Highlights
-
•
The contribution of asphaltene aggregation to bitumen viscosity is studied.
-
•
The ultrasound waves reduce the viscosity of the bitumen.
-
•
The applied frequency and the gas environment influence the bitumen viscosity reduction.
-
•
A positive correlation between the asphaltene yield and the viscosity is established.
Keywords: Asphaltene, Bitumen viscosity, Ultrasound, Sonication, Bitumen
Abstract
The present work investigates the contribution of asphaltene aggregation to bitumen viscosity subject to ultrasound irradiation. A West-African bitumen with a viscosity of 12043 cP at room temperature was sonicated at low (38 kHz) and mild frequency (200 kHz) under controlled gas environment including air, nitrogen (N2) and carbon dioxide (CO2). The rheology of the bitumen, asphaltene content analyses as well as spectral studies were conducted. Herein was found that sonicating the bitumen at 200 kHz under air-environment reduces the initial viscosity up to 2079 cP, which was twice larger than that obtained when a low frequency was used. In respect of the gas environment, it was shown that ultrasound irradiation under N2 environment could lower the bitumen viscosity up to 3274 cP. A positive correlation between the asphaltene content and the viscosity reduction was established. The results from the spectral analyses including Fast Fourier Infrared and the observations from Scanned Electron Microscope were consistent with the rheological studies and led to the argument that the viscosity reduction results from either the scission of long chain molecules attached to the aromatic rings (when the applied frequency was altered under fixed gas environment) or the self-aggregation of asphaltene monomers (when gas environment was changed at fixed frequency).
1. Introduction
Bitumen has raised a considerable interest because of its potential to compensate the depletion of conventional resources in addition to the large reserves (∼5,505 billion of barrels) reported to sit within the reach of existing fields [1]. Bitumen is an asphaltic, dense, and viscous hydrocarbon in its natural state. These attributes dictate its extraction, transportation, and to a certain extent its processing at the surface facilities [2], [3]. Thermal methods are the most advanced and understood recovery techniques as far as bitumen recovery is concerned [4].
In a classical thermal approach, the heat is supplied to the oil-bearing matrix, causes the oil to vaporize and facilitates thereby the oil flow. Unfortunately, thermal recovery methods are not only energy-demanding but also, they are sensitive to the formation heterogeneity. More importantly, these methods are challenged by the poor sweeping efficiency of injected fluid [5]. Alternative approaches have been extensively investigated as part of efforts to propose cost-effective methods [6]. These include dilution of bitumen with light compounds [7], bitumen heating [8], formation of oil-in-water emulsions [9], [10], [11], drag reduction [12] or even viscosity reduction via annular flow through pipelines [13] and more recently the electromagnetic waves[14], [15].
In respect of the potential of electromagnetic waves, the literature reports that microwaves [16], gamma irradiation [17], as well as ultrasound treatment [18], [19] have raised considerable interest; with the latter being the scope of this paper. Ultrasound waves are emitted at a frequency equal to (or greater than) 16 kHz. The fundamental mechanism of the ultrasound waves involves the stretching of the molecular spacing of fluid through which the propagation occurs [20]. The oscillations within the fluid are thus subsequent to a compression and rarefaction phenomena from which micro-bubbles are formed [21].
These microbubbles implode further violently during the compression stage releasing thereby a considerable amount of energy. The literature reports that the collapse of microbubbles creates the micro-voids, high local pressures as high as1000 atm, and high transitory temperatures that may reach up to 5000 K [22], [23]. Both the chemical and physical effects of ultrasound waves have been well exploited in chemistry (sonochemistry) as the chemical effects have the ability to generate free chemical radicals [24], while the physical effects can enhance the reactivity of a catalyst by enlarging its surface area or even by increasing a reaction rate [25].
Meribout (2018) reviewed that enhancing the oil production using ultrasonic treatment (1) was much cheaper than conventional recovery methods, (2) was less-energy demanding, and (3) allows a better control of the ultrasonic wave propagation. As per the same author, the primary challenge is the relatively limited range of ultrasonic radiation (up to 2 m) at the operational resonance frequency, which usually ranges from 20 to 40 kHz [26]. This is to say that this method still suffer from deeper understanding to make meaningful and consistent scientific elaborations despite the wealth of papers covering the topic [27], [28], [29], [30], [31], [32], [33].
To probe the mechanisms of oil viscosity reduction, one has to look at the molecular structure and/or the composition of the propagating medium, therefore [34], [35]. Montes et al. (2018), and later Cui et al. (2020), showed that combining the ultrasound irradiation with addition of nanoparticle could disrupt the viscoelasticity of asphaltene, which leads to a decrease in viscosity [36], [37]. Mansouri et al. (2021) combined ultrasonic waves with different frequencies and magnetic nanoparticles. They argued that the viscosity of heavy crude oil could be reduced from 87.2 to 65.7 cSt after sonicating the oil for 10 min at 20 °C, which was concomitant with a decrease in asphaltene concentration.
It appears from the literature that the viscosity reduction pertains to (1) asphaltene re-dissolution [18], [38], (2) conversion of asphaltene molecules by dehydrogenation [27], or (3) conversion of asphaltenes to lighter aromatics [39], [40]. At any rate, controlling the solubility of asphaltene under ultrasound irradiation, could provide significant insights on the reduction of bitumen viscosity. However, most of the reported works have emphasized either on the applied frequency or irradiation time (up to 2 h) or even the addition of foreign materials. The available literature seemingly disregards the influence of the environment under which the irradiation takes place.
This is particularly relevant if one recalls from a chemistry point of view that asphaltene monomers self-aggregate as a result of a decrease in their solubility within the dispersed medium, which itself depends strongly on the environment [41]. Thus, the present work investigates the contribution of asphaltene on viscosity reduction of bitumen. The further objective is to establish the extent to which both applied frequency as well as the gas environment impact the formation of asphaltenes and subsequently alter the viscosity.
