Abstract
Enterococci are becoming major nosocomial pathogens, and increasing resistance to vancomycin has been well documented. Conventional identification methods, which are based on culturing, require 2 to 3 days to provide results. PCR has provided a means for the culture-independent detection of enterococci in a variety of clinical specimens and is capable of yielding results in just a few hours. However, all PCR-based assays developed so far are species specific only for clinically important enterococci. We have developed a PCR-based assay which allows the detection of enterococci at the genus level by targeting the tuf gene, which encodes elongation factor EF-Tu. Initially, we compared the nucleotide sequences of the tuf gene from several bacterial species (available in public databases) and designed degenerate PCR primers derived from conserved regions. These primers were used to amplify a target region of 803 bp from four enterococcal species (Enterococcus avium, E. faecalis, E. faecium, and E. gallinarum). Subsequently, the complete nucleotide sequences of these amplicons were determined. The analysis of a multiple alignment of these sequences revealed regions conserved among enterococci but distinct from those of other bacteria. PCR primers complementary to these regions allowed amplification of genomic DNAs from 14 of 15 species of enterococci tested (E. solitarius DNA could not be amplified). There was no amplification with a majority of 79 nonenterococcal bacterial species, except for 2 Abiotrophia species and several Listeria species. Furthermore, this assay efficiently amplified all 159 clinical isolates of enterococci tested (61 E. faecium, 77 E. faecalis, 9 E. gallinarum, and 12 E. casseliflavus isolates). Interestingly, the preliminary sequence comparison of the amplicons for four enterococcal species demonstrated that there were some sequence variations which may be used to generate species-specific internal probes. In conclusion, this rapid PCR-based assay is capable of detecting all clinically important enterococci and has potential for use in clinical microbiology laboratories.
Enterococci are members of the normal flora of the gastrointestinal tract in humans and animals (24). The incidence of enterococcal infections has increased in recent years because of widespread multiresistant enterococcal strains and increasing numbers of immunosuppressed patients and catheter-related infections. In fact, enterococci are now the second most common nosocomial pathogens in the United States (4, 31). There are two major pathogenic species in humans, Enterococcus faecalis and E. faecium, with occasional infections being caused by E. durans, E. gallinarum, E. casseliflavus, E. avium, E. hirae, E. mundtii, and E. raffinosus (24, 38). Devriese et al. (8) suggested that the extensive agricultural use of glycopeptides has created an animal reservoir of resistant enterococci which may lead to more enterococcal species resistant to glycopeptides in animal sources and complicate the control of such infections. Enterococci resistant to glycopeptides have been isolated with increasing frequency and have become a major concern worldwide. Rapid identification of enterococci is important in reducing the spread of multiresistant enterococci (2, 16).
Identification of enterococci through conventional methods, i.e., by determining phenotypic characters, is complicated and often requires 24 to 48 h (7, 11, 28). The automated methods currently used are unable to reliably identify enterococci other than E. faecalis and E. faecium (29, 32, 34). Furthermore, phenotypic identification of some enterococcal species may be occasionally difficult or even impossible because these species lack typical characteristics. More rapid and accurate methods would be helpful for microbiology laboratories. Several DNA-based methods for the specific detection of E. faecalis or E. faecium have been reported (5, 10, 27, 30). Other molecular methods, such as contour-clamped homogeneous electric field electrophoresis patterns, amplified ribosomal DNA spacer polymorphisms, and randomly amplified polymorphic DNA analysis, have been used to identify enterococci at the species level (3, 9, 23, 26, 34). However, it is difficult to adapt these tests for use in clinical microbiology laboratories because of their complexity. An Enterococcus sp. assay based on the hybridization of rRNA genes (Gen-Probe, San Diego, Calif.) is commercially available for culture confirmation (6). The sensitivity of this assay is unsatisfactory for direct detection from clinical specimens.
A variety of conserved genes, including rRNA genes (3, 19, 22), the heat shock protein 60 (HSP60 or CPN60) gene (13, 14), the major cold shock protein gene (12), and the sod gene (39), have been exploited for the detection of bacteria. The tuf gene, encoding elongation factor EF-Tu, is involved in peptide chain formation and is an essential constituent of the bacterial genome (15). These characteristics make it a target of choice for diagnostic purposes. PCR-based assays in which the tuf gene serves as the target sequence have been developed for Mycoplasma fermentans (1) and M. pneumoniae (20). We report here the development of a PCR-based assay that targets the tuf gene, that can detect most enterococcal species with excellent sensitivity and acceptable specificity, and that has potential for the development of species-specific internal probes.
(This study was presented in part at the 98th General Meeting of the American Society for Microbiology 1998, Atlanta, Ga., 17 to 21 May 1998.)
MATERIALS AND METHODS
Bacterial strains.
Twenty enterococcal strains obtained from the American Type Culture Collection (ATCC), Manassas, Va., were used in this study. These strains represent the following species: E. avium (ATCC 14025), E. casseliflavus (ATCC 25788), E. cecorum (ATCC 43198), E. dispar (ATCC 51266), E. durans (ATCC 19432), E. faecalis (ATCC 19433, ATCC 29212, ATCC 33186, ATCC 49533, and ATCC 51299), E. faecium (ATCC 19434 and ATCC 51559), E. flavescens (ATCC 49996), E. gallinarum (ATCC 49573), E. hirae (ATCC 8043), E. mundtii (ATCC 43186), E. pseudoavium (ATCC 49372), E. raffinosus (ATCC 49427), E. saccharolyticus (ATCC 43076), and E. solitarius (ATCC 49428). An additional 159 clinical isolates of enterococci obtained from various sources were also used in this study (Table 1).
TABLE 1.
Sources of the 159 enterococcal clinical isolates used in this study
| Species | No. of strains from the following sourcea:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | Total | |
| E. casseliflavus | 12 | 12 | |||||||
| E. faecalis | 47 | 2 | 2 | 12 | 9 | 1 | 4 | 77 | |
| E. faecium | 7 | 1 | 38 | 10 | 1 | 4 | 61 | ||
| E. gallinarum | 1 | 7 | 1 | 9 | |||||
| Total | 55 | 2 | 12 | 3 | 57 | 19 | 3 | 8 | 159 |
A, Centre Hospitalier Universitaire de Québec (Pavillon Centre Hospitalier de l'Université Laval), Sainte-Foy, Québec, Canada; B, Centers for Disease Control and Prevention, Atlanta, Ga; C, Hôpital Charles Lemoyne, Montréal, Québec, Canada; D, Huashan Hospital, Shanghai, China; E, Laboratoire de Santé Publique du Québec, Sainte-Anne-de-Bellevue, Québec, Canada; F, Mount Sinai Hospital, Toronto, Ontario, Canada; G, Universidad de Buenos Aires, Buenos Aires, Argentina; H, University of Texas, Houston.
