Abstract
This study was conducted to investigate the influence of zinc (Zn) supplementation on growth performance, intestinal development and intestinal barrier function in Pekin ducks. A total of 480, one-day-old male Pekin ducks were divided into 6 groups with 8 replicates: 0 mg/kg Zn, 0 mg/kg Zn +0.5 mg/kg lipopolysaccharide (LPS), 30 mg/kg Zn, 30 mg/kg Zn +0.5 mg/kg LPS, 120 mg/kg Zn, 120 mg/kg Zn +0.5 mg/kg LPS. The duck primary intestinal epithelial cells (DIECs) were divided into 6 groups: D-Zn (Zinc deficiency, treated with 2 µmol/L zinc Chelator TPEN), A-Zn (Adequate Zinc, basal medium), H-Zn (High level of Zn, supplemented with 20 µmol/L Zn), D-Zn + 20 µg/mL LPS, A-Zn + 20 µg/mL LPS, H-Zn + 20 µg/mL LPS. The results were as follows: in vivo, with Zn supplementation of 120 mg/kg reduced LPS-induced decrease of growth performance and intestine damage (P < 0.05), and increased intestinal digestive enzyme activity of Pekin ducks (P < 0.05). In addition, Zn supplementation also attenuated LPS-induced intestinal epithelium permeability (P < 0.05), inhibited LPS-induced the expression of proinflammatory cytokines and apoptosis-related genes (P < 0.05), as well as reduced LPS-induced the intestinal stem cells mobilization of Pekin ducks (P < 0.05). In vitro, 20 µmol/L Zn inhibited LPS-induced expression of inflammatory factors and apoptosis-related genes (P < 0.05), promoted the expression of cytoprotection-related genes, and attenuated LPS-induced intestinal epithelium permeability in DIECs (P < 0.05). Mechanistically, 20 µmol/L Zn enhanced tight junction protein markers including CLDN-1, OCLD, and ZO-1 both at protein and mRNA levels (P < 0.05), and also increased the level of phosphorylation of TOR protein (P < 0.05) and activated the TOR signaling pathway. In conclusion, Zn improves growth performance, digestive enzyme activity, and intestinal barrier function of Pekin ducks. Importantly, Zn also reverses LPS-induced intestinal barrier damage via enhancing the expression of tight junction proteins and activating the TOR signaling pathway.
Key words: zinc, Pekin duck, growth performance, intestinal barrier, intestinal inflammation
INTROCUTION
Zinc (Zn) is an essential trace element for animals. In human proteins, there are 2,800 kinds of Zn-binding sites, accounting for about 10% of the human proteome (Andreini et al., 2006). Zn-binding protein can play an important role in life activities as metalloenzymes, growth factors, receptors and transcription factors (Andreini and Bertini, 2012). Since the 1950s, people have gradually realized that Zn is an essential trace element for poultry (Morrison and Sarett, 1958). Research on broilers found that among the 4 trace elements tested (copper, iron, manganese, and zinc), Zn is the first restrictive trace element that affects growth performance (Bao et al., 2010a). Zinc is used as a dietary additive to improve animal production performance, reproductive performance, immune function, and maintain the normal development of feathers and bones (Salim et al., 2008; Abd El-Hack et al., 2017).
In addition to being responsible for the digestion and absorption of nutrients, animal intestines are also an important barrier to prevent harmful substances from entering the body (Abo Ghanima et al., 2020). Intestinal barrier function disorder can cause intestinal antigens, harmful macromolecular substances, and intestinal bacteria to enter the animal body through paracellular route, causing enteritis and metabolic diseases (Vereecke et al., 2011; Halpern and Denning, 2015). Damage of intestinal epithelial tight junction barrier caused by pathogen contributes to the development of inflammatory bowel disease (IBD) and necrotizing enterocolitis (NEC) by an increase in intestinal permeability (Chen et al., 2015; Guo et al., 2015). Lipopolysaccharide (LPS) plays an important role in inducing intestinal and systemic inflammatory responses (Tan et al., 2015; d'Hennezel et al., 2017).
Zn plays an important role in human and animal intestinal health. When Zn is deficient, it can cause changes in the expression of tight junctions in the animal's intestines, and finally lead to changes in the permeability of the intestinal mucosa and the occurrence of many gastrointestinal diseases (Skrovanek et al., 2014). High doses of Zn can be used to control ethanol-induced intestinal leakage in rats (Zhong et al., 2015), post-weaning diarrhea in piglets (Zhang and Guo, 2009), and inflammatory enteritis in humans (Sturniolo et al., 2001). Studies have found that Zn supplementation improved the intestinal morphology of livestock and poultry and increased the expression of tight junction proteins in the intestine (Zhang and Guo, 2009; Hu et al., 2013). The effect of Zn on improving intestinal tight junctions has also been confirmed experimentally (Ranaldi et al., 2009). But its mechanistic effect on intestinal barrier function is not clear. Although meat duck breeding occupies an important position in poultry production, the research on the trace element nutrition of meat ducks is still relatively lacking. Early studies have found that Zn is an essential trace element for ducks (Wight and Dewar, 1976). Zn deficiency can cause growth retardation, multiple organ damage, feather hypoplasia, and severely affect the development and function of duck lymphatic organs (Cui et al., 2003; Cui et al., 2004). However, it has not been reported whether the level of dietary Zn has a protective effect on the intestinal barrier function of Pekin ducks. Therefore, this study intends to explore the effects of Zn supplementation on their growth performance, intestinal development, and intestinal barrier function-related gene expression under LPS stress. In addition, through the establishment of duck primary intestinal epithelial cells (DIECs) model, the protective effect of Zn on the intestinal barrier function of Pekin ducks and its mechanism were explored.
MATERIALS AND METHODS
Materials
Twenty-day-old Pekin duck embryos and 1-day-old male Pekin ducks were purchased from Sichuan Mianying Duck Industry Co., Ltd. (Mianyang, China). ZnSO4·H2O, TPEN, LPS (Lipopolysaccharides from Escherichia coli O55:B5) and FITC-D (MW 3–5 kDa; Sigma, St. Louis, MO) were obtained from Sigma-Aldrich (Saint Louis, MO). TOR and β-actin were obtained from Thermo Fisher Scientific Co., Ltd. (Shanghai, China).
Animals and Diets
The experimental procedures for animal trials were conducted in accordance with the Chinese guidelines for animal welfare and approved by the Animal Health and Care Committee of Sichuan Agricultural University (SICAU 2009-0135).
A total of 480 one-day-old male Pekin ducks were obtained from commercial hatchery. The ducks were reared in cages (2.0 × 1.0 m) in temperature-controlled room and maintained on a 24-h constant light schedule, and allowed free access to feed and water. The basal diet was formulated based on the National Research Council (1994). The basal diet meets or exceeds the requirement of Pekin ducks except Zn (Table S1). The measured concentration of Zn in each diet is displayed in Table S2. The ducks were randomly allocated into 6 treatments, 8 replicates for each treatment and 10 ducks for each replicate: 0 mg/kg Zn, 0 mg/kg Zn +0.5 mg/kg LPS, 30 mg/kg Zn, 30 mg/kg Zn +0.5 mg/kg LPS, 120 mg/kg Zn, 120 mg/kg Zn +0.5 mg/kg LPS. LPS was intraperitoneal injected to ducks at d 15, 17, 19, and 21. The nonchallenged groups were intraperitoneal injected with saline. Ducks were gavaged with FITC-D to evaluate the intestinal permeability on d 21. The samples were collected 4 h after last injection.
Data and Sample Collection
The weight of the duck and the feed consumption of feed was determined every stage throughout the experiment to calculate the body weight (BW), body weight gain (BWG), feed intake (FI), and feed to gain ratio (F/G). Then, 1.5 h after the last intraperitoneal injection of LPS, 1 duck with the average BW of each pen was chosen to administer FITC-D (4.16 mg/kg, dissolved in normal saline). After administering FITC-D for 2.5 h, blood was collected from the jugular vein to separate the serum and stored at −20°C until analysis. Then tissue samples including jejunum and jejunal mucosa were collected and immediately frozen with liquid nitrogen and stored at −80°C for RNA isolation and enzymatic activity analysis after the Pekin duck died of cervical dislocation. In addition, jejunum segments 1.5 cm in length (midway between the point of entry of the bile ducts and Meckel's diverticulum) were flushed with saline (0.9% NaCl) and fixed in 100 g/L buffered formalin (pH = 7.0) for morphology examination.
Jejunum Morphometry
The fixed intestinal samples were embedded in paraffin, then sectioned (5 μm) and stained with hematoxylin and eosin, and 20 villi in per section were examined by a light microscope (Olympus CX31, Tokyo, Japan) (El-Shall et al., 2019). The villus height (VH), crypt depth (CD), and villus height to crypt depth ratio (VCR) were determined according to Wen et al. (2018).
Determination of MUC2 Content and Enzyme Activity
The intestinal mucosa and the saline added to homogenizer in the mass ratio of 1:9 for manual grinding, and then the intestinal mucosal homogenate was prepared using an ultrasonic cell disruptor. The prepared homogenate was centrifuged at 3,000 r/min for 30 min at 4°C, and the supernatant was collected for determination of protein content and enzyme activity. The concentration of intestinal mucosal protein was detected by the BCA protein assay method. The enzyme activity was determined by colorimetric method according to the test procedure (Chen et al., 2014).
Determination of FITC-D and D-Lactate in Blood
The normal treatment group duck serum and PBS were used to prepare a 1:1 mixed solvent to prepare FITC-D concentration gradient standard solution, the fluorescence intensity of the standard solution was measured by multi-purpose microplate reader, and the FITC-D content of the sample was calculated by the standard curve. D-lactic acid in serum was measured using Shanghai enzyme-linked kit following the manufacturer's instructions (Meyer et al., 2020).
DIECs Culture and Grouping Treatment
DIECs (Duck primary intestinal epithelial cells isolated from 21 d duck embryo jejunum) were cultured in DMEM/F-12, supplemented with 10% FBS, at 37°C in a humidified atmosphere that contained 5% CO2. Cells formed a confluent monolayer after 2 d and were then used in the following experiments.
