Abstract
Background
Emergency medical services (EMS) patients with acute dyspnea require prompt treatment. Limited data describe out‐of‐hospital dyspnea treatment with non‐invasive, positive‐pressure ventilation (NIPPV), including continuous positive airway pressure (CPAP) or bi‐level positive air pressure (BPAP). We sought to determine the course and outcomes of out‐of‐hospital acute dyspnea patients treated with NIPPV.
Methods
We analyzed retrospective data on 1289 EMS agencies from the ESO Data Collaborative (ESO, Inc., Austin, TX) between January and December 2018. We defined acute dyspnea as adults with an initial respiratory rate ≥ 30 breaths/min (bpm), with a primary or secondary EMS subjective impression of a respiratory condition, who received oxygen and/or a respiratory medication and had 2 or more recordings of respiratory rate (RR). We excluded patients with trauma and those with altered mental status. We identified cases receiving care with and without NIPPV. The primary outcome was change in respiratory rate (RR), censored at 90 minutes of treatment. We compared baseline characteristics between NIPPV and non‐NIPPV patients. We compared RR changes between NIPPV and non‐NIPPV patients at 20 and 40 minutes of treatment. Using mixed linear, fractional polynomial, and multiple spline models, we examined the association of out‐of‐hospital NIPPV with overall change in RR. Secondary outcomes included whether the patient received advanced airway treatment (intubation, supraglottic airway device, and/or cricothyroidotomy).
Results
We analyzed 33,585 EMS encounters for patients with acute dyspnea, including 8,750 (26.1%) NIPPV and 24,835 (73.9%) non‐NIPPV encounters. Median treatment duration was similar between NIPPV and non‐NIPPV (23.3 minutes vs 23.6 minutes, rank‐sum P = 0.266). Common concurrent treatments included albuterol (NIPPV, 48.8%; non‐NIPPV, 46.2%), ipratropium bromide (27.9%, 24.8%), and methylprednisolone (24.9%, 18.5%). At 20 minutes, mean RR change was slightly lower for the NIPPV group than non‐NIPPV; −6.0 versus −6.8 breaths/min. At 40 minutes, mean RR change was similar between NIPPV and non‐NIPPV groups; −7.7 versus −7.9 breaths/min. On linear mixed modeling adjusted for age, sex, incident location, race, ethnicity, agency type, initial RR, and medication use, NIPPV was associated with a smaller RR decrease across time than NIPPV; [NIPPV × time] interaction P < 0.001. Out‐of‐hospital advanced airway placement (endotracheal intubation or supraglottic airway insertion) was higher for NIPPV than non‐NIPPV group (2.3% vs 1.3%, odds ratio = 2.23, 95% confidence interval = 2.01–2.47).
Conclusions
NIPPV has been proven to be an effective treatment for out‐of‐hospital patients experiencing acute dyspnea through prior studies. Our findings provide detailed insight into characteristics and use of NIPPV and highlight the commonality of this treatment modality with use in over 1 in 4 patients in respiratory distress.
Keywords: dyspnea, emergency medical services, noninvasive ventilation
1. INTRODUCTION
1.1. Background
More than 1 out of every 10 emergency medical services (EMS) encounters involves patients with acute respiratory distress. 1 EMS personnel care for patients with acute dyspnea due to conditions, such as congestive heart failure (HF), chronic obstructive pulmonary disease (COPD), asthma, and pneumonia, among others. Prompt treatment is critical in preventing deterioration and optimizing outcomes of patients with acute dyspnea. An out‐of‐hospital treatment option for acute dyspnea is non‐invasive positive‐pressure ventilation (NIPPV), encompassing continuous positive air pressure (CPAP) or bi‐level positive airway pressure (BPAP). 2
1.2. Importance
Although prior studies describe out‐of‐hospital NIPPV, these efforts have important limitations. These mainly occurred in single EMS agencies with limited sample sizes. A meta‐analysis of 10 studies totaling 190 BPAP and 610 CPAP patients included mainly EMS systems with physicians in Europe and provided limited insights of the course and outcomes of patients receiving out‐of‐hospital NIPPV. 3 Limited data characterize NIPPV in the care of acute dyspnea in United States EMS systems.
1.3. Goals of this investigation
We sought to determine the course and outcomes of out‐of‐hospital acute dyspnea patients treated with NIPPV.
2. MATERIALS AND METHODS
2.1. Study design and setting
This study was approved by the Committee for the Protection of Human Subjects at the University of Texas Health Science Center at Houston. We performed a retrospective analysis of patient care records from the ESO Data Collaborative (Austin, TX). ESO is a large out‐of‐hospital electronic health record system used by EMS agencies throughout the United States. The electronic health record (EHR) software collects a broad range of information related to the EMS encounter, including event characteristics, patient demographics, clinical signs and symptoms, interventions, vital signs, and outcomes. This software is compliant with the National EMS Information System (NEMSIS) version 3 standards. The Data Collaborative contains all patient care records from EMS agencies who have signed voluntary agreements to contribute their de‐identified data for research and benchmarking. In 2018, there were more than 1,200 EMS agencies participating in the Data Collaborative. The 2018 public use research data set used for this analysis contained more than 7.5 million EMS encounters and is made available to researchers through a proposal process and approval by an external expert review committee.
