Abstract
Background
Plant health and growth are negatively affected by pathogen invasion; however, plants can dynamically modulate their rhizosphere microbiome and adapt to such biotic stresses. Although plant-recruited protective microbes can be assembled into synthetic communities for application in the control of plant disease, rhizosphere microbial communities commonly contain some taxa at low abundance. The roles of low-abundance microbes in synthetic communities remain unclear; it is also unclear whether all the microbes enriched by plants can enhance host adaptation to the environment. Here, we assembled a synthetic community with a disease resistance function based on differential analysis of root-associated bacterial community composition. We further simplified the synthetic community and investigated the roles of low-abundance bacteria in the control of Astragalus mongholicus root rot disease by a simple synthetic community.
Results
Fusarium oxysporum infection reduced bacterial Shannon diversity and significantly affected the bacterial community composition in the rhizosphere and roots of Astragalus mongholicus. Under fungal pathogen challenge, Astragalus mongholicus recruited some beneficial bacteria such as Stenotrophomonas, Achromobacter, Pseudomonas, and Flavobacterium to the rhizosphere and roots. We constructed a disease-resistant bacterial community containing 10 high- and three low-abundance bacteria enriched in diseased roots. After the joint selection of plants and pathogens, the complex synthetic community was further simplified into a four-species community composed of three high-abundance bacteria (Stenotrophomonas sp., Rhizobium sp., Ochrobactrum sp.) and one low-abundance bacterium (Advenella sp.). Notably, a simple community containing these four strains and a thirteen-species community had similar effects on the control root rot disease. Furthermore, the simple community protected plants via a synergistic effect of highly abundant bacteria inhibiting fungal pathogen growth and less abundant bacteria activating plant-induced systemic resistance.
Conclusions
Our findings suggest that bacteria with low abundance play an important role in synthetic communities and that only a few bacterial taxa enriched in diseased roots are associated with disease resistance. Therefore, the construction and simplification of synthetic communities found in the present study could be a strategy employed by plants to adapt to environmental stress.
Video abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s40168-021-01169-9.
Keywords: Low-abundance bacteria, High-abundance bacteria, Synthetic community, Community simplification, Root rot disease
Background
The rhizosphere is known as the soil region surrounding the plant roots as well as the reservoir of soil microorganisms. It hosts a complex assemblage of microbes, including bacteria, fungi, and archaea, which collectively influence plant performance [1]. A key role of the soil microbiota is to protect plant roots and leaves from pathogens, nematodes, and pests [2–4]. Consequently, the diversity and abundance of beneficial microbes, such as plant growth–promoting bacteria (PGPB) and arbuscular mycorrhizal fungi (AMF), considerably influence plant health [5, 6].
Monoculture systems and excessive chemical fertilizer application alter soil physicochemical properties, which, in turn, influence rhizosphere microbial community structure [7–9]. The reduction in beneficial soil microbiota could lead to the accumulation of pathogens in the rhizosphere, which would promote soil-borne disease spreading in crops via negative plant–soil feedback over the long term [10]. Notably, continuous cultivation of a susceptible crop could induce the suppression of soil pathogens, such as Fusarium oxysporum, Pythium ultimum, and Rhizoctonia solani, following several severe disease outbreaks [11–14], so that plants may not exhibit disease symptoms despite pathogen presence in the disease-suppressive soil. In addition, soil disease-suppressive capacity could be augmented by soil suspension inoculation, and disease-suppressive soil can also be converted into susceptible soil by sterilization [15]. Such phenomena attribute to the enrichment of specific microorganisms in the rhizosphere of plant roots, as these microorganisms may participate in the resistance to the invasion of pathogens [2]. Under the induction of environmental factors, the physiological status and metabolic pathway of plants changed, which influences the proportions and compositions of rhizodeposits, including root exudates and complex organic compounds resulting from sloughed-off root tissues [16]. Such rhizodeposits released by roots attract specific microorganisms from the soil to colonize the rhizosphere and roots. The microorganisms then assemble into root-associated microbial communities and lead to variations in the diversity of microbial communities in the rhizosphere [17]. However, plants are exposed to various biotic and abiotic stress factors simultaneously in the course of growth under natural conditions, and microbes with different ecological functions are enriched in the rhizosphere and roots. Even under plant pathogen infestation conditions, the enriched microorganisms are not all associated with disease resistance. Consequently, the assembled microbial communities are not able to suppress disease when the plants are initially infected by pathogens; however, root-associated microbial communities could suppress diseases after successive pathogen infestation events.
Plants recruit protective microorganisms by regulating the production of root exudates through defense-related signaling molecules such as salicylic acid (SA) and jasmonic acid (JA) [16]. Previous studies have mainly focused on isolating and screening antagonists in the rhizosphere or roots that directly inhibit pathogen growth for potential application in plant disease control [17, 18]. Over the last decade, however, multiomics sequencing technologies have been extensively applied in plant microbiome studies, and they have revealed that complex microbial community assemblages in the rhizosphere rather than single microbial strains protect plants from pathogen infection [19, 20]. In attempts to mimic natural disease-suppressive soil conditions, the potential of artificial synthetic communities composed of diverse bacteria to prevent soil-borne disease has been explored. For example, a synthetic microbial community consisting of three bacterial species, including Xanthomonas sp., Stenotrophomonas sp., and Microbacterium sp., could synergistically control downy mildew in Arabidopsis thaliana [16]. Another study demonstrated that a synthetic community consisting of 38 bacterial species could influence the immune activity of A. thaliana [21]. However, the structural and functional stability of such synthetic communities are influenced considerably by microbial species, which are further influenced by the host species and genotypes, in addition to biotic and abiotic stress factors from the environment [22–25]. Consequently, determining which microbial strains in an introduced community can survive and persist in interactions among bacteria, plants, and pathogens is essential for the assembly of synthetic microbial communities with disease resistance potential.
In addition to microbial diversity, the abundance of key species could influence microbial community function. Although thousands of distinct bacterial species are found in the soil, their proportions vary extensively [26]. Numerous high-throughput sequencing studies have revealed that the relative abundances of a small number of microbial taxa are greater than 1%, while a large proportion of microorganisms that belong to rare taxa have relative abundances lower than 0.1% [27]. Generally, high-abundance microbes have important ecological functions, while low-abundance taxa are considered seed banks and exhibit low metabolic activity in the soil [28]. However, some studies have demonstrated that microorganisms with low abundance have diverse metabolic functions and participate in soil nutrient transformation, redox reactions, and bioremediation activities [29–32]. Nevertheless, no evidence has shown whether low-abundance microorganisms in synthetic bacterial communities play an important role in disease suppression.
Astragalus membranaceus Bge. var. mongholicus (Bge.) Hsiao (hereafter, Astragalus), a perennial herbaceous leguminous plant, is one of the most important traditional Chinese herbs. It is also an important cash crop in Gansu, Shanxi, Inner Mongolia, and other provinces in China [33]. Long-term monocropping leads to a high incidence of Astragalus root rot. In the present study, we studied microbial community responses to the Fusarium oxysporum infection and investigated (1) how plant-associated microbial communities respond to F. oxysporum infection, (2) whether enrichment with bacteria with low relative abundance could confer root rot disease prevention functions in synthetic communities, and (3) whether simple synthetic bacterial communities have roles similar to those of complex synthetic communities.
To address the questions above, we first analyzed differences in root-associated microbial community structure between healthy and diseased plants. Afterward, we constructed a synthetic community (SCI) consisting of 10 high-abundance and three low-abundance bacteria enriched in diseased roots. In addition, we selected nine bacterial strains (two strains for Bacillus) with decreased abundance and randomly selected four strains in diseased roots to construct synthetic community II (SCII), which served as the control. Furthermore, using a combination of PacBio sequencing and plant selection, we simplified the complex synthetic community (SCI) into a simple four-species synthetic community (SCIII) that conferred root rot disease resistance. Finally, we investigated the separate and joint effects of the four bacteria in SCIII on F. oxysporum growth, plant growth promotion, host plant–induced systemic resistance (ISR) activation, and root rot control.
Materials and methods
Sample preparation and soil physicochemical property measurement
The samples used in this work were collected from three medicinal plant fields in Tanchang County, Gansu Province, China (N34° 15.123’; E104° 09.727’). The soil organic matter, total nitrogen, total phosphorus, total potassium concentrations, and pH were 2.76 g/kg, 1.75 g/kg, 1.31 g/kg, 10.86 g/kg, and 8.17, respectively. The fields had a 4-year history of potato and Astragalus rotation before the samples were collected. In March 2019, 1-year-old Astragalus seedlings were transplanted to the fields, and 24 plants (12 healthy + 12 diseased plants) were uprooted randomly with shovels when root rot disease was determined to be severe in May 2019. Roots were shaken vigorously to remove loose soil and the 1–2-mm–thick soil layer surrounding the root was defined as the rhizosphere soil. To collect the rhizosphere soil, the roots were transferred into tubes containing 25 mL of 1× phosphate buffer. The tubes were inverted 4–5 times and vortexed for 5 min, and then roots were removed. Afterward, the tubes were centrifuged at 2500 × g for 5 min. The supernatant was discarded, and the remaining material was centrifuged at 2500 × g for 5 min. After collecting the rhizosphere soil, the remaining roots were surface-disinfected with 75% ethanol for 1 min and then 1% sodium hypochlorite for 30 s. After that, the roots were rinsed 10 times with sterile water (Fig. s1). In total, 72 samples were obtained from bulk soil, rhizosphere soil, and roots (3 compartments × 3 fields × 4 repetitions × 2 health states). All the roots and soil samples were stored at − 80 °C before DNA isolation.
DNA extraction, amplification, and sequencing
All the 72 samples were divided into two groups: the first group was transported to the laboratory at room temperature for microbial isolation, and the other group was frozen and instantly sent to the laboratory for DNA extraction. Total DNA was extracted from 0.5 g of soil or root using a FastDNA®SPIN Kit (MP Biomedicals, Solon, USA), according to the manufacturer’s protocol. The barcoded primers 515F (GTGCCAGCMGCCGCGG) /907R (CCGTCAATTCMTTTRAGT) were used to amplify the V4–V5 region of bacterial 16S rRNA, and the primers ITS1F (CTTGGTCATTTAGAGGAAGTAA)/ITS1R (GCTGCGTTCTT CATCGATGC) were used to amplify the ITS1 regions of fungi. The purified PCR products were sequenced on the Illumina MiSeq PE300 platform at Major Biomedical Technology Co., Ltd (Shanghai, China). Raw data were assembled and quality-filtered according to Caporaso et al. [34], and chimeric sequences were removed using the UCHIME tool in USEARCH [35]. The sequences matching the mitochondria and chloroplast were also removed [36], and the remaining effective sequences were clustered into operational taxonomic units (OTUs) at 97% similarity [37].
Isolation of plant-associated bacteria and pathogenic fungi from field-grown plants
Field-grown Astragalus at the vegetative stage displaying symptoms of root rot disease were used for the isolation of fungal pathogens according to Schuck et al. [38] with minor modifications (Supplementary Materials). Plant-associated bacteria were isolated as described in [39]. Bacterial 16S rDNA and fungal 18S rDNA were amplified and sequenced at Sangon Biotech Co., Ltd (Shanghai, China). Sequence alignment was performed on the NCBI website, and each microbial species was preserved in 1 mL of 30% glycerol (v/v) at − 80 °C. The healthy plants were then reinoculated with the potential pathogens, and the pathogenic capacity of each fungus was evaluated.
