Unstructured Abstract
Cardiovascular risk and functional burden, or the accumulation of cardiovascular risk factors coupled with functional decline, may be an important risk state analogy to multimorbidity. We investigated prospective associations of sedentary time (ST), light intensity physical activity (LPA), and moderate to vigorous intensity physical activity (MVPA) with cardiovascular risk and functional burden at midlife. Participants were 1,648 adults (mean ± SD age=45±4 years, 61% female, 39% Black) from Coronary Artery Risk Development in Young Adults (CARDIA) who wore accelerometers in 2005-2006 and 2015-2016. Cardiovascular risk and functional burden was defined as ≥2 cardiovascular risk factors (untreated/uncontrolled hypertension and hypercholesterolemia, type 2 diabetes, reduced kidney function) and/or functional decline conditions (reduced physical functioning and depressive symptoms). Prospective logistic regression models tested single activity, partition, and isotemporal substitution associations of accelerometer-measured ST, LPA, and MVPA with cardiovascular risk and functional burden 10 years later. In isotemporal models of baseline activity, reallocating 24 minutes of ST to MVPA was associated with 15% lower odds of cardiovascular risk and functional burden (OR: 0.85; CI: 0.75, 0.96). Reallocating 24 minutes of LPA to MVPA was associated with a 14% lower odds of cardiovascular risk and functional burden (OR: 0.86; CI: 0.75, 0.99). In longitudinal isotemporal models, similar beneficial associations were observed when 10-year increases in MVPA replaced time in ST or LPA. Findings suggest that maintaining an MVPA dose reflecting daily physical activity recommendations in early midlife is associated with lower odds of cardiovascular risk and functional burden later in midlife.
Keywords: Aging, physical activity, accelerometers, functional decline, multimorbidity
Introduction
Previous research has focused on individual associations of moderate to vigorous intensity physical activity (MVPA), sedentary time (ST), and light intensity physical activity (LPA), with cardiovascular disease (CVD) risk. Regular MVPA reduces CVD risk,1–3 however, less is known about ST and LPA and CVD risk. Accelerometer studies indicate more ST may increase CVD risk.5,6 Emerging evidence suggests any amount and intensity of daily physical activity, including LPA, may protect against incident CVD.7
Multimorbidity, or the coexistence of chronic health conditions, leads to adverse outcomes, increased hospitalizations, and higher medical costs.8–11 Previous studies have focused on identifying multimorbidity in life-late, when older adults (+65 years) have already developed multiple chronic conditions.11 Analogous to multimorbidity, having multiple risk states, such as CVD risk factor clustering12 in young adulthood13 and tracking into midlife,14 may increase risk of CVD and mortality.15 Independently, poor physical function and depression, comorbid conditions of CVD, are linked to premature mortality.16,17 An important risk state analogy to multimorbidity may be cardiovascular risk and functional burden, or the clustering of CVD risk factors with declining physical function and mental health. Self-reported physical activity is inversely associated with multimorbidity,18–20 however, we do not know if engaging in daily MVPA and LPA and limiting ST prevents the clustering of risk conditions at midlife.
Constrained by the 24-hour day, ST, LPA, and MVPA are interdependent. Thus, changing time spent in one activity, will automatically change time in another.21 Isotemporal substitution is a novel statistical technique that estimates changes in disease risk if time spent in one activity is reallocated to another.21 Isotemporal substitution investigates increases or decreases in an activity, and highlights meaningful activity substitutions. Previous isotemporal studies demonstrated shifting time in ST, LPA, and MVPA had meaningful associations with CVD risk factors.22–26 Few isotemporal studies have incorporated longitudinal data to evaluate change in activities over time and associations with CVD risk.26 Of these previous studies, Whitaker et al. observed shifts in activity over a 10-year period were prospectively associated with cardiometabolic risk factors.25 This study aims to build upon the Whitaker et al. findings, applying a longitudinal isotemporal approach and simultaneously assessing the clustering of multiple CVD and functional risk states.
Leveraging the Coronary Artery Risk Development in Young Adults (CARDIA) study our objectives are to: 1) identify the prevalence of cardiovascular risk and functional burden in a community-based sample at midlife (45-64 years); 2) evaluate cross-sectional reallocations of time spent in ST, LPA, MVPA in 2005-2006 and associations with cardiovascular risk and functional burden 10-years later (2015-2016); and 3) examine 10-year changes in time spent in ST, LPA, MVPA (from 2005-2006 to 2015-2016) and associations with cardiovascular risk and functional burden. While cross-sectional isotemporal models can estimate hypothetical changes in ST, LPA, MVPA, they do not represent real changes in activity. The prospective isotemporal models examine 10-year changes in ST, LPA, MVPA and associations with cardiovascular risk and functional burden. We hypothesize that lower average ST and higher average LPA and MVPA in 2005-2006 are associated with lower odds of cardiovascular risk and functional burden 10 years later. In 10-year change models, we hypothesize that reallocating ST to increases in LPA or MVPA, is associated with lower odds of cardiovascular risk and functional burden.
Methods
Study Sample
Between 1985-86, 5,115 black and white adults, from four locations (Birmingham, AL; Chicago, IL; Minneapolis, MN; and Oakland, CA) were enrolled in CARDIA. To obtain a representative cohort of black and white adult populations (ages 18-30), stratified recruitment was used to enroll nearly equal numbers of black and white adults, females and males, persons < and > 25 years of age, and persons with less and more than high school education. In-person clinical exams were completed every 2 to 5 years, including a year 20 (2005-2006; baseline) and year 30 (2015-2016; 10-year follow-up) exam. At these visits, the CARDIA Fitness Study (baseline; n=2,332) and CARDIA Activity Study (10-year follow-up; n=1,397) included a 7-day accelerometer protocol. CARDIA study design and follow-up have been previously reported.27 Center Institutional Review Boards approved the study and all participants provided written informed consent.