In select communities, out‐of‐hospital patient care records are linked with hospital outcomes data including diagnosis and disposition. This linkage is accomplished through ESO's proprietary bi‐directional Healthcare Data Exchange (HDE) system. For the bi‐directional exchange to occur, both the EMS agency and receiving hospital must use the ESO HDE software product. HDE use is voluntary. For participating hospitals and EMS agencies, hospital data elements, including ICD‐10 diagnosis codes and dispositions, are linked back to the prehospital record using HL7 messaging.
2.2. Selection of subjects
We included 911 calls for adult (≥18 years of age) patients treated by a paramedic level service with acute dyspnea. We defined acute dyspnea as an initial respiratory rate ≥30 breaths/min (bpm) with a primary or secondary EMS subjective impression of a respiratory condition (Appendix 1), who received an intervention involving oxygen and/or a respiratory medication, and had 2 or more recordings of RR. We excluded patients presenting with trauma or altered mental status, those patients who expired on scene, and any patient vital signs after the point of intubation (Figure 1).
FIGURE 1.
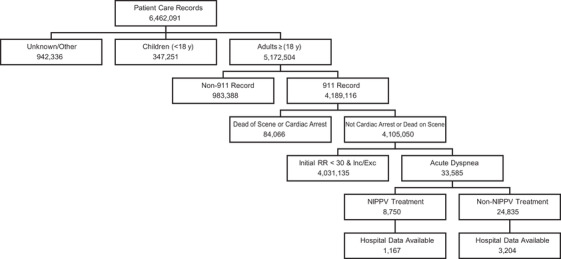
Study population. Acute dyspnea defined as adults with an initial respiratory rate ≥30 breaths/min (bpm), with a primary or secondary EMS subjective impression of a respiratory condition, who received oxygen and/or a respiratory medication, and had 2 or more recordings of respiratory rate. EMS, emergency medical services; RR, respiratory rate; NIPPV, non‐invasive positive pressure ventilation
2.3. Exposure
The primary exposure was the out‐of‐hospital use of NIPPV. We defined NIPPV as use of CPAP or BPAP. The comparison group consisted of patients with acute dyspnea that did not receive NIPPV.
The Bottom Line.
Although commonly used by paramedics, only limited data describe the course and outcomes of patients treated by out‐of‐hospital, non‐invasive, positive‐pressure ventilation (NIPPV). In this analysis of over 33,000 emergency medical service (EMS) dyspnea patients (including over 8,000 treated with NIPPV), reductions in respiratory rate were less pronounced than without NIPPV; however, inferences from these results are limited due to the non‐randomized design of the study. This is one of the largest descriptions of EMS NIPPV use.
2.4. Outcomes
The primary outcome was change in RR during the EMS encounter. We included respiratory rates censored at 90 minutes of care starting from the point of the first vital sign (>99.99% of recorded vital signs in study population). We used 20‐minute and 40‐minute time intervals, and first versus last, to determine change in RR because the majority (91%) of patients had a total treatment time of <40 minutes. We modeled the RR of the treatment group pre‐ and post‐application of the NIPPV device to determine if there was a difference with the change in RR over time. Secondary outcomes included changes in heart rate (HR), systolic blood pressure (SBP), and SpO2 and whether the patient received an advanced airway treatment (intubation, supraglottic airway device, and/or cricothyroidotomy).
Although other respiratory variables were available, including peripheral capillary oxygen saturation (SpO2) and end‐tidal carbon dioxide (ETCO2), we used RR as the primary indicator of acute dyspnea, because this measure was the most consistently available measure across EMS agencies. We did not use SpO2 as an indicator of acute dyspnea because some causes may not initially present with a decrease in oxygen saturation, and patients receiving oxygen therapy could still have dyspnea while presenting with a normal oxygen saturation. The data set does not contain subjective measures of dyspnea, such as a Borg score. 4 , 5
Where linked to hospital data, we determined ICD10 discharge diagnoses and Charlson comorbidity index score of hospitalized patients.
2.5. Data analysis
We compared patient age, sex, race, ethnicity, initial RR, agency type, and incident location between NIPPV and non‐NIPPV patients. We compared initial vital signs, response time, scene time, transport time, and treatment time between NIPPV and non‐NIPPV patients. We determined the association between NIPPV use and the change in RR using a t‐test. We used linear mixed modeling for comparison between the NIPPV and non‐NIPPV group with the change in RR as the dependent outcome and the use of NIPPV as the primary exposure. We used multivariable linear regression and fractional polynomial modeling with the change in RR as the dependent outcome and NIPPV application time as the independent variable to see the impact that NIPPV had within the treatment group. We modeled EMS agency as a random effect. The model adjusted for pertinent confounders including age, sex, race, EMS agency type, incident location, medication use, and initial respiratory rate. In a secondary analysis, we examined the primary hospital discharge diagnoses of patients with available linkage between EMS and hospital records. All analysis was performed using Stata 15.1 (College Station, TX).
