepsin. Arrowheads indicate full‐length fibronectin, the 70 kDa fragment, the 50 kDa cleavage fragment generated by hepsin, and hepsin. The schematic figure shows the full‐length fibronectin protein and the 70 KDa N‐terminal fragment. The red arrow indicates the location of the putative hepsin cleavage site. RGD indicates the RGD‐binding domain in fibronectin. Experiments in (B, C, D) are representative of at least three repeats.
Source data are available online for this figure.
To determine whether fibronectin could be a target for direct proteolytic processing by hepsin, we performed in vitro cleavage assays with purified proteins. This experiment showed that hepsin can cleave full‐length fibronectin in vitro (Fig 4D, upper panel). Using different N‐terminal and C‐terminal fragments, we mapped the hepsin cleavage site to the N‐terminal 70 kDa part of fibronectin (Figs 4D, lower panel, and EV3F–J). While the in vitro cleavage assays exposed only one direct hepsin cleavage site in fibronectin, we note that hepsin overexpression induced a smear‐like pattern of extracellular fibronectin cleavage products in MCF10A‐pIND20‐HPN cell culture supernatant (Fig 4B). Thus, it is likely that other proteases downstream of hepsin also contributed to hepsin‐dependent fibronectin degradation.
Next, we investigated whether hepsin also downmodulates fibronectin in vivo. To that end, we analyzed a panel of tissue samples from our wild‐type (Wt) and Hpn −/− mice. The results showed that Hpn −/− mammary glands, and also skin, expressed more fibronectin protein than mammary glands from Wt littermates (Fig 5A). Similar effects were not observed in other examined tissues (Fig 5A). Importantly, the increased fibronectin protein abundance in the Hpn −/− mammary glands was not accompanied by increased Fn1 mRNA expression (Fig EV3K). An inverse correlation between fibronectin and hepsin was also observed in mammary glands from another hepsin knockout mouse strain (Wu et al, 1998) (Fig 5B) and in prostate tissue from mice genetically engineered to overexpress hepsin specifically in the prostate gland (Klezovitch et al, 2004) (Fig 5C). Together, our findings demonstrate that hepsin downregulates fibronectin protein levels in cultured cells and in vivo.
Figure 5. Hepsin reduces fibronectin levels in vivo .

- Immunoblot analysis of fibronectin in indicated tissues isolated from Wt, Hpn +/− and Hpn −/− mouse (representative of at least three repeats). *As the same tissue lysates were used as in Fig 1F, the same GAPDH blot is used here as the loading control.
- Immunoblot analysis of fibronectin and β‐tubulin (loading control) in lysates from Wt and Hpn −/− mouse mammary tissue (N = 5 animals each) from another hepsin knockout mouse model (Wu et al, 1998).
- Immunoblot analysis of fibronectin, hepsin, and β‐tubulin (loading control) in prostate lysates of control mice or mice with prostate‐specific hepsin overexpression (Klezovitch et al, 2004). N = 2 mice each, the two prostate lobes were run separately (A and B).
Hepsin regulates TGFβ1 signaling via fibronectin
If our hypothesis that hepsin activates TGFβ signaling through degradation of fibronectin is correct, downregulation of fibronectin should be sufficient to increase TGFβ signaling. We assessed this using four different experiments. We silenced fibronectin in MCF10A‐pIND20‐HPN cells and assessed TGFβ signaling using Western blot for pSmad2/3. Consistent with our hypothesis, downmodulation of fibronectin levels in these cells was sufficient to increase pSmad2/3 levels (Figs 6A and EV4A shows uncut immunoblot). In an MCF10A cell line overexpressing TGFβ‐V5, silencing of fibronectin released the fully processed V5‐tagged TGFβ (12 kDa) into the culture medium and also upregulated Smad2/3 phosphorylation (Figs 6B and EV4B shows uncut immunoblot). In addition, we performed a TGFβ1 reporter assay for which we created the MCF10A‐pIND20‐HPN pL6‐TGFβ1 (SRE) TGFβ1 reporter cell line, harboring a construct with luciferase under the control of a Smad‐response element (SRE). In line with our hypothesis that fibronectin modulates TGFβ signaling, silencing fibronectin, as well as induction of hepsin expression, in this cell line increased TGFβ1 reporter activity (Figs 6C and EV4D). Lastly, we verified that knockdown of fibronectin leads to TGFβ release into the culture supernatant using a breast cancer cell line (Hs578T) that expresses high levels of endogenous TGFβ (Figs 6D and EV4C). Together, these findings suggest that reducing fibronectin levels is sufficient to promote TGFβ release from the extracellular matrix and activate signaling, consistent with our hypothesis that hepsin promotes TGFβ signaling through fibronectin degradation.
Figure 6. Hepsin‐mediated TGFβ1 signaling through the downregulation of fibronectin.
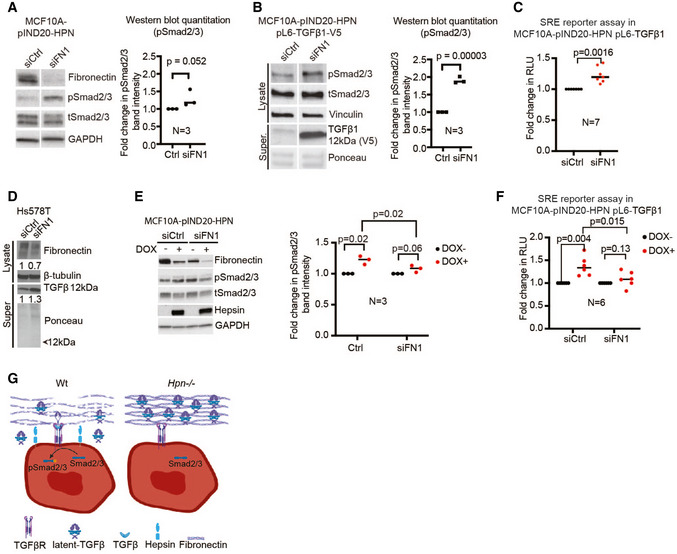
- Immunoblot analysis of fibronectin, pSmad2/3, total Smad2/3, and GAPDH (loading control) in cell lysates from MCF10A‐pIND20‐HPN cells without hepsin overexpression. The graph depicts quantification of pSmad2/3 normalized to total Smad2/3 levels, compared to siCtrl (N = 3 biological repeats).
- Immunoblot analysis of cell lysates (pSmad2/3, total Smad2/3, and vinculin as loading control) and concentrated cell culture supernatant (anti‐V5 for TGFβ) from MCF10A‐pIND20‐HPN pL6‐TGFβ1‐V5 cells without hepsin overexpression. Ponceau staining is shown as a loading control for the Western blot with concentrated culture supernatant. The graph depicts quantification of pSmad2/3 normalized to total Smad2/3 levels, compared to siCtrl (N = 3 biological repeats).
- TGFβ luciferase reporter assay of control or fibronectin silenced MCF10A‐pIND20‐HPN pL6‐TGFβ1 (SRE) TGFβ1 reporter cells with (DOX+) or without (DOX−) hepsin overexpression (N = 7 biological repeats; Y‐axis shows fold change in relative light units (RLU)).
- Immunoblot analysis of cell lysates (fibronectin and β‐tubulin (loading control)) and concentrated cell culture supernatant (TGFβ) from Hs578T cells with knockdown of fibronectin. Ponceau staining is shown as the loading control for the Western blot with concentrated culture supernatant. A representative blot from three biological repeats is shown.
- Immunoblot analysis of fibronectin, pSmad2/3, total Smad2/3, hepsin, and GAPDH (loading control) in cell lysates from MCF10A‐pIND20‐HPN cells with (DOX+) and without (DOX−) hepsin overexpression, and with or without fibronectin silencing (siFN1). The graph depicts quantification of pSmad2/3 normalized to total Smad2/3 levels (N = 3 biological repeats).