Measures
Accelerometer-measured physical activity and sedentary time
Accelerometer data were collected using ActiGraph devices (ActiGraph; Pensacola, FL) at baseline (7164) and 10-year follow-up (wGT3X-BT) using nearly identical protocols. Devices were distributed at the exam; however, a few participants received mailed devices at follow-up. Participants wore a hip-worn accelerometer for 7 consecutive days during waking hours. After 7 days, participants returned accelerometers using a pre-paid mailer. Data were downloaded, compressed to 60 second epoch files, and screened for wear-time 28, with valid wear defined as ≥10 hours per day for ≥4 days. Day-to-day intensity-specific estimates were defined using Freedson count thresholds,29,30 consistent with previous CARDIA analyses.25,31 Day-to-day estimates were summed and averaged across valid wear days to create average minutes of ST, LPA, and MVPA.
In a CARDIA calibration study, the 7164 and GT3X-BT accelerometers were compared in a subset of participants (N=100).32 A calibration factor was developed and applied to GT3X-BT values. After calibration, no differences were observed between minutes per day (min/day) for ST (513.2 versus 509.6), LPA (335.3 versus 338.7), or MVPA (33.1 versus 32.0). This analysis includes calibrated GT3X-BT ST, LPA and MVPA estimates.
Cardiovascular Risk and Functional Burden
We sought to identify cardiovascular risk and functional burden by analogy with multimorbidity, and focused on identifying individuals accumulating CVD risk factors and functional decline conditions at midlife. Conditions were selected based on those commonly included in multimorbidity definitions33–35, appropriate for middle-aged adults, and associated with increased CVD risk.36–39 Cardiovascular risk and functional burden was defined as having two or more of the following conditions: untreated or uncontrolled hypertension (systolic blood pressure ≥ 140 mm Hg or a diastolic blood pressure ≥90 mm Hg), untreated or uncontrolled hypercholesterolemia (low density lipoprotein cholesterol (LDL-C) ≥ 130 mg/dL), hypertriglyceridemia (triglycerides ≥ 150 mg/dL), type 2 diabetes (fasting glucose levels (≥126 mg/dl) and self-reported oral hypoglycemic medications or insulin at years 20, 25, and 30; 2-hour postload glucose (≥200 mg/dl) during a 75 g oral glucose tolerance test at years 20 and 25; or glycated hemoglobin (HbA1c) ≥6.5% at years 20 and 25), reduced kidney function (estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2), reduced physical function (SF-12: physical components score (PCS) <40), and depressive symptoms (Center for Epidemiologic Studies Depression Scale (CES-D) score ≥ 16).
Outcome measures were collected using standardized protocols. Prior to exams, participants fasted for at least 12 hours and refrained from smoking or physical activity for 2 hours. Blood pressure was measured using Omron blood pressure monitors. Cholesterol and triglycerides plasma concentrations were measured using enzymatic assays by Northwest Lipids Research Laboratory (Seattle, WA). LDL-C was calculated using the Friedewald equation.40 Serum glucose was measured from fasting blood samples processed by the Molecular Epidemiology and Biochemistry Research Laboratory (Minneapolis, MN). Fasting insulin was measured using radioimmunoassay by Linco Research (St. Louis, MO) at baseline and the Molecular Epidemiology and Biochemistry Research Laboratory (Minneapolis, MN) at follow-up. HbA1c was measured using a Tosoh G7 high performance liquid chromatography instrument (Tosoh Bioscience). Participants reported taking medications for diabetes treatment. The 2009 CKD Epidemiology Collaboration creatinine equation41 was used to calculate eGFR from serum creatinine measured by nephelometry. Physical functioning was assessed with the validated SF-1242 PCS score, with a score <40 representing reduced physical functioning.36 Depressive symptoms were assessed with the validated CES-D,43 with a score ≥ 16 indicating depressive symptoms, consistent with previous CARDIA analyses.38
Covariates
Covariates were selected on clinical relevance and associations with the exposure or outcome. Baseline questionnaires collected information on self-reported age, sex, race (Black or White), years of education (highest year of school completed), employment status (yes/no), health insurance status (yes/no), smoking status (never, former, current), alcohol consumption (alcohol milliliters per day), drinking status (yes/no), and self-reported sleep quality in the past month (1-5; higher scores poorer quality). Baseline body mass index (BMI) was calculated using measured height and weight (kg/m2).
Statistical Analyses
We excluded participants with possible device malfunction (>14 hours MVPA per day; n=3) and extreme MVPA (> 200 minutes/day; n=2) at baseline. For a complete case analysis, we excluded participants with missing baseline covariates (n=7) or missing outcome measures at baseline (n=131) or follow-up (n=135). Individuals with prevalent cardiovascular risk and functional burden at baseline were also excluded (N=406). The final analytic sample included 1,648 participants (Supplemental Figure 1). For the 10-year change analysis, data were from participants with valid accelerometer data at 10-year follow-up (N=759).
Descriptive statistics were calculated and stratified according to baseline MVPA quartiles. Age and sex standardized prevalence estimates of cardiovascular risk and functional burden conditions were calculated by race (standardized to analytic sample).
Single Activity and Partition models
Prior to analyses, activity estimates were divided by one standard deviation (SD), so a one-unit change represents a one SD change in minutes per day.
Single activity logistic regression models were used to examine individual associations of minutes of ST, LPA, and MVPA at baseline with cardiovascular risk and functional burden at 10-year follow-up. Models included adjustment for wear time, baseline demographics (age, sex, race, study center, education, health insurance status, and employment status), lifestyle characteristics (smoking status, alcohol consumption, drinking status, sleep quality), but not other activities.
Next, we tested independent associations of ST, LPA, and MVPA at baseline with cardiovascular risk and functional burden at 10-year follow-up. This model includes ST, LPA, and MVPA, and aforementioned covariates.
Isotemporal substitution models
Isotemporal substitution models tested associations with cardiovascular risk and functional burden of reallocating time in one activity for another. The first models evaluated cross-sectional reallocations of activity at baseline with cardiovascular risk and functional burden at 10-year follow-up. The second isotemporal models examined longitudinal changes in activity between baseline and follow-up and associations with cardiovascular risk and functional burden. Isotemporal model results are reported for one SD substitution of each activity.
Isotemporal substitution models included a variable for each activity besides the activity of interest, total wear time, and the aforementioned covariates. Omitting one activity represents replacing time in that activity with an equal amount of time from another.21 Including total wear time holds time constant, allowing for interpretation to be made about shifting time from one activity to another.21 For longitudinal change models, variables represented change in activity from baseline to follow-up. To account for differences in baseline activity, change models included adjustment for baseline ST and MVPA.
