Abstract
Background:
Congenital muscular torticollis (CMT) is a postural deformity evident shortly after birth, typically characterized by lateral flexion/side bending of the head to one side and cervical rotation/head turning to the opposite side due to unilateral shortening of the sternocleidomastoid muscle (SCM); it may be accompanied by other neurological or musculoskeletal conditions. Infants with CMT should be referred to physical therapists to treat these postural asymmetries as soon as they are identified.
Purpose:
This update of the 2013 CMT clinical practice guideline (CPG) informs clinicians and families as to whom to monitor, treat, and/or refer, and when and what to treat. It links 17 action statements with explicit levels of critically appraised evidence and expert opinion with recommendations on implementation of the CMT CPG into practice.
Results/Conclusions:
The CPG addresses: education for prevention; referral; screening; examination and evaluation; prognosis; 1st choice and supplemental interventions; consultation; discontinuation from direct intervention; reassessment and discharge; implementation and compliance audits; and research recommendations. Flow sheets for referral paths and classification of CMT severity have been updated.
DOCUMENT ORGANIZATION
This 2018 Congenital Muscular Torticollis Clinical Practice Guideline (2018 CMT CPG) is an update of the 2013 Congenital Muscular Torticollis Clinical Practice Guideline (2013 CMT CPG).1 It is intended as a reference document to guide physical therapists (PTs), families, health care professionals, and educators to improve clinical outcomes and health services for children with congenital muscular torticollis (CMT), and to inform future research. Accepted international methods of evidence-based practice were used to systematically search for peer-reviewed literature, assign levels of evidence (Table 1), summarize the literature, formulate action statements, and assign grades for each action statement (Table 2).
Table 1:
Levels of Evidence
| Level | Criteria |
|---|---|
| I | Evidence obtained from high quality diagnostic studies, prognostic or prospective studies, cohort studies or randomized controlled trials, meta analyses or systematic reviews (Critical appraisal score greater than 50% of criteria.) |
| II | Evidence obtained from lesser-quality diagnostic studies, prognostic or prospective studies, cohort studies or randomized controlled trials, meta analyses or systematic reviews (e.g. weaker diagnostic criteria and reference standards, improper randomization, no blinding, <80% follow-up)(Critical appraisal score less than 50% of criteria.) |
| III | Case controlled studies or retrospective studies |
| IV | Case studies and case series |
| V | Expert opinion |
Table 2:
Recommendation Grades for Action Statements
| Grade | Recommendation | Quality of Evidence |
|---|---|---|
| A | Strong | A preponderance of level I studies, but at least one level I study directly on the topic support the recommendation. |
| B | Moderate | A preponderance of level II studies but at least one level II study directly on topic support the recommendation. |
| C | Weak | A single level II study at less than 25% critical appraisal score or a preponderance of level III and IV studies, including consensus statements by content experts support the recommendation. |
| D | Theoretical/Foundational | A preponderance of evidence from animal or cadaver studies, from conceptual/theoretical models/principles, or from basic science/bench research, or published expert opinion in peer-reviewed journals supports the recommendation. |
| P | Best Practice | Recommended practice based on current clinical practice norms, exceptional situations where validating studies have not or cannot be performed and there is a clear benefit, harm or cost, and/or the clinical experience of the guideline development group. |
| R | Research | There is an absence of research on the topic, or higher-quality studies conducted on the topic disagree with respect to their conclusions. The recommendation is based on these conflicting or absent studies. |
Table 3 (SDC 3) summarizes the 17 action statements with their 2018 status. They are organized under 4 major headings: Education, Identification and Referral of Infants with Asymmetries/CMT; Physical Therapy Examination and Evaluation of Infants with Asymmetries/CMT; Physical Therapy Intervention for Infants with CMT; and Physical Therapy Discontinuation, Reassessment, and Discharge of Infants with CMT. Following the summary (Table 3), descriptions of the CPG purpose, scope and methods are followed by an action statement with a standardized profile of information based on the Institute of Medicine’s criteria for transparent clinical practice guidelines (http://nationalacademies.org/hmd/reports/2011/clinical-practice-guidelines-we-can-trust.aspx). Research recommendations are placed within the text where the topics arise and are collated at the end of the document. Evidence tables on measurement, the first-choice intervention, supplemental interventions, and long-term follow-up are available online at https://pediatricapta.org/clinical-practice-guidelines/.
