Abstract
Background and aims
The kidney transplant patients who receive immunosuppressive and specific medication may lead to different mortality risk factors between kidney transplant patients with COVID‐19 and the general population. We aimed to provide a model predictor and a risk analysis of mortality in kidney transplant COVID‐19 positive patients.
Methods
We performed our search using PubMed, MEDLINE, Web of Science, Scopus, and Google Scholar to identify English articles published from the beginning of December 2019 through August 2020. Excluded manuscripts had no full text, lacked information, were not the original article, or consisted of less than three cases. We gathered information about demographic information, comorbidities, COVID‐19 symptoms, lung radiographic findings, history of medication therapy, and changes in the kidney maintenance therapy after confirming their COVID‐19 on the data extraction forms.
Results
We found a total of 31 eligible articles. We set a 10% mortality rate as our cutoff point. The most common sign and symptoms were cough (53.22 [29.42]), dyspnea (50.80 [24.55]). In the bivariate analysis, fatigue (P = .04, OR of 0.92; 95% CI: 0.85‐1.00), hypertension (P = .07, OR of 1.03; 95% CI: 1.00‐1.07), and dyspnea (P = .08, OR of 1.04; 95% CI: 1.00‐1.09) showed a statistically significant relationship with increases in mortality.
In multivariate regression analysis, an independent association was only found between hypertension and mortality (P = .035; AOR of 1.064; CL: 1.004‐1.127).
Conclusion
Clinicians should pay special attention to modifiable risk factors for COVID‐19 infection mortality, such as hypertension among kidney transplant patients, because it may be possible to decrease mortality by controlling these factors.
Keywords: COVID‐19, kidney transplant, mortality
1. BACKGROUND
In December 2019, a worldwide pandemic respiratory syndrome started in Wuhan, China, which caused a multi‐organ infection named severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). 1 , 2
As the disease continued to spread rapidly, in March 2020, the World Health Organization proclaimed coronavirus disease 2019 (COVID‐19) as a global pandemic. 3 By 29 December 2020, the total number of confirmed cases had reached over 79 million, and over 1.7 million cases of confirmed patients had died. 4
In several published articles like systematic reviews and case reports about COVID‐19, an increase in age and chronic diseases such as hypertension, diabetes, chronic respiratory disease, and cardiovascular disease could increase the severity of the disease. 5 , 6 Due to a lack of curative treatment, supportive care is the only option available. 7 , 8 Immunosuppressive medication such as dexamethasone is one of the main treatments for COVID‐19. 9 , 10 , 11 , 12 The majority of kidney transplant patients receive immunosuppressive medication such as corticosteroids. There is neither an established course of the disease nor a specific absolute therapeutic method for them. 13 Hence, we decided to review published articles about kidney transplant patients infected with COVID‐19, discuss their symptoms, the different medication regimens given, and address the possible relation between any of these variables with mortality COVID‐19 positive patients.
2. METHODS
2.1. Data sources
We performed our search using PubMed, MEDLINE, Web of Science, Scopus, and Google Scholar to identify English articles published from the beginning of December 2019 through August 2020. We used keywords including “COVID‐19,” “SARS‐CoV‐2,” “Coronavirus 2,” “coronavirus 2019,” “renal transplant,” and “kidney transplant” to identify articles.
2.2. Study selection
After collecting studies, the full texts and abstracts were evaluated for excluding criteria. Every article had been reviewed by two individuals, and the final observation over the reviewed articles was performed by the third party.
Duplicated studies, the protocols and management guidelines, letters to the editor, abstracts, studies with lack of information about demographic data of the patients, radiographic findings, medication therapy, or mortality, and articles with no available full text, were excluded. Also, we excluded studies on less than three cases due to the small population and chance of bias. Any disagreements during data collection were solved through group discussion.
2.3. Data extraction
We gathered information about authors and year of publication, patient age, gender, comorbidities, COVID‐19 symptoms, lung radiographic findings, history of medication therapy, and kidney maintenance therapy changes after confirming their COVID‐19 on the data extraction forms. Our gathered data included only kidney‐transplanted recipient cases. Two independent inspectors carried out the data extraction and any disagreement during the process was solved by the third member.
