Abstract
Children with attention-deficit/hyperactivity disorder (ADHD) have previously shown a decreased magnitude of event-related desynchronization (ERD) during a finger-tapping task, with a large between-group effect. Because the neurobiology underlying several transcranial magnetic stimulation (TMS) measures have been studied in multiple contexts, we compared ERD and 3 TMS measures (resting motor threshold [RMT], short-interval cortical inhibition [SICI], and task-related up-modulation [TRUM]) within 14 participants with ADHD (ages 8–12 years) and 17 control children. The typically developing (TD) group showed a correlation between greater RMT and greater magnitude of alpha (10–13 Hz, here) ERD, and there was no diagnostic interaction effect, consistent with a rudimentary model of greater needed energy input to stimulate movement. Similarly, inhibition measured by SICI was also greater in the TD group when the magnitude of movement-related ERD was higher; there was a miniscule diagnostic interaction effect. Finally, TRUM during a response-inhibition task showed an unanticipated pattern: in TD children, the greater TMS task modulation (TRUM) was associated with a smaller magnitude of ERD during finger-tapping. The ADHD group showed the opposite direction of association: Greater TRUM was associated with larger magnitude of ERD. Prior EEG results have demonstrated specific alterations of task-related modulation of cortical physiology, and the current results provide a fulcrum for multimodal study.
Keywords: ADHD, event-related desynchronization, mirror overflow, response inhibition, TMS
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is one of the most common neurobehavioral conditions in children, affecting ~4% of the pediatric population (Vasileva et al. 2020). Despite psychopharmacology and behavioral interventions that are effective in the short term in many cases, ADHD continues to have a substantial burden of negative outcomes (Molina et al. 2009), underscoring the need for novel diagnostics and therapeutics (Ewen 2016; Sahin et al. 2018). Although diagnosis is based on the core, clinical symptoms of inattention, hyperactivity, and impulsivity (Wolraich et al. 2019), cognitive research has repeatedly identified deficits in cognitive control, including response inhibition (Mostofsky and Simmonds 2008; Crosbie et al. 2013) as well as parallel deficits in motor control, particularly motor inhibition (Denckla and Rudel 1978). These specific deficits provide a mechanistic basis for the biology of ADHD for the purpose of developing new interventions and biomarkers to improve outcomes. (Ewen and Beniczky 2018; Ewen et al. 2021, 2019; FDA-NIH Biomarker Working Group 2016)
Event-related spectral perturbation, consists of task-related increases (event-related synchronization—ERS) and decreases in oscillatory power (event-related desynchronization—ERD; Pfurtscheller and Neuper 1994; Pfurtscheller and Lopes Da Silva 1999). Differences in ERS/ERD have been demonstrated in ADHD (McAuliffe et al. 2020) and other NDDs (Murphy et al. 2014; Ewen et al. 2016a). ERD refers to the relative suppression of a particular EEG oscillation during a cognitive or motor task. Our group has studied ERD in ADHD in the context of mirror overflow, a deficit in motor inhibition often found in children with ADHD (Cole et al. 2008; MacNeil et al. 2011; McAuliffe et al. 2020). Mirror overflow refers to the involuntary production of movement on the opposite side of the body from a volitional and unilateral movement.
We examined ERD in alpha (here, 10–13 Hz) and beta bands (here, 18–28 Hz). Both alpha and beta ERD are understood to be inhibitory in effect (Kelly et al. 2006; Engel and Fries 2010). The mu band (10–28 Hz), or sensory-motor rhythm, is an oscillation typically recorded from central scalp regions and is suppressed during a variety of motor tasks. Many studies report only the alpha component of the mu rhythm, but mu is truly composed of both alpha and beta components. Importantly these components show dissociations in experimental contexts (e.g., McAuliffe et al. 2020). Alpha is believed to be generated by the postcentral gyrus (Salmelin et al. 1995), whereas beta activity is believed to be generated by precentral gyrus (Keil et al. 2014). To set the stage for the current analysis, our prior results from within a larger sample, of which the sample presented here is a subset, demonstrated an ADHD-associated decrease in left-hemisphere alpha ERD, but not left-hemisphere beta ERD, during finger-sequencing of the right hand (eliciting mirror overflow in the left hand; McAuliffe et al. 2020).
Although a good deal is known about the generators of mu-alpha and mu-beta rhythms and other cognitively-associated oscillatory activity (reviewed in Cannon et al. 2014), there is relatively little known about the mechanisms that allow for task-related modulation of them. Task-related modulation is particularly relevant, as baseline measures likely do not reflect the “real-life” implications of these cortical rhythms.
Transcranial magnetic stimulators (TMS), in contrast to EEG, has been studied in a wide range of demographic, diagnostic and pharmacological contrasts, and different TMS indices have been repeatedly demonstrated to reflect different aspects of physiology. For example, resting motor threshold (RMT) is the percentage of maximum stimulator output required to evoke a motor-evoked potential (MEP; Mills and Nithi 1997), such that a higher threshold indicates a greater energy requirement for activation and is thought of as a basic indicator of readiness of the motor cortex to depolarize. It may index terminal myelination, subcortical myelination, and developmentally regulated changes in ion channels. A longstanding finding in healthy children is that RMT is the highest in infancy and declines through childhood, reaching adult levels at approximately age 12 years (Muller et al. 1991).
Short-interval cortical inhibition (SICI) is one of the most widely studied TMS measures and utilizes paired-pulse approaches. SICI is quantified as the ratio of the conditioned (paired-pulse) MEP and unconditioned (single-pulse) MEP. SICI is understood to index GABA-A mediated inhibitory interneuronal activity acting on motor cortex (Kujirai et al. 1993). SICI is diminished in a large variety of neurodevelopmental and neurodegenerative disorders (Moll et al. 2001; Rothwell et al. 2009; Ni et al. 2013; Mimura et al. 2021). Altered SICI in ADHD is particularly robust, and correlates with parent-rated symptom severity of both hyperactive/impulsive and inattentive symptoms (Gilbert et al. 2011) and is modified by standard pharmacologic treatments (Moll et al. 2000).
The third TMS index reported was dubbed by our laboratory “TRUM”: task-related up modulation (of motor-evoked potentials) (Gilbert et al. 2019; Zea Vera et al. 2020). Interestingly, compared with rest, SICI diminishes during preparation to act and during actions (Garry and Thomson 2009; Hoegl et al. 2012; Gilbert et al. 2019) and is thus modulated by task-presence. Like the ERD, this metric is task associated. In the current set of experiments, TRUM was studied in the context of a modified, child-friendly version of the Slater Hammel stop signal task (SST; Guthrie et al. 2018; Gilbert et al. 2019), which evaluates response inhibition performance. Different phases of the task are understood to represent action-selection and response preparation. This research was based on prior observations that motor cortex excitability, and hence MEP amplitude, increases prior to cued and self-paced movements (Chen et al. 1998).