To achieve this purpose, the viscosity of the bitumen was measured at different applied ultrasonic frequencies and gas-environments. The contribution of asphaltenes were then investigated and the mechanisms pertaining to viscosity reduction are equally discussed.
2. Material and methods
2.1. Material
The bitumen sample, used in the present work, was sampled from the surface equipment in an oilfield located in the Gulf of Guinea. The properties of the candidate bitumen are outlined in Table 1.
Table 1.
Physico-chemical properties of the investigated bitumen.
| Physical Properties | API gravity (o) | 7.700 |
|---|---|---|
| Specific gravity (-) | 1.017 | |
| Chemical Properties | Total Acid number (mg KOH/g-oil) | 6.587 |
| Total Base Number (mg HCl/g-oil) | 0.435 | |
| Mercaptan Content (wt.%) | 5.742 | |
| Asphaltene Content (wt.%) | 85.04 |
2.2. Methods
2.2.1. Sonication of the candidate bitumen
The ultrasound irradiation of bitumen was performed in two different apparatuses, whose schematics are depicted in Fig. 1.
Fig. 1.
Schematic representation of ultra-sonication equipment used for bitumen irradiation; (1) submersible traducer; (2) bitumen sample; (3) thermostatic water bath; (4) thermocouple; (5) gas flowmeter; (6) gas holding tank.
A low (38 kHz) and a mild frequency (200 kHz) were considered. When the sonication was performed either at 28 or 200 kHz, the ultrasound waves were generated by an ultrasonic generator (Kaijo TA-4021) and the submersible traducer ensured the sonication (Fig. 1a). On the other hand, an Ultrasonic Cleaner (C 100D, Kaijo, Japan) was used when the sonication of the bitumen was performed at 38 kHz (Fig. 1b).
The output power for 38 and 200 kHz were computed to be19.3, and 25.2 W, respectively. These output powers were determined by calorimetric method, whose procedure is detailed elsewhere [42]. Five grams of oil were weighed, and loaded into a flat bottom flask, which was later immersed into a water bath (25 °C). The flat bottom flask was placed 3 cm above the submersible traducer; optimal distance at which the cavitation for the equipment used is maximal [24].
The bitumen was then allowed to reach the equilibrium temperature. The sonication environment was generated by flushing the gas of interest in the reactor cell through an 0.4-μm glass tube at rate of 0.2 ml.min−1 for a minimum of 15 min prior sonication. Three gases were considered including air, nitrogen (N2, 99.9% pure), and carbon dioxide (CO2, 99.9% pure). To investigate the influence of the gas environment, the applied frequency was fixed at 38 kHz. On the other hand, the effect of the applied frequency was evaluated under air environment.
In either case, ice flocs were immersed into the water bath to mitigate the increase in temperature inherent to ultra-sound irradiation. The ultrasound irradiation was performed continuously for 1, 2, and 3 h. Fig. 2 shows sample photographs of sonicated bitumen using a 200 kHz frequency under air environment.
Fig. 2.
Photographs of (a) fresh bitumen and sonicated bitumen after (b) 2 h and (c) 3hr; no efforts were performed to trap the light ends of the oil.
At the end of each respective sonication time, the rheological properties of the oil were measured as per the procedure described in the following section.
2.2.2. Rheological studies
At room temperature and before sonication, the sample appears solid (Fig. 3a).
Fig. 3.

Sample photographs of BT-1 exposed for 3 h at different temperatures.
The oil flow could only be initiated when the bitumen was heated at temperature equal or greater than 70 °C (Fig. 3b, c). For these reasons, viscosity measurements of the bitumen and those of sonicated bitumen were performed at 70 °C using a Brookfield programmable rheometer (Model DV-III) over the shear rate in the range from 4 to 30 sec-1.
2.2.3. Asphaltene extraction
To extract the asphaltenes from the bitumen (or the sonicated bitumen), 1 g of oil was accurately weighed and transferred to a 100 ml Erlenmeyer flask. The oil was then diluted with toluene at a volume ratio of 1:10, oil:toluene. The mixture was stirred at 1500 rpm using a magnetic stirrer (D Lab, MS H280-pro) for 2 h and the resulting solution was then filtered through a 0.45 µm filter paper.
One volume of the filtrate was added to 20 volumes of pentane, followed by storage overnight at atmospheric conditions. The precipitates were separated from the supernatant fluid by vacuum filtration using a pre-weighed filter paper (0.45 µm). The filtered solids (asphaltenes) were then dried at ambient conditions; after this step, no further purification of the precipitated asphaltenes was performed.
The concentration in asphaltenes was computed as the quotient of the difference of the filter paper after and before the filtration to the initial mass of oil. Both toluene and pentane were purchased from Junsei (Japan) and were used as received.
2.2.4. Spectral analyses
The spectral analyses used in this study included Fourier Transform Infrared Spectra (FT/IR) and Scanning Electron Microscopy (SEM). The former was performed on fresh and sonicated bitumen, while the latter was used for asphaltenes extracted from oils.
Fourier Transform Infrared Spectra (FTIR) of the fresh bitumen and those of the sonicated bitumen were acquired using a Jasco spectrophotometer (Model 4100, Japan). All the absorption spectra were acquired from 400 to 4000 cm−1 with 48 scans and a resolution of 1 cm−1. A baseline correction was performed prior to the analysis. It is worth mentioning that no sample preparation was needed and the FTIR spectra were obtained right after ultrasound treatment.
The surface morphology of the asphaltenes extracted from fresh and sonicated bitumen was investigated using a low vacuum high sensitivity scanning electron microscope (Model SU 3500). The extracted asphaltenes, with no further purification, were inserted in the vacuum chamber from where SEM photographs were taken by a low vacuum observation.
