Abstract
Objectives:
Obesity is associated with sarcopenia in older adults, and weight loss can lead to further muscle mass loss. Oxytocin decreases with age, and animal studies suggest that oxytocin administration has trophic effects on skeletal muscle cells and reduces adiposity. We conducted a clinical trial to examine the safety and preliminary efficacy of intranasal oxytocin for older adults with sarcopenic obesity.
Design:
A double-blind, placebo-controlled randomized controlled trial of intranasal oxytocin (24 IU 4 times per day) for 8 weeks.
Setting and Participants:
Twenty-one older (67.5 ± 5.4 years), obese (30–43 kg/m2), sedentary (<2 strenuous exercise per week) adults with slow gait speed (<1 m/s, proxy measure of sarcopenia) were recruited.
Measures:
Generalized estimating equations were used to evaluate the effect of oxytocin on safety/tolerability of oxytocin administration and whole body muscle and fat mass.
Results:
At baseline, body mass index (BMI) was 36.8 ± 3.6 kg/m2, fat mass 46.09 ± 6.99 kg, lean mass 50.98 ± 11.77 kg, fasting plasma glucose (FPG) 92.0 ± 8.9 mg/dL, hemoglobin A1c (HbA1c) 5.7% 0.4%, low density lipoprotein (LDL) 111.3 41.5 mg/dL, high-density lipoprotein (HDL) 47.85 ± 10.96 mg/dL, and triglycerides 140.55 ± 83.50 mg/dL. Oxytocin administration was well tolerated without any significant adverse events. Oxytocin led to a significant increase of 2.25 kg in whole body lean mass compared with placebo (P < .01) with a trend toward decreasing fat mass, and a significantly reduced plasma LDL cholesterol by −19.3 mg/dL (P = .023) compared against placebo. There were no significant changes in BMI, appetite scores, glycemia, plasma HDL, triglycerides, or depressive symptoms.
Conclusions and Implications:
This proof-of-concept study indicates that oxytocin may be useful for the treatment of sarcopenic obesity in older adults. Oxytocin administration may also provide additional cardiovascular benefits.
Keywords: Obesity, sarcopenia, body composition, clinical trials
In humans, muscle mass begins to decline in the third decade of life, contributing to age-related decreases in strength and function.1,2 These physiologic changes of aging increase the risk of developing obesity in older adults.3 Decreased muscle mass combined with increased fat mass can lead to sarcopenic obesity, which affects approximately 18% of older women and 40% of older men.4 The loss of muscle strength often perpetuates a cycle of sedentary behavior, which then leads to additional muscle loss and obesity. Although obesity is highly prevalent in older adults, there is controversy about whether to recommend weight loss because it can lead to decreases in lean muscle mass and bone mass in addition to fat mass, which may be detrimental in older adults who are at increased risk for debility and frailty.5
The etiology of sarcopenia of aging is unclear and likely multifactorial. Factors thought to play a role in sarcopenia of aging include alterations in muscle protein turnover, higher mitochondrial dysfunction, enhanced inflammation, motor neuron dysfunction, changes in nutrition and systemic hormones, and impaired stem cell function. A limiting step in muscle regeneration and growth appears to be impaired activation of the muscle stem cells with age.6 To regenerate muscle, these stem cells break quiescence and proliferate to form new myofibers. Stem cells from aged muscle have the potential to repair damaged muscle, but may be inhibited from doing so by the effects of aging. Emerging data suggest that aged muscle stem cells can be reactivated for tissue repair by experimental interventions, including oxytocin exposure.7
Oxytocin is a 9-amino acid polypeptide produced by the supraoptic and paraventricular hypothalamic nuclei and released into the circulation via the posterior pituitary gland. Although oxytocin is commonly known for inducing uterine contractions during delivery and promoting lactation in women, oxytocin is produced also in men and nonpregnant women, but the physiologic relevance in these groups is unclear. Oxytocin regulates feeding and metabolism at multiple sites and through various central and peripheral mechanisms.8 Some groups have reported that oxytocin suppresses appetite in rodents9 and humans.10,11 Prior studies in younger adults showed that intranasal oxytocin reduces appetite, caloric intake, body mass index (BMI), and waist circumference.10–13 In a study of 25 young (18–45 years old) men across a spectrum of weights (13 normal weight and 12 overweight/obese) by Lawson et al.,10 a 1-time intranasal dose of 24 international units (IU) of oxytocin resulted in reduced total caloric intake and reduced fat intake at a subsequent meal. Another longer term, 8-week study of 24 IU intranasal oxytocin 4 times daily in 24 younger adults (60% women, mean age 35 years) resulted in reduced body weight, BMI, and waist circumference,13 suggesting a favorable effect on metabolic parameters. These effects are possibly mediated by oxytocin receptors expressed on gastric vagal nerve endings and throughout the gastrointestinal tract.11 In vitro and animal studies also suggest that oxytocin induces lipolysis and fat oxidation, which promote fat loss.12,14,15
Oxytocin plasma concentrations decrease with age and may have effects on skeletal muscle.7 In old mice, systemic oxytocin administration restores muscle regeneration by improving aged muscle stem cell function.7 Oxytocin acts directly on muscle stem cells in vitro and in vivo by stimulating pro-myogenic pathways.6 Taken together, these data suggest that age-related decreases in oxytocin may play a role in the pathogenesis of sarcopenia and that oxytocin replacement may be helpful to improve muscle mass and function in older adults. Yet, the effect of oxytocin on skeletal muscle in humans has not been studied, to the best of our knowledge.
