Abstract
Natural or plant products, because of their structural diversity, are a potential source for identifying new anti-hepatitis B virus (HBV) agents. Here, we report the anti-HBV activity of Euphorbia schimperi and its quercetin (QRC) and kaempferol derivatives. The anti-HBV-active methanol fraction of E. schimperi was subjected to chromatographic techniques, leading to isolation of three flavonols, following their structure determination by 1H and 13C NMR spectroscopies. Their cytotoxicity and anti-HBV potential were assessed using HBV reporter HepG2.2.15 cells, and their modes of action were delineated by molecular docking. The isolated compounds identified as quercetin-3-O-glucuronide (Q3G), quercetin-3-O-rhamnoside (Q3R), and kaempferol-3-O-glucuronide (K3G) were non-cytotoxic to HepG2.2.15 cells. The viral HBsAg/HBeAg production on day 5 was significantly inhibited by K3G (∼70.2/∼73.4%), Q3G (∼67.8/∼72.1%), and Q3R (∼63.2%/∼68.2%) as compared to QRC (∼70.3/∼74.8%) and lamivudine (∼76.5/∼84.5%) used as standards. The observed in vitro anti-HBV potential was strongly supported by in silico analysis, which suggested their structure-based activity via interfering with viral Pol/RT and core proteins. In conclusion, this is the first report on the anti-HBV activity of E. schimperi-derived quercitrin-3-O-glucuronide, quercitrin-3-O-rhamnoside, and kaempferol-3-O-glucuronide, most likely through interfering with HBV proteins.
1. Introduction
The hepatitis B virus (HBV) causes acute and chronic liver diseases in about one-third of world’s population, of which ∼360 million remain at risk of developing chronic hepatitis B, liver cirrhosis, or hepatocellular carcinoma.1,2 Though several effective, safe, and well-tolerated anti-HBV drugs, notably, nucleoside analogues, are available, they have their own limitations.3 Of these, long-term treatment with lamivudine, adefovir, or famciclovir often leads to drug resistance.4 In recent decades, several herbal or natural products, mainly the Traditional Chines Medicine (TCM), have become very popular in treating chronic hepatitis B worldwide.5 However, some of these formulations have been reported to cause liver and other organ toxicity, including serious side effects.6 Recently, we have reported several medicinal plant extracts and fractions as well as isolation of anti-viral compounds for their novel anti-HBV potential.7−13
The genus Euphorbia (family: Euphorbiaceae) is recognized with over 2000 species, of which several are known for their use in traditional medicine.14 Their different extracts and isolated compounds have shown to possess anti-cancer, anti-cell proliferative, anti-malarial, anti-bacterial, anti-venom, anti-inflammatory, anti-oxidant, and anti-hepatitis properties.15,16 To date, more than 80 types of flavonoids have been isolated from the aerial parts, roots, seeds, and whole body of Euphorbia, where most are flavonols with O-substitutions, C-methylation, prenylation, glycosylation, or glycosidic linkages.15−20
Euphorbia schimperi C. Presl. has been characterized for its various in vitro biological activities such as anti-cell proliferative, free-radical scavenging, and anti-microbial potential.21,22 We have recently reported the in vivo wound healing property of the methanol extract of E. schimperi grown in Saudi Arabia.23 Several biologically active chemical constituents such as luteolin, scopoletin, kaempferol, flavonoid glycosides, and triterpenoids have been isolated from E. schimperi.21,24 Previously, extracts of E. cotinifolia, E. cestrifolia, and E. tirucalli grown in Latin America have shown in vitro anti-herpes simplex virus (HSV) activities.25 In addition, lectins isolated from E. pulcherrima and E. tirucalli have been demonstrated to have anti-HSV potential.26 Notably, flavonoids from E. humifusa have been shown for their anti-HBV activities through downregulation of viral antigen secretions in the cell culture model.27 Because of a similar replication mechanism of HBV and HSV, the anti-HSV drugs also work very well in chronic hepatitis B patients.3 With this background, we assessed the anti-HBV potential of E. schimperi and isolated flavonols quercetin and kaempferol derivatives using HBV reporter cells as well as delineated their modes of action by molecular docking.
2. Results
2.1. Identification of Compounds
The 1H and 13C NMR spectroscopy data (Figures S1–S3) of the isolated compounds were in good agreement with the published literature.24,28,29 Structures of the isolated compounds were identified as quercetin-3-O-glucuronide (Q3G), quercetin-3-O-rhamnoside (Q3R), and kaempferol-3-O-glucuronide (K3G).
Q3G—1H NMR (DMSO-d6): δ 6.21 (1H, s, H-6), 6.41 (1H, s, H-8), 7.86 (1H, br s, H-2′), 6.84 (1H, d, J = 8.4 Hz, H-5′), 7.51 (1H, d, J = 7.7 Hz, H-6′), 5.40 (1H, d, J = 6.3 Hz, H-1″), 3.28 (2H, m, H-2″, 3″), 3.33 (1H, d, J = 9.1 Hz, H-4″), 3.50 (1H, d, J = 9.1 Hz, H-5″). 12.46, 9.54 (OHs); 13C NMR (DMSO-d6): δ 157.3 (C-2), 133.9 (C-3), 177.9 (C-4), 104.4 (C-4a), 161.5 (C-5), 99.3 (C-6), 164.8 (C-7), 94.2 (C-8), 156.8 (C-8a), 121.0 (C-1′), 117.3 (C-2′), 145.3 (C-3′), 149.0 (C-4′), 115.7 (C-5′), 121.7 (C-6′), Glc—102.3 (C-1″), 74.5 (C-2″), 76.5 (C-3″), 72.0 (C-4″), 75.7 (C-5″), 171.3 (C-6″).
Q3R—1H NMR (DMSO-d6): δ 6.21 (1H, s, H-6), 6.40 (1H, s, H-8), 7.31 (1H, d, J = 2.1 Hz, H-2′), 6.87 (1H, d, J = 8.4 Hz, H-5′), 7.26 (1H, dd, J = 2.1, 2.1 Hz, H-6′), 5.26 (1H, s, H-1″), 3.97 (1H, m, H-2″), 3.51 (1H, dd, J = 3.5, 3.5 Hz, H-3″), 3.15 (1H, m, H-4″), 3.21 (1H, m, H-5″), 0.82 (3H, d, J = 6.3 Hz, −CH3), 12.67, 10.91, 9.74, 9.35 (OHs);13C NMR (DMSO-d6): δ 157.8 (C-2), 134.7 (C-3), 178.2 (C-4), 104.5 (C-4a), 161.8 (C-5), 99.1 (C-6), 164.6 (C-7), 94.1 (C-8), 156.9 (C-8a), 121.1 (C-1′), 116.1 (C-2′), 145.7 (C-3′), 148.9 (C-4′), 115.9 (C-5′), 121.5 (C-6′), Glc—102.3 (C-1″), 70.5 (C-2″), 71.1 (C-3″), 71.6 (C-4″), 70.7 (C-5″), 17.9 (C-6″).
K3G—1H NMR (DMSO-d6): δ 6.23 (1H, s, H-6), 6.45 (1H, s, H-8), 8.05 (2H, d, J = 8.4 Hz, H-2′, 6′), 6.89 (2H, d, J = 7.7 Hz, H-3′, 5′), 5.51 (1H, d, J = 7.0 Hz, H-1″), 3.25 (3H, m, H-2″, 3″, 4″), 3.60 (1H, d, J = 9.8 Hz, H-5″), 12.54, 10.93, 10.25 (OHs);13C NMR (DMSO-d6): δ 156.8 (C-2), 133.4 (C-3), 177.7 (C-4), 104.3 (C-4a), 161.7 (C-5), 99.3 (C-6), 164.6 (C-7), 94.1 (C-8), 156.9 (C-8a), 121.1 (C-1′), 131.3 (C-2′, C-6′), 160.4 (C-4′), 115.5 (C-3′, C-5′), Glc—101.7 (C-1″), 74.4 (C-2″), 76.4 (C-3″), 71.9 (C-4″), 76.1 (C-5″), 170.3 (C-6″).
