Abstract
Leucocytozoonosis is a vector-borne infection of birds, caused by members of the haemosporidian genus Leucocytozoon. The clinical presentation may range from asymptomatic to severe disease. Consequences of Leucocytozoon infection on blood profiles remain to be described, especially for different host species in the wild. In the current study, the prevalence of Leucocytozoon infection was determined in wild nestlings of three European raptor species, the common buzzard (Buteo buteo, n = 464), red kite (Milvus milvus, n = 46) and northern goshawk (Accipiter gentilis, n = 18). Among 528 nestlings, 51.9% (n = 274) were infected with Leucocytozoon spp., whereby the highest prevalence was found in common buzzards (54.9%), followed by red kites (32.6%) and northern goshawks (22.2%). For a subset of 87 individuals (50 common buzzards, 29 red kites, 8 northern goshawks), a detailed analysis of differential leukocyte counts and several blood chemistry parameters in response to infection was conducted: AP (alkaline phosphatase), AST (aspartate aminotransferase), GLDH (glutamate dehydrogenase), LDH (lactate dehydrogenase), GGT (gamma glutamyl transferase), CK (creatine kinase), BuChE (butyrylcholinesterase), BA (bile acids), ALB (albumin) and TP (total protein). Even though in the physiological range, infected nestlings displayed significantly increased levels of heterophils, aspartate aminotransferase, lactate dehydrogenase, bile acids and butyrylcholinesterase, but decreased lymphocyte and monocyte values compared to uninfected ones. Furthermore, significant species differences with regard to blood parameters, but no sex differences were found. Overall, obtained results show a high prevalence, but a low pathogenicity of Leucocytozoon spp. in wild raptor chicks, presumably resulting from coevolutionary adaptation, but show signatures of infection in the haematological and blood chemistry profiles.
Keywords: Avian malaria, Blood parasites, Buteo buteo, Milvus milvus, Accipiter gentilis, Haematology, Blood chemistry
Graphical abstract
Highlights
-
•
Leucocytozoon prevalence in nestlings of three wild raptor species was 52%.
-
•
Common buzzards (Buteo buteo) showed the highest prevalence (55%).
-
•
Examined blood parameter values varied with species, age and infection status.
-
•
Leucocytozoon infections were associated with increased AST and BuChE.
-
•
Leucocytozoon infections in Accipitridae nestlings seem to be of minor pathogenicity.
1. Introduction
Life-threatening malaria is caused by blood parasites of the genus Plasmodium while related haemosporidian parasites of the genera Haemoproteus and Leucocytozoon can cause a suite of similar conditions in diverse vertebrate hosts (Atkinson et al., 2008; Valkiunas, 2005). These haemosporidian parasites are transmitted by blood-feeding insects and occur in numerous bird species worldwide (Bensch et al., 2009; Clark et al., 2014; Valkiūnas and Iezhova, 2017), whereby Leucocytozoon is particularly widespread in raptors (Krone et al., 2008; Lierz et al., 2002).
Plasmodium, Haemoproteus and Leucocytozoon spp. have been repeatedly associated with considerable pathology in various bird species and clinical signs like anemia, anorexia, depression, neurological disorder, blindness or even sudden death, particularly in weak or injured individuals (Atkinson et al., 2008; Atkinson and van Riper, 1991; Bennett et al., 1993; Donovan et al., 2008; Raida and Jaensch, 2000; Remple, 2004). Infections may lead to tissue damage of several organs (Donovan et al., 2008; Valkiūnas and Iezhova, 2017). However, severe clinical disease may rather be an exception in most wild birds, but systematic studies of haemosporidian infections in wild hosts are scarce (Atkinson and van Riper, 1991; Bennett et al., 1993). The complex life cycle of Leucocytozoon spp. includes transmission by blood-feeding blackflies (Simuliidae), as well as blood and tissue stages in the bird host (Santiago-Alarcon et al., 2012; Valkiunas, 2005). In contrast to Plasmodium, Leucocytozoon spp. do not undergo erythrocytic merogony, but initially multiply in hepatocytes (Valkiūnas and Iezhova, 2017). Further multiplication occurs in numerous organs, with formation of so-called megalomeronts. These often establish in the spleen, while the liver is rarely affected, presumably due to local immunity initiated during hepatic merogony (Valkiūnas and Iezhova, 2017). Although their exact significance within the developmental cycle is unclear, megalomeronts can lead to an increased size of the affected organ, and potentially impair its functional capacity (Valkiunas, 2005; Valkiūnas and Iezhova, 2017). In general, haemoparasite infections in wild birds are common and improved knowledge regarding their impact on haematology and blood chemistry values can represent an important tool for diagnostics and treatment in avian medicine (Bensch et al., 2009; Clark et al., 2014; Schoenle et al., 2017). So far, Plasmodium and Haemoproteus infections have been linked with changes in blood chemistry and haematology in birds (Campos et al., 2014; Dawson and Bortolotti, 1997; Fokidis et al., 2008; Hõrak et al., 1998; Krams et al., 2013; Townsend et al., 2018; Williams, 2005), whereas Leucocytozoon infections seem to cause only minor or no changes in blood profiles (Raida and Jaensch, 2000; Townsend et al., 2018). Additionally, many Leucocytozoon spp. are rather taxon-specific and thought to infect nestlings without causing severe pathology (Chitty and Lierz, 2008). However, it remains to be elucidated whether blood cell count and blood chemistry values reflect infection status and parasitemia of raptors, and indicate how nestlings cope with the infection.
In this study, we examined chicks of three common European raptor species, the common buzzard (Buteo buteo), red kite (Milvus milvus) and the northern goshawk (Accipiter gentilis), belonging to the family Accipitridae. According to previous studies, these species are more likely to be infected with Leucocytozoon than with Plasmodium or Haemoproteus spp. (Hanel et al., 2016; Krone et al., 2008; Pérez-Rodríguez et al., 2013). Forest raptors show a particularly high Leucocytozoon prevalence (Chakarov et al., 2008; Krone et al., 2001; Lierz et al., 2002), presumably because of their habitat overlap with vectors and common within-family transmission (Chakarov et al., 2015, 2020). Due to its abundance, the common buzzard is also one of the most common raptor species presented in veterinary practices in Central Europe, indicating the need for an in-depth investigation of leucocytozoonosis in this species (Hatt and Clauss, 2016).
The purpose of this study was to investigate Leucocytozoon prevalence among wild nestlings of three different raptor species in northwestern Germany and to investigate the effects of the infection on haematological and blood chemistry profiles. on these raptors.
2. Materials and methods
2.1. Study site and sampling
The prevalence of Leucocytozoon spp. was examined in 528 raptor nestlings belonging to three species, common buzzard (n = 464), red kite (n = 46) and northern goshawk (n = 18). Sampling of nestlings was permitted by the ethics commission of the Animal Care and Use Committee of the German North Rhine-Westphalia State Office for Nature, Environment and Consumer Protection (Landesamt für Natur, Umwelt und Verbraucherschutz Nordrhein-Westfalen) under reference number 84-02.04.2017.A147.