The specificity of the PCR-based assay was verified by use of a battery of ATCC reference strains consisting of 44 gram-negative and 50 gram-positive bacterial species (Table 2). The 159 clinical isolates of enterococci (61 E. faecium, 77 E. faecalis, 9 E. gallinarum, and 12 E. casseliflavus) from various origins (Table 1) were also tested to further validate the Enterococcus-specific PCR-based assay. The reference strains as well as the clinical isolates were all identified by conventional methods or with an automated MicroScan Autoscan-4 system equipped with a Positive BP Combo Panel Type 6 (Dade Diagnostics, Mississauga, Ontario, Canada). Bacterial strains were grown from frozen stocks kept at −80°C in brain heart infusion medium containing 10% glycerol and were cultured on sheep blood agar or in brain heart infusion broth.
TABLE 2.
Specificity test performed with the Enterococcus-specific 40-cycle PCR assay and DNA from a variety of gram-positive and gram-negative bacterial species
| Gram-positive bacteria (n = 50) | PCR | Gram-negative bacteria (n = 44) | PCR | |
|---|---|---|---|---|
| Abiotrophia adiacens ATCC 49175 | + | Acinetobacter baumannii ATCC 19606 | − | |
| Abiotrophia defectiva ATCC 49176 | + | Acinetobacter haemolyticus ATCC 17906 | − | |
| Corynebacterium aquaticus ATCC 14665 | − | Bordetella pertussis ATCC 9797 | − | |
| Enterococcus avium ATCC 14025 | + | Bulkholderia cepacia ATCC 25416 | − | |
| Enterococcus casseliflavus ATCC 25788 | + | Citrobacter diversus ATCC 27028 | − | |
| Enterococcus cecorum ATCC 43198 | + | Citrobacter freundii ATCC 8090 | − | |
| Enterococcus dispar ATCC 51266 | + | Enterobacter aerogenes ATCC 13048 | − | |
| Enterococcus durans ATCC 19432 | + | Enterobacter agglomerans ATCC 27155 | − | |
| Enterococcus faecalis ATCC 19433 | + | Enterobacter cloacae ATCC 13047 | − | |
| Enterococcus faecium ATCC 19434 | + | Escherichia coli ATCC 25922 | − | |
| Enterococcus flavescens ATCC 49996 | + | Haemophilus ducreyi ATCC 33940 | − | |
| Enterococcus gallinarum ATCC 49573 | + | Haemophilus haemolyticus ATCC 33390 | − | |
| Enterococcus hirae ATCC 8043 | + | Haemophilus influenzae ATCC 9007 | − | |
| Enterococcus mundtii ATCC 43186 | + | Haemophilus parahaemolyticus ATCC 10014 | − | |
| Enterococcus pseudoavium ATCC 49372 | + | Haemophilus parainfluenzae ATCC 7901 | − | |
| Enterococcus raffinosus ATCC 49427 | + | Hafnia alvei ATCC 13337 | − | |
| Enterococcus saccharolyticus ATCC 43076 | + | Kingella indologenes ATCC 25869 | − | |
| Enterococcus solitarius ATCC 49428 | − | Klebsiella oxytoca ATCC 13182 | − | |
| Gemella haemolysans ATCC 10379 | − | Klebsiella pneumoniae ATCC 13883 | − | |
| Lactobacillus acidophilus ATCC 4356 | − | Moraxella atlantae ATCC 29525 | − | |
| Listeria grayi ATCC 19120 | − | Moraxella catarrhalis ATCC 25240 | − | |
| Listeria innocua ATCC 33090 | + | Moraxella osloensis ATCC 19976 | − | |
| Listeria ivanovii ATCC 19119 | + | Morganella morganii ATCC 25830 | − | |
| Listeria monocytogenes ATCC 15313 | + | Neisseria caviae ATCC 14659 | − | |
| Listeria murrayi ATCC 25401 | − | Neisseria elangata ATCC 25295 | − | |
| Listeria seeligeri ATCC 35967 | + | Neisseria gonorrhoeae ATCC 35201 | − | |
| Micrococcus luteus ATCC 9341 | − | Neisseria meningitidis ATCC 13077 | − | |
| Staphylococcus aureus ATCC 25923 | − | Neisseria mucosa ATCC 19696 | − | |
| Staphylococcus capitis ATCC 27840 | − | Pasteurella aerogenes ATCC 27883 | − | |
| Staphylococcus epidermidis ATCC 14990 | − | Proteus mirabilis ATCC 25933 | − | |
| Staphylococcus haemolyticus ATCC 29970 | − | Proteus vulgaris ATCC 13315 | − | |
| Staphylococcus hominis ATCC 27844 | − | Providencia alcalifaciens ATCC 9886 | − | |
| Staphylococcus lugdunensis ATCC 43809 | − | Providencia rettgeri ATCC 9250 | − | |
| Staphylococcus saprophyticus ATCC 15305 | − | Providencia rustigianii ATCC 33673 | − | |
| Staphylococcus simulans ATCC 27848 | − | Providencia stuartii ATCC 29914 | − | |
| Staphylococcus warneri ATCC 27836 | − | Pseudomonas aeruginosa ATCC 27853 | − | |
| Streptococcus agalactiae ATCC 13813 | − | Pseudomonas fluorescens ATCC 13525 | − | |
| Streptococcus anginosus ATCC 33397 | − | Pseudomonas stutzeri ATCC 17588 | − | |
| Streptococcus bovis ATCC 33317 | − | Salmonella typhimurium ATCC 14028 | − | |
| Streptococcus constellatus ATCC 27823 | − | Serratia marcescens ATCC 8100 | − | |
| Streptococcus crista ATCC 51100 | − | Shigella flexneri ATCC 12022 | − | |
| Streptococcus intermedius ATCC 27335 | − | Shigella sonnei ATCC 29930 | − | |
| Streptococcus mitis ATCC 33399 | − | Stenotrophomonas maltophilia ATCC 13843 | − | |
| Streptococcus mutans ATCC 25175 | − | Yersinia enterocolitica ATCC 9610 | − | |
| Streptococcus parasanguis ATCC 15912 | − | |||
| Streptococcus pneumoniae ATCC 6303 | − | |||
| Streptococcus pyogenes ATCC 19615 | − | |||
| Streptococcus salivarius ATCC 7073 | − | |||
| Streptococcus sanguis ATCC 10556 | − | |||
| Streptococcus suis ATCC 43765 | − |
PCR primers.
