Abstract
The genitourinary tract can be affected by several pathologies which require repair or replacement to recover biological functions. Current therapeutic strategies are challenged by a growing shortage of adequate tissues. Therefore, new options must be considered for the treatment of patients, with the use of stem cells (SCs) being attractive. Two different strategies can be derived from stem cell use: Cell therapy and tissue therapy, mainly through tissue engineering. The recent advances using these approaches are described in this review, with a focus on stromal/mesenchymal cells found in adipose tissue. Indeed, the accessibility, high yield at harvest as well as anti-fibrotic, immunomodulatory and proangiogenic properties make adipose-derived stromal/SCs promising alternatives to the therapies currently offered to patients. Finally, an innovative technique allowing tissue reconstruction without exogenous material, the self-assembly approach, will be presented. Despite advances, more studies are needed to translate such approaches from the bench to clinics in urology. For the 21st century, cell and tissue therapies based on SCs are certainly the future of genitourinary regenerative medicine.
Keywords: Genitourinary tract, Cell therapy, Tissue engineering, Stem cells
Core Tip: Considering the lack of adequate tissue to perform repair or replacement of organ/tissue for urologic patients, new strategies must be developed, and stem cell-based therapies and tissue engineering approaches seem promising therapeutic alternatives. A complete overview of stem cells used in urology will be presented with a focus on adipose-derived stem cells which have particularly drawn the attention of researchers. Finally, an innovative technique allowing tissue reconstruction without exogenous material, the self-assembly approach, will be presented.
INTRODUCTION
From the kidneys to the extremity of the urethra, urological tissues can be affected by several pathologies. These pathologies can mainly be divided in two groups: Congenital and acquired anomalies. Due to the severity of the anomaly or its recurrence, patients may require surgical reconstruction to restore the genitourinary tract's normal function. The repair and reconstruction of these damaged/abnormal tissues are still a challenge nowadays. Using transplantation of autologous tissues to restore the urogenital function remains the gold-standard[1]. However, this technique is limited by the characteristics of the donor, the secondary donor site injuries, and the adequacy of the function of the grafted tissue[2]. Indeed, it is easy to conceive that the intestinal bowel, used for ureteral reconstruction, cannot provide the required impermeability function[3] since the leading role of the intestinal bowel is to absorb nutrients, while the role of the ureters is the opposite, acting as a barrier to protect other tissues from urine. On the other hand, allogeneic transplantation is limited by the risk of tissue rejection and the availability of these tissues. The demand for transplantable tissues is increasing with the ageing of the population and the increasing incidence of several anomalies (e.g., hypospadias), while the offer remains low. Due to the lack of available tissues for these genitourinary reconstructions, efforts are invested in thinking outside the box. In the last decades, the emergence of regenerative medicine has been recognized as a promising avenue for meeting these clinical needs. Regenerative medicine allows to regenerate or replace human cells, tissues or organs, to restore a normal function[4]. This highly collaborative scientific field brings together many disciplines such as electrical, mechanical and tissue engineering, biochemistry, biophysics, cellular and molecular biology.
Regenerative medicine strategies can rely on two distinct approaches to restore the tissue functions: (1) Cell therapy: Injection of autologous or allogeneic cells or their secretome/conditioned medium to allow the regeneration of the tissues; and (2) Tissue therapy: Implantation of a synthetic or natural biomaterial, seeded or not with cells and eventually including growth factors, to improve and guide the repair process. It appears that the presence of cells in the biomaterials before grafting, including stem cells (SCs), is particularly important to the success of the implantation[5].
This review will shortly present the genitourinary tract anatomy to better understand its pathologies and why current therapies need improvement. Second, we will provide an overview of the different sources of SCs available for genitourinary regenerative medicine. The description of works in this field using SCs will be done with a particular focus on mesenchymal stem/stromal cells isolated from adipose tissue (AT) since data is now available from an increasing number of studies. Finally, an innovative technique allowing tissue reconstruction without the use of exogenous material, the self-assembly approach, will be presented.
ANATOMY, PATHOLOGIES AND CURRENT TREATMENTS
Kidneys
The kidney is one of the most challenging organs of the genitourinary system (Figure 1) to repair/reconstruct in regenerative medicine as its structure and roles are complex. Kidneys can be affected by numerous malfunctions. Chronic kidney disease (CKD) is one of the top causes of death and affects 9%-14% of the United States adult population[6,7]. CKD is defined as a glomerular filtration rate inferior to 60 mL/min per 1.73 m² or presence of kidney damage markers for at least three months[8]. It is mainly caused by diabetes, glomerulonephritis, pyelonephritis or hypertension, with a variation of its prevalence by ethnicity[8]. Congenital autosomal dominant polycystic kidney disease can also be a cause, with a prevalence of 1/400 to 1/1000 birth[9]. The quality of life of the patients and socioeconomic status are reduced as CKD progresses.
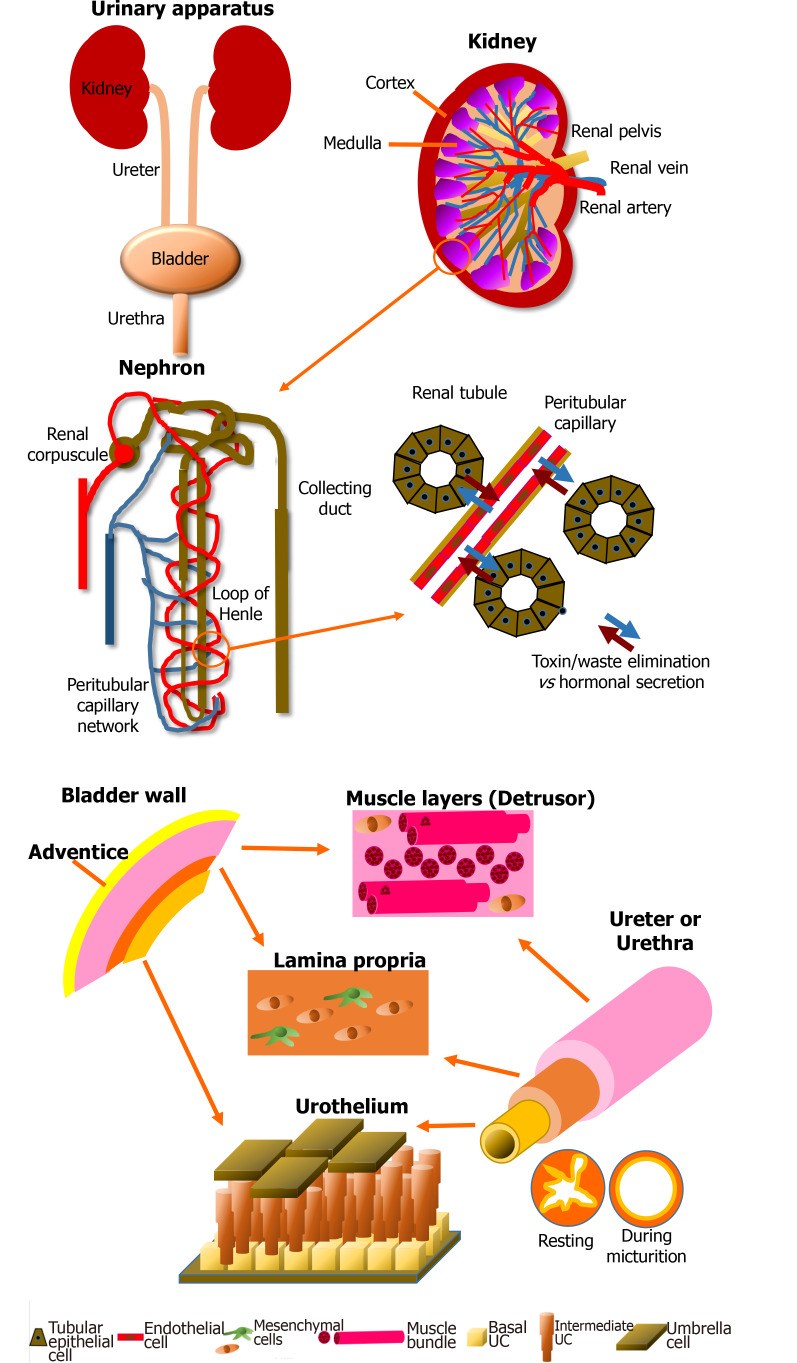
Figure 1.
Schematic of the anatomy of the urinary apparatus. Kidney: Kidneys are the blood filtration unit of the body, extracting toxins and metabolic waste to produce the urine purifying 180 L of blood every day. The nephron is the element which allows exchange between blood and urine through the renal tubules and the capillaries. Another role of the kidney is the regulation of the water balance when the body is sweating or in the presence of abundant/inadequate hydration by reabsorption of components during blood filtration. Kidneys also produce hormones essential for blood pressure regulation, such as renin, which will cause, via angiotensin II, stimulation of the secretion of aldosterone. Finally, 90% of the erythropoietin is secreted by this organ. This glycoprotein stimulates the proliferation and differentiation of erythrocyte precursors. Pathologies can impair these functions and endanger the patient’s life. Bladder: The central role of the bladder is to receive and dynamically store the urine produced by the kidneys through structural and regulatory mechanisms. The urine is brought to the bladder via the ureters. The bladder is also a complex organ composed of various layers. It is described from its outer surface to the lumen with the adventice (layer of fatty tissue), the detrusor muscle, the lamina propria (a connective tissue including a muscularis mucosae) and the epithelium, which is named the urothelium. The latter can be divided in three layers: basal, intermediate and superficial (umbrella). The impermeability is due to a thin asymmetric unitary membrane of uroplakin, expressed at the apical surface of mature urothelial cells called umbrella cells. Ureter and urethra: Ureters and urethra share a common primary role: to transport urine, allowing its excretion outside of the body. Anatomic organization of these organs are histologically related to the bladder due to their similar protective role against urine through the urothelial cell layers. From the inside to the outside, these two organs are composed of the transitional urothelial cell layer, a submucosa comprising fibroblasts and extracellular matrix, especially type I and III collagens, a muscular cell layer of smooth muscle cells, and an adventitia surrounding the organ. During the micturition, the star-shaped configuration of the urethra allows to compensate the increase in pressure. The muscular layers also play a critical role in the maintenance of the tubular shape.
Because nephrogenesis is limited to embryonic development, the intrinsic capacity to self-repair of adult kidneys is limited, causing a permanent loss of nephrons, which can lead to end-stage kidney disease[10,11]. Treatments at this stage are renal replacement or hemodialysis, but the mortality and morbidity remain high[12]. Non-drug related therapies for CKD rely mainly on lifestyle changes: Enhancing hydration and maintaining a diet rich in vegetables and fruits, smoking cessation, exercising, limiting alcohol intake, maintaining body mass index within the normal range, and limiting sodium and protein intake[13]. Treatments to control hypertension and hyperglycemia can also be administered to the patients[13].
Bladder
Bladder characteristics can be observed in Figure 1. Hormonal and metabolic factors contribute to the integrity and function of this organ. Its disturbance induces a dysfunction, which can lead from simple pain to even the need for the ablation of the bladder.
Regenerative medicine for bladder reconstruction or regeneration is necessary following various pathological conditions, such as cancer, trauma, congenital malformation, overactive bladder, interstitial cystitis, infectious cystitis (e.g., unresolved E. Coli-induced cystitis), ketamine-induced cystitis, inflammation, stress incontinence or voiding dysfunction. A loss of storage capacity or bladder compliance induces a frequent need to urinate. Chronic urinary tract infections, incontinence, and renal calculi, which can extend to renal failure, can affect these patients[14]. Despite side effects, bladder augmentation using bowel segments or “enterocystoplasty” often remains the proposed treatment to patients presenting these pathologies. This surgery is considered the "gold-standard" treatment[15]. However, short- and long-term complications often present since the leading role of the intestinal tissue is to absorb nutrients, whereas the role of the bladder is to protect against urine toxicity[16]. Thus, malignancies, metabolic complications such as reabsorption of acid, electrolyte disturbance and mucus retention, bladder calculi, bladder perforation, upper tract deterioration and chronic infections are commonly affecting patients that underwent enterocystoplasty[17,18].
Ureters and urethra
Ureters and urethra are the conduits of the urinary tract (Figure 1). These structures can be affected by both congenital and/or acquired anomalies. Due to their location, the most common causes of ureteral injuries are iatrogenic in 80% of the cases, while 20% are due to external trauma[19]. Ureteral injury occurs most commonly during gynecologic surgeries, with an estimation of 52% to 82%[19]. Depending on the pathology stage and evolution, different operations may be performed, such as endoscopic surgery, partial ablation by segmental resection or total ablation of the injured ureter with or without radical nephroureterectomy. End-to-end anastomosis is applied for patients presenting short-length ureteral deficit due to the successful outcomes of this technique[20]. For the long-segment reconstruction, the use of autologous bowel is the gold-standard[3]. However, this kind of tissue presents many potential complications, including metabolic imbalance, malabsorption of vitamins, cholelithiasis, nephrolithiasis and infections[21]. It is not unusual that multiple surgical procedures are needed over time due to stenosis or strictures at the repair site, which may require nephrectomy[3].
On the other extremity of the urinary tract, the urethra is prone to congenital anomalies. With 1/250 newborn boys affected, hypospadias represents 73.3% of congenital penile anomalies[22,23] and its prevalence is increasing[24]. This anomaly is due to a defect of the tubularization and the fusion of the urethral plate. Hypospadias is characterized by the inadequate position of the urethral meatus under the glans penis. This meatus can be localized on the ventral face of the penis in a position more or less close to the normal opening. The opposite urethral anomaly, epispadias, is less frequent with 1/10000 boys affected[25] and is characterized by a malposition of the urethral meatus from the dorsal side of the penis to the pubic symphysis. On the other side, acquired anomalies appears after birth, due to an external event (e.g., accident or a trauma). The most commonly acquired urethral anomaly is urethral stenosis (called stricture for anterior urethra), with an incidence of 0.6%, leading to more than 5000 hospital visits per year in the United States[26]. Stenosis is characterized by a urethral lumen becoming impeded by surrounding fibrotic tissues leading to a narrowing or even a total obstruction of the conduit. Stenosis is most commonly caused by an injury/trauma (e.g., motorcycle or bicycle accident), but it can also be induced by infection, surgical complications, lichen sclerosus or cancer. Depending on the severity of the pathology, few tissues can be used to repair or replace the penile anomalies. Skin grafts, tunica vaginalis[27,28], lingual or buccal mucosa have all been evaluated[29-36], but it appears that oral mucosa remains the gold standard[37]. However, these approaches are associated with many complications such as re-stenosis, numbness, submucosal scars, dry mouth, difficulty to open the mouth (contracture), neuro-sensory defects, lesions, discomfort, pain, and risk of infection. In addition, considering that a limited amount of tissue can be harvested, this is problematic for longer urethral anomalies in need of correction[38-43].
Problems related to current treatments
The paucity of native tissues available for repair, the anatomical characteristics, and the side effects such as morbidity at the donor site are significant limitations of the current treatments for severe urologic cases[3]. Moreover, long-term complications are commonly found such as fibrosis, malignancies, metabolic troubles, stenoses and fistulas[44]. To improve the quality of life of urologic patients, new and innovative therapeutic strategies need to be developed to be more efficient with less debilitating side effects. Regenerative medicine strategies using SC transplantation or their use in tissue engineering have been proposed as solutions.
REGENERATIVE MEDICINE STRATEGIES USING SCs
Stem cell sources for urologic regenerative medicine: A concise overview
SCs can be described as clonogenic self-renewing cells with the potential to differentiate into one or more cell types. These cells are maintained in an undifferentiated state in very specific microenvironment called niches[45,46]. They are classified according to their differentiation potency. Pluripotent SCs [or embryonic SCs (ESC)] are derived from the inner cell mass of the embryo blastocyst and can produce cells of the three germ layers. The use of ESCs is controversial due to ethical issues but also to their high capacity to create teratomas after injection. Interestingly, adult SCs can be harvested from many tissues (Figure 2), such as the brain, bone marrow (BM), peripheral blood, blood vessels, skeletal muscle, skin, cornea, retina, tooth dental pulp, digestive system, liver and, pancreas[47]. They can be divided in two main categories. The first one, composed of multipotent SCs able to differentiate into many cell types, include, for example, neuronal SCs (derived from the ectoderm), mesenchymal SCs (MSCs) and hematopoietic SCs (both derived from the mesoderm). The other category contains the unipotent SCs, which have a reduced range of differentiation, such as epidermal SCs (of ectodermal origin), muscle SCs (of mesodermal origin) or urothelial SCs (of endodermal origin).
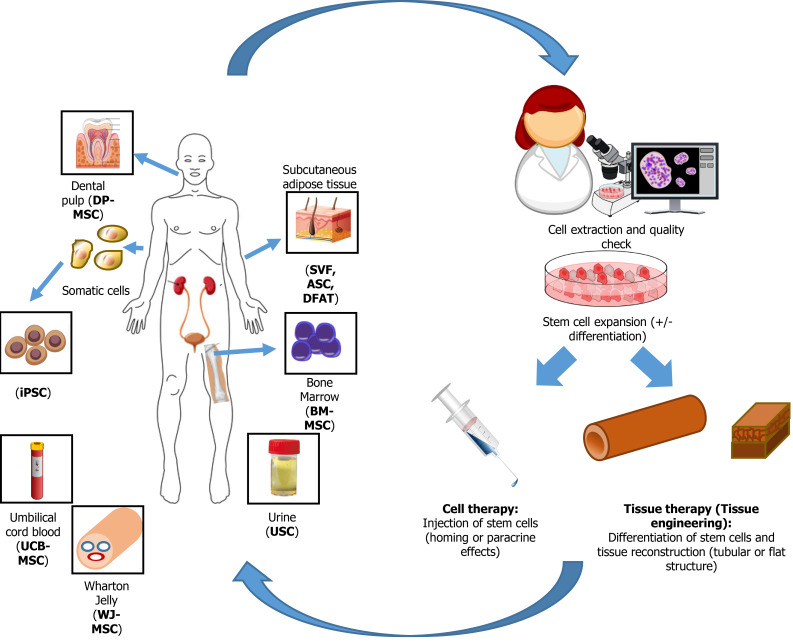
Figure 2.
Source of stem cells used for genitourinary regenerative medicine. After extraction from various tissues, stem cells are expanded and eventually differentiated, depending on their origin, before to be reinjected to the patients or used to reconstruct genitourinary engineered tissues. DP-MSC: Dental pulp-mesenchymal stem cells; BM-MSC: Bone marrow-mesenchymal stem cells; iPSC: Induced pluripotent stem cells; SVF: Stromal vascular fraction; ASC: Adipose-derived stromal/stem cells; DFAT: Dedifferentiated fat; UCB-MSC: Umbilical cord blood-mesenchymal stem cells; WJ-MSC: Wharton Jelly-mesenchymal stem cells.
Since the discovery of adherent SCs in BM by Friedenstein et al[48] 50 years ago[48], the number of MSC family members has increased considerably. These cells can now be extracted from various tissues such as bone marrow (BM-MSC), AT (adipose-derived SC or ASC), synovium (SMSC), dental pulp (DP-MSC), muscle, peripheral blood, yellow ligament, menstrual blood, endometrium, maternal milk, amniotic fluid, placenta (PDSC), umbilical cord blood (UCB-MSC), and umbilical cord Wharton’s jelly[49,50] (Figure 2). MSCs are heterogeneous populations, which can be important in regard of their differentiation efficiency in the context of animal grafting[49].
Many types of SCs have been studied (Table 1), but due to greater accessibility[51], postnatal SCs used in urology are mainly MSCs, especially but not exclusively BM-MSCs and ASCs. Indeed, through their secretome and multilineage potential of differentiation, MSCs are the basic building blocks used in regenerative medicine, including in the field of urology. For example, MSCs can differentiate into different cell types presenting features of various lineages, such as endothelial cells, epithelial cells (including urothelial cells), myoblasts, smooth muscle cells (SMCs), fibroblasts or neurogenic cells[52]. Because MSCs also have a significant cell proliferation and differentiation potential, only one sample containing multipotent SCs could theoretically allow the reconstruction of an entire urological organ. For example, human dental pulp SCs (DP-SCs) are also of interest in the urological field. Indeed, Song et al[53] successfully differentiated DP-SCs into bladder SMCs using a conditioned medium. Studies have shown that somatic SCs can be obtained from testes throughout the male lifetime[54]. Other reports have shown that even endometrial SCs (EnSCs) could be differentiated into SMCs and urothelial cells for bladder engineering[55,56]. The less invasive way to obtain these cells is by collecting menstrual blood, but an endometrial biopsy can also be performed. Harvesting EnSCs does not necessitate anesthetic procedures compared to the protocols used for ASCs or BM-MSCs[57].