The goal of the current paper is to directly explore associations between task-based and resting measures of EEG and TMS indices. As many participants participated in only one procedure or the other, prior publications from larger samples of which the one presented here is a subset have reported independently on TMS (Gilbert et al. 2019) and EEG (McAuliffe et al. 2020). Within the larger sample that received TMS, we found that, compared with typically developing (TD) children, children with ADHD showed reduced SICI at rest and during the action-selection phase of the task (selecting to go or to stop), such that reduced SICI at rest does not resolve or normalize during engagement with this task. In addition, we found that the amount of TRUM of motor cortex excitability during this task was diminished in children with ADHD (Gilbert et al. 2019). However, SICI and TRUM were only minimally correlated with one another (Zea Vera et al. 2020), supporting the notion that these measures may be differentially sensitive to distinct mechanisms of altered physiology within ADHD.
The overall analytic strategy was first to examine EEG–TMS associations within the TD group alone, given the absence of pre-existing basic knowledge about the relationship between ERD and TMS indices. We next examined diagnostic (interaction) effects, looking for associations in which ADHD diagnosis may moderate the TMS–ERD relationship, as potentially promising areas of further study. Because of a dearth of TMS–EEG comparisons to date, our working hypotheses were necessarily speculative. Because only alpha ERD showed diagnostic group differences within the left hemisphere under the task conditions studied (the only hemisphere stimulated by TMS), we limited working hypotheses to those involving “alpha” ERD, though we explored beta ERD associations as well.
TD-only hypotheses were as follows: Because RMT reflects baseline “readiness to depolarize” (higher RMT = less readiness to depolarize), and ERD is analogous to the “energetic change” in the cortex, we predicted that TD children with higher RMT would show a greater magnitude of ERD. Our second prediction was that higher levels of SICI (thus, more suppression) would portend greater ERD, as a more inhibited resting cortex would require greater activation (indexed by ERD) to generate behavior. Third, under the assumption that a joint “cortical physiology modulation” mechanism is indexed by TRUM and by ERD, we predicted that those 2 measures would correlate, despite the respective physiology being measured in the context of 2 separate tasks.
With regards to ADHD, within the larger TMS sample, RMT was not substantially decreased in the ADHD group (4.2% decrease in observed means compared with TD group, P = 0.13; Gilbert et al. 2019). Because alpha ERD was in fact different between groups, we predicted the presence of a diagnostic interaction effect. Second, the resting SICI was lower (i.e., ratio was increased) in the ADHD group (i.e., less inhibition) by 21% (P = 0.03; Gilbert et al. 2019), and alpha ERD was decreased (McAuliffe et al. 2020). We therefore expected SICI–alpha ERD correlations in both groups and no interaction effect. Finally, within the larger TMS sample, children with ADHD showed less TRUM (Gilbert et al. 2019). Within the larger ADHD sample, they showed less alpha ERD (McAuliffe et al. 2020). Therefore, we did not anticipate a group interaction effect within the combined sample (i.e., diagnosis does not moderate the TRUM–ERD relationship).
Materials and Methods
Participants
Participants reflect a subsample of those reported by Gilbert et al. (2019) and McAuliffe et al. (2020), each of which was a part of larger study with consistent recruitment criteria. Briefly, these were case-control studies of 8–12-year-old children with ADHD and TD controls. Children participated in the 2 studies within a 6-month time-period. All data collection was performed at a single site (Kennedy Krieger Institute, Baltimore, MD, USA). ADHD diagnoses were based on parent interviews using the diagnostic interview for children and adolescents, fourth edition; (DICA-IV; Reich 2000) or kiddie schedule for affective disorders and schizophrenia for school-aged children (K-SADS; Kaufman et al. 1997). TDs were excluded for any diagnosis on the DICA-IV/K-SADS or for elevated ADHD symptoms on the Conner’s Rating Scale-Revised (CPRS-R; Conners et al. 1998). Additional exclusion criteria for both groups included history of seizures, intellectual disability, neurological illness or injury, or left-handedness/mixed dominance, as assessed by the Edinburgh handedness inventory (≤0.5) (Oldfield 1971). All children with full-scale IQ scores below 80 on the Wechsler intelligence scale for children, fourth edition (WISC-IV; Wechsler 2003) or WISC-V (Kaufman et al. 2015) were excluded. Children prescribed stimulant medications had doses held at least 24 h prior to all testing sessions, and individuals on any other neuro-active medication were excluded from the study.
Finger-Tapping and EEG
Finger-Tapping Task Used for EEG–ERD
Participants were instructed to tap each finger against the thumb in successive order (index–middle–ring–little) and self-paced timing, one hand at a time, for 6 s in a trial. A start cue was presented on a computer monitor. Left-handed finger-tapping (LHFT) and right-handed finger-tapping (RHFT) trials alternated in each block, although only RHFT trials were analyzed for this study. There were 5 blocks consisting of 20 trials in each per block. Behavioral overflow was measured in the non-tapping hand via electronic goniometers (Biopac Systems Inc.); overflow was quantified per previous studies in our laboratory (MacNeil et al. 2011; McAuliffe et al. 2020).
EEG Recording, Preprocessing and ERD Analysis
EEG was recorded during finger tapping using a 47-channel, full-scalp, equidistant WaveGuard cap system, and an asa-lab amplifier (Advanced Neuro Technologies). Trials for each subject were excluded during a video analysis if children were observed not to be paying attention, moved out of compliance with visually displayed instructions, or did not complete at least 5 s of tapping within that trial. Data were recorded at a 1024-Hz sampling rate and 138-Hz anti-aliasing filter and were referenced to an average of all channels. Impedances were kept below 15 kΩ. Preprocessing was conducted in EEGLAB (Delorme and Makeig 2004). EEG data were preprocessed using asa-lab version 4 software. Data were high-pass filtered at 0.2 Hz, and visually inspected for eye-blinks, horizontal eye movements, and muscle activity. These artifacts could all be identified visually based on well-defined morphology. A principal component analysis-based method of removing artifact components within asa-lab was used to remove components that account for >90% of the variance of the artifact subspace. Not a single trial from any subject was removed in the artifact rejection step. To minimize effects of volume conduction, signals were then converted to current source density (CSD) estimates from CSD toolbox (Kayser and Tenke 2006) in MATLAB (Mathworks). Full details can be found in McAuliffe et al. (2020).
The EEG measures of interest were alpha band (empirically derived 10–13-Hz range) and beta band (empirically derived 18–28-Hz range) ERD in the scalp region approximating left M1, during right hand finger tapping (RHFT). Bands were selected via spectrogram, as reported in (McAuliffe et al. 2020). Analysis was restricted to Left M1/RHFT because the TMS procedures only interrogated left M1 via EMG captured from right hand. Data were down-sampled to 256 Hz, and ERD was calculated for each channel as follows: at each time-frequency point during the task (starting from the point of tapping onset for each trial, as measured by initial goniometer deflection in the tapping hand), a z-score was calculated relative to a distribution created from the baseline period (1 s prior to start cue). We limited our analysis to a 1.5-s window (1.5–3-s relative to tapping onset) in the middle of each tapping block to avoid EEG onset and offset (rebound) effects. ERD-related z-scores for each channel were integrated over 384 time-samples (in 1.5 s) × 8 frequency bins per Hz. ERD is a negative value, so a greater magnitude of ERD is a more negative value.