3. RESULTS
Of 5,172,504 adult 911 patients, 33,585 (0.6%) presented with acute dyspnea, including 8,750 (26.1%) NIPPV and 24,835 (73.9%) non‐NIPPV cases (Table 1). The NIPPV group included 8,696 (99.4%) patients who received CPAP and 54 (0.6%) who were treated with BPAP. NIPPV use was more common in patients >60 years (28.4% vs 21.1%). The most common primary clinical impressions for NIPPV and non‐NIPPV were respiratory and cardiac emergencies (Table 2). Pulmonary medication use was more common for NIPPV than for non‐NIPPV patients (80.1% vs 73.2%, P < 0.0001). Common concurrent pulmonary treatments for NIPPV and non‐NIPPV included the following: albuterol (48.8%, 46.2%), ipratropium bromide (27.9%, 24.8%), and methylprednisolone (24.9%, 18.5%) (Table 3).
TABLE 1.
Characteristicsof EMS patients with acute dyspnea
| Characteristic | NIPPV n (%) | Non‐NIPPV n (%) | All patients n (%) | NIPPV versus Non‐NIPPV OR (95% CI) |
|---|---|---|---|---|
| Age (y) | ||||
| 18‐30 | 109 (1.2) | 1,152 (4.6) | 1,261 (3.8) | Reference |
| 31‐40 | 166 (1.9) | 1,179 (4.7) | 1,345 (4.0) | 1.49 (1.15‐1.92) |
| 41‐50 | 432 (4.9) | 1,970 (7.9) | 2,402 (7.2) | 2.32 (1.86‐2.89) |
| 51‐60 | 1,604 (18.3) | 4,336 (17.5) | 5,940 (17.7) | 3.91 (3.19‐4.8) |
| 61‐70 | 2,495 (28.5) | 5,810 (23.4) | 8,305 (24.7) | 4.54 (3.71‐5.55) |
| >70 | 3,944 (45.1) | 10,388 (41.8) | 14,332 (42.7) | 4.01 (3.29‐4.9) |
| Sex | ||||
| Female | 4,556 (52.1) | 13,426 (54.1) | 17,982 (53.5) | Reference |
| Male | 4,153 (47.5) | 11,271 (45.4) | 15,424 (45.9) | 1.09 (1.03‐1.14) |
| Unknown | 41 (0.5) | 138 (0.6) | 179 (0.5) | 0.88 (0.62‐1.24) |
| Race | ||||
| American Indian or Alaska Native | 7 (0.1) | 35 (0.1) | 42 (0.1) | Reference |
| Asian | 44 (0.5) | 140 (0.6) | 184 (0.5) | 1.57 (0.65‐3.79) |
| Black or African American | 2,111 (24.1) | 5,094 (20.5) | 7,205 (21.5) | 2.07 (0.92‐4.67) |
| Native Hawaiian or Other Pacific Islander | 4 (0.0) | 19 (0.1) | 23 (0.1) | 1.05 (0.27‐4.06) |
| Other race | 11 (0.1) | 35 (0.1) | 46 (0.1) | 1.57 (0.55‐4.52) |
| White | 6,104 (69.8) | 18,032 (72.6) | 24,136 (71.9) | 1.58 (0.7‐3.59) |
| Unknown | 469 (5.4) | 1,480 (6.0) | 1,949 (5.8) | 1.69 (0.75‐3.81) |
| Ethnicity | ||||
| Hispanic or Latino | 282 (3.2) | 986 (4.0) | 1268 (3.8) | Reference |
| Not Hispanic or Latino | 7,569 (86.5) | 21,482 (86.5) | 29,051 (86.5) | 1.23 (1.08‐1.41) |
| Unknown | 899 (10.3) | 2,367 (9.5) | 3,266 (9.7) | 1.33 (1.14‐1.55) |
| Initial RR | ||||
| 30‐34 | 4,389 (50.2) | 14,974 (60.3) | 19,363 (57.7) | Reference |
| 35‐39 | 1,784 (20.4) | 4,532 (18.2) | 6,316 (18.8) | 1.34 (1.26‐1.43) |
| 40‐44 | 1,724 (19.7) | 3,452 (13.9) | 5,176 (15.4) | 1.7 (1.59‐1.82) |
| 45‐49 | 397 (4.5) | 718 (2.9) | 1,115 (3.3) | 1.89 (1.66‐2.14) |
| 50‐54 | 260 (3.0) | 460 (1.9) | 720 (2.1) | 1.93 (1.65‐2.25) |
| 55‐59 | 67 (0.8) | 142 (0.6) | 209 (0.6) | 1.61 (1.2‐2.16) |
| ≥60 | 129 (1.5) | 557 (2.2) | 686 (2.0) | 0.79 (0.65‐0.96) |
| Initial vital sign (IQR) | ||||
| RR | 34 (30‐40) | 32 (30‐38) | 33 (30‐38) | P < 0.001 a |
| SpO2 | 85 (76‐92) | 92 (85‐97) | 90 (83‐96) | P < 0.001 a |