2.4. Quality assessment
To ensure the quality of the selected articles, we made a checklist consisting of 15 questions (Table 1). The quality of papers was scored based on the checklist. 14 The results were reported in three groups: Low quality (0‐5), medium quality (6‐10), and high quality (11‐15). The authors discussed the qualified full texts to make the final decision. After reading the full text of these papers, the authors decided to include/exclude each of them.
TABLE 1.
Quality assessment checklist for the articles
| No. | Question |
|---|---|
| 1 | Does the study address any research question(s) or objective(s)? |
| 2 | Does the study provide any theoretical framework for the evaluation method? |
| 3 | Does the theoretical framework of the study include any health promotion theory? |
| 4 | Does the study provide a time frame for the data collection? |
| 5 | Does the study identify the country where the search was conducted? |
| 6 | Does the study mention that the reviewed current evidence was downloaded for evaluation? |
| 7 | Does the study discuss the selection criteria for current evidence to be included or excluded for review? |
| 8 | Does the study provide a clear description of the evaluation method? |
| 9 | Are there at least two independent data extractors with a consensus procedure in place in case of disagreement? |
| 10 | Is a list of the reviews of current evidence provided? |
| 11 | Does the study discuss the findings of the evaluation? |
| 12 | Does the study look at the reviewed evidence to promote or enable behavioral change? |
| 13 | Does the study discuss any limitations? |
| 14 | Does the study provide any future recommendations in general? |
| 15 | Does the study state any conflict of interest? |
2.5. Data synthesis
A quantitative synthesis of identified characteristics was done. These findings were then analyzed based on frequencies (n) to make data interpretation easier. We used SPSS Statistics software (version 22, SPSS Inc., Chicago, Ill., USA) for data analysis. We considered all variables quantitative. Besides, we used univariate, bivariate, and multivariate analysis methods in this study. Odds ratio (OR) with the corresponding 95% confidence interval (CI) was calculated for each analysis. The data were analyzed by the variables listed in Table 2.
TABLE 2.
List of the variables extracted from the articles and used in the data analysis
| Demographics | |
|---|---|
| Male | Mean age |
| Signs, symptoms, and related morbidities and outcomes | |
|---|---|
| Fever | Nausea/vomiting |
| Cough | Myalgia |
| Dyspnea | Intubated |
| Gustatory dysfunction | Still in hospital |
| Dysgeusia, Hypogeusia, Phantogeusia, Ageusia | Recover/discharge |
| Fatigue | |
| Changes in kidney transplant medications and medications for COVID‐19 | |
|---|---|
| Antimetabolite stopped | Antimetabolite dose reduction |
| Steroids stopped or lower the dose | Calcineurin stopped |
| Calcineurin dose reduction | Antiviral |
| Antibiotic | Tocilizumab |
| Hydroxychloroquine | IVIG |
| Imaging studies | |
|---|---|
| Lung involvement in CT/X‐ray | Unilateral lung involvement |
| Bilateral lung involvement | |
| Comorbidities | |
|---|---|
| Hypertension | Heart disease |
| Diabetes mellitus | Lung disease |
| Cancer | |
3. RESULTS
In this systematic review study, we found a total of 140 articles. After initial review, six duplicates were identified and excluded. The remaining articles were evaluated by their abstract and title, which excluded 103 other studies (Figure 1). The mean score of quality assessment for the remaining articles was 13.5 (range 12 to 15), which showed that the included study had a high quality (the characteristic data of included studies can be found in the Appendix S1). 1 , 7 , 11 , 12 , 18 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51
FIGURE 1.
