Abstract
BACKGROUND:
The Centers for Disease Control and Prevention (CDC) initiated the Colorectal Cancer Screening Demonstration Program (CRCSDP) to explore the feasibility of establishing a large-scale colorectal cancer screening program for underserved populations in the United States. The authors of the current report provide a detailed description of the total program costs (clinical and nonclinical) incurred during both the start-up and service delivery (screening) phases of the 4-year program.
METHODS:
Tailored cost questionnaires were completed by staff at the 5 CRCSDP sites. Cost data were collected for clinical services and nonclinical programmatic activities (program management, data collection, and tracking, etc). In-kind contributions also were measured and were assigned monetary values.
RESULTS:
Nearly $11.3 million was expended by the 5 sites over 4 years, and 71% was provided by the CDC. The proportion of funding spent on clinical service delivery and service delivery/patient support comprised the largest proportion of cost during the implementation phase (years 2–4). The per-person nonclinical cost comprised a substantial portion of total costs for all sites. The cost per person screened varied across the 5 sites and by screening method. Overall, economies of scale were observed, with lower costs resulting from larger numbers of individuals screened.
CONCLUSIONS:
Programs incur substantial variable costs related to clinical services and semivariable costs related to nonclinical services. Therefore, programs that serve large populations are likely to achieve a lower cost per person.
Keywords: colorectal cancer, cancer screening programs, clinical costs, nonclinical costs, cost assessment
INTRODUCTION
To explore the feasibility of establishing a national colorectal cancer screening program for underserved US populations, the Centers for Disease Control and Prevention (CDC) established the 4-year Colorectal Cancer Screening Demonstration Program (CRCSDP) in 2005.1 In this article, we describe the total costs, both clinical and nonclinical, of the CRCSDP activities over the course of the 4-year demonstration. We previously reported programmatic start-up costs from the CRCSDP.2 The start-up period was defined as the time between the initial funding award (August 31, 2005) and the start of clinical service delivery (screening and diagnostic services) at each program demonstration site. Because programs began screening at different times during their first year, the start-up period varied slightly across the 5 sites. The CRCSDP was established in 5 sites: Baltimore City (Baltimore City Colon Cancer Screening Program); St. Louis (Missouri Screen for Life); Nebraska (Nebraska Colon Cancer Screening Program); Suffolk County, New York (Project Suffolk County Preventive Endoscopy Project [SCOPE]); and Greater Seattle (Washington Colon Health Program). Two of the programs (Baltimore City Colon Cancer Screening Program and Project SCOPE) provided only colonoscopies as screening tools (colonoscopy only), whereas the remaining 3 programs provided a combination of fecal occult blood tests (FOBTs) and colonoscopy screenings (mixed programs). Among mixed programs, the Washington Colon Health Program provided the highest percentage of FOBTs (71.2%), and the Missouri Screen for Life program provided the highest percentage of colonoscopies (84.5%). The 5 demonstration sites have been described elsewhere in publications1,3 as well as in this supplement of Cancer.4 Table 1 summarizes the sites, the geographic areas covered, how the services were provided, and the type/number of screening test(s) the sites provided. Overall, 5603 individuals were screened, ranging from 438 in St. Louis to 2229 in Nebraska.
TABLE 1.
Overview of the Centers for Disease Control and Prevention Colorectal Cancer Screening Demonstration Program Sitesa
| Site | Grantee | Area Covered | Type of Screening | Providers | No. Screened |
|---|---|---|---|---|---|
| Baltimore City | State health department | City | Colonoscopy | Hospitals | 720 |
| St. Louis | State health department | City | 15.5% FOBT, 84.5% colonoscopy | Specialty care clinics | 438 |
| Nebraska | State health department | State | 65.7% FOBT, 34.3% colonoscopy | Multiple providers | 2229 |
| Suffolk County, NY | Academic medical center | County | Colonoscopy | University hospital | 815 |
| Greater Seattle | County health department | 3 counties | 71.2% FOBT, 0.2% sigmoidoscopy, 28.6% colonoscopy | Community health centers | 1401 |
Abbreviations: FOBT, fecal occult blood test.
Source: Centers for Disease Control and Prevention, 2012.5
The CRCSDP supported both clinical activities (ie, delivery of colorectal cancer screening and diagnostic services, including associated office visits and laboratory fees) and nonclinical activities intended to support the provision of screening and diagnostic services. The nonclinical components of the program included program management, service delivery and patient support (activities that facilitate patients’ receiving screening and diagnostic follow-up tests, such as patient navigation and provider support), public education and outreach, quality assurance and professional development, partnership development and maintenance, data collection and tracking, program evaluation, and other activities. Table 2 provides a description of the CRCSDP program activities.
TABLE 2.
