Abstract
Context
Bupleuri Radix, the dried root of Bupleurum chinense DC and Bupleurum scorzonerifolium Willd (Apiaceae), is an important medicinal herb widely used to treat cancers for hundreds of years in Asian countries. As the most antitumour component but also the main toxic component in Bupleuri Radix, saikosaponin D (SSD) has attracted extensive attention. However, no summary studies have been reported on the antitumour effects, toxicity and pharmacokinetics of this potential natural anticancer substance.
Objective
To analyse and summarise the existing findings regarding to the antitumour effects, toxicity and pharmacokinetics of SSD.
Materials and methods
We collected relevant information published before April 2021 by conducting a search of literature available in various online databases including PubMed, Science Direct, CNKI, Wanfang database and the Chinese Biological Medicine Database. Bupleurum, Bupleuri Radix, saikosaponin, saikosaponin D, tumour, toxicity, and pharmacokinetics were used as the keywords.
Results
The antitumour effects of SSD were multi-targeted and can be realised through various mechanisms, including inhibition of proliferation, invasion, metastasis and angiogenesis, as well as induction of cell apoptosis, autophagy, and differentiation. The toxicological effects of SSD mainly included hepatotoxicity, neurotoxicity, haemolysis and cardiotoxicity. Pharmacokinetic studies demonstrated that SSD had the potential to alter the pharmacokinetics of some drugs for its influence on CYPs and P-gp, and the oral bioavailability and actual pharmacodynamic substances in vivo of SSD are still controversial.
Conclusions
SSD is a potentially effective and relatively safe natural antitumour substance, but more research is needed, especially in vivo antitumour effects and pharmacokinetics of the compound.
Keywords: Bupleuri Radix, saikosaponins, phytochemicals, tumour, mechanisms, cytotoxicity, metabolism
Introduction
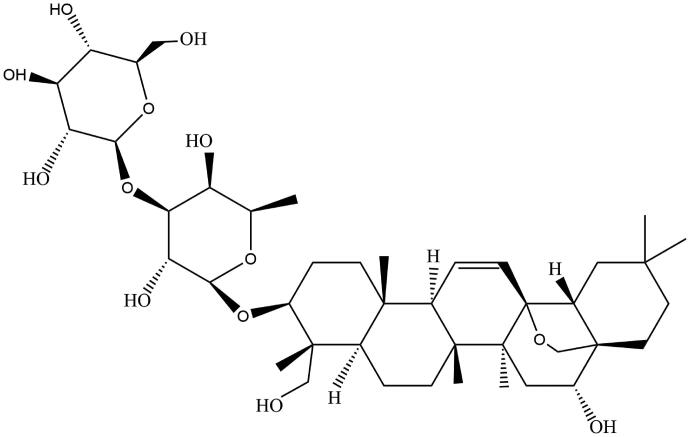
The genus of Bupleurum (Apiaceae) has about 200 species, mainly distributed in the north temperate zone (Meng et al. 2012). The roots of some species of Bupleurum are famous for being used as Bupleuri Radix, an important plant medicine widely used to treat febrile, digestive, endocrine, mental, oncological and other diseases in Asian countries, such as China, South Korea and Japan, for more than 2000 years (Ashour and Wink 2011; Jiang et al. 2020). Saikosaponins (SSs) are triterpene saponins extracted from only Bupleurum plants and have more than 100 kinds including SSA, SSB, SSC, SSD, etc. (He et al. 2019). SSs are also the main secondary metabolites of Bupleurum, accounting for 7% of the total root dry weight (Ashour and Wink 2011). Many pharmacological effects of Bupleuri Radix are directly related to SSs (Li et al. 2018; Jiang et al. 2020). According to the theory of TCM, Bupleuri Radix can dissipate tumour by promoting qi and blood circulation, so it is widely used clinically in the prescriptions for treating tumours (Lin et al. 2015; Xiao et al. 2020). As the key bioactive components of Bupleuri Radix, SSs have been extensively studied to reveal the potential antitumour mechanism of Bupleuri Radix (Li et al. 2018). SSD (Figure 1), whose molecular formula is C42H68O13 and molecular weight is 780.98, has been proved to possess the strongest antitumour activity among SSs, and exert antitumour effects on various cancer cells through multiple mechanisms (Li et al. 2015; Yuan et al. 2017; Hu et al. 2019). Many triterpenoid saponins have been found to be more sensitive to cancer cells than normal histiocytic cells, indicating their potential safety as anticarcinogens (Du et al. 2014). SSD may be of great significance for the research and development of new anticarcinogen, but unfortunately, information on the efficacy and safety of SSD as an anticarcinogen is scattered. Hence, in this paper, the relevant studies on the antitumour effects, toxicity and pharmacokinetics of SSD will be reviewed.
Figure 1.
Chemical structure of SSD.
Antitumour effects
The antitumour effects of SSD in different types of tumours will be discussed below, and it was mainly tested with cellular models in vitro, while less in vivo research has been done. The relevant information is summarised in Tables 1 and 2.
Table 1.
Antitumour effects of SSD in vitro.
| Cancer type | Cancer cells | Concn. | Suggested mechanism | Ref. |
|---|---|---|---|---|
| Hepatoma | SMMC-7721 | 3.2–19.2 µM | Inhibition of p-STAT3/HIF-1α pathway and further suppression of COX-2 expression. Inhibition of proliferation | (He et al. 2014) |
| SMMC-7721, HepG2 | 3.2–19.2 µM | Inhibition of p-STAT3 /C/EBPβ pathway and further suppression of COX-2 expression. Inhibition of proliferation. Induction of apoptosis. | (Ren et al. 2019) | |
| HepG2, Hep3B | 1–10 µM | Activation of p53 and further activation of Fas/FasL pathway. Inhibition of NF-κB pathway. Induction of G1-cell cycle arrest. Inhibition of proliferation. Induction of apoptosis. | (Hsu, Kuo, Chiang, et al. 2004) | |
| HepG2 | 10 µM | Suppression of NF-κB activation. Inhibition of proliferation, angiogenesis and invasion. Induction of apoptosis. | (Wong, Zhang, et al. 2013) | |
| SMMC-7721 | 1.28, 3.84 µM | Activation of the p53 pathway. Increase of G0/G1 arrest. Induction of G2/M-phase arrest under hypoxia. Induction of apoptosis. Inhibition of growth. Radiosensitization. | (Wang et al. 2013, 2014) | |
| SMMC-7721, MHCC97L | 3.84 µM | Suppression of mTOR pathway. Inhibition of proliferation. Induction of autophagy formation. Radiosensitization. | (Tian et al. 2019) | |
| Hep3B | 2–15 µM | Upregulation of SENP5 expression and subsequent inhibition of Gli1 SUMOylation. Inhibition of SHh pathway. Inhibition of viability, invasion and migration. Induction of apoptosis. Chemosensitization (HSVtk/GCV). | (Zhang et al. 2019) | |
| Pancreatic cancer | BxPC3 | 1–8 µM | Activation of MKK4-JNK pathway. Inhibition of proliferation. Induction of apoptosis. | (Lai et al. 2020) |
| Lung cancer | A549 | 1–20 µM | Activation of p53 pathway and Fas/FasL apoptotic system. Induction of G1-phase arrest. Induction of apoptosis. Inhibition of proliferation. | (Hsu, Kuo, et al. 2004) |
| A549, H1299 | 5–20 µM | Inhibition of STAT3 pathway. Induction of the G0/G1-phase arrest. Inhibition of proliferation. Induction of apoptosis. | (Wu et al. 2020) | |
| HCC827, H1975, PC-9, HCC827/GR | 5–40 µM | Inhibition of STAT3 pathway. Inhibition of proliferation. Induction of apoptosis. Chemosensitization (gefitinib). | (Tang et al. 2019) | |
| A549 | 2 µM | Induction of ROS accumulation. Enhancement of apoptosis. Chemosensitization (CDDP) | ( Wang, Zheng, et al. 2010) | |
| Breast cancer | HCC1937 | 13–100 µM | Inhibition of Wnt/β-catenin pathway. Inhibition of proliferation. Induction of apoptosis. | (Wang et al. 2018) |
| MDA-MB-231 | 6–15 µM | Activation of the p38 pathway. Inhibition of viability. Induction of apoptosis. | (Fu et al. 2020) | |
| MCF-7 | 10 µM | Inhibition of SERCA. Activation of the CaMKKβ-AMPK-mTOR signalling cascade, ER stress and UPR. Induction of apoptosis and autophagy. | (Wong, Li, et al. 2013) | |
| MCF-7/ADR, MCF-7 | 0.13–0.6 µM | Downregulation of MDR1/P-gp. Reversal of MDR without toxic effect. Chemosensitization (ADR) | (Li, Guan, et al. 2017) | |
