Abstract
The biotype and virulence of skin isolates of Candida parapsilosis were compared with blood isolates of the same fungus. Morphotype, resistotype, and electrophoretic karyotype determinations did not reveal any special cluster with a unique or dominant pathogenic feature among all of the isolates, regardless of their source. However, all cutaneous isolates had uniformly elevated secretory aspartyl-protease (Sap) activity, more than four times higher than the enzyme activity of the blood isolates. They were also highly vaginopathic in a rat vaginitis model, being significantly more virulent than blood isolates in this infection model. In contrast, skin isolates were nonpathogenic in systemic infection of cyclophosphamide-immunodepressed mice, while some blood isolates were, in this model, highly pathogenic (median survival time, 2 days, with internal organ invasion at autopsy). Finally, skin isolates did not differ, as a whole, from blood isolates in their adherence to plastic. This property was associated with a morphotype, as defined by a colony with continuous fringe, which was present among both skin and blood isolates. While confirming the genetic heterogenicity of C. parapsilosis, our data strongly suggest that the potential of this fungus to cause mucosal disease is associated with Sap production and is substantially distinct from that of systemic invasion.
Candida parapsilosis is a human opportunistic fungus particularly involved in candidemic episodes in patients with underlying disorders of various types (15, 18, 21, 24, 26, 38). Over the last few years, its incidence has progressively increased relative to other Candida species in the settings of malignant hemopathic patients to become, in some investigations (15), the most prevalent cause of candidemia. In contrast to Candida albicans and Candida tropicalis, studies on the virulence and experimental pathogenicity of C. parapsilosis are few and somewhat controversial (2, 15, 26, 35). We have previously highlighted the role of some biotypes of C. parapsilosis as aggressive agents of fungemia in central-venous-catheter-bearing leukemic patients (5, 15), as well as the vaginopathic potential of C. parapsilosis isolates from women with vaginal candidiasis (1, 10). These studies gave some experimental support to the notion that pathogenicity of C. parapsilosis is largely strain dependent, both for systemic and mucosal infections.
C. parapsilosis is also frequently isolated from the skin but, to our knowledge, no systematic comparative studies on the molecular characteristics and virulence of skin isolates have been performed. In view of the previously established importance of the isolation source for biotyping and virulence properties of C. parapsilosis (4, 5, 22, 29), we have now addressed the pathogenic potential of a number of C. parapsilosis isolates from the skin of human immunodeficiency virus-positive (HIV+) and HIV− subjects. This has been done in experimental systemic infections of either unmodified or cyclophosphamide-induced leukopenic mice (2) and in vaginal infection in ovariectomized estrogen-treated rats (9). In an attempt to identify potential virulence factors, we also evaluated plastic adherence properties, as well as secretion of aspartyl proteinase, a putative virulence factor (8, 16, 31) that has been shown to be critical for mucosal infection by C. albicans (9, 11).
MATERIALS AND METHODS
Yeast isolates and clinical source.
All available (total of 12) sequential skin isolates of C. parapsilosis from HIV+ and HIV− subjects were studied. No subject was affected by cutaneous candidiasis or was under treatment with antimycotics. For comparative purposes, nine isolates of the fungus from HIV− candidemic patients (representing seven strains sequentially isolated during the whole 1997 and not included in a previous study (5), plus two strains of microbial type collection) and strains of C. albicans with intact or deleted SAP2 gene were also examined. Table 1 lists the source and code of each C. parapsilosis isolate, as well as the status of each subject from whom the fungus was isolated.
TABLE 1.
C. parapsilosis isolates used throughout this study
| Isolate (laboratory code) | Sourcea | Disease status and infectionb |
|---|---|---|
| 2296 | Skin | HIV+ |
| 2446 | Skin | HIV+ |
| 2518 | Skin | HIV+ |
| 2550 | Skin | HIV+ |
| 93006 | Skin | HIV− |
| 93179 | Skin | HIV+ |
| 93858 | Skin | HIV+ |
| 94259 | Skin | HIV+ |
| 94260 | Skin | HIV− |
| 94864 | Skin | HIV− |
| 94915 | Skin | HIV− |
| 94938 | Skin | HIV− |
| 2057 | Catheter | Catheter-related candidemia |
| 2058 | Catheter | Catheter-related candidemia |
| HEM-26 | Blood | Catheter-related candidemia |
| HEM-27 | Blood | Catheter-related candidemia |
| HEM-28 | Blood | Catheter-related candidemia |
| HEM-29 | Blood | Catheter-related candidemia |
| HEM-30 | Blood | Catheter-related candidemia |
| HEM-31 | Blood | Candidemia with deep-seated invasive infection |
| HEM-32 | Blood | Candidemia with deep-seated invasive infection |
Neither HIV+ nor HIV− subjects were affected by cutaneous candidiasis.
Catheter-related candidemia and deep-seated infection were defined as in reference 15.
Determination of the morpho-resistotype.
