Graphical abstract
Abbreviations: •NO, nitric oxide radical; AP-1, activator protein 1; ATP, adenosine triphosphate; BHT, butylated hydroxytoluene; C31H28N2Na4O13S, xylenol tetrasodium; C5FeN6Na2O, sodium nitroprusside; CAT, catalase; CDNB, 1-chloro-2,4-dinitrobenzene; Cu(NO3)2.3H2O, copper II nitrate; CYCP, cyclophosphamide; DNA, deoxyribonucleic acid; DTNB, 5,5ˈ-dithiobis(2-nitrobenzoic acid); eNOS, endothelial nitric oxide synthase; FeSO4.7H2O, Iron (II) sulfate heptahydrate; G6PDH, glucose-6-phosphate dehydrogenase; GSH, reduced glutathione; GSPx, glutathione peroxidase; GSR, glutathione reductase; GSSG, oxidized glutathione; GST, glutathione-S-transferase; H2O2, hydrogen peroxide; H3PO3, phosphoric acid; HO•, hydroxyl radical; HSCs, hepatic stellate cells; i.p., intraperitoneally; K2HPO4, dipotassium hydrogen phosphate; KCl, potassium chloride; LDH, lactate dehydrogenase; MAPKs, mitogen-activated protein kinases; MDA, malondialdehyde; metHb, methemoglobin; MMP, matrix metalloprotease; mRNA, messenger ribonucleic acid; Na2HPO4, disodium hydrogen phosphate; NAD+, nicotinamide adenine dinucleotide; NADH, nicotinamide adenine dinucleotide reduced; NADPH, nicotinamide adenine dinucleotide phosphate reduced; NaH2PO4, sodium dihydrogen phosphate; NF-κB, nuclear factor kappa B; NH4OH, ammonium hydroxide; NO, nitric oxide; NO2−, nitrite; NO3−, nitrate; NOAEL, no-observed-adverse-effect level; Nrf2, nuclear factor-erythroid factor 2-related factor 2; O2•–, superoxide radical; O2HbFe2+, oxyhemoglobin; OONO−, peroxynitrite radical; PBS, phosphate-buffered saline; PUFA, Polyunsaturated fatty acids; RNS, reactive nitrogen species; ROS, reactive oxygen species; R-Smad, Smad activated receptor; SOD, superoxide dismutase; TBA, 2-thiobarbituric acid; TBARS, thiobarbituric acid reactive substances; TGF-β, transforming growth factor-β; TLR, toll-like receptor; TROOH, total hydroperoxide; VLDL, very low density lipoprotein; α-SMA, alpha smooth muscle actin
Keywords: Cyclophosphamide, Erythrocytotoxicity, Lipid profile, Oxidative stress, Antioxidants, Naringin
Highlights
-
•
Naringin inhibits and/or scavenge in vitro free radicals.
-
•
Exposure to cyclophosphamide (CYCP) invoked erythrocytotoxicity.
-
•
Erythrocytotoxicity was characterized by oxidative stress and dyslipidaemia.
-
•
LDH (a marker of anaerobic ATP generation) was also depleted.
-
•
Naringin pre-administered reversed the CYCP-induced erythrocytotoxicity.
Abstract
Earlier reports have shown that Cyclophosphamide (CYCP), an anti-malignant drug, elicited cytotoxicity; and that naringin has several beneficial potentials against oxidative stress and dyslipidaemias. We investigated the influence of naringin on free radical scavenging, cellular integrity, cellular ATP, antioxidants, oxidative stress, and lipid profiles in the CYCP-induced erythrocytotoxicity rat model. Rats were pretreated orally by gavage for fourteen consecutive days with three doses (50, 100, and 200 mg/kg) naringin before single CYCP (200 mg/kg, i.p.) administration. Afterwards, the rats were sacrificed. Naringin concentrations required for 50 % scavenging hydrogen peroxide and nitric oxide radical were 0.27 mg/mL and 0.28 mg/mL, respectively. Naringin pretreatment significantly (p < 0.05) protected erythrocytes plasma membrane architecture and integrity by abolishing CYCP-induced decrease in the activity of erythrocyte LDH (a marker of ATP). Pretreatment with naringin remarkably (p < 0.05) reversed CYCP-induced decreases in the erythrocytes glutathione levels, activities of glutathione-S-transferase, catalase, glutathione peroxidase, and glutathione reductase; attenuated CYCP-mediated increases in erythrocytes levels of malondialdehyde, nitric oxide, and major lipids (cholesterol, triacylglycerol, phospholipids, and non-esterified fatty acids). Taken together, different acute pretreatment doses of naringin might avert CYCP-mediated erythrocytes dysfunctions via its antioxidant, free-radical scavenging, and anti-dyslipidaemia properties.
1. Introduction
Podsiedlik et al. [1] have documented that exposure to xenobiotics, including some drugs, is associated with erythrocyte abnormalities, such as perturbation of erythrocytes cytoskeleton, viscosity, morphology and sizes, ion permeability, reduced erythrocytes life span, schistocytes, and haemolysis. Losses of haemoglobin compartmentalization also cause loss of arginase 1 from erythrocyte resulting in decreased systemic NO and impaired endothelial function [2]. Others include depletion in the antioxidant arsenal, intracellular ATP, and glycolytic enzymes leakage due to lysed erythrocytes. Lactate dehydrogenase (LDH, a marker of ATP generation and haemolysis) activity of erythrocytes and serum could offer information about the status of erythrocyte membrane integrity since erythrocytes depend solely on glucose metabolized through the glycolytic pathway as the only source of intracellular ATP generation [3]. Xenobiotics have been reported to elicit erythrocytes LDH and ATP depletion [4,5]. Deceased ATP, in turn, is known to be responsible for erythrocytes membrane fragmentation, deformation, vesiculation, vacuolization, and eryptosis [6,7].
All these perturbations in the constituents of erythrocytes and erythrocyte ghost enumerated above make erythrocytes serve as valuable in vitro and in vivo candidates for evaluating xenobiotics induced cytotoxicity.
Cyclophosphamide (CYCP) is a synthetic alkylating cytostatic drug used as an antitumor and immunosuppression agent [8]. CYCP cytotoxic antitumor activities through DNA and protein alkylation are associated with its metabolic product, phosphoramide mustard, whereas another metabolic product, acrolein, elicits its noxious side effects [9]. Phosphoramide mustard, acrolein, and other free reactive species mediated by CYCP majorly target erythrocytes ghost and intracellular enzymes of the circulating erythrocytes [10] and promote their antioxidant machinery depletion, which in turn promotes oxidative stress, including alteration in erythrocyte morphology and erythrocyte ghost conformation, protein cross-linking, lipid peroxidation, haemolysis, and cell death. So, the therapeutic usages of CYCP have been limited owing to its cytotoxicity and myriads adverse side effects [11]. On the other hand, acrolein induces disruption of serum lipid homeostasis characterized by hypercholesterolemia and hypertriacylglycerolemia, promoting atherosclerosis [12]. However, to the best of our knowledge, no study has been carried out on the effect of CYCP on erythrocyte lipid.
Dietary polyphenols, including naringin, has been reported for their antioxidants, anti-inflammatory, and blood lipid-lowering activities [13,14]. Naringin promotes antioxidant signalling pathways by various mechanisms. First, naringin quenches the toxic effect of free radicals by increasing the mRNA and protein levels of nuclear factor erythroid 2-related factor 2 (Nrf2), a transcription factor that activates biosynthesis of antioxidant machinery [15] and controls the transactivation of over 500 cytoprotective genes, including GSH synthesis and regeneration, NADPH generation, phase-I, II and III xenobiotics detoxifying enzymes, etc. [70]. Second, naringin prevents apoptosis by inhibiting the intrinsic (initiated by mitochondria) and extrinsic pathways [16]. Thirdly, naringin equally prevents profibrogenic pathways and reduces fibrosis by (i) inhibiting the transforming growth factor-β (TGF-β) binding to its receptors (TβRI and TβRII) and activation, (ii) reducing tissue TGF-β levels, (iii) inhibiting mitogen-activated protein kinases (MAPKs) activation, (iv) inhibiting the phosphorylation, preventing the nuclear translocation, and downregulating the mRNA expression of cytoplasmic transcription factors Smads activated receptor (R-Smads) 3/2 with the consequent inhibition of α-SMA and collagen expression, (v) inactivating hepatic stellate cells (HSCs), reducing extracellular matrix (ECM), (vi) downregulating matrix metalloprotease (MMP)-2 and MMP-9, toll-like receptors (TLRs) 4 and 2 (vii) decreasing translocation and DNA binding of nuclear factor-κB (NF-κB) and activator protein 1 (AP-1) [[16], [17], [18], [19]].
We postulated that naringin might be effective in protecting CYCP-induced erythrocytotoxicity. Hence, the current study was designed to validate the toxicity consequence of CYCP, examine the possible protective effect of naringin against CYCP-mediated erythrocytotoxicity, and explain naringin fundamental biochemical mechanisms of action against erythrocytes damage and intracellular oxidation utilizing in vitro and in vivo models.
2. Materials and methods
2.1. Chemicals and reagents
Cyclophosphamide was obtained from Pfizer International (NY, USA). Lactate dehydrogenase (LDH), total protein, cholesterol, and triacylglycerol kits were products of Randox Laboratories Limited (Admore, Crumlin, Co-Antrim, UK). Griess reagent kit was purchased from Cayman Chemical (Ann Arbor, Michigan, USA). Sodium nitroprusside (C5FeN6Na2O), hydrogen peroxide (H2O2), butylated hydroxytoluene (BHT), vitamin C, 2- thiobarbituric acid (2, 6-dihydroxypyrimidine-2-thiol; TBA), dipotassium hydrogen phosphate (K2HPO4), potassium dihydrogen phosphate (KH2PO4), potassium chloride (KCl), reduced glutathione (GSH), oxidized glutathione (GSSG), 1-chloro-2, 4-dinitrobenzene (CDNB), Ellman’s reagent DTNB [5,5ˈ-dithiobis(2-nitrobenzoic acid)], pyrogallol, reduced nicotinamide adenine dinucleotide phosphate (NADPH), and xylenol tetrasodium (C31H28N2Na4O13S), chloroform, isopropanol, triton X-100, ethanol, ammonium ferrothiocyanate, copper II nitrate (Cu(NO3)2.3H2O), triethanolamine, oxalic acid bis(cyclohexylidenehydrazide), and ammonium hydroxide (NH4OH) were procured from Aldrich Sigma Chemical Company (St. Louis, MO, USA). Iron (II) sulfate heptahydrate (FeSO4.7H2O) was imported from Merck KGaA, Darmstadt, Germany. Sulfuric acid was supplied by Ajax Finechem, Australia. All other chemicals and reagents were of analytical grade and were procured from British Drug Houses (Poole, UK).
