ABSTRACT
Introduction: Coronavirus outbreak 2019 (COVID-19) has affected all the corners of the globe and created chaos to human life. In order to put some control on the pandemic, vaccines are urgently required that are safe, cost effective, easy to produce, and most importantly induce appropriate immune responses and protection against viral infection. DNA vaccines possess all these features and are promising candidates for providing protection against SARS-CoV-2.
Area covered: Current understanding and advances in DNA vaccines toward COVID-19, especially those under various stages of clinical trials.
Expert opinion: Through DNA vaccines, host cells are momentarily transformed into factories that produce proteins of the SARS-CoV-2. The host immune system detects these proteins to develop antibodies that neutralize and prevent the infection. This vaccine platform has additional benefits compared to traditional vaccination strategies like strong cellular immune response, higher safety margin, a simple production process as per cGMP norms, lack of any infectious agent, and a robust platform for large-scale production.
KEYWORDS: SARS-CoV-2, COVID-19, vaccine, immunization, pandemic, DNA vaccine, genetic vaccine, nucleotide vaccines
1. Introduction – background of COVID-19
COVID-19 is a new infectious disease that is impacting the globe. The SARS-CoV-2 is responsible for such devastating conditions globally [1,2]. It is an enveloped, non-segmented, positive sense RNA virus which belongs to the subgenus of sarbecovirus, orthocorona virinae sub-family, and is present in humans and other mammals. Its size is 63–125 nm in diameter, encompassing single strands of RNA (Figure 1) [3]. SARS-CoV-2 contains four structural proteins, spike S1 and S2 subunits (S), envelope protein, membrane protein, and nucleocapsid protein, as well as sixteen non-structural proteins (Nsp). Notably, Nsp1 is involved in RNA production and replication. Nsp2 on the other hand involves in the host cell’s survival signaling process and Nsp3 ensures separation of the translated protein [4].
Figure 1.
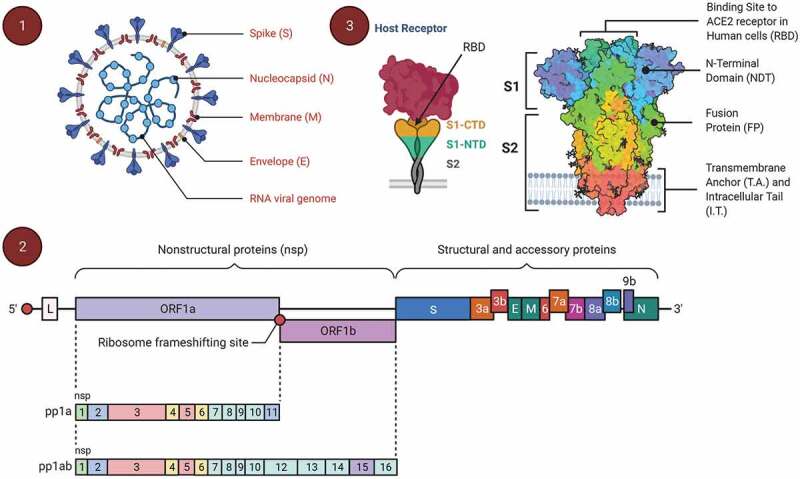
The genomic organization of SARS-CoV-2 and spike protein structure. (1) The structure of SARS-CoV-2 containing an RNA as genetic material with four distinguished structural proteins like spike glycoprotein (S), membrane protein (M), envelop protein (E), and nucleocapsid (N), (2) the genomic organization of the SARS-CoV-2 with 16 Nsps along with the structural and the accessory proteins gene (from 5ʹ end to 3ʹ end), and (3) spike glycoprotein structure with two subunits S1 and S2 that are targeted by the human enzyme Furin, and it may also cause the development of a syncytium (cell fusion) where S1 contains a receptor binding domain (RBD); S1 has a C-terminal domain (CTD) and an N-terminal domain (NTD)
The S1 subunit of the viral spike glycoprotein is having a receptor binding domain (RBD). Initially an RBD binds with the target cell’s receptor which is then transferred into endosomes [5,6]. The RNA-dependent RNA polymerase (Nsp12) and helicase (Nsp13) accelerate SARS-CoV-2 genome expansion and mRNA transcription upon entering the host cell [7,8]. At endoplasmic reticulum (ER)-golgi pathway transcription and translation process of viral mRNA lead to the formation of viral structural proteins that are essential for viral assembly formation along with other non-structural proteins and enzymes and once viral assembly is engineered the virus exits the cell through exocytosis [9,10] going on to infect other host cells. As the virus is foreign to the host, antigen presenting cells (dendritic cells, macrophages) present viral proteins to CD4 + T-helper (Th) cells via the major histocompatibility complex (MHC) class 1, resulting in the secretion of interleukin-(IL)-12 and interferon-γ to further trigger Th1 and natural killer cells [11,12]. In addition there is activation of the NF-kB signaling pathway resulting in pro-inflammatory cytokines (IL-1β, IL-6, TNF-α, IL-17, IL-8) [13,14] which aid to attract neutrophils and monocytes which stimulate other pro-inflammatory cytokines and chemokines including monocyte chemoattractant protein-1 (MCP-1) [15,16]. Concurrently, IgM antibodies are generated lasting up to 12 weeks followed by IgG antibodies providing long lasting protection as a result of viral invasion [17]. Further, sensitization to the virus promotes the stimulation of CD8+ memory cells which are long term [18,19]. As such, a vast amount of research has been focussed in the last year for COVID-19 vaccines development to stimulate primarily antibody responses, but also T cell responses.
2. COVID-19 vaccines – A global picture
The effect of vaccination on human wellbeing is one of the brightest chapters in the history of medicine. Although the concept of vaccinations dates back thousands of years, the first formal vaccine was developed over 300 years ago. Vaccines include either damaged, live, or destroyed microorganisms or virus, as well as proteins or toxins from the organism. They help escape illness from infectious diseases by boosting innate and adaptive immune systems [20]. Vaccination was a widely used term instead of the immunization because it was first extracted from a virus that afflicted cows. Edward Jenner developed the smallpox vaccine in 1796 [21] and through Louis Pasteur’s work in microbiology, advanced the idea [22].
Vaccine fundamental research was kick-started after 1950s. New methods for generating viruses resulted in rapid findings and breakthroughs vaccinations [23]. Critical pediatric infections were the focus, such as measles, mumps, and rubella, and vaccinations for these have greatly reduced burden of disease [24].
2.1. Whole inactivated (dead) vaccines
The first step is to inactivate or destroy the disease-carrying virus or bacterium, or one that is somewhat close to it, using chemicals, heat, or radiation. This technique employs technology that is effective in humans – this is how flu and polio vaccines were produced with great safety profiles [25]. By using the inactivated vaccine platform, 17 vaccine candidates against SARS-CoV-2 are in progress. The Chinese pharmaceutical company, Sinovac Research and Development Co., is conducting a phase 4 clinical trial for CoronaVac® [26]. The company has produced 200 million doses worldwide and can generate 2 billion doses each year to meet global demand (NCT04383574). Covaxin®, India’s first COVID-19 vaccine produced in-house, is a two-dose injection that uses an inactivated version of the virus. In a joint statement from Bharat biotech and co-developers, the Indian Council of Medical Research (ICMR), Covaxin® has demonstrated 100% efficacy against severe COVID-19 and hospitalization [27]. According to Phase III clinical data, Covaxin® showed 81% efficacy in preventing mild, moderate, and severe COVID-19 (NCT04918797). The serum samples from the patients who received this vaccine were able to neutralize alpha variant, gamma variant, zeta variant, kappa variant, and delta variant [28,29].
2.2. Live-attenuated vaccines
Live attenuated vaccines comprised living but a damaged form of the virus or very similar to it. This form of vaccine has been used in some traditional vaccines such as, measles mumps rubella (MMR) vaccine as well as the chickenpox and shingles vaccines [30]. Like the inactivated vaccine, this method employs similar technologies and can easily be produced in large scale. However, such vaccinations may not be appropriate for people with weakened immune systems [31]. As such, COVI-VAC, developed by Serum Institute of India in partnership with Codagenix (USA), is in phase I clinical trial (NCT04619628). Meissa Canninces, Inc. is currently conducting a phase 1 clinical trial with MV-014-212, a live attenuated vaccine for respiratory syncytial virus which expresses the S protein of SARS-CoV-2 (NCT04798001).
