Abstract
Background
Gastroesophageal reflux disease (GERD) is associated with lower esophageal sphincter (LES) incompetence. In some patients, GERD is refractory to acid reduction therapy which is the main treatment for GERD. So far, medications that can increase LES tone are few. Arecae pericarpium (A. pericarpium) is a medication in Traditional Chinese Medicine known to promote intestinal motility.
Methods
We investigated the effect of A. pericarpium extracts on porcine LES motility. In addition, we used tetrodotoxin (TTX) and atropine to study the underlying mechanism of A. pericarpium extracts-induced contractions of LES.
Results
The results of this study showed that A. pericarpium extracts and their main active ingredient, arecoline, can induce the contractions of porcine LES sling and clasp muscles in a dose-response manner. TTX did not have an inhibitory effect on the contractions induced by A. pericarpium extracts and arecoline in LES. However, atropine significantly inhibited A. pericarpium extracts- and arecoline-induced contractions of LES.
Conclusion
A. pericarpium extracts can induce the contractions of porcine LES in a dose dependent manner, possibly through muscarinic receptors, and hence, may be worth developing as an alternative therapy for GERD.
Keywords: Arecae pericarpium, Lower esophageal sphincter, Motility, Muscarinic receptor
Background
Gastroesophageal reflux disease (GERD) is a disorder involving inflammation of the lower esophagus which is mainly induced by food, bile, or acid regurgitation. Incompetence of the lower esophageal sphincter (LES), including decreased tone or repeated transient relaxation, is considered a major contributing factor to GERD [1, 2]. Up to 30% of patients show obstinate symptoms despite the use of proton pump inhibitors (PPIs), which reduce esophageal acid exposure [3]. This is partly due to the importance of LES incompetence in GERD. Presently, there are limited drug choices for the treatment of LES incompetence. Baclofen is one of the few drugs that can increase LES tone; however, due to multiple neurological side effects, the clinical application of baclofen in GERD is not feasible [4, 5].
Arecae pericarpium (A. pericarpium) is the dried pericarp of Areca catechu L. that is common in Southern China, India, Philippines, Taiwan, and Southeast Asian countries. The unripe fruit of Areca catechu L. is harvested from winter to spring, dried after cooking, and longitudinally split into two petals. Afterwards, the pericarp is peeled to obtain A. pericarpium, a medication in Traditional Chinese Medicine (TCM) embodied in Taiwan Herbal Pharmacopeia [6]. In accordance with the Compendium of Materia Medica, A. pericarpium has been used to treat constipation, abdominal distension, and edema in TCM. A. pericarpium has effect on gastric emptying and can promote the function of the small intestine in rats via muscarinic receptor [7, 8]. In addition, A. pericarpium can protect hepatic injury induced by alpha-naphthylisothiocyanate [9]. The use of A. pericarpium is considered safe and only very rare case experienced an allergic reaction after consumption. Furthermore, common dosage of A. pericarpium is 3–10 g [10].
To the best of our knowledge, there has been no study on the effect of A. pericarpium extracts on porcine LES motility to date. The aim of this study was to investigate the effect of A. pericarpium extracts on LES and the mechanism underlying A. pericarpium extracts-induced contractions of LES by using porcine LES.
Methods
Materials
This study was conducted in accordance with applicable laws and regulations of Taiwan, and E-Da hospital. All pigs weighed approximately 110 kg and were stunned with an electric shock device at 220 V for at least 3 s, followed by cutting off the main artery within 15 s of stunning, and exsanguination until death. All pigs were slaughtered in a regulated slaughterhouse supervised by the Council of Agriculture, Executive Yuan, R.O.C. (Taiwan). The stomachs and lower esophagi of pigs were purchased from this slaughterhouse in Kaohsiung City. The subjects were exempted from the review of Institutional Animal Care and Use Committee of E-DA Hospital because the esophagi and stomachs of pigs are considered pork variety meats and not live animal parts. After proximal stomachs and distal esophagi were obtained, they were delivered in an ice-cold oxygenated Kreb-Henseleit solution to the laboratory in 30 min. The Kreb-Henseleit buffer solution was composed of the following: 25 mM NaHCO3, 1.2 mM NaH2PO4, 4.7 mM KCl, 118 mM NaCl, 1.8 mM CaCl2, and 14 mM glucose, and was kept under constant pH of 7.4. The tetrodotoxin (TTX) was purchased from Tocris Cookson Inc. (Avonmouth, Bristol, UK), and arecoline hydrobromide and atropine were manufactured by Sigma-Aldrich (St. Louis, MO, USA).