Table 1.
In vivo studies for regenerative medicine of urologic tissues (excluding studies using adipose tissue-derived stem cells) (words used in the PubMed research engine (National Library of Medicine): “Urology” “regeneration” “reconstruction” “stem cells”)
|
Stem cell type
|
Organ treated
|
Pathology
|
Animal model
|
Ref.
|
| AFSC | Bladder | OAB | Rat | [203,204] |
| AFSC | Bladder | DUA | Rat | [205] |
| AFSC | Bladder | PD | Rat | [206] |
| AFSC | Bladder | Stroke | Rat | [203] |
| BM-MSC | Bladder | OAB | Rat | [207,208] |
| BM-MSC | Bladder | pBOO | Rat | [209] |
| BM-MSC | Bladder | IC/BPS | Rat | [210] |
| BM-MSC | Bladder | PD | Rat | [206,211] |
| BM-MSC | Bladder | SCI | Rat | [212-214] |
| BM-MSC | Bladder | Partial cystectomy | Rat | [215] |
| BM-MSC | Kidney | Renal regeneration | Mouse | [216] |
| BM-MSC | Kidney | CKD | Rat | [69,70] |
| BM-MSC | Kidney | AKI | Rat | [7,71] |
| BM-MSC | Kidney | AKI | Human | [83] |
| BM-MSC | Bladder | Partial cystectomy | Rat | [90] |
| BM-MSC | Urinary sphincter | SUI | Rat | [91] |
| BM-MSC | Bladder | Hemicystectomy | Dog | [97] |
| BM-MSC | Bladder | Augmentation | Rat | [98] |
| BM-MSC | Penis | ED | Rat | [217,218] |
| BM-MSC | Urethra | Urethroplasty | Rabbit | [103] |
| DP-SC | Bladder | IC/BPS | Rat | [219] |
| ESC | Kidney | Reconstruction | Rat/mouse | [88] |
| ESC-MGE | Bladder | SCI | Mouse | [220] |
| ESC-MSC | Bladder | IC/BPS | Rat | [221-223] |
| MDC | Bladder | DUA | Rat | [224,225] |
| MDC | Bladder | DUA | Mouse | [225] |
| NPC | Bladder | SCI | Rat | [226-228] |
| OM-SC | Bladder | SCI | Rat | [229] |
| Sk-MSC | Bladder | DUA | Rat | [230] |
| Sk-MSC | Urinary sphincter | SUI | Monkey | [231] |
| Sk-MSC | Urinary sphincter | SUI | Human | [92-94] |
| Sk-MSC | Urethra | Urethral defect | Rat | [102] |
| UCB-MSC | Bladder | OAB | Rat | [232] |
| UCB-MSC | Bladder | IC/BPS | Rat | [233-235] |
| UCB-MSC | Bladder | Cerebral Ischemia | Rat | [232] |
| USC | Bladder | IC/BPS | Rat | [236] |
| USC | Urethra | Urethral defect | Rabbit | [237] |
| USC | Penile Cavernous body | ED | Rat | [238] |
| USC | Kidney | AKI | Rat | [84] |
| USC | Bladder | Augmentation | Rat | [101] |
AKI: Acute kidney injury; ASCs: Adipose-derived stromal/stem cells; BM-MSC: Bone marrow-mesenchymal stem cells; UCB-MSC: Umbilical cord blood-mesenchymal stem cells; USC: Urine derived stem cells; DPSC: Dental pulp stem cells; OAB: Overactive bladder; DUA: Detrusor underactivity; PD: Peyronie’s disease; pBOO: Partial bladder outlet obstruction; IC/PBS: Interstitial cystitis/bladder pain syndrome; SCI: Spinal cord injury; SUI: Stress urinary incontinence.
Of note, in 2008, Zhang et al[58] showed for the first time that SCs present in urine, urine derived SCs (USCs) could differentiate into urothelial, SMCs, endothelial and interstitial cells, allowing a non-invasive collection of SCs for genitourinary tissue engineering[58]. This promising method generates approximately 2 to 7 progenitor cells for 100 mL of urine, which can be extensively expanded in culture. Subsequently, the differentiation of these cells toward the meso-, endo- and ectodermal lineages have been shown by using appropriate induction media, further increasing their interest[59].
Finally, a new source of SCs was created by human hands in 2006, the induced pluripotent SCs (iPSCs). After collecting somatic cells, such as fibroblasts or white blood cells, the latter are transduced using viral vectors to express transcription factors associated with pluripotency[60]. These reprogrammed cells can then be redifferentiated in vitro towards the cell type needed: e.g., into MSCs[61]. Because from one blood sample many differentiated cells can be obtained, but also because cells from patients suffering from various pathologies cannot be cultivated in vitro[62], iPSCs represent a promising SC source. However, their production appears much more technically challenging than for the previously described sources.
In the next sections, key examples of the use of SCs for genitourinary regenerative medicine will be presented before focusing on the most recent work using postnatal MSCs isolated from AT.
Regenerative medicine for kidney
The kidney function relies on complex vascular structures, and this organ is an ecosystem where 24 cell types synergize over the vascular, interstitial, glomerular and tubular compartments[63]. Reconstructing/regenerating this organ using regenerative medicine is a true challenge. Especially, the microvasculature of glomeruli and the complex tissue structure must be preserved. Even if progress has been made to support the renal function, the following section will only focus on studies using SCs as therapeutics. Two main strategies can be distinguished: (1) The repair and regeneration of the kidney using cell therapy; and (2) The reconstruction of the entire organ for transplant using tissue engineering.
Cell therapy for the kidney: BM-MSCs have been the most studied postnatal mesenchymal cell type, undoubtedly due to the demonstration of their safety in clinical trials (Table 1)[64]. Three hypotheses have been established concerning the effects of MSCs of various sources: (1) The regenerative potential of tissues is improved by the paracrine secretion of bioactive factors; (2) The regeneration of tissues is allowed by the differentiation of MSCs into resident cells to repopulate the tissue; or (3) The fusion of MSCs with resident cells[63]. Nevertheless, very few studies indicated that MSCs could fuse with resident cells[65] or transdifferentiate[66]. However, as low detectable differentiation of BM-MSCs into kidney epithelial cells to repopulate the tissue has been reported, a paracrine mechanism of action of MSCs to regenerate the kidney is suspected[67,68]. Beneficial effects were shown after exogenous administration of BM-MSCs in various models of acute and CKD. Direct injection of BM-MSCs in the kidney reduced or prevented renal dysfunction and renovascular hypertension in a CKD rat model[69,70]. Furthermore, in acute kidney injury (AKI) models and following BM-MSC injection, cellular proliferation was increased while the tubular dysfunction was reduced, including decreased apoptotic and necrotic cell death[71,72]. Supporting the paracrine hypothesis, studies have shown that most of the BM-MSC’s effects can be obtained using conditioned medium only[71,73,74]. Indeed, the immunomodulatory properties of MSCs are well-known, with an induction of anti-inflammatory factors such as interleukin (IL)-10 and a decrease in inflammatory factors (IL-1 and -6)[71,75,76]. On the other hand, the secretome of the MSCs is also characterized by growth factors such as hepatocyte growth factor (HGF), insulin-like growth factor-1, transforming growth factor (TGF)-α and -β, fibroblast growth factor (FGF) and vascular endothelial growth factors (VEGF), which can be enhanced by the preconditioning of the MSCs (e.g., hypoxic conditioning)[51,77,78]. MSCs can release these factors, also known to be produced by renal cells during kidney injury[63], either in a free-state or contained within exosomes, vesicles of 20-100 nm diameter originating from endosomes, or microvesicles, vesicles of 50-1000 nm originating from the plasma membrane[51]. These extracellular vesicles contain nucleic acids such as mRNAs and microRNAs, cytoplasmic and membrane proteins, and even bioactive lipids, all contributing to cell-cell communication and signal transmission[79].
Depending on the culture conditions (e.g., 3D microspheres), BM-MSCs can modulate their secretome, overproducing factors found in low quantity when cells are grown as monolayers, such as antiapoptotic and anticancer factors including tumor necrosis factor (TNF)-stimulated gene 6 protein (TSG-6) and IL-24[80,81]. The mechanism of action of the paracrine effects of MSCs to regenerate the kidney can be advantageous for clinical use compared to transplantation. Indeed, the large-scale culture of MSCs and harvesting of their secretory products, or the production of a recombinant mixture of proteins, can be done in good manufacturing practice (GMP) facilities and presents lower risks than direct injection of SCs[63]. However, these cell-free therapies' therapeutic efficacy has been limited by the low stability and retention of these components[82]. Indeed, cells are continuously producing factors, whereas cell-free therapies would need repeated injections. Using new technologies as supramolecular nanofibers peptides could be an alternative to improving kidney repair, enhancing, and prolonging bioavailability of these factors[82]. A clinical study on 66 patients with postoperative AKI showed that intra-aortic infusion of allogenic BM-MSCs led to a worse prognosis in the postoperative period[83]. Further studies are therefore needed to expand the use of global MSCs in clinical routine.
Interestingly, Sun et al[84] showed that USCs used as a therapy in a rat AKI model significantly improved the renal function and histological damage[84]. Furthermore, it inhibited the inflammation and apoptosis processes in the kidney while promoting tubular epithelial proliferation.
Tissue engineering for kidney reconstruction: Whole organ production has also been evaluated using tissue engineering. First, acellular structures have been produced and studied from porcine, human and rat kidneys[85]. The main advantage is the preservation of the architecture of the decellularized matrix. This architecture has been shown to impact cell morphology and differentiation[86]. Fully differentiated epithelial and endothelial renal cells can be seeded on the decellularized scaffold as done by Song et al[85] before perfusion in a whole-organ bioreactor[85]. When perfused through their intrinsic bed, the resulting substitute produced rudimentary urine in vitro. When grafted in vivo, grafts were perfused by the recipient’s circulation and produced urine through the ureteral conduit[85]. However, only a negligible excretion of urea and creatinine was measured. Similar results have been found by other teams[87]. In another study, rat kidneys were decellularized and seeded with pluripotent murine ESCs[88]. After the proliferation of the primitive precursor cells within the glomerular, vascular and tubular structures, cells were reported to express epithelial cell’s differentiation markers[88]. Despite promising preclinical outcomes obtained with animal models, such as the reduced acute and chronic kidney injuries[64,89], clinical trials remain in the early phases, mainly investigating the safety and some efficacy of allogenic MSC infusion[64,89].
Regenerative medicine for bladder
The bladder is a highly elastic hollow organ surrounded by three muscular layers forming the Detrusor. These muscular cells are essential to maintain a fully functional bladder. Moreover, neural and vascular networks are required for a healthy, competent bladder and self-control of the micturition. As for the kidneys, the bladder architecture must be preserved to ensure that efficacy is preserved.
Cell therapy for the bladder: Several studies have described phenotypic and physiologic similarities between adult MSCs and bladder SMCs. Sharma et al[90] indicated that unstimulated BM-MSCs have a similar contractile protein profile as the bladder SMCs[90]. Furthermore, no significant difference of increased magnitude of intracellular Ca2+ release has been found between both groups when stimulated. Another study using adult nude rats to evaluate bladder augmentation found that the BM-MSCs seeded on the poly 1,8 octanediol-cocitrate scaffold maintained a high level of protein expression of smooth muscle markers, significantly increasing the muscle-to-collagen ratios at ten weeks post augmentation[90]. Therefore, it has been of interest to evaluate the impact of MSC injection for patients affected by stress urinary incontinence (SUI). In an injured rat model, using serial vaginal dilatation, Dissaranan et al[91] showed that the leak point pressure (LPP) was significantly improved in animals receiving an injection of BM-MSCs compared to the controls[91]. These results suggest that factors secreted by MSCs, in general, can have therapeutic effects when injected. Local injection of autologous muscle-derived SCs has been evaluated in clinical trials and successfully improved patients' quality of life affected by SUI[92-94]. Since the main effects of MSCs have been attributed to their secretome, it will be interesting to evaluate the secretome-only injection as treatment or as prophylaxis for patients with SUI. Indeed, even without engraftment, the only use of MSC injection could result in the release of soluble factors and enhanced quality of life of patients[64]. Therefore, MSCs and their secretome could offer a safe and effective treatment for bladder dysfunction, targeting the pathophysiology instead of only targeting symptoms. However, the current state of knowledge needs more data in the long term to ensure a safe large scale and efficient use of these cells. Indeed, more studies are needed to determine the method of delivery (systemic or local injection), the dose and the most secure and efficient cell origin (ASCs, BM-MSCs, etc.).
Tissue engineering for bladder reconstruction: Reconstruction of a whole bladder to replace the affected one has been investigated by many teams. Tissue engineering for bladder reconstruction is based on the use of natural, synthetic or hybrid scaffolds. Decellularized scaffolds such as bladder acellular matrix (BAM) and small intestinal submucosa (SIS) have been studied for bladder reconstruction[2,95]. However, issues have been encountered. Indeed, the maximum distance allowing tissue regeneration by native cells using an acellular graft has been evaluated to 0.5 cm[5]. It is, therefore, necessary to seed cells on the scaffold before its implantation[5]. Nevertheless, autologous cells could not be used in patients with cancer[96] or benign end-stage bladder diseases[62]. In these cases, the use and differentiation of SCs could represent an alternative.
BM-MSCs have been evaluated as an alternative cell source. Zhang et al[97] used BM-MSCs on an SIS scaffold for bladder reconstruction in a hemicystectomy canine model[97]. They showed that BM-MSCs had a similar cell proliferation, histological appearance, and contractile phenotype as primary cultured bladder SMCs. Using an amniotic membrane, BM-MSC contributed to regenerate the detrusor and urothelium in a rat model of bladder augmentation[98]. However, proper urinary bladder function could not be achieved, and further studies are required.
Urine-derived SCs can also be considered an alternative source for bladder reconstruction as only a fresh -collected less than 24 h before- urine sample is necessary. To this end, studies showed that USCs could be differentiated in SMCs with contractile function comparable to native SMCs[99]. In another study, USCs have been differentiated in urothelial and SMCs that were then seeded on a bacterial cellulose scaffold[100]. They showed that a multilayered urothelium could be obtained with the colonization of the cells into the matrix, holding promises for urinary reconstruction using USCs. Lee et al[101] directly seeded undifferentiated USCs on a surface modified composite scaffold (polycaprolactone/pluronic bladder submucosa matrix) in a rat model to improve bladder capacity[101]. They showed a significant functional improvement of the bladder compliance compared to the control group and an increased regeneration of SMC tissues with a well-differentiated multilayered urothelium.
Regenerative medicine for the ureters and urethra
The primary function of ureters and the urethra are a strictly waterproof barrier preventing the toxic urine diffusion beneath the epithelium. The reconstruction of these structures must preserve this function otherwise fibrosis induction will occur. As these tubular organs are surrounded by a muscular layer allowing to compensate the increase of pressure during the micturition, elasticity and mechanical resistance have to be maintained in the reconstructed substitutes.
Cell therapy for ureters and urethra: Human skeletal muscle derived SCs were applied on the damaged urethral site in a rat model and improved penile functional recovery[102]. Indeed, six weeks after transplantation, a higher functional recovery was found in the transplanted group than controls (70.2% vs 39.1%). The authors indicated that transplanted human cells differentiated into skeletal muscle fibers, nerve-related Schwann cells, perineuriums, and vascular pericytes while active paracrine angiogenic cytokines were also detected.
Tissue engineering for ureter and urethra reconstruction: SCs have also been used for urethra and ureteral reconstruction. Yudintceva et al[103] seeded allogenic BM-MSCs on a bilayer poly-D,L-lactide/poly-ε-caprolactone scaffold for urethra reconstruction in a rabbit model in comparison with conventional urethroplasty, performed using an autologous buccal mucosa graft[103]. After a follow-up of 12 wk, the absence of complications, reduced fibrosis and inflammatory cell infiltration were observed in the experimental group compared to the group for which buccal mucosa was grafted.
In other studies, SCs have been first differentiated before being seeded on scaffolds. Urothelial and SMCs derived from USCs have been compared to native cells when seeded on a SIS scaffold[104]. The authors indicated that USCs expressed urothelial cell markers or SMC markers according to the differentiation protocol. Furthermore, the resulting tissues were similar to those formed when urothelial cells and SMCs derived from native ureters were used.
The optimization of the culture medium/media used to differentiate SCs is a challenge and must be different for each kind of SCs because their engagement status is different: ESCs is different from definitive endoderm SCs or urothelial cell progenitors. As an example of this kind of works, murine ESCs have been successfully differentiated into urothelial cells using chemically defined conditions. Such protocols could facilitate the generation of epithelial cells for in vitro tissue production[105].
TOWARDS CLINICAL APPLICATIONS
AT as a source of therapeutic cells
Beyond its metabolic and endocrine functions, white AT represents an important source of multipotent cells for regenerative medicine[106,107], with subcutaneous depots being most abundant and accessible in humans through lipoaspiration procedures. ASCs originate from the cultivation of cells extracted from the stromal vascular fraction (SVF) of AT. This classical way of extracting multipotent human ASCs has been mastered by Zuk et al[108,109] and reported in 2001 and 2002[108,109]. SVF corresponds to the fresh cellular pellet obtained after AT collagenase digestion and centrifugation[110] (Figure 3). SVF is heterogeneous, being composed of fibroblasts, endothelial cells, SMCs, macrophages, monocytes, preadipocytes and mesenchymal stromal/SCs[111,112]. This cell heterogeneity plays an important role conferring the SVF several important therapeutic characteristics such as pro-angiogenic, anti-inflammatory and immunomodulating properties[113-115]. However, it is also associated with a high variability of the SVF’s therapeutic potential among individuals, making it more complicated to standardize SVF-based treatments[116]. The use of SVF as treatment of urogenital disorders has been widely investigated in recent years in particular for preclinical studies for erectile disfunction and for Peyronie’s disease[117-120]. Moreover, a small number of early clinical studies using injected SVF for urogenital system related pathologies, like urinary incontinence, erectile disfunction, and Peyronie’s disease, have been performed in the last years[121-123]. Since treatments based on freshly extracted SVF cells have been recently reviewed[115,124], the following sections will focus on the use of cultured mesenchymal cells obtained from AT [ASCs and dedifferentiated (DFAT) cells] for regenerative medicine applied to the urologic system.
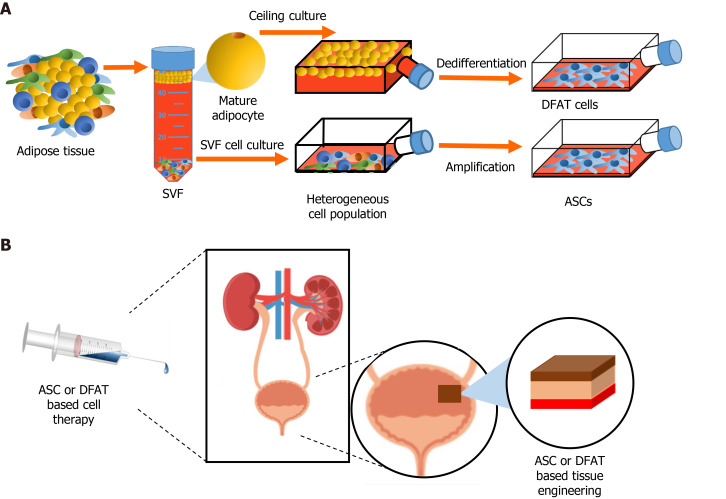
Figure 3.
