Abstract
Hepatitis C virus (HCV) chronic infection is associated with fibrosis progression, end-stage liver complications and HCC. Not surprisingly, HCV infection is a leading cause of liver-related morbidity and mortality worldwide. After sustained virological response (SVR), the risk of developing hepatocellular carcinoma is not completely eliminated in patients with established cirrhosis or with advanced fibrosis. Therefore, lifelong surveillance is currently recommended. This strategy is likely not universally cost-effective and harmless, considering that not all patients with advanced fibrosis have the same risk of developing HCC. Factors related to the severity of liver disease and its potential to improve after SVR, the molecular and epigenetic changes that occur during infection and other associated comorbidities might account for different risk levels and are likely essential for identifying patients who would benefit from screening programs after SVR. Efforts to develop predictive models and risk calculators, biomarkers and genetic panels and even deep learning models to estimate the individual risk of HCC have been made in the direct-acting antiviral agents era, when thousands of patients with advanced fibrosis and cirrhosis have reached SVR. These tools could help to identify patients with very low HCC risk in whom surveillance might not be justified. In this review, factors affecting the probability of HCC development after SVR, the benefits and risks of surveillance, suggested strategies to estimate individualized HCC risk and the current evidence to recommend lifelong surveillance are discussed.
Keywords: Hepatitis C virus, Hepatocellular carcinoma, Liver fibrosis, Surveillance, Sustained virologic response, Epigenetic changes, Predictive models, Cost-effectiveness
Core Tip: Hepatocellular carcinoma (HCC) risk is reduced after sustained viral response, but a substantial threat persists over time. Understanding the natural history of hepatitis C virus infection and the variable influence of viral eradication in the molecular and epigenetic changes that occur during infection are essential to explain the different risk of developing HCC in patients with advanced fibrosis. The definition of the appropriate tools to estimate the individual risk of HCC after antiviral treatment providing reliable recommendations about HCC surveillance is probably the most important challenge to be clarified in this field.
INTRODUCTION
Hepatitis C virus (HCV) chronic infection is a major cause of liver-related morbidity and mortality worldwide. Direct-acting antiviral agents (DAAs) have definitely changed the natural history of the disease by reducing liver-related complications in patients with advanced liver fibrosis (including those with cirrhosis) and improving the survival rate. Nonetheless, the risk of developing hepatocellular carcinoma is not completely eliminated with viral clearance. Not surprisingly, clinical guidelines still recommend life-long ultrasound surveillance in all patients with advanced fibrosis (F3) and cirrhosis (F4)[1,2].
However, the risk of HCC occurrence is not homogenous within the spectrum of compensated advanced chronic liver disease (c-ACLD). Therefore, surveillance strategies might not be cost-effective or harmless in all patients. Thus, the identification of patients who truly benefit from screening programs and for how long is a matter of debate.
HCC risk factors are associated with the severity of liver disease and the degree of improvement after sustained virological response but also with the presence of other comorbidities and preneoplastic changes induced by HCV. All of them are discussed in this review, as well as their predictive capacity to estimate individualized HCC risk. We also discuss the current evidence to recommend surveillance.
FACTORS AFFECTING HCC OCCURRENCE IN HCV PATIENTS
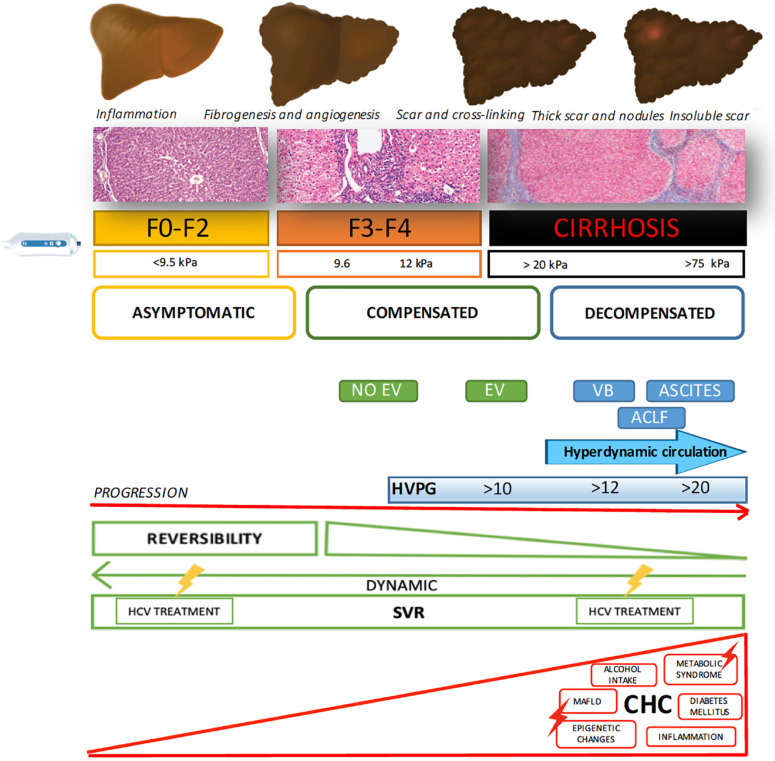
A combination of different factors, occurring either before or after SVR, is involved in the risk of HCC associated with HCV chronic infection (Figure 1).
Figure 1.
Factors involved in increasing or decreasing hepatocellular carcinoma risk, either before or after sustained virologic response. EV: Esophageal varices; VB: Variceal bleeding; ACLF: Acute on chronic liver failure; PH: Portal hypertension; SVR: Sustained virologic response; HCC: Hepatocellular carcinoma; MAFLD: Metabolic associated fatty liver disease.
Development of fibrosis during HCV infection
Chronic HCV infection typically causes damage and inflammation in the liver parenchyma, which can be followed by fibrosis deposition of different severities. Fibrogenesis is a dynamic process characterized by the synthesis of extracellular matrix (ECM), composed of a mixed complex of glycoproteins (collagen, elastin, fibronectin, laminin) and proteoglycans organized in a three-dimensional network[3]. Therefore, fibrosis is a physiological mechanism that can become pathological when viral infection and chronic hepatocellular injury persist[4].
In chronic hepatitis, including hepatitis C, active fibrosis begins around the portal areas (periportal or zone 1 fibrosis) and gradually extends out into the lobules toward the central veins (zone 3), with septum formation and then bridging fibrosis[5]. The final stage of this process constitutes cirrhosis, in which extensive fibrosis linking portal and central areas and nodular regeneration of the liver parenchyma appear. Collagen and matrix proteins are largely produced by activated hepatic stellate cells (HSCs). In contrast, activated liver sinusoidal endothelial cells (LSECs) contribute to ECM production, including the synthesis of basement membrane components, leading to perisinusoidal fibrosis. They also produce cytokines that activate HSCs and secrete factors that contribute to intrahepatic vasoconstriction and to portal hypertension in cirrhosis[6]. It has been widely demonstrated that the severity of liver fibrosis and the development of cirrhosis are the most important risk factors for HCC[7-9]. Therefore, the earlier that this process is discontinued by means of SVR, the lower that the likelihood is of HCC occurrence.