2.2. Effect of E. schimperi and Its Flavonols on Cell Viability
As revealed by the MTT assay, while the E. schimperi methanol fraction (ESF) was non-cytotoxic at 100 μg/mL, it showed significant toxicity at 150 μg/mL and above (CC50: 136.25 μg/mL). The tested compounds did not show any sign of cytotoxicity up to 50 μg/mL (CC50: 72.53–78.25 μg/mL) and enhanced the cell growth at 12.5 μg/mL and above (data not shown).
2.3. Dose-Dependent Inhibition of HBsAg Expression
At 48 h post-treatment, the estimated maximal inhibitions of HBsAg were by 100 μg/mL of ESF (∼38%) and 12.5 μg/mL each of K3G (∼48.7%), Q3G (∼46%), and Q3R (∼43.8%), including quercetin (QRC) which served as the positive control (Figure S4). Because of the observed cytotoxicity of ESF at higher concentration, doses above 100 μg/mL were not included. For isolated compounds, because concentrations at 12.5 and 25 μg/mL had comparable activity, the equal molar concentrations (12.25–13 μg/mL) were used for further analyses.
2.4. Time Course Inhibition of HBsAg Expressions
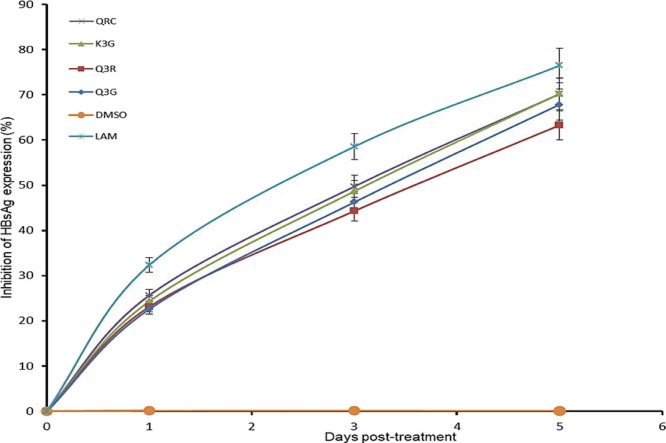
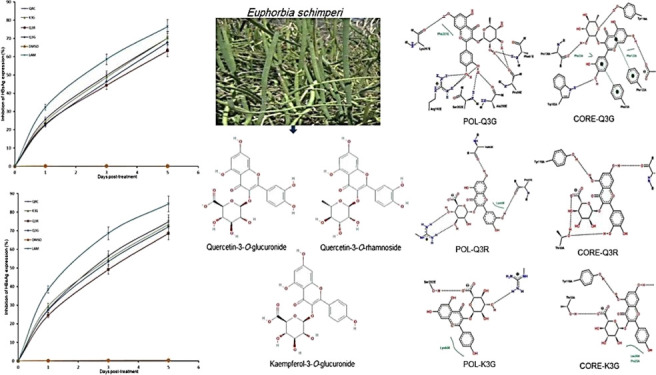
A time course study of the active flavonol compounds (27 μM each; IC50: 22.3–23.5 μM) was further carried out. Compared to days 1 and 3 post-treatment, HBsAg production on day 5 was significantly inhibited by K3G (∼70.2%), Q3G (∼67.8%), and Q3R (∼63.2%) (Figure 1). Notably, the reference drugs QRC and lamivudine (LAM) suppressed the HBV “e” antigen (HBeAg) level by ∼70.3 and ∼76.5%, respectively. The study was not extended beyond day 5 because the tested flavonols promoted cell proliferation resulting in cell overgrowth and death (data not shown).
Figure 1.
Time course inhibition of HBsAg expressions by E. schimperi-derived Q3G, Q3R, and K3G (27 μM, each) relative to untreated control in HepG2.2.15 culture supernatants at days 1, 3, and 5 post-treatment. QRC (27 μM) and LAM (2 μM) served as positive controls, while DMSO (0.1%) acted as the negative control. The values on the Y-axis are means of three determinations.
2.5. Downregulation of Virus Replication
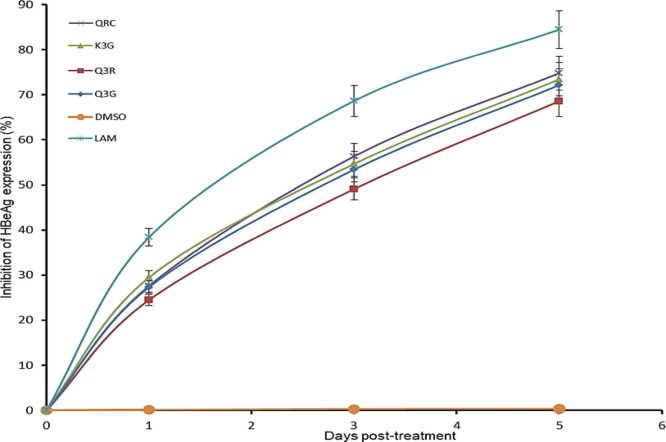
HBeAg, the small processed protein, is co-translated with HBV-Core (HBcAg) by a bicistronic RNA and therefore serves as a marker of active viral replication. Therefore, Q3G, Q3R, and K3G (27 μM each) were tested for their effects on HBeAg production. The three compounds markedly downregulated the HBeAg synthesis in a time-dependent manner. Compared to days 1 and 3, HBeAg production at day 5 was markedly downregulated by K3G (∼73.4%), Q3G (∼72.1%), and Q3R (∼68.2%) (Figure 2). Notably, the reference drugs QRC and LAM suppressed the HBeAg level by ∼74.8 and ∼84.5%, respectively. The study was not extended beyond day 5 because the tested flavonols promoted cell proliferation, resulting in cell overgrowth and death (data not shown).
Figure 2.
Time course inhibition of HBeAg expressions by E. schimperi-derived Q3G, Q3R, and K3G (27 μM, each) relative to untreated control in HepG2.2.15 culture supernatants at days 1, 3, and 5 post-treatment. QRC (27 μM) and LAM (2 μM) served as positive controls while DMSO (0.1%) acted as the negative control. The values on the Y-axis are means of three determinations.
2.6. Reporter Gene Assay
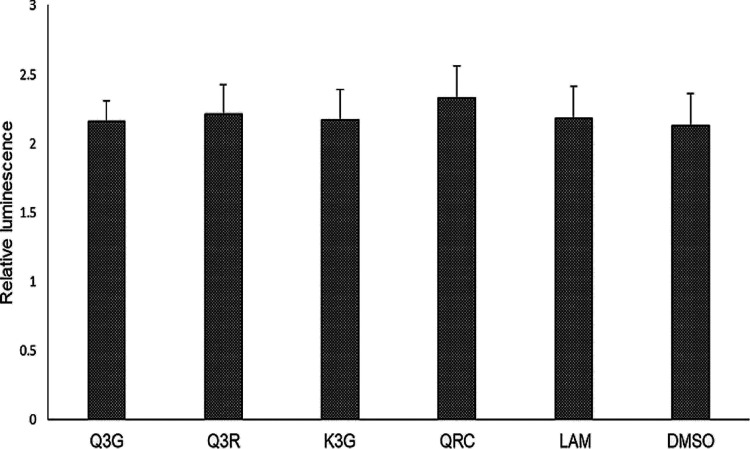
As revealed by the reporter gene assay, there were no significant changes in the levels of Renilla luciferase expressions upon treatment with Q3G, Q3R, and K3G, including the reference drugs QRC and LAM (Figure 3). This confirmed that S. schimperi-derived anti-HBV-active flavonols specifically inhibited the production of HBsAg and HBeAg but did not affect the non-viral or host protein synthesis.