Morphotype on malt extract agar and on Sabouraud-triphenyltetrazolium agar, as well as the resistotype on MacConkey agar (Oxoid, Basingstoke, United Kingdom) or different media containing 1.8 g of boric acid, 110 to 130 mg of NaCl, 20 g of urea, and 25 mg of 5-fluorocytosine (Sigma Chemical Co., St. Louis, Mo.) per liter or a citrate buffer with a pH of 2.8 ± 0.1, was determined as previously described (5). Every isolate received a code depending on the characteristics of the colonies: A, no fringe; B, continuous fringe; and C, discontinuous fringe. On Sabouraud-triphenyltetrazolium agar the texture of the colonies, their color, and the presence or absence of a mycelial halo were recorded as follows: S, smooth texture; P, pink color (P1 = pale pink, corresponding to Pantone 176C; P2 = dark pink, corresponding to Pantone 1785C); V, violet color (V1 = pale violet, corresponding to Pantone 251C; V2 = dark violet, corresponding to Pantone 262C); and N, no mycelial halo. Isolates that grew on boric acid, sodium chloride, and MacConkey media were coded as “1.” If they grew on sodium chloride and MacConkey media, they were coded as 2. Those that grew sodium chloride only were coded as 3. Code 4 was given to isolates that did not grow on any medium, and code 5 was used for any other further combination. A similar approach was used for resistotyping on the urea, citrate, and 5-fluorocytosine media. Isolates that grew on all three media were coded as “a.” Growth on urea and citrate media was coded as b, on urea only as c, and no growth on any medium as d. For biotype delineation, we used a code based on a combination of the morphotyping and resistotyping codes. Therefore, isolates with the AIV2c code had no fringe on malt extract agar, were coded SV2N on Sabouraud-triphenyl-tetrazolium agar (smooth texture, dark violet, no mycelial halo surrounding the colony), and grew on sodium chloride and MacConkey media and on urea medium.
Karyotype analysis by pulsed-field gel electrophoresis.
Cells of C. parapsilosis were grown to stationary phase in YPD medium (glucose, 2%; yeast extract, 1%; Bacto-Peptone, 2% [Difco, Detroit, Mich.]) overnight at 28°C. The cells were packed by centrifugation (3,000 rpm for 5 min) and washed in 1.2 M sorbitol solution containing 20 mM EDTA (pH 8.0). The pellet was resuspended to a cell concentration of 109/ml in a solution of EDTA-sorbitol, as described above, but containing 20 mM mercaptoethanol and was then incubated for 15 min at 37°C. The samples were then embedded in low-melting-point agar (L.M.; Bio-Rad, Richmond, Va.), and the spheroplast lysis method described by Vollrath and Davis (36) was used to prepare the chromosomal DNA. The cell-agarose mixture was transferred to plug molds. Solidified pellets were removed from the molds and placed in 1.2 M sorbitol solution containing 20 mM EDTA, 10 mM Tris-HCl (pH 7.5), and 100 μl of Zymoliase 100T (1 mg/ml) (100,000 U/g; Seikagaku, Tokyo, Japan). After incubation for 2 h at 37°C, the plugs were incubated in 1% sodium dodecyl sulfate solution containing 10 mM EDTA–10 mM Tris-HCl (pH 7.5) overnight at 37°C. The pellets were stored in 1% sarcosyl solution containing 100 mM EDTA–10 mM Tris-HCl (pH 7.5) at 4°C.
The DNA samples in agarose inserts were resolved by contour-clamped homogeneous field electrophoresis (CHEF). CHEF analysis was performed with the CHEF-DRII apparatus (Bio-Rad). The operating conditions included three consecutive runs on each gel (14.5 by 20.5 cm; 1-cm-thick 1% agarose; Bio-Rad) containing the agarose inserts of DNA immersed in running buffer (50 mM Tris-HCl, 50 mM boric acid, 1.5 mM EDTA; pH 8.2). Saccharomyces cerevisiae (Bio-Rad) was used as a standard. The parameters for each run were at 150 V and 14°C for 24 h with 90-, 120-, and 180-s switches. Gels were then stained with ethidium bromide (0.5 μg/ml; 30 min), destained, and photographed under UV light.
Secretory aspartyl proteinase production.
The abilities to secrete aspartyl proteinase in bovine serum albumin (BSA) were assessed in solid and liquid media, as previously described (9, 10). Briefly, the yeast was pregrown in YPD medium and induced to proteinase secretion in BSA agar (1.17% yeast carbon base, 0.01% yeast extract, 0.2% BSA; Sigma) (pH 5.0). Enzyme activity was measured as the diameter of a lytic area on BSA and scored as − to ++, as previously described (9–11). For proteinase activity determination in liquid YPD medium by the spectrophotometric method culture, supernatants (0.1 ml) were added to 0.9 ml of 0.1 M citrate buffer (pH 3.2) with 0.2% BSA with or without the proteinase inhibitor pepstatin A (0.5 mg/ml; Sigma) and then incubated at 37°C. The reaction was terminated after 3 min by the addition of 5% trichloroacetic acid. The mixture was centrifuged, and the supernatant was read at 280 nm. Aspartyl proteinase (Sap) activity was expressed as the change in optical density per minute per milliliter of culture, as previously described (9–11).
Adherence assay.
