Highlights
-
•
CoOx/BiFeO3 nanocomposite was prepared via a photodeposition method.
-
•
CoOx/BiFeO3 realized piezocatalytic RhB degradation by harvesting ultrasonic vibration energy.
-
•
CoOx/BiFeO3 presented much better piezocatalytic performance than BiFeO3.
-
•
The piezocatalytic activity of CoOx/BiFeO3 can be improved via optimizing reaction condiction.
-
•
CoOx nanoparticles improved the charge separation via trapping the piezoinduced holes of BiFeO3.
Keywords: BiFeO3, CoOx, Piezoelectric effect, RhB, Piezocatalytic
Abstract
Piezoelectric materials have received much attention due to their great potential in environmental remediation by utilizing vibrational energy. In this paper, a novel piezoelectric catalyst, CoOx nanoparticles anchored BiFeO3 nanodisk composite, was intentionally synthesized via a photodeposition method and applied in piezocatalytic degradation of rhodamine B (RhB) under ultrasonic vibration. The as-synthesized CoOx/BiFeO3 composite presents high piezocatalytic efficiency and stability. The RhB degradation rate is determined to be 1.29 h−1, which is 2.38 folds higher than that of pure BiFeO3. Via optimizing the reaction conditions, the piezocatalytic degradation rate of the CoOx/BiFeO3 can be further increased to 3.20 h−1. A thorough characterization was implemented to investigate the structure, piezoelectric property, and charge separation efficiency of the CoOx/BiFeO3 to reveal the nature behind the high piezocatalytic activity. It is found that the CoOx nanoparticles are tightly adhered and uniformly dispersed on the surface of the BiFeO3 nanodisks. Strong interaction between CoOx and BiFeO3 triggers the formation of a heterojunction structure, which further induces the migration of the piezoinduced holes on the BiFeO3 to CoOx nanoparticles. The recombination of electron-hole pairs is retarded, thereby increasing the piezocatalytic performance greatly. This work may offer a new paradigm for the design of high-efficiency piezoelectric catalysts.
1. Introduction
Water is a limited resource and determines the sustainable development of humans. The rapid population growth and environmental pollution have led to a sharp decline in freshwater resources, thus posing a major challenge worldwide. A feasible solution is to realize the recycling of wastewater via degrading the toxic pollutants in wastewater into non-toxic substances. Several advanced oxidation processes (AOP) are thus developed and applied in water purification, such as UV/H2O2, UV/O3, Fenton, photocatalysis, photo-Fenton, sonolysis, electrochemical oxidation, and piezocatalysis [1], [2], [3], [4]. Among them, piezoelectric catalytic technology is a new type of AOP technology developed in recent years [5], [6], [7]. It can realize the efficient degradation of organic pollutants by using the abundant low-frequency mechanical energy source in nature, and hence attached much attention.
Till to date, some classic piezoelectric materials including ZnO [8], [9], BaTiO3 [10], [11], and Pb(Zr0.52Ti0.48)O3 [12], [13] have been applied in piezocatalytic degradation of dyes. The piezocatalytic reaction process can be described as follows: the piezoelectric material deforms under external mechanical stress, causing the misalignment of the centers of positive and negative charges, thereby generating piezoelectric potential. Driven by the piezoelectric field, the thermal-excited free charges in the piezoelectric material are enriched in the opposite direction of the crystal surface, which then triggers the surface electrochemical reaction to achieve dye degradation, CO2 reduction, water decomposition, etc [14], [15], [16], [17]. The piezoelectric property is the key factor that affects the piezocatalytic behavior of the piezocatalysts. Hence, the method of controlling the morphology of catalysts is usually used to increase the piezoelectric potential, thereby affecting its piezoelectric catalytic performance. For instance, compared with ZnO nanoparticles, Ning et al. found that ZnO nanorods are easy to be deformed under mechanical vibration and generate a higher piezoelectric potential [18]. Due to the same reason, BaTiO3 nanowires exhibited much higher activity than BaTiO3 nanoparticles in piezocatalytic degradation of methyl orange (MO) [19]. In addition to the piezoelectric property, the concentration and lifetime of the thermal-excited free charge carriers can also greatly influence the piezocatalytic reaction. Bai et al. reported that the introduction of oxygen vacancy into ZnO can increase the concentration of free electrons and hence enhances the piezocatalytic activity in dyes degradation [20]. Zhou et al. loaded BiOX (X = Cl, Br, and Cl0.166Br0.834) on BaTiO3 surface [21]. The formed heterojunction structure extends the lifetime of free charge carriers and hence elevates the piezocatalytic activity of BaTiO3. The same promotion effect can be achieved by loading precious metals on the piezoelectric catalyst surface [22]. In any case, pure piezoelectric catalyst needs to be modified to obtain high piezocatalytic efficiency.
BiFeO3 is a typical ABO3-type perovskite composite with R3c space group structure. It has fascinating physical properties and has been applied in many fields including nonlinear optics, ferroelectric memory, photoelectrochemical cells, multilayer ceramic capacitors, as well as photocatalysis [23], [24]. Meanwhile, BiFeO3 is also a classic piezoelectric material with a large piezoelectric coefficient (d33) of about 100 pmV [25], indicating its good ability in converting mechanical energy to electric energy. Several researchers have reported the piezocatalytic application of BiFeO3. For example, Mushtaq et al. synthesized BiFeO3 nanorods and realized efficient degradation of RhB under ultrasonic vibration [26]. Xu et al. decorated Ag nanoparticles on BiFeO3 nanofibers via a photo-reduction reaction [27]. The synergistic effect of piezoelectric effect and localized surface plasmon resonance of Ag nanoparticles make the Ag/BiFeO3 sample present high performance in piezocatalytic and piezo-photocatalytic degradation of MO and methyl blue (MB). Liu et al. improved the piezocatalytic and piezo-photocatalytic performance of BiFeO3 by coupling TiO2 nanoparticles [28]. Currently, however, research focusing on the piezocatalytic application of BiFeO3 is still rare. For practical application, it is still worthy of studying to design and synthesize BiFeO3 based piezocatalyst for efficient degradation of organic pollutants. The construction of heterojunction structures by coupling promoters has been verified to be a convenient and efficient method for synthesizing high-efficient piezocatalysts, suggesting that the piezocatalytic activity of BiFeO3 can be enhanced via the decoration of a suitable cocatalyst. Cobalt oxide has been reported as an outstanding cocatalyst for many semiconductor photocatalysts including TiO2 [29], ZnO [30], g-C3N4 [31], and BaNbO2N [32]. Shi et al. found that the anchor of Co3O4 on BiFeO3 greatly improved the photocatalytic activity in nitrophenol isomers degradation [33], indicating that Co3O4 has matched band potential and can be a suitable promoter for BiFeO3. Raju et al. synthesized polyvinylidene fluoride/ZnSnO3/Co3O4 composite and further verified the promotion effect of Co3O4 in piezocatalytic degradation of RhB [34]. Therefore, the modification of cobalt oxide on BiFeO3 may construct an effective piezoelectric catalyst. Although a similar photocatalyst has been synthesized and applied in photocatalytic degradation of nitrophenol isomers, in the piezocatalytic field, this catalyst has not been studied.
Based on the above discussion, BiFeO3 nanodisks were synthesized via a hydrothermal method. CoOx nanoparticles were then anchored to the BiFeO3 surface by a photodeposition process. The as-synthesized CoOx/BiFeO3 was found to show high activity in piezocatalytic degradation of dyes. Under ultrasonic vibration for 90 min, 81.2% of RhB is degraded in the presence of CoOx/BiFeO3, which is more effective than pure BiFeO3 (50.76%). A thorough characterization of the structure, morphology, piezoelectric property of the CoOx/BiFeO3 composite was implemented to understand the reason for the significant increase in its activity. In addition to the piezocatalytic stability and the reactive species trapping experiment, the effects of solution pH and catalyst dosage on the piezocatalytic activity were also investigated.
2. Experimental
2.1. Preparation of piezocatalytsts
Iron chloride hexahydrate (FeCl3·6H2O), Bismuth nitrate pentahydrate (Bi(NO3)3·5H2O), Potassium hydroxide (KOH), Nitric acid (HNO3), Cobalt nitrate hexahydrate (Co(NO3)2·6H2O), and Methanol (CH3OH) were used during the preparation of CoOx/BiFeO3 piezocatalyst. All the reagents are of analytical grade and are commercially available.
BiFeO3 nanodisks were synthesized through a hydrothermal process. Typically, 2.027 g FeCl3·6H2O and 3.638 g Bi(NO3)3·5H2O were dispersed into a mixed solution of 24 mL deionized water and 6 mL HNO3. Meanwhile, 18 g KOH was dissolved in 40 mL deionized water. The obtained KOH solution was then dropped slowly into the above suspension under stirring, and the pH value of the solution was adjusted to 13–14. Finally, the mixture was transferred into a Teflon-lined stainless-steel autoclave and kept at 200 °C for 12 h. After that, the product was collected by centrifugation, washed several times with distilled water and ethanol, and dried in vacuum at 60 °C for 12 h.