3. Results and discussion
3.1. Rheological properties of fresh and sonicated bitumen
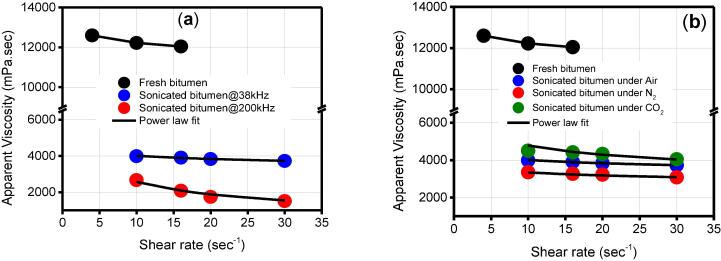
Fig. 4 shows the apparent viscosity of bitumen before and after sonication under air environment.
Fig. 4.
Apparent viscosity of the bitumen before and after sonication under at 70 °C under air environment.
It was seen that the apparent viscosity decreases over the range of investigated shear rates. Also, it was found that an extended sonication process (or period) led to a bitumen with a lower viscosity. These obvious observations were consistent with the literature, which reports that the ultrasound irradiation releases heat that dissipates into the oil leading invariably to a less viscous bitumen [21], [36], [37].
To appreciate the influence of the sonication time on the viscoelastic behavior of the oil, one must apply the empirical models that describe the behavior of a time-dependent fluid. In this study, Power law model was selected because of the shear-thinning behavior of the bitumen in addition to its prominence in oil rheology [43]. The power law model is expressed as,
| (1) |
where µ is the apparent viscosity (mPa.sec), γ is the shear rate (sec-1), K is the consistency index (mPa.sec2), n is the flow index (dimensionless) indicates the degree with which a material exhibits non-Newtonian flow behavior, the values of n close to 1 implied a Newtonian behavior and a value of n close to 0 would indicate a departure from the Newtonian behavior.
The experimental data (Fig. 3) were then fitted and the statistical parameters including the root mean square error (RMSE) and the square root (R2) were evaluated using equations (2), (3) respectively,
| (2) |
| (3) |
where N is the number of points (dimensionless). The results are shown in Table 2.
Table 2.
Parameters for rheological models used for sonicated and fresh bitumen.
| Parameters | Fresh bitumen | Sonicated bitumen | |
|---|---|---|---|
| 2 h | 3 h | ||
| K*10-2 (Pa.sec2) | 13.19 | 124.7 | 122.1 |
| n (-) | 0.967 | 0.982 | 0.922 |
| RMSE (-) | 3.040 | 15.65 | 80.64 |
| R2 (-) | 0.999 | 0.984 | 0.970 |
Under ambient conditions, Table 2 shows that a fresh bitumen behaves like a Newtonian fluid, which a n- value close to 1. These findings are consistent with the results presented by Ukwuoma and Ademodi (1999), who reported the influence of the temperature on a Nigerian oil sand Bitumen sampled from a deposit located in the same geological basin [44].
It was found that even after a prolonged sonication, the viscoelasticity of the bitumen was still fairly altered as evidenced by the values of n close to 1. This suggests at first sight that that the ultrasounds do not change the viscoelasticity of the oil. Furthermore, these preliminary observations suggested that viscosity of the bitumen even after ultrasound treatment was not low enough to be considered for an engineering application i.e., transportation. In fact, Martinez-Palou et al. (2011) reviewed that to transport bitumen, the viscosity of the oil should be lesser than 200 mPa.sec [45].
With an average bitumen viscosity equal to 10165 mPa.s after 3 h of continuous sonication at ambient conditions that is nearly 51-fold larger than the required value, it is evident that process parameters must be optimized to reach (or at least to get close to) the sought value.
In this regard, considerations were then given to both applied frequency and the gas environment. It is noteworthy to mention that the influence of either parameter will be addressed simultaneously, and the findings reported are those obtained after 3 h of continuous irradiation.
3.2. Effect of applied frequency and gas environment on bitumen viscosity reduction
The effect of applied gas and gas environment are shown in Fig. 5.
Fig. 5.
(a) Influence of applied ultra-sound frequency conducted under air environment and (b) influence of gas environment on viscosity alteration after 3 h.
The viscosity of the bitumen decreases with the applied frequency with the largest viscosity reduction observed for 200 kHz (Fig. 5a). For example, at the fixed shear rate of 20 sec- 1, the viscosity of the fresh bitumen was 9654 cP. Irradiating the same bitumen continuously for 3 h at the frequency of 38 kHz and 200 kHz gave a bitumen with an apparent viscosity of 2798 cP and 1744 cP respectively (Fig. 5a). Similar results were reported by Mohapatra and Kirpalani (2016), who argued that sonicating a Canadian Bitumen at a frequency as high as 378 kHz could lower the viscosity of the oil up to 15% from its original value [28].
The influence of the gas environment was also noticeable (Fig. 5b). On the average, the viscosity was threefold lower than that of the fresh bitumen when the irradiation was performed under either gas environment. However, it was found that the bitumen with the lowest viscosity was obtained when the irradiation was performed under N2 environment. Comparing the viscosity reduction obtained after altering either the applied frequency (Fig. 5a) or the gas environment (Fig. 5b), it appears that the former parameter is the conspicuous parameter. For instance, the bitumen sonicated with an applied frequency of 200 kHz gives a viscosity, on average, twice lower than those obtained when the gas environment was altered.
Table 3 summarizes the rheological parameters under different applied frequencies and gas-environment.
Table 3.
