Abstract
BACKGROUND
Even in the immuno-oncology era, transcatheter arterial chemoembolisation (TACE) is the most effective way to treat intermediate stage hepatocellular carcinoma (HCC). Postembolisation syndrome (PES) is the most common side effect from TACE and there is still no standard prevention guideline.
AIM
To evaluate the efficacy of single dose intravenous dexamethasone regimen to prevent PES after TACE among patients with HCC.
METHODS
This study enrolled patients with HCC who had eligible indication for TACE without macrovascular invasion/extrahepatic metastasis. Patients were randomly assigned to either an intravenous single dose of dexamethasone 8 mg or placebo one hour before TACE. The primary outcome was a negative result of PES at 48 h after TACE, which was defined as score < 2 of Southwest Oncology Group toxicity coding criteria using fever, nausea, vomiting and pain to calculated. And the secondary end point was duration of admission between two groups.
RESULTS
One hundred patients were randomly assigned 1:1. Under intention-to-treat analysis, 49 patients were randomly assigned to the dexamethasone and 51 to the placebo groups. Both groups were similar for baseline characteristics. The negative PES rate was significantly higher in the dexamethasone group than in the placebo group (63.3% vs 29.4%; P = 0.005). Mean Southwest Oncology Group toxicity coding PES was 2.14 (95%CI: 1.41-2.8) vs 3.71 (95%CI: 2.97-4.45) between the dexamethasone and placebo groups, respectively. Cumulative incidence of fever was significantly lower in dexamethasone group with P < 0.001, pain, nausea and vomiting were also lower in the dexamethasone group compared with the placebo group (P = 0.16, P = 0.11, and P = 0.49). The dexamethasone regimen was generally well tolerated by patients with HCC patients including those with hepatitis B virus infection and well-controlled diabetes mellitus.
CONCLUSION
Single dose dexamethasone was effective at preventing PES among patients with HCC treated with TACE. The study showed no adverse events of special interest related to dexamethasone.
Keywords: Hepatocellular carcinoma, Chemoembolization, Dexamethasone, Double blind method, Prevention, Postembolization syndrome
Core Tip: Even in the immuno-oncology era, transcatheter arterial chemoembolisation (TACE) is the most effective way to treat intermediate stage hepatocellular carcinoma. Postembolisation syndrome (PES) is the most common side effect from TACE and there is still no standard prevention guideline. In the present study we report the method to prevent PES, the most common adverse event after TACE in intermediate stage hepatocellular carcinoma patients, by using single dose dexamethasone.
INTRODUCTION
Even though immune-oncology has improved outcomes of many cancers including hepatocellular carcinoma (HCC), globally the third leading cause of cancer-related death is still HCC exhibiting a one-year overall survival around 17.5% without treatment[1]. Transarterial chemoembolisation (TACE) is the standard treatment option for Barcelona Clinic Liver Cancer (BCLC) stage B HCC which was shown to improve patients’ survival in many systematic reviews[2]. In Thailand, 41.6% of patients were initially treated with TACE which technically aimed to embolise vessel supplying the tumour leading to ischemic necrosis of tumour cells[3]. However, postembolisation syndrome (PES) is the most common complication after TACE and can occur in 60 to 90% of cases, manifesting as fever, nausea, vomiting or abdominal pain[4].
Although, the etiology of PES is not well understood, it is possibly related to tumour necrosis and ischemic injury of normal hepatocytes which provokes a systemic inflammatory response from the human body[5]. This self-limited syndrome can resolve within 24 h or may be prolonged up to two weeks due to many factors such as tumour size and numbers, dosage of chemotherapeutic drug and other host factors[6]. Many studies have shown that PES can increase hospital stay and when calculated Southwest Oncology Group (SWOG) toxicity coding score (Supplementary Table 1)[7], and PES score of 2 or greater may shorten the survival of patients[8].
PES is typically managed with symptomatic treatment by administering analgesic, antiemetic and antipyretic agents[9]. However, no standard prevention guidance to prevent PES has been established. The pathogenesis of PES is possibly related to systemic inflammation, so corticosteroid could play a role in preventing this syndrome. Dexamethasone and prednisolone have been confirmed to be effective against chemotherapy-induced nausea/vomiting and are recommended by the National Comprehensive Cancer Network. In the United States, some medical centers use dexamethasone for prophylaxis of PES before TACE among patients with HCC. One observational study reported that systemic corticosteroid could significantly reduce the nausea/vomiting rate[10]. Recently, a few prospective studies have demonstrated the effect of dexamethasone in preventing PES[11-13]. However, the dose and duration of dexamethasone varies. The aim of this study was to evaluate the efficacy of single dose intravenous dexamethasone regimen to prevent PES after TACE among patients with HCC.
