Abstract
Recently, murine kobuvirus (MuKV), a novel member of the family Picornaviridae, was identified in faecal samples of Rattus norvegicus in China. The limited information on the circulation of MuKV in other murine rodent species prompted us to investigate its prevalence and conduct a genetic characterization of MuKV in Rattus losea, Rattus tanezumi and Rattus norvegicus in China. Between 2015 and 2017, 243 faecal samples of these three murine rodent species from three regions in southern China were screened for the presence of MuKV. The overall prevalence was 23.0% (56/243). Three complete MuKV polyprotein sequences were acquired, and the genome organization was determined. Phylogenetic analyses suggested that our sequences were closely related to Chinese strains and belong to the species Aichivirus A in the genus Kobuvirus. Additional studies are required to understand the true prevalence of MuKV in murine rodent populations in China.
Keywords: detection, genetic characterization, MuKV, murine rodent
Data Summary
The new sequences in this study have been deposited in the GenBank database under accession numbers MW292451 to MW292482.
Introduction
Picornaviruses (family Picornaviridae) are small, non-enveloped viruses with positive-sense single-stranded RNA genomes. As of March 2020, there were 147 known species in 63 genera in the family Picornaviridae (https://www.picornaviridae.com/), many of which result in significant diseases in humans and a wide variety of animals. The genus Kobuvirus is a member of the family Picornaviridae. It has a genomic organization similar to that of other picornaviruses and has a linear genome ranging from 8.2 to 8.4 kb. Kobuvirus genomes are composed of VPg, 5′UTRIRES-V, a large ORF encoding a leader protein (L) and three other functional regions (P1-P2-P3), a 3′UTR, and a poly(A) tail. The P1 region encodes structural proteins (VP0-VP3-VP1), whereas the P2 (2AH-Box/NC-2B-2C) and P3 (3A-3BVPg-3Cpro-3Dpol) regions encode nonstructural proteins [1]. Of these, the three-dimensional (3D) region is the most conserved region and plays an essential role in viral replication [2]. The VP1 region is highly variable, encoding the most important viral capsid protein that determines viral pathogenicity and antigenicity [3].
Since the determination of the first human kobuvirus (Aichi virus) in a patient with nonbacterial diarrhoea in Japan in March 1989 [4], several additional strains isolated from a variety of animal species have been determined to be members of the genus Kobuvirus. Bovine kobuvirus was first recognized as a cytopathic agent in 2003 in the culture medium of HeLa cells that had been used for over 30 years in a laboratory in Japan [5]. Porcine kobuvirus was initially discovered in 2008 in faecal samples from domestic pigs in Hungary [6]. Subsequently, ovine kobuvirus related to bovine kobuvirus was isolated from young, healthy domestic sheep in Hungary in 2009 [7]. In the last 10 years, kobuviruses have been described worldwide in a wide range of hosts, including black goats in South Korea [8], rabbits in Hungary [9], dogs in South Korea and Africa [10, 11], cats in South Korea and China [12, 13], ferrets in the Netherlands and Sweden [14], rats [15] and bats [16] in China, and wolves [17], red foxes [18] and roe deer [19] in Italy.
According to the International Committee on Taxonomy of Viruses (ICTV), there are currently six officially recognized Kobuvirus species (Aichivirus A–F). Aichivirus A consists of Aichi virus (found in humans), murine kobuvirus (MuKV), canine kobuvirus, feline kobuvirus and a kobuvirus found in sewage [20]. Aichivirus B consists of bovine kobuvirus [5], ferret kobuvirus [14] and ovine kobuvirus [7]. Regarding Aichivirus C, two distinct types have been described, porcine and caprine kobuvirus [21]. Cattle kobuvirus [22], rabbit kobuvirus [9] and bat kobu-like viruses represent Aichivirus D, E and F, respectively.
MuKV was initially detected in the stool of a canyon mouse (Peromyscus crinitus) in the USA in 2010 [23]. Subsequently, MuKVs were identified in Hungary [3], Vietnam [24] and the USA [25] in faecal samples from several rodent species, including striped field mouse (Apodemus agrarius), Norway rat (Rattus norvegicus), Rice-field rat (Rattus losea and Rattus argentiventer) and house mouse (Mus musculus). We recently conducted an initial epidemiological study on MuKV in Rattus norvegicus in Guangdong, China [15]. However, there is a lack of information on kobuvirus strains in other murine rodent species in China. This study investigated the prevalence and genetic characterization of MuKV in Rattus losea, Rattus tanezumi and Rattus norvegicus in several regions in southern China.
Methods
Sample collection
A total of 243 fresh faecal samples were collected between October 2015 and September 2017 from rats with unknown health status (they appeared healthy at capture and dissection) captured close to human residences in three regions in China, comprising Xiamen city in Fujian province, Yiyang city in Hunan province and Malipo county in Yunnan province. Individual fresh stool samples were immediately placed in RNase-free tubes with 700 µl PBS (0.3% homogenate) and stored at −80 °C until further use. These stool specimens have been previously examined for rat bocavirus (RBoV) [26], porcine bocaviruses (PBoV) [27], and rodent torque teno virus (RoTTV) [28].
Nucleic acid extraction and cDNA synthesis
The thawed faecal samples were fully resuspended in PBS and centrifuged at 10000 g for 10 min. Viral nucleic acid was extracted from 200 µl of each supernatant using a MiniBEST Viral RNA/DNA Extraction Kit (TaKaRa, Japan). The obtained viral RNA was then reverse transcribed to synthesize cDNA using a Transcriptor First Strand cDNA Synthesis Kit (Roche, Switzerland) and random hexamer primers. The cDNA was directly used as the template for PCR or kept frozen at −20 °C.
Detection of MuKV
The presence of MuKV was detected by PCR using universal primers (UNIV-kobu-F/UNIV-kobu-R) targeting a 217 bp fragment of the partial 3D region found in all kobuvirus strains, in line with previous research [21]. Thereafter, to determine other nucleotide (nt) sequences of the 217 bp 3D region-positive samples, two sets of primers for a longer 3D region (461 bp) and the complete VP1 region (831 bp) of MuKV were used [15]. Each PCR mixture had a total volume of 25 µl, including 12.5 µl Green Master Mix (Promega, USA), 8.5 µl sterilized H2O, 2 µl cDNA template, and 1.0 µM of each primer. The mixtures were amplified using the following programme: 95 °C for 2 min, 40 cycles of 95 °C for 30 s, 56 °C for 1 min, and 72 °C for 1 min, with a final elongation step at 72 °C for 5 min. The amplified products were subjected to 1.0% agarose gel electrophoresis and sequenced using an ABI Prism 3730xl DNA Analyzer (Applied Biosystems, Foster City, CA, USA).