Stem cells from adipose tissue in regenerative medicine. A: Extraction of the stromal vascular fraction from adipose tissue and obtention of adipose-derived stromal/stem cells (ASCs) after cell culture. Ceiling culture of mature adipocytes and resulting populations of dedifferentiated fat (DFAT) cells; B: Potential uses of ASCs and/or DFAT cell-based therapies in genitourinary regenerative medicine. SVF: Stromal vascular fraction; ASC: Adipose-derived stromal/stem cells; DFAT: Dedifferentiated fat.
There are two approaches for obtaining stromal/SCs from AT (Figure 3). ASCs represent the more common source as they are obtained following culture of the fresh SVF. ASCs are described as relatively homogenous adherent cultures of cells possessing key functional properties: Important multilineage differentiation potential and relevant secretome-related therapeutic potential[109,125,126]. Since their discovery, cell therapies based on ASCs have been developed for many pathological conditions including wound healing, cardiovascular diseases, bone fractures and many other health problems, including urological-related pathologies[115,127-131]. The second and most recent method of obtaining progenitor cells from AT depots is by inducing the dedifferentiation of floating mature adipocytes obtained following collagenase digestion, leading to cell populations commonly named DFAT cells (Figure 3). In fact, as soon as 1986, Sugihara et al[132] developed a ceiling culture method that induced the dedifferentiation of mature rat adipocytes into fibroblast-like cells[132]. Later in 2004, Yagi et al[133] showed that isolated fibroblast-like cell populations obtained following the dedifferentiation of mature murine adipocytes exhibited long term viability in culture and adipogenic potential upon induction[133]. The seminal study by Matsumoto et al[134] in 2008 showed that human cells originating from dedifferentiated adipocytes extracted from subcutaneous depots exhibited critical features associated with ASCs and BM-MSCs, including cell surface markers such as CD13, CD29, CD44, CD90 and CD105, and multilineage differentiation potential towards adipogenesis, osteogenesis, chondrogenesis[134]. More recent studies also indicate that DFAT cells could acquire myogenic and neural-like cells phenotype[135,136]. Importantly, as part of the secretome of these dedifferentiated cells, many growth factors with proangiogenic action have been quantified such as the VEGF-A, HGF, FGF and Angiopoietin-1 (Ang-1)[137]. DFAT cells, has been more widely studied in recent years for different fields of regenerative medicine. Some preclinical studies have shown DFAT cardiomyocyte differentiation and pro-angiogenic potential in myocardial infarction and nerve regeneration using rat models[136,138,139]. Despite the recent characterization of DFAT cells, some preclinical studies have already shown promising therapeutic potential for these cells. The following section details the recent studies that used ASCs and DFAT cells for the treatment of urogenital diseases.
ASC-based therapies and tissue engineering approaches for treating urogenital-related diseases/pathologies
In the last five years (2016-2021), different ASC-based therapeutic approaches have been developed to treat genitourinary-related pathologies. Recent cell therapies based on ASCs injections for treating kidney, ureter, bladder and urethral related pathologies in different animal models are presented (Table 2), as well as the latest tissue engineering efforts using ASCs to reconstruct 3D urologic tissues (Table 3).
Table 2.
Adipose-derived stromal/stem cells-based preclinical studies for treating urogenital related diseases/pathologies (2016-2021)
|
Year
|
Organ/tissue
|
Disease/method
|
Animal model
|
Type of therapy (cell type/host anatomic site)
|
Cell/molecule concentration
|
Outcomes
|
Ref.
|
| 2021 | Kidney | Renal interstitial fibrosis/unilateral urethral obstruction | Nu/nu mice 6–8 week-old males, n = 40 | Injection of genetically modified SC human GDNF-ASCs and non-modified ASCs/intravenous | 5 × 105 cells in 150 μL of saline | Improvement of vascular rarefaction/Renal protection against microvascular injuries/Oxidative stress reduction | Li et al[144], 2021 |
| 2020 | Kidney injury/ischemia-reperfusion | Wistar rats 100-200 g males, n = 28 | Injection of SC rat ASCs/tail vein | 2 × 106 cells in 1 mL of PBS | Reduction of total tissue damage and urine mineral concentration/ASC anti-inflammatory effects | Changizi-Ashtiyani et al[153], 2020 | |
| 2020 | Kidney injury/ischemia-reperfusion | SD rats 8–12 week-old males, n = N/S | Injection of epididymal rat ASCs/left kidney | 2 × 106 cells in 100 μL of decellularized kidney ECMH | Epithelial differentiation of post transplanted ASCs/accelerated repair of renal tubular injury via ASC pro-angiogenic molecules | Zhou et al[152], 2020 | |
| 2020 | Sepsis-induced AKI/cecal ligation and puncture | C57/BL6 mice 6–8 week-old males, n = 140 | Injection of SC human ASCs-derived exosomes/tail vein injection | 100 μg of exosomes in 200 μL of vehicle solution | Exosome protective functions against AKI/apoptosis and inflammation reduction via Sirtuin-1 pathway regulation | Gao et al[149], 2020 | |
| 2019 | Renal interstitial fibrosis/unilateral ureteral obstruction | Nude mice, n = 12 | Injection of SC human GDNF-ASCs/tail vein | 5 × 105 cells in 150 μL of vehicle solution | Macrophage transition from inflammatory (M1) to reparative (M2) phenotype/reduction of renal fibrosis and inflammation | Wang et al[143], 2019 | |
| 2019 | Diabetic nephropathy/induced diabetes | C57BL/KsJ db/db mice 8 week-old males, n = 20 | Injection of SC murine ASCs-derived exosomes/tail vein | N/S | Attenuation of spontaneous diabetes and nephropathy by reduced proteins levels in the urine of treated mice | Jin et al[148], 2019 | |
| 2017 | Renal interstitial fibrosis/unilateral ureteral obstruction | Wistar rats 6 week-old males, n = 45 | Injection of epididymal rat ASCs/tail vein | 5 × 106 cells in 1 mL of vehicle solution | Significantly reduced EMT and inflammatory response via TGF-β1 signaling pathway inhibition in treated rats | Song et al[140], 2017 | |
| 2017 | Chronic kidney injury/adenine intoxication | Wistar rats 250 g males, n = 12 | Injection of SC human ASCs/tail vein | 2 × 106 cells in vehicle solution | Reduction of kidney fibrosis/improved creatine and urea in serum/significantly lower expression of profibrogenic genes in treated rats | Rivera-Valdes et al[141], 2017 | |
| 2017 | Acute kidney injury/ischemia-reperfusion | SD rats 220-250 g males, n = 32 | Injection of perinephric human ASCs or SVF/intra-parenchymal | 2 × 106 cells in 100 μL of PBS | SVF and ASCs equally improved renal injury by promoting cell proliferation and decreasing tubular injury and cell apoptosis | Zhou et al[146], 2017 | |
| 2016 | Acute kidney injury/ischemia-reperfusion | SD rats 250-300 g males, n = 72 | Injection of rat ASCs/tail vein | 1 × 106 cells in vehicle solution | Significantly lower kidney injury scores at days 1 and 3 post-treatment/not significant improvement at day 7 post-treatment | Sheashaa et al[145], 2016 | |
| 2016 | Acute kidney injury/IRI | SD rats 320-350 g males, n = 40 | Injection of epididymal rat ASCs and ASCs-derived exosomes/intravenous | 1.2 × 106 cells + 100 μg of ASCs-derived exosomes | Combined ASCs and exosomes confer higher kidney protection towards IRI than either one alone | Lin et al[147], 2016 | |
| 2016 | Chronic kidney disease/already present | Cats (various sex, age and breeds), n = 8 | Injection of allogenic cryopreserved feline ASCs/cephalic vein | 2 × 106 cells per kg in vehicle solution | No significant improvement of renal functions between treated and control groups/not adverse side effects noticed using allogenic ASCs | Quimby et al[150], 2016 | |
| 2018 | Urethra | Stress Urinary Incontinence/pudendal nerve transection | SD rats adult females, n = 48 | Injection of exosomes derived from SC human ASCs/peripheral urethral | 50 μg of exosomes in 50 μL of saline | Increased bladder capacity and leak point pressure/higher muscle fiber and nerve fiber regeneration | Ni et al[171], 2018 |
| 2018 | Stress Urinary Incontinence/pudendal nerve transaction | SD rats 6-7 week-old females, n = 144 | Injection of inguinal rat ASCs/transurethral sphincter | 1 × 106 cells in 400 μL of D-Hanks’s solution | ASCs in vivo viability 60 d post-implantation/higher content of striated muscle in the urethra/higher values of leak point pressure | Cui et al[170], 2018 | |
| 2018 | Urethral stricture/N/S | SD rats, N/S | Injection of miR-21 modified SC human ASCs/ urethral wall | 1 × 106 cells in 100 μL of saline | miR-27 cells increased epithelium and smooth muscle layer formation compared to normal ASCs/improve the epithelial wound healing microenvironment | Feng et al[168], 2018 | |
| 2016 | Urethral fibrosis/TGF-β1 induced model | SD rats 300 g males, n = 18 | Injection of inguinal rat ASCs/urethra | 2 × 105 cells in 50 μL of saline | Significantly decreased fibrosis evaluated by reduced collagen type I and III expression | Sangkum et al[167], 2016 | |
| 2016 | Urethral stricture/induced by TGF-β1 and surgical incision | SD rats 300-350 g males, n = 36 | Injection of SC human ASCs/urethral wall | 1 × 106 cells in 100 μL of PBS | Increased bladder capacity (50%)/wider urethral lumen/decreased expression of fibrosis-related genes | Castiglione et al[166], 2016 |
AKI: Acute kidney injury; ASCs: Adipose-derived stromal/stem cells; db/db: Spontaneous diabetes; ECMH: Extracellular matrix hydrogel; EMT: Epithelial mesenchymal transition; g: grams; GDNF: Glial-derived neurotrophic factor; IRI: ischemia-reperfusion injury; N/S: Not specified; PBS: Phosphate-buffered saline; SC: Subcutaneous; SD: Sprague-Dawley; SVF: Stromal vascular fraction; TGF-β1: Transforming growth factor-β1.
Table 3.
Preclinical studies of urogenital related pathologies/disease using adipose-derived stromal/stem cells in tissue engineering (2016-2020)
|
Year
|
Organ/tissue
|
Approach
|
Animal model
|
Substitute implantation (cell type and scaffold/host anatomic site)
|
Cell concentration per scaffold
|
Outcomes
|
Ref.
|
| 2020 | Kidney | Diabetic nephropathy/unilateral nephrectomy | SDT fatty rats 5-week-old males, n = 21 | SC rat ASCs three-layer sheets/renal capsule transplantation | 1 × 106 cells in 35-mm culture dish/sheet | 14-d survival of transplanted sheets/significantly lower urinary TNF-α levels/maintained renal tubular structure in treated rats | Takemura et al[155], 2020 |
| 2018 | Kidney reconstruction | Wistar rats 6-8 week-old males | Inguinal rat ASCs seeded onto a rat decellularized kidney/no implantation | 1 × 107 cells in 2 ml of culture medium per decellularized kidney | ASCs differentiated into endothelial and tubular cells after 5 d of culture/few cells attached to the scaffold after 10 d | Xue et al[154], 2018 | |
| 2016 | Ureter | Artificial ureter injury/surgical excision | New Zealand white rabbits3.5 kg females, n = 20 | Smooth muscle like-cells from SC rabbit ASCs seeded onto ventral aorta/decellularized matrix/graft placed over ureter defect | N/S | Seeded ASCs showed urothelial and smooth muscle-like cells phenotype in the ureter substitute 8 wk after implantation | Zhao et al[165], 2016 |
| 2020 | Bladder | Complete bladder removal/surgical excision | SD rats 300 g adult females, n = 9 | SFP human ASCs seeded onto a decellularized rat bladder matrix/bladder transplantation | 1 × 106 cells in 500 μL of cells suspension/bladder scaffold | Acquisition of a smooth muscle-like phenotype of seeded ASCs seeded/ASC paracrine effect increased vascularization and innervation | Moreno-Manzano et al[163], 2020 |
| 2020 | Sub-totally resected urinary bladder/upper two-thirds bladder excision | Athymic rats 200 g adult females, n = 9 | Smooth muscle-like cells from SC human ASCs seeded onto 3-layer PLGA sheet/bladder graft anastomosis | 1 × 106 cells mixed with 500 μL of human plasma/scaffold | Complete bladder regeneration and functionality restoration/fusion of smooth muscle-like cells in the regenerated muscular layer | Salem et al[162], 2020 | |
| 2019 | Bladder injury/surgical incision(1 cm) | SD rats 6-week-old females, n = 48 | Inguinal rat ASCs cells and PGA combined sheets/bladder patch anastomose | 1 × 105 cells/cm2 per sheet reconstruction | Patches promote urothelium, smooth muscle, neural and blood vessel regeneration/restored bladder function | Wang et al[151], 2019 | |
| 2018 | Bladder augmentation/cystotomy incision (1 cm) | SD rats 8 week-old males, n = 34 | Inguinal rat ASCs seeded onto PCL-Chitosan scaffold/bladder substitute anastomose | 15 × 107 cells/mL per scaffold | Higher smooth-muscle regeneration from ASCs/larger bladder capacity/increased angiogenesis | Zhou et al[161], 2018 | |
| 2017 | Bladder augmentation/surgical incision(1 cm) | SD rats 8 week-old females, n = 46 | SC rat ASCs seeded onto an AM-SF scaffold/bladder substitute anastomose | 10 × 107 cells/mL in 40 μL of saline per scaffold | Bladder capacity augmentation (30%)/relatively normal micturition pattern/ASC viability after 12 wk of implantation | Wang et al[157], 2017 | |
| 2017 | Bladder augmentation/surgical incision (1 cm) | SD rats 8 week-old males, n = 30 | Inguinal rat ASCs seeded onto a BAMG-SF scaffold/bladder substitute anastomosis | 50 μL of cell suspension at 1 × 108 cells/mL per scaffold | Higher bladder capacity (2.3-fold)/Enhanced VEGF angiogenic potential by ERK ½ phosphorylation | Xiao et al[160], 2017 | |
| 2017 | Augmentation cystoplasty/surgical incision (1 cm) | SD rats 8 week-old males, n = 30 | Rat ASCs encapsulated in an ADA/GEL seeded onto a porcine BAMG/bladder substitute anastomosis | 100 μL of encapsulated cells at a 1 × 106/mL concentration per scaffold | Morphological bladder restoration by enhanced scaffold degradation/enhanced VEGF-mediated angiogenesis and smooth muscle regeneration in treated rats | Xiao et al[210], 2017 | |
| 2016 | Bladder augmentation/surgical incision (1 cm) | SD rats immunocompetent 36 week-old males, n = 30 | Inguinal rat ASCs seeded onto a porcine BAMG/Bladder substitute anastomosis | 15 × 107/mL cell suspension per scaffold | Greater bladder capacity in experimental group/equal urothelial regeneration in the treated and non-treated groups at 4- and 14-wk post-implantation | Zhe et al[156], 2016 | |
| 2016 | Partial cystectomy/half upper bladder transection | Beagle dogs 10-12 Kg males, n = 12 | Human ASCs seeded onto a whole porcine BAMG/scaffold grafted onto bladders’ dome | 1 × 105 cells per cm2 of each scaffold | Complete urothelial coverage of seeded and unseeded bladder after 6 mo/higher capillary density and smooth muscle organization in treated dogs’ bladder | Hou et al[159], 2016 | |
| 2020 | Urethra | Urethral injury/surgically induced | New Zealand white rabbits 9-week-old males, n = 24 | SC rabbit ASCs seeded onto a human DAM scaffold/urethral graft | 1 × 106 cells per scaffold | Higher number of urethras healed following seeding of ASCs onto DAM | Hariastawa et al[172], 2020 |
| 2020 | Urethral injury/surgically induced (2 cm × 0.6 cm) | New Zealand white rabbits males, n = 15 | Inguinal rabbit ASCs seeded in a nanofibrous scaffold/graft placed over urethral defect | 1 × 107 cells per scaffold | Hypoxia preconditioning of ASCs increased urethral lumen diameter/preserved morphology/enhanced angiogenesis | Wan et al[173], 2020 |
ADA-GEL: Alginate dialdehyde-gelatin; AM-SF: Autologous myofibroblast-silk fibroin; ASCs: Adipose-derived stromal/stem cells; BAMG-SF: Bladder acellular matrix graft-silk fibroin; bFGF: Basic fibroblast growth factor; DAM: Dried amniotic membrane; g: gram; Kg: Kilogram; N/S: Not specified PCL: Polycaprolactone; PGA: Polyglycolic acid; PLGA: Poly(lactid-co-glycolic acid); SC: Subcutaneous; SD: Sprague-Dawley; SDT: Spontaneously diabetic Torii; SFP: Suprapatellar fat pad; TNF-α: Tumor necrosis factor-α; VEGF: Vascular endothelial growth factor.
ASC-based therapies in renal diseases: Various ASC-based therapies have been developed in the last five years to treat different aspects of CKD due to their therapeutic properties. Fibrosis is a late manifestation of CKD that is often irreversible. Therefore, numerous teams are focusing their efforts on using ASCs for the treatment of Renal Interstitial Fibrosis (RIF) (Table 2). Among recent studies, Song et al[140] described the anti-inflammatory effects of rat ASCs in RIF rat models, where lower gene expression of TNF-α and IL-1 were observed in treated rats compared to control[140]. Moreover, ASCs also mediated partial inhibition the TGF-β1 signaling axis, therefore significantly suppressing the epithelial-mesenchymal transition (EMT) of tubular epithelial cells involved in fibrosis[140]. Rivera-Valdes et al[141] also showed the potential of human ASCs in reducing RIF in rats[141]. Treated groups showed significantly reduced gene expression of COL1A1 and ACTA2, leading to 89% less collagen deposition and to a 40% reduction of fibrosis as assessed by a morphometric analysis of microphotographs of Masson trichrome and Sirius red stained tissues when compared to control groups[141].
Fibrosis typically results from chronic inflammation. The anti-inflammatory effects of MSCs have long been known as an important tool for treating fibrotic tissue phenotypes[142]. Recent research suggests that production of glial-derived neurotrophic factor (GDNF) by human ASCs can provide renoprotective effects in treated RIF mouse models[143]. Indeed, Wang et al[143] recently showed that compared to wild-type human ASC-based therapy, ASCs genetically modified to overexpress GDNF (GDNF-ASCs) enhanced tissue repair by increasing macrophage transition from an M1 inflammatory phenotype to an M2 reparative phenotype in mice[143]. More recently, Li et al[144] showed in 2021 that interstitial fibrosis mice treated with GDNF-ASC exhibit reduced capillary rarefaction, significantly reducing oxidative stress, leading to renal protection to microvascular injuries (EMT inhibition via PI3K/AKT signaling pathway), and renal fibrosis in treated mice[144]. This suggests that GDNF upregulation could be a future candidate for improving CKD-related fibrosis.
In the last years, different teams have developed ASC-based treatments for Ischemia-Reperfusion-Injury (IRI) animal models of AKI. In 2016, Sheashaa et al[145] showed in a IRI rat model that rat ASC-based systemic therapy significantly reduced creatinine levels in serum by increasing creatinine clearance for seven days after treatment. In the first three days, lower injury scores were also observed in treated rats compared to controls[145]. However, this study stipulates that injury scores did not improve further after one week of therapy and creatinine levels were not followed beyond seven days of treatment.
A study by Zhou et al[146] showed that injection of human SVF and cultured ASCs significantly reduced proinflammatory and immunomodulatory cytokine production (TNF-α and IL-10) in treated IRI rats. In this study, treated animals showed increased densities of peritubular kidney capillaries, with similar improvements achieved by SVF and ASCs[146]. Using a different approach, Lin et al[147] tested a combination of rat ASCs and ASC-derived exosomes (Ex-ASC) in IRI rat models. Their results showed that rats treated with this combined therapy showed a significant improvement of AKI features compared to ASCs and exosome only treated groups[147]. Exosomes therapeutic mechanisms of action are still not well understood. However, recent studies involving a murine model of diabetic nephropathy suggest that miR-486, carried in murine ASC-derived exosomes, downregulated mTOR activation-mediated autophagy in podocytes of treated mice[148]. This translated into a decreased podocyte autophagy disfunction-mediated injury, reducing tissue damage and improving renal function[148]. Findings in sepsis-derived AKI mice models also suggest that ASC-derived exosomes contribute to reduced renal damage by inhibiting apoptotic responses via Sirtuin-1 (SIRT1) pathway activation, reducing the inflammatory reaction[149]. However, similar to most studies available, rather short-term effects of ASC treatments have been described, and further investigations are needed to better understand and establish if long-term therapeutic outcomes can be achieved.