This experiment uses primary culture of DIECs as the experimental subject. A 3 × 2 trial design was used with 3 Zn levels. A total of 6 groups: D-Zn (Zinc deficiency, treated with 2 µmol/L zinc Chelator TPEN), A-Zn (Adequate Zinc, basal medium), H-Zn (High level of Zn, supplemented with 20 µmol/L Zn), D-Zn + 20 µg/mL LPS, A-Zn + 20 µg/mL LPS, H-Zn + 20 µg/mL LPS.
Cell Viability Tested by CCK-8 Assay
Cell viability was determined using a CCK-8 cell viability assay kit. All cells were pretreated with cultured methods as indicated for 48 h in a 96-well plate. 10 uL of cell viability assay kit solution was added to each well of the plate. After incubation for 1 h at 37°C in the dark, absorbances were measured at 450 nm using a multiwell plate reader (Lou et al., 2010).
Transepithelial Electrical Resistance
DIECs were seeded (1 × 105 cells/cm2) in a transwell chamber with 4.5-μm pores that had been placed in a 6-well plate. The other one transwell remained blank. After being confluent, cells were differentiated and polarized for 7 to 10 d in the culture medium. Transepithelial electrical resistance (TEER) was measured using an epithelial volt-ohm meter with a chopstick electrode. The electrode was immersed at a 90° angle with one tip in the basolateral chamber and the other in the apical chamber. An insert without cells was used as a blank and its mean resistance was subtracted from all samples. Unit area resistance was then calculated by dividing resistance values by the effective membrane area (4.5 cm2) ( He et al., 2019).
Quantitative Real-Time PCR
Real-time quantitative PCR was conducted using the primers listed in Table S3. The PCR system consisted of 5.0 μL of SYBR Green qPCR Mix, 0.2μL of cDNA, 0.3 μL of each primer, and 4.2 μL of double distilled water in a final volume of 20 μL. The housekeeping gene β-actin was served as a control to normalize the mRNA expression level. Relative quantities of mRNA were calculated using the 2−ΔΔCt method (Zhang et al., 2014).
Western Blot Assay
The method used for the Western blot assay has been described previously (Chen et al., 2016). Cellular extract corresponding to 30 ug of protein was loaded and separated by 10% step-gradient SDS-polyacrylamide gels electrophoresis, the separated proteins were transferred electrophoretically from the gel to nitrocellulose membrane (Millipore). Membranes were blocked for 2 h in PBST containing 3% BSA. Primary antibody was added in BSA and allowed to incubate overnight at 4°C, washed with TBS for 5 times before the secondary antibody was added and then incubated for an additional hour at room temperature. The membrane was again washed 3 times before adding Pierce Super Signal chemiluminescent substrate (Rockford, IL) and then immediately imaged on ChemiDoc (Bio-Rad, Hercules, CA). ImageJ software was used for gray scan analysis.
Statistical Analyses
Statistical analyses were performed using SPSS 21.0 (Chicago, IL). The significant difference between 2 groups was determined using one-way analysis of variance (ANOVA) and Duncan's test. All data are expressed as mean and SEM. The level of significance was set at P < 0.05.
RESULTS
Effect of Zinc Supplementation on the Growth Performance of Pekin Ducks Subjected to an Inflammatory Stimulus
As presented in Tables 1 and 2, dietary 120 mg/kg Zn supplementation increased the BW of day 14 and 21, as well as the BWG of d 1–14 and 15–21, and decreased the F/G of d 1 to 4 (P < 0.05). But it had no effect on FI (P > 0.05). LPS challenge significantly reduced the BW of d 21 and the BWG and the FI of day 15-21 (P < 0.05; Table 2). Interestingly, we also found that dietary 120 mg/kg Zn supplementation relieved the decrease in the BW of d 21 and the BWG and the FI of d 15 to 21 caused by LPS (Table 2).
Table 1.
Effect of dietary Zn on the growth performance of ducks from 1 to 14 d of age.
| Zinc (mg/kg) | 0 | 30 | 120 | SEM | P-value |
|---|---|---|---|---|---|
| 1 d BW, g | 50.21 | 50.22 | 50.14 | 0.05 | 0.537 |
| 14 d BW, g | 652.98b | 666.28b | 705.85a | 4.35 | 0.002 |
| 1–14 d BWG, g | 602.77b | 616.06b | 655.70a | 4.35 | <0.001 |
| 1–14 d FI, g | 842.58 | 845.6 | 861.19 | 4.03 | 0.128 |
| 1–14 d F/G | 1.40a | 1.37a | 1.31b | 0.01 | <0.001 |
Abbreviations: BW, body weight; BWG, body weight gain; FI, feed intake; F/G, feed to gain ratio.
Mean values (n = 16) in a row without common superscript are significantly different (P < 0.05).
Table 2.
Effect of dietary Zn on the growth performance of ducks challenged with LPS.
| Zinc level | 0 | 30 | 120 | 0 | 30 | 120 | SEM | Main effect | Zn level |
LPS |
P-value |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LPS | - | - | - | + | + | + | 0 | 30 | 120 | - | + | Zn level | LPS | Interaction | ||
| 21 d BW, g | 1,216b | 1,233b | 1,285a | 1,123d | 1,152c | 1,239b | 8.56 | 1,170c | 1192b | 1,262a | 1,244a | 1,171b | <0.001 | <0.001 | 0.017 | |
| 15–21 d BWG, g | 561 | 565 | 580 | 472 | 487 | 531 | 6.73 | 517b | 526b | 556a | 569a | 497b | <0.001 | <0.001 | 0.057 | |
| 15–21 d FI, g | 893 | 886 | 900 | 768 | 788 | 826 | 9.61 | 830 | 837 | 863 | 893a | 793b | 0.084 | <0.001 | 0.246 | |
| 15–21 d F/G | 1.59 | 1.57 | 1.55 | 1.63 | 1.62 | 1.56 | 0.02 | 1.61 | 1.6 | 1.56 | 1.57 | 1.60 | 0.343 | 0.382 | 0.877 | |
Abbreviations: BW, body weight; BWG, body weight gain; FI, feed intake; F/G, feed to gain ratio.
Mean values in a row without common superscript are significantly different (P < 0.05) (n = 8).
Effect of Zinc Supplementation on the Jejunal Histomorphology of Pekin Ducks Subjected to an Inflammatory Stimulus
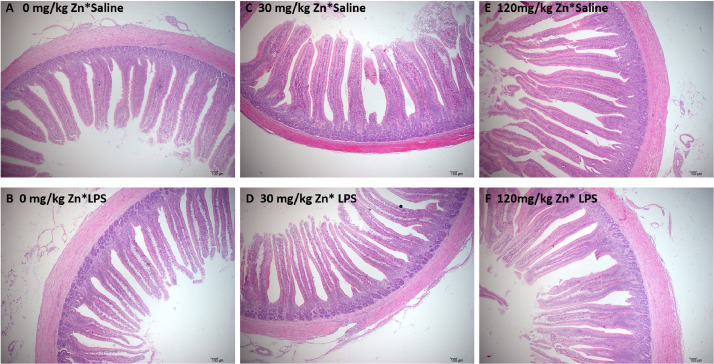
At d 21, in each Zn treatment group, the villus morphology was intact (Figure 1 A, 1C, and 1E). In the LPS group, the top of jejunal villi were shed and broken (Figure 1B and 1D). However, supplementing diets with high Zn alleviated LPS-induced histopathological changes (Figure 1F). LPS group significantly decreased the VH and the VCR and increased the CD (P < 0.05; Table 3). But dietary supplementation with 120 mg/kg Zn increased the VH and the VCR and decreased the CD (P < 0.05). Dietary 120 mg/kg Zn supplementation relieved LPS-induced decrease in the VCR and the increase in the CD (Table 3).
Figure 1.
Effect of dietary zinc on the histological structure of the jejunum in Pekin ducks challenged with LPS (magnification 10 ×). (A) Jejunum of 0 mg/kg Zn group, (B) jejunum of 0 mg/kg Zn +0.5 mg/kg LPS group, (C) jejunum of 30 mg/kg Zn group and (D) jejunum of 30 mg/kg Zn +0.5 mg/kg LPS group. (E) Jejunum of 120 mg/kg Zn group. (F) Jejunum of 120 mg/kg Zn +0.5 mg/kg LPS group. Abbreviation: LPS, lipopolysaccharide.
Table 3.
Effect of dietary Zn on the intestinal morphology of ducks challenged with LPS.
| Zinc level | 0 | 30 | 120 | 0 | 30 | 120 | SEM | Main effect | Zn level |
LPS |
P-value |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LPS | - | - | - | + | + | + | 0 | 30 | 120 | - | + | Zn level | LPS | Interaction | |||||||
| VH, um | 847.11a | 869.89a | 900.46a | 679.72c | 744.69b | 841.93a | 13.72 | 763.41c | 807.29b | 871.19a | 872.48a | 755.45b | <0.001 | <0.001 | 0.037 | ||||||
| CD, um | 122.36b | 121.59b | 112.66c | 142.57a | 136.00a | 119.44bc | 1.81 | 132.46a | 128.79a | 116.05b | 118.87b | 132.67a | <0.001 | <0.001 | 0.048 | ||||||
| VCR | 6.95b | 7.18b | 8.02a | 4.79d | 5.48c | 7.07b | 0.18 | 5.87c | 6.33b | 7.56a | 7.39a | 5.78b | <0.001 | <0.001 | 0.028 | ||||||
Abbreviations: CD, crypt depth; VCR, villus height to crypt depth ratio; VH, villus height.
Mean values in a row without common superscript are significantly different (P < 0.05) (n = 8).
Effect of Zinc Supplementation on the Intestinal Digestive Enzyme Activity of Pekin Ducks Subjected to an Inflammatory Stimulus
As presented in Table 4, LPS group significantly decreased the activity of maltase, AKP, and Na+-K+-ATPase in the jejunum of Pekin ducks (P < 0.05). There were no obvious changes (P > 0.05) in the jejunal maltase activity in response to Zn supplementation in the diet, but supplementation with 120 mg/kg Zn significantly increased (P < 0.05) the enzyme activity of jejunum AKP and Na+-K+-ATPase, and also increased the content of MUC2 (P < 0.05). However, dietary 120 mg/kg Zn supplementation relieved LPS-induced decrease in the enzyme activity of jejunum AKP and Na+-K+-ATPase caused by LPS. To evaluate the effects of Zn supplementation on the decrease of enzyme activity induced by LPS in vitro, we determined the enzyme activity of maltase, AKP and Na+-K+-ATPase of DIECs. The results showed that supplementation with Zn significantly increased (P < 0.05) the enzyme activity of AKP and Na+-K+-ATPase of DIECs, but no difference was observed for the enzyme activity of maltase. Similarly, we also found that Zn supplementation in the culture solution significantly increased the activities of AKP and Na+-K+-ATPase when DIECS was challenged by LPS (Table 5).