The study area covers more than 300 km2 in Eastern Westphalia, Germany and is characterised by an agricultural landscape interspersed with many small and large forest areas (Krüger, 2004). In March and April 2019, nests of breeding raptors were identified and revisited for sampling between May and July 2019. At the time of sampling, chicks were between two and seven weeks old. All raptor chicks were collected from their nests and lowered to the ground for sampling. Sampling was performed within 30 min as described by Chakarov et al. (2008) before chicks were returned to the nest. Tarsus length was measured with a calliper to the nearest 0.1 mm as well as wing length to the nearest 1 mm with a ruler. Weight was measured with a spring scale (Pesola, Schindellegi, Switzerland) to the nearest 5 g. The age of chicks at sampling was determined from wing length using standard growth curves for the respective species (Bijlsma, 1997; Mammen and Stubbe, 1995). In common buzzards, the sex of the individuals as well as light, intermediate and dark plumage of this polymorphic species was additionally determined (Bijlmsa, 1999, Melde, 1995). A blood sample of up to 0.5 ml was taken from the Vena ulnaris of each bird, using a 0.3 mm (30G) x 8 mm needle. Immediately after collection, two blood smears were prepared and air-dried. The remaining blood sample was instantly transferred into 1.3 ml heparinized tubes. The heparinized blood was centrifuged at 2000×g for 10 min (LabNet centrifuge, C1301), and the superficial plasma was stored at −80 °C until further investigation. The remaining cell-rich blood fraction was diluted with 1 ml phosphate buffered saline (PBS) and stored at −20 °C.
2.2. Examination of blood smears
Blood smears were fixed in absolute ethanol within 12 h, dried and stained with 10x diluted Giemsa stock solution (48900, Sigma-Aldrich, St. Louis, USA). All smears were scanned for haemosporidian parasites by light microscopy (Axioskope, Zeiss, Oberkochen, Germany) at 400x magnification. Approximately 10.000 erythrocytes per chick were scanned. Infection intensity was categorized as follows: not infected (no detectable parasites), low (1–10 parasites per 10.000 erythrocytes), medium (>10–100 parasites per 10.000 erythrocytes) and high infection intensity (>100 parasites per 10.000 erythrocytes).
2.3. Haematology and blood chemistry
A subsample of 87 nestlings was chosen for detailed comparison of haematological and blood chemistry parameters between infected and uninfected nestlings. Of these, 44 birds were infected with Leucocytozoon spp. (25 common buzzards, 15 red kites and 4 northern goshawks), and 43 were uninfected (25 common buzzards, 14 red kites and 4 northern goshawks). In the 43 uninfected individuals, absence of infection was confirmed via nested PCR, testing haemosporidian infections as described by Pérez-Rodríguez et al. (2013). Briefly, DNA was extracted from the PBS-diluted blood cells using a standard phenol-chloroform protocol (Sambrook et al., 1989). Amplification products were visualized on a 2% agarose gel stained with ethidium bromide. Furthermore, these 87 selected individuals were additionally sexed molecularly using primers 2550F (5′-GTTACTGATTCGTCTACGAGA-3′) and 2718R (5′-ATTGAAATGATCCAGTGCTTG-3′) to amplify the CHD1W (females) and CHD1Z (males) gene as previously described (Fridolfsson and Ellegren, 1999).
Per sample, 100 leukocytes where differentiated into lymphocytes, granulocytes (heterophils, eosinophils and basophils) and monocytes by morphologic and staining characteristics at 1000x magnification and the heterophil/lymphocyte ratio (H/L) was calculated (Samour, 2006). In order to estimate the total number of leukocytes per microliter blood, the number of cells counted in 10 fields at 400x magnification was multiplied by 2000 (Campbell and Ellis, 2007; Sabater and Forbes, 2014).
Blood chemistry parameters were mainly selected to represent liver function (Harr, 2006; Sabater and Forbes, 2015; Wernery et al., 2004) to detect indications of organ damage caused by developing Leucocytozoon meronts. All parameters were analysed by a commercial laboratory (SYNLAB.vet GmbH, Geesthacht, Germany). An AU680 Clinical Chemistry Analyzer (Beckman Coulter, Brea, USA) was used for photometric measurement of the following metabolite concentrations: AP (alkaline phosphatase), AST (aspartate aminotransferase), GLDH (glutamate dehydrogenase), LDH (lactate dehydrogenase), GGT (gamma-glutamyl transferase), CK (creatine kinase), BuChE (butyrylcholinesterase), BA (bile acids), ALB (albumin) and TP (total protein).
2.4. Statistical analyses
Statistical analyses were conducted using R. v. 4.0.2 (R Core Team, 2020). To determine potential factors influencing Leucocytozoon prevalence among the 528 chicks, a generalized linear mixed model (GLMM) with binomial error structure and logit link function was constructed, including bird species and age as fixed factors. Age was standardized by dividing every value by the species-specific average nestling duration (common buzzard: 45.5 days; northern goshawk: 44 days; red kite: 47.5 days) to account for variations in the length of the nestling phase (Südbeck et al., 2005). This measure is equivalent to the proportion of the entire nestling period that has been spent in the nest at sampling. Furthermore, another GLMM was calculated for the subset of common buzzards only, including the variables sex and plumage morph, which were only available for this species, in addition to body mass as fixed factors. Both models included nest ID as a random intercept to account for the fact that several individuals were sampled per brood. Full models were compared to null models including only the random intercept via likelihood ratio tests (R function “anova”).
A linear discriminant analysis (LDA) was used to investigate whether the measured blood parameters allowed discrimination between infected and non-infected nestlings in the subset of 87 individuals. Furthermore, linear models were constructed to estimate the influence of Leucocytozoon infections on the following blood parameters, which were identified as having high discriminatory power in the LDA: lymphocytes (%), monocytes (%), heterophils (%), eosinophils (%), AST, BA, BuChE, LDH and AP. Response variables were log-transformed, except for eosinophils (%). Bird species, sex and age were included as confounding factors. Interactions between predictor variables were initially included and retained in models if statistically significant (P ≤ 0.05). Multiple comparisons between levels of categorical predictors were conducted based on Tukey contrasts using the function “glht” [R package “multcomp” (Hothorn et al., 2008)] with single-step P-value adjustment. For model validation, normality and homogeneity of residuals was assessed graphically. Furthermore, full models were compared to null models including only an intercept term (R function “anova”). Finally, false discovery rate (FDR) adjustment was applied to all P-values using the Benjamini-Hochberg method.
3. Results
3.1. Prevalence of Leucocytozoon spp. in raptor nestlings
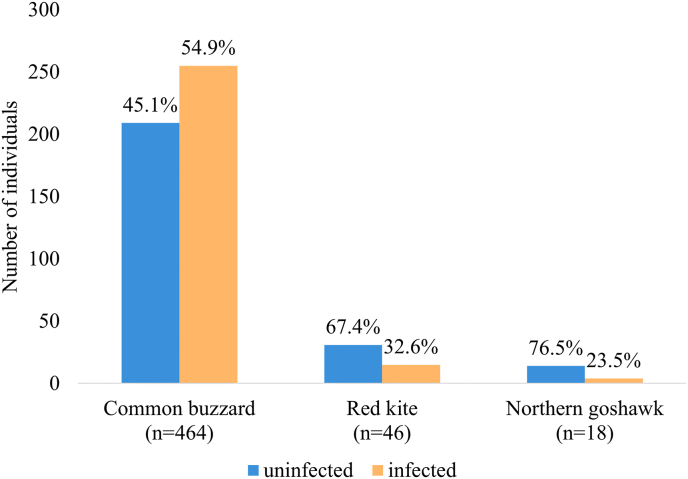
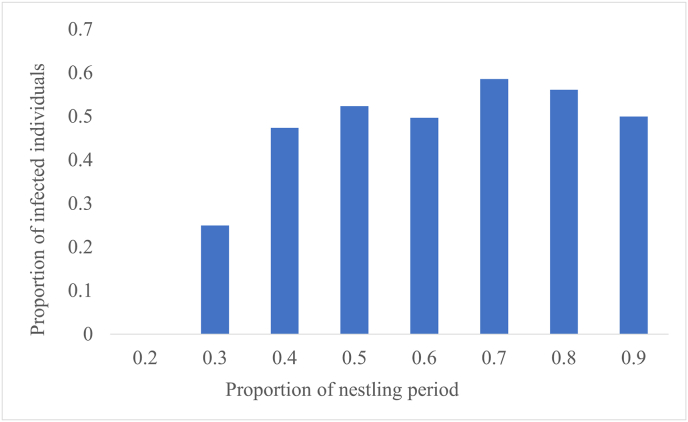
In total, 528 samples were screened for Leucocytozoon spp., comprising 464 common buzzards, 46 red kites and 18 northern goshawks. Leucocytozoon infections were detected in 51.9% (274/528) of all raptor nestlings. Prevalence in common buzzards was 54.9% (255/464), in red kites 32.6% (15/46) and in northern goshawk 22.2% (4/18) (Fig. 1). Among the 274 infected nestlings, 21.5% showed a low, 27.4% a medium and 51.1% a high infection intensity. Regarding the onset of infection, there was a steep increase in the first half of the nesting period, and by the end of this first half, more than 50% of the tested individuals were infected (Fig. 2).
