ABSTRACT
The extracellular matrix (ECM) disruption and cytoskeleton reorganization are crucial events in tumor proliferation and invasion. E-Cadherin (E-CAD) is a member of cell adhesion molecules involved in cell-cell junctions and ECM stability. The loss of E-CAD expression is associated with cancer progression and metastasis. This retrospective study aimed to assess E-CAD protein expression in ovarian cancer (OC) tissues and to evaluate its prognostic value. Patients and Methods: 143 formalin-fixed and paraffin-embedded (FFPE) blocks of primary advanced stages OC were retrieved and used to construct Tissue microarrays. Automated immunohistochemistry technique was performed to evaluate E-CAD protein expression patterns in OC. Results: E-CAD protein expression was significantly correlated with OC histological subtype (p < 0.0001), while borderline significant correlations were observed with both tumor grade (p = 0.06) and stage (p = 0.07). Interestingly, Kaplan-Meier survival analysis showed that OC patients with membranous E-CAD expression survived longer than those with no E-CAD expression mainly those at advanced stages (p < 0.009). Further in silico analysis confirms the key roles of E-CAD in OC molecular functions. Conclusion: we reported a prognosis value of membranous E-CAD in advanced stage OC patients. Further validation using larger cohorts is recommended to extract clinically relevant outcomes towards better OC management and individualized oncology.
KEYWORDS: E-Cadherin, ovarian cancer, prognostic value, immunohistochemistry, tissue microarray
1. Introduction
Ovarian cancer (OC) is a highly aggressive gynaecological malignancy causing significant global mortality among female patients. It is the fifth leading cause of cancer-related deaths among women globally. It was estimated that in 2018 about 295,414 new cases of OC were diagnosed worldwide resulting in 184,799 deaths [1]. In Saudi Arabia, the incidence of OC is about 3.5% of total cancer incidence among Saudis [2]. Remarkably, the incidence of OC has been progressively increasing over the past decade in almost all countries. Overall, OC constitutes 2.5% of all female malignancies and 5% of total female cancer deaths worldwide. The low survival rate of OC patients has been mainly attributed to the late-stage diagnosis and a unique metastatic pattern suggested for this disease [3]. Mostly, the process is clinically silent leading to late-stage diagnosis of an advanced and more aggressive tumor. As most cancer, OC is an heterogenous disease (the epithelial ovarian cancer (EOC) is the most common ovarian malignancy) with several histologic subtypes and molecular signatures. Moreover, individuals with a family history of breast cancer or OC are at higher risk for OC [4]. Several genomic predispositions, environmental factors and lifestyle choices were investigated as potential initiators of OC [5–8]. Despite the improvement in five-year survival rate in the past 10 years, the prognosis is still poor with an estimated overall five-year survival of 47% and only 29% for the advanced stages [9]. This is due to several contributing factors including non-specific symptoms, lack of reliable biomarkers, late-stage diagnosis, drug resistance and recurrence. However, the average survival time in women with stage IV disease is a dismal 3 years. The prognosis of OC patients is highly improved if the diagnosis is made at early stages (stage I) with 5-year survival reaching as high as 90%. Unfortunately, OC survival outcomes remain below 30% for advanced stages (III–IV) [10]. Therefore, novel, more sensitive, specific and highly selective markers are urgently needed for OC prevention, early diagnosis and management. Currently, the majority of OC patients have already advanced OC at first clinical presentation. Thus, in addition to early detection of OC, prognostic biomarkers associated with disease progression and dissemination are critical for designing effective treatment modalities and management for patients with metastatic disease.
The routine clinical practice relies on the serum OC biomarker cancer antigen 125 (CA125) for OC diagnosis and monitoring of disease progression, response to chemotherapy and relapse of the disease. However, the overall sensitivity of CA125 is variable. For example, 90% of OC patients with advanced-stage display elevated levels of CA125 whereas only 50% of stage I patients are positive for CA125 [11]. Considering the inconsistent results of CA125 in predicting treatment outcomes and for determining OC risk in asymptomatic populations [12,13], additional and more sensitive molecular biomarkers are required. In this context, E-Cadherin (E-CAD), a calcium-dependent tumor suppressor transmembrane glycoprotein (120kDa), has been suggested as a potential prognosis biomarker for several cancers [14]. It plays an important role in the progression and dissemination of various types of epithelium-derived carcinomas [15]. E-CAD (Molecular Weight:120 kDa) is encoded by Cadherin-1 (CDH1) gene located on human chromosome 16q22.1 consisting of 16 exons intervened by 15 introns. It is chiefly located at the cell membrane of epithelial cells. The extracellular domain of CDH1 is crucial for cellular adhesion [14], however its intracellular domain binds with the cytoskeleton through β-catenin to induce an array of intracellular signaling cascades [14,16]. E-CAD has been reported to be severely downregulated in advanced malignancies and loss of its function is suggested to promote epithelial to mesenchymal transition (EMT) and therefore metastasis [17]. The metastatic peritoneal dissemination of OC involves mainly a reduction in cell-cell adhesion in the growing tumor. The cell-cell adhesions and integrity of cell junctions are mediated by E-CAD. Reduced levels of E-CAD allow cells to engage in the EMT, detach from the primary tumor and migrate to distant locations. Therefore, altered E-CAD expression is considered as an important contributory factor in cell invasion and migration [18]. In this context, this study aimed to assess E-CAD protein expression patterns as potential molecular biomarker and OC prognosticator in Saudi OC patients. This could facilitate improved assessment of patient prognosis, enhance current management approaches and thus result in increased overall survival. Therefore, correlations between E-CAD expression patterns with Saudi OC patients’ clinicopathological features and its prognosis value were investigated and discussed on the light of its reported molecular and cellular functions.