2.3. Recombinant viral vectors
Recombinant viral vectors comprise an unrelated transformed virus such as adenovirus, incorporating an antigen of interest. The antigen is delivered into cells in the same way that an actual virus would enter, inducing robust antigen-specific cellular and humoral immune responses on their own, eliminating the need for external adjuvants [32]. There are total of six vaccine candidates approved for emergency use based on non-replicating viral vector for vaccine delivery while 20 vaccine candidates are under different stages of clinical development using same platform [33]. Additionally, there are nine vaccine candidates under clinical development based on replicating viral vectors. Russia’s Sputnik V vaccine (also known as Gam-COVID-Vac) was the first to be approved prematurely for COVID-19 therapy and uses two different engineered adenovirus vectors rAd26 and rAd5, encoding recombinant SARS-CoV-2 S protein (NCT04530396) for first and second doses respectively. The use of two varying serotypes at an interval of 21 days overcomes already prevailing adenovirus immunity in the population [34]. AZD1222 (Covishield) is presently authorized for emergency use in many countries including Europe and India. However, severe blood clotting adverse reactions have been reported in several countries, raising safety concerns [35,36]. The serum samples from the patients who received this vaccine were able to neutralize variants of the B.1.1.7 (alpha variant), P.1- B.1.1.28 (Gamma), and B.1.617 (delta variant) lineages. Another promising candidate developed using this technology is Ad26.COV.S, a single dose vaccine developed by Janssen Pharmaceuticals showing 66% efficacy in preventing COVID-19 disease in interim phase 1/2a data [37]. It is currently under global phase 3 clinical trial (NCT04505722). The serum samples from the patients who received this vaccine were able to neutralize alpha variant, gamma variant, zeta variant, kappa variant, and delta variant [38–40].
2.4. Subunit protein vaccines
Subunit vaccines include small regions of a virus (proteins) delivered with appropriate adjuvants to induce an immune response. The majority of the vaccines on the pediatric list are subunit vaccines including, whooping cough, tetanus, and diphtheria [41]. The disadvantage of protein subunit vaccines is that they have reduced immunogenic power and so incorporation of immunostimulatory molecules or adjuvants is needed to address this problem. Also, multiple doses are required to be given for achieving long-term immunity. A number of vaccines have been developed using this technology against SARS-CoV-2, to be precise 45 vaccine candidates [33]. Notably, the Novavax vaccine is in phase 3 clinical trial which uses spike protein made using moth cells and an adjuvant Matrix-M based on a saponin extracted from the soapbark tree Quillaja Saponaria (NCT04611802). The adjuvant Matrix-M helps in achieving a higher immune response with a smaller dose of spike protein. Sanofi Pasteur in collaboration with GlaxoSmithKline have also generated a subunit vaccine, VAT00002, which is currently in clinical trial (NCT04762680).
2.5. Virus like particles
Virus like particles (VLPs) are viral structural proteins that self-assemble to resemble the conformation of native viruses, although they lack the viral genome. When compared to protein subunit vaccines, VLP vaccines present the epitope in a more native virus-like conformation, resulting in more robust immune responses [42]. VLP vaccines with firmly repeated antigenic units on its surface aid in inducing a more robust antibody response by effectively cross-linking B cell surface receptors. Medicago (Canada), in collaboration with GlaxoSmithKline, is developing an adjuvanted plant-based VLP vaccine consisting of S recombinant protein and ‘coronavirus-like-particle’ technology [43]. In addition there are five more vaccine candidates that are under clinical development based on VLP platform [33].
2.6. Nucleic acid vaccines
Nucleic acid vaccines use DNA or mRNA, which encode specific pathogenic antigens to induce an immune response. Until the emergence of the COVID-19 pandemic, no vaccine was approved for human use developed with this technology. Despite the unknown safety of this platform, two COVID-19 vaccines, mRNA-1273 and BNT162b2, have received emergency use authorization in the US [26]. Nucleic acid vaccines are simple to create since they only include a genetic code for the pathogenic antigen and a delivery method. DNA is more durable than mRNA and can therefore be processed at 4°C for a longer period of time [44]. DNA can be supplied directly to dendritic cells to stimulate T cells. Proteins that have been synthesized can also enter various tissues and specifically activate local B cells [45]. ZyCoV-D from Zydus Cadila (CTRI/2021/01/030416), Takera by Osaka University (NCT04655625), and INO-4800 from Inovio Pharmaceuticals (NCT04447781, NCT04642638 and ChiCTR2000040146) are three DNA vaccine candidates for COVID-19 under the phase 3 clinical development. There are five more DNA vaccine candidates in phase 2 development while three vaccine candidates in phase 1 clinical trial [33]. In case of mRNA vaccine, a delivery platform such as lipid nanoparticles delivers mRNA into the host cell cytoplasm for protein translation. The synthesized protein then stimulates T cell and antibody responses [46]. There are three vaccine candidates with emergency use approval (EUA) viz. TAK-919 (Moderna formulation developed by japan based company Takeda), BNT162b2; Tozinameran or Comirnaty (Pfizer/BioNTech), and Spikevax/mRNA-1273 from Moderna. A total of 24 number of candidate vaccines have been developed for COVID-19 and are under different stages of clinical development [33,47].
3. DNA vaccines
Enzo Paoletti and Dennis Panicali of the New York department of health established a way to make recombinant DNA vaccines between 1979 to 1983 by employing genetic engineering to change regular smallpox vaccination into vaccinations that may be capable of preventing other illnesses [48–50]. Consequently, plasmid DNA was noted to activate immune responses to encoded antigens when administered into the skin or muscle of mice [51,52]. Since then, there has been steady improvement in understanding the mechanism of this technology, improving its immunogenic potential [51,53] and attenuating their toxicity [54,55].
3.1. Mode of eliciting host immunity
DNA vaccines are responsible for eliciting both humoral as well as cellular immunity. It consists of delivering DNA plasmids encoding immunogenic antigens and a mammalian promoter [56]. They are administered via intramuscular, subcutaneous, mucosal, or transdermal routes (Figure 2) [57]. Myocytes and antigen-presenting cells are the primary targets of DNA vaccine [58]. Here, the plasmid encoding the antigen of interest is delivered into the host cell’s nucleus, where they undergo transcription and translation process with the help of host cell machinery (Figure 2). Once proteins and peptides are formed, they are presented via MHC class I and class II to stimulate T cells [59,60]. In addition, the unmethylated CpG sequences present in the plasmid vector stimulate the innate immune response [61] and synergize with the host’s adaptive immune response. In the case of the COVID-19, DNA vaccines encoding the S protein have been developed [62]. Following administration of a DNA vaccine via the intra-muscular route, myocytes take up the plasmid vector and activate Th1 cells, IL-2 and IFN-gamma. If delivered through a gene gun or electroporation technology into the skin, Th2 immune responses have been shown [63,64]. Using DNA vaccine technology, CD4+ Th1, Th2, CD8 + T cells and antibodies have been shown to be induced to a number of antigens [63,65,66]. Thus, DNA vaccines provide balanced Th1/Th2 responses. If more Th2 based immune response is obtained then possibility of vaccine-associated enhanced respiratory disease (VAERD) increases. The alum-adjuvanted inactivated SARS-CoV vaccine in animals has caused VAERD [67,68], though no such data is obtained when used on human beings. Thus, DNA vaccine is safe and have potential for providing effective immunity.
Figure 2.