The adhesion of C. parapsilosis cells to polystyrene was quantified spectrofluorometrically. Cells in the stationary phase were inoculated into 350 ml of medium 199 (pH 6.7) (Gibco BRL/Life Technologies, Rockville, Md.) at a final concentration of 106/ml, and the medium was incubated for 2 h at 37°C in 24-well polystyrene plates. After a washing, the plates were stained with 1% calcofluor white in saline for 20 min at room temperature. The plates were washed again, and the fluorescence was read in a spectrofluorometer (Floroscan Ascent; Labsystem, Helsinki, Finland), with an excitation filter of 358 nm and an emission filter of 460 nm. Controls were used to examine possible interference from nonspecific binding and consisted of wells with medium 199. The results were expressed as the relative fluorescence, which was obtained by subtracting the reactivity obtained in the control wells from that shown by the test wells. All values are mean values derived from at least four independent assays.
Systemic infection in mice.
For experimental systemic infections, yeasts were grown in YPD medium for 24 h at 28°C on a shaker at 200 rpm (New Brunswick Scientific Co.), centrifuged, washed, counted in a hemocytometer, and diluted in saline. Of this suspension, 0.2 ml containing either 106 or 107 cells of the C. parapsilosis isolates was injected intravenously into inbred male CDF1 mice (18 to 21 g; Charles River, Calco, Italy). Groups of nine mice each were pretreated with cyclophosphamide (CY; Cytoxan; Sigma) 2 days before the infectious challenge. The drug was dissolved in sterile saline and injected intraperitoneally at a concentration of 150 mg/kg in a final volume of 0.2 ml. Survival was assessed over a period of 30 days and was expressed as the number of dead animals over the total number of animals infected (D/T) and as median survival time in days (MST).
Experimental vaginal infection in ovariectomized rats.
For experimental vaginal infection, ovariectomized female Wistar rats (80 to 100 g) (Charles River) were injected subcutaneously with 0.5 mg of estradiolbenzoate (Benzatrone-Samil, Rome, Italy) every 2 days. At 6 days after the first estradiol dose, the animals were inoculated intravaginally with 107 cells (0.1-ml volume) of each isolate tested. Yeast cells had been grown in YPD medium at 28°C, centrifuged, washed, suspended in sterile saline, and injected into the vaginal cavity through a syringe with a multipurpose calibrated tip (Combitip PBI, Milan, Italy). Enumeration of C. parapsilosis in the vaginal cavity was achieved by taking 1-μl samples by use of a calibrated plastic loop (Disponoic; PBI) from each animal every 2 days and culturing it on Sabouraud agar containing chloramphenicol (50 μg/ml). Cultures were incubated at 28°C for 48 h.
Statistics.
Differences between mean values of continuous variables were assessed by the Student's t test, whereas differences between proportions were assessed by the Fisher's exact test or the χ2 method, as appropriate.
RESULTS
Molecular and phenotypic biotyping.
We analyzed the electrophoretic karyotype of both skin and blood isolates of C. parapsilosis by the CHEF technique, which gave an optimal resolution of the chromosome bands, particularly those between molecular sizes of 1.4 and 0.8 Mb. Common to almost all isolates were three well-defined chromosome-sized DNA molecules of relatively large molecular size (>3.0 to 1.9). In the lower-molecular-size range there were ample variations in the number and size of electrophoretic bands, and these constituted the source of the isolate distinctiveness. Overall, the numbers of chromosome-sized bands were seven (in 14 isolates) and eight (in 5 isolates).
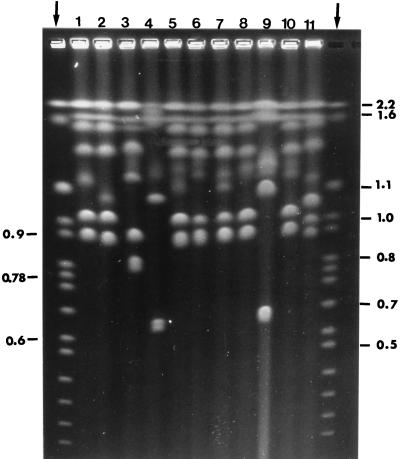
Considering both the number and size of the chromosomal bands, there was some apparent relatedness among isolates. For instance, the skin isolates 2518, 94260, 2296, 93858, 94938, 94259, 93179, and 94915 and the blood strains HEM-23 and HEM-26 had identical seven-chromosome patterns (Fig. 1, lane 1). On the other hand, the skin isolate 93006 and the three blood isolates 2057, 2058, and HEM-28 also had the seven-chromosome pattern but the fifth chromosomal band had a smaller (∼1,050 kb, first three isolates) or a higher (∼1,180 kb, the latter isolate) molecular mass than the fifth band of the former group of isolates (∼1,200 kb) (lanes 2 and 8, respectively, in Fig. 1). All other strains (with two exceptions, see below) had eight distinct chromosome-sized bands, with differences among them regarding the molecular size of the bands in the range of 1.6 to 0.8 (Fig. 1, lanes 3, 5 to 7, and 10 to 11).
FIG. 1.
Representative electrophoretic karyotype patterns of C. parapsilosis. Each numbered lane corresponds to a cluster of overlapping karyotypes, as defined in the text. Arrowed lanes at left and right are S. cerevisiae chromosomal markers, and their molecular size is expressed in megabases. For other details, see the text.
Two blood strains (HEM-30 and HEM-31) had rather peculiar chromosomal profiles, characterized by two bands (or two doublets) in the 2.2- to 1.5-Mb section, and, mostly, the absence of the prominent chromosomes in the 1.2- to 0.9-Mb size section, replaced by an apparent doublet or intense single-chromosome-sized band in a very low molecular mass region (ca. 0.65 Mb) where no other isolate of C. parapsilosis showed any band (Fig. 1, lanes 4 and 9).