2. Patients and methods
Clinicopathological features and follow up data. The study cohort is composed of 143 formalin-fixed and paraffin-embedded (FFPE) tissue samples of primary OC of advanced stages consent patients. FFPE blocks were retrieved from the archives of the Department of Pathology, King Abdulaziz University. Patients were diagnosed and treated mainly at the Departments of Pathology, and Obstetrics and Gynecology, King Abdulaziz University Hospital (KAUH), between 1999 and 2014. Only specimens containing more than 80% of tumor cells were used for analysis. The histopathological features of the carcinoma specimens were classified according to the tumor, node, metastasis (TNM) classification system. The clinical and pathological data of the patients were collected from the patients’ medical KAUH records. All patients were followed up until death or when last seen alive at their clinical visit (month, year) with the median follow-up time of months (range: 1–484 month, mean: 62 months). Ethical approval was obtained from the Biomedical Research Ethics Committee at KAUH (Ref. number: KAU-189-14). The main clinicopathological data of the patients are shown in Table 1.
Table 1.
Correlations between membraneous E-CAD expression at advanced stages (III, IV) and OC clinical pathological features using the cut-off point (no expression (0) vs (positive expression (1+,2+,3+))
| Clinico-pathological Features | Number Of samples | Significance |
|---|---|---|
| Age | Number (%) | p-value |
| ‹ 50 | 64 (56%) | 0.518 |
| › 50 | 51 (44%) | |
| Lymph Node status | ||
| Positive | 19 (32%) | 0.844 |
| Negative | 41 (68%) | |
| Histological subtypes | ||
| Serous | 52 (47%) | 0.0001 |
| Mucinous | 23 (21%) | |
| Other types | 36 (32%) | |
| Tumor Size | ||
| 1–5 cm | 21 (19%) | 0.441 |
| 6–10 cm | 33 (29%) | |
| >10 cm | 59 (52%) | |
| Tumor Grade | ||
| Grade 1 | 14 (14%) | 0.060 |
| Grade 2 | 16 (17%) | |
| Grade 3 | 67 (69%) | |
| Parity | ||
| Parous | 51 (60%) | 0.077 |
| Nulliparous | 34 (40%) | |
| Body mass index (BMI) | ||
| < 23 | 7 (8%) | 0.623 |
| 23–26 | 23 (27%) | |
| >26 | 55 (65%) | |
| Recurrence | ||
| Negative | 46 (53%) | 0.665 |
| positive | 40 (47%) | |
| Tumor Stage | ||
| Low Stage | 37 (36%) | 0.069 |
| High Stage | 66 (64%) | |
| Endpoint Status | ||
| Deceased | 34 (35%) | 0.394 |
| Living | 63 (65%) |
Tissue Microarray. Using tissue microarray technique (TMA), approximately 245 FFPE blocks of ovarian cancer were successfully transferred to construct TMA slides for evaluating the expression pattern of E-CAD protein expression as previously described [19]. Out of 245, 143 samples were from patients at advanced OC stages (III, IV). Our TMA slides and protocols were previously validated using both IHC for protein profiling and BDISH for copy number variations in colorectal and bladder cancers [20,21]. It is a reliable and cost-effective technique for biomarkers discovery and validation in solid tumors.
Immunohistochemistry (IHC). Expression of E-CAD protein was detected using an automated IHC Ventana staining system and concentrated E-CAD mouse monoclonal antibody (M3612, Dako) was used. The detailed protocol of the IHC procedure was performed as described elsewhere [21,22].
Evaluation of E-CAD protein expression patterns. E-CAD staining was blindly evaluated using a regular light microscope at the magnification of x40 by two independent certified pathologists to prevent bias. Membranous and cytoplasmic staining were evaluated separately. Expression was categorized into 4 levels: no expression (0), weak (1+), moderate (2+) and strong (3+). The evaluators of E-CAD expression patterns were blinded to the clinical follow-up data. However, the expression patterns profile of E-CAD was scored using the Index Score method, an objective and validated method designed by Lipponen and Collan [23,24]. The reproducibility of the evaluation of E-CAD staining indices was tested by employing intra-observer reproducibility. The best cut off discriminator for E-CAD expression patterns was No expression (0) versus E-CAD positive expression (1+, 2+, 3+).
Statistical analysis. Statistical analyses have been performed by the SPSS® (IMB NY, USA) software packages (PASW Statistics for Windows, model 19). Frequency tables have been analyzed by using the Chi-Square test, with likelihood ratio (LR) or Fischer’s exact test to evaluate the importance of the correlation between the different variables. The disease-specific survival (DSS) and disease-free survival (DFS) were calculated as the time from diagnosis to death (due to disease) or to the date of last seen alive, and time from diagnosis to the appearance of recurrent disease or date of last seen disease-free, respectively. In calculating DSS, patients died of other or unknown causes were censored. The univariate analysis of both DFS and DSS outcomes were calculated based on Kaplan-Meier survival analysis method, with long-rank (Mantel-Cox) comparison test. In all tests, the value (p < 0.05) was considered as the statistically significant cut off point.