Parameters of rheological model for fresh and sonicated bitumen at different frequencies and gas environment.
| Parameters | Fresh bitumen | Applied frequency | Gas-environment | |||
|---|---|---|---|---|---|---|
| 38 kHz | 200 kHz | Air | N2 | CO2 | ||
| K*10-2 (Pa.sec2) | 13.19 | 46.56 | 75.06 | 46.56 | 39.3 | 68.27 |
| n (-) | 0.967 | 0.935 | 0.535 | 0.935 | 0.929 | 0.845 |
| RMSE (-) | 3.040 | 12.6 | 76.89 | 12.6 | 37.58 | 25.52 |
| R2 (-) | 0.999 | 0.991 | 0.967 | 0.991 | 0.999 | 0.999 |
Table 3 suggests that simulating the flow by altering either the frequency or the gas environment, the bitumen would flow easily. Also, as the applied frequency increases, it could be seen that the bitumen departs from the Newtonian behavior. This trend, however, was less noticeable when the gas environment was altered. For all the cases, bitumen behaved like a Newtonian fluid.
Considering the sonication process as the conversion of electrical power into vibrational energy i.e., heat and cavitation [46], these results suggested that the extent to which the viscosity is altered dependent somewhat on either heat supplied or induced cavitation, which are sensitive to operating conditions. Therefore, it was then sought to probe the extent to which former form of energy (heat) contributes to the viscosity alteration.
3.3. Probing the mechanisms of viscosity reduction
Both the temperature of bitumen and that of the water bath in which the sonication was carried were recorded to investigate the influence of the heat supplied to the oil by either the applied frequency or the gas environment. The difference between the two temperatures was then plotted against the apparent viscosity. The results are shown in Fig. 6.
Fig. 6.
Viscosity alteration as function of temperature of (a) applied ultra-sound frequency and (b) gas environment.
It could be seen that the apparent viscosity decreases exponentially with the increase in temperature. The curvature of the decay appeared less pronounced when the influence of the applied frequency was investigated (Fig. 6a), which contrasted with the influence of the gas environment (Fig. 6b). Therein, the decay approaches seemingly an equilibrium. Eyring (1936) argued that viscosity decay implies the existence of an energy, termed as activation energy of flow (Ea) [47].
Per the literature, Ea is a measure of the height of a potential energy barrier associated with the force needed to produce elemental quantum steps [48]. In this study, the flow activation energy should be then regarded as the minimum energy required by the molecules to be set in motion against the frictional forces subsequent either to a change in gas environment or applied frequency. From the viscosity theory of Eyring viscosity, the Arrhenius equation was then employed to compute Ea,
| (4) |
where μs and μ0 are the apparent viscosity of sonicated and fresh bitumen at 16 sec-1, respectively (in mPa.sec), Ea is the activation energy of flow (in kJ/mol), R is the gas constant (R = 8.314 J.mol- 1. K−1), Ts is the temperature of the bitumen after sonication (in K), and To is the initial temperature of the bitumen (in K).
Ea is determined from the plot versus . The results are shown in Fig. 7.
Fig. 7.
Influence of (a) applied frequency and (b) gas sonication environment on activation energy estimated from Arrhenius equation (Eq. (4)).
The minimum energy requires to initiate the flow increased with the applied frequency (Fig. 7a). It could be seen that applying a frequency equal to 38 kHz yielded the lowest activation energy (35.6 kJ/mol), which was four-fold lower than that of a mild frequency (200 kHz). As opposed to the applied frequency, changing the gas environment did not have a striking influence on the activation energy (Fig. 7b). Nonetheless, the lowest energy of activation was obtained for when the irradiation was conducted under CO2-rich environment (∼29 kJ/mol). Looking at the results from Fig. 5 and Fig. 7, the viscosity reduction seems to be inversely proportional to the activation energy of flow. The probable reasons could be drawn out from the chemical effects of ultrasound waves.
Suslick (1989, 1999) reported that the ultrasound breaks the long chain molecules and dissolves the suspended soluble particles, presumably asphaltenes [21], [22]. This would mean that the decrease in energy of activation (Fig. 6) should be regarded rather as an increase in solubility of heavy components initially in suspension. With the knowledge that bitumen is highly asphaltic, it is reasonable to think that the viscosity reduction pertains perhaps to the solubilization of asphaltenes suspended in the oil as either monomers or nanoaggregates [34].
This statement could be verified qualitatively by monitoring the intensity of aromatic carbon peak (sp2 C = C) and that of bonds from aliphatic carbon (sp3 C–H) on FTIR spectra [49], [50]. Thus, FTIR spectra of the fresh bitumen and those of sonicated bitumen under different gas-environment and applied frequency were acquired (Fig. 8).
Fig. 8.
Infrared spectra of fresh and sonicated bitumen under different (a) applied frequency and (b) different gas-environment; the spectra were acquired after 1 h of sonication.
The spectrum of the native bitumen and those of the sonicated bitumen features characteristic stretching peaks at (i) ∼ 3100 cm- due to an aromatic sp2 C–H, (ii) ∼ 2900 cm−1 assigned to an aliphatic sp3 C–H, (iii) ∼ 1600 cm- 1 due to a sp2 C = C. Also, bending peaks characteristic of an aliphatic (or alkyl) sp3 C–H and aromatic sp2 C–H were observed respectively at ∼ 1450 cm−1 and ∼ 750 cm−1 respectively. It could be seen that the vibration stretches of C = O bonds from a functional group appearing around ∼ 1700 cm- 1 was noticeable for all the samples.
Rogel (2015, 2017) proposed that computing the ratio of the integrated intensity of the peak of sp2 C = C vibrating at ∼ 1600 cm−1 to that of sp3 C–H appearing at ∼ 2900 cm−1 could enable the quantification of the molar ratio of aromatic rings to aliphatic side chains [51], [52]. Alhreez and Wen (2019) proposed that the ratio 3100 cm−1/2900 cm−1 is an indicator of the number of carbon atoms per alkyl chain [53]. However, it should be noted the determination of these ratios would assume that the extinction coefficients of the functional groups do not change among the fraction [51], [54].