| MCF-7/ADR | 0.13–0.6 µM | Inhibition of P-gp expression. Reversal of MDR without toxic effect. Chemosensitization (doxorubicin) | (Li, Xue, et al. 2017) | |
| Ovarian cancer | SKOV3 | 2 µM | Induction of intracellular ROS accumulation. Enhancement of apoptosis. Chemosensitization (CDDP). | (Wang, Zheng, et al. 2010) |
| A2780s, A2780cp, Hey, SKOV3 | 1, 2 µM | Increase of Ca2+concentration. Induction of MMP loss. Activation of CaMKI. Inhibition of PPM1D. Promotion of mitochondrial fission. Induction of G2/M arrest. Chemosensitization (CDDP). | (Tsuyoshi et al. 2017) | |
| Cervical cancer | HeLa | 10 µM | Inhibition of SERCA. Activation of CaMKK-AMPK-mTOR kinase signalling cascade, ER stress and UPR. Induction of apoptosis and autophagy. | (Wong, Li, et al. 2013) |
| HeLa | 10 µM | Inhibition of NF-κB pathway and its target oncogenic genes expression. Inhibition of proliferation, angiogenesis and invasion. Induction of apoptosis. Chemosensitization (TNF-α) | (Wong, Zhang, et al. 2013) | |
| HeLa, Siha | 2 µM | Induction of intracellular ROS accumulation. Enhancement of apoptosis. Chemosensitization (CDDP). | (Wang, Zheng, et al. 2010) | |
| Renal cancer | 769-P, 786-O | 10–20 µM | Inhibition of EGFR/p38 pathway. Upregulation of p53. Induction of apoptosis. Induction of G0/G1-phase arrest. Inhibition of proliferation. | (Cai et al. 2017) |
| Prostate cancer | DU145 | 2.5–50 µM | Upregulation of p53. Inhibition of proliferation. Induction of G0/G1-phase arrest. Induction of apoptosis. | (Yao et al. 2014) |
| DU145, CWR22Rv1 | 5, 10 µM | Inhibition of GSK3β/β-catenin pathway in CWR22Rv1. Suppression of proliferation, metastasis and invasion. | (Zhong et al. 2016) | |
| Glioma | U87 | 1–8 µM | Downregulation of PI3K/Akt and ERK pathway. Activation of JNK. Inhibition of proliferation. Enhancement of apoptosis. | (Li, Cai, et al. 2017) |
| C6 | 2.8–128 µM | Induction of differentiation. Inhibition of growth. | (Tsai et al. 2002) | |
| Osteosarcoma | 143B, MG-63 | 80 µM | Activation of the p53 pathway. Induction of apoptosis. Induction of G0/G1-phase arrest. Inhibition of proliferation. | (Gao et al. 2019) |
| U2 | 5–20 µM | Inhibition of Akt and ERK pathway. Inhibition of proliferation, invasion, and migration. Induction of apoptosis. | (Zhao et al. 2019) | |
| Thyroid carcinoma | ARO, 8305C, SW1736 | 5–20 µM | Activation of p53 pathway. Inhibition of proliferation. Induction of G1-phase arrest. Induction of apoptosis. | (Liu and Li 2014) |
| Leukaemia | HL60 | 12.8–19.2 µM | Upregulation of GR mRNA expression. Induction of G0/G1-phase arrest. Inhibition of proliferation. | (Bu et al. 2000) |
| Melanoma | A375.S2 | 5–20 µM | Activation of JNK, p38 and p53. Inhibition of proliferation. Induction of apoptosis. | (Hu et al. 2016) |
Table 2.
Antitumour effects of SSD in vivo.
| Cancer type | Animal models | Concn. | Administration | Duration | Suggested mechanism | Ref. |
|---|---|---|---|---|---|---|
| Hepatoma | HSVtk/Hep3B cells xenograft tumour in nude mice | 10 mg/kg | Intraperitoneal injection | Every other day for 33 days | Inhibition of growth. Promotion of apoptosis. Chemosensitization (HSVtk/GCV) | (Zhang et al. 2019) |
| Lung cancer | HCC827/GR cells xenograft tumour in nude mice | 5, 10 mg/kg | Not mentioned | Every day for 14 days | Inhibition of growth. Promotion of apoptosis. Chemosensitization (gefitinib) | (Tang et al. 2019) |
| Breast cancer | MCF-7/ADR cells xenograft tumour in nude mice | 5 mg/kg | Intraperitoneal injection | Every other day for 20 days | Inhibition of growth. Inhibition of P-gp expression. Reversal of MDR without toxic effect. | (Li, Xue et al. 2017) |
| Thyroid carcinoma | ARO cells xenograft tumour in nude mice | 5–20 mg/kg | Oral gavage | Every day for 4 weeks | Inhibition of growth. | (Liu and Li 2014) |
Liver cancer
Bupleuri Radix is mainly used to treat liver disease, as it is believed to affect Liver Meridian most strongly in TCM. The effect of SSD on liver cancer cells has also become a hotspot in the research of its antitumour effects. Cyclooxygenase-2 (COX-2) is considered to be involved in the occurrence and development of malignant tumours through different ways, including promoting cancer cell proliferation, angiogenesis, metastasis and apoptosis inhibition (Möbius et al. 2005; Cheng and Fan 2013; Zhu et al. 2020). Anti-COX-2 is an important direction of cancer treatment (Hashemi Goradel et al. 2019; Mahboubi Rabbani and Zarghi 2019; Zhu et al. 2020). The proliferation of SMMC-7721 cells in vitro was found to be suppressed by SSD (2.5–15 μg/mL; 3.2–19.2 μM) in a concentration and time-dependent manner, and the potential mechanism was connected with the suppression of COX-2 expression through inhibiting the phospho-signal transducer and activator of transcription 3 (p-STAT3)/hypoxia-inducible factor-1α (HIF-1α) pathway (He et al. 2014). Another in vitro study revealed that SSD (2.5–15 μg/mL; 3.2–19.2 μM) restrained proliferation and promoted apoptosis in SMMC-7721 cells and HepG2 cells in a concentration and time-dependent manner through COX-2 inhibition mediated by blocking p-STAT3/CCAAT/enhancer binding protein β (C/EBPβ) pathway (Ren et al. 2019). Besides COX-2 expression inhibition, the treatment of SSD (1–10 μM) has been reported to exert concentration-dependent antiproliferative effect on HepG2 cells in vitro through induction of apoptosis and G1-cell cycle arrest by stimulating p53 and further up-regulating the expression of p21/WAF1, Fas/APO-1 and its two ligands, as well as Bcl-2 associated X protein (Bax).
SSD also suppressed the survival of HepG2 cells and Hep3B cells in vitro by increasing the expression of inhibitor kappa B α (IκBα) and inhibiting the expression and activity of nuclear factor kappa B (NF-κB), subsequently reducing the amount of B-cell lymphoma-extra large (Bcl-XL) (Hsu, Kuo, Chiang, et al. 2004). Furthermore, several studies have shown that SSD can be used as an ideal sensitiser for the radiotherapy and chemotherapy of liver cancer. An in vitro study indicated that SSD (10 μM) significantly enhanced apoptosis mediated by tumour necrosis factor-α (TNF-α) in HepG2 cells through inhibiting NF-κB activation and the expression of its target genes involving cancer cells proliferation, angiogenesis, invasion and survival (Wong, Zhang, et al. 2013). Besides, there is evidence to show that radiation combined with SSD (1 and 3 μg/mL; 1.28 and 3.84 μM) exerted concentration and time-dependent synergies on the growth inhibition and apoptosis induction of SMMC-7721 cells in vitro, and these actions may be related to the activation of p53 pathway (Wang et al. 2013, 2014). SSD (3 μg/mL; 3.84 μM) was proved to be quite effective to inhibit growth and promote radiosensitivity in SMMC-7721 cells and MHCC97L cells in vitro through inducing autophagy, and this effect can be partially reversed by the addition of chloroquine, an autophagy inhibitor, or the mammalian target of rapamycin mammalian target of rapamycin (mTOR) agonist, indicating that the suppression of mTOR pathway plays a role in SSD-mediated autophagy (Tian et al. 2019). Hypoxia can increase the entrin/small ubiquitin-like modifier (SUMO) modification of the glioma-associated oncogene (Gli) family proteins, which are the key molecules mediating the sonic hedgehog (SHh) pathway, leading to cell invasion, metastasis and chemotherapeutic resistance (Giroux-Leprieur et al. 2018).