MATERIALS AND METHODS
Patient population
We conducted a randomised, double blind, placebo controlled trial at Phramongkutklao Hospital, Bangkok, Thailand from September 2017 to October 2019. Eligible individuals were inpatients aged more than 18 years with a diagnosis of HCC without main portal vein invasion and extrahepatic metastasis and fit to undergo TACE treatment. Criteria for diagnosis of HCC was presence of histologically confirmed or radiologically diagnosed HCC (fulfilling the criteria for lesions with typical imaging according to American Association for the Study of Liver Diseases guidelines)[14]. The key exclusion criteria included those described below: Presence of Child-Pugh class C disease; active gastro-intestinal bleeding; extrahepatic metastasis; patients with encephalopathy; patients with refractory ascites; patients with intrahepatic duct dilatation; patients with major portal vein thrombosis; total bilirubin level > 2.0 mg/dL, and patients unable to undergo arterialised intervention or who develop severe allergic or anaphylactoid reaction. All consecutive patients were screened and approached for enrollment by an internist in outpatient clinics at Phramongkutklao Hospital. Information on medical history, performance status, current use of medications and causes of cirrhosis were recorded on forms for each patient. Number of tumours and size were calculated and staged by BCLC staging system, Milan criteria, TNM staging by 7th American Joint Commission on Cancer and Okuda Staging. Laboratory evaluations including hemoglobin, platelet count, prothrombin time (PT) and international normalised ratio albumin, bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), sodium, blood urea nitrogen and serum glucose were collected from all participants. Child-Pugh score (CTP) score, model for end stage liver disease (MELD) and MELD-Na scores were calculated to assess severity of liver impairment.
This randomised, double-blind, placebo-controlled trial was conducted according to the good clinical practice guidelines at Phramongkutklao Hospital. The study was registered in the Thai Clinical Trials Registry (TCTR20170906004). The investigators collected the data, and the Institutional Review Board of the Royal Thai Army Medical Department approved and monitored the study. All patients had been properly informed and consented to participate in this trial by signing the informed consent regulation provided by Institutional Review Board of the Royal Thai Army Medical Department committee. All authors had access to the study data and reviewed and approved the proposal and final manuscript. All methods were conducted according to the relevant guidelines and regulations by the Institutional Review Board of the Royal Thai Army Medical Department which uses Guideline for Good Clinical Practice: ICH Harmonised Tripartite Guideline and Declaration of Helsinki as regulations.
Study design, randomisation, and interventions
All patients who agreed to participate were randomly assigned in a 1:1 ratio to receive either 8 mg dexamethasone plus normal saline up to 5 mL intravenously one hour before TACE or a placebo regimen (intravenous normal saline 5 mL). The randomisation sequence in a block of four for treatment allocation was generated by computer located in the Clinical Research Center. All patients were blinded to treatment assignment and allocation was kept in opaque sealed envelopes. The allocation coordinators at the Clinical Research Center enrolled patients using informed consent and assigned them to the trial groups. The study drugs were prepared by nonblinded clinical pharmacists in Phramongkutklao Hospital and distributed to the investigators. The nonblinded clinical pharmacists were not involved in the rest of the study. All study investigators were masked to treatment group allocation.
All patients were admitted to the hospital for preprocedural evaluation and preparation at least 24 h before the pre-scheduled intervention. TACE was performed using a super-selective technique by two interventional radiologists who were blinded to randomisation assignment. In brief, the femoral artery was catheterised under local anesthesia and infused with a mixture of ethiodised oil (lipiodol) 3 to 20 mg and chemotherapeutic agents (doxorubicin dose 30 to 75 mg/m2 or mitomycin dose 8 to 12 mg/m2) emulsion in the feeding arteries of the tumour. All patients received intravenous antibiotic prophylaxis (ceftriaxone 2 mg) on the day of TACE procedure. When severe allergic or anaphylactoid reaction occurred, treatment was permanently stopped, and the patient was treated with standard protocol of severe allergic reaction and excluded from the study.
After completing procedures, the following parameters were recorded: Symptoms according to SWOG toxicity coding (such as nausea, vomiting, fever, and abdominal discomfort), vital signs, and adverse events. Laboratory parameters were collected before and at 24 h after the procedure including CBC, PT, serum creatinine, liver function test, serum glucose and serum alpha-fetoprotein. Patients with chronic hepatitis B infection were appointed for outpatient visit 30 d after TACE to collect biochemistry laboratory parameters such as AST, alanine transaminase, ALT, albumin, and PT in order to evaluate incidence of hepatitis B reactivation.
Outcome measurement
Primary outcome was a negative result of PES, which was defined as score < 2 of SWOG toxicity coding using fever, nausea, vomiting and pain to calculate within 48 h after the procedure.
Secondary outcomes included duration of admission between two groups and cumulative incidences of adverse reactions using Common Terminology Criteria for Adverse Events (CTCAE, Version 4.0; Supplementary Table 2) such as fever, anorexia, nausea and vomiting in each group.
