Abstract
Background:
Increasing evidence suggests that hypertension is a risk factor for cognitive impairment and dementia. The relationship between blood pressure and cognition in a racially and ethnically diverse population remains unclear.
Objective:
To study association of blood pressure with cognition cross-sectionally and longitudinally in the elderly.
Methods:
Participants are stroke-free individuals from the racially and ethnically diverse Northern Manhattan Study (NOMAS) (n = 1215). General linear models are constructed to examine blood pressure in relation to cognition cross-sectionally and longitudinally at a five-year follow-up.
Results:
We found a cross-sectional association of systolic blood pressure (SBP) with word fluency/semantic memory, executive function, and processing speed/visual motor integration (VMI) function. This association was independent of demographics, vascular risk factors, white matter hyperintensity volume (WMHV), and carotid intima-media thickness (cIMT). The cross-sectional association of SBP with processing speed/VMI and executive function was attenuated after adjusting anti-hypertension medications in the models. Baseline SBP was associated with the change of processing speed/VMI function after adjusting vascular risk factors, WMHV, and cIMT at a 5-year follow-up. This longitudinal association was not found after adjusting anti-hypertension medications in the models. Further analyses revealed that individuals with category SBP from < 120mmHg to ≥ 140mmHg had a linear decline in processing speed/VMI function at a 5-year follow-up.
Conclusion:
We show that SBP is negatively associated with cognition cross-sectionally and longitudinally in the elderly. Anti-hypertension treatment eliminates the negative association of SBP with processing speed/VMI function longitudinally. Our findings support the treatment of stage 1 systolic hypertension in the elderly.
Keywords: Cognition, northern manhattan study (NOMAS), race/ethnicity, systolic blood pressure
INTRODUCTION
Increasing evidence suggests that hypertension is a risk factor for cognitive impairment and dementia [1, 2]. Individuals with high blood pressure are associated with lower scores on Mini-Mental State Examination (MMSE), slowed processing speed, and impaired executive function [3-5]. High blood pressure in midlife is a strong predictor of poor cognitive performance in later life [3, 6-9]. Our study shows that blood pressure, among other metabolic syndrome factors, is a strong correlate of cognitive performance in the Northern Manhattan Study (NOMAS) cohort [10]. On the contrary, no association of hypertension with cognitive performance has also been reported [11-13].
In the elderly, isolated systolic hypertension is the most common form of hypertension [14]. It is the leading cause of cardiovascular mortality and morbidity in old adults [15-17]. The relationship between blood pressure and cognition in the elderly is particularly complex as older people accumulate other vascular risk factors of cognitive impairment in addition to hypertension and aging [1, 18, 19].
In racial/ethinic minorities, the disparities in hypertension prevalence, morbidity, and mortality have been reported [20]. Midlife systolic blood pressure (SBP), but not late-life, is associated with steeper cognitive decline as blood pressure increased in white individuals not in African American in a 20-year follow-up [21]. Another study shows mean SBP level in Black individuals is associated with significantly faster decline in global cognition and memory but slower declines in executive function [22]. Among Hispanics, the individuals with history of hypertension are reported to be associated with poorer executive function as compared to non-Hispanic whites [23]. Taken together, the relationship between blood pressure and cognition among multi-ethnic and racial groups remains to be investigated.
Recent SPRINT-MIND study reports that intensive blood pressure control significantly reduces the risk of mild cognitive impairment but not a risk of probable dementia [24]. A meta-analysis of 14 randomized clinical trials (96, 158 participants) shows lowing blood pressure with anti-hypertensive drugs is significantly associated with a low risk of both incident dementia or cognitive impairment [25]. Given that hypertension is a highly modifiable factor for various medical conditions, further research examining the relationship between blood pressure and cognitive function in racially/ethnically diverse elderly is warranted.
We hypothesized that there was a relationship between blood pressure and cognition in the racially/ethnically diverse elderly. We examined the associations between blood pressure and cognition, the latter organized by individual domains at baseline and follow-up visits in a racially and ethnically diverse population, using baseline and follow-up cognitive data.
MATERIALS AND METHODS
The northern Manhattan study (NOMAS)
NOMAS is an ongoing, prospective NIH-funded population-based cohort study [26]. The participants were eligible if never diagnosed with clinical stroke, ≥ 40 years age, and resident of Northern Manhattan ≥ 3 months in a household with a telephone. Initial visit data were collected between 1993 and 2001 by trained bilingual research assistants. There were a total of 3,298 participants enrolled. All participants underwent a baseline evaluation of demographic characteristics, health behaviors, and health status, including comprehensive medical history, physical/neurologic examination, medical record review, and fasting blood samples. Standardized questions were adapted from the Centers for Disease Control and Prevention Behavioral Risk Factor Surveillance System. From 2003 to 2008, 1,290 stroke-free participants, aged 50 years and older, who were eligible for MRI were enrolled in the MRI sub-study. For this study at baseline visit (MRI study), there were 1,215 participants who completed all of assessment required for the analyses. For the analyses at follow-up visit, there are 830 participants. The characteristics of the participants at baseline visit, the participants at follow-up visit, and excluded cases are reported in Table 1 and Supplementary Table 1. All participants signed informed consent and the institutional review boards of Columbia University and the University of Miami approved the study.
Table 1.
Characteristics of the NOMAS Cognition/MRI-Sub-study Sample (N = 1,215)
| Age (y) | 71 ± 9 |
| Education (y) | 10 ± 5 |
| Male (%) | 40 |
| White (%) | 15 |
| Hispanic (%) | 66 |
| Black (%) | 17 |
| other (%) | 2 |
| Hypertension (%) | 73 |
| SBP (mm Hg) | 136 ± 17 |
| DBP (mm Hg) | 78 ± 10 |
| PP (mm Hg) | 58 ± 15 |
| Diabetes (%) | 24 |
| Hyperlipidemia (%) | 39 |
| APOE 4 (%) | 25 |
| BMI (mean ± SD) | 28 ± 5 |
| cIMT (mean ± SD) | 0.92 ± 0.09 |
| WMHV (mean ± SD) | 0.68 ± 0.84 |
| MMSE (mean ± SD) | 27 ± 3 |
SBP, systolic blood pressure; DBP, diastolic blood pressure; PP, pulse pressure; BMI, body mass index; cIMT, carotid intima media thickness; WMH, white matter hyperintensity volume corrected by total cranial volume.