Fig. 1.
Prevalence of Leucozytozoon spp. infection in nestlings of common buzzards (Buteo buteo), red kites (Milvus milvus) and northern goshawks (Accipiter gentilis). Asterisks refer to P-values ≤0.05, determined by GLMM. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 2.
Proportion of infected individuals of common buzzard (Buteo buteo), red kite (Milvus milvus) and northern goshawk (Accipiter gentilis) nestlings (n = 528) in relation to the proportion of the nestling period, determined by age and the species-specific average nestling duration. Calculated nestling periods were grouped into steps of ten percent (0.2–0.9). Each step represents the proportion of all individuals examined within this period. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Leucocytozoon prevalence in goshawks and red kites was significantly lower than in common buzzards (GLMM, P = 0.034 and P = 0.013, respectively, Table 1). Furthermore, the likelihood of infection increased with the standardised age (P = 0.027, Table 1). Among the 447 common buzzards for which these variables were available, the effect of standardised age was similar in magnitude, but did not reach statistical significance (P = 0.077). Furthermore, the likelihood of infection was not explained by sex or plumage morph (Supplementary Table 1).
Table 1.
Results of the binomial GLMM testing the effect of raptor species and age on the probability of Leucocytozoon infection among 528 raptor nestlings. The full model was significantly different from a null model containing only the random factor “nest ID” (χ2 = 14.05, Df = 3, P = 0.003). Significant P-values are printed in bold.
| Estimate | Std. Error | z | P | |
|---|---|---|---|---|
| Intercept | −1.00 | 0.58 | −1.71 | 0.08767 |
| Age, standardiseda | 2.07 | 0.94 | 2.19 | 0.02852 |
| Speciesb | ||||
| A. gentilis vs. B. buteo | −1.83 | 0.75 | −2.45 | 0.0344 |
| M. milvus vs. B. buteo | −1.23 | 0.44 | −2.79 | 0.0135 |
| M. milvus vs. A. gentilis | 0.60 | 0.84 | 0.72 | 0.7399 |
Standardised by dividing every value by the species-specific average nestling duration.
Multiple comparisons between species were conducted based on Tukey contrasts using the function “glht” (R package “multcomp”; Hothorn et al., 2008) with single-step P-value adjustment.
3.2. Effects of Leucocytozoon infection on haematological and blood chemistry parameters
Eighty-seven samples were analysed for 12 haematological and 10 blood chemistry parameters. Of the 87 individuals, a total of 48 chicks were identified as male and 39 as female. Among common buzzards, 30 were male and 20 female, among red kites 15 were male and 14 female, and among goshawks 3 were male and 5 female. Obtained haematological values are presented in Table 2, and blood chemistry values in Table 3. Due to the low levels of variation in GLDH and basophil values, these were not considered in this analysis.
Table 2.
Haematology values (means ± standard deviation) of uninfected and Leucocytozoon-infected raptor nestlings.
| Variable |
B. buteo (n = 50) |
M. milvus (n = 29) |
A. gentilis (n = 8) |
|||
|---|---|---|---|---|---|---|
| uninfected | infected | uninfected | infected | uninfected | infected | |
| White blood cells/μl | 15424 ± 7701.8 | 13520 ± 4565.08 | 16700 ± 5187.41 | 21000 ± 6457.77 | 14750 ± 6184.66 | 13000 ± 4033.2 |
| Lymphocytes/μl | 4327.12 ± 2226.76 | 2742.72 ± 1156.6 | 4043.57 ± 1706.03 | 4210 ± 2720.37 | 4627.5 ± 2393.93 | 2078 ± 336.53 |
| Lymphocytes % | 28.88 ± 9.28 | 21.08 ± 8.04 | 24.29 ± 6.52 | 20.6 ± 11.79 | 31.25 ± 10.66 | 16.75 ± 3.59 |
| Monocytes/μl | 605.12 ± 535.4 | 302.4 ± 297.16 | 958.86 ± 328.3 | 825.87 ± 589.78 | 160 ± 201.99 | 134 ± 91.94 |
| Monocytes % | 3.56 ± 1.78 | 2.12 ± 2.01 | 5.86 ± 1.46 | 3.73 ± 1.91 | 1 ± 1.41 | 1 ± 0.82 |
| Heterophils/μl | 5033.2 ± 2816.62 | 5100.96 ± 2206.5 | 6459.71 ± 2001.63 | 9262.8 ± 2839.82 | 4342.5 ± 1582.87 | 5083 ± 1488.69 |
| Heterophils % | 33.36 ± 8.69 | 37.44 ± 8.7 | 39.21 ± 7.26 | 45 ± 10.68 | 30 ± 2.94 | 39.25 ± 1.89 |
| H/L ratioa | 1.29 ± 0.58 | 2.13 ± 1.19 | 1.76 ± 0.69 | 3.56 ± 3.22 | 1.05 ± 0.4 | 2.43 ± 0.53 |
| Eosinophils/μl | 5447.04 ± 3278.82 | 5363.52 ± 2317.34 | 5223.86 ± 2918.04 | 6672.4 ± 4538.32 | 5580 ± 3160.13 | 5705 ± 2330.51 |
| Eosinophils % | 34.12 ± 11.34 | 39.28 ± 8.83 | 30.57 ± 9.91 | 30.53 ± 11.44 | 37.5 ± 11.09 | 43 ± 5.35 |
| Basophils/μl | 11.52 ± 40.47 | 10.4 ± 36.04 | 14 ± 52.38 | 28.93 ± 76.4 | 40 ± 80 | 0 ± 0 |
| Basophils % | 0.08 ± 0.28 | 0.08 ± 0.28 | 0.07 ± 0.27 | 0.13 ± 0.35 | 0.25 ± 0.5 | 0 ± 0 |
Heterophil/lymphocyte ratio.
Table 3.
Blood chemistry values (means ± standard deviation) of uninfected and Leucocytozoon-infected raptor nestlings.