Assembly and simplification of synthetic communities
Based on the root-associated bacterial community composition and diversity in diseased plants, some bacteria that were significantly enriched and depleted in the rhizosphere or roots were selected as candidate strains for bacterial community construction. Following measurement of the P dissolution, K dissolution, indole acetic acid (IAA) production, and F. oxysporum antagonism by the candidate bacteria, we selected 13 bacterial species with plant growth promotion or pathogenic fungal growth inhibition capacity, which were obviously enriched in Astragalus diseased roots (Table s1), and equal volumes of each strain (~ 108 cells/mL) were mixed to establish synthetic community I (SCI).
The abundant genera (relative abundance > 0.1%) accounted for 98.02% of all OTUs. Therefore, we classified the genera with abundance > 0.1% and abundance < 0.1% as high-abundance bacteria and low-abundance bacteria, respectively [27]. Consequently, SCI contained 10 high- and three low-abundance bacteria. In addition, synthetic community II (SCII) was assembled using nine depleted bacteria and four random bacteria found in diseased roots (Table s1). Then, the effects of synthetic communities on the incidence of root rot disease were investigated using pot experiments, and sterile water was used as a negative control. In addition, the effects of synthetic communities on root length, shoot length, plant fresh weight, and plant dry weight were also determined. For detailed methods, see Supplementary Materials.
To determine which bacteria from the synthetic bacterial communities were retained and performed functions in the rhizosphere or roots during interactions among plants, F. oxysporum, and bacterial communities, we inoculated 7-day-old Astragalus seedlings with SCI and grew them in a greenhouse for 30 days. DNA extraction and near full-length 16S rRNA library preparation were performed as described previously [40]. Sequencing of near full-length 16S rRNA amplicons and the assembly of reads were performed on the PacBio platform at Novogene Technology Co., Ltd (Beijing, China). A four-species community (SCIII) was constructed using strains that could colonize the rhizosphere or roots, based on the 16S rRNA full-length sequencing results.
Disease control by synthetic bacterial communities
The capacity of synthetic bacterial communities to control Astragalus root rot was evaluated in vivo. A planting bag was filled with 500 g of substrate (soil:vermiculite:peat = 3:2:1, v/v) for autoclaving. Subsequently, each bag was inoculated with 10 mL of SCI, SCII, or SCIII; sealed; and incubated for 7 days. Soil medium supplemented with the same volume of sterile water was used as the control, and each treatment contained 10 bags. Astragalus seeds were surface-sterilized with 75% ethanol for 3 min and 3% sodium hypochlorite solution for 8 min, followed by six-time rinsing with deionized distilled water. Sterilized seeds were germinated on wet filter paper in Petri dishes for 48 h. Ten germinated seedlings were sown in a planting bag. After 7 days, the seedlings were thinned, and five uniform plants were maintained. The remaining plants were inoculated again with 10 mL of the synthetic communities, and the plants were grown continuously for 5 days before adding a 10-mL spore suspension of F. oxysporum (~ 105 spores per milliliter). All the plants were grown in a greenhouse at 25 °C 16 h light/8 h darkness). Root rot incidence and plant mortality were assessed every 3 days. The antagonistic activity of SCIII against pathogenic fungi was examined by assessing fungal growth inhibition using a confrontation bioassay.
Induced systemic resistance assay
Four-week-old plants were inoculated with 10 mL of sterile water, Stenotrophomonas sp., Rhizobium sp., Advenella sp., Ochrobactrum sp., and different synthetic communities, as mentioned above. Plants were grown in planting bags placed in a greenhouse for 10 days. Plant samples were harvested at 8, 24, 72, 120, and 168 h after inoculation with different bacterial or synthetic communities. Subsequently, phenylalanine ammonia lyase (PAL), polyphenol oxidase (PPO), peroxidase (POD), lipoxygenase (LOX), chitinase activity, and jasmonic acid (JA) contents were measured. For detailed methods, see Supplemental Materials.
Effect of bacteria of different abundance in synthetic communities on plant health and ISR
To verify that Ochrobactrum sp. and Advenella sp. from SCIII could perform functions at low abundance, Stenotrophomonas sp., Rhizobium sp., Ochrobactrum sp., and Advenella sp. was mixed in 10:10:1:1 and 100:100:1:1 proportions. Additionally, Stenotrophomonas:Rhizobium:Ochrobactrum:Advenella at 600:200:10:1 was also included according to the 16S amplicon sequence (Table s4). Four-week-old plants were inoculated with 10 mL of different bacterial mixtures, and a similar volume of sterile water was used as the control. Then, the root rot incidence, PAL, PPO, POD, LOX, chitinase activities, and JA content were measured as mentioned above.
Statistical analyses
All statistical analyses were performed in the R environment (version: V3.6.0, http://www.r-project.org/). Alpha diversity indices, including the Pielou index, Shannon index, Simpson index, and Chao1 index, were analyzed using the “vegan” package in R. The results were visualized using the “ggplot2” package. The alpha diversity indices of different samples were analyzed using analysis of variance (ANOVA). Permutational multivariate analysis of variance (PERMANOVA) with 999 permutations was used to evaluate the effect of F. oxysporum infection on bacterial community structure using the Adonis function (vegan package) in R [41]. Differences in the abundance and enrichment of bacterial genera were examined using Kruskal–Wallis tests, and ternary plots were created using the “vcd” package and visualized by “ggplot2” in R. Spearman correlations were calculated between microbial and pathogen parameters using the “ggcor” package in R [42]. The enriched OTUs and depleted OTUs in roots between the healthy and diseased roots were analyzed using the “EdgeR” package in R [43]. The distinct and shared bacteria were analyzed with Venn diagrams using the “VennDiagram” package in R V3.6.0. PICRUSt software was used to predict KEGG ortholog functional profiles of bacterial communities in rhizospheres and roots using the 16S rRNA sequences [44]. Phylogenetic trees of bacteria isolated from rhizospheres and roots were drawn using MEGA 6, based on 16S rDNA.
Results
Isolation and verification of fungal pathogens from diseased plants
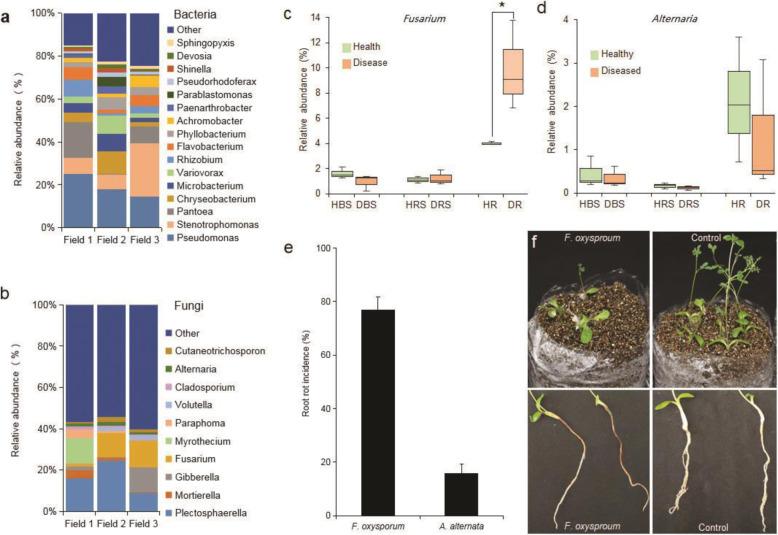
As illustrated in Fig. 1, Pseudomonas, Stenotrophomonas, Pantoea, and Chryseobacterium had relatively high abundance in the bacterial communities of diseased roots, while Plectosphaerella, Cephalotrichum, and Fusarium had relatively high abundance in the fungal communities of diseased roots. According to previous studies, Ralstonia solanacearum, Cylindrocarpon destructans, Fusarium oxysporum, Fusarium solani, Alternaria sp., and Phytophthora incognitae are the potential pathogens causing root rot [45–47]. However, no potentially pathogenic bacteria that could be clustered into known genera from diseased roots were observed among the highly abundant OTUs (Fig. 1a). In contrast, Fusarium (8.79%) and Alternaria (1.30%) accounted for a relatively high abundance of fungi in diseased roots (Fig. 1b). Fusarium was significantly enriched in the roots of the diseased plants; however, there was no difference in Alternaria abundance between the healthy and diseased roots (Fig. 1c and d). In addition, 53 culturable fungi, including F. oxysporum, F. solani, and Alternaria alternata, were isolated from diseased Astragalus. According to the pot experiment results, only F. oxysporum caused high disease incidence, and plants displayed typical root rot symptoms: curly leaves, short plants, and brown and decaying roots (Fig. 1e and f). Therefore, this fungus was used as an indicator in subsequent experiments.
Fig. 1.
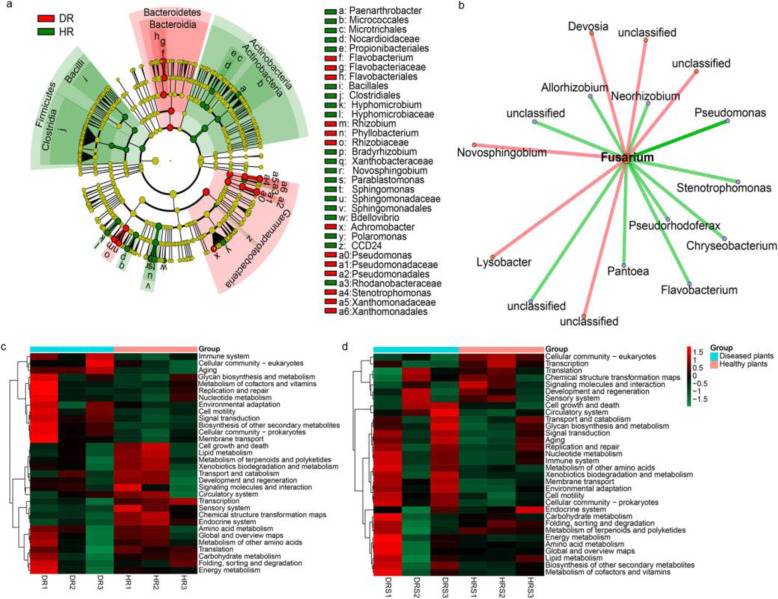
Microbial community composition in diseased roots and pathogenic capacity of potential fungal pathogens. a Dominant bacterial genera in field-grown plants with root rot symptoms. b Dominant fungal genera in field-grown plants with root rot symptoms. Two fungal genera (Fusarium and Alternaria) related to root rot disease were observed. c and d Relative abundance of Fusarium and Alternaria in healthy and diseased roots, rhizosphere, and bulk soil; HBS, healthy bulk soil; DBS, diseased bulk soil; HRS, healthy rhizosphere; DRS, diseased rhizosphere; HR, healthy root; DR, diseased root. e Root rot disease incidence in Astragalus with fungal pathogen infection. f Shoot and root symptoms of plants with and without F. oxysporum infection
Effect of fungal pathogens on the structure of root-associated bacterial communities
According to the 16S amplicon sequencing results, a total of 2,287,677 high-quality bacterial reads were obtained. After removing the low-quality and plant-derived reads, the remaining reads were clustered into 7547 bacterial OTUs at ≥ 97% sequence identity. The majority of the bacterial OTUs discovered in the healthy and diseased roots were also present in the rhizosphere. However, a considerable proportion of the bacteria was observed solely in the diseased rhizosphere and root (9.2% and 0.6%, respectively), although a majority of the OTUs associated with diseased plants were also present in the rhizosphere and roots of healthy plants (Fig. s2).