We conducted a proof-of-concept randomized clinical trial to test the hypothesis that intranasal oxytocin administration would decrease adiposity without lowering muscle mass in older adults with sarcopenic obesity. The primary outcomes were safety/tolerability of intranasal oxytocin administration and effect on fat and lean mass. Secondary outcomes included changes in body weight, physical function, appetite, mood, cholesterol concentrations, and glycemia (HbA1c and glucose tolerance during an oral glucose tolerance test).
Methods
Design Overview
This was an 8-week double-blind, randomized, placebo-controlled trial conducted at 3 Texas academic institutions (UT Health San Antonio, UT Health Houston, and University of Texas Medical Branch at Galveston). The study was approved by a central institutional review board (IRB) at UT Health San Antonio which served as the relying IRB for other sites. Written consent was obtained from all participants. The protocol was approved and monitored by a Data Safety Monitoring Board. Approval for the use of the oxytocin in this trial was obtained through an Food and Drug Administration Investigational New Drug (132460). The study is registered with clinicaltrials.gov (NCT03119610).
Study Population
Participants were community-dwelling adults aged ≥60 years with obesity and slow gait speed. Recruitment methods included media advertisements, community events, and query of electronic medical records for potentially eligible subjects. Inclusion and exclusion criteria are summarized in Supplementary Table 1. We enrolled men and women with BMI 30.0 to 43.0 kg/m2 and slow gait speed (<1 m/s). Subjects had a stable body weight (±2%) and were sedentary in the past 3 months (≤ 2 strenuous exercise per week), and had hematocrit ≥34%, glomerular filtration rate ≥30 mL/min, and normal electrolytes, urinalysis, coagulation, and liver function tests. Subjects with diabetes based on hemoglobin A1c (HbA1c) ≥6.5, those taking medications known to affect glucose homeostasis, those with significant medical comorbidities, including heart disease, poorly controlled hypertension, cognitive impairment, unstable depression, or a history of eating disorders were also excluded.
Study Procedures
Screening
Participants underwent telephone screening to rule out exclusionary diseases or conditions. Those without exclusionary criteria were invited to an in-person screening visit. At the screening visit and after informed consent, participants underwent medical history and physical examination, electrocardiogram, screening laboratory assays and measurements of vital signs, height, weight, and waist circumference. Screening for cognitive impairment was performed with the Mini-Cog and for depressive symptoms with the Geriatric Depression Scale.16 Gait speed was measured over a 15-foot course; individuals with gait speed less than 1 m/s were eligible. We defined sarcopenia as those with slow gait speed, as gait speed has been shown to be useful as an initial screening measure for sarcopenia and can mediate the effects of sarcopenia on dependency in activities of daily living (ADLs).17,18
Baseline assessments
Individuals without an exclusionary criterion were assessed with the Montreal Cognitive Assessment,19 Center for Epidemiologic Studies Depression Scale,20 2-hour oral glucose tolerance test, physical performance [Short Physical Performance Battery (SPPB),21 Timed Up and Go,22 grip strength], and appetite assessment measured by the Council on Nutrition and Appetite Questionnaire.23 Participants underwent assessment of body composition via dual energy x-ray absorptiometry (DXA). Additional laboratory measurements included lipid profile, coagulation tests, urinalysis, and serum osmolality.
Randomization
Participants were randomized 1:1 to intranasal oxytocin or placebo (saline). All participants and study team members were blinded, except for the research pharmacist at each site and the biostatistician. A randomization schedule for each site was prepared by the study statistician and provided to each research pharmacist.
Oxytocin administration
Participants were given in-person instructions for the self-administration of 24 IU of oxytocin or placebo (3 spays to each nostril) 4 times a day (before meals and at bedtime). The study drug oxytocin (Syntocinon) was compounded for intranasal use by Victoria Apotheke (Zurich, Switzerland). All subjects were trained on appropriate administration of the study drug. Study drug compliance was tracked by the participants self-recording of their administration on a log as well as a count of the number of oxytocin bottles used.
Follow-up
Participants were evaluated in-person at the clinical research units of each site 1 week after randomization and approximately every 4 weeks thereafter for monitoring of adverse events and safety laboratory tests. Between in-person visits, participants were called each week to assess for adverse events. At 8 weeks, participants underwent repeat measurement of all baseline assessments.