- TGFβ luciferase reporter assay of control or fibronectin silenced MCF10A‐pIND20‐HPN pL6‐TGFβ1 (SRE) TGFβ1 reporter cells, with (DOX+) and without (DOX−) hepsin overexpression. (N = 6 biological repeats; Y‐axis shows fold change in relative light units (RLU)).
- Model figure depicting how hepsin regulates TGFβ signaling. Under Wt conditions, hepsin promotes degradation of fibronectin, which releases latent‐TGFβ from ECM stores, thus resulting in the induction of TGFβ signaling. In hepsin knockout mammary glands, TGFβ signaling is compromised as latent‐TGFβ cannot be released from ECM stores (Created with BioRender.com). Statistical analyses in (A, B, C, E, F) were done using Student’s t‐test. Data in (A, B, C, E, F) are presented as dot plots where black lines represent the mean.
Source data are available online for this figure.
Figure EV4. Effects of fibronectin knockdown, hepsin overexpression on TGFβ signaling.
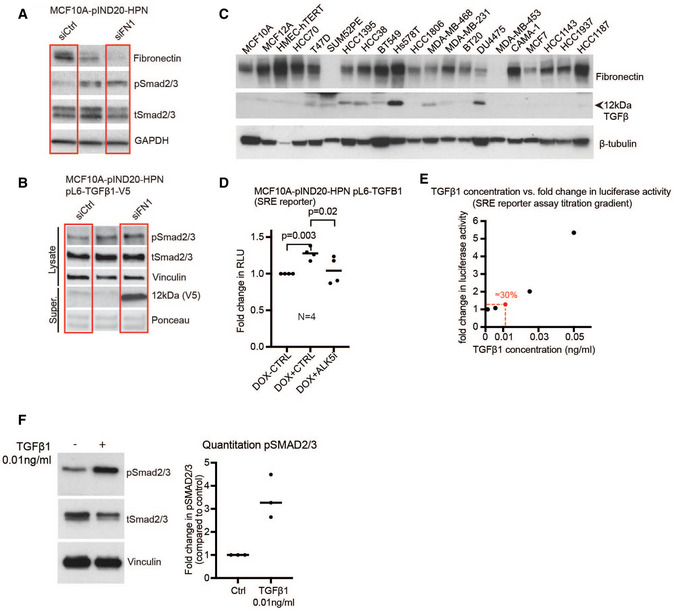
- Uncropped Western blot corresponding to Fig 6A.
- Immunoblot analysis of fibronectin and the 12 kDa form of TGFβ in a panel of normal breast and breast cancer cell lines. β‐Tubulin was used as the loading control.
- TGFβ luciferase reporter assay of MCF10A‐pIND20‐HPN pL6‐TGFβ1 reporter cells harboring a Smad‐response element (SRE), with (DOX+) and without (DOX−) hepsin overexpression, and with or without galunisertib (TGFβR/ALK5 inhibitor). (N = 4 biological repeats; Y‐axis shows fold change in relative light units (RLU)). The black line denotes the average. Paired Student’s t‐test was used for statistical analyses.
- Titration of the TGFβ1 concentration in a luciferase assay with the MCF10A‐pIND20‐HPN pL6‐TGFβ1 (SRE) reporter cell line. Figure shows one biological repeat as a representative of three biological repeats; Y‐axis shows fold change in relative light units (RLU).
- Immunoblot for pSmad2/3, total Smad2/3, and vinculin as loading control from 0.01 ng/ml TGFβ1 and control treated MCF10A‐pIND20‐HPN pL6‐TGFβ1 reporter cells. The graph depicts quantification of pSmad2/3 normalized to total Smad2/3 levels (N = 3 biological repeats)
Source data are available online for this figure.
If hepsin‐dependent TGFβ pathway activation is due to fibronectin proteolysis, reduced fibronectin levels should dampen hepsin‐induced TGFβ signaling activation. To investigate this, we examined the effect of fibronectin silencing on hepsin‐dependent induction of pSmad2/3 in MCF10A‐pIND20‐HPN cells. We found that in the absence of fibronectin, hepsin shows reduced capacity to activate TGFβ signaling indicated by pSmad2/3 levels (Fig 6E). These results were confirmed using a TGFβ1 reporter assay with control or fibronectin silenced MCF10A‐pIND20‐HPN pL6‐TGFβ1 (SRE) TGFβ1 reporter cells with (DOX+) or without (DOX−) hepsin overexpression (Fig 6F). Importantly, a TGFβ titration experiment with the MCF10A‐pIND20‐HPN pL6‐TGFβ1 (SRE) TGFβ1 reporter cell line showed that incubation with low levels (0.01 ng/ml) of TGFβ1 increased SRE reporter activity by approximately 30% (Fig EV4E), similar to the increased SRE reporter activity induced by silencing FN1 (Fig 6C) or DOX‐induced hepsin expression (Fig 6F). Similar to the experiments with silencing FN1 (Fig 6B) or DOX‐induced hepsin expression (Fig 6E), this 0.01 ng/ml of TGFβ1 induced robust induction of pSMAD2/3 levels in this cell line (Fig EV4F), thus providing evidence that the changes in SRE reporter activity and pSMAD2/3 levels are in concordance.
Together, our results demonstrate that hepsin‐mediated TGFβ signaling depends on fibronectin, consistent with a role for hepsin in fibronectin degradation and subsequent activation of latent‐TGFβ (see a schematic model in Fig 6G).
Discussion
In this study, we identified the protease hepsin as a promoter of TGFβ signaling in the mammary gland and mammary tumors. We provide evidence that hepsin promotes TGFβ signaling through downregulation of fibronectin levels and subsequent release of latent‐TGFβ from the ECM storage compartment.
The oncogenic role of hepsin overexpression has been well‐established (Klezovitch et al, 2004; Tervonen et al, 2016), but the mechanisms through which hepsin promotes initiation and progression of tumors are still largely unclear. The especially frequent hepsin overexpression in breast cancer (up to 70% (Tervonen et al, 2016)) warrants further studies of the role of hepsin in mouse models of breast cancer. To facilitate such studies, we generated a new hepsin knockout mouse in the inbred FVB/N strain using CRISPR/Cas9 gene editing, since many common mouse models of breast cancer designed to study multistage carcinogenesis, such as MMTV‐PyT (directs polyomavirus middle T antigen to mammary tissue), C3(1)‐Tag (drives SV40 large T antigen to prostate and mammary tissue), and Wap‐Myc (directs Myc to luminal mammary epithelial cells), have been generated in this strain. We anticipate that these Hpn −/− mice will facilitate future studies aimed to clarify the role of hepsin in multistage tumorigenesis through syngraft approaches, as presented here (Fig 3C).
The FVB/N Hpn −/− mice displayed hearing loss and reduced liver size, consistent with the phenotypes reported for earlier hepsin knockout mouse models (Guipponi et al, 2007; Li et al, 2020), demonstrating the validity of our knockout model. In addition to these phenotypes, we observed increased epithelial branching in Hpn −/− mammary glands. This novel mammary gland phenotype resembles the phenotype reported for Tgfβ +/− females, which show a branching phenotype at the age of 6 weeks (Ewan et al, 2002; Ingman & Robertson, 2008). Tgfβ −/− mice develop multiple severe phenotypes, such as neurodegeneration, developmental defects, and autoimmunity (Shull et al, 1992; Kulkarni et al, 1993; Dickson et al, 1995; Proetzel et al, 1995; Sanford et al, 1997; Brionne et al, 2003). The absence of these phenotypes in Hpn −/− knockout mice shows that hepsin is not required for overall TGFβ function in vivo but it is plausible that hepsin is either a tissue‐specific regulator of TGFβ function or that other proteases compensate the loss of hepsin outside the mammary gland. This selective role of hepsin in the regulation of extracellular TGFβ signaling opens up possibilities to modulate the TGFβ pathway in a mammary tissue‐selective manner with the existing neutralizing antibodies or small molecule inhibitors of hepsin (Ganesan et al, 2012; Koschubs et al, 2012; Pant et al, 2018b; Damalanka et al, 2019).