Table 4.
Effect of dietary Zn on the activities of digestive enzyme and the content of MUC2 in jejunum mucosa of ducks challenged with LPS.
| Zinc level | 0 | 30 | 120 | 0 | 30 | 120 | SEM | Main effect | Zn level |
LPS |
P-value |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LPS | - | - | - | + | + | + | 0 | 30 | 120 | - | + | Zn level | LPS | Interaction | ||||||
| Maltase | 108.12 | 115.56 | 117.23 | 97.31 | 99.75 | 104.64 | 2.361 | 102.72 | 107.65 | 110.93 | 113.64a | 100.56b | 0.325 | 0.005 | 0.898 | |||||
| AKP | 116.65ab | 119.91ab | 129.74a | 73.70d | 94.45c | 114.65b | 3.267 | 95.17c | 107.18b | 122.20a | 122.10a | 94.27b | <0.001 | <0.001 | 0.016 | |||||
| Na+-K+-ATPase | 2.6 | 2.46 | 2.8 | 1.69 | 1.88 | 2.36 | 0.071 | 2.14b | 2.17b | 2.58a | 2.62a | 1.97b | <0.001 | <0.001 | 0.089 | |||||
| MUC2 | 0.178 | 0.188 | 0.247 | 0.277 | 0.303 | 0.333 | 0.012 | 0.23b | 0.25ab | 0.29a | 0.20b | 0.30a | 0.027 | <0.001 | 0.809 | |||||
Abbreviations: AKP, alkaline phosphatase; MUC2, mucin2.
Mean values in a row without common superscript are significantly different (P < 0.05) (n = 8).
Table 5.
Effect of Zn levels on the digestive enzymes of DIECs challenged with LPS.
| Zinc level | TPEN | CON | ZINC | TPEN | CON | ZINC | SEM | Main effect | Zn level |
LPS |
P-value |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LPS | - | - | - | + | + | + | TPEN | CON | ZINC | - | + | Zn level | LPS | Interaction | ||
| Maltase | 1,2.46 | 1,2.93 | 1,4.56 | 1,2.43 | 1,2.14 | 1,3.44 | 0.47 | 12.45 | 12.54 | 14 | 13.32 | 12.67 | 0.374 | 0.521 | 0.901 | |
| AKP | 47.9 | 53.16 | 52.72 | 34.45 | 35.89 | 46.33 | 1.75 | 41.17b | 44.53b | 49.59a | 51.26a | 38.89b | 0.005 | <0.001 | 0.072 | |
| Na+-K+-ATPase | 7.67ab | 8.56a | 8.48a | 5.28c | 7.14b | 8.36ab | 0.28 | 6.48b | 7.85a | 8.42a | 8.24a | 7.09b | <0.001 | 0.001 | 0.038 | |
Abbreviation: AKP, alkaline phosphatase.
TPEN = D-Zn (Zinc deficiency, treated with 2 µmol/L zinc Chelator TPEN), Control = Zn (Adequate Zinc, basal medium), Zinc = H-Zn (High level of Zn, supplemented with 20 µmol/L Zn), TPEN + LPS = D-Zn + 20 µg/mL LPS, LPS = A-Zn + 20 µg/mL LPS, Zinc + LPS = H-Zn + 20 µg/mL LPS.
Mean values in a row without common superscript are significantly different (n = 8) (P < 0.05).
Effect of Zinc Supplementation on the Intestinal Permeability of Pekin Ducks Subjected to an Inflammatory Stimulus
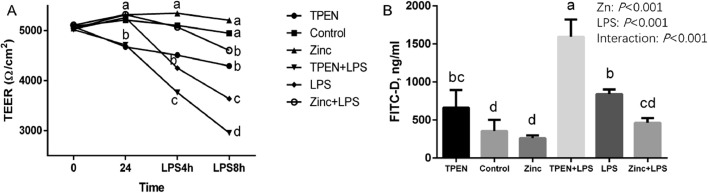
As shown in Table 6, dietary 120 mg/kg Zn supplementation significantly reduced LPS-induced increase in the content of FITC-D and D-lactic acid in the serum of Pekin ducks (P < 0.05). Then to evaluate the effects of Zn supplementation on the increase of intestinal permeability induced by LPS in vitro, we measured the TEER value in the DIECs. As shown in Figure 2A, there was no significant difference in TEER values in each group before treatment (P > 0.05). But Zn treatment for 24 h had a significant effect on the TEER of DIECs, and the TEER value in the TPEN groups were significantly lower than that of the control and Zn treatment groups (P < 0.05). LPS groups significantly decreased TEER by 4 h and 8 h (P < 0.05), and TPEN groups aggravated LPS-induced decrease of TEER value. Whereas, Zn supplementation relieved LPS-induced decrease in TEER value of DIECs (P < 0.05). Additionally, we also discovered that Zn supplementation relieved the increase in FITC-D content of DIECs caused by LPS (P < 0.05; Figure 2B).
Table 6.
Effect of dietary Zn on the intestinal permeability of ducks challenged with LPS.
| Zinc level | 0 | 30 | 120 | 0 | 30 | 120 | SEM | Main effect | Zn level |
LPS |
P-value |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LPS | - | - | - | + | + | + | 0 | 30 | 120 | - | + | Zn level | LPS | Interaction | |||||||
| FITC-D | 0.118c | 0.112c | 0.081d | 0.204a | 0.167b | 0.123c | 0.006 | 0.161a | 0.139b | 0.102c | 0.104b | 0.165a | <0.001 | <0.001 | 0.001 | ||||||
| D-Lactate | 41.15cd | 42.34cd | 37.00d | 62.34a | 49.41b | 46.34bc | 1.48 | 51.75a | 45.88a | 41.67b | 40.17b | 52.70a | <0.001 | <0.001 | 0.008 | ||||||
Abbreviation: FITC-D, fluorescein isothiocyanate dextran.
Mean values in a row without common superscript are significantly different (P < 0.05) (n = 8).
Figure 2.
Effects of zinc levels on intestinal barrier function of DIECs challenged with LPS (n = 4). (A) TEER of DIECs with different treatment; (B) FITC-D of DIECs with different treatment. Bars with different letters indicate P < 0.05. TPEN = D-Zn (Zinc deficiency, treated with 2 µmol/L zinc Chelator TPEN), Control = Zn (Adequate Zinc, basal medium), Zinc = H-Zn (High level of Zn, supplemented with 20 µmol/L Zn), TPEN +LPS = D-Zn + 20 µg/mL LPS, LPS =A-Zn + 20 µg/mL LPS, Zinc+ LPS = H-Zn + 20 µg/mL LPS. Abbreviations: DIECs, duck primary intestinal epithelial cells; LPS, lipopolysaccharide; TEER, transepithelial electrical resistance.
Effect of Zinc Supplementation on Gene and Protein Expression of Tight Junction in Pekin Ducks Subjected to an Inflammatory Stimulus
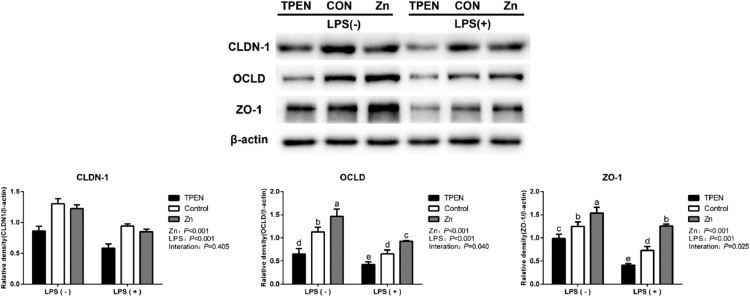
As shown in Tables 7 and 8, Zn increased expression of CLDN-1, OCLD, ZO-1, and ZO-3, and decreased expression of CLDN-2 and MLCK in Pekin duck jejunum or DIECs (P < 0.05). In contrast, as we expected, LPS downregulated the mRNA levels of CLDN-1, OCLD, ZO-1, ZO-3, EPCAM, and JAM2 and upregulated the mRNA levels of CLDN-2 and MLCK in Pekin duck jejunum or DIECs (P < 0.05). However, mRNA levels of CLDN-1 and ZO-1 with co-treatment of Zn and LPS were significantly enhanced and mRNA levels of MLCK was significantly decreased compared with the treatment of LPS alone (P < 0.05). To double confirm these results, protein levels of expression of CLDN, OCLD, and ZO-1 with the treatment of Zn in DIECs were determined by western blot as well. As shown in Figure 3, Zn increased CLDN-1, OCLD, and ZO-1 expression in the protein level while LPS decreased tight junctions (P < 0.05). Similar to the results of RT-PCR, we found that co-treatment of Zn and LPS reversed LPS-induced decrease of OCLD and ZO-1 at protein levels (P < 0.05), indicating Zn promoted the synthesis of tight junction protein.
Table 7.