CoOx/BiFeO3 piezocatalysts were synthesized via a photodeposition method. Specifically, 0.3 g of the synthesized BiFeO3 nanodisks and 0.7407 g of Co(NO3)2·6H2O were dispersed in a mixed solution of 80 mL deionized water and suspended 20 mL methanol. After being stirred in dark for 60 min, the suspension was exposed to the irradiation of Xe lamp for n h (n = 1.0, 2.0, 3.0, and 4.0 h). The precipitate was collected by centrifugation, washed by deionized water and alcohol in sequence, and dried at 60 °C in a vacuum oven. The obtained piezocatalyst was expressed as nCoOx/BiFeO3, where n presents the irradiation time.
2.2. Piezocatalytic reaction and characterization of catalysts
The piezocatalytic reaction of RhB dye under ultrasonic vibration was implemented to investigate the piezocatalytic activity of the prepared CoOx/BiFeO3 composite. The detailed information about the piezocatalytic reaction and the characterization of the CoOx/BiFeO3 was provided in the Supplementary Materials.
3. Result and discussion
3.1. Characterization of CoOx/BiFeO3 composite
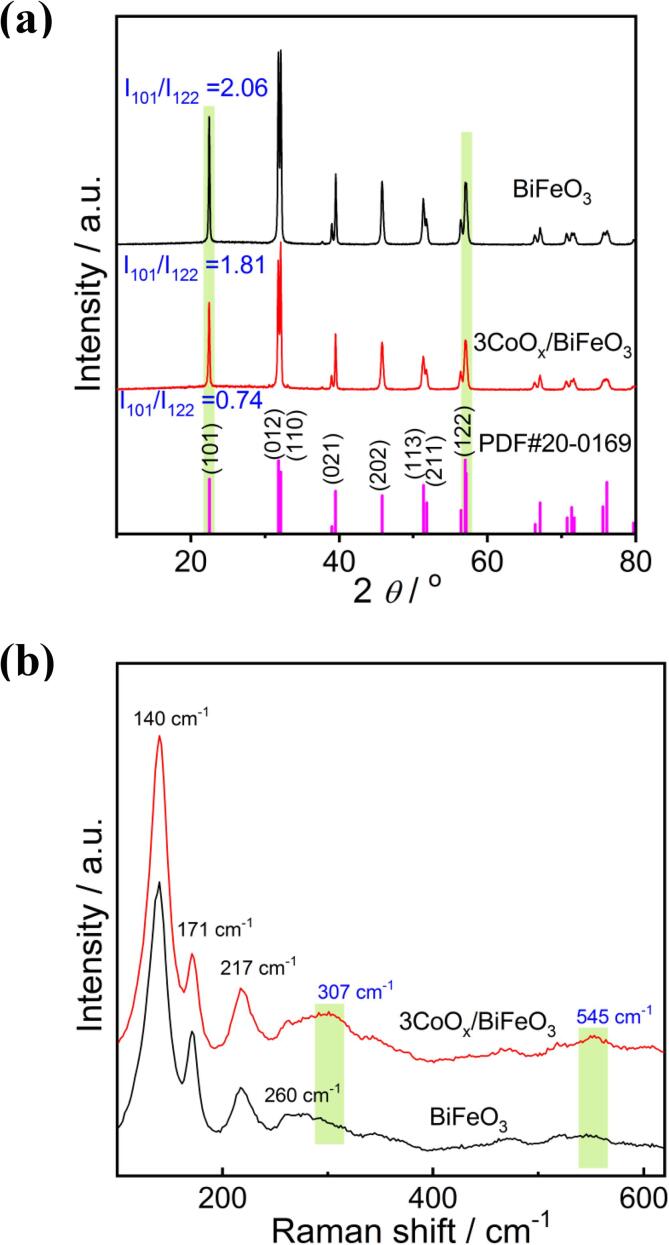
XRD and Raman experiments were implemented to investigate the structure of CoOx/BiFeO3. The 3CoOx/BiFeO3 piezocatalyst was selected as the representative sample. As Fig. 1 shows, BiFeO3 sample displays strong diffraction peaks at 22.4, 31.7, 32.1, 39.5, 45.7, 51.3, and 57.0°, corresponding to the (1 0 1), (0 1 2), (1 1 0), (0 2 1), (2 0 2), (1 1 3), and (1 2 2) planes of the rhombohedral structure of BiFeO3 (PDF# 20–0169). No diffraction peaks corresponding to other substances are observed, suggesting the synthesized sample is pure. An interesting phenomenon is that the diffraction peak intensity of the synthesized BiFeO3 is inconsistent with the standard card. Take the peaks at 22.4 and 57.0° as an example, the peak intensity ratio of the two peaks (I101/I122) for the standard card is 0.74. For BiFeO3, the I101/I122 value increases to 2.06, indicating that the piezocatalyst may have special anisotropic growth along the (1 0 1) plane and may show a special nanostructure morphology [26]. The XRD patterns of 3CoOx/BiFeO3 sample are almost the same as that of BiFeO3, but the peak intensity is slightly decreased. The I101/I122 value is still significantly higher than that of the standard card, indicating that the structure and morphology of BiFeO3 change little. No cobalt oxide species is observed in the XRD pattern, which may be due to its low concentration. Fig. 1b demonstrates the Raman spectra of BiFeO3 and 3CoOx/BiFeO3 samples. Pure BiFeO3 presents the characteristic peaks at 140, 171, and 217 cm−1, which can be ascribed to to A1-1, A1-2, and A1-3 modes, respectively [35]. For CoOx/BiFeO3 sample, in addition to the characteristic peaks of BiFeO3, two weak Raman signals appear at about 307 and 545 cm−1. The peak at 307 cm−1 may be assigned to the CoO species [36], while the 545 cm−1 peak is close to the Raman active modes of Co(OH)2 and Co3O4 [36], [37]. Therefore, the photodeposition of cobalt oxide species on the BiFeO3 surface is definite. However, due to the low content, the loaded Co species is difficult to be identified. So, it is expressed as CoOx.
Fig. 1.
XRD patterns (a) and Raman (b) spectra of BiFeO3 and 3CoOx/BiFeO3.
Fig. 2 shows the XPS spectra of BiFeO3 and CoOx/BiFeO3. All signals of Bi, Fe, and O are detected in the XPS spectrum of BiFeO3, confirming its elemental composition. The Bi4f7/2 and 4f5/2 peaks appear at 158.5 and 163.8 eV, respectively (Fig. 2a). The valence state of Bi is determined to be + 3 based on the reported literature [38]. For 3CoOx/BiFeO3 sample, the Bi 4f peaks slightly shift to the lower binding energy (BE) direction, indicating that the chemical environment of Bi may be changed by the introduced CoOx. This influence can also be observed in the Fe2p and O1s XPS spectra. As Fig. 2b shows, the Fe2p3/2 and 2p1/2 peaks of BiFeO3 are located at 710.5 and 724.1 eV, respectively, corresponding to the + 3 valence state of the Fe element [39]. The Fe2p BE of 3CoOx/BiFeO3 is determined to be 710.2 and 723.8 eV, which are 0.3 eV lower than that of BiFeO3. Fig. 3c displays the O1s XPS spectra of the two samples. The O1s peaks of BiFeO3 appear at 529.3 eV and 531.6 eV, which can be attributed to the lattice oxygen and the surface OH species, respectively [40]. The OH species of the 3CoOx/BiFeO3 sample still appears at 531.6 eV. Nevertheless, the peak of lattice oxygen slightly moves to 529.0 eV, confirming that the electron density of BiFeO3 is decreased due to the introduced CoOx. CoOx is a well-known hole trapper in composite photocatalysts [41], [42], [43]. The loaded CoOx may fabricate a heterojunction structure with BiFeO3, which induces the electron migration from CoOx to BiFeO3 thereby changing the surrounding of BiFeO3. The Co2p XPS spectrum of 3CoOx/BiFeO3 is presented in Fig. 2d. The BE of Co2p3/2 is determined to be 781.2 eV, while the peak at 785.5 eV is the satellite signal [44], [45]. Considering that the BE of Co2+ and Co3+ are very close [44], [45], it is more reasonable to ascribe the peak to the mixed-valence states of + 2 and + 3. The presence of CoOx species on the piezocatalyst surface is undoubted. Based on the peak area and the correction factor of Co2p, the Co atomic content is estimated to be 2.5% in the 3CoOx/BiFeO3 piezocatalyst surface.
Fig. 2.
XPS spectra of BiFeO3 and 3CoOx/BiFeO3. (a) Bi4f; (b) Fe2p; (c) O1s; (d) Co2p.
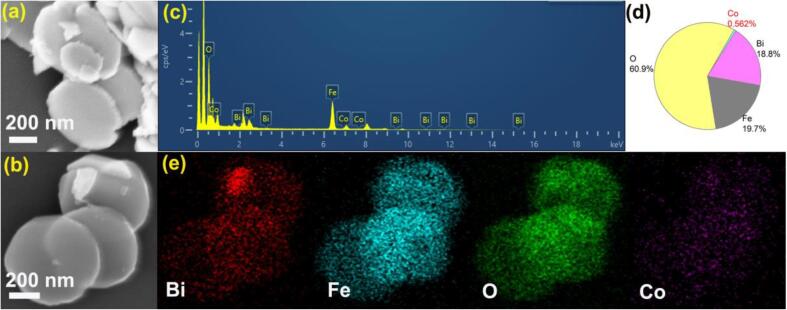
Fig. 3.