TGFβ signaling is regulated by the ECM, in which three components are directly involved in the storage of latent‐TGFβ: LTBPs, fibrillins, and fibronectin (ten Dijke & Arthur, 2007). Interestingly, some human conditions caused by dysregulation of TGFβ signaling are linked either to genetic alterations of TGFβ‐binding fibrillins, or proteolytic alterations of LTBPs, indicating that release of the growth factor complex from the ECM may regulate pathway activation and provide a niche for a tissue‐specific regulatory layer (Neptune et al, 2003; Ge & Greenspan, 2006; Quarto et al, 2012; Beaufort et al, 2014).
One important mechanism to activate latent‐TGFβ is mediated by integrins (Robertson & Rifkin, 2016), which can bind LAP/LTBP and activate TGFβ by either pulling latent‐TGFβ apart through mechanical forces generated by the actin cytoskeleton that are transmitted to the fibronectin‐containing ECM by the integrins or presenting latent‐TGFβ to metalloproteases for proteolytic cleavage (Fontana et al, 2005; Mamuya & Duncan, 2012). In addition to integrin‐mediated TGFβ activation, various integrin‐independent TGFβ activation pathways are known, for example, through reactive oxygen species, pH, and proteolytic cleavage (Robertson & Rifkin, 2016). Our data identify hepsin as a novel activator of TGFβ signaling that mediates the release of latent‐TGFβ from the ECM (Fig 2A–G) through degradation of fibronectin (Fig 6A–F) and does not activate TGFβ signaling through direct or indirect cleavage of LAP (Fig 3F–H). In biological settings, the activation of latent‐TGFβ is likely to occur through an interplay of different mechanisms, for example, depending on the tissue, physiological, or pathophysiological context.
The most established function of fibronectin is to mediate the interaction between cells and various ECM components, therefore acting as a “glue” and a structural component (Mouw et al, 2014). The N‐terminal part of fibronectin, for example, directly interacts with fibrillins, which is critical for the maturation of ECM fibers and incorporation of latent‐TGFβ into ECM stores (Dallas et al, 2005). Therefore, cleavage of fibronectin, especially the N‐terminus, which we demonstrate is directly cleaved by hepsin in vitro, may affect ECM storage of TGFβ and thus TGFβ signaling. It is likely, however, that other proteases also play a role in hepsin‐mediated TGFβ signaling in vivo as hepsin can activate other proteases, such as MMPs (Reid et al, 2017; Wilkinson et al, 2017) and serine proteases (Andreasen et al, 1997; Moran et al, 2006; Reid et al, 2017), reported to regulate the cleavage of fibronectin (Moran et al, 2006; Borgoño et al, 2007; Doucet & Overall, 2011; Zhang et al, 2012). Such proteolytic cascades may result, for example, from hepsin‐induced downregulation of the general serine protease inhibitor HAI‐1 (Tervonen et al, 2016). Studies with other proteases capable of cleaving fibronectin have demonstrated that accumulation of fibronectin may lead to alterations of collagen fibers in vivo and thus changes in the overall ECM structure (Taylor et al, 2015). Whether changes in fibronectin levels, due to the deregulation of hepsin, result in gross structural changes in overall ECM architecture remains to be investigated.
In conclusion, we show that hepsin mediates the release of latent‐TGFβ from the ECM storage specifically in the mammary tissue, which leads to increased TGFβ signaling. This suggests a novel role for hepsin as a mediator between ECM proteolysis and TGFβ growth factor signaling.
Materials and Methods
Reagents
Table 1 lists purified proteins used in this study.
Table 1.
Purified proteins used in the study.
| Protein | Company | Catalog |
|---|---|---|
| Purified plasma fibronectin | Merck | F0895 |
| Fibronectin proteolytic fragment from human plasma, 70 kDa | Merck | F0287 |
| Fibronectin proteolytic fragment from human plasma, 30 kDa | Merck | F9911 |
| Fibronectin 40 kDa α chymotryptic fragment (heparin‐binding region) | Merck | F1903 |
| Fibronectin proteolytic fragment from human plasma, 45 kDa | Merck | F0162 |
| Fibronectin 120 kDa α chymotryptic fragment (cell attachment region) | Merck | F1904 |
| hLatent‐TGFβ1 | CST | #5154 |
| Recombinant human hepsin protein, CF | R&D Systems | 4776‐SE |
Cell lines
All cell lines were regularly tested for mycoplasma contamination. The SUM52PE cell line was a generous donation from Dr. SJ Cook and Dr. PR Gavine (Chell et al, 2013) and was cultured in Hams F12 (Invitrogen), insulin 5 μg/ml, hydrocortisone 1 μg/ml, L‐glutamine, and antibiotics. All other cell lines were obtained from American Type Culture Collection (ATCC). HMEC‐hTERT cell media was MCDB 170 (US Biological) supplemented with insulin 5 μg/ml, bovine pituitary extract (BPE) 70 μg/ml, hydrocortisone 0.5 μl/ml, EGF 5 ng/ml, human transferrin 5 μg/ml, and isoproterenol 0.01 μM and 5% FBS (Biowest). Cell lines MCF7, T47D, MCF10A, and Hs578T were cultured as described in Tervonen et al, 2016. MCF12A was cultured in DMEM/F12 (Invitrogen) with 10% FBS (Biowest), EGF 20 ng/ml, hydrocortisone 1 μg/ml, insulin 10 μg/ml, and human transferrin 5 μg/ml. Cell lines HCC70, HCC1395, HCC38, HCC1806, DU4475, HCC1143, HCC1937, and HCC1187 were cultured in RPMI‐1640 (Gibco) supplemented with 10% FBS (Biowest), and BT‐549 in RPMI‐1640 with 10% FBS (Biowest) and 10 μg/ml insulin. The MDA‐MB‐231 cell line was cultured in Dulbecco's modified Eagle's medium (Sigma), 10% FBS (Biowest); BT‐20 and CAMA‐1 cell lines were cultured in Eagle's minimum essential medium (Lonza) supplemented with 10% FBS (Biowest). MDA‐MB‐436 and MDA‐MB‐453 were cultured in Leibovitz's L‐15 Medium (Lonza) with 10% FBS (Biowest).
siRNA knockdown
Fibronectin knockdown was performed with siRNA pool (siTOOLS Biotech) and HIPerFect (Qiagen) transfection reagent, with 100 nM siRNA final concentration. Hs578T cells were switched to serum‐free media after transfection, for supernatant analysis. Supernatants were concentrated as described elsewhere (see Mass Spectrometry).
Virus transduction, TGFβ reporter cell lines
Cell line transduction was carried out by incubating plated cells with virus‐containing supernatant O/N in the presence of 8 μg/ml of polybrene (MOI = 1). We generated the TGFβ reporter cell lines by transducing MCF10A cells with SRE construct harboring lentivirus particles (MOI=10). Reporter construct virus particles were obtained commercially (Kerafast #FCT228). The luciferase signal was detected by using Pierce™ Firefly Luciferase Glow Assay Kit (Thermo Scientific, PI16177) according to the manufacturer's instructions. Readings were obtained with FLUOstar OMEGA multiplate reader (BMG LabTech).