For observing the antitumour activities of SSD in vivo, a herpes simplex virus thymidine kinase (HSVtk)/Hep3B xenograft tumour mouse model was developed, and the intraperitoneal injection of SSD (10 mg/kg) every other day for 33 days was found to inhibit proliferation and enhance the chemosensitivity to HSVtk/GCV (ganciclovir) in Hep3B cells. Meanwhile, in vitro, SSD (2–15 µM) inhibited the viability, migration and invasion of Hep3B cells in a concentration and time-dependent manner and enhanced HSVtk/GCV-induced apoptosis through the upregulation of SENP5 (SUMO-specific protease 5) expression and subsequent inhibition of Gli1 SUMOylation under hypoxia (Zhang et al. 2019).
Pancreatic cancer
BxPC3 cell lines were tested in vitro and the results demonstrated that SSD (1–8 μM) possessed concentration and time-dependent antitumour effects in pancreatic cancer cells via proliferation inhibition and the induction of apoptosis. The underlying mechanisms was proved to be the activation of the mitogen-activated protein kinase kinase 4 (MKK4)-c-Jun N-terminal protein kinase (JNK) pathway (Lai et al. 2020).
Lung cancer
SSD (1–20 μM) was found to concentration and time dependently induce the G1-cell cycle arrest and apoptosis of A549 cells in vitro, and the effect was associated with the activation of p53 and further stimulation of Fas/FasL apoptotic system (Hsu, Kuo, et al. 2004). Besides, SSD (5–20 µM) exhibited concentration-dependent decrease of proliferation and induction of apoptosis in A549 cells and H1299 cells in vitro via inhibiting the activation of STAT3 pathway (Wu et al. 2020). Another study indicated that SSD (5–40 μM) enhanced the in vitro inhibitory effect of gefitinib on non-small cell lung cancer (NSCLC) cells by inhibiting proliferation and inducing apoptosis in a concentration-dependent manner. Besides, a HCC827/GR cells xenograft tumour mouse model was established to assess the in vivo effect of SSD in combination with gefitinib in resistant cancer cells, and SSD (5 and 10 mg/kg) was found to exert synergistic effects on gefitinib-induced apoptosis and growth inhibition. Further exploration suggested that the underlying mechanism were associated with the suppression of STAT3/Bcl-2 pathway both in vitro and in vivo (Tang et al. 2019). SSD (2 µM) was also reported to sensitise A549 cells to cisplatin (CDDP)-induced apoptosis in vitro by promoting the accumulation of reactive oxygen species (ROS) (Wang, Zheng, et al. 2010).
Breast cancer
SSD was identified as the most effective component against triple-negative breast cancer from Bupleuri Radix in vitro. SSD (13–100 µM) concentration and time dependently inhibited proliferation and induced apoptosis in HCC1937 cells by the suppression of Wnt/β-catenin pathway, and compared to taxol, a clinically used anticancer drug, SSD showed a much higher potency (Wang et al. 2018). Another in vitro study suggested that SSD (6–15 µM) inhibited the viability of MDA-MB-231 cells in a concentration-dependent manner via inducing apoptosis, and a significant increase of phospho-p38 (p-p38) expression levels was observed, furthermore, the addition of inhibitor, significantly attenuated the induced apoptotic rate, indicating that the antitumour effect was involved with the activation of p38 pathway (Fu et al. 2020). Additionally, SSD (10 µM) induced both apoptosis and autophagy of MCF-7 cells in vitro through direct inhibition of sarcoplasmic/endoplasmic reticulum Ca2+ ATPase (SERCA), causing increased intracellular Ca2+ levels and activating the Ca2+/calmodulin-dependent kinase kinase-β (CaMKKβ)-AMP-activated protein kinase (AMPK)-mTOR pathway, endoplasmic reticulum (ER) stress and unfolded protein responses (UPR) (Wong, Li, et al. 2013).
Tumour cells often gain multidrug resistance (MDR) through the overexpression of P-glycoprotein (P-gp) encoded by the MDR1 gene, while SSD (<0.6 μM) can be used as a reversal agent for P-gp-mediated MDR with low toxicity. SSD (0.1–0.5 μg/mL; 0.13–0.6 μM) has been proved to be non-toxic and effectively reverse P-gp-mediated MDR and enhanced the sensitivity of MCF-7/adriamycin (ADR) cells to ADR or doxorubicin in vitro by inhibiting P-gp expression in a concentration-dependent manner. Furthermore, studies in MCF-7/ADR xenograft mice confirmed that the intraperitoneal injection of SSD (5 mg/kg) reversed MDR without increasing toxic effects (Li, Guan, et al. 2017; Li, Xue, et al., 2017).
Ovarian cancer
SSD (2 μM) was reported to make SKOV3 cells more sensitive to CDDP-induced apoptosis through induction of intracellular ROS accumulation in vitro (Wang, Zheng, et al. 2010). Another in vitro study suggested that SSD (2 μM) alone induced apoptosis potently in all ovarian cancer cells including A2780s, A2780cp, Hey and SKOV3. Although SSD (1 μM) alone was ineffective, it can sensitise chemoresistant ovarian cancer cells to CDDP through promoting mitochondrial fission and inhibiting G2/M transition in a p53-independent status. The mitochondrial fission was associated with the increase of cytosolic Ca2+ concentration, and subsequent mitochondrial membrane potential (MMP) loss and phosphorylation of Ca2+/calmodulin-dependent protein kinase I (CaMKI). G2/M arrest induction was mediated by inhibition of protein phosphatase magnesium-dependent 1 D (PPM1D), whose overexpression can inhibit the action of p53 and checkpoint kinase 1 (Chk1) (Tsuyoshi et al. 2017).
Cervical cancer
In HeLa cells, SSD (10 μM) was demonstrated to promote apoptosis and autophagy in vitro. Further studies confirmed that SSD inhibited SERCA directly, and then inducing disruption of calcium homeostasis, causing activation of CaMKKβ-AMPK-mTOR pathway, ER stress and UPR (Wong, Li, et al. 2013). Treatment with SSD (10 μM) in vitro effectively potentiated the antitumour activities of TNF-α in HeLa cells. In this case the synergistic effect were attributed to suppression of NF-κB activation and its target oncogenic genes expression involving proliferation, angiogenesis and invasion (Wong, Zhang, et al. 2013). Additionally, SSD (2 μM) was proved to potently enhance CDDP-induced apoptosis in HeLa and Siha cells in vitro through inducing ROS accumulation (Wang, Zheng, et al. 2010).
Renal cancer
A concentration and time-dependent antiproliferative effect on renal cell carcinoma cells was observed upon treatment with SSD (10–20 μM) in vitro, which is very likely to be mediated by apoptosis induction and G0/G1 cell cycle arrest through the inhibitory activation of epidermal growth factor receptor (EGFR)/p38 pathway and subsequent upregulation of p53 protein expression (Cai et al. 2017).
Prostate cancer
SSD (2.5–50 μM) exhibited concentration-dependent inhibitory action on the proliferation of DU145 cells in vitro by triggering the mitochondrial pathway of cell apoptosis and G0/G1-phase cell cycle arrest via upregulation of p53 (Yao et al. 2014). Furthermore, SSD (5 and 10 μM) suppressed the proliferation, metastasis, invasion and cancer stem cell phenotypes of prostate cancer cells in a concentration and time-dependent manner. These in vitro effects were attributed to the inhibition of glycogen synthase kinase-3β (GSK3β)/β-catenin pathway in CWR22Rv1 cells, while this mechanism was not confirmed in DU145 cells (Zhong et al. 2016).
Glioma
SSD (1–8 µM) was found to concentration-dependently suppress the proliferation of human malignant glioma U87 cells and enhance apoptosis in vitro. The underlying mechanisms of these effects were demonstrated to be associated with the downregulation of phosphatidylinositol 3-kinase (PI3K)/Akt and extracellular signal-regulated kinases (ERK) pathway, and activation of JNK (Li, Cai, et al. 2017). Another in vitro study indicated that SSD (10–100 µg/mL; 12.8–128 µM) could not only inhibit cell growth but also induce C6 glioma cells to differentiate into astrocytes, suggesting that SSD may be used as a differentiation inducer in cancer therapy. However, the mechanism of SSD-mediated differentiation induction remains unclear (Tsai et al. 2002).
Osteosarcoma
An in vitro study report suggested that SSD (5–20 µM) concentration dependently inhibited cell proliferation, invasion, migration and induced apoptosis in osteosarcoma U2 cells, and these effects were stronger than those of JNK inhibitor SP600125 at the same concentration. Further detection showed that the expression of p-Akt, p-ERK, myeloid cell leukaemia-1 (Mcl-1), Bcl-2, procaspase-3, -9, and -8 were downregulated, while cytochrome C release and the expression of Bax and cleaved caspase-3 were upregulated, indicating that the antitumour effects of SSD are involved in the inhibition of Akt pathway and ERK pathway (Gao et al. 2019). However, human osteosarcoma 143B cells and MG-63 cells were not as sensitive to the cytotoxicity of SSD as U2 cells, only when the concentration is as high as 80 µM in vitro did SSD significantly induced proliferative inhibition, G0/G1-phase arrest and apoptosis via activating p53 and then regulating its downstream targets (Zhao et al. 2019).
