Abstract
Interpenetrating polymer network (IPN)-based bead formulations were exploited by cross-linking different hydrophilic polymers in different combinations and at different ratios. Polyvinyl alcohol, xanthan gum, guar gum, gellan gum, and sodium alginate (Na-alginate) were used in this work as hydrophilic polymers to enhance the solubility of diclofenac sodium and also to target the delivery at preferred locations. IPN beads based on polysaccharides were prepared by the ionic gelation method. Differential scanning calorimetry, powder X-ray diffraction, scanning electron microscopy, and Fourier transform infrared spectroscopy data showed that the IPN microbeads solubilized and encapsulated the drug within the network. We found over 83% encapsulation efficiency of the drug delivery system for the drug, and this efficiency increased with the concentration of the polymer. Ex vivo experiments using the goat intestine revealed that the IPN microbeads were able to adhere to the intestinal epithelium, a mucoadhesive behavior that could be beneficial to the drug pharmacokinetics, while in vitro experiments in phosphate buffer showed that the IPN enabled significant drug release. We believe that these IPN microbeads are an excellent drug delivery system to solubilize drug molecules and ensure adhesion to the intestinal wall, thereby localizing the drug release to enhance bioavailability of poorly soluble drugs.
1. Introduction
The most convenient way to deliver a drug is the oral route.1 The main limitation in utilization of the oral route of delivery is that bioavailability is very low as the drug transport though the epithelium in the intestine is poor due to the harsh physiological and biochemical conditions in the gastrointestinal tract (GIT). Some of these challenges can be overcome by well-designed delivery formulations; this can be done by increasing the bioavailability, which improves the therapeutic activity effectively.2,3 To effectively deliver the drug through the oral route, nowadays, microspheres are emerging to be the best solution as a multiparticulate drug delivery system. To reduce side effects, increase bioavailability, prolong the action of the drug, and also to ensure both predictable and reproducible pharmacodynamics and pharmacokinetic responses, a multiparticulate drug delivery system can be engineered into both controlled and sustained type so as to achieve all these4−7 above-said benefits. To precisely and effectively target the biological sites, it may be important to formulate the beads as a long-acting dosage system.8−10 Advantage of an orally controlled multiparticulate system based on hydrophilic polymers over the single-unit dosage form has been discussed by other researchers too.11 This multiparticulate drug delivery system is able to act as a controlled and targeted drug delivery system.
For formulation of microbeads, a combination of two or more polymers is involved or required. Interpenetrating polymeric network (IPN) microbeads are prepared with more than one polymer; for example, when two or more polymers are used it does not mean that they will cross-link, but if another chemical entity is present, then, in the presence of that entity, a cross-linked network will form.12 Recent studies are more focused on hydrophilic polymer-derived IPN-based multiparticulate drug delivery systems because they have broad regulatory acceptance, they have well-established processing and synthesis procedure, and they are cost-effective.13−18 Sometimes in IPNs, the polymeric networks are entangled together due to which they cannot be broken apart but not chemically bonded.19,20
To deliver a broad spectrum of therapies, which includes hydrophobic drugs and drugs with low half-life, hydrophilic polymers are the suitable candidates for drug delivery systems. Hydrophilic polymers will be able to deliver hydrophobic drugs in a sustained way and also increase solubility of hydrophobic drugs by creating an aqueous microenvironment around the microsphere.
In our project, diclofenac sodium (DS) is used as a model drug. DS is a nonsteroidal anti-inflammatory drug. It is a derivative of benzoic acid, and it has an extensive array for pharmacological effects such as analgesic, anti-inflammatory, antipyretic activities, and so forth. Due to its potent analgesic activity, it is being used as a medicine for arthritis, joint pain, neuralgia, and so forth.
As the half-life of the drug is very low, hence, with the help of IPNs, the bioavailability of the drug can be enhanced by prolonging its release time. Due to the use of hydrophilic polymers, solubility of DS can be enhanced due to the presence of water throughout the polymeric network where the drug remained in a dispersed form. For design of IPN-based beads, a number of different hydrophilic polymers are exploited such as sodium alginate, polyvinyl alcohol (PVA), xanthan gum, gellan gum, and guar gum. Xanthan gum due to its biosafety reports and also because of its use in IPN-based drug delivery systems for sustaining release activity encouraged us to use it in the present work.21 Gellan gum and guar gum also have been reported for their sustained release profile and nontoxicity.22,23 Therefore, their combination with sodium alginate and PVA will be helpful to get a targeted and sustained release profile.
In the present study, instrumental studies such as differential scanning calorimetry (DSC), powder X-ray diffraction (XRD), scanning electron microscopy (SEM), and Fourier transform infrared spectroscopy (FTIR) analysis were performed as characterization studies. Furthermore, drug dissolution studies, ex vivo mucoadhesion, and swelling studies of the prepared beads were conducted.
2. Results and Discussion
2.1. Formulation of IPN Beads
Different formulations of IPN beads were prepared by the ionic gelation method using different polymers; whereas the base polymer was sodium alginate, the other polymers used were PVA, xanthan gum, gellan gum, and guar gum. DS was used as model drug. A total of 10 different formulations were prepared (Table 1) which were marked as F-1, F-2, F-3, F-4, F-5, F-6, F-7, F-8, F-9, and F-10; here, the ratio of the polymers was altered among them, but the cross-linking time and cross-linking agent concentration were kept constant in all of the formulations. There were no difficulties in the formulation of the beads that were obtained with a slight tailing. Formulation F-1 was kept as the base formulation prepared using only drug and sodium alginate. The ratio of the polymers in formulations F-2, F-5, and F-8 was the same, but the only change was one of the polymers, which were xanthan gum for F-2, gellan gum for F-5, and guar gum for F-7. The ratios of polymers were then changed for formulations F-3, F-6, and F-9 and for formulations F-4, F-7, and F-10 but keeping polymers the same as for F-2, F-5, and F-8. It is shown in several studies that for the enhancement of drug delivery, microspheres have played a major role as Kim and Lee24 and Kulkarni25 stated that when formulations are prepared using only sodium alginate, they erode, but when other polymers are used, then they are effective in altering the drug release behavior.26 Different polymers have different effects on the microspheres as when sodium alginate is blended with carrageenan, it improves the behavior of drug delivery, whereas when polyacrylamide is used, it increases its mechanical strength.27 Also, using hydrophilic polymers for IPN preparation eliminates the use of organic solvents.