Cloning
The pLenti6‐TGFβ1‐V5 overexpression construct was generated via Gateway cloning from the ORFeome library by the Genome Biology Unit supported by HiLIFE and the Faculty of Medicine, University of Helsinki, and Biocenter Finland.
Tissue, organ weight, and body composition analysis
Carmine alum staining (Sigma, #C1022‐5G) of whole mammary glands was performed in inguinal mammary glands from 6‐week‐old females were spread on glass slips and fixed with Kahle's fixative (Patel et al, 2019) O/N at room temperature followed by one wash with 70% ethanol and gradual change to Milli‐Q water. Glands were stained with carmine alum O/N, gradually transferred into 100% ethanol, and cleared in xylene. Upon clearing glands were transferred into 100% ethanol, followed by 100% glycerol. The branching was quantitated using ImageJ 1.46r.
H&E staining was performed as described previously (Partanen et al, 2012).
Body composition for liquid, fat, and lean mass percentage and organ weight was measured in 6‐week‐old mice with Bruker minispec LF50 Body Composition Analyzer (Brüker). Statistical significance was derived from a one‐sided t‐test. All the groups had at least N = 3.
RNASeq
RNA was extracted from up to 6‐week‐old mouse mammary glands using hard‐tissue beads (Precellys) and TRIzol (Thermo Fisher Scientific). RNA was then DNase treated and purified using RNeasy Mini Kit from Qiagen. Before the library preparation for sequencing, 1 μg of total RNA was treated with NEBNext rRNA Depletion Kit (Human/Mouse/Rat) that removes the ribosomal RNA from the total RNA. The ribosomal depleted RNA was purified with RNeasy mini Elute columns (Qiagen). The absence of rRNA and the quantity of mRNA were measured with Agilent TapeStation 4200. Samples were sequenced with Illumina NextSeq500 (Illumina, San Diego, CA, USA) at the Biomedicum Functional Genomics Unit, University of Helsinki, using NEBNext Ultra Directional RNA Library Prep Kit. The sequencing was performed as single‐end sequencing for read length 75 bp. GSEA analysis and statistical analysis were performed in GSEA 4.0.3 software (Subramanian et al, 2005). Gene set clustering was analyzed with Cytoscape (Merico et al, 2010). Genomic PCR products were Sanger sequenced (Sequencing Unit at the Finnish Institute for Molecular Medicine, HiLIFE, University of Helsinki and Biocenter Finland) using the same reverse primer used also for genomic PCR (above).
Proteins and hepsin cleavage
Recombinant human hepsin was incubated at various concentrations with 1 μg/μl substrate proteins (latent‐TGFβ1, fibronectin fragments) for 1 h, 37°C in PBS, separated on gradient gels and silver stained (GE Healthcare, GE17‐1150‐01). Silver staining was performed by the Meilahti Clinical Proteomics Core Facility.
Antibodies and inhibitors
The inhibitors and antibodies used in this study were Galunisertib (Selleckchem, S2230), anti‐sheep HRP (Upstate cell signaling solutions, #12‐342), anti‐goat HRP (Millipore, AP106P), anti‐rabbit HRP (Millipore, AP132P), anti‐mouse (Millipore, AP160P), anti‐hepsin (Santa Cruz, sc‐33542), anti‐hepsin (R&D Systems, AF4776), anti‐Fibronectin (Abcam, ab45688), anti‐TGFβ1 LAP peptide (Abcam, ab155264), anti‐ pSmad2/3 (CST#8828), anti‐Smad2/3 (CST#8685) anti‐TGFβ (CST#3711S), anti‐SNAIL (CST#3879S), anti‐GAPDH (CST#2118S), anti‐β‐tubulin (Abcam, ab6046), anti‐PAI‐1 (Abcam, ab66705), and anti‐V5 (Invitrogen, #R96025).
Animal models and experiments
All work and sample isolation from 11‐month‐old Hpn −/− (Wu et al, 1998), 6‐month‐old LPB‐Hepsin (Kasper et al, 1998) mice were performed at Lerner Research Institute, Cleveland Clinic, USA; Division of Human Biology, Fred Hutchinson Cancer Research Center, Seattle, USA, respectively.
All mice used for the experiments in Helsinki were housed in individually ventilated cages under the optimal conditions of temperature and humidity. The experiments were conducted according to 3R principles, and animal welfare was regularly monitored.
Wap‐Myc mammary/tumor cell isolations were performed like previously described (Partanen et al, 2012; Tervonen et al, 2016). Experiments were approved by The National Animal Ethics Committee of Finland (ESAVI/3678/04.10.07/2016).
One day before transplantation, Wap‐Myc epithelial cells were isolated and transduced with the pInducer21‐HPN (Tervonen et al, 2016), lentiviral construct, MOI = 10. On transplantation day, 105 cells were injected into the cleared fat pads of 3‐week‐old recipient mice. The experimental mice started receiving doxycycline in drinking water, supplemented with 5% sucrose, 3 days after the transplantation, the control mice received drinking water with sucrose only. Tumors were induced at week 8 by two sequential pregnancies. Mice were sacrificed when the tumor diameter reached 1 cm.
Transplantation experiment of Wt Wap‐Myc tumor cells and into Wt and Hpn −/− recipients: 3‐week‐old Wt FVB recipient mice were obtained from Janvier Labs. Cells were isolated 1 day before transplantation from Wt Wap‐Myc tumors and kept in floating culture O/N in growth media (Partanen et al, 2012). On transplantation day, viability was estimated with trypan blue, 105/gland cells were injected into cleared fat pads of 4‐week‐old recipient mice (2 glands/mouse). Mice were sacrificed when the tumor diameter reached 2 cm.
Generation of Hpn knockout mice by CRISPR/Cas9 editing
gRNAs for in vivo CRISPR/Cas9 genome editing were in vitro transcribed from mouse genome‐wide arrayed lentiviral CRISPR/Cas9 gRNA libraries (Sigma‐Aldrich/Merck). Sanger gRNA ID 1822530, target sequence with PAM was 5’‐AAGGTGGCAGCTCTCATTGTGG‐3’ in exon 4 (exon ID: ENSMUSE00001198671). For in vitro transcription, primers were from Sigma‐Aldrich/Merck and the sequence for gRNA synthesis forward primer was 5’‐TAATACGACTCACTATAGAAGGTGGCAGCTCTCA‐3’ and reverse primer 5’‐TTCTAGCTCTAAAACCAATGAGAGCTGCCACCTT‐3’. The gRNA was produced with GeneArt™ Precision gRNA Synthesis Kit (Thermo Fischer Scientific) according to the manufacturer’s instructions. Injections into mouse zygotes were performed with the purified gRNA and Cas9 mRNA. Altogether, 209 zygotes were injected, 178 transferred into founding mothers (FVB strain), and 100 live pups were born. Mice were genotyped from ear samples taken after weaning by genomic PCR that was performed with the following primers: forward 5’‐TGTCATCGGAAAGGAGTGGC‐3’ and reverse 5’‐GGAGAACAGGCGGGTTGTAA‐3’ flanking the gRNA‐targeting sequence (product length was 493 bp). PCR mix contained a final concentration of 2 mM dNTP mix (Bioline, London, UK), 200 nM of primers (oligos from Sigma‐Aldrich/Merck), 1 mM MgCl2, 1 × Phusion HF PCR Buffer (Thermo Fischer Scientific), Phusion High‐Fidelity DNA polymerase (Thermo Fischer Scientific), and 2 ng/μl DNA template. Genomic PCR products were Sanger sequenced (Biomedicum Sequencing Unit and Sequencing Unit, FIMM, HiLIFE life science research infrastructures, University of Helsinki and Biocenter Finland) using the same reverse primer used also for genomic PCR (above). We found that 15 mice (16% of live‐born F0 cohort) were mutant for Hpn. We selected F0 male with 50 bp deletion in Hpn exon 4 as a founding father of the strain. This male was paired with wild‐type females to obtain F1 generation. The strain was maintained as heterozygotes (no apparent phenotype), which mated to produce wild‐type and Hpn −/− littermates.