Figure 3.
Reporter gene assay showing relative expressions of luciferase in transfected HepG2.2.15 cells treated with Q3G, Q3R, and K3G (27 μM, each). QRC (27 μM) and LAM (2 μM) served as positive controls, while DMSO (0.1%) acted as the negative control. The values on the Y-axis are means of three determinations.
2.7. Molecular Docking
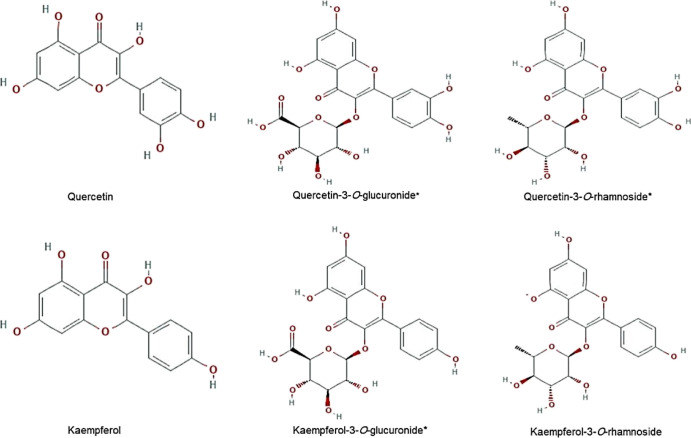
Because of their common flavonol skeleton, the E. schimperi-derived anti-HBV-active Q3G, Q3R, and K3G along with kaempferol-3-O-rhamnoside, QRC, and kaempferol (Figure 4) were subjected to docking analysis against both HBV-Pol and HBV-Core proteins to determine their general mode of action and to evaluate the types of molecular interactions.
Figure 4.
2D structures of quercetin, kaempferol, quercetin-3-O-glucuronide*, quercetin-3-O-rhamnoside*, kaempferol-3-O-glucuronide*, and kaempferol-3-O-rhamnoside used in molecular docking analysis. *E. schimperi derived.
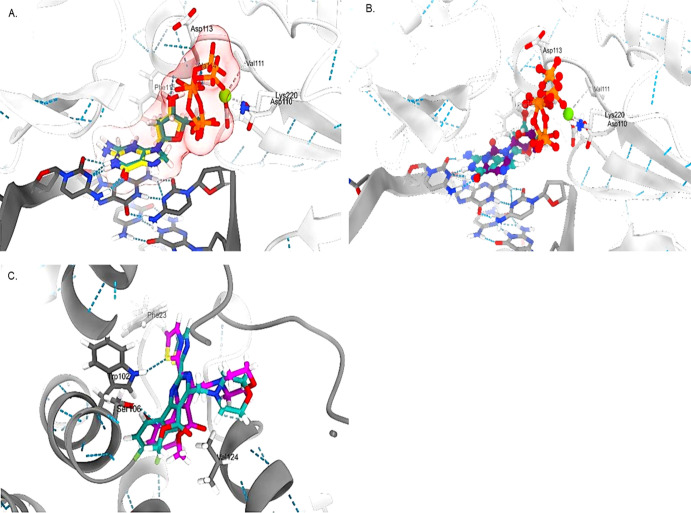
The docking validation revealed good re-alignment of the ligands inside the binding site with low root-mean-square deviation (RMSD) values (<2) for the standard LAM (Figure 5A) and heteroaryldihydropyrimidine (HAP) (Figure 5C). Further superimposition of LAM inside the binding pocket gave a quite similar conformation adopted by the co-crystallized 2-deoxyguanosine-5-triphosphate (Figure 5B).
Figure 5.
3D representations of the (A) co-crystallized ligand 2-deoxyguanosine-5-triphosphate before (light sea green) and after docking (yellow) with HBV-Pol, (B) alignment of LAM (purple) compared to 2-deoxyguanosine-5-triphosphate (light sea green), and (C) HAP with HBV-Core before (light sea green) and after docking (magenta).
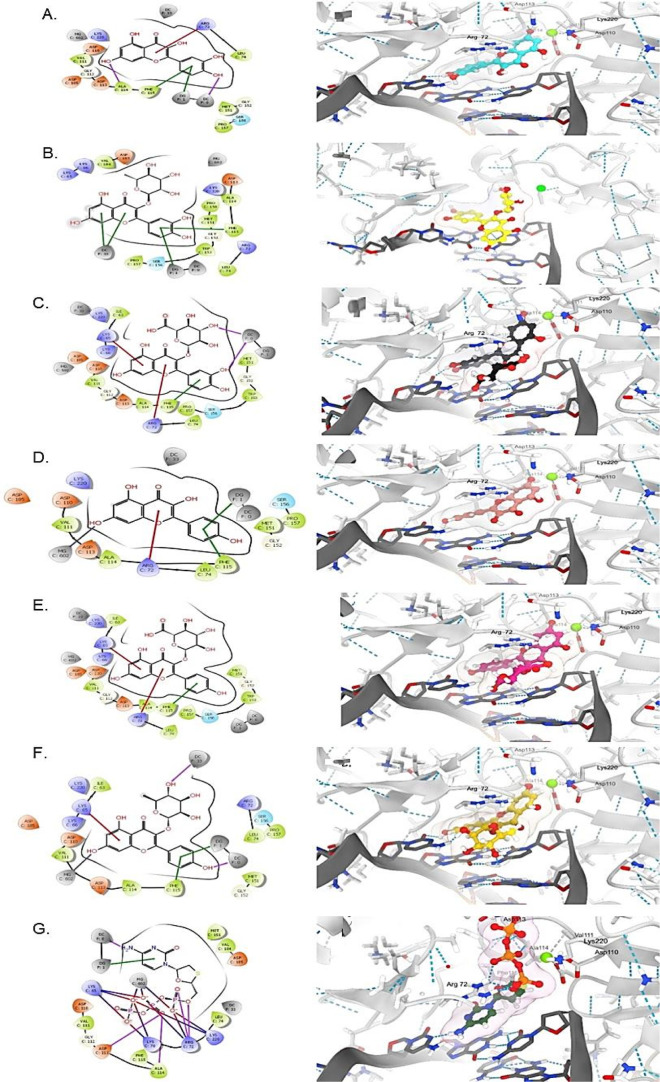
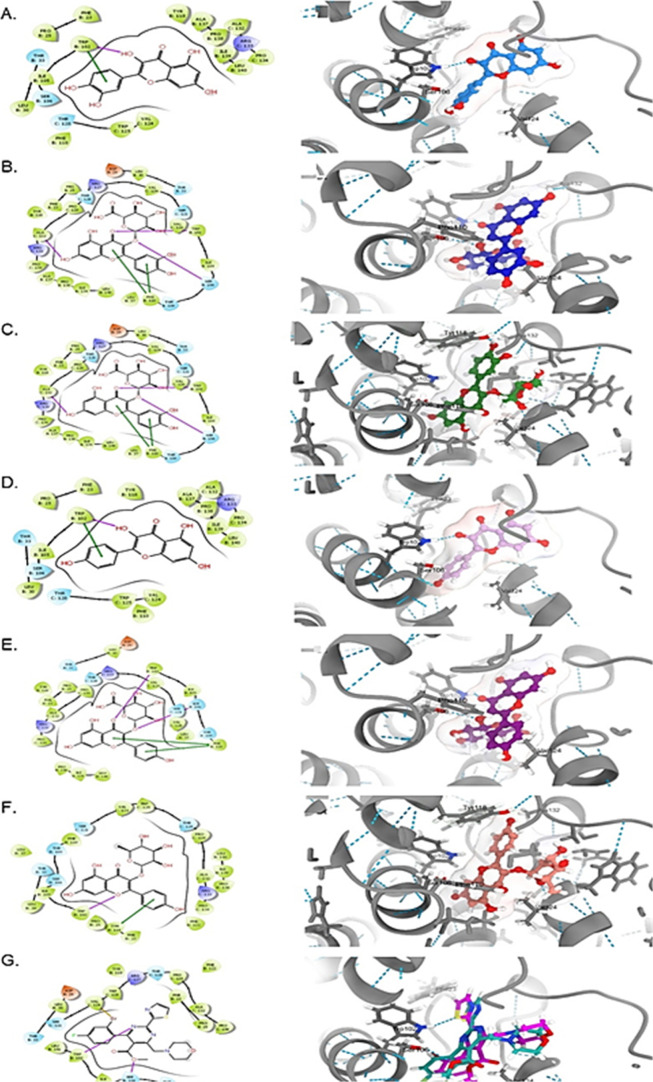
The docking results showed that all the tested compounds were finely docked into both targets, and they had comparable binding affinities compared to the standards (Figures 6 and 7). Of these, kaempferol-3-O-rhamnoside and kaempferol showed the best docking score toward HBV-Pol and HBV-Core proteins, respectively (Table S1). Overall, all flavonols showed relatively similar orientations and alignments inside the active site of these targets, forming mainly hydrogen bonds and π-stacking interactions with residues and nucleotides of the active site. These expected similarities were largely due to their common general skeleton.