Statistical Analysis
Descriptive statistics for each treatment group are summarized in terms of means and standard deviation for continuous variables, and frequency for categorical variables. In exploratory analysis, Wilcoxon rank sum tests were used for comparing continuous variables between treatment groups, and Fisher exact tests for categorical variables. All outcomes were considered before and after oxytocin or placebo administration. Repeated measures of each continuous measure were modeled as a linear function of time, oxytocin, and oxytocin*time interaction with a random effect was used to account for within patient correlation. Generalized estimating equations (GEE) were used for model estimation to provide a consistent estimate of the outcomes, which is robust to violation of normality or possible covariance. The effect of oxytocin was assessed by whether the model coefficient associated with oxytocin*time (ie, differential change in each measure between oxytocin versus placebo) was significantly different from 0. Statistical significance was determined by P < .05 based on a 2-sided test. Normal transformed score of each outcome was used in the analyses when appropriate. As there is no prior data related to the effect of oxytocin on lean mass, sample size calculations were not performed for this pilot study. All analyses were conducting using SAS, version 9.3 software (SAS Institute, Inc., Cary, NC).
Results
Baseline Characteristics
A total of 107 participants were prescreened and 35 participated in informed consent and in-person screening for eligibility. Of these, 24 were eligible. One participant withdrew consent and 23 were randomized. After randomization, 2 participants (8.7%) voluntarily exited the study or was lost to follow-up and 21 completed; 12 participants in the oxytocin group and 9 in the placebo group. See Supplementary Figure 1 for consort diagram. Table 1 presents characteristics of the study population at baseline and follow-up stratified by treatment group. Participants were 67.5 ± 5.3 years, 71% female, 19% African American, and 81% white (91% were non-Hispanic white and 10% were Hispanic). Mean BMI was 36.8 kg/m2 in both groups. There were no significant differences between the oxytocin and control groups at baseline with regard to demographics, laboratory values, anthropomorphic measures (BMI), DXA results (lean mass, fat mass), physical function (SPPB), appetite questionnaires, functional measurements, or cognitive assessments.
Table 1.
Baseline Characteristics of Study Cohort by Treatment Arm
| Oxytocin | Placebo | P value* | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Age, y | 68.3 | 5.8 | 66.3 | 4.8 | .44 |
| Sex, male | 41.7% | 11.1% | .15 | ||
| Ethnic group, Latino | 8.3% | 11.1% | .69 | ||
| Body weight, kg | 105.7 | 16.6 | 93.9 | 10.4 | .19 |
| Body mass index, kg/m2 | 36.8 | 3.5 | 36.8 | 3.6 | 1.00 |
| Waist circumference, cm | 117.9 | 10.2 | 116.5 | 9.1 | .50 |
| Fasting glucose, mg/dL | 91.9 | 9.0 | 92.0 | 8.8 | .97 |
| 2-hour glucose, mg/dL | 150.4 | 34.9 | 148.3 | 44.7 | .71 |
| Hemoglobin A1c, % | 5.7 | 0.3 | 5.7 | 0.5 | .65 |
| Total cholesterol, mg/dL | 195.7 | 50.1 | 177.0 | 42.2 | .43 |
| High-density lipoprotein, mg/dL | 45.8 | 9.6 | 50.3 | 12.6 | .50 |
| Low-density lipoprotein, mg/dL | 123.8 | 43.6 | 95.9 | 35.1 | .24 |
| Triglycerides, mg/dL | 128.9 | 49.9 | 154.8 | 114.2 | .82 |
| Appetite, score | 31.0 | 1.8 | 29.6 | 4.0 | .34 |
| CES-D, score | 3.9 | 3.3 | 5.4 | 4.5 | .44 |
| MOCA, score | 27.3 | 2.5 | 28.1 | 2.8 | .43 |
| SPPB, score | 9.3 | 2.3 | 9.1 | 1.5 | .89 |
| Timed Up and Go, seconds | 11.9 | 3.6 | 12.2 | 2.7 | .52 |
| Fat mass, kg | 47.9 | 6.2 | 43.6 | 7.6 | .44 |
| Lean mass, kg | 54.0 | 13.9 | 46.8 | 6.9 | .40 |
| Fat, % | 156.2 | 110.4 | 143.4 | 107.9 | 1.00 |
| Serum osmolality, mOsm/kg | 327.27 | 107.20 | 292.33 | 5.39 | .27 |
CES-D, Centers for Epidemiologic Studies Depression scale; MOCA, Montreal Cognitive Assessment.
Wilcoxon Test for independent samples was used for the comparison of continuous variables; Fisher exact test was used for comparison of binary variables.
Safety
Adverse events are presented in Supplementary Table 2. The most common reported adverse events among all participants were dizziness, headache, and nausea. There were no significant differences in reported adverse events between groups.
Treatment Effects
Table 2 shows unadjusted change in outcomes between baseline and posttreatment in the oxytocin and placebo groups. Compared with baseline, there were no significant changes in BMI, waist circumference, fasting glucose, HbA1c, 2-hour glucose on oral glucose tolerance testing, physical function (SPPB), muscle strength, gait speed, appetite score, or depression score. There was an increase in unadjusted lean body mass by 1.019 kg in the oxytocin group, whereas there was a loss of 0.214 kg in the placebo group (Figure 1). There was a trend toward lowered low density lipoprotein (LDL) cholesterol in the oxytocin group (−8.7 mg/dL) along with increase in the placebo group (4.1 mg/dL); however, this was not significant.