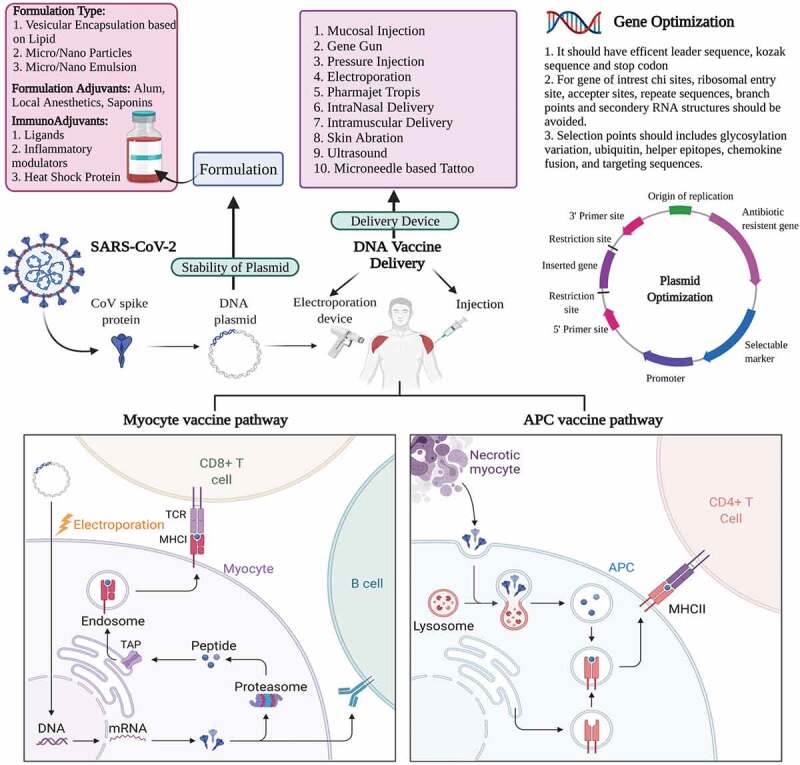
Mechanism of action of DNA based vaccines for SARS-CoV-2. To make the DNA vaccine, single-stranded RNA (ssRNA) of SARS-CoV-2 spike protein (as an example) is extracted, synthesized into double-stranded (dsDNA), and cloned into a plasmid. This plasmid is then injected intramuscularly in conjunction with an electroporation device to facilitate uptake. Within the muscles, myocytes take up the plasmid and express the protein of interest. This will lead to either CD8 + T cell activation through MHC class I or B cell activation. Antigen presenting cells like macrophages will endocytose spike proteins from necrotizing myocytes and activate CD4 + T cells through MHC class II presentation. Overall, leading to the recruitment of multiple immune subsets
3.2. DNA vaccine features
In this pandemic era, the rapid development of vaccines is a critical need. DNA vaccines that use plasmids to deliver antigen of interest are more straightforward to construct than the complex processes required in live, attenuated or recombinant vaccines [69]. Here, exposure to live pathogens is avoided as the constructs can be synthesized chemically to be considered safe and non-dangerous (Table 1). These plasmids are reasonably stable at room temperature, and there is no need for cold storage for transportation world-wide [63,70].
Table 1.
Features of DNA-based vaccines
| Feature | Comment |
|---|---|
| Make-up | Easy construction and manipulation of plasmid DNA |
| Production | Can be easily produced on large scale, less time consuming, and consistent product obtained. |
| Safety | No harmful effects associated with those of live attenuated or killed (dead) vaccines; use of toxic treatment is not required |
| Shelf- life | Long and thermostable and no requirement for cold chain storage for transportation |
| Immunogenic potential | Both antibody and T cell immune responses have been shown. |
The plasmid constructs can be made to contain the proteins of interest, with improved immunogenicity and safety profile [71]. An essential aspect for manufacturing DNA vaccines is the selection of the promoter region in the plasmid. It is mainly responsible for expressing the gene of interest. The most commonly used promoter is the mammalian CMV promoter, although other host tissue-specific promoters can also be a choice of interest. For proper completion of the transcription process, a termination site or poly (A) signal site is also incorporated, thereby releasing the formed mRNA into the cytosol [72,73]. The most commonly used termination sequences are bovine growth hormone terminator sequence or sequences that downstream from the ORF of gene of interest [59].
Formulation of DNA vaccines need to address two issues. One is protection from degradation, and the second is to increase its ability to transfect the cells in vivo [74]. Both these problems can be overcome by delivering the vaccine through the use of devices like mucosal injections, gene guns, electroporation techniques, pressure needle-free injections [75,76]. Injecting through gene guns or Pharmajet devices like Tropis and Stratis directly results in the transfection of skin or muscle cells [77]. Using the electroporation technique, pores are formed in the cells on the application of an electric field which is temporary for ease of transfection of the DNA plasmid in cells [78]. Another approach could involve the encapsulation of the vaccine formulation into nanoparticles, liposomes or virus-like particles to be taken up by dendritic cells for entry into the cytosol of the cells [79]. Plasmid DNA can be trapped on the surface of the polymers, like polylactic-co-glycolides, cationic polymers, or chitosan, and can be administered parenterally or directly to mucosal surfaces (orally or via the respiratory tract), where they are taken up by dendritic cells [80,81] and induce systemic and mucosal immunity. Liposome vehicles are used to prevent DNA degradation during its transfection across the membrane of cells [82] and have been shown to induce cellular and humoral immunity activation [83,84]. More recently, microneedle patches have been considered the better formulation option for DNA vaccines with advantages such as improved immunogenicity, elimination of needles, no need for reconstitution, simplified supply chain, ease of use, reducing the need for healthcare providers, and greater acceptability compared to traditional hypodermic injections [85,86]. Thus, in the condition of an emergency, the manufacturing of vaccines based on DNA is easy, rapid, and straightforward, provided the viral genomic sequence is known.
3.3. DNA vaccine formulation improvements
To have increased efficacy from DNA vaccines the manufacturing platform can be optimized in many ways such as, (1) plasmid optimization to improve the expression of the gene of interest, (2) gene optimization by various methods like incorporating species-specific codon, (3) supplementing the vaccine with formulation adjuvants like alum, encapsulation in liposomes, microemulsions, etc., (4) plasmid adjuvants such as cytokines and toll-receptor ligands, (5) use of novel delivery methods which can improve the transfection ability of the DNA into cells like electroporation, needle-free delivery system, etc. In regards to the route of administration, the most popular route is intramuscular, where myocytes are predominantly targeted [87]. However, targeting skin dendritic cells is more beneficial and this could be achieved via microneedles, jet injection and electroporation [88–90]. Oral and mucosal routes are also being explored to increase the efficacy of the DNA vaccines, which are currently being conducted by Symvivo and Mediphage Bioceuticals, respectively [91].
3.4. DNA vaccines for COVID-19
A number of DNA vaccine candidates in phase I, II, III clinical trials for COVID-19 are under evaluation as shown in Table 2.
Table 2.