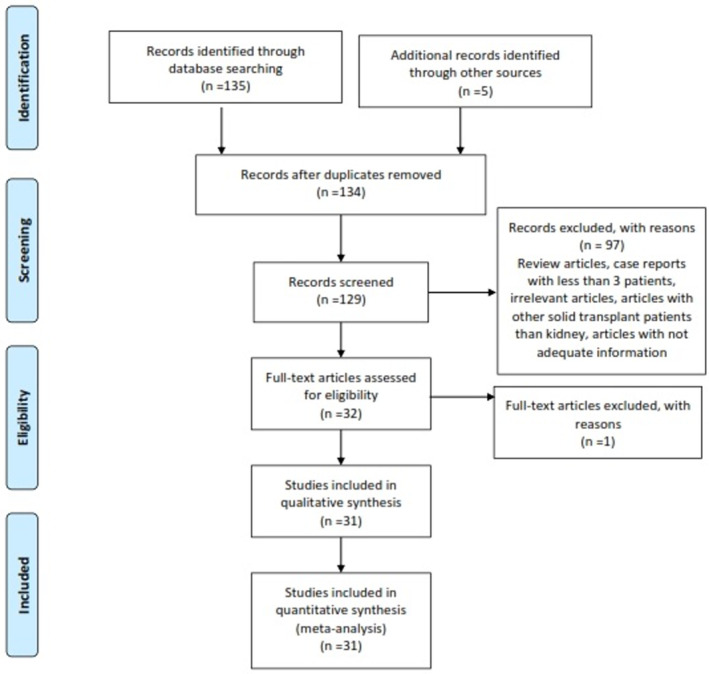
PRISMA flowchart for extracted articles for investigation of mortality risk factors in kidney‐transplanted patients with COVID‐19
Our included studies resulted in finding 903 kidney‐transplanted patients with confirmed COVID‐19 PCR tests. For this research, based on the clinical findings from a similar study, we set a 10% mortality rate as the cutoff point. 5 , 6
3.1. The most common signs and symptoms
The seven most common sign and symptoms with the highest mean among included articles were cough (53.22 [29.42]), dyspnea (50.80 [24.55]), myalgia (26.21 [23.96]), fatigue (8.04 [19.79]), fever (79.13 [17.78]), gustatory dysfunction (79.13 [17.78]), and nausea/vomiting (4.59 [8.77]). Table 3 shows detailed information.
TABLE 3.
Different variables and their associations with the hospital mortality of COVID‐19 in kidney‐transplanted patients in the bivariate analysis
| Characteristics | Number of studies | Mean ± SD | Odds ratio (95% CI) a | P value |
|---|---|---|---|---|
| Demographics | ||||
| Male (%) | 31 | 65.71 (13.91) | 1.01 (0.95‐1.07) | .75 |
| Mean age (year) | 31 | 64.0 (12.99) | 0.93 (0.86‐1.00) | .53 |
| Comorbidities | ||||
| Hypertension (%) | 27 | 74.93 (26.69) | 1.03 (1.00‐1.07) | .07 |
| Diabetes mellitus (%) | 23 | 31.21 (22.95) | 0.98 (0.94‐1.02) | .37 |
| Cancer (%) | 11 | 6.86 (10.39) | 0.98 (0.90‐1.06) | .60 |
| Heart disease (%) | 18 | 16.45 (19.45) | 1.04 (0.97‐1.12) | .26 |
| Lung disease (%) | 11 | 5.14 (7.99) | 1.04 (0.91‐1.19) | .53 |
| Imaging studies | ||||
| Lung involvement in CT/X‐ray (%) | 19 | 81.20 (24.12) | 0.96 (0.89‐1.03) | .23 |
| Unilateral lung involvement (%) | 12 | 17.94 (16.09) | 1.03 (0.96‐1.11) | .37 |
| Bilateral lung involvement (%) | 19 | 67.74 (23.18) | 0.97 (0.93‐1.02) | .31 |
| Changes in kidney transplant medications and medications for Covid‐19 | ||||
| Antimetabolite stopped (%) | 22 | 73.00 (30.09) | 0.98 (0.94‐1.02) | .27 |
| Antimetabolite dose reduction (%) | 6 | 13.83 (30.85) | 1.04 (0.96‐1.12) | .37 |
| Steroids stopped or lower the dose (%) | 11 | 15.72 (27.60) | 1.00 (0.97‐1.03) | .96 |
| Calcineurin stopped (%) | 22 | 42.96 (34.74) | 1.00 (0.98‐1.03) | .82 |
| Calcineurin dose reduction (%) | 5 | 24.62 (38.17) | 0.99 (0.96‐1.03) | .71 |
| Antiviral (%) | 19 | 41.15 (38.03) | 1.00 (0.98‐1.03) | .94 |
| Antibiotic (%) | 20 | 55.78 (41.46) | 1.00 (0.98‐1.03) | .75 |