SEM image of BiFeO3 (a) and 3CoOx/BiFeO3 (b), EDS analysis of 3CoOx/BiFeO3 piezocatlayst (c–e).
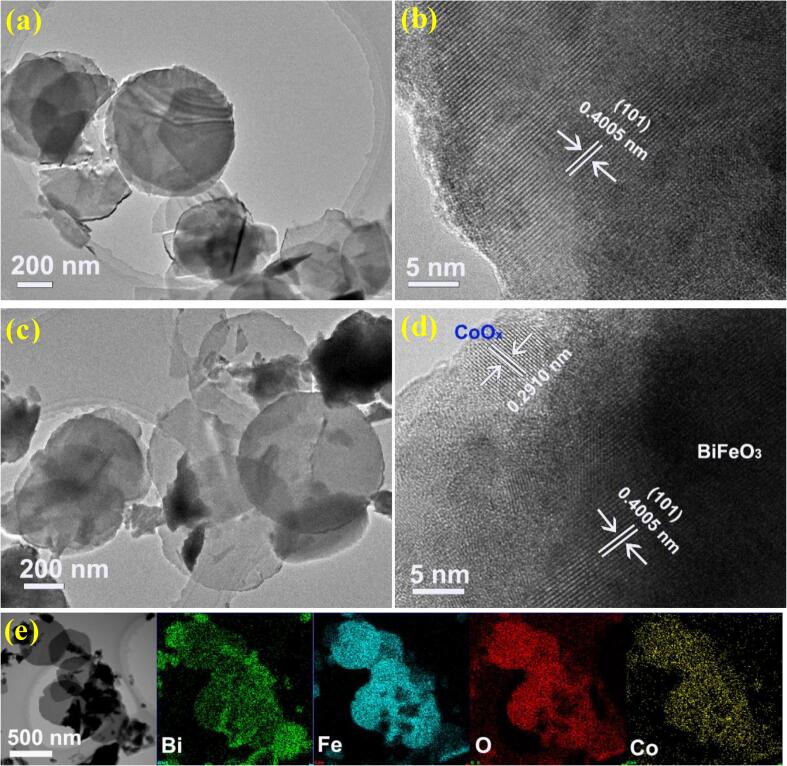
Fig. 3 displays the SEM images of BiFeO3 and 3CoOx/BiFeO3 samples. As predicted in the XRD analysis, BiFeO3 presents a special morphology of nanodisk with a diameter of 500–600 nm (Fig. 3a). The photodeposition of CoOx does not change the appearance of BiFeO3 (Fig. 3b). CoOx species is not observed, which may be ascribed to its small size or low content. Nevertheless, the EDS analysis definitely verifies that CoOx species is uniformly dispersed on the surface of BiFeO3 nanodisk, which is consistent with the XPS result. The Co atomic concentration is estimated to be 0.56% (Fig. 3c), which is smaller than that of XPS. This result may be attributed to the fact that the XPS technique evaluates the surface composition of the catalyst, while the EDS result reflects the phase composition of the CoOx/BiFeO3 catalyst. The nanodisk morphology of BiFeO3 is clearer in its TEM picture (Fig. 4a). The transparent nature of the disk further indicates that the BiFeO3 nanodisk is thin. The HR-TEM image of the BiFeO3 shows a clear lattice fringe of 0.4005 nm, which can be assigned to the (1 0 1) plane of BiFeO3. The 3CoOx/BiFeO3 sample presents a similar appearance as pure BiFeO3. Nevertheless, the high-resolution TEM picture indicates that some nanoparticles with a lattice fringe of 0.2910 nm are observed on the surface of the BiFeO3 nanodisk. Considering that the crystal plane in BiFeO3 which has the closest interplanar spacing to the detected value is the (0 1 2) plane (d = 0.2810 nm), we don’t think that these nanoparticles can be assigned to the BiFeO3 phase. Relatively, this lattice fringe is more likely to match the (0 0 2) plane (d = 0.2870 nm) of Co2O3 (PDF# 020770) and the (2 2 0) plane (d = 0.2860 nm) of Co3O4 (PDF#42–1467). Combined the XPS and SEM analysis, it is more reasonable to assign the detected nanoparticles to CoOx species. Fig. 4e shows the EDS mapping of 3CoOx/BiFeO3 piezocatalyst. The observed result is similar to that of Fig. 3e. Moreover, the larger scanning range further enhances the credibility of the universal dispersion of CoOx in the piezocatalyst.
Fig. 4.
TEM images of BiFeO3 (a, b) and 3CoOx/BiFeO3 (c, d). EDS mapping (e) of 3CoOx/BiFeO3 sample.
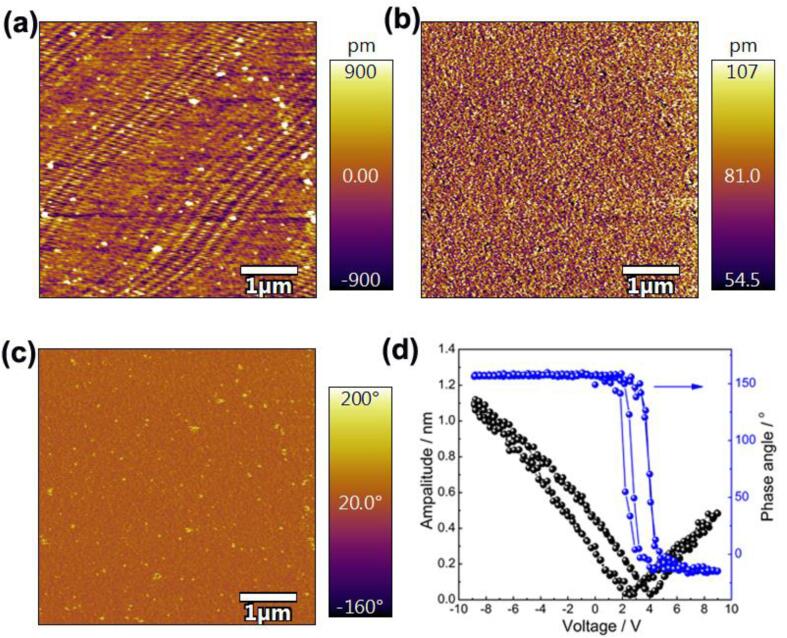
The piezoelectric force microscope (PFM) analysis was carried out to investigate the local piezoelectric response of the CoOx/BiFeO3 composite, and the result is shown in Fig. 5. Due to the limitation of the equipment, the detected images do not show the fine morphological information of the CoOx/BiFeO3 composite. The low magnification makes the catalyst more like nanoparticles. However, the local hysteresis loop (Fig. 5d) indicates the CoOx/BiFeO3 composite presents a phase angle change of about 160° under the reversal of the 10 V DC bias field. The butterfly-shaped hysteresis loop indicates the excellent local piezoelectric response of the CoOx/BiFeO3 sample, demonstrating that the composite can drive the piezoelectric catalytic reaction.
Fig. 5.
The AFM picture (a), PFM amplitude image (b), PFM phase image (b), and hysteresis loop (d) of the prepared 3CoOx/BiFeO3 composite.