The Centers for Disease Control and Prevention Colorectal Cancer Screening Demonstration Program Activities
| Clinical activities |
| Clinical service delivery |
| Provide CRC prescreening (EKG and blood work), office visit, bowel preparation, screening, administration of anesthesia, diagnostic follow-up, and surveillance colonoscopy services |
| Nonclinical activities |
| Program management |
| Recruit, hire, and train staff |
| Develop a fiscal system |
| Collaborate with CDC |
| Establish and manage non-service delivery contracts |
| Identify and contract with local physicians and clinics to deliver screening services |
| Develop administrative-related policies and procedures |
| Manage programmatic/administrative/reporting issues |
| Travel for program meetings |
| Establish the necessary administrative billing and reimbursement system. |
| Service delivery support/patient support |
| Identify providers, manage contracts, and facilitate processes to provide screening and diagnostic testing |
| Develop a plan, including identifying funding sources, for assuring treatment services for individuals diagnosed with cancer or experiencing medical complications |
| Establish and implement a patient support system to assure that appropriate screening, diagnostic, and treatment services are offered (eg case management, including patient enrollment, scheduling, discussing screening procedures, providing results) |
| Public education and outreach |
| Develop and implement public education and outreach strategies, including: |
| Develop and plan public education and outreach activities |
| Conduct outreach/in-reach activities |
| Conduct and facilitate related training |
| Collaborate with related partners |
| Quality assurance and professional development |
| Convene a medical advisory committee |
| Develop quality control standards and mechanisms |
| Develop clinical policies and procedures |
| Develop or enhance training for health care professionals |
| Partnership development and maintenance |
| Develop and maintain partnerships (ie, Comprehensive Cancer Control Program, community-based organizations, medical health care systems, businesses, etc) |
| Data collection and tracking |
| Develop and adapt data collection and reporting system |
| Establish surveillance system to track and follow clients who have abnormal screening results or who are diagnosed with cancer |
| Program evaluation |
| Participate in meetings to discuss the CDC evaluation plan, and provide feedback to CDC regarding CDC evaluation plan design |
| Participate in CDC evaluation efforts |
| Plan and implement site-specific evaluation activities. |
| Other activities |
| Activities not included above |
Abbreviations: CDC, Centers for Disease Control and Prevention; EKG, electrocardiogram.
Numerous studies have used decision modeling to assess the cost effectiveness of colorectal cancer screening.6–9 These assessments have consistently concluded that screening for colorectal cancer using tests recommended by clinical guidelines, such as the guaiac-based stool test or colonoscopy, is cost effective. However, those studies have not accounted for the costs of developing and implementing a comprehensive colorectal cancer screening program, including both clinical service delivery and nonclinical programmatic costs. Nonclinical programmatic costs are an essential component that should be included in the economic assessment of colorectal cancer screening. In addition, to our knowledge, there have been no published evaluations that provide details on the cost of implementing a colorectal cancer screening program for underserved populations.
The CRCSDP provided an ideal opportunity to collect detailed cost data from the 5 funded sites and to perform a comprehensive economic assessment that would inform federal-funded and state-funded cancer screening initiatives. For the assessment, we analyzed 4 years of clinical and nonclinical program costs, including the start-up period, when program planning was performed, and the implementation phase. In this article, we report clinical service delivery costs (those costs attributable both to the provision of screening and to diagnostic follow-up services) and nonclinical costs (those costs that support nonclinical program components, including public education and outreach, program management, and data collection). Furthermore, for both the start-up and implementation periods, we analyzed trends in costs according to funding source and program activities. In a companion article in this supplement, we also compare the screening and diagnostic costs related to FOBT and colonoscopy that provide “real-world” estimates to inform future program planning.10
MATERIALS AND METHODS
A cost-assessment tool was developed and completed by the CDC and RTI International.11 Staff at the CRCSDP-funded sites submitted information annually beginning in 2006 (1 year after the start of the demonstration) to report costs incurred in each program year. To ensure that data collection methods were standardized across all 5 sites, the sites were provided with training, a user’s guide giving detailed definitions of each activity included in the demonstration, and ongoing technical assistance to address any questions about data collection and reporting. RTI International and the CDC also corresponded with sites regarding any data questions and outliers, and the sites reviewed and approved all data summaries. All data were collected using Microsoft Excel (Microsoft Corporation, Redmond, Wash), which the program staff could use on an ongoing basis to enter cost and resource data. The cost data collection was performed from a programmatic perspective; therefore, details on all resources used by the programs, both monetary and in-kind contributions, were collected.