Table 1. Composition, Average Particle Size, and Drug Entrapment Efficiency of Different Formulations.
| codes | sodium alginate (g) | PVA (g) | gum used (g) | drug (g) | DL (%) | EE (%) | particle size (mm) |
|---|---|---|---|---|---|---|---|
| Xanthan Gum | |||||||
| F-1 | 0.4 | 0.05 | 10.89 | 98.02 | 1.002 ± 0.17 | ||
| F-2 | 0.3 | 0.1 | 0.010 | 0.05 | 9.78 | 90.01 | 0.950 ± 0.12 |
| F-3 | 0.3 | 0.3 | 0.015 | 0.05 | 6.63 | 88.23 | 0.910 ± 0.12 |
| F-4 | 0.3 | 0.15 | 0.010 | 0.05 | 9.26 | 86.14 | 0.890 ± 0.16 |
| Gellan | |||||||
| F-5 | 0.3 | 0.1 | 0.010 | 0.05 | 9.91 | 91.22 | 1.020 ± 0.20 |
| F-6 | 0.3 | 0.3 | 0.015 | 0.05 | 6.78 | 90.18 | 0.984 ± 0.08 |
| F-7 | 0.3 | 0.15 | 0.010 | 0.05 | 9.38 | 87.22 | 0.920 ± 0.18 |
| Guar Gum | |||||||
| F-8 | 0.3 | 0.1 | 0.010 | 0.05 | 9.84 | 90.56 | 1.038 ± 0.14 |
| F-9 | 0.3 | 0.3 | 0.015 | 0.05 | 6.73 | 89.54 | 1.058 ± 0.08 |
| F-10 | 0.3 | 0.15 | 0.010 | 0.05 | 9.5 | 86.03 | 0.951 ± 0.14 |
2.2. Particle Size, Drug Loading Content, Drug Entrapment Efficiency, Angle of Repose, and External Morphology of the IPN Beads
The average particle size of the beads increased as there was an increase in the ratio of the polymers. The increase in the mean particle size may be due to the increase in the concentration of the solution.28 The microspheres were found out be smooth and were spherical in shape. The particle size was found to be ranging from 0.8 to 1 mm. The cross-linking time and amount of cross-linking agent were kept fixed for all formulations. We can consider that any ratio of different polymers with 2.5% CaCl2 and 1 h as cross-linking time were ideal requirements for formulation of IPN microspheres. The extent of cross-linking within the network system of different polymers can be predicted by the particle size. The spherical morphology of the microspheres was not dependent on any of the factors such as the ratio of different polymers, number of polymers used, or amount of the cross-linker and cross-linking time. The molecular size interaction and intimate contact between the polymers leads to a rigid structuring and the microstructure of the formulations.
The drug loading content varied from 6.73 to 10.83%. The entrapment efficiency of the IPN-based beads varied with the different polymers used and different ratios used. The drug entrapment efficiency was observed to be ranging from 86.03 to 98.02%. The different values in entrapment efficiency were caused due to the presence of polymers and different polymeric ratios that were used for the preparation of the formulation. Encapsulation of the drug was easier and higher as we worked with a low amount of the drug and a higher amount of the polymer. However, it showed that IPN-based microspheres had a great scope for maximizing the load of drugs. The polymer concentration and drug-to-polymer ratio both had significant effect on encapsulation. It was noticed that more amount of the polymer and low concentration of the drug increased drug loading and encapsulation efficiency. It can be explicated that a higher concentration of the polymer due to higher viscosity generated a dense internal structure, preventing loss of the drug due to leaching35 and also delayed diffusion process of the drug through the polymeric network.29
The angle of repose of the beads showed that the value ranging between 31 and 35° implies good flow property.
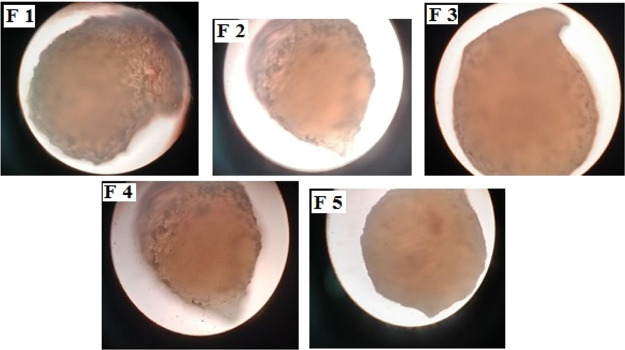
The images obtained by a trinocular microscope showed the beads to be spherical in shape, and the beads were observed to have a smooth surface and has a rigid sphere (Figure 1). This suggests that polymeric chains that are cross-linked between different types of polymers are tightly packed, which results in a flawless microstructure. For the study of drug release behavior, the morphology of the bead is an important characteristic.30 There were no drug crystals present on the surface of the beads, which states that there was an efficient encapsulation of drug within the network of polymers. Slight tailing was observed for all formulation as dropping the speed for controlling the polymer mixture was done manually. An automatic method will avert this tailing.
Figure 1.
External surface morphology of F-1, F-2, F-3, F-4, and F-5 formulations captured by the trinocular microscope.
2.3. Swelling Study of the IPN Beads
In phosphate buffer of pH 6.8, the swelling properties of the drug-loaded beads were studied. The water uptake was high in the given pH. The maximum swelling occurred up to 5 h, and then, the breakdown of the beads occurred. This swelling study confirmed integrity of prepared formulations for a longer duration.
2.4. FTIR Spectroscopy Analysis
The FTIR spectra of DS, F-3, F-6, and F-9 are shown in Figure 2. The important FTIR peaks of DS at 1282.43 and 1304.89 cm–1 resulted from C–N stretching, whereas peaks at 1555.31 and 1572.66 cm–1 resulting from C=C stretching and C=O stretching of the carboxyl group, respectively31 were detected in all formulations. Thereby, the FTIR spectroscopy study confirmed chemical stability of the drug in the polymeric network after formulation. In all three formulations, few characteristic peaks of pure PVA were detected such as between 1628 and 1622 cm–1 due to the asymmetric N–H bending, and the peak in the range of 1560–1552 cm–1 was indicative of bending vibrations of CH2. In F-3, the characteristic peak at 1008.75 was indicative of symmetric carboxylate anion stretching of xanthan gum. The characteristic peak of 2955.38 cm–1 in F-6 indicated C–H stretching of gellan gum. However, characteristic peaks of gaur gum were missing in F-9. The FTIR spectrum of IPN-based beads (F-3, F-6, and F-9) showed OH stretching peaks with lowered intensity observed at 3343.96, 3366.14, and 3387.85 cm–1, respectively, which confirms the presence of H-bonding between alginate and PVA polymers. Additionally, a notable stretching band was observed for IPN-based beads at about ν = 1449–1448 cm–1 of −CH2 groups, which are regarded as feature groups attributed to cross-linking of PVA and SA in the PVA–SA blend IPN network.32 The characteristic peak located at ν = 1119–1115 cm–1 was due to the stretching vibration of C–O–C due to the ether linkage and acetal linkage of IPN.33 This confirmed that the IPN-based beads were formed by gum cross-linking, which acted as a bifunctional cross-linker and formed ether/acetal linkage between the hydroxyl group of PVA and alginate.
Figure 2.
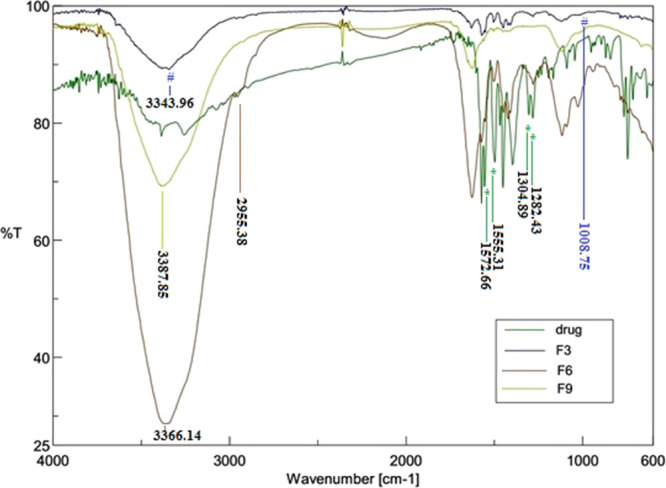
FTIR spectra of drug; F-3; F-6; F-9.