The study by Quimby et al[150] in 2016 evaluated longer term effects of ASCs in a CKD feline model by performing three allogenic injections of cat ASCs at two, four, and six weeks. Although this study highlighted the safety of repeated allogenic ASC injections, treated cats showed no improvement compared to controls (n = 4 per group)[150]. However, important parameters must be taken into consideration, such as the cat’s sex, age, and breed, which were considerably different between the experimental groups. In addition, the cells used for each of the three injections were obtained from different cat donors. These conditions complicate the analyses and conclusions that can be drawn.
ASC’s delivery vehicle is an essential component of the SCs therapies since it can act on cell viability and therapeutic potential by improving cell retention and reducing cell stress[151]. Embedding cells into an acellular matrix can have such protective effects. Indeed, the use of a decellularized matrix hydrogel (DMH) was shown to improve ASC local delivery and survival in a IRI rat model. After 30 d of ASC-DMH injection into the kidney, tubular epithelial-like cells differentiation and viability of ASCs were observed in the kidney of treated mice[152]. This suggests that the use of decellularized matrix may induced ASC differentiation into an epithelial-like phenotype, promotes growth factor secretion and improved ASCs long term viability in treated rats after IRI. In 2020, Changizi-Ashtiyani et al[153], showed the impact of rat ASCs injected before inducing IRI induced CKD. Results revealed the renoprotective effects of ASC-based therapy in a IRI rat model. Compared to non-treated animals, treated rats showed 2.5 times lower urea levels in the blood, higher urine osmolarity, significantly reduced oxidative stress levels and higher protection of kidney tissue 48 h after treatment[153].
As mentioned before, organ transplantation is one of the most common therapeutic approaches for advanced CKD. Therefore, many researchers, in addition to developing ASC-related cell therapies, have also undertaken the development of tissue engineering approaches using ASCs as the primary therapeutic component. Selected studies are described in Table 3. For example, Xue et al[154] recently developed a reconstructed rat kidney model by seeding rat ASCs in a decellularized rat whole kidney. The authors concluded that ASCs underwent differentiation towards endothelial-like and tubular cells with high cellular adherence to the scaffold induced by the action of stromal cell-derived factor 1 (SDF-1) and the cell-scaffold interactions[154]. Therefore, even if in vivo implantation was not performed in this study, it suggests ASCs can be beneficial when used in recellularization approaches for future clinical applications[154]. Although complete kidney reconstruction seems like an ideal goal, research is still far from the development of an entire functional kidney to be used in clinical trials. However, different tissue engineering approaches could still contribute to kidney disease improvement. Takemura et al[155] recently showed that grafting reconstructed sheets produced from rat ASCs significantly lowered the secretion of proinflammatory molecules, reduced renal tubules atrophy, and contributed to the maintenance of typical renal tubular structures in diabetic nephropathy treated mice[155]. However, ASC survival was only followed and observed for 14 d after implantation of the cell sheets in treated mice. Additional follow-up studies showing are thus needed to understand better the therapeutic potential of ASCs reconstructed sheets.
ASC-based tissue engineering approaches targeting bladder disorders: Many studies aim to achieve bladder reconstruction as a clinical alternative for treating different bladder-related diseases. Either complete or partial bladder reconstruction with MSCs have been suggested as promising approaches using bladder augmentation different animal models[52]. Among those studies, selected research from last five years using ASC-based tissue engineering approaches are shown in Table 3.
Zhe et al[156] used a porcine BAM scaffold seeded with undifferentiated rat ASCs, using an incubation period in the peritoneal cavity in a rat bladder augmentation model. This study showed that 14 wk postoperatively, treated rats displayed significant signs of improved regeneration of bladder SMCs, nerve cells, as well as increased bladder capacity compared to the BAM only controls[156]. Of note, seeded ASCs were not detected in the graft after 14 wk post-implantation. Increasing ASCs long-term viability and maintenance still represent a challenge for bladder engineering.
BAM graft is commonly used as a scaffold for achieving bladder tissue reconstruction, but new scaffolds have been tested lately. For example, Wang et al[157] in 2017 showed a novel autologous myofibroblast (AM)-silk fibroin (SF) scaffold obtained by incubating pig BAM treated with a SF solution[157]. This scaffold was implanted subcutaneously in the back of female SD rats. Their results showed that using an AM-SF scaffold contributed to bladder augmentation in rats with ASC viability still detected 12 wk after implantation[157]. Similar results were also obtained by this team using a pig BAMG-SF for bladder reconstruction in rats[158].
A recent preclinical study in larger animals, namely a dog model (n = 13) evaluated the therapeutic properties of human ASCs for bladder reconstruction when seeded in a pig BAM scaffold[159]. This study showed significantly increased bladder volume and compliance, in addition to an increased bladder regeneration by smooth muscle differentiation and improved vascularization in treated dogs six months after implantation compared to BAM only controls[159]. Different biomaterials and seeding methods have been developed in recent years, such as encapsulation of rat ASCs in an alginate dialdehyde gelatin (ADA/Gel) hydrogel[160] or polycaprolactone/chitosan scaffolds[161] promoting bladder regeneration in rats with a contribution of ASCs cells to differentiation into smooth muscle-like cells in vivo, as shown in previous studies.
The more recent work of Salem et al[162] highlighted the potential of human ASCs when the latter were predifferentiated into SMCs and seeded in a triple layered poly(lactid-co-glycolic acid) (PLGA) sheet for bladder reconstruction[162]. This study showed that short-term results (two weeks) of the implanted grafts in athymic rats were similar among ASC-PLGA and PLGA only treated groups. However, in the follow-up analyses after 12 wk, only the ASC-PLGA treated group showed regeneration within the main bladder layers (mucosal, stratified urothelium, submucosal and muscular layer) with a significantly restoration of bladder functions[162]. The study by Moreno-Manzano et al[163] showed similar results of bladder regeneration in a model of partial cystectomy in rats after treatment with a BAM seeded with undifferentiated human ASCs[163]. Taken together, these studies support a promising use of ASCs, warranting further research on their mechanisms of action, before future clinical trials are designed.
ASC-based cellular therapies and tissue engineering for ureteral and urethral diseases: The ureters and the urethra are fibromuscular tubes that can be affected by physiological defects such as ureteral and urethral fibrosis, urethral stricture (US), among others, that can compromise these organs' optimal function, justifying the need for new therapeutic strategies[164].
To our knowledge, cell therapy by ASC injection has not been performed yet for ureters. However, tissue reconstruction has been achieved (Table 3). For example, in 2016, Zhao et al[165] showed that rabbit ASCs seeded onto decellularized matrix obtained from rabbit ventral aorta and cultured in vitro for six weeks produced a well-structured ureter eight weeks post-transplantation in a rabbit[165]. This study suggested a five-step strategy for ureteral tissue engineering such as ASCs culture, urothelium and smooth muscle phenotype induction, sandwich co-culture of the vessel extracellular matrix scaffold and the differentiated cells followed by ureteral maturation within the omentum before transplantation[165].
For urethral therapies, ASCs have been used to treat US and fibrosis in the last years (Table 2). First, in 2016 Castiglione et al[166] showed that the injection of human ASCs directly in the urethral wall of stricture in rats resulted in an increased bladder capacity (50%) with a wider urethral lumen in addition to decreased expression of fibrosis-related genes compared to the non-treated control[166]. In the same year, Sangkum et al[167] showed in a rat model of urethral fibrosis that injection of rat ASCs directly onto the urethra of treated animals significantly decreased submucosal fibrosis and collagen type I and III protein production[167]. Taken together, these studies suggest that ASCs injection could represent a potential treatment for preventing scar formation in US disease. Recent studies aimed at increasing the therapeutic potential of ASCs. For example, Feng et al[168] used genetically modified miR-21 human ASCs for treating US in rat models[168]. This study showed that miR-21-ASCs significantly increased proangiogenic gene expression, such as hypoxia inducible factor-α, VEGF, bFGF, HGF-1, stem cell factor and SDF-1. Furthermore, miR-21-ASCs therapy also improved the epithelial wound healing microenvironment, smooth muscle layer formation and enhanced SC survival compared to normal ASC-treated rats[168]. This work thus suggests a new approach for enhancing urethral repair for future urethroplasty interventions.
Urinary incontinence is also a common medical condition and is related to a lowered basal LPP below 60 cm H2O or a maximal urethral closure pressure below 20 cm H2O[169]. Recent studies using ASC injections have been developed using a urinary incontinence rat model to better understand the therapeutic effects of ASCs. First in 2018, Cui et al[170] showed that injection of rat ASCs was associated with higher content of striated muscle in the urethra and higher values of LPP compared to non-treated rats[170]. ASCs survival was also detected 60 d post-implantation. Later that same year, Ni et al[171], using a different approach, studied the therapeutic potential of human ASC-derived exosomes[171]. Their study showed an enhanced proliferation of skeletal muscle and Schwann cell lines in vitro upon exosome exposure. When injected in vivo, a higher bladder capacity and LPP were observed, associated with enhanced muscle fibers and peripheral nerve fibers regeneration in the urethra of the exosome-treated rats, eight weeks after injection. These results suggest that local injection of exosomes derived from human ASCs can improve functional and histological recovery of the urethra of incontinent rats[171].
Finally, ASC-based tissue engineering approaches have also been developed for urethral reconstruction (Table 3). Hariastawa et al[172] study showed that rabbit ASCs seeded onto a human dried amniotic membrane (DAM) increased urethral healing in a surgically induced urethral injury rabbit[172]. This study showed that 28 d after implantation, rabbits treated with ASC-DAM exhibited less features of fibrosis with decreased fistula presence in the healed tissue compared to DAM only treated and untreated groups, suggesting this novel ASC-DAM seeded scaffold as a potential graft for urethral reconstruction. Also in 2020, Wan et al[173] tested a nanofibrous blend scaffold (PLLA/PCL/PLGA) seeded with rat ASCs preconditioned by hypoxia for urethral reconstruction in an induced urethral injury rabbit model[173].
This study showed that hypoxia preconditioning of ASCs, combined with the nanofibrous scaffold, led to larger urethral lumen diameter, preserved urethral morphology, and increased angiogenesis by enhanced VEGF secretion compared to the normoxia ASCs treated group. This study showed that hypoxia preconditioning of ASCs mediated an upregulation of angiogenesis in comparison to the use of non-preconditioned ASCs seeded into the scaffold.
DFAT cells for treatment of urogenital diseases
An increasing number of studies have characterized DFAT cell properties to gain insights into the biology of this unique cell population[174]. Since their discovery, only a few preclinical studies have been performed using DFAT cells as therapeutics, with a handful having investigated their use for urogenital-related conditions (Table 4).
Table 4.
Preclinical studies for treatment of urogenital related diseases using dedifferentiated fat cells
|
Year
|
Disease/injury
|
Animal model
|
Type of therapy (cell type/injection site)
|
Cell concentration
|
Outcomes
|
Ref.
|
| 2016 | VUR | SD rats 8 week-old females weighing 200 g, n = 10 | Injection of undifferentiated rat DFAT cells/bilateral vesicoureteral junction | 1 × 106 cells in 30 μL of saline | Significant amelioration of VUR in treated rats/nephroprotective effects in rats | Ikado et al[178], 2016 |
| 2015 | Immunologically induced glomerulonephritis and adriamycin induced nephropathy | Wistar rats, males weighing 250 g, n = 64 | Injection of undifferentiated rat DFAT cells/RA or TV | 1 × 106 cells in 20 μL of saline | TV DFAT cell injection showed lower proteinuria and renal degeneration than direct cell implantation/DFAT immunosuppressive effects significantly reduced glomerulonephritis in treated rats | Maruyama et al[177], 2015 |
| 2011 | Urethral sphincter injury by VD | SD rats 8 week-old females, n = 16 | Injection of undifferentiated rat DFAT cells/paraurethral connective tissue at mid-urethra | 1 × 106 cells in 20 μL of saline | Sphincter muscle regeneration by DFAT cell therapy/improvement of “lowered leak point” | Obinata et al[176], 2011 |
| 2009 | Cryo-injured bladder wall (2 mm diameter) | C57BL/6 mice 8-9 week-old males, n = 10 | Injection of smooth muscle-like cells differentiated from human DFAT cells/bladder wall | 1 × 106 cells in 20 μL of Hanks’ balanced solution | DFAT differentiation potential into smooth muscle-like cells/approximately 2-fold higher αSMA expressing cells in scar tissue 30 d post-injection in treated mice | Sakuma et al[175], 2009 |
αSMA: alpha smooth muscle actin; DFAT: Dedifferentiated fat; RA: Renal artery; SD: Sprague-Dawley; TV: Tail vein; VD: Vaginal distension; VUR: Vesicoureteral reflux.
In 2009, Sakuma et al[175] reported a study based on the use of murine DFAT cells to promote smooth muscle-like differentiation in a mouse bladder injury model[175]. Their work showed a significant contribution of DFAT cells to bladder tissue regeneration, as assessed 30 d after cell transplantation. Treated mice had almost twice higher levels of α-smooth muscle actin (α-SMA) expressing cells in the injured areas compared to untreated controls, indicating favorable wound healing by reducing scar tissue with smooth muscle like cells[175]. Then in 2011, a study performed by Obinata et al[176] showed the contribution of undifferentiated rat DFAT cells for the treatment of urethral sphincter atrophy caused by vaginal distention[176]. The study showed two groups of Sprague-Dawley rats that underwent vaginal distention causing urethral injury with reduced LPP of the urethral sphincter. DFAT cell transplantation resulted in a significant improvement of LPP (DFAT group: 37.3 ± 6.4 vs control group: 21.7 ± 5.7 mmHg, P < 0.01). Immunohistochemistry quantification revealed that the striated muscle thickness as well as α-SMA positive areas were significantly increased in the DFAT injected group than in the control group[176]. Therefore, this study suggests that undifferentiated DFAT cells can differentiate in vivo after injection to help rebuild damaged smooth muscle tissue. DFAT cell therapy was then evaluated in 2015 for its potential to improve glomerulonephritis related disease in Wistar rats with immunological and non-immunological induced renal injury[177]. This study showed that systemic tail vein injection of rat DFAT cells generally led to high numbers of cells trapped in the lungs, DFAT cells significantly reduced proteinuria (P < 0.01), as well as interstitial fibrosis, in association with decreased expression of kidney-injury molecule 1 in the non-immunological renal injury model. However, DFAT cell-based therapy did not show significant renal improvement in the non-immunological induced renal injury model[177]. The authors suggested that DFAT cells trapped in the lungs might secrete anti-inflammatory and/or immunosuppressive substances that contribute to the renal injury healing process.
Finally, DFAT cells were used by Ikado et al[178] in 2016 in their study investigating vesicoureteral reflux in rats that underwent urethral clamping and placement of cystostomy followed by intravesical pressurization[178]. In this study, undifferentiated rat DFAT cells were transplanted in treated rats, resulting in significantly lower vesicoureteral reflux grade and reduced hydronephrosis leading to lower renal scaring during the healing process[178]. This work suggested that DFAT cells expression of TGF-β1 and tissue inhibitors of metalloproteinases, contributed to extracellular matrix production and stabilization in the scar tissue. Interesting advantages have been observed so far using DFAT cell-based therapy for treating urogenital-related pathologies. However, more extensive studies are needed to evaluate DFAT cell’s mechanisms of action and long-term outcomes.
THE SELF-ASSEMBLY APPROACH FOR GENITOURINARY TISSUE ENGINEERING
As we have seen, tissue engineering strategies rely on scaffolds such as synthetic or natural biomaterials, including decellularized tissues[179]. Studies indicate that cellularized structures have better potential than acellular ones[44]. Furthermore, obtention of a better long-term outcome highly depends on preserving the pool of SCs[180,181]. As they naturally reside in privileged locations called niches[182], SC conservation depends on the biomaterial's capacity to recreate this unique SC-type specific structure. Twenty years ago, Dr. François A. Auger and his team at the LOEX research center developed a unique and innovative strategy used since then to reconstruct a wide variety of tissues, including urologic tissues. This technique is called the "self-assembly" approach using tissue engineering[183]. It relies on mesenchymal cells' ability to secrete and assemble their own ECM when cultivated long-term in the presence of serum and ascorbate, a cofactor of lysyl- and prolyl-hydroxylase, enzymes involved in the process of maturation of collagen fibers[184,185]. Because cells produce their own microenvironment, the physicochemical conditions allowing SCs to be preserved are thus recreated. This technique was used in the clinic to produce reconstructed skin for patients with severe burns[186] and those suffering from chronic ulcers[187]. In this context, preservation of epithelial SCs was demonstrated for this organ undergoing monthly cell renewal[188].
The self-assembly approach has then been similarly applied to urologic tissues. Porcine, then human urethra substitutes have been produced[189], matured[190] then grafted subcutaneously into mice[191]. Bladder substitutes have also been reconstructed[192,193] and further matured using bioreactors[194]. The need for organ-specific stroma has been demonstrated in this model by obtaining a better epithelial differentiation after replacing skin fibroblasts with bladder mesenchymal cells[195]. In these substitutes, the urothelium presented characteristics close to native tissue as evidenced by functional tests (permeability of urea using Franz's diffusion cells).
Furthermore, electron microscopy indicated the maturation of uroplakin plaques and the presence of discoid and fusiform vesicles near the plasma membranes, and immunostaining of uroplakins, tight junction proteins, and various markers such as keratins. These reconstructed tissues were used as models to study several pathologies such as bladder cancer[196], ketamine-induced cystitis[197] or the effect of metallic stent use[198]. Soon after, the preservation of epithelial SCs (i.e. urothelial cell progenitors) was studied, not only during the expansion of cells before reconstruction but during the engineering phase itself, and showed significant improvement under hypoxic conditions[199].
The use of ASCs for urological tissue reconstruction was also evaluated to further improve the substitutes reconstructed by the self-assembly technique. This was performed with the final goal of increasing the potential of graft take through enhanced angiogenesis and limiting surgery-induced inflammation. In this study, different combinations of reconstructed stroma were evaluated[200]. The use of skin fibroblast and ASC sheets gave compelling results with improved mechanical properties, mainly required for surgical manipulation. These features need to be confirmed by performing in vivo experiments. Also, the combination of bladder mesenchymal cells and ASC remains to be evaluated. Self-assembly protocols based on ASCs also lead to the production of tubular structures[201], supporting their future use for urethra or ureter engineering using this approach. The self-assembly approach is thus very promising based on these results showing that reconstructed tissues feature structural and functional properties closely resembling those of native tissues and support long-term preservation of SC pool required for positive graft outcomes.
CONSIDERATIONS ON THE USE OF SC FROM VARIOUS ORIGINS
As detailed above, promising studies have been performed using SCs to provide therapeutic solutions to urologists, with the goal of reducing side effects for the patients. It is expected that improvements in the knowledge and use of SCs to treat kidney, bladder or ureters/urethra pathologies will continue to be increasingly evaluated and published soon. The risks associated with SC-based therapies, however, should not be minimized. Most preclinical studies have been performed at a laboratory scale, with few animals and SCs extracted and amplified under conditions that will have to be adjusted to cGMP guidelines to respect safety and regulatory issues. Translating the preclinical findings to routine use in urology, with thousands or even millions of patients over the years, will necessitate collaborative efforts in designing the most appropriate clinical trials based on reliable and trusted SC sources. Indeed, depending on their source and their degree of potency, SCs use can be associated to various degrees of risk. Researchers and clinicians must keep in mind that SCs can share properties associated with cancer cells, such as an enhanced ability to proliferate. This is best illustrated by the formation of teratomas after injection of ESCs or iPSCs into animals, for example, during tumorigenicity assays. It is essential to devise strict protocols and safety assays to ensure a tight regulation of SC fate after induction of differentiation towards the desired cell type and the elimination of residual pluripotent undifferentiated cells. The use of defined culture media and the engineering of a microenvironment (e.g., ECM, cytokines) inducing the proper target cell differentiation will likely contribute to the success of increased cell safety and efficacy. However, in the near future, iPSCs could represent a great alternative as the potential of urothelial cells extracted from diseased bladders has been shown to be impacted[62].