DNA vaccines under different clinical stage of development against SARS-CoV-2
| Name of company/developer/ researcher |
Development Stage | Description of product | Delivery method* | Clinical Trial Identification No. | Start Date/Completion Date/Study location | Antigen encoded in the vaccine | Ref |
|---|---|---|---|---|---|---|---|
| Zydus Cadila Healthcare Limited, India | EUA in India | ZyCoV-D (pVAX-1 plasmid DNA vaccine vector) | Intradermal – Needle free delivery using Pharmajet Tropis@ device | CTRI/2021/01/030416 | 20 January 2021/ September-22/India | Spike protein (S) with RBD responsible for binding with angiotensin converting enzyme (ACE-2) receptor. | [33,92–95] |
| Inovio Pharmaceuticals U.S.A |
Phase II/III | INO-4800 (pGX001plasmid DNA vaccine vector) | Intradermal using Electroporation technique using CELLECTRA 2000 device | NCT04642638, ChiCTR2000040146 and NCT04447781 | 30 November 2020/ Sep-22/ U.S.A | Spike protein (S) with RBD responsible for binding with ACE-2 receptor | [33,44,96,97] |
| AnGes Inc/Osaka University/ Takara Bio, Japan | Phase II/III | AG0301 & AG0302 (pVAX-1 plasmid vaccine vector + alum adjuvant | Intramuscular delivery | NCT04655625 and NCT04527081 | 23 November 2020/ 31 March 2022/Japan | The spike glycoprotein containing the RBD, heptad repeat 1 (HR1), heptad repeat 2 (HR2), the transmembrane domain, and the cytosolic domain. | [33,98] |
| GeneOne Life Science, Korea | Phase I/II | GLS-5310 | Intradermal delivery | NCT04673149 | 23 December 2020/ 31 December 2022/Korea | Spike (S) protein and a second antigenic target (ORF3a, protein 3a) of SARS-CoV-2 | [33,98] |
| Genexine Consortium, Korea | Phase I/II | GX-19 N | Intramuscular delivery | NCT04715997 and NCT04445389 | 30 December 2020/ 30 March 2022/ Korea | S-protein antigen including the Nucleocapsid protein (NP) antigen | [33,52] |
| Takis/Rottapharm Biotech, Italy | Phase I/II | COVID-eVax | Intramuscular or Intramuscular + electroporation using Cliniporator and EPSGun | EUCTR2020-003734-20 and NCT04788459 | 25 February 2021/ Jun-2022/ Italy | Monomeric form of SARS-CoV-2 S protein RBD | [33,99] |
| OncoSec Medical Incorporated/Providence Health and Services U.S.A |
Phase I | CORVax12 (TAVO™ (plasmid IL-12) with a DNA-encodable version of the SARS-CoV-2 spike or ‘S’ glycoprotein) | Intradermal route using Cliniporator | NCT04627675 | 30 December 2020/May-2022/ U.S.A | SARS-CoV-2 spike or ‘S’ glycoprotein | [33,100] |
| Symvivo Corporation, Australia | Phase I | bacTRL-Spike | Oral route | NCT04334980 | 2 November 2020/ 28 February 2022/ Australia | Spike protein from SARS-CoV-2 | [33] |
| University of Sydney, Bionet Co., Ltd Technovalia, Australia |
Phase I | COVIGEN | Subcutaneous or intramuscular delivery using PharmaJet Stratis needle free delivery system | NCT04742842 | 15 February 2021/ 31 December 2022/ Australia | Not revealed | [33] |
| Entos Pharmaceuticals/ Cytiva, Canada | Phase I | Covigenix VAX-001 – DNA vaccines + proteo-lipid vehicle (PLV) formulation | Intramuscular route using Fusogenix delivery system | NCT04591184 | 7 April 2021/ Aug-2022/ Canada | Spike protein from SARS-CoV-2 | [33] |
| Chula Vaccine Research Center, Thailand | Pre-clinical | DNA plasmid | Electroporation | Not available | Not Available | Not revealed | [33] |
| Ege University, Turkey | Pre-clinical | DNA plasmid | - | Not available | Not Available | Not revealed | [33] |
| Globe Biotech Limited, Bangladesh | Pre-clinical | Plasmid vaccine | - | Not available | Not Available | Not revealed | [33] |
| Immunomic Therapeutics/ EpiVax/ PharmaJet, U.S.A | Pre-clinical | Plasmid vaccine | Needle-free intradermal delivery using PharmaJet Tropis@ device | Not available | Not Available | Not revealed | [33] |
| Mediphage Bioceuticals/ University of Waterloo/ Lambton College, Canada | Pre-clinical | msDNA-VLP | Ministering DNA encoding Virus like particle derived from SARS-COV-2 genome for intranasal delivery. | Not Available | Not Available | Not revealed | [33] |
| National Research Center, Egypt | Pre-clinical | plasmid vaccine S, S1, S2, RBD & N | - | Not Available | Not Available | Spike protein with RBD domain and N protein | [33] |
| National Institute of Chemistry, Slovenia | Pre-clinical | pcDNA3.1 (+) Plasmid DNA vector, nanostructured RBD | - | Not Available | Not Available | Nanostructured RBD domain | [33] |
| Karolinska Institute/Cobra Biologics (OPENCORONA Project), Sweden | Pre-clinical | DNA plasmid | Electroporation | Not Available | Not Available | Not revealed | [33] |
| Scancell/ University of Nottingham/ Nottingham Trent University, U.K. | Pre-clinical | SN14 (plasmid vaccine RBD&N) | - | Not Available | Not Available | RBD domain and N protein | [33] |
| Statens Serum Institute, Denmark | Pre-clinical | CoVAXIX plasmid vaccine | - | Not Available | Not Available | Not revealed | [33] |
| University of Cambridge/ DIOSynVax/ PharmaJet U.K. |
Pre-clinical | DIOS-CoVax2 synthetic gene inserts | Intradermal delivery using PharmaJet Tropis delivery system | Not Available | Not Available | Not revealed | [33] |
| Center of Genomics and Bioinformatics of Academy of Science of Republic of Uzbekistan | Pre-clinical | The 3 regions of SARS-Cov-2 Spike-protein: NTD, RBD and HR1-HR2 inserted into the plasmid of PcDNA3.1 (+) | - | Not Available | Not Available | NTD, RBD and HR1-HR2 regions of Spike protein | [33] |
*All these vaccines required two doses for providing sufficient immune protection
The S protein of the SARS-CoV-2 virus is responsible for entry into host cells via the ACE-2 receptor, so it is a favorable and obvious target utilized for the preparation of most vaccine candidates [101]. In the development of the DNA vaccines, the IgE signal sequence is commonly incorporated into the plasmid vector followed by insertion of the chemically synthesized S gene region. In the case of ZyCoV-D, pVAX-1 vector is used with a sequence encoding S protein from the Wuhan Hu-1 isolate (Genebank Accession No. MN908947.3). Zydus Cadila submitted its results of an adaptive phase I/II dose-escalation clinical trial in over 1,000 healthy volunteers to the Indian regulatory authorities at the end of December 2020 [95]. The vaccine was shown to be safe and effective and Zydus Cadila was the first organization to initiate a large-scale phase III clinical trial for DNA -based anti-SARS-CoV-2 vaccine in India in the month of January 2021 [94]. The study was conducted on over 28,000 volunteers across more than 50 clinical sites pan India. The results of the study indicated that it is a safe and effective vaccine especially against the new mutant strain the delta variant. In addition, the trial was successfully carried out on 1000 children in age group of 12–18 years and the plasmid DNA vaccine was found to be safe. From the interim analysis in symptomatic RT-PCR positive cases primary efficacy of about 66.6% was found of ZyCoV-D. Administration of third dose of the vaccine showed 100% efficacy for moderate disease. The ZyCoV-D vaccine will be administered using the PharmaJet device known as Tropis®. This needle-free injection system can deliver vaccine intramuscularly or subcutaneously employing a narrow, high-velocity fluid jet, which penetrates the skin [92,102,103]. Earlier it was devised for a three-dose regimen but recently they conducted another study where two doses were administered each dose of 3 mg and the immunogenicity results were found to be equivalent to the three-dose regimen. Thus, ZyCoV-D has become the first plasmid DNA vaccine to get DCGI approval for Emergency Use Authorization [93].
With regards to the immunogenicity potential of ZyCoV-D, it was demonstrated well from the preclinical studies carried out in mice, guinea pig and rabbit models by intradermal route at dose of 25, 100 and 500 microgram respectively [92]. The vaccine was able to induce production of neutralizing antibodies (NAB) as well as Th-1 response in animal models which was confirmed by the elevated levels of interferon- ϒ. The phase I clinical studies of ZyCoV-D were done between July 2020 to October 2020 on 48 healthy adults aged between 18 to 55 years in a single center, open-label, non-randomized manner [93]. As mentioned earlier it was a three-dose regimen administered at a gap of 28 days and each subject was followed up up-to 28 days post vaccination for monitoring the safety and immunogenicity. No adverse effects were reported. The study regime consists of four treatment arms where volunteers in first and third arm received the dose through 1 mg and 2 mg needle-based injection system whereas second and fourth treatment arm received dose through 1 mg and 2 mg needle free injection system (Tropis). In the study the dose administered was 0.5 ml of ZyCoV-D vaccine which contained 5 mg of DNA plasmid with spike protein gene region insert suspended in phosphate buffer saline. The seroconversion rate in volunteers was based on IgG titers found at day 84 and around 36.36%, 33.33%, 100%, and 80% proportion of the subjects were found to be seroconverted in all four treatment arms successively [93].