Stop-Signal Task and TMS
Slater Hammel SST
Participants operated a standard game controller with their right, dominant hand for this response inhibition task. GO and STOP Stimuli were presented on a computer monitor via Presentation (v.10.0; Neurobehavioral Systems). Ulnar aspects of both arms and hands rested on a body-surrounding pillow (The Boppy Company, LLC) so the palmar surface faced medially. TMS data at rest and during the task were monitored continuously in real time on a separate monitor by the operator. The appearance of movement artifact during trials was immediately noted and feedback provided to the participant to maintain relaxation of the hand. Trials with visible artifact 100 ms prior to the TMS pulse were tagged offline and discarded. In addition, for all trials, the EMG artifact during this epoch was measured as an area under the curve (rectified difference from 0) for every trial and this value included as a covariate in the mixed model analysis.
The dominant hand operated the game controller with a fully extended index finger. Surface EMG electrodes recorded the first dorsal interosseous (FDI) muscle. The participant initiated each trial by adducting (pushing down) the index finger on the game controller button, activating the finger flexors (antagonistic to the FDI), causing a racecar at the left side of the screen to audibly start its “engine,” and then traverse a straight, 1000 ms “racetrack” across the screen. The car kept going only as long as the finger is adducted. The “go action” of this task required lifting the finger, that is, activating FDI, when the car was as close as possible to the 800-ms mark, without going past it. However, in 25% of trials, at random, the car stopped itself spontaneously 300–700 ms after trial onset. This was the “Stop Cue.” The child was instructed that if the car stops itself early, they should suppress their finger lift action and maintain their finger pressed down until they saw a checkered flag (which occurs at 1000 ms). Successful stopping is “not lifting the finger at the 800 ms mark,” and maintaining finger adduction for greater than 1000 ms. The stop cue timing shifted by 50-ms increments depending on success or failure, allowing the stop trial times to converge to indicate response inhibition efficiency (Coxon et al. 2006; Guthrie et al. 2018). The behavioral variables were the average “go-action” time average (see above) and the stop signal reaction time (SSRT). SSRT is the difference between the mean “go-action” time and the average (final 4) stop-cue times, such that later average stop cue times yield lower SSRTs, indicating more efficient stopping. Not all participants underwent SSRT testing due to participant schedule, the stimulator overheating, and the long duration of SSRT testing. There is no indication that missingness was related to group, ADHD severity or other dependent/independent variables.
The game was played with three 40 trial blocks (30 go with 10 stop randomly intermixed). A full description and demonstration of the task is available open-access (Guthrie et al. 2018).
TMS in Resting Motor Cortex (RMT and SICI)
Dominant (left) hemisphere M1 physiology was assessed using a Magstim 200 TMS (Magstim Co.) connected through a Bistim module to a round 90-mm coil and Signal processing software as described previously (Gilbert et al. 2011; Guthrie et al. 2018). TMS utilizes magnetic fields to generate an electric field that can induce depolarization in neurons within range of the coil. Single suprathreshold intensity pulses over M1 can generate a MEP measurable in anatomically localized muscles with surface EMG. Pairing suprathreshold pulses with preceding subthreshold TMS pulses can consistently inhibit or activate motor cortex interneurons, reducing or increasing the amplitude of the MEP. TMS coil placement was flat at the vertex, with the handle directly posterior. This technique and coil were chosen to enhance stability in hyperkinetic children (compared with the more common tangential placement of the figure of 8 coil). All protocols for active and resting motor thresholds (AMT and RMT; Mills and Nithi 1997) and paired-pulse TMS for SICI (Kujirai et al. 1993; Rothwell et al. 2009) are in standard use, implemented by our laboratories in 8–12-year-old children, as previously described (Gilbert et al. 2011). In brief, threshold measures were performed first, to habituate children, starting with pulses at 10% maximal stimulator output, increasing by 10% until a consistent MEP was observed, then decreasing the intensity until a minimum point was reached where 3 of 6 pulses produced no MEP and 3 produced an MEP of approximately at least 50 microvolts, at rest (RMT). RMT was indexed by the maximum stimulator output of the TMS stimulator being used; the maximum is 100%. Participants were excluded from SICI and further analysis if their RMT was greater than the stimulator maximum divided by 1.2 (here, 83).
SICI at rest was evaluated using 10 single test pulses and 10-paired pulses, with conditioning pulses at 0.6 × RMT and test pulses at 1.2 × RMT, at an interstimulus interval of 3 ms and an intertrial interval of 6 s, ±5%. Test pulses were administered at 1.2 × RMT with a goal of evoking MEPs averaging 0.5–1.5 mV per trial. SICI is expressed as ratios of paired to single pulses. For SICI, ratios closer to 1.0 indicate less inhibition by the 3-ms pair, relative to the single pulse, that is, less SICI. 17 TD participants and 14 with ADHD underwent SICI testing.
TRUM
TRUM was measured during the SST; there were 2 blocks of 40 trials. Single (at 1.2 × RMT) and 3-ms-paired (at 0.6 and 1.2 × RMT) TMS pulses were delivered randomly across trials. For go trials, TMS pulses were administered at the time of expected action selection—150 ms prior to the finger lift; for stop trials, TMS pulses were administered at the time of expected action suppression—not lifting the finger, 150 ms after the stop cue. TRUM is the ratio of MEP amplitudes during the response inhibition task compared with MEP amplitudes at rest. Eight TD participants and 9 with ADHD underwent TRUM testing.
Statistical Approach
This is a post hoc, exploratory analysis from an intersecting subsample of previously published data from 2 unique studies involving analysis of well-characterized children with ADHD and TD controls (Gilbert et al. 2019; McAuliffe et al. 2020). Diagnostic group comparisons were performed with unpaired t-tests for continuous variables and χ2 for categorical variables. We calculated group distributional effect sizes as Cohen’s d.
The relationship between RMT and ERD (alpha and beta, separately) was tested using linear models separately in each group, adjusting for sex, age (which is known to affect RMT), and general intellectual ability (General Ability Index of the Wechsler tests: GAI), which showed nearly significant differences between groups.
The point estimate of SICI was calculated as the average of paired pulse TMS-evoked MEP amplitudes divided by average of single pulse TMS-evoked MEP amplitudes. SICI-ERD associations were tested using repeated measures mixed models with MEP amplitude as the dependent variable, ERD as a predictor variable, and subject as a random effect, incorporating all trials instead of averaging within subjects (Guthrie et al. 2018; Gilbert et al. 2019). Age, sex, and GAI were also included in the models. Log transformation was used to optimize residuals. In repeated measures, SICI was estimated from the PulseType (paired vs., single), and the SICI-ERD association as PulseType × ERD. Rather than stratifying, diagnostic effects were then estimated from interaction terms Diagnosis × PulseType, where Diagnosis was a categorical term. The test for diagnostic difference was thus a 3-way interaction term: MEP = Diagnosis × PulseType × ERD.
TRUM was calculated using analogous models, except that the TRUM effect was defined by the Block (task vs., rest) variable, the TRUM-ERD association as Block × ERD, and the test for diagnostic difference as the significance of the 3-way term: MEP = Diagnosis × Block × ERD.
P values < 0.05 were considered significant, and no correction was made for multiple comparisons. All models were analyzed using SAS statistical software version 9.4 (SAS Institute Inc.). Relationships with ERD were determined by including alpha and beta ERD as factors in separate models.