All isolates were also phenotyped by assessing the colony morphology and resistance to various chemicals in different media (a combination designated as a morpho-resistotype), according to a previously established, useful scheme (5, 17). By this method, all skin isolates were grouped into eight categories (A to H), the most numerous of which included only three isolates. Overall, there were more morpho-resistotypes (eight) than electrophoretic karyotypes (five) among all the skin isolates. Concerning the important feature of colonial morphology on malt extract (see also below), the isolates were equally distributed between those with no fringe colonies (A) and those with continuous fringe colonies (B) (six per group).
Sap production.
Skin isolates of C. parapsilosis were assayed for Sap production by measuring BSA hydrolysis both on solid and liquid media. As shown in Table 2, both methods coherently indicated that all skin isolates, independently of their isolation from HIV+ or HIV− subjects, were high Sap producers, with top scores on BSA agar and uniformly elevated enzyme activity in BSA broth. In contrast, Sap production by the nine isolates of C. parapsilosis from candidemic patients showed low score in BSA-agar and averaged a score of 0.85 ± 0.07 (range, 0.77 to 1.02) in BSA broth (four times less than the Sap production by skin isolates). This confirms previous results showing the low Sap production by other blood isolates of this fungus (5) (Table 2).
TABLE 2.
Sap production by skin and blood isolates of C. parapsilosis
| Source (n) | HIV status | Sap production on:
|
|||
|---|---|---|---|---|---|
| BSA agara
|
BSA brothb (mean U ± SD) | Pd | |||
| No. ++/total | Pc | ||||
| Skin (7) | HIV+ | 7/7 | 3.84 ± 0.15 | ||
| Skin (5) | HIV− | 5/5 | 3.80 ± 0.17 | ||
| Blood (9) | HIV− | 0/9 | <0.01 | 0.85 ± 0.07 | <0.01 |
Measured as the diameter of BSA clear zone (0, none; +, 1 to 2 mm; ++, 3 to 5 mm) (see also references 9 and 10).
P < 0.01 (Fisher exact test), comparing Sap production by skin isolates (only HIV+ or else HIV+ plus HIV−) to that by blood isolates.
P < 0.01 (Student's t test), comparing Sap production as in footnote c.
Plastic adherence.
Since the pathogenic potential of C. parapsilosis (mostly regarding the isolates from candidemic patients) has often been related to its adhesive properties (3, 38), we measured adhesion to plastic of all skin isolates of this fungus, again in comparison to the isolates from the candidemic subjects. The adhesion degree of skin isolates varied as much as from 0.1 to 3.4 with a mean (± the standard deviation [SD]) of 1.82 ± 1.16. The plastic adherence of the nine blood isolates also varied in the same range (0.1 to 3.6, with a mean ± SD of 1.78 ± 1.45). Thus, adherence to plastic was substantially similar between the two categories of the isolates. Interestingly, a comparison between the two basic morphotype categories of skin isolates (B, continuous fringe colonies; A, no fringe around colony), and plastic adherence values showed that the isolates of the first category were, as a group, significantly more adhesive (by >3-fold) to the plastic than those belonging to the no-fringe colony group (2.73 ± 0.5 versus 0.85 ± 0.9, respectively; P < 0.001, Student's t test).
Experimental pathogenicity in systemic infection of mice.
Skin isolates of C. parapsilosis were assessed for their experimental systemic pathogenicity in normal and in cyclophosphamide (Cy)-immunodepressed mice. The pathogenicity was evaluated both as MST and as the number of animals dead out of the total challenged, (D/T) over 30 days. The isolates of C. parapsilosis were collectively nonlethal upon intravenous challenge for both types of mice. Some occasional animal deaths were observed with isolate 94259, which killed one Cy-treated and one normal mouse (of the nine inoculated with 107 cells) and 2446 (one normal mouse died with 107 cells). The animals were not observed for organ invasion.
Eight of the nine hematologic isolates of C. parapsilosis were also tested in this model. Interestingly, two of them (HEM-31 and HEM-32) were highly pathogenic for leukopenic animals, with a median survival time of 2 days. The specific mortality was confirmed by the finding of high Candida burden in mice organs (kidney and heart) (not shown).
Experimental vaginal infection in rats.
Ovariectomized, estradiol-treated rats were used to reproduce an experimental vaginal infection with C. parapsilosis. Seven fungal strains were randomly selected from all skin isolates and matched for their vaginopathic potential with similarly selected blood isolates. A high Sap producer strain of C. albicans and a Sap nonproducer mutant of the same species were also used as positive and negative controls, respectively, of the vaginopathic potential, as previously shown (9–11). Table 3 shows the cumulated viable counts of all fungal cells detected in the vagina over a 3-week period and the number of rats infected (>103 cells per ml of vaginal fluid) of the total after a vaginal challenge of 107 cells. Overall, all skin isolates of C. parapsilosis gave a sustained vaginal infection with a high number of viable fungal cells for 14 days, with general kinetics of fungal clearance that did not substantially differ from that typically obtained with vaginopathic strains of C. albicans (9, 11) (see also below). C. parapsilosis Sap was detected in the vaginal fluid of most of the rats tested during the first week of infection (data not shown). In contrast, the blood isolates were cleared from the vagina much earlier, by the first to the second week postchallenge (Table 3). One strain (HEM-27) was eliminated by day 5 postchallenge (not shown).