Thyroid carcinoma
SSD (5–20 µM) treatment showed a concentration and time-dependent antiproliferative effect on anaplastic thyroid cancer cells in vitro, which was attributed to G1-phase cell cycle arrest and apoptosis enhancement induced by the activation of p53 pathway. Additionally, data from ARO cells xenograft tumorigenesis in nude mouse model indicated that daily oral gavage of SSD (5–20 mg/kg) for 4 weeks significantly decreased the volume and weight of tumour in a concentration-dependent manner (Liu and Li 2014).
Leukaemia
The activity of SSD against human acute promyelocytic leukaemia cells has been studied in vitro, and SSD (10–15 µg/mL; 12.8–19.2 µM) was found to have a concentration and time-dependent antiproliferative effect on HL60 cells via the cell cycle arrest at G0/G1 phase caused by the upregulation of glucocorticoid receptor (GR) mRNA (Bu et al. 2000).
Melanoma
A study evaluated the anti-melanoma activity of different SSs, including SSA, SSC and SSD. SSD was identified as the most potent anti-melanoma component, and showed cytotoxicity to A375.S2 cells at a dose of 5 µM. Further investigation of the molecular mechanism revealed that the antiproliferative effect was related to the activation of JNK, p38 and p53 (Hu et al. 2016).
Toxicity
There is a saying in TCM, “Bupleuri Radix consumes liver Yin,” which means that Bupleuri Radix can harm the liver Yin if used too much or for a long time. However, because the toxicity of Bupleuri Radix was not clearly recorded in the ancient Chinese medical literature, it did not arouse people's attention. Bupleuri Radix has long been regarded as an effective and non-toxic herb, and its toxicity is even not recorded in the Chinese pharmacopoeia. It was not until the clinical reports of drug toxicity damage caused by Bupleuri Radix and its compound preparations appeared successively in modern clinical application, especially the Sho-saiko-to poisoning incident in Japan that extensive attention and research on the toxicity of Bupleuri Radix was aroused. In Japan, Bupleuri Radix and its compound preparations are the hottest aspect in toxicity research of Kampo medicines (Ikegami et al. 2003). Modern studies have shown that SSs are not only the main effective components, but also the main toxic components of Bupleuri Radix (Lv et al. 2009; Huang and Sun 2010), therefore, it is necessary to rationally apply SSD in the context of in-depth understanding of its toxic effects. The toxicological effects of SSD reported presently were mainly hepatotoxicity, neurotoxicity, haemolysis and cardiotoxicity, and the mechanisms of these toxicities are summarised in Table 3.
Table 3.
Toxicity of SSD in vitro/in vivo.
| Toxicity | Study type | Models | Dose/Concn. | Administration | Duration | Suggested mechanism | Ref. |
|---|---|---|---|---|---|---|---|
| Hepatotoxicity | In vitro | LO2 cells | 0.4–2 μM | Incubation | 24 h | Suppression of PDGF-βR/P38 pathway. Induction of mitochondrial apoptosis. | (Chen et al. 2013) |
| In vitro | LO2 cells | 0.8–2 μM | Incubation | 24 h | Activation of both the death receptor apoptosis pathway and mitochondrial apoptosis pathway. | (Zhang et al. 2016) | |
| In vivo | ICR mice | 300 mg/kg | Oral gavage | Every day for 7 days | Induction of apoptosis and liver injury. | (Zhang et al. 2016) | |
| Neurotoxicity | In vivo | ICR mice | 4, 8 mg/kg | Oral gavage | Every day for 7 days | Suppression of Akt/FoxG1 pathway. Inhibition of hippocampal neurogenesis. Impairment of cognitive ability. | (Xu et al. 2018). |
| In vitro | Primary neuronal progenitor cells | 2, 4 μM | Incubation | 24 h | Suppression of GSK3β/β-catenin pathway. Inhibition of proliferation and survival. | (Qin et al. 2019) | |
| In vivo | C57BL/6J mice | 16 mg/kg | Oral gavage | Every day for 14 days | Suppression of GSK3β/β-catenin pathway. Inhibition of cell proliferation and adult neurogenesis. Impairment of cognitive ability. | (Qin et al. 2019) | |
| In vivo | C57BL/6J mice | 16 mg/kg | Oral gavage | Every day for 14 days | Induction of cellular Ca2+ overload and further disorder of BDNF pathway. Activation of p75NTR cell death signalling. Suppression of TrkB signalling. Inhibition of survival and hippocampal neurogenesis. Induction of cognitive dysfunction. | (Qin et al. 2020) | |
| In vitro | Cultured murine neocortical neurons | 1.28–19.2 μM | Incubation | 24 h | Enhancement of cell membrane permeability. Induction of extracellular Ca2+ influx and cellular Ca2+ overload. Induction of apoptosis. | (Zheng et al. 2019) | |
| Haemolysis | In vitro | Human erythrocytes | 0.64–1.92 μM | Incubation | 3 min | Decrease of ATP level in erythrocytes. Change of membrane transport. | (Abe et al. 1978) |
| In vitro | Sheep erythrocytes | ≥1.28 μM | Incubation | 30 min | / | (Nose et al. 1989) | |
| Cardiotoxicity | In vitro | Neonatal rat cardiomyocytes | 10 μΜ | Incubation | 30 min | Inhibition of SERCA. Blockage of myocardial beating activities | (Wang et al. 2017) |
Hepatotoxicity
SSs are considered as the main material basis of hepatic toxicity of Bupleuri Radix (Lv et al. 2009). Human LO2 hepatocyte cell is an in vitro model widely used in hepatotoxicity studies. In the in vitro studies about SSD-induced liver injury, SSD (0.4–2 μM) treatment showed a significant concentration-dependent inhibitory effect on LO2 cell activity at the IC50 value of 2.14 μM. The effect was associated with the induction of mitochondrial apoptosis mediated by platelet-derived growth factor-β receptor (PDGF-βR)/P38 pathway disruption (Chen et al. 2013). In the later studies about SSD-induced liver injury by the same team, the results of experiments in vitro suggested that SSD (0.8–2 μM) concentration-dependently promoted the activation of both the death receptor apoptosis pathway and mitochondrial apoptosis pathway in LO2 cells. Meanwhile, animal experiments showed that oral administration of SSD (300 mg/kg) for one week caused liver injury and hepatocyte apoptosis in mice, and Bax was significantly upregulated concomitant with the significant downregulation of Bcl-2 (Zhang et al. 2016).
Neurotoxicity
Accumulating evidence in recent years suggests that SSD possess neurotoxicity. A cognitive decline in mice was observed after intragastric administration of SSD (4 and 8 mg/kg) for 7 days, and this neurotoxicity was in connection with the inhibition of hippocampal neurogenesis mediated by Akt/forkhead box G1 (FoxG1) pathway suppression (Xu et al. 2018). SSD (2 and 4 μM) was reported to inhibit the proliferation and survival of primary neuronal stem/progenitor cells in vitro. Besides, daily intragastric administration of SSD (16 mg/kg) for 14 days caused cognitive deficits in mice, and an inhibitory effect on cell proliferation and adult neurogenesis was observed. Further studies indicated that the cytotoxic effects in vitro and in vivo were both involved with the GSK3β/β-catenin pathway (Qin et al. 2019). In another study conducted by the same team, the neural cytotoxicity was demonstrated to be associated with the activation of p75 neurotrophin receptor (p75NTR) cell death signalling and suppression of tyrosine receptor kinase B (TrkB) signalling, engaged by disordered brain-derived neurotrophic factor (BDNF) pathway dependent on the cytosolic Ca2+ dysfunction (Qin et al. 2020). Moreover, SSD was considered to have the strongest neurotoxicity among SSs, and SSD (1–15 µg/mL; 1.28–19.2 μM) caused apoptosis of murine neocortical neurons in vitro through enhancing the permeability of cell membrane and further improving the intracellular Ca2+ concentration. The EC50 value of SSD-induced neuronal death was calculated to be 2.92 µM (Zheng et al. 2019).
Haemolysis
Many plant saponins are known for their haemolytic activity (Lorent et al. 2014). SSs can also induce strong haemolysis through its influence on erythrocyte membrane. In an in vitro study, SSD (0.5–1.5 µg/mL; 0.64–1.92 μM) was demonstrated to cause concentration-dependent haemolysis in human erythrocytes (Abe et al. 1978). Another in vitro research indicated that SSD and its intestinal metabolites, prosaikogenin G (PSG), showed significant haemolytic activity for their possession of an α-hydroxyl function at C16, and SSD began to exhibit haemolysis activity in sheep erythrocytes at 1 µg/mL (1.28 μM) (Nose et al. 1989).