| Hydroxychloroquine (%) | 22 | 70.44 (38.17) | 1.02 (0.99‐1.04) | .17 |
| Tocilizumab (%) | 15 | 19.31 (27.60) | 1.05 (0.97‐1.14) | .23 |
| IVIG (%) | 10 | 12.96 (21.52) | 0.99 (0.95‐1.03) | .49 |
| Signs and symptoms | ||||
| Fever (%) | 28 | 79.13 (17.78) | 0.99 (0.93‐1.05) | .69 |
| Cough (%) | 24 | 53.22 (29.42) | 0.98 (0.94‐1.02) | .33 |
| Dyspnea (%) | 26 | 50.80 (24.55) | 1.04 (1.00‐1.09) | .08 |
| Gustatory dysfunction (%) | 2 | 25.30 (17.42) | 1.02 (0.96‐1.09) | .55 |
| Myalgia (%) | 19 | 26.21 (23.96) | 1.02 (0.97‐1.07) | .41 |
| Fatigue (%) | 6 | 8.04 (19.79) | 0.92 (0.85‐1.00) | .04 |
| Nausea/vomiting (%) | 7 | 4.59 (8.77) | 0.95 (0.86‐1.06) | .37 |
| Morbidities and outcomes | ||||
| Intubated (%) | 17 | 23.11 (21.25) | 1.11 (0.98‐1.26) | .09 |
| Recover/discharge (%) | 27 | 54.63 (20.24) | 0.98 (0.93‐1.04) | .56 |
| Still in hospital (%) | 11 | 12.71 (20.56) | 0.97 (0.92‐1.01) | .14 |
Two‐sided 95% CI.
3.2. Bivariate analysis
In the bivariate analysis, applying a cutoff point of P < .10 for significance, among signs and symptoms, fatigue (P = .04, OR of 0.92; 95% CI: 0.85‐1.00), hypertension (P = .07, OR of 1.03; 95% CI: 1.00‐1.07), and dyspnea (P = .08, OR of 1.04; 95% CI:1.00‐1.09) showed a statistically significant relationship with increases in mortality.
It also showed that being intubated while in hospital (P = .09, OR of 1.11; 95% CI:0.98‐1.26) was associated with mortality rate. None of the chest X‐ray findings, mean age, or different medication therapy showed significant association with mortality rate (Table 3).
3.3. Multivariate regression analysis
We additionally conducted a multivariate regression analysis of the variables that had an association with COVID‐19. In this analysis, our threshold of significance (P value) was <.05. An independent association was found between hypertension and mortality (P = .035; AOR of 1.064; 95% CI:1.004‐1.127).
4. DISCUSSION
In this systematic review, we found hypertension as a major risk factor of the mortality rate in kidney transplant COVID‐19 positive patients. Statistic results showed that other comorbidities, medications, or symptoms were not effective in increasing or decreasing the mortality rate of these patients. Also, cessation or dose reduction of drugs related to kidney transplant may not have a significant effect on mortality of COVID‐19.