Figure 6.
Molecular docking analysis showing 2D (left) and 3D (right) interactions of HBV-Pol with (A) QRC, (B) Q3G*, (C) Q3R*, (D) kaempferol, (E) K3G*, (F) kaempferol-3-O-rhamnoside, and (G) lamivudine phosphate. *E. schimperi derived.
Figure 7.
Molecular docking analysis showing 2D (left) and 3D (right) interactions of HBV-Core with (A) QRC, (B) Q3G*, (C) Q3R*, (D) kaempferol, (E) K3G*, (F) kaempferol-3-O-rhamnoside, and (G) HAP. *E. schimperi derived.
Molecular docking of flavonols into HBV-Pol showed that they had comparable estimated docking scores in comparison with LAM (−9.15 kcal/mol; Table S1) except for Q3R (−6.49 kcal/mol; Table S1), which adopted a different alignment inside the binding pocket (Figure 6C). The most important interaction shown by the majority of these compounds was the π-cation with Lys65 and/or Arg75 and the π-stacking with the nucleotides facing the binding site. These interactions were similar to LAM and 2-deoxyguanosine-5-triphosphate, though the interactions of the negative charge of their triphosphate groups with Lys65 and Arg75 residues are ionic type. Q3R did not show a π-cation interaction with neither Lys65 nor Arg75 residues (Figure 6C), which may contribute to its lower docking scores compared to others (Table S1). In contrast, some flavonols such as kaempferol and kaempferol-3-O-rhamnoside showed a T-shaped edge-to-face π–π interaction with Phe115 and face–face π–π interaction with the nucleotide (Figure 6D,F). The magnesium cation imbedded into the active site was in close contact with one or two hydroxy groups of 2-deoxyguanosine-5-triphosphate and LAM (Figure 5A,B), showing a possibility of chelation in kaempferol and kaempferol-3-O-rhamnoside.
Docking of the flavonols and HAP with HBV-Core shared an interaction with Trp102 through hydrogen bonding. Of these, Q3R and kaempferol exhibited the best binding affinity with docking scores of −9.01 and −9.12 kcal/mol, respectively (Table S1). The ligand–protein complexes of both were also stabilized by the π–π interaction with Trp118 in the case of Q3R (Figure 7C) while with Trp102 in the case of kaempferol (Figure 7D). Furthermore, Q3R showed an extra interaction with Trp118 and Ala132 through the H-bond.
QRC, K3G, and kaempferol-3-O-rhamnoside showed lower binding scores compared to HAP (Table S1). Of the tested flavonols, Q3G had the lowest docking score. Notably, the ligands with the sugar moiety adopted a similar conformation inside the target according to the sugar type rather than the aglycone types. Also, those without sugar components showed the same behavior. Other residues that surrounded the ligands and may make a contribution to the complex stability include Phe32, Ser106, Phe110, and Val124.
Taken together, our in silico analysis strongly endorsed the in vitro anti-HBV potential of quercetin and kaempferol derivatives along with Q3G, Q3R, and K3G through interactions with HBV-Pol and HBV-Core proteins.
3. Discussion
Natural products or plant secondary metabolites, because of their structural diversity, are a potential source for identifying anti-HBV agents with novel structures and mechanisms of action. In the last 2 decades, several bioactive natural compounds belonging to different classes such as flavonoids, alkaloids, lignans, and anthraquinones have been identified as promising anti-HBV agents in vitro and in vivo.5,30,31 Flavonoids and their glucosides, galactosides, arabinosides, rutinosides, and rhamnosides constitute the largest source of antiviral compounds.30 Of these, quercetin and kaempferol are among the most ubiquitous natural flavonols found as glucoside derivatives rather than free form, which impact their bioactivities.31 In continuation of our natural anti-HBV product discoveries,7−13 we have recently reported the novel anti-HBV potential of Guiera senegalensis and identified quercetin and myricetin-3-rhamnoside (myricitrin), possibly via inhibitions of HBV-Pol and HBV-Core.32 Over 80 types of flavonoids have been isolated from different species of Euphorbia, mostly flavonols with O-substitutions, C-methylation, prenylation, glycosylation, and so forth.15−20 Of these, quercetin and kaempferol derivatives such as Q3R, K3G, kaempferol 3-O-glucoside, kaempferol-3-l-rhamnoside, and kaempferol-3-O-α-rhamnoside-O-β-D-glucopyranoside have been reported.15,33,34 Notably, E. humifusa-derived apigenin-7-O-glucopyranoside markedly inhibited in vitro HBsAg and HBeAg productions, whereas quercetin-3-O-α-L-rhamnosy-β-D-galactoside, quercetin-3-O-β-D-glucopyranoside, and quercetin-3-O-β-D-galactoside had no anti-HBV effects.27 In the present study, we report the novel anti-HBV potential of locally grown E. schimperi and identified quercitrin-3-O-glucuronide, quercitrin-3-O-rhamnoside, and K3G as the active principles.
Quercetin is the aglycon form of other flavonoid glycosides with antiviral activities against many RNA and DNA viruses, including HIV, HSV, and HBV.35−40 Its structurally close rosmarinic acid has also been shown to inhibit HBV replication via targeting HBV-Pol.41 Previously, we have demonstrated the high anti-HBV potential of quercetin that significantly inhibited the HBsAg and HBeAg synthesis in HepG2.2.15 cells.13 In addition, we have very recently reported the potent anti-HBV activity of G. senegalensis-derived quercetin that inhibited HBsAg and HBeAg by 60% and 62%, respectively.32 In addition, quercetin derivatives such as Q3G and quercetin-7-O-rhamnoside have been reported for their efficacy against the porcine epidemic diarrhea virus and influenza A virus, respectively.42,43 Quercetin and Q3R have been shown to have strong anti-HBV activity in cultured HepG2.2.15 cells.44 Also, quercetin, quercetin-3-O-acetylglucuronide, and quercetin-3-O-acetylglucuronide methyl ester have shown significant anti-HBV activity assayed by anti-HBsAg production.45 We in this study therefore, included quercetin as a natural anti-HBV standard along with lamivudine. Our data on anti-HBV activity was supported by molecular docking analysis where Q3G strongly interacted with the HBV-Pol and HBV-Core proteins indicative of its mode of antiviral action.