Effect of dietary Zn on the tight junction related gene expression in jejunum of ducks challenged with LPS.
| Zinc level | 0 | 30 | 120 | 0 | 30 | 120 | SEM | Main effect | Zn level |
LPS |
P-value |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LPS | - | - | - | + | + | + | 0 | 30 | 120 | - | + | Zn level | LPS | Interaction | ||
| CLDN-1 | 1.00c | 1.27b | 1.50a | 0.66d | 0.80d | 1.33b | 0.083 | 0.83c | 1.03b | 1.41a | 1.26a | 0.93b | <0.001 | <0.001 | 0.046 | |
| CLDN-2 | 1.01 | 0.84 | 0.85 | 1.32 | 1.22 | 0.92 | 0.040 | 1.12a | 1.04b | 0.81b | 0.90b | 1.08a | 0.001 | <0.001 | 0.084 | |
| OCLD | 1.01 | 1.20 | 1.44 | 0.73 | 0.85 | 1.34 | 0.045 | 0.87c | 1.02b | 1.39a | 1.27a | 0.97b | <0.001 | <0.001 | 0.163 | |
| ZO-1 | 1.01c | 1.25b | 1.67a | 0.68d | 1.18b | 1.56c | 0.052 | 0.84c | 1.21b | 1.62a | 1.31a | 1.14b | <0.001 | <0.001 | 0.029 | |
| ZO-2 | 1.00 | 1.06 | 1.11 | 0.93 | 1.04 | 1.02 | 0.028 | 0.97 | 1.05 | 1.07 | 1.06 | 1.00 | 0.291 | 0.264 | 0.846 | |
| ZO-3 | 1.01 | 1.49 | 1.63 | 0.69 | 1.17 | 1.46 | 0.050 | 0.85c | 1.33b | 1.55a | 1.37a | 1.11b | <0.001 | <0.001 | 0.208 | |
| EPCAM | 1.01 | 1.07 | 1.10 | 0.75 | 0.94 | 0.95 | 0.030 | 0.88 | 1.00 | 1.02 | 1.06a | 0.88b | 0.068 | 0.002 | 0.594 | |
| JAM2 | 1.01 | 1.19 | 1.08 | 0.81 | 0.84 | 1.06 | 0.032 | 0.91 | 1.02 | 1.07 | 1.10a | 0.90b | 0.063 | 0.001 | 0.067 | |
| JAM3 | 1.00 | 1.11 | 1.10 | 0.89 | 0.98 | 1.07 | 0.031 | 0.95 | 1.05 | 1.09 | 1.07 | 0.98 | 0.187 | 0.148 | 0.831 | |
| MLCK | 1.01d | 0.76d | 0.65d | 3.95a | 3.50b | 2.31c | 0.198 | 2.48a | 2.13b | 1.48c | 0.81b | 3.25a | <0.001 | <0.001 | <0.001 | |
Abbreviations: CLDN-1, claudin1; CLDN-2, claudin 2; EPCAM, epithelial cell adhesion molecule; JAM2, junctional adhesion molecule-2; JAM3, junctional adhesion molecule-3; MLCK, myosin light-chain kinase; OCLD, occluding; ZO-1, zonula occludens-1; ZO-2, zonula occludens-2; ZO-3, zonula occludens-3.
Mean values in a row without common superscript are significantly different (P < 0.05) (n = 8).
Table 8.
Effect of Zn levels on the tight junction related gene expression of DIECs challenged with LPS.
| Zinc level | TPEN | CON | ZINC | TPEN | CON | ZINC | SEM | Main effect | Zn level |
LPS |
P-value |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LPS | - | - | - | + | + | + | TPEN | CON | ZINC | - | + | Zn level | LPS | Interaction | ||
| CLDN-1 | 0.78c | 1.01b | 1.41a | 0.53d | 0.79c | 0.87c | 0.043 | 0.66c | 0.90b | 1.14a | 1.07a | 0.73b | <0.001 | <0.001 | 0.002 | |
| CLDN-2 | 1.36 | 1.00 | 0.72 | 1.7 | 1.45 | 1.1 | 0.053 | 1.53a | 1.23b | 0.91c | 1.03b | 1.42a | <0.001 | <0.001 | 0.687 | |
| OCLD | 0.82c | 1.01c | 1.97a | 0.81c | 0.93c | 1.23b | 0.064 | 0.82c | 0.97b | 1.60a | 1.27a | 0.99b | <0.001 | <0.001 | < 0.001 | |
| ZO-1 | 0.65d | 1.01b | 1.68a | 0.48e | 0.67cd | 0.81c | 0.06 | 0.57c | 0.84b | 1.25a | 1.11a | 0.66b | <0.001 | <0.001 | <0.001 | |
| ZO-2 | 0.9 | 1.00 | 0.97 | 1.05 | 1.03 | 1.06 | 0.024 | 0.97 | 1.01 | 1.02 | 0.96 | 1.05 | 0.685 | 0.074 | 0.586 | |
| ZO-3 | 0.79 | 1.01 | 1.26 | 0.63 | 0.76 | 0.85 | 0.038 | 0.71c | 0.89b | 1.06a | 1.02a | 0.75b | <0.001 | <0.001 | 0.143 | |
| EPCAM | 0.86c | 1.01b | 1.26a | 0.74c | 0.82c | 1.17a | 0.032 | 0.81c | 0.92b | 1.21a | 1.04a | 0.91b | <0.001 | 0.001 | 0.556 | |
| JAM2 | 0.82 | 1.01 | 1.24 | 0.84 | 0.9 | 0.94 | 0.032 | 0.83b | 0.95b | 1.09a | 1.02a | 0.90b | 0.001 | 0.023 | 0.052 | |
| JAM3 | 0.92 | 1.01 | 1.05 | 0.85 | 0.96 | 0.94 | 0.021 | 0.89 | 0.99 | 0.99 | 0.92 | 0.99 | 0.062 | 0.065 | 0.828 | |
| MLCK | 1.75d | 1.01e | 0.74e | 5.50a | 4.50b | 3.44c | 0.269 | 3.63a | 2.75b | 2.09c | 1.17b | 4.48a | < 0.001 | < 0.001 | 0.012 | |
Abbreviations: CLDN-1, claudin1; CLDN-2, claudin 2; EPCAM, epithelial cell adhesion molecule; JAM2, junctional adhesion molecule-2; JAM3, junctional adhesion molecule-3; MLCK, myosin light-chain kinase; OCLD, occluding; ZO-1, zonula occludens-1; ZO-2, zonula occludens-2; ZO-3, zonula occludens-3.
Mean values in a row without common superscript are significantly different (P < 0.05) (n = 8).
Figure 3.
Effects of zinc levels on the expression of tight junction protein of DIECs challenged with LPS (n = 4). Bars with different letters indicate P < 0.05. TPEN = D-Zn (Zinc deficiency, treated with 2 µmol/L zinc Chelator TPEN), Control=Zn (Adequate Zinc, basal medium), Zinc = H-Zn (High level of Zn, supplemented with 20 µmol/L Zn), TPEN +LPS = D-Zn + 20 µg/mL LPS, LPS = A-Zn + 20 µg/mL LPS, Zinc+ LPS = H-Zn + 20 µg/mL LPS. Abbreviations: DIECs, duck primary intestinal epithelial cells; LPS, lipopolysaccharide.
Effect of Zinc Supplementation on the Inflammatory Responses and Apoptosis of Pekin Ducks Subjected to an Inflammatory Stimulus
The impact of zinc supplementation on the inflammatory responses and apoptosis are shown in Tables 9 and 10. Zn supplementation downregulated the mRNA levels of IL-2, IL-6, IL-8, TNF-α, iNOS, and TLR4 and upregulated the mRNA levels of IL-10 in Pekin duck jejunum or DIECs (P < 0.05). LPS increased the mRNA levels of IL-2, IL-6, IL-8, IL-10, INF-γ, TNF-α, iNOS, and TLR4 (P < 0.05). However, mRNA levels of IL-2, TNF-α, TLR4, and iNOS with co-treatment of Zn and LPS were significantly decreased and mRNA level of IL-10 was significantly increased compared with the treatment of LPS alone (P < 0.05). Moreover, 120 mg/kg Zn significantly reduced LPS-induced increase of Caspase-3 and Caspase-8 mRNA levels in the jejunum of Pekin ducks (P < 0.05; Table 9). In addition, we found that LPS increased the mRNA levels of Caspase-3, Caspase-8, BAX, A20 and MT in DIECs (P < 0.05). But mRNA levels of A20 and MT with co-treatment of Zn and LPS were significantly upregulated and mRNA levels of Caspase-3 were significantly downregulated compared with the treatment of LPS alone (P < 0.05; Table 11).
Table 9.
Effect of dietary Zn on the inflammation and the apoptosis related gene expression in jejunum of ducks challenged with LPS.
| Zinc level | 0 | 30 | 120 | 0 | 30 | 120 | SEM | Main effect | Zn level |
LPS |
P-value |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LPS | - | - | - | + | + | + | 0 | 30 | 120 | - | + | Zn level | LPS | Interaction | ||
| IL-2 | 1.01d | 1.10d | 0.96d | 4.45a | 3.50b | 2.79c | 0.219 | 2.73a | 2.30ab | 1.88b | 1.02b | 3.58a | 0.003 | <0.001 | 0.005 | |
| IL-6 | 1.00c | 1.10c | 0.91c | 3.54a | 3.85a | 2.73b | 0.187 | 2.27a | 2.48a | 1.82b | 1.01b | 3.37a | <0.001 | <0.001 | 0.003 | |
| IL-8 | 1.00c | 1.11c | 1.13c | 2.83a | 2.23b | 2.09b | 0.110 | 1.92a | 1.67b | 1.61b | 1.08b | 2.38a | 0.020 | <0.001 | 0.001 | |
| IL-10 | 1.01c | 1.21c | 1.25c | 2.40b | 2.53b | 3.38a | 0.134 | 1.70b | 1.87b | 2.32a | 1.16b | 2.77a | <0.001 | <0.001 | 0.002 | |
| INF-α | 1.01 | 1.15 | 1.22 | 1.02 | 0.93 | 1.02 | 0.096 | 1.02 | 1.04 | 1.12 | 1.12 | 0.99 | 0.902 | 0.509 | 0.877 | |
| INF-β | 1.01 | 1.05 | 1.02 | 1.15 | 1.05 | 1.09 | 0.022 | 1.08 | 1.05 | 1.06 | 1.03 | 1.10 | 0.860 | 0.111 | 0.381 | |
| INF-γ | 1.01c | 1.12c | 1.07c | 2.38a | 1.91b | 1.75b | 0.086 | 1.69a | 1.52b | 1.41b | 1.07b | 2.02a | 0.046 | <0.001 | 0.008 | |
| TNF-α | 1.01d | 1.14d | 0.93d | 2.73a | 2.43b | 2.07c | 0.112 | 1.87a | 1.78a | 1.50b | 1.03b | 2.41a | 0.001 | <0.001 | 0.015 | |
| TLR4 | 1.01c | 1.03c | 1.07c | 4.60a | 4.17a | 3.43b | 0.234 | 2.81a | 2.60a | 2.25b | 1.04b | 4.07a | 0.003 | <0.001 | 0.001 | |
| iNOS | 1.00c | 0.89c | 0.73c | 2.84a | 2.01b | 1.72b | 0.113 | 1.92a | 1.45b | 1.23c | 0.87b | 2.19a | <0.001 | <0.001 | <0.001 | |
| Caspase-3 | 1.00d | 0.98d | 1.05d | 4.58a | 3.61b | 2.87c | 0.216 | 2.79a | 2.30b | 1.96c | 1.01b | 3.69a | <0.001 | <0.001 | <0.001 | |
| Caspase-8 | 1.00d | 1.14d | 1.06d | 3.13a | 2.50b | 1.92c | 0.129 | 2.07a | 1.82a | 1.49b | 1.07b | 2.52a | <0.001 | <0.001 | <0.001 | |
Abbreviations: IL-2, interleukin 2; IL-6, interleukin 6; IL-8, interleukin 8; IL-10, interleukin 10; INFα, interferon-α; INFβ, interferon-β; INFγ, interferon-γ; iNOS, inducible nitric oxide synthase; TNFα, tumor necrosis factor-α; TLR4, Toll-like receptor 4.