We did not adjust for BMI in analyses due to potential for BMI to be in the causal pathway for outcome conditions. To test if associations were independent of BMI, we examined single activity and partition models with additional adjustment for BMI. In sensitivity analyses, we reran isotemporal models stratifying the sample on number of baseline prevalent conditions (0 or 1) to determine if results differed by health status. Additionally, we tested for interactions by sex and race. Analyses were performed using R software version 3.6.44
Results
At baseline, 19.7% (n=406) of participants had prevalent cardiovascular risk and functional burden. Compared to excluded CARDIA Fitness Study participants, included participants were more likely to be female, white, have health insurance, be non-smokers, and have lower BMI. (Supplemental Table 1).
Participants were on average 45.2 (SD: ± 3.6) years at baseline. Approximately 61% of participants were female, 39% were black and the average BMI was 28.1 (SD: ± 6.0) kg/m2 (Table 1). At baseline, participants spent on average 486 (SD: ± 102.0) minutes per day (min/day) in ST, 364 (SD: ± 84.3) min/day in LPA, and 36.2 (SD: ± 23.9) min/day in MVPA. From baseline to follow-up, average ST minutes increased while average LPA and MVPA minutes decreased (Supplemental Table 2; results previously reported31). Compared to participants with the lowest MVPA, participants with the highest daily MVPA were more likely to be male, have more education, less likely to be smokers, and have a lower BMI. At 10-year follow-up, 482 participants (29%) developed cardiovascular risk and functional burden. Of these participants, approximately 20% had two, 7% had three, and 2% had 4 or more conditions. Participants with ≥2 conditions were more likely to be black (Figure 1a.). Over the 10-year period, 34.3% developed untreated or uncontrolled hypercholesterolemia and 17.6% had depressive symptoms. For black participants, the standardized prevalence was higher for every condition, except hypercholesterolemia and hypertriglyceridemia (Figure 1b). The most common combination of conditions was hypertension and hypertriglyceridemia followed by hypercholesterolemia and depressive symptoms (Supplemental Table 3).
Table 1.
CARDIA Study participant characteristics at baseline (2005-2006) by quartiles of moderate to vigorous intensity activity (N=1648)
| MVPA Quartiles (mins/day) | |||||
|---|---|---|---|---|---|
|
| |||||
| Overall | Q1 | Q2 | Q3 | Q4 | |
|
| |||||
| <18.5 | 18.5 – 31.0 | 31.1 – 48.9 | ≥49 | ||
| Demographics | |||||
|
| |||||
| Age, years | 45.2 ± 3.6 | 44.9 ± 3.8 | 45.2 ± 3.7 | 45.6 ± 3.5 | 45.1 ± 3.4 |
| Female sex, n (%) | 1010 (61.3) | 320 (77.7) | 270 (65.5) | 224 (54.4) | 196 (47.6) |
| Black, n (%) | 639 (38.8) | 220 (53.4) | 171 (41.5) | 127 (30.8) | 121 (29.4) |
| Education, years | 15.5 ± 2.5 | 14.9 ± 2.4 | 15.4 ± 2.4 | 15.7 ± 2.6 | 15.9 ± 2.6 |
| Unemployment status, n (%) | 160 (9.7) | 47 (11.4) | 44 (10.7) | 33 (8.0) | 36 (8.7) |
| Health Insurance, n (%) | 1477 (89.6) | 366 (88.8) | 367 (89.1) | 372 (90.3) | 372 (90.3) |
|
| |||||
| Lifestyle characteristics | |||||
|
| |||||
| Smoking, n (%) | |||||
| Never | 1079 (65.5) | 275 (66.7) | 262 (63.6) | 260 (63.1) | 282 (68.4) |
| Former | 345 (20.9) | 74 (18.0) | 98 (23.8) | 96 (23.3) | 77 (18.7) |
| Current | 224 (13.6) | 63 (15.3) | 52 (12.6) | 56 (13.6) | 53 (12.9) |
| Current Drinker, n (%) | 1340 (81.3) | 313 (77.3) | 339 (83.1) | 341 (83.8) | 347 (85.5) |
| Alcohol consumption, mL/day, median [IQR] | 2.7 [14.5] | 0.0 [7.2] | 2.4 [14.3] | 4.9 [16.7] | 7.2 [19.5] |
| Self-reported sleep quality score (1-5) | 2.4 ± 1.0 | 2.4 ± 1.0 | 2.3 ± 0.9 | 2.4 ± 1.0 | 2.3 ± 1.9 |
| BMI, kg/m2 | 28.1 ± 6.0 | 29.4 ± 7.1 | 28.7 ± 6.3 | 27.5 ± 5.3 | 26.7 ± 4.9 |
|
| |||||
| Accelerometer-measured activity, minutes/day | |||||
|
| |||||
| Total wear time | 887.0 ± 82.9 | 866.7 ± 83.1 | 885.7 ± 83.7 | 892.3 ± 83.1 | 903.5 ± 77.5 |
| ST | 486.3 ± 102.0 | 508.5 ± 95.6 | 497.6 ± 101.2 | 485.7 ± 103.7 | 453.6 ± 99.3 |
| LPA | 364.5 ± 84.3 | 346.2 ± 86.6 | 363.6 ± 78.3 | 367.4 ± 83.9 | 380.7 ± 84.9 |
| MVPA | 36.2 ± 23.9 | 12.0 ± 4.3 | 24.4 ± 3.6 | 39.2 ± 5.0 | 69.3 ± 20.3 |
|
| |||||
| Cardiovascular risk and functional burden conditions | |||||
|
| |||||
| Systolic blood pressure, mm Hg | 113.1 ± 12.9 | 113.2 ± 12.9 | 113.2 ± 13.2 | 112.9 ± 12.9 | 113.3 ± 12.4 |
| Diastolic blood pressure, mm Hg | 70.4 ± 9.9 | 71.9 ± 9.9 | 71.1 ± 10.2 | 69.2 ± 9.2 | 69.2 ± 9.8 |
| LDL-C, mg/dL | 106.2 ± 27.7 | 107.7 ± 28.0 | 107.6 ± 27.6 | 105.7 ± 27.6 | 103.7 ± 27.3 |
| Glucose, mg/dL | 95.2 ± 12.8 | 95.7 ± 14.5 | 95.1 ± 12.4 | 95.5 ± 12.9 | 94.6 ± 11.4 |
| Triglycerides, mg/dL, median [IQR] | 78.0 [50.0] | 77.5 [49.0] | 78.0 [57.2] | 79.0 [48.0] | 77.0 [50.0] |
| eGFR, mL/min/1.73 m2 | 96.1 ± 22.2 | 99.5 ± 22.8 | 94.7 ± 19.5 | 94.3 ± 23.2 | 95.9 ± 22.8 |
| Physical Functioning Score (0-100) | 53.3 ± 5.2 | 52.2 ± 5.5 | 53.2 ± 4.9 | 53.5 ± 5.5 | 54.2 ± 5.0 |
| CES-D Depression Scale (0-60) | 7.6 ± 6.2 | 7.8 ± 6.6 | 7.7 ± 5.9 | 7.4 ± 6.7 | 7.3 ± 5.7 |
Data presented as mean ± SD unless otherwise specified; BMI: Body Mass Index; ST: sedentary time; LPA: light intensity physical activity; MVPA: moderate to vigorous intensity physical activity; LDL-C: low density lipoprotein cholesterol; eGFR: estimated glomerular filtration rate, CES-D: Center for Epidemiologic Studies Depression Scale
Figure 1a.