Kidney‐transplanted patients may have numerous comorbidities, which may be the reason for their kidney injury. 15 Among all comorbidities, we only found hypertension as a risk factor to raise mortality risk. In the Pranata meta‐analysis study, they also found that hypertension is a risk factor for increasing the mortality rate among COVID‐19 patients. 16 In a study, Wu and McGoogan suggested that hypertension and cardiovascular disease impact mortality. 17 Besides, in another study conducted by Cravedi et al, hypertension was mentioned as the most common comorbidity affecting 95% of COVID‐19 patients with kidney transplantation. 18 Zaki et al reviewed that using antihypertensive drugs such as calcium channel blockers (CCBs), angiotensin II receptor blockers (ARBs), and angiotensin‐converting enzyme inhibitors (ACEIs) can reduce the risk of mortality in COVID‐19 patients with hypertension. 19 Surprisingly, it has been reported that utilization of ARBs and ACEI in patients with diabetes and hypertension leads to upregulation of angiotensin‐converting enzyme 2 (ACE2) in Fang's study. SARS‐CoV‐2 invades the body using ACE2 receptors. Therefore, it has been hypothesized that upregulation of ACE2 in these patients may predispose them to more severe infection. 20 , 21 However, several investigations and systematic reviews have mentioned hypertension as a major comorbidity that affects mortality significantly even without medical history of kidney transplant. 19 , 22 Nevertheless, more investigations are demanded to clarify the exact mechanism of hypertension in deteriorating respiratory infections.
Lung involvement is one of the significant and life‐threatening consequences of the COVID‐19 infection. Some studies found lung involvement as a risk factor for predicting the outcome of COVID‐19 patients, 23 but the current study did not support this finding and did not show any relation between lung involvement in COVID‐19 patients who had a kidney transplant. In Zhu's study in China, they concluded that kidney‐transplanted patients have severe lung involvement, but it just increases their clinical course duration and has no effect on their mortality rate. 12 In Chen's study, they also showed that although most of their patients had chest X‐ray involvement, it did not increase the mortality rate. 1 Other studies support our findings as well. 5
Our‐regression analysis did not show a significant effect between stoppage or reduction of kidney transplant's medications and fatality of the COVID‐19 infection. One of the medications is immunosuppressant, which reduces the risk of transplant rejection. As the COVID‐19 virus pandemic started, there were several attempts to reduce or change the immunosuppressive medicines to decrease the mortality rate in these patients. This systematic review did not show any relation between reducing or stopping antimetabolite (methotrexate) and calcineurin inhibitor medication with reducing mortality. Also, an unclear effect of immunosuppressant as a risk factor on morbidity and mortality of COVID‐19 patients has been mentioned in several published articles. 18 , 24 Besides, a meta‐analysis study conducted by Tassone et al concluded that immunosuppressants cannot be a significant risk factor for COVID‐19 infection. 25 Our analysis also showed no mortality risk enhancement with starting antiviral or antibiotic drugs as well. Nair et al study in New York, which was done on 10 patients, stopped giving antibiotics as a routine treatment plan to their patients, as it did not show any impact on their outcomes. 26 Trujillo had reported that even after reduction of kidney transplant's drugs, there was no observation of organ rejection or increment of donor‐specific antibodies. 27
5. LIMITATIONS AND STRENGTHS
We did an immense systematic review of mortality in kidney‐transplanted patients who had COVID‐19. We extracted and analyzed a vast group of articles. Some studies had a small study population, though, which did not help us obtain adequate information from them. We omitted these studies to prevent any bias in our analysis results. Due to the novelty of COVID‐19, we also had a scarcity of information regarding treatment protocols and published articles regarding some risk factors and possible effective therapy. Availability of medication and facilities to all the population was a debate. The authors did not have access to primary data, and they worked on secondary data. Thus, in some articles, the authors had just median, and in some others, they had mean and SD.
6. CONCLUSION
Our analysis showed that 24.12% of cases showed lung involvement in their chest radiography or CT scan. Among those, 23.18% had bilateral involvement. Our statistical analysis findings suggest that hypertension, fatigue, dyspnea, and intubation in the course of hospitalization, have increased the mortality rate among kidney transplant patients who are COVID‐19 positive. However, in the multivariate analysis, only hypertension showed an independent relationship with increased mortality. We suggest that further research be done to examine other risk factors with COVID‐19 in kidney‐transplanted patients.
FUNDING
All affiliations with or financial involvement in any entity with a financial interest in, or in competition with, the manuscript's subject matter are disclosed. This includes stock ownership, employment, consultancies, honoraria, grants, patents, and royalties. This research did not receive any specific assistance from funding agencies in the public, commercial, or not‐for‐profit sectors.
CONFLICT OF INTEREST
There is no conflict of interest regarding the publication of this manuscript.