Well established methods of collecting cost data for program evaluation, such as the ingredient approach, were considered in creating the tool.12–14 The tool was designed to collect data on amounts and sources of funding as well as expenditure data. The tool collects information primarily on the following budget categories: staff salaries, contract expenditures, purchases of materials and equipment, and administration or overhead activities, such as telephone and rent. To appropriately allocate the expenditures, the questionnaire captured details on the distribution of both labor and nonlabor costs for all activities performed. Program staff then allocate costs to the following CRCSDP activities: program management, clinical service delivery (cost of screening and diagnostic testing), service delivery support/patient support, patient education and outreach, quality assurance/professional development, partnership development/maintenance, data collection and tracking, and evaluation. We estimated labor costs using the following information: 1) the number of hours worked by staff per month on various activities, 2) the proportion of staff salaries paid through CRCSDP funds, 3) data on the percentage of time that staff members worked, and 4) staff salaries. We computed the hourly rate for each staff member and used the hours spent on each program activity to allocate parts of the total salary to the activities performed. We then aggregated the labor costs for each activity and assigned in-kind labor contributions to each program activity.
Similarly, we aggregated the costs of consultants, materials, equipment, and supplies for each activity and derived the total overhead costs related to the service delivery period by using detailed information provided by the sites on rent, utility payments, and other indirect costs. Although the general approach in economic assessments is to use an appropriate allocation methodology to assign indirect costs to program activities,15 we chose to present administrative or overhead costs as a separate cost category to allow for greater accuracy when comparing the 5 sites with each other. Because overhead costs can differ greatly across multiple sites, reporting these costs separately allowed us to assess the magnitude of the administrative costs in relation to other costs and to understand the effect of these costs on overall program costs.
To assess clinical service delivery costs while accounting for the number of individuals screened by each site, we estimated the cost per person screened. Clinical costs were calculated from the payments made by the sites for the screening and diagnostic services, which are often based on the Medicare Fee Schedule. We obtained information on the number of individuals screened and the type of screening tests performed (FOBT or colonoscopy) from the clinical data elements reported to the CDC.4 We calculated the cost per person screened by dividing the total cost by the total number of individuals who received clinical services in each of the sites. To facilitate comparison across the sites, we stratified costs into clinical service delivery (clinical cost of screening and diagnostic testing), service delivery support/patient support, and other nonclinical costs. We separated out service delivery support/patient support cost from other nonclinical costs because the sites incurred substantial costs for service delivery and patient support activities. To explore potential economies of scale—that is, a reduction in cost per person by delivery of services to a large number of individuals—we generated a scatter plot to observe the relation between the average total cost per person screened and the number of individuals screened for each implementation year.
We performed the cost analysis with the intent of measuring total programmatic costs expended in this effort. RTI International collected data related to all funding sources for the sites, including not only funds from CDC CRCSDP but also funds from the state and from other sources, and reported on how all costs were allocated, combined together regardless of the funding source. The sites also were asked to include data on in-kind contributions and their estimated monetary value to assess the total funding required to replicate similar programs (eg, source and hourly wages used to derive the cost of in-kind labor contributions). The questionnaire collected details of the methods used to assign monetary value to in-kind contributions. The cost of clinical complications resulting from the colonoscopy procedure and the cost of treatment for cancer were not included, because complications and treatment costs were covered by the individual sites rather than by the CDC, and the associated cost information was not reported to the CDC. The clinical findings, including complications, are summarized in separate reports in this supplement.4,16
To ensure valid comparisons, we adjusted the costs to reflect differences in the cost of living in the geographic locations of the 5 sites. We adjusted the clinical service delivery costs by using the regional medical care component of the Consumer Price Index (CPI), and all other costs were adjusted by using the overall CPI.17 Our unit of analysis was program fiscal year. We reported start-up costs and implementation costs. Year 1 was divided into the start-up period and the clinical service delivery period. Much of year 1 was devoted to start-up activities (the first 9 to 11 months of the demonstration, depending on the site). Clinical service delivery ranged from 1 to 3 months in the first year. In each subsequent year, the sites reported costs for a 12-month period. We reported the costs either for each site separately or as aggregated from all sites, as appropriate. We also reported the cost of each program activity as a proportion of the total cost. This study was considered exempt by the Institutional Review Board of RTI International.
RESULTS
Start-up costs, as previously reported2 and based on all funding sources, ranged from $60,602 to $337,715, with $855,694 on average incurred across all 5 sites combined. In total, nearly $11.3 million was expended by the CRCSDP-funded sites during the implementation period, 71% of which was funded by the CDC. In-kind contributions (eg, time donated by physicians participating in the Medical Advisory Committee and senior health department staff who supervised the programs) across all years made up $2.6 million of the expenditures (23%) followed by 6% from other funding sources, such as state funds and funds from the Comprehensive Cancer Control Program and the US Multisociety Task Force on Colorectal Cancer.
Figure 1 displays the percentage distribution of costs by program activities for all sites during the start-up and implementation periods. The start-up period consisted mostly of program management, data collection and tracking, and administrative costs, which, combined, comprised 62% of all start-up costs. In the implementation years (years 2–4), most costs were for clinical service delivery (41%), followed by service delivery support/patient support (18%). Program management and administration each comprised 10% of costs.