2.2. Determination of hydrogen peroxide (H2O2) scavenging activity of naringin
Hydrogen peroxide scavenging activity of naringin was determined as described by Ruch et al. [20]. Briefly, a control solution of hydrogen peroxide (40 mM) was prepared in phosphate buffer (50 mM, pH 7.4). The absorbance of H2O2 was determined by absorption at 230 nm using a Jenway 615UV/VIS Spectrophotometer (Staffordshire, UK). Naringin (500 μL, concentrations 0.1, 0.2, 0.3, 0.4 and 0.5 mg/mL) in distilled water was added to H2O2. After 10 min incubation, absorbance was determined at 230 nm against a blank solution containing phosphate buffer without H2O2. The percentage of H2O2 scavenging was calculated by the formula:
| % H2O2 scavenged = (A0 – A1/A0) x 100 |
Where A0 was the absorbance of the control and A1 was the absorbance of the test.
2.3. Determination of nitric oxide radical (•NO) scavenging activity of naringin
Nitric oxide radical generated from the decomposition of sodium nitroprusside (C5FeN6Na2O) in an aqueous solution (physiological pH 7.2) is oxidized by oxygen to nitrite and nitrate. Nitrite level is a measure of nitric oxide produced; the nitrite reacts with a colour developing agent such as Griess reagent [sulphanilamide (1 mL, 2%) in H3PO3 (5%) and N-(1-naphthyl)ethylenediamine dihydrochloride (1 mL, 0.2 %)] to form a purple azoic compound, [•NO → NO2−, + sulphanilamide → diazonium salt, + N-(1-naphthyl)ethylenediamine → Azo dye] which gives the concentration of •NO in the solution; hence, the ability of naringin to scavenge •NO was determined according to the method described by Green et al. [21]. Briefly, sodium nitroprusside (2 mL, 10 mM) was dissolved in phosphate buffer saline (1.0 mL, pH 7.4) and mixed with naringin [2.0 mL, (0.1−0.5 mg/mL)]. The resultant mixture was incubated at 25 °C for 60 min. A similar procedure was repeated without naringin as blank, which served as control. Incubated sample (5.0 mL) and Griess reagent (5.0 mL) were mixed and then incubated again at room temperature for 30 min, after which the absorbance of the chromophore formed was measured at 540 nm. Percentage inhibition of the nitrite oxide generated was measured by relating the absorbance values of control (ascorbic acid) and naringin. The amount of nitric oxide radical inhibition was calculated by the formula:
| % inhibition of •NO = [A0 - A1]/A0 x 100 |
Where A0 was the absorbance before reaction and A1 was the absorbance after the reaction has taken place with Griess reagent.
2.4. Animal model
Experimental animals, female albino Wistar rats weighing 150 g – 200 g, were inbred at the Animal House, Department of Biochemistry, Federal University of Agriculture, Abeokuta, Nigeria. They were housed in a plastic suspended cage placed in a well-ventilated, temperature-controlled (25 °C) rat house with standard 12-h light/12-h dark cycles. The rats were provided with standard pellet chow and given fresh water ad libitum. All animals were acclimatized for one week before the commencement of the experiments. All the animals received humane care according to the conditions outlined in the ‘Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Science (NAS) and published by the National Institute of Health [22]. The institution approved an experimental number of the researcher is 12/2373.
2.5. Experimental protocol
Twenty-five rats were divided into five groups (n = 5), using a simple randomization method. Five rats per group were used based on 3R (replacement, reduction and refinement) principles [23]. Before the experiments, naringin was mixed with the vehicle (sweetened condensed milk diluted in water in a 1:6 ratio). Aliquots of different concentrations (50, 100, and 200 mg/kg bw naringin) were administered orally to the animals with a gavage needle daily for fourteen consecutive days at 8.00–9.00 hours. These various naringin dosages have been stated to prevent oxidative stress in rats [24]. Meanwhile, the normal control and CYCP alone rats received the vehicle, as detailed in Table 1. Afterwards, a single dose of CYCP (200 mg/kg) prepared in sterile injection water was administered intraperitoneally (i.p.) to all the groups (II–V) except normal control group I, which received only sterile injection water i.p.
Table 1.
Groups and treatments administered in the experiment.
| Groups | Treatments |
|---|---|
| Normal control (n = 5) | Administered vehicle p.o. for 14 d then sterile injection water i.p. |
| CYCP alone (n = 5) | Administered vehicle p.o. for 14 d then CYCP (200 mg/kg, i.p.) |
| Naringin 50 + CYCP (n = 5) | Administered naringin (50 mg/kg bw, p.o.) for 14 d then CYCP (200 mg/kg, i.p.) |
| Naringin 100 + CYCP (n = 5) | Administered naringin (100 mg/kg bw, p.o.) for 14 d then CYCP (200 mg/kg, i.p.) |
| Naringin 200 + CYCP (n = 5) | Administered naringin (200 mg/kg bw, p.o.) for 14 d then CYCP (200 mg/kg, i.p.) |
2.6. Preparation of serum and erythrocyte suspension
At the end of the experiment, 24 h after the CYCP administration, within which the CYCP administered, would have been completely metabolized; the animals were sacrificed by cervical dislocation and dissected. Blood samples were collected via cardiac puncture into plain centrifuge tubes. The blood samples were centrifuged at 5000 rpm for 10 min. The serum, which was the clear supernatant removed and was used for the estimation of serum enzymes. The buffy coat was equally removed by careful suction to obtain the erythrocytes.
The erythrocytes were mixed and washed with phosphate-buffered saline (PBS) [NaCl (150 mM), NaH2PO4 (1.9 mM), and Na2HPO4 (8.1 mM), pH 7.4], after which the mixture was centrifuged at 5000 rpm for 10 min at 4 °C. The supernatant was again removed and discarded by careful suction, and a few erythrocytes were forfeited to remove any residual buffy layer. This washing procedure was repeated twice. This method removed practically all the leucocytes from the final erythrocyte preparation. The washed erythrocytes were laked by suspending it in isotonic Tris−HCl buffer pH 7.6 to an approximate haematocrit of 50 %, and the samples were kept at -5 ℃ until further biochemical assays.
2.7. In vivo biochemical assays
Appropriate aliquots of the 50 % erythrocyte suspensions (haemolysate) were made and used for various biochemical assays.
2.8. Determination of serum and erythrocytes lactate dehydrogenase (LDH) activity (a marker of erythrocyte integrity and cellular ATP)
LDH catalyzes the conversion of pyruvate and NADH to lactate and NAD+. LDH activity in the serum and erythrocytes was assayed spectrophotometrically at 420 nm by monitoring the conversion of NAD+ to NADH according to the available kit manual manufacturer’s instructions [25]. LDH specific activity was expressed as Units/g haemoglobin.
Determination of erythrocytes lipid peroxidation concentration
Lipid peroxidation in the erythrocytes was assayed spectrophotometrically at 532 nm by measuring malondialdehyde (MDA, an end product of cell membrane lipid peroxides/oxidative damage), as the formation of thiobarbituric acid reactive substances (TBARS) (TBA + MDA → TBA-MDA adduct) by the method of Wright et al. [26]. TBARS contents were expresses as nmol MDA formed/mg haemoglobin using MDA molar extinction coefficient (Σ) of 1.56 × 105 M−1 cm−1.
2.9. Determination of erythrocytes total hydroperoxide concentration
Total (amino acid-, peptide-, protein-, and lipid-derived) hydroperoxide was determined by a method described by Hawkins et al. [27]. FOX (ferrous oxidation – xylenol orange) assay. This process employs the hydroperoxide-induced oxidation of a Fe(II)-xylenol orange to the Fe(III) form, the amount of the latter is then determined spectrophotometrically at 560 nm.
2.10. Determination of erythrocytes total nitric oxide radical (•NO)
Nitric oxide radical is produced in biological tissues by nitric oxide synthase, which metabolizes arginine to citrulline with the formation of •NO via a five electron oxidative reaction. •NO is oxidized by oxygen to nitrite (NO2−) and nitrate (NO3−), which are stable final products of •NO metabolism and may be used as indirect cellular markers of •NO presence. The sum of cellular nitrite and nitrate levels is a measure of nitric oxide produced and hence the activity of nitric oxide synthase. Nitrate is reduced to nitrite. The nitrite reacts with a colour developing agent such as Griess reagent [2% sulphanilamide in 5% phosphoric acid and 0.2 % N-(1-naphthyl)ethylenediamine dihydrochloride] then generate a purple azoic compound, [•NO → NO2−, + sulphanilamide → diazonium salt, + N-(1-naphthyl)ethylenediamine → Azo dye] which gives the concentration of •NO in the erythrocytes as described by Green et al. [21].
2.11. Determination of antioxidants
Antioxidants were determined in a suitably diluted erythrocyte lysate.
Glutathione-S-transferase (GST) activity was determined spectrophotometrically at 340 nm by monitoring the rate of conjugation formation between GSH and 1-chloro-2, 4-dinitrobenzene (CDNB), (GSH + CDNB → GS-CDNB conjugate + HCl), according to the method of Habig et al. [28]. The specific activity of GST was expressed as Units/g haemoglobin or mmol GS-CDNB conjugate formed/min/g haemoglobin using GS-CDNB molar extinction coefficient (Σ) of 9.6 mM−1 cm−1.
Reduced glutathione (GSH) concentration was determined spectrophotometrically at 412 nm by measuring the rate of formation of chromophoric product 2-nitro-5-thiobenzoate (TNB) as a result of reduction of Ellman’s reagent DTNB [5,5ˈ-dithiobis(2-nitrobenzoic acid)] by the free sulphydryl group of reduced glutathione (2GSH + DTNB → TNB + GSSG); the intensity of the yellow-coloured complex formed is directly proportional to the amount of –SH groups, as described by the method of Jollow et al. [29]. GSH values were expressed as μgGSH/g haemoglobin using GSH molar extinction coefficient (Σ) of 9.6 0.017 mM−1 cm−1.
Superoxide dismutase (SOD) activity was determined spectrophotometrically at 420 nm by measuring the inhibition of autoxidation of pyrogallol, a superoxide-reacting indicator molecule (SRIM) that compete with SOD for the reaction with superoxide in alkaline medium (pyrogallol/SOD + O2• − + 2H+ → H2O2 + O2), according to the method described by Marklund and Marklund [30]. The specific activity of SOD was expressed as Units/g haemoglobin or pyrogallol 50 % oxidation auto-inhibition/min/g haemoglobin using pyrogallol molar extinction coefficient (Σ) of 8.0 × 105 M−1 cm−1.
Catalase activity was determined spectrophotometrically at 374 nm by measuring the rate of decomposition of hydrogen peroxide (2H2O2 → 2H2O + O2), according to the method of Hadwan and Abed [31]. The specific activity of catalase was expressed as Units/g haemoglobin or mmol H2O2 degraded/min/g haemoglobin using the H2O2 molar extinction coefficient (Σ) of 43.6 M−1 cm−1.