| Variable |
Buteo buteo (N = 50) |
Milvus milvus (N = 29) |
Accipiter gentilis (N = 8) |
||||
|---|---|---|---|---|---|---|---|
| uninfected | infected | uninfected | infected | uninfected | infected | ||
| AST | (U/l) | 202.24 ± 52.62 | 311.72 ± 118.09 | 356.79 ± 94.4 | 375.2 ± 160.96 | 491.75 ± 259.92 | 578.75 ± 81.68 |
| (μkat/l) | 3.37 ± 0.88 | 5.2 ± 1.97 | 5.95 ± 1.57 | 6.25 ± 2.68 | 8.2 ± 4.33 | 9.65 ± 1.36 | |
| LDH | (U/l) | 699.24 ± 330.56 | 1037.64 ± 465.49 | 807.5 ± 435.22 | 693.93 ± 245.24 | 1778.75 ± 731.58 | 1916.25 ± 405.82 |
| (μkat/l) | 11.66 ± 5.51 | 17.3 ± 7.76 | 13.46 ± 7.26 | 11.57 ± 4.09 | 29.65 ± 12.2 | 31.94 ± 6.77 | |
| CK | (U/l) | 2349.04 ± 994.93 | 2295.96 ± 745.72 | 1654.71 ± 443.36 | 1282.87 ± 663.94 | 2988.75 ± 520.17 | 2667.5 ± 523.28 |
| (μkat/l) | 39.16 ± 16.59 | 38.27 ± 12.43 | 27.58 ± 7.39 | 21.39 ± 11.07 | 49.82 ± 8.67 | 44.47 ± 8.72 | |
| BA | (μmol/I) | 24.39 ± 12.29 | 31.41 ± 14.02 | 34.47 ± 19.1 | 46.74 ± 17.97 | 21.23 ± 12.66 | 36.21 ± 18.97 |
| BuChE | (KU/I) | 1.7 ± 0.3 | 1.95 ± 0.28 | 1.37 ± 0.22 | 1.36 ± 0.33 | 1.76 ± 0.27 | 2.23 ± 0.51 |
| (μkat/l) | 28.37 ± 4.96 | 32.51 ± 4.64 | 22.79 ± 3.73 | 22.64 ± 5.42 | 29.38 ± 4.48 | 37.09 ± 8.57 | |
| GGT | (U/I) | 7.04 ± 1.93 | 7.36 ± 2.74 | 7 ± 2.6 | 8.33 ± 4.15 | 9 ± 6.16 | 7 ± 2.83 |
| (μkat/l) | 0.12 ± 0.03 | 0.12 ± 0.05 | 0.12 ± 0.04 | 0.14 ± 0.07 | 0.15 ± 0.1 | 0.12 ± 0.05 | |
| GLDH | (U/I) | 2 ± 0 | 3.06 ± 3.87 | 2.61 ± 2.27 | 2 ± 0 | 2 ± 0 | 5.75 ± 7.5 |
| (μkat/l) | 0.03 ± 0 | 0.05 ± 0.06 | 0.04 ± 0.04 | 0.03 ± 0 | 0.03 ± 0 | 0.1 ± 0.13 | |
| AP | (U/l) | 425.24 ± 192.69 | 381.84 ± 124.75 | 391.21 ± 82.72 | 324.8 ± 57.64 | 571.25 ± 112.35 | 587.75 ± 26.59 |
| (μkat/l) | 7.09 ± 3.21 | 6.37 ± 2.08 | 6.52 ± 1.38 | 5.41 ± 0.96 | 9.52 ± 1.87 | 9.8 ± 0.44 | |
| TP | (g/dl) | 3.25 ± 0.53 | 3.28 ± 0.3 | 3.71 ± 0.45 | 3.61 ± 0.62 | 3.9 ± 0.84 | 4.1 ± 0.47 |
| (g/l) | 32.48 ± 5.28 | 32.8 ± 3.03 | 37.07 ± 4.51 | 36.07 ± 6.17 | 39 ± 8.45 | 41 ± 4.69 | |
| ALB | (g/dl) | 0.95 ± 0.21 | 0.96 ± 0.18 | 1.16 ± 0.31 | 1.14 ± 0.27 | 1.21 ± 0.65 | 1.35 ± 0.29 |
| (g/l) | 9.46 ± 2.14 | 9.56 ± 1.8 | 11.55 ± 3.13 | 11.39 ± 2.71 | 12.05 ± 6.54 | 13.53 ± 2.85 | |
*detection limit: 2 U/l.
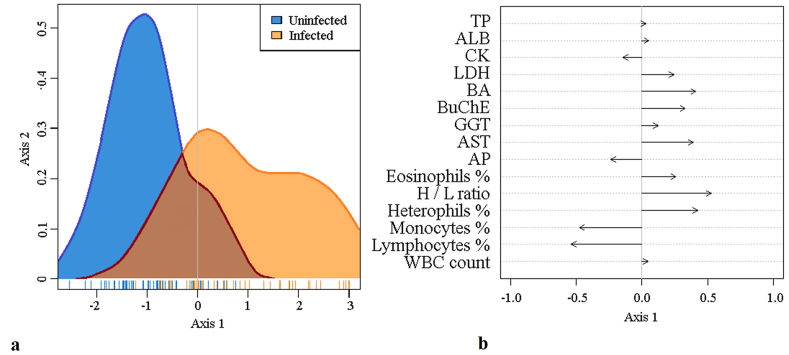
Overall, there was a considerable overlap in most blood parameter values between infected and uninfected nestlings (Table 3). However, the LDA indicated that the blood profiles differed slightly between uninfected and infected nestlings (Fig. 3). The variance-covariance matrix was homogeneous and the multivariate distribution of the variables followed a normal distribution. An error rate of 30.1% [standard deviation (SD): 0.78] was estimated in predicting infection status (infected or uninfected) in seven subsets randomly selected from the complete dataset, using a cross validation method.
Fig. 3.
Results of linear discriminant analysis (LDA) comparing 15 selected blood parameters between uninfected and infected raptor nestlings. a) Distribution of the LDA according to uninfected and infected nestlings. b) Correlation plot of the first axis of the 15 selected variables.
Based on the LDA result, linear models were employed to quantify the influence of infection on nine predominantly affected blood parameters (lymphocytes, monocytes, heterophils, eosinophils, AST, BA, BuChE, LDH, AP), taking bird species, sex and age into consideration. Significant differences in blood parameter values with respect to infection status were observed for seven parameters (Table 4, Supplementary Table 2). Infection was significantly associated with elevated heterophil, AST, LDH, BA and BuChE, but decreased lymphocyte and monocyte values. Regarding these seven parameters, all were within the species-specific standard range, with the exception of LDH in northern goshawks, where the values of uninfected also deviated from references (Chitty and Lierz, 2008; Gelli et al., 2009; Hanauska-Brown et al., 2003; Hernandez et al., 1990; Stout et al., 2010).
Table 4.
Summary of the results of linear models assessing the influence of Leucocytozoon infections on nine individual blood parameters in raptor nestlings, taking bird species, sex and age as well as interactions among those factors into account. All response variables, except eosinophils and AP, were log-transformed before analysis. All P-values are FDR-adjusted. Detailed model results are provided in Supplementary Table 2.
| Blood parameter | Estimate (P-Value) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Infection | Species | Age | Sex | Infection * Age | Infection * Species | Infection * Sex | Species * Age | |
| Lymphocytes (%) | −0.34 (0.001) | NS | NS | NS | NS | NS | NS | NS |
| Monocytes (%) | −0.464 (<0.001) | −4.204 (0.012)2 | NS | NS | NS | NS | NS | 0.126 (0.016)4 |
| −4.869 (<0.003)3 | ||||||||
| Heterophils (%) | 0.109 (0.046) | 0.252 (<0.001)1 | −0.01 (0.012) | NS | NS | NS | NS | NS |
| Eosinophils (%) | NS | −8.729 (0.010)1 | 0.445 (0.046) | NS | NS | NS | NS | NS |
| 10.67 (0.046)3 | ||||||||
| AST (U/I) | 1.399 (<0.001) | 0.369 (<0.001)1 | 0.017 (0.035) | NS | −0.041 (<0.001) | NS | NS | NS |
| 0.797 (<0.001)2 | ||||||||
| 0.428 (0.005)3 | ||||||||
| BA (U/I) | 0.313 (0.012) | 0.447 (0.008)1 | NS | NS | NS | NS | NS | NS |
| BuChE (KU/I) | 0.126 (0.035) | −0.348 (<0.001)1 | NS | NS | NS | NS | NS | NS |
| 0.501 (<0.001)3 | ||||||||
| LDH (U/I) | 0.384 (0.004) | −0.364 (0.046)1 | NS | NS | NS | −0.486 (0.023)5 | NS | NS |
| 0.682 (0.018)2 | ||||||||
| 1.045 (<0.001)3 | ||||||||
| −77.090 (0.017)1 | ||||||||
| AP (U/I) | NS | 150.900 (0.001)2 | 7.178 (0.001) | NS | NS | NS | NS | NS |
| 228.000 (<0.001)3 | ||||||||
NS = not significant, 1Milvus milvus vs. Buteo buteo, 2Accipiter gentilis vs. Buteo buteo, 3Accipiter gentilis vs. Milvus milvus, 4age in Accipiter gentilis vs. Buteo buteo, 5infection in Milvus milvus vs. Buteo buteo.