Mean values in a row without common superscript are significantly different (P < 0.05) (n = 8).
Table 10.
Effect of Zn levels on the inflammatory response gene expression of DIECs challenged with LPS.
| Zinc level | TPEN | CON | ZINC | TPEN | CON | ZINC | SEM | Zn level |
LPS |
P-value |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LPS | - | - | - | + | + | + | Main effect | TPEN | CON | ZINC | - | + | Zn level | LPS | Interaction | |
| IL-2 | 1.38c | 1.01c | 0.81c | 4.68a | 2.75b | 3.11b | 0.215 | 3.03a | 1.88b | 1.96b | 1.06b | 3.52a | <0.001 | <0.001 | 0.001 | |
| IL-6 | 2.29 | 1.01 | 0.93 | 4.77 | 3.84 | 2.95 | 0.214 | 3.53a | 2.46b | 1.94c | 1.41b | 3.86a | <0.001 | <0.001 | 0.062 | |
| IL-8 | 1.36 | 1.00 | 0.94 | 4.03 | 3.23 | 2.96 | 0.186 | 2.69a | 2.17b | 1.92b | 1.10b | 3.38a | <0.001 | <0.001 | 0.062 | |
| IL-10 | 1.20c | 1.01c | 1.15c | 2.58b | 2.27b | 3.47a | 0.145 | 1.89b | 1.64b | 2.31a | 1.12b | 2.78a | <0.001 | <0.001 | 0.003 | |
| INF-γ | 1.67d | 1.01e | 0.89e | 3.46a | 2.89b | 2.10c | 0.14 | 2.57a | 1.95b | 1.50c | 1.19b | 2.81a | <0.001 | <0.001 | 0.001 | |
| TNF-α | 2.15c | 1.01e | 0.88e | 4.29a | 3.12b | 1.63d | 0.182 | 3.22a | 2.06b | 1.25c | 1.35b | 3.01a | <0.001 | <0.001 | <0.001 | |
| iNOS | 15.99b | 1.01d | 0.86d | 20.57a | 3.28c | 2.08cd | 1.175 | 18.28a | 2.14b | 1.47c | 5.95b | 8.65a | <0.001 | <0.001 | 0.015 | |
| TLR4 | 1.40c | 1.01c | 1.12c | 4.32a | 3.94a | 2.81b | 0.203 | 2.86a | 2.48b | 1.96c | 1.18b | 3.69a | <0.001 | <0.001 | <0.001 | |
Abbreviations: IL-2, interleukin 2; IL-6, interleukin 6; IL-8, interleukin 8; IL-10, interleukin 10; INFγ, interferon-γ; iNOS, inducible nitric oxide synthase; TNFα, tumor necrosis factor-α; TLR4, Toll-like receptor 4.
Mean values (n = 8) in a row without common superscript are significantly different (n = 8) (P < 0.05).
Table 11.
Effect of Zn levels on the apoptosis related gene expression of DIECs challenged with LPS.
| Zinc level | TPEN | CON | ZINC | TPEN | CON | ZINC | SEM | Main effect | Zn level |
LPS |
P-value |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LPS | - | - | - | + | + | + | TPEN | CON | ZINC | - | + | Zn level | LPS | Interaction | ||
| Caspase-3 | 1.58b | 1.01a | 0.81a | 4.12a | 3.02b | 2.50c | 0.177 | 2.85a | 2.02b | 1.65c | 1.13b | 3.22a | <0.001 | <0.001 | 0.007 | |
| Caspase-8 | 2.03 | 1.01 | 0.88 | 3.53 | 2.64 | 1.90 | 0.145 | 2.78a | 1.83b | 1.39c | 1.31b | 2.69a | <0.001 | <0.001 | 0.170 | |
| BAX | 1.77 | 1.01 | 0.74 | 2.16 | 1.61 | 1.23 | 0.078 | 1.97a | 1.21b | 0.99b | 1.17b | 1.60a | <0.001 | <0.001 | 0.901 | |
| A20 | 0.75d | 1.01c | 1.56b | 0.85cd | 1.54b | 2.01a | 0.070 | 0.80c | 1.28b | 1.78a | 1.10b | 1.47a | <0.001 | <0.001 | 0.003 | |
| MT | 0.01d | 1.01d | 15.59b | 0.01d | 6.18c | 19.93a | 1.177 | 0.01c | 3.59b | 17.76a | 5.54b | 8.71a | <0.001 | <0.001 | <0.001 | |
| AKP | 0.84c | 1.03b | 1.36a | 0.74d | 0.88c | 0.90c | 0.035 | 0.79c | 0.98b | 1.13a | 1.08a | 0.84b | <0.001 | <0.001 | 0.003 | |
Abbreviations: AKP, alkaline phosphatase; A20, tumor necrosis factor, alpha-induced protein 3; BAX, Bcl-2 associated X protein; MT, metallothionein.
Mean values (n = 8) in a row without common superscript are significantly different (n = 8) (P < 0.05).
Effect of Zinc Supplementation on the Expression of Related Genes in Intestinal Stem Cells of in Pekin Ducks Subjected to an Inflammatory Stimulus
Table 12 demonstrated that Zn increased expression of AKP, villin, MUC2, LYZ, and Sox9 in Pekin duck jejunum (P < 0.05). However, LPS down-regulated the mRNA levels of AKP and villin and upregulated the mRNA levels of MUC2, LYZ, Sox9, Lgr5, Bmi1, Dll1, and Tert in Pekin duck jejunum (P < 0.05). Surprisingly, Zn supplementation relieved the decrease of AKP mRNA level and the increase of Lgr5, Bmi1, Tert, and β-catenin mRNA levels caused by LPS (P < 0.05). Additionally, Zn supplementation also promoted the increase of MUC2, LYZ, and Sox9 mRNA levels caused by LPS (P < 0.05).
Table 12.
Effect of dietary Zn on the intestinal epithelial stem cell marker gene expression in jejunum of ducks challenged with LPS.
| Zinc level | 0 | 30 | 120 | 0 | 30 | 120 | SEM | Main effect | Zn level |
LPS |
P-value |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LPS | - | - | - | + | + | + | 0 | 30 | 120 | - | + | Zn level | LPS | Interaction | ||
| AKP | 1.01c | 1.32b | 1.50a | 0.67d | 0.96c | 1.33b | 0.044 | 0.84c | 1.14b | 1.42a | 1.28a | 0.99b | <0.001 | <0.001 | 0.042 | |
| Villin | 1.01 | 1.02 | 1.22 | 0.70 | 0.78 | 0.87 | 0.031 | 0.85b | 0.90b | 1.04a | 1.08a | 0.78b | <0.001 | <0.001 | 0.419 | |
| MUC2 | 1.01d | 1.52c | 1.37c | 2.39b | 2.36b | 2.76a | 0.099 | 1.70b | 1.94a | 2.06a | 1.30b | 2.50a | 0.001 | <0.001 | 0.005 | |
| LYZ | 1.01d | 1.45c | 1.67bc | 1.96a | 1.75ab | 1.82ab | 0.055 | 1.48b | 1.60ab | 1.74a | 1.38b | 1.85a | 0.011 | <0.001 | <0.001 | |
| SOX9 | 1.01d | 1.07d | 1.30c | 2.78b | 2.92b | 3.51a | 0.150 | 1.89b | 1.99b | 2.41a | 1.11b | 3.07a | <0.001 | <0.001 | 0.022 | |
| Lgr5 | 1.00d | 1.12d | 1.11d | 2.28a | 2.08b | 1.70c | 0.078 | 1.64b | 1.60b | 1.41a | 1.08b | 2.02a | 0.002 | <0.001 | <0.001 | |
| Bmi1 | 1.01c | 1.11c | 1.08c | 4.13a | 3.99a | 2.74b | 0.208 | 2.57a | 2.55a | 1.91b | 1.07a | 3.62b | < 0.001 | <0.001 | <0.001 | |
| Dll1 | 1.01 | 1.13 | 1.08 | 3.30 | 3.07 | 3.00 | 0.158 | 2.16 | 2.1 | 2.04 | 1.07b | 3.12a | 0.646 | <0.001 | 0.254 | |
| Tert | 1.03c | 1.14c | 1.03c | 2.23a | 1.89b | 1.91b | 0.790 | 1.63 | 1.52 | 1.47 | 1.06b | 2.01a | 0.208 | <0.001 | 0.045 | |
| β-catenin | 1.00d | 1.03d | 1.04d | 2.90a | 2.41b | 1.68c | 0.118 | 1.95b | 1.72b | 1.36a | 1.03b | 2.33a | <0.001 | <0.001 | <0.001 | |
Abbreviations: AKP, alkaline phosphatase; Bmi1, bmi1 polycomb ring finger oncogene; DLL1, Delta-like 1; Lgr5, leucine-rich-repeat-containing G-protein-coupled receptor 5; Muc2, Mucin2; LYZ, lysozyme; Sox9, SRY-related high mobility group-box gene 9; Tert, telomerase reverse transcriptase.
Mean values in a row without common superscript are significantly different (P < 0.05) (n = 8).
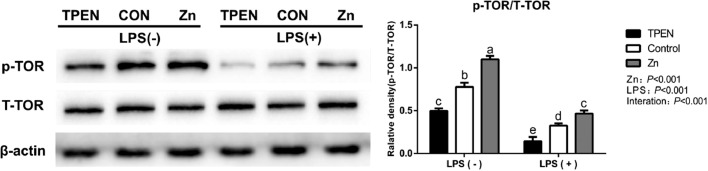
Importantly, Figure 4 demonstrated that LPS decreased phosphorylated TOR expression while Zn significantly increased TOR expression (P < 0.05). The results from con-treatment of Zn and LPS exhibited the profound effect of Zn on relieving LPS-induced decrease in protein synthesis of DIECs (P < 0.05).