Glutathione peroxidase (GSPx) activity was determined spectrophotometrically at 420 nm by measuring the residual GSH content during the decomposition of hydrogen peroxide using GSH as co-factor (H2O2 + 2GSH →2H2O + GSSG), according to the method of Mohandas et al. [32]. GSPx specific activity was expressed as Units/g haemoglobin or nmol of residual GSH /min/g haemoglobin using GSH molar extinction coefficient (Σ) of 9.6 0.017 mM−1 cm−1.
Glutathione reductase (GSR) activity was determined spectrophotometrically at 340 nm by monitoring the rate of oxidation of NADPH to NADP+ as a decrease in absorbance (GSSG + NADPH → 2GSH + NADP+), according to the method of Carlberg and Mannervik [33]. GSR specific activity was expressed as Units/g haemoglobin or nmol NADPH oxidized/min/g haemoglobin using the NADPH molar extinction coefficient (Σ) of 6220 M−1 cm−1.
2.12. Determination of erythrocyte lipid profile
Since the Folch extraction [34] formed lipid extracts that were exceedingly pigmented, a better method for the extraction of lipids from erythrocytes using chloroform:isopropanol (7:11, v/v) described by Rose and Oklander [35] was engaged to remove the haemoglobin by precipitation. Briefly, chloroform/isopropanol (0.9 mL) (7:11, v/v) was added to 0.1 mL of erythrocytes; the mixture was thoroughly mixed using a vortex mixer every 5 min for 30 min at 25℃. The mixture was then centrifuged; the supernatant was taken, and 0.1 mL of 0.05 M KCl was added to it. This again was thoroughly mixed using a vortex mixer and allowed to stand at room temperature for 5 min. The mixture was then centrifuged again, and the chloroform (lower) layer was taken into clean Eppendorf tubes.
For cholesterol assay, an aliquot of the chloroform-isopropanol extract was evaporated to dryness at 60 °C. Triton X-100 / chloroform mixture (1:1, v/v, 20 μL) was added to resolve the lipids, and again the solvent was evaporated. Then commercially available cholesterol kit reagent (1 mL) was added and vortexed. After incubation in the dark at room temperature for 30 min, cholesterol concentration was quantified spectrophotometrically at 500 nm [36].
For the triacylglycerol assay, an aliquot of the chloroform-isopropanol extract was evaporated to dryness at 60 °C. Next, the dried extracts were redissolved in ethanol (0.1 mL, 90 %). Then commercially available cholesterol kit reagent (1 mL) was added and vortexed. After incubation in the dark at room temperature for 30 min, triacylglycerol concentration was quantified spectrophotometrically at 500 nm [37].
For phospholipids assay, an aliquot of the chloroform-isopropanol extract was evaporated to dryness at 60 °C. The dried extracts were redissolved in chloroform (2 mL) followed by the addition of ammonium ferrothiocyanate (2 mL), and the mixture vortexed for 1 min. They were left for 10 min for the phases to separate. The chloroform layer was carefully removed by suction and absorbance read at 488 nm. Phospholipid concentrations were then quantified using a phospholipid standard as a reference described by Stewart [38].
For non-esterified acids (FFAs), the assay was carried out as described by Soloni and Sardina [39]. Briefly, an aliquot of the chloroform-isopropanol extract was evaporated to dryness at 60 °C. The dried extracts were redissolved in chloroform (2 mL) followed by the addition copper reagent [Cu(N03)2.3H20 (40 g/l), triethanolamine (120 mL/L), (1:1, v/v, 0.3 mL)]. The mixture was vortexed for 10 min and centrifuged at 5000 rpm for 10 min. After centrifugation, the chloroform layer was removed, and to this was added 1 mL of cuprizone [0.4 g oxalic acid bis(cyclohexylidenehydrazide) in 1 L of isopropanol] and 0.1 mL of ammonia reagent (58 % NH4OH, v/v). The contents were thoroughly vortexed and incubated for 10 min, after which absorbance was read at 620 nm. A standard curve of palmitic acid taken through the same procedure was used to extrapolate the concentrations of FFAs in the erythrocytes.
2.13. Statistical analysis
Data were expressed as the mean ± SEM of five replicates in each group. Analysis of Variance (ANOVA) was carried out to test for the level of homogeneity among the groups. Where heterogeneity occurred, the groups were separated using Duncan Multiple Range Test (DMRT). A p-value of less than 0.05 was considered statistically significant. All the statistics were carried out by SPSS (Statistical Package for Social Sciences) software for Windows version 20 (SPSS Inc., Chicago, Illinois, USA). Graphs were plotted using GraphPad Prism 8 Software (GraphPad Software Inc., San Diego, USA).
3. Results
3.1. In vitro antioxidant assay
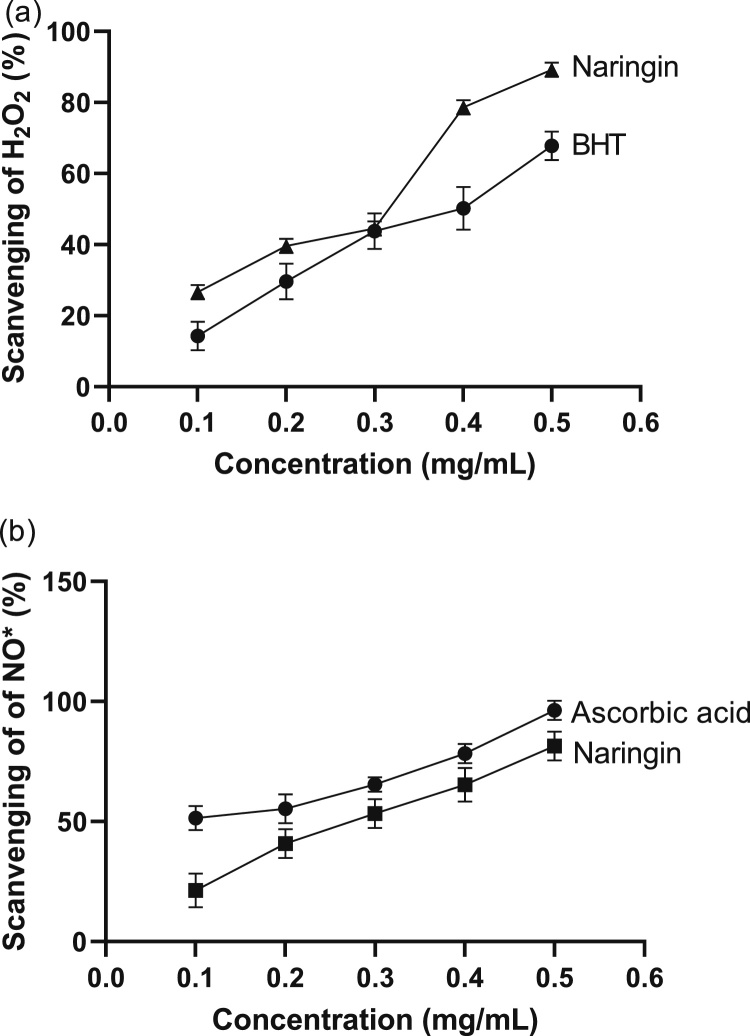
Hydrogen peroxide scavenging strength of naringin and standard antioxidant [(butylated hydroxytoluene (BHT)] is shown in Fig. 1a. At 0.1 mg/mL of naringin and BHT, H2O2 scavenging activities were 26.7 % and 14.3 %, respectively. At 0.2 mg/mL, naringin was also a better scavenger of H2O2 compared to BHT. H2O2 scavenging activities were 1.3 times significantly (p < 0.05) higher in naringin than in the BHT treatment group. At 0.3 mg/mL concentration, there was no significant (p > 0.05) difference in H2O2 scavenging activities of the naringin and BHT treatment group. The highest % H2O2 inhibition by the naringin and the BHT treatment group was noted at 0.5 mg/mL. The concentrations required for 50 % decrease (IC50) were 0.27 mg/mL and 0.37 mg/mL for naringin and BHT treatment group respectively.
Fig. 1.
(a) Scavenging effects of naringin and standard synthetic antioxidant [butylated hydroxytoluene (BHT)] on hydrogen peroxide (H2O2). (b) Scavenging effects of naringin and standard antioxidant (ascorbic acid) on nitric oxide radical (•NO). Values are mean ± SEM of three replicates.
The ability of the naringin and the standard antioxidant (ascorbic acid) to scavenge nitric oxide radical (•NO) is shown in Fig. 1b. The •NO scavenging effect of naringin and ascorbic acid increased with increasing concentration (0.1 – 0.5 mg/mL) of the naringin and ascorbic acid. The naringin and ascorbic acid showed respective 21.3–81.5% and 51.4–96.3% inhibitory effects at 0.1 – 0.5 mg/mL. The inhibitory concentrations at 50 % (IC50) of the naringin and ascorbic acid were 0.28 mg/mL and 0.13 mg/mL respectively.
3.2. Naringin pretreatment conserved erythrocyte integrity
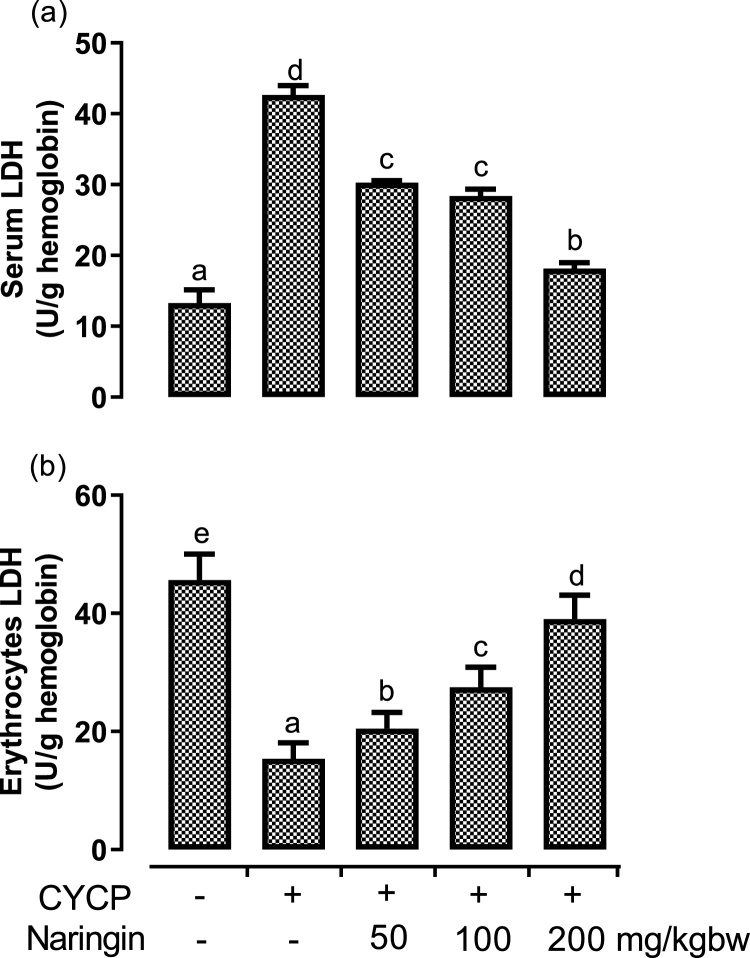
The protective outcome of naringin on CYCP-mediated erythrocyte damage was measured by evaluating the activities of LDH in both the serum and erythrocytes (Fig. 2). CYCP administration initiated a remarkable (p < 0.05) rise in the activity of serum LDH (Fig. 2a) by 220.8 % when compared with the corresponding negative control (normal) group. CYCP administration, however, invoked a significant (p < 0.05) reduction in the activity of erythrocyte LDH (Fig. 2b) by 66.4 % when juxtaposed with the corresponding negative control (normal) group.