3.3. The influence of sex, species and age on blood parameters of raptor nestlings
Neither infection status nor haematological and blood chemistry parameters differed between male and female nestlings in any of the three raptor species (Table 4). In contrast, species differed significantly regarding all haematological parameters, except lymphocyte proportion. The heterophil fraction was approximately 28% higher in red kites compared to common buzzards (Supplementary Table 2). Red kites also had higher BA values, whereas their eosinophil and BuChE levels were lower than in both common buzzards and northern goshawks. Moreover, significantly lower proportions of monocytes were observed in northern goshawk than in the other two species, while significant differences in AST, AP and LDH levels appeared between all examined species. In addition, age had a significant positive effect on AST, AP and eosinophil counts.
Furthermore, significant interaction effects were detected. In older raptor chicks, the effect of Leucocytozoon infections on AST was weaker than in younger individuals. Also, the difference in monocyte levels between northern goshawks and common buzzards decreased with age in both infected and uninfected nestlings. In red kites, the effect of Leucocytozoon infections on LDH was smaller compared to buzzards. No apparent sex-infection interactions appeared to explain any additional variation in blood parameters.
4. Discussion
Studies on the prevalence of Leucocytozoon and other haemosporidian parasites in raptors are numerous and mostly suggest high prevalence, in some cases up to 100% (Jeffries et al., 2015; Valkiunas, 2005). Recent studies have shown Leucocytozoon infections to be highly prevalent also in passerine species with similar or shorter altricial periods than the studied raptors (Schmid et al., 2017; Schumm et al., 2019; Shurulinkov et al., 2018). In this study, Leucocytozoon infections were detected in nestlings of all three studied raptor species, as expected from related results (Bensch et al., 2009). Preliminary genetic data showed that in all three species the infecting parasites belonged to the Leucocytozoon toddi-group. The genetic lineages represented in kites and buzzards were MILANS04 and MILVUS01, while ACGE02 of the species Leucocytozoon mathisi was responsible for the infections of goshawks (unpublished results). Since at the end of the first half of the nestling period more than 50% of the at this time examined nestlings tested positive, transmission by simuliid vector appears to be very effective in the first weeks of life. Another interesting finding was that 51% of all infected nestlings showed a high infection intensity. However, haemosporidian prevalence and infection intensities can be quite variable between years, geographic locations and depend mostly on the occurrence of vectors (Isaksson et al., 2013; Shurulinkov and Chakarov, 2006; Sol et al., 2000; Yohannes et al., 2009). Seasonal effects can also be of great importance, as adult birds affected by stress and sexual hormones can experience spring relapses during the breading season, thus facilitating parasite transmission to the offspring (Ashford et al., 1990; Krone et al., 2001).
Among the raptor species, we observed significantly higher infection rates in common buzzards (55%) compared to 33% in red kites and 22% in northern goshawks. This confirms previous studies showing a substantially higher Leucocytozoon prevalence in buzzards than in kites (Krone et al., 2001; Lierz et al., 2002). This difference may result from the different habitat niches used by these sympatric species, as the microhabitats used by buzzards in our study area are also preferred by simuliid vectors of Leucocytozoon spp. (Chakarov et al., 2020). Although black flies occur almost globally, the specific habitat and location of the host nest appears to play a major role in the infection probability of raptor nestlings, even on a small spatial scale (Crosskey, 1990; Tella et al., 1999). Additionally, raptor species differ in their immunological response to Leucocytozoon infections, facilitating differences in prevalence rates in the raptor species populating our study area. Notable, due to the considerably smaller number of tested red kites and northern goshawks, these prevalences are subject to greater uncertainty. In addition to species differences, the spent nestling period significantly predicted the infection probability. An increasing risk of infection with age has been shown for common buzzards and sparrowhawks before, presumably due to the cumulatively longer exposure to the vectors (Svobodová et al., 2015), whereas no correlation was evident in great tits with a considerably shorter nestling period and relatively simuliid-protected cavity nesting (Norte et al., 2009; Chakarov et al., 2021). Previously reported effects of sex on haemosporidian prevalence are inconsistent. Similar to the present investigation, most studies found no difference in infection prevalence between sexes (Granthon and Williams, 2017; Krone et al., 2001; Lierz et al., 2002; Norte et al., 2009), but several exceptions exist (Dey et al., 2010; Schmid et al., 2017). Overall, variability in the infection prevalence of different raptor species requires further research, e.g. with regard to the preferences of blackflies in host selection (Chakarov et al., 2021).
Blood parameter analyses revealed significant differences in blood cell counts and blood chemistry values between the three study species as well as correlations with age, whereas sex did not appear to affect examined blood parameters. However, blood parameter differences between species, even between closely related ones, are common (Fudge, 2000) and there is substantial variation of reference values within each species [e.g. Hernandez et al. (1990), Gelli et al. (2009) for common buzzards; Hanauska-Brown et al. (2003), Stout et al. (2010) for northern goshawks]. Besides species variations, we found significant blood parameter differences between uninfected raptor chicks and those infected with Leucocytozoon species. Nevertheless, with the exception of eosinophils and LDH of northern goshawks, significantly differing values were still within the described standard range, if available for these species (Chitty and Lierz, 2008; Gelli et al., 2009; Hanauska-Brown et al., 2003; Hernandez et al., 1990; Stout et al., 2010). However, since Leucocytozoon prevalence is usually high in most Accipitriformes populations, it is important to note that such infections may increase the variation of within- and between-population reference values.
In mammals, parasitaemia leads to an increase of eosinophilic granulocytes (Behm and Ovington, 2000; Dombrowicz and Capron, 2001), but this is not generally the case in birds (Campbell and Ellis, 2007; Fudge, 2000; Wernery et al., 2004). Some previous studies point to higher eosinophil counts during parasite infections (Clark and Kerry, 1993; Samour et al., 1996). In the present study, eosinophils were among the blood parameter values potentially discriminating infected and non-infected birds based on the LDA analysis, however, the effect of infection on eosinophil proportion was not significant after FDA correction of P-values. Therefore, the possible link between increasing eosinophil proportion and Leucocytozoon spp. infection in birds needs to be investigated further. Compared to reference values by Hanauska-Brown et al. (2003) and Chitty and Lierz (2008), our eosinophil counts are higher in infected but also in uninfected northern goshawks, and, furthermore, eosinophil counts increased with increasing age of the nestlings. However, the above reference values are based on adult birds, which show lower counts compared to nestlings (Hernández and Margalida, 2010). Our study also demonstrated a clear increase of the H/L ratio with infection. This cell ratio is known as an indicator for physiological stress and illness in birds (Davis et al., 2008). Nevertheless, we presume Leucocytozoon infections to be a mild stressor in nestlings, as increased H/L ratios are based on the relative numbers of heterophils and lymphocytes, while overall levels of WBCs in infected chicks did not show considerable changes in our study, most likely due to decreased lymphocytes and monocytes despite the infection. Regarding monocytes, decreased levels in connection with blood parasite infection have not yet been reported. Possibly, low monocyte levels might result from a relatively early stage of infection (Campos et al., 2014; Motta et al., 2013). However, these slow-reacting immune cells (Fudge, 2000) show a very low density and can therefore not be used as informative for Leucocytozoon infections.