AUTHOR CONTRIBUTIONS
Conceptualization: Yasaman Navari, Amir Behzad Bagheri
Data Curation: Yasaman Navari, Amir Behzad Bagheri
Formal Analysis: Arash Akhavan Rezayat
Investigation: Sara Najafi, Arash Akhavan Rezayat
Methodology: SeyedAhmad SeyedAlinaghi, Arash Akhavan Rezayat
Software: Sara Najafi, SeyedAhmad SeyedAlinaghi
Supervision: Ali Asadollahi‐Amin, SeyedAhmad SeyedAlinaghi
Validation: Alireza Barzegary, Sara Najafi
Visualization: Alireza Barzegary
Writing—Original Draft: Ali Asadollahi‐Amin, Yasaman Navari, Amir Behzad Bagheri
Writing—Review and Editing: Ali Asadollahi‐Amin
All authors have read and approved the final version of the manuscript.
Corresponding author had full access to all of the data in this study and takes complete responsibility for the integrity of the data and the accuracy of the data analysis. This article has not been published and is not under consideration for publication elsewhere.
TRANSPARENCY STATEMENT
Ali Asadollahi‐Amin affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
ETHICS STATEMENT
We also declare that the study was performed according to the international, national, and institutional rules considering animal experiments, clinical studies, and biodiversity rights.
Supporting information
Appendix S1. Supporting Information.
ACKNOWLEDGMENT
None.
Navari Y, Bagheri AB, Akhavan Rezayat A, et al. Mortality risk factors in kidney‐transplanted patients with COVID‐19: A systematic review and regression analysis. Health Sci Rep. 2021;4:e427. doi: 10.1002/hsr2.427
Yasaman Navari and Amir Behzad Bagheri contributed equally to this study.
DATA AVAILABILITY STATEMENT
All information provided in this article can be obtained from the author on request.
REFERENCES
- 1. Chen TY, Farghaly S, Cham S, et al. COVID‐19 pneumonia in kidney transplant recipients: focus on immunosuppression management. Transpl Infect Dis. 2020;22(5):e13378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Raja MA, Mendoza MA, Villavicencio A, et al. COVID‐19 in solid organ transplant recipients: a systematic review and meta‐analysis of current literature. Transplant Rev. 2021;35(1):100588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sohrabi C, Alsafi Z, O'neill N, et al. World health organization declares global emergency: a review of the 2019 novel coronavirus (COVID‐19). Int J Surg. 2020;76:71‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization . Weekly epidemiological update ‐ 29 December 2020. https://www.who.int/publications/m/item/weekly-epidemiological-update---29-december-2020.
- 5. Mehraeen E, Karimi A, Barzegary A, et al. Predictors of mortality in patients with COVID‐19–a systematic review. Eur J Integr Med. 2020;40:101226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arpali E, Akyollu B, Yelken B, Tekin S, Turkmen A, Kocak B. Case report: a kidney transplant patient with mild COVID‐19. Transpl Infect Dis. 2020;22(4):e13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang H, Chen Y, Yuan Q, et al. Identification of kidney transplant recipients with coronavirus disease 2019. Eur Urol. 2020;77(6):742–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ge H, Wang X, Yuan X, et al. The epidemiology and clinical information about COVID‐19. Eur J Clin Microbiol Infect Dis: Off Publ Eur Soc Clin Microbiol. 2020;39(6):1011‐1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. RECOVERY Collaborative Group , Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid‐19 ‐ preliminary report. N Engl J Med. 2021;384(8):693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang W, Zhao Y, Zhang F, et al. The use of anti‐inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID‐19): the perspectives of clinical immunologists from China. Clin Immunol. 2020;214:108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alberici F, Delbarba E, Manenti C, et al. Management of patients on dialysis and with kidney transplant during SARS‐COV‐2 (COVID‐19) pandemic in Brescia, Italy. Kidney Int Rep. 2020;5(5):580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhu L, Gong N, Liu B, et al. Coronavirus disease 2019 pneumonia in immunosuppressed renal transplant recipients: a summary of 10 confirmed cases in Wuhan, China. Eur Urol. 2020;77(6):748–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rodríguez Faba O, Boissier R, Budde K, et al. European Association of Urology guidelines on renal transplantation: update 2018. Eur Urol Focus. 2018;4(2):208‐215. [DOI] [PubMed] [Google Scholar]