Fig. 2.
(a-b) The effects of naringin pretreatment on (a) CYCP-induced increase in the activity of serum lactate dehydrogenase; and (b) CYCP-mediated decrease in the activity of erythrocyte lactate dehydrogenase. Bars represent mean ± SEM (n = 5). Bars with different letters are significantly different at P < 0.05.
Nevertheless, naringin administered at doses of 50, 100, and 200 mg/kg to the CYCP-intoxicated group significantly (p < 0.05) diminished the altered activity of LDH that were released into serum as a result of CYCP-mediated erythrocyte injury. Likewise, the various doses of naringin significantly (p < 0.05) enhanced the erythrocyte functional activities of LDH by increasing its erythrocyte activities when compared with CYCP alone administered groups. In the two compartments, the protective effect of naringin on CYCP-induced erythrocytotoxicity was concentration-dependent.
3.3. Naringin pretreatment prevents the modifications of the antioxidants invoked by CYCP in rat erythrocytes
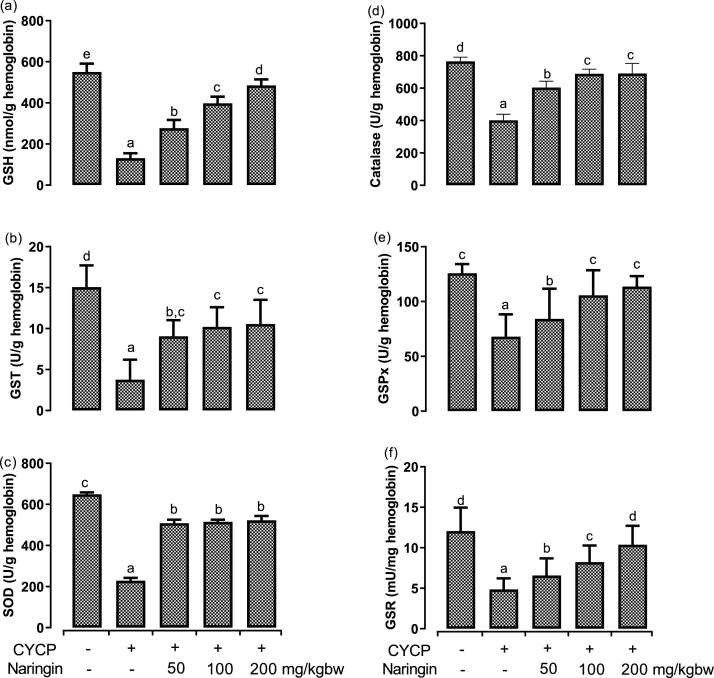
The effects of 200 mg/kg CYCP (single dose); pretreatment with 50, 100, and 200 mg/kg naringin for 14 consecutive days, on the levels of reduced glutathione; and the activities of glutathione-S-transferase, superoxide dismutase, catalase, glutathione peroxidase, glutathione reductase were assessed in the erythrocytes (Fig. 3). CYCP pretreatment elicited a remarkable (p < 0.05) reduction in intracellular erythrocyte GSH levels (Fig. 3a) by 76.2 % when compared with the negative control (normal) group. Likewise, CYCP-induced significant (p < 0.05) decreases in the activities of glutathione-S-transferase (Fig. 3b), superoxide dismutase (Fig. 3c), catalase (Fig. 3d), glutathione peroxidase (Fig. 3e), and glutathione reductase (Fig. 3f) by 75.1 %, 64.8 %, 47.6 %, 45.9 % and 59.8 %, respectively, when compared with the corresponding negative control (normal) group. Oral administration of the rats with naringin annul remarkably (p < 0.05) the CYCP-induced decreases in erythrocytes antioxidants contents to varying degrees. The protective effects of naringin on CYCP-induced decreases in erythrocytes antioxidants followed a dose-dependent except in SOD when juxtaposed with the positive control (CYCP alone treated) group.
Fig. 3.
(a-f) Effects of naringin pretreatment on CYCP-mediated decreases in antioxidants in rat erythrocytes. (a) the levels of reduced glutathione, (b) the activities of glutathione-S-transferase, (c) the activities of superoxide dismutase, (d) the activities of catalase, (e) the activities of glutathione peroxidase, and (f) the activities of glutathione reductase. Bars represent mean ± SEM (n = 5). Bars with different letters are significantly different at P < 0.05.
3.4. Naringin pretreatment attenuated CYCP-induced oxidative stress markers
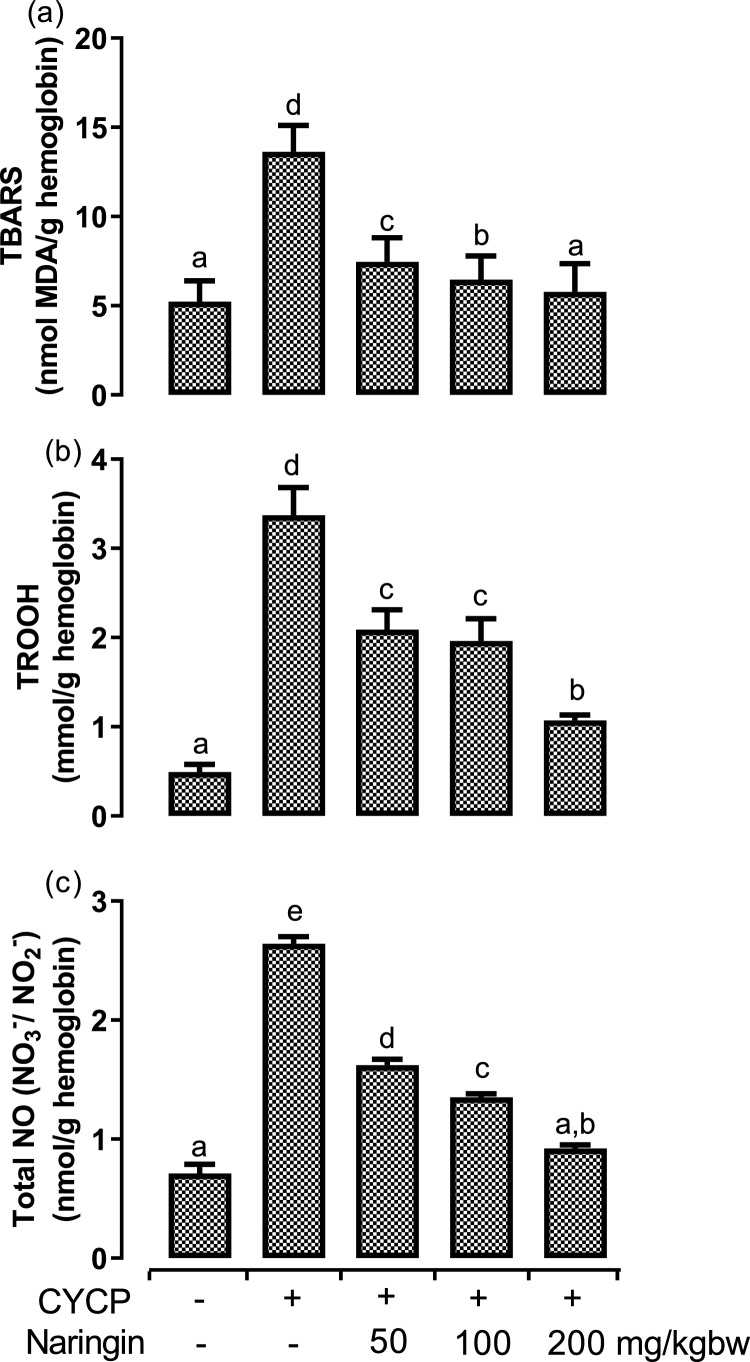
The levels of oxidative stress markers, including TBARS, total hydroperoxide, and total nitric oxide, were quantified in the erythrocytes (Fig. 4). CYCP administration elicited an incredible (p < 0.05) increase in the production of TBARS (Fig. 4a), hydroperoxide (Fig. 4b), and nitric oxide (Fig. 4c) by 160.6 %, 587.8 %, and 271.8 %, respectively when compared with the corresponding negative control (normal) group. Nevertheless, oral pretreatment of the rats with various doses of naringin rescind significantly (p < 0.05) the CYCP-mediated elevation in oxidative stress parameters to a varying extent. The action of three doses of naringin was concentration-dependent.
Fig. 4.
(a-c) Effects of naringin pretreatment on CYCP-mediated increases in oxidative stress markers in rat erythrocytes. (a) the levels of thiobarbituric acid reactive substances, and (b) the levels of total hydroperoxide, and (c) the levels of total nitric oxide. Bars represent mean ± SEM (n = 5). Bars with different letters are significantly different at P < 0.05.