Regarding blood chemistry profiles, the enzymes selected in the present study do not have a known function in the vascular system, so elevated levels are typically used as indicators of increased synthesis or tissue damage (Prinzinger et al., 2012). To differentiate between liver and muscle damage, AST and LDH should be evaluated in combination with CK (Sabater and Forbes, 2015). We found a significant increase of AST and LDH but not CK in infected nestlings, representing an indication for liver cell damage (Wernery et al., 2004). However, as already mentioned, most values were within the wide standard range for these raptors (Gelli et al., 2009; Stout et al., 2010). Less frequently evaluated but strongly specific, increased levels of BA along with AST, as detected in infected nestlings in the present study, provide further indication of liver dysfunction (Horowitz et al., 2016). However, preceding food intake, for which we could not control, is known to have a considerable impact on BA levels (Lumeij, 1997; Lumeij and Remple, 1992). A significant postprandial increase occurs in birds as well as mammals, as BA is released from the gall bladder for intestinal fat digestion (Lumeij, 1997). Nevertheless, liver damage can be differentiated from food intake by significantly higher values (Cray et al., 2008), but reference values of plasma BA are not yet available for most bird species.
Our study demonstrates an increase of AST and AP production in older chicks, corresponding to increasing consumption and production during growth (Harr, 2006; Joseph, 1999). While values of LDH significantly differed between infected and uninfected chicks, the direction was not consistent between species. Therefore, this parameter cannot be generalized as informative of infection status across species. Based on interactions between infection, species and age, we also suspect that a variety of different factors influence blood chemistry values.
In contrast to leucocytozoonosis, infections with the genus Plasmodium have been shown to induce stronger effects on blood parameters in birds (Townsend et al., 2018; Williams, 2005). Potentially, this may be an effect of the host generality of many Plasmodium species, while many Haemoproteus and the Leucocytozoon lineages studied here have more closely coevolved with their hosts, resulting in minimal damage. Based on the overall rather minor shifts in blood parameters in our study, we conclude that Leucocytozoon is of relatively low pathogenicity in Accipitridae nestlings aged between two and seven weeks. Although changes in blood parameters between infected and uninfected chicks were significantly different, these are not in the range indicating serious organ damage. Correspondingly, the tissue damage caused by Leucocytozoon may be low and readily compensated in growing birds (Valkiūnas and Iezhova, 2017). This is supported by long-term observations showing no correlation between nestling infection status and fledging success (unpublished results), while juvenile or adult and especially injured and weakened birds may show more obvious alterations due to blood parasitism (Valkiunas, 2005).
Our findings imply that Leucocytozoon infections are not reliably detected based on a single blood parameter. However, increased levels of AST and BA might be suggestive of a present Leucocytozoon infection and in such cases, we suggest to perform molecular or blood smear examinations of Leucocytozoon parasites. In general, due to the high infection frequency in the wild, young raptors in rehabilitation should be tested and treated if necessary.
5. Conclusions
The present study showed leucocytozoonosis to be highly prevalent in raptor nestlings, especially in common buzzards. However, according to the blood profile results, the pathogenicity of the parasite appears to be only low. In addition to differences in blood parameters between uninfected and infected individuals, we also found variance with age and among raptor species. Future long-term studies are necessary to evaluate further and prolonged effects of Leucocytozoon infections in fledged raptor hosts.
Declarations of competing interest
None.
Acknowledgements
We wish to thank the Wirtschaftsgenossenschaft deutscher Tierärzte (WDT) for donating heparin tubes and slides. We are grateful to Karl Rohn for the help with statistical analyses, and to all field workers helping to collect samples. Furthermore, we want to thank Elke Hippauf, Ann-Christin Polikeit and Katrin Lehmann for excellent technical assistance. This publication was supported by Deutsche Forschungsgemeinschaft and University of Veterinary Medicine Hannover, Foundation within the funding programme Open Access Publishing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijppaw.2021.10.009.
Funding
Parts of the study were funded by the Deutsche Forschungsgemeinschaft (DFG - German Research Foundation) as part of the SFB TRR 212 “A Novel Synthesis of Individualisation across Behaviour, Ecology and Evolution: Niche Choice, Niche Conformance, Niche Construction (NC3)” (project number 396780709) and a research grant for the project “Short- and long-term consequences of malaria-like infections in birds” (project number 398434413).
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Ashford R., Wyllie I., Newton I. Leucocytozoon toddi in british sparrowhawks Accipiter nisus: observations on the dynamics of infection. J. Nat. Hist. 1990;24:1101–1107. [Google Scholar]
- Atkinson C.T., Thomas N.J., Hunter D.B. Wiley-Blackwell; Ames, Iowa: 2008. Parasitic Diseases of Wild Birds. [Google Scholar]
- Atkinson C.T., van Riper C., III . In: Bird-parasite Interactions: Ecology, Evolution, and Behaviour. Loye J.E., Zuk M., editors. Oxford University Press; Oxford: 1991. Pathogenicity and epizootiology of avian haematozoa: Plasmodium, leucocytozoon, and haemoproeus; pp. 19–48. [Google Scholar]
- Behm C., Ovington K. The role of eosinophils in parasitic helminth infections: insights from genetically modified mice. Parasitol. Today. 2000;16:202–209. doi: 10.1016/s0169-4758(99)01620-8. [DOI] [PubMed] [Google Scholar]
- Bennett G., Peirce M., Ashford R. Avian haematozoa: mortality and pathogenicity. J. Nat. Hist. 1993;27:993–1001. [Google Scholar]
- Bensch S., Hellgren O., Pérez-Tris J. MalAvi: a public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol. Ecol. Resour. 2009;9:1353–1358. doi: 10.1111/j.1755-0998.2009.02692.x. [DOI] [PubMed] [Google Scholar]
- Bijlsma R.G. KNNV Uitgeverij; Utrecht, NL: 1997. Handleiding Veldonderzoek Roofvogels. [Google Scholar]
- Bijlmsa R. Sex determination of nestling common buzzards Buteo buteo. Limosa. 1999;72:1–10. [Google Scholar]
- Campbell T.W., Ellis C.K. third ed. Blackwell Publishing; Ames, Iowa: 2007. Avian and Exotic Animal Hematology and Cytology. [Google Scholar]
- Campos S.D., Pires J.R., Nascimento C.L., Dutra G., Torres-Filho R.A., Toma H.K., Brener B., Almosny N.R. Analysis of hematologic and serum chemistry values of Spheniscus magellanicus with molecular detection of avian malarial parasites (Plasmodium spp.) Pesqui. Vet. Bras. 2014;34:1236–1242. [Google Scholar]