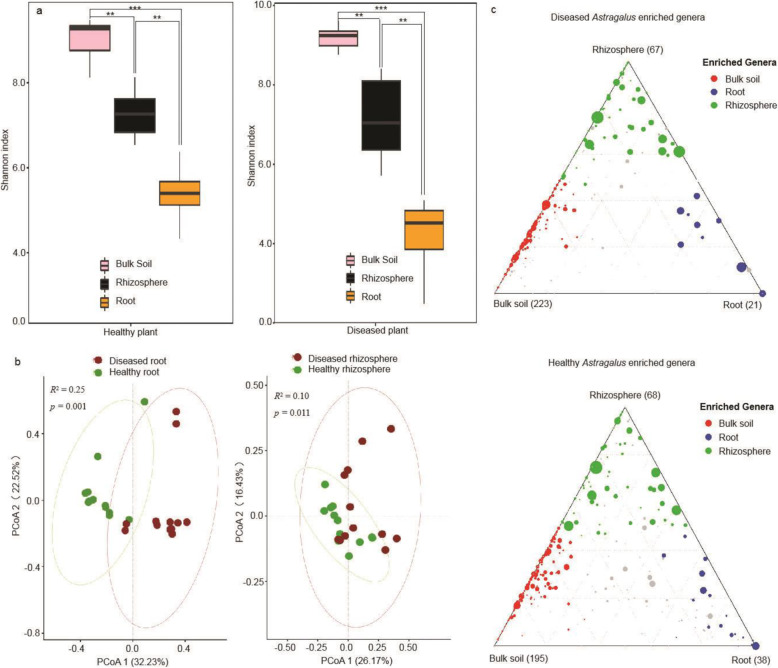
As shown in Fig. 2a, the Shannon α-diversity of the bacterial community decreased gradually from bulk soil to roots in the presence and absence of F. oxysporum (healthy roots: F2, 33 = 90.96, P < 0.001; diseased root: F2, 33 = 84.78, P < 0.001), which indicated that the plants had a strong selective effect on bacterial colonization in roots. In addition, the invasion of F. oxysporum decreased the Shannon α-diversity of bacterial communities in roots without influencing the α-diversity in bulk soil and rhizosphere (rhizosphere: F1, 22 = 0.48, P < 0.50; root: F1, 22 = 9.71, P = 0.005) (Fig. s3 and Table s2). Principal coordinate analysis (PCoA) based on Bray-Curtis revealed significant differences in the composition of bacterial communities in roots and rhizospheres between healthy and diseased plants. In addition, the difference in endophytic community composition between the healthy and diseased roots was greater than that in the rhizosphere (root: R2 = 0.25, P = 0.001; rhizosphere: R2 = 0.10, P = 0.011) (Fig. 2b). Ternary plots also showed that many bacterial OTUs enriched in diseased roots and rhizospheres were significantly different from those in the healthy plants (Fig. 2c). However, no differences were observed in bulk soil samples. The results suggested that the plants recruited certain microbes to colonize the rhizosphere and roots under F. oxysporum stress.
Fig. 2.
Effect of F. oxysporum on bacterial community structure in the rhizosphere and roots. a Shannon diversity of bacteria in bulk soil, rhizosphere, and roots of healthy and diseased plants. Asterisks indicate statistically significant (*P < 0.05; **P < 0.01) differences based on analysis of variance with Dunn’s multiple comparison test. b Principal coordinates analysis (PCoA) of root microbial communities based on Bray-Curtis distance. Fungal pathogen infection altered the bacterial community structure. c Differences in the abundance and enrichment of bacterial genera in three compartments of healthy and diseased plants analyzed using the Kruskal–Wallis test. The enriched bacterial genera in the diseased roots and the rhizosphere were different from those in the healthy samples
Plant-associated microbial community composition
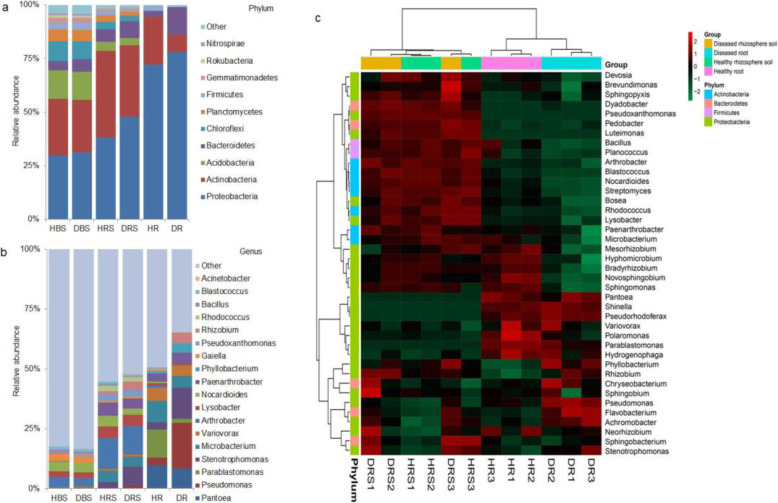
Based on the OTU classification results, Proteobacteria, Actinobacteria, and Bacteroidetes were the dominant phyla in the roots and rhizosphere, accounting for 86.7–98% of the total reads, while Proteobacteria (30.4%), Actinobacteria (25.6%), Acidobacteria (13.1%), and Chloroflexi (8.8%) dominated the bulk soil bacterial communities (Fig. 3a). Compared with the bulk soil, the rhizosphere was significantly enriched in Proteobacteria and Actinobacteria, while the roots were obviously enriched in Proteobacteria (Fig. s4). Fusarium oxysporum infection could also affect the root-associated bacterial community composition. The bacterial phyla that were significantly affected by pathogen infection were Proteobacteria and Bacteroidetes in the roots, and Actinobacteria and Bacteroidetes in the rhizosphere. However, bacterial phylum abundance in bulk soil was not affected by F. oxysporum (Fig. 4 and Fig. s5).
Fig. 3.
Plant-associated bacterial community composition in different compartments between healthy and diseased plants. a Distribution and abundance of major bacterial phyla in different compartments of healthy and diseased plants. b Effect of fungal pathogen infection on bacterial community composition and the abundance of major bacterial phyla in different compartments. c Heat map of the bacterial community composition with cluster analysis. Similar samples were clustered horizontally, and vertical patterns illustrate the phylogenetic relationships among the top 40 bacterial genera across samples. HBS, healthy bulk soil; HRS, healthy rhizosphere; HR, healthy root; DBS, diseased bulk soil; DRS, diseased rhizosphere; DR, diseased root
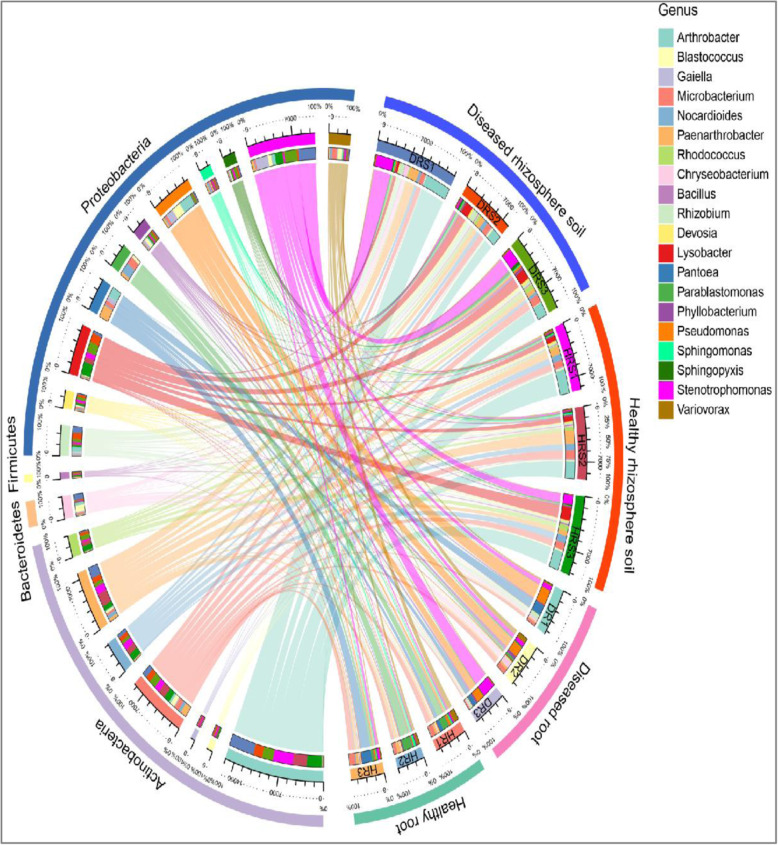
Fig. 4.
Effects of fungal pathogens on bacterial composition in healthy and diseased plants. The dominant bacterial phyla and genera in diseased and healthy roots and rhizosphere. The left half circle from the outside to the inside represents the dominant phyla, dominant genera, and proportions of each genus in different samples. The right half circle represents the different samples
At the genus level, the most abundant bacterial genera in bulk soil were Nocardioides (3.9%), Arthrobacter (3.8%), and Gaiella (2.8%). Conversely, Arthrobacter and Stenotrophomonas were the dominant genera in the rhizosphere, while Pseudomonas, Stenotrophomonas, and Pantoea were the most dominant genera in the roots (Fig. 3b).
The heat map of the top 40 genera revealed that the bacterial communities in the healthy and diseased roots were clustered separately. However, bacterial communities in the rhizosphere were not distinguishable between the healthy and diseased plants (Fig. 3c), indicating that infection with F. oxysporum greatly impacted the bacterial community structure in roots more than it did that of rhizospheres. To investigate which bacteria were recruited by plant roots during fungal pathogen infection, we analyzed the distinct bacteria between the healthy and diseased plants. The results showed that Stenotrophomonas, Pseudomonas, and Flavobacterium were highly enriched in diseased roots (Fig. 5a), while Rhizobiaceae and Flavobacteriales were enriched in diseased rhizospheres (Fig. s6).
Fig. 5.
Taxonomic and functional characteristics of bacterial communities after pathogen infection. Least discriminant analysis (LDA) effect size taxonomic cladogram comparing bacteria from healthy and diseased samples. The different classification levels are presented from the inside to the outside. The red nodes indicate enriched genera in diseased roots, yellow nodes indicate no difference, and green nodes indicate enriched genera in healthy roots. HR, healthy roots; DR, diseased roots. b The relationship between root-associated bacteria (relative abundance > 0.001) and Fusarium was calculated using Spearman correlations. Bacteria with a correlation coefficient greater than 0.6 were used to construct the network. Red and green lines indicate that the relative abundance of the bacteria was positively or negatively correlated with Fusarium. c The root and d rhizosphere functional profiles. The color scale represents enrichment or reduction of the predicted function
The functional traits of the bacterial communities in diseased rhizospheres and roots
The 16S rRNA sequencing results showed that the bacterial community composition of diseased roots was significantly different from that of healthy roots. To investigate whether bacteria recruited from diseased plants alter the functional profiles of bacterial communities, we first predicted the community functions via PICRUSt software. The bacterial community functional profiles were different between healthy and diseased rhizospheres and roots. In diseased rhizospheres and roots, the functions were related to “environmental adaption”, “cell motility”, “signal transduction,” and “biosynthesis of secondary metabolites” (Fig. 5c and d and Table s3). Next, we analyzed the relationship between the enriched OTUs in diseased roots and fungal pathogens. Interacting network analysis results indicated that Stenotrophomonas, Flavobacterium, and Pseudomonas abundances were negatively correlated with Fusarium growth (Fig. 5b).