Table 2.
Change in Measures From Baseline to Follow-up by Treatment Arm, Unadjusted
| Oxytocin | Placebo | Difference* | P value | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| Body weight, kg | 0.54 | 1.6 | .04 | 2.5 | −0.59 | 2.1 | 1.00 |
| Body mass index, kg/m2 | −0.0400 | 0.6649 | −0.0944 | 1.2407 | −0.0544 | 0.9954 | .49 |
| Waist circumference, cm | −.6958 | 4.8662 | −1.372 | 2.1997 | −0.6764 | 3.9682 | .89 |
| Fasting glucose, mg/dL | 3.3636 | 10.181 | 2.8571 | 6.2830 | −0.5065 | 8.9212 | .62 |
| 2-hour glucose, mg/dL | −3.514 | 31.126 | −3.375 | 14.877 | 0.1386 | 25.710 | .66 |
| Hemoglobin A1c, % | −0.0500 | 0.1857 | 0.0286 | 0.1254 | 0.0786 | 0.1657 | .45 |
| Total cholesterol, mg/dL | −11.18 | 17.417 | −1.143 | 16.056 | 10.039 | 16.920 | .48 |
| Low density lipoprotein, mg/dL | −8.727 | 16.224 | 4.1429 | 11.539 | 12.870 | 14.644 | .095 |
| High-density lipoprotein, mg/dL | 1.0000 | 2.8983 | 0.1429 | 3.8048 | −0.8571 | 3.2678 | .56 |
| Triglycerides, mg/dL | −16.82 | 23.828 | −26.29 | 46.903 | −9.468 | 34.348 | .89 |
| Appetite score | −1.375 | 2.1860 | 0.2222 | 2.7285 | 1.5972 | 2.4292 | .15 |
| CES-D score | −0.6667 | 3.4989 | 2.1111 | 7.0079 | 2.7778 | 5.2694 | .18 |
| MOCA score | 1.0000 | 1.5954 | 0.4444 | 1.3333 | −0.5556 | 1.4907 | .47 |
| SPPB score | 0.5000 | 1.4460 | 0.6250 | 1.5980 | 0.1250 | 1.5069 | .64 |
| Timed Up and Go, seconds | −0.5383 | 2.8716 | −.8857 | 2.4939 | −0.3474 | 2.7443 | .71 |
| Fat mass, kg | −0.2 | 0.99 | −0.1 | 0.92 | 0.07 | 0.96 | 1.00 |
| Lean mass, kg | 1.0 | 1.4 | −0.2 | 1.2 | −1.2 | 1.4 | .058 |
| Fat, % | −1.764 | 2.9262 | −1.150 | 4.7994 | 0.6136 | 3.8107 | .33 |
| Serum osmolality, mOsm/kg | −35.64 | 106.99 | 2.28 | 7.27 | −37.92 | 40.95 | .094 |
CES-D, Centers for Epidemiologic Studies Depression scale; MOCA, Montreal Cognitive Assessment.