Results
Participants
There were 17 children in the TD group (75% male; age 10.9 ± 1.4; 10.2–11.6 years; and 59% Caucasian, 6% African American, 18% Asian, 18% Biracial, and 0% Hispanic ethnicity) and 14 in the ADHD group (57% male; age 10.7 ± 1.2; 10.1–11.4 years; and 71% Caucasian, 7% African American, 0% Asian, 0%, 21% Biracial, and 14% Hispanic ethnicity) who had both EEG and TMS data available (see Table 1). Eleven ADHD and 3 TD participants were excluded due to RMT being higher than the allowable threshold. It was possible to examine ERD values for 5 of the participants excluded from the ADHD group and all 3 from the TD group; excluded ERD values had similar central tendencies to the included values.
Table 1.
Participants
| ADHD | TD Control | P -values | |||||
|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | t-test | |
| Age | 14 | 10.7 | 1.2 | 17 | 10.9 | 1.4 | 0.74 |
| ADHD severity | |||||||
| Conners T score hyper/impulsive | 14 | 75 | 15 | 17 | 45 | 6.8 | <.0001 |
| Conners T score inattentive | 14 | 75 | 11 | 17 | 45 | 9.8 | <.0001 |
| ADHD-RS hyper/impulsive | 13 | 11 | 7.6 | 17 | 2.0 | 2.4 | 0.0005 |
| ADHD-RS inattentive | 13 | 18 | 4.6 | 17 | 2.7 | 2.9 | <.0001 |
| ADHD-RS total | 13 | 30 | 10 | 17 | 4.8 | 4.8 | <.0001 |
| Cognitive | |||||||
| Full scale IQ | 13 | 107 | 10 | 13 | 116 | 12 | 0.047 |
| GAI | 14 | 104 | 13 | 17 | 112 | 13 | 0.081 |
| Other | |||||||
| Hollingshead socio-economic | 12 | 54 | 11 | 16 | 59 | 6 | 0.18 |
Note: ADHD-RS, attention-deficit hyperactivity disorder-rating scale; IQ, intelligence quotient; GAI, general intellectual ability.
Between-Group Effect Sizes
Of the various physiological metrics, alpha ERD during finger-tapping showed the greatest between-group separation (Cohen’s d = 0.89, “large” effect, by convention). RMT showed d = 0.5, and TRUM during the SST d = 0.42, both “medium” effect sizes. Baseline SICI and beta ERD both showed effect sizes of 0.35 and 0.31, respectively, both in the “small to medium” range.
RMT and ERD
Unadjusted mean RMTs for the 2 groups were 65.2 ± 8.6 for the TD group and 60.6 ± 8.6 for the ADHD group (Cohen’s d = 0.5 and P = 0.15). Given the number of children excluded from the ADHD group due to high RMT, it is likely that a sample with a greater maximum allowable RMT would show a smaller diagnostic difference. Consistent with our working hypothesis, children with higher resting thresholds had greater ERD in the alpha range, after adjusting for age, sex and GAI (see Fig. 1, reporting RMT residualized for age, sex, and GAI). This association was statistically significant in the TD group (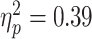 ; P = 0.024; and one outlier removed). Although it did not quite reach significance in children with ADHD, the measured effect size was similar (
; P = 0.024; and one outlier removed). Although it did not quite reach significance in children with ADHD, the measured effect size was similar (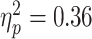 ; P = 0.068; and one outlier removed), and there was no significant diagnostic interaction effect (
; P = 0.068; and one outlier removed), and there was no significant diagnostic interaction effect (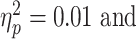 P = 0.62), contrary to our predictions (Fig. 1). The association between RMT and beta ERD was marginal at best in TDs (
P = 0.62), contrary to our predictions (Fig. 1). The association between RMT and beta ERD was marginal at best in TDs (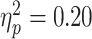 ; P = 0.14; and one outlier removed) and nonsignificant in the ADHD group (
; P = 0.14; and one outlier removed) and nonsignificant in the ADHD group (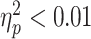 ; P = 0.97; and one outlier removed). There was no significant diagnostic-group interaction (
; P = 0.97; and one outlier removed). There was no significant diagnostic-group interaction (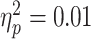 and P = 0.65; Fig. 2).
and P = 0.65; Fig. 2).
Figure 1 .
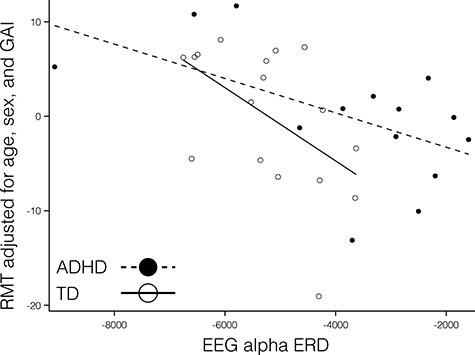
There was a significant association between RMT (adjusted) and alpha ERD in the TD children (hollow circles and solid line; 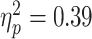 ; P = 0.024; with one highly influential point removed). The association in the ADHD group did not reach significance though the measured effect size was similar (
; P = 0.024; with one highly influential point removed). The association in the ADHD group did not reach significance though the measured effect size was similar (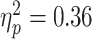 ; P = 0.068; with one highly influential point removed). There was no significant diagnostic interaction effect (
; P = 0.068; with one highly influential point removed). There was no significant diagnostic interaction effect (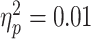 and P = 0.62), contrary to our predictions.
and P = 0.62), contrary to our predictions.
Figure 2 .
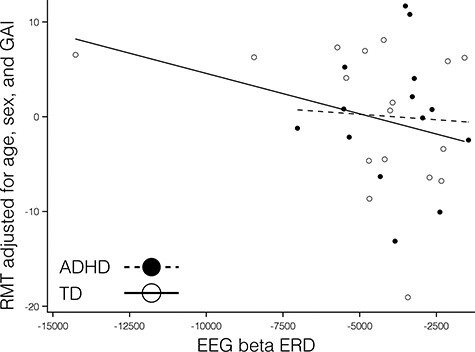
There was no statistically-significant association between RMT and beta ERD in TDs (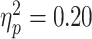 ; P = 0.14; and one influential point removed) or in the ADHD group (
; P = 0.14; and one influential point removed) or in the ADHD group (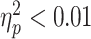 ; P = 0.97; and one influential point removed). There was no significant diagnostic-group interaction (
; P = 0.97; and one influential point removed). There was no significant diagnostic-group interaction (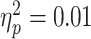 and P = 0.65). Note that the outlier to the negative end of the x-axis was not influential; removing it did not substantially alter the calculated statistical results.
and P = 0.65). Note that the outlier to the negative end of the x-axis was not influential; removing it did not substantially alter the calculated statistical results.