Epigenetic changes involved in hepatitis C infection
Cirrhosis of all causes can be complicated by the appearance of liver tumors, but the risk is higher in patients with chronic liver disease of a viral etiology[7]. In 2018, a large cohort study aimed to compare HCC risk according to the etiology of liver disease and showed that patients with HCV-related cirrhosis had a 3-fold increased HCC risk compared to ALD or NAFLD-related cirrhosis, suggesting that hepatitis C virus itself might have a direct carcinogenic effect[10].
HCV is an RNA virus with limited potential for integration into the host genome and therefore requires continuous replication to maintain chronic infection. HCV directly contributes to hepatocarcinogenesis, interrupting the signal transduction pathways that affect cell survival, proliferation, and transformation via HCV protein or RNA and indirectly by inducing chronic inflammation[8,11].
Epigenetic regulation is an indispensable process for the normal development and preservation of tissue-specific gene expression profiles. Thus, any perturbation of the epigenetic landscape can lead to shifted gene function and malignant cellular transformation. The changed epigenome of HCC is characterized by gene-specific hypermethylation or hypomethylation, global genomic hypomethylation, abnormal expression of DNA methyltransferases and histone modifying enzymes, altered histone modification patterns, and aberrant expression of microRNAs, which can affect the expression of oncogenes, tumor suppressor genes and other tumor-related genes altering the cancer development pathways over time[12]. In fact, a recent study showed a clear, positive correlation between epigenetic changes and fibrosis stages in HCV chronic infection[13], which persist after SVR.
Reversibility of liver fibrosis
Effective antiviral treatment has proved to change the natural history of HCV-related liver disease, reducing the risk of liver events and HCC, even in patients with advanced liver disease and cirrhosis. A recent meta-analysis[14] showed a significant absolute risk reduction in HCC after SVR, which was even greater in patients with cirrhosis (22%; 95%CI: 13-31) than in patients with any stage of fibrosis (6.7%; 95%CI: 5-8). It has been suggested that regression of fibrosis is one of the key mechanisms. In contrast to what was previously believed, there is currently substantial evidence indicating that the removal of fibrosis, hepatocyte repopulation and microvasculature remodeling occur after SVR following several cellular processes[15,16]. Mechanisms of the resolution of liver fibrosis involve senescence and apoptosis of activated HSCs/myofibroblasts caused by deprivation of fibrogenic cytokines. Reversal of these cells to an inactive phenotype during liver fibrosis regression[6,15] has also been described. However, regression of liver disease depends on the severity of fibrosis before antiviral therapy, with total regression being more likely in patients with mild/moderate fibrosis than in those with established cirrhosis due to the presence of a stronger cross-linking matrix, which is more difficult to remove by methaloproteases. Furthermore, the presence of architectural distortion with vascular shunts also contributes to the persistence of architectural changes[17-21]. However, the “point of no return” is controversial. A Canadian study[16] examined explants of patients with cirrhosis or precirrhosis who underwent transplantation, in which the causative agent of the liver disease was controlled or had been removed. The authors found that regression involves two main processes: removal of fibrosis and repopulation of scarred regions with hepatocytes, concluding that reversibility is possible in all stages of fibrosis, including precirrhotic stages and even macronodular cirrhosis. Interestingly, “sinusoidal capillarization” was considered the “point of no return”. Moreover, advanced stages of cirrhosis with thicker septa and smaller micronodules are associated with the presence of clinically significant portal hypertension and therefore with a lower likelihood of reversibility[20].
An Italian study including patients with cirrhosis with paired biopsies after achieving SVR with PEGINF/RBV showed that, in more than half of the patients, regression of cirrhosis was observed during follow-up. Patients who did not change their METAVIR scores after SVR also presented a decrease in the amount of collagen fibers, coinciding with the transformation of micronodular cirrhosis into a macronodular form or incomplete septal cirrhosis[21]. Finally, a Spanish study published in 2018 evaluated the regression of fibrosis using paired biopsies in 112 patients with posttransplant recurrence of HCV infection after treatment with DAAs[22]. Fibrosis regression occurred in 72-85% of the patients without liver cirrhosis (F1-F3) and in 43% of the patients with cirrhosis. Interestingly, in this study, more than 50% of the cirrhotic patients had a history of decompensation, suggesting that patients with liver cirrhosis without clinically significant portal hypertension are more likely to have improved liver injury, likely decreasing the risk of developing HCC after SVR.
Therefore, among patients with advanced fibrosis and cirrhosis, the risk of HCC seems to be lower in patients with less severe disease, who are in fact those who benefit the most after SVR.
One important issue is how to assess fibrosis regression since liver biopsy is an invasive procedure, does not distinguish early from advanced stages of cirrhosis and cannot be performed repeatedly after SVR. Not surprisingly, noninvasive elastographic and direct or indirect serological markers have been widely used to assess fibrosis regression[23]. However, in regressed cirrhosis, macronodules and aberrant vasculature with capillarization of the sinusoids can persist despite a decrease in liver stiffness assessed by TE. The study from D’ Ambrosio et al[24] revealed that, after 61 mo of follow-up, 38% of patients with biopsy-proven F4 had liver stiffness < 12 kPa, resulting in low predictive power of TE to diagnose cirrhosis after viral eradication. Thus, a combination of TE and serological noninvasive markers might improve the capacity to assess fibrosis regression.
Inflammation and liver cancer
Another factor specifically affecting HCC risk during HCV chronic infection is the presence of inflammation. Various types of cancer arise in the setting of chronic inflammation, indicating a strong link between inflammation and cancer. It has been estimated that approximately 15% of all human cancers are associated with inflammation and chronic infections[25]. During chronic viral hepatitis, host immune responses to HBV or HCV are often not sufficiently strong to completely eradicate the infection, inducing persistent stimulation of antigen-specific immune responses. Host immune cells are known to destroy virus-infected liver cells, resulting in the production of different cytokines and growth factors, consequently inducing compensatory regeneration of hepatocytes. The persistent cycle of hepatocyte necroinflammation and regeneration has a synergistic effect with the severity of liver fibrosis and cirrhosis, promoting architectural distortion and portal hypertension with reduced sinusoidal perfusion favoring hypoxia, which is the substrate for the formation of hypervascular tumors. These factors increase the risk of genetic changes in hepatocytes, promoting the survival and expansion of the initiated cells and leading to dysregulated hepatocyte proliferation, which contributes to the development and progression of liver cancer. Furthermore, oxidative stress accelerates hepatocarcinogenesis through several mechanisms, including transcription and activation of cytokines and growth factors, oxidative DNA damage, DNA methylation, and hepatocyte injury[26-30]. Therefore, HCC risk is expected to decrease after eradicating infection and the subsequent decrease in inflammation mechanisms.
HCC RISK CAN PERSIST AFTER SUSTAINED VIROLOGICAL RESPONSE
Although SVR is associated with a reduction in some of the HCC pathogenetic factors mentioned above, there are many other contributors to HCC occurrence that can persist after viral eradication, related either to the stage of liver disease (e.g., Child B, portal hypertension, low platelet count) or to the presence of comorbidities, such as diabetes, alcohol consumption, smoking and older age[31-34].