3.2. Piezocatalytic activity of CoOx/BiFeO3 composite
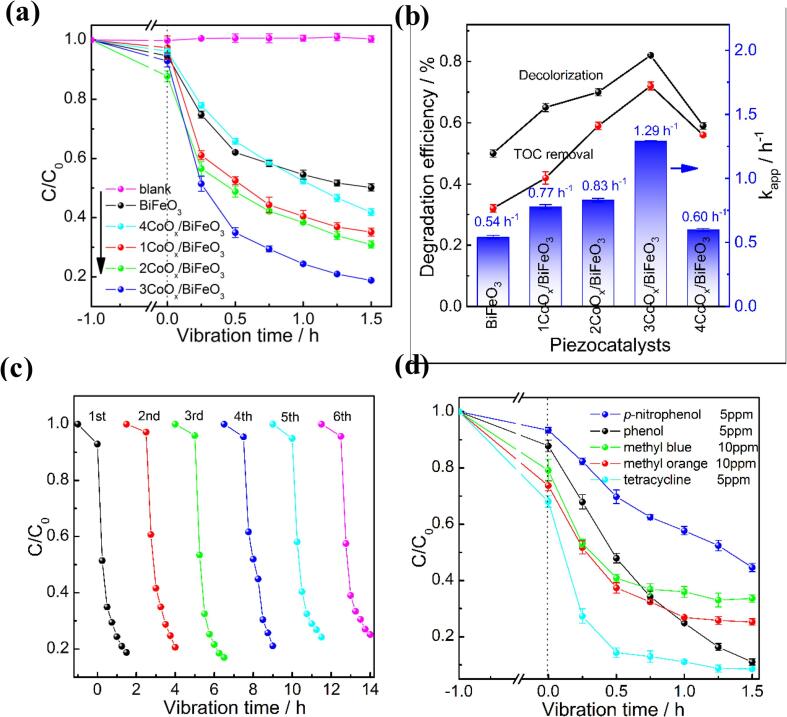
The piezocatalytic activity of BiFeO3 and CoOx/BiFeO3 composite was evaluated via piezocatalytic degradation of RhB under ultrasonic vibration of a digital ultrasonic generator (40 kHz, 120 W). No RhB is degraded in the absence of piezocatalysts, indicating that the contribution of ultrasonic decomposition can be ignored. When BiFeO3 or CoOx/BiFeO3 piezocatalyst is added to the reaction solution, the RhB content is obviously reduced as the vibration time increases (Fig. 6a). This result demonstrates that the synthesized catalysts can induce the piezocatalytic reaction, which is consistent with the PFM analysis. After ultrasonic vibration for 1.5 h, about 50% of RhB is degraded by BiFeO3, and the degradation rate is estimated to be 0.54 h−1 (Fig. 6b). The CoOx/BiFeO3 composite has a higher piezocatalytic activity than pure BiFeO3, indicating that there is a synergy effect of CoOx and BiFeO3. The piezocatalytic activity of the CoOx/BiFeO3 composites is closely related to the light irradiation time. The 3CoOx/BiFeO3 sample which is irradiated by a Xe lamp for 3 h presents the best piezocatalytic performance. About 82% of RhB is degraded during the 1.5 h of ultrasonic vibration, and the degradation rate is 1.29 h−1, which is 2.38 times higher than that of BiFeO3. In addition to the decolorization efficiency, the total organic carbon (TOC) removal efficiency also shows a similar trend. As the light irradiation time increases from one hour to four hours, the TOC removal rate of the as-prepared samples increases first and then decreases. The 3CoOx/BiFeO3 piezocatalyst is still the best sample with a TOC removal efficiency of 72.0%. This appearance can be ascribed to the variation of the CoOx concentration. The light irradiation time is closely related to the concentration of the deposited species [46], [47]. XPS analysis result clearly reveals that the CoOx content enhances with the increase of light exposure time (Fig. S1). The inductively coupled plasma-optical emission spectroscopy (ICP-OES) analysis further confirmed that the CoOx atomic concentrations in the CoOx/BiFeO3 sample prepared by light irradiation for 1, 2, 3, and 4 h are 0.17%, 0.28%, 0.44%, and 0.94%, respectively. According to the reported literature [40], [41], [42], [46], [47], the content of the promoter has a great influence on its dispersion in the catalyst. A high content of the promoter may cause serious agglomeration and reduce the number of heterojunction structures in the composite materials, which is not beneficial to the piezocatalytic reaction. In the current case, 3 h of light time may be the equilibrium point, which results in the 3CoOx/BiFeO3 piezocatalyst having a suitable CoOx concentration and hence exhibiting the best piezocatalytic performance.
Fig. 6.
Piezocatalytic activity of CoOx/BiFeO3 composite (a) and the corresponding degradation rate (b), the performance of 3CoOx/BiFeO3 in the cyclic test of RhB degradation (c) and the piezocatalytic degradation of different organic pollutants (d).
In addition to the piezocatalytic activity, the stability of the CoOx/BiFeO3 catalyst was also examined by the cyclic test. The result indicates that the synthesized CoOx/BiFeO3 still shows high piezocatalytic activity after five cyclic tests (Fig. 6c). Considering that the ultrasonic vibration may destroy the composite piezocatalyst via stripping the CoOx nanoparticles, the data in Fig. 6c undoubtedly proves the good stability of the CoOx/BiFeO3 catalyst. The piezocatalytic activity of the 3CoOx/BiFeO3 catalyst in the presence of p-benzoquinone (BQ), KI, and isopropanol (IPA) is shown in Fig. S2. The inhibitory effect of the sacrificial agent on the piezoelectric catalytic reaction follows the order of BQ (•O2– scavenger) > IPA (•OH scavenger) > KI (h+ scavenger). Given that the addition of KI slightly reduces the piezocatalytic activity, the main active radicals of the CoOx/BiFeO3 in the piezocatalytic degradation of RhB are •O2– and •OH. Fig. 6d displays the piezocatalytic activity of 3CoOx/BiFeO3 in the degradation of different organic pollutants. The 3CoOx/BiFeO3 is also efficient in piezocatalytic degradation of MO (anionic dye) and MB (anionic dye) even though they are two different types of dyes. Phenol and p-nitrophenol are two common pollutants that are more difficult to be degraded than dyes. Under ultrasonic vibration for 90 min, 3CoOx/BiFeO3 sample can reduce the concentration of Phenol and p-nitrophenol by 89% and 55%, respectively. For tetracycline, a common antibiotic contaminant, the degradation efficiency of 3CoOx/BiFeO3 reaches 92%. The data in Fig. 6d suggests that the CoOx/BiFeO3 piezocatalyst has a wide range of application potential in water purification due to its high degradation efficiency for different organic pollutants.
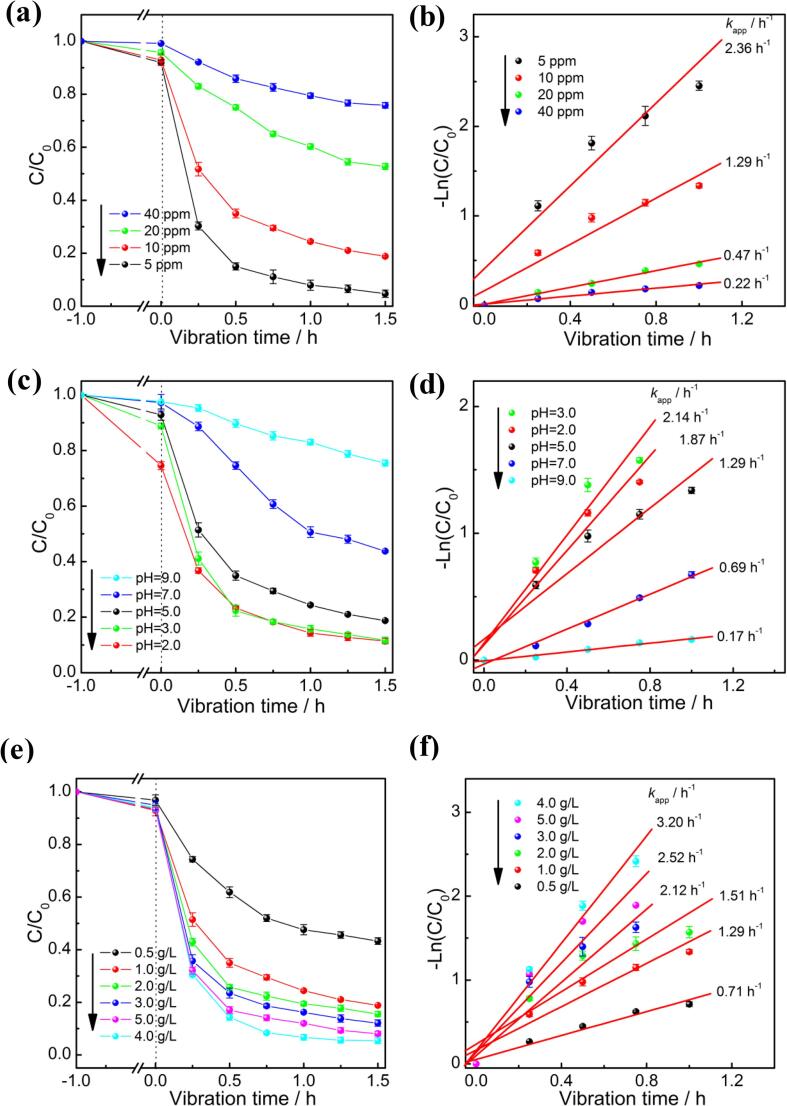
In addition to the catalyst, the reaction conditions also have a great influence on the piezoelectric catalytic reaction. Therefore, the effect of RhB content, solution pH, and catalyst dosage were investigated. Fig. 7a and b present the piezocatlaytic activity of 3CoOx/BiFeO3 composite in reaction solution containing different RhB content. As shown in Fig. 7a, RhB removal efficiency at 1.5 h is 96%, 82%, 48%, and 35% when the RhB content is 5 ppm, 10 ppm, 20 ppm, and 30 ppm, respectively. The corresponding RhB degradation rate also indicates that the increase of the RhB concentration reduces the piezocatalytic efficiency (Fig. 7b). Considering that the concentration of the reactive species keeps constant, the excessive RhB in the reaction solution will undoubtedly lead to a decrease in the piezocatalytic efficiency [48], [49]. The effect of the pH value of the reaction solution on the piezocatalytic activity of 3CoOx/BiFeO3 is shown in Fig. 7c. The acidic environment is conducive to the degradation of RhB. When the pH value decreases from 5.0 to 3.0, the piezocatalytic activity is improved and the RhB degradation rate increases to 2.14 h−1. The further decrease in pH value does not show the promotion effect, which may be due to the partial dissolution of the CoOx/BiFeO3 catalyst in a strong acid environment [50]. Correspondingly, the increase of the pH value greatly reduces the piezocatalytic efficiency. When the pH value of the reaction solution is elevated to 9.0, the RhB degradation rate obviously decreases to 0.17 h−1. Only about 15% of RhB is decolorized after ultrasonic vibration for 1.5 h. Similar phenomenon has been reported by Hengky et al [51], [52]. The different RhB adsorption capability is the reason why the piezocatalytic activity of the CoOx/BiFeO3 varies at different pH. Generally, a tight and overcrowded co-ions screening layer exists on the catalyst surface, which would hinder the adsorption of dye molecular and the subsequent degradation by charge carriers [51]. The OH– content on the catalyst surface is closely related to the thickness of the screening layer. At lower pH values, the decreased OH– concentration would reduce the screening layer and allows RhB to be directly adsorbed on the CoOx/BiFeO3 surface. The piezocatalytic degradation process is thus hastened. On the contrary, at higher pH values, the increased OH– content would prevent the piezocatalytic degradation process. The adsorption procedure in the dark provides a direct proof for the above inference. When the pH value is 2.0, the concentration of RhB drops the most when the adsorption–desorption equilibrium is reached. When the pH value is equal to 9.0, only a small amount of RhB is adsorbed.