Patients were evaluated for the presence of fever, anorexia, and nausea/vomiting for 48 h to measure the primary outcome and were followed up after seven days to evaluate the adverse event after the procedure. Laboratory tests including hematologic parameters, blood chemistry and hemoculture were conducted at baseline and day 2. Rescue therapy such as antipyretic or anti-emetic therapies were allowed to patients developing fever, anorexia, or nausea/vomiting in consultation with the treating physician. The SWOG score was evaluated before patients received any rescue therapy. All serious adverse events were reported to the Institutional Review Board of the Royal Thai Army Medical Department.
Statistical analysis
The statistical methods of this study were reviewed by Worarachanee Imjaijitt from Department of Clinical Epidemiology and Biostatistics, Phramongkutklao College of Medicine. Superiority of the dexamethasone regimen over the control regimen was defined as a 25% decrease in PES incidence with the dexamethasone regimen compared with the control regimen.
The results were expressed as mean with 95%CI or median with range. Student’s t test or the Mann–Whitney U test was performed to compare between two groups. For categorical data, Chi-square test or Fisher’s exact test was applied. A value of P < 0.05 was taken as significant. A logistic regression analysis was performed for analysing the impact of gender, age, tumour size, intervention and all other factors listed in the baseline characteristics table on development of PES.
Sample size calculation
This study used rate of development of PES after TACE to calculate sample size for primary endpoint. Based on previous results, the incidence of PES among patients with HCC after receiving TACE was more than 60%[4,15]. Superiority of the dexamethasone regimen over the control regimen was defined as a 25% decrease in PES with the dexamethasone regimen compared with the control regimen. Intravenous dexamethasone was hypothesised to reduce the incidence of PES by 20%. This study used a two-tailed test which calculated at least 44 patients were required in each group, for a P < 0.05 with an alpha error of 5% and beta error of 20%[11].
RESULTS
Study population
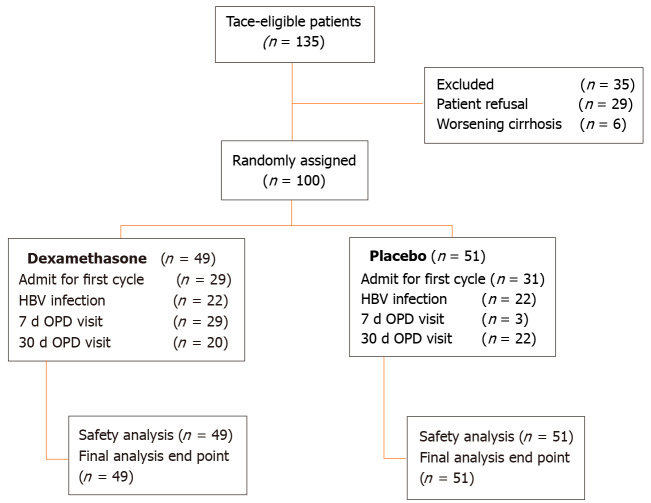
From September 2017 to October 2019, 135 patients with diagnosis of HCC were screened. Twenty-nine patients refused to participate in the study and six patients progressed to CTP class C who were excluded, leaving 100 patients enrolled and randomly assigned to two groups. Forty-nine patients were in the dexamethasone group and 51 patients were in the control group as depicted in Figure 1. The baseline demographic characteristics of the study population did not significantly differ between the dexamethasone and placebo groups as shown in Table 1. Mean age of patients was 61 years and the majority was male (82%). The most common cause of cirrhosis in the study was chronic hepatitis B virus infection (44%). Most patients were CTP class A cirrhosis (87%). After all the data were grouped by Milan classification, the number of patients who were classified within the Milan criteria were slightly higher in the dexamethasone group compared with that of the placebo group (38.8% vs 17.6%; P < 0.05). However, the mean largest diameter of tumour did not significantly differ between the two groups. No statistically significant differences were found in biochemical values between the two groups. A total of 100 patients were included in intention-to-treat analysis.
Figure 1.
Consort diagram. OPD: Operationalized psychodynamic diagnosis; HBV: Hepatitis B virus.
Table 1.