3. Results
Expression Patterns of E-CAD protein profiling in advanced stage ovarian cancer tissues. Our results showed that the cellular localization of E-CAD expression was both membranous and cytoplasmic. The expression patterns are illustrated in Figure 1. Most patients’ samples (73%) showed E-CAD membranous E-CAD expression, while no expression was observed in 27% of the cohort.
Figure 1.
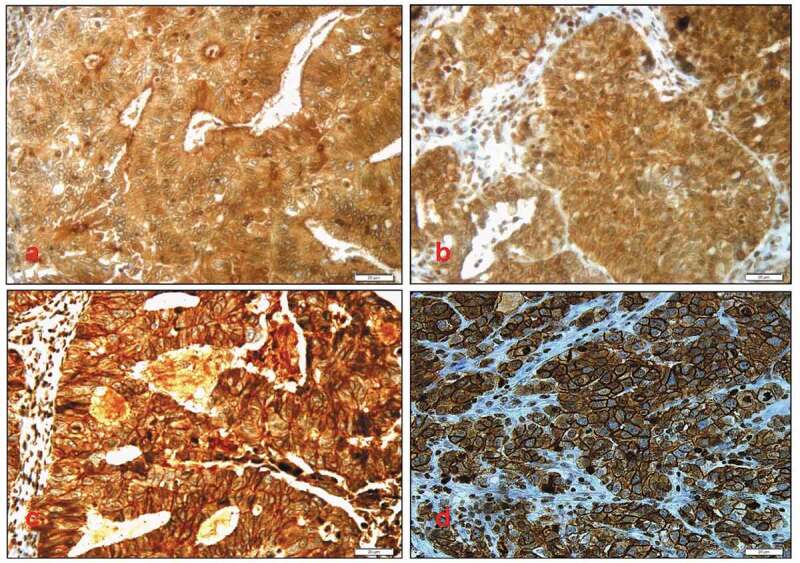
Different membranous E-Cadherin expression patterns: (A) no (0) expression pattern of E-cadherin protein, (B) weak (1+) membranous expression pattern, (C) moderate (2+) membranous expression pattern, (D) strong (3+) membranous expression pattern of E-cadherin protein. Cytoplasmic E-cadherin expression pattern was also noticed. Magnification X40
Correlation of E-CAD protein expression patterns with clinicopathological. The correlation of E-CAD protein expression in advanced stage OC tissues with the patients’ clinicopathological characteristics by using different cut-offs showed that no E-CAD expression (0) versus varying expression cut-off (No expression (0) versus positive expression (1+, 2+, 3+).) was the most powerful discriminator. For histological subtypes, 56% of OC serous subtype showed significantly higher E-CAD expression whereas 44% showed low expression. Similarly, 87% of OC mucinous subtypes displayed higher expression of E-CAD (p < 0.0001). Borderline significant correlations between E-CAD expression and both tumor grade (p = 0.06) and stage (p = 0.07) as well as parity (p = 0.07) were observed. In fact, 100% of the intermediate grade showed positive E-CAD expression compared to the high grade where the E-CAD expression was observed in just 73% of them. Interestingly, the E-CAD expression intensity decreased with advanced stages to reach a level where no overexpression pattern (3+) was observed at stage IV.
The other clinicopathological features did not show significant correlations with E-CAD expression profiles including parity (0.07), age (p = 0.5), tumor size (p = 0.4), body mass index (BMI) (p = 0.6) and lymph node status (p = 0.8) (Table 1).
Correlations of E-CAD protein expression with survival outcomes. Kaplan-Meier Survival analysis performed on the whole patients’ cohort showed that OC patients with higher membranous E-CAD expression showed a trend of longer survivals than those with no E-CAD expression (Figure 2A and B). For example, at 3 years follow up time, approximately 80% of OC patients with higher E-CAD expression were alive compared to 40% death rate for the OC patients of all stages with no E-CAD expression (Figure 2B, p = 0.1, log-rank).
Figure 2.
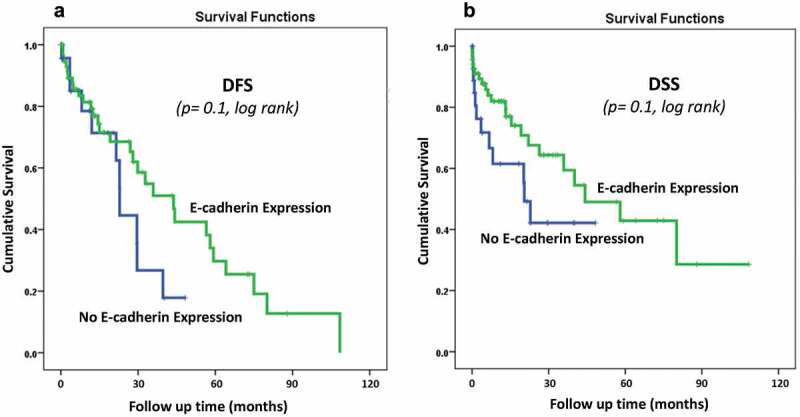
E-Cadherin membraneous protein expression pattern status in the whole OC cohort using the cut-off (No expression (0) vs Positive expression (1 + 2+,3+)) as a determinant of (A): disease-free survival (DFS) (p = 0.1, log-rank), and (B): disease-specific survival (DSS) (p = 0.1, log-rank) in univariate (Kaplan-Meier) analysis
Interestingly, the assessment of DSS by stratifying the patients according to their stage status, using the (no expression vs. positive expression) cut-off point and following the patient more than 10 years showed a significant prognostic power. In that, patients with advanced stage OC tissues that expressed E-CAD lived longer (Figure 3B). Interestingly, after 10 years of follow up time, about 55% of OC patients who had advanced-stage tumors with E-CAD expression were still alive compared to only 15% of advanced cancer patients with no E-CAD expression (p < 0.009, log-rank) (Figure 3B).