- Chakarov N., Boerner M., Krüger O. Fitness in common buzzards at the cross‐point of opposite melanin–parasite interactions. Funct. Ecol. 2008;22:1062–1069. [Google Scholar]
- Chakarov N., Kampen H., Wiegmann A., Werner D., Bensch S. Blood parasites in vectors reveal a united blackfly community in the upper canopy. Parasites Vectors. 2020;13:1–8. doi: 10.1186/s13071-020-04177-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakarov N., Linke B., Boerner M., Goesmann A., Krüger O., Hoffman J.I. Apparent vector‐mediated parent‐to‐offspring transmission in an avian malaria‐like parasite. Mol. Ecol. 2015;24:1355–1363. doi: 10.1111/mec.13115. [DOI] [PubMed] [Google Scholar]
- Chakarov N., Veiga J., Ruiz-Arrondo I., Valera F. Atypical behavior of a black fly species connects cavity-nesting birds with generalist blood parasites in an arid area of Spain. Parasites Vectors. 2021;14:1–9. doi: 10.1186/s13071-021-04798-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitty J., Lierz M. Small Animal Veterinary Association; Quedgeley: 2008. BSAVA Manual of Raptors, Pigeons and Passerine Birds. [Google Scholar]
- Clark J., Kerry K. Diseases and parasites of penguins. Korean J. Polar Res. 1993:79–96. [Google Scholar]
- Clark N.J., Clegg S.M., Lima M.R. A review of global diversity in avian haemosporidians (Plasmodium and Haemoproteus: haemosporida): new insights from molecular data. Int. J. Parasitol. 2014;44:329–338. doi: 10.1016/j.ijpara.2014.01.004. [DOI] [PubMed] [Google Scholar]
- Cray C., Gautier D., Harris D.J., Arheart K.L. Changes in clinical enzyme activity and bile acid levels in psittacine birds with altered liver function and disease. J. Avian Med. Surg. 2008;22:17–24. doi: 10.1647/2006-011R.1. [DOI] [PubMed] [Google Scholar]
- Crosskey R.W. Wiley; Chichester: 1990. The Natural History of Blackflies. [Google Scholar]
- Davis A., Maney D., Maerz J. The use of leukocyte profiles to measure stress in vertebrates: a review for ecologists. Funct. Ecol. 2008;22:760–772. [Google Scholar]
- Dawson R.D., Bortolotti G.R. Are avian hematocrits indicative of condition? American kestrels as a model. J. Wildl. Manag. 1997:1297–1306. [Google Scholar]
- Dey A., Begum N., Paul S., Noor M., Islam K. Prevalence and pathology of blood protozoa in pigeons reared at Mymensingh district, Bangladesh. Int. J. Biores. 2010;2:25–29. [Google Scholar]
- Dombrowicz D., Capron M. Eosinophils, allergy and parasites. Curr. Opin. Immunol. 2001;13:716–720. doi: 10.1016/s0952-7915(01)00284-9. [DOI] [PubMed] [Google Scholar]
- Donovan T.A., Schrenzel M., Tucker T.A., Pessier A.P., Stalis I.H. Hepatic hemorrhage, hemocoelom, and sudden death due to Haemoproteus infection in passerine birds: eleven cases. J. Vet. Diagn. Invest. 2008;20:304–313. doi: 10.1177/104063870802000307. [DOI] [PubMed] [Google Scholar]
- Fokidis H.B., Greiner E.C., Deviche P. Interspecific variation in avian blood parasites and haematology associated with urbanization in a desert habitat. J. Avian Biol. 2008;39:300–310. [Google Scholar]
- Fridolfsson A.-K., Ellegren H. A simple and universal method for molecular sexing of non-ratite birds. J. Avian Biol. 1999:116–121. [Google Scholar]
- Fudge A.M. Saunders; Philadelphia: 2000. Laboratory Medicine: Avian and Exotic Pets. [Google Scholar]
- Gelli D., Ferrari V., Franceschini F., Lai O., Laricchiuta P., Zanella A., Bernardini D., Romagnoli S. Serum biochemistry and electrophoretic patterns in the Eurasian buzzard (Buteo buteo): reference values. J. Wildl. Dis. 2009;45:828–833. doi: 10.7589/0090-3558-45.3.828. [DOI] [PubMed] [Google Scholar]
- Granthon C., Williams D.A. Avian malaria, body condition, and blood parameters in four species of songbirds. Wilson J. Ornithol. 2017;129:492–508. [Google Scholar]
- Hanauska-Brown L.A., Dufty A.M., Jr., Roloff G.J. Blood chemistry, cytology, and body condition in adult Northern goshawks (Accipiter gentilis) J. Raptor Res. 2003;37:299–306. [Google Scholar]
- Hanel J., Doležalová J., Stehlíková Š., Modrý D., Chudoba J., Synek P., Votýpka J. Blood parasites in northern goshawk (Accipiter gentilis) with an emphasis to Leucocytozoon toddi. Parasitol. Res. 2016;115:263–270. doi: 10.1007/s00436-015-4743-1. [DOI] [PubMed] [Google Scholar]
- Harr K.E. In: Clinical Avian Medicine. Harrison G.J., Lightfoot T.L., editors. Spix Pub; Palm Beach, FL: 2006. Diagnostic value of biochemistry; pp. 611–629. [Google Scholar]
- Hatt J.M., Clauss M. Tierärztliche Betreuung von Greifvögeln: entwicklungen im Zeitraum von 1985 bis 2015. Schweiz. Arch. Tierheilk. 2016;158:639–645. doi: 10.17236/sat00084. [DOI] [PubMed] [Google Scholar]
- Hernández M., Margalida A. Hematology and blood chemistry reference values and age-related changes in wild bearded vultures (Gypaetus barbatus) J. Wildl. Dis. 2010;46:390–400. doi: 10.7589/0090-3558-46.2.390. [DOI] [PubMed] [Google Scholar]
- Hernandez M., Martin S., Fores P. Clinical hematology and blood chemistry values for the common buzzard. J. Raptor Res. 1990;24:113–119. [Google Scholar]
- Hõrak P., Ots I., Murumägi A. Haematological health state indices of reproducing great tits: a response to brood size manipulation. Funct. Ecol. 1998;12:750–756. [Google Scholar]
- Horowitz I.H., Yanco E.G., Landau S., Nadler-Valency R., Anglister N., Bueller-Rosenzweig A., Apelbom-Halbersberg T., Cuneah O., Hanji V., Bellaiche M. Whole blood cholinesterase activity in 20 species of wild birds. J. Avian Med. Surg. 2016;30:122–126. doi: 10.1647/2014-044. [DOI] [PubMed] [Google Scholar]
- Hothorn T., Bretz F., Westfall P. Simultaneous inference in general parametric models. Biom. J. 2008;50:346–363. doi: 10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
- Isaksson C., Sepil I., Baramidze V., Sheldon B.C. Explaining variance of avian malaria infection in the wild: the importance of host density, habitat, individual life-history and oxidative stress. BMC Ecol. 2013;13:1–11. doi: 10.1186/1472-6785-13-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffries M.I., Miller R.A., Laskowski M.D., Carlisle J.D. High prevalence of leucocytozoon parasites in nestling northern goshawks (Accipiter gentilis) in the Northern Great Basin, USA. J. Raptor Res. 2015;49:294–302. [Google Scholar]
- Joseph V. Raptor hematology and chemistry evaluation. Vet. Clin. North Am. Exot. Anim. Pract. 1999;2:689–699. doi: 10.1016/s1094-9194(17)30116-0. [DOI] [PubMed] [Google Scholar]
- Krams I., Suraka V., Rantala M., Sepp T., Mierauskas P., Vrublevska J., Krama T. Acute infection of avian malaria impairs concentration of haemoglobin and survival in juvenile altricial birds. J. Zool. 2013;291:34–41. [Google Scholar]
- Krone O., Priemer J., Streich J., Sommer P., Langgemach T., Lessow O. Haemosporida of birds of prey and owls from Germany. Acta Protozool. 2001;40:281–290. [Google Scholar]
- Krone O., Waldenström J., Valkiūnas G., Lessow O., Müller K., Iezhova T., Fickel J., Bensch S. Haemosporidian blood parasites in European birds of prey and owls. J. Parasitol. 2008;94:709–715. doi: 10.1645/GE-1357.1. [DOI] [PubMed] [Google Scholar]
- Krüger O. The importance of competition, food, habitat, weather and phenotype for the reproduction of buzzard Buteo buteo. Bird Stud. 2004;51:125–132. [Google Scholar]