Baseline characteristics
|
Dexamethasome (n = 49)
|
Placebo (n = 51)
|
P value | |||
|
|
n
|
%
|
n
|
%
|
|
| Sex | 0.721 | ||||
| Male | 40 | 81.60 | 43 | 84.30 | |
| Female | 9 | 18.40 | 8 | 15.70 | |
| Age (yr) | 0.679 | ||||
| mean ± SD | 61.18 ± 11.13 | 61.82 ± 10.68 | |||
| Size (cm) | 0.154 | ||||
| Median (min-max) | 3.9 | (0.40-18.30) | 5.4 | (0.80-18.00) | 0.061 |
| > 3 cm | 30 | 61.20 | 40 | 78.40 | |
| Etiology | 0.209 | ||||
| Hepatitis B | 22 | 44.90 | 22 | 43.10 | |
| Hepatitis C | 17 | 34.70 | 14 | 27.50 | |
| Cryptogenic | 3 | 6.10 | 11 | 21.60 | |
| Alcoholic cirrhosis | 5 | 10.20 | 3 | 5.90 | |
| NASH | 1 | 2.00 | 0 | 0 | |
| BCLC staging | 0.154 | ||||
| A | 11 | 22.40 | 7 | 13.70 | |
| B | 36 | 73.50 | 44 | 86.30 | |
| ECOG performance status | 0.845 | ||||
| 0 | 7 | 14.30 | 10 | 19.60 | |
| 1 | 39 | 79.60 | 39 | 76.50 | |
| Child-Pugh class | 0.511 | ||||
| A | 41 | 83.70 | 45 | 88.20 | |
| B | 8 | 16.30 | 6 | 11.80 | |
| AFP level | 0.72 | ||||
| None | 46 | 93.90 | 50 | 98.00 | |
| > 400 ng/mL | 10 | 20.40 | 9 | 17.60 | |
| No of TACE | 0.744 | ||||
| 1 | 29 | 59.20 | 31 | 60.80 | |
| 2 | 9 | 18.40 | 9 | 17.60 | |
| Embolization agent | 0.619 | ||||
| Lipiodol plus doxorubicin | 15 | 30.60 | 18 | 35.30 | |
| Lipiodol plus mitomycin-C | 34 | 69.40 | 33 | 64.70 | |
| Lipiodol dose | 0.483 | ||||
| mean ± SD | 10.67 ± 3.01 | 10.14 ± 1.60 | |||
| Level of embolisation | 0.612 | ||||
| Right branch | 30 | 61.20 | 36 | 70.60 | |
| Left branch | 11 | 22.40 | 8 | 15.70 | |
| Main trunk | 7 | 14.30 | 7 | 13.70 | |
| Diabetes mellitus | 0.806 | ||||
| None | 30 | 61.20 | 30 | 58.80 | |
| Diabetes mellitus | 19 | 38.80 | 21 | 41.20 | |
NASH: Non-alcoholic steatohepatitis; BCLC: Barcelona Clinic Liver Cancer; ECOG: Eastern Cooperative Oncology Group; AFP: Alpha-fetoprotein; TACE: Transarterial chemoembolization.
Primary outcome
The negative PES rate was defined by less than 2 scores of combined fever, nausea, vomiting and abdominal pain score by SWOG toxicity coding. The negative PES rate was significantly greater in the dexamethasone group than in the placebo group (63.3% vs 29.4%; P < 0.01; Figure 2). Receiving dexamethasone was a protective factor against PES with an OR of 0.24 (0.10-0.55, P = 0.001) from binary logistic regression. Mean SWOG PES score was 2.14 (95%CI: 1.41-2.8), vs 3.71 (95%CI: 2.97-4.45) between dexamethasone and placebo groups respectively.
Figure 2.
The negative postembolisation syndrome rate. PES: Postembolisation syndrome.
Secondary outcome
Considering each parameter using Common Terminology Criteria for Adverse Events (CTCAE, Version 4.0), the incidence of fever (body temperature more than or equal 38.5 °C) was significantly lower in the dexamethasone group (49.1% vs 18.4%; P < 0.01). Patients with more than one grade incidence of pain, nausea and vomiting were 56%, 50.9%, and 19% in the placebo group and 36%, 30%, and 14% in the dexamethasone group, respectively (P = 0.16, P = 0.11, and P = 0.49, respectively; Figure 3). The median duration of admission was four days (IQR 3-7 d) in patients receiving placebo similar to those receiving dexamethasone (IQR 3-5 d; P = 0.24). The mean duration of admission was 5.33 d (95%CI: 4.4-6.13) longer among patients receiving placebo than those receiving dexamethasone, namely, 4.9 d (95%CI: 4-5.8).
Figure 3.
The incidences of common terminology criteria for adverse events.
Predictors of PES
From univariate analysis, tumour diameter more than 3 cm of the HCC mass and receiving intravenous dexamethasone were associated with developing PES after TACE (Table 2). Receiving dexamethasone was a protective factor against PES with an OR of 0.24 (0.10-0.55, P = 0.001) from binary logistic regression. Also, using multivariate analysis, both factors were independently associated with developing PES (Table 2). Patients with HCC diameter more than 3 cm were associated with developing postembolization syndrome after TACE with an OR of 3.66 (1.39-9.6, P = 0.008) and receiving dexamethasone was a protective factor against PES with an OR of 0.27 (0.11-0.64, P = 0.003). All other factors were compare using univariate analysis showed in Supplementary Tables 3 and 4.
Table 2.