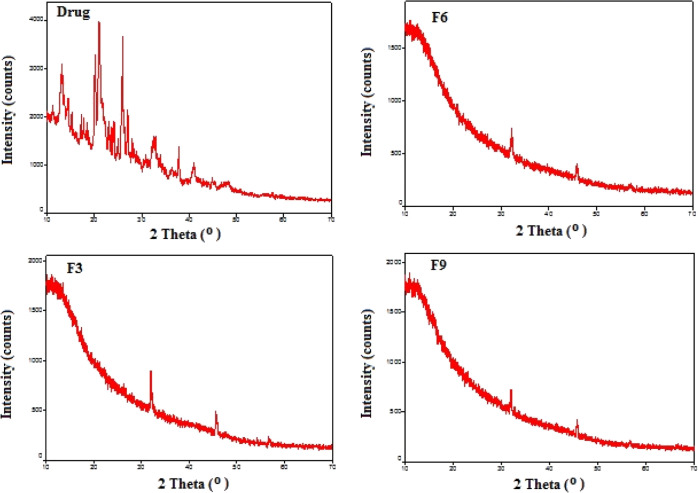
2.5. Powder XRD Analysis
The X-ray diffractograms of the pure drug and drug-loaded IPN based beads are presented in Figure 3. The characteristic peaks appearing at 13.092, 20.154, 21.035, 21.58, 25.729, and 25.939 2θ values in the XRD pattern of DS clearly revealed the crystalline nature of the drug. The absence of prominent drug peaks in the diffractogram of IPN-based formulations (F-3, F-6, and F-9) confirmed the existence of the drug in an amorphous form in the polymer matrix. This suggested that the drug existed as a solid–solid solution in the IPN matrix, confirming that the drug is molecularly dispersed in the beads in an amorphous form. This molecular dispersion of drug within matrix is a clear indication of enhancement of drug solubility in aqueous environments.
Figure 3.
XRD spectra of the drug; F-3; F-6; F-9.
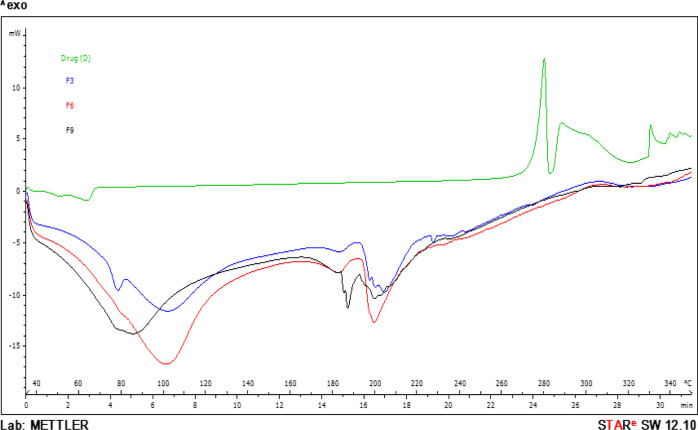
2.6. DSC Analysis
From the DSC study, it was observed that the exothermic peaks at about 280 and 288 °C in the DSC thermogram of the pure drug are the outcome of drug decomposition just prior to the melting point with formation of a related indole cyclic amide.34 Each bead exhibited (Figure 4) similar thermal behavior with the absence of endothermal or exothermal peaks for DCP near 280–288 °C. This suggested possible molecular dispersion of DCP in IPN matrices with transformation of the drug from the crystalline to amorphous state35 The molecular dispersion of diclofenac in the IPN matrix seemed to have protected the drug from decomposition by formation of hydrogen bonding with the drug.36 The wide endothermic peaks at 80 to 110 °C correspond to moisture loss from the microspheres. In all three formulations, PVA showed its characteristic melting peak at about 192 °C, and a degradation peak was observed at about 322 °C.37
Figure 4.
DSC spectra of the drug; F-3; F-6; F-9.
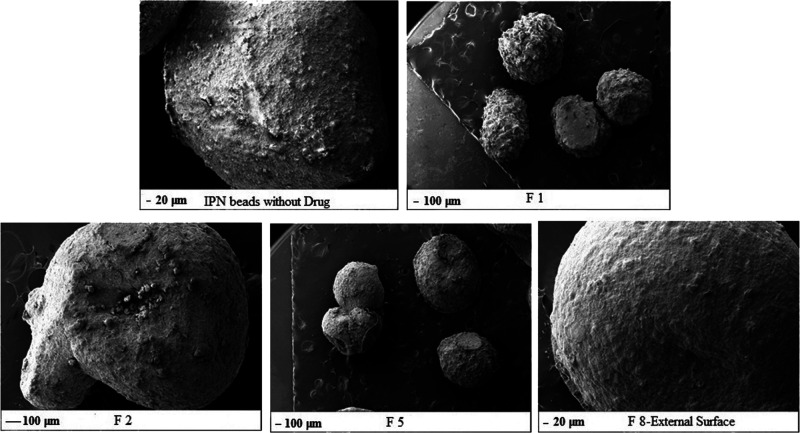
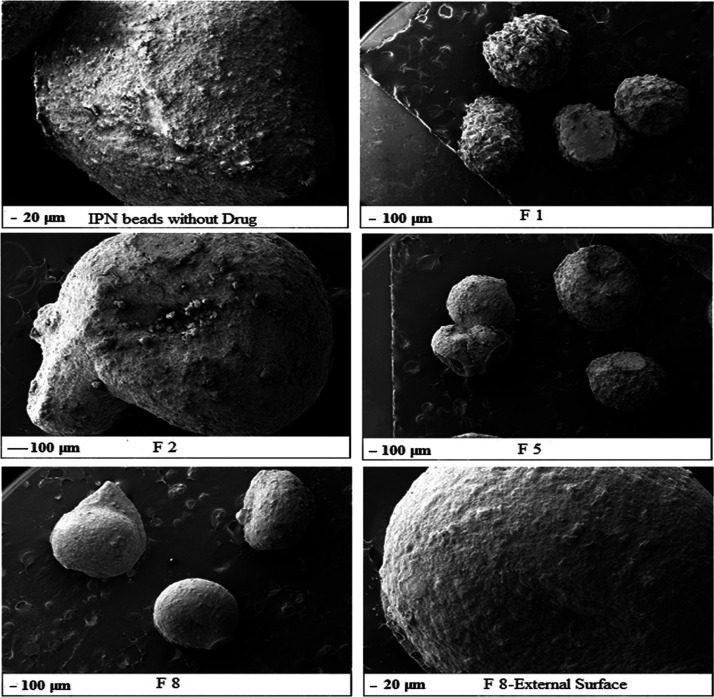
2.7. Scanning Electron Microscopy
SEM analysis was done to observe microspheres or formulations at a higher magnification. It was evident that the sizes of the microspheres were all nearly the same in all formulations. In the formulation without the drug, beads (Figure 5) were oval in shape with smooth surfaces. In formulation F-1, it was evident that a spherical shape was obtained but not a smooth surface as some amount of the drug gets adhered to the surface. In all other formulations, xanthan gum, guar gum, or gellan gum were blended separately with Na-alginate and PVA for F-2, F-5, and F-8, respectively. The beads obtained from F-2, F-5, and F-8 were all spherical in shape and had a smooth surface, which shows us that the microspheres formulated using different polymers and with different ratios interacted well among them and bonded well; the drug was encapsulated well inside the system, and there was no leakage of the drug, which was confirmed by the smooth surface. Hence, in the presence of other hydrophilic polymers (xanthan, gellan, or guar gum), encapsulation of the drug occurred within the network. We can conclude that IPN microspheres with the encapsulated drug were formed.