Therefore, the peaks appearing within the FTIR band 1400–1700 cm−1 and 2700–3100 cm−1 were baseline-corrected, deconvoluted, and fitted using Gaussian functions (Fig. S1, Fig. S2 supplementary file). The ratios 1600 cm−1/2900 cm−1 and 3100 cm−1/2900 cm−1 were then computed from the integrated peaks (Table 4).
Table 4.
Molar ratio of aromatic rings to aliphatic side chains (1600 cm−1/2900 cm−1) and the number of carbon atoms per alkyl chain (3100 cm−1/2900 cm−1) for fresh and sonicated bitumen.
| IR band | Ratio | Fresh bitumen | Applied frequency | Gas-environment | |||
|---|---|---|---|---|---|---|---|
| 38 kHz | 200 kHz | Air | N2 | CO2 | |||
| 1600 cm−1/2900 cm−1 | sp2 C = C/sp3 CH | 0.050 | 0.047 | 0.062 | 0.047 | 0.049 | 0.055 |
| 3100 cm−1/2900 cm−1 | sp2 C–H/sp3 C–H | 0.218 | 0.231 | 0.034 | 0.231 | 0.398 | 0.287 |
As per Table 4, it seems that the molar ratio of aromatic rings to aliphatic side chains (i.e., 1600 cm−1/2900 cm−1) is fairly altered by either the change in applied frequency or gas environment evidenced by a value ∼ 0.050. A different trend was observed for the number of carbon atoms per aromatic ring. In the fresh bitumen, the number of carbon atoms per alkyl chain was computed to be 0.22.
The sonication at a mild frequency decreases the number of carbons per alkyl side chain to 0.03, suggesting either the presence of a large concentration in carbon atoms in the alkyl chain or a decrease in the size of the aromatic rings. Considering that the molar ratio of aromatic ring to aliphatic side chain was fairly altered, it is reasonable to think that a value of 0.03 implies a scission of long chain molecules attached to the aromatic rings, which is supported but the findings of Luo and Gu (2007).
They argued that the energy supplied by the ultrasound degrades the hydrogen bonds between the resins and the asphaltene particles at the outmost layers [38]. In respect of the gas environment, one could observe that that the gas-environment has a reverse effect on number of carbon atoms per alkyl chain. In fact, it was found an average increase of 40% from the initial value This suggests that the gas environment prompts the formation of aggregates.
Table 4 confirms further a direct correlation between the asphaltene content and the viscosity reduction.
3.4. Contribution of asphaltene content and sonochemical enhancement
3.4.1. Monitoring the asphaltene concentration
Table 5 summarizes the asphaltene concentration monitored during the sonication at different applied frequencies and gas-environment.
Table 5.
Monitoring of asphaltene concentration under different applied frequencies and gas-environmenta.
| Sonication time (min) | Fresh bitumen | Applied frequency (38 kHz) | Air-environment | |||
|---|---|---|---|---|---|---|
| Air | CO2 | N2 | 38 kHz | 200 kHz | ||
| 0 | 3.21 | 3.21 | 3.21 | 3.21 | 3.21 | 3.21 |
| 60 | 0.73 | 11.3 | 13.6 | 13.9 | 11.3 | 8.39 |
| 120 | 2.22 | 24.2 | 12.8 | 6.99 | 24.2 | 25.6 |
| 180 | 2.37 | 7.94 | 15.1 | 16.3 | 7.94 | 1.15 |
All concentrations are expressed in weight per cent (wt.%)
For all the investigated samples, the concentration in asphaltene increases with the sonication time, which is somewhat consistent with the results presented in Table 4. In respect of the gas environment, the asphaltene concentration at the end of the experiments (3 h of irradiation) was computed to be six-fold larger compared to the fresh bitumen; the largest concentration was obtained when the sonication was performed under N2-environment.
As opposed to the gas influence, the concentration in asphaltenes decreases when a mild frequency was applied. These observations agree well with the results from FTIR and the literature [55], [56], [57]. It could be further highlighted that aggregation (or dissolution of asphaltene) and thus the viscosity reduction depends on operating conditions as it could be seen in Fig. 9 and Fig. 10.
Fig. 9.
SEM photographs of asphaltene precipitation at different ultrasound frequencies.
Fig. 10.
SEM images of the surface morphology of asphaltenes under different gas environment.
Therefrom, large flocs and macro-aggregates could be observed when a low frequency was applied to the bitumen (Fig. 9a), which differ from the porous appearance when the gas was changed (Fig. 10). The different shapes of the extracted asphaltenes could be attributed either to the scission of long chain molecules [57] or the self-aggregation of asphaltene monomers, which leads to the formation of macro aggregates [58]. Either reason seems to corroborate with FTIR spectra.
3.4.2. Viscosity reduction, asphaltene yield, and sonochemical enhancement
Herein was evaluated the relationship between ultrasound irradiation, the oil viscosity reduction and the asphaltene aggregation. To do so, two dimensionless parameters were introduced namely viscosity reduction and asphaltene yield. Both terms were selected by analogy to sonochemical enhancement, which expresses the quotient of the product yield with sonication to that without sonication [59]. The mathematical expressions are shown in equations (4), (5) respectively,
| (5) |
where µ@16sec-1 is the apparent viscosity of the bitumen (cP)expresses the apparent
| (6) |
Per Eqs. (4), (5), a viscosity reduction (or an asphaltene yield) equal or close to 0 would express a poor influence of the sonication, while a value close to 1 would imply otherwise. The results of the viscosity reduction and those of the asphaltene yield when the applied frequency (or the gas environment) were plotted against the sonication time (Fig. 11).
Fig. 11.
Effect of applied frequency (b, c) and gas enviroment (d, e, f)on viscosity enhancement and asphaltene yield.