Univariate and multivariate analysis of factors associated with the development of postembolization syndrome
| Factors |
Univariate analysis
|
Multivariate analysis
|
||||
|
OR
|
95%CI
|
P
value
|
OR
|
95%CI
|
P
value
|
|
| Size > 3 cm | 4.2 | 1.672-10.553 | 0.002a | 3.661 | 1.395-9.605 | 0.008a |
| Dexamethasone | 0.242 | 0.105-0.559 | 0.001a | 0.271 | 0.114-0.647 | 0.003a |
P < 0.05.
Safety
No serious adverse events were associated with dexamethasone and the TACE procedure. Regarding other specific adverse events that seemed to be associated with dexamethasone, patients had more than grade 3 hyperglycemia, higher than that of the placebo group, but without statistical significance (22.4% vs 15.7%; P = 0.743). Incidence of more than grade 3 transient transaminitis within 48 h using AST and ALT in dexamethasone and placebo groups was 26.5% vs 43.1%, P = 0.26 and 18.4% vs 23.5%, P = 0.53, respectively. Only one patient in the dexamethasone group had more than grade 3 transient hyperbilirubinemia, but the patient recovered during their admission (Supplementary Table 2). We also used two bottles of hemoculture to confirm that no patients had an acute bacterial infection during hospital stay despite developing fever. After the procedure, we compared albumin-bilirubin (ALBI) grading system at 48 h between both groups, based on the following equation: ALBI score = [log10 bilirubin (µmol/L) × 0.66] + [albumin (g/L) × (-0.0852)][16]. The equation could determine the prognosis of patients with intermediate stage liver cancer undergoing embolisation treatment. In a recent study patients with grade 1 ALBI score had superior outcomes in terms of overall survival over other grades[17]. Our data showed that patients in the dexamethasone group had grade 1 ALBI score at 40.8% more than that of the placebo group which was 21.6%, but without significance (P = 0.112).
In all, 44 patients had chronic hepatitis B infection, divided equally into two groups. Thirty-day postintervention visits were appointed to collect biochemical values and report any adverse outcome. No hepatitis B flare occurred among any of the 42 patients who visited the outpatient department after admission for post-intervention visit. The placebo group developed more than grade 3 elevated aspartate transaminase 13.6% more than 10.0% in the dexamethasone group; P = 0.34. One patient in each group had more than grade 3 elevated serum ALT and no more than grade 3 hyperbilirubinemia occurred in both groups.
DISCUSSION
The main finding of this study was administering a single dose of intravenous dexamethasone one hour before TACE was effective and safe to reduce the incidence of PES among patients with HCC. This constituted a well-designed, randomised, double-blind, placebo-controlled trial that verified the endpoints with definition of PES using SWOG toxicity coding score. Additionally, enrolled patients who were candidates for treating with TACE in our study represented the real-world situation, in which the majority of patient characteristics were BCLC stage B, and cirrhosis Child-Pugh A. Presently, no clear criteria are available to diagnose PES. For this reason, many related studies of PES used many different definitions for PES criteria. The SWOG toxicity coding score system was shown to predict longer duration of stay among patients with more than 2 points using fever, nausea, vomiting and abdominal pain. Lately, many trials have shown interest in determining the effect of steroids to prevent PES. It has been proposed that this syndrome is possibly related to a release of inflammatory cytokines in the bloodstream making corticosteroid a reasonable choice due to the nature of this drug class in reducing systemic inflammatory effects[18].
One retrospective study clearly showed that patients with prophylactic dexamethasone tended to receive lower doses of anti-emetic agents than those who did not after post-TACE procedures[10]. A double-blinded, randomised controlled trial demonstrated that the use of dexamethasone with total dose of 27 mg combined with ginsenosides pre- and post-procedure for six days was effective in controlling nausea, vomiting, fever and pain after TACE[13]. Another double-blinded, randomised controlled trial used high dose dexamethasone regimen comprising 36 mg for 3 d which significantly reduced the cumulative incidence rates of fever, anorexia and nausea/vomiting during the initial 120 h following TACE. The number of patients achieving complete remission in both acute and delayed phases was higher among those following the dexamethasone regimen[12]. Furthermore, a double-blinded, randomised control trial by using only 12 mg of dexamethasone before TACE showed a 19.5% reduction of PES in the dexamethasone group[11]. Although direct comparisons between related reports and this study are difficult, injecting a single dose of dexamethasone was simpler and safer than applying multiple doses. An 8 mg dexamethasone single dose was selected in this study due to the effectiveness in preventing analgesia-related nausea and vomiting in postoperative patients and also minimising the side effects of corticosteroid including hyperglycemia and sepsis by using the lowest effective dose[19]. Because pharmacokinetic effects of dexamethasone take 12 to 24 h to reach the most optimal effect and 36 to 72 h to eliminate from the body, single dose pre-prophylactic regimen was preferred to prevent PES after single TACE procedure[20]. The findings of this study were similar to those in related studies that evaluated the use of higher dose steroids for prophylaxis of PES after TACE for HCC. Several worldwide studies have demonstrated other regimens to control PES. Another single study from Thailand used N-acetylcysteine (NAC) given before and after TACE, which has antioxidant properties that could prevent liver injury among patients with acetaminophen overdose. NAC was able to reduce the incidence of PES from 48.2 to 26.4%[21]. However, NAC regimens use a high volume of saline infusion and NAC did not prevent post-TACE liver decompensation.