Difference = (change in placebo group) – (change in oxytocin group).
Fig. 1.
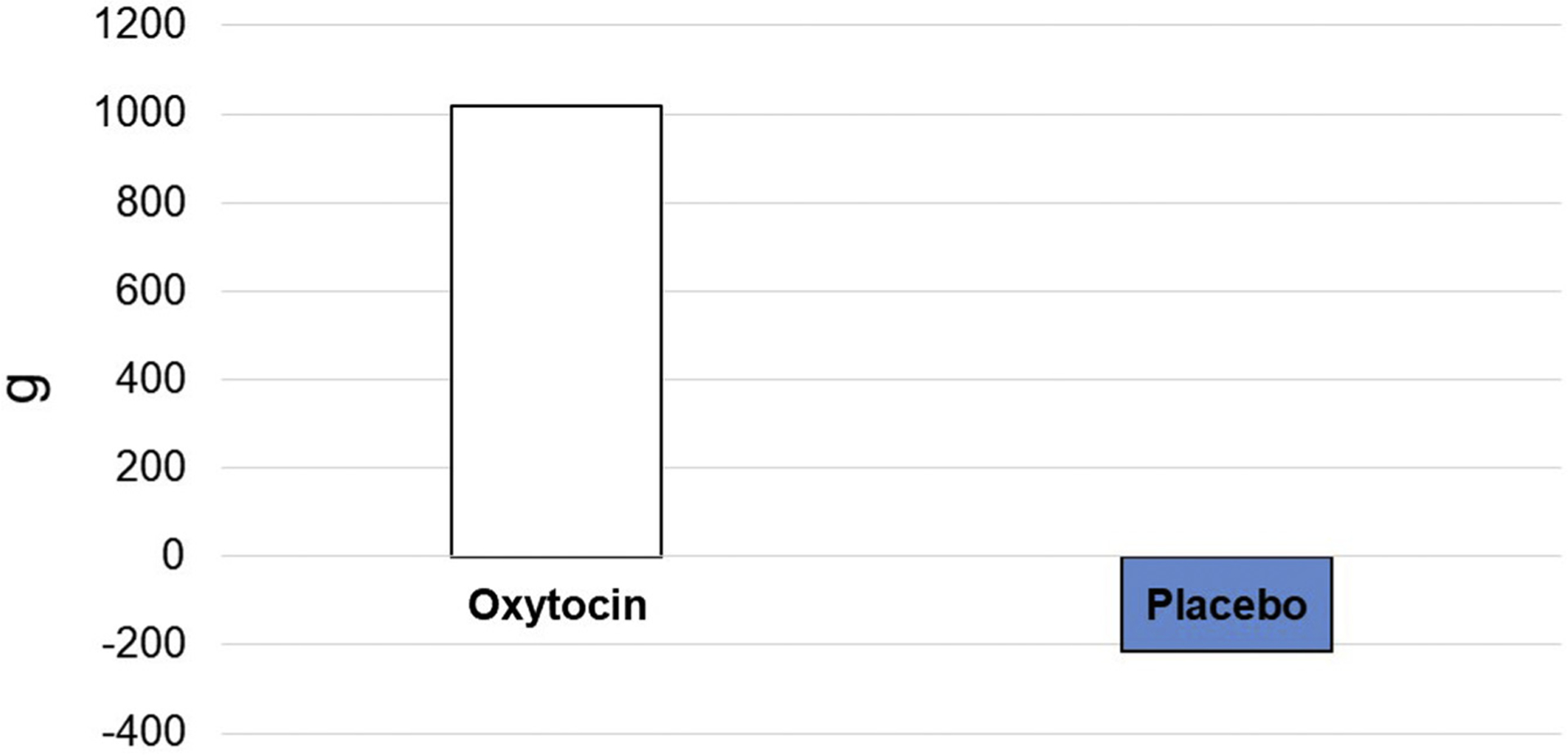
Change in lean mass (grams) by treatment group, oxytocin versus placebo.
Table 3 presents the differential change in repeated measures from baseline between the oxytocin and control groups as assessed by GEE accounting for baseline measures and adjusting for covariates. GEE analysis demonstrated that the change in lean mass was increased by 2.250 kg greater in the oxytocin compared with the placebo group (P = .0116). This analysis also demonstrated a significant reduction in LDL cholesterol in the oxytocin relative to the placebo group by 19.7 mg/dL (P = .0226) (Figure 2).
Table 3.
Differential Change From Baseline Between Oxytocin and Placebo Groups Based on GEEs, Adjusting for Time, Oxytocin Group Status, and Oxytocin*Time Interaction
| Parameter Estimate | Standard Error | Prob Z | |
|---|---|---|---|
| Weight, kg | 0.1526 | 3.3185 | 0.96 |
| BMI, kg/m2 | −0.1058 | 0.7458 | 0.89 |
| Waist circumference, cm | 0.6764 | 1.5122 | 0.65 |
| HbA1c, % | 0.0334 | 0.0979 | 0.73 |
| Fasting glucose, mg/dL | 1.1385 | 3.3722 | 0.74 |
| 2-hour glucose, mg/dL | 3.6991 | 11.3868 | 0.75 |
| Appetite score | −1.5972 | 1.0489 | 0.13 |
| CES-D score | −2.7778 | 2.4053 | 0.25 |
| MOCA score | 0.5556 | 0.6083 | 0.36 |
| SPPB | 0.1389 | 0.6317 | 0.83 |
| Timed Up and Go | −0.1208 | 1.1730 | 0.92 |
| Total cholesterol | −16.8106 | 8.9720 | 0.061 |
| High-density lipoprotein | 4.9437 | 3.5170 | 0.16 |
| Low density lipoprotein | −19.7150 | 8.6434 | 0.023 |
| Triglycerides | −10.559 | 16.3309 | 0.52 |
| Lean mass, kg | 2.3 | 0.89 | 0.012 |
| Fat mass, kg | −1.6 | 1.2 | 0.20 |
| Fat, % | −0.6327 | 14.2586 | 0.96 |
| Serum osmolality, mOsm/kg | −37.2846 | 30.8676 | 0.22 |
Fig. 2.