Acquisition of complete polyprotein sequences
Three MuKV-positive samples were randomly selected to undergo amplification of the full-length polyprotein sequences using ten pairs of primers designed in this study using the Benchling website (https://benchling.com), based on the Chinese sequence Wencheng-Rt386-2 (accession no. MF352432.1). The primer sequences are shown in Table S1 (available in the online version of this article). After sequencing, the full-length polyprotein sequences were assembled using Lasergene SeqMan software (DNASTAR, Madison, WI, USA) and are available in GenBank under accession no. MW292480–MW292482.
Genetic and phylogenetic analyses
The MuKV nt sequences identified in this study were compared to the corresponding sequences of other kobuvirus strains in GenBank using Basic Local Alignment Search Tool (blast; https://www.ncbi.nlm.nih.gov). Multiple sequence alignment was conducted using ClustalW mega v7.0 (Oxford Molecular, UK) [29], involving corresponding regions of kobuvirus reference sequences in GenBank. The pairwise nt and amino acid (aa) identities among all sequences were calculated using MegAlign (DNASTAR, Madison, WI, USA). The ORFs were predicted for the identified sequences using ORF finder (https://www.ncbi.nlm.nih.gov/orffinder). A similarity plot analysis of the full-length polyprotein sequences was performed using SimPlot v3.5.1 [30, 31]. The 5′ and 3′UTR RNA secondary structures were predicted using Mfold [32]. Phylogenetic analyses were conducted using the maximum-likelihood method with 1000 bootstrap replicates in mega v7.0 and further visualized in FigTree v1.4.0. The model selections were based on the results of ‘Find best DNA/Protein models’ in mega v7.0.
Results
Detection of MuKV in stool samples
Using PCR with a universal primer pair targeting a partial 3D region found in all kobuvirus strains, 243 faecal samples of murine rodents from Xiamen (n=96), Malipo (n=48), and Yiyang (n=99) in southern China were screened for the presence of MuKV. The overall prevalence was 23.0% (56/243). The prevalence was 28.1%(41/146) in Rattus norvegicus, followed by 23.7 %(9/38) in Rattus tanezumi and 10.2% (6/59) in Rattus losea (Table S2).
Phylogenetic analyses of 3D and VP1 region sequences
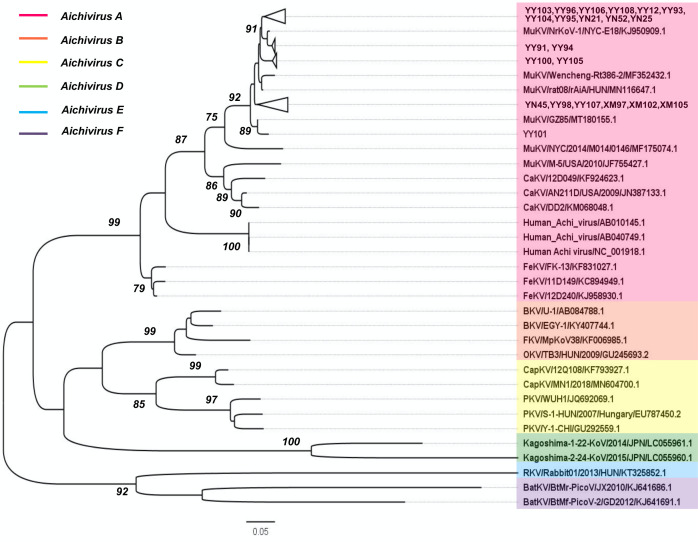
Twenty-two sequences of the partial 3D region (461 bp) were randomly selected for further PCR amplification and sequencing within the 56 of positive samples. Our MuKV sequences that were isolated from three different rodent species captured in different geographic locations were found to be highly conserved in relation to each other (92.7–99.5% at the nt level and 95–100% at the aa level). Maximum-likelihood phylogenetic analysis based on the partial 3D regions was generated using representative kobuvirus strains from multiple species. The analysis showed that our sequences belonged to Aichivirus A and clustered with MuKVs. Interestingly, only one of our sequences, YY101, was closely related to the Chinese MuKV sequence (GZ85) isolated from Rattus norvegicus [15]. The other 21 sequences formed a major group with MuKVs from USA, China and Hungary (Fig. 1).
Fig. 1.
Phylogenetic analysis of the MuKVs based on the nucleotide sequences of partial 3D regions. The tree was generated by the maximum-likelihood method based on the Kimura 2-parameter model (gamma distributed and partial deletion) with 1000 bootstrap replicates. Statistics values >70% are displayed above the tree branches. Lateral blank triangles indicate the MuKVs identified in this study, and YY101 (MW292457) is the other MuKV identified in this study. The sequences YY12 (MW292465), YY91 (MW292464), YY93 (MW292463), YY95 (MW292461), YY96 (MW292460), YY98 (MW292459), YY100 (MW292458), YY101, YY103 (MW292456), YY104 (MW292455), YY106 (MW292453), YY108 (MW292451), YN21 (MW292469), YN25 (MW292468), YN45 (MW292467) and YN52 (MW292466) were isolated from Rattus norvegicus. The sequences YY94 (MW292462), YY105 (MW292454), YY107 (MW292452) and XM105 (MW292470) were isolated from Rattus tanezumi. The sequences XM97 (MW292472) and XM102 (MW292471) were isolated from Rattus losea. BKV, bovine kobuvirus; BatKV, bat kobuvirus; CaKV, canine kobuvirus; CapKV, caprine kobuvirus; FKV, ferret kobuvirus; FeKV, feline kobuvirus; PKV, porcine kobuvirus; RKV, rabbit kobuvirus. (For a better view of the sequences with different colours, refer to the web version of this article.).