| Heart rate | 113 (97‐129) | 106 (90‐122) | 109 (91‐124) | P < 0.001 a |
| Systolic blood pressure | 162 (139‐190) | 148 (12‐170) | 150 (130‐177) | P < 0.001 a |
| EMS agency type | ||||
| Community, non‐profit | 5,405 (61.8) | 15,786 (63.6) | 21,191 (63.1) | Reference |
| Fire department | 1,104 (12.6) | 3,045 (12.3) | 4,149 (12.4) | 1.06 (0.98‐1.14) |
| Governmental, non‐fire | 1,776 (20.3) | 4,407 (17.7) | 6,183 (18.4) | 1.18 (1.1‐1.25) |
| Private, non‐hospital | 465 (5.3) | 1,597 (6.4) | 2,062 (6.1) | 0.85 (0.76‐0.95) |
| Median min (interquartile range) | ||||
| Response time | 7 (5‐10) | 7 (5‐10) | 7 (5‐10) | P < 0.32 a |
| On scene time | 18 (14‐23) | 17 (13‐22) | 18 (13‐22) | P < 0.00 a |
| Transport time | 11 (7‐17) | 13 (8‐19) | 12 (8‐19) | P < 0.001 a |
| Incident location | ||||
| Healthcare facility | 1,274 (14.6) | 4,140 (16.7) | 5,414 (16.1) | Reference |
| Public location | 251 (2.9) | 1,268 (5.1) | 1,519 (4.5) | 0.64 (0.55‐0.75) |
| Residence | 6,963 (79.6) | 18,252 (73.5) | 25,215 (75.1) | 1.24 (1.16‐1.33) |
| Outdoor/street/highway | 115 (1.3) | 533 (2.1) | 648 (1.9) | 0.7 (0.57‐0.87) |
| Unknown | 0 (0.0) | 1 (0.0) | 1 (0.0) | N/A |
| Other | 147 (1.7) | 641 (2.6) | 788 (2.3) | 0.75 (0.29‐0.33) |
Abbreviations: COPD, chronic obstructive pulmonary disease; EMS, emergency medical services; IQR, interquartile range; NIPPV, non‐invasive, positive‐pressure ventilation; OR, odds ratio; RR, respiratory rate.
Includes 8750 NIPPV and 24,835 non‐NIPPV. ORs determined using logistic regression.
Wilcoxon rank‐sum test.
TABLE 2.
EMS primary clinical impression of patients with acute dyspnea
| Suspected illness | NIPPV n (%) | Non‐NIPPV n (%) | All patients n (%) |
|---|---|---|---|
| Airway obstruction | 0 (0.0) | 7 (0.0) | 7 (0.0) |
| Behavioral | 1 (0.0) | 180 (0.7) | 181 (0.5) |
| Cardiac arrest | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Cardiac symptoms | 764 (8.7) | 2,763 (11.1) | 3,527 (10.5) |
| Diabetes/endocrine | 5 (0.1) | 50 (0.2) | 55 (0.2) |
| Environmental | 3 (0.0) | 70 (0.3) | 73 (0.2) |
| Foreign body | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Gastrointestinal | 1 (0.0) | 36 (0.1) | 37 (0.1) |
| Hemorrhage | 5 (0.1) | 27 (0.1) | 32 (0.1) |
| Infection | 33 (0.4) | 342 (1.4) | 375 (1.1) |
| Injury/trauma | 3 (0.0) | 13 (0.1) | 16 (0.0) |
| Neurological | 1 (0.0) | 63 (0.3) | 64 (0.2) |
| Obstetric/neonatal | 1 (0.0) | 1 (0.0) | 2 (0.0) |
| Pain | 2 (0.0) | 43 (0.2) | 45 (0.1) |
| Poisoning/overdose | 5 (0.1) | 213 (0.9) | 218 (0.6) |
| Renal | 1 (0.0) | 24 (0.1) | 25 (0.1) |
| Respiratory a | 7,903 (90.3) | 20,696 (83.3) | 28,599 (85.2) |
| Respiratory distress | 5,671 (64.8) | 14,189 (57.1) | 19,860 (59.1) |
| Emphysema/COPD | 655 (7.5) | 1,500 (6.0) | 2,155 (6.4) |
| Shortness of breath | 297 (3.4) | 1,741 (7.0) | 2,038 (6.1) |
| Shock | 11 (0.1) | 55 (0.2) | 66 (0.2) |
| Other | 11 (0.1) | 252 (1.0) | 263 (0.8) |
Abbreviations: COPD, chronic obstructive pulmonary disease; EMS, emergency medical services; NIPPV, non‐invasive, positive‐pressure ventilation.