When using postnatal SCs such as MSCs, their reduced multilineage potency is associated with an advantageous safety profile. However, many parameters must be taken into considerations including the choice of the culture media used for expansion, the number of cell passages required to obtain enough cells per patient, the method of implantation (systemic or local injection, tissue engineering strategies) that can all affect their therapeutic properties and secretory profile. Therefore, more regulated clinical trials need to be initiated, ideally as multicenter studies, to determine not only the safety but also the efficacy of a specific SC-based treatment for a specific indication. The notion that a single SC type, prepared and implanted is a specific fashion could treat a wide variety of unrelated diseases can lead to hasty conclusions on SCs potential and to unsafe clinical practices, such as stem cell tourism[202].
CONCLUSION
With the rapid progress of knowledge, technical breakthroughs, and reliable SC manufacturing, we can hope that the present century will be one of regenerative medicine and that SCs will play an essential role in the development of safe and efficient therapies available to a greater number of patients. There is no doubt that therapies using SCs necessitate close patient’s follow-up with competent personnel. These new medications could allow prescription of treatment which better meet the patient’s need with less side-effects.
Footnotes
Conflict-of-interest statement: The authors declare no conflict of interests for this article.
Manuscript source: Invited manuscript
Peer-review started: April 30, 2021
First decision: June 16, 2021
Article in press: September 14, 2021
Specialty type: Cell and tissue engineering
Country/Territory of origin: Canada
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Collart-Dutilleul PY, Montero-Vilchez T, Najman SJ S-Editor: Fan JR L-Editor: A P-Editor: Xing YX
Contributor Information
Christophe Caneparo, Centre de Recherche en Organogénèse Expérimentale de l'Université Laval/LOEX, Centre de Recherche du CHU de Québec-Université Laval, Axe Médecine Régénératrice, Quebec G1J1Z4, Canada.
Luis Sorroza-Martinez, Centre de Recherche en Organogénèse Expérimentale de l'Université Laval/LOEX, Centre de Recherche du CHU de Québec-Université Laval, Axe Médecine Régénératrice, Quebec G1J1Z4, Canada.
Stéphane Chabaud, Centre de Recherche en Organogénèse Expérimentale de l'Université Laval/LOEX, Centre de Recherche du CHU de Québec-Université Laval, Axe Médecine Régénératrice, Quebec G1J1Z4, Canada.
Julie Fradette, Centre de Recherche en Organogénèse Expérimentale de l'Université Laval/LOEX, Centre de Recherche du CHU de Québec-Université Laval, Axe Médecine Régénératrice, Quebec G1J1Z4, Canada; Department of Surgery, Faculty of Medicine, Université Laval, Quebec G1V0A6, Canada.
Stéphane Bolduc, Centre de Recherche en Organogénèse Expérimentale de l'Université Laval/LOEX, Centre de Recherche du CHU de Québec-Université Laval, Axe Médecine Régénératrice, Quebec G1J1Z4, Canada; Department of Surgery, Faculty of Medicine, Université Laval, Quebec G1V0A6, Canada. stephane.bolduc@fmed.ulaval.ca.
References
- 1.Bhargava S, Chapple CR. Buccal mucosal urethroplasty: is it the new gold standard? BJU Int. 2004;93:1191–1193. doi: 10.1111/j.1464-410X.2003.04860.x. [DOI] [PubMed] [Google Scholar]
- 2.Zhang C, Murphy SV, Atala A. Regenerative medicine in urology. Semin Pediatr Surg. 2014;23:106–111. doi: 10.1053/j.sempedsurg.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Janke HP, de Jonge PKJD, Feitz WFJ, Oosterwijk E. Reconstruction Strategies of the Ureter and Urinary Diversion Using Tissue Engineering Approaches. Tissue Eng Part B Rev. 2019;25:237–248. doi: 10.1089/ten.TEB.2018.0345. [DOI] [PubMed] [Google Scholar]
- 4.Mason C, Dunnill P. A brief definition of regenerative medicine. Regen Med. 2008;3:1–5. doi: 10.2217/17460751.3.1.1. [DOI] [PubMed] [Google Scholar]
- 5.Dorin RP, Pohl HG, De Filippo RE, Yoo JJ, Atala A. Tubularized urethral replacement with unseeded matrices: what is the maximum distance for normal tissue regeneration? World J Urol. 2008;26:323–326. doi: 10.1007/s00345-008-0316-6. [DOI] [PubMed] [Google Scholar]
- 6.Ene-Iordache B, Perico N, Bikbov B, Carminati S, Remuzzi A, Perna A, Islam N, Bravo RF, Aleckovic-Halilovic M, Zou H, Zhang L, Gouda Z, Tchokhonelidze I, Abraham G, Mahdavi-Mazdeh M, Gallieni M, Codreanu I, Togtokh A, Sharma SK, Koirala P, Uprety S, Ulasi I, Remuzzi G. Chronic kidney disease and cardiovascular risk in six regions of the world (ISN-KDDC): a cross-sectional study. Lancet Glob Health. 2016;4:e307–e319. doi: 10.1016/S2214-109X(16)00071-1. [DOI] [PubMed] [Google Scholar]
- 7.Miyoshi T, Hiratsuka K, Saiz EG, Morizane R. Kidney organoids in translational medicine: Disease modeling and regenerative medicine. Dev Dyn. 2020;249:34–45. doi: 10.1002/dvdy.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Webster AC, Nagler EV, Morton RL, Masson P. Chronic Kidney Disease. Lancet. 2017;389:1238–1252. doi: 10.1016/S0140-6736(16)32064-5. [DOI] [PubMed] [Google Scholar]
- 9.Bergmann C, Guay-Woodford LM, Harris PC, Horie S, Peters DJM, Torres VE. Polycystic kidney disease. Nat Rev Dis Primers. 2018;4:50. doi: 10.1038/s41572-018-0047-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, McMahon AP, Bonventre JV. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell. 2008;2:284–291. doi: 10.1016/j.stem.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 11.Little MH, McMahon AP. Mammalian kidney development: principles, progress, and projections. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a008300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collins AJ, Foley RN, Herzog C, Chavers BM, Gilbertson D, Ishani A, Kasiske BL, Liu J, Mau LW, McBean M, Murray A, St Peter W, Guo H, Li Q, Li S, Peng Y, Qiu Y, Roberts T, Skeans M, Snyder J, Solid C, Wang C, Weinhandl E, Zaun D, Arko C, Chen SC, Dalleska F, Daniels F, Dunning S, Ebben J, Frazier E, Hanzlik C, Johnson R, Sheets D, Wang X, Forrest B, Constantini E, Everson S, Eggers PW, Agodoa L. Excerpts from the US Renal Data System 2009 Annual Data Report. Am J Kidney Dis. 2010;55:S1–420, A6. doi: 10.1053/j.ajkd.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drawz P, Rahman M. Chronic kidney disease. Ann Intern Med. 2015;162:ITC1–IT16. doi: 10.7326/AITC201506020. [DOI] [PubMed] [Google Scholar]
- 14.Iannaccone PM, Galat V, Bury MI, Ma YC, Sharma AK. The utility of stem cells in pediatric urinary bladder regeneration. Pediatr Res. 2018;83:258–266. doi: 10.1038/pr.2017.229. [DOI] [PubMed] [Google Scholar]
- 15.Jeong SJ, Oh SJ. The Current Positioning of Augmentation Enterocystoplasty in the Treatment for Neurogenic Bladder. Int Neurourol J. 2020;24:200–210. doi: 10.5213/inj.2040120.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bankhead RW, Kropp BP, Cheng EY. Evaluation and treatment of children with neurogenic bladders. J Child Neurol. 2000;15:141–149. doi: 10.1177/088307380001500301. [DOI] [PubMed] [Google Scholar]
- 17.Budzyn J, Trinh H, Raffee S, Atiemo H. Bladder Augmentation (Enterocystoplasty): the Current State of a Historic Operation. Curr Urol Rep. 2019;20:50. doi: 10.1007/s11934-019-0919-z. [DOI] [PubMed] [Google Scholar]
- 18.Mousa NA, Abou-Taleb HA, Orabi H. Stem cell applications for pathologies of the urinary bladder. World J Stem Cells. 2015;7:815–822. doi: 10.4252/wjsc.v7.i5.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elliott SP, McAninch JW. Ureteral injuries: external and iatrogenic. Urol Clin North Am. 2006;33:55–66, vi. doi: 10.1016/j.ucl.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 20.Mammen C, Chilaka V, Cust MP. Urological surgical techniques. Best Pract Res Clin Obstet Gynaecol. 2006;20:139–156. doi: 10.1016/j.bpobgyn.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 21.Martini A, Villari D, Nicita G. Long-term complications arising from bowel interposition in the urinary tract. Int J Surg. 2017;44:278–280. doi: 10.1016/j.ijsu.2017.07.030. [DOI] [PubMed] [Google Scholar]
- 22.Caione P. Prevalence of hypospadias in European countries: is it increasing? Eur Urol. 2009;55:1027–9; discussion 1029. doi: 10.1016/j.eururo.2009.01.051. [DOI] [PubMed] [Google Scholar]
- 23.Boillot B, Teklali Y, Moog R, Droupy S. [Penile congenital abnormalities] Prog Urol. 2013;23:664–673. doi: 10.1016/j.purol.2013.01.022. [DOI] [PubMed] [Google Scholar]
- 24.Nelson CP, Park JM, Wan J, Bloom DA, Dunn RL, Wei JT. The increasing incidence of congenital penile anomalies in the United States. J Urol. 2005;174:1573–1576. doi: 10.1097/01.ju.0000179249.21944.7e. [DOI] [PubMed] [Google Scholar]
- 25.Lloyd JC, Wiener JS, Gargollo PC, Inman BA, Ross SS, Routh JC. Contemporary epidemiological trends in complex congenital genitourinary anomalies. J Urol. 2013;190:1590–1595. doi: 10.1016/j.juro.2013.04.034. [DOI] [PubMed] [Google Scholar]
- 26.Mangera A, Osman N, Chapple C. Evaluation and management of anterior urethral stricture disease. F1000Res. 2016;5 doi: 10.12688/f1000research.7121.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsivian A, Sidi AA. Dorsal graft urethroplasty for female urethral stricture. J Urol. 2006;176:611–3; discussion 613. doi: 10.1016/j.juro.2006.03.055. [DOI] [PubMed] [Google Scholar]
- 28.Caldamone AA, Edstrom LE, Koyle MA, Rabinowitz R, Hulbert WC. Buccal mucosal grafts for urethral reconstruction. Urology. 1998;51:15–19. doi: 10.1016/s0090-4295(98)00088-0. [DOI] [PubMed] [Google Scholar]
- 29.Fu Q, Deng CL. Ten-year experience with composite bladder mucosa-skin grafts in hypospadias repair. Urology. 2006;67:1274–7; discussion 1277. doi: 10.1016/j.urology.2005.12.048. [DOI] [PubMed] [Google Scholar]
- 30.Bhargava S, Patterson JM, Inman RD, MacNeil S, Chapple CR. Tissue-engineered buccal mucosa urethroplasty-clinical outcomes. Eur Urol. 2008;53:1263–1269. doi: 10.1016/j.eururo.2008.01.061. [DOI] [PubMed] [Google Scholar]
- 31.Ehrlich RM, Alter G. Split-thickness skin graft urethroplasty and tunica vaginalis flaps for failed hypospadias repairs. J Urol. 1996;155:131–134. [PubMed] [Google Scholar]
- 32.Song LJ, Xu YM, Hu XY, Zhang HZ. Urethral substitution using autologous lingual mucosal grafts: an experimental study. BJU Int. 2008;101:739–743. doi: 10.1111/j.1464-410X.2007.07230.x. [DOI] [PubMed] [Google Scholar]
- 33.Barbagli G, Selli C, Tosto A, Palminteri E. Dorsal free graft urethroplasty. J Urol. 1996;155:123–126. [PubMed] [Google Scholar]
- 34.McAninch JW. Urethral reconstruction: a continuing challenge. J Urol. 2005;173:7. doi: 10.1097/01.ju.0000148540.57463.22. [DOI] [PubMed] [Google Scholar]
- 35.Dublin N, Stewart LH. Oral complications after buccal mucosal graft harvest for urethroplasty. BJU Int. 2004;94:867–869. doi: 10.1111/j.1464-410X.2004.05048.x. [DOI] [PubMed] [Google Scholar]
- 36.Djordjevic ML. Graft surgery in extensive urethral stricture disease. Curr Urol Rep. 2014;15:424. doi: 10.1007/s11934-014-0424-3. [DOI] [PubMed] [Google Scholar]
- 37.Korneyev I, Ilyin D, Schultheiss D, Chapple C. The first oral mucosal graft urethroplasty was carried out in the 19th century: the pioneering experience of Kirill Sapezhko (1857-1928) Eur Urol. 2012;62:624–627. doi: 10.1016/j.eururo.2012.06.035. [DOI] [PubMed] [Google Scholar]
- 38.Aldaqadossi HA, Shaker H, Youssof H, Kotb Y, Eladawy M. Outcomes of staged lingual mucosal graft urethroplasty for redo hypospadias repair. J Pediatr Urol. 2019;15:519.e1–519.e7. doi: 10.1016/j.jpurol.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 39.Spilotros M, Sihra N, Malde S, Pakzad MH, Hamid R, Ockrim JL, Greenwell TJ. Buccal mucosal graft urethroplasty in men-risk factors for recurrence and complications: a third referral centre experience in anterior urethroplasty using buccal mucosal graft. Transl Androl Urol. 2017;6:510–516. doi: 10.21037/tau.2017.03.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nelson CP, Bloom DA, Kinast R, Wei JT, Park JM. Patient-reported sexual function after oral mucosa graft urethroplasty for hypospadias. Urology. 2005;66:1086–9; discussion 1089. doi: 10.1016/j.urology.2005.05.057. [DOI] [PubMed] [Google Scholar]
- 41.Markiewicz MR, DeSantis JL, Margarone JE 3rd, Pogrel MA, Chuang SK. Morbidity associated with oral mucosa harvest for urological reconstruction: an overview. J Oral Maxillofac Surg. 2008;66:739–744. doi: 10.1016/j.joms.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 42.Kim SJ, Lee J, Park CH, Park JY, Song SH, Kim KS, Kim HG. Urethral defect due to periurethral abscess treated with a tunica vaginalis flap: A case report. Medicine (Baltimore) 2018;97:e13249. doi: 10.1097/MD.0000000000013249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hmida W, Othmen MB, Bako A, Jaidane M, Mosbah F. Penile skin flap: a versatile substitute for anterior urethral stricture. Int Braz J Urol. 2019;45:1057–1063. doi: 10.1590/S1677-5538.IBJU.2018.0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Orabi H, Bouhout S, Morissette A, Rousseau A, Chabaud S, Bolduc S. Tissue engineering of urinary bladder and urethra: advances from bench to patients. ScientificWorldJournal. 2013;2013:154564. doi: 10.1155/2013/154564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- 46.Watt FM, Hogan BL. Out of Eden: stem cells and their niches. Science. 2000;287:1427–1430. doi: 10.1126/science.287.5457.1427. [DOI] [PubMed] [Google Scholar]
- 47.Sharma S, Gupta DK. Tissue Engineering and Stem Cell Therapy in Pediatric Urology. J Indian Assoc Pediatr Surg. 2019;24:237–246. doi: 10.4103/jiaps.JIAPS_77_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Friedenstein AJ, Deriglasova UF, Kulagina NN, Panasuk AF, Rudakowa SF, Luriá EA, Ruadkow IA. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol. 1974;2:83–92. [PubMed] [Google Scholar]
- 49.Andrzejewska A, Lukomska B, Janowski M. Concise Review: Mesenchymal Stem Cells: From Roots to Boost. Stem Cells. 2019;37:855–864. doi: 10.1002/stem.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hayward CJ, Fradette J, Morissette Martin P, Guignard R, Germain L, Auger FA. Using human umbilical cord cells for tissue engineering: a comparison with skin cells. Differentiation. 2014;87:172–181. doi: 10.1016/j.diff.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 51.Bochon B, Kozubska M, Surygała G, Witkowska A, Kuźniewicz R, Grzeszczak W, Wystrychowski G. Mesenchymal Stem Cells-Potential Applications in Kidney Diseases. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20102462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Horst M, Eberli D, Gobet R, Salemi S. Tissue Engineering in Pediatric Bladder Reconstruction-The Road to Success. Front Pediatr. 2019;7:91. doi: 10.3389/fped.2019.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Song B, Jiang W, Alraies A, Liu Q, Gudla V, Oni J, Wei X, Sloan A, Ni L, Agarwal M. Bladder Smooth Muscle Cells Differentiation from Dental Pulp Stem Cells: Future Potential for Bladder Tissue Engineering. Stem Cells Int. 2016;2016:6979368. doi: 10.1155/2016/6979368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Chiara L, Famulari ES, Fagoonee S, van Daalen SKM, Buttiglieri S, Revelli A, Tolosano E, Silengo L, van Pelt AMM, Altruda F. Characterization of Human Mesenchymal Stem Cells Isolated from the Testis. Stem Cells Int. 2018;2018:4910304. doi: 10.1155/2018/4910304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shoae-Hassani A, Sharif S, Seifalian AM, Mortazavi-Tabatabaei SA, Rezaie S, Verdi J. Endometrial stem cell differentiation into smooth muscle cell: a novel approach for bladder tissue engineering in women. BJU Int. 2013;112:854–863. doi: 10.1111/bju.12195. [DOI] [PubMed] [Google Scholar]
- 56.Shoae-Hassani A, Mortazavi-Tabatabaei SA, Sharif S, Seifalian AM, Azimi A, Samadikuchaksaraei A, Verdi J. Differentiation of human endometrial stem cells into urothelial cells on a three-dimensional nanofibrous silk-collagen scaffold: an autologous cell resource for reconstruction of the urinary bladder wall. J Tissue Eng Regen Med. 2015;9:1268–1276. doi: 10.1002/term.1632. [DOI] [PubMed] [Google Scholar]
- 57.Ulrich D, Muralitharan R, Gargett CE. Toward the use of endometrial and menstrual blood mesenchymal stem cells for cell-based therapies. Expert Opin Biol Ther. 2013;13:1387–1400. doi: 10.1517/14712598.2013.826187. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Y, McNeill E, Tian H, Soker S, Andersson KE, Yoo JJ, Atala A. Urine derived cells are a potential source for urological tissue reconstruction. J Urol. 2008;180:2226–2233. doi: 10.1016/j.juro.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 59.Bharadwaj S, Liu G, Shi Y, Wu R, Yang B, He T, Fan Y, Lu X, Zhou X, Liu H, Atala A, Rohozinski J, Zhang Y. Multipotential differentiation of human urine-derived stem cells: potential for therapeutic applications in urology. Stem Cells. 2013;31:1840–1856. doi: 10.1002/stem.1424. [DOI] [PubMed] [Google Scholar]
- 60.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 61.Lian Q, Zhang Y, Zhang J, Zhang HK, Wu X, Lam FF, Kang S, Xia JC, Lai WH, Au KW, Chow YY, Siu CW, Lee CN, Tse HF. Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation. 2010;121:1113–1123. doi: 10.1161/CIRCULATIONAHA.109.898312. [DOI] [PubMed] [Google Scholar]