Blood pressure measurement
Blood pressure was measured twice using mercury sphygmomanometers when the participants were enrolled in the MRI sub-study. Blood pressure was usually measured the same day when participants had neuro-psychological testing and brain MRI scan. Averaged SBP and diastolic blood pressure (DBP) were used in this analysis.
Hypertension was defined as SBP ≥ 140; DBP ≥ 90 mm Hg; or use of anti-hypertensive medications as reported in the study questionnaire. Pulse pressure (PP) was obtained by subtracting DBP from SBP.
Carotid intima media thickness (cIMT)
Carotid ultrasound was performed according to the standard scanning and reading protocols by a trained and certified sinologist. Measurement of cIMT was performed outside the areas of carotid plaque. Excellent reliability in our measures of cIMT was reported previously [27]. cIMT was measured using an automated computerized edge tracking software (M’Ath, Intelligence in Medical Technologies, Inc, Paris, France), which improved precision and reduced variance of the measurements. The carotid artery segments were defined as follows: 1) near and far wall of the segment extending from 10 to 20 mm proximal to the tip of the flow divider into the common carotid artery; 2) near and far wall of the carotid bifurcation beginning at the tip of the flow divider and extending 10 mm proximal to the flow divider tip; and 3) near and far wall of the proximal 10 mm of the internal carotid artery. The composite IMT was calculated as the means of near and far wall IMT of all carotid segments.
Neuropsychological testing and cognitive domain scores
At the MRI sub-study enrollment visits, all participants who underwent the brain MRI also completed a neuropsychological (NP) battery in English or Spanish based on their preferred language. To determine cognitive domains, we explored interrelationships among individual NP test scores with factor analysis and used a Scree plot of eigenvalues to determine the number of constructs (domains). We computed composite scores for each of four domains by averaging individual z-scores transformed from raw test scores, as previously described: 1) episodic memory: sub-scores from a 12-word 5-trial list-learning task; 2) executive function: time to complete Color Trails, color trail 2 minus Color Trails 1 and the sum of Odd-Man-Out subtests 2 and 4; 3) processing speed/visual motor integration (VMI): non-dominant hand Grooved Pegboard times, Color Trails 1 time, and Visual-Motor Integration test scores (copy test of 6 figures, max points = 18); 4) semantic memory/word fluency: picture naming (modified Boston Naming), category fluency (Animal Naming), and phonemic fluency (letters C, F, L in English speakers and P, S, V in Spanish speakers) [28-30]. Participants were invited to be re-assessed with the same neuropsychological test battery 5 years after their original testing, provided they could still give informed consent and were well enough to sit for testing. A total of 989 subjects completed the follow-up NP testing at an average of 5 years (5.2 ± 0.4 years). A total of 830 subjects was included in the analyses as these subjects completed all of other measures.
To measure change in cognitive performance between the two NP testing sessions, we subtracted the z-scores of the second NP test scores from the first ones.
APOE genotyping
DNA samples were extracted from peripheral blood white cells using HhaI digestion and amplified by polymerase chain reaction, as previously described [31]. APOE ε4 carriers were categorized as individuals with a genotype of APOE ε4/ε2, APOE ε4/ε4, or APOE ε4/ε3.
Brain MRI measurements
Brain MRI scans were obtained from 2003 to 2008, usually on the same day as the neuropsychological testing, depending on scanner availability. We used a 1.5 T Philips Intera scanner (Philips, Best, the Netherlands) at Columbia University Medical Center. Images were transferred electronically to University of California, Davis for morpho-metric analysis of total cerebral volume and white matter hyperintensity volume (WMHV) using T1 and fluid- attenuated inversion recovery sequences. WMHV was calculated as the sum of voxels ≥ 3.5 SDs above the mean image intensity multiplied by pixel dimensions and section thickness, and adjusted for total cranial volume, as described previously [28, 32].
Statistical analyses
To examine the association between blood pressure and cognitive composite scores at time of first neuropsychological assessment and follow-up, we built a series of linear regression models adjusting for potential confounders. The continuous variables of SBP, DBP, and PP were used as independent variables for cognitive domains including episodic memory, word fluency/semantic memory, processing speed/VMI and executive function. For the cross-sectional study, model 1 was adjusted for age, sex, educational attainment, APOE status, and race/ethnicity with or without anti-hypertension medications in the model; model 2 was adjusted for other vascular risk factors including blood glucose levels, cholesterol levels, body mass index, and smoking status with or without anti-hypertension medications in the model; model 3 was adjusted for WMHV with or without anti-hypertension medications in the model; model 4 was adjusted for carotid intima-media thickness with or without anti-hypertension medications in the model. For the longitudinal study, we used SBP at the time of the first neuropsychological assessment as a predictor and cognitive change score after 5 years as an outcome. The follow-up duration and baseline cognitive scores were included in each model of longitudinal study with or without anti-hypertension medications in the models. All data analyses were performed using statistical software SAS version 9.3 (SAS institute, Cary, NC). The level of statistical significance was set at p < 0.05.
RESULTS
Demographic information on study participants
Table 1 describes the demographics of our sample at MRI (baseline) visit. There were 1,215 stroke-free participants in this study. The mean age of the participants was 71 ± 9 years old. SBP and DBP were 136 ± 17 mm Hg and 78 ± 10 mm Hg, respectively. At baseline, 40% of the participants were male, 73% of the participants had a diagnosis of hypertension, 24% of the participants had a diagnosis of diabetes, and 39% of the participants had a diagnosis of hyperlipidemia. Mean body mass index was 28 ± 5 and MMSE score was 27 ± 3.