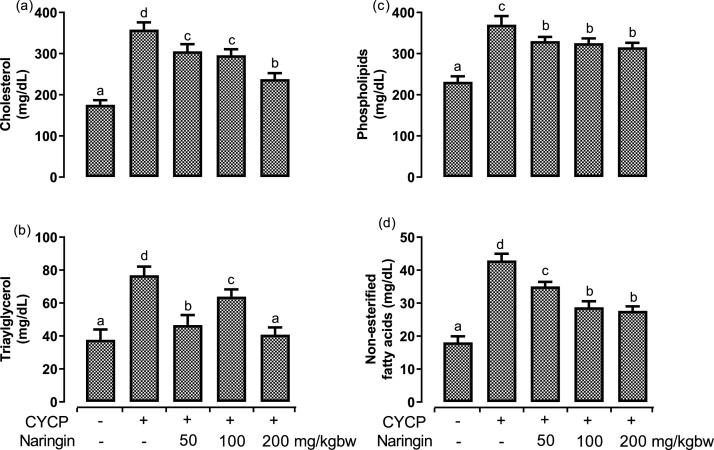
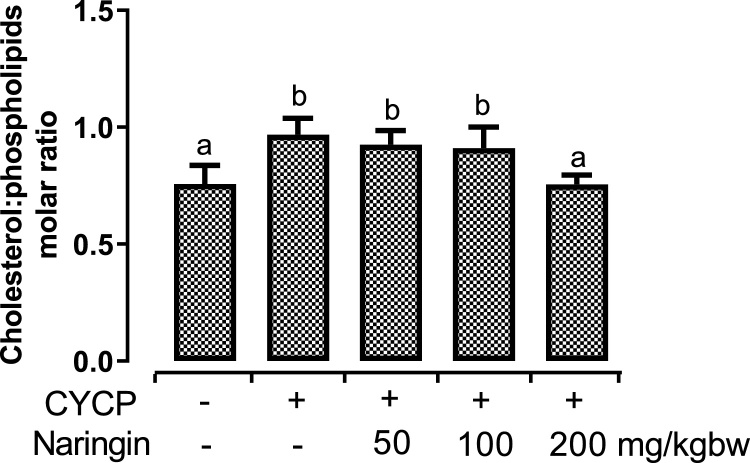
3.5. Naringin pretreatment abrogated CYCP-induced dyslipidaemias
Fig. 5, Fig. 6 illustrate the effects of CYCP and pretreatment with various doses of naringin on major erythrocytes lipids (cholesterol, triacylglycerol, phospholipids, and non-esterified fatty acids) and cholesterol-phospholipids molar ratio. Administration of CYCP resulted in statistically significant (p < 0.05) elevation in the entire major lipids. CYCP pretreatment provoked a remarkable (p < 0.05) up-regulation of the concentrations of the erythrocyte cholesterol by 104.1 % (Fig. 5a), erythrocyte triacylglycerol by 103.9 % (Fig. 5b), erythrocyte phospholipids by 59.8 % (Fig. 5c), erythrocyte non-esterified fatty acids by 137.3 % (Fig. 5d), and erythrocyte cholesterol-phospholipids molar ratio by 27.7 % (Fig. 6) when compared with their corresponding negative control (normal) group.
Fig. 5.
(a-d) Effects of naringin pretreatment on CYCP-induced increases in rat erythrocytes major lipids. (a) the concentration of cholesterol, (b) the concentration of triacylglycerol, (c) the concentration of phospholipids, and (d) the concentration of non-esterified fatty acids. Bars represent mean ± SEM (n = 5). Bars with different letters are significantly different at P < 0.05.
Fig. 6.
Effects of naringin pretreatment on CYCP-induced increase in rat erythrocytes cholesterol:phospholipids molar ratio. Bars represent mean ± SEM (n = 5). Bars with different letters are significantly different at P < 0.05.
However, oral pretreatment with naringin abrogated (p < 0.05) the CYCP-induced dyslipidaemias to varying extents.
4. Discussion
In this research, we employed two established in vitro assays to assess the free radical scavenging and antioxidant abilities of naringin. Moreover, we confirmed the capability of a single dose of CYCP (200 mg/kg, i.p.) administration to induce erythrocytotoxicity in a rat model; we similarly evaluated the effects of naringin on free radical scavenging, cellular integrity, cellular ATP, antioxidants, oxidative stress parameters, and lipid profile in erythrocytes. Our outcomes specified that naringin might reduce CYCP-mediated erythrocytotoxicity by acting as specific free radical scavengers, improved cellular integrity, decreased intracellular ATP, improved the antioxidants, reduced oxidative stress parameters, and abrogated dyslipidemia, thus underlying naringin mechanism of action on CYCP-induced acute erythrocytotoxicity rats.
We evaluated the H2O2 scavenging ability of naringin. Our findings indicated that naringin, a citrus bioflavonoid elicited radical stabilization or metal chelating action as one of its mechanisms of action by scavenging hydrogen peroxide (H2O2). Our results also specified that naringin had an upper capacity to inhibit and/or mop up H2O2 than synthetic antioxidant BHT; thus, the observed lower concentration (0.27 mg/mL vs 0.37 mg/mL) of half-maximum inhibitory concentration (IC50). Conversely, a reverse was observed when the naringin nitric oxide radical (•NO) scavenging ability was compared with ascorbic acid, IC50 of 0.28 mg/mL vs 0.13 mg/mL were obtained. The discrepancy in mopping up H2O2 and NO by naringin might be because of its antioxidant and free radical chelating specificity [40]. H2O2 reacts with transition metals, including iron II and copper I, to generate unstable and highly reactive free radicals such as hydroxyl radical (HO•), which can induce the oxidation of macromolecules. Also, a remarkable elevation of •NO has been implicated in dyslipidaemias and hypotension [41]. Therefore, our in vitro results supported the notion that naringin could offer protection by acting as a dietary antioxidant and oxidants scavenger via donating protons or electrons, thereby preventing oxidant-mediated cytotoxicity [24].
Dietary antioxidants, including polyphenols, have been reported to be a double-edged sword since they have been reported to elicit both beneficial and harmful effects. Specifically, N-acetylcysteine (NAC), a precursor substrate of reduced glutathione and vitamin E, promotes increased GSH concentrations, stimulates tumor progression by reducing ROS and in turn down-regulates p53 expression (ROS-p53 axis) [71]. The observed positive in vitro polyphenols antioxidant results do not always complement the in vivo results during validation experiment since plant antioxidants undergo some physiological processes, such as metabolism, when administered in living organisms [71]. Nonetheless, the findings of in vitro studies are usually inappropriately extrapolated to organisms in the absence of a substantial number of in vivo investigations [72]. Naringin (50–500 mg/Kg bw) is frequently consumed in combination with common clinical drugs [73]. The highest dose used in this work is 200 mg/kg bw, which is remarkably below a safe therapeutic dose (no-observed-adverse-effect level; NOAEL) of 500 mg/kg bw recently reported by Cheng et al. [73].
To this end, we investigated some in vivo biomarkers. We observed a marked increase in serum lactate dehydrogenase (LDH) functional activities in CYCP alone administered rats. A remarkable increase in serum lactate dehydrogenase (LDH) is associated with injury to the liver, lungs, kidney, cardiac muscle, and skeletal muscle [42]. So, we additionally assessed the LDH activity in the erythrocytes. Our results showed that erythrocytes LDH functions and activities in the CYCP alone group decreased significantly compared with the counterpart negative control (normal) group. The observed dynamics in both serum and erythrocytes compartments might be ascribed to erythrocyte deformity initiated by CYCP-induced erythrocyte ghost non-enzymatic oxidative degradation of PUFAs and protein cross-linking, oxidative stress, cytoplasmic cellular leakage, deterioration in the functional integrity of erythrocytes ghost, and eryptosis [4,5,43]. This observation agrees with the earlier study that CYCP intoxication remarkably elevated serum LDH [44]. However, naringin administration to rats inhibited CYCP-induced decrease in LDH activity, an enzyme responsible for erythrocyte plasma membrane architecture and integrity.
Our findings from this study also showed decreased erythrocytes antioxidants parameters such as GSH, GST, SOD, CAT, GSPx, and GSR activities in CYCP alone administered animals. Diminution of these antioxidants could be a result of their increased functional activities to either inhibit or scavenge CYCP reactive metabolites and/or CYCP-mediated free radicals [8,45].
In aerobic respiration, up to 100 % of used oxygen moves to the mitochondria, where it is reduced to water by accepting four electrons, one at a time, with concomitant production of energy (ATP). However, roughly 1–2 % of the transferred electrons may escape the respiratory chain, which permits the generation of partially reduced oxygen intermediates such as ROS (superoxide anion radicals, hydrogen peroxide, hydroxyl radicals) [74]. These by-products of basal cellular metabolism are deleterious agents, especially at high concentrations.
Erythrocytes are sheltered from oxidative damage by myriads of non-enzymatic and enzymatic antioxidants [46]. SOD scavenges superoxide and catalyzes its conversion to H2O2 and O2. SOD contains zinc and copper, which are respectively responsible for SOD stability and maintenance of its activity. Hemolysis-induced haemoglobin release is hazardous since the released haemoglobin could react with H2O2 to form methaemoglobin and/or be degraded with hydroxyl radical formation. Both methaemoglobin and hydroxyl radical are potent promoters of lipid peroxidation, oxidative stress, Toll-like receptor 4 activation, and NFkB inducer [47]. The majority (98 %) of blood catalase is located in the erythrocytes and is responsible for the removal of both extracellular and intracellular H2O2. CAT catalyzes the breakdown of H2O2 to water. SOD and CAT depletion results in the accumulation of O2•– and H2O2, respectively [48,75,76].
GSH has been reported as one of the conventional markers of oxidative stress [77]. Schafer and Buettner [49] and Polyxeni et al. [76] hypothesized that reduced glutathione, GSH, is the principal and most crucial non-protein thiol that protects erythrocytes ghost and its intracellular component from free radical-induced oxidation. GSH does not cross the erythrocytes ghost passively, and its de novo synthesis is the sole source of GSH in erythrocytes. GSH is synthesized from three substrates, of which two (cysteine and glycine) are conveyed into the cells; however, the third substrate, glutamate, is synthesized from aspartate and alanine by aspartate aminotransferase and alanine aminotransferase. GSH de novo synthesis is ATP-dependent, while its reduction from GSSG to GSH involves NADPH. Hence ATP depletion in erythrocytes would impair GSH de novo synthesis [50]. Likewise, GSH depletion causes a decrease in GST, GSPx, GSR, and G6PDH activities [51]. The decreases in the activities of GST, GSPx, GSR observed in this study could result from CYCP-induced decreases in ATP, GSH or as a result of CYCP itself and/or its metabolites. Concerted activities of all the above mentioned endogenous antioxidant defences prevent free radical-mediated necrosis, DNA and RNA fragmentation, proteins modification, and lipids peroxidation of erythrocytes ghost [52]. GSH protects erythrocytes cytoskeleton and intracellular macromolecules against oxidation and cell injury by reacting and scavenging ROS, controlling SOD transcription and reactivating GST and GSPx [53,82]. So, depletion of GSH, GST, GSPx, and GSR could increase oxidative and/or nitrosative stress and cause alteration in various compartments, including the erythrocytes composition.