Cardiotoxicity
The decrease of SERCA activity contributes to Ca2+ overload, reduced contractility and arrhythmias (Kiess and Kockskämper 2019). As a reported SERCA inhibitor, the cardiotoxicity of SSD has been noted (Wong, Li, et al. 2013). SSD was found to possess higher affinity with SERCA by molecular docking, and the exposure of SSD (10 μΜ) was verified to block the whole period of myocardial beating activities of neonatal rat cardiomyocytes in vitro (Wang et al. 2017).
Pharmacokinetics
The bioavailability of drugs is an important aspect that affects their efficacy, and the structural characteristics of molecules are closely related to their bioavailability. As a triterpenoid saponin compound, SSD is characterised by poor water solubility, which leads to the fact that SSD is generally considered to be difficult to use as an effective oral preparation for its low absorption rate in human gastrointestinal tract. However, the claim is controversial as in one study, a sensitive method was performed to detect the plasma concentration of SSD in rats after oral administration of Bupleurum chinense DC extract, and the result showed that SSD was absorbed rapidly with a Tmax less than 30 min (Xu et al. 2012). In addition, some studies suggested that the pharmacological effects of SSD oral administration may be closely related to the secondary metabolic derivatives transformed by SSD in the gastrointestinal tract.
Under the influence of transformation factors in vivo, including intestinal flora, gastric acids and enzymes, SSD can be hydrolysed to prosaikogenins (PSGs) and saikogenins (SGs) with smaller relative molecular weight, stronger membrane permeability and easier intestinal absorption. SSD was completely transformed into saikosaponin B2 (SSB2) after incubation in gastric juice of rats for 30 min, and after incubation in intestinal juice of rats, SSD was transformed into PSG and saikogenin G (SGG), while SSB2 was transformed into prosaikogenin D (PSD) and saikogenin D (SGD) (Shimizu et al. 1985). Studies exploring the effects of human gut microbiota on the hydrolysis of SSD also found the same transformation relationship (Meselhy 1999; Tang et al. 2020). Although the membrane permeability of SGs is improved compared with that of SSD, the bioavailability of SGs was found to be still low, and the efficacy of SSD might depend on the further transformation of secondary metabolites (Liu et al. 2019). The in vitro metabolism of SSD, as well as its two derivatives (PSG and SGG) in the gastrointestinal tract, was studied respectively in the liver microsomes of rats to explore their further metabolism after entering the circulatory system. Experimental results indicated that the predominant observed metabolic routes were kinds of oxidation, including hydroxylation, carboxylation and so on, and SSD, PSG, SGG were all transformed into Phase-I metabolites mediated by liver microsomal cytochrome P450 (CYPs) enzymes (Yu et al. 2017).
The evaluation of interaction potential with other drugs of new drug candidates is an important step in the drug development (Prueksaritanont et al. 2013). CYPs family mediates the metabolism of most important drugs, and many drug‐drug interactions are related to it (Guengerich et al. 2016). SSD (5–10 μM) was found to significantly increase the expression of CYP1A2 and CYP2D6 mRNA and protein as well as the relative enzyme activity in a concentration-dependent manner, while inhibit the expression of CYP3A4 mRNA and protein as well as the enzyme activity (Li et al. 2020, 2021). Therefore, when SSD is co-administered with drugs metabolised by CYP1A2, CYP2D6 and CYP3A4, especially drugs with a narrow therapeutic range, it is necessary to pay attention to the safety and efficacy due to drug‐drug interactions. Additionally, P-gp is an efflux transporter that affects the absorption, distribution and elimination of various compounds (Elmeliegy et al. 2020). It has been previously discussed that SSD can inhibit P-gp expression and reverse P-gp-mediated MDR both in vitro and in vivo. Thus, SSD may also alter the pharmacokinetics of some drugs due to its influence on P-gp. Nevertheless, research showed that the combination of SSD (5 mg/kg) reversed MDR without any effect on the pharmacokinetics of doxorubicin in MCF-7/ADR cell xenograft mouse model (Li, Xue, et al., 2017).
Discussion and conclusions
The development of new anticarcinogens has become a hotspot in pharmaceutical research area due to the excessive serious adverse reactions of traditional anticarcinogens and the drug resistance problem that seriously affects the efficacy of anticarcinogens (Kirtane et al. 2013; Katz and Shaked 2015; Bar-Zeev et al. 2017). Traditional medication experience is accumulated on the basis of long-term human drug use. The increasing level of molecular research and the in-depth understanding of the genesis and development of tumours as well as drug action mechanisms enable us to explore the mechanisms behind these traditional therapeutic experience at a deeper level, and provide us with inspirations and breakthroughs for drug development (Cragg and Newman 2005; Efferth et al. 2007). As the most antitumour substance in Bupleuri Radix, SSD has attracted extensive attention and been proved to play an antitumour role in multiple cancers. Meanwhile, the antitumour effects of SSD are multi-targeted and can be realised through various mechanisms, including inhibition of proliferation, invasion, metastasis and angiogenesis, as well as induction of cell apoptosis, autophagy and differentiation, which can largely avoid the problem that tumour cells are prone to develop drug resistance to single-targeted drugs. Furthermore, studies data indicated that SSD even showed a higher antitumour potency than some known antitumour drugs in vitro, such as taxol and SP600125. Therefore, the researchers identified SSD as a promising natural substance for the treatment of these cancers due to its definite antitumour activities. However, current reports mainly focus on in vitro experiments, and the antitumour activities and mechanisms of SSD in vivo still need more research and verification.
Drug resistance of cancer cells is a major obstacle in the current treatment of malignancy (Hussain et al. 2019). SSD has been found to play a synergistic role with some chemotherapeutics, and ease drug resistance via improving the sensitivity of cancer cells to some chemotherapeutic drugs. At concentrations below 0.6 μM, SSD not only reversed drug resistance, but also did not produce toxic effects. This suggests that lower concentrations of SSD have the potential to be developed as a safe chemotherapeutic sensitiser.
From the literature above, SSD was reported to induce apoptosis in breast cancer cells through activating p38 pathway, while in renal cell carcinoma cells, it was found to restrain proliferation and enhance apoptosis by inhibiting p38 pathway. The specific role of p38 in SSD-mediated apoptosis of cancer cells is puzzling, but perhaps it can be explained by the particularity of p38 pathway. P38 is one of the MAPK pathways that different from others, it exhibits distinct or even opposite effects in different cancers, and also exerts different activities in different stages of the same tumour (Maik-Rachline et al. 2020; Martínez-Limón et al. 2020). Current data show that there are more than 100 downstream substrates of p38, which regulate C/EBPβ, p53, STAT and other pathways (Sanchez-Prieto et al. 2000; Platanias 2003; Zhang et al. 2011; Trempolec et al. 2013). The p38 MAPK sub-family is composed of four main isoforms, including p38α, p38β, p38γ and p38δ. Different isoforms selectively act on different particular substrates, and they are also tissue-specific in their distribution: P38α and p38β are widely found in various tissue cells, p38γ is found in only skeletal muscle cells and nervous system, while p38δ is mainly found in glands (Yokota and Wang 2016). The selectivity of different isomers to substrates and the specificity of their tissue distribution result in different or even completely opposite effects of p38 activation in different tumour cells. Up to now, the regulation mechanisms of p38 in the occurrence, proliferation, differentiation, metastasis and apoptosis of different tumours are still too complex to be clearly understood (Martínez-Limón et al. 2020). Therefore, it may be meaningful to explore the antitumour mechanisms of SSD in different cancer cells by using more specific antibody of corresponding isoform of p38.
Although studies have demonstrated that SSD played an antitumour role in female reproductive system tumours, it should be noted that SSD was found to be structurally similar to oestrogens and exert oestrogen-like action through the stimulation of oestrogen receptors α (ERα) -mediated pathway, further exerting proliferative promotion on MCF-7 cells (Wang, Ren, et al. 2010). This finding means that we should pay attention to the potential health risk of SSD in patients with ERα-positive cancers and further studies on the effects of SSD in these tumours are also need to be conducted.
Although SSD is a natural substance with great antitumour potential, we should use it on the premise of certain understanding of its toxicity to ensure the safety of medication. Currently, the toxicological effects of SSD found in studies include hepatotoxicity, neurotoxicity, haemolysis and cardiotoxicity, and according to the related research results, these toxic effects are mainly mediated by cytotoxic mechanisms. It is not difficult to see that the mechanisms of the toxic and adverse effects of SSD discovered are basically the same as those of its antitumour activities. As is known to all, the use of anticarcinogen to kill cancer cells will inevitably cause damage to some normal cells. Moreover, natural chemicals derived from plants are thought to be less toxic to normal cells in organisms than chemosynthetic drugs and have greater potential safety as anticarcinogens (Li et al. 2015; Gezici and Şekeroğlu 2019).