Figure 3.
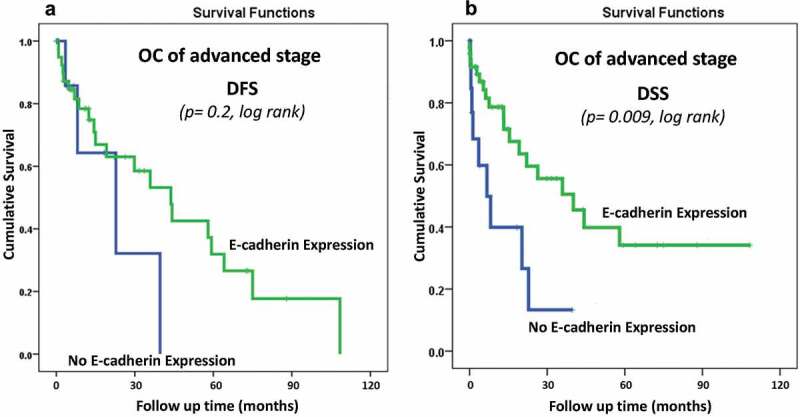
E-Cadherin membraneous protein expression pattern status in advanced stages OC cohort using the cut-off (No expression (0) vs Positive expression (1 + 2+,3+)) as a determinant of (A): disease-free survival (DFS) (p = 0.2, log-rank), and (B): disease-specific survival (DSS) (p = 0.009, log-rank) in univariate (Kaplan-Meier) analysis
On the other hand, for the DFS, Kaplan-Meier Survival analysis did not reveal any significant correlations between E-CAD expression and survival outcomes either for the whole cohort (p = 0.1, Figure 2A) or on patients with advanced-stages OC (p = 0.2) (Figure 3A). However, a clear trend of less recurrence is observed in patients with E-CAD expression compared to those with no E-CAD expression (Figure 2A, Figure 3A)
4. Discussion
The development of precision high-throughput technologies and big data have enabled us to significantly advance our understanding of cancer biology through dissecting the disease progression and dissemination at genomics, transcriptomics, proteomics and/or metabolomics levels. However, cancer-related deaths remain a major concern worldwide. Moreover, the cost of these high-throughput technologies, mainly genomic profiling, has become exceptionally time and cost-effective for clinical application. Therefore, OMICs-based prognostic approaches have also been used for biomarkers discovery to strengthen existing screening approaches to enhance prognostic power of cancer patients’ outcomes [25,26]. In fact, the major challenges in cancer management include early diagnosis, prognosis, prediction, development of resistance to anticancer therapeutics and disease recurrence. This study focuses on ovarian cancer which is still the deadly women cancer worldwide. The reasons for these OC high mortality rates are mainly advanced stage at diagnosis and frequent recurrence following surgical resection and adjuvant therapy [27]. However, the major challenge for clinicians is how to accurately discriminate the OC patients that are most likely to obtain considerable survival advantage. Some biomarkers such as CA125, HE4 and ROMA have been utilized to predict treatment outcomes, recurrence and survival. However, these biomarkers are still lacking sensitivity and specificity; and challenged particularly by the heterogeneous nature of OC tumors. Therefore, it is currently well recognized that there is a need for more accurate molecular stratification of OC patients to offer them the best conventional and molecular therapeutic strategies. Such molecular stratification of OC relies on the effective identification of more sensitive and selective biomarkers. In this context, E-CAD has been reported to be associated with the progression and metastasis of a variety of cancer types including OC [15,28,29]. So far, the clinical and prognostic relevance of E-CAD in OC remains not well-investigated worldwide, especially in Saudi Arabia. Based on this fact, this study was performed to investigate the E-CAD expression patterns in OC and assess its prognostic value in Saudi patients.
In our study cohort of 245 OC patients, membranous E-CAD protein expression was detected in the majority of patients (73%) which is in line with previously reported studies [30]. In our association analysis, E-CAD expression correlated well with histologic subtypes since most serous and mucinous subtypes showed higher E-CAD expression compared to other histologic subtypes. In fact, E-CAD expression was found to be a good diagnostic marker to distinguish between serous tumors and mucinous tumors from other histologic tumors. This observation is in agreement with recently published studies [14,31]. However, higher stage tumors demonstrated a diminishing trend in E-CAD expression level. These findings suggest that E-CAD plays an important role in the suppression of tumor progression through maintaining cell-cell adherence [14,32]; and the decrease of E-CAD expression seems to favour tumor dissemination [33].