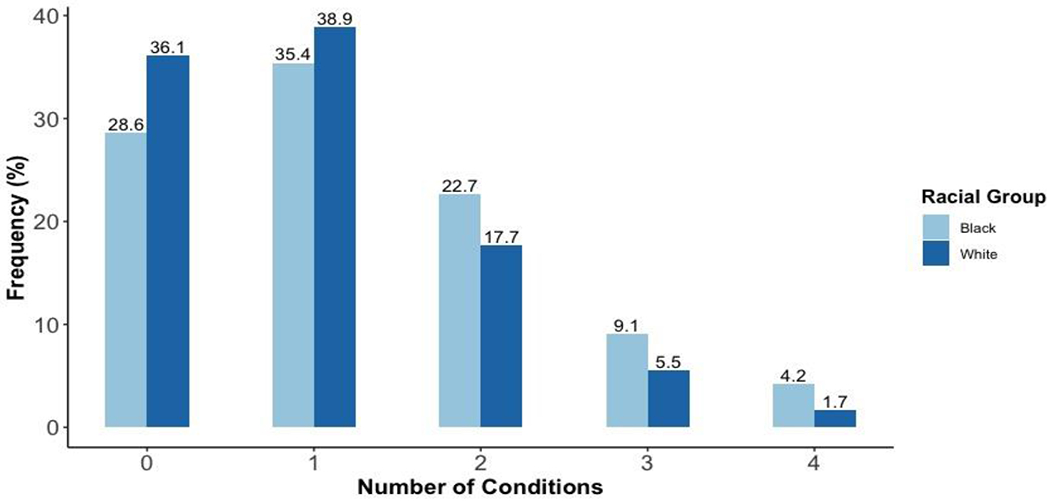
Number of cardiovascular risk and functional burden conditions at 10-year follow-up by race
Figure 1b.

Age and sex standardized prevalence of cardiovascular risk and functional burden conditions by race
In single activity models, baseline ST and MVPA were associated with cardiovascular risk and functional burden at 10-year follow-up (Table 2). After adjustment for demographics and lifestyle characteristics, ST was associated with 15% higher odds (Odds Ratio [OR]: 1.15, 95% Confidence Interval [CI]: 1.01 – 1.32) and MVPA was associated with 16% lower odds of cardiovascular risk and functional burden (OR: 0.84, CI: 0.74 – 0.95). In the partition model, MVPA remained associated with lower odds of cardiovascular risk and functional burden 10 years later (OR: 0.85, CI: 0.75 - 0.96). With adjustment for ST and LPA, each additional 24 minutes of MVPA was associated with 15% lower odds of cardiovascular risk and functional burden; though not statistically significant, associations for ST and LPA were in hypothesized directions.
Table 2.
Individual and independent associations of baseline (2005-2006) accelerometer-measured physical activity and sedentary time with cardiovascular risk and functional burden at 10-year follow-up (2015-2016), CARDIA study (N=1648)
| Single activity modelsa | OR (95% CI) | |
|---|---|---|
| ST (per 1 SD) c | ||
|
| ||
| Crude | 1.00 (0.90, 1.11) | |
| Adjusted | 1.15 (1.01, 1.32) | |
| LPA (per 1 SD) d | ||
|
| ||
| Crude | 0.98 (0.88, 1.09) | |
| Adjusted | 0.91 (0.81, 1.03) | |
| MVPA (per 1 SD) e | ||
|
| ||
| Crude | 0.83 (0.74, 0.92) | |
| Adjusted | 0.84 (0.74, 0.95) | |
| Partition Modelb | OR (95% CI) | |
|
| ||
| ST | 1.00 (0.87, 1.16) | |
| LPA | 0.94 (0.82, 1.08) | |
| MVPA | 0.85 (0.75, 0.96) | |
Single activity models include individual activity and not the other activities with adjustments for age, sex, race, study center, education, health insurance, employment status, smoking status, alcohol consumption, drinking status, sleep quality, and wear time;
Partition model includes ST, LIPA, MVPA (no wear time variable) in one model with adjustment for age, sex, race, study center, education, health insurance, employment status, smoking status, alcohol consumption, drinking status and sleep quality ST: sedentary time; LPA: light intensity physical activity; MVPA: moderate to vigorous intensity physical activity;
1 SD of ST: 102 minutes;
1 SD of LPA: 84 minutes;
1 SD of MVPA: 24 minutes
In baseline isotemporal models after adjustment for demographics and lifestyle characteristics, reallocating 24 minutes of ST to MVPA, while keeping LPA constant, was associated with 15% lower odds of cardiovascular risk and functional burden (OR: 0.85, CI: 0.75 - 0.96; Table 3). Replacing LPA with 24 minutes of MVPA was associated with 14% lower odds of cardiovascular risk and functional burden (OR: 0.86, CI: 0.75 – 0.99). There was no association with cardiovascular risk and functional burden when ST was replaced with LPA (OR: 0.94, CI: 0.83 – 1.06).