The tuf gene sequences available from public databases were analyzed with GCG programs (version 8.0) (Genetics Computer Group, Madison, Wis.). Based on multiple sequence alignments, regions of the tuf gene highly conserved among eubacteria were chosen, and PCR primers were derived from these regions with Oligo primer analysis software (version 5.0) (National Biosciences, Plymouth, Minn.). When required, the primers contained inosines or degeneracies at one or more variable positions. Oligonucleotide primers were synthesized with a model 391 DNA synthesizer (Perkin-Elmer Corp., Applied Biosystems Division, Mississauga, Ontario, Canada). PCR primers used in this study are listed in Table 3.
TABLE 3.
PCR primers used in this study
| Primer | Sequence | Nucleotide positionsa | Product length (bp) |
|---|---|---|---|
| Universal amplification | 803 | ||
| U1 | 5′-AAYATGATIACIGGIGCIGCICARATGGA-3′ | 271–300 | |
| U2 | 5′-AYRTTITCICCIGGCATIACCAT-3′ | 1051–1073 | |
| Enterococcus specific | 112 | ||
| Ent1 | 5′-TACTGACAAACCATTCATGATG-3′ | 618–639 | |
| Ent2 | 5′-AACTTCGTCACCAACGCGAAC-3′ | 708–729 | |
| Internal control | 252 | ||
| IC1 | 5′-TCTCGAGCTCTGTACATGTCC-3′ | ||
| IC2 | 5′-GTTCTAGAGGTACCGGTTGTT-3′ |
The nucleotide positions given are for the E. coli tuf sequence (GenBank accession no. J01690).
DNA sequencing.
An 803-bp portion of the tuf gene was sequenced for E. avium, E. faecalis, E. faecium, and E. gallinarum. Amplification was performed with 1 ng of genomic DNA prepared by use of a G NOME DNA kit (Bio 101, Vista, Calif.). The 20-μl PCR mixtures used to generate PCR products for sequencing contained 1.0 μM each universal primer (U1 and U2; Table 3), 200 μM each deoxyribonucleoside triphosphate (Pharmacia Biotech Inc., Baie d'Urfé, Québec, Canada), 10 mM Tris-HCl (pH 9.0), 50 mM KCl, 0.1% Triton X-100, 2.5 mM MgCl2, 0.5 U of Taq polymerase (Promega Corp., Madison, Wis.), and TaqStart antibody (Clontech Laboratories Inc., Palo Alto, Calif.). The TaqStart antibody, which is a neutralizing monoclonal antibody for Taq DNA polymerase, was added to all PCR mixtures to enhance the efficiency of the amplifications (18).
The PCR mixtures were subjected to thermal cycling (3 min at 95°C and then 35 cycles of 30 s at 95°C, 30 s at 55°C, and 1 min at 72°C, with a 7-min final extension at 72°C) with a PTC-200 DNA Engine thermocycler (MJ Research Inc., Watertown, Mass.). The amplified PCR mixtures were resolved by electrophoresis through 1.5% agarose gels at 4 V/cm for 90 min; the gels were then stained with ethidium bromide and visualized under 312-nm UV light. Subsequently, PCR products having the predicted sizes were recovered from the gels with a QIAquick gel extraction kit (QIAGEN Inc., Mississauga, Ontario, Canada).
The purified DNA fragments were cloned into pCR2.1 vector (Invitrogen Corp., Carlsbad, Calif.). Plasmids were isolated from transformed Escherichia coli with a QIAGEN plasmid mini-kit. The presence of DNA inserts in the recombinant plasmids was confirmed by digesting purified plasmid DNA with EcoRI (New England Biolabs, Ltd., Mississauga, Ontario, Canada), which allowed excision of the inserted fragments. Both strands of the DNA inserts for each of the selected recombinant plasmids were sequenced with a PRISM Ready Reaction DyeDeoxy Terminator cycle sequencing kit and an Applied Biosystems 373A sequencer (Perkin-Elmer). In order to exclude the possibility of sequencing errors attributable to misincorporations by Taq polymerase, each strand of the insert was sequenced from three different clones.
PCR amplification.
For all bacterial species, amplification was performed from purified genomic DNA or from a bacterial suspension whose turbidity was adjusted to that of a 0.5 McFarland standard, which corresponds to approximately 1.5 × 108 bacteria per ml. One nanogram of genomic DNA or 1 μl of standardized bacterial suspension was transferred directly to a 19-μl PCR mixture containing 50 mM KCl, 10 mM Tris-HCl (pH 9.0), 0.1% Triton X-100, 2.5 mM MgCl2, 0.2 μM each Enterococcus-specific primer (Ent1 and Ent2; Table 3), 200 μM each deoxynucleoside triphosphate (Pharmacia Biotech), 3.3 μg of bovine serum albumin (BSA) (Sigma-Aldrich Canada Ltd., Oakville, Ontario, Canada) per μl, 0.5 U of Taq polymerase (Promega), and TaqStart antibody (Clontech). PCR amplification and agarose gel analysis of the amplified products were performed as previously described (21).
The Superlinker phagemid pSL1180 (Pharmacia Biotech) linearized by digestion with EcoRI (New England Biolabs) and the primers (IC1 and IC2; Table 3) derived from the multiple cloning sites of this plasmid were used to provide an internal control for all Enterococcus-specific PCR-based assays. These primers can amplify a 252-bp product. The internal control was integrated into the PCR-based assays to verify the efficiency of the amplifications and to ensure that significant PCR inhibition was absent. Four thousand copies of the linearized plasmid were added to each PCR. The concentrations of the internal control primers were adjusted to ensure that there was no detrimental effect on the Enterococcus-specific amplification. We found that concentrations of 0.1 and 0.04 μM were optimal for 30- and 40-cycle PCRs, respectively.