Includes 8750 NIPPV and 24,835 non‐NIPPV
Top 3 respiratory categories.
TABLE 3.
Pharmacologic interventions for acute dyspnea
| Intervention | NIPPV n (%) | Non‐NIPPV n (%) | All patients n (%) |
|---|---|---|---|
| n = 8750 | n = 24,835 | n = 33,585 | |
| Albuterol | 4,266 (48.8) | 11,469 (46.2) | 15,735 (46.9) |
| Bumetanide | 1 (0.0) | 0 (0.0) | 1 (0.0) |
| Combivent | 1,473 (16.8) | 4,036 (16.3) | 5,509 (16.4) |
| Dobutamine | 3 (0.0) | 1 (0.0) | 4 (0.0) |
| Dopamine | 5 (0.1) | 7 (0.0) | 12 (0.0) |
| Ipratropium | 2,443 (27.9) | 6,160 (24.8) | 8,603 (25.6) |
| Furosemide | 257 (2.9) | 186 (0.7) | 443 (1.3) |
| Levosalbutamol | 143 (1.6) | 450 (1.8) | 593 (1.8) |
| Magnesium sulfate | 621 (7.1) | 668 (2.7) | 1,289 (3.8) |
| Methylprednisolone | 2,179 (24.9) | 4,606 (18.5) | 6,785 (20.2) |
| Morphine | 36 (0.4) | 144 (0.6) | 180 (0.5) |
| Nitroglycerine | 2,038 (23.3) | 2,644 (10.6) | 4,682 (13.9) |
| Norepinephrine | 3 (0.0) | 8 (0.0) | 11 (0.0) |
| Other | 1,655 (18.9) | 5,493 (22.1) | 7,148 (21.3) |
Abbreviation: NIPPV, non‐invasive, positive‐pressure ventilation.
Includes 8750 NIPPV and 24,835 non‐NIPPV. Excludes repeat treatments. Patients may have received more than 1 intervention.
Mean initial RR was slightly higher for NIPPV than non‐NIPPV patients (36.1 bpm vs 35.4 bpm), mean initial SpO2 was lower for NIPPV than non‐NIPPV (83.1% vs 89.4%), mean initial HR was higher for NIPPV than non‐NIPPV (112.7 beats/min vs 106.9 beats/min), and mean initial SBP was higher for NIPPV than non‐NIPPV (164.7 mm Hg vs 149.6 mm Hg).
Median treatment duration was similar between NIPPV and non‐NIPPV (23.3 minutes vs 23.6 minutes, rank‐sum P = 0.266). Mean RR change at 20 minutes was greater for non‐NIPPV patients than NIPPV (−6.8 bpm vs−6.0 bpm) patients; this difference resolved at 40 minutes of care (−7.9 bpm vs −7.7 bpm). The mean RR change between the first and last recorded values was greater for non‐NIPPV than NIPPV (−7.4 bpm vs−6.5 bpm) (Table 4). On adjusted linear mixed modeling, non‐NIPPV treatment was associated with a larger decrease in RR across time than NIPPV treatment; [NIPPV × time] interaction P < 0.001 (Figure 2). Multiple splines and fractional polynomial modeling of only the NIPPV group suggested a greater decline in RR after application of NIPPV (Figure 3).
TABLE 4.
Vital sign changes over course of EMS care
| Vital sign | Initial vital sign | Last vital sign | Mean difference in first versus last measurement (95% CI) | Percent of patients with vital sign recorded (%) |
|---|---|---|---|---|
| NIPPV | ||||
| RR (breaths/min) | 36.1 | 29.6 | −6.5 (−6.3 to −6.7) | 100.0 |
| SPO2 (% saturation) | 83.1 | 92.8 | 9.7 (9.5 to 10.0) | 91.2 |
| Systolic blood pressure (mm Hg) | 164.7 | 155.7 | −9.0 (−8.3 to −9.7) | 80.5 |
| Pulse rate (beats/min) | 112.7 | 109.9 | −2.8 (−2.4 to −3.2) | 94.7 |
| Non‐NIPPV | ||||
| RR (breaths/min) | 35.4 | 28.0 | −7.4 (−7.2 to −7.5) | 100.0 |
| SPO2 (% saturation) | 89.4 | 95.6 | 6.2 (6.1 to 6.3) | 90.1 |
| Systolic blood pressure (mm Hg) | 149.6 | 142.8 | −6.8 (−6.5 to −7.2) | 83.6 |
| Pulse rate (beats/min) | 106.9 | 103.6 | −3.3 (−3.1 to −3.6) | 95.4 |
Abbreviations: CI, confidence interval; EMS, emergency medical services; NIPPV, non‐invasive, positive‐pressure ventilation; RR, respiratory rate.