Comparison of cognitive performance between individuals with hypertension and those without hypertension
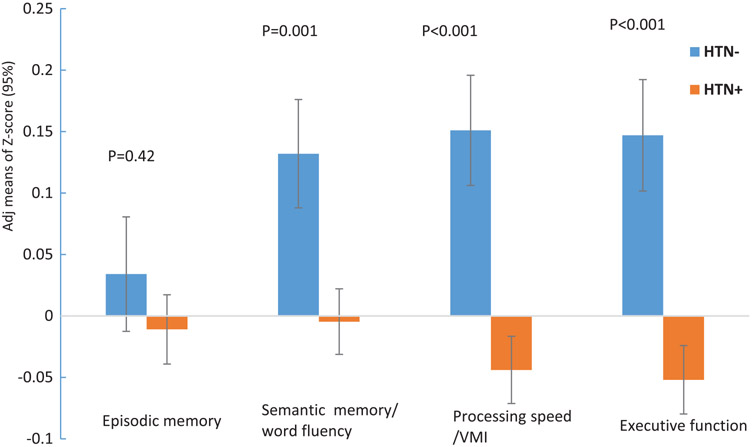
Figure 1 shows cognitive performance in participants with and without hypertension. The mean z-scores of cognitive performance were adjusted for age, education, sex, APOE genotype, and race-ethnicity. Those with hypertension had poorer cognitive performance in word fluency/semantic memory (p = 0.001), processing speed/VMI (p < 0.001), and executive function (p < 0.001) as compared to those without hypertension.
Fig. 1.
Cognitive performance in individuals with hypertension and those without hypertension. The Z-score means of cognitive performance were adjusted by age, education, sex, APOE genotype, and race-ethnicity.
Cross-sectional association of SBP with cognitive performance at baseline visit
Table 2 shows SBP and PP, but not DBP, were in versely associated with word fluency/semantic memory, processing speed/VMI, and executive function independent of age, education, sex, APOE status, and race-ethnicity in the participants without anti-hypertension meds. Further analyses of association of SBP with cognitive performance by domains were conducted in a series of multivariate linear regression models (Table 3). SBP was inversely associated with cognitive performance across all domains in an unadjusted model (data not shown). After adjusting for age, education, sex, APOE status, and race-ethnicity, SBP (per 10 mmHg) was associated with cognitive domains of word fluency/semantic memory, processing speed/VMI and executive function (β = −0.048, p = 0.0003; β = −0.046, p = 0.0007; and β = −0.042, p = 0.003), but not episodic memory (β = −0.006, p = 0.68) (Table 3). In model 2, SBP remained associated with word fluency/semantic memory, processing speed/VMI, and executive function after adjusting for vascular risk factors (β = −0.054, p < 0.0001; β = −0.040, p = 0.004; and β = −0.039, p = 0.006). SBP remained independently associated with word fluency, processing speed/VMI and executive function after adjusting for white matter hyperintensity volume in model 3; and after adjustment for carotid artery intimal medial thickness in model 4 (β = −0.044, p = 0.001; β = −0.034, p = 0.016 and β = −0.031, p = 0.034). In addition, we included insurance status as a social disadvantage confounding variable in the models to analyze the association of SBP with processing speed/VMI function. The significant association of SBP with processing speed/VMI function remained no change (data not shown).
Table 2.
Cross-sectional association of blood pressure with cognitive performance adjusted by demographic information (N = 1,215)
| Cognitive Domains (Beta 95% CI, per 10 mm Hg) | ||||
|---|---|---|---|---|
| Episodic memory | Word fluency/Semantic memory | Processing speed/VMI | Executive function | |
| Blood pressure parameters | β | β | β | β |
| SBP | 0.0058 (−0.0221; 0.0336) | −0.0483 (−0.0746; −0.0220)*** | −0.0464 (−0.0733; −0.0195)*** | −0.0417 (−0.0691; −0.0141)** |
| DBP | 0.0283 (−0.0213; −0.0779) | −0.0454 (−0.0926; 0.0018) | −0.0141 (−0.0626; 0.0343) | 0.0119 (−0.0377; 0.0615) |
| PP | −0.0044 (−0.0375; 0.0287) | −0.0480 (−0.0793; −0.0168) ** | −0.0592 (−0.0911; −0.0273)*** | −0.0638 (−0.0963; −0.0312)*** |
p≤0.05
p≤0.01
p≤0.001
SBP, systolic blood pressure, DBP, diastolic blood pressure; PP, pulse pressure. The models are adjusted for age, education, sex, race-ethnicity, and APOE genotype.
Table 3.
Cross-sectional association of systolic blood pressure with cognition at MRI visit (N = 1215)
| Cognitive domains (Beta 95% CI, per 10 mmHg SBP) | ||||
|---|---|---|---|---|
| Episodic memory | Processing speed/VMI | |||
| Hypertension meds | − | + | − | + |
| Model 1 | −0.0058 (−0.0220; 0.0336) | 0.0075 (−0.0211; 0.0360) | −0.0464 (−0.0732; −0.0195)*** | −0.0362 (−0.0636; −0.0088) ** |
| Model 2 | −0.0003 (−0.0285; 0.0290) | 0.0013 (−0.0281; 0.0307) | −0.0400 (−0.0673; −0.0126)** | −0.0313 (−0.0592; −0.0034)* |
| Model 3 | 0.0038 (−0.0247; 0.0324) | 0.0040 (−0.0252; 0.0033) | −0.0370 (−0.0639; −0.0095)** | −0.0289 (−0.0566; −0.0011)* |
| Model 4 | 0.0158 (−0.0132; 0.0450) | 0.0159 (−0.0140; 0.0458) | −0.0341 (−0.0620; −0.0063)* | −0.0265 (−0.0549; 0.0020) |
| Word fluency/semantic memory | Executive function | |||
| Hypertension meds | − | + | − | + |
| Model 1 | −0.0483 (−0.0746; −0.0220)*** | −0.0422 (−0.0690; −0.0154)** | −0.0417 (−0.0692; −0.0141)*** | −0.0351 (−0.0633; −0.0070)** |
| Model 2 | −0.0540 (−0.0809; −0.0272)*** | −0.0477 (−0.0752; −0.0201)*** | −0.0393 (−0.0675; −0.0111)** | −0.0363 (−0.0652; −0.0073)** |
| Model 3 | −0.0518 (−0.0786;. −0.0250)*** | −0.0460 (−0.0734; −0.0185)*** | −0.0380 (−0.0660; −0.0098)** | −0.0352 (−0.0642; −0.0062)* |
| Model 4 | −0.0442 (−0.0716; −0.0169)** | −0.0385 (−0.0666; −0.0023)** | −0.0313 (−0.0603; −0.0023)* | −0.0286 (−0.0583; 0.0012) |
p≤0.05
p≤0.01
p≤0.001.