Fig. 11a shows the results of the fresh and unsonicated bitumen. Fig. 10b-c show those obtained when the applied frequency was changed under air-environment, and Fig. 11 d-f are the results obtained when the gas environment was changed at the applied frequency of 38 kHz. Irrespective of the investigated process parameter, the decrease in viscosity during sonication treatment was found proportional to aggregation in asphaltenes.
At ambient conditions, the yield in asphaltene was the lowest in addition to the fair alteration in viscosity (Fig. 10a). This suggests a poor scission of long chains of aliphatic groups attached to the asphaltenes or a poor solubilization (Table 4). However, in the event in which the sonication is conducted under a controlled gas-environment i.e., Fig. 11 d-f, it could be seen that for the bitumen to flow, more asphaltenes must be segregated from the bulk oil.
Fig. 11 suggests that the bitumen that flows consists of lighter molecules that have been separated from the asphaltic materials. Within the limit of the present work, it appears that either mild frequency (200 kHz) or an N2-environment are suitable for bitumen viscosity reduction and it could be extrapolated to crude oil upgrading [60].
Storm et al.(1995), and later Rahimi et al. (2017) reported that the viscosity improvement also pertains to a separation of resins from asphaltene particles [18], [61]. This means that beyond asphaltene content, concerns should be also given to the resins, which will be addressed in a future work.
4. Conclusions
The present work investigated the contribution of the asphaltene aggregation to the viscosity reduction of bitumen sampled from an oilfield located in Gulf of Guinea. The effects of applied frequency (38 kHz, 200 kHz) and the gas environment (air, nitrogen, and carbon dioxide) were investigated.
It was shown that the viscosity of bitumen decreased proportionally with the sonication time and the asphaltene aggregation. With an apparent viscosity of 12043cP at ambient conditions, the bitumen viscosity could be as low as 2853, and 3274 cP upon sonication with an applied frequency of 200 kHz under air-environment and 38 kHz under nitrogen environment, respectively.
At any rate, the yield in asphaltene (and thus the viscosity reduction) was larger for a mild frequency and the sonication gas environment respectively due to due to the scission of long chain molecules attached to the aromatic rings and the self-aggregation of asphaltene monomers. This study argued that ultrasound irradiation at ambient conditions or a low frequency are less efficient.
CRediT authorship contribution statement
Ronald Nguele: Conceptualization, Writing – original draft. Hirokazu Okawa: Writing – review & editing, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
The authors are grateful to Dr. Olalekan Alade (King Fahd University of Petroleum and Minerals, Saudi Arabia) for supplying kindly the bitumen used in this study.
Funding
This work was supported by JSPS KAKENHI Grant Number JP 15K06638.
Author Contribution
All authors have given approval to the final version of the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2021.105811.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.R. Nguele, K. Nchimi Nono, K. Sasaki, Nanocomposite and Nanofluids: Towards a Sustainable Carbon Capture, Utilization, and Storage, in: S. M. Sohel Murshed (Ed.), Adv. Microfluid. Nanofluids, IntechOpen, 2021.
- 2.Hart A. A review of technologies for transporting heavy crude oil and bitumen via pipelines. J. Pet. Explor. Prod. Technol. 2014;4(3):327–336. [Google Scholar]
- 3.Wang J., Bai Y., Sui H., Li X., He L. Understanding the effects of salinity on bitumen-calcite interactions. Fuel Process. Technol. 2021;213 [Google Scholar]
- 4.Velayati A., Nouri A. Emulsification and emulsion flow in thermal recovery operations with a focus on SAGD operations: A critical review. Fuel. 2020;267 [Google Scholar]
- 5.Shah A., Fishwick R., Wood J., Leeke G., Rigby S., Greaves M. A review of novel techniques for heavy oil and bitumen extraction and upgrading. Energy Environ. Sci. 2010;3:700. [Google Scholar]
- 6.Buslaev G., Morenov V., Konyaev Y., Kraslawski A. Reduction of carbon footprint of the production and field transport of high-viscosity oils in the Arctic region. Chem. Eng. Process. - Process Intensif. 2021;159 [Google Scholar]
- 7.Ghannam M.T., Hasan S.W., Abu-Jdayil B., Esmail N. Rheological properties of heavy & light crude oil mixtures for improving flowability. J. Pet. Sci. Eng. 2012;81:122–128. [Google Scholar]
- 8.Ghannam M.T., Esmail N. Flow enhancement of medium-viscosity crude oil. Pet. Sci. Technol. 2006;24(8):985–999. [Google Scholar]
- 9.Alade O.S., Sasaki K., Sugai Y., Ademodi B., Nakano M. Bitumen emulsification using a hydrophilic polymeric surfactant: Performance evaluation in the presence of salinity. J. Pet. Sci. Eng. 2016;138:66–76. [Google Scholar]
- 10.Martínez-Palou R., Reyes J., Cerón-Camacho R., Ramírez-de-Santiago M., Villanueva D., Vallejo A.A., Aburto J. Study of the formation and breaking of extra-heavy-crude-oil-in-water emulsions—A proposed strategy for transporting extra heavy crude oils. Chem. Eng. Process. Process Intensif. 2015;98:112–122. [Google Scholar]