Figure 5.
SEM images of IPN beads without the drug; F-1, F-2; F-5; and F-8.
2.8. In Vitro Dissolution Study and Kinetics
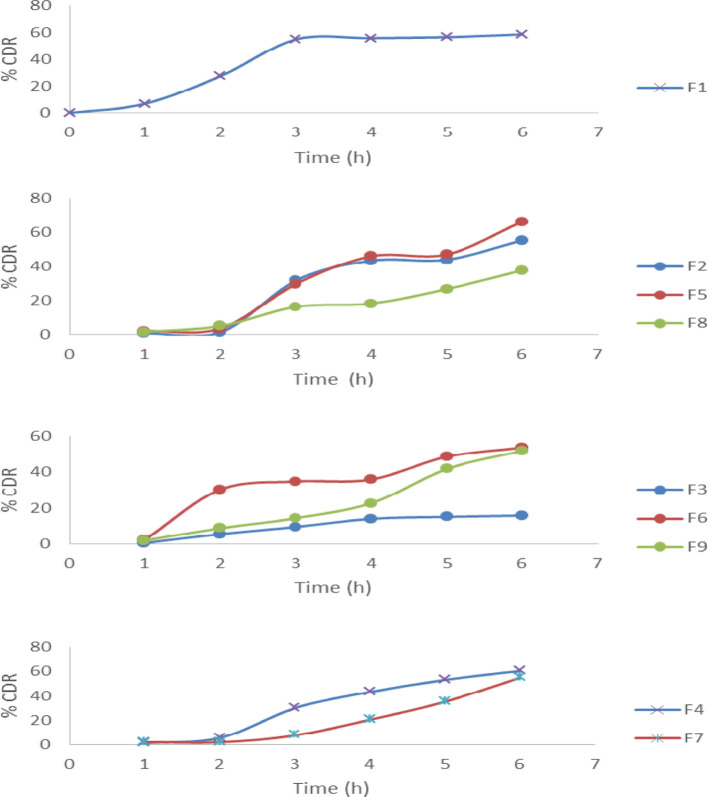
The in vitro dissolution test was done to know the effect of pH on the release of the microsphere.38−40 The main polymer used for the preparation of IPN microspheres here is sodium alginate. In stomach, at low pH, sodium alginate gets protonated; due to this, the polymeric chain interaction increases, thus restricting the drug release from the beads in gastric pH and under acidic conditions, whereas at higher pH, under basic conditions, sodium alginate gets deprotonated, and thus, the polymeric chain interaction decreases, which results in enabling the intestinal fluids to enter into the polymeric network so that it swells and the drug entrapped in them gets diffused efficiently in the GIT. Here, we have taken sodium alginate as the base polymer and the in vitro study of formulation F-1 was a basis to compare the result of the other formulations. In this study, different formulations and different ratios of different polymers were used. Formulations F-2, F-5, and F-8 had the same amount of polymers used with a change in the type of the polymer, which were xanthan gum, gellan gum, and guar gum, respectively. From the in vitro study (% cumulative drug release vs time, i.e., % CDR vs time), it was concluded that all three formulations showed sustained release characteristics when there was little or no difference between F-2 and F-5 due to the change in the polymers as the ratio or the amount of the polymers were the same and the basic polymers used for making IPN were the same in all three, that is, sodium alginate, although release from F-8 was more sustained. When different formulations such as F-3, F-6, and F-9 in which the amount of the base polymers were kept constant but the amounts of xanthan gum, gellan gum, and guar gum were slightly increased, respectively, were studied, the PVA concentration was also increased for all formulations. Here, we observed that formulation F-3 was more sustained than F-6 and F-9, which may be because xanthan gum has a more branched polysaccharide structure than guar gum and gellan gum has a linear structure, due to which the drug gets entrapped in xanthan gum and diffusion of the drug in it decreases more when compared to the other two; when the other two formulations were compared, there was less difference in their results. In formulations F-4, F-7, and F-10, the amount of sodium alginate was kept fixed and the amount of xanthan gum, gellan gum, and guar gum was lowered with the amount of PVA in comparison to F-3, F-6, and F-9. This showed that the release rate was quite higher (Figure 6). Here, formulation F-8 showed the best result when compared to marketed formulation (data not shown). In case of F-10, the release in the first few hours was very less and after sometime, the release increased drastically, due to which the result and the data collection could not be done properly.
Figure 6.
In vitro drug release from IPN beads (n = 3).
As discussed in Section 3.2, the diffusion process of the drug occurred through the dense polymeric network.41 Slow diffusion of the drug through the polymeric network led to a constant and stable drug release profile. IPN-based microspheres are capable of controlling and targeting the drug release profile, as discussed by other researchers.42,43 Due to excellent controlled swelling ability, specificity, and mechanical strength of the IPN-based drug system imparted by various polymers, this kind of a drug delivery system will be able to maintain a constant and stable release profile. After fitting all the release data in different model equations of release kinetics, it was perceived that release of DS from different formulations revealed different mechanisms. The release constants, the correlation coefficients (r2), and exponents (n) for different formulations are shown in Table 2. Release patterns of DS from different formulations were fitted well with the Higuchi (r2, 0.9206–0.9467) model equations. In case of the Korsmeyer–Peppas equation44−46 (r2, 0.8053–0.9911), release exponents (n) ranging from 1.19 to 2.53 indicated that the drug release from all the formulations was super-case II-type of release. The case of F-9 (r2 = 0.9811) was best explained by the first-order equation. The F-8 formulation was best suited by the Korsmeyer–Peppas equation (r2 = 0.9911), and the drug release was super-case II-type of release.
Table 2. Drug Release Kinetics for Different IPN Beads.