In the case of INO-4800 (Inovio Pharmaceuticals, US), the pGX001 vector is used with sequence encoding the S protein with an N -terminal IgE along with the eukaryotic cytomegalovirus (CMV) promoter and bovine growth hormone polyadenylation signal. The resulting plasmid was designated as pGX9501, designed to encode the SARS-CoV-2 S protein. The research was funded by the Coalition for Epidemic Preparedness (CEPI)/Gates Foundation and US Department of Defense. This vaccine is given intradermally through its proprietary device, CELLECTRA® [96,104], to stimulate local dendritic cells and induce immune responses [105]. Using this technique, short electrical pulses are given on the site of interest, which leads to a more efficacious immune response due to enhanced cell membrane permeability and better absorption of the antigen [96,97]. The have created two plasmids viz. pGX9501 and pGX9503 encoding SARS-CoV-2 IgE-spike protein for better transfection efficacy and immunogenicity which is further characterized in vitro by RT-PCR and western blotting [44]. Further, it was characterized in mice that demonstrated both B cell and T cell mediated immune response. In preclinical testing in guinea pigs neutralizing antibodies and T-cell responses were generated [44]. Anti-SARSCoV- 2 binding antibodies were detected in lung washes of INO- 4800-immunized mice and guinea pigs [96]. The outcomes of the phase I trial results were published in The Lancet’s preprint server in December 2020 and noted that INO-4800 induced good safety and tolerability and elicited immune responses in all 38 vaccinated individuals [97]. On 23 April 2021, Inovio announced that given the universal eligibility for authorized COVID-19 vaccines in the US, the Department of Defense Joint Program Executive Office for Chemical, Biological, Radiological, and Nuclear Defense had discontinued funding for the candidate’s phase III trial (NCT04642638) [106].
The third DNA vaccine in Phase III clinical trial is AG0301/AG0302 developed by AnGes, Takaro Bio, and Osaka University, and utilizes pVAX-1 as plasmid vector along with alum as an adjuvant and injected intramuscularly [107]. A phase I/II trial of the AG0301 vaccine is planned to recruit 30 healthy individuals in a non-randomized open-label non controlled study to determine its safety and immunogenicity (NCT04463472). The start date was in June 2020 with primary completion date in September 2020 and study end date 2021. The AG0302 vaccine is also recruiting 30 health individuals with 2 mg dose at either 2 or 4 week intervals, and the completion data is expected to be by September 2021 (NCT04527081) [98,108].
4. Concluding remarks
In order to eradicate the COVID-19 pandemic, there is an urgent need of an efficient vaccine against it. DNA vaccines are part of the cutting edge vaccination strategy that doesn’t require carrier and the viral protein encoding gene are transfected to in vivo cell processing to induce adaptive immunity. DNA vaccination does have a strong safety and efficacy profile in comparison to many other vaccine platforms because DNA plasmids need not replicate or elicit vector-directed immune responses in hosts. Once the DNA is inside host cells fragment are transcribed into an RNA capable of inducing protein formation. Using the viral Spike protein from SARS-CoV-2 which serves as the virus’s entrance key, to enter host cells is an obvious target for neutralizing antibody formation. Conventional vaccines could be replaced with DNA vaccines due to their potential to induce humoral and cellular immune responses. The fundamental idea behind DNA vaccines is to use a DNA plasmid that encodes for a protein from the virus. Plasmid DNA is cheap, durable, and clean, making the non-viral platform a viable alternative for gene delivery. Amongst the vaccines in clinical trials for COVID-19, there are a number of vaccines that are DNA-based. Patient with mild to moderate symptoms of SARS-CoV-2 does not result in long-lasting humoral immunity, implying that an effective COVID-19 vaccine would induce a more significant immune response than a typical infection. Also, it is an ideal platform to deal with COVID-19 where the virus is continuously mutating as the vaccine is based on plug and play technology and thus make it adaptable to deal with it. Thus, due to good clinical safety profile, low manufacturing and transportation costs, and ability to induce strong immunity, plasmid DNA-based vaccines show promise as an effective vaccine strategy against COVID-19.
5. Expert opinion – Future prospects
The plasmid DNA vaccine platform is thought to have many complementary properties that make it a suitable choice for COVID-19. The DNA plasmid manufacturing process enables modular product development and can avoid the complexities of traditional vaccine productions, cell cultures, purification and scale-ups [44]. DNA vaccines are attractive as they are usually non-frozen and are stable for a number of years at 2°C–8°C and room temperatures and about 1 year at 37°C, whilst preserving effectiveness at temperatures up to 60°C [109]. The potential of these vaccine candidates to elicit humoral and cellular immune responses makes them an appealing candidates in vaccine formulations. With continuous vaccine formulation advancements in DNA Vaccine; it is thoroughly evaluated at developmental as well as during clinical development. Many such vaccines have been approved for veterinary use. This can be a breakthrough path for the strong commercial viability of DNA vaccine for human use, defining impactful research in this area. Using this platform, a number of vaccines have quickly be developed against SARS-CoV-2 virus and translated into human clinical trials with promising outcomes. While the slow vaccination rate in industrialized economies points to the need for simpler vaccination methods, DNA-based vaccines may alleviate these limitations of conventional vaccination procedures, especially in resource-limited settings. However, DNA-based vaccines for SARS-CoV-2 are currently unavailable, owing to a lack of existing manufacturing and regulatory infrastructure required for rapid production. Continued and increased investment in emerging vaccine delivery systems, such as DNA vaccines, is required to ensure that the technologies and facilities meet potential pandemic needs. On 20 August 2021, Drug Controller General of India approved ZyCoV-D from Zydus Cadila with EUA for people 12 years and above and it is the first DNA vaccine ever approved for mankind [110].
Acknowledgments
The authors would like to acknowledge Dr. Lalit Vora (Queen’s University, Belfast, UK) for peer-reviewing the manuscript and providing valuable input to refine the manuscript. Vasso Apostolopoulos would like to thank the Greek Orthodox Archdiocese of Australia, the donors to the Victoria University vaccine appeal, the Pappas Family and the Immunology and Translational Research Group, Victoria University Australia for support and/or valuable insights.
Funding Statement
This paper was not funded.
Article highlights
DNA vaccine platform has many benefits over traditional vaccination strategies like a strong cellular immune response along with generation of neutralizing antibodies, higher safety margin, a simple production process as per cGMP norms, lack of any infectious agent, and a robust platform for large-scale production.
The short amount of time needed from design to clinical trials is a significant benefit of current advances in DNA vaccine development. As a result, this could eventually be able to test alternative antigen variants that cover ubiquitous mutations in the very same vaccination.
In human clinical trials in healthy individuals, DNA-based COVID-19 vaccines have shown better safety profile, and a strong immune response generation in the investigated time frames.
This manuscript covers information related to all DNA vaccines for COVID-19 including EUA vaccine from Zydus.
Declaration of interest
The author(s) have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Author contributions
Vivek P Chavda conceptualized the manuscript. Radhika Pandya and Vivek P Chavda wrote the manuscript, Vasso Apostolopoulos revised the manuscript. All authors contributed equally and reviewed the final version of the manuscript. The figures of the manuscript were prepared using BioRender.com; https://app.biorender.com/biorender-templates
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Huang Y, Yang C, Xu X F, et al. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol Sin. 2020;41(9):1141–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chavda VP, Vora LK, Pandya AK, et al. Intranasal vaccines for SARS-CoV-2: from challenges to potential in COVID-19 management. Drug Discov Today [Internet]. 2021; 10.1016/j.drudis.2021.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu R, Zhao X, Li J, et al., Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 395(10224): 565–574. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This review provides genomic understanding of SARS-CoV-2.
- 4.Wang M-Y, Zhao R, Gao L-J, et al. SARS-CoV-2: structure, Biology, and Structure-Based Therapeutics Development. Front Cell Infect Microbiol [Internet]. 2020;10:724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang N, Shang J, Jiang S, et al. Subunit vaccines against emerging pathogenic human coronaviruses. Front Microbiol. 2020;11:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Snijder EJ, Decroly E, Ziebuhr J.. The nonstructural proteins directing coronavirus RNA synthesis and processing. Adv Virus Res. 2016;96:59–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Du L, He Y, Zhou Y, et al. The spike protein of SARS-CoV–a target for vaccine and therapeutic development. Nat Rev Microbiol. 2009;7(3):226–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romano M, Ruggiero A, Squeglia F, et al. A Structural View of SARS-CoV-2 RNA Replication Machinery: RNA Synthesis, Proofreading and Final Capping. Cells [Internet]. 2020;9:1267. 1267: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang L, Liu S, Liu J, et al. COVID-19: immunopathogenesis and Immunotherapeutics. Signal Transduct Target Ther. 2020;5(1):128. [Internet]. ;:.Available from [DOI] [PMC free article] [PubMed] [Google Scholar]; •• It is comprehensive review to understand the COVID replication and pathogenesis process inside host.