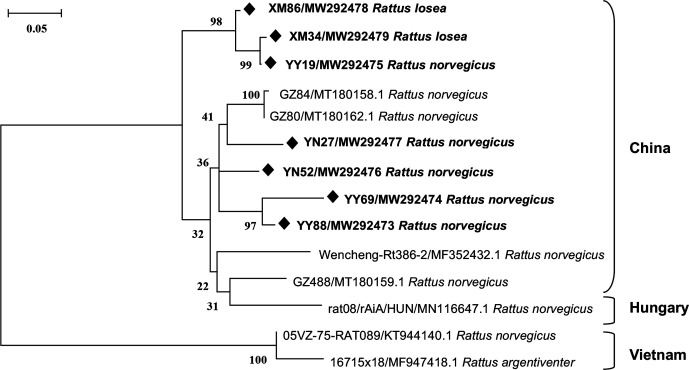
Seven complete VP1 regions (917 nt) were successfully determined, which had 88.7–99.3% nt and 95.1–100% aa identities with one another. Our sequences shared the highest nt sequence similarity (91.3–93.9%) with the Chinese MuKV MM33 (accession no. MN648600.1). A phylogenetic tree was constructed based on our seven VP1 sequences and the available complete MuKV VP1 nt sequences from three countries, indicating that our VP1 sequences clustered with two MuKV sequences, GZ80 and GZ84, previously described in Guangdong, China. The other kobuvirus reference sequences from China, Hungary and Vietnam formed another group (Fig. 2). The partial 3D and complete VP1 nt sequences of MuKV identified in this study have been submitted to GenBank (accession no. MW292451–MW292472 and MW292473–MW292479, respectively).
Fig. 2.
Phylogenetic tree of the MuKVs based on the complete nucleotide sequences of VP1. The tree was generated by the maximum-likelihood method based on the general time Reversible (GTR) model (gamma distributed and partial deletion) with 1000 bootstrap replicates. Black diamonds indicate the MuKV sequences identified in this study.
Genomic and phylogenetic analyses of complete polyprotein sequences
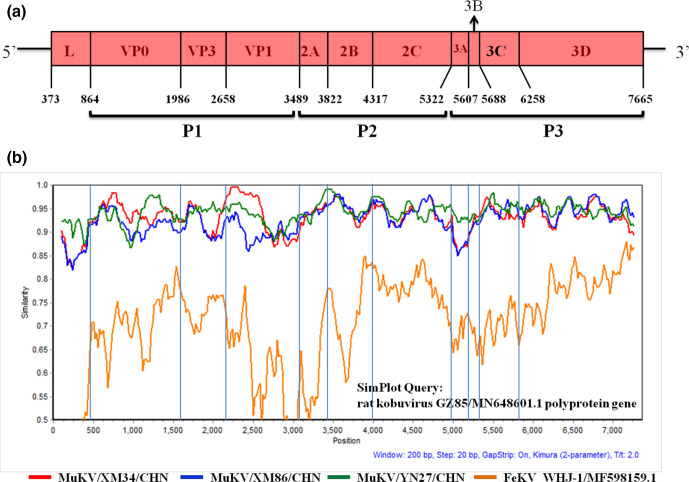
Three complete MuKV polyprotein sequences (except the 5′ and 3′UTR) were successfully obtained using ten primer pairs. They were designated MuKV/XM34/CHN (accession no. MW292482), MuKV/XM86/CHN (accession no. MW292481) and MuKV/YN27/CHN (accession no. MW292480). These sequences were 7714, 7714 and 7711 nt in length and each had a single complete ORF (7293, 7293 and 7290 nt) encoding a potential polyprotein of 2430, 2430 and 2429 aa, respectively. We performed additional multiple sequence alignments with these three sequences, and they shared 92.5–97.1% nt and 97.1–98.1% aa identities with each other. Interestingly, there was a single amino acid deletion in the 3A region of MuKV/YN27/CHN. The predicted genome organization of our sequences was the same as in other previously identified kobuviruses, and the detailed information is shown in Fig. 3(a). Further, the predicted RNA secondary structure of the partial 3′UTR displayed a characteristic ‘barbell-like’ structure (Fig. S1).
Fig. 3.
Genome organization and genetic characterization of the MuKV sequences identified in this study. (a) Genome organization of MuKV/XM34/CHN, with initial nucleotide positions labelled for each functional region. The partial 5’UTR is located at positions 1–372 and the partial 3′UTR is located at positions 7666–7714. P1 represents the structural proteins, and P2 and P3 represent the nonstructural proteins. (b) Similarity plot analysis of the complete polyprotein sequences of MuKV/XM34/CHN (red line), MuKV/XM86/CHN (blue line) and MuKV/YN27/CHN (green line), with feline kobuvirus sequence WHJ-1/MF598159.1 (orange line) as the out-group sequence and MuKV sequence GZ85/MN648601.1 as the query sequence, using the Kimura 2-parameter model in Simplot v3.5.1.
According to blastn analysis, our sequences showed the highest similarity to the previously published MuKV strain GZ85 (MN648601.1), with identities of 93.2–94.5% nt. Moreover, our sequences were also closely related to other previously described MuKVs. Consequently, we conducted a comparative sequence analysis for each functional region between our sequences and representative kobuvirus sequences in GenBank. Our complete polyprotein sequences showed aa identities with those of rat (96.0–98.3%), human (80.1–80.7%), canine (80.1–80.5%) and feline (80.1–80.3%) kobuvirus reference sequences. Regarding MuKV/XM34/CHN and MuKV/XM86/CHN, the highest nt identities (94.6 and 94.7%, respectively) were found for the P2 region of MuKV sequence GZ85, and the highest aa identities (both 98.7%) were found for the P2 region of rat08/rAiA/HUN. Regarding MuKV/YN27/CHN, the highest nt (95.4%) and aa (99.8%) identities were found for the P2 and P1 regions of GZ85, respectively (Table 1). In addition, a similarity plot analysis was conducted to further analyse the genetic characteristics of the complete polyprotein nt sequences, with the feline kobuvirus sequence WHJ-1 (MF598159.1) used as the out-group sequence and the MuKV reference sequence GZ85 (MN648601.1) used as the query sequence. The findings showed that the VP0, VP3, 2A-2C, 3C and 3D regions displayed relatively high similarities to the query sequence, whereas low similarities were exhibited in the L, VP1, 3A and 3B regions. Additionally, MuKV/YN27/CHN had considerably higher similarities in the L, 3A and 3B regions and the 2A-2B junction than MuKV/XM34/CHN and MuKV/XM86/CHN (Fig. 3b).
Table 1.