A recent publication suggested that regression of liver damage after SVR can last for years, with HCC risk persisting during this period; in other patients, liver injury is not reversed due to advanced cirrhosis stage, or it progresses because of the coexistence of other factors, such as obesity, diabetes, and alcohol intake[35]. In addition, older age contributes, even years after SVR, to the progression of liver fibrosis and to an increased risk of HCC.
Epigenetic memory is another of the possible mechanisms involved in HCC risk persistence after SVR. As mentioned above, HCV infection induces epigenetic alterations. DAA treatment eliminates the virus inside cells, but it is not able to restore the concomitant epigenetic signatures already produced and associated with the risk of HCC. Available data suggest that, when infection has already induced epigenetic changes, gene expression is conserved in cells; therefore, the presence of the virus is no longer necessary to exert oncogenic effects on host cells, producing what is known as epigenetic memory or persistent epigenetic changes[13,36].
HCC SURVEILLANCE AFTER SVR: CURRENT RECOMMENDATIONS
Current guidelines from EASL[1] and AASLD[37] agree regarding the recommendation of hepatocellular carcinoma surveillance after SVR in all patients with cirrhosis. However, EASL recommends indefinite HCC screening in patients with advanced fibrosis (F3) by ultrasound every six months, whereas AASLD does not. These differences are likely related to controversy regarding the risk of developing HCC in F3 patients due to the heterogeneity of this population, which could include misclassified patients (over- or underestimating the severity of fibrosis).
ACCURACY IN THE DIAGNOSIS OF ADVANCED FIBROSIS AND CIRRHOSIS
Considering that advanced fibrosis and especially cirrhosis are the main factors contributing to HCC risk, an accurate diagnosis prior to antiviral therapy is mandatory to predict individual risk after SVR. Liver biopsy remains the gold standard for the assessment of hepatic fibrosis, although noninvasive methods for estimating liver fibrosis are increasingly used. However, the accuracy of fibrosis staging in the noninvasive assessment era is imperfect -- even more so after sustained virological response. This fact is especially important in patients with advanced fibrosis but without cirrhosis, in which liver stiffness measurements (LSMs) can occasionally overestimate fibrosis, especially when marked inflammation is present[38]. Another problem is the definition of F3 stage by LSM, with cutoffs varying from 9.5 kPa to 14.5 kPa according to Castera’s[39] study or up to 12.5 kPa as Ziol et al[40] suggested. Therefore, a substantial proportion of patients might be misclassified, overestimating or underestimating fibrosis and leading to an indefinite link to medical care or, alternatively, mistaken discharge. Thus, other clinical or serological markers should be available to accurately define the severity of compensated advanced liver disease and the remaining HCC risk after SVR.
COST-EFFECTIVENESS, BENEFITS AND RISKS OF HCC SURVEILLANCE
HCC surveillance aims to prolong patient survival and quality of life by improving early diagnosis and curative therapy. Based on estimated tumor doubling times, current guidelines recommend ultrasound every six months, and they establish an incidence of at least 1.5% per year to justify HCC surveillance. Although this threshold is likely too high due to the clinical benefits induced by DAA after SVR and the improvement in HCC therapies, it is not clear whether HCC surveillance remains cost effective in all patients with advanced fibrosis after SVR. Regarding this point, a study suggested that HCC surveillance is unlikely to be cost effective in patients with F3 fibrosis, whereas both annual and biannual modalities are likely to be cost effective for patients with cirrhosis compared with no surveillance[41]. The study suggested that an annual HCC incidence greater than 0.5% might be currently cost-effective, and it proved that both an APRI greater than 2 or an FIB-4 greater than 3.25 allow for the identification of patients for whom HCC surveillance becomes cost effective, suggesting that patients with values less than these thresholds should be discharged from follow-up evaluation. Another study evaluated the cost-effectiveness of risk-stratified HCC screening in cirrhosis based on a combination of biomarkers and clinical variables, including epidermal growth factor single-nucleotide polymorphism, age, sex, smoking status, alkaline phosphatase level, and platelet count. The study showed that HCC surveillance strategies targeting high- and intermediate-risk patients with cirrhosis are cost-effective. Finally, the authors suggested that omitting screening in the lowest-risk subjects was cost-effective compared with biannual screening, without sacrificing net survival benefit[42].
Moreover, in a recent opinion article, Jepsen et al[43] argued that randomized trials of HCC surveillance vs no surveillance are necessary to make formal recommendations involving thousands of patients. The authors made their arguments indicating that universal surveillance could negatively impact patients’ quality of life by generating anxiety about the possibility of a cancer diagnosis. Furthermore, the problems associated with false positives in screening procedures and the need to be connected for life to hospital care in patients with negligible HCC risk are also matters of concern. Therefore, accurate models including predictive factors to identify different risk levels are likely the key to adequate follow-up of patients after SVR.
PREDICTIVE FACTORS OF HCC RISK
Multiple models including clinical, serological, molecular and elastographic variables have attempted to stratify HCC risk in patients with advanced fibrosis. Importantly, there are controversies about the markers that should be used and when they should be measured, considering the dynamic changes that occur after SVR. Table 1 summarizes some studies that have assessed the risk of HCC according to both baseline and dynamic risk factors.
Table 1.
Studies assessing the risk of hepatocellular carcinoma according to baseline and dynamic risk factors
| Ref. | Country | n | Population at risk | Diagnosis of cirrhosis | Treatment regimens | Baseline risk factors | Dynamic risk factors |
| Lleo et al[33], 2019 | Italy | 1766 | All cirrhosis 11.4% Child B/C | 1 or more of the following: Stage 4 fibrosis by METAVIR score, esophageal and/or gastric varices at endoscopy, LSM > 12.5 kPa | All were treated with DAA | Age > 50 years old and presence of esophageal varices, platelets < 110000/L and LSM > 25 kPa | Lack of SVR |
| Ioannou et al[44], 2018 | United States | 45810 | 23% cirrhosis | Clinical, based on ICD-9 or 10 codes for cirrhosis or its complications | Either IFN or DAA | Cirrhosis, SVR, ALT, AST, platelets, albumin, age | |
| Ioannou et al[45], 2019 | United States | 48135 | 9784 with pretreatment cirrhosis | Clinical, based on ICD-9 or 10 codes for cirrhosis or its complications | Either IFN or DAA | Cirrhosis and FIB-4 ≥ 3.25 before treatment | FIB-4 ≥ 3.25 post-SVR |
| Ravaioli et al[47], 2018 | Italy | 139 | All cirrhosis; Included previous HCC 11.5% Child B | All were treated with DAA | History of previous HCC | Child B and LSM reduction after DAA treatment < 30% | |
| Pons et al[48], 2019 | Spain | 572 | All LSM ≥ 10 kPa; All compensated | cACLD defined by LSM ≥ 10 kPa | All were treated with DAA | High risk: baseline albumin < 4 g/dL; Low risk: Baseline albumin ≥ 4 g/dL | High risk: LSM ≥ 20 kPa or LSM 10-20 kPa and albumin < 4.4 g/dL; Low risk: LSM < 10 kPa or LSM 10-20 kPa and albumin ≥ 4.4 g/dL |
| Fan et al[50], 2020 | China | 3566 | 75.3% cirrhosis compensated and decompensated | Histological and/or radiological | Either IFN or DAA | aMAP score; Low risk: 0-50; Intermediate risk: 50-60; High risk: 60-100 | |
| Alonso et al[49], 2020 | Spain | 993 | Advanced fibrosis or compensated cirrhosis | Clinical or histological; advanced fibrosis defined by a LSM by TE > 9.5 Kpa | All were treated with DAA | Albumin < 4.2 g/dL; LSM > 17,3 kPaFIB-4 > 3.7 | Delta LSM < 25.5%; FIB-4 > 3.3; GGT > 42 IU |
| Ioannou et al[62], 2020 | United States | 48151 | All cirrhosis compensated and decompensated | Clinical, based on ICD-9 or 10 codes for cirrhosis or its complications | Either IFN or DAA | Deep learning RNN model | |
| Cirrhosis diagnosis, sex, race and HCV genotype 3 | Development of cirrhosis, SVR, BMI, AST, ALT, bilirubin, FIB-4, APRI, platelets | ||||||
LSM: Liver stiffness measurement; TE: Transient elastography; INF: Interferon, DAA: Direct-acting antiviral agent; HCV: Hepatitis C virus; SVR: Sustained virologic response; FIB-4: Fibrosis 4; HCC: Hepatocellular carcinoma; c-ACLD: Compensated advanced chronic liver disease; CP: Child-Pugh; FU: Follow-up; amap: age, male, ALBI and platelets; GGT: Gamma-glutamyltransferase; RNN: Recurrent neural networks; APRI: AST to platelet ratio index; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; BMI: Body mass index.