SICI and ERD
Alpha ERD
Baseline SICI was significantly associated with alpha ERD in the TD control group, after adjusting for age, sex, GAI, and artifact (P = 0.0024 and n = 17), with a greater magnitude of ERD corresponding to a greater SICI ratio (interpreted as greater inhibition). SICI was not associated with alpha ERD in the ADHD group (P = 0.27, n = 14). Although the diagnostic interaction effect reached statistical significance (P = 0.017), the estimated effect size was small (Fig. 3). An approximation of 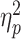 for this repeated-measures model based on the F-statistic divided by the quantity (F-statistic + degrees-of-freedom of the denominator) came to a value of 0.01. Note that the numerator degrees of freedom for all F-statistics was equal to 1.
for this repeated-measures model based on the F-statistic divided by the quantity (F-statistic + degrees-of-freedom of the denominator) came to a value of 0.01. Note that the numerator degrees of freedom for all F-statistics was equal to 1.
Figure 3 .
Regression Mixed-Model alpha ERD/baseline SICI relationships in n = 17 TD children and n = 14 children with ADHD. In these figures, the upper (dashed) line is single pulse MEP amplitudes, the lower (solid) line is 3-ms paired (inhibitory) MEP amplitudes. A greater distance between the 2 lines indicates a greater SICI ratio (i.e., more inhibition), and a more negative ERD value indicates a higher magnitude of ERD. The baseline SICI-alpha ERD association reached statistical significance in the control group (P = 0.0024) but not in the ADHD group (P = 0.27), with a statistically significant but very-small-magnitude diagnostic interaction effect (P = 0.017).
Beta ERD
We found no evidence of an association between magnitude of beta ERD and baseline SICI in the TD group after adjusting for age, sex, GAI, and artifact (P = 0.85), however there was a significant association in the ADHD group (P = 0.045; Fig. 4), with a greater magnitude of ERD corresponding to a greater SICI ratio (interpreted as greater inhibition). There was no significant diagnostic-group interaction effect (approximate 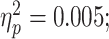 P = 0.11), though the groups did show different directions of observed effect, with the TD group showing a smaller SICI ratio associated with a greater magnitude of ERD (Fig. 4).
P = 0.11), though the groups did show different directions of observed effect, with the TD group showing a smaller SICI ratio associated with a greater magnitude of ERD (Fig. 4).
Figure 4 .
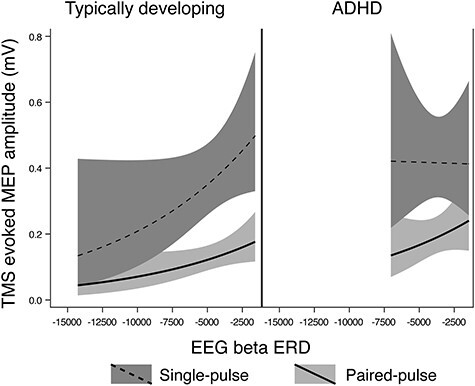
Regression Mixed-Model beta ERD/SICI relationships in n = 17 TD children and n = 14 children with ADHD. In these figures, the upper (dashed) line is single pulse MEP amplitudes, the lower (solid) line is 3 ms paired (inhibitory) MEP amplitudes, with a greater distance between the 2 lines indicating a larger SICI effect. A more negative ERD value indicates a greater magnitude of ERD. There was no statistical association between SICI and beta ERD in the TD group (P = 0.85), however there was a significant association in the ADHD group (P = 0.045). There was no significant diagnostic-group interaction effect (P = 0.11), though the plot illustrates an opposite direction of association between groups, with TD showing a smaller SICI ratio associated with a larger magnitude of ERD and ADHD showing a larger SICI ratio associated with a larger magnitude of ERD. The nonlinear shape in TD is due to logarithmic transformation to optimize residuals.
TRUM and ERD
Performance on the Slater Hammel task was comparable between groups as a consequence of the adaptive nature of the task. Moreover, task difficulty as a consequence of this adaptation was similar between groups. Go-action times were TD 839 ± 32 ms; ADHD: 838 ± 18; Cohen’s d = 0.04; and P = 0.92) SSRTs were TD: 301 ± 99 ms; ADHD: 314 ± 77 ms; Cohen’s d = 0.17; and P = 0.75).
Alpha
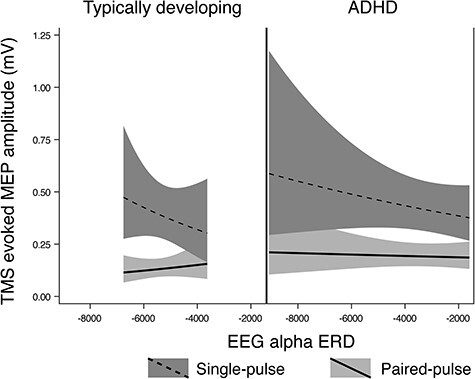
After removing one outlier (TD group), higher alpha ERD in left M1 during right-hand finger tapping was associated with lower magnitude of TRUM (P < 0.001) after adjusting for age, sex, GAI, and artifact, that is, in the direction opposite from our hypotheses. By contrast, for children with ADHD, higher alpha ERD was associated with a “greater” magnitude of TRUM (opposite direction to TD group) (P < 0.001). There was a large diagnostic interaction effect (P < 0.001), contrary to our predictions (Fig. 5).
Figure 5 .
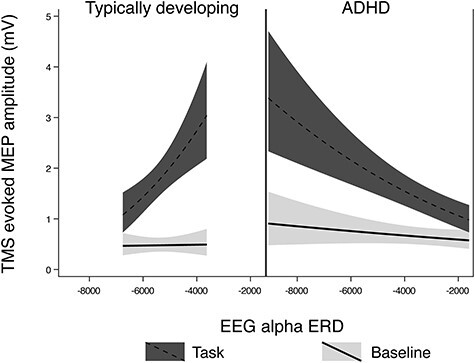
Regression Mixed-Model alpha ERD/TRUM relationships in n = 8 TD children and n = 9 children with ADHD. In these figures, the upper (red) line is MEP amplitudes during the response inhibition task trials, the lower (blue) line is the MEP amplitudes at rest. Among TD children with more left M1 alpha ERD when finger tapping, there is less left M1 TRUM (the ratio of the red to the blue line value) (P < 0.001). The relationship is opposite children with ADHD (P < 0.001), and the diagnostic interaction effect was large (P < 0.001). The nonlinear shape is due to logarithmic transformation to optimize residuals.
Beta: Left M1
In TD children, there was a statistical association between TRUM and beta ERD (P < 0.001), with greater TRUM associated with a “decreased” magnitude of ERD, as also seen in the “alpha” ERD–TRUM relationship. In the ADHD group, the relationship between TRUM and beta ERD was again similar to that seen with alpha ERD, with a greater magnitude of TRUM associated with a greater magnitude of ERD (P = 0.021). There was a large TRUM–beta ERD diagnostic interaction effect (P < 0.001; Fig. 6).
Figure 6 .