Figure 1.
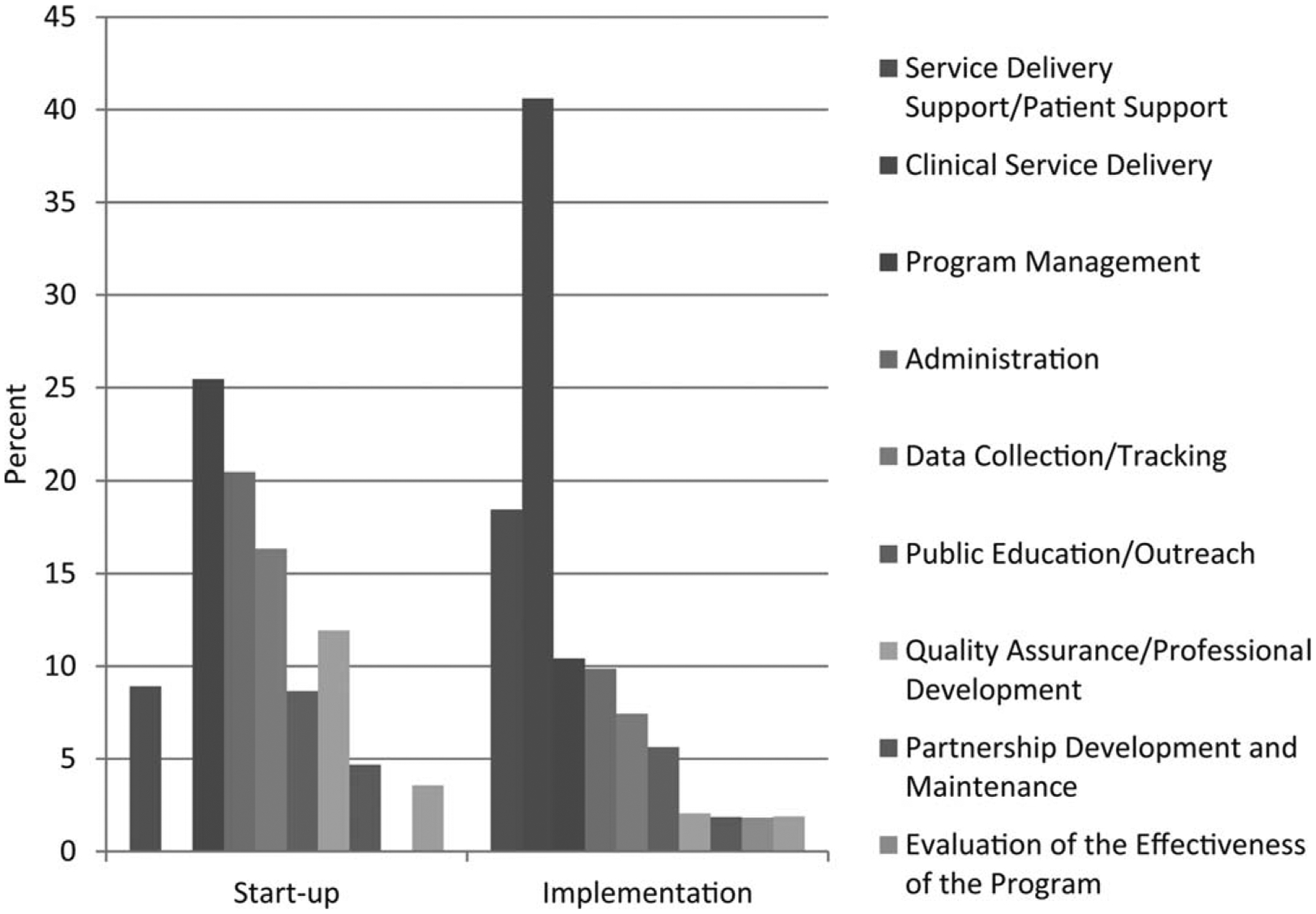
This chart illustrates the distribution of costs by program activity during the start-up and implementation phases. Costs are adjusted using the regional Consumer Price Index to allow for systematic comparisons across sites.
Clinical service delivery costs comprised the largest proportion of costs per patient during the implementation phase. The clinical service delivery costs were influenced by the type of test used (eg, guaiac-based fecal tests, colonoscopies, or a combination of these tests). The mean cost of clinical service delivery per person (those who completed a test), averaged across all screening test types and for all tests combined, was $695. Service delivery support/patient support was the second largest cost component per person, and other large costs were program management and administrative costs ($178 and $127 per person, respectively).