The study by Ioannou et al[44] introduced the need for risk modeling, suggesting that screening strategies based on HCC risk models were superior to “screen-all” or “screen-none” strategies. The proposed HCC risk model, which included the presence of cirrhosis, SVR, baseline ALT, AST, platelets, albumin and age, allowed us to calculate the individual risk of HCC. However, the definition of cirrhosis was made based on the presence of portal hypertension signs and/or clinical complications, suggesting that perhaps patients with advanced fibrosis or with early stages of cirrhosis could be misclassified as not having cirrhosis.
In a further study, the authors used a different evaluation of liver disease severity, introducing not only baseline data but also changes in FIB-4 over time, to assess different HCC risk levels. In this second study, the authors showed that patients in whom FIB-4 was less than 3.25 before treatment and after SVR had a lower risk of HCC[45].
Other predictive models have included different combinations of laboratory and elastographic parameters. A study from Italy[33] aimed to evaluate the risk of HCC occurrence and recurrence in cirrhotic patients after DAA therapy. The authors identified a subgroup of patients with SVR and the “Extended Baveno Criteria”[46] (> 110000 platelets and LSM < 25 kPa), with an HCC incidence of 0.5%/per year, suggesting that the Baveno Criteria could be an appropriate tool for stratification, advising less frequent follow-up in this population.
Models that include dynamic changes after SVR could be especially relevant since they can identify patients with less severe disease more likely to regress or improve after SVR. The dynamic change in LSM as an HCC risk factor was previously described in a study showing that a reduction in liver stiffness > 30% at the end of treatment was associated with a significantly lower risk of HCC at one year after EOT[47]. In this study, TE was likely performed too early to detect regression or improvement, although it could identify those patients with overestimated fibrosis at baseline, in whom the resolution of inflammatory activity accounts for the rapid decrease in liver stiffness.
A Spanish study developed a model to estimate HCC risk in patients with c-ACLD after SVR. This study revealed that the combination of follow-up LSM and serum albumin levels at one year after SVR was able to identify different HCC risk groups. The authors observed that patients with LSM < 10 kPa or with LSM between 10-20 kPa and high albumin levels at follow-up had an incidence rate of HCC of less than 1/100 patients per year; thus, the authors considered them a low-risk group[48].
Furthermore, a recent large, multicenter cohort study performed in our unit[49] confirmed the impact of both baseline and dynamic changes in noninvasive markers on the risk of HCC development. We constructed two simple models: the first one included baseline albumin (g/dL) and LSM (Kpa) and the percentage of LSM variation one year after EOT. Patients with baseline albumin > 4.2 g/dL, baseline LSM ≤ 17.3 kPa, and 1-year DeltaLSM > 25.5% had the lowest HCC risk (cumulative incidence of HCC at 3 years of 0%). Considering that LSM might not be universally available, we also built a second model that exclusively included noninvasive serological markers. Similarly, the FIB-4-based model identified patients with baseline albumin > 4.2 g/dL, baseline FIB-4 ≤ 3.7, 1-year FIB-4 score ≤ 3.3, and 1-year GGT ≤ 42 IU/mL as the group with the lowest HCC risk (cumulative incidence of 0.4% at 3 years). Notably, our results suggested that baseline and dynamic LSM (or FIB-4, when TE is not available) could identify patients with a lower incidence of HCC after SVR that does not justify continuous HCC screening. Approximately 20% of the patients were considered to have low or very low HCC risk, and according to our findings, they could be safely discharged from surveillance.
Another recently validated predictive model is the aMAP score[50], which includes laboratory and clinical parameters such as the albumin-bilirubin score (ALBI score) platelets, age and sex. The score identifies two different risk groups, suggesting that only patients belonging to the high-risk group (aMAP score > 60) should undergo intensive surveillance to detect early HCC. This prognostic tool was externally validated in patients with different cirrhosis etiologies from 11 global prospective studies; interestingly, the score properly discriminated 5-year HCC irrespective of etiology of liver disease and ethnicity.
Thus, predictive models and risk scores, preferably based on baseline data and dynamic changes, are likely the best approach for determining the individual risk of HCC after SVR.
LONG-TERM RISK OF HCC: DOES IT DECREASE OVER TIME?
Different long-term studies in HCV patients treated with IFN-based regimens have documented a reduction in the incidence of HCC by 75% in patients with SVR[51-53]. Currently, there is growing evidence that the same occurs after DAA therapy. Several data have shown that SVR after DAAs does not have a significant impact on the development of HCC in the short or medium term since many of the patients were treated with a more advanced disease, it but reduces the risk of HCC in the medium and long term, like what occurs in patients treated with IFN-based regimens[9].
Conversely, it has been suggested that HCC risk persists for up to 10 years after HCV eradication in patients with baseline cirrhosis or high FIB-4 scores[45]. In this study, HCC incidence remained > 2% per year even 10 years after antiviral treatment in patients with a high baseline FIB-4 ≥ 3.25, especially if it remained ≥ 3.25 after SVR. Conversely, patients with baseline FIB-4 < 3.25 had an HCC incidence < 1%.
Moreover, aging and comorbidities that can occur or progress over time could have an additive effect on HCC risk. Consequently, there is no strong evidence to support a dynamic assessment of HCC risk over time, and the best strategy is likely to continue screening patients with intermediate and high HCC risk until they reach an age when it is no longer cost effective.
HCC SCREENING: WHO DOES BENEFIT AND FOR HOW LONG?