Acoustic startle reflex (ASR) test
The test is based on Willott et al (2003). Shortly, mice were placed in a transparent plastic tube (Ø 4.5 cm, length 8 cm) that was put in the startle chamber (Med Associates) with a background white noise of 65 dB and left undisturbed for 5 min. Acoustic startle stimuli (20‐ms white noise bursts) were presented in random order with 8–15 s between the subsequent trials. Altogether, 36 trials with the following noise intensities were randomly applied: 68, 72, 75, 78, 82, 86, 90, 100, and 110 dB. The startle response was recorded for 65 ms starting with the onset of the startle stimulus. The maximum startle amplitude recorded during the 65‐ms sampling window was used as the dependent variable and averaged over four trials with given stimulus intensity. The test was performed by Mouse Behavioral Phenotyping Facility (MBPF) at Neuroscience Center, Helsinki Institute of Life Science (HiLIFE), University of Helsinki and Biocenter Finland (N).
Immunoblot, antibody array, RNA expression analysis, silver staining, and ELISA
Immunoblots were quantitated using ImageJ. Statistical significance was determined via a one‐sided t‐test, unless specified otherwise in the figure legends. All lysates for immunoblot analysis were prepared by lysing cells in PBS‐1% Triton X‐100 buffer supplemented with Protease Inhibitor Cocktail and Phosphatase inhibitor (Roche). Thereafter, the resulting suspension was incubated on ice for 10 min, centrifuged, and the supernatant collected. Tissue lysates were made in the mentioned lysis buffer by using Precellys 24 tissue homogenizer (Bertin instruments) and the Precellys kit (#KT03961‐1‐003.2). Proteins were separated on gradient SDS gels (Bio‐Rad) and transferred to nitrocellulose membranes (Bio‐Rad #1704158). Transfer quality was evaluated with Ponceau staining (Sigma, #P7170). For antibody array experiments, the whole mammary lysates were prepared from 6‐week‐old virgin females according to the manufacturer’s instructions (R&D Systems) and analyzed with Mouse XL Cytokine Antibody Array Kit (RPPA). Signal intensity was quantitated with MATLAB. The proteins from LPB‐hepsin prostate samples were extracted from ventral lobes in RIPA buffer (50 mM Tris–HCl pH7.5, 100 mM NaCl, 1% NP‐40, 1% Na deoxycholate, 0.1% SDS, 1 mM EDTA, protease/phosphatase inhibitors). The active and total TGFβ ELISA was performed with Human TGF‐beta 1 Quantikine ELISA Kit (R&D Systems) according to manufacturer’s instructions, except for the activation step, which was substituted with heat activation (Brown et al, 1990). In order to detect the absolute levels of TGFβ and not only the active form, we performed hear mediated activation, which liberates the active TGFβ from the small latent complex not detectable by the assay (Brown et al, 1990). Readings were obtained with FLUOstar OMEGA multiplate reader (BMG Labtech) at 450 nm.
Mass spectrometry
Culture supernatants were concentrated with Amicon Ultra‐0.5 ml 3 K Centrifugal Filters according to the manufacturer’s instructions (Millipore). Lysates and supernatants were boiled with lysis buffer with (R)/without β‐mercaptoethanol (NR) and separated on gradient gels (Bio‐Rad).
Gels were stained o/n RT with Coomassie stain (10% acetic acid, 50% ethanol, Coomassie Brilliant Blue‐R250 in MQ), followed by destaining (10% acetic acid, 50% ethanol in MQ).
Bands that were altered by hepsin overexpression (DOX−/DOX+) were excised and analyzed by MALDI‐TOF as described before (Vaarala et al, 2014), and the results were plotted against the proteome of the Mammalian taxon (66651 sequences). Mass spectrometric analysis was performed by the Meilahti Clinical Proteomics Core Facility.
Author contributions
DB conceptualized; provided resources; curated the data; involved in formal analysis; investigated; contributed to methodology; and wrote the original draft, review, and editing. SMP provided resources; curated data; validated; investigated; contributed to methodology; and wrote the original draft, review, editing, and illustrations. PM conceptualized; provided resources; curated the data; involved in formal analysis; investigated the study; contributed to methodology; and wrote the original draft. IS involved in formal analysis; visualized the study; and wrote the original draft. KB curated the data; involved in formal analysis; investigated the study; contributed to methodology; and wrote the original draft. H‐AH provided resources and methodology. JE provided resources and methodology, and wrote the original draft. TR provided resources and methodology. OK, VV, SL, and QW provided resources and wrote the original draft. OM, SK, and PL provided resources, curated the data, involved in funding acquisition, and wrote the original draft. JP conceptualized the study; involved in funding acquisition; visualized the study; contributed to methodology; and wrote the original draft, review, and editing. TT conceptualized the study; provided resources; curated the data; involved in formal analysis; supervised the study; involved in funding acquisition; investigated the study; visualized the study; contributed to methodology; wrote the original draft; and contributed to project administration, review, and editing. JK conceptualized the study; provided resources; curated the data; involved in formal analysis; supervised the study; involved in funding acquisition; validated the study; investigated the study, visualized the study; contributed to methodology; wrote the original draft; involved in project administration; review and editing.
Conflict of interest
Dr. Klefström’s research projects received funding from AbbVie, Orion Pharma, and Roche/Genentech. Dr. Klefström has served as a member of scientific advisory board or consultant to AbbVie, Astra‐Zeneca, MSD, Orion Pharma, Pfizer, Roche/Genentech, and UPM Biomedicals. Dr. Pouwels has consulted for Biomedicum Genomics. Other authors declare no conflicts of interest.
Supporting information
Expanded View Figures PDF
Table EV1
Source Data for Figure EV4
Source Data for Figure 1
Source Data for Figure 4
Source Data for Figure 6
Acknowledgements
We are grateful to Biomedicum Functional Genomics Unit/Libraries (FuGU/Libraries), Finnish Genome Editing Center (FinGEEC), Biomedicum Virus Core Unit (BVC), Genome Biology Unit (GBU) and Biomedicum Imaging Unit (BIU), Meilahti Clinical Proteomics Core Facility, and Laboratory Animal Center (LAC) (all from HiLIFE, University of Helsinki and Biocenter Finland) for their services. We the Klefström laboratory personnel for discussions and critical comments on the manuscript. We thank for the technical assistance provided by M. Merisalo‐Soikkeli, K. Karjalainan, and T.Välimäki. We thank for financial support the Finnish Cancer Institute (FCI). This work was funded by grants from The Academy of Finland, Business Finland, EU H2020 RESCUER, Archimedes Foundation, Ida Montinin Foundation, Cancer Society of Finland, Finnish Cancer Organizations, Sigrid Juselius Foundation, K. Albin Johanssons stiftelse, Jane and Aatos Erkko Foundation, and iCAN Digital Precision Cancer Medicine Flagship. T.A.T. was funded by the Helsinki Institute of Life Science Infrastructures (HiLIFE) of the University of Helsinki.
EMBO reports (2021) 22: e52532.
Data availability
The raw RNAseq data for the pIND21‐Wap‐Myc tumors GSE164510 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE164510), Hpn wild‐type, and knockout mammary glands GSE164509 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE164509) are publically available at the GEO repository.