Model 1: adjusted for age, education, sex, race-ethnicity, APOE genotype with/without anti-hypertension medications. Model 2: model 2 additionally adjusted for BMI, smoking status, blood glucose, and cholesterol levels with/without anti-hypertension medications. Model 3: model 3 additionally adjusted for white matter hyperintensity volume (WMHV) corrected by total cranial volume, with/without anti-hypertension medications. Model 4: model 4 additionally adjusted for total carotid artery intima media thickness (cIMT), with/without anti-hypertension medications.
Association of SBP with processing speed/VMI, and executive function was attenuated but remained significant in the presence of anti-hypertension medications status in the models (Table 3).
Longitudinal association of SBP with cognitive performance
Table 4 provides a longitudinal association between SBP at MRI visit (baseline) and cognitive change scores at a 5-year follow-up using multivariate linear regression models. There was no longitudinal association of SBP with episodic memory, word fluency/semantic memory, and executive function. The longitudinal association of SBP with processing speed/VMI function was found after adjusting demographic information and vascular risk factors (β = −0.026, p = 0.046) (model 2). The association of SBP with processing speed/VMI function remained significant after adjusting WMHV in model 3 (β = −0.026, p = 0.050) and carotid artery intima media thickness in model 4 (β = −0.029, p = 0.032). In addition, insurance status as a social disadvantage cofounder was included in the models. Association of SBP with processing speed/VMI remained significant (data not shown).
Table 4.
Longitudinal association of systolic blood pressure with cognition change at a 5-year follow-up (N = 830)
| Cognitive domains (Beta 95% CI, per 10 mmHg SBP) | ||||
|---|---|---|---|---|
| Episodic memory | Processing speed/VMI | |||
| Hypertension meds | − | + | − | + |
| Model 1 | −0.0134 (−0.0443, 0.0175) | −0.0129 (−0.0446; 0.0188) | −0.0243 (−0.0495, 0.0008) | −0.0200 (−0.0457; 0.0057) |
| Model 2 | −0.0222 (−0.0540, 0.0097) | −0.0206 (−0.0532; 0.0121) | −0.0262 (−0.0520, −0.0006)* | −0.0219 (−0.0481; 0.0044) |
| Model 3 | −0.0200 (−0.0517, 0.0117) | −0.0190 (−0.0514; 0.0135) | −0.0258 (−0.0515, −0.00004)* | −0.0215 (−0.0477; 0.0047) |
| Model 4 | −0.0193 (−0.0513, 0.0127) | −0.0184 (−0.0512; 0.0143) | −0.0290 (−0.0555, −0.0025)* | −0.0239 (−0.0509; 0.0031) |
| Word fluency/semantic memory | Executive function | |||
| Hypertension meds | − | + | − | + |
| Model 1 | −0.0113 (−0.0362, 0.0137) | −0.0131 (−0.0386; 0.0124) | 0.0142 (−0.0162, 0.0447) | 0.0156 (−0.0156; 0.0468) |
| Model 2 | −0.0150 (−0.0408, 0.0108) | −0.0156 (−0.0419; 0.0107) | 0.0189 (−0.0123, 0.0501) | 0.0197 (−0.0122; 0.0517) |
| Model 3 | −0.0138 (−0.0395, 0.0119) | −0.0148 (−0.0410; 0.0114) | 0.0195 (−0.0118, 0.0507) | 0.0202 (−0.0118; 0.0522) |
| Model 4 | −0.0149 (−0.0414, 0.0116) | −0.0161 (−0.0431; 0.0109) | 0.0183 (−0.0136, 0.0502) | 0.0170 (−0.0158; 0.0497) |
p≤0.05
p≤0.01
p<0.001.
Model 1: adjusted for age, education, sex, race-ethnicity, APOE genotype with/without anti-hypertension medications. Model 2: model 2 additionally adjusted for BMI, smoking status, blood glucose, and cholesterol levels with/without anti-hypertension medications. Model 3: model 3 additionally adjusted for white matter hyperintensity volume (WMHV) corrected by total cranial volume, with/without anti-hypertension medications. Model 4: model 4 additionally adjusted for total carotid artery intima media thickness (cIMT), with/without anti-hypertension medications.
In addition, we analyzed the association of SBP with cognitive change in the presence of anti-hypertension medications status in these models. There was no association of SBP with processing speed/VMI function in the presence of anti-hypertension medications status in the models (Table 4).
Longitudinal decline of processing speed/VMI in the participants with different category SBP
In order to characterize the longitudinal decline of processing speed/VMI function in association with category SBP, the participants aged ≥ 60 years and MMSE score >24 were classified into 4 groups (SBP < 120 mmHg, n = 98; SBP 120–129 mmHg, n = 176; SBP 130–139 mmHg, n = 207; and SBP ≥ 140 mmHg, n = 317). Figure 2 shows mean z-scores of processing speed/VMI function is linearly declined as SBP increases from < 120 mmHg to ≥ 140 mmHg, independent from age, education, sex, APOE status, and race-ethnicity (p for trend = 0.02).
Fig. 2.