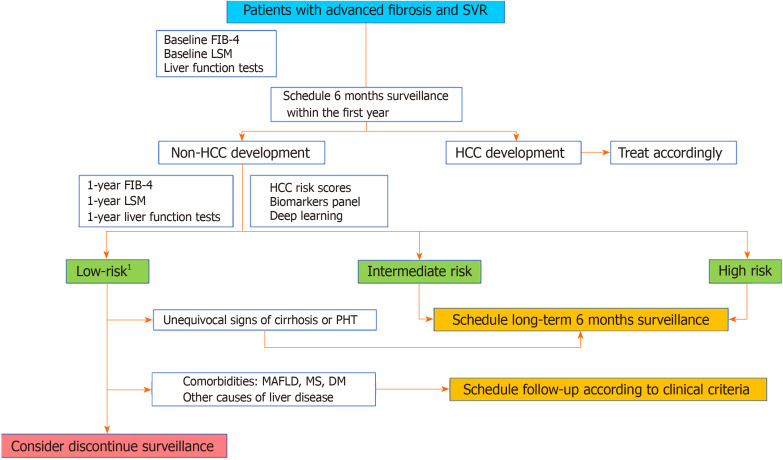
It seems clear that the current recommendations regarding HCC screening after SVR in HCV patients with advanced fibrosis at baseline should be modified in the future. While changes become formal, we provide below a proposal based on the current data (Figure 2).
Figure 2.
Surveillance hepatocellular carcinoma algorithm proposed. 1Most likely, annual incidence < 0.5% per year. FIB-4: Fibrosis 4; LSM: Liver stiffness measurement; TE: Transient elastography; PHT: Portal hypertension; DM: Diabetes mellitus; MS: Metabolic syndrome; MAFLD: Metabolic associated fatty liver disease.
Cirrhotic patients with baseline decompensated or compensated advanced liver disease with significant portal hypertension. HCC risk in this population remains greater than the accepted threshold for surveillance; therefore, biannual US screening is recommended.
Patients with findings of advanced chronic liver disease without clear evidence of baseline portal hypertension. In our opinion, these patients should be screened for HCC at least 1 year after EOT to evaluate early dynamic changes and make more accurate estimations of the individual HCC risk.
FUTURE STRATEGIES IN SURVEILLANCE
As previously stated, several questions remain concerning surveillance strategies. Most likely, the most important concern is that we do not have accurate information about whether HCC risk persists constantly over the long term and therefore for how long patients with medium or high risk should continue HCC surveillance.
It is possible that, in the near future, patients will benefit from a more specific biomarker panel, genetic and molecular profiles and even deep learning models to predict the risk of developing HCC.
Circulating biomarkers are promising tools for better stratification of patients[54-56]. Biomarker panels, such as the GALAD score, are excellent tools for the detection of early-stage HCC, including tumors with negative AFP (AUC of 0.96 for detection of early-stage HCC)[57,58]. As previously exposed, HCV induces epigenetic alterations persisting after DAA cure[13], so the opportunity to detect epigenetic changes of histones bound to circulating DNA in plasma represents a new opportunity to uncover biomarkers of HCC risk. These approaches could represent personalized, noninvasive and cost-effective alternatives based on clinical and biological findings for HCC screening.
The use of deep learning models, which have also been successfully applied in other settings to predict clinical events[59], could also be relevant. Various types of model architectures, such as recurrent neural networks, have been used to capture temporal dynamics and long-term information over time[60]. Recently, it was suggested that some machine-learning algorithms accurately stratify the risk of HCC in patients with cirrhosis, identifying those at high risk for developing HCC[61]. Additionally, it has been shown that, in terms of cost-effectiveness, deep learning and recurrent neural network models were able to improve HCC surveillance strategies in HCV patients, thereby identifying high-risk cases[62].
CONCLUSION
In conclusion, HCC surveillance in HCV patients should likely be based on an evaluation of individualized risk rather than exclusively based on baseline fibrosis stage. Currently available risk scores should be improved and validated, likely by including novel approaches, such as personalized biomarkers and deep learning methods.
Footnotes
Conflict-of-interest statement: Adriana Ahumada has received fees for serving as a speaker and a grant from Abbvie and personal fees for serving as a speaker and for Gilead. Laura Rayón, Clara Usón and Rafael Bañares have nothing to disclose. Sonia Alonso has received fees for serving as a speaker and consultant from Abbvie and Gilead.
Manuscript source: Invited manuscript
Peer-review started: April 11, 2021
First decision: June 13, 2021
Article in press: September 19, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Djuric O S-Editor: Wang LL L-Editor: A P-Editor: Yu HG
Contributor Information
Adriana Ahumada, Liver Unit, Hospital General Universitario Gregorio Marañón, Madrid 28007, Spain; Liver Unit, Instituto de Investigación Sanitaria Gregorio Marañón (IiSGM), Madrid 28007, Spain.
Laura Rayón, Liver Unit, Hospital General Universitario Gregorio Marañón, Madrid 28007, Spain.
Clara Usón, Liver Unit, Hospital General Universitario Gregorio Marañón, Madrid 28007, Spain.
Rafael Bañares, Liver Unit, Hospital General Universitario Gregorio Marañón, Madrid 28007, Spain; Liver Unit, Instituto de Investigación Sanitaria Gregorio Marañón (IiSGM), Madrid 28007, Spain; Medicine, Universidad Complutense de Madrid, Madrid 28006, Spain; Centro de Investigación Biomédica En Red de Enfermedades Hepáticas y Digestivas (CIBERehd), Instituto de Salud Carlos III, Madrid 28029, Spain.
Sonia Alonso Lopez, Liver Unit, Hospital General Universitario Gregorio Marañón, Madrid 28007, Spain; Liver Unit, Instituto de Investigación Sanitaria Gregorio Marañón (IiSGM), Madrid 28007, Spain; Centro de Investigación Biomédica En Red de Enfermedades Hepáticas y Digestivas (CIBERehd), Instituto de Salud Carlos III, Madrid 28029, Spain. salonsol@telefonica.net.