Nucleotide and putative amino acid sequence identity of the complete polyprotein sequences and L, P1-P3 regions between MuKV/XM34/XM86/YN27/CHN and other Kobuvirus reference sequences*
|
Gene region |
Rat |
Human |
Canine |
Feline |
||||
|---|---|---|---|---|---|---|---|---|
|
MuKV/XM86/CHN |
MuKV/YN27/CHN |
GZ85 |
Wencheng-Rt386-2 |
rat08/rAiA/HUN |
A846/88 |
CH-1 |
WHJ-1 |
|
|
MuKV/XM34/CHN |
||||||||
|
L |
99.0/98.8 |
89.4/94.2 |
89.9/93.0 |
88.4/90.7 |
84.5/87.2 |
64.7/60.5 |
57.3/51.2 |
57.3/48.8 |
|
P1 |
96.0/97.5 |
92.2/98.3 |
94.0/98.3 |
91.4/97.9 |
92.0/98.1 |
74.7/77.4 |
74.4/77.4 |
75.0/79.5 |
|
P2 |
98.3/99.3 |
93.8/97.9 |
94.6/98.2 |
94.2/98.2 |
94.5/98.7 |
77.9/82.5 |
79.8/85.3 |
78.6/84.9 |
|
P3 |
97.4/97.8 |
93.5/97.1 |
90.4/93.5 |
92.9/97.1 |
93.1/97.1 |
79.3/86.3 |
81.7/86.2 |
78.9/83.8 |
|
Polyprotein |
97.2/98.2 |
92.8/97.5 |
92.7/96.4 |
92.4/97.2 |
92.4/97.1 |
76.3/80.4 |
76.9/80.3 |
75.9/80.1 |
|
MuKV/XM86/CHN |
||||||||
|
L |
100/100 |
88.8/94.2 |
89.4/93.0 |
88.2/90.7 |
84.3/87.2 |
64.5/61.0 |
56.9/50.6 |
57.4/49.4 |
|
P1 |
100/100 |
91.0/96.7 |
91.9/96.5 |
90.6/96.3 |
90.9/96.4 |
74.0/76.3 |
73.9/76.5 |
74.6/78.6 |
|
P2 |
100/100 |
93.9/97.9 |
94.7/98.2 |
93.9/98.2 |
94.5/98.7 |
78.1/82.5 |
79.9/85.4 |
78.8/85.4 |
|
P3 |
100/100 |
93.3/97.7 |
91.0/94.5 |
93.6/97.8 |
93.5/97.9 |
79.7/86.7 |
82.0/86.4 |
79.4/84.4 |
|
Polyprotein |
100/100 |
92.3/97.1 |
92.1/96.0 |
92.2/96.9 |
92.2/96.8 |
76.3/80.1 |
76.8/80.1 |
76.0/80.1 |
|
MuKV/YN27/CHN |
||||||||
|
L |
88.8/94.2 |
100/100 |
92.3/96.5 |
90.5/94.2 |
86.5/90.7 |
63.5/62.2 |
59.2/51.2 |
56.3/48.3 |
|
P1 |
91.0/96.7 |
100/100 |
93.9/99.8 |
92.4/99.6 |
91.3/99.2 |
74.3/77.5 |
74.2/77.8 |
75.3/79.9 |
|
P2 |
93.9/97.9 |
100/100 |
95.4/98.9 |
93.6/98.7 |
93.5/98.5 |
78.0/82.8 |
79.9/85.1 |
78.8/84.8 |
|
P3 |
93.3/97.7 |
100/100 |
91.4/95.0 |
93.0/97.4 |
93.0/97.6 |
79.1/86.5 |
81.3/86.4 |
78.4/84.4 |
|
Polyprotein |
92.3/97.1 |
100/100 |
93.3/98.0 |
92.8/98.3 |
92.0/97.9 |
76.1/80.7 |
76.8/80.5 |
75.8/80.3 |
Values were described as nucleotide identity (%) /amino acid identity (%).
*Reference Kobuvirus sequences include: GZ85 (accession no. MN648601.1), Wencheng-Rt386–2 (accession no. MF352432.1), rat08/rAiA/HUN (accession no. MN116647.1), A846/88 (accession no. AB010145.1), CH-1 (accession no. JQ911763.1) and WHJ-1 (accession no. MF598159.1).
Table 2 shows the predicted protease-cleavage sites of our sequences and the reference kobuviruses, including Q/G, Q/H, Q/T, Q/S, Q/A, A/Q and L/T. The predicted protease-cleavage sites between L and VP0 (Q/G), VP0 and VP3 (Q/H), 2A and 2B (Q/G), 2B and 2C (Q/G), 2C and 3A (Q/G), and 3C and 3D (Q/S) are conserved among kobuviruses isolated from different species. In comparison, the protease-cleavage site between 3A and 3B is Q/A for all the kobuvirus sequences except for porcine kobuvirus (Q/G). The protease cleavage sites in our sequences are highly conserved with those typically found across the genus [3].
Table 2.
Protease-cleavage sites of MuKV sequences identified in this study and other Kobuvirus reference sequences from several species
|
Cleavage between |
Rat |
Human |
Bovine |
Canine |
Feline |
Porcine |
|||
|---|---|---|---|---|---|---|---|---|---|
|
MuKV/XM34/CHN |
MuKV/XM86/CHN |
MuKV/YN27/CHN |
rat08/rAiA/HUN |
A846/88 |
U-1 |
CH-1 |
WHJ-1 |
S-1-HUN |
|
|
L/VP0 |
LQRQ/GNST |
LQRQ/GNST |
LQRQ/GNST |
LQRQ/GNST |
LQRQ/GNSV |
IISQ/GQSQ |
LKPQ/GNSV |
IKPQ/GNST |
IVRQ/GNST |
|
VP0/VP3 |
VAPQ/HWKT |
VAPQ/HWKT |
VAPQ/HWKT |
VAPQ/HWKT |
LAPQ/HWKT |
VSKQ/HWKT |
VAPQ/HWKT |
VAPQ/HWKT |
VQTQ/HWKI |
|
VP3/VP1 |
LSSQ/TLSE |
LSSQ/SLSE |
LSSQ/SLSE |
LSSQ/TLSE |
LTSQ/TLSE |
LALQ/ADGD |
LTSQ/ANSE |
LSSQ/GNSE |
FQIQ/AADD |
|
VP1/2A |
VVRQ/GANY |
VVRQ/GANY |
VVRQ/GANY |
VVRQ/GANY |
VIKQ/GAAS |
RQCA/GESL |
VVKQ/GAHT |
VKRQ/GAPA |
RQCL/TNDV |
|
2A/2B |
IRRQ/GLLT |
IRRQ/GLLT |
IRRQ/GLLT |
IRRQ/GLLT |
IRRQ/GLLT |
ITRQ/GLLT |
IKRQ/GLLT |
IKRQ/GLLT |
IIRQ/GLLS |
|
2B/2C |
LEHQ/GLKD |
LEHQ/GLKD |
LEHQ/GLKD |
LEHQ/GLKD |
LEPQ/GLKD |
LEPQ/GVRD |
LEPQ/GLKD |
LEPQ/GLKD |
VEHQ/GVRD |
|
2C/3A |
IKRQ/GNRV |
IKRQ/GNRV |
IKRQ/ GRVI |
IKRQ/GNRV |
IRRQ/GNRV |
IKRQ/GAHS |
IKRQ/GRTI |
IKRQ/GRTI |
VKRQ/GLTI |
|
3A/3B |
SSAQ/AAYS |
SSAQ/AAYS |
SSTQ/AAYS |
SSSQ/AAYS |
QEPQ/AAYS |
DEPQ/AAYS |
SETQ/AAYS |
SAAQ/AAYS |
DKSQ/GAYS |
|
3B/3C |
IQRQ/GISP |
IQRQ/ GISP |
IQRQ/ GISP |
IQRQ/ GISP |
IQRQ/ GISP |
VVRQ/AGPS |
IQRQ/GLSP |
IQRQ/GLSP |
VVRQ/SLSP |
|
3C/3D |
TTHQ/SQII |
TTHQ/SQII |
TTHQ/SQII |
TTHQ/SQII |
TTQQ/SLIV |
TECQ/SLII |
TTHQ/SLIV |
TTQQ/SLII |
IDQQ/SIII |
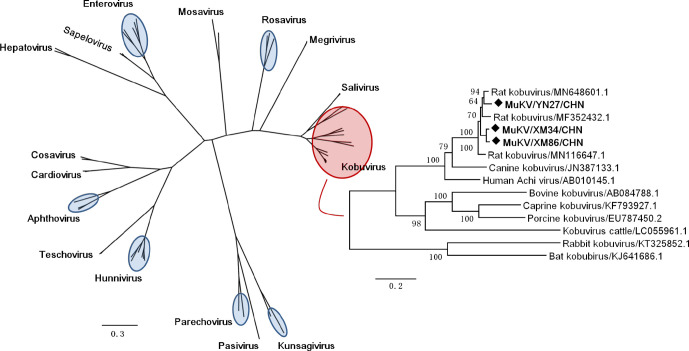
Maximum-likelihood phylogenetic analysis was conducted based on our complete polyprotein nt sequences and those from representative picornaviruses. The results indicated that our sequences were located at the same branch as the members of the genus Kobuvirus, and other MuKV sequences represented the closest relatives (Fig. 4).
Fig. 4.