For determination of the sensitivities of the PCR-based assays, twofold dilutions of purified genomic DNA were used to determine the minimal number of genomes which can be detected.
Nucleotide sequence accession numbers.
GenBank accession numbers for the 751-bp partial sequence of the tuf gene (excluding the sequences of the two universal amplification primers) are as follows: AF124220 for E. avium, AF124221 for E. faecalis, AF124222 for E. faecium, AF124223 for E. gallinarum, AF124224 for Abiotrophia adiacens, and AF124225 for A. defectiva.
RESULTS
Sequencing of a portion of the tuf gene from four enterococcal species.
The tuf sequences from a number of selected bacterial species, including E. coli (J01690), M. genitalium (U39732), Haemophilus influenzae (U32746), Neisseria gonorrhoeae (L36380), Salmonella typhimurium (X55116), and Micrococcus luteus (M17788), were aligned and compared. Two highly conserved regions were identified, and a pair of primers (U1 and U2) amplifying a region of 803 bp was designed (Table 3). Several degeneracies and inosines were incorporated into these two primers because some positions are variable among eubacteria. These primers allowed the amplification of tuf sequences from a wide variety of bacteria, including 14 enterococcal species. By using these primers, we were able to amplify the 803-bp portion of tuf for four enterococcal species: E. avium, E. faecalis, E. faecium, and E. gallinarum. After purification from agarose gels, the 803-bp PCR product was cloned into a TA cloning vector. Subsequently, the sequence of the inserted DNA fragment was determined by sequencing of three randomly selected clones for each enterococcal species to ensure that no sequencing errors were attributable to misincorporation by the Taq polymerase. In order to facilitate the selection of Enterococcus-specific primers, we conducted a multiple sequence analysis using the sequences mentioned above (available in public databases) as well as partial tuf sequences from staphylococci, streptococci, enterococci, Listeria spp., and Abiotrophia spp. (all obtained from our laboratory; unpublished data). By using this approach, we were able to identify regions conserved in the four enterococcal species but variable in the other bacterial species. The Enterococcus-specific PCR primers were derived from these regions. A similarity comparison of the tuf sequences for the enterococcal species E. avium, E. faecalis, E. faecium, and E. gallinarum as well as for two Abiotrophia species is given in Table 4. The sequence similarities for the 803-bp fragment in these species ranged from 85 to 91%.
TABLE 4.
Sequence homology for an 803-bp portion of the tuf gene and Enterococcus-specific PCR products for four enterococcal species and two Abiotrophia species
| Species | % of sequence similaritya for:
|
|||||
|---|---|---|---|---|---|---|
| A. adiacens | A. defectiva | E. avium | E. faecalis | E. faecium | E. gallinarum | |
| A. adiacens | 85 | 89 | 89 | 89 | 87 | |
| A. defectiva | 89.8 | 87 | 85 | 86 | 85 | |
| E. avium | 92.7 | 97.1 | 91 | 90 | 90 | |
| E. faecalis | 92.7 | 91.3 | 92.7 | 89 | 90 | |
| E. faecium | 95.7 | 89.8 | 91.3 | 91.3 | 90 | |
| E. gallinarum | 94.2 | 95.7 | 95.7 | 95.7 | 94.2 | |
The numbers in the upper right triangle are the percentage of similarity for the sequenced 803-bp fragment, and those in the lower left triangle represent the percentage of similarity for the 69-bp sequences flanked by the two Enterococcus-specific PCR primers. The similarity scores were obtained by use of the PILEUP program of the GCG software package.
The selected primers revealed no more than two mismatches within the tuf sequences of the enterococcal species, except for E. faecium and two Abiotrophia species, in which three mismatches were present at the 5′ end of the 5′ primer. Importantly, more than five mismatches were found in the corresponding regions of the other bacterial species mentioned above. Since the mismatches in the other bacteria were clustered at the 3′ end of the primers, a position critical for discriminatory PCR amplification, the amplification of bacterial species other than enterococci could be efficiently prevented.
Amplifications with the Enterococcus-specific PCR assay.
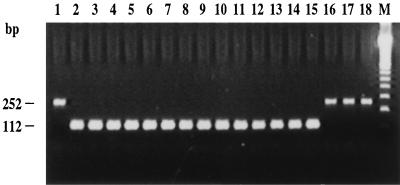
The specificity of the assay was assessed by performing 30-cycle and 40-cycle PCR amplifications with the panel of gram-positive (50 species from 9 genera) and gram-negative (44 species from 21 genera) bacterial species listed in Table 2. The PCR assay was able to detect 14 of 15 enterococcal species tested in both 30-cycle and 40-cycle regimens. E. solitarius was the only enterococcal species tested that was not amplified by the Enterococcus-specific assay (Fig. 1). For 30-cycle PCR, all bacterial species tested other than enterococci were negative, except for two Abiotrophia species, A. adiacens and A. defectiva. For 40-cycle PCR, four Listeria species, Listeria innocua, L. ivanovii, L. monocytogenes, and L. seeligeri, were also positive in addition to the enterococci and Abiotrophia species. The other species tested remained negative. The internal control was always efficiently amplified when no target DNA was present, thereby showing the absence of PCR inhibitors. On the contrary, the internal control was not amplified when target DNA was present in a sample (Fig. 1). This result is explained by the fact that the concentrations of the internal control primers were limited in order to favor the amplification of the target DNA. Tests of a collection of clinical isolates comprising E. faecalis (n = 77), E. faecium (n = 61), E. gallinarum (n = 9), and E. casseliflavus (n = 12) showed a uniform amplification signal with both the 30-cycle and the 40-cycle PCR assays and a perfect relationship between the genotype and classical means of identification.
FIG. 1.