References
- Andreasen PA, Kjøller L, Christensen L, Duffy MJ (1997) The urokinase‐type plasminogen activator system in cancer metastasis: a review. Int J cancer 72: 1–22 [DOI] [PubMed] [Google Scholar]
- Antalis TM, Buzza MS, Hodge KM, Hooper JD, Netzel‐Arnett S (2010) The cutting edge: membrane‐anchored serine protease activities in the pericellular microenvironment. Biochem J 428: 325–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaufort N, Scharrer E, Kremmer E, Lux V, Ehrmann M, Huber R, Houlden H, Werring D, Haffner C, Dichgans M (2014) Cerebral small vessel disease‐related protease HtrA1 processes latent TGF‐β binding protein 1 and facilitates TGF‐β signaling. Proc Natl Acad Sci USA 111: 16496–16501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellomo C, Caja L, Moustakas A (2016) Transforming growth factor β as regulator of cancer stemness and metastasis. Br J Cancer 115: 761–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgoño CA, Michael IP, Shaw JLV, Luo L‐Y, Ghosh MC, Soosaipillai A, Grass L, Katsaros D, Diamandis EP (2007) Expression and functional characterization of the cancer‐related serine protease, human tissue kallikrein 14. J Biol Chem 282: 2405–2422 [DOI] [PubMed] [Google Scholar]
- Brionne TC, Tesseur I, Masliah E, Wyss‐Coray T (2003) Loss of TGF‐beta 1 leads to increased neuronal cell death and microgliosis in mouse brain. Neuron 40: 1133–1145 [DOI] [PubMed] [Google Scholar]
- Brown PD, Wakefield LM, Levinson AD, Sporn MB (1990) Physicochemical activation of recombinant latent transforming growth factor‐beta’s 1, 2, and 3. Growth Factors 3: 35–43 [DOI] [PubMed] [Google Scholar]
- Chang C, Werb Z (2001) The many faces of metalloproteases: cell growth, invasion, angiogenesis and metastasis. Trends Cell Biol 11: S37–S43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chell V, Balmanno K, Little AS, Wilson M, Andrews S, Blockley L, Hampson M, Gavine PR, Cook SJ (2013) Tumour cell responses to new fibroblast growth factor receptor tyrosine kinase inhibitors and identification of a gatekeeper mutation in FGFR3 as a mechanism of acquired resistance. Oncogene 32: 3059–3070 [DOI] [PubMed] [Google Scholar]
- Chen Z, Fan Z, McNeal JE, Nolley R, Caldwell MC, Mahadevappa M, Zhang Z, Warrington JA, Stamey TA (2003) Hepsin and maspin are inversely expressed in laser capture microdissectioned prostate cancer. J Urol 169: 1316–1319 [DOI] [PubMed] [Google Scholar]
- Cichon MA, Moruzzi ME, Shqau TA, Miller E, Mehner C, Ethier SP, Copland JA, Radisky ES, Radisky DC (2016) MYC is a crucial mediator of TGFβ‐induced invasion in basal breast cancer. Cancer Res 76: 3520–3530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallas SL, Sivakumar P, Jones CJP, Chen Q, Peters DM, Mosher DF, Humphries MJ, Kielty CM (2005) Fibronectin regulates latent transforming growth factor‐beta (TGF beta) by controlling matrix assembly of latent TGF beta‐binding protein‐1. J Biol Chem 280: 18871–18880 [DOI] [PubMed] [Google Scholar]
- Damalanka VC, Han Z, Karmakar P, O’Donoghue AJ, La Greca F, Kim T, Pant SM, Helander J, Klefström J, Craik CS et al (2019) Discovery of selective matriptase and Hepsin serine protease inhibitors: useful chemical tools for cancer cell biology. J Med Chem 62: 480–490 [DOI] [PubMed] [Google Scholar]
- Del Rosso M, Fibbi G, Pucci M, D’Alessio S, Del Rosso A, Magnelli L, Chiarugi V (2002) Multiple pathways of cell invasion are regulated by multiple families of serine proteases. Clin Exp Metastasis 19: 193–207 [DOI] [PubMed] [Google Scholar]
- Derynck R, Budi EH (2019) Specificity, versatility, and control of TGF‐β family signaling. Sci Signal 12: eaav5183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanasekaran SM, Barrette TR, Ghosh D, Shah R, Varambally S, Kurachi K, Pienta KJ, Rubin MA, Chinnaiyan AM (2001) Delineation of prognostic biomarkers in prostate cancer. Nature 412: 822–826 [DOI] [PubMed] [Google Scholar]
- Dickson MC, Martin JS, Cousins FM, Kulkarni AB, Karlsson S, Akhurst RJ (1995) Defective haematopoiesis and vasculogenesis in transforming growth factor‐beta 1 knock out mice. Development 121: 1845–1854 [DOI] [PubMed] [Google Scholar]
- ten Dijke P, Arthur HM (2007) Extracellular control of TGFbeta signalling in vascular development and disease. Nat Rev Mol Cell Biol 8: 857–869 [DOI] [PubMed] [Google Scholar]
- Doucet A, Overall CM (2011) Broad coverage identification of multiple proteolytic cleavage site sequences in complex high molecular weight proteins using quantitative proteomics as a complement to edman sequencing. Mol Cell Proteomics 10: M110.003533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst T, Hergenhahn M, Kenzelmann M, Cohen CD, Bonrouhi M, Weninger A, Klären R, Gröne EF, Wiesel M, Güdemann C et al (2002) Decrease and gain of gene expression are equally discriminatory markers for prostate carcinoma: a gene expression analysis on total and microdissected prostate tissue. Am J Pathol 160: 2169–2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewan KB, Shyamala G, Ravani SA, Tang Y, Akhurst R, Wakefield L, Barcellos‐Hoff MH (2002) Latent transforming growth factor‐beta activation in mammary gland: regulation by ovarian hormones affects ductal and alveolar proliferation. Am J Pathol 160: 2081–2093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Chen Y, Prijatelj P, Sakai T, Fässler R, Sakai LY, Rifkin DB (2005) Fibronectin is required for integrin alphavbeta6‐mediated activation of latent TGF‐beta complexes containing LTBP‐1. FASEB J Off Publ Fed Am Soc Exp Biol 19: 1798–1808 [DOI] [PubMed] [Google Scholar]
- Fukushima T, Uchiyama S, Tanaka H, Kataoka H (2018) Hepatocyte growth factor activator: a proteinase linking tissue injury with repair. Int J Mol Sci 19: 3435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan R, Kolumam GA, Lin SJ, Xie M‐H, Santell L, Wu TD, Lazarus RA, Chaudhuri A, Kirchhofer D (2011) Proteolytic activation of pro‐macrophage‐stimulating protein by hepsin. Mol Cancer Res 9: 1175–1186 [DOI] [PubMed] [Google Scholar]
- Ganesan R, Zhang Y, Landgraf KE, Lin SJ, Moran P, Kirchhofer D (2012) An allosteric anti‐hepsin antibody derived from a constrained phage display library. Protein Eng Des Sel 25: 127–133 [DOI] [PubMed] [Google Scholar]
- Ge G, Greenspan DS (2006) BMP1 controls TGFbeta1 activation via cleavage of latent TGFbeta‐binding protein. J Cell Biol 175: 111–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KA, Lund LR (2005) ECM degrading proteases and tissue remodelling in the mammary gland. BioEssays 27: 894–903 [DOI] [PubMed] [Google Scholar]
- Guipponi M, Tan J, Cannon PZF, Donley L, Crewther P, Clarke M, Wu Q, Shepherd RK, Scott HS (2007) Mice deficient for the type II transmembrane serine protease, TMPRSS1/hepsin, exhibit profound hearing loss. Am J Pathol 171: 608–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herter S, Piper D, Aaron W, Gabriele T, Cutler G, Cao P, Bhatt A, Choe Y, Craik C, Walker N et al (2005) Hepatocyte growth factor is a preferred in vitro substrate for human hepsin, a membrane‐anchored serine protease implicated in prostate and ovarian cancers. Biochem J 390: 125–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashiyama S, Nanba D, Nakayama H, Inoue H, Fukuda S (2011) Ectodomain shedding and remnant peptide signalling of EGFRs and their ligands. J Biochem 150: 15–22 [DOI] [PubMed] [Google Scholar]