| zero-order |
first-order |
Hixson–Crowell |
Higuchi |
Korsmeyer–Peppas |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| batch code | K0 (h–1) | r2 | K1 (h–1) | r2 | KHC (h–1/3) | r2 | KH (h–1/2) | r2 | KKP (h–n) | r2 | n |
| F-1 | 9.8951 | 0.7529 | –0.0695 | 0.7815 | –0.5830 | 0.7360 | 27.7553 | 0.9300 | 0.0953 | 0.8643 | 1.19 |
| F-2 | 11.7629 | 0.9019 | –0.0730 | 0.9326 | –0.6720 | 0.8868 | 24.6718 | 0.9263 | 0.0083 | 0.8587 | 2.53 |
| F-3 | 3.1557 | 0.9239 | –0.0151 | 0.9315 | –0.4117 | 0.8403 | 30.7839 | 0.9325 | 0.0087 | 0.8903 | 1.86 |
| F-4 | 12.8971 | 0.9633 | –0.0855 | 0.9811 | –0.6702 | 0.9217 | 30.6813 | 0.9353 | 0.0157 | 0.9566 | 2.22 |
| F-5 | 13.3434 | 0.9456 | –0.0918 | 0.9387 | –0.6698 | 0.9171 | 31.5819 | 0.9351 | 0.0190 | 0.9064 | 2.07 |
| F-6 | 8.96 | 0.8612 | –0.0581 | 0.9155 | –0.5772 | 0.7721 | 24.9156 | 0.9311 | 0.0432 | 0.8053 | 1.59 |
| F-7 | 10.7271 | 0.9012 | –0.0653 | 0.8505 | –0.5987 | 0.9680 | 27.5439 | 0.9206 | 0.0129 | 0.8662 | 1.94 |
| F-8 | 7.0802 | 0.9697 | –0.0384 | 0.9522 | –0.5198 | 0.9217 | 30.6269 | 0.9467 | 0.0183 | 0.9805 | 1.71 |
| F-9 | 10.2180 | 0.9564 | –0.0623 | 0.9219 | –0.5857 | 0.9457 | 27.5957 | 0.9286 | 0.0212 | 0.9911 | 1.79 |
2.9. Mucoadhesion Study
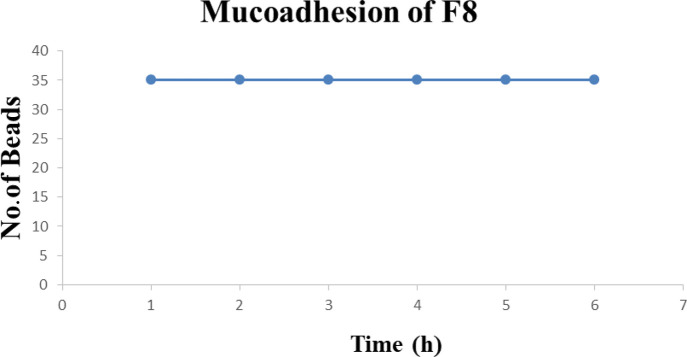
The mucoadhesive property of microspheres to their target sites was investigated in this study to properly understand the adhesion of the microspheres in their respective target sites. Due to peristalsis in the GIT, the microspheres after oral administration47 get propelled away from their target sites. For achieving localized drug delivery systems, polymers with good mucoadhesive properties can be used or are suitable for oral delivery of the drug as these polymers adhere to the site to target drug release at desired areas. In this study, we see how the mucoadhesive property of the IPN is affected under influence of pH, polymer concentration, and types of polymers. It was observed that the mucoadhesive property is good in basic pH, which shows that the mucoadhesive property is pH-dependent. In phosphate buffer of pH 6.8, it was observed that the beads adhered for around 6 h to the mucus layer. Our study resembles the mucoadhesive study of Gombotz and Wee,48 which states that alginate exhibits mucoadhesive property but also contradicts that of Gåserød et al.,49 which states that when alginate beads become hydrated and swollen, they get attached to the mucosa at a low pH. It is also seen in several studies that due to the presence of higher pH in the duodenum, the beads swell and lead to a phenomenon due to which the functional group present in the polymers has more chances of interaction with the mucosa, thus increasing adhesion. We can conclude that at pH 6.8, the functional group of the polymers interacted with the intestinal mucosa upon getting access, thus showing a prolonged and better mucoadhesive property. In a period of 6 h, there was no detachment of the microspheres in phosphate buffer (pH 6.8). There was excellent mucoadhesion in phosphate buffer, as shown in Figure 7. The beads in Figure 8 have been used for the mucoadhesive study (only F-3 mucoadhesion result is shown here; other formulations have shown almost the same result). The same study was carried out in acidic pH. It was found that not a single bead adhered to the mucosa, which confirmed that the prepared IPN beads are able to retard release of the drug in the stomach, reducing ulceration, destruction of gastric mucosa, and hemorrhage. Therefore, IPN-based drug delivery systems would be expected not to show any toxicity as formulations did not not swell in acidic pH, did not attach to the mucosa, and did not release any drug in acidic pH. Anti-inflammatory drugs such as ibuprofen, diclofenac, and so forth are always preferred to be concealed from the stomach region. The mucoadhesion study showed that IPN-based beads prepared in this research were successful for achieving this goal.
Figure 7.
Mucoadhesion study of formulation F-8.
Figure 8.
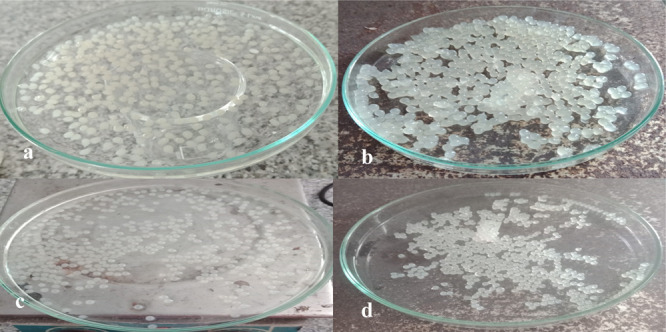
Prepared microspheres; (a) preparation of microspheres with only sodium alginate and PVA; (b) preparation of microspheres with sodium alginate, PVA, and xanthan gum; (c) preparation of microspheres with sodium alginate, PVA, and gellan gum; (d) preparation of microspheres with sodium alginate, PVA, and guar gum.
3. Materials and Methods
3.1. Materials
DS (MW: 318.1 g/mol) was purchased bulk from a local supplier. Sodium alginate (low viscosity; viscosity of 2% solution at 25 °C, 250 cps) and PVA were purchased from Merck, India. Xanthan gum, gellan gum, and guar gum were purchased from a local supplier in Kolkata. Double-distilled water was used for all the experiments. All the chemicals were used in this research work as received from the suppliers.
3.2. Preparation of IPN Beads
IPN beads (F1) were prepared by cross-linking Na-alginate and PVA polymer blends. For F-2–F-4, IPN beads were prepared by cross-linking blends of Na-alginate, PVA, and xanthan gum. For F-5 to F-7, IPN beads were prepared by cross-linking blends of Na-alginate, PVA, and guar gum. Blends of Na-alginate, PVA, and guar gum were prepared by the ionic gelation method for preparation of F-8–F-10 IPN beads. After the sodium alginate was dissolved, PVA (cold water-soluble) was added in the system with constant stirring and was allowed to dissolve completely. Once the PVA was dissolved, then, the required amount of the drug (DS) was added and was allowed to dissolve. After that, according to Table 1, either xanthan gum, guar gum, or gellan gum was added into the mixture at different ratios. For every formulation, the amount of distilled water used to dissolve the polymers was fixed to 15 mL. Twenty milliliters of 5% calcium chloride solution was prepared, which acts as a cross-linker.50 Then, the polymer and drug mixture was filled into a syringe and an 18-gauge needle was placed in its mouth. The beaker containing the cross-linking solution was placed below the needle, and the mixture was poured into the solution dropwise to form the microbeads. The microbeads were then filtered after 1 h with the help of a Whatman filter paper. The beads were then allowed to dry using a tray dryer, and thus, the microbeads were prepared.
3.3. Particle Size, Angle of Repose, and Preliminary Morphological Determination of Beads
The lower jaws of the dial calipers on the outside were opened by moving the movable jaws of the aerospace caliper. The beads to be measured were kept in the open space of the dial caliper, and the measuring jaws were moved on the beads that were to be measured. Then, the measured value of the particle size of the beads was taken into account.
To determine the angle of repose, the beads were taken and poured through a funnel; the funnel was fixed in such a way that the lower-end tip of the funnel was at a height of 2 cm above the surface.51 Until the upper end of the beads touched the lower end of the funnel, the beads were poured into it.