Figure 2 presents the cost per person screened for all years combined by site. Over the 4-year period of the program, for all sites combined and for all screening methods, the total per-person cost was $1856, including $144 for start-up, $695 for clinical activities, $316 for service delivery support/patient support, and $701 for other nonclinical activities. The costs differed by site, and Baltimore City and Suffolk County, New York—both colonoscopy-only sites—had the highest total costs ($3393 and $3522 per person, respectively). Nebraska and Greater Seattle—both mixed tests sites with large volume of FOBTs—had the lowest total costs ($991 and $1265 per person, respectively).
Figure 2.
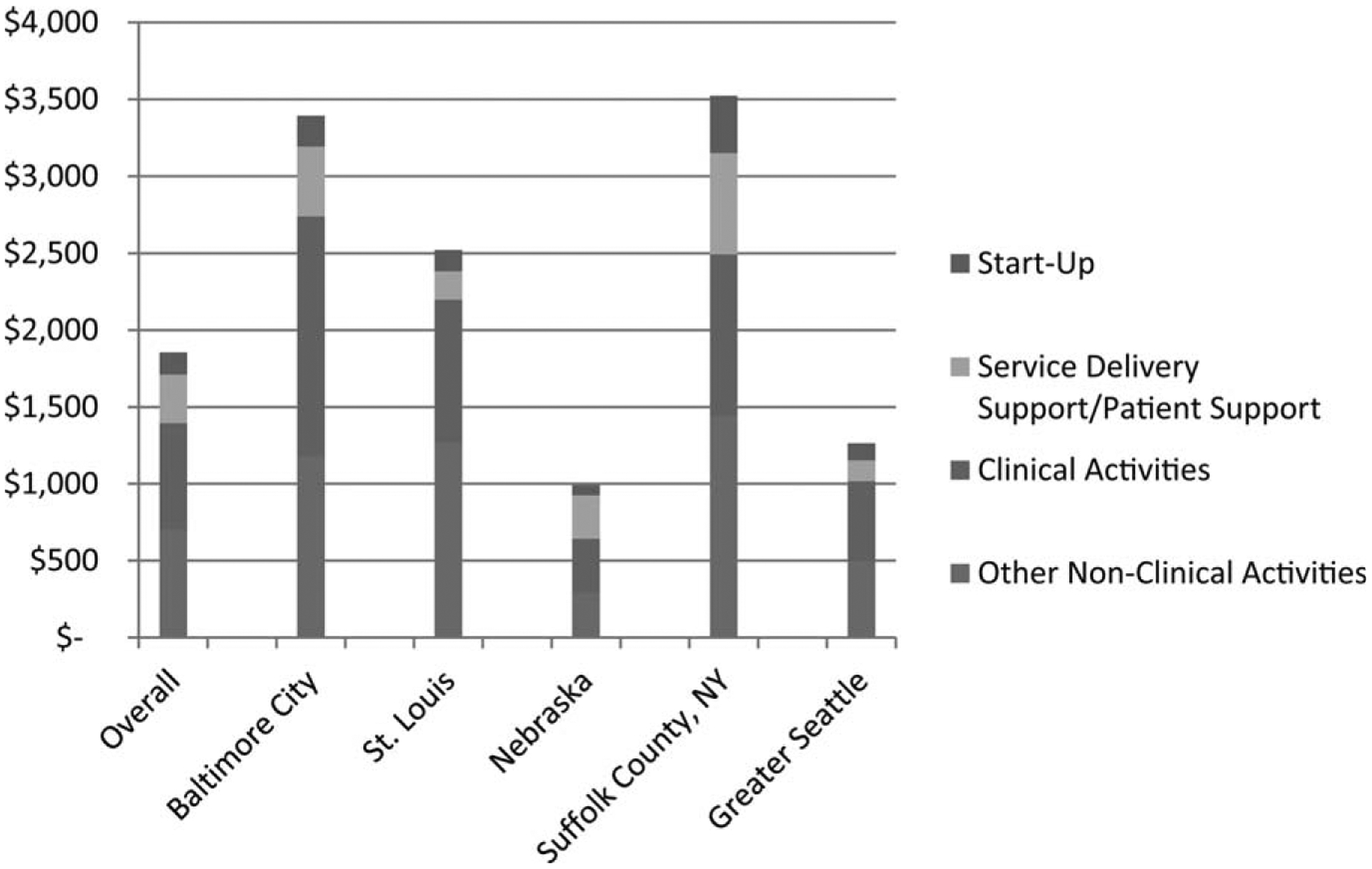
This chart illustrates the cost per person screened overall and by site for the start-up and implementation phases. Costs are adjusted using the regional Consumer Price Index to allow for systematic comparisons across sites. Baltimore City and Suffolk County, New York were colonoscopy programs, and the others provided a mix of fecal occult blood testing and FOBT and colonoscopy.