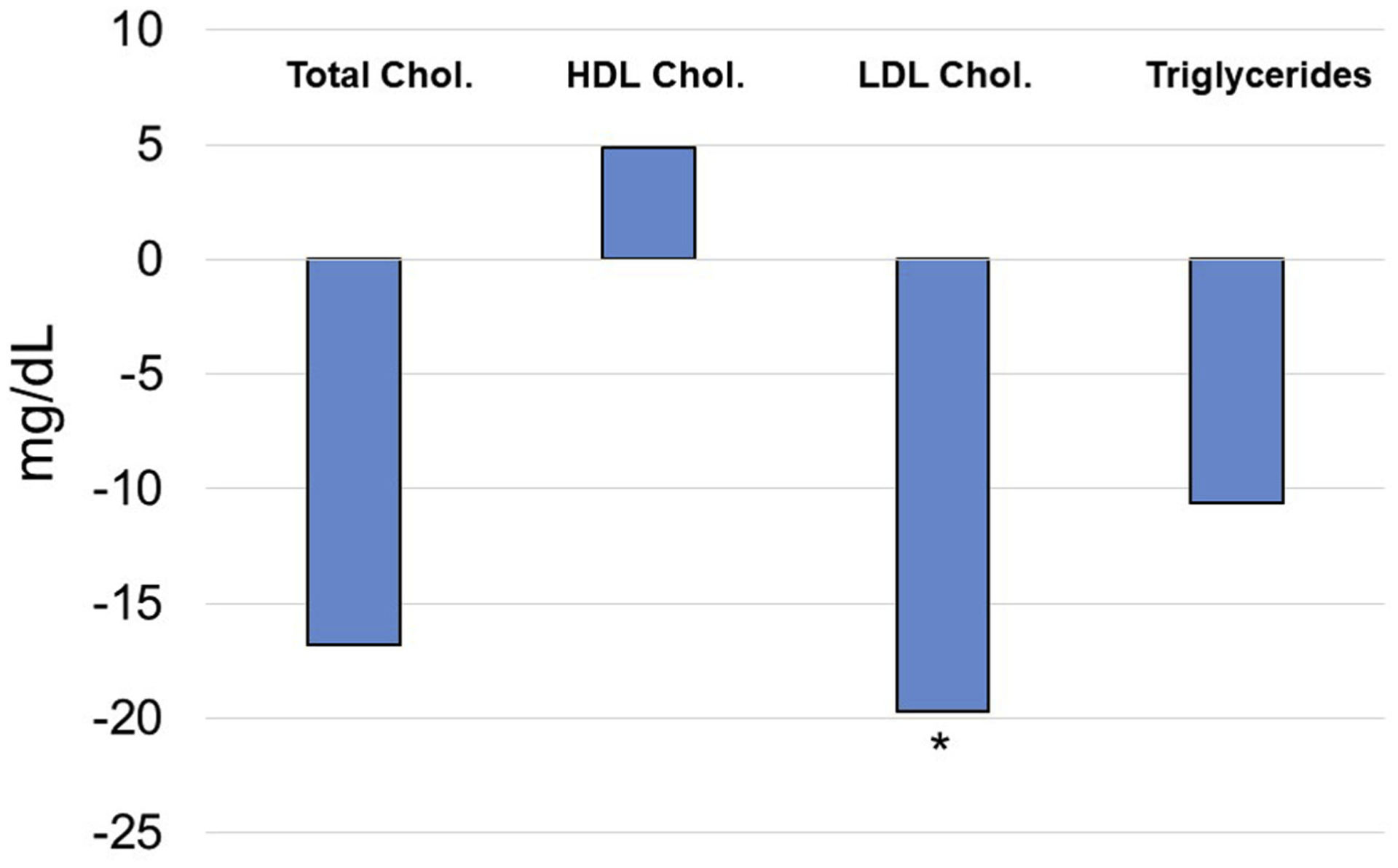
Differential change from baseline between oxytocin and placebo based on GEE analysis. *P < .05.
Discussion
Sarcopenic obesity in older adults is increasingly prevalent and there are limited therapeutic options.4,24,25 Based on this proof-of-concept study, we found that lean body mass increased after oxytocin administration compared with placebo. Although oxytocin administration increased lean mass, it was not accompanied by a concomitant increase in physical function as measured by SPPB. This may be due to the limitation of the SPPB test to detect functional changes in individuals like our subjects whose physical function was still relatively high at baseline (ceiling effect). It is also possible that the relatively short duration of the treatment did not allow for the increase in lean mass to translate into increased physical function. Further, the lean mass gain due to oxytocin may require the addition of exercise to improve physical function.
More research is required to determine the mechanisms by which oxytocin promotes muscle growth in human subjects. Animal studies have shown that the oxytocin receptor is expressed in myoblasts and that oxytocin has trophic effects on muscle by improving myogenic progenitor cell proliferation and stem cell function through activation of the MAPK/ERK signaling pathway.7 In cultured muscle cells, oxytocin also stimulates AMP-activated protein kinase, an energy-sensing enzyme that controls numerous cellular and metabolic processes.26 Further, pharmacologic attenuation of oxytocin receptor signaling decreases myogenic proliferation, and oxytocin knockout mice display a premature aging phenotype. Therefore, future studies in humans should evaluate if, as seen in mice,7 oxytocin improves aged muscle stem cell function and stimulates muscle regeneration, and to determine the signaling pathways that mediate this effect. It will also be important to examine whether oxytocin has an anabolic effect on muscle protein.