This study encountered several limitations. This study was conducted in a single center, and all patients received only super-selective TACE with two different chemotherapy regimens with doxorubicin or mitomycin-c. Nevertheless, the effects of systemic absorption from chemotherapy used in TACE are low. However, those agents may affect nausea/vomiting symptoms. Therefore, intravenous dexamethasone is able to reduce incidence and severity of chemotherapy induced nausea and vomiting similar to pre-medication. For chemotherapeutic agents, mitomycin-c has been widely used for TACE in our center compared with doxorubicin. Moreover, mitomycin-c is associated with less adverse events of nausea/vomiting than doxorubicin. Therefore, dexamethasone prophylaxis might not be appropriate for high emetogenic chemotherapy. In addition, the association between GI side effects of each chemotherapeutic agent and PES should be further studied. Our data did not demonstrate prophylactic effects of dexamethasone after drug-eluting beads (DEBs) TACE which were more expensive. In fact, DEBs-TACE is not better than conventional TACE in terms of efficacy and safety[22]. Therefore, we may suggest using dexamethasone prophylaxis for both DEBs-TACE and conventional TACE. However, further study of using dexamethasone prophylaxis for particular DEBs-TACE technique should be explored. The number of patients who were enrolled in this study was relatively small. Therefore, adequate subgroup analyses could not be performed and we could not demonstrate statistical significance of anti-emetic effects of dexamethasone. Interestingly, in our study, the secondary endpoints also did not include any inflammatory laboratory analysis such as changes of serum cytokines (IL-1β, IL-6 and TNF-α) which may have informed us about the pathophysiology of this syndrome. Also, our study did not record the quality of life of any patients, but our composite SWOG score may represent the symptoms of all patients. Finally, this study did not plan survival assessments after four weeks. Therefore, it could be hypothesised that dexamethasone could reduce the inflammation and counterbalance the anti-tumour effect of TACE. Additional analyses are required to evaluate the short and long-term influence of dexamethasone on patient’s survival after TACE. Therefore, further studies are required to establish a standard of care for dexamethasone use as prophylaxis for PES among patients with HCC undergoing TACE.
CONCLUSION
In conclusion, we found a significant reduction of PES in the dexamethasone-containing prophylactic regimen among patients with HCC receiving TACE. This study demonstrated the efficacy and safety of single dose dexamethasone for the prophylaxis of PES based on a well-designed randomised, placebo-controlled trial. Our results provide an effective regimen for future investigation options to prevent TACE-induced PES.
ARTICLE HIGHLIGHTS
Research background
Corticosteroids are used empyrically to prevent postembolisation syndrome (PES) after transcatheter arterial chemoembolisation (TACE).
Research motivation
Effects of corticosteroids administration before TACE on the prevention of PES have not been demonstrated in detail.
Research objectives
We conducted the present study to examine the utility and safety of steroid use in the prevention of PES following TACE.
Research methods
This study was a well-designed prospective randomized control trial answering the important clinical question in hepatocellular carcinoma patients undergo TACE.
Research results
The result of this study showed that single dose 8 milligrams of intravenous dexamethasone one hour before TACE was significantly reduce the incidence of PES in 48 h.
Research conclusions
We conclude that the administration of single dose of intravenous dexamethasone was useful for the prevention of adverse events after TACE in patients with hepatocellular carcinoma.
Research perspectives
Further examination is needed to confirm the utility and tolerability of dexamethasone for prevention of PES with respect to TACE in a large study trial.
ACKNOWLEDGEMENTS
Authors thank Mr. Thomas Mc Manamon a medical English specialist from The Office of Research and Development Phramongkutklao College of Medicine and Mr. Stephen Pinder a medical English specialist from the Department of Clinical Epidemiology and Biostatistics, Faculty of Medicine Ramathibodi Hospital, Mahidol University for English Language Editing. Both of them are native-speaking medical English specialists who conducted an English language review of our manuscript.
Footnotes
Institutional review board statement: Institutional Review Board of the Royal Thai Army Medical Department committee used World Medical Association: DELCARATION OF HELSINKI, GUIDELINE FOR GOOD CLINICAL PRACTICE: ICH Harmonised Tripartite Guideline, Council for International Organizations of Medical Sciences (CIOMS), CODE of FEDERAL REGULATIONS: Title 45 Public Welfare; Part 46 Protection of Human Subjects and The Belmont Report to regulate the ethical concern in publication Informed consent was obtained from all subjects, and all methods were conducted according to the relevant guidelines and regulations.