The flavonol kaempferol has been reported to have broad antiviral activities against influenza A virus,46 Japanese encephalitis virus,47 enterovirus E7,48 and dengue virus.49 Further, kaempferol and kaempferol-7-O-glucoside have been shown to have potent anti-HSV activity and anti-HIV activity in vitro.50,51 Kaempferol-3-O-rhamnopyranoside is shown to significantly inhibit the replication of influenza A virus in vitro.52 Also, kaempferol and its rhamnose derivatives are reported for their good antiviral candidates for coronaviruses.53 Notably, the anti-HIV activities of kaempferol and kaempferol-3-O-acetylrhamnosides have been shown via inhibition of viral Pol/RT.54,55 Another derivative, kaempferol-3,7-bisrhamnoside, has been shown to have potent activity against hepatitis C virus via inhibition of NS3 protease.56 Notably, kaempferol has been demonstrated to have significant anti-HBV activity in cultured HepG2.2.15 cells.57 Our data on anti-HBV activity was well endorsed by molecular docking, where K3G strongly interacted with HBV-Pol, indicative of its mode of antiviral action.
HBV-Pol is the most favored antiviral target where its inhibition leads to cessation of HBV replication. In our docking analysis for HBV-Pol, Q3G and kaempferol-3-O-rhamnoside showed the best docking scores than Q3R and K3G. However, as compared to LAM, all tested flavonols showed better affinities toward HBV-Pol. HBV-Core protein forms the viral capsid and has emerged as a promising antiviral target. Our docking analysis showed a higher binding affinity of HBV-Core with Q3R and kaempferol-3-O-rhamnoside as compared to quercetin-3-O-glucuronide and K3G. HAP is a novel class of HBV-Core inhibitor58,59 that however showed lower binding affinity than the tested flavonols. In brief, kaempferol-3-O-rhamnoside seemed to have the best docking scores for both HBV-Pol and HBV-Core proteins. Most of the tested flavonols showed closely similar orientations and alignments inside the active site of both standards, forming mainly hydrogen bonds and π-stacks, which are attributed to their common general skeleton. In addition, flavonols with a sugar moiety adopted similar conformation inside the target according to the sugar type rather than the aglycone types, and those without sugar also showed the same behavior. Overall, our in silico data strongly supported the in vitro anti-HBV activity of E. schimperi-derived quercetin and kaempferol derivatives through interfering with the viral polymerase and capsid proteins.
4. Conclusions
This work reports on the in vitro anti-HBV potential of E. schimperi as well as its bioactive quercetin and kaempferol derivatives: quercitrin-3-O-glucuronide, quercitrin-3-O-rhamnoside, and kaempferol-3-O-glucuronide. Further in silico analysis of quercetin, kaempferol, and its derivatives strongly endorsed their antiviral activities through interfering with HBV-Pol and HBV-Core proteins. Our data therefore warrants extended studies on E. schimperi toward further identifications of novel anti-HBV constituents.
5. Materials and Methods
5.1. Plant Collection, Extraction, and Isolation of Compounds
The whole flowering plant of E. schimperi was collected from the Wadi-e-Gama region of Saudi Arabia in February 2014. The plant was identified by Dr. Mohammad Yousuf, an expert plant taxonomist at the College of Pharmacy, King Saud University, Riyadh, and a voucher specimen (no. 16322) was deposited. The processing of the plant including extraction, fractionation, isolation of compounds, and structure determinations was performed essentially as described in our previous report.24 Briefly, the bioactive methanol fraction was subjected to various chromatographic techniques, leading to the isolation of three compounds quercitrin-3-O- glucuronide (Q3G), quercitrin-3-O-rhamnoside (Q3R), and kaempferol-3-O-glucuronide (K3G). Their chemical structures were further verified by 1H and 13C NMR spectroscopy data obtained in DMSO on a Bruker Avance spectrometer operating at 700 MHz for 1H and 175 MHz for 13C.
5.2. Cell Culture, Compounds, and Drugs
The HBV reporter hepatoma cell line HepG2.2.15 cells (kind gift of Dr. S. Jameel, International Center for Genetic Engineering & Biotechnology, New Delhi, India) were grown and maintained in RPMI-1640 medium (Gibco, USA) supplemented with 10% fetal bovine serum (Gibco, USA), 1× penicillin–streptomycin mix, and 1× sodium pyruvate (HyClone Laboratories, USA) at 37 °C in a humid chamber with 5% CO2 supply. The approved nucleoside analogue-based anti-HBV drug lamivudine (Sigma-Aldrich, Germany) and the anti-HBV-active natural compound quercetin (Sigma-Aldrich, Germany) were used as standard or positive controls.32,60 Stocks of E. schimperi methanol extract, the isolated compounds, and standards were prepared in RPMI after dissolving in DMSO (<1%, final) as mentioned elsewhere.13 DMSO served as the vehicle or negative control.
5.3. Cell Proliferative Assay of E. schimperi Fraction and Isolated Compounds
HepG2.2.15 cells (0.5 × 105/100 μL/well) were seeded in flat bottom 96-well tissue culture plates (Corning, USA) and incubated overnight. Next day, the cells were replenished with fresh RPMI media containing different doses of ESF (25, 50, 100, 150, and 200 μg/mL) or the isolated compounds (6.25, 12.5, 25, 50, and 100 μg/mL each) or DMSO (<0.1% final). After 48 h of incubation, the MTT cell proliferation assay (Terbigen, USA) was performed to determine their non-cytotoxic concentrations. All samples were tested in triplicate and repeated. Briefly, all cells were treated with MTT reagent (10 μL/well) and incubated for 3–4 h until a purple color appeared, following addition of detergent solution (100 μL) and incubated for 1 h. The absorbance (A; λ = 570 nm) was recorded in a microplate reader (BioTek, ELx800), and non-linear regression analysis (Excel, Microsoft, USA) was performed to determine the maximal concentration resulting in 50% inhibition of cell proliferation or viral activity (IC50).
5.4. Dose-Dependent Inhibition Assay of HBV Surface Antigen (HBsAg)
HepG2.2.15 cells were seeded in 96-well plates (0.5 × 105/well) and incubated overnight at 37 °C. Next day, the cells were treated with various doses of ESF (25, 50, and 100 μg/mL) or test compounds, including QRC (6.25, 12.5, and 25 μg/mL, each).11 After 48 h of incubation, culture supernatants were collected and analyzed for the inhibition of viral HBsAg synthesis (MonolisaHBsAg ULTRA, BioRad, USA) as per the manufacturer’s manual. All samples were tested in triplicate and repeated. Briefly, the absorbance was recorded (Microplate reader; BioTek, ELx800), and nonlinear regression analysis was performed.
5.5. Time Course Inhibition Assay of HBsAg
Based on the optimal dose-dependent (12.5 μg/mL) HBsAg inhibition data, the equal molar concentrations (≈12.25–13 μg/mL) of the tested compounds were used for time course (days 1, 3, and 5) analyses. Quercitrin-3-O-glucuronide (13 μg/mL), quercitrin-3-O-rhamnoside (12.25 μg/mL), and K3G (12.5 μg/mL) were reconstituted to furnish ≈27 μM each. QRC (8.25 μg/mL, ≈27 μM) and LAM (2.0 μM)58 were included as positive controls, whereas DMSO (0.1%) served as the negative control. All samples were tested in triplicate and repeated.