The beads were taken and placed on a clean glass slide. The slide with the beads was mounted on the stage of a trinocular microscope, and external morphology was observed.
3.4. Analysis of Drug Loading Content (DL) and Entrapment Efficiency (EE)
In each formulation, 50 mg of the drug was added during IPN preparation and the amount of the drug that was entrapped was determined by encapsulation efficiency. The amount of the drug present in a specific amount of microspheres was the drug loading content (DL). A definite amount of beads was weighed and was dissolved in phosphate buffer pH 6.8. The solution was then kept overnight under stirring, and then, it was filtered and further dilutions were done. Then, the absorbance of the solution was measured using an UV–vis spectrophotometer (Shimadzu 1202 UV–vis spectrophotometer, Japan) at 276 nm against phosphate buffer of pH 6.8 as the blank and the percentage of the drug present in the sample was calculated with the help of the following formula
3.5. Swelling Study
The swelling study was conducted by taking a known amount of beads and then immersing them in 50 mL of phosphate buffer of pH 6.8.
3.6. Solid-State Characterization
3.6.1. FTIR Spectroscopy
FTIR spectroscopy of DS and drug microspheres was performed using attenuated total-reflectance–FTIR spectroscopy (FT/IR-4600, JASCO, Japan) to confirm the formation of DS-loaded IPN beads and compatibility of different ingredients of the formulations. The samples were mounted on a zinc selenide (ZnSe) window, and the spectra were recorded with a resolution of 4 cm–1 between 4000 and 600 cm–1 spectral ranges with an average of 32 scans.
3.6.2. Powder XRD Analysis
X-ray diffraction patterns of the pure drug and drug-loaded IPN beads were scanned using a powder diffractometer (Rigaku Ultima IV) using Cu Kα radiation (λ = 1.54051 Å) with a 2°/min scanning speed. The 2θ scans were recorded at room temperature ranging from 10 to 90 °C in a continuous scan mode.
3.6.3. DSC Analysis
The DSC thermograms of the pure drug and IPN formulations were obtained by a differential scanning calorimeter (DSC-1, Mettler Toledo; Software-Star E, SNR-18289) in a nitrogen atmosphere within the range of 30–350 °C at a constant heating rate of 10 °C/min. Each sample (3–7 mg) was accurately weighed into a 40 mL aluminum pan in a hermetically sealed condition.
3.6.4. SEM Analysis
The IPN beads were fixed onto stubs using a double-sided adhesive tape and sputter-coated with a palladium layer in a JFC-1600 auto fine coater so as to make them conductive. The coated beads were then observed under a scanning electron microscope (JSM-6701F, JEOL, Japan). All the micrographs were captured at an excitation voltage of 10 kV using different magnifications.52
3.7. In Vitro Drug Release Study and Kinetics of Drug Release
The in vitro drug release study was done using a USP-II rotating paddle-type dissolution test apparatus using phosphate buffer of pH 6.8 as the medium at a stirring speed of 50 rpm and 37 °C temperature. A specific amount of beads was taken and placed in a dissolution bowl, and 500 mL of phosphate buffer of pH 6.8 was added to it. At a regular time interval, 1 mL of the sample was withdrawn, and the same volume of freshly prepared dissolution medium was replaced in the dissolution bowl. After proper dilution, the samples were analyzed with the help of a double-beam UV–vis spectrophotometer (Shimadzu 1202 UV–vis spectrophotometer, Japan) at 276 nm wavelength.53 From the absorbance, the cumulative percentage drug release (% CDR) was calculated, and % CDR was plotted against time to find the pattern of drug release. After this, different kinetic models such as zero-order, first-order, Higuchi, Hixson–Crowell, and Korsmeyer–Peppas were used to find out the drug release mechanism.54
3.8. Ex Vivo Mucoadhesion Study
The upper part of the small intestine of a goat was taken at first, and then, it was cut into a specific length to expose the mucus layer. Then, it was placed and tied over a glass scale and was attached to it using a thread. A known amount of the beads was then placed in the mucus layer, then, a small amount of buffer was applied above the beads, and then, after some time, the beads were gently pressed.55 The glass scale with attached beads was afterward tied to the stem of the disintegration apparatus and was kept in phosphate buffer of pH 6.8, and the machine was switched on. The beads were then observed to see how many beads detached from the mucus layer at a regular time interval, and the number of beads that were detached against time was noted down.
4. Conclusions
A suitable delivery system is necessary for better therapeutic efficacy. To get the desired bioavailability in the system is the major role of any delivery system. IPN beads nowadays are being used for drug delivery due to their small size, and they are capable of incorporating large-molecular-weight particles; they can also be engineered for targeted and sustained release of drug into the system to attain the desired drug release profile. The IPN beads were formulated successfully. The preparation procedure of the IPN beads was easy and reproducible. IPN-based beads of drug DS were successfully prepared. The studies that were done here showed that the DS-loaded beads exhibited a sustained drug release profile. High encapsulation efficiency of the drug was observed. The microspheres exhibited good mucoadhesive property and exhibited a release behavior that was pH-dependent.
Acknowledgments
We are very thankful to Dr. B. C. Roy College of Pharmacy and AHS for helping us with resources.
The authors declare no competing financial interest.