Phylogenetic relationships between kobuviruses and other picornaviruses based on complete polyprotein nucleotide sequences. The tree was generated by the maximum-likelihood method based on the GTR model (gamma distributed with Invariant sites [G+I]) and partial deletion) with 1000 bootstrap replicates. Representative members of the family Picornaviridae are marked with letters and some are marked with blue circles. Black diamonds indicate the MuKV sequences identified in this study.
Discussion
Kobuvirus circulation in rats has been reported in Guangdong, China, but only in Rattus norvegicus [15]. To the best of our knowledge, this study represents the first study to investigate the prevalence of MuKV in the other two murine rodent species (Rattus losea and Rattus tanezumi) in southern China.
The detection results indicated that 56 MuKV sequences were identified in faecal samples from these murine rodents, with a detection rate of 23.0% (56/243). The prevalence of MuKV in Rattus losea, Rattus tanezumi and Rattus norvegicus was 10.2% (6/59), 23.7% (9/38) and 28.1% (41/146), respectively, highlighting the circulation of MuKV within the three murine rodent populations. In comparison, MuKVs were detected in 50 and 55.5% of Rattus norvegicus faecal samples in the USA and Hungary, respectively [33, 34]. Likewise, a previous study in Vietnam reported that the occurrence of rodent-derived kobuvirus sequences was 10.9% in six murine species [24]. Even though our findings confirmed that murine rodent species are hosts of MuKV, additional studies are required to fully understand the true prevalence of MuKV in murine rodents in China.
As mentioned above, the faecal samples in this study had been previously used for RBoV, PBoV and RoTTV detection, with the respective prevalence of 43.6, 44.4 and 25.5%. Notably, coinfections of MuKV and RBoV were found in 10.3% of the 243 specimens, coinfections of MuKV and PBoV in 10.3%, and coinfections of MuKV and RoTTV in 8.6%. Several studies have reported that coinfections of porcine kobuvirus and other viruses were very common [35, 36], whereas limited data are available on the coinfections of MuKV and other pathogens. Although we reported that MuKV co-infected with RBoV, PBoV and RoTTV, many other pathogens have not been ruled out in these specimens. Additional studies are needed to consider the coinfections of MuKV and other pathogens in rats.
Previous studies have detected kobuviruses in humans and animals with diarrhoea and other clinical manifestations, suggesting that kobuviruses are a causative agent of gastroenteritis [13, 37–43]. However, as in this study, others have also identified kobuviruses in asymptomatic animals or individuals co-infected with other pathogens [4, 13, 19, 37–39, 41, 44, 45]. Consequently, additional studies are warranted to determine the actual influence of MuKV and other kobuviruses on animal health. The presence of MuKV in rodent populations living near humans poses a potential threat to public health. Susceptible animal models should be established to verify the clinical significance of kobuviruses in gastrointestinal disease. Taken together, our epidemiological evidence will help to promote experimental studies on MuKV pathogenesis and clinical manifestations, especially regarding gastroenteritis.
Phylogenetic analysis based on a partial 3D region confirmed our sequences to be members of the species Aichivirus A in the genus Kobuvirus. Furthermore, the sequences clustered with MuKV, canine and feline kobuviruses, and human Aichi viruses. The 21 MuKV 3D region sequences identified in this study formed a large group with the US, Chinese and Hungarian MuKV sequences. This is in accordance with previous research indicating no geographic clustering [46]. Besides those 21, the other, one sequence (YY101) exhibited the closest relationship to the Chinese MuKV sequence GZ85. Moreover, our sequences tended to phylogenetically cluster at a branch associated with their host species (Fig. 1).
The VP1 protein is the most immunodominant portion of the kobuvirus capsid, determining the viral antigenicity and pathogenicity; it is also the most variable kobuvirus structural protein [3, 47]. The phylogenetic analysis based on our complete VP1 nt sequences (with the murine rodent host species shown in italics) indicates that our VP1 sequences clustered closely with those from China but were separated from MuKV VP1 sequences from Hungary and Vietnam (Fig. 2). This demonstrates that the MuKV sequences from China may share a similar evolutionary background and circulate among the various murine rodent populations in China [48]. This provides evidence of geographic clustering of the analysed VP1 sequences, though geographic clustering was not observed regarding the 3D region. Whether the MuKV sequences from different geographic regions impact pathogenicity and antigenicity warrants attention in the future.