Example of multiplex PCR amplifications with the Enterococcus-specific PCR assay and the internal control. PCR assays (40 cycles) were performed with 1 μl of genomic DNA (1 ng/μl) from various bacteria and 4,000 copies of linearized pSL1180 as an internal control. The internal control was not amplified when target DNA was present due to competitive inhibition by amplification of Enterococcus DNA. Lanes: 2, E. avium ATCC 14025; 3, E. casseliflavus ATCC 25788; 4, E. cecorum ATCC 43198; 5, E. durans ATCC 19432; 6, E. dispar ATCC 51266; 7, E. faecalis ATCC 19433; 8, E. faecium ATCC 19434; 9, E. flavescens ATCC 49996; 10, E. gallinarum ATCC 49573; 11, E. hirae ATCC 8043; 12, E. mundtii ATCC 43186; 13, E. pseudoavium ATCC 49372; 14, E. raffinosus ATCC 49427; 15, E. saccharolyticus ATCC 43076; 16, E. solitarius ATCC 49428; 17, E. coli ATCC 25922; 1 and 18, controls to which no DNA was added; M, 100-bp molecular size standard ladder.
The sensitivity of our Enterococcus-specific assay with 30-cycle and 40-cycle PCR protocols was determined by using purified genomic DNA from 14 enterococcal species. For PCR with 30 cycles, we found a detection limit ranging from 330 to 1,700 copies of genomic DNA, depending on the enterococcal species tested (Table 5). In order to enhance the sensitivity of the assay, we increased the number of cycles. For PCR with 40 cycles, the detection limit was lowered to two to eight genome copies (Table 5).
TABLE 5.
Detection limits of the Enterococcus-specific PCR assays for 14 enterococcal species
| Species | Detection limit (no. of genomes) for PCR with the following no. of cyclesa:
|
|
|---|---|---|
| 30 | 40 | |
| E. avium | 1,700 | 8 |
| E. cecorum | 1,700 | 2 |
| E. casseliflavusb | 670 | 4 |
| E. dispar | ND | 8 |
| E. durans | 330 | 4 |
| E. faecalis | 670 | 2 |
| E. faecium | 670 | 8 |
| E. flavescensb | ND | 8 |
| E. gallinarum | 330 | 8 |
| E. hirae | 330 | 4 |
| E. mundtii | 1,700 | 8 |
| E. pseudoavium | 330 | 2 |
| E. raffinosus | 1,700 | 8 |
| E. saccharolyticus | 330 | 2 |
The genome size for all enterococcal species was considered the same as that of E. faecalis (3.0 Mb). Serial twofold dilutions of genomic DNA from each enterococcal species were tested to determine the detection limit of the assay for each species. ND, not determined.
E. casseliflavus and E. flavescens are now considered the same species (33).
DISCUSSION
Enterococci can be encountered throughout the environment, from human, animal, and food sources. The emergence and dissemination of vancomycin-resistant enterococci (VRE) underscore the importance of the rapid detection of these organisms (16). Conventional identification of some enterococci is difficult because no phenotypic criteria are available to unequivocally separate the genus Enterococcus from the other genera of gram-positive cocci. A number of tests, such as Lancefield group D antigen, the ability to grow in 6.5% NaCl broth, the pyrolidonylarylamidase test, the leucinearylamidase test, and the bile esculin test, are all valuable for the identification of enterococci. Unfortunately, none of these tests alone or in combinations provides a phenotype unique to the enterococci (7). Furthermore, such tests require 24 to 48 h to provide results after pure cultures are obtained. Even with automated systems, incubation for up to 24 h is routinely required, and some additional tests may have to be carried out for species differentiation (29, 32). Since the vancomycin resistance genes are transferable among different enterococcal species or even among different genera (38), the inability to detect enterococci promptly may cause delays in reporting VRE; this situation may lead to complex and costly containment efforts to eliminate VRE colonization and infection.
The development of rapid and sensitive DNA-based assays which are applicable for the direct detection of enterococci from clinical specimens may improve the rapidity and the accuracy of the diagnosis of enterococcal infections. The validity and versatility of PCR in applications for the detection of nucleic acids from a variety of infectious agents have been well reviewed by Whelen and Persing (36). In the present study, we have developed a rapid PCR-based assay to improve the diagnosis of enterococcal infections. Initially, PCR primers complementary to highly conserved regions of the tuf gene among eubacteria were designed and used to amplify an 803-bp portion of the tuf gene from 14 enterococcal species. By sequencing four enterococcal species, which represented four different enterococcal subgroups based on 16S rRNA gene analysis, we have obtained adequate sequence information to design a pair of Enterococcus-specific PCR primers. Subsequently, a PCR-based assay was set up and optimized to be simple and rapid. Among 50 species of gram-positive (representing 9 genera) and 44 species of gram-negative (representing 21 genera) clinically important bacteria tested, this PCR-based assay was able to detect all enterococcal species tested, except for E. solitarius, which in fact does not appear to be a member of the genus Enterococcus based on a phylogenetic analysis (25, 37). Two Abiotrophia species were also amplified efficiently under the same amplification conditions. Moreover, amplification of four Listeria species was also observed when the number of PCR cycles was increased from 30 to 40. It should be noted that DNA from Listeria species was amplified about 100 times less efficiently than enterococcal DNA. However, most clinically important bacteria can be easily differentiated from enterococci by this assay, indicating its usefulness for the detection of enterococci. The concomitant use of species-specific internal probes should allow exclusion of Abiotrophia and Listeria species by increasing the specificity of the Enterococcus-specific PCR-based assay.
To elucidate the fact that the developed PCR-based assay failed to detect E. solitarius, which rarely causes infections in humans, we amplified and sequenced an 890-bp portion of tuf encompassing the 803-bp region by using another pair of primers (unpublished data). The sequence similarity between E. solitarius and other enterococci ranged from 79 to 81%, while that between the other enterococci ranged from 89 to 91%. This finding supports 16S rRNA data reported by others (25, 37) suggesting that E. solitarius is not a member of the genus Enterococcus (93.0 to 94.8% homology) but is phylogenetically more closely related to the genus Tetragenococcus (97.8% homology). Sequence data revealed that there were six mismatches at the Enterococcus-specific 3′-primer binding site which led to a failure in the amplification of E. solitarius by the developed PCR-based assay.