Preparation of A. pericarpium extracts
A. pericarpium was purchased from Da-Tian Chinese Medicine pharmacy (Kaohsiung, Taiwan, ROC) and authenticated by Dr. Li-Wei Lin (The School of Chinese Medicine for Post-Baccalaureate, I-Shou University) according to Taiwan Herbal Pharmacopeia [6]. A herbarium sample (code number, ISU-MCMM-199) was preserved in the School of Chinese Medicine for Post-Baccalaureate, I-Shou University (Kaohsiung, Taiwan, ROC) for future reference. Dried A. pericarpium (30 g) was chopped and soaked in 300 ml of 95% ethanol for 24 h and the mixture was filtered with gauze. The resulting filtrate is referred to as extract 1 of A. pericarpium. Residual A. pericarpium was soaked and filtered repeatedly in the same way as described above, to produce extracts 2 and 3, respectively. Additionally, 1 g of A. pericarpium was powdered, soaked in 20 mL of 95% ethanol, vortexed using ultrasonic power 200 W at 30 °C for 1 h, and the resulting solution filtered to produce extract 4. These extracts were concentrated under vacuum with a rotary evaporator to produce around 30 ml of dense plasters, and the plasters were then freeze-dried [11, 12].
High performance liquid chromatography (HPLC) analysis of arecoline in A. pericarpium extracts
The chromatographic system consisted of a Shimadzu SIL-10 AD VP auto injector, a Shimadzu LC-20 AD prominence liquid chromatography and a Shimadzu SPD-M10A VP diode array detector (Shimadzu, Kyoto, Japan). A Cosmosil 5C18-PAQ 4.6 mm × 250 mm column (Nacalai tesque, CA, USA) was used for the separation and the temperature was controlled at 30 °C. The mobile phase consisted of 0.5% aqueous phosphoric acid and 99.5% acetonitrile at volumetric ratios. The detection was monitored at 215 nm. The mobile phase was delivered at a rate of 1.0 mL/min and the volume of injection loop was 10 μL. According to Taiwan Herbal Pharmacopeia, arecoline is the active ingredient of Areca catechu L. [6]. Arecoline (1 mg/mL) was diluted to 0.4, 0.2, 0.1, 0.05, 0.025, and 0.01X and analyzed using HPLC. We also dissolved 1 mg powder of extracts of A. pericarpium in 1 ml of 10% ethanol for HPLC analysis. The extraction yield of arecoline from A. pericarpium was calculated as
Measurement of A. pericarpium extracts-induced contractions of porcine LES
In contrast to the circular LES of rats, pigs share similar anatomy of LES with humans as they are both composed of sling and clasp muscles [13]. For this reason, swine LES is commonly used for the study of human gastroesophageal reflux and esophageal motility disorder [14–17]. According to previously reported method, one end of the muscle strips was attached to an isometric transducer with surgical wire (FORT10g; Grass Technologies, RI, USA). The transducer was connected to an amplifier (Gould Instrument Systems, OH, USA), the signal obtained was then recorded by a computer recording system (BIOPAC Systems, CA, USA) [16, 17]. The muscle’s basal tone was set to 1.0 g for this study. After a 30-min equilibration period, 1 × 10− 6 M carbachol was added to the organ bath to induce contraction of the muscle strip, and the carbachol was washed away. One mg powders from extracts 1 and 2 respectively were used and dissolved in 1 ml 10% ethanol in order to check the effect of A. pericarpium extracts on LES. One hundred μL (= 100 ng/L) of extract 1 of A. pericarpium was added after another 30-min equilibration period, and 200 μL (= 200 ng/L) of extract 1 of A. pericarpium was added (cumulative dose = 300 ng/L) when the contraction reached a peak equilibration induced by 100 μL of extract 1 of A. pericarpium. In the same way, the extract 2 of A. pericarpium was also used to check its effect on LES.
Measurement of arecoline-induced contractions of porcine LES
In order to check the effect of arecoline on LES, 300 nM arecoline was added after a 30-min equilibration period, and 1 μM arecoline was added when the contraction reaches a peak equilibration induced by 300 nM arecoline.
Effect of TTX on A. pericarpium extracts- and arecoline-induced contractions of porcine LES
To investigate the mechanism of A. pericarpium extracts- and arecoline-induced contractions of LES, the isolated LES was pretreated with TTX (10− 6 M) into the organ bath. After 15 min, A. pericarpium extract (100 ng/L and 200 ng/L) or arecoline (300 nM and 1 μM) was added sequentially to the organ bath as earlier described [16, 18].
Effect of atropine on A. pericarpium extracts- and arecoline-induced contractions of porcine LES
To investigate the mechanism of A. pericarpium extracts- and arecoline-induced contractions of LES, the isolated LES was also pre-treated with atropine (10− 6 M), for 6 min followed by the addition of A. pericarpium extract (100 ng/L and 200 ng/L) or arecoline (300 nM and 1 μM) sequentially to the organ bath as earlier described [16, 18].
Data analysis
The data are expressed as the means ± SEM. Statistical analysis of the results was performed by using Mann-Whitney U test. The minimum sample size was 4. In all cases, differences were considered significant at p < 0.05. All analyses were performed using the SPSS statistical software version 24 (IBM Corp., NY, USA).