Numerous studies have shown that cholesterol is the key factor in saponin-induced cytotoxicity. Saponins aggregate with membrane cholesterol, resulting in the change of cell membrane permeability, destruction of the lipid raft and mitochondrial membrane, and further activation of programmed cell death related signalling pathways (Lorent et al. 2014). Tumour cells tend to be more sensitive to the cytotoxicity of saponins because they contain higher levels of intracellular cholesterol and lipid rafts than normal cells (King et al. 2009; Waheed et al. 2012; Shaheen et al. 2018; Mollinedo and Gajate 2020). An in vitro test showed that the cytotoxicity of SSD to breast cancer H1299 cells (IC50 = 30.2 μM) was three times that of human normal lung fibroblast CCD19Lu (IC50 = 10.8 μM) (Wong, Zhang, et al. 2013). The selective cytotoxicity of SSD between normal and cancer cells makes it relatively safe as an anticancer agent. Thus, these toxic and adverse effects do not affect our judgement of SSD as an ideal new antitumour compound.
SSD may have a higher bioavailability than our expectation, despite belonging to the triterpenoid saponins with poor water solubility. Meanwhile, studies on the metabolism of SSD have shown that it can be converted into certain metabolites under the action of various transformation factors in vivo. Currently, there is still a lack of adequate understanding of the actual pharmacodynamic substances after SSD enters the body. Whether SSD enters the systemic circulation to play a role in the form of prototype or metabolites such as PSGs and SGs, and the differences in antitumour activity and toxicity between SSD and these metabolites in vivo need to be confirmed by many studies.
Disclosure statement
The authors declare that there is no conflict of interest about this article.
References
- Abe H, Sakaguchi M, Konishi H, Tani T, Arichi S.. 1978. The effects of saikosaponins on biological membranes. 1. The relationship between the structures of saikosaponins and haemolytic activity. Planta Med. 34(2):160–166. [DOI] [PubMed] [Google Scholar]
- Ashour ML, Wink M.. 2011. Genus Bupleurum: a review of its phytochemistry, pharmacology and modes of action. J Pharm Pharmacol. 63(3):305–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Zeev M, Livney YD, Assaraf YG.. 2017. Targeted nanomedicine for cancer therapeutics: towards precision medicine overcoming drug resistance. Drug Resist Updat. 31:15–30. [DOI] [PubMed] [Google Scholar]
- Bu S, Xu J, Sun J.. 2000. Effect of saikosaponin-D on up-regulating GR mRNA expression and inhibiting cell growth in human leukemia cells. Zhongguo Zhong Xi Yi Jie He Za Zhi. 20(5):350–352. [PubMed] [Google Scholar]
- Cai C, Zhang H, Ou Y, Jiang Y, Zhong D, Qi H, Dang Q.. 2017. Saikosaponin-D suppresses cell growth in renal cell carcinoma through EGFR/p38 signaling pathway. Neoplasma. 64(4):518–525. [DOI] [PubMed] [Google Scholar]
- Chen L, Zhang F, Kong D, Zhu X, Chen W, Wang A, Zheng S.. 2013. Saikosaponin D disrupts platelet-derived growth factor-β receptor/p38 pathway leading to mitochondrial apoptosis in human LO2 hepatocyte cells: a potential mechanism of hepatotoxicity. Chem Biol Interact. 206(1):76–82. [DOI] [PubMed] [Google Scholar]
- Cheng J, Fan XM.. 2013. Role of cyclooxygenase-2 in gastric cancer development and progression. World J Gastroenterol. 19(42):7361–7368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg GM, Newman DJ.. 2005. Plants as a source of anti-cancer agents. J Ethnopharmacol. 100(1–2):72–79. [DOI] [PubMed] [Google Scholar]
- Du JR, Long FY, Chen C.. 2014. Research progress on natural triterpenoid saponins in the chemoprevention and chemotherapy of cancer. Enzymes. 36:95–130. [DOI] [PubMed] [Google Scholar]
- Efferth T, Li PC, Konkimalla VS, Kaina B.. 2007. From traditional Chinese medicine to rational cancer therapy. Trends Mol Med. 13(8):353–361. [DOI] [PubMed] [Google Scholar]
- Elmeliegy M, Vourvahis M, Guo C, Wang DD.. 2020. Effect of p-glycoprotein (p-gp) inducers on exposure of p-gp substrates: review of clinical drug-drug interaction studies. Clin Pharmacokinet. 59(6):699–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu R, Zhang L, Li Y, Li B, Ming Y, Li Z, Xing H, Chen J.. 2020. Saikosaponin D inhibits autophagosome-lysosome fusion and induces autophagy-independent apoptosis in MDA-MB-231 breast cancer cells. Mol Med Rep. 22(2):1026–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao T, Zhao P, Yu X, Cao S, Zhang B, Dai M.. 2019. Use of saikosaponin D and JNK inhibitor SP600125, alone or in combination, inhibits malignant properties of human osteosarcoma U2 cells. Am J Transl Res. 11(4):2070–2080. [PMC free article] [PubMed] [Google Scholar]
- Gezici S, Şekeroğlu N.. 2019. Current perspectives in the application of medicinal plants against cancer: novel therapeutic agents. Anticancer Agents Med Chem. 19(1):101–111. [DOI] [PubMed] [Google Scholar]
- Giroux-Leprieur E, Costantini A, Ding VW, He B.. 2018. Hedgehog signaling in lung cancer: from oncogenesis to cancer treatment resistance. IJMS. 19(9):2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guengerich FP, Waterman MR, Egli M.. 2016. Recent structural insights into cytochrome P450 function. Trends Pharmacol Sci. 37(8):625–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemi Goradel N, Najafi M, Salehi E, Farhood B, Mortezaee K.. 2019. Cyclooxygenase-2 in cancer: a review. J Cell Physiol. 234(5):5683–5699. [DOI] [PubMed] [Google Scholar]
- He S, Lu G, Hou H, Zhao Z, Zhu Z, Lu X, Chen J, Wang Z.. 2014. Saikosaponin-D suppresses the expression of cyclooxygenase-2 through the phospho-signal transducer and activator of transcription 3/hypoxia-inducible factor-1α pathway in hepatocellular carcinoma cells. Mol Med Rep. 10(5):2556–2562. [DOI] [PubMed] [Google Scholar]
- He Y, Hu Z, Li A, Zhu Z, Yang N, Ying Z, He J, Wang C, Yin S, Cheng S.. 2019. Recent advances in biotransformation of saponins. Molecules. 24:2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YL, Kuo PL, Chiang LC, Lin CC.. 2004. Involvement of p53, nuclear factor kappaB and Fas/Fas ligand in induction of apoptosis and cell cycle arrest by saikosaponin D in human hepatoma cell lines. Cancer Lett. 213(2):213–221. [DOI] [PubMed] [Google Scholar]
- Hsu YL, Kuo PL, Lin CC.. 2004. the proliferative inhibition and apoptotic mechanism of saikosaponin D in human non-small cell lung cancer A549 cells. Life Sci. 75(10):1231–1242. [DOI] [PubMed] [Google Scholar]
- Hu SC, Lai YC, Lin CL, Tzeng WS, Yen FL.. 2019. Inclusion complex of saikosaponin-D with hydroxypropyl-β-cyclodextrin: improved physicochemical properties and anti-skin cancer activity. Phytomedicine. 57:174–182. [DOI] [PubMed] [Google Scholar]
- Hu SC, Lee IT, Yen MH, Lin CC, Lee CW, Yen FL.. 2016. Anti-melanoma activity of Bupleurum chinense, Bupleurum kaoi and nanoparticle formulation of their major bioactive compound saikosaponin-D. J Ethnopharmacol. 179:432–442. [DOI] [PubMed] [Google Scholar]
- Huang W, Sun R.. 2010. Study on hepatotoxicity on rats caused by crude extracts of total saikosaponins and correlation with oxidative damage mechanism. Zhongguo Zhong Yao Za Zhi. 35:1745–1749. [DOI] [PubMed] [Google Scholar]
- Hussain S, Singh A, Nazir SU, Tulsyan S, Khan A, Kumar R, Bashir N, Tanwar P, Mehrotra R.. 2019. Cancer drug resistance: a fleet to conquer. J Cell Biochem. 120(9):14213–14225. [DOI] [PubMed] [Google Scholar]
- Ikegami F, Fujii Y, Ishihara K, Satoh T.. 2003. Toxicological aspects of Kampo medicines in clinical use. Chem Biol Interact. 145(3):235–250. [DOI] [PubMed] [Google Scholar]