Nonetheless, naringin pretreatment exerted protection by reversing CYCP-induced decreases in GSH, SOD, CAT, GSPx, and GSR could be by preventing the in vivo generated CYCP reactive metabolites and free radicals, removing active metal ions and suppressing the oxidation of non-transition metals, supplying hydrogen atoms or electrons to stabilize the in vivo generated CYCP reactive metabolites to cause their stability, and detoxifying (scavenging and/or mopping up) toxic CYCP reactive metabolites and ROS. The presence of metal ions, including iron (II) ion (Fe2+) has been implicated in Fenton and Haber–Weiss reactions. In the Fenton reaction, Fe2+ reacts with H2O2 to produce hydroxide anion (OH−) and hydroxyl radical (HO•) radical. While in Haber–Weiss reaction, OH− and HO •are generated from the reaction of H2O2 and superoxide ion (O2•–) catalyzed by Fe2+. HO •is a hazardous radical and could cause decreased CAT and GSPx activities [70,74,77]. Hence, compounds capable of lightening their formation and/or reactivity are required as valuable candidates against numerous redox-pertinent pathological conditions. Kanno et al. [83] demonstrated that naringin attenuated H2O2-induced cytotoxicity and apoptosis in mouse leukemia P388 cells via its antioxidant and anti-apoptotic properties. Our result is similar to the one reported by Priftis et al. [78], where they reported that consumption of caffeine polyphenols for two weeks strengthened redox status in the erythrocyte lysates and various visceral by upregulating the γ-GCLc, CAT, and SOD mRNA/gene expression, and correspondingly increasing GSH, CAT, and SOD protein levels, as well as GST and GSPx levels. GSH elevation can be due to increased recycling rate expedited by GSPx and GSR, and/or increased biosynthetic rate mediated by γ-glutamylcysteine ligase catalytic subunit (γ-GCLc) and glutathione synthetase [78,79]. GSH quenches H2O2 via a GSPx-catalyzed reaction, thereby inhibiting OH •production [80], whereas GST conjugates electrophilic compounds to GSH, facilitating their elimination and excretion from the body [81]. The combination of these enzymes may reduce the formation of HO•.
We observed that the CYCP-intoxication provoked a noticeable elevation in the levels of erythrocytes MDA and total (amino acids, peptides, proteins, and lipids) hydroperoxide (TROOH), thus established CYCP-induced erythrocytes lipid peroxidation. Casanova et al. [54] have documented that CYCP-intoxication initiates erythrocytes lipid peroxidation. Nevertheless, pretreatment of naringin remarkably decreased CYCP-induced increases in the erythrocytes levels of MDA and TROOH and protected against the accumulation of noxious lipid peroxidation products MDA and TROOH. Furthermore, naringin offered protection against CYCP-induced peroxidative and TROOH toxicities by supplying protons or electrons to stabilize the in vivo generated ROS, break radical chain reaction within the cell membrane and hence quenches lipid peroxidation, protect the PUFA side chain of membrane lipids from undergoing autocatalytic lipid peroxidation initiation and propagation, preserve cell membrane integrity, and prevent the inactivation and depletion of the plasma membrane [24,53,55,79].
This current investigation shows that the rats treated with CYCP showed a remarkable increase in the levels of erythrocytes •NO; this agrees with previous reports that CYCP-mediated significant increase in erythrocytes •NO and NOS levels [43].
Apart from RBCs involved in the transportation of O2, CO2, it equally carries a functional endothelial nitric oxide synthase (eNOS), which controls vascular or circulating NO and NO metabolites pool, nitrite homeostasis, blood pressure, and cardioprotection in an ischemia/reperfusion injury. RBCs take up and inactivate endothelium-derived NO by reacting and converting oxyhemoglobin (O2HbFe2+) to methemoglobin (metHb, HbFe3+) and nitrate (NO3−), thereby decreasing NO available for vasodilatation [56]. Under hypoxia, RBCs induced NO-dependent vasodilation by partaking in the synthesis, storage, and transportation of NO metabolic products [57,58]. In addition, the RBCs eNOS has erythrocrine (exocrine) function such as regulation of cardiovascular system (hemostasis, blood pressure control, etc.), erythrocytes deformability [59]. Low levels of erythrocyte NO increase RBC deformability, membrane fluidity, and RBC filterability.
However, elevated erythrocytes NO level exerts detrimental consequences such as hypotension, endothelial dysfunction, oxidative stress on the cellular environment, mitochondrial dysfunction, airway hyperresponsiveness, etc. [60,61]. Besides, a remarkable increase in NO levels could vigorously combine with superoxide anion, another free radical, to form a stable and robust oxidant called peroxynitrite radical (OONO−). RNS such as NO and/or OONO− interact with cellular biomolecules such as lipids, proteins, and nucleic acids following the depletion of the antioxidant defence system and elicit tissue perturbation and injury via induction of nitrosative (RNS) stress [62]. In this study, we observed remarkable suppression in erythrocyte NO levels of CYCP exposed rats after the pretreatment of naringin; this suggests the notion that naringin could reverse CYCP-induced toxicities due to its intrinsic antioxidant properties [24,63]. Nonetheless, in this research, we observed that naringin attenuated elevated NO and thus decreased erythrocyte deformability.
One of the key findings of the current investigation was that CYCP elicited significant disruption of lipid homeostasis characterized by up-regulation in the mean concentrations of erythrocyte cholesterol, triacylglycerol, phospholipids, non-esterified fatty, and cholesterol-phospholipids molar ratio. This observation is characteristic changes that have been reported during xenobiotics, including CYCP administration [[64], [65], [66], [67]].
The observed CYCP-induced hypertriacylglycerolemia in this study could result from either increased VLDL production or decreased VLDL clearance [68]. Increased cholesterol is equally associated with increased cholesterol anabolism and/or decreased cholesterol catabolism [65]. The current research demonstrated a CYCP-induced significant increase in phospholipids. Hyperphospholipidaemia is characterized by the availability of non-esterified fatty and increased cholesterol [67]. The results from this investigation show that the two above-stated mechanisms might be responsible for the observed hyperphospholipidaemia. Increased erythrocyte cholesterol, phospholipids, non-esterified fatty, and cholesterol-phospholipids molar ratio have been implicated in erythrocyte ghost membrane fluidity and viscosity. Pronounce elevation of erythrocyte and erythrocyte ghost cholesterol-phospholipids molar ratio is correlated with decreased erythrocytes fluidity and deformability and ultimately impair the systemic movement of the erythrocyte [69]. However, this study, detected that naringin down-regulates the CYCP-induced up-regulation in the mean concentrations of erythrocyte cholesterol, triacylglycerol, phospholipids, free fatty acids, and cholesterol-phospholipids molar ratio.
This study has demonstrated that naringin pretreatment remarkably protected erythrocytes plasma membrane architecture and integrity by reversing cyclophosphamide invoked decreases in the activity of erythrocyte LDH, erythrocytes glutathione levels, activities of antioxidant enzymes including glutathione-S-transferase, catalase, glutathione peroxidase, and glutathione reductase; and equally repudiate cyclophosphamide-induced increases in erythrocytes levels of malondialdehyde, nitric oxide, and major lipids (cholesterol, triacylglycerol, phospholipids, and non-esterified fatty acids).
5. Conclusion
Taken together, our results support the notion that different doses of naringin pretreatment for as few as 14 days could prevent CYCP-induced erythrocytotoxicity by acting as specific ROS and RNS scavengers, preserve erythrocytes morphology and size, prevent haemolysis, oxidative stress, endothelial dysfunction, ATP depletion, and dyslipidaemia in a rat model through its antioxidant, free-radical scavenging, and anti-dyslipidaemia properties.
CRediT authorship contribution statement
Adio J. Akamo: Conceptualization, Methodology, Supervision, Validation, Writing - original draft, Writing - review & editing, Funding acquisition. Dorcas I. Akinloye: Resources, Supervision, Project administration, Funding acquisition. Regina N. Ugbaja: Resources, Supervision, Project administration, Funding acquisition. Oluwagbemiga O. Adeleye: Resources, Supervision, Project administration. Oluwatosin A. Dosumu: Resources, Supervision, Project administration. Ofem E. Eteng: Resources, Supervision, Project administration. Moses C. Antiya: Resources, Supervision, Project administration. Gogonte Amah: Resources, Supervision, Project administration. Oluwafunke A. Ajayi: Investigation, Formal analysis, Writing - original draft, Project administration, Funding acquisition. Samuel O. Faseun: Investigation, Formal analysis, Writing - original draft, Project administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Handling Editor: Dr. Aristidis Tsatsakis
References
- 1.Podsiedlik M., Markowicz-Piasecka M., Sikora J. Erythrocytes as model cells for biocompatibility assessment, cytotoxicity screening of xenobiotics and drug delivery. Chem. Biol. Interact. 2020:109305. doi: 10.1016/j.cbi.2020.109305. [DOI] [PubMed] [Google Scholar]
- 2.Kuhn V., Diederich L., Keller T.S., IV, Kramer C.M., Lückstädt W., Panknin C., Suvorava T., Isakson B.E., Kelm M., Cortese-Krott M.M. Red blood cell function and dysfunction: redox regulation, nitric oxide metabolism, anemia. Antioxid. Redox Signal. 2017;26(13):718–742. doi: 10.1089/ars.2016.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kato G.J., McGowan V., Machado R.F., Little J.A., Taylor J., Morris C.R., Nichols J.S., Wang X., Poljakovic M., Morris S.M., Gladwin M.T. Lactate dehydrogenase as a biomarker of hemolysis-associated nitric oxide resistance, priapism, leg ulceration, pulmonary hypertension, and death in patients with sickle cell disease. Blood. 2006;107(6):2279–2285. doi: 10.1182/blood-2005-06-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Makni M., Chtourou Y., Fetoui H., Garoui E.M., Barkallah M., Marouani C., Zeghal N. Erythrocyte oxidative damage in rat treated with CCl4: protective role of vanillin. Toxicol. Ind. Health. 2012;28(10):908–916. doi: 10.1177/0748233711427055. [DOI] [PubMed] [Google Scholar]