Table 3.
Isotemporal associations for substitutions in activity with cardiovascular risk and functional burden
| Substitution of one SD* of activity at baseline (n=1648) | |||
|---|---|---|---|
|
| |||
| OR (95% CI) | |||
|
|
|||
| Replace ST with 84 mins of LPA | Replace ST with 24 mins of MVPA | Replace LPA with 24 mins of MVPA | |
| Cardiovascular risk and functional burden | 0.94 (0.83, 1.06) | 0.85 (0.75, 0.96) | 0.86 (0.75, 0.99) |
| Substitution of one SD* change in activity from baseline to 10-year follow-upb (n=759) | |||
|
| |||
| OR (95% CI) | |||
|
|
|||
| Replace ST with 84 mins of LPA | Replace ST with 24 mins of MVPA | Replace LPA with 24 mins of MVPA | |
|
| |||
| Cardiovascular risk and functional burden | 1.02 (0.84, 1.24) | 0.62 (0.49, 0.79) | 0.62 (0.48, 0.79) |
Models adjusted for 2 activities (besides activity being replaced) age, sex, race, study center, education, health insurance, employment status, smoking status, alcohol consumption, drinking status, sleep quality, and total wear time;
10-year change models also adjusted for baseline ST and MVPA;
ST: sedentary time; LPA: light intensity physical activity; MVPA: moderate to vigorous intensity physical activity; SD: Standard Deviation; mins: minutes;
1 SD of LPA: 84 minutes; 1 SD of MVPA: 24 minutes
In longitudinal isotemporal models, with additional adjustment for baseline ST and MVPA, a 24-minute increase in MVPA, replacing a 24-minute increase in ST or LPA, was associated with lower odds of cardiovascular risk and functional burden, with similar magnitude of associations (ST: OR: 0.62, CI: 0.49 – 0.79; LPA: OR: 0.62, CI: 0.48 - 0.79).
After additional adjustment for BMI, results remained mostly unchanged (Supplemental Table 4). In sensitivity analyses, the pattern of associations observed in baseline isotemporal models remained consistent across baseline health status (Supplemental Table 5). In longitudinal isotemporal models, among individuals with zero conditions at baseline, 10-year reallocations to MVPA had substantially larger beneficial associations with cardiovascular risk and functional burden as compared to the same activity reallocation among individuals with one condition at baseline. There was no evidence of effect modification by sex or race.
Discussion
Identifying lifestyle opportunities to reduce the risk of accumulating cardiovascular risk factors and functional decline conditions (cardiovascular risk and functional burden) is critical before multiple conditions develop and pharmaceutical intervention is needed. We sought to examine individual, independent, and isotemporal substitution associations of ST, LPA, and MVPA with cardiovascular risk and functional burden at midlife in a community-based sample of black and white adults. From our analysis, three key findings emerged. First, cardiovascular risk and functional burden was highly prevalent in our sample of adults at midlife, especially among black participants. Second, daily MVPA was consistently associated with cardiovascular risk and functional burden 10 years later. Third, isotemporal results suggest reallocating time to MVPA is associated with lower odds of future cardiovascular risk and functional burden. Our results support the importance of maintaining MVPA at midlife as a cardiovascular risk and functional decline reduction strategy.
We investigated cardiovascular risk and functional burden as an early indicator of multimorbidity, with a focus on identifying individuals accumulating midlife CVD risk and functional decline conditions. This multi-risk population poses a unique challenge to developing effective prevention strategies,9 therefore identifying at-risk individuals and modifiable lifestyle factors may highlight opportunities for prevention. At baseline, 20% of participants, 45 years old on average, had prevalent cardiovascular risk and functional burden and were excluded from analyses. Over the 10-year study, 30% of the remaining sample transitioned from having < 2 to ≥ 2 conditions. These results indicate a critical period for cardiovascular risk and functional burden prevention may be early adulthood prior to midlife,46 and future studies should consider identifying cardiovascular risk and functional burden to detect at-risk populations.
The protective effects of MVPA against accumulation of CVD risk factors are well supported,3,47 however evidence is needed to understand the effect of ST and LPA on cardiovascular health.6,48 Previous studies relied upon self-reported measures of activity prone to measurement error.49 In our analysis with accelerometer-measured ST, we found the association of ST with cardiovascular risk and functional burden was not independent of MVPA. While our results do not support the harmful effects of ST, the 2018 Physical Activity Guidelines for Americans (PAG) encourage limiting daily ST to decrease future cardiovascular risk.50 The PAG also encourage daily minutes of physical activity at any intensity. Contrary to our hypothesis, LPA was not associated with lower odds of cardiovascular risk and functional burden. Taken together with disparate findings suggesting LPA is related to lower CVD risk,3,51 our findings indicate daily LPA may be more important for some age groups, including older adults. More accelerometer assessment of LPA and ST is needed to better understand associations with CVD risk.
Previous isotemporal studies explored cross-sectional reallocations of daily ST, LPA, and MVPA with cardiovascular health.22–24,52 While cross-sectional isotemporal studies can estimate associations of hypothetical changes in activity, our longitudinal analysis estimates associations of actual 10-year changes in activities. In our longitudinal models, we found beneficial associations of reallocating time to MVPA. Our results are consistent with longitudinal results from Whitaker, et al., in the CARDIA cohort.25 However, Whitaker, et al., also found beneficial associations when ST minutes was reallocated to LPA, suggesting beneficial effects of LPA on cardiometabolic health.25 We did not find beneficial associations for substitution of ST with LPA, but we do not suggest substituting ST with LPA is less important, but rather our results may be explained by our composite outcome. Previous isotemporal studies found substituting ST with LPA is beneficially associated with CVD risk factors,22,24,25 but not consistently associated with measures of physical function or depressive symptoms.53–56 A prospective isotemporal analysis by Mekary et al. found self-reported intensity of activity mattered when replacing television viewing for risk of incident depression. Few longitudinal isotemporal studies have included accelerometer-measured ST, LPA, and MVPA with prospective outcomes.26 Our study extends upon the Whitaker and Mekary findings and contributes to this literature gap, by investigating longitudinal changes in accelerometer-measured ST, LPA, and MVPA with future cardiovascular risk and functional burden. Our findings support the importance of decreasing ST and increasing or maintaining MVPA throughout midlife. Of note, the magnitude of association found when ST or LPA was replaced with MVPA was greater in the longitudinal isotemporal models than in the cross-sectional models. These findings are hypothesis generating and may suggest cross-sectional isotemporal models underestimate the association of daily activities with CVD risk factors and functional decline conditions. Lastly, with no variation in associations across sex or race groups, our results indicate daily MVPA is important for all middle-aged adults.