Results
HPLC analysis of arecoline in A. pericarpium extracts
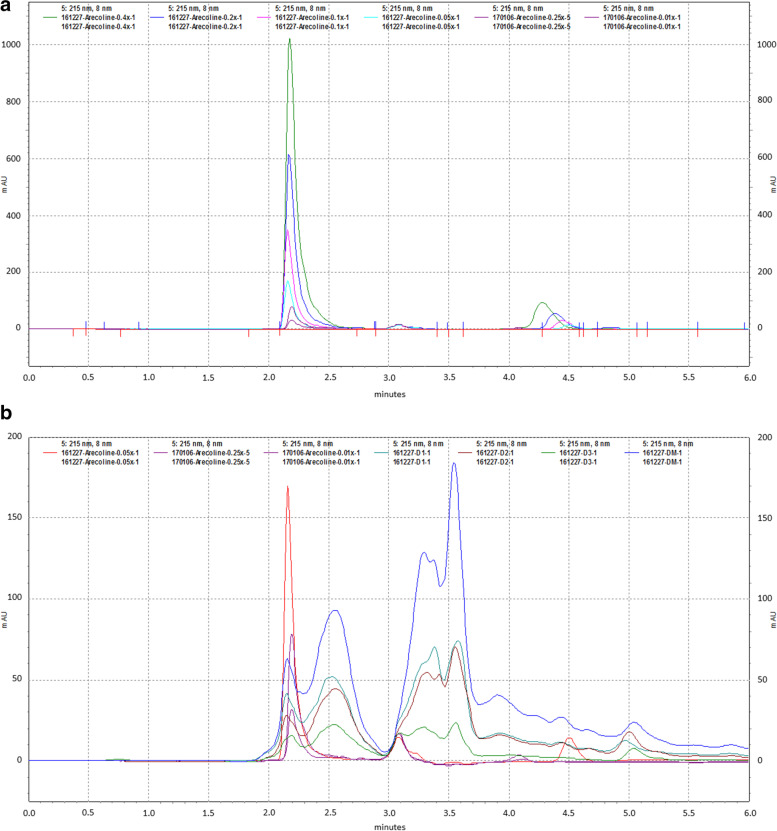
The chromatogram of the standard compound arecoline and that of the extracts of A. pericarpium is shown in Fig. 1A and B, respectively. The sample calibration curve for arecoline was linear (r2 = 0.9982) within the range 0–50 mg/mL. Intra- and inter-day coefficients of variation of the assays were less than 5% (n = 5). The content of arecoline was detected by HPLC and quantified as 1.3, 0.6, 0.3 and 2.0 μg/mL in extracts 1, 2, 3, and 4 of A. pericarpium, respectively. Furthermore, because 0.47, 0.57, 0.81 and 0.16 g powders were obtained from extracts 1, 2, 3, and 4, respectively, the extraction yield of arecoline from A. pericarpium was 0.02, 0.011, 0.011, and 0.32% in extracts 1, 2, 3, and 4, respectively.
Fig. 1.
HPLC of arecoline and Arecae pericarpium (A. pericarpium) extracts. The chromatograms reveal (A) the repeatability and reproducibility of the arecoline standard, and (B) arecoline in separations of A. pericarpium extracts 1–4 (D1, D2, D3, and DM represent extracts 1, 2, 3, and 4, respectively)
Measurement of A. pericarpium extracts-induced contractions of porcine LES
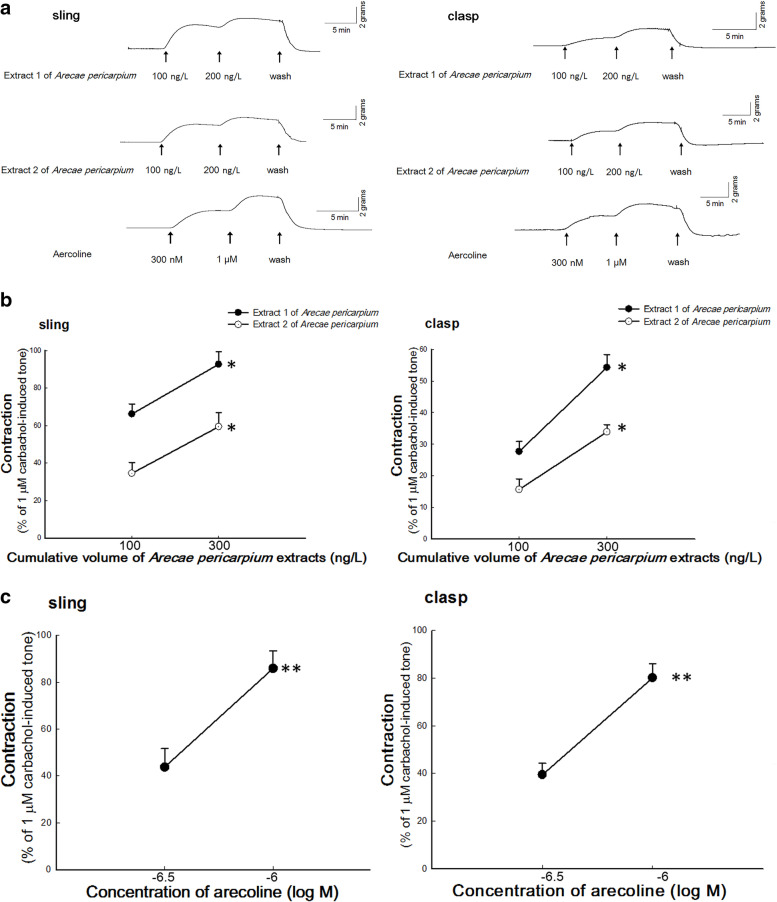
Figure 2A shows typical tracings of A. pericarpium extracts-induced tonic contractions of sling and clasp muscles. Contractile responses were produced in sling and clasp muscles by the application of increasing dosages of A. pericarpium extracts. As shown in Fig. 2B, cumulative doses of 100 and 300 ng/L of extract 1 of A. pericarpium caused significant contractile responses of 66.19 ± 5.44% and 92.71 ± 6.61% while extract 2 caused 34.54 ± 5.79% and 59.43 ± 7.43% responses to 1 μM carbachol-induced contractions in sling muscles, respectively. There was a significant difference between the two contractions induced by different volumes of either extract 1 or 2 (p = 0.02 and 0.04 respectively, both n = 4). In addition, cumulative doses of 100 and 300 ng/L of A. pericarpium extract 1 caused significant contractions of 27.72 ± 3.18% and 54.30 ± 4.09% while extract 2 caused 15.69 ± 3.36% and 33.90 ± 2.32% responses to 1 μM carbachol-induced contractions in clasp muscles, respectively. Cumulative dose of 300 ng/L of either extract 1 or 2 induced an increased significantly contractions than 100 ng/L respectively (p = 0.002 and 0.005 respectively, both n = 4).