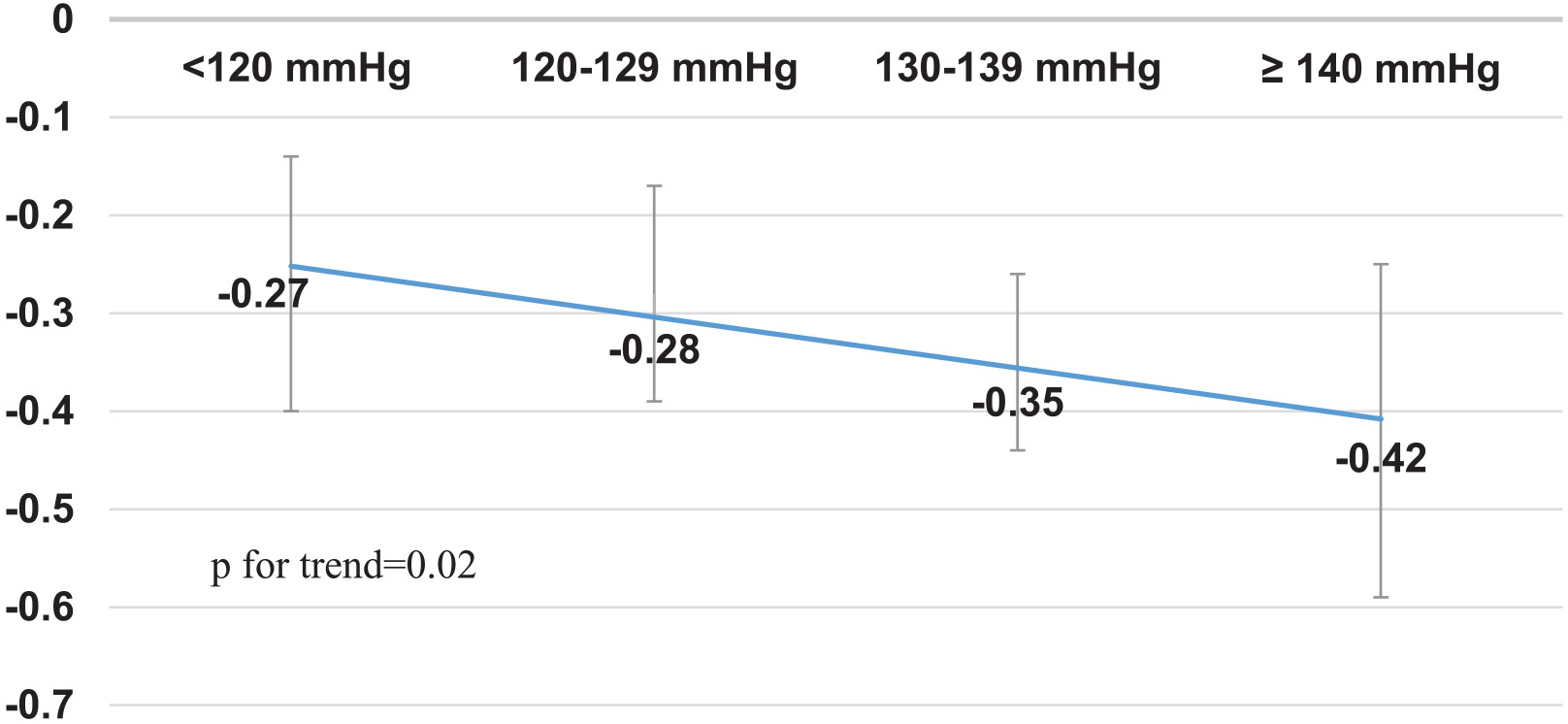
Longitudinal decline of processing speed/VMI in the participants with category SBP at a 5-year follow-up (95% CI). The mean change of z-score of processing speed/VMI function was adjusted for age, education, sex, APOE genotype and race-ethnicity in SBP category participants. The participants were ≥60 years old and MMSE >24. SBP <120 mmHg, n = 98; SBP 120–129 mmHg, n = 176; SBP 130–139 mmHg, n = 207; and SBP ≥140 mmHg, n = 317.
DISCUSSION
In this multi-ethnic and stroke-free elderly population, SBP but not DBP is inversely associated with cognitive performance, including word fluency/semantic memory, processing speed/VMI, and executive function cross-sectionally. High SBP at baseline is associated with the decline of processing speed/VMI function after adjusting vascular risk factors at a 5-year follow-up in this cohort.
In the NOMAS, we previously examined the relationship between metabolic syndrome and cognition cross-sectionally. Among the vascular risk factors included in the metabolic syndrome, blood pressure was the strongest correlate with cognitive performance after adjusting demographics and other vascular risk factors [10]. In this study, we have extended the previous findings by showing a linear negative association between SBP and cognitive domains cross-sectionally and between SPB and processing speed/VMI longitudinally in this elderly population.
The finding that SBP rather than DBP affects cognitive performance in this cohort is supported by several studies. As SBP progressively increases and DBP decreases after age 55 years, isolated systolic hypertension is the most common form of hypertension in elderly [14]. SBP is an important risk predictor for cardiovascular disease, stroke and end-stage renal disease in the elderly [15-17]. Recently, a large population study (512,891 adults, mean age at 64 years) shows that SBP is continuously related to major vascular disease with no evidence of a threshold down to 120mmHg at a 9- year follow-up [33]. Taken together, it is suggested that SBP plays an important role in modulating heart, brain, and renal function in the elderly. Regarding the role of SBP in cognition, the Honolulu-Asia Aging Study reports the male subjects with midlife elevated SBP predict a future decline in cognitive function at later life [7]. A longitudinal population study shows that the subjects > 65 years with SBP ≥ 160 mmHg or SBP < 130 mmHg made more errors in a cognitive screening test, demonstrating a U-shape association between SBP and cognition [34]. Further, it has also been shown that cognitive decline is apparent among older (80 years) individuals with higher SBP [35]. Our findings are consistent with these findings [7, 34, 35]. Additionally, we have shown a negative linear association of SBP and cognition in multi-ethnic elderly population. We have also demonstrated that examining the relationship between SBP and cognitive domains is a more sensitive measure than that of global cognition in studying the relationship between blood pressure and cognition in elderly.