- 10.V’kovski P, Kratzel A, Steiner S, et al. Coronavirus biology and replication: implications for SARS-CoV-2. Nat Rev Microbiol. 2021;19(3):155–170.Internet]. ;:. Available from [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar S, Nyodu R, Maurya VK, et al. Host immune response and immunobiology of human SARS-CoV-2 infection. Saxena SKeditor. Coronavirus Dis 2019 Epidemiol Pathog Diagnosis, Ther [Internet]. 2020;43–53. Available from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7189399/ [Google Scholar]
- 12.Wang Y, Wang Y, Chen Y, et al. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J Med Virol. 2020;92(6):568–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Darif D, Hammi I, Kihel A, et al. The pro-inflammatory cytokines in COVID-19 pathogenesis: what goes wrong? Microb Pathog [Internet]. 2021;153:104799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chavda VP, Apostolopoulos V. Mucormycosis – an opportunistic infection in the aged immunocompromised individual: a reason for concern in COVID-19. Maturitas [Internet]. 2021; 10.1016/j.maturitas.2021.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li G, Fan Y, Lai Y, et al. Coronavirus infections and immune responses. J Med Virol. 2020;92(4):424–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X, Geng M, Peng Y, et al. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal. 2020;10(2):102–108. Xi’an Jiaotong University. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The Promise AJ and Peril of antibody testing for COVID-19.JAMA.2020;323(19):1881–1883;[Internet]. ;:. Available from [DOI] [PubMed] [Google Scholar]
- 18.Walls AC, Park YJ, Tortorici MA, et al. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(281–292.e6):281–292.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang F, Quan Y, Xin Z-T, et al. Lack of peripheral memory B cell responses in recovered patients with severe acute respiratory syndrome: a six-year follow-up study. J Immunol. 2011;186(12):7264–7268. [DOI] [PubMed] [Google Scholar]
- 20.Plotkin S. History of vaccination. Proc Natl Acad Sci U S A. [Internet]. 2014 August 18. 2014;111:12283–12287. Available from. ;(34):. https://pubmed.ncbi.nlm.nih.gov/25136134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baxby D. Edward Jenner’s inquiry after 200 years. BMJ. 1999;318(7180):390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plotkin SA, Plotkin SL. The development of vaccines: how the past led to the future. Nat Rev Microbiol. 2011;9(12):889–893. England. [DOI] [PubMed] [Google Scholar]
- 23.Hawken J, Troy SB. Adjuvants and inactivated polio vaccine: a systematic review. Vaccine. [Internet]. 2012 October 03. 2012;30:6971–6979. Available from. ;(49):. https://pubmed.ncbi.nlm.nih.gov/23041122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grassly NC. Immunogenicity and effectiveness of routine immunization with 1 or 2 doses of inactivated poliovirus vaccine: systematic review and meta-analysis. J Infect Dis.2014;210(suppl_1):S439–S446;[Internet]. ;:. Available from [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bolles M, Deming D, Long K, et al. A double-inactivated severe acute respiratory syndrome coronavirus vaccine provides incomplete protection in mice and induces increased eosinophilic proinflammatory pulmonary response upon challenge. J Virol. 2011;85(23):12201–12215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chavda VP, Vora LK, Dr V. COVAX-19Ⓡ vaccine: completely blocks virus transmission to non-immune individuals. Clin Complement Med Pharmacol [Internet]. 2021;1:100004. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Batty CJ, Heise MT, Bachelder EM, et al. Vaccine formulations in clinical development for the prevention of severe acute respiratory syndrome coronavirus 2 infection. Adv Drug Deliv Rev. 2021;169:168–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parkins K. Covaxin: india’s homegrown Covid-19 vaccine brings hope and controversy [Internet]. 2021 [cited 2021. Jun 1]. p. Last Updated 2021 Apr 27th. Available from: https://www.clinicaltrialsarena.com/analysis/covaxin-indias-homegrown-covid-19-vaccine-brings-hope-and-controversy/.
- 29.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021;385(7):585–594.[Internet]. ;:. Available from [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minor PD. Live attenuated vaccines: historical successes and current challenges. Virology. 2015;479-480:379–392. [DOI] [PubMed] [Google Scholar]
- 31.Kaur SP, Gupta VCOVID-19. Vaccine: a comprehensive status report. Virus Res. [Internet]. 2020 August 13. 2020;288:198114. Available from. [];:. : https://pubmed.ncbi.nlm.nih.gov/32800805. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This review provide consolidated summary of the vaccine candidates for COVID-19.
- 32.Rauch S, Jasny E, Schmidt KE, et al. New vaccine technologies to combat outbreak situations. Front Immunol. 2018;9:1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGill COVID19 vaccine tracker team. VACCINES CANDIDATES BY TRIAL PHASE [Internet]. 2021 [cited 2021. Aug 21]. p. Last Updated 2021 Aug 20. Available from: https://covid19.trackvaccines.org/vaccines/.
- 34.Jones I, Sputnik RP. V COVID-19 vaccine candidate appears safe and effective. Lancet.2021;397(10275):642–643;Internet]. ;:. Available from [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hofman K, Shenoy GN, Chak V, et al. Pharmaceutical aspects and clinical evaluation of COVID-19 vaccines. Immunol Invest [Internet]. 2021;1–37. Available from. ;. 10.1080/08820139.2021.1904977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sadoff J, Le Gars M, Shukarev G, et al. Interim results of a phase 1-2a trial of Ad26.COV2.S Covid-19 vaccine. N Engl J Med. 2021;384(19):1824–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carl Zimmer, Jonathan Corum, and Sui-Lee, Wee. Coronavirus Vaccine Tracker . The New York Times. 2021. 12 October 2021 https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html.
- 39.Burki T. Understanding variants of SARS-CoV-2. Lancet.2021;397(10273):462;Internet]. ;:. Available from [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abdool Karim SS, de Oliveira T. New SARS-CoV-2 Variants — clinical, public health, and vaccine implications. N Engl J Med.2021;384(19):1866–1868;[Internet]. ;:. Available from [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chua BY, Sekiya T, Jackson DC. Opinion: making inactivated and subunit-based vaccines work. Viral Immunol. 2018;31(2):150–158. [DOI] [PubMed] [Google Scholar]
- 42.Qian C, Liu X, Xu Q, et al. Recent progress on the versatility of virus-like particles. Vaccines (Basel) [Internet]. 2020 [cited 2021. Apr 27];8:139. 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Medicago . Medicago and gsk start phase 3 trial of adjuvanted COVID-19 vaccine candidate [Internet]. March 16, 2021. 2021 [cited 2021 Apr 10]. Available from: https://www.medicago.com/en/media-room/medicago-and-gsk-start-phase-3-trial-of-adjuvanted-covid-19-vaccine-candidate/
- 44.Smith TRF, Patel A, Ramos S, et al. Immunogenicity of a DNA vaccine candidate for COVID-19. Nat Commun. 2020;11(1):2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Nmgp Q, Marinho FV, Chagas MA, et al. Vaccines for COVID-19: perspectives from nucleic acid vaccines to BCG as delivery vector system. Microbes Infect. 2020;22(10):515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pardi N, Hogan MJ, Weissman D. Recent advances in mRNA vaccine technology. Curr Opin Immunol. 2020;65:14–20. [DOI] [PubMed] [Google Scholar]
- 47.Yoo JH. What we do know and do not yet know about COVID-19 vaccines as of the beginning of the year 2021. J Korean Med Sci. [Internet]. 2021;36:e54–e54. ;(6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Panicali D, Davis SW, Weinberg RL, et al. Construction of live vaccines by using genetically engineered poxviruses: biological activity of recombinant vaccinia virus expressing influenza virus hemagglutinin. Proc Natl Acad Sci [Internet]. 1983;80:5364–5368. 17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakano E, Panicali D, Paoletti E. Molecular genetics of vaccinia virus: demonstration of marker rescue. Proc Natl Acad Sci U S A. [Internet]. 1982;79:1593–1596. (5): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Panicali D, Paoletti E. Construction of poxviruses as cloning vectors: insertion of the thymidine kinase gene from herpes simplex virus into the DNA of infectious vaccinia virus. Proc Natl Acad Sci U S A. 1982;79(16):4927–4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Son H-Y, Apostolopoulos V, Chung J-K, et al. Protective efficacy of a plasmid DNA vaccine against transgene-specific tumors by Th1 cellular immune responses after intradermal injection. Cell Immunol [Internet]. 2018;329:17–26. [DOI] [PubMed] [Google Scholar]
- 52.Seo YB, Suh YS, Ryu JI, et al. Soluble spike DNA vaccine provides long-term protective immunity against SARS-CoV-2 in mice and nonhuman primates. Vaccines (Basel). 2021;9(4):307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ferraro B, Morrow MP, Hutnick NA, et al. Clinical applications of DNA vaccines: current progress. Clin Infect Dis [Internet]. 2011;53:296–302. 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lopes A, Vandermeulen G, Cancer PV. DNA vaccines: current preclinical and clinical developments and future perspectives. J Exp Clin Cancer Res.2019;38(1):146;Internet]. ;:. Available from [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Apostolopoulos V, Weiner DB. Development of more efficient and effective DNA vaccines. Expert Rev Vaccines.2009;8(9):1133–1134;[Internet]. ;:. Available from [DOI] [PubMed] [Google Scholar]
- 56.Khan KH. DNA vaccines: roles against diseases. Germs. Internet]. 2013;3:26–35. (1): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lewis PJ, Babiuk LA. DNA vaccines: a review. Adv Virus Res. 1999;54:129–188. [DOI] [PubMed] [Google Scholar]
- 58.Braathen R, HCL S, Hinke DM, et al. A DNA vaccine that encodes an antigen-presenting cell-specific heterodimeric protein protects against cancer and influenza. Mol Ther - Methods Clin Dev [Internet]. 2020;17:378–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kutzler MA, Weiner DB. DNA vaccines: ready for prime time? Nat Rev Genet. 2008;9(10):776–788. [DOI] [PMC free article] [PubMed] [Google Scholar]; • The reference highlights the future of DNA products and the currently licensed DNA therapies available.