TABLE 3.
Vaginal infection by skin and blood isolates of C. parapsilosisa
| Days | Mean CFU (103) ± SE/ml of vaginal fluid
|
No. of rats infected/totalb
|
||
|---|---|---|---|---|
| Skin | Blood | Skin | Blood | |
| 1 | >100 | 92 ± 6 | 35/35 | 35/35 |
| 7 | 52 ± 20*c | 9 ± 7* | 35/35 | 35/35 |
| 14 | 8 ± 2* | <1* | 13/35* | 0/35* |
| 21 | <1 | <1 | 3/35 | 0/35 |
Cumulative results of randomly selected seven isolates from skin and seven isolates from blood. Each isolate was used to infect five rats, with an inoculum size of 107 fungal cells in 0.1 ml of saline. Sampling on day 0 (1 h postchallenge) showed that all rats had >105 cells/ml of vaginal fluid.
Rat infected, >103 CFU/ml of vaginal fluid.
The differences between each pair of values marked with an asterisk in the same row are highly significant (P < 0.01, two-tailed test) according to the Mann-Whitney U test for CFU and the χ2 test for rats infected/total number of rats.
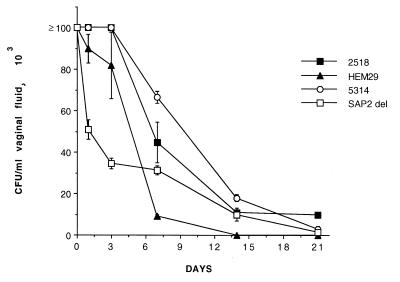
Figure 2 shows the different vaginopathic potentials of representative skin and blood isolates of C. parapsilosis, compared both to one another and to vaginopathic and nonvaginopathic strains of C. albicans.
FIG. 2.
Kinetics of vaginal infection by representative, high (■) and low (▴) Sap producer strains of C. parapsilosis isolated from the skin or blood, respectively, compared to a prototype C. albicans strain (5314 [○]) and its SAP2 gene null mutant (□). For experimental details, see the text and references 9 to 11.
DISCUSSION
C. parapsilosis is now recognized among the pathogenic species of the genus Candida, and several authors have recently reviewed its increasing incidence in various disease settings, including nosocomial bloodstream infections and neonatal candidemia in intensive care units (15, 19, 21, 28, 37, 38). We have previously demonstrated a close clinical association between vaginal isolation of C. parapsilosis from women with active vaginitis and the vaginopathic potential in an estrogen-dependent rat vaginitis model (1, 10), an observation that has subsequently been confirmed by others (34). These vaginopathic strains, at variance with blood isolates of the same fungus, were high producers of Sap and were equal to C. albicans, a well-known cause of candidal vaginitis, in their experimental vaginopathic potential (1, 10). However, the low-Sap-producing blood isolates of the fungus included strains with rather remarkable pathogenicity in a systemic infection model of nonimmunodepressed mice (5, 15), a property that is usually possessed only by the most pathogenic Candida species, such as C. tropicalis and C. albicans (2, 26).
Much less is known regarding skin isolates of C. parapsilosis despite the fact that this species is rather commonly isolated from the skin, which has also been implicated in nosocomial transmission of fungemia, in particular from the hands of hospital personnel (20, 33). In addition, there is more than circumstantial evidence of the importance of the skin isolates in exogenously disseminated candidiasis in immunosuppressed patients (26, 37). On this basis, we have addressed biotyping and in vitro-in vivo virulence properties of various skin isolates of C. parapsilosis and compared them with the properties of recent blood isolates of the same fungus.
It is known that C. parapsilosis is genotypically heterogeneous, its karyotype extending from six to nine chromosome-sized bands in all different source groups (3, 4, 13, 22, 23, 29, 30). Variability in the chromosomal asset has been confirmed here with the skin isolates, although none of them had nine chromosomal bands or showed the peculiar features of some blood isolates with the absence of a whole chromosome-sized region at less than 1.6 Mb or the presence of unusually low-molecular-weight bands. Overall, these variations support the idea of a possible separation of C. parapsilosis isolates into three separate groups, or even species, as previously suggested (5, 22) and as more recently supported by DNA-relatedness studies (30). Noteworthy is that one of the two blood isolates with low-molecular-weight bands resembled other previously studied biotypes with the same peculiar karyotype (5), and was likewise highly pathogenic in systemic infection. Of interest is that no special electrophoretic karyotype was found among the skin isolates studied here, all of which originated from the same geographical region and clinical source. There was indeed a major cluster of eight isolates belonging to a single class, but two blood isolates also belonged to this group.
More association was previously found among morpho-resistotype, source, and experimental pathogenicity. In particular, the morpho-resistotype group BIV2c of blood isolates included the most pathogenic strains in experimental bloodstream infections (5). Interestingly, none of the C. parapsilosis skin isolates belonged to this morpho-resistotype, and none was virulent for systemic mouse infection. On the other hand, no relationship could be found between any particular morpho-resistotype and vaginopathic potential since all skin isolates belonging to seven morpho-resistotype groups were equally vaginopathic. This suggests that, as for C. albicans (12), the determinants of superficial infections by C. parapsilosis may be very different from those of systemic or deep-seated infections, although it is not yet clear which specific virulence determinants or host-adaptation factors are differentially expressed on the mucosa or systemically.