- Hooper JD, Clements JA, Quigley JP, Antalis TM (2001) Type II transmembrane serine proteases. Insights into an emerging class of cell surface proteolytic enzymes. J Biol Chem 276: 857–860 [DOI] [PubMed] [Google Scholar]
- Hsu Y‐C, Huang H‐P, Yu I‐S, Su K‐Y, Lin S‐R, Lin W‐C, Wu H‐L, Shi G‐Y, Tao M‐H, Kao C‐H et al (2012) Serine protease hepsin regulates hepatocyte size and hemodynamic retention of tumor cells by hepatocyte growth factor signaling in mice. Hepatology 56: 1913–1923 [DOI] [PubMed] [Google Scholar]
- Ingman WV, Robertson SA (2008) Mammary gland development in transforming growth factor beta1 null mutant mice: systemic and epithelial effects. Biol Reprod 79: 711–717 [DOI] [PubMed] [Google Scholar]
- Jenkins G (2008) The role of proteases in transforming growth factor‐beta activation. Int J Biochem Cell Biol 40: 1068–1078 [DOI] [PubMed] [Google Scholar]
- Kasper S, Sheppard PC, Yan Y, Pettigrew N, Borowsky AD, Prins GS, Dodd JG, Duckworth ML, Matusik RJ (1998) Development, progression, and androgen‐dependence of prostate tumors in probasin‐large T antigen transgenic mice: a model for prostate cancer. Lab Invest 78: i–xv [PubMed] [Google Scholar]
- Klezovitch O, Chevillet J, Mirosevich J, Roberts RL, Matusik RJ, Vasioukhin V (2004) Hepsin promotes prostate cancer progression and metastasis. Cancer Cell 6: 185–195 [DOI] [PubMed] [Google Scholar]
- Koschubs T, Dengl S, Dürr H, Kaluza K, Georges G, Hartl C, Jennewein S, Lanzendörfer M, Auer J, Stern A et al (2012) Allosteric antibody inhibition of human hepsin protease. Biochem J 442: 483–494 [DOI] [PubMed] [Google Scholar]
- Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, Flanders KC, Roberts AB, Sporn MB, Ward JM, Karlsson S (1993) Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci USA 90: 770–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Peng J, Wang H, Zhang W, Brown JM, Zhou Y, Wu Q (2020) Hepsin enhances liver metabolism and inhibits adipocyte browning in mice. Proc Natl Acad Sci USA 117: 12359–12367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R‐M, Desai LP (2015) Reciprocal regulation of TGF‐β and reactive oxygen species: A perverse cycle for fibrosis. Redox Biol 6: 565–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Takai K, Weaver VM, Werb Z (2011) Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol 3: a005058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Duggan DJ, Chen Y, Sauvageot J, Ewing CM, Bittner ML, Trent JM, Isaacs WB (2001) Human prostate cancer and benign prostatic hyperplasia: molecular dissection by gene expression profiling. Cancer Res 61: 4683–4688 [PubMed] [Google Scholar]
- Lyons RM, Keski‐Oja J, Moses HL (1988) Proteolytic activation of latent transforming growth factor‐beta from fibroblast‐conditioned medium. J Cell Biol 106: 1659–1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons RM, Gentry LE, Purchio AF, Moses HL (1990) Mechanism of activation of latent recombinant transforming growth factor beta 1 by plasmin. J Cell Biol 110: 1361–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JA, Araki T, Patil S, Ehrig T, True L, Humphrey PA, Catalona WJ, Watson MA, Milbrandt J (2001) Expression profiling reveals hepsin overexpression in prostate cancer. Cancer Res 61: 5692–5696 [PubMed] [Google Scholar]
- Mamuya FA, Duncan MK (2012) aV integrins and TGF‐β‐induced EMT: a circle of regulation. J Cell Mol Med 16: 445–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, Kadel EE III, Koeppen H, Astarita JL, Cubas R et al (2018) TGFβ attenuates tumour response to PD‐L1 blockade by contributing to exclusion of T cells. Nature 554: 544–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merico D, Isserlin R, Stueker O, Emili A, Bader GD (2010) Enrichment map: a network‐based method for gene‐set enrichment visualization and interpretation. PLoS One 5: e13984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao J, Mu D, Ergel B, Singavarapu R, Duan Z, Powers S, Oliva E, Orsulic S (2008) Hepsin colocalizes with desmosomes and induces progression of ovarian cancer in a mouse model. Int J cancer 123: 2041–2047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazono K, Hellman U, Wernstedt C, Heldin CH (1988) Latent high molecular weight complex of transforming growth factor beta 1. Purification from human platelets and structural characterization. J Biol Chem 263: 6407–6415 [PubMed] [Google Scholar]
- Mohammed FF, Khokha R (2005) Thinking outside the cell: proteases regulate hepatocyte division. Trends Cell Biol 15: 555–563 [DOI] [PubMed] [Google Scholar]
- Moran P, Li W, Fan B, Vij R, Eigenbrot C, Kirchhofer D (2006) Pro‐urokinase‐type plasminogen activator is a substrate for hepsin. J Biol Chem 281: 30439–30446 [DOI] [PubMed] [Google Scholar]
- Moses H, Barcellos‐Hoff MH (2011) TGF‐beta biology in mammary development and breast cancer. Cold Spring Harb Perspect Biol 3: a003277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouw JK, Ou G, Weaver VM (2014) Extracellular matrix assembly: a multiscale deconstruction. Nat Rev Mol Cell Biol 15: 771–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC (2003) Dysregulation of TGF‐beta activation contributes to pathogenesis in Marfan syndrome. Nat Genet 33: 407–411 [DOI] [PubMed] [Google Scholar]
- Olinger E, Lake J, Sheehan S, Schiano G, Takata T, Tokonami N, Debaix H, Consolato F, Rampoldi L, Korstanje R et al (2019) Hepsin‐mediated processing of uromodulin is crucial for salt‐sensitivity and thick ascending limb homeostasis. Sci Rep 9: 12287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall CM, Blobel CP (2007) In search of partners: linking extracellular proteases to substrates. Nat Rev Mol Cell Biol 8: 245–257 [DOI] [PubMed] [Google Scholar]
- Pant SM, Belitskin D, Ala‐Hongisto H, Klefström J, Tervonen TA (2018a) Analyzing the type II transmembrane serine protease hepsin‐dependent basement membrane remodeling in 3D cell culture. Methods Mol Biol 1731: 169–178 [DOI] [PubMed] [Google Scholar]
- Pant SM, Mukonoweshuro A, Desai B, Ramjee MK, Selway CN, Tarver GJ, Wright AG, Birchall K, Chapman TM, Tervonen TA et al (2018b) Design, synthesis, and testing of potent, selective hepsin inhibitors via application of an automated closed‐loop optimization platform. J Med Chem 61: 4335–4347 [DOI] [PubMed] [Google Scholar]