- 62.Subramaniam R, Hinley J, Stahlschmidt J, Southgate J. Tissue engineering potential of urothelial cells from diseased bladders. J Urol. 2011;186:2014–2020. doi: 10.1016/j.juro.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 63.Borges FT, Schor N. Regenerative medicine in kidney disease: where we stand and where to go. Pediatr Nephrol. 2018;33:1457–1465. doi: 10.1007/s00467-017-3754-9. [DOI] [PubMed] [Google Scholar]
- 64.Squillaro T, Peluso G, Galderisi U. Clinical Trials With Mesenchymal Stem Cells: An Update. Cell Transplant. 2016;25:829–848. doi: 10.3727/096368915X689622. [DOI] [PubMed] [Google Scholar]
- 65.Held PK, Al-Dhalimy M, Willenbring H, Akkari Y, Jiang S, Torimaru Y, Olson S, Fleming WH, Finegold M, Grompe M. In vivo genetic selection of renal proximal tubules. Mol Ther. 2006;13:49–58. doi: 10.1016/j.ymthe.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 66.Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz DM, Nakano Y, Meyer EM, Morel L, Petersen BE, Scott EW. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002;416:542–545. doi: 10.1038/nature730. [DOI] [PubMed] [Google Scholar]
- 67.Dekel B, Shezen E, Even-Tov-Friedman S, Katchman H, Margalit R, Nagler A, Reisner Y. Transplantation of human hematopoietic stem cells into ischemic and growing kidneys suggests a role in vasculogenesis but not tubulogenesis. Stem Cells. 2006;24:1185–1193. doi: 10.1634/stemcells.2005-0265. [DOI] [PubMed] [Google Scholar]
- 68.Dziedzic K, Pleniceanu O, Dekel B. Kidney stem cells in development, regeneration and cancer. Semin Cell Dev Biol. 2014;36:57–65. doi: 10.1016/j.semcdb.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 69.Caldas HC, de Paula Couto TA, Fernandes IM, Baptista MA, Kawasaki-Oyama RS, Goloni-Bertollo EM, Braile DM, Abbud-Filho M. Comparative effects of mesenchymal stem cell therapy in distinct stages of chronic renal failure. Clin Exp Nephrol. 2015;19:783–789. doi: 10.1007/s10157-015-1079-1. [DOI] [PubMed] [Google Scholar]
- 70.Oliveira-Sales EB, Maquigussa E, Semedo P, Pereira LG, Ferreira VM, Câmara NO, Bergamaschi CT, Campos RR, Boim MA. Mesenchymal stem cells (MSC) prevented the progression of renovascular hypertension, improved renal function and architecture. PLoS One. 2013;8:e78464. doi: 10.1371/journal.pone.0078464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang X, Wu S, Zhu Y, Chu CQ. Long-term durable repaired cartilage induced by SOX9 in situ with bone marrow-derived mesenchymal stem cells. Int J Med Sci. 2021;18:1399–1405. doi: 10.7150/ijms.52510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhuo W, Liao L, Xu T, Wu W, Yang S, Tan J. Mesenchymal stem cells ameliorate ischemia-reperfusion-induced renal dysfunction by improving the antioxidant/oxidant balance in the ischemic kidney. Urol Int. 2011;86:191–196. doi: 10.1159/000319366. [DOI] [PubMed] [Google Scholar]
- 73.Bruno S, Grange C, Collino F, Deregibus MC, Cantaluppi V, Biancone L, Tetta C, Camussi G. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS One. 2012;7:e33115. doi: 10.1371/journal.pone.0033115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen TS, Lai RC, Lee MM, Choo AB, Lee CN, Lim SK. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res. 2010;38:215–224. doi: 10.1093/nar/gkp857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wise AF, Williams TM, Kiewiet MB, Payne NL, Siatskas C, Samuel CS, Ricardo SD. Human mesenchymal stem cells alter macrophage phenotype and promote regeneration via homing to the kidney following ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2014;306:F1222–F1235. doi: 10.1152/ajprenal.00675.2013. [DOI] [PubMed] [Google Scholar]
- 76.Fiorina P, Jurewicz M, Augello A, Vergani A, Dada S, La Rosa S, Selig M, Godwin J, Law K, Placidi C, Smith RN, Capella C, Rodig S, Adra CN, Atkinson M, Sayegh MH, Abdi R. Immunomodulatory function of bone marrow-derived mesenchymal stem cells in experimental autoimmune type 1 diabetes. J Immunol. 2009;183:993–1004. doi: 10.4049/jimmunol.0900803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Razban V, Lotfi AS, Soleimani M, Ahmadi H, Massumi M, Khajeh S, Ghaedi M, Arjmand S, Najavand S, Khoshdel A. HIF-1α Overexpression Induces Angiogenesis in Mesenchymal Stem Cells. Biores Open Access. 2012;1:174–183. doi: 10.1089/biores.2012.9905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nigam eS, Lieberthal W. Acute renal failure. III. The role of growth factors in the process of renal regeneration and repair. Am J Physiol Renal Physiol. 2000;279:F3–F11. doi: 10.1152/ajprenal.2000.279.1.F3. [DOI] [PubMed] [Google Scholar]
- 79.Shi J, Zhao YC, Niu ZF, Fan HJ, Hou SK, Guo XQ, Sang L, Lv Q. Mesenchymal stem cell-derived small extracellular vesicles in the treatment of human diseases: Progress and prospect. World J Stem Cells. 2021;13:49–63. doi: 10.4252/wjsc.v13.i1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bartosh TJ, Ylöstalo JH, Bazhanov N, Kuhlman J, Prockop DJ. Dynamic compaction of human mesenchymal stem/precursor cells into spheres self-activates caspase-dependent IL1 signaling to enhance secretion of modulators of inflammation and immunity (PGE2, TSG6, and STC1) Stem Cells. 2013;31:2443–2456. doi: 10.1002/stem.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bartosh TJ, Ylöstalo JH, Mohammadipoor A, Bazhanov N, Coble K, Claypool K, Lee RH, Choi H, Prockop DJ. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc Natl Acad Sci U S A. 2010;107:13724–13729. doi: 10.1073/pnas.1008117107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang C, Shang Y, Chen X, Midgley AC, Wang Z, Zhu D, Wu J, Chen P, Wu L, Wang X, Zhang K, Wang H, Kong D, Yang Z, Li Z. Supramolecular Nanofibers Containing Arginine-Glycine-Aspartate (RGD) Peptides Boost Therapeutic Efficacy of Extracellular Vesicles in Kidney Repair. ACS Nano. 2020;14:12133–12147. doi: 10.1021/acsnano.0c05681. [DOI] [PubMed] [Google Scholar]
- 83.Swaminathan M, Stafford-Smith M, Chertow GM, Warnock DG, Paragamian V, Brenner RM, Lellouche F, Fox-Robichaud A, Atta MG, Melby S, Mehta RL, Wald R, Verma S, Mazer CD ACT-AKI investigators. Allogeneic Mesenchymal Stem Cells for Treatment of AKI after Cardiac Surgery. J Am Soc Nephrol. 2018;29:260–267. doi: 10.1681/ASN.2016101150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sun B, Luo X, Yang C, Liu P, Yang Y, Dong X, Yang Z, Xu J, Zhang Y, Li L. Therapeutic Effects of Human Urine-Derived Stem Cells in a Rat Model of Cisplatin-Induced Acute Kidney Injury In Vivo and In Vitro. Stem Cells Int . 2019;2019:8035076. doi: 10.1155/2019/8035076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Song JJ, Guyette JP, Gilpin SE, Gonzalez G, Vacanti JP, Ott HC. Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat Med. 2013;19:646–651. doi: 10.1038/nm.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.O'Neill JD, Freytes DO, Anandappa AJ, Oliver JA, Vunjak-Novakovic GV. The regulation of growth and metabolism of kidney stem cells with regional specificity using extracellular matrix derived from kidney. Biomaterials. 2013;34:9830–9841. doi: 10.1016/j.biomaterials.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Guan Y, Liu S, Sun C, Cheng G, Kong F, Luan Y, Xie X, Zhao S, Zhang D, Wang J, Li K, Liu Y. The effective bioengineering method of implantation decellularized renal extracellular matrix scaffolds. Oncotarget. 2015;6:36126–36138. doi: 10.18632/oncotarget.5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ross EA, Williams MJ, Hamazaki T, Terada N, Clapp WL, Adin C, Ellison GW, Jorgensen M, Batich CD. Embryonic stem cells proliferate and differentiate when seeded into kidney scaffolds. J Am Soc Nephrol. 2009;20:2338–2347. doi: 10.1681/ASN.2008111196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Monsel A, Zhu YG, Gennai S, Hao Q, Liu J, Lee JW. Cell-based therapy for acute organ injury: preclinical evidence and ongoing clinical trials using mesenchymal stem cells. Anesthesiology. 2014;121:1099–1121. doi: 10.1097/ALN.0000000000000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sharma AK, Hota PV, Matoka DJ, Fuller NJ, Jandali D, Thaker H, Ameer GA, Cheng EY. Urinary bladder smooth muscle regeneration utilizing bone marrow derived mesenchymal stem cell seeded elastomeric poly(1,8-octanediol-co-citrate) based thin films. Biomaterials. 2010;31:6207–6217. doi: 10.1016/j.biomaterials.2010.04.054. [DOI] [PubMed] [Google Scholar]
- 91.Dissaranan C, Cruz MA, Kiedrowski MJ, Balog BM, Gill BC, Penn MS, Goldman HB, Damaser MS. Rat mesenchymal stem cell secretome promotes elastogenesis and facilitates recovery from simulated childbirth injury. Cell Transplant. 2014;23:1395–1406. doi: 10.3727/096368913X670921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Carr LK, Steele D, Steele S, Wagner D, Pruchnic R, Jankowski R, Erickson J, Huard J, Chancellor MB. 1-year follow-up of autologous muscle-derived stem cell injection pilot study to treat stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:881–883. doi: 10.1007/s00192-007-0553-z. [DOI] [PubMed] [Google Scholar]
- 93.Stangel-Wojcikiewicz K, Piwowar M, Jach R, Majka M, Basta A. Quality of life assessment in female patients 2 and 4 years after muscle-derived cell transplants for stress urinary incontinence treatment. Ginekol Pol. 2016;87:183–189. doi: 10.17772/gp/61330. [DOI] [PubMed] [Google Scholar]
- 94.Arjmand B, Safavi M, Heidari R, Aghayan H, T Bazargani S, Dehghani S, Goodarzi P, Mohammadi-Jahani F, Heidari F, Payab M, Pourmand G. Concomitant Transurethral and Transvaginal-Periurethral Injection of Autologous Adipose Derived Stem Cells for Treatment of Female Stress Urinary Incontinence: A Phase One Clinical Trial. Acta Med Iran. 2017;55:368–374. [PubMed] [Google Scholar]
- 95.Lin HK, Godiwalla SY, Palmer B, Frimberger D, Yang Q, Madihally SV, Fung KM, Kropp BP. Understanding roles of porcine small intestinal submucosa in urinary bladder regeneration: identification of variable regenerative characteristics of small intestinal submucosa. Tissue Eng Part B Rev. 2014;20:73–83. doi: 10.1089/ten.teb.2013.0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Donnenberg VS, Zimmerlin L, Rubin JP, Donnenberg AD. Regenerative therapy after cancer: what are the risks? Tissue Eng Part B Rev. 2010;16:567–575. doi: 10.1089/ten.teb.2010.0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang Y, Lin HK, Frimberger D, Epstein RB, Kropp BP. Growth of bone marrow stromal cells on small intestinal submucosa: an alternative cell source for tissue engineered bladder. BJU Int. 2005;96:1120–1125. doi: 10.1111/j.1464-410X.2005.05741.x. [DOI] [PubMed] [Google Scholar]
- 98.Adamowicz J, Juszczak K, Bajek A, Tworkiewicz J, Nowacki M, Marszalek A, Thor PJ, Chlosta P, Drewa T. Morphological and urodynamic evaluation of urinary bladder wall regeneration: muscles guarantee contraction but not proper function--a rat model research study. Transplant Proc. 2012;44:1429–1434. doi: 10.1016/j.transproceed.2012.01.144. [DOI] [PubMed] [Google Scholar]
- 99.Lang R, Liu G, Shi Y, Bharadwaj S, Leng X, Zhou X, Liu H, Atala A, Zhang Y. Self-renewal and differentiation capacity of urine-derived stem cells after urine preservation for 24 hours. PLoS One. 2013;8:e53980. doi: 10.1371/journal.pone.0053980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bodin A, Bharadwaj S, Wu S, Gatenholm P, Atala A, Zhang Y. Tissue-engineered conduit using urine-derived stem cells seeded bacterial cellulose polymer in urinary reconstruction and diversion. Biomaterials. 2010;31:8889–8901. doi: 10.1016/j.biomaterials.2010.07.108. [DOI] [PubMed] [Google Scholar]
- 101.Lee JN, Chun SY, Lee HJ, Jang YJ, Choi SH, Kim DH, Oh SH, Song PH, Lee JH, Kim JK, Kwon TG. Human Urine-derived Stem Cells Seeded Surface Modified Composite Scaffold Grafts for Bladder Reconstruction in a Rat Model. J Korean Med Sci. 2015;30:1754–1763. doi: 10.3346/jkms.2015.30.12.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nakajima N, Tamaki T, Hirata M, Soeda S, Nitta M, Hoshi A, Terachi T. Purified Human Skeletal Muscle-Derived Stem Cells Enhance the Repair and Regeneration in the Damaged Urethra. Transplantation. 2017;101:2312–2320. doi: 10.1097/TP.0000000000001613. [DOI] [PubMed] [Google Scholar]
- 103.Yudintceva NM, Nashchekina YA, Mikhailova NA, Vinogradova TI, Yablonsky PK, Gorelova AA, Muraviov AN, Gorelov AV, Samusenko IA, Nikolaev BP, Yakovleva LY, Shevtsov MA. Urethroplasty with a bilayered poly-D,L-lactide-co-ε-caprolactone scaffold seeded with allogenic mesenchymal stem cells. J Biomed Mater Res B Appl Biomater. 2020;108:1010–1021. doi: 10.1002/jbm.b.34453. [DOI] [PubMed] [Google Scholar]
- 104.Wu S, Liu Y, Bharadwaj S, Atala A, Zhang Y. Human urine-derived stem cells seeded in a modified 3D porous small intestinal submucosa scaffold for urethral tissue engineering. Biomaterials. 2011;32:1317–1326. doi: 10.1016/j.biomaterials.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Boumelhem BB, Fraser ST, Assinder SJ. Differentiation of Urothelium from Mouse Embryonic Stem Cells in Chemically Defined Conditions. Methods Mol Biol. 2019;2029:103–115. doi: 10.1007/978-1-4939-9631-5_9. [DOI] [PubMed] [Google Scholar]
- 106.Zhang J, Liu Y, Chen Y, Yuan L, Liu H, Wang J, Liu Q, Zhang Y. Adipose-Derived Stem Cells: Current Applications and Future Directions in the Regeneration of Multiple Tissues. Stem Cells Int. 2020;2020:8810813. doi: 10.1155/2020/8810813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lindroos B, Suuronen R, Miettinen S. The potential of adipose stem cells in regenerative medicine. Stem Cell Rev Rep. 2011;7:269–291. doi: 10.1007/s12015-010-9193-7. [DOI] [PubMed] [Google Scholar]
- 108.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 109.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rodbell M. Metabolism of isolated fat cell. I. effects of hormones on glucose metabolism and lipiolysis. J Biol Chem. 1964;239:375–380. [PubMed] [Google Scholar]
- 111.Williams SK, Wang TF, Castrillo R, Jarrell BE. Liposuction-derived human fat used for vascular graft sodding contains endothelial cells and not mesothelial cells as the major cell type. J Vasc Surg. 1994;19:916–923. doi: 10.1016/s0741-5214(94)70019-2. [DOI] [PubMed] [Google Scholar]
- 112.Dong Z, Peng Z, Chang Q, Lu F. The survival condition and immunoregulatory function of adipose stromal vascular fraction (SVF) in the early stage of nonvascularized adipose transplantation. PLoS One. 2013;8:e80364. doi: 10.1371/journal.pone.0080364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ramakrishnan VM, Boyd NL. The Adipose Stromal Vascular Fraction as a Complex Cellular Source for Tissue Engineering Applications. Tissue Eng Part B Rev. 2018;24:289–299. doi: 10.1089/ten.teb.2017.0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ghiasloo M, Lobato RC, Díaz JM, Singh K, Verpaele A, Tonnard P. Expanding Clinical Indications of Mechanically Isolated Stromal Vascular Fraction: A Systematic Review. Aesthet Surg J. 2020;40:NP546–NP560. doi: 10.1093/asj/sjaa111. [DOI] [PubMed] [Google Scholar]
- 115.Bateman ME, Strong AL, Gimble JM, Bunnell BA. Concise Review: Using Fat to Fight Disease: A Systematic Review of Nonhomologous Adipose-Derived Stromal/Stem Cell Therapies. Stem Cells. 2018;36:1311–1328. doi: 10.1002/stem.2847. [DOI] [PubMed] [Google Scholar]
- 116.Nguyen A, Guo J, Banyard DA, Fadavi D, Toranto JD, Wirth GA, Paydar KZ, Evans GR, Widgerow AD. Stromal vascular fraction: A regenerative reality? J Plast Reconstr Aesthet Surg. 2016;69:170–179. doi: 10.1016/j.bjps.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 117.Yin GN, Wang L, Lin XN, Shi L, Gao ZL, Han FC, Li P, Jin YC, Suh JK, Ryu JK, Wang X, Jin HR. Combination of stromal vascular fraction and Ad-COMP-Ang1 gene therapy improves long-term therapeutic efficacy for diabetes-induced erectile dysfunction. Asian J Androl. 2018;20:465–472. doi: 10.4103/aja.aja_16_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Song KM, Jin HR, Park JM, Choi MJ, Kwon MH, Kwon KD, Batbold D, Yin GN, Kim WJ, Koh GY, Ryu JK, Suh JK. Intracavernous delivery of stromal vascular fraction restores erectile function through production of angiogenic factors in a mouse model of cavernous nerve injury. J Sex Med. 2014;11:1962–1973. doi: 10.1111/jsm.12597. [DOI] [PubMed] [Google Scholar]
- 119.Castiglione F, Hedlund P, Weyne E, Hakim L, Montorsi F, Salonia A, Bivalacqua TJ, De Ridder D, Milenkovic U, Ralph D, Garaffa G, Muneer A, Joniau S, Albersen M Trauma and Reconstructive Urology Working Party of the European Association of Urology (EAU) Young Academic Urologists (YAU) Intratunical injection of stromal vascular fraction prevents fibrosis in a rat model of Peyronie's disease. BJU Int. 2019;124:342–348. doi: 10.1111/bju.14570. [DOI] [PubMed] [Google Scholar]
- 120.Hakim L, Fiorenzo S, Hedlund P, Montorsi F, Bivalacqua TJ, De Ridder D, Weyne E, Ralph D, Garaffa G, Muneer A, Joniau S, Albersen M, Castiglione F Trauma and Reconstructive Urology Working Party of the European Association of Urology (EAU) Young Academic Urologists (YAU) Intratunical injection of autologous adipose stromal vascular fraction reduces collagen III expression in a rat model of chronic penile fibrosis. Int J Impot Res. 2020;32:281–288. doi: 10.1038/s41443-019-0136-9. [DOI] [PubMed] [Google Scholar]
- 121.Andia I, Maffulli N, Burgos-Alonso N. Stromal vascular fraction technologies and clinical applications. Expert Opin Biol Ther. 2019;19:1289–1305. doi: 10.1080/14712598.2019.1671970. [DOI] [PubMed] [Google Scholar]
- 122.Keshel SH, Rahimi A, Hancox Z, Ebrahimi M, Khojasteh A, Sefat F. The promise of regenerative medicine in the treatment of urogenital disorders. J Biomed Mater Res A. 2020;108:1747–1759. doi: 10.1002/jbm.a.36942. [DOI] [PubMed] [Google Scholar]