Acquiring complete polyprotein sequences in this study allowed us to obtain comprehensive information on the genetic characteristics of MuKVs circulating in murine rodents in southern China. The three sequences were designated MuKV/XM34/CHN (7296 nt), MuKV/XM86/CHN (7296 nt) and MuKV/YN27/CHN (7293 nt). Genomic analysis revealed that our polyprotein sequences exhibited a similar genome organization to those of other kobuviruses, including L, VP0, VP3, VP1, 2A, 2B, 2C, 3A, 3B, 3C and 3D (Fig. 3a). Interestingly, a single amino acid deletion was found in the deduced 3A region of MuKV/YN27/CHN, similar to the canine strain CH-1 and the feline strain WHJ-1. According to previous research, a single amino acid deletion was also found in the VP0 region of feline kobuvirus from a cat with diarrhoea [13]. In contrast, there was a thirty-amino-acid deletion in the 2B region of porcine kobuvirus from healthy piglets, which might be associated with the pathogenicity of porcine kobuvirus [49]. Natural mutation and recombination occurs in picornaviruses, playing an important role in genetic diversity [50]. Whether the amino acid deletions influence the pathogenicity of kobuviruses from different species requires further investigation via structural prediction and genomic analysis.
The 49-nt partial 3′UTR of the MuKVs identified in this study showed the highest sequence identities (98.0–100 %) to the Chinese sequences Wencheng-Rt386-2 (accession no. MF352432.1). The predicted partial 3′UTR RNA secondary structure had a characteristic ‘barbell-like’ structure with a conserved AGGGAAC motif, as in the MuKV reference sequences in GenBank. Barbell-like structures have been identified in members of the genus Kobuvirus at different nt positions in the 3′UTR [33, 51]. Future studies should determine the functions of this structure.
The predicted cleavage sites generate a typical picornavirus gene order of VP0–VP3–VP1-2A1-2A2-2B–2C–3A–3B–3C–3D [52]. Prior studies have reported the hypothetical protease cleavage sites in feline kobuvirus [51, 53], canine kobuvirus [54] and even different rodent picornavirus lineages [55]. This study predicted the cleavage site positions in the MuKV polyprotein by sequence alignment with those putative or demonstrated cleavage sites in kobuviruses. Predicted cleavage sites of our MuKV sequences mainly comprised Q/G, Q/H, Q/T, Q/S and Q/A amino acid sequences, except for A/G and L/T between VP1 and 2A. We further compared the protease cleavage sites among kobuvirus sequences isolated from humans and various animal hosts. The findings supported a previous review indicating predicted cleavage sites are similar to those in other picornaviruses [3].
A phylogenetic analysis of Kobuvirus and other picornaviruses demonstrated that our sequences belong to the genus Kobuvirus (Fig. 4). The phylogenetic relationships between our complete polyprotein sequences and other kobuvirus sequences (Aichivirus A–F species) were also shown in Fig. 4, illustrating that our MuKV sequences shared a common root with the kobuviruses identified in canine and humans but related more distantly to bovine kobuvirus, as indicated by the nucleotide and amino acid homology analyses. In addition, this result suggests that cross-species kobuvirus transmission might occur due to frequent contact between rats, dogs and humans in the natural environment, in line with a study demonstrating that cross-species transmission may occur within and among mammalian species [24, 56]. These findings increase understanding of Kobuvirus evolution and genomic characteristics in murine rodent populations.
Conclusions
For the first time, we identified MuKV in Rattus losea and Rattus tanezumi in China, demonstrating the expanded host range of kobuviruses. We identified the molecular characteristics of MuKV and provided evidence of its circulation in three murine rodent species. A limitation of the study is the small sample size. Therefore, more detailed analyses, including epidemiological and experimental investigations, are needed to investigate the pathogenicity, genetic diversity and potential risk to human health of MuKV.
Supplementary Data
Funding information
This work was supported by the National Natural Science Foundation of China (grant no. 81973107).
Acknowledgements
We are grateful for the generous support of our colleagues regarding sample collection and technical assistance.
Author contributions
M.Z.: conceptualization, investigation, writing-original draft preparation; F.Y.: methodology, investigation; F.W.: investigation; H.H.: visualization; Q.L.: investigation; Q.C.: conceptualization, writing-review and editing, supervision.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Ethical statement
The research protocol was approved by the Animal Ethics and Welfare Committee of the School of Public Health, Southern Medical University, China, and met the guidelines for the Rules for the Implementation of Laboratory Animal Medicine (1998) from the Ministry of Health, China.
Footnotes
Abbreviations: aa, amino acid; BLAST, Basic Local Alignment Search Tool; 3D, three-dimensional; ICTV, International Committee on Taxonomy of Viruses; MuKV, murine kobuvirus; nt, nucleotide; PBoV, porcine bocavirus; RBoV, rat bocavirus; RoTTV, rodent torque teno virus.
Two supplementary tables and one supplementary figures are available with the online version of this article.