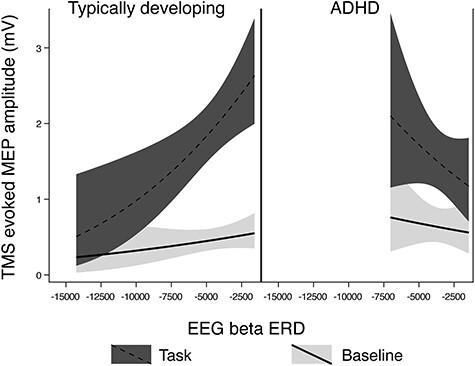
Regression Mixed-Model beta ERD/TRUM relationships in n = 8 TD children and n = 9 children with ADHD. In these figures, the upper (red) line is MEP amplitudes during the response inhibition task trials, the lower (blue) line is the MEP amplitudes at rest. The pattern of results was similar to that seen with alpha ERD: that is, TD children showed a greater magnitude of ERD when the magnitude of TRUM was smallest (P < 0.001), and the children with ADHD showed a greater magnitude of ERD when the magnitude of TRUM was the largest (P = 0.021). The was a diagnostic interaction effect (P < 0.001). The nonlinear shape in the TD group is due to logarithmic transformation to optimize residuals.
Discussion
Our primary findings in the TD-only analyses were consistent with 2 of 3 of our working hypotheses for that population group: RMT correlated with both alpha and beta ERD, such that a higher TMS field strength needed to generate a MEP was associated with a greater magnitude of ERD during the finger-tapping task. Similarly, SICI and alpha ERD correlated, such that greater SICI (i.e., greater inhibition) at rest was associated with a greater magnitude of alpha ERD. And although TRUM was statistically associated with both alpha and beta ERD, the direction was opposite to the one predicted for TD children: Greater task-related up-modulation of MEP from rest to task engagement was associated with a “smaller” magnitude of alpha ERD during the finger-tapping task.
Regarding effects of diagnosis (ADHD vs., TD), for our primary RMT-alpha ERD finding, there was no significant diagnosis × physiology interaction in either the alpha or beta band, with significant positive associations in both ADHD and TD children. For SICI, there was also no evidence of an interaction effect (though ADHD post-hoc testing showed no evidence of a SICI-alpha ERD association; the presence of a statistical association in TD but absence in ADHD could be due to greater heterogeneity in the ADHD group). The TRUM-ERD × diagnosis effect was pronounced, and the association was unexpectedly in the opposite direction for the 2 groups, with greater task-related up-modulation of MEP from rest to task engagement associated with a larger magnitude of both alpha and beta ERD during the finger-tapping task.
RMT, SICI, and ERD
Our working model relating RMT and ERD assumed that RMT and ERD reflected aspects of a single mechanism and suggested that a larger RMT would indicate less “readiness to depolarize,” and a greater magnitude of ERD would be required to activate the cortex. The results are consistent with this hypothesis. However, the results presented here further assumed that all participants “reach the same point” of activation during finger tapping. It may be warranted, in future work using larger samples, to use additional task-related physiological measurements or behavioral measurements to quantify the degree of task-related activation on an individual-participant or even trial-by-trial basis. Such a model would test the notion that RMT + ERD = degree of task-related activation.
The SICI–ERD results also showed an association with alpha ERD, but only in the TD group. As SICI is believed to be mediated by GABA-ergic interneuron input into motor cortex pyramidal cell output, this suggests that the nature of SICI/ERD interactions might be clarified in future multimodal studies in which participants also undergo GABA measurements with MRS (Harris et al. 2021). However, given the lack of association of RMT and SICI in the TMS supra-sample (Gilbert et al. 2019), this suggests that alpha ERD magnitude is likely dependent on 2 separate mechanisms, one indexed by RMT and the other by SICI. Failure to find this SICI–ERD relationship in ADHD is difficult to interpret but may related to heterogeneity of ADHD as a categorical diagnosis reducing the signal-to-noise ratio.
TRUM and ERD
The most robust and strikingly unexpected findings in this dataset was highly significant diagnostic interaction effect, such that: 1) opposite to what we predicted, for TD children, higher alpha ERD in left M1 during right-hand finger tapping was associated with “lower” magnitude of TRUM; yet, and 2) consistent with what we predicted for the TD group, for children with ADHD, higher alpha ERD was associated with a “greater” magnitude of TRUM. There are a few conclusions that we can derive from these results. First, TRUM and ERD do not reflect the same underlying process, as we initially assumed. This is borne out by the anticorrelation within the TD group as well as the diagnosis-related interaction effect. However, the presence of TRUM-ERD statistical associations demonstrates that these processes interact. What these processes are, how they interact and how dependent they may be on the difference in cognitive-motor task between the TMS (SST) experiment and the EEG (finger-tapping) experiment needs to be explored with additional data and additional tasks.
The literature linking task-related modulation of TMS and EEG (i.e., TRUM and ERD) is sparse. Lepage et al. (2008) systematically examined the dissociation between task-related TMS modulation and “alpha” ERD results in 16 healthy adults, albeit during a different series of motor tasks (as opposed to our cognitive control task), finding, as did we, a lack of correlations between TMS modulation indices and alpha ERD. Given strong evidence for reliability and validity of each measure, and assuming that TMS modulation and ERD reflect the same processes, they attributed the lack of correlation to the proposition that alpha ERD is more reflective of postcentral activation, whereas beta (which they had not measured) was reflective of precentral activation. They predicted that future work examining beta ERD (reflective of the physiology of the precentral gyrus) would indeed find correlations with task-related TMS modulation. Our results belie that hypothesis. Taken together, both our results suggest that TMS measures of modulation and ERD are sensitive to different task-engaged processes. At this stage, any attempt at explanation for the dissociation between physiological modulation during the finger-tapping task (as measured by ERD) and modulation during the SST (as measured by TRUM) is necessarily speculative; however we note that the SST likely has a larger contribution of top-down control from prefrontal regions (e.g., right dorsolateral prefrontal cortex [DLPFC]) than does the finger-tapping task. The diagnostic interaction in the TRUM–ERD relationship may be attributable to ADHD-related effects of DLPFC influence (Westwood et al. 2021). As therapeutically-oriented stimulation of both DLPFC (Schroeder et al. 2020) and primary sensory-motor cortex (Kwon and Kwon 2013) have been shown to affect response inhibitory tasks, further clarification of the primary locus of impairment in ADHD will be important for guiding emerging neurostimulatory interventions.
What is clear in our dataset is that the largest between-group effect size comes from strong diagnosis-associated effects associated with comparing “task-related modulation” of both EEG and TMS. As noted in the Introduction, the phenomenon of altered task-related modulation of brain physiology has been identified within not only ADHD (McAuliffe et al. 2020) but also autism spectrum disorder (ASD; Murphy et al. 2014; Ewen et al. 2016b; Pillai et al. 2017; Harvy et al. 2019), even in instances where baseline measures did not differ (or differ much) between groups. Although our basic neuroscientific understanding is strong in relation to many aspects of resting brain physiology, including the TMS measures covered above as well as the generation of EEG oscillations (Cannon et al. 2014), the associated understanding of mechanisms of task-related modulation of brain physiology is limited. The strong diagnosis-specific signal from the TRUM–ERD analyses appears to support mechanisms of cortical modulation as a promising area of targeted investigation, particularly as it relates to NDD, and could lead to novel biomarkers and treatments. Trans-diagnostic research is key to demonstrating what aspects of these various modulatory effects are task-specific (as also in Lepage et al. 2008), diagnosis-related or related to the comorbidity among these conditions.