Initial vital signs limited to measurements within first 5 minutes of care.
FIGURE 2.
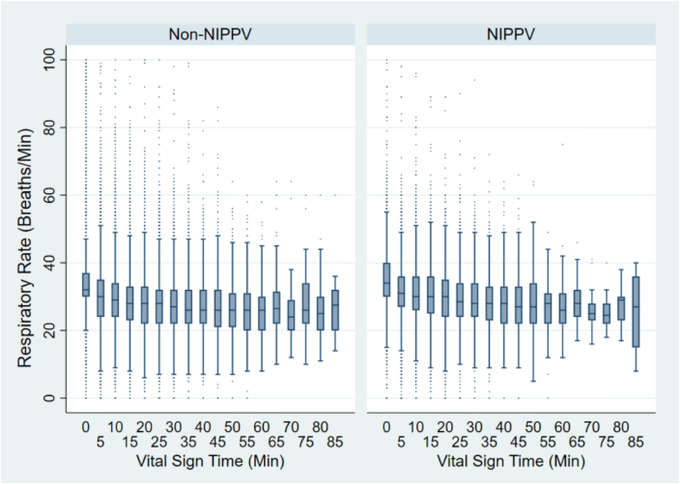
Respiratory rate over course‐of‐care for acute dyspnea patients stratified by NIPPV use
FIGURE 3.
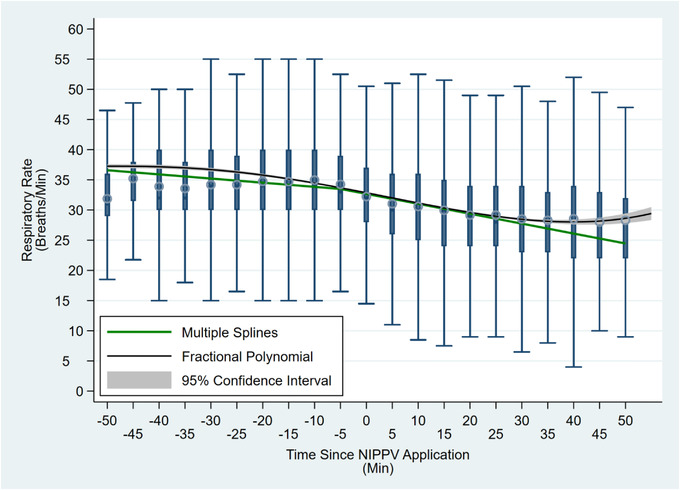
Fractional polynomial and spline models of respiratory rate over time for patients receiving NIPPV. Time = 0 minutes reflects point of NIPPV application
Out‐of‐hospital advanced airway placement (endotracheal intubation or supraglottic airway insertion) was higher for NIPPV than non‐NIPPV group (2.3% vs 1.3%, odds ratio = 2.23, 95% confidence interval = 2.01–2.47). There were no significant differences in respiratory rate changes (or intubation rates (2.4% vs 5.9%, P = 0.113) between CPAP and BiPAP patients.
Hospital data were available for 13.0% of included cases. The most common diagnoses for NIPPV patients were respiratory failure (84.1%), COPD (50.3%), and congestive heart failure (CHF) (47.7%), and respiratory failure (48.0%), COPD (41.2%), and CHF (29.2%) for non‐NIPPV patients (Table 5). Charlson comorbidity index score, calculated through ICD10 codes, were found to be 1.27 for non‐NIPPV and 1.59 NIPPV patients.
TABLE 5.
Hospital diagnosis of EMS patients with acute dyspnea
| Intervention | NIPPV n (%) | Non‐NIPPV n (%) | All patients n (%) |
|---|---|---|---|
| n = 1167 | n = 3204 | n = 4371 | |
| Asthma | 88 (7.5) | 360 (11.2) | 448 (10.2) |
| Bronchitis | 15 (1.3) | 43 (1.3) | 58 (1.3) |
| CHF | 557 (47.7) | 935 (29.2) | 1492 (34.1) |
| COPD | 587 (50.3) | 1319 (41.2) | 1906 (43.6) |
| Emphysema | 27 (2.3) | 63 (2) | 90 (2.1) |
| Pneumonia | 290 (24.9) | 783 (24.4) | 1073 (24.5) |
| Pulmonary edema | 128 (11) | 118 (3.7) | 246 (5.6) |
| Respiratory failure | 981 (84.1) | 1539 (48) | 2520 (57.7) |
| Other respiratory disease | 231 (19.8) | 721 (22.5) | 952 (21.8) |
Abbreviations: CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; EMS, emergency medical services; NIPPV, non‐invasive, positive‐pressure ventilation.
Includes 4,371 of 33,585 (13.0%) successfully linked with hospital records. Patients may have received more than 1 diagnosis.