- 123.NIH ClinicalTrials.gov is a database of privately and publicly funded clinical studies conducted around the world. [cited 10 March 2021]. Available from: https://www.clinicaltrials.gov/
- 124.Haney NM, Gabrielson A, Kohn TP, Hellstrom WJG. The Use of Stromal Vascular Fraction in the Treatment of Male Sexual Dysfunction: A Review of Preclinical and Clinical Studies. Sex Med Rev. 2019;7:313–320. doi: 10.1016/j.sxmr.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 125.Storti G, Scioli MG, Kim BS, Orlandi A, Cervelli V. Adipose-Derived Stem Cells in Bone Tissue Engineering: Useful Tools with New Applications. Stem Cells Int. 2019;2019:3673857. doi: 10.1155/2019/3673857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bacakova L, Zarubova J, Travnickova M, Musilkova J, Pajorova J, Slepicka P, Kasalkova NS, Svorcik V, Kolska Z, Motarjemi H, Molitor M. Stem cells: their source, potency and use in regenerative therapies with focus on adipose-derived stem cells - a review. Biotechnol Adv. 2018;36:1111–1126. doi: 10.1016/j.biotechadv.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 127.Hassanshahi A, Hassanshahi M, Khabbazi S, Hosseini-Khah Z, Peymanfar Y, Ghalamkari S, Su YW, Xian CJ. Adipose-derived stem cells for wound healing. J Cell Physiol. 2019;234:7903–7914. doi: 10.1002/jcp.27922. [DOI] [PubMed] [Google Scholar]
- 128.Gir P, Oni G, Brown SA, Mojallal A, Rohrich RJ. Human adipose stem cells: current clinical applications. Plast Reconstr Surg. 2012;129:1277–1290. doi: 10.1097/PRS.0b013e31824ecae6. [DOI] [PubMed] [Google Scholar]
- 129.Si Z, Wang X, Sun C, Kang Y, Xu J, Hui Y. Adipose-derived stem cells: Sources, potency, and implications for regenerative therapies. Biomed Pharmacother. 2019;114:108765. doi: 10.1016/j.biopha.2019.108765. [DOI] [PubMed] [Google Scholar]
- 130.Dai R, Wang Z, Samanipour R, Koo KI, Kim K. Adipose-Derived Stem Cells for Tissue Engineering and Regenerative Medicine Applications. Stem Cells Int. 2016;2016:6737345. doi: 10.1155/2016/6737345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Câmara DAD, Shibli JA, Müller EA, De-Sá-Junior PL, Porcacchia AS, Blay A, Lizier NF. Adipose Tissue-Derived Stem Cells: The Biologic Basis and Future Directions for Tissue Engineering. Materials (Basel) 2020;13 doi: 10.3390/ma13143210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sugihara H, Yonemitsu N, Miyabara S, Yun K. Primary cultures of unilocular fat cells: characteristics of growth in vitro and changes in differentiation properties. Differentiation. 1986;31:42–49. doi: 10.1111/j.1432-0436.1986.tb00381.x. [DOI] [PubMed] [Google Scholar]
- 133.Yagi K, Kondo D, Okazaki Y, Kano K. A novel preadipocyte cell line established from mouse adult mature adipocytes. Biochem Biophys Res Commun. 2004;321:967–974. doi: 10.1016/j.bbrc.2004.07.055. [DOI] [PubMed] [Google Scholar]
- 134.Matsumoto T, Kano K, Kondo D, Fukuda N, Iribe Y, Tanaka N, Matsubara Y, Sakuma T, Satomi A, Otaki M, Ryu J, Mugishima H. Mature adipocyte-derived dedifferentiated fat cells exhibit multilineage potential. J Cell Physiol. 2008;215:210–222. doi: 10.1002/jcp.21304. [DOI] [PubMed] [Google Scholar]
- 135.Kishimoto N, Honda Y, Momota Y, Tran SD. Dedifferentiated Fat (DFAT) cells: A cell source for oral and maxillofacial tissue engineering. Oral Dis. 2018;24:1161–1167. doi: 10.1111/odi.12832. [DOI] [PubMed] [Google Scholar]
- 136.Jumabay M, Boström KI. Dedifferentiated fat cells: A cell source for regenerative medicine. World J Stem Cells. 2015;7:1202–1214. doi: 10.4252/wjsc.v7.i10.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Watanabe H, Goto S, Kato R, Komiyama S, Nagaoka Y, Kazama T, Yamamoto C, Li Y, Konuma N, Hagikura K, Matsumoto T. The neovascularization effect of dedifferentiated fat cells. Sci Rep. 2020;10:9211. doi: 10.1038/s41598-020-66135-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ohta Y, Takenaga M, Tokura Y, Hamaguchi A, Matsumoto T, Kano K, Mugishima H, Okano H, Igarashi R. Mature adipocyte-derived cells, dedifferentiated fat cells (DFAT), promoted functional recovery from spinal cord injury-induced motor dysfunction in rats. Cell Transplant. 2008;17:877–886. doi: 10.3727/096368908786576516. [DOI] [PubMed] [Google Scholar]
- 139.Matsumine H, Takeuchi Y, Sasaki R, Kazama T, Kano K, Matsumoto T, Sakurai H, Miyata M, Yamato M. Adipocyte-derived and dedifferentiated fat cells promoting facial nerve regeneration in a rat model. Plast Reconstr Surg. 2014;134:686–697. doi: 10.1097/PRS.0000000000000537. [DOI] [PubMed] [Google Scholar]
- 140.Song Y, Peng C, Lv S, Cheng J, Liu S, Wen Q, Guan G, Liu G. Adipose-derived stem cells ameliorate renal interstitial fibrosis through inhibition of EMT and inflammatory response via TGF-β1 signaling pathway. Int Immunopharmacol. 2017;44:115–122. doi: 10.1016/j.intimp.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 141.Rivera-Valdes JJ, Garcia-Banuelos J, Salazar-Montes A, Garcia-Benavides L, Dominguez-Rosales A, Armendariz-Borunda J, Sandoval-Rodriguez A. Human adipose derived stem cells regress fibrosis in a chronic renal fibrotic model induced by adenine. PLoS One. 2017;12:e0187907. doi: 10.1371/journal.pone.0187907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Semedo P, Correa-Costa M, Antonio Cenedeze M, Maria Avancini Costa Malheiros D, Antonia dos Reis M, Shimizu MH, Seguro AC, Pacheco-Silva A, Saraiva Camara NO. Mesenchymal stem cells attenuate renal fibrosis through immune modulation and remodeling properties in a rat remnant kidney model. Stem Cells. 2009;27:3063–3073. doi: 10.1002/stem.214. [DOI] [PubMed] [Google Scholar]
- 143.Wang Z, Li S, Wang Y, Zhang X, Chen L, Sun D. GDNF enhances the anti-inflammatory effect of human adipose-derived mesenchymal stem cell-based therapy in renal interstitial fibrosis. Stem Cell Res. 2019;41:101605. doi: 10.1016/j.scr.2019.101605. [DOI] [PubMed] [Google Scholar]
- 144.Li S, Wang Y, Wang Z, Chen L, Zuo B, Liu C, Sun D. Enhanced renoprotective effect of GDNF-modified adipose-derived mesenchymal stem cells on renal interstitial fibrosis. Stem Cell Res Ther. 2021;12:27. doi: 10.1186/s13287-020-02049-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sheashaa H, Lotfy A, Elhusseini F, Aziz AA, Baiomy A, Awad S, Alsayed A, El-Gilany AH, Saad MA, Mahmoud K, Zahran F, Salem DA, Sarhan A, Ghaffar HA, Sobh M. Protective effect of adipose-derived mesenchymal stem cells against acute kidney injury induced by ischemia-reperfusion in Sprague-Dawley rats. Exp Ther Med. 2016;11:1573–1580. doi: 10.3892/etm.2016.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhou L, Song Q, Shen J, Xu L, Xu Z, Wu R, Ge Y, Zhu J, Wu J, Dou Q, Jia R. Comparison of human adipose stromal vascular fraction and adipose-derived mesenchymal stem cells for the attenuation of acute renal ischemia/reperfusion injury. Sci Rep. 2017;7:44058. doi: 10.1038/srep44058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lin KC, Yip HK, Shao PL, Wu SC, Chen KH, Chen YT, Yang CC, Sun CK, Kao GS, Chen SY, Chai HT, Chang CL, Chen CH, Lee MS. Combination of adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes for protecting kidney from acute ischemia-reperfusion injury. Int J Cardiol. 2016;216:173–185. doi: 10.1016/j.ijcard.2016.04.061. [DOI] [PubMed] [Google Scholar]
- 148.Jin J, Shi Y, Gong J, Zhao L, Li Y, He Q, Huang H. Exosome secreted from adipose-derived stem cells attenuates diabetic nephropathy by promoting autophagy flux and inhibiting apoptosis in podocyte. Stem Cell Res Ther. 2019;10:95. doi: 10.1186/s13287-019-1177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gao F, Zuo B, Wang Y, Li S, Yang J, Sun D. Protective function of exosomes from adipose tissue-derived mesenchymal stem cells in acute kidney injury through SIRT1 pathway. Life Sci. 2020;255:117719. doi: 10.1016/j.lfs.2020.117719. [DOI] [PubMed] [Google Scholar]
- 150.Quimby JM, Webb TL, Randall E, Marolf A, Valdes-Martinez A, Dow SW. Assessment of intravenous adipose-derived allogeneic mesenchymal stem cells for the treatment of feline chronic kidney disease: a randomized, placebo-controlled clinical trial in eight cats. J Feline Med Surg. 2016;18:165–171. doi: 10.1177/1098612X15576980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wang X, Rivera-Bolanos N, Jiang B, Ameer G. Advanced functional biomaterials for stem cell delivery in regenerative engineering and medicine. Advan Func Mater. 2019;29:1809009. [Google Scholar]
- 152.Zhou C, Zhou L, Liu J, Xu L, Xu Z, Chen Z, Ge Y, Zhao F, Wu R, Wang X, Jiang N, Mao L, Jia R. Kidney extracellular matrix hydrogel enhances therapeutic potential of adipose-derived mesenchymal stem cells for renal ischemia reperfusion injury. Acta Biomater. 2020;115:250–263. doi: 10.1016/j.actbio.2020.07.056. [DOI] [PubMed] [Google Scholar]
- 153.Changizi-Ashtiyani S, Hafazeh L, Ghasemi F, Najafi H, Babaei S, JalallyMashayekhi F, Hoseini SJ, Bastani B. The effect of adipose-derived mesenchymal stem cells on renal function and histopathology in a rat model of ischemia-reperfusion induced acute kidney injury. Iran J Basic Med Sci. 2020;23:999–1006. doi: 10.22038/ijbms.2020.40334.9601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Xue A, Niu G, Chen Y, Li K, Xiao Z, Luan Y, Sun C, Xie X, Zhang D, Du X, Kong F, Guo Y, Zhang H, Cheng G, Xin Q, Guan Y, Zhao S. Recellularization of well-preserved decellularized kidney scaffold using adipose tissue-derived stem cells. J Biomed Mater Res A. 2018;106:805–814. doi: 10.1002/jbm.a.36279. [DOI] [PubMed] [Google Scholar]
- 155.Takemura S, Shimizu T, Oka M, Sekiya S, Babazono T. Transplantation of adipose-derived mesenchymal stem cell sheets directly into the kidney suppresses the progression of renal injury in a diabetic nephropathy rat model. J Diabetes Investig. 2020;11:545–553. doi: 10.1111/jdi.13164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhe Z, Jun D, Yang Z, Mingxi X, Ke Z, Ming Z, Zhong W, Mujun L. Bladder Acellular Matrix Grafts Seeded with Adipose-Derived Stem Cells and Incubated Intraperitoneally Promote the Regeneration of Bladder Smooth Muscle and Nerve in a Rat Model of Bladder Augmentation. Stem Cells Dev. 2016;25:405–414. doi: 10.1089/scd.2015.0246. [DOI] [PubMed] [Google Scholar]
- 157.Wang Q, Xiao DD, Yan H, Zhao Y, Fu S, Zhou J, Wang Z, Zhou Z, Zhang M, Lu MJ. The morphological regeneration and functional restoration of bladder defects by a novel scaffold and adipose-derived stem cells in a rat augmentation model. Stem Cell Res Ther. 2017;8:149. doi: 10.1186/s13287-017-0597-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Xiao D, Wang Q, Yan H, Lv X, Zhao Y, Zhou Z, Zhang M, Sun Q, Sun K, Li W, Lu M. Adipose-derived stem cells-seeded bladder acellular matrix graft-silk fibroin enhances bladder reconstruction in a rat model. Oncotarget. 2017;8:86471–86487. doi: 10.18632/oncotarget.21211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hou X, Shi C, Chen W, Chen B, Jia W, Guo Y, Ma C, Ye G, Kang J, Dai J. Transplantation of human adipose-derived mesenchymal stem cells on a bladder acellular matrix for bladder regeneration in a canine model. Biomed Mater. 2016;11:031001. doi: 10.1088/1748-6041/11/3/031001. [DOI] [PubMed] [Google Scholar]
- 160.Xiao D, Yan H, Wang Q, Lv X, Zhang M, Zhao Y, Zhou Z, Xu J, Sun Q, Sun K, Li W, Lu M. Trilayer Three-Dimensional Hydrogel Composite Scaffold Containing Encapsulated Adipose-Derived Stem Cells Promotes Bladder Reconstruction via SDF-1α/CXCR4 Pathway. ACS Appl Mater Interfaces. 2017;9:38230–38241. doi: 10.1021/acsami.7b10630. [DOI] [PubMed] [Google Scholar]
- 161.Zhou Z, Yan H, Liu Y, Xiao D, Li W, Wang Q, Zhao Y, Sun K, Zhang M, Lu M. Adipose-derived stem-cell-implanted poly(ϵ-caprolactone)/chitosan scaffold improves bladder regeneration in a rat model. Regen Med. 2018;13:331–342. doi: 10.2217/rme-2017-0120. [DOI] [PubMed] [Google Scholar]
- 162.Salem SA, Rashidbenam Z, Jasman MH, Ho CCK, Sagap I, Singh R, Yusof MR, Md Zainuddin Z, Haji Idrus RB, Ng MH. Incorporation of Smooth Muscle Cells Derived from Human Adipose Stem Cells on Poly(Lactic-co-Glycolic Acid) Scaffold for the Reconstruction of Subtotally Resected Urinary Bladder in Athymic Rats. Tissue Eng Regen Med. 2020;17:553–563. doi: 10.1007/s13770-020-00271-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Moreno-Manzano V, Mellado-López M, Morera-Esteve MJ, Alastrue-Agudo A, Bisbal-Velasco V, Forteza-Vila J, Serrano-Aroca Á, Vera-Donoso CD. Human adipose-derived mesenchymal stem cells accelerate decellularized neobladder regeneration. Regen Biomater. 2020;7:161–169. doi: 10.1093/rb/rbz049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hickling DR, Sun TT, Wu XR. Anatomy and Physiology of the Urinary Tract: Relation to Host Defense and Microbial Infection. Microbiol Spectr. 2015;3 doi: 10.1128/microbiolspec.UTI-0016-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhao Z, Yu H, Fan C, Kong Q, Liu D, Meng L. Differentiate into urothelium and smooth muscle cells from adipose tissue-derived stem cells for ureter reconstruction in a rabbit model. Am J Transl Res. 2016;8:3757–3768. [PMC free article] [PubMed] [Google Scholar]
- 166.Castiglione F, Dewulf K, Hakim L, Weyne E, Montorsi F, Russo A, Boeri L, Bivalacqua TJ, De Ridder D, Joniau S, Albersen M, Hedlund P. Adipose-derived Stem Cells Counteract Urethral Stricture Formation in Rats. Eur Urol. 2016;70:1032–1041. doi: 10.1016/j.eururo.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 167.Sangkum P, Yafi FA, Kim H, Bouljihad M, Ranjan M, Datta A, Mandava SH, Sikka SC, Abdel-Mageed AB, Hellstrom WJ. Effect of adipose tissue-derived stem cell injection in a rat model of urethral fibrosis. Can Urol Assoc J. 2016;10:E175–E180. doi: 10.5489/cuaj.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Feng Z, Chen H, Fu T, Zhang L, Liu Y. miR-21 modification enhances the performance of adipose tissue-derived mesenchymal stem cells for counteracting urethral stricture formation. J Cell Mol Med. 2018;22:5607–5616. doi: 10.1111/jcmm.13834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Agarwal A, Rathi S, Patnaik P, Shaw D, Jain M, Trivedi S, Dwivedi US. Does preoperative urodynamic testing improve surgical outcomes in patients undergoing the transobturator tape procedure for stress urinary incontinence? Korean J Urol. 2014;55:821–827. doi: 10.4111/kju.2014.55.12.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Cui L, Meng Q, Wen J, Gao Z, Yan Z, Tian Y, Xu P, He X, Yu H, Lian P. A Functional Comparison of Treatment of Intrinsic Sphincter Deficiency with Muscle-Derived and Adipose Tissue-Derived Stem Cells. IUBMB Life. 2018;70:976–984. doi: 10.1002/iub.1896. [DOI] [PubMed] [Google Scholar]
- 171.Ni J, Li H, Zhou Y, Gu B, Xu Y, Fu Q, Peng X, Cao N, Jin M, Sun G, Wang J, Jin Y, Liu F. Therapeutic Potential of Human Adipose-Derived Stem Cell Exosomes in Stress Urinary Incontinence - An in Vitro and in Vivo Study. Cell Physiol Biochem. 2018;48:1710–1722. doi: 10.1159/000492298. [DOI] [PubMed] [Google Scholar]
- 172.Hariastawa IGBA, Rantam FA, Hardjowijoto S. The application of dried amniotic membrane scaffold with adipose derived-mesenchymal stem cell seeding as graft in urethral reconstruction (experiment on rabbit) Int J Surg. 2020;24:32–37. [Google Scholar]
- 173.Wan X, Xie MK, Xu H, Wei ZW, Yao HJ, Wang Z, Zheng DC. Hypoxia-preconditioned adipose-derived stem cells combined with scaffold promote urethral reconstruction by upregulation of angiogenesis and glycolysis. Stem Cell Res Ther. 2020;11:535. doi: 10.1186/s13287-020-02052-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Côté JA, Ostinelli G, Gauthier MF, Lacasse A, Tchernof A. Focus on dedifferentiated adipocytes: characteristics, mechanisms, and possible applications. Cell Tissue Res. 2019;378:385–398. doi: 10.1007/s00441-019-03061-3. [DOI] [PubMed] [Google Scholar]
- 175.Sakuma T, Matsumoto T, Kano K, Fukuda N, Obinata D, Yamaguchi K, Yoshida T, Takahashi S, Mugishima H. Mature, adipocyte derived, dedifferentiated fat cells can differentiate into smooth muscle-like cells and contribute to bladder tissue regeneration. J Urol. 2009;182:355–365. doi: 10.1016/j.juro.2009.02.103. [DOI] [PubMed] [Google Scholar]
- 176.Obinata D, Matsumoto T, Ikado Y, Sakuma T, Kano K, Fukuda N, Yamaguchi K, Mugishima H, Takahashi S. Transplantation of mature adipocyte-derived dedifferentiated fat (DFAT) cells improves urethral sphincter contractility in a rat model. Int J Urol . 2011;18:827–834. doi: 10.1111/j.1442-2042.2011.02865.x. [DOI] [PubMed] [Google Scholar]
- 177.Maruyama T, Fukuda N, Matsumoto T, Kano K, Endo M, Kazama M, Kazama T, Ikeda J, Matsuda H, Ueno T, Abe M, Okada K, Soma M, Matsumoto K, Kawachi H. Systematic implantation of dedifferentiated fat cells ameliorated monoclonal antibody 1-22-3-induced glomerulonephritis by immunosuppression with increases in TNF-stimulated gene 6. Stem Cell Res Ther. 2015;6:80. doi: 10.1186/s13287-015-0069-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ikado Y, Obinata D, Matsumoto T, Murata Y, Kano K, Fukuda N, Yamaguchi K, Takahashi S. Transplantation of mature adipocyte-derived dedifferentiated fat cells for the treatment of vesicoureteral reflux in a rat model. Int Urol Nephrol. 2016;48:1951–1960. doi: 10.1007/s11255-016-1426-5. [DOI] [PubMed] [Google Scholar]
- 179.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 180.Yin Y, Li X, He XT, Wu RX, Sun HH, Chen FM. Leveraging Stem Cell Homing for Therapeutic Regeneration. J Dent Res. 2017;96:601–609. doi: 10.1177/0022034517706070. [DOI] [PubMed] [Google Scholar]