It is important to recognize several limitations of these data. First is the potential for variance errors due to the relatively small sample size. This limitation is most immediate in the TRUM–ERD analysis. Further studies will substantially larger sample sizes will be needed to increase confidence in the reproducibility of the results. Additionally, the relatively long between-measurement interval in some groups may present confounds associated with maturation within the interval. As this would likely introduce power-reducing statistical noise (i.e., increasing risk for type II and not type I error), this interval is not a strong threat to validity. Finally, we note potential sources of bias in order to design future studies that are more insensitive to confounds and more generalizable. Some participants (particularly those with ADHD) were excluded due to high RMT and stimulator capacity. Although those excluded did not appear to have different ERD values, future research with a greater n than was possible in this sample size should consider statistical methods to adjust for RMT capacity or to reduce sensitivity to potential bias. Moreover, the current results were measured within a limited age range, and understanding the trajectory of physiology over the lifespan of individuals with ADHD should be a goal to increase our understanding of the scope of this effect.
Contributor Information
Joshua B Ewen, Department of Neurology and Developmental Medicine, Kennedy Krieger Institute, Baltimore, MD 21205, USA; Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, MD 21205, USA.
Nicolaas A Puts, Neurodevelopmental Sciences, King’s College London, Strand, London WC2R 2LS, United Kingdom.
Stewart H Mostofsky, Neurodevelopmental and Imaging Research, Kennedy Krieger Institute, Baltimore, MD 21205, USA; Pediatrics and Neurology, Johns Hopkins University School of Medicine, Baltimore, MD 21205, USA.
Paul S Horn, Department of Neurology, Cincinnati Children’s Hospital Medical Center and University of Cincinnati, Cincinnati, OH 45229, USA.
Donald L Gilbert, Department of Neurology, Cincinnati Children’s Hospital Medical Center and University of Cincinnati, Cincinnati, OH 45229, USA.
Funding
National Institutes of Health (R01 MH08532 and R01 MH078160-08S1 to S.H.M.; R01MH095014 to D.L.G. and P.S.H., and P50 HD103538 supporting the effort of J.B.E.).
Notes
We appreciate the generous involvement of our research participants and their families as well as the laboratory members who were involved in data collection and analysis. We thank Mr Jack Adamek and Ms Deana Crocetti for their assistance with analyses.
Conflict of Interest: None declared.
References
- Cannon J, McCarthy MM, Lee S, Lee J, Borgers C, Whittington MA, Kopell N. 2014. Neurosystems: brain rhythms and cognitive processing. Eur J Neurosci. 39:705–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Yaseen Z, Cohen LG, Hallett M. 1998. Time course of corticospinal excitability in reaction time and self-paced movements. Ann Neurol. 44:317–325. [DOI] [PubMed] [Google Scholar]
- Cole WR, Mostofsky SH, Larson JC, Denckla MB, Mahone EM. 2008. Age-related changes in motor subtle signs among girls and boys with ADHD. Neurology. 71:1514–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners CK, Sitarenios G, Parker JD, Epstein JN. 1998. The revised Conners' Parent Rating Scale (CPRS-R): factor structure, reliability, and criterion validity. J Abnorm Child Psychol. 26:257–268. [DOI] [PubMed] [Google Scholar]
- Coxon JP, Stinear CM, Byblow WD. 2006. Intracortical inhibition during volitional inhibition of prepared action. J Neurophysiol. 95:3371–3383. [DOI] [PubMed] [Google Scholar]
- Crosbie J, Arnold P, Paterson A, Swanson J, Dupuis A, Li X, Shan J, Goodale T, Tam C, Strug LJ, et al. 2013. Response inhibition and ADHD traits: correlates and heritability in a community sample. J Abnorm Child Psychol. 41:497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, Makeig S. 2004. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 134:9–21. [DOI] [PubMed] [Google Scholar]
- Denckla MB, Rudel RG. 1978. Anomalies of motor development in hyperactive boys. Ann Neurol. 3:231–233. [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P. 2010. Beta-band oscillations--signalling the status quo? Curr Opin Neurobiol. 20:156–165. [DOI] [PubMed] [Google Scholar]
- Ewen JB. 2016. The eternal promise of EEG-based biomarkers: getting closer? Neurology. 87:2288–2289. [DOI] [PubMed] [Google Scholar]
- Ewen JB, Beniczky S. 2018. Validating biomarkers and diagnostic tests in clinical neurophysiology: developing strong experimental designs and recognizing confounds. In: Schomer DL, Lopes da Silva FH, editors. Niedermeyer's electroencephalography. 7th ed. New York: Oxford University Press. [Google Scholar]
- Ewen JB, Lakshmanan BM, Pillai AS, McAuliffe D, Nettles C, Hallett M, Crone NE, Mostofsky SH. 2016a. Decreased modulation of EEG oscillations in high-functioning autism during a motor control task. Front Hum Neurosci. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewen JB, Pillai AS, McAuliffe D, Lakshmanan BM, Ament K, Hallett M, Crone NE, Mostofsky SH. 2016b. Practicing novel, praxis-like movements: physiological effects of repetition. Front Hum Neurosci. 10:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewen JB, Potter WZ, Sweeney JA. 2021. Biomarkers and neurobehavioral diagnosis. Biomarkers Neuropsychiatry. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewen JB, Sweeney JA, Potter WZ. 2019. Conceptual, regulatory and strategic imperatives in the early days of EEG-based biomarker validation for neurodevelopmental disabilities. Front Integr Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garry MI, Thomson RH. 2009. The effect of test TMS intensity on short-interval intracortical inhibition in different excitability states. Exp Brain Res. 193:267–274. [DOI] [PubMed] [Google Scholar]
- Gilbert DL, Huddleston DA, Wu SW, Pedapati EV, Horn PS, Hirabayashi K, Crocetti D, Wassermann EM, Mostofsky SH. 2019. Motor cortex inhibition and modulation in children with ADHD. Neurology. 93:e599–e610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert DL, Isaacs KM, Augusta M, MacNeil LK, Mostofsky SH. 2011. Motor cortex inhibition: A marker of ADHD behavior and motor development in children. Neurology. 76:615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA-NIH Biomarker Working Group . 2016. In: Silver Spring, MD, Bethesda MD , editors. BEST (Biomarkers, EndpointS, and other Tools) resource. Food and Drug Administration and National Institutes of Health (US). [PubMed] [Google Scholar]
- Guthrie MD, Gilbert DL, Huddleston DA, Pedapati EV, Horn PS, Mostofsky SH, Wu SW. 2018. Online transcranial magnetic stimulation protocol for measuring cortical physiology associated with response inhibition. J Vis Exp. e56789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A, Gilbert D, Horn P, Crocetti D, Cecil K, Edden R, Huddleston D, Mostofsky S, Puts N. 2021. Relationship between GABA levels and task-dependent cortical excitability in children with attention-deficit/hyperactivity disorder. Clin Neurophysiol. 132:1163–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvy J, Ewen JB, Thakor N, Bezerianos A, Li J. 2019. Cortical functional connectivity during praxis in autism spectrum disorder. Annu Int Conf IEEE Eng Med Biol Soc . 333–336. [DOI] [PubMed]