- 60.Løvås T-O, Bruusgaard JC, Øynebråten I. Løvås T-O, Bruusgaard JC, Øynebråten I, et al . DNA vaccines: MHC II-targeted vaccine protein produced by transfected muscle fibres induces a local inflammatory cell infiltrate in mice. SadeghNasseri S, editor. . SadeghNasseri S, editor. PLoS One [Internet]. 2014. [cited 2021 Jun 1;9:e108069. 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Williams JA, Carnes AE, Hodgson CP. Plasmid DNA vaccine vector design: impact on efficacy, safety and upstream production. Biotechnol Adv. [Internet]. 2009. 2009 February 20;27:353–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dong Y, Dai T, Wei Y, et al. OPEN A systematic review of SARS-CoV-2 vaccine candidates. Signal Transduct Target Ther. 2020;5(1). 10.1038/s41392-020-00352-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Silveira MM, Moreira GMSG, Mendonça M. DNA vaccines against COVID-19: perspectives and challenges. Life Sci [Internet]. 2020 December 19. 2021;267:118919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eschenburg G, Stermann A, Preer R, et al. DNA Vaccination: using the Patient’s Immune System to Overcome Cancer. Rezaei N, editor. Clin Dev Immunol [Internet]. 2010;2010:169484. Available from 10.1155/2010/169484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Awate S, Babiuk LA, Mutwiri G. Mechanisms of action of adjuvants. Front Immunol. [Internet]. 2013;4:114. Available from. ;:. https://pubmed.ncbi.nlm.nih.gov/23720661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wennhold K, Shimabukuro-Vornhagen A, von Bergwelt-Baildon M. B cell-based cancer immunotherapy. Transfus Med Hemother. [Internet]. 2019;46:36–46. (1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li -D-D, Q-h L. SARS-CoV-2: vaccines in the pandemic era. Mil Med Res. 2021;8(1). 10.1186/s40779-020-00296-y [Internet]. ;:. Available from [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bettini E, Locci M. SARS-CoV-2 mRNA Vaccines: immunological Mechanism and Beyond. Vaccines (Basel). [Internet]. 2021;9:147. (2): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu MA. A comparison of plasmid DNA and mRNA as vaccine technologies. Vaccines (Basel). Internet]. 2019;7:37. (2): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Prompetchara E, Ketloy C, Tharakhet K, et al. DNA vaccine candidate encoding SARS-CoV-2 spike proteins elicited potent humoral and Th1 cell-mediated immune responses in mice. PLoS One. 2021;16(3):e0248007.[Internet]. ;:. Available from [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li H, Yang Y, Hong W, et al. Applications of genome editing technology in the targeted therapy of human diseases: mechanisms, advances and prospects. Signal Transduct Target Ther. [Internet]. 2020;5:1. Available from. ;(): 10.1038/s41392-019-0089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Flingai S, Czerwonko M, Goodman J, et al. Synthetic DNA vaccines: improved vaccine potency by electroporation and co-delivered genetic adjuvants. Front Immunol [Internet]. 2013;4:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li L, Petrovsky N. Molecular mechanisms for enhanced DNA vaccine immunogenicity. Expert Rev Vaccines [Internet]. 2015 December 28. 2015;15:313–329. 3 [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Important for understanding the mechanism of working of DNA vaccine and its development.
- 74.Bolhassani A, Yazdi SR. DNA immunization as an efficient strategy for vaccination. Avicenna J Med Biotechnol [Internet]. 2009;1:71–88. Available fromhttps://pubmed.ncbi.nlm.nih.gov/23407787 [PMC free article] [PubMed] [Google Scholar]
- 75.Barolet D, Benohanian A. Current trends in needle-free jet injection: an update. Clin Cosmet Investig Dermatol [Internet]. 2018;11:231–238. Available fromhttps://pubmed.ncbi.nlm.nih.gov/29750049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ravi AD, Sadhna D, Nagpaal D, et al. Needle free injection technology: a complete insight. Int J Pharm Investig Internet]. 2015;5:192–199. 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brocato RL, Kwilas SA, Josleyn MD, et al. Small animal jet injection technique results in enhanced immunogenicity of hantavirus DNA vaccines. bioRxiv [Internet]. 2020;2020 November 09.374439. Available from. ; :. http://biorxiv.org/content/early/2020/11/09/2020.11.09.374439.abstract [DOI] [PubMed] [Google Scholar]
- 78.Sokołowska E, Au B-Z. A critical review of electroporation as a plasmid delivery system in mouse skeletal muscle. Int J Mol Sci. [Internet]. 2019;20:2776. (11): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gao Y, Wijewardhana C, Mann JFS, et al. Liposome, and polymeric particle-based vaccines against HIV-1. Front Immunol. [Internet]. 2018;9:345. Available from. ;:. https://pubmed.ncbi.nlm.nih.gov/29541072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ak G, Rath G, Garg T. Nanotechnological approaches for genetic immunization. Erdmann VA, Barciszewski J, editors. DNA RNA Nanobiotechnologies Med Diagnosis Treat Dis [Internet]. 2013;67–120. Available from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7121080/ [Google Scholar]
- 81.Farris E, Brown DM, Ramer-Tait AE, et al. Micro- and nanoparticulates for DNA vaccine delivery. Exp Biol Med (Maywood). [Internet]. 2016 April 04. 2016;241:919–929. 9: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu C, Zhang L, Zhu W, et al. Barriers and strategies of cationic liposomes for cancer gene therapy. Mol Ther Methods Clin Dev [Internet]. 2020;18:751–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang N, Chen M, Wang T. Liposomes used as a vaccine adjuvant-delivery system: from basics to clinical immunization. J Control Release [Internet]. 2019 May 03. 2019;303:130–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bozzuto G, Molinari A. Liposomes as nanomedical devices. Int J Nanomedicine. [Internet]. 2015;10:975–999. Available from. ;:. https://pubmed.ncbi.nlm.nih.gov/25678787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang F-Y, Chen Y, Huang -Y-Y, et al. Transdermal drug delivery systems for fighting common viral infectious diseases. Drug Deliv Transl Res. 2021;11(4):1498–1508.[Internet]. ; Available from [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Arya J, Prausnitz MR. Microneedle patches for vaccination in developing countries. J Control Release [Internet]. 2015 November 18. 2016;240:135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang B, Jeang J, Yang A, et al. DNA vaccine for cancer immunotherapy. Hum Vaccin Immunother [Internet]. 2014;10:3153–3164. 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hasson SSAA, JKZ A-B, Sallam TA. The past, current and future trends in DNA vaccine immunisations. Asian Pac J Trop Biomed. [Internet]. 2015;5:344–353. (5): [Google Scholar]
- 89.Hobernik D, Bros M. DNA vaccines-how far from clinical use? Int J Mol Sci. [Internet]. 2018;19:3605. (11): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu MA. DNA vaccines: an historical perspective and view to the future. Immunol Rev. 2011;239(1):62–84. [DOI] [PubMed] [Google Scholar]; •• It highlights the history and development of DNA vaccine with focus on its future prospects especially for emerging pandemics.