Regarding the relationship between SBP and cognitive domains, we showed a significant association of SBP with processing speed/VMI function both cross-sectionally and longitudinally. Interestingly, the longitudinal association of SBP with processing speed/VMI was found after adjusting vascular risk factors in the models. It is suggested that a combination of SBP with other vascular risk factors could accelerate cognitive decline in later life although we did not observe interaction between vascular risk factors and SBP. The speed of information processing and VMI function are reported to be sensitive to aging [36-38]. The observed longitudinal association of SBP with processing speed/VMI function may also represent aging related cognitive change in the presence of vascular risk factors in the elderly. Neuropsychologically, a measure of process speed/VMI function can be a cognitive biomarker to detect early cognitive change in the individuals with hypertension. In this study, we were not able to detect the association of SBP with memory function. It is possible that the memory test we used was not sensitive enough to detect vascular pathology-related subtle memory change in this stroke free population.
In this cohort, the mean years of education are 10 ± 5 years. This population represents a racially and ethnically diverse community with low education. Previous studies have reported that individuals with less education show lower level of cognitive function and have increased risk for dementia [39]. As individuals with low education have less cognitive reserve and are prone to the effects of vascular damage [40]. It will be important to investigate the incidence of dementia in relation to vascular risk factors in this cohort in the future.
In this study, we also found that anti-hypertension therapy attenuated the association of SBP with executive function and processing speed/VMI function cross-sectionally and eliminated the association of SBP with processing speed/VMI function longitudinally. It is suggested that lowing blood pressure therapy in the elderly has beneficial effect in cognitive function. Our result is consistent with previous studies that anti-hypertension therapy reduced the risk of cognitive impairment and dementia [24, 25, 41, 42].
Although this study shows a linear negative association of SBP (continuous variables) with longitudinal decline in processing speed/VMI function, the clinical implication of this association is not clear. We further analyzed this longitudinal relationship between category SBP and processing speed/VMI. We showed that processing speed/VMI function was linearly declined in the elderly with SBP from > 120 mmHg to SBP ≥ 140 mmHg, especially in the elderly with SBP ≥ 130 (p for trend = 0.02). This finding supports national blood pressure target for management of hypertension [43]. We provide the evidence that treatment of stage 1 systolic hypertension in the elderly favors cognitive function.
In this study, we did not find a difference in association of SBP with cognition among racial/ethnic groups although disparities in prevalence, management and outcomes of hypertension are reported in racial/ethnic groups [43]. Previous studies showed that compared with white individuals, mean SBP level in black individuals was associated with significantly faster decline in global cognition and memory but slower declines in executive function [22]. The discrepancy between our and their studies is likely due to different sample size and follow-up duration. Our study is consistent with the findings from a large Hispanic Community Health Study which shows SBP is negatively associated with cognitive function [44]. We have extended their study by showing a longitudinal association of SBP with processing speed in Hispanic population. There was no difference in association of SBP with cognition between white individuals and Hispanics in this study. Although the rate of elevated SBP is higher in Hispanics and black individuals as compared to white individuals in this population, the effects of SBP on cognition appear to be the same. The findings raise the question if there is different cognitive resilience to elevated SBP among different ethnic groups.
Finally, we explored the mechanism involved in the association of SBP with cognition. The association of SBP with cognition is independent of vascular risk factors, small and large vessel disease markers (WMHV and cIMT) in the models. Therefore, the mechanism underlying the association of SBP with cognition warrants further investigation.
Limitations of this study include possible unmeasured confounding variables that are not included in the analyses. This cohort is population-based, but is limited by eligibility for MRI and therefore may be less generalizable to the Northern Manhattan community than the original random sample. In addition, the subjects of this population have low education level (10 ± 5 years). It is suggested that the subjects might be susceptible to aging and pathology insults due to poor cognitive resilience. This cohort does not necessarily mirror a general community sample. Also, the relatively short follow-up interval to assess cognitive change may explain why the observed cognitive decline was limited to processing speed/VMI in this cohort. Longer than 5-year follow-up of cognitive change might show more robust cognitive changes. Additionally, it is possible that attrition resulting in a smaller sample size may contribute to the lack of observed association between SBP and other cognitive domains. Given the fact that some of the participants with older age and poorer education (Supplementary Table 1) were lost for the follow-up study, the bias could further lead to dilution of an association between SBP and cognition.
In summary, our study shows that SBP is negatively associated with processing speed/VMI cross-sectionally and longitudinally. Anti-hypertension therapy in the elderly could attenuate or eliminate the negative association of SBP with cognition. Our findings support the treatment of stage 1 systolic hypertension in elderly for reducing the risk of cognitive impairment. Given the fact that processing speed /VMI function may be the first change of cognitive decline in the elderly, monitoring processing speed/VMI function might be a sensitive marker to assess cognitive decline in the elderly in association with systolic hypertension.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the National Institute of Neurological Disorders and Stroke (R37NS29993 to R.L.S./MSVE) and the Evelyn F. McKnight Brain Institute. The authors thank the participants and their families for this study. The authors thank Dr. Carolina Marinovic Gutierrez for coordinating NOMAS project and facilitating academic discussion of this study.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/21-0252r1).
Footnotes
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-210252.