Figure 4.
Effects of zinc levels on the phosphorylation of total target of rapamycin (T-TOR) protein of DIECs challenged with LPS (n = 4). Bars with different letters indicate P < 0.05. TPEN = D-Zn (Zinc deficiency, treated with 2 µmol/L zinc Chelator TPEN), Control = Zn (Adequate Zinc, basal medium), Zinc=H-Zn (High level of Zn, supplemented with 20 µmol/L Zn), TPEN +LPS=D-Zn + 20 µg/mL LPS, LPS = A-Zn + 20 µg/mL LPS, Zinc+ LPS = H-Zn + 20 µg/mL LPS. Abbreviations: DIECs, duck primary intestinal epithelial cells; LPS, lipopolysaccharide.
DISCUSSION
As the development of poultry feeding and breeding industry, the growth rate of poultry was faster than three decades ago, but the requirement of trace minerals requirement is still not updated. The current (National Research Council, 1994) Zn recommended level for optimal performance in duck is 60 mg Mn/kg for 0 to 2 wk (National Research Council, 1994). It has been clearly established that dietary nutrients includes trace minerals of diets can affect their development and performance in feeding period (Yang et al., 2021). Dietary Zn supplementation significantly increased the BW and BWG and decreased the F/G of Pekin ducks. The BW, BWG and FI of Pekin ducks significantly reduced after intraperitoneal injection of LPS, consistent with Zhang et al. (2013), observed that the growth performance of broilers were reduced by LPS challenge. The reason may be that immune stress can suppress the appetite of the animal, and the limited nutrients in the animal's body are mobilized to participate in the immune response, which in turn leads to the reduction of animal performance. However, Zn supplementation can alleviate LPS-induced decrease in growth performance of Pekin ducks to a certain extent. Similar results were reported that the growth performance of broilers was reduced by Salmonella infection (Shao et al., 2014).
Intestinal morphology is directly related to the surface area and capacity of intestinal digestion and absorption, higher VH and lower CD enable the intestines to have a stronger ability to digest and absorb nutrients (Jia et al., 2010). In this study, intraperitoneal injection of LPS significantly impaired the morphological structure of duck jejunum, significantly reduced the VCR of Pekin ducks, increased the CD, and reduced the activity of digestive enzymes, indicating that LPS can destroy the morphology and structure of intestinal tissues in Pekin ducks and injure to the digestion and absorption function of the intestines, which leading to lower BWG of Pekin ducks. These results are consistent with past results of Hu et al. (2011) and Zhang et al. (2017). However, in this study, Zn supplementation in the diet has the effect of alleviating the intestinal morphological damage caused by LPS. The possible reason is that Zn alleviates the apoptosis of intestinal epithelial cells in Pekin ducks caused by LPS, maintains the integrity of the intestinal epithelium, and does not need to mobilize more intestinal epithelial cells for compensatory proliferation, thus showing higher VH and lower CD (de Queiroz et al., 2014). A similar study found that Zn supplementation improved the morphology of jejunal villi in lactose-induced diarrhea rats (Shao et al., 2014).
AKP is distributed in the intestinal epithelium of animals. It can participate in the absorption of a variety of nutrients. Studies have found that AKP is involved in the expression and localization of tight junction proteins and the regulation of intestinal permeability (Liu et al., 2016). The Na+-K+-ATPase of intestinal epithelial cells participates in the construction of Na+ concentration gradient to help the coordinated absorption of amino acids and glucose. In addition, Na+-K+-ATPase is also involved in the assembly of intestinal tight junction proteins (Rajasekaran and Rajasekaran, 2009). Studies have found that the use of Na+-K+-ATPase inhibitors can inhibit the Na+-K+-ATPase activity of intestinal epithelial T84 cells (Sugi et al., 2001). In this study, Zn supplementation can alleviate the decrease in the activity of digestive enzymes such as AKP and Na+-K+-ATPase caused by LPS, indicating Zn promoted the digestion and absorption of nutrients in Pekin ducks.
TEER and macromolecular permeability are important indicators to evaluate the physical barrier function of intestinal epithelial cells. It indicates the integrity of the intestinal epithelial tight junctions and the integrity of the monolayer, and the intestinal epithelial cell tight junctions are through complete cells and cells (Lee et al., 2018). D-lactic is a bacterial metabolite, usually only a small amount enters the animal's body, and only when the tight junction of the intestine is destroyed can it bypass the cell into the animal's body (Wu et al., 2013). Studies have shown that LPS can significantly reduce the TEER of IPEC-J2 cell lines (Yang et al., 2015). Zn supplementation alleviates the decrease in TEER and the increase in FITC-D of Caco-2 cells caused by Salmonella infection (Shao et al., 2017a). In this study, LPS stress significantly increased the serum FITC-D and D-lactic acid concentrations of Pekin ducks, lowered the TEER value of DIECs, and reduced the physical barrier function of Pekin ducks and DIECs. Zn supplementation can alleviate Pekin ducks and DIECs caused by LPS, which consistent with the results of previous studies and indicating reverses LPS-induced intestinal barrier damage.
The integrity of the structure and function of the intestinal physical barrier depends on the expression of tight junction proteins. Studies have found that intraperitoneal injection of LPS or Zn deficiency can significantly reduce the expression of CLDN-1, ZO-1, OCLD mRNA in the intestine, increase the expression of CLDN-2 mRNA, and then destroy the animal's intestinal barrier function (Wang et al., 2013). In this study, the increase in dietary Zn levels promoted the expression of tight junction proteins CLDN-1, OCLD, ZO-1, and ZO-3 in Pekin ducks, and alleviated the adverse effects of LPS stress on the expression of tight junction-related tight junctions in Pekin ducks, and reduced the expression of CLDN-2 and MLCK induced by LPS, thereby maintaining the integrity of the tight junctions of the intestines of Pekin ducks. The results of this experiment are consistent with the finding that Zn can resist alcohol-based diets to reduce the expression of CLDN-1 and ZO-1 in the rat intestine (Zhong et al., 2015). In addition, LPS also reduced the expression of tight junction protein, and promoted the expression of MLCK and the leaky protein CLDN-2. And Zn supplementation can alleviate this result. The same report is also seen in Zn supplementation to alleviate the reduction of tight junction protein expression and TEER in Caco-2 cells caused by Salmonella infection (Shao et al., 2017a). The above results indicate that Zn supplementation can alleviate LPS-induced the decrease in the expression of tight junction proteins in the intestine and DIECs of Pekin ducks, and reduce the damage of LPS to the physical barrier function of animal intestines.
LPS stress or Zn deficiency can significantly increase the expression of intestinal inflammatory factors (Bao et al., 2010b; Zhang et al., 2017). Inflammatory factors can activate the ERK1/2 signaling pathway and promote the expression of MLCK, which will reduce the expression of tight junction proteins and destroy their localization, increasing the permeability of intestinal epithelial cells (Al-Sadi et al., 2013). Zn supplementation can inhibit the excessive activation of the intestinal NF-κB signaling pathway under various pathological conditions, reduce the expression of proinflammatory factors, and increase the expression of anti-inflammatory factors (de Queiroz et al., 2014). The results of this study showed that Zn supplementation alleviated LPS-induced the increase in the expression of proinflammatory factors such as IL-2, IL-6, IL-8, INF-γ, and TNF-α in Pekin duck jejunum and DIECs, and increased the expression of anti-inflammatory factor IL-10. A20 is a target gene of NF-κB, which can inhibit the excessive activation of NF-κB signaling pathway through a negative feedback mechanism (Coornaert et al., 2009). Studies have reported that Zn can promote the expression of A20 stimulated by PMA, and knocking out A20 will lead to the disappearance of the inhibitory effect of Zn on PMA-induced TNF-α and IL-1β expression (Prasad et al., 2011). The LPS receptor TLR4 is positively regulated by NF-κB and plays an important role in the bypass permeability of intestinal epithelial cells. Interfering with the expression of TLR4 can block the decrease in TEER and the enhance of macromolecular permeability of intestinal epithelial cells caused by LPS challenge (Guo et al., 2013). Studies have reported that LPS stress or Zn deficiency can cause cell apoptosis by activating Caspase-3 and Caspase-8, causing tight junction protein damage (Seth et al., 2015). In this experiment, Zn supplementation can significantly reduce the increase in the expression of TLR4 and Caspase-3 caused by LPS. These results were consistent with the classical role of Zn in cell apoptosis (Ranaldi et al., 2013). Animals will activate the NF-κB signaling pathway under stress conditions and induce the synthesis of nitric oxide synthase (inducible NO synthase, iNOS), the product of which can destroy the intestinal barrier function (Han et al., 2004). Studies have reported that Zn supplementation can alleviate the expression of iNOS and the synthesis of NO in endothelial cells induced by inflammatory factors (Cortese-Krott et al., 2014). Zn supplementation significantly increased the expression of Caco-2 and MT, alleviated the oxidative stress caused by ochratoxin and its damage to the intestinal barrier function (Ranaldi et al., 2009). In this study, Zn supplementation significantly reduced LPS-induced the increase in the expression of TLR4, iNOS and Caspase-3, and promoted the expression of MT. The above results indicated that Zn alleviated the damage of oxidative and inhibited LPS-induced the expression of proinflammatory cytokines and apoptosis-related genes via alleviating the activation of the TLR4 signaling pathway induced by LPS.