Fig. 2.
The Arecae pericarpium (A. pericarpium) extracts- and arecoline-induced contractions of porcine lower esophageal sphincters (LES). A Typical tracing of the contractions of porcine LES sling and clasp muscles in response to cumulative addition of A. pericarpium extracts and arecoline. Arrows indicate the addition of A. pericarpium extracts to LES as cumulative dosage. Dose-response curves of (B) extracts 1 and 2 of A. pericarpium-induced (n = 4) or (C) arecoline-induced (n ≥ 4) contractions of porcine sling and clasp muscles. *,** represent significant differences (p < 0.05) from the response caused by 100 ng/L corresponding A. pericarpium extract or 300 nM arecoline, respectively
Measurement of arecoline-induced contractions of porcine LES
Figure 2A shows typical tracings of arecoline-induced tonic contractions of sling and clasp muscles. As shown in Fig. 2C, the contractile responses of LES with cumulative doses of 300 nM and 1 μM arecoline were 43.82 ± 7.91% and 85.94 ± 7.58% while 39.41 ± 4.81% and 80.15 ± 5.92% contractions was induced by 1 μM carbachol in the sling and clasp muscles, respectively. There was a significant difference between the two contractions induced by 300 nM and 1 μM arecoline in LES sling and clasp muscles (p = 0.002 and 0.0002, n = 4 and 7 respectively).
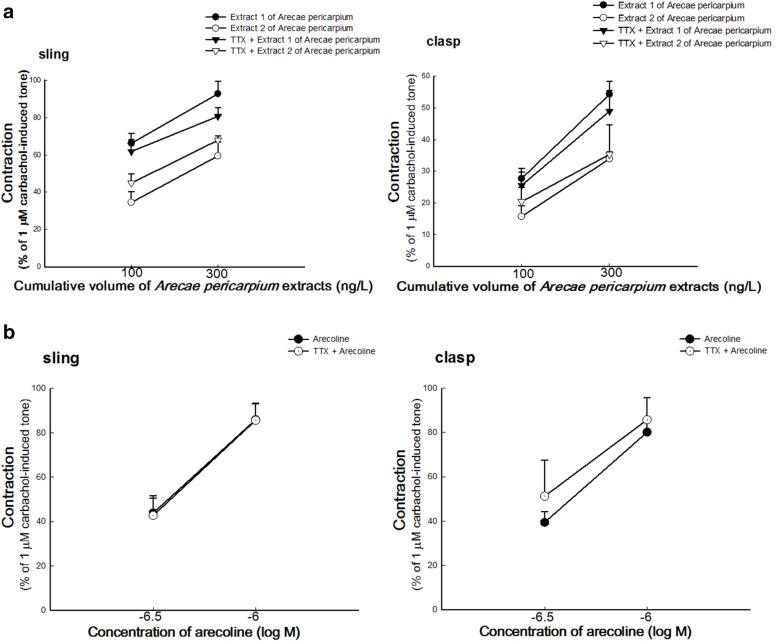
Effect of TTX on A. pericarpium extracts- and arecoline-induced contractions of porcine LES
As shown in Fig. 3A, the dose-response contractions of A. pericarpium extracts were almost unaffected by pretreatment with TTX (p > 0.05, compared to the corresponding A. pericarpium extracts alone, n = 4). In addition, the effect of TTX on arecoline-induced contractions of LES sling and clasp muscles was not significant, compared to arecoline alone in Fig. 3B (p > 0.05, n ≥ 4).