- 5.Ramdani G., Langsley G. ATP, an extracellular signaling molecule in red blood cells: a messenger for malaria? Biomed. J. 2014;37(5) doi: 10.4103/2319-4170.132910. [DOI] [PubMed] [Google Scholar]
- 6.Farag M.R., Alagawany M. Erythrocytes as a biological model for screening of xenobiotics toxicity. Chem. Biol. Interact. 2018;279:73–83. doi: 10.1016/j.cbi.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Pagano M., Faggio C. The use of erythrocyte fragility to assess xenobiotic cytotoxicity. Cell Biochem. Funct. 2015;33(6):351–355. doi: 10.1002/cbf.3135. [DOI] [PubMed] [Google Scholar]
- 8.Ali A.H.A., Al-Ghamdi S., Alanazi G.G., Alsomait M.A., Alaskar A.N., El-Enazi A.K., Alashqar H.M., Ahmad G. Protective effects of ginger extract against the toxicity of cyclophosphamide on testes: an experimental laboratory-based study. Int. J. Med. hth. Sci. 2020;9(1):27–33. [Google Scholar]
- 9.Kern J.C., Kehrer J.P. Acrolein-induced cell death: a caspase-influenced decision between apoptosis and oncosis/necrosis. Chem. Biol. Interact. 2002;139(1):79–95. doi: 10.1016/s0009-2797(01)00295-2. [DOI] [PubMed] [Google Scholar]
- 10.O’Brien P.J., Siraki A.G., Shangari N. Aldehyde sources, metabolism, molecular toxicity mechanisms, and possible effects on human health. Crit. Rev. Toxicol. 2005;35(7):609–662. doi: 10.1080/10408440591002183. [DOI] [PubMed] [Google Scholar]
- 11.Papaldo P., Lopez M., Marolla P., Cortesi E., Antimi M., Terzoli E., Calabresi F. Impact of five prophylactic filgrastim schedules on hematologic toxicity in early breast cancer patients treated with epirubicin and cyclophosphamide. J. Clin. Oncol. 2005;23(28):6908–6918. doi: 10.1200/jco.2005.03.099. [DOI] [PubMed] [Google Scholar]
- 12.Karema El M.S., Attia A.M., El-Banna S.G., Azab A.E., Yahya R.A. Anti-dyslipidemic effect of zinc oxide nanoparticles against cyclophosphamide induced dyslipidemia in male albino rats. Gazette Medic. Sci. 2020;1(1):055–063. https://thegms.co/pharmacology/pharmaco-ra-20050901.pdf [Google Scholar]
- 13.Adebiyi O.A., Adebiyi O.O., Owira P.M. Naringin reduces hyperglycemia-induced cardiac fibrosis by relieving oxidative stress. PLoS One. 2016;11(3):e0149890. doi: 10.1371/journal.pone.0149890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yi L., Ma S., Ren D. Phytochemistry and bioactivity of Citrus flavonoids: a focus on antioxidant, anti-inflammatory, anticancer and cardiovascular protection activities. Phytochem. Rev. 2017;16(3):479–511. https://link.springer.com/article/10.1007/s11101-017-9497-1 [Google Scholar]
- 15.Martinez R.M., Pinho-Ribeiro F.A., Steffen V.S., Caviglione C.V., Vignoli J.A., Barbosa D.S. Naringenin inhibits UVB irradiation-induced inflammation and oxidative stress in the skin of hairless mice. J. Nat. Prod. 2015;78:1647e1655. doi: 10.1021/acs.jnatprod.5b00198. [DOI] [PubMed] [Google Scholar]
- 16.Ghanbari-Movahed M., Jackson G., Farzaei M.H., Bishayee A. A systematic review of the preventive and therapeutic effects of naringin against human malignancies. Front. Pharmacol. 2021;12:250. doi: 10.3389/fphar.2021.639840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y., Chen D., Chu C., Li S., Chen Y., Wu C. Naringenin inhibits dendritic cell maturation and has therapeutic effects in a murine model of collagen-induced arthritis. J. Nutr. Biochem. 2015;26:1467–1478. doi: 10.1016/j.jnutbio.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 18.Yen H.R., Liu C.J., Yeh C.C. Naringenin suppresses TPA-induced tumor invasion by suppressing multiple signal transduction pathways in human hepatocellular carcinoma cells. Chem. Biol. Interact. 2015;235:1e9. doi: 10.1016/j.cbi.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Jung J.W., Park I.H., Cho J.S., Lee H.M. Naringenin inhibits extracellular matrix production via extracellular signal-regulated kinase pathways in nasal polyp-derived fibroblasts. Phytother. Res. 2013;27 doi: 10.1002/ptr.4735. [DOI] [PubMed] [Google Scholar]
- 20.Ruch R.J., Cheng S.J., Klaunig J.E. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis. 1989;10(6):1003–1008. doi: 10.1093/carcin/10.6.1003. [DOI] [PubMed] [Google Scholar]
- 21.Green L.C., Wagner D.A., Glogowski J., Skipper P.L., Wishnok J.S., Tannenbaum S.R. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal. Chem. 1982;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 22.Albus U. 2012. Guide for the Care and Use of Laboratory Animals (8th edn)http://dx.crossref.org/10.17226/12910 [Google Scholar]
- 23.Maestri E. The 3Rs principle in animal experimentation: a legal review of the state of the art in Europe and the case in Italy. Biotechnology. 2021;10(2):9. doi: 10.3390/biotech10020009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caglayan C., Temel Y., Kandemir F.M., Yildirim S., Kucukler S. Naringin protects against cyclophosphamide-induced hepatotoxicity and nephrotoxicity through modulation of oxidative stress, inflammation, apoptosis, autophagy, and DNA damage. Environ Sci. Pollut. Res. 2018;25(21):20968–20984. doi: 10.1007/s11356-018-2242-5. [DOI] [PubMed] [Google Scholar]
- 25.Kornberg A. Academic Press; New York: 1955. Methods in Enzymology; pp. 441–443. [Google Scholar]
- 26.Wright J.R., Colby H.D., Miles P.R. Cytosolic factors which affect microsomal lipid peroxidation in lung and liver. Arch. Biochem. Biophys. 1981;206(2):296–304. doi: 10.1016/0003-9861(81)90095-3. [DOI] [PubMed] [Google Scholar]
- 27.Hawkins C.L., Morgan P.E., Davies M.J. Quantification of protein modification by oxidants. Free Radic. Biol. Med. 2009;46(8):965–988. doi: 10.1016/j.freeradbiomed.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 28.Habig W.H., Pabst M.J., Jakoby W.B. Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974;249(22):7130–7139. [PubMed] [Google Scholar]
- 29.Jollow D.J., Mitchell J.R., Zampaglione N.A., Gillette J.R. Bromobenzene-induced liver necrosis: protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmcology. 1974;11(3):151–169. doi: 10.1159/000136485. [DOI] [PubMed] [Google Scholar]
- 30.Marklund S., Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974;47(3):469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 31.Hadwan M.H., Abed H.N. Data supporting the spectrophotometric method for the estimation of catalase activity. Data Brief. 2016;6:194–199. doi: 10.1016/j.dib.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohandas J., Marshall J.J., Duggin G.G., Horvath J.S., Tiller D.J. Differential distribution of glutathione and glutathione-related enzymes in rabbit kidney: possible implications in analgesic nephropathy. Biochem. Pharmacol. 1984;33(11):1801–1807. doi: 10.1016/0006-2952(84)90353-8. [DOI] [PubMed] [Google Scholar]
- 33.Carlberg I.N.C.E.R., Mannervik B.E.N.G.T. Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J. Biol. Chem. 1975;250(14):5475–5480. [PubMed] [Google Scholar]
- 34.Folch L., Lees M., Stanley G.S. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957;226(1):497–509. [PubMed] [Google Scholar]
- 35.Rose H.G., Oklander M. Improved procedure for the extraction of lipids from human erythrocytes. J. Lipid Res. 1965;6(3):428–431. [PubMed] [Google Scholar]
- 36.Allain C.C., Poon L.S., Chan C.S., Richmond W.F.P.C., Fu P.C. Enzymatic determination of total serum cholesterol. Clin. Chem. 1974;20(4):470–475. [PubMed] [Google Scholar]
- 37.Bucolo G., David H. Quantitative determination of serum triglycerides by the use of enzymes. Clin. Chem. 1973;19(5):476–482. [PubMed] [Google Scholar]
- 38.Stewart J.C.M. Colorimetric determination of phospholipids with ammonium ferrothiocyanate. Anal. Biochem. 1980;104(1):10–14. doi: 10.1016/0003-2697(80)90269-9. [DOI] [PubMed] [Google Scholar]
- 39.Soloni F.G., Sardina L.C. Colorimetric microdetermination of free fatty acids. Clin. Chem. 1973;19(4):419–424. [PubMed] [Google Scholar]
- 40.Adesanoye O.A., Farombi E.O. In vitro Antioxidant properties of methanolic leaf extract of Vernonia amygdalina Del, Niger. J. Physiol. Sci. 2014;29(2):93–101. https://www.ajol.info/index.php/njps/article/view/120070 [PubMed] [Google Scholar]
- 41.Senaphan K., Kukongviriyapan U., Sangartit W., Pakdeechote P., Pannangpetch P., Prachaney P., Greenwald S.E., Kukongviriyapan V. Ferulic acid alleviates changes in a rat model of metabolic syndrome induced by high-carbohydrate, high-fat diet. Nutrients. 2015;7(8):6446–6464. doi: 10.3390/nu7085283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akamo J.A., Ugbaja R.N., Amah G.H., Fabuluje I., Edaferiemu J.O., Lepzem N.G., Oyekale K.O. The relationship between indices of hepatocellular injury and anthropometric measurements in some Babcock University Students, Ilisan, Ogun State, Nigeria. Braz. J. Biol. Sci. 2015;2(3):23–37. http://revista.rebibio.net/v2n3/v02n03a04a.html [Google Scholar]
- 43.Temel Y., Çağlayan C., Ahmed B.M., Kandemir F.M., Çiftci M. The effects of chrysin and naringin on cyclophosphamide-induced erythrocyte damage in rats: biochemical evaluation of some enzyme activities in vivo and in vitro. Naunyn-Schmiedebergs Arch. Pharmacol. 2020;394:645–654. doi: 10.1007/s00210-020-01987-y. https://link.springer.com/article/10.1007/s00210-020-01987-y [DOI] [PubMed] [Google Scholar]
- 44.Nagi M.N., Al‐Shabanah M.O.A., Hafez M.M., Sayed‐Ahmed M.M. Thymoquinone supplementation attenuates cyclophosphamide‐induced cardiotoxicity in rats. J. Biochem. Mol. Toxicol. 2011;25(3):135–142. doi: 10.1002/jbt.20369. [DOI] [PubMed] [Google Scholar]
- 45.Fouad A.A., Qutub H.O., Al-Melhim W.N. Punicalagin alleviates hepatotoxicity in rats challenged with cyclophosphamide. Environ. Toxicol. Pharmacol. 2016;45:158–162. doi: 10.1016/j.etap.2016.05.031. [DOI] [PubMed] [Google Scholar]
- 46.van Rossen M.E.E., Sluiter W., Bonthuis F., Jeekel H., Marquet R.L., van Eijck C.H. Scavenging of reactive oxygen species leads to diminished peritoneal tumor recurrence. Cancer Res. 2000;60(20):5625–5629. [PubMed] [Google Scholar]