- 11.Mohsin M., Meribout M. Oil–water de-emulsification using ultrasonic technology. Ultrason. Sonochem. 2015;22:573–579. doi: 10.1016/j.ultsonch.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 12.Nesyn G.V., Sunagatullin R.Z., Shibaev V.P., Malkin A.Y. Drag reduction in transportation of hydrocarbon liquids: From fundamentals to engineering applications. J. Pet. Sci. Eng. 2018;161:715–725. [Google Scholar]
- 13.Bensakhria A., Peysson Y., Antonini G. Experimental Study of the Pipeline Lubrication for Heavy Oil Transport. Oil Gas Sci. Technol. 2004;59(5):523–533. [Google Scholar]
- 14.Pilipovik V., Riverol C. Electromagnetic radiation in heavy oil recovery process. Assessing a numerical model to predict the oil mobility. J. Pet. Explor. Prod. Technol. 2016;6(2):293–296. [Google Scholar]
- 15.Doust A.M., Rahimi M., Feyzi M. Effects of solvent addition and ultrasound waves on viscosity reduction of residue fuel oil. Chem. Eng. Process. Process Intensif. 2015;95:353–361. [Google Scholar]
- 16.Mutyala S., Fairbridge C., Paré J.R.J., Bélanger J.M.R., Ng S., Hawkins R. Microwave applications to oil sands and petroleum: A review. Fuel Process. Technol. 2010;91(2):127–135. [Google Scholar]
- 17.Mouazen M., Poulesquen A., Bart F., Masson J., Charlot M., Vergnes B. Rheological, structural and chemical evolution of bitumen under gamma irradiation. Fuel Process. Technol. 2013;114:144–153. [Google Scholar]
- 18.Rahimi M.A., Ramazani S.A.A., Alijani Alijanvand H., Ghazanfari M.H., Ghanavati M. Effect of ultrasonic irradiation treatment on rheological behaviour of extra heavy crude oil: A solution method for transportation improvement. Can. J. Chem. Eng. 2017;95:83–91. [Google Scholar]
- 19.Razavifar M., Qajar J. Experimental investigation of the ultrasonic wave effects on the viscosity and thermal behaviour of an asphaltenic crude oil. Chem. Eng. Process. - Process Intensif. 2020;153 [Google Scholar]
- 20.Adewuyi Y.G. Sonochemistry: Environmental Science and Engineering Applications. Ind. Eng. Chem. Res. 2001;40:4681–4715. [Google Scholar]
- 21.Suslick K.S., Didenko Y., Fang M.M., Hyeon T., Kolbeck K.J., McNamara W.B., III, Mdleleni M.M., Wong M. Acoustic cavitation and its consequences. Philos. Trans. R. Soc. A. 1999;357:335–353. [Google Scholar]
- 22.Suslick K.S. The Chemical Effects of Ultrasound. Sci. Am. 1989;260(2):80–86. [Google Scholar]
- 23.Serpone N., Colarusso P., Sonochemistry I. Effects of ultrasounds on heterogeneous chemical reactions – a useful tool to generate radicals and to examine reaction mechanisms. Res. Chem. Intermed. 1994;20:635–679. [Google Scholar]
- 24.Kitamura Y., Okawa H., Kato T., Sugawara K. Effect of ultrasound intensity on the size and morphology of synthesized scorodite particles. Adv. Powder Technol. 2016;27(3):891–897. [Google Scholar]
- 25.Nakamura T., Okawa H., Kawamura Y., Sugawara K. Solid–liquid separation by sonochemistry: A new approach for the separation of mineral suspensions. Ultrason. Sonochem. 2011;18(1):85–91. doi: 10.1016/j.ultsonch.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 26.Meribout M. On Using Ultrasonic-assisted Enhanced Oil Recovery (EOR): Recent Practical Achievements and Future Prospects. IEEE Access. 2018;6:51110–51118. [Google Scholar]
- 27.Dunn K., Yen T.F. A plausible reaction pathway of asphaltene under ultrasound. Fuel Process. Technol. 2001;73(1):59–71. [Google Scholar]
- 28.Mohapatra D.P., Kirpalani D.M. Bitumen heavy oil upgrading by cavitation processing: effect on asphaltene separation, rheology, and metal content. Appl. Petrochemical Res. 2016;6:107–115. [Google Scholar]
- 29.Okawa H., Saito T., Yasuda S., Kawamura Y., Kato T., Sugawara K., Babadagli T. Enhancement of bitumen recovery from the oil sand in an alkaline solution using ultrasound irradiation and carbon dioxide. Jpn. J. Appl. Phys. 2020;59(SK):SKKD02. doi: 10.35848/1347-4065/ab79ec. [DOI] [Google Scholar]
- 30.Champion B., van der Bas F., Nitters G. The Application of High-Power Sound Waves for Wellbore Cleaning. SPE Prod. Facil. 2004;19:113–121. [Google Scholar]
- 31.Gizem Gunal O., Islam M.R. Alteration of asphaltic crude rheology with electromagnetic and ultrasonic irradiation, in. J. Pet. Sci. Eng., Elsevier. 2000;26(1-4):263–272. [Google Scholar]
- 32.Cataldo F. Ultrasound-induced cracking and pyrolysis of some aromatic. Ultrason. Sonochem. 2000;7:35–43. doi: 10.1016/s1350-4177(99)00019-x. [DOI] [PubMed] [Google Scholar]
- 33.Hamida T., Babadagli T. Effects of ultrasonic waves on the interfacial forces between oil and water. Ultrason. Sonochem. 2008;15(4):274–278. doi: 10.1016/j.ultsonch.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 34.Shalygin A.S., Kozhevnikov I.V., Kazarian S.G., Martyanov O.N. Spectroscopic imaging of deposition of asphaltenes from crude oil under flow. J. Pet. Sci. Eng. 2019;181 [Google Scholar]
- 35.(Jenny) Hristova E., Stoyanov S.R. Bitumen froth treatment in the transition region between paraffinic and naphthenic process conditions. Fuel. 2021;286 [Google Scholar]
- 36.Montes D., Cortés F.B., Franco C.A. Reduction of heavy oil viscosity through ultrasound cavitation assisted by NiO nanocrystals-functionalized SiO2 nanoparticles. DYNA. 2018;85(207):153–160. [Google Scholar]
- 37.Cui J., Zhang Z., Liu X., Liu L., Peng J. Analysis of the viscosity reduction of crude oil with nano-Ni catalyst by acoustic cavitation. Fuel. 2020;275 [Google Scholar]
- 38.P. Luo, Y. Gu, Effects of Asphaltene Content and Solvent Concentration on Heavy Oil Viscosity, in: SPE Int. Therm. Oper. Heavy Oil Symp., Society of Petroleum Engineers, 2005.