- 14. BinDhim NF, Hawkey A, Trevena L. A systematic review of quality assessment methods for smartphone health apps. Telemed J E Health: Off J Am Telemed Assoc. 2015;21(2):97‐104. [DOI] [PubMed] [Google Scholar]
- 15. Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020;5(7):811‐818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pranata R, Lim MA, Huang I, Raharjo SB, Lukito AA. Hypertension is associated with increased mortality and severity of disease in COVID‐19 pneumonia: a systematic review, meta‐analysis and meta‐regression. J Renin Angiotensin Aldosterone Syst. 2020;21(2):1470320320926899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239‐1242. [DOI] [PubMed] [Google Scholar]
- 18. Cravedi P, Mothi SS, Azzi Y, et al. COVID‐19 and kidney transplantation: results from the TANGO International Transplant Consortium. Am J Transplant. 2020;20(11):3140‐3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zaki N, Alashwal H, Ibrahim S. Association of hypertension, diabetes, stroke, cancer, kidney disease, and high‐cholesterol with COVID‐19 disease severity and fatality: a systematic review. Diabetes Metab Syndr Clin Res Rev. 2020;14(5):1133‐1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID‐19 infection? Lancet Respir Med. 2020;8(4):e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. ERA‐EDTA Council; ERACODA Working Group . Chronic kidney disease is a key risk factor for severe COVID‐19: a call to action by the ERA‐EDTA. Nephrol Dial Transplant. 2021;36(1):87‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fraser J, Mousley J, Testro A, Smibert OC, Koshy AN. Clinical presentation, treatment, and mortality rate in liver transplant recipients with coronavirus disease 2019: a systematic review and quantitative analysis. Transplant Proc. 2020;52:2676‐2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tian W, Jiang W, Yao J, et al. Predictors of mortality in hospitalized COVID‐19 patients: a systematic review and meta‐analysis. J Med Virol. 2020;92(10):1875‐1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Price KN, Frew JW, Hsiao JL, Shi VY. COVID‐19 and immunomodulator/immunosuppressant use in dermatology. J Am Acad Dermatol. 2020;82(5):e173‐e175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tassone D, Thompson A, Connell W, et al. Immunosuppression as a risk factor for COVID‐19: a meta‐analysis. Intern Med J. 2021;51(2):199‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nair V, Jandovitz N, Hirsch JS, et al. COVID‐19 in kidney transplant recipients. Am J Transplant. 2020;20(7):1819–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Trujillo H, Caravaca‐Fontán F, Sevillano Á, et al. SARS‐CoV‐2 infection in hospitalized patients with kidney disease. Kidney Int Rep. 2020;5(6):905‐909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Abolghasemi S, Mardani M, Sali S, Honarvar N, Baziboroun M. COVID‐19 and kidney transplant recipients. Transpl Infect Dis. 2020;22(6):e13413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Abrishami A, Samavat S, Behnam B, Arab‐Ahmadi M, Nafar M, Taheri MS. Clinical course, imaging features, and outcomes of COVID‐19 in kidney transplant recipients. Eur Urol. 2020;78(2):281‐286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Abuzeineh M, Muzaale AD, Crews DC, Avery RK, Brotman DJ, Brennan DC, Segev DL, Al Ammary F. Telemedicine in the care of kidney transplant recipients with coronavirus disease 2019. 2020. p 2620–2625. [DOI] [PMC free article] [PubMed]