References
- 1.Khamrin P, Maneekarn N, Okitsu S, Ushijima H. Epidemiology of human and animal kobuviruses. Virusdisease. 2014;25:195–200. doi: 10.1007/s13337-014-0200-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lescar J, Canard B. RNA-dependent RNA polymerases from flaviviruses and Picornaviridae . Curr Opin Struct Biol. 2009;19:759–767. doi: 10.1016/j.sbi.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 3.Reuter G, Boros A, Pankovics P. Kobuviruses - a comprehensive review. Rev Med Virol. 2011;21:32–41. doi: 10.1002/rmv.677. [DOI] [PubMed] [Google Scholar]
- 4.Yamashita T, Sakae K, Ishihara Y, Isomura S, Utagawa E. Prevalence of newly isolated, cytopathic small round virus (Aichi strain) in Japan. J Clin Microbiol. 1993;31:2938–2943. doi: 10.1128/jcm.31.11.2938-2943.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamashita T, Ito M, Kabashima Y, Tsuzuki H, Fujiura A, et al. Isolation and characterization of a new species of kobuvirus associated with cattle. J Gen Virol. 2003;84:3069–3077. doi: 10.1099/vir.0.19266-0. [DOI] [PubMed] [Google Scholar]
- 6.Reuter G, Boldizsár A, Kiss I, Pankovics P. Candidate new species of Kobuvirus in porcine hosts. Emerg Infect Dis. 2008;14:1968–1970. doi: 10.3201/eid1412.080797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reuter G, Boros A, Pankovics P, Egyed L. Kobuvirus in domestic sheep, Hungary. Emerg Infect Dis. 2010;16:869–870. doi: 10.3201/eid1605.091934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee M-H, Jeoung H-Y, Lim J-A, Song J-Y, Song D-S, et al. Kobuvirus in South Korean black goats. Virus Genes. 2012;45:186–189. doi: 10.1007/s11262-012-0745-6. [DOI] [PubMed] [Google Scholar]
- 9.Pankovics P, Boros Á, Bíró H, Horváth KB, Phan TG, et al. Novel picornavirus in domestic rabbits (Oryctolagus cuniculus var. domestica. Infect Genet Evol. 2016;37:117–122. doi: 10.1016/j.meegid.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oem J-K, Choi J-W, Lee M-H, Lee K-K, Choi K-S. Canine kobuvirus infections in Korean dogs. Arch Virol. 2014;159:2751–2755. doi: 10.1007/s00705-014-2136-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olarte-Castillo XA, Heeger F, Mazzoni CJ, Greenwood AD, Fyumagwa R, et al. Molecular characterization of canine kobuvirus in wild carnivores and the domestic dog in Africa. Virology. 2015;477:89–97. doi: 10.1016/j.virol.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Cho Y-Y, Lim S-I, Kim YK, Song J-Y, Lee J-B, et al. Molecular characterization of the full kobuvirus genome in a cat. Genome Announc. 2014;2 doi: 10.1128/genomeA.00420-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niu T-J, Yi S-S, Wang X, Wang L-H, Guo B-Y, et al. Detection and genetic characterization of kobuvirus in cats: The first molecular evidence from Northeast China. Infect Genet Evol. 2019;68:58–67. doi: 10.1016/j.meegid.2018.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smits SL, Raj VS, Oduber MD, Schapendonk CME, Bodewes R, et al. Metagenomic analysis of the ferret fecal viral flora. PLoS One. 2013;8:e71595. doi: 10.1371/journal.pone.0071595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.You F-F, Zhang M-Y, He H, He W-Q, Li Y-Z, et al. Kobuviruses carried by Rattus norvegicus in Guangdong, China. BMC Microbiol. 2020;20:94. doi: 10.1186/s12866-020-01767-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu Z, Yang L, Ren X, He G, Zhang J, et al. Deciphering the bat virome catalog to better understand the ecological diversity of bat viruses and the bat origin of emerging infectious diseases. ISME J. 2016;10:609–620. doi: 10.1038/ismej.2015.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melegari I, Sarchese V, Di Profio F, Robetto S, Carella E, et al. First molecular identification of kobuviruses in wolves (Canis lupus) in Italy. Arch Virol. 2018;163:509–513. doi: 10.1007/s00705-017-3637-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Martino B, Di Profio F, Melegari I, Robetto S, Di Felice E, et al. Molecular evidence of kobuviruses in free-ranging red foxes (Vulpes vulpes. Arch Virol. 2014;159:1803–1806. doi: 10.1007/s00705-014-1975-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Martino B, Di Profio F, Melegari I, Di Felice E, Robetto S, et al. Molecular detection of kobuviruses in European roe deer (Capreolus capreolus) in Italy. Arch Virol. 2015;160:2083–2086. doi: 10.1007/s00705-015-2464-5. [DOI] [PubMed] [Google Scholar]
- 20.Ng TFF, Marine R, Wang C, Simmonds P, Kapusinszky B, et al. High variety of known and new RNA and DNA viruses of diverse origins in untreated sewage. J Virol. 2012;86:12161–12175. doi: 10.1128/JVI.00869-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reuter G, Boldizsár A, Pankovics P. Complete nucleotide and amino acid sequences and genetic organization of porcine kobuvirus, a member of a new species in the genus Kobuvirus, family Picornaviridae . Arch Virol. 2009;154:101–108. doi: 10.1007/s00705-008-0288-2. [DOI] [PubMed] [Google Scholar]
- 22.Otomaru K, Naoi Y, Haga K, Omatsu T, Uto T, et al. Detection of novel kobu-like viruses in Japanese black cattle in Japan. J Vet Med Sci. 2016;78:321–324. doi: 10.1292/jvms.15-0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phan TG, Kapusinszky B, Wang C, Rose RK, Lipton HL, et al. The fecal viral flora of wild rodents. PLoS Pathog. 2011;7:e1002218. doi: 10.1371/journal.ppat.1002218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu L, Van Dung N, Ivens A, Bogaardt C, et al. Genetic diversity and cross-species transmission of kobuviruses in Vietnam. Virus Evol. 2018;4:vey002. doi: 10.1093/ve/vey002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams SH, Che X, Garcia JA, Klena JD, Lee B, et al. Viral diversity of house mice in New York City. mBio. 2018;9 doi: 10.1128/mBio.01354-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiong Y-Q, Zhou J-H, Zhang M-Y, You F-F, Li D-L, et al. Presence of rat bocavirus in oropharyngeal and fecal samples from murine rodents in China. Arch Virol. 2018;163:3099–3103. doi: 10.1007/s00705-018-3943-2. [DOI] [PubMed] [Google Scholar]
- 27.Xiong Y-Q, You F-F, Chen X-J, Chen Y-X, Wen Y-Q, et al. Detection and phylogenetic analysis of porcine bocaviruses carried by murine rodents and house shrews in China. Transbound Emerg Dis. 2019;66:259–267. doi: 10.1111/tbed.13011. [DOI] [PubMed] [Google Scholar]
- 28.Xiong Y-Q, Mo Y, Chen M-J, Cai W, He W-Q, et al. Detection and phylogenetic analysis of torque teno virus (TTV) carried by murine rodents and house shrews in China. Virology. 2018;516:189–195. doi: 10.1016/j.virol.2018.01.017. [DOI] [PubMed] [Google Scholar]