3.1. LIMITATIONS
Our study was retrospective and subject to recall and reporting bias. Confounding variables may not have been identified or documented in the patient care report. Confounding by indication likely influenced paramedics’ decision to initiate NIPPV. Initial respiratory rates were higher for patients treated with NIPPV, so the opportunity to improve have might disproportionately favored this group. Medical interventions and treatments differed from patient to patient. EMS treatment and documentation protocols varied across services. The EMS agencies included have varying protocols and practices, ranging from variations in standing delegated orders to the distance of the receiving hospital. We attempted to mitigate the variation in level of service by limiting our analysis to only those agencies offering paramedic level of care.
EMS agencies did not consistently document common indicators of acute dyspnea, such as tidal volume, SpO2, and ETCO2. EMS personnel subjectively documented whether the patient had a respiratory complaint using ESO's preselected categorizations that could have led to cases being missed. We chose RR as the primary indicator of acute dyspnea due to consistent documentation of this value in the ESO dataset. Vital sign measurement intervals varied widely from patient to patient. Although many agencies likely imported vital signs from their patient care monitors, the source of the vital signs was not included in the dataset and, therefore, respiratory rates may have been objectively measured. We estimated the RR at 20‐minute and 40‐minute intervals using a linear regression between the last recorded RR prior to the interval and the first recorded RR after the interval but acknowledge that additional changes may have occurred beyond this point that were not captured in our analysis. We included the change between the first and last recorded respiratory rate as well but the length of treatment time varied between patients.
Hospital outcomes were available for only a subset of patients in our study. Future prospective study is needed to further evaluate out‐of‐hospital treatment with hospital course and outcomes.
4. DISCUSSION
NIPPV is used widely in the out‐of‐hospital treatment of acute dyspnea. We present one of the largest descriptions of out‐of‐hospital NIPPV use in the United States, encompassing 8,750 patients. Although these data represent a sample of convenience, the included records are national in scope, spanning care from over 1,200 EMS agencies.
More than 1 in 4 patients presenting with acute dyspnea were treated with NIPPV. Our study has important limitations and should not be used to formulate conclusions regarding the efficacy of NIPPV. Although we observed larger reduction in respiratory rate with non‐NIPPV, our series was not randomized and vulnerable to confounding by indication. Confounders likely influencing the association between NIPPV use and RR include comorbidities, severity of illness, and caregiver selection bias. We note that patients were older and baseline vital signs were worse in the NIPPV than non‐NIPPV groups. Another limitation was our use of RR as the primary outcome, which we selected because it is widely reported by EMS personnel. Alternate measures such as the Borg score may better reflect respiratory distress but were not available in the data set. We elected not to use SpO2 as an indicator of acute dyspnea because patients undergoing oxygen resuscitation may exhibit a normal SpO2 even in the face of severe dyspnea. Likewise, ETCO2 values are reported inconsistently among spontaneously breathing patients. Further, the observed difference of <1 bpm between the non‐NIPPV and NIPPV groups, though statistically significant, may not be clinically relevant. Our analysis provides the best retrospective description of out‐of‐hospital NIPPV given the limitations of the available data. Randomization of NIPPV use and the systematic collection of respiratory measures such as Borg scores, RR, SaO2, ETCO2, and rate of intubation would be necessary to overcome these important limitations. 6
Few studies have described NIPPV in the out‐of‐hospital setting. Published works have focused mainly on patients experiencing acute dyspnea due to specific etiologies such as pneumonia, COPD and/or CHF exacerbations, examining mostly intubation rates and hospital survival. 3 In a randomized controlled trial of 70 out‐of‐hospital dyspnea patients, Thompson et al 7 found out‐of‐hospital NIPPV use on properly selected patients led to a 30% absolute reduction in intubation rates and a 21% absolute reduction in mortality. A meta‐analysis by Goodacre et al 3 confirmed a reduction in intubation rates and mortality for the group of patients who received CPAP compared with controls. Although not performed in the out‐of‐hospital setting, Arsude et al 8 found that there was a significant improvement in Borg score at 4, 12, and 24 hours after initial presentation. Although our study was limited to the use of respiratory rate, we were able to include a larger selection of patients with a range of etiologies for acute dyspnea. We were also able to provide more detailed insight into patient demographics and clinical course associated with out‐of‐hospital NIPPV treatment.
NIPPV plays a practical role in the out‐of‐hospital care of dyspnea. NIPPV is non‐invasive, easy to implement, and relatively inexpensive. NIPPV can even be implemented by basic life support personnel. 9 Pharmacologic treatments available for the out‐of‐hospital treatment of acute dyspnea generally include the use of nitroglycerin, diuretics, beta‐agonists, steroids, magnesium sulfate, and epinephrine; NIPPV provides an important adjunct to these therapies and creates an alternative to endotracheal intubation in cases of worsened respiratory distress or failure. Acknowledging its limitations, our study adds to existing knowledge regarding NIPPV use, supporting its use across a range of clinical settings in the United States. Future study is important to help delineate the role, methods and indications for out‐of‐hospital NIPPV as well as the best indicators of benefit.