References
- 1.European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C 2018. J Hepatol. 2018;69:461–511. doi: 10.1016/j.jhep.2018.03.026. [DOI] [PubMed] [Google Scholar]
- 2.Singal AG, Lim JK, Kanwal F. AGA Clinical Practice Update on Interaction Between Oral Direct-Acting Antivirals for Chronic Hepatitis C Infection and Hepatocellular Carcinoma: Expert Review. Gastroenterology. 2019;156:2149–2157. doi: 10.1053/j.gastro.2019.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schuppan D, Ruehl M, Somasundaram R, Hahn EG. Matrix as a modulator of hepatic fibrogenesis. Semin Liver Dis. 2001;21:351–372. doi: 10.1055/s-2001-17556. [DOI] [PubMed] [Google Scholar]
- 4.Marcellin P, Asselah T, Boyer N. Fibrosis and disease progression in hepatitis C. Hepatology. 2002;36:S47–S56. doi: 10.1053/jhep.2002.36993. [DOI] [PubMed] [Google Scholar]
- 5.Goodman ZD, Ishak KG. Histopathology of hepatitis C virus infection. Semin Liver Dis. 1995;15:70–81. doi: 10.1055/s-2007-1007264. [DOI] [PubMed] [Google Scholar]
- 6.Jung YK, Yim HJ. Reversal of liver cirrhosis: current evidence and expectations. Korean J Intern Med. 2017;32:213–228. doi: 10.3904/kjim.2016.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 8.Bandiera S, Billie Bian C, Hoshida Y, Baumert TF, Zeisel MB. Chronic hepatitis C virus infection and pathogenesis of hepatocellular carcinoma. Curr Opin Virol. 2016;20:99–105. doi: 10.1016/j.coviro.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rinaldi L, Nevola R, Franci G, Perrella A, Corvino G, Marrone A, Berretta M, Morone MV, Galdiero M, Giordano M, Adinolfi LE, Sasso FC. Risk of Hepatocellular Carcinoma after HCV Clearance by Direct-Acting Antivirals Treatment Predictive Factors and Role of Epigenetics. Cancers (Basel) 2020;12 doi: 10.3390/cancers12061351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ioannou GN, Green P, Lowy E, Mun EJ, Berry K. Differences in hepatocellular carcinoma risk, predictors and trends over time according to etiology of cirrhosis. PLoS One. 2018;13:e0204412. doi: 10.1371/journal.pone.0204412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoshida Y, Villanueva A, Sangiovanni A, Sole M, Hur C, Andersson KL, Chung RT, Gould J, Kojima K, Gupta S, Taylor B, Crenshaw A, Gabriel S, Minguez B, Iavarone M, Friedman SL, Colombo M, Llovet JM, Golub TR. Prognostic gene expression signature for patients with hepatitis C-related early-stage cirrhosis. Gastroenterology. 2013;144:1024–1030. doi: 10.1053/j.gastro.2013.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y. Detection of epigenetic aberrations in the development of hepatocellular carcinoma. Methods Mol Biol. 2015;1238:709–731. doi: 10.1007/978-1-4939-1804-1_37. [DOI] [PubMed] [Google Scholar]
- 13.Hamdane N, Jühling F, Crouchet E, El Saghire H, Thumann C, Oudot MA, Bandiera S, Saviano A, Ponsolles C, Roca Suarez AA, Li S, Fujiwara N, Ono A, Davidson I, Bardeesy N, Schmidl C, Bock C, Schuster C, Lupberger J, Habersetzer F, Doffoël M, Piardi T, Sommacale D, Imamura M, Uchida T, Ohdan H, Aikata H, Chayama K, Boldanova T, Pessaux P, Fuchs BC, Hoshida Y, Zeisel MB, Duong FHT, Baumert TF. HCV-Induced Epigenetic Changes Associated With Liver Cancer Risk Persist After Sustained Virologic Response. Gastroenterology. 2019;156:2313–2329.e7. doi: 10.1053/j.gastro.2019.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Messori A, Badiani B, Trippoli S. Achieving Sustained Virological Response in Hepatitis C Reduces the Long-Term Risk of Hepatocellular Carcinoma: An Updated Meta-Analysis Employing Relative and Absolute Outcome Measures. Clin Drug Investig. 2015;35:843–850. doi: 10.1007/s40261-015-0338-y. [DOI] [PubMed] [Google Scholar]
- 15.Sun M, Kisseleva T. Reversibility of liver fibrosis. Clin Res Hepatol Gastroenterol. 2015;39 Suppl 1:S60–S63. doi: 10.1016/j.clinre.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wanless IR, Nakashima E, Sherman M. Regression of human cirrhosis. Morphologic features and the genesis of incomplete septal cirrhosis. Arch Pathol Lab Med. 2000;124:1599–1607. doi: 10.5858/2000-124-1599-ROHC. [DOI] [PubMed] [Google Scholar]
- 17.Shiratori Y, Imazeki F, Moriyama M, Yano M, Arakawa Y, Yokosuka O, Kuroki T, Nishiguchi S, Sata M, Yamada G, Fujiyama S, Yoshida H, Omata M. Histologic improvement of fibrosis in patients with hepatitis C who have sustained response to interferon therapy. Ann Intern Med. 2000;132:517–524. doi: 10.7326/0003-4819-132-7-200004040-00002. [DOI] [PubMed] [Google Scholar]
- 18.Poynard T, McHutchison J, Manns M, Trepo C, Lindsay K, Goodman Z, Ling MH, Albrecht J. Impact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology. 2002;122:1303–1313. doi: 10.1053/gast.2002.33023. [DOI] [PubMed] [Google Scholar]
- 19.Romero-Gómez M, Del Mar Viloria M, Andrade RJ, Salmerón J, Diago M, Fernández-Rodríguez CM, Corpas R, Cruz M, Grande L, Vázquez L, Muñoz-De-Rueda P, López-Serrano P, Gila A, Gutiérrez ML, Pérez C, Ruiz-Extremera A, Suárez E, Castillo J. Insulin resistance impairs sustained response rate to peginterferon plus ribavirin in chronic hepatitis C patients. Gastroenterology. 2005;128:636–641. doi: 10.1053/j.gastro.2004.12.049. [DOI] [PubMed] [Google Scholar]
- 20.Nagula S, Jain D, Groszmann RJ, Garcia-Tsao G. Histological-hemodynamic correlation in cirrhosis-a histological classification of the severity of cirrhosis. J Hepatol. 2006;44:111–117. doi: 10.1016/j.jhep.2005.07.036. [DOI] [PubMed] [Google Scholar]
- 21.D'Ambrosio R, Aghemo A, Rumi MG, Ronchi G, Donato MF, Paradis V, Colombo M, Bedossa P. A morphometric and immunohistochemical study to assess the benefit of a sustained virological response in hepatitis C virus patients with cirrhosis. Hepatology. 2012;56:532–543. doi: 10.1002/hep.25606. [DOI] [PubMed] [Google Scholar]
- 22.Mauro E, Crespo G, Montironi C, Londoño MC, Hernández-Gea V, Ruiz P, Sastre L, Lombardo J, Mariño Z, Díaz A, Colmenero J, Rimola A, Garcia-Pagán JC, Brunet M, Forns X, Navasa M. Portal pressure and liver stiffness measurements in the prediction of fibrosis regression after sustained virological response in recurrent hepatitis C. Hepatology. 2018;67:1683–1694. doi: 10.1002/hep.29557. [DOI] [PubMed] [Google Scholar]