Compositional data analysis (CODA) is an alternative statistical approach to examining the interrelationships of daily activities.57–59 CODA recognizes that together ST, LPA, MVPA, and sleep make up the 24-hour day, and therefore relative contributions should be analyzed. Using NHANES data, Chastin et al. found the composition of the 24-hour day was associated with cardiometabolic markers, and for some markers, replacing ST with LPA was as beneficial as replacing ST with MVPA.59 CODA and isotemporal substitution models may differ theoretically (relative vs absolute time in activity), however they often produce materially similar results.60 In our study, we only measured waking day activity with accelerometers, without sleep, and for this reason we opted not to standardize the day (e.g. 10 hours) to form waking day compositions. Future studies with 24-hour accelerometer data should explore 24-hour activity CODA reallocations with cardiovascular risk and functional burden to further test our results.
Study strengths include accelerometer-measured ST, LPA, and MVPA at two timepoints and thorough measures of cardiovascular risk and functional decline conditions. However, several study limitations should be noted. Over the 10-year period, we are unable to investigate whether changes in activity or the outcome occurred first, thus we draw no conclusions on causality. Although waist-worn accelerometry provides accurate physical activity measurement, its use to estimate ST is a limitation. Waist-worn accelerometers do not capture standing without ambulation, potentially overestimating ST. Accelerometers were worn only for waking wear, thus we were not able to include sleep in analyses. Lastly, differences between the excluded and analytic samples were observed, and selection bias may be present.
Conclusions
In summary, early adulthood, prior to midlife, may be a critical time to prevent the development of cardiovascular risk and functional burden. Over a 10-year period, replacing ST or LPA with MVPA had consistent beneficial associations with cardiovascular risk and functional burden among black and white adults at midlife. Our findings support developing effective strategies to help young adults decrease ST and increase or maintain daily MVPA to minimize future cardiovascular risk and functional decline.
Supplementary Material
Highlights.
Cardiovascular risk and functional burden may be an early marker of multimorbidity
Daily MVPA was linked to lower odds of cardiovascular risk and functional burden
Prospective isotemporal substitution showed benefits of reducing sedentary time
Early adulthood may be a critical period for preventing clustering of risk factors
Acknowledgements
The authors thank the investigators, the staff, and the participants of the CARDIA study for their valuable contributions.
Funding
The Coronary Artery Risk Development in Young Adults Study (CARDIA) is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with the University of Alabama at Birmingham (HHSN268201800005I & HHSN268201800007I), Northwestern University (HHSN268201800003I), University of Minnesota (HHSN268201800006I), and Kaiser Foundation Research Institute (HHSN268201800004I). CARDIA is also partially supported by the Intramural Research Program of the National Institute on Aging (NIA) and an intra-agency agreement between NIA and NHLBI (AG0005). The CARDIA Fitness Study was supported by the National Institutes of Health (R01 HL078972 to BS & SS) as was the CARDIA Activity Study (R56 HL125423 to KPG, BS/SS). Full is supported by an NHLBI training grant T32 HL007779.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Publisher's Disclaimer: Disclaimer
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services
References
- 1.Parker ED, Schmitz KH, Jacobs DR, Dengel DR, Schreiner PJ. Physical Activity in Young Adults and Incident Hypertension Over 15 Years of Follow-Up: The CARDIA Study. Am J Public Health. 2007;97(4):703–709. doi: 10.2105/AJPH.2004.055889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith AD, Crippa A, Woodcock J, Brage S. Physical activity and incident type 2 diabetes mellitus: a systematic review and dose-response meta-analysis of prospective cohort studies. Diabetologia. 2016;59(12):2527–2545. doi: 10.1007/s00125-016-4079-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.LaMonte MJ, Lewis CE, Buchner DM, et al. Both Light Intensity and Moderate-to-Vigorous Physical Activity Measured by Accelerometry Are Favorably Associated With Cardiometabolic Risk Factors in Older Women: The Objective Physical Activity and Cardiovascular Health (OPACH) Study. doi: 10.1161/(ISSN)2047-9980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prince SA, Adamo KB, Hamel M, Hardt J, Connor Gorber S, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. 2008;5(1):56. doi: 10.1186/1479-5868-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellettiere J, LaMonte MJ, Evenson KR, et al. Sedentary Behavior and Cardiovascular Disease in Older Women. Circulation. 2019;139(8):1036–1046. doi: 10.1161/CIRCULATIONAHA.l18.035312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young DR, Hivert M-F, Alhassan S, et al. Sedentary Behavior and Cardiovascular Morbidity and Mortality. Circulation. 2016:CIR.0000000000000440. doi: 10.1161/CIR.0000000000000440. [DOI] [PubMed] [Google Scholar]
- 7.LaCroix AZ, Bellettiere J, Rillamas-Sun E, et al. Association of Light Physical Activity Measured by Accelerometry and Incidence of Coronary Heart Disease and Cardiovascular Disease in Older Women. JAMA Netw Open. 2019;2(3):e190419. doi: 10.1001/jamanetworkopen.2019.0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lehnert T, Heider D, Leicht H, et al. Review: Health Care Utilization and Costs of Elderly Persons With Multiple Chronic Conditions. Med Care Res Rev. 2011;68(4):387–420. doi: 10.1177/1077558711399580. [DOI] [PubMed] [Google Scholar]