5.6. Time Course Inhibition Assays of HBV Pre-core Antigen (HBeAg)
The three test compounds (27 μM, each) showing promising inhibitory effects on HBsAg production, including proper controls, were further subjected to time course (days 1, 3, and 5) analysis of HBeAg expression in culture supernatants (HBeAg/Anti-HBe Elisa Kit; DIASource, Belgium) as per the manufacturer’s manual. All samples were tested in triplicate and repeated.
5.7. Luciferase Reporter Gene Assay
To rule out the inhibitory effects, if any, of the tested compounds on cellular proteins, luciferase assay was performed as described elsewhere.12 Briefly, HepG2.2.15 cells were transfected with the Renilla-luciferase reporter plasmid (pRL-TK; Promega, USA) using FuGENE6 (Promega, USA) in a 48-well flat-bottom culture plate a day before treatment with the anti-HBV-active compounds (27 μM, each) including controls and incubated for another 2 days (i.e., day 3 post-transfection). Cell lysates were prepared in cold condition and luciferase expression was measured (Luciferase Reporter Assay System; Promega, USA), and data were presented in relation to the negative control. All samples were tested in triplicate and repeated.
5.8. Molecular Docking Studies
Based on their promising in vitro anti-HBV activity, the three flavonols Q3G, Q3R, and K3G were subjected to molecular docking analysis to delineate their plausible mode of antiviral actions. Also, because they share a common flavonol skeleton, QRC, kaempferol, and kaempferol-3-O-rhamnoside were also included in the in silico analysis. The two HBV proteins, polymerase/reverse transcriptase (Pol/RT) and capsid/core (Core), were used as targets, whereas LAM (HBV-Pol inhibitor) and HAP (HBV-Core inhibitor) were used as standard ligands as described elsewhere.58,59 The crystallographic structure for HBV-Core (PDB code: 5E0I; resolution: 1.60 Å) was retrieved from the Protein Data Bank (https://www.rcsb.org/).
In the absence of a 3D model for HBV-Pol in protein database, we constructed our own homology-based model using a template with a co-crystallized nucleoside ligand (2-deoxyguanisine-5-triphosphate). Because the standard LAM (2′,3′-dideoxy-3′-thiacytidine triphosphate) is not the original co-crystallized ligand present in the used template, we further superposed LAM on 2-deoxyguanosine-5-triphosphate inside the binding pocket. Also, for HBV-Pol analysis, the DNA was imported and placed into its cavity by homology. All ligands were imported from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/), solvent molecules and co-crystallized ligands from the proteins were removed, and hydrogen atoms were added. The active site of each target was determined based on a literature review and confirmed using the automated assembly tool SEINA.60 Preparation and energy minimization of each protein were performed with Maestro.61 The 2D and 3D visualizations of the ligand–target interactions were generated using Maestro and UCSF ChimeraX,62 respectively. The compound set was docked on the previous targets’ binding sites by using the software AutoDock Vina 1.2.0 (2021) operated in Linux OS.63 The docking protocol was validated by re-docking the co-crystallized ligands into the binding site and then inspected visually, and the RMSD was calculated.
5.9. Statistical Analysis
All data in triplicates were presented as mean ± standard error and analyzed using one-way analysis of variance. Differences between two groups were compared using Student’s t-test. p < 0.05 was considered significant. All statistical analyses were performed with SPSS software (Version 25; IBM, USA).
Acknowledgments
The project was supported by the Deanship of Scientific Research, King Saud University, Riyadh (grant no. RG-1435-053).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c04320.
1H and 13C NMR spectra of the isolated compounds; dose-dependent anti-HBV effect of E. schimperi and isolated compounds; and molecular docking predicted ligand–target interaction scores (PDF)
Author Contributions
M.K.P., S.A., and M.S.A.-D. conceived and designed the experiments. M.K.P., S.A., M.S.A.-D., M.A.S.A., A.H.A., and M.M.A.-Q. performed the experiments and data acquisition. M.K.P., S.A., M.S.A.-D., and A.J.A.-R. analyzed the data, interpreted the results, and wrote the manuscript. The final manuscript was read and approved by all authors.
The authors declare no competing financial interest.
Supplementary Material
References
- Shepard C. W.; Simard E. P.; Finelli L.; Fiore A. E.; Bell B. P. Hepatitis B virus infection: epidemiology and vaccination. Epidemiol. Rev. 2006, 28, 112–125. 10.1093/epirev/mxj009. [DOI] [PubMed] [Google Scholar]
- Teo C.-G.; Locarnini S. A. Potential threat of drug-resistant and vaccine-escape HBV mutants to public health. Antiviral Ther. 2010, 15, 445–449. 10.3851/imp1556. [DOI] [PubMed] [Google Scholar]
- Parvez M. K.; Mechkarska M. Currently available anti-hepatitis viruses drugs. J. Gastroenterol. Hepatol. Res. 2020, 9, 3155–3157. [Google Scholar]
- Devi U.; Locarnini S. Hepatitis B antivirals and resistance. Curr. Opin. Virol. 2013, 3, 495–500. 10.1016/j.coviro.2013.08.006. [DOI] [PubMed] [Google Scholar]
- Cui X.; Wang Y.; Kokudo N.; Fang D.; Tang W. Traditional Chinese medicine and related active compounds against hepatitis B virus infection. BioSci. Trends 2010, 4, 39–47. [PubMed] [Google Scholar]
- Parvez M. K.; Al-Dosari M. S.; S. Al-Dosari M. CAM/Drug-induced hepatotoxicity: implications in viral hepatitis. J. Gastroenterol. Hepatol. Res. 2016, 5, 1921–1923. 10.17554/j.issn.2224-3992.2016.05.552. [DOI] [Google Scholar]
- Arbab A. H.; Parvez M. K.; Al-Dosari M. S.; Al-Rehaily A. J. In vitro evaluation of novel antiviral activities of 60 medicinal plants extracts against hepatitis B virus. Exp. Ther. Med. 2017, 14, 626–634. 10.3892/etm.2017.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbab A. H.; Parvez M. K.; Al-Dosari M. S.; Al-Rehaily A. J.; Al-Sohaibani M.; Zaroug E. E.; AlSaid M. S.; Rafatullah S. Hepatoprotective and antiviral efficacy of Acacia mellifera leaves fractions against hepatitis B virus. Biomed Res. Int. 2015, 2015, 929131. 10.1155/2015/929131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam P.; Parvez M. K.; Arbab A. H.; Al-Dosari M. S. Quantitative analysis of rutin, quercetin, naringenin, and gallic acid by validated RP- and NP-HPTLC methods for quality control of anti-HBV active extract of Guiera senegalensis. Pharm. Biol. 2017, 55, 1317–1323. 10.1080/13880209.2017.1300175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvez M. K.; Alam P.; Arbab A. H.; Al-Dosari M. S.; Alhowiriny T. A.; Alqasoumi S. I. Analysis of antioxidative and antiviral biomarkers β-amyrin, β-sitosterol, lupeol, ursolic acid in Guiera senegalensis leaves extract by validated HPTLC methods. Saudi Pharm. J. 2018, 26, 685–693. 