REFERENCES
- [1].Iadecola C, Yaffe K, Biller J, Bratzke LC, Faraci FM, Gorelick PB, Gulati M, Kamel H, Knopman DS, Launer LJ, Saczynski JS, Seshadri S, Zeki Al Hazzouri A, American Heart Association Council on Hypertension; Council on Clinical Cardiology; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Stroke Council (2016) Impact of hypertension on cognitive function: A scientific statement from the American Heart Association. Hypertension 68, e67–e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Qiu C, Winblad B, Fratiglioni L (2005) The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol 4, 487–499. [DOI] [PubMed] [Google Scholar]
- [3].Kilander L, Nyman H, Boberg M, Hansson L, Lithell H (1998) Hypertension is related to cognitive impairment: A 20-year follow-up of 999 men. Hypertension 31, 780–786. [DOI] [PubMed] [Google Scholar]
- [4].Kuo HK, Sorond F, Iloputaife I, Gagnon M, Milberg W, Lipsitz LA (2004) Effect of blood pressure on cognitive functions in elderly persons. J Gerontol A Biol Sci Med Sci 59, 1191–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Obisesan TO, Obisesan OA, Martins S, Alamgir L, Bond V, Maxwell C, Gillum RF (2008) High blood pressure, hypertension, and high pulse pressure are associated with poorer cognitive function in persons aged 60 and older: The Third National Health and Nutrition Examination Survey. J Am Geriatr Soc 56, 501–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Elias MF, Wolf PA, D’Agostino RB, Cobb J, White LR (1993) Untreated blood pressure level is inversely related to cognitive functioning: The Framingham Study. Am J Epidemiol 138, 353–364. [DOI] [PubMed] [Google Scholar]
- [7].Launer LJ, Masaki K, Petrovitch H, Foley D, Havlik RJ (1995) The association between midlife blood pressure levels and late-life cognitive function. The Honolulu-Asia Aging Study. JAMA 274, 1846–1851. [PubMed] [Google Scholar]
- [8].Swan GE, Carmelli D, Larue A (1998) Systolic blood pressure tracking over 25 to 30 years and cognitive performance in older adults. Stroke 29, 2334–2340. [DOI] [PubMed] [Google Scholar]
- [9].Yaffe K, Vittinghoff E, Pletcher MJ, Hoang TD, Launer LJ, Whitmer R, Coker LH, Sidney S (2014) Early adult to midlife cardiovascular risk factors and cognitive function. Circulation 129, 1560–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Levin BE, Llabre MM, Dong C, Elkind MS, Stern Y, Rundek T, Sacco RL, Wright CB (2014) Modeling metabolic syndrome and its association with cognition: The Northern Manhattan study. J Int Neuropsychol Soc 20, 951–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Farmer ME, White LR, Abbott RD, Kittner SJ, Kaplan E, Wolz MM, Brody JA, Wolf PA (1987) Blood pressure and cognitive performance. The Framingham Study. Am J Epidemiol 126, 1103–1114. [DOI] [PubMed] [Google Scholar]
- [12].Scherr PA, Hebert LE, Smith LA, Evans DA (1991) Relation of blood pressure to cognitive function in the elderly. Am J Epidemiol 134, 1303–1315. [DOI] [PubMed] [Google Scholar]
- [13].van Boxtel MP, Gaillard C, Houx PJ, Buntinx F, de Leeuw PW, Jolles J (1997) Can the blood pressure predict cognitive task performance in a healthy population sample? J Hypertens 15, 1069–1076. [DOI] [PubMed] [Google Scholar]
- [14].Franklin SS, Jacobs MJ, Wong ND, L’Italien GJ, Lapuerta P (2001) Predominance of isolated systolic hypertension among middle-aged and elderly US hypertensives: Analysis based on National Health and Nutrition Examination Survey (NHANES) III. Hypertension 37, 869–874. [DOI] [PubMed] [Google Scholar]
- [15].Dong C, Della-Morte D, Rundek T, Wright CB, Elkind MS, Sacco RL (2016) Evidence to Maintain the Systolic Blood Pressure Treatment Threshold at 140 mm Hg for Stroke Prevention: The Northern Manhattan Study. Hypertension 67, 520–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Collins R, Peto R, MacMahon S, Hebert P, Fiebach NH, Eberlein KA, Godwin J, Qizilbash N, Taylor JO, Hennekens CH (1990) Blood pressure, stroke, and coronary heart disease. Part 2, Short-term reductions in blood pressure: Overview of randomised drug trials in their epidemiological context. Lancet 335, 827–838. [DOI] [PubMed] [Google Scholar]
- [17].Hsu CY, McCulloch CE, Darbinian J, Go AS, Iribarren C (2005) Elevated blood pressure and risk of end-stage renal disease in subjects without baseline kidney disease. Arch Intern Med 165, 923–928. [DOI] [PubMed] [Google Scholar]
- [18].Guo Z, Fratiglioni L, Winblad B, Viitanen M (1997) Blood pressure and performance on the Mini-Mental State Examination in the very old. Cross-sectional and longitudinal data from the Kungsholmen Project. Am J Epidemiol 145, 1106–1113. [DOI] [PubMed] [Google Scholar]
- [19].Tadic M, Cuspidi C, Hering D (2016) Hypertension and cognitive dysfunction in elderly: Blood pressure management for this global burden. BMC Cardiovasc Disord 16, 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Deere BP, Ferdinand KC (2020) Hypertension and race/ethnicity. Curr Opin Cardiol 35, 342–350. [DOI] [PubMed] [Google Scholar]
- [21].Gottesman RF, Schneider AL, Albert M, Alonso A, Bandeen-Roche K, Coker L, Coresh J, Knopman D, Power MC, Rawlings A, Sharrett AR, Wruck LM, Mosley TH (2014) Midlife hypertension and 20-year cognitive change: The atherosclerosis risk in communities neurocognitive study. JAMA Neurol 71, 1218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Levine DA, Gross AL, Briceno EM, Tilton N, Kabeto MU, Hingtgen SM, Giordani BJ, Sussman JB, Hayward RA, Burke JF, Elkind MSV, Manly JJ, Moran AE, Kulick ER, Gottesman RF, Walker KA, Yano Y, Gaskin DJ, Sidney S,Yaffe K, Sacco RL, Wright CB, Roger VL, Allen NB, Galecki AT (2020) Association between blood pressure and later-life cognition among black and white individuals. JAMA Neurol 77, 810–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Stickel A, McKinnon A, Ruiz J, Grilli MD, Ryan L, Alzheimer’s Disease Neuroimaging Initiative (2019) The impact of cardiovascular risk factors on cognition in Hispanics and non-Hispanic whites. Learn Mem 26, 235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Group SMIftSR, Williamson JD, Pajewski