- Jiang H, Yang L, Hou A, Zhang J, Wang S, Man W, Zheng S, Yu H, Wang X, Yang B, et al. 2020. Botany, traditional uses, phytochemistry, analytical methods, processing, pharmacology and pharmacokinetics of Bupleuri Radix: a systematic review. Biomed Pharmacother. 131:110679. [DOI] [PubMed] [Google Scholar]
- Katz OB, Shaked Y.. 2015. Host effects contributing to cancer therapy resistance. Drug Resist Updat. 19:33–42. [DOI] [PubMed] [Google Scholar]
- Kiess TO, Kockskämper J.. 2019. SERCA activity controls the systolic calcium increase in the nucleus of cardiac myocytes. Front Physiol. 10:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King FW, Fong S, Griffin C, Shoemaker M, Staub R, Zhang YL, Cohen I, Shtivelman E.. 2009. Timosaponin AIII is preferentially cytotoxic to tumor cells through inhibition of mTOR and induction of ER stress. PLoS One. 4(9):e7283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirtane AR, Kalscheuer SM, Panyam J.. 2013. Exploiting nanotechnology to overcome tumor drug resistance: challenges and opportunities. Adv Drug Deliv Rev. 65(13–14):1731–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M, Ge Y, Chen M, Sun S, Chen J, Cheng R.. 2020. Saikosaponin D inhibits proliferation and promotes apoptosis through activation of MKK4-JNK signaling pathway in pancreatic cancer cells. Onco Targets Ther. 13:9465–9479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Guan X, Xue H, Wang P, Wang M, Gai X.. 2017. Reversal of P-glycoprotein-mediated multidrug resistance is induced by saikosaponin D in breast cancer MCF-7/adriamycin cells. Pathol Res Pract. 213(7):848–853. [DOI] [PubMed] [Google Scholar]
- Li C, Xue HG, Feng LJ, Wang ML, Wang P, Gai XD.. 2017. The effect of saikosaponin D on doxorubicin pharmacokinetics and its MDR reversal in MCF-7/adr cell xenografts. Eur Rev Med Pharmacol Sci. 21(19):4437–4445. [PubMed] [Google Scholar]
- Li DQ, Wu J, Liu LY, Wu YY, Li LZ, Huang XX, Liu QB, Yang JY, Song SJ, Wu CF.. 2015. Cytotoxic triterpenoid glycosides (saikosaponins) from the roots of Bupleurum chinense. Bioorg Med Chem Lett. 25(18):3887–3892. [DOI] [PubMed] [Google Scholar]
- Li H, Tang Y, Wang Y, Wei W, Yin C, Tang F.. 2020. Effects of saikosaponin D on CYP1A2 and CYP2D6 in HepaRG Cells. Drug Des Devel Ther. 14:5251–5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Tang Y, Wei W, Yin C, Tang F.. 2021. Effects of saikosaponin-D on CYP3A4 in HepaRG cell and protein-ligand docking study. Basic Clin Pharmacol Toxicol. 128(5):661–668. [DOI] [PubMed] [Google Scholar]
- Li X, Li X, Huang N, Liu R, Sun R.. 2018. A comprehensive review and perspectives on pharmacology and toxicology of saikosaponins. Phytomedicine. 50:73–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Cai T, Zhang W, Zhu W, Lv S.. 2017. Effects of saikosaponin D on apoptosis in human U87 glioblastoma cells. Mol Med Rep. 16(2):1459–1464. [DOI] [PubMed] [Google Scholar]
- Lin HJ, Kao ST, Siao YM, Yeh CC.. 2015. The Chinese medicine Sini-San inhibits HBx-induced migration and invasiveness of human hepatocellular carcinoma cells. BMC Complement Altern Med. 15:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Xue Y, Sun J, Fu R, Ren S, Zhang Z, Song R.. 2019. Pharmacokinetics and oral bioavailability studies of three saikogenins in rats using a validated UFLC-MS/MS method. J Chromatogr B Analyt Technol Biomed Life Sci. 1124:265–272. [DOI] [PubMed] [Google Scholar]
- Liu RY, Li JP.. 2014. Saikosaponin-D inhibits proliferation of human undifferentiated thyroid carcinoma cells through induction of apoptosis and cell cycle arrest. Eur Rev Med Pharmacol Sci. 18(17):2435–2443. [PubMed] [Google Scholar]
- Lorent JH, Quetin-Leclercq J, Mingeot-Leclercq MP.. 2014. The amphiphilic nature of saponins and their effects on artificial and biological membranes and potential consequences for red blood and cancer cells. Org Biomol Chem. 12(44):8803–8822. [DOI] [PubMed] [Google Scholar]
- Lv L, Huang W, Yu X, Ren H, Sun R.. 2009. Comparative research of different Bupleurum chinense composition to influence of hepatotoxicity of rats and oxidative damage mechanism. Zhongguo Zhong Yao Za Zhi. 34(18):2364–2368. [PubMed] [Google Scholar]
- Mahboubi Rabbani SMI, Zarghi A.. 2019. Selective COX-2 inhibitors as anticancer agents: a patent review (2014-2018). Expert Opin Ther Pat. 29(6):407–427. [DOI] [PubMed] [Google Scholar]
- Maik-Rachline G, Lifshits L, Seger R.. 2020. Nuclear p38: roles in physiological and pathological processes and regulation of nuclear translocation. IJMS. 21(17):6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Limón A, Joaquin M, Caballero M, Posas F, de Nadal E.. 2020. The p38 pathway: from biology to cancer therapy. IJMS. 21(6):1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J, Yao R, Chen X, Yang X, Li Z.. 2012. Advances in studies on classification of Bupleurum. Zhongguo Zhong Yao Za Zhi. 37(11):1523–1526. [PubMed] [Google Scholar]
- Meselhy M. 1999. Human intestinal bacteria responsible for the metabolism of saikosaponins. J Trandition Med. 17:1–11. [Google Scholar]
- Möbius C, Stein HJ, Spiess C, Becker I, Feith M, Theisen J, Gais P, Jütting U, Siewert JR.. 2005. COX2 expression, angiogenesis, proliferation and survival in Barrett's cancer. Eur J Surg Oncol. 31(7):755–759. [DOI] [PubMed] [Google Scholar]
- Mollinedo F, Gajate C.. 2020. Lipid rafts as signaling hubs in cancer cell survival/death and invasion: implications in tumor progression and therapy: thematic review series: biology of lipid rafts. J Lipid Res. 61(5):611–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nose M, Amagaya S, Ogihara Y.. 1989. Effects of saikosaponin metabolites on the hemolysis of red blood cells and their adsorbability on the cell membrane. Chem Pharm Bull (Tokyo)). 37(12):3306–3310. [DOI] [PubMed] [Google Scholar]
- Platanias LC. 2003. The p38 mitogen-activated protein kinase pathway and its role in interferon signaling. Pharmacol Ther. 98(2):129–142. [DOI] [PubMed] [Google Scholar]
- Prueksaritanont T, Chu X, Gibson C, Cui D, Yee KL, Ballard J, Cabalu T, Hochman J.. 2013. Drug-drug interaction studies: regulatory guidance and an industry perspective. Aaps J. 15(3):629–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin T, Fu X, Yu J, Zhang R, Deng X, Fu Q, Ma Z, Ma S.. 2019. Modification of GSK3β/β-catenin signaling on saikosaponins-D-induced inhibition of neural progenitor cell proliferation and adult neurogenesis. Toxicology. 424:152233. [DOI] [PubMed] [Google Scholar]
- Qin T, Yuan Z, Yu J, Fu X, Deng X, Fu Q, Ma Z, Ma S.. 2020. Saikosaponin-D impedes hippocampal neurogenesis and causes cognitive deficits by inhibiting the survival of neural stem/progenitor cells via neurotrophin receptor signaling in mice. Clin Transl Med. 10(8):e243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren M, McGowan E, Li Y, Zhu X, Lu X, Zhu Z, Lin Y, He S.. 2019. Saikosaponin-D suppresses COX2 through p-STAT3/C/EBPβ signaling pathway in liver cancer: a novel mechanism of action. Front Pharmacol. 10:623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Prieto R, Rojas JM, Taya Y, Gutkind JS.. 2000. A role for the p38 mitogen-acitvated protein kinase pathway in the transcriptional activation of p53 on genotoxic stress by chemotherapeutic agents. Cancer Res. 60(9):2464–2472. [PubMed] [Google Scholar]
- Shaheen U, Ragab EA, Abdalla AN, Bader A.. 2018. Triterpenoidal saponins from the fruits of Gleditsia caspica with proapoptotic properties. Phytochemistry. 145:168–178. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Amagaya S, Ogihara Y.. 1985. Structural transformation of saikosaponins by gastric juice and intestinal flora. J Pharmacobiodyn. 8(9):718–725. [DOI] [PubMed] [Google Scholar]