- 9.Marengoni A, Angleman S, Melis R, et al. Aging with multimorbidity: A systematic review of the literature. Ageing Res Rev. 2011;10(4):430–439. doi: 10.1016/J.ARR.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Condelius A, Edberg A-K, Jakobsson U, Hallberg IR. Hospital admissions among people 65+ related to multimorbidity, municipal and outpatient care. Arch Gerontol Geriatr. 2008;46(1):41–55. doi: 10.1016/J.ARCHGER.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Violan C, Foguet-Boreu Q, Flores-Mateo G, et al. Prevalence, determinants and patterns of multimorbidity in primary care: A systematic review of observational studies. PLoS One. 2014;9(7):3–11. doi: 10.1371/journal.pone.0102149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Genest J, Cohn JS. Clustering of cardiovascular risk factors: Targeting high-risk individuals. Am J Cardiol. 1995;76(1-2 SUPPL. 1). doi: 10.1016/S0002-9149(05)80010-4. [DOI] [PubMed] [Google Scholar]
- 13.Manolio TA, Savage PJ, Burke GL, et al. Association of fasting insulin with blood pressure and lipids in young adults: The CARDIA Study. Arterioscler Thromb Vasc Biol. 1990;10(3):430–436. doi: 10.1161/01.ATV.10.3.430. [DOI] [PubMed] [Google Scholar]
- 14.Wilson PWF, Kannel WB, Silbershatz H, D’Agostino RB. Clustering of Metabolic Factors and Coronary Heart Disease. Arch Intern Med. 1999;159(10):1104. doi: 10.1001/archinte.159.10.1104. [DOI] [PubMed] [Google Scholar]
- 15.Yusuf HR, Giles WH, Croft JB, Anda RF, Casper ML. Impact of Multiple Risk Factor Profiles on Determining Cardiovascular Disease Risk. Prev Med (Baltim). 1998;27(1):1–9. doi: 10.1006/PMED.1997.0268. [DOI] [PubMed] [Google Scholar]
- 16.Christensen GT, Maartensson S, Osler M. The association between depression and mortality – a comparison of survey- and register-based measures of depression. J Affect Disord. 2017;210:111–114. doi: 10.1016/J.JAD.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 17.Sokka T, Pincus T. Poor physical function, pain and limited exercise: risk factors for premature mortality in the range of smoking or hypertension, identified on a simple patient self-report questionnaire for usual care. BMJ Open. 2011;1(1):e000070–e000070. doi: 10.1136/bmjopen-2011-000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dhalwani NN, O’Donovan G, Zaccardi F, et al. Long terms trends of multimorbidity and association with physical activity in older English population. Int J Behav Nutr Phys Act. 2016;13(1):1–9. doi: 10.1186/sl2966-016-0330-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cimarras-Otal C, Calderón-Larrañaga A, Poblador-Plou B, et al. Association between physical activity, multimorbidity, self-rated health and functional limitation in the Spanish population. BMC Public Health. 2014;14(1):1–10. doi: 10.1186/1471-2458-14-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vancampfort D, Koyanagi A, Ward PB, et al. Chronic physical conditions, multimorbidity and physical activity across 46 low and middle income countries. Int J Behav Nutr Phys Act. 2017;14(1):1–13. doi: 10.1186/sl2966-017-0463-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mekary RA, Willett WC, Hu FB, Ding EL. Isotemporal substitution paradigm for physical activity epidemiology and weight change. Am J Epidemiol. 2009;170(4):519–527. doi: 10.1093/aje/kwp163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ekblom-Bak E, Ekblom Ö, Bergström G, Börjesson M. Isotemporal substitution of sedentary time by physical activity of different intensities and bout lengths, and its associations with metabolic risk. Eur J Prev Cardiol. 2016;23(9):967–974. doi: 10.1177/2047487315619734. [DOI] [PubMed] [Google Scholar]
- 23.Hamer M, Stamatakis E, Steptoe A. Effects of substituting sedentary time with physical activity on metabolic risk. Med Sci Sports Exerc. 2014;46(10):1946–1950. doi: 10.1249/MSS.0000000000000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buman MP, Winkler E a H, Kurka JM, et al. Reallocating time to sleep, sedentary behaviors, or active behaviors: Associations with cardiovascular disease risk biomarkers, NHANES 2005–2006. Am J Epidemiol. 2014;179(3):323–334. doi: 10.1093/aje/kwt292. [DOI] [PubMed] [Google Scholar]
- 25.Whitaker KM, Gabriel KP, Buman MP, et al. Associations of accelerometer-measured sedentary time and physical activity with prospectively assessed cardiometabolic risk factors: The CARDIA study. J Am Heart Assoc. 2019;8(1):1–11. doi: 10.1161/JAHA.l18.010212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grgic J, Dumuid D, Bengoechea EG, et al. Health outcomes associated with reallocations of time between sleep, sedentary behaviour, and physical activity: A systematic scoping review of isotemporal substitution studies. Int J Behav Nutr Phys Act. 2018;15(1):1–68. doi: 10.1186/sl2966-018-0691-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friedman GD, Cutter GR, Donahue RP, et al. Cardia: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41(11):1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 28.Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, Mcdowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 29.Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc. 1998;30(5):777–781. doi: 10.1097/00005768-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 30.Kozey-keadle S, Libertine A, Lyden K, Staudenmayer J, Freedson PS. Validation of Wearable Monitors for Assessing Sedentary Behavior. Med Sci Sport Exerc. 2011;43(8):1561–1567. doi: 10.1249/MSS.0b013e31820cel74. [DOI] [PubMed] [Google Scholar]
- 31.Pettee Gabriel K, Sidney S, Jacobs DR, et al. Ten-Year Changes in Accelerometer-Based Physical Activity and Sedentary Time During Midlife. Am J Epidemiol. 2018;187(10):2145–2150. doi: 10.1093/aje/kwyll7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whitaker KM, Pettee Gabriel K, Jacobs DR, Sidney S, Sternfeld B, Stemfeld B. Comparison of Two Generations of ActiGraph Accelerometers: The CARDIA Study. Med Sci Sports Exerc. 2018;50(6):1333–1340. doi: 10.1249/MSS.0000000000001568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goodman RA, Posner SF, Huang ES, Parekh AK, Koh HK. Defining and Measuring Chronic Conditions: Imperatives for Research, Policy, Program, and Practice. Prev Chronic Dis. 2013;10:120239. doi: 10.5888/pcdl0.120239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ward BW, Schiller JS, Goodman RA. Multiple chronic conditions among US adults: a 2012 update. Prev Chronic Dis. 2014;11(3):E62. doi: 10.5888/pcdl1.130389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380(9836):37–43. doi: 10.1016/S0140-6736(12)60240-2. [DOI] [PubMed] [Google Scholar]