Clinical trial registration statement: The study was registered in the Thai Clinical Trials Registry (TCTR20170906004).
Informed consent statement: All subjects have been properly instructed and have consented to participate in this trial by signing the informed consent regulation provided by Institutional Review Board of the Royal Thai Army Medical Department committee. Informed consent was obtained by signature of all participants from all subjects to inform all the information about publication.
Conflict-of-interest statement: The authors declare that they have no conflict of interest.
CONSORT 2010 statement: The authors have read the CONSORT 2010 statement, and the manuscript was prepared and revised according to the CONSORT 2010 statement.
Manuscript source: Unsolicited manuscript
Peer-review started: July 4, 2021
First decision: July 26, 2021
Article in press: September 10, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Thailand
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tamori A S-Editor: Wang JJ L-Editor: A P-Editor: Wu RR
Contributor Information
Panot Sainamthip, Department of Pharmacology, Chulalongkorn University, Bangkok 10330, Thailand.
Chutcharn Kongphanich, Department of Radiology, Phramongkutklao College of Medicine, Bangkok 10400, Thailand.
Naiyarat Prasongsook, Division of Medical Oncology, Department of Medicine, Phramongkutklao College of Medicine, Bangkok 10400, Thailand.
Sakkarin Chirapongsathorn, Division of Gastroenterology and Hepatology, Department of Medicine, Phramongkutklao College of Medicine, Jatujak 10900, Thailand. sakkarin.chi@pcm.ac.th.
Data sharing statement
The datasets used during the current study are available from the corresponding author on reasonable request.
References
- 1.Cabibbo G, Enea M, Attanasio M, Bruix J, Craxì A, Cammà C. A meta-analysis of survival rates of untreated patients in randomized clinical trials of hepatocellular carcinoma. Hepatology. 2010;51:1274–1283. doi: 10.1002/hep.23485. [DOI] [PubMed] [Google Scholar]
- 2.Frenkel JK. Diagnosis, incidence, and prevention of congenital toxoplasmosis. Am J Dis Child. 1990;144:956–958. doi: 10.1001/archpedi.1990.02150330014008. [DOI] [PubMed] [Google Scholar]
- 3.Somboon K, Siramolpiwat S, Vilaichone RK. Epidemiology and survival of hepatocellular carcinoma in the central region of Thailand. Asian Pac J Cancer Prev. 2014;15:3567–3570. doi: 10.7314/apjcp.2014.15.8.3567. [DOI] [PubMed] [Google Scholar]
- 4.Blackburn H, West S. Management of Postembolization Syndrome Following Hepatic Transarterial Chemoembolization for Primary or Metastatic Liver Cancer. Cancer Nurs. 2016;39:E1–E18. doi: 10.1097/NCC.0000000000000302. [DOI] [PubMed] [Google Scholar]
- 5.Paye F, Farges O, Dahmane M, Vilgrain V, Flejou JF, Belghiti J. Cytolysis following chemoembolization for hepatocellular carcinoma. Br J Surg. 1999;86:176–180. doi: 10.1046/j.1365-2168.1999.01014.x. [DOI] [PubMed] [Google Scholar]
- 6.Li CP, Chao Y, Chen LT, Lee RC, Lee WP, Yuan JN, Yen SH, Lee SD. Fever after transcatheter arterial chemoembolization for hepatocellular carcinoma: incidence and risk factor analysis. Scand J Gastroenterol. 2008;43:992–999. doi: 10.1080/00365520801971744. [DOI] [PubMed] [Google Scholar]
- 7.Green S, Weiss GR. Southwest Oncology Group standard response criteria, endpoint definitions and toxicity criteria. Invest New Drugs. 1992;10:239–253. doi: 10.1007/BF00944177. [DOI] [PubMed] [Google Scholar]
- 8.Wigmore SJ, Redhead DN, Thomson BN, Currie EJ, Parks RW, Madhavan KK, Garden OJ. Postchemoembolisation syndrome--tumour necrosis or hepatocyte injury? Br J Cancer. 2003;89:1423–1427. doi: 10.1038/sj.bjc.6601329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hesketh PJ, Kris MG, Basch E, Bohlke K, Barbour SY, Clark-Snow RA, Danso MA, Dennis K, Dupuis LL, Dusetzina SB, Eng C, Feyer PC, Jordan K, Noonan K, Sparacio D, Somerfield MR, Lyman GH. Antiemetics: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2017;35:3240–3261. doi: 10.1200/JCO.2017.74.4789. [DOI] [PubMed] [Google Scholar]