- Tang C, Fu Q, Chen X, Hu Y, Renaud H, Ma C, Rao T, Chen Y, Tan Z, Klaassen CD, et al. 2020. The biotransformation of Bupleuri Radix by human gut microbiota. Xenobiotica. 50(9):1011–1022. [DOI] [PubMed] [Google Scholar]
- Tang JC, Long F, Zhao J, Hang J, Ren YG, Chen JY, Mu B.. 2019. The effects and mechanisms by which saikosaponin-D enhances the sensitivity of human non-small cell lung cancer cells to gefitinib. J Cancer. 10(26):6666–6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian YD, Lin S, Yang PT, Bai MH, Jin YY, Min WL, Ma HB, Wang BF.. 2019. Saikosaponin-D increases the radiosensitivity of hepatoma cells by adjusting cell autophagy. J Cancer. 10(20):4947–4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trempolec N, Dave-Coll N, Nebreda AR.. 2013. Snapshot: p38 MAPK substrates. Cell. 152(4):924–924.e921. [DOI] [PubMed] [Google Scholar]
- Tsai YJ, Chen IL, Horng LY, Wu RT.. 2002. Induction of differentiation in rat C6 glioma cells with saikosaponins. Phytother Res. 16(2):117–121. [DOI] [PubMed] [Google Scholar]
- Tsuyoshi H, Wong VKW, Han Y, Orisaka M, Yoshida Y, Tsang BK.. 2017. Saikosaponin-D, a calcium mobilizing agent, sensitizes chemoresistant ovarian cancer cells to cisplatin-induced apoptosis by facilitating mitochondrial fission and G2/M arrest. Oncotarget. 8(59):99825–99840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waheed A, Barker J, Barton SJ, Owen CP, Ahmed S, Carew MA.. 2012. A novel steroidal saponin glycoside from Fagonia indica induces cell-selective apoptosis or necrosis in cancer cells. Eur J Pharm Sci. 47(2):464–473. [DOI] [PubMed] [Google Scholar]
- Wang BF, Dai ZJ, Wang XJ, Bai MH, Lin S, Ma HB, Wang YL, Song LQ, Ma XL, Zan Y, et al. 2013. Saikosaponin-D increases the radiosensitivity of SMMC-7721 hepatocellular carcinoma cells by adjusting the G0/G1 and G2/M checkpoints of the cell cycle. BMC Complement Altern Med. 13:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang BF, Lin S, Bai MH, Song LQ, Min WL, Wang M, Yang P, Ma HB, Wang XJ.. 2014. Effects of SSD combined with radiation on inhibiting SMMC-7721 hepatoma cell growth. Med Sci Monit. 20:1340–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Qi H, Zhang X, Si W, Xu F, Hou T, Zhou H, Wang A, Li G, Liu Y, et al. 2018. Saikosaponin D from Radix Bupleuri suppresses triple-negative breast cancer cell growth by targeting β-catenin signaling . Biomed Pharmacother. 108:724–733. [DOI] [PubMed] [Google Scholar]
- Wang P, Ren J, Tang J, Zhang D, Li B, Li Y.. 2010. Estrogen-like activities of saikosaponin-D in vitro: a pilot study. Eur J Pharmacol. 626(2–3):159–165. [DOI] [PubMed] [Google Scholar]
- Wang Q, Zheng X, Yang L, Shi F, Gao L, Zhong Y, Sun H, He F, Lin Y, Wang X.. 2010. Reactive oxygen species-mediated apoptosis contributes to chemosensitization effect of saikosaponins on cisplatin-induced cytotoxicity in cancer cells. J Exp Clin Cancer Res. 29:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Zhang Y, Zhang Q, Peng S, Shen C, Yu Y, Zhang M, Yang W, Wu Q, Zhang Y, et al. 2017. Content decline of SERCA inhibitors saikosaponin A and D attenuates cardiotoxicity and hepatotoxicity of vinegar-baked Radix bupleuri. Environ Toxicol Pharmacol. 52:129–137. [DOI] [PubMed] [Google Scholar]
- Wong VK, Li T, Law BY, Ma ED, Yip NC, Michelangeli F, Law CK, Zhang MM, Lam KY, Chan PL, et al. 2013. Saikosaponin-D, a novel SERCA inhibitor, induces autophagic cell death in apoptosis-defective cells. Cell Death Dis. 4:e720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong VK, Zhang MM, Zhou H, Lam KY, Chan PL, Law CK, Yue PY, Liu L2.. 2013. Saikosaponin-D enhances the anticancer potency of TNF-α via overcoming its undesirable response of activating NF-Kappa B signalling in cancer cells. Evid Based Complement Alternat Med. 2013:745295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Chen W, Liu K, Ren F, Zheng D, Xu F, Wu H.. 2020. Saikosaponin D inhibits proliferation and induces apoptosis of non-small cell lung cancer cells by inhibiting the STAT3 pathway. J Int Med Res. 48(9):300060520937163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao K, Li K, Long S, Kong C, Zhu S.. 2020. Potential molecular mechanisms of Chaihu-Shugan-San in treatment of breast cancer based on network pharmacology. Evid Based Complement Alternat Med. 2020:3670309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Ji Z, Guo L, Zhang R, Qu R, Ma S.. 2018. Saikosaponin-D-mediated downregulation of neurogenesis results in cognitive dysfunction by inhibiting Akt/Foxg-1 pathway in mice. Toxicol Lett. 284:79–85. [DOI] [PubMed] [Google Scholar]
- Xu L, Song R, Tian JX, Tian Y, Liu GQ, Zhang ZJ.. 2012. Analysis of saikosaponins in rat plasma by anionic adducts-based liquid chromatography tandem mass spectrometry method. Biomed Chromatogr. 26(7):808–815. [DOI] [PubMed] [Google Scholar]
- Yao M, Yang J, Cao L, Zhang L, Qu S, Gao H.. 2014. Saikosaponin-D inhibits proliferation of DU145 human prostate cancer cells by inducing apoptosis and arresting the cell cycle at G0/G1 phase. Mol Med Rep. 10(1):365–372. [DOI] [PubMed] [Google Scholar]
- Yokota T, Wang Y.. 2016. p38 MAP kinases in the heart. Gene. 575(2 Pt 2):369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P, Qiu H, Wang M, Tian Y, Zhang Z, Song R.. 2017. In vitro metabolism study of saikosaponin D and its derivatives in rat liver microsomes. Xenobiotica. 47(1):11–19. [DOI] [PubMed] [Google Scholar]
- Yuan B, Yang R, Ma Y, Zhou S, Zhang X, Liu Y.. 2017. A systematic review of the active saikosaponins and extracts isolated from Radix Bupleuri and their applications. Pharm Biol. 55(1):620–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CY, Jiang ZM, Ma XF, Li Y, Liu XZ, Li LL, Wu WH, Wang T.. 2019. Saikosaponin-D inhibits the hepatoma cells and enhances chemosensitivity through SENP5-dependent inhibition of Gli1 SUMOylation under hypoxia. Front Pharmacol. 10:1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Chen L, Jin H, Shao J, Wu L, Lu Y, Zheng S.. 2016. Activation of Fas death receptor pathway and Bid in hepatocytes is involved in saikosaponin D induction of hepatotoxicity. Environ Toxicol Pharmacol. 41:8–13. [DOI] [PubMed] [Google Scholar]
- Zhang G, Jin B, Li YP.. 2011. C/EBPβ mediates tumour-induced ubiquitin ligase atrogin1/MAFbx upregulation and muscle wasting. Embo J. 30(20):4323–4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Li J, Sun ZB, Sun C, Yu ZH, Guo X.. 2019. Saikosaponin D inhibits proliferation of human osteosarcoma cells via the p53 signaling pathway. Exp Ther Med. 17(1):488–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Chen J, Zou X, Zhao F, Guo M, Wang H, Zhang T, Zhang C, Feng W, Pessah IN, et al. 2019. Saikosaponin D causes apoptotic death of cultured neocortical neurons by increasing membrane permeability and elevating intracellular Ca2+ concentration. Neurotoxicology. 70:112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong D, Zhang HJ, Jiang YD, Wu P, Qi H, Cai C, Zheng SB, Dang Q.. 2016. Saikosaponin-D: A potential chemotherapeutics in castration resistant prostate cancer by suppressing cancer metastases and cancer stem cell phenotypes. Biochem Biophys Res Commun. 474(4):722–729. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Shi C, Zeng L, Liu G, Jiang W, Zhang X, Chen S, Guo J, Jian X, Ouyang J, et al. 2020. High COX-2 expression in cancer-associated fibiroblasts contributes to poor survival and promotes migration and invasiveness in nasopharyngeal carcinoma. Mol Carcinog. 59(3):265–280. [DOI] [PMC free article] [PubMed] [Google Scholar]