Potential in vivo-inducible virulence factors of C. parapsilosis are considered to include adherence and slime production, an attribute of special importance for adhesion to plastic and therefore for catheter-related candidemia. We have examined adhesion to plastic by our skin and blood isolates and found a great variation in this property among them, with adhesion values ranging from very low (0.09 ± 0.03) to high (3.42 ± 0.21), in both categories. These results are in agreement with previous data of others, who found rather large variations in the capability of C. parapsilosis isolates from blood and catheter cultures to produce large amounts of viscid slime material in glucose-containing solutions (3). However, we have also observed that certain morpho-resistotypes, namely, those showing colonies on malt extract agar with continuous fringes and shared by skin and blood categories, are endowed with a higher capacity to adhere to polystyrene. These isolates grew on malt extract agar predominantly as pseudomycelial cells, in contrast to the no-fringe isolates, which grew as yeasts. Since in C. albicans the fringe is due to the growth in the filamentous phase (germ tubes) (17), which has increased capacity to adhere to polystyrene (32) and since cell length appears to increase in the more-adhesive strains of C. parapsilosis (27), it is tempting to speculate that the increased capacity to adhere to polystyrene shown by C. parapsilosis skin isolates belonging to biotypes with a continuous fringe is due to their ability to produce abundant pseudomycelium on that medium. However, since among the strains used for experimental vaginal infections, which were almost equally vaginopathic, adhesiveness to plastic varied over a wide range (5), it is clear that adhesion to plastic, as measured by the current methods, bears no direct and unequivocal relationship to vaginopathic potential or systemic infection, although adherence to cells in vivo may well be relevant for both kinds of infection.
What seems clear from our data is that the production of aspartyl proteinase (Sap) is associated with vaginopathic potential. All skin isolates of C. parapsilosis were uniformly high Sap producers, averaging more than four times the enzyme production by the blood isolates and significantly higher than previously studied vaginal isolates (10). High Sap production was common to all skin isolates of C. parapsilosis regardless of the variations in their electrophoretic karyotype and morpho-resistotype, suggesting that it was a common property and not a particular feature of some special biotypes of the fungus. Interestingly, high Sap production did not confer upon the strains any appreciable capacity for systemic infection, even in strongly leukopenic, cyclophosphamide-immunodepressed mice. Thus, among all the putative virulence features of this fungus, only Sap production appeared to reflect the vaginopathic property of the skin isolates. In their study of C. parapsilosis complex, Lin et al. (22) showed the variability of Sap production by isolates of C. parapsilosis from various sources, and this is here confirmed with regard to a comparison between blood and skin isolates of the fungus. Importantly, of the 10 isolates with the highest Sap score, 7 were from a source directly or indirectly bound to the skin or mucosa, such as vaginal fluid, ear, leg wound, or vitreous fluid (22).
C. parapsilosis possesses two SAP genes, which are partly homologous to the aspartyl proteinase of C. tropicalis (14, 16, 25). The vaginal environment has several properties that favor the induction and expression of this activity inclusive of the acidic pH reached under estrogen treatment and the abundance of proteinaceous secretions. Studies of C. albicans with deleted SAP genes have clearly demonstrated the role of some of these enzymes in vaginitis (11). Moreover, Sap inhibitors, inclusive of HIV-protease inhibitors (6, 7), have exerted a therapeutic effect in experimental vaginitis. The role of Sap in superficial candidiasis may be somewhat advantageously studied in C. parapsilosis since this fungus does not seem to possess other penetrant virulence attributes, has a less varied clinical spectrum of diseases, and apparently possesses fewer Sap enzymes than those secreted by C. albicans.
ACKNOWLEDGMENTS
This work was granted in part by grant UPV 093.327-G01/98 from the Universidad del Paìs Vasco and by the National AIDS Project-ISS (Rome, Italy) under contract 50B/B.
We are grateful to A. Girolamo for excellent technical assistance and to F. Girolamo and A. Botzios for manuscript preparation.