- Lierz M., Göbel T., Schuster R. Untersuchungen zum Vorkommen von Parasiten bei heimischen Greifvögeln und Eulen. Berl. Münchener Tierärztliche Wochenschr. 2002;115:43–52. [PubMed] [Google Scholar]
- Lumeij J. In: Clinical Biochemistry of Domestic Animals. Kaneko J.J., Harvey J.W., Bruss M.L., editors. Elsevier; 1997. Avian clinical biochemistry; pp. 857–883. [Google Scholar]
- Lumeij J., Remple J. Plasma bile acid concentrations in response to feeding in peregrine falcons (Falco peregrinus) Avian Dis. 1992:1060–1062. [PubMed] [Google Scholar]
- Mammen U., Stubbe M. Alterseinschätzung und Brutbeginn des Rotmilans (Milvus milvus) Vogel Umw. 1995;8:91–98. [Google Scholar]
- Melde M. Der Mäusebussard - Buteo buteo. Westarp/Spektrum Akademischer Verlag; Magdeburg: 1995. [Google Scholar]
- Motta R.O.C., Marques M.V.R., Junior F.C.F., de Assis Andery D., Horta R.S., Peixoto R.B., Lacorte G.A., de Abreu Moreira P., Leme F.d.O.P., Melo M.M. Does haemosporidian infection affect hematological and biochemical profiles of the endangered Black-fronted piping-guan (Aburria jacutinga)? PeerJ. 2013;1:e45. doi: 10.7717/peerj.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norte A.C., Araujo P.M., Sampaio H.L., Sousa J.P., Ramos J.A. Haematozoa infections in a great tit Parus major population in central Portugal: relationships with breeding effort and health. Ibis. 2009;151:677–688. [Google Scholar]
- Pérez-Rodríguez A., de la Puente J., Onrubia A., Pérez-Tris J. Molecular characterization of haemosporidian parasites from kites of the genus Milvus (Aves: Accipitridae) Int. J. Parasitol. 2013;43:381–387. doi: 10.1016/j.ijpara.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Prinzinger R., Misovic A., Nagel B. Cuvillier Verlag; Göttingen: 2012. Aviäre Hämatologie: Das Vogelblut: Struktur, Funktion, Diagnose und Parasiten. [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2020. R: A Language and Environment for Statistical Computing. [Google Scholar]
- Raida S., Jaensch S.M. Central nervous disease and blindness in Nankeen kestrels (Falco cenchroides) due to a novel Leucocytozoon-like infection. Avian Pathol. 2000;29:51–56. doi: 10.1080/03079450094289. [DOI] [PubMed] [Google Scholar]
- Remple J.D. Intracellular hematozoa of raptors: a review and update. J. Avian Med. Surg. 2004;18:75–88. [Google Scholar]
- Sabater M., Forbes N. Avian haematology and biochemistry 1. haematology. In Pract. 2014;36:510–518. [Google Scholar]
- Sabater M., Forbes N. Avian haematology and biochemistry 2. biochemistry. In Pract. 2015;37:139–142. [Google Scholar]
- Sambrook J., Fritsch E., Maniatis T. Cold Spring Harbor Laboratory Press, Cold Spring Harbor; New York, USA: 1989. Molecular Cloning: A Laboratory Manual. [Google Scholar]
- Samour J. In: Clinical Avian Medicine. Harrison G.J., Lightfoot T.L., editors. Spix Pub; Palm Beach, FL: 2006. Diagnostic value of hematology; pp. 587–609. [Google Scholar]
- Samour J., D'Aloia M.-A., Howlett J. Normal haematology of captive saker falcons (Falco cherrug) Comp. Haematol. Int. 1996;6:50–52. [Google Scholar]
- Santiago‐Alarcon D., Palinauskas V., Schaefer H.M. Diptera vectors of avian haemosporidian parasites: untangling parasite life cycles and their taxonomy. Biol. Rev. 2012;87:928–964. doi: 10.1111/j.1469-185X.2012.00234.x. [DOI] [PubMed] [Google Scholar]
- Schmid S., Fachet K., Dinkel A., Mackenstedt U., Woog F. Carrion crows (Corvus corone) of southwest Germany: important hosts for haemosporidian parasites. Malar. J. 2017;16:369. doi: 10.1186/s12936-017-2023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenle L.A., Kernbach M., Haussmann M.F., Bonier F., Moore I.T. An experimental test of the physiological consequences of avian malaria infection. J. Anim. Ecol. 2017;86:1483–1496. doi: 10.1111/1365-2656.12753. [DOI] [PubMed] [Google Scholar]
- Schumm Y.R., Wecker C., Marek C., Wassmuth M., Bentele A., Willems H., Reiner G., Quillfeldt P. Blood parasites in Passeriformes in central Germany: prevalence and lineage diversity of haemosporida (Haemoproteus, Plasmodium and Leucocytozoon) in six common songbirds. PeerJ. 2019;6 doi: 10.7717/peerj.6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shurulinkov P., Chakarov N. Prevalence of blood parasites in different local populations of reed warbler (Acrocephalus scirpaceus) and great reed warbler (Acrocephalus arundinaceus) Parasitol. Res. 2006;99:588–592. doi: 10.1007/s00436-006-0202-3. [DOI] [PubMed] [Google Scholar]
- Shurulinkov P., Spasov L., Stoyanov G., Chakarov N. Blood parasite infections in a wild population of ravens (Corvus corax) in Bulgaria. Malar. J. 2018;17:33. doi: 10.1186/s12936-018-2179-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sol D., Jovani R., Torres J. Geographical variation in blood parasites in feral pigeons: the role of vectors. Ecography. 2000;23:307–314. [Google Scholar]
- Stout J.D., Brinker D.F., Driscoll C.P., Davison S., Murphy L.A. Serum biochemistry values, plasma mineral levels, and whole blood heavy metal measurements in wild northern goshawks (Accipiter gentilis) J. Zoo Wildl. Med. 2010;41:649–655. doi: 10.1638/2009-0258.1. [DOI] [PubMed] [Google Scholar]
- Südbeck P., Andretzke H., Gedeon K., Schikore T., Schröder K., Fischer S., Sudfeldt C. Max-Planck-Institut für Ornithologie Radolfzell; 2005. Methodenstandards zur Erfassung der Brutvögel Deutschlands. [Google Scholar]
- Svobodová M., Weidinger K., Peške L., Volf P., Votýpka J., Voříšek P. Trypanosomes and haemosporidia in the buzzard (Buteo buteo) and sparrowhawk (Accipiter nisus): factors affecting the prevalence of parasites. Parasitol. Res. 2015;114:551–560. doi: 10.1007/s00436-014-4217-x. [DOI] [PubMed] [Google Scholar]
- Tella J.L., Blanco G., Forero M.G., Gajón Á., Donázar J.A., Hiraldo F. Habitat, world geographic range, and embryonic development of hosts explain the prevalence of avian hematozoa at small spatial and phylogenetic scales. Proc. Natl. Acad. Sci. U.S.A. 1999;96:1785–1789. doi: 10.1073/pnas.96.4.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend A.K., Wheeler S.S., Freund D., Sehgal R.N., Boyce W.M. Links between blood parasites, blood chemistry, and the survival of nestling American crows. Ecol. Evol. 2018;8:8779–8790. doi: 10.1002/ece3.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valkiunas G. CRC press; Boca Raton, Florida, USA: 2005. Avian Malaria Parasites and Other Haemosporidia. [Google Scholar]
- Valkiūnas G., Iezhova T.A. Exo-erythrocytic development of avian malaria and related haemosporidian parasites. Malar. J. 2017;16:101. doi: 10.1186/s12936-017-1746-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernery U., Wernery R., Kinne J., Samour J. Schluetersche; Hannover: 2004. Colour Atlas of Falcon Medicine. [Google Scholar]
- Williams R. Avian malaria: clinical and chemical pathology of Plasmodium gallinaceum in the domesticated fowl Gallus gallus. Avian Pathol. 2005;34:29–47. doi: 10.1080/03079450400025430. [DOI] [PubMed] [Google Scholar]
- Yohannes E., Križanauskienė A., Valcu M., Bensch S., Kempenaers B. Prevalence of malaria and related haemosporidian parasites in two shorebird species with different winter habitat distribution. J. Ornithol. 2009;150:287–291. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.