- Partanen JI, Tervonen TA, Myllynen M, Lind E, Imai M, Katajisto P, Dijkgraaf GJP, Kovanen PE, Makela TP, Werb Z et al (2012) Tumor suppressor function of Liver kinase B1 (Lkb1) is linked to regulation of epithelial integrity. Proc Natl Acad Sci USA 109: E388–E397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel Y, Soni M, Awgulewitsch A, Kern MJ, Liu S, Shah N, Singh UP, Chen H (2019) Overexpression of miR‐489 derails mammary hierarchy structure and inhibits HER2/neu‐induced tumorigenesis. Oncogene 38: 445–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proetzel G, Pawlowski SA, Wiles MV, Yin M, Boivin GP, Howles PN, Ding J, Ferguson MWJ, Doetschman T (1995) Transforming growth factor–β3 is required for secondary palate fusion. Nat Genet 11: 409–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarto N, Leonard B, Li S, Marchand M, Anderson E, Behr B, Francke U, Reijo‐Pera R, Chiao E, Longaker MT (2012) Skeletogenic phenotype of human Marfan embryonic stem cells faithfully phenocopied by patient‐specific induced‐pluripotent stem cells. Proc Natl Acad Sci USA 109: 215–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid JC, Matsika A, Davies CM, He Y, Broomfield A, Bennett NC, Magdolen V, Srinivasan B, Clements JA, Hooper JD (2017) Pericellular regulation of prostate cancer expressed kallikrein‐related peptidases and matrix metalloproteinases by cell surface serine proteases. Am J Cancer Res 7: 2257–2274 [PMC free article] [PubMed] [Google Scholar]
- Robertson IB, Horiguchi M, Zilberberg L, Dabovic B, Hadjiolova K, Rifkin DB (2015) Latent TGF‐β‐binding proteins. Matrix Biol 47: 44–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson IB, Rifkin DB (2016) Regulation of the bioavailability of TGF‐β and TGF‐β‐related proteins. Cold Spring Harb Perspect Biol 8: a021907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford LP, Ormsby I, Gittenberger‐de Groot AC, Sariola H, Friedman R, Boivin GP, Cardell EL, Doetschman T (1997) TGFbeta2 knockout mice have multiple developmental defects that are non‐overlapping with other TGFbeta knockout phenotypes. Development 124: 2659–2670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Rifkin DB (1989) Inhibition of endothelial cell movement by pericytes and smooth muscle cells: activation of a latent transforming growth factor‐beta 1‐like molecule by plasmin during co‐culture. J Cell Biol 109: 309–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D (1992) Targeted disruption of the mouse transforming growth factor‐beta 1 gene results in multifocal inflammatory disease. Nature 359: 693–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sounni NE, Dehne K, van Kempen L, Egeblad M, Affara NI, Cuevas I, Wiesen J, Junankar S, Korets L, Lee J et al (2010) Stromal regulation of vessel stability by MMP14 and TGFbeta. Dis Model Mech 3: 317–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamey TA, Warrington JA, Caldwell MC, Chen Z, Fan Z, Mahadevappa M, McNeal JE, Nolley R, Zhang Z (2001) Molecular genetic profiling of Gleason grade 4/5 prostate cancers compared to benign prostatic hyperplasia. J Urol 166: 2171–2177 [PubMed] [Google Scholar]
- Stephan C, Yousef GM, Scorilas A, Jung K, Jung M, Kristiansen G, Hauptmann S, Kishi T, Nakamura T, Loening SA et al (2004) Hepsin is highly over expressed in and a new candidate for a prognostic indicator in prostate cancer. J Urol 171: 187–191 [DOI] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander, ES , et al (2005) Gene set enrichment analysis: a knowledge‐based approach for interpreting genome‐wide expression profiles. Proc Natl Acad Sci USA 25: 15545–15550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimoto H, Yan Y, Clarke J, Korourian S, Shigemasa K, Parmley TH, Parham GP, O’Brien TJ (1997) Hepsin, a cell surface serine protease identified in hepatoma cells, is overexpressed in ovarian cancer. Cancer Res 57: 2884–2887 [PubMed] [Google Scholar]
- Tauriello DVF, Palomo‐Ponce S, Stork D, Berenguer‐Llergo A, Badia‐Ramentol J, Iglesias M, Sevillano M, Ibiza S, Cañellas A, Hernando‐Momblona X et al (2018) TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 554: 538–543 [DOI] [PubMed] [Google Scholar]
- Taylor SH, Yeung C‐Y, Kalson NS, Lu Y, Zigrino P, Starborg T, Warwood S, Holmes DF, Canty‐Laird EG, Mauch C et al (2015) Matrix metalloproteinase 14 is required for fibrous tissue expansion. eLife 4: e09345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tervonen TA, Belitškin D, Pant SM, Englund JI, Marques E, Ala‐Hongisto H, Nevalaita L, Sihto H, Heikkilä P, Leidenius M et al (2016) Deregulated hepsin protease activity confers oncogenicity by concomitantly augmenting HGF/MET signalling and disrupting epithelial cohesion. Oncogene 35: 1832–1846 [DOI] [PubMed] [Google Scholar]
- Tripathi M, Nandana S, Yamashita H, Ganesan R, Kirchhofer D, Quaranta V (2008) Laminin‐332 is a substrate for hepsin, a protease associated with prostate cancer progression. J Biol Chem 283: 30576–30584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utz B, Turpin R, Lampe J, Pouwels J, Klefström J (2020) Assessment of the WAP‐Myc mouse mammary tumor model for spontaneous metastasis. Sci Rep 10: 18733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaarala O, Vuorela A, Partinen M, Baumann M, Freitag TL, Meri S, Saavalainen P, Jauhiainen M, Soliymani R, Kirjavainen T et al (2014) Antigenic differences between AS03 adjuvanted influenza A (H1N1) pandemic vaccines: implications for pandemrix‐associated narcolepsy risk. PLoS One 9: e114361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh JB, Sapinoso LM, Su AI, Kern SG, Wang‐Rodriguez J, Moskaluk CA, Frierson HFJ, Hampton GM (2001) Analysis of gene expression identifies candidate markers and pharmacological targets in prostate cancer. Cancer Res 61: 5974–5978 [PubMed] [Google Scholar]
- Wilkinson DJ, Desilets A, Lin H, Charlton S, Del Carmen Arques M, Falconer A, Bullock C, Hsu Y‐C, Birchall K, Hawkins A et al (2017) The serine proteinase hepsin is an activator of pro‐matrix metalloproteinases: molecular mechanisms and implications for extracellular matrix turnover. Sci Rep 7: 16693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willott JF, Tanner L, O’Steen J, Johnson KR, Bogue MA, Gagnon L (2003) Acoustic startle and prepulse inhibition in 40 inbred strains of mice. Behav Neurosci 117: 716–727 [DOI] [PubMed] [Google Scholar]
- Wiseman BS, Sternlicht MD, Lund LR, Alexander CM, Mott J, Bissell MJ, Soloway P, Itohara S, Werb Z (2003) Site‐specific inductive and inhibitory activities of MMP‐2 and MMP‐3 orchestrate mammary gland branching morphogenesis. J Cell Biol 162: 1123–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Yu D, Post J, Halks‐Miller M, Sadler JE, Morser J (1998) Generation and characterization of mice deficient in hepsin, a hepatic transmembrane serine protease. J Clin Invest 101: 321–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung T‐L, Leung CS, Wong K‐K, Samimi G, Thompson MS, Liu J, Zaid TM, Ghosh S, Birrer MJ, Mok SC (2013) TGF‐β modulates ovarian cancer invasion by upregulating CAF‐derived versican in the tumor microenvironment. Cancer Res 73: 5016–5028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Stamenkovic I (2000) Cell surface‐localized matrix metalloproteinase‐9 proteolytically activates TGF‐beta and promotes tumor invasion and angiogenesis. Genes Dev 14: 163–176 [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Chen CT, Bhargava M, Torzilli PA (2012) A comparative study of fibronectin cleavage by MMP‐1, ‐3, ‐13, and ‐14. Cartilage 3: 267–277 [DOI] [PMC free article] [PubMed] [Google Scholar]