- 36.Saquib N, Brunner R, Kubo J, et al. Self-perceived physical health predicts cardiovascular disease incidence and death among postmenopausal women. BMC Public Health. 2013;13(1):468. doi: 10.1186/1471-2458-13-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keyes CLM. The nexus of cardiovascular disease and depression revisited: the complete mental health perspective and the moderating role of age and gender. Aging Ment Health. 2004;8(3):266–274. doi: 10.1080/13607860410001669804. [DOI] [PubMed] [Google Scholar]
- 38.Davidson K, Jonas BS, Dixon KE, Markovitz JH. Do Depression Symptoms Predict Early Hypertension Incidence in Young Adults in the CARDIA Study? Arch Intern Med. 2000;160(10):1495. doi: 10.1001/archinte.160.10.1495. [DOI] [PubMed] [Google Scholar]
- 39.Benjamin EJ, Muntner P, Alonso A, et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Vol 139.; 2019. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 40.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, Without Use of the Preparative Ultracentrifuge. Clin Chem. 1972;18(6):499–502. doi: 10.1093/clinchem/18.6.499. [DOI] [PubMed] [Google Scholar]
- 41.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ware J, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey : Construction of Scales and Preliminary Tests of Reliability and Validity Author (s): John E . Ware , Jr ., Mark Kosinski and Susan D . Keller Published by : Lippincott Williams & Wilkins Stable URL : http://www.jstor. Med Care. 1996;34(3):220–233. [DOI] [PubMed] [Google Scholar]
- 43.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale). Am J Prev Med. 1994;10(2):77–84. http://www.ncbi.nlm.nih.gov/pubmed/8037935. Accessed February 22, 2015. [PubMed] [Google Scholar]
- 44.R Core Team. R: A language and environment for statistical computing. 2013. http://www.r-project.org/.
- 45.Sakakibara BM, Obembe AO, Eng JJ. The prevalence of cardiometabolic multimorbidity and its association with physical activity, diet, and stress in Canada: evidence from a population-based cross-sectional study. BMC Public Health. 2019; 19(1): 1361. doi: 10.1186/sl2889-019-7682-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pencina MJ, Navar AM, Wojdyla D, et al. Quantifying Importance of Major Risk Factors for Coronary Heart Disease. Circulation. 2019;139(13):1603–1611. doi: 10.1161/CIRCULATIONAHA.l17.031855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.He D, Xi B, Xue J, Huai P, Zhang M, Li J. Association between leisure time physical activity and metabolic syndrome: a meta-analysis of prospective cohort studies. Endocrine. 2014;46(2):231–240. doi: 10.1007/s12020-013-0110-0. [DOI] [PubMed] [Google Scholar]
- 48.Füzéki E, Engeroff T, Banzer W. Health Benefits of Light-Intensity Physical Activity: A Systematic Review of Accelerometer Data of the National Health and Nutrition Examination Survey (NHANES). Sport Med. 2017;47(9):1769–1793. doi: 10.1007/s40279-017-0724-0. [DOI] [PubMed] [Google Scholar]
- 49.Ekelund U, Tarp J, Steene-Johannessen J, et al. Dose-response associations between accelerometry measured physical activity and sedentary time and all cause mortality: systematic review and harmonised meta-analysis. BMJ. 2019;366:14570. doi: 10.1136/bmj.14570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Piercy KL, Troiano RP, Ballard RM, et al. The Physical Activity Guidelines for Americans. JAMA. 2018;320(19):2020. doi: 10.1001/jama.2018.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.LaCroix A Accelerometer-measured Light Physical Activity is Heart Healthy in Older Women: The OPACH Study. TBD. 2018. [Google Scholar]
- 52.Stamatakis E, Rogers K, Ding D, et al. All-cause mortality effects of replacing sedentary time with physical activity and sleeping using an isotemporal substitution model: a prospective study of 201,129 mid-aged and older adults. Int J Behav Nutr Phys Act. 2015;12(1):121. doi: 10.1186/sl2966-015-0280-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dillon CB, McMahon E, O’Regan G, Perry IJ. Associations between physical behaviour patterns and levels of depressive symptoms, anxiety and well-being in middle-aged adults: A cross-sectional study using isotemporal substitution models. BMJ Open. 2018;8(1):1–8. doi: 10.1136/bmjopen-2017-018978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rethorst CD, Moncrieft AE, Gellman MD, et al. Isotemporal analysis of the association of objectively measured physical activity with depressive symptoms: Results from hispanic community health study/study of Latinos (HCHS/SOL). J Phys Act Heal. 2017;14(9):733–739. doi: 10.1123/jpah.2016-0648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yasunaga A, Shibata A, Ishii K, et al. Associations of sedentary behavior and physical activity with older adults’ physical function: an isotemporal substitution approach. BMC Geriatr. 2017;17(1):280. doi: 10.1186/sl2877-017-0675-l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mekary RA, Lucas M, Pan A, et al. Isotemporal substitution analysis for physical activity, television watching, and risk of depression. Am J Epidemiol. 2013;178(3):474–483. doi: 10.1093/aje/kws590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dumuid D, Pediši Ž, Antoni J. Compositional Data Analysis in Time-Use Epidemiology : What, Why, How. Int J Environ Res Public Health. 2020;17(7):2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dumuid D, Stanford TE, Martin-Fernández J-A, et al. Compositional data analysis for physical activity, sedentary time and sleep research. Stat Methods Med Res. 2018;27(12):3726–3738. doi: 10.1177/0962280217710835. [DOI] [PubMed] [Google Scholar]
- 59.Chastin SFM, Palarea-Albaladejo J, Dontje ML, Skelton DA. Combined Effects of Time Spent in Physical Activity, Sedentary Behaviors and Sleep on Obesity and Cardio-Metabolic Health Markers: A Novel Compositional Data Analysis Approach. PLoS One. 2015;10(10):e0139984. doi: 10.1371/journal.pone.0139984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mekary RA, Ding EL. Isotemporal substitution as the gold standard model for physical activity epidemiology: Why it is the most appropriate for activity time research. Int J Environ Res Public Health. 2019;16(5):11–13. doi: 10.3390/ijerph16050797. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