Construction of synthetic communities and determination of disease resistance
To further evaluate the effects of the recruited bacteria on Astragalus health, we isolated 423 bacterial isolates from the rhizosphere and roots of healthy and diseased Astragalus using 0.1 ×, 0.2 ×, and 0.5 × TSA, R2A and LB plates. The isolates were from 74 genera, according to the 16S rRNA sequencing results. Among them, 44 isolates could solubilize organic P, 65 isolates could solubilize inorganic P, 40 isolates could solubilize K, and 80 isolates could produce IAA (Fig. s7).
With a relative abundance ratio of 2 as the threshold, 39 bacterial genera were enriched, and 137 bacterial genera were depleted in diseased roots (Tables s4 and s5). F. oxysporum infection led to a significant increase in bacterial genera such as Pseudomonas, Strenotrophomonas, Chryseobacterium, Achromobacter, and Flavobacterium in roots and a decrease in Pantoea, Parablastomonas, and Microbacterium (Fig. 5a). We isolated 16 out of the 39 enriched genera and 11 out of the 137 depleted genera, and each genus contained two to eight species. We then selected one species from 10 high-abundance (> 0.1%) and three low-abundance genera (< 0.1%) based on their antagonistic or plant growth–promoting characteristics. We then mixed all the species with equal-volume suspensions (~ 108 cells/L) and generated synthetic community I (SCI, Fig. s8 and Table s1). To investigate whether non-enriched bacteria could prevent fungal pathogens from infecting Astragalus, we also selected nine species with decreased relative abundance and four random species to construct synthetic community II (SCII) (Fig. 6a, Fig. s9, and Table s1).
Fig. 6.
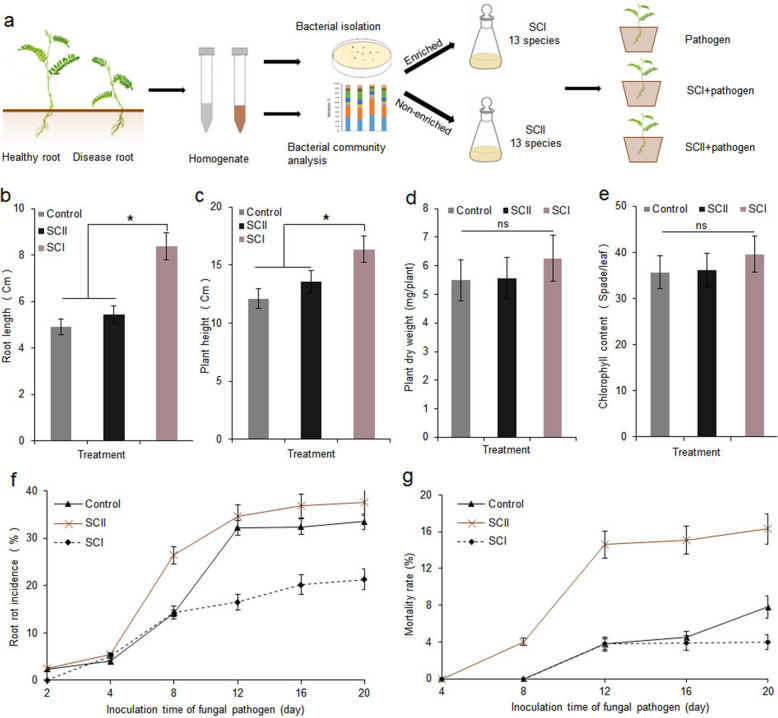
Synthetic bacterial community assembly and the regulatory effects of synthetic communities on Astragalus root rot. a Processes of establishing synthetic bacterial communities. Bacteria were isolated from the rhizosphere and roots by culturable methods as much as possible, and the different strains of healthy and diseased roots were analyzed. Subsequently, the synthetic community I (SCI) and synthetic community II (SCII) were assembled with enriched and non-enriched bacteria in diseased roots, respectively. b–e Effects of SCI and SCII on root length, plant height, chlorophyll content (n = 20), and plant dry weight (n = 4) 20 days after inoculation. The synthetic bacterial communities were inoculated 5 days before the fungal pathogens were introduced into the soil, and each plant was inoculated with a 2-mL suspension of synthetic communities (~ 108 CFU/mL). Asterisks indicate significant differences (P < 0.05). f–g Effects of SCI and SCII on root rot incidence and plant mortality at different time points after inoculation (n = 45). Each plant was inoculated with a 2-mL spore suspension of F. oxysporum (~ 105 spores/L)
Plants treated with the two synthetic communities grew for 20 days, and the root lengths in SCI-treated plants were 70.50% and 54.33% greater than the lengths of the uninoculated and SCII-treated plants, respectively, while the plant heights of SCI-treated plants were 34.48% and 20.56% greater than the heights in the control and SCII-treated plants, respectively (Fig. 6b and c). There were no significant differences in root length or plant height between the SCII-treated and control plants. In addition, neither SCI nor SCII treatment influenced plant fresh weight, dry weight, and chlorophyll contents (Fig. 6d and e).
SCI and SCII also had different effects on root rot incidence (Fig. 6f and g), based on the results of the pot experiments. After 12 days of inoculation with F. oxysporum, SCI significantly reduced the root rot incidence in Astragalus. The average incidence and mortality of SCI-treated plants were 26.32% and 23.64% lower than those of the control at 20 days after pathogen inoculation.
Simplification of the complex bacterial community
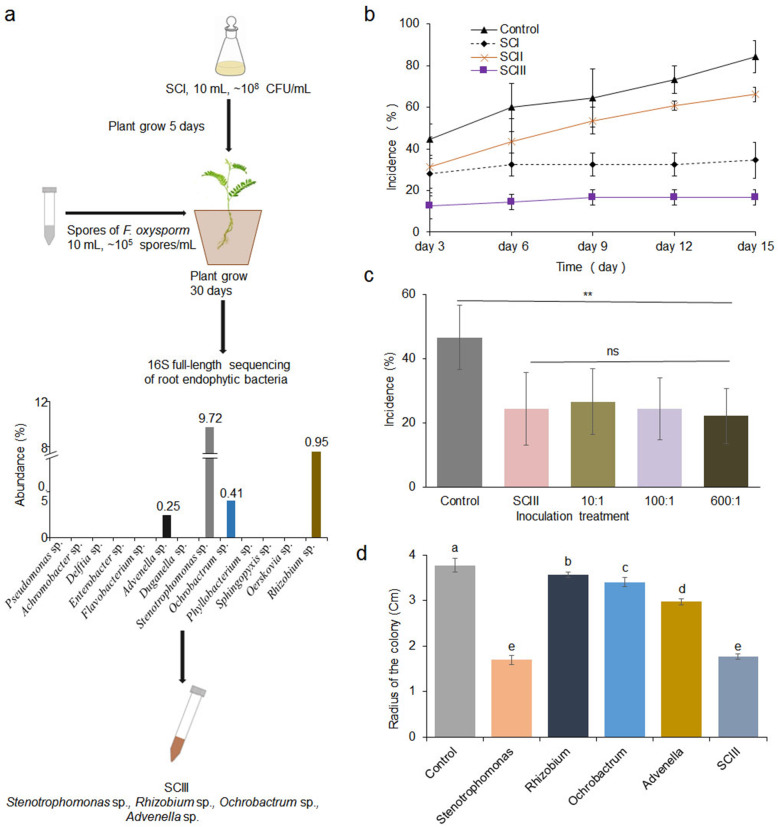
After SCI was inoculated in soil for 30 days, only Stenotrophomonas sp., Rhizobium sp., Advenella sp., and Ochrobactrum sp. could be recovered from the roots and rhizospheres, and Stenotrophomonas sp. was the dominant species (Fig. 7a). However, it is interesting that the simple community (SCIII) assembled with the four species could also decrease root rot incidence and plant mortality (Fig. 7b, n = 12, P < 0.001, Tukey test). Furthermore, the numbers of diseased seedlings treated with SCI and SCIII were significantly lower than those in the untreated control and SCII-treated plants. No obvious differences in the number of diseased seedlings were found between the SCI- and SCIII-treated plants (Fig. 7b and Fig. s10). We also tested the control effects of the mixture of high-abundance bacteria and low-abundance bacteria at different proportions when the simple synthetic community was established. The results showed that reducing the amount of Advenella sp. and Ochrobactrum sp. did not affect the regulatory effects of simple synthetic communities on Astragalus root rot (Fig. 7c).
Fig. 7.
Simplification of synthetic bacterial community I and disease control effects. a SCIII was constructed by mixing Stenotrophomonas sp., Rhizobium sp., Advenella sp., and Ochrobactrum sp. that survived joint selection by the host plant and F. oxysporum. The bar graph shows the abundance of the four bacterial strains in roots. b Root rot disease incidence in plants inoculated with different bacterial communities for 15 days. SCI, synthetic community I; SCII, synthetic community II; SCIII, four-species simple community. c Colony radii of F. oxysporum treated with different bacteria after 6 days of growth (n = 3). d Root rot incidence of Astragalus inoculated with mixed bacteria at different ratios. Asterisks indicate significant differences (Tukey test, P < 0.01). 10:1, Stenotrophomonas:Rhizobium:Ochrobactrum:Advenella = 10:10:1:1; 100:1, Stenotrophomonas:Rhizobium:Ochrobactrum:Advenella = 100:100:1:1; 600:1, Stenotrophomonas:Rhizobium:Ochrobactrum:Advenella = 600:200:10:1
Fungal pathogen growth inhibition and plant disease control by the four bacteria
Stenotrophomonas sp. could directly inhibit the growth of fungal mycelia; however, Advenella sp., Rhizobium sp., and Ochrobactrum sp. had a minimal negative effect on F. oxysporum growth, which was consistent with interacting network analysis showing that Stenotrophomonas, Flavobacterium, and Pseudomonas abundance was negatively correlated with Fusarium growth (Figs. 5b and 7d). Although the colony radii of fungal pathogens treated with Advenella sp., Rhizobium sp., and Ochrobactrum sp. were smaller than those of the control fungi after 6 days of growth, no inhibitory effect was observed on Day 10 (Fig. s10). Interestingly, SCIII and Stenotrophomonas sp. alone had similar inhibitory efficiency against F. oxysporum, indicating that the inhibitory effect of SCIII on fungal growth did not increase with the addition of three bacterial strains (Advenella sp., Rhizobium sp., and Ochrobactrum sp.) in PDA agar plates. Therefore, we speculated that Stenotrophomonas sp. protected Astragalus by inhibiting F. oxysporum growth, while the other three strains prevented the fungal pathogens from invading Astragalus by different means, instead of inhibiting fungal mycelial growth in plants.