Figure 3 is a scatter plot indicating the number of individuals screened by each site on the horizontal axis and the cost per person screened on the vertical axis. In general, the larger the number of individuals screened by the site, the lower the total cost per person. Both Nebraska and Greater Seattle had the lowest costs ($925 and $1154, respectively) while screening the largest number of individuals, and New York and Maryland screened fewer individuals but had higher costs ($3152 and $3193, respectively).
Figure 3.
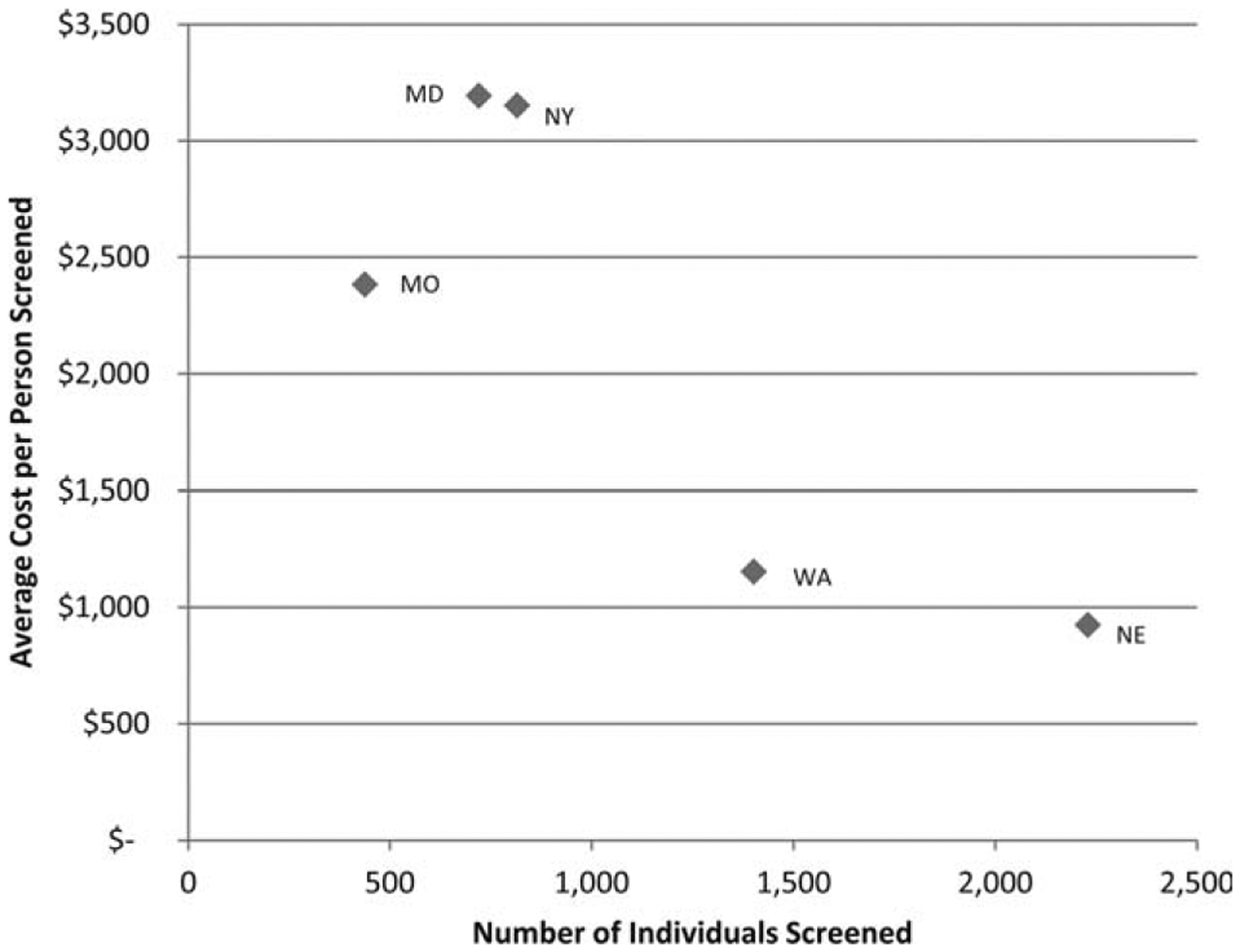
This chart illustrates the average cost per person screened according to the number screened by site during the implementation phase. Costs are adjusted using the regional Consumer Price Index to allow for systematic comparisons across sites. Baltimore City and Suffolk County, New York were colonoscopy programs, and the others provided a mix of fecal occult blood testing and colonoscopy.
DISCUSSION
In this report, we present details of the actual costs incurred during the start-up and implementation phases of the 5 CRCSDP sites. Although the CDC CRCSDP provided most of the funding for the sites, in-kind contributions and other sources, such as state funds, were important for the successful execution of these programs. Overall, start-up costs were about 10% of the total for the 4-year demonstration, and costs incurred during start-up should be considered for successful planning and development of future public health programs.