- 23.Poynard T, Moussalli J, Munteanu M, Thabut D, Lebray P, Rudler M, Ngo Y, Thibault V, Mkada H, Charlotte F, Bismut FI, Deckmyn O, Benhamou Y, Valantin MA, Ratziu V, Katlama C FibroFrance-GHPS group. Slow regression of liver fibrosis presumed by repeated biomarkers after virological cure in patients with chronic hepatitis C. J Hepatol. 2013;59:675–683. doi: 10.1016/j.jhep.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 24.D'Ambrosio R, Aghemo A, Fraquelli M, Rumi MG, Donato MF, Paradis V, Bedossa P, Colombo M. The diagnostic accuracy of Fibroscan for cirrhosis is influenced by liver morphometry in HCV patients with a sustained virological response. J Hepatol. 2013;59:251–256. doi: 10.1016/j.jhep.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 25.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang YH, Huang JH, Tian ZF, Zhou YF, Yang J. The role of CXC cytokines as biomarkers and potential targets in hepatocellular carcinoma. Math Biosci Eng. 2019;17:1381–1395. doi: 10.3934/mbe.2020070. [DOI] [PubMed] [Google Scholar]
- 27.Nakagawa H, Maeda S. Inflammation- and stress-related signaling pathways in hepatocarcinogenesis. World J Gastroenterol. 2012;18:4071–4081. doi: 10.3748/wjg.v18.i31.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marra M, Sordelli IM, Lombardi A, Lamberti M, Tarantino L, Giudice A, Stiuso P, Abbruzzese A, Sperlongano R, Accardo M, Agresti M, Caraglia M, Sperlongano P. Molecular targets and oxidative stress biomarkers in hepatocellular carcinoma: an overview. J Transl Med. 2011;9:171. doi: 10.1186/1479-5876-9-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bishayee A. The role of inflammation and liver cancer. Adv Exp Med Biol. 2014;816:401–435. doi: 10.1007/978-3-0348-0837-8_16. [DOI] [PubMed] [Google Scholar]
- 30.Morse MA, Sun W, Kim R, He AR, Abada PB, Mynderse M, Finn RS. The Role of Angiogenesis in Hepatocellular Carcinoma. Clin Cancer Res. 2019;25:912–920. doi: 10.1158/1078-0432.CCR-18-1254. [DOI] [PubMed] [Google Scholar]
- 31.Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825–832. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- 32.Finkelmeier F, Dultz G, Peiffer KH, Kronenberger B, Krauss F, Zeuzem S, Sarrazin C, Vermehren J, Waidmann O. Risk of de novo Hepatocellular Carcinoma after HCV Treatment with Direct-Acting Antivirals. Liver Cancer. 2018;7:190–204. doi: 10.1159/000486812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lleo A, Aglitti A, Aghemo A, Maisonneuve P, Bruno S, Persico M collaborators. Predictors of hepatocellular carcinoma in HCV cirrhotic patients treated with direct acting antivirals. Dig Liver Dis. 2019;51:310–317. doi: 10.1016/j.dld.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 34.van der Meer AJ, Feld JJ, Hofer H, Almasio PL, Calvaruso V, Fernández-Rodríguez CM, Aleman S, Ganne-Carrié N, D'Ambrosio R, Pol S, Trapero-Marugan M, Maan R, Moreno-Otero R, Mallet V, Hultcrantz R, Weiland O, Rutter K, Di Marco V, Alonso S, Bruno S, Colombo M, de Knegt RJ, Veldt BJ, Hansen BE, Janssen HLA. Risk of cirrhosis-related complications in patients with advanced fibrosis following hepatitis C virus eradication. J Hepatol. 2017;66:485–493. doi: 10.1016/j.jhep.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 35.Ioannou GN. HCC surveillance after SVR in patients with F3/F4 fibrosis. J Hepatol. 2021;74:458–465. doi: 10.1016/j.jhep.2020.10.016. [DOI] [PubMed] [Google Scholar]
- 36.Perez S, Kaspi A, Domovitz T, Davidovich A, Lavi-Itzkovitz A, Meirson T, Alison Holmes J, Dai CY, Huang CF, Chung RT, Nimer A, El-Osta A, Yaari G, Stemmer SM, Yu ML, Haviv I, Gal-Tanamy M. Hepatitis C virus leaves an epigenetic signature post cure of infection by direct-acting antivirals. PLoS Genet. 2019;15:e1008181. doi: 10.1371/journal.pgen.1008181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghany MG, Morgan TR AASLD-IDSA Hepatitis C Guidance Panel. Hepatitis C Guidance 2019 Update: American Association for the Study of Liver Diseases-Infectious Diseases Society of America Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Hepatology. 2020;71:686–721. doi: 10.1002/hep.31060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coco B, Oliveri F, Maina AM, Ciccorossi P, Sacco R, Colombatto P, Bonino F, Brunetto MR. Transient elastography: a new surrogate marker of liver fibrosis influenced by major changes of transaminases. J Viral Hepat. 2007;14:360–369. doi: 10.1111/j.1365-2893.2006.00811.x. [DOI] [PubMed] [Google Scholar]
- 39.Castéra L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, Darriet M, Couzigou P, De Lédinghen V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343–350. doi: 10.1053/j.gastro.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 40.Ziol M, Handra-Luca A, Kettaneh A, Christidis C, Mal F, Kazemi F, de Lédinghen V, Marcellin P, Dhumeaux D, Trinchet JC, Beaugrand M. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005;41:48–54. doi: 10.1002/hep.20506. [DOI] [PubMed] [Google Scholar]
- 41.Farhang Zangneh H, Wong WWL, Sander B, Bell CM, Mumtaz K, Kowgier M, van der Meer AJ, Cleary SP, Janssen HLA, Chan KKW, Feld JJ. Cost Effectiveness of Hepatocellular Carcinoma Surveillance After a Sustained Virologic Response to Therapy in Patients With Hepatitis C Virus Infection and Advanced Fibrosis. Clin Gastroenterol Hepatol. 2019;17:1840–1849.e16. doi: 10.1016/j.cgh.2018.12.018. [DOI] [PubMed] [Google Scholar]
- 42.Goossens N, Singal AG, King LY, Andersson KL, Fuchs BC, Besa C, Taouli B, Chung RT, Hoshida Y. Cost-Effectiveness of Risk Score-Stratified Hepatocellular Carcinoma Screening in Patients with Cirrhosis. Clin Transl Gastroenterol. 2017;8:e101. doi: 10.1038/ctg.2017.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jepsen P, West J. We need stronger evidence for (or against) hepatocellular carcinoma surveillance. J Hepatol. 2021;74:1234–1239. doi: 10.1016/j.jhep.2020.12.029. [DOI] [PubMed] [Google Scholar]
- 44.Ioannou GN, Green PK, Beste LA, Mun EJ, Kerr KF, Berry K. Development of models estimating the risk of hepatocellular carcinoma after antiviral treatment for hepatitis C. J Hepatol. 2018;69:1088–1098. doi: 10.1016/j.jhep.2018.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ioannou GN, Beste LA, Green PK, Singal AG, Tapper EB, Waljee AK, Sterling RK, Feld JJ, Kaplan DE, Taddei TH, Berry K. Increased Risk for Hepatocellular Carcinoma Persists Up to 10 Years After HCV Eradication in Patients With Baseline Cirrhosis or High FIB-4 Scores. Gastroenterology. 2019;157:1264–1278.e4. doi: 10.1053/j.gastro.2019.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Augustin S, Pons M, Maurice JB, Bureau C, Stefanescu H, Ney M, Blasco H, Procopet B, Tsochatzis E, Westbrook RH, Bosch J, Berzigotti A, Abraldes JG, Genescà J. Expanding the Baveno VI criteria for the screening of varices in patients with compensated advanced chronic liver disease. Hepatology. 2017;66:1980–1988. doi: 10.1002/hep.29363. [DOI] [PubMed] [Google Scholar]