Fig. 7.
Effect of RhB content (a,b), solution pH (c,d), and catalyst amount (e,f) on the piezocatalytic activity of 3CoOx/BiFeO3.
Fig. 7e and f display the piezocatalytic activity of 3CoOx/BiFeO3 composite with different catalyst dosages. The increase in the amount of the catalyst is usually beneficial to the RhB degradation because more active sites participate in the catalytic reaction. Hence, as the catalyst dosage increases from 0.5 g/L to 4.0 g/L, the piezocatalytic activity of the 3CoOx/BiFeO3 composite improves gradually. Correspondingly, the RhB degradation rate enhances to 3.20 h−1, and about 95% of RhB is decolorized under ultrasonic vibration for one hour. However, when the catalyst amount in the reaction solution is high (5.0 g/L), the collision between the catalysts will lead to the recombination of piezoelectric induced charges, thereby reducing the piezoelectricity [53]. Anyway, the result in Fig. 7 further indicates that the synthesized CoOx/BiFeO3 is an excellent piezocatalyst. The optimization of reaction conditions can further improve the piezocatalytic performance of the CoOx/BiFeO3. In order to reasonably evaluate the piezocatalytic activity of the CoOx/BiFeO3 catalyst, a comparison of the dye degradation performance between the prepared sample and some reported piezocatalysts is implemented [18], [19], [22], [53], [54], [55], [56], [57], [58], [59], [60]. As shown in Table 1, the CoOx/BiFeO3 works better than many piezoelectric catalysts in terms of degradation efficiency and degradation rate, indicating that it has a great potential in purifying water through piezocatalysis.
Table 1.
Comparison of the dye degradation performance of different piezocatalysts.
| Piezocatalyst | Dyes | Dye content | Ultrasonic source | Reaction time/min | Degradation efficiency/% | kapp/min−1 |
|---|---|---|---|---|---|---|
| BaTiO3[19] | MO | 5 ppm | 40 kHz, 80 W | 160 | 92 | 0.017 |
| KNbO3/ZnO [54] | MO | 10 ppm | 40 kHz, 120 W | 90 | 49 | 0.008 |
| K0.5Na0.5NbO3[53] | MB | 5 ppm | 40 kHz, 180 W | 160 | 95.7 | 0.020 |
| ZnO [18] | MB | 10 ppm | 40 kHz, 150 W | 120 | 38.0 | – |
| Ag@LiNbO3/PVDF [55] | MB | 5 ppm | 40 kHz, 70 W | 120 | 89 | 0.018 |
| BiOBr [56] | RhB | 10 ppm | 40 kHz, 120 W | 120 | 45 | 0.005 |
| BiOCl [57] | RhB | 50 ppm | 40 kHz, 120 W | 96 | 26 | 0.003 |
| NaNbO3[58] | RhB | 5 ppm | 40 kHz, 120 W | 120 | 80 | – |
| Bi0.5Na0.5TiO3[59] | RhB | 10 ppm | 28 kHz, 200 W | 60 | 75 | – |
| BaTiO3-PDMS [60] | RhB | 5 ppm | 40 kHz, 400 W | 120 | 94 | 0.040 |
| Ag/BiFeO3[22] | RhB | 10 ppm | 40 kHz, 120 W | 90 | 82 | 0.016 |
| CoOx/BiFeO3 (present work) | RhB | 10 ppm | 40 kHz, 120 W | 90 | 82 | 0.022 |
| RhB | 5 ppm | 40 kHz, 120 W | 90 | 95.7 | 0.039 |
3.3. Possible mechanism of CoOx/BiFeO3 piezocatalyst
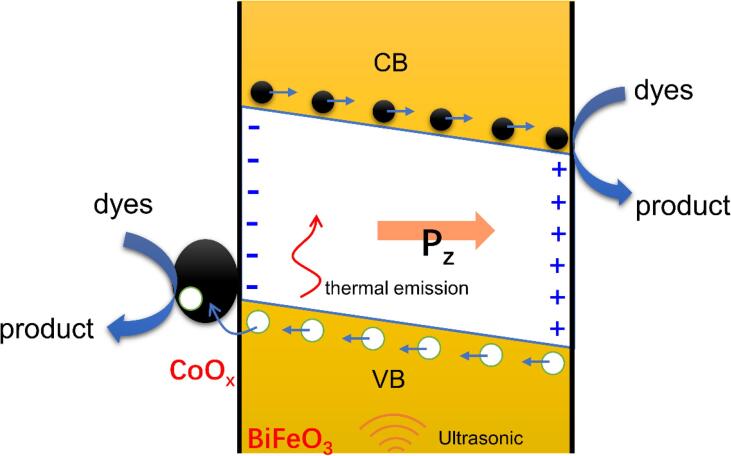
The structure characterization has clarified the composition and morphology of the synthesized CoOx/BiFeO3 piezocatalyst. CoOx nanoparticles are found to adhere to the surface of BiFeO3 nanodisks. BiFeO3 is classic piezoelectric material with good piezoelectric properties. It is no doubt that BiFeO3 can induce the piezocatalytic degradation of dyes. Under ultrasonic vibration, the cavitation effect induces a high-frequency mechanical shock on the BiFeO3 and triggers the generation of piezoelectric potential in the crystal. Meanwhile, the special morphology of the BiFeO3 makes the generation of the piezoelectric field easier because the nanodisk is easily deformed under force. Under the action of the piezoelectric potential, the thermal excited free charges are accumulated on the piezocatalyst surface and react with hydroxyl or dissolved oxygen to form reactive species, which then induces the surface piezocatalytic degradation of dyes molecules. An interesting thing is that the synergetic effect of BiFeO3 and CoOx during the piezocatalytic reaction was observed. Although Ikeda et al. reported that Co3O4 presented mechanical catalytic property in water splitting [61], our result indicates that the piezocatalytic activity of Co3O4 in RhB degradation can be ignored (Fig. S3). Additionally, considering the low content of CoOx in the CoOx/BiFeO3 composite, it is more reasonable to define CoOx as a promoter. Cobalt oxide has been proven to be an outstanding promoter for many photocatalysts [41], [42], [43], [62]. It usually acts as a hole trapper to hinder the recombination of electron-hole pairs, thereby increasing the photocatalytic activity. In the current case, we believe that CoOx may act as the same role. DRS analysis has indicated that BiFeO3 nanodisk has a band gap of 1.97 eV (Fig. S4), which is consistent with the reported value [26]. The valence band (VB) XPS analysis shows that the VB potential of BiFeO3 is estimated to be about 2.0 eV (Fig. S5). Considering that the VB position of CoOx locates at about 1.1 eV [62], it can be inferred that the free holes on the BiFeO3 can migrate to the CoOx VB, and thus promotes the separation of charge carriers (Fig. 8). This process endows the CoOx/BiFeO3 piezocatalyst high efficiency in charge separation, thereby resulting in excellent piezocatalytic activity in RhB degradation.
Fig. 8.
Possible mechanism of 3CoOx/BiFeO3 piezocatalyst under ultrasonic vibration.