- 181.Li Y, Xiao Y, Liu C. The Horizon of Materiobiology: A Perspective on Material-Guided Cell Behaviors and Tissue Engineering. Chem Rev. 2017;117:4376–4421. doi: 10.1021/acs.chemrev.6b00654. [DOI] [PubMed] [Google Scholar]
- 182.Gattazzo F, Urciuolo A, Bonaldo P. Extracellular matrix: a dynamic microenvironment for stem cell niche. Biochim Biophys Acta. 2014;1840:2506–2519. doi: 10.1016/j.bbagen.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Saba I, Jakubowska W, Bolduc S, Chabaud S. Engineering Tissues without the Use of a Synthetic Scaffold: A Twenty-Year History of the Self-Assembly Method. Biomed Res Int. 2018;2018:5684679. doi: 10.1155/2018/5684679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Switzer BR, Summer GK. Collagen synthesis in human skin fibroblasts: effects of ascorbate, -ketoglutarate and ferrous ion on proline hydroxylation. J Nutr. 1972;102:721–728. doi: 10.1093/jn/102.6.721. [DOI] [PubMed] [Google Scholar]
- 185.Hata R, Senoo H. L-ascorbic acid 2-phosphate stimulates collagen accumulation, cell proliferation, and formation of a three-dimensional tissuelike substance by skin fibroblasts. J Cell Physiol. 1989;138:8–16. doi: 10.1002/jcp.1041380103. [DOI] [PubMed] [Google Scholar]
- 186.Germain L, Larouche D, Nedelec B, Perreault I, Duranceau L, Bortoluzzi P, Beaudoin Cloutier C, Genest H, Caouette-Laberge L, Dumas A, Bussière A, Boghossian E, Kanevsky J, Leclerc Y, Lee J, Nguyen MT, Bernier V, Knoppers BM, Moulin VJ, Auger FA. Autologous bilayered self-assembled skin substitutes (SASSs) as permanent grafts: a case series of 14 severely burned patients indicating clinical effectiveness. Eur Cell Mater. 2018;36:128–141. doi: 10.22203/eCM.v036a10. [DOI] [PubMed] [Google Scholar]
- 187.Boa O, Cloutier CB, Genest H, Labbé R, Rodrigue B, Soucy J, Roy M, Arsenault F, Ospina CE, Dubé N, Rochon MH, Larouche D, Moulin VJ, Germain L, Auger FA. Prospective study on the treatment of lower-extremity chronic venous and mixed ulcers using tissue-engineered skin substitute made by the self-assembly approach. Adv Skin Wound Care. 2013;26:400–409. doi: 10.1097/01.ASW.0000433102.48268.2a. [DOI] [PubMed] [Google Scholar]
- 188.Lavoie A, Fugère C, Beauparlant A, Goyer B, Larouche D, Paquet C, Desgagné M, Sauvé S, Robitaille H, Dunnwald M, Kim DH, Pouliot R, Fradette J, Germain L. Human epithelial stem cells persist within tissue-engineered skin produced by the self-assembly approach. Tissue Eng Part A. 2013;19:1023–1038. doi: 10.1089/ten.TEA.2012.0117. [DOI] [PubMed] [Google Scholar]
- 189.Magnan M, Lévesque P, Gauvin R, Dubé J, Barrieras D, El-Hakim A, Bolduc S. Tissue engineering of a genitourinary tubular tissue graft resistant to suturing and high internal pressures. Tissue Eng Part A. 2009;15:197–202. doi: 10.1089/ten.tea.2007.0303. [DOI] [PubMed] [Google Scholar]
- 190.Cattan V, Bernard G, Rousseau A, Bouhout S, Chabaud S, Auger FA, Bolduc S. Mechanical stimuli-induced urothelial differentiation in a human tissue-engineered tubular genitourinary graft. Eur Urol. 2011;60:1291–1298. doi: 10.1016/j.eururo.2011.05.051. [DOI] [PubMed] [Google Scholar]
- 191.Imbeault A, Bernard G, Rousseau A, Morissette A, Chabaud S, Bouhout S, Bolduc S. An endothelialized urothelial cell-seeded tubular graft for urethral replacement. Can Urol Assoc J. 2013;7:E4–E9. doi: 10.5489/cuaj.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Magnan M, Berthod F, Champigny MF, Soucy F, Bolduc S. In vitro reconstruction of a tissue-engineered endothelialized bladder from a single porcine biopsy. J Pediatr Urol. 2006;2:261–270. doi: 10.1016/j.jpurol.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 193.Bouhout S, Perron E, Gauvin R, Bernard G, Ouellet G, Cattan V, Bolduc S. In vitro reconstruction of an autologous, watertight, and resistant vesical equivalent. Tissue Eng Part A. 2010;16:1539–1548. doi: 10.1089/ten.TEA.2009.0473. [DOI] [PubMed] [Google Scholar]
- 194.Bouhout S, Gauvin R, Gibot L, Aubé D, Bolduc S. Bladder substitute reconstructed in a physiological pressure environment. J Pediatr Urol. 2011;7:276–282. doi: 10.1016/j.jpurol.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 195.Bouhout S, Chabaud S, Bolduc S. Organ-specific matrix self-assembled by mesenchymal cells improves the normal urothelial differentiation in vitro. World J Urol. 2016;34:121–130. doi: 10.1007/s00345-015-1596-2. [DOI] [PubMed] [Google Scholar]
- 196.Ringuette Goulet C, Bernard G, Chabaud S, Couture A, Langlois A, Neveu B, Pouliot F, Bolduc S. Tissue-engineered human 3D model of bladder cancer for invasion study and drug discovery. Biomaterials. 2017;145:233–241. doi: 10.1016/j.biomaterials.2017.08.041. [DOI] [PubMed] [Google Scholar]
- 197.Bureau M, Pelletier J, Rousseau A, Bernard G, Chabaud S, Bolduc S. Demonstration of the direct impact of ketamine on urothelium using a tissue engineered bladder model. Can Urol Assoc J. 2015;9:E613–E617. doi: 10.5489/cuaj.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Paramitha D, Chabaud S, Bolduc S, Hermawan H. Biological Assessment of Zn-Based Absorbable Metals for Ureteral Stent Applications. Materials (Basel) 2019;12 doi: 10.3390/ma12203325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Chabaud S, Saba I, Baratange C, Boiroux B, Leclerc M, Rousseau A, Bouhout S, Bolduc S. Urothelial cell expansion and differentiation are improved by exposure to hypoxia. J Tissue Eng Regen Med. 2017;11:3090–3099. doi: 10.1002/term.2212. [DOI] [PubMed] [Google Scholar]
- 200.Rousseau A, Fradette J, Bernard G, Gauvin R, Laterreur V, Bolduc S. Adipose-derived stromal cells for the reconstruction of a human vesical equivalent. J Tissue Eng Regen Med. 2015;9:E135–E143. doi: 10.1002/term.1717. [DOI] [PubMed] [Google Scholar]
- 201.Vallières K, Laterreur V, Tondreau MY, Ruel J, Germain L, Fradette J, Auger FA. Human adipose-derived stromal cells for the production of completely autologous self-assembled tissue-engineered vascular substitutes. Acta Biomater. 2015;24:209–219. doi: 10.1016/j.actbio.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 202.Master Z, Resnik DB. Stem-cell tourism and scientific responsibility. Stem-cell researchers are in a unique position to curb the problem of stem-cell tourism. EMBO Rep. 2011;12:992–995. doi: 10.1038/embor.2011.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Liang CC, Shaw SW, Huang YH, Lin YH, Lee TH. Bladder Transplantation of Amniotic Fluid Stem Cell may Ameliorate Bladder Dysfunction After Focal Cerebral Ischemia in Rat. Stem Cells Transl Med. 2017;6:1227–1236. doi: 10.1002/sctm.16-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Liang CC, Shaw SS, Lin YH, Lee TH. Amniotic fluid stem cells ameliorate bladder dysfunction induced by chronic bladder ischemia in rat. Neurourol Urodyn. 2018;37:123–131. doi: 10.1002/nau.23316. [DOI] [PubMed] [Google Scholar]
- 205.Liang CC, Shaw SS, Huang YH, Lin YH, Lee TH. Improvement in bladder dysfunction after bladder transplantation of amniotic fluid stem cells in diabetic rats. Sci Rep. 2018;8:2105. doi: 10.1038/s41598-018-20512-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Soler R, Füllhase C, Hanson A, Campeau L, Santos C, Andersson KE. Stem cell therapy ameliorates bladder dysfunction in an animal model of Parkinson disease. J Urol. 2012;187:1491–1497. doi: 10.1016/j.juro.2011.11.079. [DOI] [PubMed] [Google Scholar]
- 207.Chen S, Zhang HY, Zhang N, Li WH, Shan H, Liu K, Yang Y. Treatment for chronic ischaemia-induced bladder detrusor dysfunction using bone marrow mesenchymal stem cells: an experimental study. Int J Mol Med. 2012;29:416–422. doi: 10.3892/ijmm.2011.846. [DOI] [PubMed] [Google Scholar]
- 208.Kanno Y, Mitsui T, Sano H, Kitta T, Moriya K, Nonomura K. Contribution of bone marrow-derived mesenchymal stem cells to the morphological changes in the bladder after partial outlet obstruction: a preliminary study. Int J Urol. 2014;21:714–718. doi: 10.1111/iju.12406. [DOI] [PubMed] [Google Scholar]
- 209.Wiafe B, Adesida AB, Churchill T, Kadam R, Carleton J, Metcalfe PD. Mesenchymal stem cell therapy inhibited inflammatory and profibrotic pathways induced by partial bladder outlet obstruction and prevented high-pressure urine storage. J Pediatr Urol. 2019;15:254.e1–254.e10. doi: 10.1016/j.jpurol.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 210.Xiao Y, Song YJ, Song B, Huang CB, Ling Q, Yu X. TGF-β/MAPK signaling mediates the effects of bone marrow mesenchymal stem cells on urinary control and interstitial cystitis after urinary bladder transplantation. Am J Transl Res. 2017;9:1193–1202. [PMC free article] [PubMed] [Google Scholar]
- 211.Campeau L, Soler R, Sittadjody S, Pareta R, Nomiya M, Zarifpour M, Opara EC, Yoo JJ, Andersson KE. Effects of allogeneic bone marrow derived mesenchymal stromal cell therapy on voiding function in a rat model of Parkinson disease. J Urol. 2014;191:850–859. doi: 10.1016/j.juro.2013.08.026. [DOI] [PubMed] [Google Scholar]
- 212.Sandner B, Ciatipis M, Motsch M, Soljanik I, Weidner N, Blesch A. Limited Functional Effects of Subacute Syngeneic Bone Marrow Stromal Cell Transplantation After Rat Spinal Cord Contusion Injury. Cell Transplant. 2016;25:125–139. doi: 10.3727/096368915X687679. [DOI] [PubMed] [Google Scholar]
- 213.Hu Y, Liao LM, Ju YH, Fu G, Zhang HY, Wu HX. Intravenously transplanted bone marrow stromal cells promote recovery of lower urinary tract function in rats with complete spinal cord injury. Spinal Cord. 2012;50:202–207. doi: 10.1038/sc.2011.128. [DOI] [PubMed] [Google Scholar]
- 214.Temeltas G, Dagci T, Evren V, Lekili M. Effects of neuronal and glial restricted precursor cells transplantation on erectile function after experimentally induced spinal cord injury. J Sex Med. 2009;6:3265–3273. doi: 10.1111/j.1743-6109.2009.01376.x. [DOI] [PubMed] [Google Scholar]
- 215.Chung SY, Krivorov NP, Rausei V, Thomas L, Frantzen M, Landsittel D, Kang YM, Chon CH, Ng CS, Fuchs GJ. Bladder reconstitution with bone marrow derived stem cells seeded on small intestinal submucosa improves morphological and molecular composition. J Urol. 2005;174:353–359. doi: 10.1097/01.ju.0000161592.00434.c1. [DOI] [PubMed] [Google Scholar]
- 216.Poulsom R, Forbes SJ, Hodivala-Dilke K, Ryan E, Wyles S, Navaratnarasah S, Jeffery R, Hunt T, Alison M, Cook T, Pusey C, Wright NA. Bone marrow contributes to renal parenchymal turnover and regeneration. J Pathol. 2001;195:229–235. doi: 10.1002/path.976. [DOI] [PubMed] [Google Scholar]
- 217.Sun C, Lin H, Yu W, Li X, Chen Y, Qiu X, Wang R, Dai Y. Neurotrophic effect of bone marrow mesenchymal stem cells for erectile dysfunction in diabetic rats. Int J Androl. 2012;35:601–607. doi: 10.1111/j.1365-2605.2012.01250.x. [DOI] [PubMed] [Google Scholar]
- 218.Sun DZ, Abelson B, Babbar P, Damaser MS. Harnessing the mesenchymal stem cell secretome for regenerative urology. Nat Rev Urol. 2019;16:363–375. doi: 10.1038/s41585-019-0169-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Hirose Y, Yamamoto T, Nakashima M, Funahashi Y, Matsukawa Y, Yamaguchi M, Kawabata S, Gotoh M. Injection of Dental Pulp Stem Cells Promotes Healing of Damaged Bladder Tissue in a Rat Model of Chemically Induced Cystitis. Cell Transplant. 2016;25:425–436. doi: 10.3727/096368915X689523. [DOI] [PubMed] [Google Scholar]
- 220.Fandel TM, Trivedi A, Nicholas CR, Zhang H, Chen J, Martinez AF, Noble-Haeusslein LJ, Kriegstein AR. Transplanted Human Stem Cell-Derived Interneuron Precursors Mitigate Mouse Bladder Dysfunction and Central Neuropathic Pain after Spinal Cord Injury. Cell Stem Cell. 2016;19:544–557. doi: 10.1016/j.stem.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 221.Kim A, Yu HY, Lim J, Ryu CM, Kim YH, Heo J, Han JY, Lee S, Bae YS, Kim JY, Bae DJ, Kim SY, Noh BJ, Hong KS, Lee SW, Song M, Chung HM, Kim JK, Shin DM, Choo MS. Improved efficacy and in vivo cellular properties of human embryonic stem cell derivative in a preclinical model of bladder pain syndrome. Sci Rep. 2017;7:8872. doi: 10.1038/s41598-017-09330-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Lee SW, Ryu CM, Shin JH, Choi D, Kim A, Yu HY, Han JY, Lee HY, Lim J, Kim YH, Heo J, Lee S, Ju H, Kim S, Hong KS, Song M, Chung HM, Kim JK, Shin DM, Choo MS. The Therapeutic Effect of Human Embryonic Stem Cell-Derived Multipotent Mesenchymal Stem Cells on Chemical-Induced Cystitis in Rats. Int Neurourol J. 2018;22:S34–S45. doi: 10.5213/inj.1836014.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Ryu CM, Yu HY, Lee HY, Shin JH, Lee S, Ju H, Paulson B, Kim S, Lim J, Heo J, Hong KS, Chung HM, Kim JK, Shin DM, Choo MS. Longitudinal intravital imaging of transplanted mesenchymal stem cells elucidates their functional integration and therapeutic potency in an animal model of interstitial cystitis/bladder pain syndrome. Theranostics. 2018;8:5610–5624. doi: 10.7150/thno.27559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Kwon D, Minnery B, Kim Y, Kim JH, de Miguel F, Yoshimura N, Chancellor MB. Neurologic recovery and improved detrusor contractility using muscle-derived cells in rat model of unilateral pelvic nerve transection. Urology. 2005;65:1249–1253. doi: 10.1016/j.urology.2005.01.037. [DOI] [PubMed] [Google Scholar]
- 225.Pruchnic R, Yokoyama T, Lee JY, Huard J, Chancellor MB. Muscle derived cell mediated ex vivo gene transfer to the lower urinary tract: comparison of viral vectors. Urol Res. 2002;30:310–316. doi: 10.1007/s00240-002-0273-2. [DOI] [PubMed] [Google Scholar]
- 226.Jin Y, Bouyer J, Shumsky JS, Haas C, Fischer I. Transplantation of neural progenitor cells in chronic spinal cord injury. Neuroscience. 2016;320:69–82. doi: 10.1016/j.neuroscience.2016.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Neuhuber B, Barshinger AL, Paul C, Shumsky JS, Mitsui T, Fischer I. Stem cell delivery by lumbar puncture as a therapeutic alternative to direct injection into injured spinal cord. J Neurosurg Spine. 2008;9:390–399. doi: 10.3171/SPI.2008.9.10.390. [DOI] [PubMed] [Google Scholar]
- 228.Mitsui T, Neuhuber B, Fischer I. Acute administration of AMPA/Kainate blocker combined with delayed transplantation of neural precursors improves lower urinary tract function in spinal injured rats. Brain Res. 2011;1418:23–31. doi: 10.1016/j.brainres.2011.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Cho YS, Ko IG, Kim SE, Lee SM, Shin MS, Kim CJ, Kim SH, Jin JJ, Kim KH. Oral mucosa stem cells alleviates spinal cord injury-induced neurogenic bladder symptoms in rats. J Biomed Sci. 2014;21:43. doi: 10.1186/1423-0127-21-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Nitta M, Tamaki T, Tono K, Okada Y, Masuda M, Akatsuka A, Hoshi A, Usui Y, Terachi T. Reconstitution of experimental neurogenic bladder dysfunction using skeletal muscle-derived multipotent stem cells. Transplantation. 2010;89:1043–1049. doi: 10.1097/TP.0b013e3181d45a7f. [DOI] [PubMed] [Google Scholar]
- 231.Williams JK, Badlani G, Dean A, Lankford S, Poppante K, Criswell T, Andersson KE. Local versus intravenous injections of skeletal muscle precursor cells in nonhuman primates with acute or chronic intrinsic urinary sphincter deficiency. Stem Cell Res Ther. 2016;7:147. doi: 10.1186/s13287-016-0411-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Liang CC, Lee TH, Chang SD. Effect of umbilical cord blood stem cells transplantation on bladder dysfunction induced by cerebral ischemia in rats. Taiwan J Obstet Gynecol. 2016;55:672–679. doi: 10.1016/j.tjog.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 233.Song M, Lim J, Yu HY, Park J, Chun JY, Jeong J, Heo J, Kang H, Kim Y, Cho YM, Kim SW, Oh W, Choi SJ, Jang SW, Park S, Shin DM, Choo MS. Mesenchymal Stem Cell Therapy Alleviates Interstitial Cystitis by Activating Wnt Signaling Pathway. Stem Cells Dev. 2015;24:1648–1657. doi: 10.1089/scd.2014.0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Xie J, Liu B, Chen J, Xu Y, Zhan H, Yang F, Li W, Zhou X. Umbilical cord-derived mesenchymal stem cells alleviated inflammation and inhibited apoptosis in interstitial cystitis via AKT/mTOR signaling pathway. Biochem Biophys Res Commun. 2018;495:546–552. doi: 10.1016/j.bbrc.2017.11.072. [DOI] [PubMed] [Google Scholar]
- 235.Kim A, Yu HY, Heo J, Song M, Shin JH, Lim J, Yoon SJ, Kim Y, Lee S, Kim SW, Oh W, Choi SJ, Shin DM, Choo MS. Mesenchymal stem cells protect against the tissue fibrosis of ketamine-induced cystitis in rat bladder. Sci Rep. 2016;6:30881. doi: 10.1038/srep30881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Li J, Luo H, Dong X, Liu Q, Wu C, Zhang T, Hu X, Zhang Y, Song B, Li L. Therapeutic effect of urine-derived stem cells for protamine/lipopolysaccharide-induced interstitial cystitis in a rat model. Stem Cell Res Ther. 2017;8:107. doi: 10.1186/s13287-017-0547-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Liu Y, Zheng Y, Li S, Xue H, Schmitt K, Hergenroeder GW, Wu J, Zhang Y, Kim DH, Cao Q. Human neural progenitors derived from integration-free iPSCs for SCI therapy. Stem Cell Res. 2017;19:55–64. doi: 10.1016/j.scr.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Yang Q, Chen X, Zheng T, Han D, Zhang H, Shi Y, Bian J, Sun X, Xia K, Liang X, Liu G, Zhang Y, Deng C. Transplantation of Human Urine-Derived Stem Cells Transfected with Pigment Epithelium-Derived Factor to Protect Erectile Function in a Rat Model of Cavernous Nerve Injury. Cell Transplant. 2016;25:1987–2001. doi: 10.3727/096368916X691448. [DOI] [PubMed] [Google Scholar]