On the other side, our results did not show any significant relationship between E-CAD expression and other clinicopathological features including patient age, tumor size, grade, body mass index and lymph node status (p > 0.05). Our results are in line with previously published studies that reported similar observations [33,34].
While OC risk has been reported to increase with age and therefore higher in older women, our results showed an interesting aspect where 56% of our patients’ cohort were below 50 years (Table 1). This early onset of OC in the Saudi population is still unexplained phenomenon compared to western countries and could be associated with genomic roots, environmental factors and/or lifestyles choices. For instance, in the UK, according to Cancer Research UK, 53% of OC cases were diagnosed at 65 and over. These findings highlight the need for additional studies to demystify the early onset problem of OC in the Arabic peninsula. Additionally, 64% of our cohort were diagnosed at advanced stages (III, IV). This late diagnosis of OC is the main reason for the high mortality rates of this disease worldwide. In fact and given its asymptomatic aspect, OC is also called the “silent killer’ with about 75% of OC patients are diagnosed at advanced stages [35,36]. This is mainly due to its anatomical position in the pelvic cavity where OC symptoms are often confused with urogenital and gastrointestinal tracts’ pain/discomfort [36–40].
In Kaplan-Meier Survival analysis, disease-free survival (DFS) analysis did not reveal any significant correlation between E-CAD expression and survival outcomes either for the whole cohort or advanced-stage cases. However, disease-specific survival (DSS) showed a general trend where OC patients with higher E-CAD expression survived longer compared to those with negative E-CAD expression (Figure 3). These findings support that E-CAD expression in OC is a good general prognosticator in our patients’ cohort, and seems to be involved in critical molecular events that prevent OC aggressive metastatic progression as suggested elsewhere [14]. After that, we refined the analysis by focusing mainly on the advanced-stage OC patients since they are the most vulnerable fraction of the cohort. Strikingly, E-CAD protein expression was a highly significant prognosticator of OC patients at the advanced-stages (III, IV) (p < 0.009, log-rank) (Figure 3). In fact, for more than 10 years of follow-up, about 40% of OC patients with both advanced-stage tumors and E-CAD protein overexpression were alive while all those with no E-CAD died. Our findings are concomitant with previous studies showing that E-CAD protein overexpression helps in maintaining tissue integrity and normal morphogenesis. However, its down-expression lead to severe perturbations in cytoskeleton structure and tissue architecture during tumor dissemination [33]. In line with our findings, negative E-CAD expression has been reported to be associated with loss of cell-cell adhesion, and therefore increase of both epithelial OC cell migration and dissemination. Moreover, E-CAD down-expression is also considered as a hallmark of epithelial-to-mesenchymal transition (EMT) and a pro-metastatic event [14,17]. Additionally, negative E-CAD expression in OC has been reported to be associated with aggressive tumors, advanced-stages and extensive metastasis, poor prognosis and shorter survivals [36–38]. Furthermore, E-CAD is involved in the molecular shifting phenomenon namely ‘Cadherin Isoform Switching’ from E-Cadherin (CDH1) to N-Cadherin (CDH2) occurs during embryonic development and the normal physiological processes to allow different cell types to separate from each other [41]. However during the EMT, tumor cells adopt this ‘Cadherin Isoform Switching’ mechanism to upregulate the CDH2 as well as metalloproteinases (MMPs); and subsequently downregulate CDH1 [42,43]. This ‘Cadherin Switching’ has significantly been correlated with ECM remodeling, increased migration and invasion of cancer cells, tumor stemness, metastasis, and higher mortality rates in cancer patients. Hence, the EMT is crucial for cancer progression, cancer drug resistance, and stemness of tumors that facilitates the initiation of metastasis [14,44,45]. That’s why, the inhibition or prevention of EMT is considered as one of the essential strategies to either prevent or ameliorate the progression of various types of human malignancies.
Our preliminary Ingenuity Pathway Analysis (IPA) (Qiagen, USA) of 959 genes, obtained from the open targets platform and associated with the development of ovarian cancer (OC) shown that the CDH1 expression was reduced and the CDH2 levels were upregulated in OC (Figure 4).
Figure 4.