Fig. 3.
Effects of tetrodotoxin (TTX) on the contractions of porcine lower esophageal sphincters (LES) induced by Arecae pericarpium (A. pericarpium) extracts and arecoline. A concentration of 10− 6 M TTX had no significant inhibitory effect on the contractions of sling and clasp muscles induced by (A) extracts 1 and 2 of A. pericarpium (n = 4) or (B) arecoline (n ≥ 4)
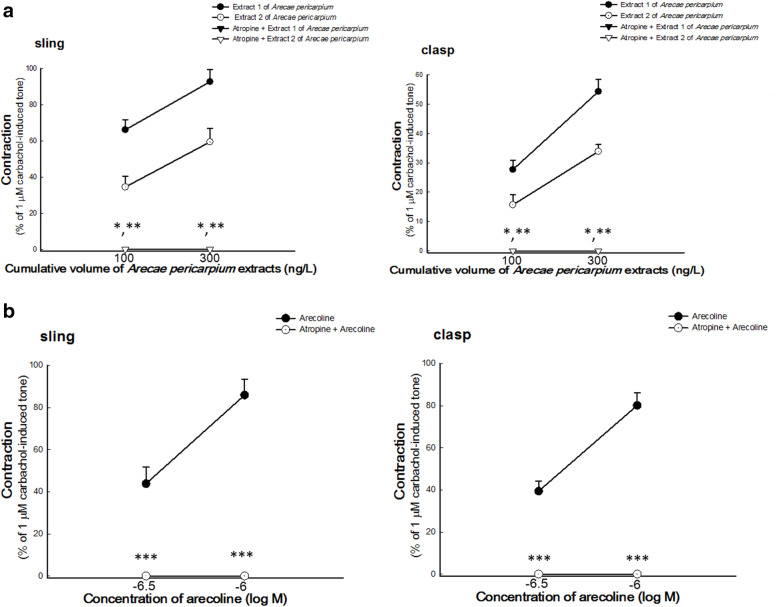
Effect of atropine on A. pericarpium extracts- and arecoline-induced contractions of porcine LES
As shown in Fig. 4A, pretreatment with atropine had a significant inhibitory effect on A. pericarpium extracts-induced contractions of LES (p = 0.001 (100 ng/L) and 0.0007 (300 ng/L) in sling muscles and 0.009 (100 ng/L) and 0.004 (300 ng/L) in clasp muscles, compared to A. pericarpium extracts alone, all n = 4). In addition, a significant inhibitory effect of atropine on arecoline-induced contractions of LES sling and clasp muscles was also observed, compared to arecoline alone as shown in Fig. 4B (p = 0.005 (300 nM) and 0.0003 (1 μM) in sling muscles and 0.0001 (300 nM) and 0.00001 (1 μM) in clasp muscles, n = 4 and 7 in sling and clasp muscles respectively).
Fig. 4.
Effects of atropine on the contractions of porcine lower esophageal sphincters (LES) induced by Arecae pericarpium (A. pericarpium) extracts and arecoline. Atropine significantly inhibited (A) extracts 1 and 2 of A. pericarpium-induced (n = 4) and (B) arecoline-induced (n ≥ 4) contractions of porcine LES sling and clasp muscles. *, **, *** represent significant differences (p < 0.05) from the response caused by the corresponding cumulative volume of extract 1, 2, or arecoline only, respectively
Discussion
These results reveal that A. pericarpium extracts and their main active ingredient, arecoline, caused the contractions of porcine LES sling and clasp muscles in a dose-response manner. In addition, LES contraction curves induced by A. pericarpium extracts and arecoline returned to the prestimulation levels after the removal of A. pericarpium extracts or arecoline, suggesting that these contractile effects probably did not occur via toxic effects.
Excitatory postganglionic myenteric neurons in the LES may release acetylcholine and its derivatives, which activate muscarinic receptors, thereby causing an increase in cytosolic [Ca2+] [19]. In addition, the activation of Ca2+ channels facilitates extracellular Ca2+ influx into cells, thereby increasing cytosolic [Ca2+]. Cytosolic Ca2+ binds to calmodulin, resulting in the activation of myosin light chain kinase (MLCK). MLCK phosphorylates the 20 kDa light chain of myosin, and myosin conjugates with actin to initiate cross-bridge cycling which induces smooth muscle contraction [20, 21].