10.1016/j.jsps.2018.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvez M. K.; Tabish Rehman M.; Alam P.; Al-Dosari M. S.; Alqasoumi S. I.; Alajmi M. F. Plant-derived antiviral drugs as novel hepatitis B virus inhibitors: cell culture and molecular docking study. Saudi Pharm. J. 2019, 27, 389–400. 10.1016/j.jsps.2018.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvez M. K.; Al-Dosari M. S.; Arbab A. H.; Niyazi S. The in vitro and in vivo anti-hepatotoxic, anti-hepatitis B virus and hepatic CYP450 modulating potential of Cyperus rotundus. Saudi Pharm. J. 2019, 27, 558–564. 10.1016/j.jsps.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvez M. K.; Al-Dosari M. S.; Alam P.; Rehman M. T.; Alajmi M. F. The anti-hepatitis B virus therapeutic potential of anthraquinones derived from Aloe vera. Phytother. Res. 2019, 33, 2960–2970. 10.1002/ptr.6471. [DOI] [PubMed] [Google Scholar]
- Johari S.; Kumar A. Euphorbia spp. and their use in traditional medicines: A review. World J. Pharm. Res. 2020, 9, 1477–1485. 10.20959/wjpr202014-19231. [DOI] [Google Scholar]
- Magozwi D. K.; Dinala M.; Mokwana N.; Siwe-Noundou X.; Krause R. W. M.; Sonopo M.; McGaw L. J.; Augustyn W. A.; Tembu V. J. Flavonoids from the Genus Euphorbia: Isolation, Structure, Pharmacological Activities and Structure-Activity Relationships. Pharmaceuticals 2021, 14, 428. 10.3390/ph14050428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas R.; Tafolla-Arellano J. C.; Martínez-Ávila G. C. G. Euphorbia antisyphilitica Zucc: A Source of Phytochemicals with Potential Applications in Industry. Plants 2021, 10, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safwat N. A.; Kashef M. T.; Aziz R. K.; Amer K. F.; Ramadan M. A. Quercetin 3-O-glucoside recovered from the wild Egyptian Sahara plant, Euphorbia paralias L., inhibits glutamine synthetase and has antimycobacterial activity. Tuberculosis 2018, 108, 106–113. 10.1016/j.tube.2017.11.005. [DOI] [PubMed] [Google Scholar]
- Zhang W.; Yue-Wei G. Chemical studies on the constituents of the chinese medicinal herb Euphorbia helioscopia L.. Chem. Pharm. Bull. 2006, 54, 1037–1039. [DOI] [PubMed] [Google Scholar]
- Liu X.; Ye W.; Yu B.; Zhao S.; Wu H.; Che C. Two new flavonol glycosides from Gymnema sylvestre and Euphorbia ebracteolata. Carbohydr. Res. 2004, 339, 891–895. 10.1016/j.carres.2003.12.017. [DOI] [PubMed] [Google Scholar]
- Nishimura T.; Li-Yan W.; Kouji K.; Susumu K. Flavonoids that mimic human ligands from the whole plants of Euphorbia lunulata. Chem. Pharm. Bull. 2005, 53, 305–308. [DOI] [PubMed] [Google Scholar]
- Abdel-Monem A. R.; Abdel-Sattar E.; Harraz F. M.; Petereit F. Chemical investigation of Euphorbia schimperi C. Presl.. Rec. Nat. Prod. 2008, 2, 39–45. [Google Scholar]
- Mothana R. A.; Gruenert R.; Bednarski P. J.; Lindequist U. Evaluation of the in vitro anticancer, antimicrobial and antioxidant activities of some Yemeni plants used in folk medicine. Pharmazie 2009, 64, 260–268. [PubMed] [Google Scholar]
- Ahmed S.; Nur-e-alam M.; Mothana R. A.; Yousaf M.; Al-Rehaily A. J. Activity guided isolation of chemical constituents from the biologically active methanol extract of Euphorbia schimperi C. Presl.. Bull. Chem. Soc. Ethiop. 2017, 31, 471–479. [Google Scholar]
- Ahmed S.; Yousaf M.; Mothana R. A.; Al-Rehaily A. J. Studies on wound healing activity of some Euphorbia species on experimental rats. Afr. J. Tradit., Complementary Altern. Med. 2016, 13, 145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancur-Galvis L.; Morales G.; Forero J.; Roldan J. Cytotoxic and antiviral activities of Colombian medicinal plant extracts of the Euphorbia genus. Mem. Inst. Oswaldo Cruz 2002, 97, 541–546. 10.1590/s0074-02762002000400017. [DOI] [PubMed] [Google Scholar]
- Torky Z. A. Antiviral activity of Euphorbia lectin against herpes simplex virus 1 and its antiproliferative activity against human cancer cell-line. J. Antivirals Antiretrovirals 2016, 8, 107–116. 10.4172/jaa.1000145. [DOI] [Google Scholar]
- Tian Y.; Li-Min S.; Xi-Qiao L.; Bin Li Q.; Jun-Xing D. Anti-HBV active flavone glucosides from Euphorbia humifusa Willd. Fitoterapia 2010, 81, 799–802. [DOI] [PubMed] [Google Scholar]
- Ma X.; Tian W.; Wu L.; Cao X.; Ito Y. Isolation of quercetin-3-O-l-rhamnoside from Acer truncatum Bunge by high-speed counter-current chromatography. J. Chromatogr. 2005, 1070, 211–214. 10.1016/j.chroma.2005.02.052. [DOI] [PubMed] [Google Scholar]
- Nugroho A.; Song B. M.; Lee K. T.; Park H. J. Quantification of antidepressant Miquelianin in mature and immature fruits of Korean Rubus species. Nat. Prod. Sci. 2014, 20, 258–261. [Google Scholar]
- Wang G.; Zhang L.; Bonkovsky H.-L. Chinese medicine for treatment of chronic hepatitis B. Chin. J. Integr. Med. 2012, 18, 253–255. 10.1007/s11655-012-1064-4. [DOI] [PubMed] [Google Scholar]
- Parvez M. K.; Arbab A. H.; Al-Dosari M. S.; Al-Rehaily A. J. Antiviral natural products against chronic hepatitis B: recent developments. Curr. Pharm. Des. 2016, 22, 286–293. 10.2174/1381612822666151112152733. [DOI] [PubMed] [Google Scholar]
- Parvez M. K.; Al-Dosari M. S.; Arbab A. H.; Al-Rehaily A. J.; Abdelwahid M. A. S. Bioassay-guided isolation of anti-hepatitis B virus flavonoid myricetin-3-O-rhamnoside along with quercetin from Guiera senegalensis leaves. Saudi Pharm. J. 2020, 28, 550–559. 10.1016/j.jsps.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smara O.; Julia A.; Moral-Salmi C.; Vigor C.; Joseph V.; Legseir B. Flavonoïds from Euphorbia guyoniana Boissier & Reuter. J. Life Sci. 2014, 8, 544–551. [Google Scholar]
- Mallavadhani U. V.; Sahu G.; Narasimhan K.; Muralidhar J. Quantitative estimation of an antidiarrhoeic marker in Euphorbiahirta samples. Pharm. Bio. 2002, 40, 103–106. 10.1076/phbi.40.2.103.5841. [DOI] [Google Scholar]
- Kaul T. N.; Middleton E.; Ogra P. L. Antiviral effect of flavonoids on human viruses. J. Med. Virol. 1985, 15, 71–79. 10.1002/jmv.1890150110. [DOI] [PubMed] [Google Scholar]
- Vrijsen R.; Everaert L.; Boeye A. Antiviral activity of flavones and potentiation by ascorbate. J. Gen. Virol. 1998, 69, 1749–1751. [DOI] [PubMed] [Google Scholar]
- Choi H.-J.; Kim J.-H.; Lee C.-H.; Ahn Y.-J.; Song J.-H.; Baek S.-H.; Kwon D.-H. Antiviral activity of quercetin 7-rhamnoside against porcine epidemic diarrhea virus. Antiviral Res. 2009, 81, 77–81. 10.1016/j.antiviral.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucsi I. Combined antiviral effects of flavonoids and 5-ethyl-2′-deoxyuridine on the multiplication of herpesviruses. Acta Virol. 1984, 28, 395–400. [PubMed] [Google Scholar]