- 29.Kumar S, Stecher G, Tamura K. mega7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol Biol Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lole KS, Bollinger RC, Paranjape RS, Gadkari D, Kulkarni SS, et al. Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J Virol. 1999;73:152–160. doi: 10.1128/JVI.73.1.152-160.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Souza Luna LK, Baumgarte S, Grywna K, Panning M, Drexler JF, et al. Identification of a contemporary human parechovirus type 1 by VIDISCA and characterisation of its full genome. Virol J. 2008;5:26. doi: 10.1186/1743-422X-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003;31:3406–3415. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boros Á, Orlovácz K, Pankovics P, Szekeres S, Földvári G, et al. Diverse picornaviruses are prevalent among free-living and laboratory rats (Rattus norvegicus) in Hungary and can cause disseminated infections. Infect Genet Evol. 2019;75:103988. doi: 10.1016/j.meegid.2019.103988. [DOI] [PubMed] [Google Scholar]
- 34.Firth C, Bhat M, Firth MA, Williams SH, Frye MJ, et al. Detection of zoonotic pathogens and characterization of novel viruses carried by commensal Rattus norvegicus in New York City. mBio. 2014;5:e01933–14. doi: 10.1128/mBio.01933-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Z, Jin W, Zhao Z, Lin W, Zhang D, et al. Genetic characterization of porcine kobuvirus and detection of coinfecting pathogens in diarrheic pigs in Jiangsu Province, China. Arch Virol. 2014;159:3407–3412. doi: 10.1007/s00705-014-2204-2. [DOI] [PubMed] [Google Scholar]
- 36.Zhao Z-P, Yang Z, Lin W-D, Wang W-Y, Yang J, et al. The rate of co-infection for piglet diarrhea viruses in China and the genetic characterization of porcine epidemic diarrhea virus and porcine kobuvirus. Acta Virol. 2016;60:55–61. doi: 10.4149/av_2016_01_55. [DOI] [PubMed] [Google Scholar]
- 37.Kong N, Zuo Y, Wang Z, Yu H, Zhou E-M, et al. Molecular characterization of new described kobuvirus in dogs with diarrhea in China. Springerplus. 2016;5:2047. doi: 10.1186/s40064-016-3738-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park S-J, Kim H-K, Moon H-J, Song D-S, Rho S-M, et al. Molecular detection of porcine kobuviruses in pigs in Korea and their association with diarrhea. Arch Virol. 2010;155:1803–1811. doi: 10.1007/s00705-010-0774-1. [DOI] [PubMed] [Google Scholar]
- 39.Collins PJ, McMenamy MJ, McClintock J, Lagan-Tregaskis P, McCabe L, et al. Molecular detection of kobuviruses in livestock in Northern Ireland and the Republic of Ireland. Arch Virol. 2017;162:1275–1279. doi: 10.1007/s00705-017-3223-6. [DOI] [PubMed] [Google Scholar]
- 40.Wang L, Fredrickson R, Duncan M, Samuelson J, Hsiao S-H. Bovine Kobuvirus in Calves with Diarrhea, United States. Emerg Infect Dis. 2020;26:176–178. doi: 10.3201/eid2601.191227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ribeiro J, Headley SA, Diniz JA, Pereira AHT, Lorenzetti E, et al. Extra-intestinal detection of canine kobuvirus in a puppy from Southern Brazil. Arch Virol. 2017;162:867–872. doi: 10.1007/s00705-016-3164-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamashita T, Kobayashi S, Sakae K, Nakata S, Chiba S, et al. Isolation of cytopathic small round viruses with BS-C-1 cells from patients with gastroenteritis. J Infect Dis. 1991;164:954–957. doi: 10.1093/infdis/164.5.954. [DOI] [PubMed] [Google Scholar]
- 43.Yamashita T, Ito M, Tsuzuki H, Sakae K. Identification of Aichi virus infection by measurement of immunoglobulin responses in an enzyme-linked immunosorbent assay. J Clin Microbiol. 2001;39:4178–4180. doi: 10.1128/JCM.39.11.4178-4180.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khamrin P, Maneekarn N, Hidaka S, Kishikawa S, Ushijima K, et al. Molecular detection of kobuvirus sequences in stool samples collected from healthy pigs in Japan. Infect Genet Evol. 2010;10:950–954. doi: 10.1016/j.meegid.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 45.Moreira ASD, Raabis SM, Graham ME, Dreyfus JM, Sibley SD, et al. Identification by next-generation sequencing of Aichivirus B in a calf with enterocolitis and neurologic signs. J Vet Diagn Invest. 2017;29:208–211. doi: 10.1177/1040638716685597. [DOI] [PubMed] [Google Scholar]
- 46.Milićević V, Kureljušić B, Maksimović-Zorić J, Savić B, Spalević L, et al. Molecular detection and characterization of Porcine Kobuvirus in domestic pigs and wild boars in Serbia. Res Vet Sci. 2020;132:404–406. doi: 10.1016/j.rvsc.2020.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen L, Zhu L, et al. Molecular and phylogenetic analysis of the porcine kobuvirus VP1 region using infected pigs from Sichuan Province, China. Virol J. 2013;10:281. doi: 10.1186/1743-422X-10-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Y, Cui Y, Li Y, Wang X, Yang K, et al. Identification and full-genome sequencing of canine kobuvirus in canine fecal samples collected from Anhui Province, eastern China. Arch Virol. 2020;165:2495–2501. doi: 10.1007/s00705-020-04773-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jin W-J, Yang Z, Zhao Z-P, Wang W-Y, Yang J, et al. Genetic characterization of porcine kobuvirus variants identified from healthy piglets in China. Infect Genet Evol. 2015;35:89–95. doi: 10.1016/j.meegid.2015.07.035. [DOI] [PubMed] [Google Scholar]
- 50.Lukashev AN. Recombination among picornaviruses. Rev Med Virol. 2010;20:327–337. doi: 10.1002/rmv.660. [DOI] [PubMed] [Google Scholar]
- 51.Choi J-W, Lee M-H, Lee K-K, Oem J-K. Genetic characteristics of the complete feline kobuvirus genome. Virus Genes. 2015;50:52–57. doi: 10.1007/s11262-014-1144-y. [DOI] [PubMed] [Google Scholar]
- 52.Kapoor A, Victoria J, Simmonds P, Wang C, Shafer RW, et al. A highly divergent picornavirus in a marine mammal. J Virol. 2008;82:311–320. doi: 10.1128/JVI.01240-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lu G, Zhang X, Luo J, Sun Y, Xu H, et al. First report and genetic characterization of feline kobuvirus in diarrhoeic cats in China. Transbound Emerg Dis. 2018;65:1357–1363. doi: 10.1111/tbed.12916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kapoor A, Simmonds P, Dubovi EJ, Qaisar N, Henriquez JA, et al. Characterization of a canine homolog of human Aichivirus. J Virol. 2011;85:11520–11525. doi: 10.1128/JVI.05317-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Du J, Lu L, Liu F, Su H, Dong J, et al. Distribution and characteristics of rodent picornaviruses in China. Sci Rep. 2016;6:34381. doi: 10.1038/srep34381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meerburg BG, Singleton GR, Kijlstra A. Rodent-borne diseases and their risks for public health. Crit Rev Microbiol. 2009;35:221–270. doi: 10.1080/10408410902989837. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.