References
- Li J.; Mooney D. J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. 10.1038/natrevmats.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbitt M. W.; Dahlman J. E.; Langer R. Emerging frontiers in drug delivery. J. Am. Chem. Soc. 2016, 138, 704–717. 10.1021/jacs.5b09974. [DOI] [PubMed] [Google Scholar]
- Lordi N. G.Sustained release dosage forms. In The Theory and Practice of Industrial Pharmacy; Lachman L., Liberman H. A., Kanig I. L., Eds.; Lea and Febiger: Philadelphia, 1986; pp 430–456. [Google Scholar]
- Mumper R. J.; Huffman A. S.; Puolakkainen P. A.; Bouchard L. S.; Gombotz W. R. Calcium-alginate beads for the oral delivery of transforming growth factor-β1 (TGF-β1): stabilization of TGF-β1 by the addition of polyacrylic acid within acid-treated beads. J. Controlled Release 1994, 30, 241–251. 10.1016/0168-3659(94)90030-2. [DOI] [Google Scholar]
- Shin B. Y.; Cha B. G.; Jeong J. H.; Kim J. Injectable macroporous ferrogel microbeads with a high structural stability for magnetically actuated drug delivery. ACS Appl. Mater. Interfaces 2017, 9, 31372–31380. 10.1021/acsami.7b06444. [DOI] [PubMed] [Google Scholar]
- Bannerman D.; Wan W. Multifunctional microbeads for drug delivery in TACE. Expert Opin. Drug Delivery 2016, 13, 1289–1300. 10.1080/17425247.2016.1192122. [DOI] [PubMed] [Google Scholar]
- Kulkarni A. R.; Soppimath K. S.; Aminabhavi T. M.; Rudzinski W. E. In-vitro release kinetics of cefadroxil-loaded sodium alginate interpenetrating network beads. Eur. J. Pharm. Biopharm. 2001, 51, 127–133. 10.1016/s0939-6411(00)00150-8. [DOI] [PubMed] [Google Scholar]
- Roy P.; Shahiwala A. Multiparticulate formulation approach to pulsatile drug delivery: current perspectives. J. Controlled Release 2009, 134, 74–80. 10.1016/j.jconrel.2008.11.011. [DOI] [PubMed] [Google Scholar]
- Sood A.; Panchagnula R. Design of controlled release delivery systems using a modified pharmacokinetic approach: a case study for drugs having a short elimination half-life and a narrow therapeutic index. Int. J. Pharm. 2003, 261, 27–41. 10.1016/s0378-5173(03)00267-9. [DOI] [PubMed] [Google Scholar]
- Asghar L. F. A.; Chandran S. Multiparticulate Formulation approach to colon specific drug delivery current perspectives. J. Pharm. Pharm. Sci. 2006, 9, 327–338. [PubMed] [Google Scholar]
- Banerjee S.; Siddiqui L.; Bhattacharya S. S.; Kaity S.; Ghosh A.; Chattopadhyay P.; Pandey A.; Singh L. Interpenetrating polymer network (IPN) hydrogel microspheres for oral controlled release application. Int. J. Biol. Macromol. 2012, 50, 198–206. 10.1016/j.ijbiomac.2011.10.020. [DOI] [PubMed] [Google Scholar]
- Butler J.; Cumming I.; Brown J.; Wilding I.; Devane J. G. A novel multiunit controlled-release system. Pharm. Technol. 1998, 22, 122–138. [Google Scholar]
- Yin Z.-C.; Wang Y.-L.; Wang K. A pH-responsive composite hydrogel beads based on agar and alginate for oral drug delivery. J. Drug Delivery Sci. Technol. 2018, 43, 12–18. 10.1016/j.jddst.2017.09.009. [DOI] [Google Scholar]
- Yang W.; Fortunati E.; Bertoglio F.; Owczarek J. S.; Bruni G.; Kozanecki M.; Kenny J. M.; Torre L.; Visai L.; Puglia D. Polyvinyl alcohol/chitosan hydrogels with enhanced antioxidant and antibacterial properties induced by lignin nanoparticles. Carbohydr. Polym. 2018, 181, 275–284. 10.1016/j.carbpol.2017.10.084. [DOI] [PubMed] [Google Scholar]
- Park J.-S.; Park J.-W.; Ruckenstein E. A dynamic mechanical and thermal analysis of unplasticized and plasticized poly (vinyl alcohol)/methylcellulose blends. J. Appl. Polym. Sci. 2001, 80, 1825–1834. 10.1002/app.1278. [DOI] [Google Scholar]
- Traore Y. L.; Fumakia M.; Gu J.; Ho E. A. Dynamic mechanical behaviour of nanoparticle loaded biodegradable PVA films for vaginal drug delivery. J. Biomater. Appl. 2018, 32, 1119–1126. 10.1177/0885328217739451. [DOI] [PubMed] [Google Scholar]
- Mendes A. C.; Strohmenger T.; Goycoolea F.; Chronakis I. S. Electrostatic self-assembly of polysaccharides into nanofibers. Colloids Surf., A 2017, 531, 182–188. 10.1016/j.colsurfa.2017.07.044. [DOI] [Google Scholar]
- Shekarforoush E.; Ajalloueian F.; Zeng G.; Mendes A. C.; Chronakis I. S. Electrospun xanthan gum-chitosan nanofibers as delivery carrier of hydrophobic bioactives. Mater. Lett. 2018, 228, 322–326. 10.1016/j.matlet.2018.06.033. [DOI] [Google Scholar]
- Soman A.; Mathew F.; Chacko A. J.; Alias M.; Vinoda Poosan G. Interpenetrating polymer network (Ipn) – hydrogels. Pharma Innovation 2014, 3, 59–66. [Google Scholar]
- Lohani A.; Singh G.; Bhattacharya S. S.; Verma A. Interpenetrating polymer networks as innovative drug delivery systems. J. Drug Delivery 2014, 2014, 1–11. 10.1155/2014/583612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya S. S.; Shukla S.; Banerjee S.; Chowdhury P.; Chakraborty P.; Ghosh A. Tailored IPN hydrogel bead of sodium carboxymethyl cellulose and sodium carboxymethyl xanthan gum for controlled delivery of diclofenac sodium. Polym.-Plast. Technol. Eng. 2013, 52, 795–805. 10.1080/03602559.2013.763361. [DOI] [Google Scholar]
- Reddy K. M.; Babu V. R.; Sairam M.; et al. Development of chitosan-guar gum semi-interpenetrating polymer network microspheres for controlled release of cefadroxil. Des. Monomers Polym. 2006, 9, 491–501. 10.1163/156855506778538047. [DOI] [Google Scholar]
- Vashisth P.; Singh H.; Pruthi P. A.; Pruthi V.. Gellan as novel pharmaceutical excipient. Handbook of Polymers for Pharmaceutical Technologies: Structure and Chemistry; John Wiley & Sons, Inc., 2015; Vol. 1, pp 1–21. [Google Scholar]
- Kim C.-K.; Lee E.-J. The controlled release of blue dextran from alginate beads. Int. J. Pharm. 1992, 79, 11–19. 10.1016/0378-5173(92)90088-j. [DOI] [Google Scholar]
- Kulkarni A. R.; Soppimath K. S.; Aminabhavi T. M.; Dave A. M. Polymeric sodium alginate interpenetrating network beads for the controlled release of chlorpyrifos. J. Appl. Polym. Sci. 2002, 85, 911–918. 10.1002/app.10364. [DOI] [Google Scholar]
- Garoushi S.; Vallittu P. K.; Watts D. C.; Lassila L. V. J. Polymerization shrinkage of experimental short glass fiber-reinforced composite with semi-inter penetrating polymer network matrix. Dent. Mater. 2008, 24, 211–215. 10.1016/j.dental.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Pongjanyakul T.; Puttipipatkhachorn S. Xanthan–alginate composite gel beads: molecular interaction and in vitro characterization. Int. J. Pharm. 2007, 331, 61–71. 10.1016/j.ijpharm.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Guo T.; Zhang N.; Huang J.; Pei Y.; Wang F.; Tang K. A facile fabrication of core–shell sodium alginate/gelatin beads for drug delivery systems. Polym. Bull. 2019, 76, 87–102. 10.1007/s00289-018-2377-z. [DOI] [Google Scholar]