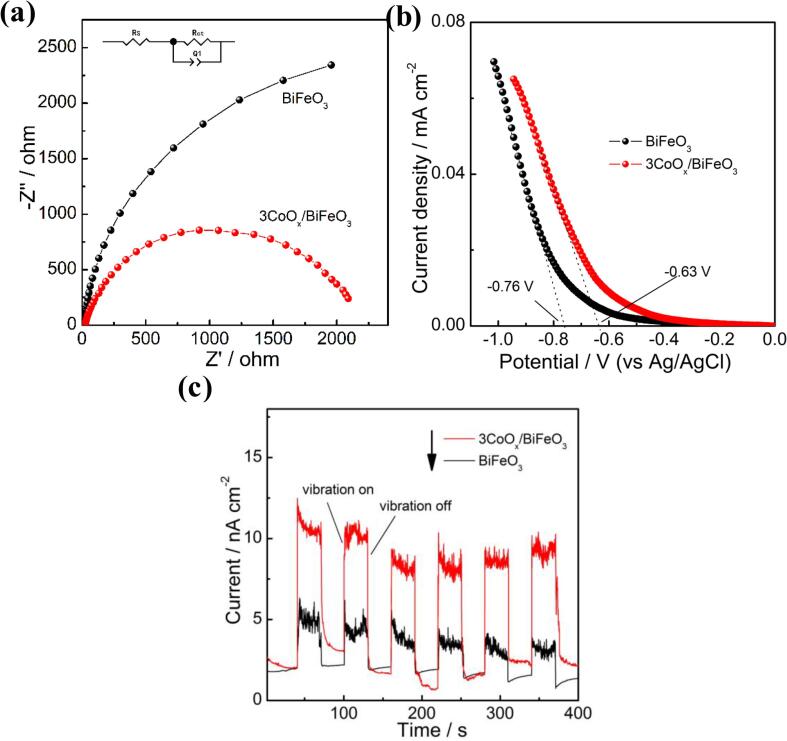
To verify the above inference, a serial of electrochemical experiments was implemented to verify the variation of charge separation in the CoOx/BiFeO3 composite. Fig. 9a shows the electrochemical impedance spectroscopy (EIS) curves of BiFeO3 and 3CoOx/BiFeO3 samples. In general, a smaller arc size of the Nyquist circles indicates a lower electron transfer interface resistance, which is conducive to the charge separation [63], [64]. The 3CoOx/BiFeO3 electrode displays a smaller arc radius than pure BiFeO3, indicating its lower interface resistance and confirming the synergistic effect of CoOx and BiFeO3. The linear sweep voltammetry (LSV) curves of the two electrodes in 0.5 M NaSO4 electrolyte provide a similar result (Fig. 9b). An overpotential of − 0.63 V is observed for the 3CoOx/BiFeO3 sample, which is lower than that of BiFeO3 (−0.76 V). This result suggests that the anchor of CoOx species on BiFeO3 nanodisk changes the electron migration and accelerates the catalytic reaction [65], [66], which is consistent with the suggested mechanism in Fig. 8.
Fig. 9.
EIS (a), LSV (b), and transient piezoelectric current response (c) profiles of BiFeO3 and 3CoOx/BiFeO3 piezocatalyst.
Fig. 9c presents the transient piezoelectric current response of BiFeO3 and 3CoOx/BiFeO3 composite under ultrasonic vibration. An obvious current response is observed under periodic ultrasonic vibration, confirming the existence of the piezoinduced charge carriers in the two electrodes. The value of the detected piezoelectric current is only a few nanoampere, which can be ascribed to the low content of heat-excited free electrons in the piezocatalyst. The 3CoOx/BiFeO3 composite generates a higher piezoelectric current than BiFeO3. This result definitely proves that the decoration of CoOx promotes the separation of piezoelectric induced charge carriers in the BiFeO3 [67], [68], and agrees well with the suggested mechanism in Fig. 8.
4. Conclusion
In summary, a novel CoOx/BiFeO3 piezocatalyst was designed and prepared via a facile photodeposition method. CoOx nanoparticles are anchored on the surface of BiFeO3 nanodisk. Under ultrasonic vibration, the generated piezoelectric field in the BiFeO3 triggers the heat-excited free electron and hole accumulation on the catalyst surface. The holes are further directionally migrated to the CoOx nanoparticles, thereby resulting in a much higher charge separation efficiency. As a result, the CoOx/BiFeO3 piezocatalyst exhibits much higher efficiency than BiFeO3 in the piezocatalytic degradation of RhB. The CoOx/BiFeO3 composite also presents high stability and adaptability for different dyes. Via optimizing the reaction conditions, the piezocatalytic performance of CoOx/BiFeO3 can be further increased several times. The work not only offers an efficient piezocatalyst for water purification, but also provides a new paradigm for the design of high-efficiency mechanical energy conversion systems.
CRediT authorship contribution statement
Linkun Wang: Writing – original draft, Investigation, Formal analysis, Data curation. Junfeng Wang: Investigation, Validation. Chenyin Ye: Investigation. Kaiqi Wang: Investigation. Chunran Zhao: Validation. Ying Wu: Conceptualization, Resources, Methodology, Writing – review & editing. Yiming He: Conceptualization, Supervision, Project administration, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The work was financially supported by National Natural Science Foundation of China (Grant No. 22172144) and Nature Science Foundation of Zhejiang Province (Grant No. LY20B030004).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2021.105813.
Contributor Information
Ying Wu, Email: yingwu@zjnu.cn.
Yiming He, Email: hym@zjnu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Gholami P., Dinpazhoh L., Khataee A., Hassani A., Bhatnagar A. Facile hydrothermal synthesis of novel Fe-Cu layered double hydroxide/biochar nanocomposite with enhanced sonocatalytic activity for degradation of cefazolin sodium. J. Hazard. Mater. 2020;381 doi: 10.1016/j.jhazmat.2019.120742. [DOI] [PubMed] [Google Scholar]
- 2.Hassandoost R., Pouran S.R., Khataee A., Orooji Y., Joo S.W. Hierarchically structured ternary heterojunctions based on Ce3+/Ce4+ modified Fe3O4 nanoparticles anchored onto graphene oxide sheets as magnetic visible-light-active photocatalysts for decontamination of oxytetracycline. J. Hazard. Mater. 2019;376:200–211. doi: 10.1016/j.jhazmat.2019.05.035. [DOI] [PubMed] [Google Scholar]
- 3.Ghasemi M., Khataee A., Gholami P., Soltani R.D.C., Hassani A., Orooji Y. In-situ electro-generation and activation of hydrogen peroxide using a CuFeNLDH-CNTs modified graphite cathode for degradation of cefazolin. J. Environ. Manage. 2020;267 doi: 10.1016/j.jenvman.2020.110629. [DOI] [PubMed] [Google Scholar]
- 4.Kanakaraju D., Glass B.D., Oelgemoller M. Advanced oxidation process-mediated removal of pharmaceuticals from water: A review. J. Environ. Manage. 2018;219:189–207. doi: 10.1016/j.jenvman.2018.04.103. [DOI] [PubMed] [Google Scholar]
- 5.Cherifi Y., Addad A., Vezin H., Barras A., Ouddane B., Chaouchi A., Szunerits S., Boukherroub R. PMS activation using reduced graphene oxide under sonication: Efficient metal-free catalytic system for the degradation of rhodamine B, bisphenol A, and tetracycline. Ultrason. Sonochem. 2019;52:164–175. doi: 10.1016/j.ultsonch.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 6.Joseph C.G., Li Puma G., Bono A., Krishnaiah D. Duduku Krishnaiah, Sonophotocatalysis in advanced oxidation process: a short review. Ultrason. Sonochem. 2009;16(5):583–589. doi: 10.1016/j.ultsonch.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Liang Z., Yan C.-F., Rtimi S., Bandara J. Piezoelectric materials for catalytic/photocatalytic removal of pollutants: recent advances and outlook. Appl. Catal. B: Environ. 2019;241:256–269. [Google Scholar]
- 8.Xu X., Jia Y., Xiao L., Wu Z. Strong vibration-catalysis of ZnO nanorods for dye wastewater decolorization via piezo-electro-chemical coupling. Chemosphere. 2018;193:1143–1148. doi: 10.1016/j.chemosphere.2017.11.116. [DOI] [PubMed] [Google Scholar]
- 9.Khataee A., Karimi A., Arefi-Oskoui S., Darvishi Cheshmeh Soltani R., Hanifehpour Y., Soltani B., Joo S.W. Sonochemical synthesis of Pr-doped ZnO nanoparticles for sonocatalytic degradation of Acid Red 17. Ultrason. Sonochem. 2015;22:371–381. doi: 10.1016/j.ultsonch.2014.05.023. [DOI] [PubMed] [Google Scholar]
- 10.Xu X., Wu Z., Xiao L., Jia Y., Ma J., Wang F., Wang L., Wang M., Huang H. Strong piezo-electro-chemical effect of piezoelectric BaTiO3 nanofibers for vibration-catalysis. J. Alloy Compd. 2018;762:915–921. [Google Scholar]
- 11.Lin E., Wu J., Qin N., Yuan B., Bao D. Silver modified barium titanate as a highly efficient piezocatalyst. Catal. Sci. Technol. 2018;8:4788–4796. [Google Scholar]
- 12.Lin H., Wu Z., Jia Y.M., Li W.J., Zheng R.K., Luo H.S. Piezoelectrically induced mechano-catalytic effect for degradation of dye wastewater through vibrating Pb(Zr0.52Ti0.48)O3 fibers. Appl. Phys. Lett. 2014;104 [Google Scholar]
- 13.Jin C.C., Liu D.M., Li M., Wang Y., He Z.W., Xu M.X., Li X., Ying H.T., Wu Y.T., Zhang Q. Preparation of multifunctional PLZT nanowires and their applications in piezocatalysis and transparent flexible films. J. Alloy Compd. 2019;811 [Google Scholar]
- 14.Tu S.C., Guo Y.X., Zhang Y.H., Hu C., Zhang T.R., Ma T.Y., Huang H.W. Piezocatalysis and piezo-photocatalysis: Catalysts classification and modification strategy, reaction mechanism, and practical application. Adv. Funct. Mater. 2020;30:2005158. [Google Scholar]