- Cheng Z.; Sun G.; Guo W.; Huang Y.; Sun W.; Zhao F.; Hu K. Inhibition of hepatitis B virus replication by quercetin in human hepatoma cell lines. Virol. Sin. 2015, 30, 261–268. 10.1007/s12250-015-3584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiow K. H.; Phoon M. C.; Putti T.; Tan B. K. H.; Chow V. T. Evaluation of antiviral activities of Houttuynia cordata Thunb. extract, quercetin, quercetrin and cinanserin on murine coronavirus and dengue virus infection. Asian Pac. J. Trop. Med. 2016, 9, 1–7. 10.1016/j.apjtm.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto Y.; Ikeda S.; Uwai K.; Taguchi R.; Chayama K.; Sakaguchi T.; Narita R.; Yao W.-L.; Takeuchi F.; Otakaki Y.; Watashi K.; Wakita T.; Kato H.; Fujita T. Rosmarinic acid is a novel inhibitor for Hepatitis B virus replication targeting viral epsilon RNA-polymerase interaction. PLoS One 2018, 13, e0197664 10.1371/journal.pone.0197664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D.; Zhou X.; Zhao C.; Chen H.; Zhao Y.; Gong X. Anti-inflammatory, antiviral and quantitative study of quercetin-3-O-β-D-glucuronide in Polygonum perfoliatum L. Fitoterapia 2011, 82, 805–810. 10.1016/j.fitote.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Song J. H.; Shim J. K.; Choi H. J. Quercetin 7-rhamnoside reduces porcine epidemic diarrhea virus replication via independent pathway of viral induced reactive oxygen species. Virol. J. 2011, 8, 460–468. 10.1186/1743-422x-8-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.; Huang H.; Zhou W.; Feng M.; Zhou P. Anti-hepatitis B virus activities of Geranium carolinianum L. extracts and identification of the active components. Biol. Pharm. Bull. 2008, 31, 743–747. 10.1248/bpb.31.743. [DOI] [PubMed] [Google Scholar]
- Zhang L.-J.; Yeh S.-F.; Yu Y.-T.; Kuo L.-M. Y.; Kuo Y.-H. Antioxidative flavonol glucuronides and Anti-HBsAg flavonol from Rotala rotundifolia. J. Tradit. Complement. Med. 2011, 1, 57–63. 10.1016/s2225-4110(16)30057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong H. J.; Ryu Y. B.; Park S.-J.; Kim J. H.; Kwon H.-J.; Kim J. H.; Park K. H.; Rho M.-C.; Lee W. S. Neuraminidase inhibitory activities of flavonols isolated from Rhodiola rosea roots and their in vitro anti-influenza viral activities. Bioorg. Med. Chem. 2009, 17, 6816–6823. 10.1016/j.bmc.2009.08.036. [DOI] [PubMed] [Google Scholar]
- Zhang T.; Wu Z.; Du J.; Hu Y.; Liu L.; Yang F.; Jin Q. Anti- Japanese-Encephalitis-Viral Effects of Kaempferol and Daidzin and Their RNA-Binding Characteristics. PLoS One 2012, 7, e30259 10.1371/journal.pone.0030259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai W.; Bi J.; Li F.; Wang S.; Huang X.; Meng X.; Sun B.; Wang D.; Kong W.; Jiang C.; Su W. Antiviral Efficacy of Flavonoids against Enterovirus 71 Infection in Vitro and in Newborn Mice. Viruses 2019, 11, 625. 10.3390/v11070625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Care C.; Sornjai W.; Jaratsittisin J.; Hitakarun A.; Wikan N.; Triwitayakorn K.; Smith D. R. Discordant activity of kaempferol towards dengue virus and Japanese encephalitis virus. Molecules 2020, 25, 1246. 10.3390/molecules25051246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behbahani M.; Shanehsazzadeh M.; Shokoohinia Y.; Soltani M. Evaluation of anti-herpetic activity of methanol seed extract and fractions of Securigera securidaca in vitro. J. Antivirals Antiretrovirals 2013, 5, 72–76. [Google Scholar]
- Behbahani M.; Sayedipour S.; Pourazar A.; Shanehsazzadeh M. In vitro anti-HIV-1 activities of kaempferol and kaempferol-7-O-glucoside isolated from Securigera securidaca. Res. Pharm. Sci. 2014, 9, 463. [PMC free article] [PubMed] [Google Scholar]
- Ha S.-Y.; Youn H.; Song C.-S.; Kang S. C.; Bae J. J.; Kim H. T.; Lee K. M.; Eom T. H.; Kim I. S.; Kwak J. H. Antiviral effect of flavonol glycosides isolated from the leaf of Zanthoxylum piperitum on influenza virus. J. Microbiol. 2014, 52, 340–344. 10.1007/s12275-014-4073-5. [DOI] [PubMed] [Google Scholar]
- Schwarz S.; Sauter D.; Wang K.; Zhang R.; Sun B.; Karioti A.; Bilia A.; Efferth T.; Schwarz W. Kaempferol derivatives as antiviral drugs against the 3a channel protein of coronavirus. Planta Med. 2014, 80, 177–182. 10.1055/s-0033-1360277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min B.-S.; Tomiyama M.; Ma C.-M.; Nakamura N.; Hattori M. Kaempferol acetylrhamnosides from the rhizome of Dryopteris crassirhizoma and their inhibitory effects on three different activities of human immunodeficiency virus-1 reverse transcriptase. Chem. Pharm. Bull. 2001, 49, 546–550. 10.1248/cpb.49.546. [DOI] [PubMed] [Google Scholar]
- Yang L.; Lin J.; Zhou B.; Liu Y.; Zhu B. Activity of compounds from Taxillus sutchuenensis as inhibitors of HCV NS3 serine protease. Nat. Prod. Res. 2017, 31, 487–491. 10.1080/14786419.2016.1190719. [DOI] [PubMed] [Google Scholar]
- Dabeek W. M.; Marra M. V. Dietary quercetin and kaempferol: bioavailability and potential cardiovascular-related bioactivity in humans. Nutrients 2019, 11, 2288. 10.3390/nu11102288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.-H. Naturally derived anti-hepatitis B virus agents and their mechanism of action. World J. Gastroenterol. 2016, 22, 188–204. 10.3748/wjg.v22.i1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvez M. K.; Sehgal D.; Sarin S. K.; Basir F. B.; Jameel S. Inhibition of hepatitis B virus DNA replicative intermediate forms by recombinant interferon-γ. World J. Gastroenterol. 2006, 12, 3006–3014. 10.3748/wjg.v12.i19.3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z.; Hu T.; Zhou X.; Wildum S.; Garcia-Alcalde F.; Xu Z.; Wu D.; Mao Y.; Tian X.; Zhou Y.; Shen F.; Zhang Z.; Tang G.; Najera I.; Yang G.; Shen H. C.; Young J. A. T.; Qin N. Heteroaryldihydropyrimidine (HAP) and sulfamoylbenzamide (SBA) inhibit hepatitis B virus replication by different molecular mechanisms. Sci. Rep. 2017, 7, 42374. 10.1038/srep42374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bietz S.; Rarey M. SIENA: Efficient Compilation of Selective Protein Binding Site Ensembles. J. Chem. Inf. Model. 2016, 56, 248–259. 10.1021/acs.jcim.5b00588. [DOI] [PubMed] [Google Scholar]
- Maestro . Schrödinger Release 2021-3; Maestro, Schrödinger, LLC: New York, NY, 2021. [Google Scholar]
- Pettersen E. F.; Goddard T. D.; Huang C. C.; Meng E. C.; Couch G. S.; Croll T. I.; Morris J. H.; Ferrin T. E. UCSF ChimeraX : Structure visualization for researchers, educators, and developers. Protein Sci. 2021, 30, 70–82. 10.1002/pro.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhardt J.; Santos-Martins D.; Tillack A. F.; Forli S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891. 10.1021/acs.jcim.1c00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.