- Hoegl T, Heinrich H, Barth W, Losel F, Moll GH, Kratz O. 2012. Time course analysis of motor excitability in a response inhibition task according to the level of hyperactivity and impulsivity in children with ADHD. PloS One. 7:e46066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman AS, Raiford SE, Coalson DL. 2015. Intelligent testing with the WISC-V. John Wiley & Sons. [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. 1997. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 36:980–988. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE. 2006. Principal components analysis of Laplacian waveforms as a generic method for identifying ERP generator patterns: II. Adequacy of low-density estimates. Clin Neurophysiol. 117:369–380. [DOI] [PubMed] [Google Scholar]
- Keil J, Timm J, Sanmiguel I, Schulz H, Obleser J, Schonwiesner M. 2014. Cortical brain states and corticospinal synchronization influence TMS-evoked motor potentials. J Neurophysiol. 111:513–519. [DOI] [PubMed] [Google Scholar]
- Kelly SP, Lalor EC, Reilly RB, Foxe JJ. 2006. Increases in alpha oscillatory power reflect an active retinotopic mechanism for distracter suppression during sustained visuospatial attention. J Neurophysiol. 95:3844–3851. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. 1993. Corticocortical inhibition in human motor cortex. J Physiol. 471:501–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon YH, Kwon JW. 2013. Response inhibition induced in the stop-signal task by transcranial direct current stimulation of the pre-supplementary motor area and primary sensoriomotor cortex. J Phys Ther Sci. 25:1083–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepage JF, Saint-Amour D, Theoret H. 2008. EEG and neuronavigated single-pulse TMS in the study of the observation/execution matching system: are both techniques measuring the same process? J Neurosci Methods. 175:17–24. [DOI] [PubMed] [Google Scholar]
- MacNeil LK, Xavier P, Garvey MA, Gilbert DL, Ranta ME, Denckla MB, Mostofsky SH. 2011. Quantifying excessive mirror overflow in children with attention-deficit/hyperactivity disorder. Neurology. 76:622–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuliffe D, Hirabayashi K, Adamek JH, Luo Y, Crocetti D, Pillai AS, Zhao Y, Crone NE, Mostofsky SH, Ewen JB. 2020. Increased mirror overflow movements in ADHD are associated with altered EEG alpha/beta band desynchronization. Eur J Neurosci. 51:1815–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KR, Nithi KA. 1997. Corticomotor threshold to magnetic stimulation: normal values and repeatability. Muscle Nerve. 20:570–576. [DOI] [PubMed] [Google Scholar]
- Mimura Y, Nishida H, Nakajima S, Tsugawa S, Morita S, Yoshida K, Tarumi R, Ogyu K, Wada M, Kurose S, et al. 2021. Neurophysiological biomarkers using transcranial magnetic stimulation in Alzheimer's disease and mild cognitive impairment: a systematic review and meta-analysis. Neurosci Biobehav Rev. 121:47–59. [DOI] [PubMed] [Google Scholar]
- Molina BS, Hinshaw SP, Swanson JM, Arnold LE, Vitiello B, Jensen PS, Epstein JN, Hoza B, Hechtman L, Abikoff HB, et al. 2009. The MTA at 8 years: prospective follow-up of children treated for combined-type ADHD in a multisite study. J Am Acad Child Adolesc Psychiatry. 48:484–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll GH, Heinrich H, Trott G, Wirth S, Bock N, Rothenberger A. 2001. Children with comorbid attention-deficit-hyperactivity disorder and tic disorder: evidence for additive inhibitory deficits within the motor system. Ann Neurol. 49:393–396. [PubMed] [Google Scholar]
- Moll GH, Heinrich H, Trott G, Wirth S, Rothenberger A. 2000. Deficient intracortical inhibition in drug-naive children with attention-deficit hyperactivity disorder is enhanced by methylphenidate. Neurosci Lett. 284:121–125. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Simmonds DJ. 2008. Response inhibition and response selection: two sides of the same coin. J Cogn Neurosci. 20:751–761. [DOI] [PubMed] [Google Scholar]
- Muller K, Homberg V, Lenard HG. 1991. Magnetic stimulation of motor cortex and nerve roots in children. Maturation of cortico-motoneuronal projections. Electroencephalogr Clin Neurophysiol. 81:63–70. [DOI] [PubMed] [Google Scholar]
- Murphy JW, Foxe JJ, Peters JB, Molholm S. 2014. Susceptibility to distraction in autism spectrum disorder: probing the integrity of oscillatory alpha-band suppression mechanisms. Autism Res. 7:442–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Z, Bahl N, Gunraj CA, Mazzella F, Chen R. 2013. Increased motor cortical facilitation and decreased inhibition in Parkinson disease. Neurology. 80:1746–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield R. 1971. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 9:97–113. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes Da Silva FH. 1999. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol. 110:1842–1857. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Neuper C. 1994. Event-related synchronization of mu rhythm in the EEG over the cortical hand area in man. Neurosci Lett. 174:93–96. [DOI] [PubMed] [Google Scholar]
- Pillai AS, McAuliffe D, Lakshmanan BM, Mostofsky SH, Crone NE, Ewen JB. 2017. Altered task-related modulation of long-range connectivity in children with autism. Autism Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich W. 2000. Diagnostic interview for children and adolescents (DICA). J Am Acad Child Adolesc Psychiatry. 39:59–66. [DOI] [PubMed] [Google Scholar]
- Rothwell JC, Day BL, Thompson PD, Kujirai T. 2009. Short latency intracortical inhibition: one of the most popular tools in human motor neurophysiology. J Physiol. 587:11–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin M, Jones SR, Sweeney JA, Berry-Kravis E, Connors BW, Ewen JB, Hartman AL, Levin AR, Potter WZ, Mamounas LA. 2018. Discovering translational biomarkers in neurodevelopmental disorders. Nat Rev Drug Discov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmelin R, Hamalainen M, Kajola M, Hari R. 1995. Functional segregation of movement-related rhythmic activity in the human brain. Neuroimage. 2:237–243. [DOI] [PubMed] [Google Scholar]
- Schroeder PA, Schwippel T, Wolz I, Svaldi J. 2020. Meta-analysis of the effects of transcranial direct current stimulation on inhibitory control. Brain Stimul. 13:1159–1167. [DOI] [PubMed] [Google Scholar]
- Vasileva M, Graf RK, Reinelt T, Petermann U, Petermann F. 2020. Research review: a meta-analysis of the international prevalence and comorbidity of mental disorders in children between 1 and 7 years. J Child Psychol Psychiatry. [DOI] [PubMed] [Google Scholar]
- Wechsler DL. 2003. Wechsler Intelligence Scale for Children - Fourth Edition (WISC-IV). San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Westwood SJ, Radua J, Rubia K. 2021. Noninvasive brain stimulation in children and adults with attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. J Psychiatry Neurosci. 46:E14–E33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolraich ML, Chan E, Froehlich T, Lynch RL, Bax A, Redwine ST, Ihyembe D, Hagan JF Jr. 2019. ADHD diagnosis and treatment guidelines: a historical perspective. Pediatrics. 144. [DOI] [PubMed] [Google Scholar]
- Zea Vera A, Horn PS, Mostofsky SH, Gilbert DL. 2020. Correlations of possible TMS biomarkers of cognitive and emotional dysfunction in ADHD (1224). Neurology. 94:1224. [Google Scholar]