The largest single cost category for the program was the clinical service delivery cost associated with providing screening and diagnostic services, but there was substantial variation among the sites. This variation could be caused by several factors, including the mix of low-cost and high-cost screening tests used, which varied across program years; reimbursement rates negotiated by the sites with the providers; and underlying patient characteristics, such as the risk of developing colorectal cancer,4 which affected how many follow-up tests were required. In addition to the cost of clinical service delivery, the cost of service delivery support/patient support activities also was substantial across all sites. This clearly highlights the finding that sites invested substantial resources in recruiting underserved populations, assessing patient eligibility, enrolling and educating patients, referring patients with gastrointestinal symptoms out of the screening program, assisting patients as they received screening and diagnostic services, and treating diagnosed cancers. These activities are generally not reimbursable by health insurers but are required by colorectal cancer screening sites to facilitate the clinical service delivery process and ensure screening adherence. These are essential functions of a successful program, regardless of the funding source.
Overall, the sites spent a significant proportion of their funding on nonclinical activities. This highlights the considerable expenditure involved in implementing comprehensive colorectal cancer screening programs, especially to underserved populations that, traditionally, may have lower health literacy, face more significant barriers to screening, and have less access to medical services.18,19 In budgeting for future programs, sites need to anticipate the funding needs related to nonclinical program components, including program management, service delivery support/patient support, public education and outreach, quality assurance and professional development, partnership development and maintenance, data collection and tracking, program evaluation (ie, data analysis), and other activities. These are all critical components of a successful public health program, and their importance cannot be overstated.
In addition, there is evidence that economies of scale exist in nonclinical activities costs. The average cost per person screened by sites generally decreases as the number of individuals screened increases. Clearly, nonclinical costs, such as program management, data collection and tracking, and partnership development and maintenance, are fixed costs and are not linearly related to the number of individuals screened, because there is a fixed component to these activities (for example, some level of staffing is needed before any of these services can be performed). Therefore, these costs should decrease on a per-person basis as the number of individuals screened increases. Future colorectal cancer screening programs should aim to serve adequately large populations to realize the benefits of economies of scale. However, with large sites, it is also possible that diseconomies of scale (services provided at increased per-person cost) could occur, which would reduce the overall efficiency of the programs. Diseconomies could be experienced, for example, because of difficulties in managing several providers who may have different processes. Further research is needed to identify the potential threshold (ie, the number of individuals screened) at which diseconomies may set in.
Although we took specific steps to ensure that the estimation methods were comparable among the sites, there are a few limitations to this analysis. First, we adjusted for differences in the cost of living by using the regional CPI, but this may not have controlled adequately for all variations among the sites. Second, our findings are based on only 5 sites, and differences among the sites in terms of patient population, provider supply, partnerships, and other factors that were not specifically included in this assessment could result in the cost differences that we have identified. Third, we did not include the cost of treating colonoscopy-related complications in this assessment, because these costs were not assessed. Fourth, program-level data did not separate screening and diagnostic costs for each grantee. Fifth, our assessment of clinical service delivery costs did not incorporate the recommended screening intervals of 1 year for FOBT and 10 years for colonoscopy, because our unit of analysis was not the patient but the program year. Such assessments have been performed using decision analytic models and clinical costs over a lifetime.7,8 The nonclinical activities costs identified in this study could be incorporated into these models to provide a more comprehensive assessment of the total cost of program operations. Finally, we did not break down costs by specific screening test type but, instead, included them in another article in this supplement,10 which focuses on the individual and comparative clinical costs in each program site.
Our findings illustrate the importance of including economic evaluation during the design phase of future colorectal cancer screening programs. Substantial costs can be incurred in performing nonclinical activities that are essential to support clinical service delivery. In addition, service delivery support activities are a large part of the total cost of screening programs and should be taken into consideration when making funding decisions. Future studies should assess how these activities can be performed in a more cost-effective manner to ensure the design and implementation of effective and efficient colorectal cancer screening programs.
FUNDING SUPPORT
The Colorectal Cancer Screening Demonstration Program evaluated in this supplement was funded by the Centers for Disease Control and Prevention Funding Opportunity Number RFA AA030. Funding for the economic evaluation was also provided by the Centers for Disease Control and Prevention (Contract No. 200-2002-00575, Task order 009).
Footnotes
The articles in this supplement were commissioned based on participation in evaluating the Centers for Disease Control and Prevention-funded Colorectal Cancer Screening Demonstration Program.
The opinions or views expressed in this supplement are those of the authors and do not necessarily reflect the opinions or recommendations of the journal editors, the American Cancer Society, John Wiley & Sons, Inc., or the Centers for Disease Control and Prevention.