AKP enzyme activity is closely related to intestinal digestion and absorption capacity. Studies have found that LPS stress significantly reduces the expression level of intestinal AKP and reduces the digestion and absorption capacity of the intestine (Zhang et al., 2013). Intestinal goblet cells play an important role in the intestinal barrier by secreting mucin. Studies have reported that Zn supplementation can significantly increase the number of goblet cells in the mouse intestine and promote mucin secretion (de Queiroz et al., 2014). Lysozyme (LYZ) is an important part of the intestinal immune barrier (Wang et al., 2016). LPS can promote the expression of LYZ mRNA in chicken intestinal primary intestinal epithelial cells (Bar Shira and Friedman, 2018). Sox9 is an essential gene for Paneth cell differentiation. Studies have found that Zn plays an important role in the differentiation of Paneth cells. Knockout of the Zn transporter ZIP4 of intestinal epithelial cells can affect the differentiation process of intestinal epithelial cells and weaken the absorption of intestinal cell marker molecules. Fatty acid binding protein 2 (FABP2) expression and Paneth cell marker LYZ expression, and this process can be alleviated by supplementing Zn (Geiser et al., 2012). The normal proliferation and differentiation of intestinal stem cells are of great significance to the intestinal health of animals. Studies have shown that when mice are stressed, they activate the Wnt/β-catenin signaling pathway to increase the number of intestinal stem cells Lgr5, Bmi1, Tert, and proliferating cells (Liu et al., 2010). In this study, Zn supplementation can alleviate the decrease in AKP mRNA level and the increase in Lgr5, Bmi1, Tert, and β-catenin mRNA levels caused by LPS. In addition, it also promotes the expression of MUC2, LYZ, and Sox9 mRNA. The above results indicate that Zn supplementation reduced LPS-induced the intestinal stem cells mobilization by inhibiting LPS-induced the expression of proinflammatory cytokines and apoptosis-related genes. mTOR is involved in the regulation of protein anabolism. Zn can promote cell proliferation, increase tight junction protein expressions and improve barrier function by activating the mTOR signaling pathway (Shao et al., 2017b). In this experiment, LPS decreased the level of phosphorylation of TOR protein, but Zn supplementation reversed LPS-induced the decrease of the level of phosphorylation of TOR protein via activating the TOR signaling pathway.
In conclusion, our study demonstrates the important role of Zn in regulating growth performance, digestive enzyme activity, intestinal barrier function and expression of tight junction proteins. One hundred and twenty mg/kg Zn improves growth performance, digestive enzyme activity and intestinal barrier function of Pekin ducks. Importantly, 20 µmol/L Zn also reverses LPS-induced intestinal barrier damage via enhancing the expression of tight junction proteins and activating the TOR signaling pathway. Based on our findings, supplementation with 120 mg/kg Zn might be advantageous for the Pekin ducks’ intestinal barrier function under LPS Challenge.
ACKNOWLEDGMENTS
This work was supported by Sichuan Longda Animal Husbandry Science and Technology Co., Ltd. (No.009H2200).
Authorship statement: Gang Jia managed the entire trial. Yueqin Xie and Min Wen revised the manuscript. Guangmang Liu and Xiaoling Chen involved in study design and helped with data collection and analysis. Hua Zhao and Gang Tian assisted with laboratory analyses. Jingyi Cai provided the original idea and critical review.
DISCLOSURES
The authors declare no conflicts of interest.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2021.101462.
Appendix. Supplementary materials
REFERENCES
- Abd El-Hack M.E., Alagawany M., Arif M., Chaudhry M.T., Emam M., Patra A. Organic or inorganic zinc in poultry nutrition: a review. World Poult. Sci. J. 2017;73:904–915. [Google Scholar]
- Abo Ghanima M.M., El-Hack A., Mohamed E., Taha A.E., Tufarelli V., Laudadio V., Naiel M.A. Assessment of stocking rate and housing system on performance, carcass traits, blood indices, and meat quality of French Pekin ducks. Agriculture. 2020;10:273. [Google Scholar]
- Al-Sadi R., Guo S., Ye D., Ma T.Y. TNF-α modulation of intestinal epithelial tight junction barrier is regulated by ERK1/2 activation of Elk-1. Am. J. Pathol. 2013;183:1871–1884. doi: 10.1016/j.ajpath.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreini C., Banci L., Bertini I., Rosato A. Counting the zinc-proteins encoded in the human genome. J. Proteome Res. 2006;5:196–201. doi: 10.1021/pr050361j. [DOI] [PubMed] [Google Scholar]
- Andreini C., Bertini I. A bioinformatics view of zinc enzymes. J. Inorg. Biochem. 2012;111:150–156. doi: 10.1016/j.jinorgbio.2011.11.020. [DOI] [PubMed] [Google Scholar]
- Bao Y.M., Choct M., Iji P.A., Bruerton K. Trace mineral interactions in broiler chicken diets. Br. Poult. Sci. 2010;51:109–117. doi: 10.1080/00071660903571904. [DOI] [PubMed] [Google Scholar]
- Bao S., Liu M.J., Lee B., Besecker B., Lai J.P., Guttridge D.C., Knoell D.L. Zinc modulates the innate immune response in vivo to polymicrobial sepsis through regulation of NF-Κb. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010;298:L744–L754. doi: 10.1152/ajplung.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar Shira E., Friedman A. Innate immune functions of avian intestinal epithelial cells: response to bacterial stimuli and localization of responding cells in the developing avian digestive tract. PLoS One. 2018;13 doi: 10.1371/journal.pone.0200393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S.W., Wang P.Y., Zhu J., Chen G.W., Zhang J.L., Chen Z.Y., Pan Y.S. Protective effect of 1, 25-dihydroxyvitamin d3 on lipopolysaccharide-induced intestinal epithelial tight junction injury in caco-2 cell monolayers. Inflammation. 2015;38:375–383. doi: 10.1007/s10753-014-0041-9. [DOI] [PubMed] [Google Scholar]
- Chen T., Xie M.Y., Sun J.J., Ye R.S., Cheng X., Sun R.P., Zhang Y.L. Porcine milk-derived exosomes promote proliferation of intestinal epithelial cells. Sci. Rep. 2016;6:1–12. doi: 10.1038/srep33862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Xie J., Wang B., Tang J. Effect of γ-aminobutyric acid on digestive enzymes, absorption function, and immune function of intestinal mucosa in heat-stressed chicken. Poult. Sci. 2014;93:2490–2500. doi: 10.3382/ps.2013-03398. [DOI] [PubMed] [Google Scholar]
- Coornaert B., Carpentier I., Beyaert R. A20: central gatekeeper in inflammation and immunity. J. Biol. Chem. 2009;284:8217–8221. doi: 10.1074/jbc.R800032200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese-Krott M.M., Kulakov L., Opländer C., Kolb-Bachofen V., Kröncke K.D., Suschek C.V. Zinc regulates iNOS-derived nitric oxide formation in endothelial cells. Redox. Biol. 2014;2:945–954. doi: 10.1016/j.redox.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui H., Jing F., Xi P. Pathology of the thymus, spleen and bursa of Fabricius in zinc-deficient ducklings. Avian Pathol. 2003;32:259–264. doi: 10.1080/10307945031000097840. [DOI] [PubMed] [Google Scholar]
- Cui H., Xi P., Junliang D., Debing L., Guang Y. Pathology of lymphoid organs in chickens fed a diet deficient in zinc. Avian Pathol. 2004;33:519–524. doi: 10.1080/03079450400003528. [DOI] [PubMed] [Google Scholar]
- de Queiroz C.A., Fonseca S.G.C., Frota P.B., Figueiredo Í.L., Aragão K.S., Magalhães C.E.C., Oriá R.B. Zinc treatment ameliorates diarrhea and intestinal inflammation in undernourished rats. BMC Gastroenterol. 2014;14:1–14. doi: 10.1186/1471-230X-14-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Hennezel E., Abubucker S., Murphy L.O., Cullen T.W. Total lipopolysaccharide from the human gut microbiome silences toll-like receptor signaling. Msystems. 2017;2 doi: 10.1128/mSystems.00046-17. e00046-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Shall N.A., Awad A.M., El-Hack M., Naiel E.A., Mohammed A.E., Othman S.I., Allam A.A., Sedeik M.E. The simultaneous administration of a probiotic or prebiotic with live salmonella vaccine improves growth performance and reduces fecal shedding of the bacterium in salmonella-challenged broilers. Animals. 2019;10:70. doi: 10.3390/ani10010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiser J., Venken K.J., De Lisle R.C., Andrews G.K. A mouse model of acrodermatitis enteropathica: loss of intestine zinc transporter ZIP4 (Slc39a4) disrupts the stem cell niche and intestine integrity. PLos Genet. 2012;8 doi: 10.1371/journal.pgen.1002766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S., Al-Sadi R., Said H.M., Ma T.Y. Lipopolysaccharide causes an increase in intestinal tight junction permeability in vitro and in vivo by inducing enterocyte membrane expression and localization of TLR-4 and CD14. Am. J. Pathol. 2013;182:375–387. doi: 10.1016/j.ajpath.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S., Nighot M., Al-Sadi R., Alhmoud T., Nighot P., Ma T.Y. Lipopolysaccharide regulation of intestinal tight junction permeability is mediated by TLR4 signal transduction pathway activation of FAK and MyD88. J. Immunol. 2015;195:4999–5010. doi: 10.4049/jimmunol.1402598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern M.D., Denning P.W. The role of intestinal epithelial barrier function in the development of NEC. Tissue Barriers. 2015;3 doi: 10.1080/21688370.2014.1000707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X., Fink M.P., Yang R., Delude R.L. Increased iNOS activity is essential for intestinal epithelial tight junction dysfunction in endotoxemic mice. Shock. 2004;21:261–270. doi: 10.1097/01.shk.0000112346.38599.10. [DOI] [PubMed] [Google Scholar]