- 10.Kogut MJ, Chewning RH, Harris WP, Hippe DS, Padia SA. Postembolization syndrome after hepatic transarterial chemoembolization: effect of prophylactic steroids on postprocedure medication requirements. J Vasc Interv Radiol. 2013;24:326–331. doi: 10.1016/j.jvir.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 11.Yang H, Seon J, Sung PS, Oh JS, Lee HL, Jang B, Chun HJ, Jang JW, Bae SH, Choi JY, Yoon SK. Dexamethasone Prophylaxis to Alleviate Postembolization Syndrome after Transarterial Chemoembolization for Hepatocellular Carcinoma: A Randomized, Double-Blinded, Placebo-Controlled Study. J Vasc Interv Radiol. 2017;28:1503–1511.e2. doi: 10.1016/j.jvir.2017.07.021. [DOI] [PubMed] [Google Scholar]
- 12.Ogasawara S, Chiba T, Ooka Y, Kanogawa N, Motoyama T, Suzuki E, Tawada A, Nagai K, Nakagawa T, Sugawara T, Hanaoka H, Kanai F, Yokosuka O. A randomized placebo-controlled trial of prophylactic dexamethasone for transcatheter arterial chemoembolization. Hepatology. 2018;67:575–585. doi: 10.1002/hep.29403. [DOI] [PubMed] [Google Scholar]
- 13.Yinglu F, Changquan L, Xiaofeng Z, Bai L, Dezeng Z, Zhe C. A new way: alleviating postembolization syndrome following transcatheter arterial chemoembolization. J Altern Complement Med. 2009;15:175–181. doi: 10.1089/acm.2008.0093. [DOI] [PubMed] [Google Scholar]
- 14.Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 15.Chan AO, Yuen MF, Hui CK, Tso WK, Lai CL. A prospective study regarding the complications of transcatheter intraarterial lipiodol chemoembolization in patients with hepatocellular carcinoma. Cancer. 2002;94:1747–1752. doi: 10.1002/cncr.10407. [DOI] [PubMed] [Google Scholar]
- 16.Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, O'Beirne J, Fox R, Skowronska A, Palmer D, Yeo W, Mo F, Lai P, Iñarrairaegui M, Chan SL, Sangro B, Miksad R, Tada T, Kumada T, Toyoda H. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550–558. doi: 10.1200/JCO.2014.57.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gui B, Weiner AA, Nosher J, Lu SE, Foltz GM, Hasan O, Kim SK, Gendel V, Mani NB, Carpizo DR, Saad NE, Kennedy TJ, Zuckerman DA, Olsen JR, Parikh PJ, Jabbour SK. Assessment of the Albumin-Bilirubin (ALBI) Grade as a Prognostic Indicator for Hepatocellular Carcinoma Patients Treated With Radioembolization. Am J Clin Oncol. 2018;41:861–866. doi: 10.1097/COC.0000000000000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kudo M, Han G, Finn RS, Poon RT, Blanc JF, Yan L, Yang J, Lu L, Tak WY, Yu X, Lee JH, Lin SM, Wu C, Tanwandee T, Shao G, Walters IB, Dela Cruz C, Poulart V, Wang JH. Brivanib as adjuvant therapy to transarterial chemoembolization in patients with hepatocellular carcinoma: A randomized phase III trial. Hepatology. 2014;60:1697–1707. doi: 10.1002/hep.27290. [DOI] [PubMed] [Google Scholar]
- 19.Lee Y, Lai HY, Lin PC, Lin YS, Huang SJ, Shyr MH. A dose ranging study of dexamethasone for preventing patient-controlled analgesia-related nausea and vomiting: a comparison of droperidol with saline. Anesth Analg. 2004;98:1066–1071, table of contents. doi: 10.1213/01.ANE.0000105875.05357.A0. [DOI] [PubMed] [Google Scholar]
- 20.Holte K, Kehlet H. Perioperative single-dose glucocorticoid administration: pathophysiologic effects and clinical implications. J Am Coll Surg. 2002;195:694–712. doi: 10.1016/s1072-7515(02)01491-6. [DOI] [PubMed] [Google Scholar]
- 21.Siramolpiwat S, Punjachaipornpon T, Pornthisarn B, Vilaichone RK, Chonprasertsuk S, Tangaroonsanti A, Bhanthumkomol P, Phumyen A, Yasiri A, Kaewmanee M. N-Acetylcysteine Prevents Post-embolization Syndrome in Patients with Hepatocellular Carcinoma Following Transarterial Chemoembolization. Dig Dis Sci. 2019;64:3337–3345. doi: 10.1007/s10620-019-05652-0. [DOI] [PubMed] [Google Scholar]
- 22.Raoul JL, Forner A, Bolondi L, Cheung TT, Kloeckner R, de Baere T. Updated use of TACE for hepatocellular carcinoma treatment: How and when to use it based on clinical evidence. Cancer Treat Rev. 2019;72:28–36. doi: 10.1016/j.ctrv.2018.11.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used during the current study are available from the corresponding author on reasonable request.