- 15.Lin Y.T., Lai S.N., Wu J.M. Simultaneous Piezoelectrocatalytic hydrogen-evolution and degradation of water pollutants by quartz microrods@ few-layered MoS2 hierarchical heterostructures. Adv. Mater. 2020;32:2002875. doi: 10.1002/adma.202002875. [DOI] [PubMed] [Google Scholar]
- 16.Chung Y.J., Yang C.S., Lee J.T., Wu G.H., Wu J.M. Coupling effect of piezo–flexocatalytic hydrogen evolution with hybrid 1T-and 2H-phase few-layered MoSe2 nanosheets. Adv. Energy Mat. 2020;10:2002082. [Google Scholar]
- 17.Li X., Wang J., Zhang J., Zhao C., Wu Y., He Y. Cadmium sulfide modified zinc oxide heterojunction harvesting ultrasonic mechanical energy for efficient decomposition of dye wastewater. J. Colloid Interf. Sci. 2022;607:412–422. doi: 10.1016/j.jcis.2021.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Ning X., Hao A., Cao Y., Hu J., Xie J., Jia D. Effective promoting piezocatalytic property of zinc oxide for degradation of organic pollutants and insight into piezocatalytic mechanism. J. Colloid Interf. Sci. 2020;577:290–299. doi: 10.1016/j.jcis.2020.05.082. [DOI] [PubMed] [Google Scholar]
- 19.Wu J., Qin N.i., Bao D. Effective enhancement of piezocatalytic activity of BaTiO3 nanowires under ultrasonic vibration. Nano Energy. 2018;45:44–51. [Google Scholar]
- 20.Bai Y., Zhao J., Lv Z., Lu K. Enhanced piezocatalytic performance of ZnO nanosheet microspheres by enriching the surface oxygen vacancies. J. Mater. Sci. 2020;55(29):14112–14124. [Google Scholar]
- 21.Zhou X.F., Yan F., Wu S.H., Shen B., Zeng H.R., Zhai J.W. Remarkable piezophoto coupling catalysis behavior of BiOX/BaTiO3 (X = Cl, Br, Cl0.166Br 0.834) piezoelectric composites. Small. 2020;16:2001573. doi: 10.1002/smll.202001573. [DOI] [PubMed] [Google Scholar]
- 22.Li Z.Y., Zhang Q.L., Wang L.K., Yang J.Y., Wu Y., He Y.M. Novel application of Ag/PbBiO2I nanocomposite in piezocatalytic degradation of rhodamine B via harvesting ultrasonic vibration energy. Ultrason. Sonochem. 2021;78 doi: 10.1016/j.ultsonch.2021.105729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Catalan G., Scott J.F. Physics and applications of bismuth ferrite. Adv. Mater. 2009;21(24):2463–2485. [Google Scholar]
- 24.X. Zhu Q. Hang Z. Xing Y. Yang J. Zhu Z. Liu N. Ming P. Zhou Y.e. Song Z. Li T. Yu Z. Zou Microwave hydrothermal synthesis, structural characterization, and visible-light photocatalytic activities of single-crystalline bismuth ferric nanocrystals 94 8 2011 2688 2693.
- 25.You H., Wu Z., Zhang L., Ying Y., Liu Y., Fei L., Chen X., Jia Y., Wang Y., Wang F., Ju S., Qiao J., Lam C.-H., Huang H. Harvesting the vibration energy of BiFeO3 nanosheets for hydrogen evolution. Angew. Chem. Int. Ed. 2019;58(34):11779–11784. doi: 10.1002/anie.201906181. [DOI] [PubMed] [Google Scholar]
- 26.Mushtaq F., Chen X.Z., Hoop M., Torlakcik H., Pellicer E., Sort J., Gattinoni C., Nelson B.J., Pane S. Piezoelectrically enhanced photocatalysis with BiFeO3 nanostructures for efficient water remediation. iScience. 2018;4:236–246. doi: 10.1016/j.isci.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu J., Qin T.J., Chen W.M., Lv J.B., Zeng X.R., Sun J.Y., Li Y.Y., Zhou J. Synergizing piezoelectric and plasmonic modulation of Ag/BiFeO3 fbrous heterostructure toward boosted photoelectrochemical energy conversion. Nano Energy. 2021;89 [Google Scholar]
- 28.Liu Y.-L., Wu J.M. Synergistically catalytic activities of BiFeO3/TiO2 core-shell nanocomposites for degradation of organic dye molecule through piezophototronic effect. Nano Energy. 2019;56:74–81. [Google Scholar]
- 29.Jo W.K., Kumar S., Isaacs M.A., Lee A.F., Karthikeyan S. Cobalt promoted TiO2/GO for the photocatalytic degradation of oxytetracycline and Congo Red. Appl. Catal. B Environ. 2017;201:159–168. [Google Scholar]
- 30.Kanakkillam S.S., Shaji S., Krishnan B., Vazquez-Rodriguez S., Aguilar Martinez J.A., Palma M.I.M., Avellaneda D.A. Nanoflakes of zinc oxide:cobalt oxide composites by pulsed laser fragmentation for visible light photocatalysis. Appl. Surf. Sci. 2020;501 [Google Scholar]
- 31.Zheng J., Zhang L. Designing 3D magnetic peony flower-like cobalt oxides/g-C3N4 dual Z-scheme photocatalyst for remarkably enhanced sunlight driven photocatalytic redox activity. Chem. Eng. J. 2019;369:947–956. [Google Scholar]
- 32.Hisatomi T., Katayama C., Moriya Y., Minegishi T., Katayama M., Nishiyama H., Yamada T., Domen K. Photocatalytic oxygen evolution using BaNbO2N modified with cobalt oxide under photoexcitation up to 740 nm. Energy Environ. Sci. 2013;6(12):3595. doi: 10.1039/c3ee42951b. [DOI] [Google Scholar]
- 33.Shi X., Quan S., Yang L., Shi G., Shi F. Facile synthesis of magnetic Co3O4/BFO nanocomposite for effective reduction of nitrophenol isomers. Chemosphere. 2019;219:914–922. doi: 10.1016/j.chemosphere.2018.12.045. [DOI] [PubMed] [Google Scholar]
- 34.Raju T.D., Veeralingam S., Badhulika S. Polyvinylidene fluoride/ZnSnO3 nanocube/Co3O4 nanoparticle thermoplastic composites for ultrasound-assisted piezo-catalytic dye degradation. ACS Appl. Nano Mater. 2020;3(5):4777–4787. [Google Scholar]
- 35.Wu H., Zhu X. Microstructures, magnetic, and dielectric properties of Ba-doped BiFeO3 nanoparticles synthesized via molten salt route. J Am. Ceram. Soc. 2019;102(8):4698–4709. [Google Scholar]
- 36.Ji X.u., Cheng S., Yang L., Jiang Y.u., Jiang Z.-J., Yang C., Zhang H., Liu M. Phase transition–induced electrochemical performance enhancement of hierarchical CoCO3/CoO nanostructure for pseudocapacitor electrode. Nano Energy. 2015;11:736–745. [Google Scholar]
- 37.Letsholathebe D., Them F.T., Mphale K., Mohamed H.E.A., Holonga K.J., Ketlhwaafetse R., Chimidza S. Optical and structural stability of Co3O4 nanoparticles for photocatalytic applications. Mater. Today: Pro. 2021;36:499–503. [Google Scholar]
- 38.Chen L., Dai X.Q., Li X.J., Wang J.F., Chen H.F., Hu X., Lin H.J., He Y.M., Wu Y., Fan M.H. Novel Bi2S3/KTa0.75Nb0.25O3 nanocomposite with high efficiency for photocatalytic and piezocatalytic N2 fixation. J. Mater. Chem. A. 2021;9:13344–13354. [Google Scholar]
- 39.Yin J., Liao G., Zhou J., Huang C., Ling Y.u., Lu P., Li L. High performance of magnetic BiFeO3 nanoparticle-mediated photocatalytic ozonation for wastewater decontamination. Sep. Purif. Technol. 2016;168:134–140. [Google Scholar]
- 40.Zhang W., Xing P., Zhang J., Chen L.u., Yang J., Hu X., Zhao L., Wu Y., He Y. Facile preparation of novel nickel sulfide modified KNbO3 heterojunction composite and its enhanced performance in photocatalytic nitrogen fixation. J. Colloid Interf. Sci. 2021;590:548–560. doi: 10.1016/j.jcis.2021.01.086. [DOI] [PubMed] [Google Scholar]
- 41.Han C.C., Ge L., Chen C.F., Li Y.J., Xiao X.L., Zhang Y.N., Guo L.L. Novel visible light induced Co3O4-g-C3N4 heterojunction photocatalysts for efficient degradation of methyl orange. Appl. Catal. B Environ. 2014;147:546–553. [Google Scholar]
- 42.Li L.i., She X., Yi J., Pan L.i., Xia K., Wei W., Zhu X., Chen Z., Xu H., Li H. Integrating CoOx cocatalyst on hexagonal α-Fe2O3 for effective photocatalytic oxygen evolution. Appl. Surf. Sci. 2019;469:933–940. [Google Scholar]
- 43.Zhang J., Yu Z., Gao Z., Ge H., Zhao S., Chen C., Chen S., Tong X., Wang M., Zheng Z., Qin Y. Porous TiO2 nanotubes with spatially separated platinum and CoOx cocatalysts produced by atomic layer deposition for photocatalytic hydrogen production. Angew. Chem. Int. Ed. 2017;56(3):816–820. doi: 10.1002/anie.201611137. [DOI] [PubMed] [Google Scholar]
- 44.H.H. Zhong, L.A. Estudillo-Wong, Y. Gao, Y.J. Feng, Ni. Alonso-Vante, Oxygen vacancies engineering by coordinating oxygen-buffering CeO2 with CoOx nanorods as efficient bifunctional oxygen electrode electrocatalyst, J. Energy Chem. 59 (2021) 615–625.