TTX acts as a sodium channel blocker which blocks voltage-dependent sodium channels in motor neurons. However, the contractions of LES sling and clasp muscles induced by A. pericarpium extracts and arecoline were not blocked by TTX in this study. This indicates that A. pericarpium extracts- and arecoline-induced contractions of LES were not induced by the effect of these substances on nerve fibers.
The non-selective muscarinic receptor antagonist atropine can block the muscarinic receptors (M1–5). Atropine inhibited A. pericarpium extracts- and arecoline-induced contractions of LES sling and clasp muscles. These results indicate that A. pericarpium extracts- and arecoline-induced contractions were possibly mediated by muscarinic receptors.
Extracts from several medicinal plants, such as Ceratonia silique, Myrtus communis, Salvia miltiorrhiza, and Cydonia oblonga, have been reported to be useful for the management of GERD, in both animal and human studies. In these animal studies, the mechanisms underlying the beneficial effects of the extracts were considered to be related to anti-oxidation, anti-inflammation, improvement of gastric mucus and barrier function, or a reduction in gastric acid [22]. However, inducing LES contraction to avoid reflux is more helpful for patients with PPI-refractory GERD than other treatment effects. To date, only Salvia miltiorrhiza has been reported to induce tonic contraction of the LES in Sprague-Dawley rats [18]. However, the effect of A. pericarpium extract on porcine LES is more dominant than that of Salvia miltiorrhiza extract in rats because of the similarities between the porcine and the human LES.
Previous study demonstrated that arecoline can excite the colonic smooth muscle motility in rabbits via muscarinic receptor [23]. In addition, A. pericarpium can promote gastrointestinal motility in rats via muscarinic receptor [7, 8]. However, the LES is a specialized smooth muscle that is different from the muscularis propria of the gastrointestinal tract, because LES always remains contracted and temporarily opens only during swallowing. A key novel finding of this study is that A. pericarpium extracts and arecoline can induce contractions in this specialized smooth muscle.
Arecoline is one of several active ingredients of Areca catechu L. and can cause side effects such as the promotion of oral submucosal fibrosis (OSF). However, the development of OSF takes time, and it is observed that 3.5 and 6.5 years of chewing areca nut was necessary to develop OSF in younger and older cohorts, respectively [24]. Furthermore, the half-maximal inhibitory concentration (IC50) of arecoline was approximately 210 μM in HaCaT keratinocytes and buccal mucosal fibroblasts (BMFs) [25, 26]. The concentrations of arecoline used in studies on OSF were reported to be around 160 μM in HaCaT cells and 0 to 672 μM in BMFs [27, 28]. However, in this study, the working concentration of arecoline applied to LES tissues (300 nM to 1 μM) was much lower than previously reported IC50 concentrations or those used for studying OSF. Moreover, arecoline has different effects depending on the concentration, such as increasing cell proliferation rate but inducing cell cycle arrest, apoptosis, and DNA damage at lower and higher concentrations, respectively, in oral squamous cell carcinoma cells [29]. Previous studies have focused on the relationship between the dosage and toxicity of arecoline, but it is important to determine the difference between pharmacological dosages with an appropriate effect and toxic doses of arecoline [30].
In addition, epigallocatechin-3-gallate (extracted from green tea), Ganoderma microsporum immunomodulatory protein (extracted from Ganoderma microsporum), and hinokitiol (derived from Chamacyparis taiwanensis) can suppress arecoline-induced OSF [31–34]. These findings provide an opportunity for further research to develop a multicomponent herbal preparation that not only mitigates against unwanted side effects of arecoline but which can also be used for the management of GERD.
Conclusions
A. pericarpium extracts can induce the contractions of porcine LES in a dose dependent manner, and the contractions are possibly related to muscarinic receptors. These results suggest that A. pericarpium may be exploited as a potential alternative therapy for GERD.
Acknowledgements
The authors thanks Ph.D. Shih-Che Huang for helpful suggestions and technical consultation.
Abbreviations
- GERD
Gastroesophageal reflux disease
- PPI
Proton pump inhibitor
- LES
Lower esophageal sphincters; A. pericarpium: Arecae pericarpium
- TCM
Traditional Chinese Medicine
- HPLC
High performance liquid chromatography
- MLCK
Myosin light chain kinase
- TTX
Tetrodotoxin
- IC50
Half-maximal inhibitory concentration
Authors’ contributions
CCT and LWL conceived the idea of this study. LWL and LCC performed extraction. SLT, CYL and FRC conducted HPLC. CCT, YLC and SLT performed ex vivo muscular experiments. SNY, CCT and LCC analyzed the data. CCT and SLT wrote the paper. CCT revised the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by intramural funding, provided by the E-Da hospital (EDAHP105031, EDAHP106072, EDAHI107004, and EDAHP107042). The funding body had no role in the design of the study or collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
All relevant data are included within the manuscript and are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was exempted from the review of Institutional Animal Care and Use Committee of E-DA Hospital because the study didn’t involve live animals.