Oxytocin administration did not result in a decrease in total body mass in this study. This is explained by the increase in lean mass with a concomitant decrease in fat mass, albeit changes in adiposity did not reach statistical significance. It is unclear why the effect on adiposity observed in the present study was smaller compared with those from others,11 but differences in study population, particularly in age, may help to explain the different outcomes. We also found that LDL cholesterol decreased with oxytocin, a finding that is in line with previous studies.13,27,28
Depression and anxiety are highly prevalent in obese and older adults. Here we observed a trend toward a lower depression score in the oxytocin group, which is consistent with previous studies indicating that oxytocin plays a role in mood.29,30 In addition to its reproductive functions, oxytocin modulates emotionality and stress response likely through anatomic overlap between oxytocin and serotonin regulated centers in the hypothalamus and amygdala,31,32 and circulating oxytocin level has been shown to be inversely correlated with depression and anxiety.33 Intranasal oxytocin is now being investigated as potential treatment for various mood disorders.34
Intranasal oxytocin was marketed in the United States beginning in 1957 and used in the 1960s in the postpartum period for milk let-down. It was taken off the market in 1995 in the United States, but is still available in Europe. In a review article examining the use of intranasal oxytocin for conditions other than lactation, which included 38 trials and 3 case reports of both men and women, only few mild adverse events were reported.35 In this study, we demonstrated safety and tolerability of intranasal oxytocin with no significant differences in adverse events between treatment and control groups, and no serious adverse events.
A potential limitation of this pilot study is that we used obesity combined with slow gait as a proxy definition for sarcopenic obesity. We chose these criteria because slow gait speed has been shown to be useful as an initial screening measure for sarcopenia and can mediate the effects of sarcopenia on dependency in ADLs.17,18
Conclusions and Implications
This proof-of-concept pilot trial supports oxytocin as a potential novel therapy to promote health span and provides effect size information needed for a larger, more definitive trial. Future, larger studies are necessary to confirm these findings in individuals with sarcopenic obesity using criteria based on appendicular lean mass, including persons aged 75 and older, those of varied ethnicities, and those with age-related conditions commonly seen in older persons. Longer studies that include diet and exercise interventions will also help determine whether the observed effect on lean mass is accompanied by improvements in strength and physical function, metabolic outcomes, cardiovascular health, mood, and general well-being.
Supplementary Material
Acknowledgments
We thank the volunteers in this study and the research staff who conducted recruitment, study assessments, and provided regulatory support and study coordination. The sponsors had no role in study design, data collection, analysis, decision to publish, or preparation of the manuscript.
This work was supported by the National Institute on Aging (National Institutes of Health) through the Claude D. Pepper Older Americans Independence Center at University of Texas Health Science Center at San Antonio (Grant P30 AG044271) and at University of Texas Medical Branch (Grant P30 AG024832). It was also supported by the National Institutes of Health through Clinical Translational Science Awards to the University of Texas Health Science Center at San Antonio (Grant UL1 TR002645), the University of Texas Medical Branch (Grant UL1 TR001439), and the University of Texas Health Science Center at Houston (Grant UL1 TR00037). Administrative and infrastructure support was provided by the Geriatrics Research, Education and Clinical Center at the South Texas Veterans Health Care System.
Footnotes
The authors of have no conflicts of interest to declare in relation to this work.
References
- 1.Kane RL, Shamliyan T, Talley K, Pacala J. The association between geriatric syndromes and survival. J Am Geriatr Soc 2012;60:896–904. [DOI] [PubMed] [Google Scholar]
- 2.Kallman DA, Plato CC, Tobin JD. The role of muscle loss in the age-related decline of grip strength: cross-sectional and longitudinal perspectives. J Gerontol 1990;45:M82–M88. [DOI] [PubMed] [Google Scholar]
- 3.Batsis JA, Villareal DT. Sarcopenic obesity in older adults: Aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol 2018;14:513–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Batsis JA, Mackenzie TA, Barre LK, et al. Sarcopenia, sarcopenic obesity and mortality in older adults: Results from the National Health and Nutrition Examination Survey III. Eur J Clin Nutr 2014;68:1001–1007. [DOI] [PubMed] [Google Scholar]
- 5.Frimel TN, Sinacore DR, Villareal DT. Exercise attenuates the weight-loss-induced reduction in muscle mass in frail obese older adults. Med Sci Sports Exerc 2008;40:1213–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blau HM, Cosgrove BD, Ho AT. The central role of muscle stem cells in regenerative failure with aging. Nat Med 2015;21:854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elabd C, Cousin W, Upadhyayula P, et al. Oxytocin is an age-specific circulating hormone that is necessary for muscle maintenance and regeneration. Nat Commun 2014;5:4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leng G, Ludwig M. Intranasal oxytocin: Myths and delusions. Biol Psychiatry 2016;79:243–250. [DOI] [PubMed] [Google Scholar]