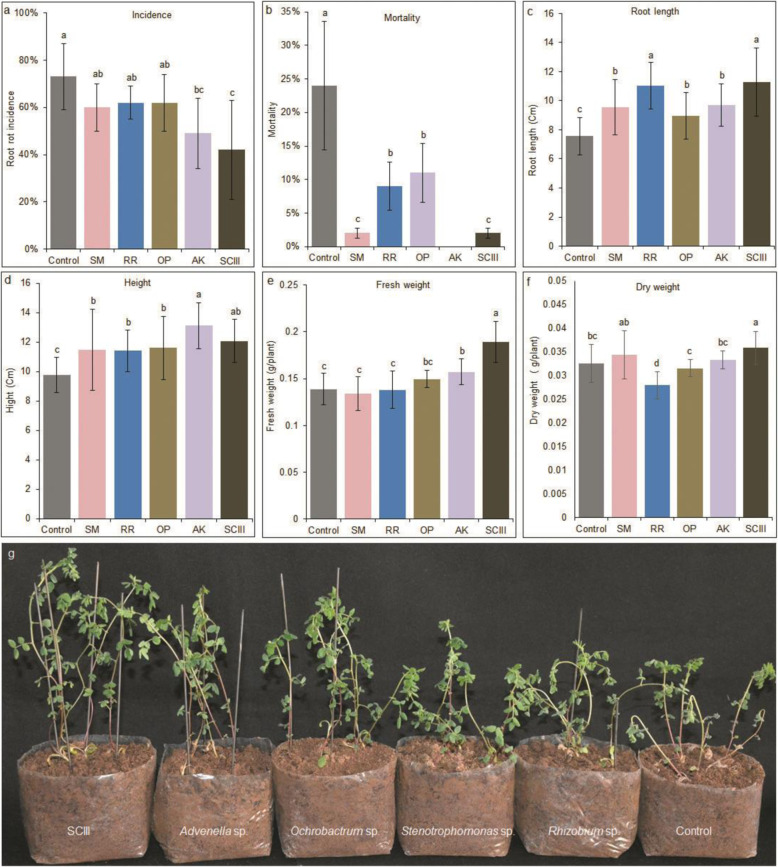
It seems that Stenotrophomonas sp. is the only bacterial species from SCIII that can inhibit the growth of F. oxysporum. We were curious to know if the presence of the three other bacterial species, Advenella sp., Rhizobium sp., and Ochrobactrum sp., plays a role in the control of root rot disease. Therefore, we examined the effects of root rot incidence in Astragalus by using single species in comparison to SCIII. Although all four species led to an obvious reduction in plant mortality, Stenotrophomonas sp., Rhizobium sp., and Ochrobactrum sp. did not significantly decrease the root rot disease incidence in host plants (Fig. 8a and b). Only Advenella sp., with the lowest abundance of all four species, significantly reduced plant disease incidence (~ 33.3%) and mortality (~ 100%). However, the effects of root rot incidence of SCIII with a decrease of 42.7% were superior to those of any other single bacteria, suggesting that the synthetic community worked better than single species in defending against pathogen infection. Moreover, all inoculation treatments could promote plant growth. Rhizobium sp., Advenella sp., and SCIII increased plant height and root length the most (Fig. 8c and d); in addition, SCIII could significantly improve plant fresh and dry weights (Fig. 8e and f).
Fig. 8.
Effect of SCIII and single bacterium inoculation on plant growth, root rot incidence, and mortality. a Root rot disease incidence and b mortality of plants inoculated with a single bacterium or SCIII after 10 days of inoculation (n = 45). Each plant was inoculated with 2 mL of bacterial suspension (~ 108 CFU/mL). After 5 days, each plant was inoculated with a 2-mL spore suspension of F. oxysporum (~ 105 spores/L). c–f Effects of different single-bacterium groups and SCIII on root length, height, fresh weight, and dry weight of Astragalus. SM, Stenotrophomonas sp.; RR, Rhizobium sp.; OP, Ochrobactrum sp.; AK, Advenella sp.; SCIII, four-species synthetic community. Letters indicate significant differences (n = 20, P < 0.05). Each plant was inoculated with 2-mL bacterial suspensions and grown for 15 days. g Phenotypes of Astragalus inoculated with single bacteria or SCIII after 15 days of growth in a greenhouse
Induction of systemic resistance against Astragalus by synthetic communities
We noticed that single species, SCI, and SCIII could significantly increase endogenous JA contents in plants. Although JA contents fluctuated in each treatment at different times, they remained much higher in the plants treated with either Advenella sp. or Ochrobactrum sp. than in the plants treated with Stenotrophomonas sp. or Rhizobium sp. SCIII induced more JA than any of the single species (Fig. s11 and Table s6).
We also examined the activities of PAL, PPO, and chitinase after inoculating the plants with synthetic communities or single species. All treatments gradually increased the activities of PAL, PPO, and chitinase and peaked at 168 h after inoculation. The activities of PAL, PPO, and chitinase in plants inoculated with Advenella sp. and Ochrobactrum sp. were higher than those of plants inoculated with Stenotrophomonas sp.. and Rhizobium sp. could also induce higher PAL activity only after 72 and 168 h and higher PPO activity than nontreated plants after 24 h. SCIII was found to increase the PAL, PPO, and chitinase activities when compared with the nontreated control by 31.9%, 40.8%, and 21.4%, respectively (Fig. s11b-s11d and Table s6).
SCI, SCIII, and single species can also rapidly increase POD and LOX enzyme activities after 8 h post inoculation, followed by a decrease. At 168 h post inoculation, the activities of POD and LOX reached their maximum. Advenella sp. and SCIII could significantly increase the activities of POD and LOX in the plants when compared to other single species and nontreated controls. At 168 h post inoculation, the activities of POD in Advenella sp. and SCIII-treated plants increased by 22.5% and 29.4%, respectively, and the activities of LOX increased by 46.1% and 39.7%, respectively (Fig. s11e–s11f, Table s6).
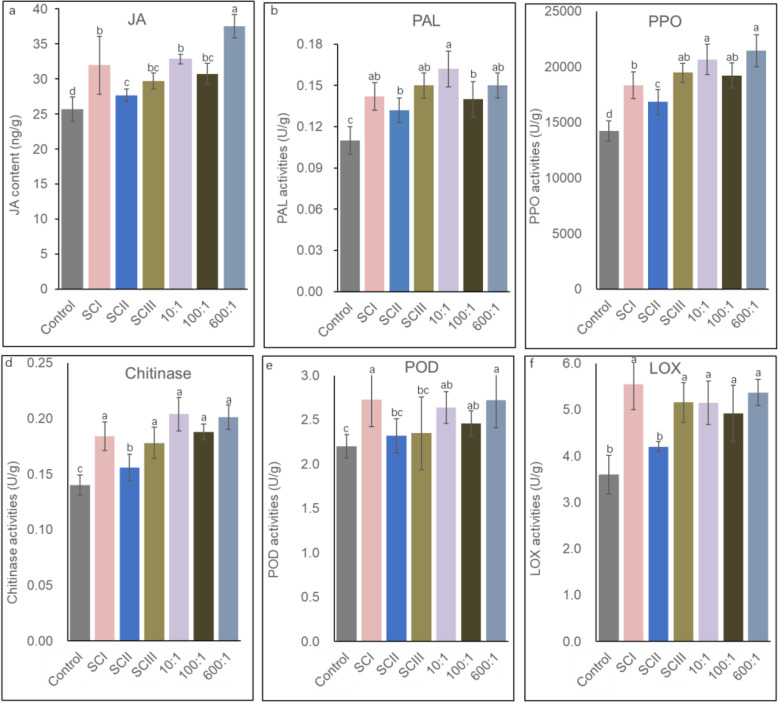
We then compared the activation of plant ISR by SCI, SCII, SCIII, SCIII-10, SCIII-100, and SCIII-600 (high- and low-abundance bacteria mixed at ratios of 10:1, 100:1, and 600:1). Five days post inoculation, no significant differences in POD, PPO, PAL, LOX, and chitinase activities were found among the plants treated with SCI, SCIII, and SCIII with different mixing ratios. Although all the enzyme activities of SCII-treated plants were higher than those of the nontreated control, they were obviously lower than those in other synthetic community-treated plants (Fig. 9).
Fig. 9.
Effects of different synthetic communities on the ISR of Astragalus. JA contents and PAL, POD, PPO, LOX, and chitinase activities of plants on the 5th day after inoculation with sterile water, SCI, SCII, SCIII, and different mixing ratios of four bacterial species. The letters above the bars denote differences based on Duncan’s multiple range test (n = 3, P < 0.05). JA, jasmonic acid; PAL, phenylalanine ammonia lyase; PPO, polyphenol oxidase; POD, peroxidase; LOX, lipoxygenase; ISR, induced systemic resistance; 10:1, Stenotrophomonas:Rhizobium:Ochrobactrum:Advenella = 10:10:1:1; 100:1, Stenotrophomonas:Rhizobium:Ochrobactrum:Advenella = 100:100:1:1; 600:1, Stenotrophomonas:Rhizobium:Ochrobactrum:Advenella = 600:200:10:1
Discussion
Recruitment of beneficial bacteria by host plants under fungal pathogen infection
Environmental conditions such as drought, fertility, pH, pathogenic fungi, and nematodes can alter the metabolic pathways of plants and induce the production of specific root exudates, which selectively recruit microorganisms from the surrounding soil to colonize the rhizosphere or roots [48, 49]. The resulting complex microbial communities could enhance the host plant’s resistance or tolerance to adverse environments. The composition and assembly of the microbial community in the rhizosphere of wheat, maize, paddy, sorghum, and other plants under biotic and abiotic stress have been extensively studied [50–52]. However, it remains unclear which members of the microbial communities directly influence plant performance. A potential approach for determining the key microbes influencing plant performance under such conditions is to simulate natural conditions, establish synthetic microbiota, and analyze their correlation with host plant growth, metabolism, and stress tolerance.
In the present study, we found that bacterial community alpha diversity in roots was significantly lower than that in the rhizosphere and bulk soil regardless of the presence or absence of F. oxysporum, which is consistent with the findings in a previous study showing that plant root systems had a strong filtration effect on soil microorganisms [53]. Pathogenic fungal infection significantly decreased the Shannon diversity of root endophytic bacterial communities. This could be due to the high enrichment of bacteria associated with disease defense in the roots. Compared with the bacterial communities in healthy plants, Pseudomonas, Stenotrophomonas, Arthrobacter, and Flavobacterium were highly enriched in the roots of diseased Astragalus plants. Pseudomonas and Stenotrophomonas were the most enriched species, with their abundance increasing from 3.33% and 2.97% in healthy roots to 19.17% and 13.19% in diseased roots, respectively. The top 10 enriched bacterial genera in diseased roots accounted for ~ 59.6% of the root endophytic bacterial communities. Many strains in these genera have been reported to promote plant growth and improve plant resistance to disease [54, 55]. The functional profiles of the root-associated bacterial communities showed different patterns among the samples. We found that some functions of root-associated communities were related to the pathogen resistance of the plant, including “biofilm formation”, “streptomycin biosynthesis”, “polyketide biosynthesis”, and “lipopolysaccharide biosynthesis”. Biofilm can help plants absorb nutrients and act as biocontrol agents to fight against diseases. Downy mildew infection in Arabidopsis leads to the formation of a three-species bacterial consortium that interacts synergistically in biofilm formation and decreases disease incidence [16]. Polyketide and streptomycin are commonly used antibiotics for inhibiting the growth of bacteria and fungi, and the expression of polyketide cyclase genes is significantly increased under pathogen stress [56]. Lipopolysaccharides produced by gram-negative bacteria can act as elicitors that can induce defense responses in plants [57]. The results indicate that F. oxysporum infection could alter the assembly of root-associated microbial communities and recruit specific microorganisms that control root rot disease in Astragalus. In our study, most of the bacteria were isolated from both diseased roots and healthy roots, indicating that the communities assembled on the roots of healthy plants also have the potential to control the disease. However, plants are exposed to stress from various biotic and abiotic factors simultaneously when they grow in natural conditions. In the present study, microbes with different ecological functions were enriched in the rhizospheres and roots. Even under pathogen inoculation, the microorganisms enriched in plants were not all associated with disease resistance. The combination of different bacteria and determination of the compatible and ecologically stable communities are laborious and time-consuming tasks. By analyzing the microbial community composition of diseased plants, we can select microbial strains for establishing appropriate combinations, which could improve the efficiency of assembling disease-resistant microbial communities.