- 47.Adesanoye O.A., Molehin O.R., Delima A.A., Adefegha A.S., Farombi E.O. Modulatory effect of methanolic extract of Vernonia amygdalina (MEVA) on tert‐butyl hydroperoxide–induced erythrocyte haemolysis. Cell Biochem. Funct. 2013;31(7):545–550. doi: 10.1002/cbf.2933. [DOI] [PubMed] [Google Scholar]
- 48.Góth L., Vitai M. The effects of hydrogen peroxide promoted by homocysteine and inherited catalase deficiency on human hypocatalasemic patients. Free Radic. Biol. Med. 2003;35(8):882–888. doi: 10.1016/s0891-5849(03)00435-0. [DOI] [PubMed] [Google Scholar]
- 49.Schafer F.Q., Buettner G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001;30(11):1191–1212. doi: 10.1016/S0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 50.Bogdanova A., Lutz H.U. Mechanisms tagging senescent red blood cells for clearance in healthy humans. Front. Physiol. 2013;4:387. doi: 10.3389/fphys.2013.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pham-Huy L.A., He H., Pham-Huy C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008;4(2):89–96. [PMC free article] [PubMed] [Google Scholar]
- 52.Ighodaro O.M., Akinloye O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defence grid. Alexandria J. Med. 2018;54(4):287–293. doi: 10.1016/j.ajme.2017.09.001. [DOI] [Google Scholar]
- 53.Bansal A., Simon M.C. Glutathione metabolism in cancer progression and treatment resistance. J. Cell Biol. 2018;217(7):2291–2298. doi: 10.1083/jcb.201804161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Casanova N.A., Simoniello M.F., López Nigro M.M., Carballo M.A. Modulator effect of watercress against cyclophosphamide-induced oxidative stress in mice. Medicina (B Aires) 2017;77(3):201–206. [PubMed] [Google Scholar]
- 55.Adesanoye O.A., Abolaji A.O., Faloye T.R., Olaoye H.O., Adedara A.O. Luteolin-Supplemented Diets Ameliorates Bisphenol A-Induced Toxicity in Drosophila melanogaster. Food Chem. Toxicol. 2020:111478. doi: 10.1016/j.fct.2020.111478. [DOI] [PubMed] [Google Scholar]
- 56.Cortese-Krott M.M., Kelm M. Endothelial nitric oxide synthase in red blood cells: key to a new erythrocrine function? Redox Biol. 2014;2:251–258. doi: 10.1016/2Fj.redox.2013.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cosby K., Partovi K.S., Crawford J.H., Patel R.P., Reiter C.D., Martyr S., Yang B.K., Waclawiw M.A., Zalos G., Xu X., Huang K.T., Gladwin M.T. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat. Med. 2003;9(12):1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 58.Webb A.J., Milsom A.B., Rathod K.S., Chu W.L., Qureshi S., Lovell M.J., Lecomte F.M., Perrett D., Raimondo C., Khoshbin E., Ahluwalia A. Mechanisms underlying erythrocyte and endothelial nitrite reduction to nitric oxide in hypoxia: role for xanthine oxidoreductase and endothelial nitric oxide synthase. Circ. Res. 2008;103(9):957–964. doi: 10.1161/CIRCRESAHA.108.175810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Horn P., Cortese-Krott M.M., Keymel S., Kumara L., Burghoff S., Schrader J., Kelm M., Kleinbongard P. Nitric oxide influences red blood cell velocity independently of changes in the vascular tone. Free Radic. Res. 2011;45(6):653–661. doi: 10.3109/10715762.2011.574288. [DOI] [PubMed] [Google Scholar]
- 60.Beckman J.S., Beckman T.W., Chen J., Marshall P.A., Freeman B.A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. 1990;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Prado C.M., Martins M.A., Tibério I.F. Nitric oxide in asthma physiopathology. ISRN Allergy. 2011 doi: 10.5402/2011/832560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Buchwalow I., Schnekenburger J., Samoilova V., Boecker W., Neumann J., Tiemann K. New insight into the role of nitric oxide pathways in pancreas. Acta Histochem. Cytochem. 2018;51(6):167–172. doi: 10.1267/2Fahc.18028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yen F.L., Wu T.H., Lin L.T., Cham T.M., Lin C.C. Naringenin-loaded nanoparticles improve the physicochemical properties and the hepatoprotective effects of naringenin in orally-administered rats with CCl4-induced acute liver failure. Pharm. Res. 2009;26(4):893–902. doi: 10.1007/s11095-008-9791-0. [DOI] [PubMed] [Google Scholar]
- 64.Abe A., Hiraoka M., Shayman J.A. A role for lysosomal phospholipase A2 in drug induced phospholipidosis. Drug Metab. Lett. 2007;1(1):49–53. doi: 10.2174/187231207779814292. [DOI] [PubMed] [Google Scholar]
- 65.Das R., Khuraijam S.D., Dutta S., Das A., Das P., Devi K.K.P. Effects of Ipomoea aquatica Forsk. In cyclophosphamide induced dyslipidaemia in albino rats. Int. J. Basic Clin. Pharmacol. 2017;6:2743–2748. doi: 10.18203/2319-2003.ijbcp20174799. [DOI] [Google Scholar]
- 66.Halliwell W.H. Cationic amphiphilic drug-induced phospholipidosis. Toxicol. Pathol. 1997;25(1):53–60. doi: 10.1177/2F019262339702500111. [DOI] [PubMed] [Google Scholar]
- 67.Sawada H., Takami K., Asahi S. A toxicogenomic approach to drug-induced phospholipidosis: analysis of its induction mechanism and establishment of a novel in vitro screening system. Toxicol. Sci. 2005;83(2):282–292. doi: 10.1093/toxsci/kfh264. [DOI] [PubMed] [Google Scholar]
- 68.Tiirola T., Jauhiainen M., Erkkilä L., Bloigu A., Leinonen M., Haasio K., Laitinen K., Saikku P. Effect of pravastatin treatment on Chlamydia pneumoniae infection, inflammation and serum lipids in NIH/S mice. Int. J. Antimicrob. Agents. 2007;29(6):741–742. doi: 10.1016/j.ijantimicag.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 69.Becatti M., Marcucci R., Mannucci A., Gori A.M., Giusti B., Sofi F., Mannini L., Cellai A.P., Liotta A.A., Mugnaini M., Fiorillo C. Erythrocyte membrane fluidity alterations in sudden sensorineural hearing loss patients: the role of oxidative stress. Thromb. Haemost. 2017;117(12):2334–2345. doi: 10.1160/TH17-05-0356. [DOI] [PubMed] [Google Scholar]
- 70.Panieri E., Buha A., Telkoparan-Akillilar P., Cevik D., Kouretas D., Veskoukis A., Skaperda Z., Tsatsakis A., Wallace D., Suzen S., Saso L. Potential applications of NRF2 modulators in cancer therapy. Antioxidants. 2020;9(3):193. doi: 10.3390/antiox9030193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kouka P., Tekos F., Papoutsaki Z., Stathopoulos P., Halabalaki M., Tsantarliotou M., Zervos I., Nepka C., Liesivuori J., Rakitskii V.N., Tsatsakis A. Olive oil with high polyphenolic content induces both beneficial and harmful alterations on rat redox status depending on the tissue. Toxicol. Rep. 2020;7:421–432. doi: 10.1016/j.toxrep.2020.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kasote D.M., Katyare S.S., Hegde M.V., Bae H. Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int. J. Biol. Sci. 2015 doi: 10.7150/ijbs.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cheng K., Zeng X., Wu H., Su W., Fan W., Bai Y., Li P. Effects of naringin on the activity and mRNA expression of CYP isozymes in rats. Nat. Prod. Commun. 2019;14(12) doi: 10.1177/2F1934578X19894180. 1934578X19894180. [DOI] [Google Scholar]
- 74.Juránek I., Nikitovic D., Kouretas D., Hayes A.W., Tsatsakis A.M. Biological importance of reactive oxygen species in relation to difficulties of treating pathologies involving oxidative stress by exogenous antioxidants. Food Chem. Toxicol. 2013;61:240–247. doi: 10.1016/j.fct.2013.08.074. [DOI] [PubMed] [Google Scholar]
- 75.Docea A.O., Gofita E., Goumenou M., Calina D., Rogoveanu O., Varut M., Olaru C., Kerasioti E., Fountoucidou P., Taitzoglou I., Zlatian O. Six months exposure to a real life mixture of 13 chemicals’ below individual NOAELs induced non monotonic sex-dependent biochemical and redox status changes in rats. Food Chem. Toxicol. 2018;115:470–481. doi: 10.1016/j.fct.2018.03.052. [DOI] [PubMed] [Google Scholar]
- 76.Fountoucidou P., Veskoukis A.S., Kerasioti E., Docea A.O., Taitzoglou I.A., Liesivuori J., Tsatsakis A., Kouretas A.D. A mixture of routinely encountered xenobiotics induces both redox adaptations and perturbations in blood and tissues of rats after a long-term low-dose exposure regimen: the time and dose issue. Toxicol. Let. 2019;317:24–44. doi: 10.1016/j.toxlet.2019.09.015. [DOI] [PubMed] [Google Scholar]
- 77.Stagos D., Goutzourelas N., Ntontou A.M., Kafantaris I., Deli C.K., Poulios A., Jamurtas A.Z., Bar-Or D., Kouretas D. Assessment of eccentric exercise-induced oxidative stress using oxidation-reduction potential markers. Oxid. Med. Cell. Longev. 2015:204615. doi: 10.1155/2015/204615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Priftis A., Soursou A., Makiou A.S., Tekos F., Veskoukis A.S., Tsantarliotou M.P., Taitzoglou I.A., Kouretas D. A lightly roasted coffee extract improves blood and tissue redox status in rats through enhancement of GSH biosynthesis. Food Chem. Toxicol. 2019;125:305–312. doi: 10.1016/j.fct.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 79.Tsatsakis A., Docea A.O., Constantin C., Calina D., Zlatian O., Nikolouzakis T.K., Stivaktakis P.D., Kalogeraki A., Liesivuori J., Tzanakakis G., Neagu M. Genotoxic, cytotoxic, and cytopathological effects in rats exposed for 18 months to a mixture of 13 chemicals in doses below NOAEL levels. Toxicol. Lett. 2019;316:154–170. doi: 10.1016/j.fct.2018.12.043. [DOI] [PubMed] [Google Scholar]
- 80.Brigelius-Flohe R., Maiorino M. Glutathione peroxidases. Biochim. Biophys. Acta Gen. Subj. 2013;1830:3289–3303. doi: 10.1016/j.bbagen.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 81.Hayes J.D., Flanagan J.U., Jowsey I.R. Glutathione transferases. Annu. Rev. Pharmacol. Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45. [DOI] [PubMed] [Google Scholar]
- 82.Tsitsimpikou C., Tzatzarakis M., Fragkiadaki P., Kovatsi L., Stivaktakis P., Kalogeraki A., Kouretas D., Tsatsakis A.M. Histopathological lesions, oxidative stress and genotoxic effects in liver and kidneys following long term exposure of rabbits to diazinon and propoxur. Toxicology. 2013;307:109–114. doi: 10.1016/j.tox.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 83.Kanno S.I., Shouji A., Asou K., Ishikawa M. Effects of naringin on hydrogen peroxide-induced cytotoxicity and apoptosis in P388 cells. Int. J. Pharmacol. Screen. Methods. 2003;92(2):166–170. doi: 10.1254/jphs.92.166. [DOI] [PubMed] [Google Scholar]