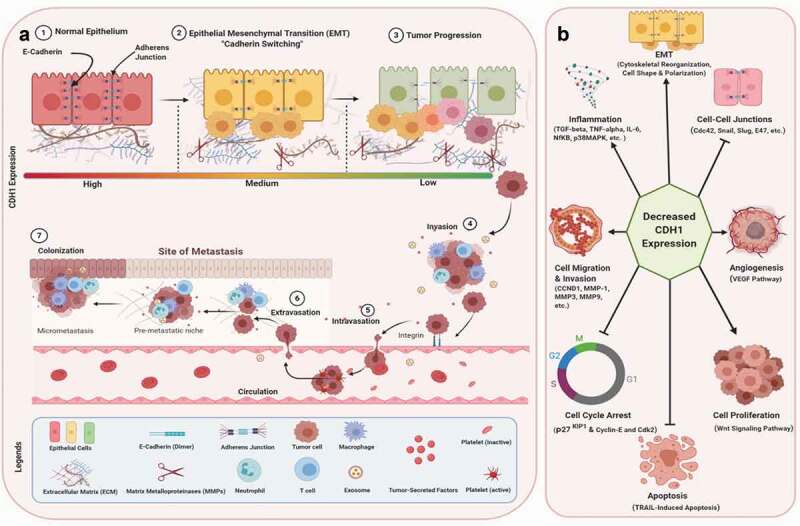
Overview of E-Cadherin (CDH1) involvement in OC pathogenesis from EMT to metastasis, and the main molecular and cellular functions associated with CDH1 down-expression. (A): ‘Cadherin Isoform Switching’ is the downregulation of E-cadherins (CDH1) and upregulation of N-cadherins in EMT. The adherens junctions mediated by CDH1 detach due to the downregulation of E-cadherin. This ‘Cadherin Isoform Switching’ has significantly been associated with increased migration and invasion of cancer cells, tumor stemness, metastasis, and reduced survival or higher mortality rate in cancer patients. The EMT is critical for tumor progression, drug resistance, and stemness of tumors that subsequently accelerate the initiation of metastasis. (B): The reduction or loss of CDH1 leads to the activation of an array of signaling pathways such as pro-inflammatory pathways (TGF-beta, TNF-alpha, IL-6, NfKB, p38 MAPK, ERK1/2, etc.), disruption of cell-cell junctions (Cdc42, Snail1, Snail2, Slug, E47, etc.), reorganization of cytoskeleton and cell shape (EMT), cell migration and invasion (CCND1, MMP1, MMP3, MMP9, etc.), angiogenesis (VEGF), cell proliferation (Wnt/β-catenin), inhibition of TRAIL-induced apoptosis, and cell cycle arrest (cyclin-E, Cdk2, p27KIP1, etc.) leading to cancer progression, cancer stemness, and metastasis
This downregulation or loss of CDH1 activates an array of signaling molecules leading to pro-inflammatory pathways, disruption of cell-cell junctions, reorganization of cytoskeleton and cell shape (EMT), cell migration and invasion, Matrix Metalloproteinases, angiogenesis, cell proliferation, and the inhibition of both TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis, and cell cycle arrest leading to cancer progression, increased stemness, and metastasis (Figure 4).
Taken together, these accumulating evidences suggest that the loss of E-CAD expression promotes OC dissemination through multiple cellular and molecular mechanisms starting from EMT to metastasis [39].
Since about 65% of our cohort was at the advanced-stages (III, IV), negative E-CAD expression seems to predict a poor prognosis outcome mainly in OC patients with advanced-stages. Such unfavourable clinical outcome predicted at the OC advanced stages was also confirmed by some previous studies [46]. Taken together, it seems that the loss of transmembrane E-CAD (120 kDa) may be considered as a meaningful prognostic biomarker at the advanced-stages’ OC patients and might, once validated, help in clinical setting.
However, some inconsistencies have been reported linking either high or low E-CAD expression with decreased tumor progression and dissemination [34,47,48]. In fact, these studies suggest an additional role of E-CAD in tumor progression apart from its cell-cell adhesion function. In fact and besides the transmembrane E-CAD (120 kDa) isoform, studies showed the presence of another soluble/cytoplasmic form of E-CAD isoform (sE-CAD; 80 kDa protein) that also seems to play an important role in the process of tumor growth by promoting angiogenesis through heterodimerization with VE-CAD (vascular endothelial Cadherin) and subsequently activating downstream β-catenin and NFkB (nuclear factor-kB) signalling cascades (Figure 4). The role of this new suggested sE-CAD isoform should be investigated further to shed light on its exact molecular roles and specific prognosis value in OC.
5. Conclusions
This study reports a prognosis power of membranous E-CAD in Saudi OC patients mainly those at the advanced-stages. However, the small size of our cohort may be a limitation of this study and therefore further research using larger cohorts is warranted to validate these findings.
Future IHC-based studies focusing on the assessment of E-CAD cellular localization, expression and prognostic value should use isoform-specific antibodies in larger OC patients’ cohorts. A suitable assessment of E-CAD expression patterns and the associated survival results will contribute to extract personalized and tangible clinically relevant outcomes towards better OC management and individualized oncology.
Funding Statement
This work was supported by the Prof. Abdullah Basalamh’s Scientific Chair for Women’s Tumors, [# 28-04/2014].
Author Contributions
KS, MA & AB: Contributed in study design, statistical analysis, and manuscript drafting; MAJ, MAE and AEO: Contributed in data analysis and helped in results & discussions drafting; JAM, NA and HB: Contributed in TMA design and construction, immunostaining and manuscript revision; SS, MS and SM: Contributed in clinicopathological data collection, statistical analysis and manuscript proofreading; KS: Study design and supervision of the whole team; All authors critically reviewed and agreed on the final version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Abbreviations
- OC
ovarian cancer
- TNM
Tumor, node and Metastasis
- IHC
Immunohistochemistry
- TMA
Tissue MicroArray
- ASCO
American Society of Clinical Oncology
- CAP
College of American Pathologists
- E-CAD
E-Cadherin (CDH1)
- DFS
Disease-free survival
- DSS
Disease specific survival
References
- [1].Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–9. [DOI] [PubMed] [Google Scholar]
- [2].Saudi Cancer Registry S : Cancer incidence report Saudi Arabia 2013. Saudi Cancer Registry; 2016. p. 1–88.