- 45.Kang M., Song M.W., Lee C.H. Catalytic carbon monoxide oxidation over CoOx/CeO2 composite catalysts. Appl. Catal. A: Gen. 2003;251:143–156. [Google Scholar]
- 46.Xing P.X., Wu S.J., Chen Y.J., Chen P.F., Hu X., Lin H.J., Zhao L.H., He Y.M. New application and excellent performance of Ag/KNbO3 nanocomposite in photocatalytic NH3 synthesis. ACS Sustain. Chem. Eng. 2019;7:12408–12418. [Google Scholar]
- 47.Xing P.X., Zhang W.Q., Chen L., Dai X.Q., Zhang J.L., Zhao L.H., He Y.M. Preparation of NiO/KNbO3 nanocomposite via photodeposition method and its superior performance in photocatalytic N2 fixation. Sustain. Energy Fuels. 2020;4:1112–1117. [Google Scholar]
- 48.Li Y.J., Sun S.G., Ma M.Y., Ouyang Y.Z., Yan W.B. Kinetic study and model of the photocatalytic degradation of rhodamine B (RhB) by a TiO2-coated activated carbon catalyst: Effects of initial RhB content, light intensity and TiO2 content in the catalyst. Chem. Eng. J. 2008;142:147–155. [Google Scholar]
- 49.Gong Cheng, Chen Fei, Yang Qi, Luo Kun, Yao Fubing, Wang Shana, Wang Xiaolin, Wu Jiawei, Li Xiaoming, Wang Dongbo, Zeng Guangming. Heterogeneous activation of peroxymonosulfate by Fe-Co layered doubled hydroxide for efficient catalytic degradation of Rhoadmine B. Chem. Eng. J. 2017;321:222–232. [Google Scholar]
- 50.Krishnakumar B., Subash B., Swaminathan M. AgBr-ZnO An efficient nano-photocatalyst for the mineralization of Acid Black 1 with UV light. Sep. Purif. Technol. 2012;85:35–44. doi: 10.1016/j.saa.2012.12.035. [DOI] [PubMed] [Google Scholar]
- 51.Hengky Chang, Moya Xavier, Mathur Neil D., Dunn Steve. Evidence of high rate visible light photochemical decolourisation of Rhodamine B with BiFeO3 nanoparticles associated with BiFeO3 photocorrosion. RSC Adv. 2012;2(31):11843. doi: 10.1039/c2ra22211f. [DOI] [Google Scholar]
- 52.Song Zhou, Wang Nan, Zhu Lihua, Huang Aizhen, Zhao Xiaorong, Tang Heqing. Efficient oxidative degradation of triclosan by using an enhanced Fenton-like process. Chem. Eng. J. 2012;198-199:379–387. [Google Scholar]
- 53.Zhang An, Liu Zhiyong, Geng Xinhui, Song Wenfeng, Lu Jinshan, Xie Bing, Ke Shanming, Shu Longlong. Ultrasonic vibration driven piezocatalytic activity of lead-free K0.5Na0.5NbO3 materials. Ceram. Int. 2019;45(17):22486–22492. [Google Scholar]
- 54.Li Y., Chen H.F., Wang L.K., Wu T.T., Wu Y., He Y.M. KNbO3/ZnO heterojunction harvesting ultrasonic mechanical energy and solar energy to efficiently degrade methyl orange. Ultrason. Sonochem. 2021;78 doi: 10.1016/j.ultsonch.2021.105754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qian Weiqi, Zhao Kun, Zhang Ding, Bowen Chris R., Wang Yuanhao, Yang Ya. Piezoelectric material-polymer composite porous foam for efficient dye degradation via the piezo-catalytic effect. ACS Appl. Mater. Interfaces. 2019;11(31):27862–27869. doi: 10.1021/acsami.9b07857. [DOI] [PubMed] [Google Scholar]
- 56.Lei H., Zhang H., Zou Y., Dong X., Jia Y., Wang F. Synergetic photocatalysis/piezocatalysis of bismuth oxybromide for degradation of organic pollutants. J. Alloys Compd. 2019;809 [Google Scholar]
- 57.Ismail Muhammad, Wu Zheng, Zhang Luohong, Ma Jiangping, Jia Yanmin, Hu Yongming, Wang Yaojin. High-efficient synergy of piezocatalysis and photocatalysis in bismuth oxychloride nanomaterial for dye decomposition. Chemosphere. 2019;228:212–218. doi: 10.1016/j.chemosphere.2019.04.121. [DOI] [PubMed] [Google Scholar]
- 58.Wang Shensong, Wu Zheng, Chen Jie, Ma Jiangping, Ying Jingshi, Cui Shouchen, Yu Shigang, Hu Yongming, Zhao Jinhe, Jia Yanmin. Lead-free sodium niobate nanowires with strong piezo-catalysis for dye wastewater degradation. Ceram. Int. 2019;45(9):11703–11708. [Google Scholar]
- 59.Zhou X.F., Sun Q.W., Zhai D., Xue G.L., Luo H., Zhang D. Excellent catalytic performance of molten-salt-synthesized Bi0.5Na0.5TiO3 nanorods by the piezo-phototronic coupling effect. Nano Energy. 2021;84 [Google Scholar]
- 60.Singh Gurpreet, Sharma Moolchand, Vaish Rahul. Flexible Ag@LiNbO3/PVDF composite film for piezocatalytic dye/pharmaceutical degradation and bacterial disinfection. ACS Appl. Mater. Interfaces. 2021;13(19):22914–22925. doi: 10.1021/acsami.1c01314. [DOI] [PubMed] [Google Scholar]
- 61.Ikeda Shigeru, Takata Tsuyoshi, Kondo Takeshi, Hitoki Go, Hara Michikazu, Kondo Junko N., Domen Kazunari, Hosono Hideo, Kawazoe Hiroshi, Tanaka Akira. Mechano-catalytic overall water splitting. Chem. Commun. 1998;(20):2185–2186. doi: 10.1039/a804549f. [DOI] [Google Scholar]
- 62.Liu Y., Ding S.P., Shi Y.Q., Liu X.F., Wu Z.Z., Jiang Q.Q., Zhou T.F., Liu N.K., Hu J.C. Construction of CdS/CoOx core-shell nanorods for efficient photocatalytic H2 evolution. Appl. Catal. B Environ. 2018;234:109–116. [Google Scholar]
- 63.Chen L., Zhang W.Q., Wang J.F., Li X.J., Li Y., Hu X., Zhao L.H., Wu Y., He Y.M. High Piezo/photocatalytic efficiency of Ag/Bi5O7I nanocomposite using mechanical and solar energy for N2 fixation and methyl orange degradation. Green Energy Environ. 2021 doi: 10.1016/j.gee.2021.04.009. [DOI] [Google Scholar]
- 64.Maimaitizi H., Abulizi A., Zhang T., Okitsu K., Zhu J.J. Facile photo-ultrasonic assisted synthesis of flower-like Pt/N-MoS2 microsphere as an efficient sonophotocatalyst for nitrogen fixation. Ultrason. Sonochem. 2020;63 doi: 10.1016/j.ultsonch.2019.104956. [DOI] [PubMed] [Google Scholar]
- 65.El-Bery H.M., Abdelhamid H.N. Photocatalytic hydrogen generation via water splitting using ZIF-67 derived Co3O4@C/TiO2. J. Environ. Chem. Eng. 2021;9 [Google Scholar]
- 66.Dai Xiaoquan, Chen Lu, Li Ziyu, Li Xiaojing, Wang Junfeng, Hu Xin, Zhao Leihong, Jia Yanmin, Sun Shi-Xin, Wu Ying, He Yiming. CuS/KTa0.75Nb0.25O3 nanocomposite utilizing solar and mechanical energy for catalytic N2 fixation. J Colloid Interf. Sci. 2021;603:220–232. doi: 10.1016/j.jcis.2021.06.107. [DOI] [PubMed] [Google Scholar]
- 67.Tian W.R., Qiu J.H., Li N.J., Chen D.Y., Xu Q.F., Li H., He J.H., Lu J.M. Efficient piezocatalytic removal of BPA and Cr(VI) with SnS2/CNFs membrane by harvesting vibration energy. Nano Energy. 2021;86 [Google Scholar]
- 68.Cheng Lili, Huang Danyao, Zhang Yun, Wu Ying. Preparation and piezoelectric catalytic performance of HT-Bi2MoO6 microspheres for dye degradation. Adv. Powder Technol. 2021;32(9):3346–3354. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.