Construction and simplification of synthetic bacterial communities
Microbial diversity in soil is much greater than that observed from 16S high-throughput sequencing. It remains unclear how many types of microbes can be recruited by plants from the soil to perform specific functions. In early studies, the number of bacteria in synthetic communities can range from several to dozens [21, 58]. It is also not clear whether some or all the recruited bacteria are involved in plant disease resistance. Therefore, the construction of synthetic bacterial communities could help answer the questions posed above.
In the present study, we found that an artificial synthetic bacterial community (SCI) composed of 13 out of 39 bacterial genera significantly enriched in diseased Astragalus roots could considerably alleviate plant disease. Further investigations revealed that the control effect of the synthetic community on plant root rot remained with only four bacterial species from SCI, suggesting that not all the enriched bacteria participated in the resistance to fungal pathogens. In our study, we found that Stenotrophomonas sp., Rhizobium sp., Advenella sp., and Ochrobactrum sp. were the key species in the control of plant root rot disease. The relative abundance ratio of Stenotrophomonas sp., Rhizobium sp., Ochrobactrum sp., and Advenella sp. was approximately 600:200:10:1. We also tested different ratios of the four species on root rot and found that this four-species community was effective in preventing root rot despite variable proportions. This result indicated that some bacteria could also play a role at low concentrations. To attract associated microbes in the soil, plants need to excrete 5–21% of photosynthetic products via their roots [59, 60]. In addition, the more microorganisms are enriched in the rhizosphere or roots, the greater resources are required to maintain the growth and reproduction of the microorganisms; thus, it is resource intensive for plants to host numerous microbes. In addition, although the colonization of the roots or the rhizosphere by microbial communities is an essential step for the control of soil-borne disease [61], the presence of numerous strains in a community could increase the competition among them. Therefore, plants tend to enrich a few microorganisms to perform specific functions. For example, Arabidopsis with downy mildew recruits Microbacterium sp., Stenotrophomonas sp., and Xanthomonas sp. in the rhizosphere [16], and Niu et al. observed that a seven-strain bacterial community delayed the growth of Fusarium verticillioides [58]. Therefore, the optimal strain combination rather than more strains should be emphasized when constructing a synthetic community.
Several species were obtained within a genus in the process of isolating the plant-recruited bacteria through culturable methods; consequently, it is essential to select the appropriate bacterial species for synthetic community assembly. Considering that beneficial microorganisms can improve plant disease resistance through growth promotion and antagonism, we selected bacteria with such characteristics for use in the assembly of bacterial communities. SCI was remarkably effective in the control of root rot disease. However, SCII did not alleviate root rot disease in Astragalus even though it contains Bacillus velezensis and Paenibacillus terrae, species that can inhibit the growth of pathogenic fungi by producing bioactive substances [62, 63]. Although SCI could decrease the incidence of Astragalus root rot disease, we found that the synthetic community containing four species had the same effect on root rot disease control. Thus, whether the other bacterial species from SCI could participate in the alleviation of root rot disease needs to be verified. Today, numerous biocontrol agents are prepared on the premise that the candidate strains are antagonistic against pathogens in the laboratory; however, such effects may not be observed in the field. Therefore, the study of the assembly of synthetic bacterial communities based on bacteria recruited by the plant facilitates the stress resistance of the host plant [16, 64].
Low- and high-abundance bacteria in synthetic communities jointly enhance plant disease resistance
Plants recruit diverse microbes to control plant disease via antibiotic production, competition for nutrients, plant growth promotion, or systemic resistance induction [65–68]. Some studies have reported that a decrease in rhizosphere microbial community diversity and the abundance of some species made the plants more susceptible to pathogen infection [69, 70]. However, the influence of the relative abundances of the components in synthetic communities cannot be excluded [21, 57]. The existence of low-abundance microorganisms also helps to accommodate more types of microbes with limited root exudates, which may be beneficial for plant growth, stress tolerance, and overall plant health [71]. In addition to their roles in inhibiting fungal pathogens and promoting plant growth, bacterial communities can also activate the immunity of plants to prevent pathogen infections. Therefore, we have also studied the effects of different synthetic communities on the ISR of Astragalus. Except for SCII, SCI and SCIII can significantly activate the ISR of plants and reducing the abundance of Ochrobactrum sp. and Advenella sp. did not weaken the control effect of SCIII. Although the low abundance of Ochrobactrum sp. and Advenella sp. could not directly inhibit F. oxysporum growth, it conferred a greater activation effect on the JA and ISR-related enzyme activities in plants than Stenotrophomonas sp., suggesting that these two low-abundance bacteria may induce plant resistance via the JA signaling pathway. Plants lacking the JA synthesis metabolic pathway are more susceptible to pathogen infection, while the incidence rates of infection can be significantly reduced by increasing JA accumulation [72].
It has been reported that some beneficial rhizosphere bacteria can activate the JA synthesis pathway during interactions with plants [73]. JA-induced pathways also produce chitinases and oxidative enzymes (for example, PAL, POD, PPO, and LOX). Increased PAL and POD activities could enhance the production of phenolics and lignification to prevent pathogen invasion [74–76].
Overall, our findings suggest that high-abundance bacteria could protect hosts by promoting plant growth and inhibiting pathogenic fungal growth, while low-abundance bacteria control diseases by enhancing plant ISR. Moreover, the combination of both high- and low-abundance bacterial species induced greater systemic resistance than any single species. Our findings could provide a reference to facilitate the assembly of functional synthetic communities and highlight a potential solution that could be applied to achieve sustainable agriculture.
Conclusions
Using next-generation sequencing methods, we showed that the composition of the root endophytic bacterial community of diseased plants was distinctly different from that of healthy plants. Particularly, under F. oxysporum challenge, the plant roots recruit some beneficial microbes, such as Pseudomonas, Strenotrophomonas, Chryseobacterium, Achromobacter, and Flavobacterium. The assembly of three low-abundance and 10 high-abundance bacteria enriched in diseased roots can effectively control Astragalus root rot disease as a synthetic community. A simplified synthetic community based on the above community but containing only four bacteria maintained the original effect. Further investigation showed that the high-abundance bacteria were responsible for inhibiting the growth of fungal pathogens, while the low-abundance bacteria were mainly associated with activating plant ISR to alleviate Astragalus root rot disease. The findings suggest that bacteria with different ecological functions can be assembled as synthetic bacterial communities for soil-borne disease control. Such functional synthetic communities can be used as a possible solution for sustainable agriculture.
Supplementary Information
Additional file 1: Supplementary materials. Fig. S1. Schematic diagram of root and soil sample collection. Fig. S2. The distinct and shared bacterial genera in different sample compartments. Fig. S3. Effect of fungal pathogen on Shannon diversity of bacterial communities in bulk soil, rhizosphere and root. Fig. S4. Enrichment of bacterial phyla by Astragalus roots. Fig. S5. Effect of F. oxysporum infection on the abundance of major bacterial phyla. Fig. S6. Least discriminant analysis (LDA) effect size taxonomic cladogram comparing healthy and diseased rhizosphere samples. Fig. S7. Plant growth-promoting properties of plant-associated bacteria. Fig. S8. Relative abundance of bacteria in SCI found in healthy and diseased roots. Fig. S9. Relative abundance of bacteria in SCII found in healthy and diseased roots. Fig. S10. Inhibition of four bacteria in simple synthetic community (SCIII) on the growth of F. oxysporum. Fig. S11. Effect of SCIII and single bacterium inoculation on the ISR of Astragalus.
Additional file 2: Table S1. Characteristics of bacteria in two synthetic communities.
Additional file 3: Table S2. Diversity index of bacterial community in different compartment of A. mongholicus.
Additional file 4: Table S3. Root and rhizosphere community KEGG pathways.
Additional file 5: Table S4. Abundance of bacterial genera increased in diseased roots.
Additional file 6: Table S5. Abundance of bacterial genera decreased in diseased roots.
Additional file 7: Table S6. Effects of different inoculation and treatment time on Astragalus JA content and ISR-related enzyme activities.
Acknowledgments
We thank Liru Jian from Northwest A & F University for determining the IAA production capacity of bacterial isolations.
Abbreviations
- JA
Jasmonic acid
- POD
Peroxidase
- PPO
Polyphenol oxidase
- PAL
Phenylalanine ammonia lyase
- LOX
Lipoxygenase
- IAA
Indole-3-acetic acid
- PDA
Potato dextrose agar
- ISR
Induced systemic resistance
- Ir
Inhibition ratio
- SCI
Synthetic community I
- SCII
Synthetic community II
- SCIII
Four-species community
Authors’ contributions
ZL wrote and edited the text; XB conducted the experiments and prepared the figures; SJ reviewed and edited the final text; YL, YY, and HZ conducted the experiments; ZL and GW did the conception and design of the study. The authors read and approved the final version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (41830755), Major Science and Technology Special Project of Shaanxi Province (2020zdzx03-02-01), and the Natural Science Basic Research Plan of Shaanxi Province (2018JM3004). The funders played no role in the design of the study, analysis, and interpretation of data or in writing the manuscript.
Availability of data and materials
Raw data used in this study are available in the NCBI Sequence Read Archive (SRA), accession numbers PRJNA762455 and PRJNA764034.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhefei Li and Xiaoli Bai contributed equally to this work.
Contributor Information
Zhefei Li, Email: lizhefei@hotmail.com.
Gehong Wei, Email: weigehong@nwafu.edu.cn.