- 47.Ravaioli F, Conti F, Brillanti S, Andreone P, Mazzella G, Buonfiglioli F, Serio I, Verrucchi G, Bacchi Reggiani ML, Colli A, Marasco G, Colecchia A, Festi D. Hepatocellular carcinoma risk assessment by the measurement of liver stiffness variations in HCV cirrhotics treated with direct acting antivirals. Dig Liver Dis. 2018;50:573–579. doi: 10.1016/j.dld.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 48.Pons M, Rodríguez-Tajes S, Esteban JI, Mariño Z, Vargas V, Lens S, Buti M, Augustin S, Forns X, Mínguez B, Genescà J. Non-invasive prediction of liver-related events in patients with HCV-associated compensated advanced chronic liver disease after oral antivirals. J Hepatol. 2020;72:472–480. doi: 10.1016/j.jhep.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 49.Alonso López S, Manzano ML, Gea F, Gutiérrez ML, Ahumada AM, Devesa MJ, Olveira A, Polo BA, Márquez L, Fernández I, Cobo JCR, Rayón L, Riado D, Izquierdo S, Usón C, Real Y, Rincón D, Fernández-Rodríguez CM, Bañares R. A Model Based on Noninvasive Markers Predicts Very Low Hepatocellular Carcinoma Risk After Viral Response in Hepatitis C Virus-Advanced Fibrosis. Hepatology. 2020;72:1924–1934. doi: 10.1002/hep.31588. [DOI] [PubMed] [Google Scholar]
- 50.Fan R, Papatheodoridis G, Sun J, Innes H, Toyoda H, Xie Q, Mo S, Sypsa V, Guha IN, Kumada T, Niu J, Dalekos G, Yasuda S, Barnes E, Lian J, Suri V, Idilman R, Barclay ST, Dou X, Berg T, Hayes PC, Flaherty JF, Zhou Y, Zhang Z, Buti M, Hutchinson SJ, Guo Y, Calleja JL, Lin L, Zhao L, Chen Y, Janssen HLA, Zhu C, Shi L, Tang X, Gaggar A, Wei L, Jia J, Irving WL, Johnson PJ, Lampertico P, Hou J. aMAP risk score predicts hepatocellular carcinoma development in patients with chronic hepatitis. J Hepatol. 2020;73:1368–1378. doi: 10.1016/j.jhep.2020.07.025. [DOI] [PubMed] [Google Scholar]
- 51.D'Ambrosio R, Della Corte C, Colombo M. Hepatocellular Carcinoma in Patients with a Sustained Response to Anti-Hepatitis C Therapy. Int J Mol Sci. 2015;16:19698–19712. doi: 10.3390/ijms160819698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158:329–337. doi: 10.7326/0003-4819-158-5-201303050-00005. [DOI] [PubMed] [Google Scholar]
- 53.Nahon P, Bourcier V, Layese R, Audureau E, Cagnot C, Marcellin P, Guyader D, Fontaine H, Larrey D, De Lédinghen V, Ouzan D, Zoulim F, Roulot D, Tran A, Bronowicki JP, Zarski JP, Leroy V, Riachi G, Calès P, Péron JM, Alric L, Bourlière M, Mathurin P, Dharancy S, Blanc JF, Abergel A, Serfaty L, Mallat A, Grangé JD, Attali P, Bacq Y, Wartelle C, Dao T, Benhamou Y, Pilette C, Silvain C, Christidis C, Capron D, Bernard-Chabert B, Zucman D, Di Martino V, Thibaut V, Salmon D, Ziol M, Sutton A, Pol S, Roudot-Thoraval F ANRS CO12 CirVir Group. Eradication of Hepatitis C Virus Infection in Patients With Cirrhosis Reduces Risk of Liver and Non-Liver Complications. Gastroenterology. 2017;152:142–156.e2. doi: 10.1053/j.gastro.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 54.Qiu L, Xu H, Ji M, Shang D, Lu Z, Wu Y, Tu Z, Liu H. Circular RNAs in hepatocellular carcinoma: Biomarkers, functions and mechanisms. Life Sci. 2019;231:116660. doi: 10.1016/j.lfs.2019.116660. [DOI] [PubMed] [Google Scholar]
- 55.Mücke VT, Thomas D, Mücke MM, Waidmann O, Zeuzem S, Sarrazin C, Pfeilschifter J, Vermehren J, Finkelmeier F, Grammatikos G. Serum sphingolipids predict de novo hepatocellular carcinoma in hepatitis C cirrhotic patients with sustained virologic response. Liver Int. 2019;39:2174–2183. doi: 10.1111/liv.14178. [DOI] [PubMed] [Google Scholar]
- 56.Pascut D, Cavalletto L, Pratama MY, Bresolin S, Trentin L, Basso G, Bedogni G, Tiribelli C, Chemello L. Serum miRNA Are Promising Biomarkers for the Detection of Early Hepatocellular Carcinoma after Treatment with Direct-Acting Antivirals. Cancers (Basel) 2019;11 doi: 10.3390/cancers11111773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johnson PJ, Pirrie SJ, Cox TF, Berhane S, Teng M, Palmer D, Morse J, Hull D, Patman G, Kagebayashi C, Hussain S, Graham J, Reeves H, Satomura S. The detection of hepatocellular carcinoma using a prospectively developed and validated model based on serological biomarkers. Cancer Epidemiol Biomarkers Prev. 2014;23:144–153. doi: 10.1158/1055-9965.EPI-13-0870. [DOI] [PubMed] [Google Scholar]
- 58.Yang JD, Addissie BD, Mara KC, Harmsen WS, Dai J, Zhang N, Wongjarupong N, Ali HM, Ali HA, Hassan FA, Lavu S, Cvinar JL, Giama NH, Moser CD, Miyabe K, Allotey LK, Algeciras-Schimnich A, Theobald JP, Ward MM, Nguyen MH, Befeler AS, Reddy KR, Schwartz M, Harnois DM, Yamada H, Srivastava S, Rinaudo JA, Gores GJ, Feng Z, Marrero JA, Roberts LR. GALAD Score for Hepatocellular Carcinoma Detection in Comparison with Liver Ultrasound and Proposal of GALADUS Score. Cancer Epidemiol Biomarkers Prev. 2019;28:531–538. doi: 10.1158/1055-9965.EPI-18-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shickel B, Tighe PJ, Bihorac A, Rashidi P. Deep EHR: A Survey of Recent Advances in Deep Learning Techniques for Electronic Health Record (EHR) Analysis. IEEE J Biomed Health Inform. 2018;22:1589–1604. doi: 10.1109/JBHI.2017.2767063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xiao C, Choi E, Sun J. Opportunities and challenges in developing deep learning models using electronic health records data: a systematic review. J Am Med Inform Assoc. 2018;25:1419–1428. doi: 10.1093/jamia/ocy068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singal AG, Mukherjee A, Elmunzer BJ, Higgins PD, Lok AS, Zhu J, Marrero JA, Waljee AK. Machine learning algorithms outperform conventional regression models in predicting development of hepatocellular carcinoma. Am J Gastroenterol. 2013;108:1723–1730. doi: 10.1038/ajg.2013.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ioannou GN, Tang W, Beste LA, Tincopa MA, Su GL, Van T, Tapper EB, Singal AG, Zhu J, Waljee AK. Assessment of a Deep Learning Model to Predict Hepatocellular Carcinoma in Patients With Hepatitis C Cirrhosis. JAMA Netw Open. 2020;3:e2015626. doi: 10.1001/jamanetworkopen.2020.15626. [DOI] [PMC free article] [PubMed] [Google Scholar]