In summary, NIPPV has been proven to be an effective treatment for out‐of‐hospital patients experiencing acute dyspnea through prior studies. Our findings provide detailed insight into characteristics and use of NIPPV and highlight the commonality of this treatment modality with use in over 1 in 4 patients in respiratory distress.
AUTHOR CONTRIBUTIONS
DCW and HEW designed the study. DCW, HEW, HKC, and REC worked on data acquisition and analysis. All study authors assisted with data interpretation. DCW and HEW prepared the manuscript. All authors worked on review and revision of the manuscript, and gave approval for final version to be published. DCW takes responsibility for the paper as a whole.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest. Dr. Wang was not involved in the review or acceptance of this manuscript.
Biography
Daniel C. Walter, BS, is a medical student at McGovern Medical School in Houston, Texas. He holds a Bachelor of Science in Petroleum Engineering from Texas A&M University College Station.

RESPIRATORY CONDITIONS REPORTED BY EMS PERSONNEL
1.
| Respiratory condition |
| Acute bronchitis |
| Acute bronchospasm |
| Altitude sickness |
| Asthma |
| Atelectasis |
| Chest pain (non‐cardiac) |
| Chest pain on breathing |
| Emphysema/COPD |
| Hyperventilation |
| Hyponasality |
| Other respiratory |
| Other tracheostomy complication |
| Pneumonia |
| Pneumothorax |
| Pneumothorax (traumatic) |
| Possible SIDS |
| Primary pulmonary hypertension |
| Pulmonary edema, acute |
| Pulmonary embolism |
| Respiratory arrest |
| Respiratory condition due to chemicals, gases, fumes, and vapors |
| Respiratory disorder |
| Respiratory distress |
| Respiratory failure |
| Severe acute respiratory syndrome (SARS) |
| Shortness of breath |
| Tracheostomy problem |
| Acute bronchitis |
Walter DC, Chan HK, Crowe RP, Osborn L, Jarvis J, Wang HE. Out‐of‐hospital, non‐invasive, positive‐pressure ventilation for acute dyspnea. JACEP Open. 2021;2:e12542. 10.1002/emp2.12542
Supervising Editor: Michael Blaivas, MD, MBA
Funding and support: By JACEP Open policy, all authors are required to disclose any and all commercial, financial, and other relationships in any way related to the subject of this article as per ICMJE conflict of interest guidelines (see www.icmje.org ). The authors have stated that no such relationships exist.
REFERENCES
- 1. Wang HE, Mann NC, Jacobson KE, et al. National characteristics of emergency medical services responses in the United States. Prehol Emerg Care. 2013;17(1). [DOI] [PubMed] [Google Scholar]
- 2. Hörmann C, Baum M, Putensen C, Mutz NJ, Benzer H. Biphasic positive airway pressure (BIPAP)–a new mode of ventilatory support. Eur J Anaesthesiol. 1994;11(1).): [PubMed] [Google Scholar]
- 3. Goodacre S, Stevens JW, Pandor A, et al. Prehospital noninvasive ventilation for acute respiratory failure: systematic review, network meta‐analysis, and individual patient data meta‐analysis. Acad Emerg Med . 2014;21(9). [DOI] [PubMed] [Google Scholar]
- 4. Sarullo FM, D'Alfonso G, Brusca I, et al. [Efficacy and safety of non‐invasive positive pressure ventilation therapy in acute pulmonary edema]. Monaldi arch chest dis = Arch Monaldi per le malattie del torace. 2004;62(1). [PubMed] [Google Scholar]
- 5. Mador MJ, Rodis A, Magalang UJ. Reproducibility of Borg scale measurements of dyspnea during exercise in patients with COPD. Chest. 1995;107(6):1590‐1597. [DOI] [PubMed] [Google Scholar]
- 6. Sengupta R, Loftus TM, Doers M, et al. Resting Borg score as a predictor of safe discharge of chronic obstructive pulmonary disease from the emergency department observation unit. Acad Emerg Med. 2020;27(12). [DOI] [PubMed] [Google Scholar]
- 7. Thompson J, Petrie DA, Ackroyd‐Stolarz S, Bardua DJ. Out‐of‐hospital continuous positive airway pressure ventilation versus usual care in acute respiratory failure: a randomized controlled trial. Ann Emerg Med. 2008;52(3). [DOI] [PubMed] [Google Scholar]
- 8. Arsude S, Sontakke A, Jire A. Outcome of noninvasive ventilation in acute respiratory failure. Indian J Crit Care Med. 2019;23(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. National Association of State EMS Officials . National EMS Scope of Practice Model 2019. Report No. DOT HS 812‐666. Washington, DC. [Google Scholar]