References
- 1.Batten KM, Scow KM, Espeland EK. Soil microbial community associated with an invasive grass differentially impacts native plant performance. Microb Ecol. 2008;55(2):220–228. doi: 10.1007/s00248-007-9269-3. [DOI] [PubMed] [Google Scholar]
- 2.Carrion VJ, Perez-Jaramillo J, Cordovez V, Tracanna V, de Hollander M, Ruiz-Buck D, et al. Pathogen-induced activation of disease-suppressive functions in the endophytic root microbiome. Science. 2019;366(6465):606–612. doi: 10.1126/science.aaw9285. [DOI] [PubMed] [Google Scholar]
- 3.Hubbard CJ, Li BH, McMinn R, Brock MT, Maignien L, Ewers BE, Kliebenstein D, Weinig C. The effect of rhizosphere microbes outweighs host plant genetics in reducing insect herbivory. Mol Ecol. 2019;28(7):1801–1811. doi: 10.1111/mec.14989. [DOI] [PubMed] [Google Scholar]
- 4.Hussain M, Hamid MI, Tian JQ, Hu JY, Zhang XL, Chen JS, et al. Bacterial community assemblages in the rhizosphere soil, root endosphere and cyst of soybean cyst nematode-suppressive soil challenged with nematodes. FEMS Microbiol Ecol. 2018;94(10). 10.1093/femsec/fiy142. [DOI] [PubMed]
- 5.Towe S, Albert A, Kleineidam K, Brankatschk R, Dumig A, Welzl G, et al. Abundance of microbes involved in nitrogen transformation in the rhizosphere of Leucanthemopsis alpina (L.) Heywood grown in soils from different sites of the Damma glacier forefield. Microb Ecol. 2010;60:762–770. doi: 10.1007/s00248-010-9695-5. [DOI] [PubMed] [Google Scholar]
- 6.Zubek S, Majewska ML, Błaszkowski J, Stefanowicz AM, Nobis M, Kapusta P. Invasive plants affect arbuscular mycorrhizal fungi abundance and species richness as well as the performance of native plants grown in invaded soils. Biol Fertil Soils. 2016;52(6):879–893. doi: 10.1007/s00374-016-1127-3. [DOI] [Google Scholar]
- 7.Zhou X, Wang Z, Jia H, Li L, Wu F. Continuously monocropped Jerusalem artichoke changed soil bacterial community composition and ammonia-oxidizing and denitrifying bacteria abundances. Front Microb. 2018;9:705. doi: 10.3389/fmicb.2018.00705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zuppinger-Dingley D, Schmid B, Petermann JS, Yadav V, De Deyn GB, Dan FBF. Selection for niche differentiation in plant communities increases biodiversity effects. Nature. 2014;515(7525):108–111. doi: 10.1038/nature13869. [DOI] [PubMed] [Google Scholar]
- 9.Zhang X, Zhang Q, Liang B, Li J. Changes in the abundance and structure of bacterial communities under long-term fertilization treatments in a peanut monocropping system. Appl Soil Ecol. 2017;121:82–89. doi: 10.1016/j.apsoil.2017.08.016. [DOI] [Google Scholar]
- 10.van der Putten WH, Bardgett RD, Bever JD, Martijn Bezemer T, Casper BB, et al. Plant-soil feedbacks: the past, the present and future challenges. J Ecol. 2013;101(2):265–276. doi: 10.1111/1365-2745.12054. [DOI] [Google Scholar]
- 11.Siegel-Hertz K, Edel-Hermann V, Chapelle E, Terrat S, Raaijmakers JM, Steinberg C. Comparative microbiome analysis of a Fusarium wilt suppressive soil and a Fusarium wilt conducive soil from the Chateaurenard Region. Front Microbiol. 2018;9:568. doi: 10.3389/fmicb.2018.00568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hagn A, Engel M, Kleikamp B, Munch JC, Schloter M, Bruns C. Microbial community shifts in Pythium ultimum-inoculated suppressive substrates. Biol Fertil Soils. 2008;44(3):481–490. doi: 10.1007/s00374-007-0230-x. [DOI] [Google Scholar]
- 13.Postma J, Scheper RWA, Schilder MT. Effect of successive cauliflower plantings and Rhizoctonia solani AG 2-1 inoculations on disease suppressiveness of a suppressive and a conducive soil. Soil Biol Biochem. 2010;42:804–812. doi: 10.1016/j.soilbio.2010.01.017. [DOI] [Google Scholar]
- 14.Mendes R, Kruijt M, de Bruijn I, Dekkers E, van der Voort M, Schneider JHM, Piceno YM, DeSantis TZ, Andersen GL, Bakker PAHM, Raaijmakers JM. Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science. 2011;332(6033):1097–1100. doi: 10.1126/science.1203980. [DOI] [PubMed] [Google Scholar]
- 15.van der Voort M, Kempenaar M, van Driel M, Raaijmakers JM, Mendes R. Impact of soil heat on reassembly of bacterial communities in the rhizosphere microbiome and plant disease suppression. Ecol Lett. 2016;19(4):375–382. doi: 10.1111/ele.12567. [DOI] [PubMed] [Google Scholar]
- 16.Berendsen RL, Vismans G, Yu K, Song Y, de Jonge R, Burgman WP, Burmølle M, Herschend J, Bakker PAHM, Pieterse CMJ. Disease-induced assemblage of a plant-beneficial bacterial consortium. ISME J. 2018;12(6):1496–1507. doi: 10.1038/s41396-018-0093-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moya P, Barrera V, Cipollone J, Bedoya C, Kohan L, Toledo A, et al. New isolates of Trichoderma spp. as biocontrol and plant growth–promoting agents in the pathosystem Pyrenophora teres-barley in Argentina. Biol Control. 2020. 10.1016/j.biocontrol.2019.104152.
- 18.Arfaoui A, Adam LR, Bezzahou A, Daayf F. Isolation and identification of cultivated bacteria associated with soybeans and their biocontrol activity against Phytophthora sojae. Biocontrol. 2018;63(4):607–617. doi: 10.1007/s10526-018-9873-9. [DOI] [Google Scholar]
- 19.Ritpitakphong U, Falquet L, Vimoltust A, Berger A, Metraux J, Lharidon F. The microbiome of the leaf surface of Arabidopsis protects against a fungal pathogen. New Phytol. 2016;210(3):1033–1043. doi: 10.1111/nph.13808. [DOI] [PubMed] [Google Scholar]
- 20.Jack AL, Nelson EB. A seed-recruited microbiome protects developing seedlings from disease by altering homing responses of Pythium aphanidermatum zoospores. Plant Soil. 2018;422(1-2):209–222. doi: 10.1007/s11104-017-3257-2. [DOI] [Google Scholar]
- 21.Lebeis SL, Paredes SH, Lundberg DS, Breakfield N, Gehring J, McDonald M, Malfatti S, Glavina del Rio T, Jones CD, Tringe SG, Dangl JL. Salicylic acid modulates colonization of the root microbiome by specific bacterial taxa. Science. 2015;349(6250):860–864. doi: 10.1126/science.aaa8764. [DOI] [PubMed] [Google Scholar]
- 22.Dawson W, Hor J, Egert M, van Kleunen M, Pester M. A small number of low-abundance bacteria dominate plant species-specific responses during rhizosphere colonization. Front Microbiol. 2017;8:975. doi: 10.3389/fmicb.2017.00975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veach AM, Morris R, Yip DZ, Yang ZK, Engle NL, Cregger MA, Tschaplinski TJ, Schadt CW. Rhizosphere microbiomes diverge among Populus trichocarpa plant-host genotypes and chemotypes, but it depends on soil origin. Microbiome. 2019;7(1):76. doi: 10.1186/s40168-019-0668-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu L, Naylor D, Dong ZB, Simmons T, Pierroz G, Hixson KK, Kim YM, Zink EM, Engbrecht KM, Wang Y, Gao C, DeGraaf S, Madera MA, Sievert JA, Hollingsworth J, Birdseye D, Scheller HV, Hutmacher R, Dahlberg J, Jansson C, Taylor JW, Lemaux PG, Coleman-Derr D. Drought delays development of the sorghum root microbiome and enriches for monoderm bacteria. P Natl Acad Sci USA. 2018;115(18):E4284–E4293. doi: 10.1073/pnas.1717308115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei Z, Hu J, Gu Y, Yin SX, Xu YC, Jousset A, et al. Ralstonia solanacearum pathogen disrupts bacterial rhizosphere microbiome during an invasion. Soil Biol Biochem. 2018;118:8–17. doi: 10.1016/j.soilbio.2017.11.012. [DOI] [Google Scholar]
- 26.Worner S, Zecchin S, Dan JG, Todorova NH, Loy A, Conrad R, et al. Gypsum amendment to rice paddy soil stimulated bacteria involved in sulfur cycling but largely preserved the phylogenetic composition of the total bacterial community. Env Microbiol Rep. 2016;8(3):413–423. doi: 10.1111/1758-2229.12413. [DOI] [PubMed] [Google Scholar]
- 27.Quero GM, Luna GM. Diversity of rare and abundant bacteria in surface waters of the Southern Adriatic Sea. Mar Genom. 2014;17:9–15. doi: 10.1016/j.margen.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Hugoni M, Taib N, Debroas D, Domaizon I, Dufournel IJ, Bronner G, et al. Structure of the rare archaeal biosphere and seasonal dynamics of active ecotypes in surface coastal waters. P Natl Acad Sci USA. 2013;110(15):6004–6009. doi: 10.1073/pnas.1216863110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurm V, van der Putten WH, de Boer W, Naus-Wiezer S, Hol WHG. Low abundant soil bacteria can be metabolically versatile and fast growing. Ecology. 2017;98(2):555–564. doi: 10.1002/ecy.1670. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Dong SK, Gao QZ, Ganjurjav H, Wang XX, Geng W. “Rare biosphere” plays important roles in regulating soil available nitrogen and plant biomass in alpine grassland ecosystems under climate changes. Agr Ecosyst Environ. 2019;279:187–193. doi: 10.1016/j.agee.2018.11.025. [DOI] [Google Scholar]
- 31.Hausmann B, Knorr KH, Schreck K, Tringe SG, del Rio TG, Loy A, et al. Consortia of low-abundance bacteria drive sulfate reduction-dependent degradation of fermentation products in peat soil microcosms. ISME J. 2016;10(10):2365–2375. doi: 10.1038/ismej.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pascoal F, Magalhaes C, Costa R. The link between the ecology of the prokaryotic rare biosphere and its biotechnological potential. Front Microbiol. 2020;11:231. doi: 10.3389/fmicb.2020.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang M, Li Z, Liu L, Bo A, Zhang C, Li M. Ecological niche modeling of Astragalus membranaceus var. mongholicus medicinal plants in Inner Mongolia, China. Sci Rep, 2020. 10:12482. [DOI] [PMC free article] [PubMed]
- 34.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7(5):335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27(16):2194–2200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beckers B, Op De Beeck M, Weyens N, Boerjan W, Vangronsveld J. Structural variability and niche differentiation in the rhizosphere and endosphere bacterial microbiome of field-grown poplar trees. Microbiome. 2017;5(1):25. doi: 10.1186/s40168-017-0241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods. 2013;10(10):996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- 38.Schuck S, Weinhold A, Luu VT, Baldwin IT. Isolating fungal pathogens from a dynamic disease outbreak in a native plant population to establish plant-pathogen bioassays for the ecological model plant Nicotiana attenuata. PLoS ONE. 2014;9(7):e102915. doi: 10.1371/journal.pone.0102915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haiyambo DH, Chimwamurombe PM, Reinhold-Hurek B. Isolation and screening of rhizosphere bacteria from grasses in east kavango region of Namibia for plant growth promoting characteristics. Curr Microbiol. 2015;71(5):566–571. doi: 10.1007/s00284-015-0886-7. [DOI] [PubMed] [Google Scholar]
- 40.Pootakham W, Mhuantong W, Yoocha T, Putchim L, Sonthirod C, Naktang C, Thongtham N, Tangphatsornruang S. High resolution profiling of coral-associated bacterial communities using full-length 16S rRNA sequence data from PacBio SMRT sequencing system. Sci Rep. 2017;7(1):2774. doi: 10.1038/s41598-017-03139-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ginestet C. ggplot2: Elegant graphics for data analysis. J R Stat Soc a Stat. 2011;174(1):245–246. doi: 10.1111/j.1467-985X.2010.00676_9.x. [DOI] [Google Scholar]