- [3].Howlader N, Noone A, Krapcho M, et al. SEER cancer statistics review, 1975–2017, National Cancer Institute. Bethesda, MD; 2020. https://seer.cancer.gov/csr/1975_2017/, posted to the SEER web site, April 2020. Bethesda, MD. [Google Scholar]
- [4].Prat J. Ovarian carcinomas: five distinct diseases with different origins, genetic alterations, and clinicopathological features. Virchows Arch. 2012;460(3):237–249. [DOI] [PubMed] [Google Scholar]
- [5].Bolton KL, Chenevix-Trench G, Goh C, et al. Association between BRCA1 and BRCA2 mutations and survival in women with invasive epithelial ovarian cancer. JAMA. 2012;307(4):382–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Menon U, Karpinskyj C, Gentry-Maharaj A. Ovarian cancer prevention and screening. Obstet Gynecol. 2018;131(5):909–927. [DOI] [PubMed] [Google Scholar]
- [7].Reid A, de Klerk N, Musk AW. Does exposure to asbestos cause ovarian cancer? A systematic literature review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2011;20(7):1287–1295. [DOI] [PubMed] [Google Scholar]
- [8].Walker JL, Powell CB, Chen L-M, et al. Society of Gynecologic Oncology recommendations for the prevention of ovarian cancer. Cancer. 2015;121(13):2108–2120. [DOI] [PubMed] [Google Scholar]
- [9].Peres LC, Cushing-Haugen KL, Köbel M, et al. Invasive epithelial ovarian cancer survival by histotype and disease stage. JNCI. 2018;111(1):60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Leung F, Bernardini MQ, Brown MD, et al. Validation of a novel biomarker panel for the detection of ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2016;25(9):1333–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nustad K, Bast RC Jr, Brien TJ, et al. Specificity and affinity of 26 monoclonal antibodies against the CA 125 antigen: first report from the ISOBM TD-1 workshop. Tumor Biol. 1996;17(4):196–219. [DOI] [PubMed] [Google Scholar]
- [12].Nossov V, Amneus M, Su F, et al. The early detection of ovarian cancer: from traditional methods to proteomics. Can we really do better than serum CA-125?. Am J Obstet Gynaecol. 2008;199:215–223. [DOI] [PubMed] [Google Scholar]
- [13].Skates SJ. Calculation of the risk of ovarian cancer from serial CA-125 values for preclinical detection in postmenopausal women. J clin oncol. 2003;21(90100):206s–210. [DOI] [PubMed] [Google Scholar]
- [14].Rosso M, Majem B, Devis L, et al. E-cadherin: a determinant molecule associated with ovarian cancer progression, dissemination and aggressiveness. PloS One. 2017;12(9):e0184439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wong SHM, Fang CM, Chuah L-H, et al. E-cadherin: its dysregulation in carcinogenesis and clinical implications. Crit Rev Oncol Hematol. 2018;121:11–22. [DOI] [PubMed] [Google Scholar]
- [16].Van Roy F, Berx G. The cell-cell adhesion molecule E-cadherin. Cell Mol Life Sci. 2008;65(23):3756–3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Dai C, Cao J, Zeng Y, et al. E-cadherin expression as a prognostic factor in patients with ovarian cancer: a meta-analysis. Oncotarget. 2017;8(46):81052–81061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cheung KJ, Padmanaban V, Silvestri V, et al. Polyclonal breast cancer metastases arise from collective dissemination of keratin 14-expressing tumor cell clusters. Proc Nat Acad Sci. 2016;113(7):E854–E863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Al-Maghrabi J, Emam E, Gomaa W, et al. c-MET immunostaining in colorectal carcinoma is associated with local disease recurrence. BMC Cancer. 2015;15(1):676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Karim S, Al-Maghrabi JA, Farsi HM, et al. Cyclin D1 as a therapeutic target of renal cell carcinoma-a combined transcriptomics, tissue microarray and molecular docking study from the Kingdom of Saudi Arabia. BMC Cancer. 2016;16(S2):741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Nedjadi T, Al-Maghrabi J, Assidi M, et al. Prognostic value of HER2 status in bladder transitional cell carcinoma revealed by both IHC and BDISH techniques. BMC Cancer. 2016;16(1):653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Assidi M, Gomaa W, Jafri M, et al. Prognostic value of Osteopontin (SPP1) in colorectal carcinoma requires a personalized molecular approach. Tumour Biol. 2019;41(9):1010428319863627. [DOI] [PubMed] [Google Scholar]
- [23].Buhmeida A, Elzagheid A, Algars A, et al. Expression of the cell-cell adhesion molecule beta-catenin in colorectal carcinomas and their metastases. Apmis. 2008;116(1):1–9. [DOI] [PubMed] [Google Scholar]
- [24].Lipponen PK, and Collan Y. Simple quantitation of immunohistochemical staining positivity in microscopy for histopathology routine. Acta Stereol. 1992. 11 1 125–132 https://popups.uliege.be/0351-580x/index.php?id=1872&lang=en https://popups.uliege.be/0351-580x/index.php?id=1872&lang=en Journal name today: Image Analysis & Stereology (IAS) ; ISSN: 1580-3139; the old/former name of this journal was: Acta Stereologica . [Google Scholar]