- 39.Sawarkar A.N., Pandit A.B., Samant S.D., Joshi J.B. Use of ultrasound in petroleum residue upgradation. Can. J. Chem. Eng. 2009;87(3):329–342. doi: 10.1002/cjce:v87:310.1002/cjce:20169. [DOI] [Google Scholar]
- 40.Oldham D., Qu X., Wang H., Fini E.H. Investigating Change of Polydispersity and Rheology of Crude Oil and Bitumen Due to Asphaltene Oxidation. Energy and Fuels. 2020;34(8):10299–10305. [Google Scholar]
- 41.Hoepfner M.P., Limsakoune V., Chuenmeechao V., Maqbool T., Fogler H.S. A Fundamental Study of Asphaltene Deposition. Energy & Fuels. 2013;27(2):725–735. [Google Scholar]
- 42.Koda S., Kimura T., Kondo T., Mitome H. A standard method to calibrate sonochemical efficiency of an individual reaction system. Ultrason. Sonochem. 2003;10(3):149–156. doi: 10.1016/S1350-4177(03)00084-1. [DOI] [PubMed] [Google Scholar]
- 43.Bazyleva A.B., Hasan MD.A., Fulem M., Becerra M., Shaw J.M. Bitumen and Heavy Oil Rheological Properties: Reconciliation with Viscosity Measurements. J. Chem. Eng. Data. 2010;55(3):1389–1397. [Google Scholar]
- 44.Ukwuoma O., Ademodi B. The effects of temperature and shear rate on the apparent viscosity of Nigerian oil sand bitumen. Fuel Process. Technol. 1999;60(2):95–101. [Google Scholar]
- 45.Martínez-Palou R., Mosqueira María.de.L., Zapata-Rendón B., Mar-Juárez E., Bernal-Huicochea César, de la Cruz Clavel-López J., Aburto J. Transportation of heavy and extra-heavy crude oil by pipeline: A review. J. Pet. Sci. Eng. 2011;75(3-4):274–282. [Google Scholar]
- 46.Suslick K.S. Wiley; 2000. Kirk-Othmer Encyclopedia of Chemical Technology. [Google Scholar]
- 47.Eyring H. Viscosity, Plasticity, and Diffusion as Examples of Absolute Reaction Rates. J. Chem. Phys. 1936;4(4):283–291. [Google Scholar]
- 48.Barnes H.A. The University of Wales Institute of Non-Newtonian Fluid; Aberystwyth: 2000. A Handbook of Elementrary Rheology. [Google Scholar]
- 49.Rogel E., Ovalles C., Moir M. Asphaltene Chemical Characterization as a Function of Solubility: Effects on Stability and Aggregation. Energy and Fuels. 2012;26(5):2655–2662. [Google Scholar]
- 50.Rogel E., León O., Espidel Y., González Y. Asphaltene Stability in Crude Oils. SPE Prod. Facil. 2001;16:84–88. [Google Scholar]
- 51.Rogel E., Miao T., Vien J., Roye M. Comparing asphaltenes: Deposit versus crude oil. Fuel. 2015;147:155–160. [Google Scholar]
- 52.Rogel E., Moir M. Effect of precipitation time and solvent power on asphaltene characteristics. Fuel. 2017;208:271–280. [Google Scholar]
- 53.Alhreez M., Wen D. Molecular structure characterization of asphaltene in the presence of inhibitors with nanoemulsions. RSC Adv. 2019;9(34):19560–19570. doi: 10.1039/c9ra02664a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bellamy L.J., editor. The Infra-red Spectra of Complex Molecules. Springer Netherlands; Dordrecht: 1975. [Google Scholar]
- 55.J. Argillier, L. Barre, F. Brucy, J. Dournaux, I. Henaut, R. Bouchard, Influence of Asphaltenes Content and Dilution on Heavy Oil Rheology, in: Proc. SPE Int. Therm. Oper. Heavy Oil Symp., Society of Petroleum Engineers, 2001: pp. 1–8.
- 56.Najafi I., Mousavi S.M.R., Ghazanfari M.H., Ghotbi C., Ramazani A., Kharrat R., Amani M. Quantifying the role of ultrasonic wave radiation on kinetics of asphaltene aggregation in a toluene-pentane mixture. Pet. Sci. Technol. 2011;29(9):966–974. [Google Scholar]
- 57.Mousavi S.M., Ramazani A., Najafi I., Davachi S.M. Effect of ultrasonic irradiation on rheological properties of asphaltenic crude oils. Pet. Sci. 2012;9(1):82–88. [Google Scholar]
- 58.Mullins O.C., Sabbah H., Eyssautier J., Pomerantz A.E., Barré L., Andrews A.B., Ruiz-Morales Y., Mostowfi F., McFarlane R., Goual L., Lepkowicz R., Cooper T., Orbulescu J., Leblanc R.M., Edwards J., Zare R.N. Advances in Asphaltene Science and the Yen-Mullins Model. Energy & Fuels. 2012;26(7):3986–4003. [Google Scholar]
- 59.Berlan J., Mason T.J. Sonochemistry: from research laboratories to industrial plants. Ultrasonics. 1992;30:203–212. [Google Scholar]
- 60.Weissman J.G. Review of processes for downhole catalytic upgrading of heavy crude oil. Fuel Process. Technol. 1997;50(2-3):199–213. [Google Scholar]
- 61.Storm D.A., Sheu E.Y. Characterization of colloidal asphaltenic particles in heavy oil. Fuel. 1995;74(8):1140–1145. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.