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
REFERENCES
- 1.Seeff LC, DeGroff A, Tangka F, et al. Development of a federally funded demonstration colorectal cancer screening program [serial online]. Prev Chronic Dis. 2008;5:A64. [PMC free article] [PubMed] [Google Scholar]
- 2.Tangka FK, Subramanian S, Bapat B, et al. Cost of starting colorectal cancer screening programs: results from 5 federally funded demonstration programs [serial online]. Prev Chronic Dis. 2008;5:A47. [PMC free article] [PubMed] [Google Scholar]
- 3.DeGroff A, Holden D, Goode Green S, Boehm J, Seeff LC, Tangka F. Start-up of the colorectal cancer screening demonstration program [serial online]. Prev Chronic Dis. 2008;5:A38. [PMC free article] [PubMed] [Google Scholar]
- 4.Seeff LC, Royalty J, Helsel WE, et al. Clinical outcomes from CDC’s Colorectal Cancer Screening Demonstration Program. Cancer. 2013;119(suppl 15):2820–2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC). Colorectal Cancer Clinical Data Elements. Atlanta, GA: CDC; 2012. Available at: http://www.cdc.gov/cancer/crccp/pdf/CCDE_Data_DefinitiontableOMB_T.pdf. [Accessed January 15, 2012.] [Google Scholar]
- 6.Vijan S, Hwang I, Inadomi J, et al. The cost-effectiveness of CT colonography in screening for colorectal neoplasia. Am J Gastroenterol. 2007;102:380–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Subramanian S, Bobashev G, Morris RJ. Modeling the cost-effectiveness of colorectal cancer screening: policy guidance based on patient preferences and compliance. Cancer Epidemiol Biomarkers Prev. 2009;18:1971–1978. [DOI] [PubMed] [Google Scholar]
- 8.Pignone M, Russell L, Wagner J, eds. Economic Models of Colorectal Cancer Screening in Average-Risk Adults: Workshop Summary. Washington, DC: Institute of Medicine and National Research Council; 2005. [PubMed] [Google Scholar]
- 9.Zauber A, Lansdorp-Vogelaar I, Wilschut J, van Ballegooijen M, Kuntz K. Cost-Effectiveness of DNA Stool Testing to Screen for Colorectal Cancer: Report to AHRQ and CMS from the Cancer Intervention and Surveillance Modeling Network (CISNET) for MISCAN and SimCRC Models. Rockville, MD: Agency for Health-care Research and Quality; 2007. [PubMed] [Google Scholar]
- 10.Tangka FKL, Subramanian S, Beebe MC, Hoover S, Royalty J, Seeff LC. Clinical costs of colorectal cancer screening in five federally funded demonstration programs. Cancer. 2013;119(suppl 15):2863–2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Subramanian S, Ekwueme DU, Gardner JG, Bapat B, Kramer C. Identifying and controlling for program-level differences in comparative cost analysis: lessons from the economic evaluation of the National Breast and Cervical Cancer Early Detection Program. Eval Program Plann. 2008;31:136–144. [DOI] [PubMed] [Google Scholar]
- 12.Anderson DW, Bowland BJ, Cartwright WS, Bassin G. Service-level costing of drug abuse treatment. J Subst Abuse Treat. 1998;15:201–211. [DOI] [PubMed] [Google Scholar]
- 13.French MT, Dunlap LJ, Zarkin GA, McGeary KA, McLellan AT. A structured instrument for estimating the economic cost of drug abuse treatment: the Drug Abuse Treatment Cost Analysis Program (DATCAP). J Subst Abuse Treat. 1997;14:445–455. [DOI] [PubMed] [Google Scholar]
- 14.Salome HJ, French MT, Miller M, McLellan AT. Estimating the client costs of addiction treatment: first findings from the client drug abuse treatment cost analysis program (Client DATCAP). Drug Alcohol Depend. 2003;71:195–206. [DOI] [PubMed] [Google Scholar]
- 15.Drummond M, Schulpher M, Torrance G, O’Brien B, Stoddard G, eds. Methods for the Economic Evaluation of Health Care Programme. 3rd ed. Oxford, United Kingdom: Oxford University Press; 2005. [Google Scholar]
- 16.Castro G, Seeff L, Azrak F, et al. Outpatient colonoscopy complications in the CDC’s Colorectal Cancer Screening Demonstration Program: a prospective analysis. Cancer. 2013;119(suppl 15):2849–2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.US Bureau of Labor Statistics. Consumer Price Index for All Urban Consumers (CPI-U): Regions, by Expenditure Category and Commodity and Service Group. Washington, DC: US Bureau of Labor Statistics; 2008. [Google Scholar]
- 18.Swan J, Breen N, Graubard BI, et al. Data and trends in cancer screening in the United States: results from the 2005 National Health Interview Survey. Cancer. 2010;116:4872–4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54:78–93. [DOI] [PubMed] [Google Scholar]