- 42.Dixon P. VEGAN, a package of R functions for community ecology. J Veg Sci. 2003;14(6):927–930. doi: 10.1111/j.1654-1103.2003.tb02228.x. [DOI] [Google Scholar]
- 43.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26(1):139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Langille MGI, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, Clemente JC, Burkepile DE, Vega Thurber RL, Knight R, Beiko RG, Huttenhower C. Predictive functional profling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol. 2013;31(9):814–821. doi: 10.1038/nbt.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Vries FT, Williams A, Stringer F, Willcocks R, McEwing R, Langridge H, et al. Changes in root-exudate-induced respiration reveal a novel mechanism through which drought affects ecosystem carbon cycling. New Phytol. 2019;224(1):132–145. doi: 10.1111/nph.16001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bodah ET. Root rot diseases in plants: a review of common causal agents and management strategies. Agri Res & Tech: Open Access J. 2017;5(3):555661. [Google Scholar]
- 47.Wicker E, Grassart L, Coranson-Beaudu R, Mian D, Guilbaud C, Fegan M, Prior P. Ralstonia solanacearum strains from Martinique (French West Indies) exhibiting a new pathogenic potential. Appl Environ Microbiol. 2007;73(21):6790–6801. doi: 10.1128/AEM.00841-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Farh ME, Kim Y, Kim YJ, Yang D. Cylindrocarpon destructans/Ilyonectria radicicola-species complex: causative agent of ginseng root-rot disease and rusty symptoms. J Ginseng Res. 2018;42(1):9–15. doi: 10.1016/j.jgr.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yuan J, Zhao J, Wen T, Zhao ML, Li R, Goossens P, Huang Q, Bai Y, Vivanco JM, Kowalchuk GA, Berendsen RL, Shen Q. Root exudates drive the soil-borne legacy of aboveground pathogen infection. Microbiome. 2018;6(1):156. doi: 10.1186/s40168-018-0537-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walters WA, Jin Z, Youngblut N, Wallace JG, Sutter J, Zhang W, González-Peña A, Peiffer J, Koren O, Shi Q, Knight R, Glavina del Rio T, Tringe SG, Buckler ES, Dangl JL, Ley RE. Large-scale replicated field study of maize rhizosphere identifies heritable microbes. P Natl Acad Sci USA. 2018;115(28):7368–7373. doi: 10.1073/pnas.1800918115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Edwards J, Johnson C, Santos-Medellin C, Lurie E, Podishetty NK, Bhatnagar S, et al. Structure, variation, and assembly of the root-associated microbiomes of rice. P Natl Acad Sci USA. 2015;112(8):E911–E920. doi: 10.1073/pnas.1414592112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schlemper TR, van Veen JA, Kuramae EE. Co-variation of bacterial and fungal communities in different sorghum cultivars and growth stages is soil dependent. Microb Ecol. 2018;76(1):205–214. doi: 10.1007/s00248-017-1108-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dibbern D, Schmalwasser A, Lueders T, Totsche KU. Selective transport of plant root-associated bacterial populations in agricultural soils upon snowmelt. Soil Biol Biochem. 2014;69:187–196. doi: 10.1016/j.soilbio.2013.10.040. [DOI] [Google Scholar]
- 54.Yasmin S, Hafeez FY, Rasul G. Evaluation of Pseudomonas aeruginosa Z5 for biocontrol of cotton seedling disease caused by Fusarium oxysporum. Biocontrol Sci and Techn. 2014;24(11):1227–1242. doi: 10.1080/09583157.2014.932754. [DOI] [Google Scholar]
- 55.Rivasgarcia T, Murilloamador B, Nietogaribay A, Rinconenriquez G, Chiquitocontreras RG, Hernandezmontiel LG. Enhanced biocontrol of fruit rot on muskmelon by combination treatment with marine Debaryomyces hansenii and Stenotrophomonas rhizophila and their potential modes of action. Postharvest Biol Tec. 2019;151:61–67. doi: 10.1016/j.postharvbio.2019.01.013. [DOI] [Google Scholar]
- 56.Hayden HL, Savin KW, Wadeson J, Gupta VVSR, Mele PM. Comparative metatranscriptomics of wheat rhizosphere microbiomes in disease suppressive and non-suppressive soils for Rhizoctonia solani AG8. Front. Microbiol. 2018;9:859. doi: 10.3389/fmicb.2018.00859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Erbs G, Molinaro A, Dow J, Newman MA. Lipopolysaccharides and plant innate immunity. In: Wang X, Quinn P, editors. Endotoxins: Structure, Function and Recognition. Subcellular Biochemistry. Dordrecht: Springer; 2010. [Google Scholar]
- 58.Niu B, Paulson JN, Zheng X, Kolter R. Simplified and representative bacterial community of maize roots. P Natl Acad Sci USA. 2017;114(12):E2450–E2459. doi: 10.1073/pnas.1616148114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Voges MJ, Bai Y, Schulzelefert P, Sattely ES. Plant-derived coumarins shape the composition of an Arabidopsis synthetic root microbiome. P Natl Acad Sci USA. 2019;116(25):12558–12565. doi: 10.1073/pnas.1820691116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Massalha H, Korenblum E, Tholl D, Aharoni A. Small molecules below-ground: the role of specialized metabolites in the rhizosphere. Plant J. 2017;90(4):788–807. doi: 10.1111/tpj.13543. [DOI] [PubMed] [Google Scholar]
- 61.Maurer KA, Zachow C, Seefelder S, Berg G. Initial steps towards biocontrol in hops: successful colonization and plant growth promotion by four bacterial biocontrol agents. Agronomy. 2013;3(4):583–594. doi: 10.3390/agronomy3040583. [DOI] [Google Scholar]
- 62.Rabbee MF, Ali MS, Choi J, Hwang BS, Jeong SC, Baek KH. Bacillus velezensis: a valuable member of bioactive molecules within plant microbiomes. Molecules. 2019;24(6):1046. doi: 10.3390/molecules24061046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu WQ, Zheng GP, Qiu DW, Yan FC, Liu WZ, Liu WX. Paenibacillus terrae NK3-4: a potential biocontrol agent that produces β-1,3-glucanase. Biol Control. 2019;129:92–101. doi: 10.1016/j.biocontrol.2018.09.019. [DOI] [Google Scholar]
- 64.Rudrappa T, Czymmek KJ, Pare PW, Bais HP. Root-secreted malic acid recruits beneficial soil bacteria. Plant Physiol. 2008;148(3):1547–1556. doi: 10.1104/pp.108.127613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jangir M, Pathak R, Sharma S, Sharma S. Biocontrol mechanisms of Bacillus sp., isolated from tomato rhizosphere, against Fusarium oxysporum f. sp lycopersici. Biol Control. 2018;123:60–70. doi: 10.1016/j.biocontrol.2018.04.018. [DOI] [Google Scholar]
- 66.Fira D, Dimkic I, Beric T, Lozo J, Stankovic S. Biological control of plant pathogens by Bacillus species. J Biotechnol. 2018;285:44–55. doi: 10.1016/j.jbiotec.2018.07.044. [DOI] [PubMed] [Google Scholar]
- 67.Vinale F, Sivasithamparam K, Ghisalberti EL, Marra R, Woo SL, Lorito M. Trichoderma-plant-pathogen interactions. Soil Biol Biochem. 2008;40(1):1–10. doi: 10.1016/j.soilbio.2007.07.002. [DOI] [Google Scholar]
- 68.Pieterse CMJ, Zamioudis C, Berendsen RL, Weller DM, Van Wees SCM, Bakker PAHM. Induced systemic resistance by beneficial microbes. Annu Rev Phytopathol. 2014;52(1):347–375. doi: 10.1146/annurev-phyto-082712-102340. [DOI] [PubMed] [Google Scholar]
- 69.Hamid MI, Wu YP, Zhang XL, Xiang MC, Liu XZ. Successive soybean-monoculture cropping assembles rhizosphere microbial communities for the soil suppression of soybean cyst nematode. Fems Microbiol Ecol. 2017;93(1). 10.1093/femsec/fiw222. [DOI] [PubMed]
- 70.van Elsas JD, Chiurazzi M, Mallon CA, Elhottova D, Kristufek V, Salles JF. Microbial diversity determines the invasion of soil by a bacterial pathogen. P Natl Acad Sci USA. 2012;109(4):1159–1164. doi: 10.1073/pnas.1109326109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saravanakumar K, Dou K, Lu ZX, Wang XH, Li YQ, Chen J. Enhanced biocontrol activity of cellulase from Trichoderma harzianum against Fusarium graminearum through activation of defense-related genes in maize. Physiol Mol Plant P. 2018;103:130–136. doi: 10.1016/j.pmpp.2018.05.004. [DOI] [Google Scholar]
- 72.Zhang H, Zhang Q, Zhai H, Gao SP, Yang L, Wang Z, Xu Y, Huo J, Ren Z, Zhao N, Wang X, Li J, Liu Q, He S. IbBBX24 promotes the jasmonic acid pathway and enhances Fusarium wilt resistance in sweet potato. Plant Cell. 2020;32(4):1102–1123. doi: 10.1105/tpc.19.00641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gimenez-Ibanez S, Chini A, Solano R. How microbes twist jasmonate signaling around their little fingers. Plants-Basel. 2016;5(1):9. doi: 10.3390/plants5010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mariutto M, Duby F, Adam A, Bureau C, Fauconnier ML, Ongena M, Thonart P, Dommes J. The elicitation of a systemic resistance by Pseudomonas putida BTP1 in tomato involves the stimulation of two lipoxygenase isoforms. BMC Plant Biol. 2011;11(1):1–15. doi: 10.1186/1471-2229-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Christopoulos MV, Tsantili E. Participation of phenylalanine ammonia-lyase (PAL) in increased phenolic compounds in fresh cold stressed walnut (Juglans regia L.) kernels. Postharvest Biol Tec. 2015;104:17–25. doi: 10.1016/j.postharvbio.2015.03.003. [DOI] [Google Scholar]
- 76.Mohamed BFF, Sallam NMA, Alamri SAM, Abo-Elyousr KAM, Mostafa YS, Hashem M. Approving the biocontrol method of potato wilt caused by Ralstonia solanacearum (Smith) using Enterobacter cloacae PS14 and Trichoderma asperellum T34. Egypt J Biol Pest Co. 2020;30(1):61. doi: 10.1186/s41938-020-00262-9. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary materials. Fig. S1. Schematic diagram of root and soil sample collection. Fig. S2. The distinct and shared bacterial genera in different sample compartments. Fig. S3. Effect of fungal pathogen on Shannon diversity of bacterial communities in bulk soil, rhizosphere and root. Fig. S4. Enrichment of bacterial phyla by Astragalus roots. Fig. S5. Effect of F. oxysporum infection on the abundance of major bacterial phyla. Fig. S6. Least discriminant analysis (LDA) effect size taxonomic cladogram comparing healthy and diseased rhizosphere samples. Fig. S7. Plant growth-promoting properties of plant-associated bacteria. Fig. S8. Relative abundance of bacteria in SCI found in healthy and diseased roots. Fig. S9. Relative abundance of bacteria in SCII found in healthy and diseased roots. Fig. S10. Inhibition of four bacteria in simple synthetic community (SCIII) on the growth of F. oxysporum. Fig. S11. Effect of SCIII and single bacterium inoculation on the ISR of Astragalus.
Additional file 2: Table S1. Characteristics of bacteria in two synthetic communities.
Additional file 3: Table S2. Diversity index of bacterial community in different compartment of A. mongholicus.
Additional file 4: Table S3. Root and rhizosphere community KEGG pathways.
Additional file 5: Table S4. Abundance of bacterial genera increased in diseased roots.
Additional file 6: Table S5. Abundance of bacterial genera decreased in diseased roots.
Additional file 7: Table S6. Effects of different inoculation and treatment time on Astragalus JA content and ISR-related enzyme activities.
Data Availability Statement
Raw data used in this study are available in the NCBI Sequence Read Archive (SRA), accession numbers PRJNA762455 and PRJNA764034.