The finding that A. adiacens and A. defectiva, formerly referred to as nutritionally variant streptococci (17), were also positive in our Enterococcus-specific PCR assay indicates a high level of similarity of the tuf genes at the primer binding sites in the genus Abiotrophia and the genus Enterococcus. Sequencing of the 803-bp region of tuf showed that the sequence similarities between the enterococcal and Abiotrophia spp. ranged from 85 to 89%, values which are quite high. For comparison, the tuf sequences among enterococci are 89 to 91% similar. Therefore, it is not surprising that PCR primers derived from the tuf sequence amplify DNA from Abiotrophia spp. as well. In fact, there are only two mismatches at the 5′ end of the Enterococcus-specific primers compared to the sequences of A. defectiva and A. adiacens. Consequently, the sensitivity level achieved for the two Abiotrophia species was similar to that obtained for the enterococcal species. In addition, several Listeria species were detectable when 40-cycle PCR was performed, even though three mismatches located near the 3′ end of the upper primer were found between the four Listeria species and enterococci. However, it should be noted that Listeria DNA was amplified approximately 100 times less efficiently than enterococcal DNA. The four Listeria species, L. monocytogenes, L. innocua, L. ivanovii, and L. seelegeri, showed sequence similarities to enterococci ranging from 82 to 83% (data not shown). Based on the level of sequence divergence for the tuf gene, it should be possible to develop enterococcal species-specific internal probes which allow discrimination of members of the genera Abiotrophia and Listeria from those of the genus Enterococcus.
Others have developed E. faecalis-specific and E. faecium-specific PCR-based assays by targeting various genes. The target genes include (i) ddl (coding for d-alanine-d-alanine ligase) for the species-specific detection of E. faecalis and E. faecium (10, 30) and (ii) PBP5 (coding for penicillin binding protein) for the species-specific detection of E. faecalis (27). A PCR-based assay amplifying different vanC genes (coding for intrinsic vancomycin resistance in E. gallinarum, E. casseliflavus, and E. flavescens) has also been developed to specifically detect E. gallinarum, E. casseliflavus, and E. flavescens (10). A sequence of unknown coding potential, selected from an E. faecium genomic library, has been used to develop an E. faecium-specific PCR-based assay (5). Although E. faecalis and E. faecium account for a majority of enterococcal infections, other enterococci may be associated with infections. However, currently available systems based on culturing for the identification of gram-positive cocci are often unable to correctly identify these less frequently encountered enterococci (29, 32, 34).
Tyrrell et al. (35) reported using an internally transcribed spacer region PCR to identify enterococcal species based on characteristic amplicon profiles and the different patterns of Sau3A-digested PCR amplicons. Donabedian et al. (9) demonstrated that contour-clamped homogeneous electric field electrophoresis patterns and DNA-DNA hybridization with biotin-labeled genomic DNAs from type strains of enterococci used as probes may be suitable for species differentiation of some enterococci. However, neither method is feasible for routine use in the clinical laboratory because of high complexity and must be used in conjunction with phenotypic tests to provide reliable results (9, 35). Recently, Quednau et al. (26) and Monstein et al. (23) identified enterococci to the species or species group level by using randomly amplified polymorphic DNA methods. A simple test for the detection of Enterococcus spp. has been developed by Gen-Probe for use as a culture confirmation assay; this test was 100% accurate in identifying enterococcal isolates from cultures by hybridization to rRNA (6). However, this assay does not offer potential for enterococcal species identification. Moreover, the sensitivity of the Gen-Probe assay is not sufficient for direct detection of enterococci from clinical specimens.
Besides identification of bacterial species by species-specific PCR-based assays, identification may also be performed by coupling the amplification of a highly conserved gene with hybridization to internal probes or DNA sequencing (1, 14, 20, 22). Many conserved genes have been selected as targets for this purpose. Among them, the 16S rRNA gene has been used to detect a wide variety of eubacteria because the presence of conserved regions and variable regions in this gene provides the possibility of developing PCR-based assays suitable for detecting and identifying bacteria at the species level or higher taxonomic levels (22). Other genes have been exploited for similar purposes. The sod gene, coding for superoxide dismutase, has been used as a target to amplify 28 species of mycobacteria and to differentiate one from another with probes recognizing species-specific regions (39). A similar approach has been used for the detection of staphylococci by targeting the chaperonin 60 (cpn60) gene (13, 14). These methods are specific, but their sensitivity remains unclear. The latter is critical when such tests are used to detect bacteria directly from clinical specimens. Berg et al. (1) have developed a PCR-based detection system for M. fermentans by targeting the tuf gene; the system has a high sensitivity. A similar assay has also been used for the identification of M. pneumoniae (20).
The tuf gene acts in translation to bring aminoacylated tRNA molecules to the ribosome. This gene represents an ideal candidate target for diagnostic purposes because it is highly conserved at the nucleotide level and ubiquitous in bacteria. By analyzing a rather small tuf sequence data set available in public databases, we were able to design PCR primers which could amplify an 803-bp portion of the tuf gene from a variety of bacteria, including members of the genera Enterococcus, Streptococcus, and Staphylococcus. We found that there were more nucleotide sequence variations in the tuf gene sequences than in the corresponding 16S rRNA gene sequences for four enterococcal species (i.e., E. faecalis, E. faecium, E. avium, and E. gallinarum). An analysis of the tuf sequences from gram-positive bacteria allowed the development of a PCR-based assay amplifying 14 of 15 enterococcal species tested. The PCR-based assay described here differentiates enterococci from most clinically relevant bacteria, indicating that the tuf gene is a target of choice for the molecular detection of enterococci. Furthermore, the amplicon sequence polymorphism should be sufficient to provide discriminatory internal probes specific for clinically important enterococcal species. The sensitivity of the 30-cycle PCR-based assay varies from 330 to 1,700 copies of enterococcal genomes for the 14 enterococcal species detected. This sensitivity level is sufficient for culture confirmation assays. It is possible to efficiently increase the sensitivity of the assay. For example, in sensitivity assays performed with 40-cycle PCR, the detection limit was reduced to about two to eight copies of enterococcal genomes for the same 14 enterococcal species. This sensitivity level should be sufficient for the direct detection of enterococci in clinical specimens, such as fecal or urine samples. The use of this PCR-based assay coupled with species-specific internal probes specific for clinically important enterococci and other PCR assays targeting vancomycin resistance genes should provide a useful screening test for VRE from rectal swabs. Such an assay is currently under development in our laboratory.