REFERENCES
- 1.Agatensi L, Franchi F, Mondello F, Bevilacqua R L, Ceddia T, De Bernardis F, Cassone A. Vaginopathic and proteolytic Candida species in outpatients attending a gynecology clinic. J Clin Pathol. 1991;44:826–830. doi: 10.1136/jcp.44.10.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bistoni F, Vecchiarelli A, Cenci E, Sbaraglia G, Perito S, Cassone A. A Comparison of experimental pathogenicity of Candida species in cyclophosphamide-immunodepressed mice. Sabouraudia. 1984;22:409–418. doi: 10.1080/00362178485380661. [DOI] [PubMed] [Google Scholar]
- 3.Branchini M L, Pfaller M A, Rhine-Chalberg J, Frempong T, Isenberg H D. Genotypic variation and slime production among blood and catheter isolates of Candida parapsilosis. J Clin Microbiol. 1994;32:452–456. doi: 10.1128/jcm.32.2.452-456.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carruba G, Pontieri E, De Bernardis F, Martino P, Cassone A. DNA fingerprinting and electrophoretic karyotype of environmental and clinical isolates of Candida parapsilosis. J Clin Micobiol. 1991;29:916–922. doi: 10.1128/jcm.29.5.916-922.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cassone A, De Bernardis F, Pontieri E, Carruba G, Girmenia C, Martino P, Fernandez-Rodriguez M, Quindos G, Ponton J. Biotype diversity of Candida parapsilosis and its relationship to the clinical source and experimental pathogenicity. J Infect Dis. 1995;171:967–975. doi: 10.1093/infdis/171.4.967. [DOI] [PubMed] [Google Scholar]
- 6.Cassone A, De Bernardis F, Torosantucci A, Tacconelli E, Tumbarello M, Cauda R. In vitro and in vivo anticandidal activity of HIV protease inhibitors. J Infect Dis. 1999;180:448–453. doi: 10.1086/314871. [DOI] [PubMed] [Google Scholar]
- 7.Cauda R, Tacconelli E, Tumbarello M, Morace G, Torosantucci A, De Bernardis F, Cassone A. The role of protease inhibitors in preventing recurrent oral candidosis in patients with HIV infection. A prospective case-control study. J Acquired Immune Defic Syndr Hum Retrovirol. 1999;21:20–25. doi: 10.1097/00126334-199905010-00003. [DOI] [PubMed] [Google Scholar]
- 8.Cutler J E. Putative virulence factors of Candida albicans. Annu Rev Microbiol. 1991;45:187–218. doi: 10.1146/annurev.mi.45.100191.001155. [DOI] [PubMed] [Google Scholar]
- 9.De Bernardis F, Agatensi L, Ross K I, Emerson G W, Lorenzini R, Sullivan P A, Cassone A. Evidence for a role for secreted aspartate proteinase of Candida albicans in vulvovaginal candidiasis. J Infect Dis. 1989;161:1276–1283. doi: 10.1093/infdis/161.6.1276. [DOI] [PubMed] [Google Scholar]
- 10.De Bernardis F, Lorenzini R, Verticchio R, Agatensi L, Cassone A. Isolation, acid proteinase secretion, and experimental pathogenicity of Candida parapsilosis from outpatients with vaginitis. J Clin Microbiol. 1989;27:2598–2603. doi: 10.1128/jcm.27.11.2598-2603.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Bernardis F, Arancia S, Morelli L, Hube B, Sanglard D, Schafer W, Cassone A. Evidence that members of the secretory aspartyl proteinases gene family (SAP), in particular SAP2, are virulence factors for Candida vaginitis. J Infect Dis. 1999;179:201–208. doi: 10.1086/314546. [DOI] [PubMed] [Google Scholar]
- 12.De Bernardis F, Muehsctlegel F A, Cassone A, Fonzi W A. The pH of the host niche controls gene expression in and virulence of Candida albicans. Infect Immun. 1998;66:3317–3325. doi: 10.1128/iai.66.7.3317-3325.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernando P H P, Samaranayake L P. Chromosome length polymorphism in clinical isolates of Candida parapsilosis. APMIS. 1998;106:941–946. [PubMed] [Google Scholar]
- 14.Fusek M, Smith E A, Monod M, Foundling S I. Candida parapsilosis expresses and secretes two aspartic proteinases. FEBS Lett. 1993;327:108–112. doi: 10.1016/0014-5793(93)81050-a. [DOI] [PubMed] [Google Scholar]
- 15.Girmenia C, Martino P, De Bernardis F, Gentile G, Boccanera M, Monaco M, Antonucci G, Cassone A. Rising incidence of Candida parapsilosis fungemia in patients with hematologic malignancies: clinical aspects, predisposing factors and differential pathogenicity of the causative strains. Clin Infect Dis. 1996;23:506–514. doi: 10.1093/clinids/23.3.506. [DOI] [PubMed] [Google Scholar]
- 16.Hube B. Candida albicans secreted aspartyl proteinases. Curr Top Med Mycol. 1996;7:55–69. [PubMed] [Google Scholar]
- 17.Hunter P R, Fraser C, Mackenzie D W R. Morphotype markers of virulence in human candidal infections. J Med Microbiol. 1989;28:85–91. doi: 10.1099/00222615-28-2-85. [DOI] [PubMed] [Google Scholar]
- 18.Komshian S V, Uwaydah A K, Sobel J D, Crane L R. Fungemia caused by Candida species and Torulopsis glabrata in the hospitalized patient: frequency, characteristics and evaluation of factors influencing outcome. Rev Infect Dis. 1989;11:379–390. doi: 10.1093/clinids/11.3.379. [DOI] [PubMed] [Google Scholar]