Consent for publication
All authors read and approved the final manuscript.
Competing interests
The authors declare no conflict of interest or competing financial interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mittal R, Vaezi MF. Esophageal motility disorders and gastroesophageal reflux disease. N Engl J Med. 2020;383:1961–1972. doi: 10.1056/NEJMra2000328. [DOI] [PubMed] [Google Scholar]
- 2.Fuchs KH, DeMeester TR, Otte F, Broderick RC, Breithaupt W, Varga G, et al. Severity of GERD and disease progression. Dis Esophagus. 2021;34:doab006. 10.1093/dote/doab006. [DOI] [PubMed]
- 3.Naik RD, Meyers MH, Vaezi MF. Treatment of refractory gastroesophageal reflux disease. Gastroenterol Hepatol (N Y) 2020;16:196–205. [PMC free article] [PubMed] [Google Scholar]
- 4.Roman S, Mion F. Refractory GERD, beyond proton pump inhibitors. Curr Opin Pharmacol. 2018;43:99–103. doi: 10.1016/j.coph.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Clarke JO, Fernandez-Becker NQ, Regalia KA, Triadafilopoulos G. Baclofen and gastroesophageal reflux disease: seeing the forest through the trees. Clin Transl Gastroenterol. 2018;9:137. doi: 10.1038/s41424-018-0010-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taiwan Herbal Pharmacopeia . Taiwan Herbal Pharmacopeia 2nd edition English version. Taipei: Ministry of health and welfare; 2016. pp. 28–29. [Google Scholar]
- 7.Li Y. Foundation and clinical research on the effect of traditional Chinese herbs on gastrointestinal motility. Chin J Integr Med. 2009;15:86–88. doi: 10.1007/s11655-009-0086-z. [DOI] [PubMed] [Google Scholar]
- 8.Wang HL, Li Y, Bai H, Zhang J. Effect of qi-regulating Chinese medicine on gastrointestinal motility. World J Dig. 2004;12:1136–1138. [Google Scholar]
- 9.Ohta S, Sato N, Tu SH, Shinoda M. Protective effects of Taiwan crude drugs on experimental liver injuries. Yakugaku Zasshi. 1993;113:870–880. doi: 10.1248/yakushi1947.113.12_870. [DOI] [PubMed] [Google Scholar]
- 10.Wang W, Xu L. Chinese Materia Medica: Combinations and Applications. Potters Bar: Donica; 2002. Herbs for regulating Qi: Da Fu Pi; p. 288. [Google Scholar]
- 11.Tsai MH, Huang GS, Hung YC, Bin L, Liao LT, Lin LW. Psoralea corylifolia extract ameliorates experimental osteoporosis in ovariectomized rats. Am J Chin Med. 2007;35:669–680. doi: 10.1142/S0192415X07005168. [DOI] [PubMed] [Google Scholar]
- 12.Dechayont B, Phuaklee P, Chunthorng-Orn J, Juckmeta T, Prajuabjinda O, Jiraratsatit K. Antibacterial, anti-inflammatory and antioxidant activities of Mahanintangtong and its constituent herbs, a formula used in Thai traditional medicine for treating pharyngitis. BMC Complement Med Ther. 2021;21:105. doi: 10.1186/s12906-021-03274-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farré R, Aulí M, Lecea B, Estrada O, Suñol X, Clavé P. Mechanisms controlling function in the clasp and sling regions of porcine lower oesophageal sphincter. Br J Surg. 2007;94:1427–1436. doi: 10.1002/bjs.5831. [DOI] [PubMed] [Google Scholar]
- 14.Korn O, Stein HJ, Richter TH, Liebermann-Meffert D. Gastroesophageal sphincter: a model. Dis Esophagus. 1997;10:105–109. doi: 10.1093/dote/10.2.105. [DOI] [PubMed] [Google Scholar]
- 15.Schopf BW, Blair G, Dong S, Troger KA. A porcine model of gastroesophageal reflux. J Investig Surg. 1997;10:105–114. doi: 10.3109/08941939709032140. [DOI] [PubMed] [Google Scholar]
- 16.Tsai CC, Chang LC, Lin KJ, Tey SL, Su YT, Liu CW, Tsai TR, Huang SC. Mechanism of bombesin-induced tonic contraction of the porcine lower esophageal sphincter. Sci Rep. 2015;5:15879. doi: 10.1038/srep15879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsai CC, Tey SL, Chang LC, Su YT, Lin KJ, Huang SC. Estradiol mediates relaxation of porcine lower esophageal sphincter. Steroids. 2018;136:56–62. doi: 10.1016/j.steroids.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Tsai CC, Chang LC, Huang SC, Tey SL, Hsu WL, Su YT, Liu CW, Tsai TR. Salvia miltiorrhiza induces tonic contraction of the lower esophageal sphincter in rats via activation of extracellular Ca2+ influx. Molecules. 2015;20:14504–14521. doi: 10.3390/molecules200814504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hornby PJ, Abrahams TP. Central control of lower esophageal sphincter relaxation. Am J Med. 2000;108:90S–98S. doi: 10.1016/S0002-9343(99)00345-9. [DOI] [PubMed] [Google Scholar]