In conclusion, we have developed a PCR-based diagnostic assay which is simple to conduct and reliable for the detection of enterococci. This assay offers an alternative to currently used methods and should allow the identification of clinically important enterococcal species if coupled with species-specific probes complementary to internal regions of the amplicons. This new diagnostic tool may lead to the early diagnosis of enterococcal infections, which is essential for the prevention and control of transmission of the infections.
ACKNOWLEDGMENTS
We thank Louise Coté, director of the microbiology laboratory of CHUQ, Pavillon CHUL, for free access to the laboratory and for providing enterococcal and other clinical isolates. We thank Jean-Luc Simard, Martin Gagnon, Marie-Josée Boily, Caroline Paquet, Nicolas Lansac, Marie-Claude Bergeron, and Gisèle Chassé for help. We also thank Louise Jetté (Laboratoire de Santé Publique du Québec), Donald E. Low (Mount Sinai Hospital), Barbara E. Murray (University of Texas, Houston), Fred C. Tenover (Centers for Disease Control and Prevention), Wang Fu (Huashan Hospital), and Daniela Centron-Garcia (Universidad de Buenos Aires) for providing enterococcal strains. We thank Maurice Boissinot for critical comments regarding the manuscript.
Marc Ouellette is an MRC scientist and a recipient of the Burroughs Wellcome Fund new investigator award in molecular parasitology. This study was supported by grant PA-15586 from the Medical Research Council of Canada and by Infectio Diagnostic (I.D.I) Inc., Sainte-Foy, Québec, Canada.
REFERENCES
- 1.Berg S, Luneberg E, Frosch M. Development of an amplification and hybridization assay for the specific and sensitive detection of Mycoplasma fermentans DNA. Mol Cell Probes. 1996;10:7–14. doi: 10.1006/mcpr.1996.0002. [DOI] [PubMed] [Google Scholar]
- 2.Bergeron M G, Ouellette M. Preventing antibiotic resistance through rapid genotypic identification of bacteria and of their antibiotic resistance genes in the clinical microbiology laboratory. J Clin Microbiol. 1998;36:2169–2172. doi: 10.1128/jcm.36.8.2169-2172.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Betzl D, Ludwig W, Schleifer K H. Identification of lactococci and enterococci by colony hybridization with 23S rRNA-targeted oligonucleotide probes. Appl Environ Microbiol. 1990;56:2927–2929. doi: 10.1128/aem.56.9.2927-2929.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Nosocomial enterococci resistant to vancomycin—United States, 1989–1993. Morbid Mortal Weekly Rep. 1993;42:597–599. [PubMed] [Google Scholar]
- 5.Cheng S, McCleskey F K, Gress M J, Petroziello J M, Liu R, Namdari H, Beninga K, Salmen A, Del Vecchio V G. A PCR assay for identification of Enterococcus faecium. J Clin Microbiol. 1997;35:1248–1250. doi: 10.1128/jcm.35.5.1248-1250.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daly J A, Clifton N L, Seskin K C, Gooch W M. Use of rapid, nonradioactive DNA probes in culture confirmation tests to detect Streptococcus agalactiae, Haemophilus influenzae, and Enterococcus spp. from pediatric patients with significant infections. J Clin Microbiol. 1991;29:80–82. doi: 10.1128/jcm.29.1.80-82.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devriese L A, Pot B, Collins M D. Phenotypic identification of the genus Enterococcus and differentiation of phylogenetically distinct enterococcal species and species groups. J Appl Bacteriol. 1993;75:399–408. doi: 10.1111/j.1365-2672.1993.tb02794.x. [DOI] [PubMed] [Google Scholar]
- 8.Devriese L A, Ieven M, Goossens H, Vandamme P, Pot B, Hommez J, Haesebrouck F. Presence of vancomycin-resistant enterococci in farm and pet animals. Antimicrob Agents Chemother. 1996;40:2285–2287. doi: 10.1128/aac.40.10.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donabedian S, Chow J W, Shlaes D M, Green M, Zervos M J. DNA hybridization and contour-clamped homogeneous electric field electrophoresis for identification of enterococci to the species level. J Clin Microbiol. 1995;33:141–145. doi: 10.1128/jcm.33.1.141-145.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dutka-Malen S, Evers S, Courvalin P. Detection of glycopeptide resistance genotypes and identification to the species level of clinically relevant enterococci by PCR. J Clin Microbiol. 1995;33:24–27. doi: 10.1128/jcm.33.1.24-27.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Facklam R R, Collins M D. Identification of Enterococcus species isolated from human infections by a conventional test scheme. J Clin Microbiol. 1989;27:731–734. doi: 10.1128/jcm.27.4.731-734.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Francis K P, Stewart G S. Detection and speciation of bacteria through PCR using universal major cold-shock protein primer oligomers. J Ind Microbiol Biotechnol. 1997;19:286–293. doi: 10.1038/sj.jim.2900463. [DOI] [PubMed] [Google Scholar]
- 13.Goh S H, Potter S, Wood J O, Hemmingsen S M, Reynolds R P, Chow A W. HSP60 gene sequences as universal targets for microbial species identification: studies with coagulase-negative staphylococci. J Clin Microbiol. 1996;34:818–823. doi: 10.1128/jcm.34.4.818-823.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goh S H, Santucci Z, Kloos W E, Faltyn M, George C G, Driedger D, Hemmingsen S M. Identification of Staphylococcus species and subspecies by the chaperonin 60 gene identification method and reverse checkerboard hybridization. J Clin Microbiol. 1997;35:3116–3121. doi: 10.1128/jcm.35.12.3116-3121.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grunberg-Manago M. Regulation of the expression of aminoacyl-tRNA synthetases and translation factors. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 2. Washington, D.C: ASM Press; 1996. pp. 1432–1457. [Google Scholar]
- 16.Hospital Infection Control Practices Advisory Committee. Recommendations for preventing the spread of vancomycin resistance. Infect Control Hosp Epidemiol. 1995;16:105–113. doi: 10.1086/647066. [DOI] [PubMed] [Google Scholar]