- 9.Iwasaki Y, Maejima Y, Suyama S, et al. Peripheral oxytocin activates vagal afferent neurons to suppress feeding in normal and leptin-resistant mice: A route for ameliorating hyperphagia and obesity. Am J Physiol Regul Integr Comp Physiol 2015;308:R360–R369. [DOI] [PubMed] [Google Scholar]
- 10.Lawson EA, Marengi DA, DeSanti RL, et al. Oxytocin reduces caloric intake in men. Obesity 2015;23:950–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borg J, Simren M, Ohlsson B. Oxytocin reduces satiety scores without affecting the volume of nutrient intake or gastric emptying rate in healthy subjects. Neurogastroenterol Motil 2011;23:56. [DOI] [PubMed] [Google Scholar]
- 12.Lawson EA, Olszewski PK, Weller A, Blevins JE. The role of oxytocin in regulation of appetitive behaviour, body weight and glucose homeostasis. J Neuroendocrinol 2020;32:e12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang H, Wu C, Chen Q, et al. Treatment of obesity and diabetes using oxytocin or analogs in patients and mouse models. PLoS One 2013;8:e61477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blevins JE, Ho JM. Role of oxytocin signaling in the regulation of body weight. Rev Endocr Metab Disord 2013;14:311–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deblon N, Veyrat-Durebex C, Bourgoin L, et al. Mechanisms of the anti-obesity effects of oxytocin in diet-induced obese rats. PLoS One 2011;6:e25565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montorio I, Izal M. The Geriatric Depression Scale: A review of its development and utility. Int Psychogeriatr 1996;8:103–112. [DOI] [PubMed] [Google Scholar]
- 17.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perez-Sousa MA, Venegas-Sanabria LC, Chavarro-Carvajal DA, et al. Gait speed as a mediator of the effect of sarcopenia on dependency in activities of daily living. J Cachexia Sarcopenia Muscle 2019;10:1009–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–699. [DOI] [PubMed] [Google Scholar]
- 20.Vilagut G, Forero CG, Barbaglia G, Alonso J. Screening for depression in the general population with the Center for Epidemiologic Studies Depression (CES-D): A systematic review with meta-analysis. PLoS One 2016;11: e0155431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994;49:M85–M94. [DOI] [PubMed] [Google Scholar]
- 22.Podsiadlo D, Richardson S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991;39:142–148. [DOI] [PubMed] [Google Scholar]
- 23.Wilson MM, Thomas DR, Rubenstein LZ, et al. Appetite assessment: Simple appetite questionnaire predicts weight loss in community-dwelling adults and nursing home residents. Am J Clin Nutr 2005;82:1074–1081. [DOI] [PubMed] [Google Scholar]
- 24.Batsis JA, Mackenzie TA, Lopez-Jimenez F, Bartels SJ. Sarcopenia, sarcopenic obesity, and functional impairments in older adults: National Health and Nutrition Examination Surveys 1999–2004. Nutr Res 2015;35: 1031–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirani V, Naganathan V, Blyth F, et al. Longitudinal associations between body composition, sarcopenic obesity and outcomes of frailty, disability, institutionalisation and mortality in community-dwelling older men: The Concord Health and Ageing in Men Project. Age Ageing 2016;46:413–420. [DOI] [PubMed] [Google Scholar]
- 26.Lee ES, Uhm K-O, Lee YM, et al. Oxytocin stimulates glucose uptake in skeletal muscle cells through the calcium–CaMKK–AMPK pathway. Regul Pept 2008; 151:71–74. [DOI] [PubMed] [Google Scholar]
- 27.Lawson EA. The effects of oxytocin on eating behaviour and metabolism in humans. Nat Rev Endocrinol 2017;13:700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burt RL, Leake NH, Dannenburg WN. Effect of synthetic oxytocin on plasma nonesterified fatty acids, triglycerides, and blood glucose. Obstet Gynecol 1963;21:708–712. [PubMed] [Google Scholar]
- 29.Olff M, Frijling JL, Kubzansky LD, et al. The role of oxytocin in social bonding, stress regulation and mental health: An update on the moderating effects of context and interindividual differences. Psychoneuroendocrinology 2013;38: 1883–1894. [DOI] [PubMed] [Google Scholar]
- 30.Striepens N, Kendrick KM, Maier W, Hurlemann R. Prosocial effects of oxytocin and clinical evidence for its therapeutic potential. Front Neuroendocrinol 2011; 32:426–450. [DOI] [PubMed] [Google Scholar]
- 31.Lee H-J, Macbeth AH, Pagani JH, Young WS 3rd. Oxytocin: the great facilitator of life. Prog Neurobiol 2009;88:127–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Emiliano AB, Cruz T, Pannoni V, Fudge JL. The interface of oxytocin-labeled cells and serotonin transporter-containing fibers in the primate hypothalamus: A substrate for SSRIs therapeutic effects? Neuropsychopharmacology 2007;32: 977–988. [DOI] [PubMed] [Google Scholar]
- 33.Scantamburlo G, Hansenne M, Fuchs S, et al. Plasma oxytocin levels and anxiety in patients with major depression. Psychoneuroendocrinology 2007;32: 407–410. [DOI] [PubMed] [Google Scholar]
- 34.Cochran DM, Fallon D, Hill M, Frazier JA. The role of oxytocin in psychiatric disorders: A review of biological and therapeutic research findings. Harv Rev Psychiatry 2013;21:219–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacDonald E, Dadds MR, Brennan JL, et al. A review of safety, side-effects and subjective reactions to intranasal oxytocin in human research. Psychoneuroendocrinology 2011;36:1114–1126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