- Yandrapu S.; Kompella U. B. Development of sustained-release microspheres for the delivery of SAR 1118, an LFA-1 antagonist intended for the treatment of vascular complications of the eye. J. Ocul. Pharmacol. Ther. 2013, 29, 236–248. 10.1089/jop.2012.0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamboa J. M.; Leong K. W. In vitro and in vivo models for the study of oral delivery of nanoparticles. Adv. Drug Delivery Rev. 2013, 65, 800–810. 10.1016/j.addr.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak A. K.; Pal D. Development of pH-sensitive tamarind seed polysaccharide–alginate composite beads for controlled diclofenac sodium delivery using response surface methodology. Int. J. Biol. Macromol. 2011, 49, 784–793. 10.1016/j.ijbiomac.2011.07.013. [DOI] [PubMed] [Google Scholar]
- Kamoun E. A.; Kenawy E.-R. S.; Tamer T. M.; El-Meligy M. A.; Mohy Eldin M. S. Poly (vinyl alcohol)-alginate physically crosslinked hydrogel membranes for wound dressing applications: Characterization and bio-evaluation. Arabian J. Chem. 2015, 8, 38–47. 10.1016/j.arabjc.2013.12.003. [DOI] [Google Scholar]
- Liu C.; Liu H.; Xiong T.; Xu A.; Pan B.; Tang K. Graphene oxide reinforced alginate/PVA double network hydrogels for efficient dye removal. Polymers 2018, 10, 835. 10.3390/polym10080835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudja P.; Khan M. Z. I.; Meštrovic E.; Horvat M.; Golja P. Thermal behaviour of diclofenac sodium: decomposition and melting characteristics. Chem. Pharm. Bull. 2001, 49, 1245–1250. 10.1248/cpb.49.1245. [DOI] [PubMed] [Google Scholar]
- Ray S.; Banerjee S.; Maiti S.; Laha B.; Barik S.; Sa B.; Bhattacharyya U. K. Novel interpenetrating network microspheres of xanthan gum-poly(vinyl alcohol) for the delivery of diclofenac sodium to the intestine-in vitro and in vivo evaluation. Drug Delivery 2010, 17, 508–519. 10.3109/10717544.2010.483256. [DOI] [PubMed] [Google Scholar]
- Mohapatra R.; Mallick S.; Nanda A.; Sahoo R. N.; Pramanik A.; Bose A.; Das D.; Pattnaik L. Analysis of steady state and non-steady state corneal permeation of diclofenac. RSC Adv. 2016, 6, 31976–31987. 10.1039/c6ra03604j. [DOI] [Google Scholar]
- Kaity S.; Ghosh A. Facile preparation of acrylamide grafted locust bean gum-poly (vinyl alcohol) interpenetrating polymer network microspheres for controlled oral drug delivery. J. Drug Delivery Sci. Technol. 2016, 33, 1–12. 10.1016/j.jddst.2016.02.005. [DOI] [Google Scholar]
- Ghosal K.; Rajabalaya R.; Maiti A. K.; Chowdhury B.; Nanda A. Evaluation of physicochemical properties and in-vitro release profile of glipizide-matrix patch. Braz. J. Pharm. Sci. 2010, 46, 213–218. 10.1590/s1984-82502010000200007. [DOI] [Google Scholar]
- Ghosal K.; Ray S. D. Alginate/hydrophobic HPMC (60M) particulate systems: new matrix for site-specific and controlled drug delivery. Braz. J. Pharm. Sci. 2011, 47, 833–844. 10.1590/s1984-82502011000400021. [DOI] [Google Scholar]
- Sankalia M. G.; Mashru R. C.; Sankalia J. M.; Sutariya V. B. Papain entrapment in alginate beads for stability improvement and site-specific delivery: physicochemical characterization and factorial optimization using neural network modeling. AAPS PharmSciTech 2005, 6, E209–E222. 10.1208/pt060231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray R.; Maity S.; Mandal S.; Chatterjee T. K.; Sa B. Studies on the release of ibuprofen from Al3+ ion cross-linked homopolymeric and interpenetrating network hydrogel beads of carboxymethyl xanthan and sodium alginate. Adv. Polym. Technol. 2011, 30, 1–11. 10.1002/adv.20199. [DOI] [Google Scholar]
- Pescosolido L.; Vermonden T.; Malda J.; et al. In situ forming IPN hydrogels of calcium alginate and dextran-HEMA for biomedical applications. Acta Biomater. 2011, 7, 1627–1633. 10.1016/j.actbio.2010.11.040. [DOI] [PubMed] [Google Scholar]
- Lohani A.; Singh G.; Bhattacharya S. S.; Verma A. Interpenetrating polymer networks as innovative drug delivery systems. J. Drug Delivery 2014, 2014, 583612. 10.1155/2014/583612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ei-Arini S. K.; Leuenberger H. Modeling of drug release from polymer matrices: effect of drug loading. Int. J. Pharm. 1995, 121, 141–148. 10.1016/0378-5173(94)00418-5. [DOI] [Google Scholar]
- Costa P.; Lobo J. M. S. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. 10.1016/s0928-0987(01)00095-1. [DOI] [PubMed] [Google Scholar]
- Ghosal K.; Chandra A.; Rajabalaya R.; Chakraborty S.; Nanda A. Mathematical modeling of drug release profiles for modified hydrophobic HPMC based gels. Pharmazie 2012, 67, 147–155. [PubMed] [Google Scholar]
- Silva B. M. A.; Borges A. F.; Silva C.; Coelho J. F. J.; Simões S. Mucoadhesive oral films: the potential for unmet needs. Int. J. Pharm. 2015, 494, 537–551. 10.1016/j.ijpharm.2015.08.038. [DOI] [PubMed] [Google Scholar]
- Gombotz W.; Wee S. Protein release from alginate matrices. Adv. Drug Delivery Rev. 1998, 31, 267–285. 10.1016/s0169-409x(97)00124-5. [DOI] [PubMed] [Google Scholar]
- Gåserød O.; Jolliffe I. G.; Hampson F. C.; Dettmar P. W.; Skjåk-Bræk G. The enhancement of the bioadhesive properties of calcium alginate gel beads by coating with chitosan. Int. J. Pharm. 1998, 175, 237–246. 10.1016/s0378-5173(98)00277-4. [DOI] [Google Scholar]
- Lanzerstorfer C. Dusts from dry off-gas cleaning: comparison of flowability determined by angle of repose and with shear cells. Granular Matter 2017, 19, 58. 10.1007/s10035-017-0745-2. [DOI] [Google Scholar]
- Keely S.; Rullay A.; Wilson C.; Carmichael A.; Carrington S.; Corfield A.; Haddleton D. M.; Brayden D. J. In vitro and ex vivo intestinal tissue models to measure mucoadhesion of poly(methacrylate) and N-trimethylated chitosan polymer. Pharm. Res. 2005, 22, 38–49. 10.1007/s11095-004-9007-1. [DOI] [PubMed] [Google Scholar]
- Giri T. K.; Choudhary C.; Alexander A.; Ajazuddin; Badwaik H.; Tripathy M.; Tripathi D. K. Sustained release of diltiazem hydrochloride from cross-linked biodegradable IPN hydrogel beads of pectin and modified xanthan gum. Indian J. Pharm. Sci. 2013, 75, 619–627. [PMC free article] [PubMed] [Google Scholar]
- Nayak A. K.; Pal D.; Santra K. Development of calcium pectinate-tamarind seed polysaccharide mucoadhesive beads containing metformin HCl. Carbohydr. Polym. 2014, 101, 220–230. 10.1016/j.carbpol.2013.09.024. [DOI] [PubMed] [Google Scholar]
- Sahoo S. K.; Sahoo S. K.; Behera A.; Patil S. V.; Panda S. K. Formulation, in vitro drug release study and anticancer activity of 5-fluorouracil loaded gellan gum microbeads. Acta Pol. Pharm. 2013, 70, 123–127. [PubMed] [Google Scholar]
- Hua S.; Ma H.; Li X.; Yang H.; Wang A. pH-sensitive sodium alginate/poly(vinyl alcohol) hydrogel beads prepared by combined Ca2+ crosslinking and freeze-thawing cycles for controlled release of diclofenac sodium. Int. J. Biol. Macromol. 2010, 46, 517–523. 10.1016/j.ijbiomac.2010.03.004. [DOI] [PubMed] [Google Scholar]