- 17.Kawamura Y, Hou X G, Sultana F, Liu S J, Yamamoto H, Ezaki T. Transfer of Streptococcus adjacens and Streptococcus defectivus to Abiotrophia gen. nov. as Abiotrophia adiacens comb. nov. and Abiotrophia defectiva comb. nov., respectively. Int J Syst Bacteriol. 1995;45:798–803. doi: 10.1099/00207713-45-4-798. [DOI] [PubMed] [Google Scholar]
- 18.Kellogg D E, Rybalkin I, Chen S, Mukhamedova N, Vlasik T, Siebert P D, Chenchik A. TaqStart Antibody™: “hot start” PCR facilitated by a neutralizing monoclonal antibody directed against Taq DNA polymerase. BioTechniques. 1994;16:2888–2893. [PubMed] [Google Scholar]
- 19.Ludwig W, Dorn S, Springer N, Kirchhof G, Schleifer K-H. PCR-based preparation of 23S rRNA-targeted group-specific polynucleotide probes. Appl Environ Microbiol. 1994;60:3236–3244. doi: 10.1128/aem.60.9.3236-3244.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luneberg E, Jensen J S, Frosch M. Detection of Mycoplasma pneumoniae by polymerase chain reaction and nonradioactive hybridization in microtiter plates. J Clin Microbiol. 1993;31:1088–1094. doi: 10.1128/jcm.31.5.1088-1094.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martineau F, Picard F J, Roy P H, Ouellette M, Bergeron M G. Species-specific and ubiquitous DNA-based assays for rapid identification of Staphylococcus aureus. J Clin Microbiol. 1998;36:618–623. doi: 10.1128/jcm.36.3.618-623.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCabe K M, Khan G, Zhang Y-H, Mason E O, McCabe E R B. Amplification of bacterial DNA using highly conserved sequences: automated analysis and potential for molecular triage of sepsis. Pediatrics. 1995;95:165–169. [PubMed] [Google Scholar]
- 23.Monstein H-J, Quednau M, Samuelsson A, Ahrné S, Isaksson B, Jonasson J. Division of the genus Enterococcus into species groups using PCR-based molecular typing methods. Microbiology. 1998;144:1171–1179. doi: 10.1099/00221287-144-5-1171. [DOI] [PubMed] [Google Scholar]
- 24.Murray B E. The life and times of the Enterococcus. Clin Microbiol Rev. 1990;3:46–65. doi: 10.1128/cmr.3.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel R, Piper K E, Rouse M S, Steckelberg J M, Uhl J R, Kohner P, Hopkins M K, Cockerill III F R, Kline B C. Determination of 16S rRNA sequences of enterococci and application to species identification of nonmotile Enterococcus gallinarum isolates. J Clin Microbiol. 1998;36:3399–3407. doi: 10.1128/jcm.36.11.3399-3407.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quednau M, Ahrné S, Petersson A C, Molin G. Identification of clinically important species of Enterococcus within 1 day with randomly amplified polymorphic DNA (RAPD) Curr Microbiol. 1998;36:332–336. doi: 10.1007/s002849900318. [DOI] [PubMed] [Google Scholar]
- 27.Robbi C, Signoretto C, Boaretti M, Canepari P. The gene coding for penicillin-binding protein 5 of Enterococcus faecalis is useful for the development of a species-specific DNA probe. Microb Drug Resist. 1996;2:215–218. doi: 10.1089/mdr.1996.2.215. [DOI] [PubMed] [Google Scholar]
- 28.Ruoff K L, De La Maza L, Murtagh M J, Spargo J D, Ferraro M J. Species identification of enterococci isolated from clinical specimens. J Clin Microbiol. 1990;28:435–437. doi: 10.1128/jcm.28.3.435-437.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sader H S, Biedenbach D, Jones R N. Evaluation of Vitek and API20S for species identification of enterococci. Diagn Microbiol Infect Dis. 1995;22:315–319. doi: 10.1016/0732-8893(95)00146-5. [DOI] [PubMed] [Google Scholar]
- 30.Satake S, Clark N, Rimland D, Nolte F S, Tenover F C. Detection of vancomycin-resistant enterococci in fecal samples by PCR. J Clin Microbiol. 1997;35:2325–2330. doi: 10.1128/jcm.35.9.2325-2330.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schaberg D R, Culver D H, Gaynes R P. Major trends in the microbial etiology of nocosomial infection. Am J Med. 1991;91:72S–75S. doi: 10.1016/0002-9343(91)90346-y. [DOI] [PubMed] [Google Scholar]
- 32.Singer D A, Jochimsen E M, Gielerak P, Jarvis W R. Pseudo-outbreak of Enterococcus durans infections and colonization associated with introduction of an automated identification system software update. J Clin Microbiol. 1996;34:2685–2687. doi: 10.1128/jcm.34.11.2685-2687.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teixeira L M, Carvalho M G S, Merquior V L C, Steigerwalt A G, Teixeira M G M, Brenner D J, Facklam R R. Recent approaches on the taxonomy of the enterococci and some related microorganisms. Adv Exp Med Biol. 1997;418:397–400. doi: 10.1007/978-1-4899-1825-3_95. [DOI] [PubMed] [Google Scholar]
- 34.Tritz D M, Iwen P C, Woods G L. Evaluation of MicroScan for identification of Enterococcus species. J Clin Microbiol. 1990;28:1477–1478. doi: 10.1128/jcm.28.6.1477-1478.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tyrrell G J, Bethune R N, Willey B, Low D E. Species identification of enterococci via intergenic ribosomal PCR. J Clin Microbiol. 1997;35:1054–1060. doi: 10.1128/jcm.35.5.1054-1060.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whelen A C, Persing D H. The role of nucleic acid amplification and detection in the clinical microbiology laboratory. Annu Rev Microbiol. 1996;50:349–373. doi: 10.1146/annurev.micro.50.1.349. [DOI] [PubMed] [Google Scholar]
- 37.Williams A M, Rodrigues U M, Collins M D. Intragenic relationships of enterococci as determined by reverse transcriptase sequencing of small-subunit rRNA. Res Microbiol. 1991;142:67–74. doi: 10.1016/0923-2508(91)90098-u. [DOI] [PubMed] [Google Scholar]
- 38.Woodford N J, Morrison A P, Speller D, David C E. Current perspectives on glycopeptide resistance. Clin Microbiol Rev. 1995;8:585–615. doi: 10.1128/cmr.8.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zolg J W, Philippi-Schulz S. The superoxide dismutase gene, a target for detection and identification of mycobacteria by PCR. J Clin Microbiol. 1994;32:2801–2812. doi: 10.1128/jcm.32.11.2801-2812.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]