- 19.Kossoff E H, Bucherer E S, Karlowicz M G. Candidemia in a neonatal intensive care unit trends during fifteen years and clinical features of 111 cases. Pediatr Infect Dis J. 1998;17:504–508. doi: 10.1097/00006454-199806000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Levin A S, Costa S F, Mussi N S, Basso M, Sinto S I, Machado C, Geiger D C, Villares M C, Schreiber A Z, Barone A A, Branchini M L. Candida parapsilosis fungemia associated with implantable and semi-implantable central venous catheters and the hands of health care workers. Diagn Microbiol Infect Dis. 1998;30:243–249. doi: 10.1016/s0732-8893(98)00006-6. [DOI] [PubMed] [Google Scholar]
- 21.Levy I, Rubin L G, Vasishtha S, Tucci V, Sood S K. Emergence of Candida parapsilosis as the predominant species causing candidemia in children. Clin Infect Dis. 1998;26:1086–1088. doi: 10.1086/520277. [DOI] [PubMed] [Google Scholar]
- 22.Lin D, Wu L C, Rinaldi M G, Lehmann P F. Three distinct genotypes within Candida parapsilosis from clinical sources. J Clin Microbiol. 1995;33:1815–1821. doi: 10.1128/jcm.33.7.1815-1821.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lott T J, Kuykendall R J, Welber S F, Pramanik A, Lasker B A. Genomic heterogenicity in the yeast Candida parapsilosis. Curr Genet. 1993;23:463–467. doi: 10.1007/BF00312635. [DOI] [PubMed] [Google Scholar]
- 24.Meunier-Carpentier F, Kiehn T E, Armstrong D. Fungemia in the immunocompromised host: changing patterns, antigenemia, high mortality. Am J Med. 1981;71:263–270. doi: 10.1016/0002-9343(81)90162-5. [DOI] [PubMed] [Google Scholar]
- 25.Monod M, Togui G, Hube B, Sanglard D. Multiplicity of genes encoding secreted aspartyl proteinases in candida species. Mol Microbiol. 1999;13:357–368. doi: 10.1111/j.1365-2958.1994.tb00429.x. [DOI] [PubMed] [Google Scholar]
- 26.Odds F C. Candida and candidosis. 2nd ed. London, England: Baillière-Tindall; 1988. [Google Scholar]
- 27.Panagoda G J, Samaranayake L P. The relationship between the cell length, adhesion to acrylic and relative cell surface hydrophobicity of Candida parapsilosis. Med Mycol. 1998;36:373–378. doi: 10.1080/02681219880000591. [DOI] [PubMed] [Google Scholar]
- 28.Plouffe J F, Brown D G, Silva J J, Eck T, Stricof R L, Fekety F R. Nosocomial outbreak of Candida parapsilosis fungemia related to intravenous infusions. Arch Intern Med. 1977;137:1686–1689. [PubMed] [Google Scholar]
- 29.Pontieri E, Gregori L, Gennarelli M, Ceddia T, Navelli G, Dallapiccola B, De Bernardis F, Carruba G. Correlation of SfiI macrorestriction endonuclease fingerprint analysis of Candida parapsilosis isolates with source of isolation. J Med Microbiol. 1996;45:173–178. doi: 10.1099/00222615-45-3-173. [DOI] [PubMed] [Google Scholar]
- 30.Roy B, Meyer S A. Confirmation of the distinct genotype groups within the form species Candida parapsilosis. J Clin Microbiol. 1998;36:216–218. doi: 10.1128/jcm.36.1.216-218.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruchel R, Boning B, Borg M. Characterization of a secretory proteinase of Candida and evidence for the absence of the enzyme during infection in vitro. Infect Immun. 1986;53:411–419. doi: 10.1128/iai.53.2.411-419.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.San Millàn R, Ezkurra P, Quindòs G, Robert R, Senet J M, Pontòn J. Effect of monoclonal antibodies directed against Candida albicans cell wall antigens on the adhesion of the fungus to polystyrene. Microbiology. 1996;142:2271–2277. doi: 10.1099/13500872-142-8-2271. [DOI] [PubMed] [Google Scholar]
- 33.Strausbaugh L, Sewell D L, Ward T T, Pfaller A, Heitzmann T, Tjoelker R. High frequency of yeast carriage on hands of hospital personnel. J Clin Microbiol. 1994;32:2299–2300. doi: 10.1128/jcm.32.9.2299-2300.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vazquez S A, Beckley A, Donabedian S, Sobel S D, Zervas M S. Comparison of restriction enzyme analysis versus pulsed-field gradient gel electrophoresis as a typing system for Torulopsis glabrata and Candida species other than C. albicans. J Clin Microbiol. 1993;31:2021–2030. doi: 10.1128/jcm.31.8.2021-2030.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vecchiarelli A, Bistoni F, Cenci E, Perito S, Cassone A. In vitro killing of Candida species by murine immunoeffectors and its relationship to the experimental pathogenicity. Sabouraudia. 1985;23:377–387. doi: 10.1080/00362178585380541. [DOI] [PubMed] [Google Scholar]
- 36.Vollrath D, Davis R W. Resolution of DNA molecules greater than 5 megabases by contour-clamped homogeneous electric field. Nucleic Acids Res. 1987;15:7865–7876. doi: 10.1093/nar/15.19.7865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weems J J, Chamberland M E, Ward J, Willy M, Padhye A A, Solomon S L. Candida parapsilosis fungemia associated with parenteral nutrition and contaminated blood pressure transducers. J Clin Microbiol. 1987;25:1029–1032. doi: 10.1128/jcm.25.6.1029-1032.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weems J J., Jr Candida parapsilosis: epidemiology, pathogenicity, clinical manifestations, and antimicrobial susceptibility. Clin Infect Dis. 1992;14:757–766. doi: 10.1093/clinids/14.3.756. [DOI] [PubMed] [Google Scholar]