- 31. Husain SA, Dube G, Morris H, et al. Early outcomes of outpatient management of kidney transplant recipients with coronavirus disease 2019. Clin J Am Soc Nephrol. 2020;15(8):1174‐1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Banerjee D, Popoola J, Shah S, Ster IC, Quan V, Phanish M. COVID‐19 infection in kidney transplant recipients. Kidney Int. 2020;97(6):1076‐1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Benotmane I, Gautier‐Vargas G, Wendling MJ, et al. In‐depth virological assessment of kidney transplant recipients with COVID‐19. Am J Transplant. 2020;20(11):3162‐3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bossini N, Alberici F, Delbarba E, et al. Kidney transplant patients with SARS‐CoV‐2 infection: the Brescia Renal COVID Task force experience. Am J Transplant. 2020;20(11):3019‐3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Columbia University Kidney Transplant Program . Early description of coronavirus 2019 disease in kidney transplant recipients in New York. J Am Soc Nephrol. 2020;31(6):1150‐1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Crespo M, Pérez‐Sáez MJ, Redondo‐Pachón D, et al. COVID‐19 in elderly kidney transplant recipients. Am J Transplant. 2020;20(10):2883‐2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Demir E, Uyar M, Parmaksiz E, et al. COVID‐19 in kidney transplant recipients: a multicenter experience in Istanbul. Transpl Infect Dis. 2020;22(5):e13371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Devresse A, Belkhir L, Vo B, et al. COVID‐19 infection in kidney transplant recipients: a single‐center case series of 22 cases from Belgium. Kidney Med. 2020;2(4):459‐466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Favà A, Cucchiari D, Montero N, et al. Clinical characteristics and risk factors for severe COVID‐19 in hospitalized kidney transplant recipients: a multicentric cohort study. Am J Transplant. 2020;20(11):3030‐3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fernández‐Ruiz M, Andrés A, Loinaz C, et al. COVID‐19 in solid organ transplant recipients: a single‐center case series from Spain. Am J Transplant. 2020;20(7):1849‐1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ghaffari Rahbar M, Nafar M, Khoshdel A, et al. Low rate of COVID‐19 pneumonia in kidney transplant recipients—A battle between infection and immune response? Transpl Infect Dis. 2020;22(5):e13406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hartzell S, Bin S, Benedetti C, et al. Evidence of potent humoral immune activity in COVID‐19‐infected kidney transplant recipients. Am J Transplant. 2020;20(11):3149‐3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lubetzky M, Aull MJ, Craig‐Schapiro R, et al. Kidney allograft recipients, immunosuppression, and coronavirus disease‐2019: a report of consecutive cases from a New York City transplant center. Nephrol Dial Transplant. 2020;35(7):1250‐1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Maritati F, Cerutti E, Zuccatosta L, et al. SARS‐CoV‐2 infection in kidney transplant recipients: experience of the italian marche region. Transpl Infect Dis. 2020;22(5):e13377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mehta SA, Leonard J, Labella P, et al. Outpatient management of kidney transplant recipients with suspected COVID‐19—Single‐center experience during the New York City surge. Transpl Infect Dis. 2020; 22(6):e13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mella A, Mingozzi S, Gallo E, et al. Case series of six kidney transplanted patients with COVID‐19 pneumonia treated with tocilizumab. Transpl Infect Dis. 2020;22(6):e13348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Monfared A, Dashti‐Khavidaki S, Jafari R, et al. Clinical characteristics and outcome of COVID‐19 pneumonia in kidney transplant recipients in Razi hospital, Rasht, Iran. Transpl Infect Dis. 2020;22(6):e13420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pascual J, Melilli E, Jiménez‐Martín C, et al. COVID‐19–related mortality during the first 60days after kidney transplantation. Eur Urol. 2020;78(4):641–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pérez‐Sáez MJ, Blasco M, Redondo‐Pachón D, et al. Use of tocilizumab in kidney transplant recipients with COVID‐19. Am J Transplant. 2020;20(11):3182‐3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rodriguez‐Cubillo B, de la Higuera MAM, Lucena R, et al. Should cyclosporine be useful in renal transplant recipients affected by SARS‐CoV‐2? Am J Transplant. 2020;20(11):3173‐3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Silva F, Cipriano A, Cruz H, et al. SARS‐CoV‐2 infection in kidney transplant recipients: early report of five cases. Transpl Infect Dis. 2021;23(1):e13394. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information.
Data Availability Statement
All information provided in this article can be obtained from the author on request.