- 91.Vela Ramirez JE, Sharpe LA, Peppas NA. Current state and challenges in developing oral vaccines. Adv Drug Deliv Rev [Internet]. 2017 April 22. 2017;114:116–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dey A, Chozhavel Rajanathan TM, Chandra H, et al. Immunogenic potential of DNA vaccine candidate, ZyCoV-D against SARS-CoV-2 in animal models. bioRxiv [Internet]. 2021;2021 January 26.428240. Available from http://biorxiv.org/content/early/2021/01/26/2021.01.26.428240.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]; • It highlights the studies conducted on animal models and the results obtained of ZyCov-D DNA vaccine. Focus on pre-clinical aspect of development.
- 93.Momin T, Kansagra K, Patel H, et al. Safety and immunogenicity of a DNA SARS-CoV-2 vaccine (ZyCoV-D): results of an open-label, non-randomized phase I part of phase I/II clinical study by intradermal route in healthy subjects in India. EClinicalMedicine [Internet]. 2021;38. 10.1016/j.eclinm.2021.101020. [DOI] [PMC free article] [PubMed] [Google Scholar]; • It highlights the studies conducted on animal models and the results obtained of ZyCov-D DNA vaccine. Focus on pre-clinical aspect of development.
- 94.Cadila Healthcare Ltd . Zydus Cadila receives approvals from the DCGI to start Phase III Clinical Trial of ZyCoV-D – fully indigenously developed vaccine [Internet]. 2021. [cited 2021 Jun 1]. 03 January, 2021. Available from: https://zyduscadila.com/public/pdf/pressrelease/Zydus_Cadila_receives_approvals_from_the_DCGI_to_start_Phase_III_Clinical_Trial_of_ZyCoV_D_fully_indigenously_developed_vaccine_-3_1_2021.pdf.
- 95.Cadila Healthcare Ltd . Zydus Cadila submits Phase I/II clinical trial data of ZyCoV-D, seeks nod to start Phase III Clinical Trials [Internet]. 2020. [cited 2021 Jun 1].December 24, 2020. Available from: https://www.google.com/search?q=https%3A%2F%2Fzyduscadila.com%2Fpublic%2Fpdf%2Fpressrelease%2FZydus_Cadila_submits_Phase_II_clinical_trial_data_of_ZyCoV_D_seeks_nod_to_start_Phase_III_Clinical_Trials_24_12_2020.pdf+&rlz=1C1CHBD_enIN909IN909&sxsrf=ALeKk03R.
- 96.Mammen MP, Tebas P, Agnes J, et al. Safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV-2: a preliminary report of a randomized, blinded, placebo-controlled, Phase 2 clinical trial in adults at high risk of viral exposure. medRxiv [Internet]. 2021;2021 May 07.21256652. Available from. ; :. http://medrxiv.org/content/early/2021/05/07/2021.05.07.21256652.abstract [Google Scholar]
- 97.Tebas P, Yang SP, Boyer JD, et al. Safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV-2: a preliminary report of an open-label, Phase 1 clinical trial. EClinicalMedicine. 2021;31:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Staff R. Japan’s AnGes begins phase 2/3 clinical trial of DNA-based COVID-19 vaccine [Internet]. Healthc. PHARMA. 2021. [cited 2021 Jun 1]. Available from: https://www.reuters.com/article/us-anges-covid-vaccine-idUSKBN28I0EA.
- 99.Conforti A, Marra E, Palombo F, et al. COVID-<em>e</em>Vax, an electroporated plasmid DNA vaccine candidate encoding the SARS-CoV-2 Receptor Binding Domain, elicits protective immune responses in animal models of COVID-19. bioRxiv [Internet]. 2021;2021 June 14.448343. Available from. ; :. http://biorxiv.org/content/early/2021/06/14/2021.06.14.448343.abstract [Google Scholar]
- 100.Algazi AP, Twitty CG, Tsai KK, et al. Phase II trial of IL-12 plasmid transfection and PD-1 blockade in immunologically quiescent melanoma. Clin Cancer Res an off J Am Assoc Cancer Res. 2020;26(12):2827–2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Samrat SK, Tharappel AM, Li Z, et al. Prospect of SARS-CoV-2 spike protein: potential role in vaccine and therapeutic development. Virus Res Internet]. 2020 August 23. 2020;288:198141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Motamedi H, Ari MM, Dashtbin S, et al. An update review of globally reported SARS-CoV-2 vaccines in preclinical and clinical stages. Int Immunopharmacol [Internet]. 2021 May 06. 2021;96:107763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pushparajah D, Jimenez S, Wong S, et al. Advances in gene-based vaccine platforms to address the COVID-19 pandemic. Adv Drug Deliv Rev [Internet]. 2021 January 07. 2021;170:113–141. Available from. ; :. https://pubmed.ncbi.nlm.nih.gov/33422546 [DOI] [PMC free article] [PubMed] [Google Scholar]; • Important for information related to all types of COVID 19 vaccines currently in development/clinical trial process
- 104.Sardesai NY, Weiner DB. Electroporation delivery of DNA vaccines: prospects for success. Curr Opin Immunol. [Internet]. 2011 April 27. 2011;23:421–429. Available from. ;(3):. https://pubmed.ncbi.nlm.nih.gov/21530212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Young JL, Dean DA. Electroporation-mediated gene delivery. Adv Genet. Internet]. 2014 December 11. 2015;89:49–88. Available from; :. :https://pubmed.ncbi.nlm.nih.gov/25620008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Richardson J. INOVIO planning for ex-US global phase 3 trial for INO-4800 [Internet]. 2021 [cited 2021. Jun 1]. p. Updated on 2021 Apr 23. Available from: https://ir.inovio.com/news-releases/news-releases-details/2021/INOVIO-Planning-for-ex-US-Global-Phase-3-Trial-for-INO-4800/default.aspx.
- 107.Rawat K, Kumari P, Saha L. COVID-19 vaccine: a recent update in pipeline vaccines, their design and development strategies. Eur J Pharmacol. 2021;892:173751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Carlson R, Reiter D, Lutmer H. AG0301 COVID-19 vaccine [Internet]. precisionvaccinations. 2021 cited 2021 Jun 1. p. Updated April 22, 2021. Available from https://www.precisionvaccinations.com/vaccines/ag0301-covid-19-vaccine
- 109.Howlett SE, Castillo HS, Gioeni LJ, et al. Evaluation of DNAstable for DNA storage at ambient temperature. Forensic Sci Int Genet. 2014;8(1):170–178. [DOI] [PubMed] [Google Scholar]
- 110.Singh S. India gives emergency approval for world’s first COVID-19 DNA vaccine [Internet]. Ed. by Shounak Dasgupta Maju Samuel. 2021 [cited 2021 Aug 21]. p. August 20, 2021. Available from: https://www.reuters.com/business/healthcare-pharmaceuticals/india-approves-zydus-cadilas-covid-19-vaccine-emergency-use-2021-08-20/. ears safe and effectiveAvailable from: 10.1016/j.eclinm.2021.101020. [DOI]; • It highlights the studies conducted on animal models and the results obtained of ZyCov-D DNA vaccine. Focus on pre-clinical aspect of development.