- He C., Deng J., Hu X., Zhou S., Wu J., Xiao D., Yang X. Vitamin A inhibits the action of LPS on the intestinal epithelial barrier function and tight junction protein. Food Funct. 2019;10:1235–1242. doi: 10.1039/c8fo01123k. [DOI] [PubMed] [Google Scholar]
- Hu X., Guo Y., Li J., Yan G., Bun S., Huang B. Effects of an early lipopolysaccharide challenge on growth and small intestinal structure and function of broiler chickens. Can. J. Anim. Sci. 2011;91:379–384. [Google Scholar]
- Hu C.H., Qian Z.C., Song J., Luan Z.S., Zuo A.Y. Effects of zinc oxide-montmorillonite hybrid on growth performance, intestinal structure, and function of broiler chicken. Poult. Sci. 2013;92:143–150. doi: 10.3382/ps.2012-02250. [DOI] [PubMed] [Google Scholar]
- Jia G., Yan J.Y., Cai J.Y., Wang K.N. Effects of encapsulated and non-encapsulated compound acidifiers on gastrointestinal pH and intestinal morphology and function in weaning piglets. J. Anim. Feed Sci. 2010;19:81–92. [Google Scholar]
- Lee B., Moon K.M., Kim C.Y. Tight junction in the intestinal epithelium: its association with diseases and regulation by phytochemicals. J. Immunol. Res. 2018;2018 doi: 10.1155/2018/2645465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Hu D., Huo H., Zhang W., Adiliaghdam F., Morrison S., Hodin R.A. Intestinal alkaline phosphatase regulates tight junction protein levels. J. Am. Coll. Surg. 2016;222:1009–1017. doi: 10.1016/j.jamcollsurg.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Lu R., Wu S., Sun J. Salmonella regulation of intestinal stem cells through the Wnt/β-catenin pathway. FEBS Lett. 2010;584:911–916. doi: 10.1016/j.febslet.2010.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou J., Chu G., Zhou G., Jiang J., Huang F., Xu J., He J. Comparison between two kinds of cigarette smoke condensates (CSCs) of the cytogenotoxicity and protein expression in a human B-cell lymphoblastoid cell line using CCK-8 assay, comet assay and protein microarray. Mutat. Res.-Gen. Tox. En. 2010;697:55–59. doi: 10.1016/j.mrgentox.2010.01.014. [DOI] [PubMed] [Google Scholar]
- Meyer M.M., Fries-Craft K.A., Bobeck E.A. Composition and inclusion of probiotics in broiler diets alter intestinal permeability and spleen immune cell profiles without negatively affecting performance. J. Anim. Sci. 2020;98:skz383. doi: 10.1093/jas/skz383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison A.B., Sarett H.P. Studies on zinc deficiency in the chick. J. Nutr. 1958;65:267–280. doi: 10.1093/jn/65.2.267. [DOI] [PubMed] [Google Scholar]
- National Research Council . Natl. Acad. Press; Washington, DC: 1994. Nutrient Requirements of Poultry. 9th rev. ed. [Google Scholar]
- Prasad A.S., Bao B., Beck F.W., Sarkar F.H. Zinc-suppressed inflammatory cytokines by induction of A20-mediated inhibition of nuclear factor-κB. Nutrition. 2011;27:816–823. doi: 10.1016/j.nut.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Rajasekaran S.A., Rajasekaran A.K. Na, K-ATPase and epithelial tight junctions. Front. Biosci. 2009;14:2130–2148. doi: 10.2741/3367. [DOI] [PubMed] [Google Scholar]
- Ranaldi G., Caprini V., Sambuy Y., Perozzi G., Murgia C. Intracellular zinc stores protect the intestinal epithelium from Ochratoxin A toxicity. Toxicol. In Vitro. 2009;23:1516–1521. doi: 10.1016/j.tiv.2009.08.012. [DOI] [PubMed] [Google Scholar]
- Ranaldi G., Ferruzza S., Canali R., Leoni G., Zalewski P.D., Sambuy Y., Murgia C. Intracellular zinc is required for intestinal cell survival signals triggered by the inflammatory cytokine TNFα. J. Nutr. Biochem. 2013;24:967–976. doi: 10.1016/j.jnutbio.2012.06.020. [DOI] [PubMed] [Google Scholar]
- Salim H.M., Jo C., Lee B.D. Zinc in broiler feeding and nutrition. Avian Biol. Res. 2008;1:5–18. [Google Scholar]
- Seth R., Corniola R.S., Gower-Winter S.D., Morgan T.J., Jr, Bishop B., Levenson C.W. Zinc deficiency induces apoptosis via mitochondrial p53-and caspase-dependent pathways in human neuronal precursor cells. J. Trace Elem. Med. Bio. 2015;30:59–65. doi: 10.1016/j.jtemb.2014.10.010. [DOI] [PubMed] [Google Scholar]
- Shao Y.X., Lei Z., Wolf P.G., Gao Y., Guo Y.M., Zhang B.K. Zinc supplementation, via GPR39, upregulates PKCζ to protect intestinal barrier integrity in Caco-2 cells challenged by salmonella enterica serovar typhimurium. J. Nutr. 2017;147:1282–1289. doi: 10.3945/jn.116.243238. [DOI] [PubMed] [Google Scholar]
- Shao Y., Lei Z., Yuan J., Yang Y., Guo Y., Zhang B. Effect of zinc on growth performance, gut morphometry, and cecal microbial community in broilers challenged with Salmonella enterica serovar typhimurium. J. Microbiol. 2014;52:1002–1011. doi: 10.1007/s12275-014-4347-y. [DOI] [PubMed] [Google Scholar]
- Shao Y., Wolf P.G., Guo S., Guo Y., Gaskins H.R., Zhang B. Zinc enhances intestinal epithelial barrier function through the PI3K/AKT/mTOR signaling pathway in Caco-2 cells. J. Nutr. Biochem. 2017;43:18–26. doi: 10.1016/j.jnutbio.2017.01.013. [DOI] [PubMed] [Google Scholar]
- Skrovanek S., DiGuilio K., Bailey R., Huntington W., Urbas R., Mayilvaganan B., Mullin J.M. Zinc and gastrointestinal disease. World J. Gastr. Pathol. 2014;5:496. doi: 10.4291/wjgp.v5.i4.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturniolo G.C., Di Leo V., Ferronato A., D'Odorico A., D'Incà R. Zinc supplementation tightens “leaky gut” in Crohn's disease. Inflamm. Bowel Dis. 2001;7:94–98. doi: 10.1097/00054725-200105000-00003. [DOI] [PubMed] [Google Scholar]
- Sugi K., Musch M.W., Chang E.B., Field M. Inhibition of Na+, K+-ATPase by interferon γ down-regulates intestinal epithelial transport and barrier function. Gastroenterology. 2001;120:1393–1403. doi: 10.1053/gast.2001.24045. [DOI] [PubMed] [Google Scholar]
- Tan Y., Zanoni I., Cullen T.W., Goodman A.L., Kagan J.C. Mechanisms of Toll-like receptor 4 endocytosis reveal a common immune-evasion strategy used by pathogenic and commensal bacteria. Immunity. 2015;43:909–922. doi: 10.1016/j.immuni.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vereecke L., Beyaert R., van Loo G. Enterocyte death and intestinal barrier maintenance in homeostasis and disease. Trends Mol. Med. 2011;17:584–593. doi: 10.1016/j.molmed.2011.05.011. [DOI] [PubMed] [Google Scholar]
- Wang L., Li J., Li J., Jr, Li R.X., Lv C.F., Li S., Zhang C.Q. Identification of the Paneth cells in chicken small intestine. Poult. Sci. 2016;95:1631–1635. doi: 10.3382/ps/pew079. [DOI] [PubMed] [Google Scholar]
- Wang X., Valenzano M.C., Mercado J.M., Zurbach E.P., Mullin J.M. Zinc supplementation modifies tight junctions and alters barrier function of CACO-2 human intestinal epithelial layers. Dig. Dis. Sci. 2013;58:77–87. doi: 10.1007/s10620-012-2328-8. [DOI] [PubMed] [Google Scholar]
- Wen M., Zhao H., Liu G., Chen X., Wu B., Tian G., Jia G. Effect of zinc supplementation on growth performance, intestinal development, and intestinal barrier-related gene expression in Pekin ducks. Biol. Trace Elem. Res. 2018;183:351–360. doi: 10.1007/s12011-017-1143-7. [DOI] [PubMed] [Google Scholar]
- Wight P.A., Dewar W.A. The histopathology of zinc deficiency in ducks. J. Pathol. 1976;120:183–191. doi: 10.1002/path.1711200308. [DOI] [PubMed] [Google Scholar]
- Wu Q.J., Zhou Y.M., Wu Y.N., Zhang L.L., Wang T. The effects of natural and modified clinoptilolite on intestinal barrier function and immune response to LPS in broiler chickens. Vet. Immunol. Immunopathol. 2013;153:70–76. doi: 10.1016/j.vetimm.2013.02.006. [DOI] [PubMed] [Google Scholar]
- Yang T., Wang X., Wen M., Zhao H., Liu G., Chen X., Jia G. Effect of manganese supplementation on the carcass traits, meat quality, intramuscular fat, and tissue manganese accumulation of Pekin duck. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F., Wang A., Zeng X., Hou C., Liu H., Qiao S. Lactobacillus reuteri I5007 modulates tight junction protein expression in IPEC-J2 cells with LPS stimulation and in newborn piglets under normal conditions. BMC Microbiol. 2015;15:1–11. doi: 10.1186/s12866-015-0372-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Chen Y., Li Y., Yang L., Wang J., Wang T. Medium-chain TAG attenuate hepatic oxidative damage in intra-uterine growth-retarded weanling piglets by improving the metabolic efficiency of the glutathione redox cycle. Br. J. Nutr. 2014;112:876–885. doi: 10.1017/S000711451400155X. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Eicher S.D., Ajuwon K.M., Applegate T.J. Development of a chicken ileal explant culture model for measurement of gut inflammation induced by lipopolysaccharide. Poult. Sci. 2017;96:3096–3103. doi: 10.3382/ps/pex160. [DOI] [PubMed] [Google Scholar]
- Zhang B., Guo Y. Supplemental zinc reduced intestinal permeability by enhancing occludin and zonula occludens protein-1 (ZO-1) expression in weaning piglets. Br. J. Nutr. 2009;102:687–693. doi: 10.1017/S0007114509289033. [DOI] [PubMed] [Google Scholar]
- Zhang H., Zhang B., Zhang X., Wang X., Wu K., Guan Q. Effects of cathelicidin-derived peptide from reptiles on lipopolysaccharide-induced intestinal inflammation in weaned piglets. Vet. Immunol. Immunopathol. 2017;192:41–53. doi: 10.1016/j.vetimm.2017.09.005. [DOI] [PubMed] [Google Scholar]
- Zhang X., Zhao L., Cao F., Ahmad H., Wang G., Wang T. Effects of feeding fermented Ginkgo biloba leaves on small intestinal morphology, absorption, and immunomodulation of early lipopolysaccharide-challenged chicks. Poult. Sci. 2013;92:119–130. doi: 10.3382/ps.2012-02645. [DOI] [PubMed] [Google Scholar]
- Zhong W., Li Q., Sun Q., Zhang W., Zhang J., Sun X., Zhou Z. Preventing gut leakiness and endotoxemia contributes to the protective effect of zinc on alcohol-induced steatohepatitis in rats. J. Nutr. 2015;145:2690–2698. doi: 10.3945/jn.115.216093. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.