NM, Auchus AP, Bryan RN, Chelune G, Cheung AK, Cleveland ML, Coker LH, Crowe MG, Cushman WC, Cutler JA, Davatzikos C, Desiderio L, Erus G, Fine LJ, Gaussoin SA, Harris D, Hsieh MK, Johnson KC, Kimmel PL, Tamura MK, Launer LJ, Lerner AJ, Lewis CE, Martindale-Adams J, Moy CS, Nasrallah IM, Nichols LO, Oparil S, Ogrocki PK, Rahman M, Rapp SR, Reboussin DM, Rocco MV, Sachs BC, Sink KM, Still CH, Supiano MA, Snyder JK, Wadley VG, Walker J, Weiner DE, Whelton PK, Wilson VM, Woolard N, Wright JT Jr., Wright CB (2019) Effect of intensive vs standard blood pressure control on probable dementia: A randomized clinical trial. JAMA 321, 553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hughes D, Judge C, Murphy R, Loughlin E, Costello M,Whiteley W, Bosch J, O’Donnell MJ, Canavan M (2020) Association of blood pressure lowering with incident dementia or cognitive impairment: A systematic review and meta-analysis. JAMA 323, 1934–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sacco RL, Boden-Albala B, Gan R, Chen X, Kargman DE, Shea S, Paik MC, Hauser WA (1998) Stroke incidence among white, black, and Hispanic residents of an urban community: The Northern Manhattan Stroke Study. Am J Epidemiol 147, 259–268. [DOI] [PubMed] [Google Scholar]
- [27].Rundek T, Elkind MS, Pittman J, Boden-Albala B, Martin S, Humphries SE, Juo SH, Sacco RL (2002) Carotid intima-media thickness is associated with allelic variants of stromelysin-1, interleukin-6, and hepatic lipase genes: The Northern Manhattan Prospective Cohort Study. Stroke 33, 1420–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Dong C, Nabizadeh N, Caunca M, Cheung YK, Rundek T,Elkind MS, DeCarli C, Sacco RL, Stern Y, Wright CB (2015) Cognitive correlates of white matter lesion load and brain atrophy: The Northern Manhattan Study. Neurology 85, 441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Siedlecki KL, Rundek T, Elkind MS, Sacco RL, Stern Y, Wright CB (2012) Using contextual analyses to examinethe meaning of neuropsychological variables across samples of english-speaking and spanish-speaking older adults. J Int Neuropsychol Soc 18, 223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Siedlecki KL, Stern Y, Reuben A, Sacco RL, Elkind MS, Wright CB (2009) Construct validity of cognitive reserve in a multiethnic cohort: The Northern Manhattan Study. J Int Neuropsychol Soc 15, 558–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hixson JE, Vernier DT (1990) Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res 31, 545–548. [PubMed] [Google Scholar]
- [32].Wright CB, Paik MC, Brown TR, Stabler SP, Allen RH, Sacco RL, DeCarli C (2005) Total homocysteine is associated with white matter hyperintensity volume: The Northern Manhattan Study. Stroke 36, 1207–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lacey B, Lewington S, Clarke R, Kong XL, Chen Y, Guo Y, Yang L, Bennett D, Bragg F, Bian Z, Wang S, Zhang H, Chen J, Walters RG, Collins R, Peto R, Li L, Chen Z, China Kadoorie Biobank collaborative group (2018) Age-specific association between blood pressure and vascular and non-vascular chronic diseases in 0.5 million adults in China: A prospective cohort study. Lancet Glob Health 6, e641–e649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Glynn RJ, Beckett LA, Hebert LE, Morris MC, Scherr PA, Evans DA (1999) Current and remote blood pressure and cognitive decline. JAMA 281, 438–445. [DOI] [PubMed] [Google Scholar]
- [35].Waldstein SR, Giggey PP, Thayer JF, Zonderman AB (2005) Nonlinear relations of blood pressure to cognitive function: The Baltimore Longitudinal Study of Aging. Hypertension 45, 374–379. [DOI] [PubMed] [Google Scholar]
- [36].Eckert MA, Keren NI, Roberts DR, Calhoun VD, Harris KC (2010) Age-related changes in processing speed: Unique contributions of cerebellar and prefrontal cortex. Front Hum Neurosci 4, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kim E, Park YK, Byun YH, Park MS, Kim H (2014) Influence of aging on visual perception and visual motor integration in Korean adults. J Exerc Rehabil 10, 245–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Salthouse TA, Ferrer-Caja E (2003) What needs to be explained to account for age-related effects on multiple cognitive variables? Psychol Aging 18, 91–110. [DOI] [PubMed] [Google Scholar]
- [39].Lovden M, Fratiglioni L, Glymour MM, Lindenberger U, Tucker-Drob EM (2020) Education and cognitive functioning across the life span. Psychol Sci Public Interest 21, 6–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Howard G, Cushman M, Moy CS, Oparil S, Muntner P, Lackland DT, Manly JJ, Flaherty ML, Judd SE, Wadley VG, Long DL, Howard VJ (2018) Association of clinical and social factors with excess hypertension risk in black compared with white US adults. JAMA 320, 1338–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Tzourio C, Anderson C, Chapman N, Woodward M, Neal B, MacMahon S, Chalmers J, PROGRESS Collaborative Group (2003) Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch Intern Med 163, 1069–1075. [DOI] [PubMed] [Google Scholar]
- [42].Forette F, Seux ML, Staessen JA, Thijs L, Babarskiene MR, Babeanu S, Bossini A, Fagard R, Gil-Extremera B, Laks T, Kobalava Z, Sarti C, Tuomilehto J, Vanhanen H, Webster J, Yodfat Y, Birkenhager WH, Systolic Hypertension in Europe Investigators (2002) The prevention of dementia with antihypertensive treatment: New evidence from the Systolic Hypertension in Europe (Syst-Eur) study. Arch Intern Med 162, 2046–2052. [DOI] [PubMed] [Google Scholar]
- [43].Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr., Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr., Williamson JD, Wright JT Jr. (2018) 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 71, 1269–1324. [DOI] [PubMed] [Google Scholar]
- [44].Tarraf W, Rodriguez CJ, Daviglus ML, Lamar M, Schneiderman N, Gallo L, Talavera GA, Kaplan RC, Fornage M, Conceicao A, Gonzalez HM (2017) Blood pressure and Hispanic/Latino cognitive function: Hispanic community health study/study of Latinos results. J Alzheimers Dis 59, 31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.