- [25].Clifford C, Vitkin N, Nersesian S, et al. Multi-omics in high-grade serous ovarian cancer: biomarkers from genome to the immunome. Am J Reprod Immunol. 2018;80(2):e12975. [DOI] [PubMed] [Google Scholar]
- [26].Fu A, Chang HR, Zhang Z-F. Integrated multiomic predictors for ovarian cancer survival. Carcinogenesis. 2018;39(7):860–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Torre LA, Trabert B, DeSantis CE, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68:284–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Elzagheid A, Buhmeida A, Laato M, et al. Loss of E-cadherin expression predicts disease recurrence and shorter survival in colorectal carcinoma. APMIS. 2012;120(7):539–548. [DOI] [PubMed] [Google Scholar]
- [29].Horne HN, Oh H, Sherman ME, et al. E-cadherin breast tumor expression, risk factors and survival: pooled analysis of 5,933 cases from 12 studies in the breast cancer association consortium. Sci Rep. 2018;8(1):6574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Koensgen D, Freitag C, Klaman I, et al. Expression and localization of e-cadherin in epithelial ovarian cancer. Anticancer Res. 2010;30:2525–2530. [PubMed] [Google Scholar]
- [31].Liew P-L, Hsu C-S, Liu W-M, et al. Prognostic and predictive values of Nrf2, Keap1, p16 and E-cadherin expression in ovarian epithelial carcinoma. Int J Clin Exp Pathol. 2015;8:5642–5649. [PMC free article] [PubMed] [Google Scholar]
- [32].Daraï E, Scoazec J-Y, Walker-Combrouze F, et al. Expression of cadherins in benign, borderline, and malignant ovarian epithelial tumors: a clinicopathologic study of 60 cases. Hum Pathol. 1997;28(8):922–928. [DOI] [PubMed] [Google Scholar]
- [33].Faleiro-Rodrigues C, Macedo-Pinto I, Pereira D, et al. Prognostic value of E-cadherin immunoexpression in patients with primary ovarian carcinomas. Ann Oncol. 2004;15(10):1535–1542. [DOI] [PubMed] [Google Scholar]
- [34].Davidson B, Holth A, Hellesylt E, et al. The clinical role of epithelial-mesenchymal transition and stem cell markers in advanced-stage ovarian serous carcinoma effusions. Hum Pathol. 2015;46(1):1–8. [DOI] [PubMed] [Google Scholar]
- [35].Ebell MH, Culp MB, Radke TJ. A systematic review of symptoms for the diagnosis of ovarian cancer. Am J Prev Med. 2016;50(3):384–394. [DOI] [PubMed] [Google Scholar]
- [36].Prazak K, Gahres J. Ovarian cancer: practice essentials. Phys Assist Clin. 2016;1:479-+. [Google Scholar]
- [37].Bower M, Waxman J. Ovarian Cancer. In: Lecture Notes: oncology. Wiley; 2016. [Google Scholar]
- [38].Khan A, Sultana K. Presenting signs and symptoms of ovarian cancer at a tertiary care hospital. J Pak Med Assoc. 2010;60:260–262. [PubMed] [Google Scholar]
- [39].Rooth C. Ovarian cancer: risk factors, treatment and management. Br J Nurs. 2013;22(Sup17):S23–30. [DOI] [PubMed] [Google Scholar]
- [40].Verheijen RHM, Zweemer RP. Screening to improve ovarian cancer prognosis? Lancet. 2016;387(10022):921–923. [DOI] [PubMed] [Google Scholar]
- [41].Thiery JP, Acloque H, Huang RYJ, et al. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890. [DOI] [PubMed] [Google Scholar]
- [42].Aleskandarany MA, Negm OH, Green AR, et al. Epithelial mesenchymal transition in early invasive breast cancer: an immunohistochemical and reverse phase protein array study. Breast Cancer Res Treat. 2014;145(2):339–348. [DOI] [PubMed] [Google Scholar]
- [43].Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Investig. 2009;119(6):1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Araki K, Shimura T, Suzuki H, et al. E/N-cadherin switch mediates cancer progression via TGF-β-induced epithelial-to-mesenchymal transition in extrahepatic cholangiocarcinoma. Br J Cancer. 2011;105(12):1885–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Mrozik KM, Blaschuk OW, Cheong CM, et al. N-cadherin in cancer metastasis, its emerging role in haematological malignancies and potential as a therapeutic target in cancer. BMC Cancer. 2018;18(1). DOI: 10.1186/s12885-018-4845-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Bačić B, Haller H, Mrklić I, et al. Prognostic role of E-cadherin in patients with advanced serous ovarian cancer. Arch Gynecol Obstet. 2012;287:1219–1224. [DOI] [PubMed] [Google Scholar]
- [47].Davidson B, Berner A, Nesland JM, et al. and Ann Florenes V: e-cadherin and α-, β-, and γ-catenin protein expression is up-regulated in ovarian carcinoma cells in serous effusions. J Pathol. 2000;192(4):460–469. [DOI] [PubMed] [Google Scholar]
- [48].Hudson LG, Zeineldin R, Stack MS. Phenotypic plasticity of neoplastic ovarian epithelium: unique cadherin profiles in tumor progression. Clin Exp Metastasis. 2008;25(6):643–655. [DOI] [PMC free article] [PubMed] [Google Scholar]