- 20.Sanders KM. Regulation of smooth muscle excitation and contraction. Neurogastroenterol Motil. 2008;20 Suppl: 1:39–53. doi: 10.1111/j.1365-2982.2008.01108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perrino BA. Calcium sensitization mechanisms in gastrointestinal smooth muscles. J Neurogastroenterol Motil. 2016;22:213–225. doi: 10.5056/jnm15186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salehi M, Karegar-Borzi H, Karimi M, Rahimi R. Medicinal plants for Management of Gastroesophageal Reflux Disease: a review of animal and human studies. J Altern Complement Med. 2017;23:82–95. doi: 10.1089/acm.2016.0233. [DOI] [PubMed] [Google Scholar]
- 23.Xie DP, Chen LB, Liu CY, Zhang CL, Liu KJ, Wang PS. Arecoline excites the colonic smooth muscle motility via M3 receptor in rabbits. Chin J Physiol. 2004;47:89–94. [PubMed] [Google Scholar]
- 24.Ranganathan K, Devi MU, Joshua E, Kirankumar K, Saraswathi TR. Oral submucous fibrosis: a case-control study in Chennai, South India. J Oral Pathol Med. 2004;33:274–277. doi: 10.1111/j.0904-2512.2004.00116.x. [DOI] [PubMed] [Google Scholar]
- 25.Li L, Gu L, Yao Z, Wang Y, Tang Z, Wu X. Arecoline suppresses epithelial cell viability by upregulating tropomyosin-1 through the transforming growth factor-β/Smad pathway. Pharm Biol. 2020;58:1244–1251. doi: 10.1080/13880209.2020.1851729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adtani PN, Narasimhan M, Punnoose AM, Kambalachenu HR. Antifibrotic effect of Centella asiatica Linn and asiatic acid on arecoline-induced fibrosis in human buccal fibroblasts. J Investig Clin Dent. 2017;8. 10.1111/jicd.12208. [DOI] [PubMed]
- 27.Lee SS, Chen YJ, Tsai CH, Huang FM, Chang YC. Elevated transglutaminase-2 expression mediates fibrosis in areca quid chewing-associated oral submucocal fibrosis via reactive oxygen species generation. Clin Oral Investig. 2016;20:1029–1034. doi: 10.1007/s00784-015-1579-0. [DOI] [PubMed] [Google Scholar]
- 28.Tsai CH, Lee SS, Chang YC. Hypoxic regulation of plasminogen activator inhibitor-1 expression in human buccal mucosa fibroblasts stimulated with arecoline. J Oral Pathol Med. 2015;44:669–673. doi: 10.1111/jop.12284. [DOI] [PubMed] [Google Scholar]
- 29.Tu HF, Chen MY, Lai JC, et al. Arecoline-regulated ataxia telangiectasia mutated expression level in oral cancer progression. Head Neck. 2019;41:2525–2537. doi: 10.1002/hed.25718. [DOI] [PubMed] [Google Scholar]
- 30.Shen YW, Shih YH, Fuh LJ, Shieh TM. Oral submucous fibrosis: a review on biomarkers, pathogenic mechanisms, and treatments. Int J Mol Sci. 2020;21:7231. doi: 10.3390/ijms21197231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsieh YP, Chen HM, Chang JZ, Chiang CP, Deng YT, Kuo MY. Arecoline stimulated early growth response-1 production in human buccal fibroblasts: suppression by epigallocatechin-3-gallate. Head Neck. 2015;37:493–497. doi: 10.1002/hed.23614. [DOI] [PubMed] [Google Scholar]
- 32.Chu C, Deng J, Man Y, Qu Y. Green tea extracts Epigallocatechin-3-gallate for different treatments. Biomed Res Int. 2017;2017:5615647. doi: 10.1155/2017/5615647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee PH, Hsieh PL, Liao YW, Yu CC. Inhibitory effect of GMI, an immunomodulatory protein from Ganoderma microsporum, on myofibroblast activity and proinflammatory cytokines in human fibrotic buccal mucosal fibroblasts. Environ Toxicol. 2018;33:32–40. doi: 10.1002/tox.22489. [DOI] [PubMed] [Google Scholar]
- 34.Yang HW, Lu MY, Chiu YW, Liao YW, Huang YF, Ju Chueh P, Hsieh PL, Yu CC. Hinokitiol ablates myofibroblast activation in precancerous oral submucous fibrosis by targeting snail. Environ Toxicol. 2018;33:454–462. doi: 10.1002/tox.22531. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are included within the manuscript and are available from the corresponding author on reasonable request.