Table 3:
SUMMARY AND STATUS OF ACTION STATEMENTS FOR THE 2018 CONGENITAL MUSCULAR TORTICOLLIS CLINICAL PRACTICE GUIDELINE
| Action Statement | Status | Page |
|---|---|---|
| EDUCATION, IDENTIFICATION AND REFERRAL OF INFANTS WITH CONGENITAL MUSCULAR TORTICOLLIS (CMT) | ||
| P. Action Statement 1: EDUCATE EXPECTANT PARENTS AND PARENTS OF NEWBORNS TO PREVENT ASYMMETRIES/CMT. Physicians, nurse midwives, prenatal educators, obstetrical nurses, lactation specialists, nurse practitioners or physical therapists should educate and document instruction to all expectant parents and parents of newborns, within the first 2 days of birth, on the importance supervised prone/tummy play when awake 3 or more times daily, full active movement throughout the body, prevention of postural preferences, and the role of pediatric physical therapists in the comprehensive management of postural preference and optimizing motor development. (Evidence quality: V; Recommendation strength: Best Practice) | New. | TBA |
| A. Action Statement 2: ASSESS NEWBORN INFANTS FOR ASYMMETRIES/CMT. Physicians, nurse midwives, obstetrical nurses, nurse practitioners, lactation specialists, physical therapists or any clinician or family member must assess and document the presence of neck and/or facial or cranial asymmetry within the first 2 days of birth, using passive cervical rotation and/or visual observation as their respective training supports, when in the newborn nursery or at site of delivery. (Evidence Quality: I, Recommendation Strength: Strong) | Revised and updated. | TBA |
| B. Action Statement 3: REFER INFANTS WITH ASYMMETRIES/CMT TO PHYSICIAN AND PHYSICAL THERAPIST. Physicians, nurse midwives, obstetrical nurses, nurse practitioners, lactation specialists, physical therapists or any clinician or family member should refer infants identified as having postural preference, reduced cervical range of motion, sternocleidomastoid masses, and/or craniofacial asymmetry to their primary physician and a physical therapist with expertise in infants as soon as the asymmetry is noted. (Evidence Quality: II, Recommendation Strength: Moderate) | Reaffirmed and updated. | TBA |
| PHYSICAL THERAPY EXAMINATION AND EVALUATION OF INFANTS WITH ASYMMETRIES/CMT | ||
| B. Action Statement 4: DOCUMENT INFANT HISTORY. Physical therapists should obtain and document a general medical and developmental history of the infant, including 9 specific health history factors, prior to an initial screening. (Evidence Quality: II, Recommendation Strength: Moderate) | Revised and updated. | TBA |
| B. Action Statement 5: SCREEN INFANTS FOR NON-MUSCULAR CAUSES OF ASYMMETRY AND CONDITIONS ASSOCIATED WITH CMT. When infants present with or without physician referral, and a professional, or the parent or caregiver indicates concern about head or neck posture and/or developmental progression, physical therapists with infant experience should perform and document screens of the neurological, musculoskeletal, integumentary and cardiopulmonary systems, including screens of vision, gastrointestinal history, postural preference and the structural and movement symmetry of the neck, face and head, trunk, hips, upper and lower extremities, consistent with state practice acts. (Evidence Quality: II-IV, Recommendation Strength: Moderate) | Revised and updated. | TBA |
| B. Action Statement 6: REFER INFANTS FROM PHYSICAL THERAPIST TO PHYSICIAN IF INDICATED BY SCREEN. Physical therapists should document referral of infants to their physicians for additional diagnostic testing when a screen identifies: non-muscular causes of asymmetry (e.g. poor visual tracking, abnormal muscle tone, extra-muscular masses); associated conditions (e.g. cranial deformation); asymmetries inconsistent with CMT; or if the infant is older than 12 months and either facial asymmetry and/or 10–15 degrees of difference exists in passive or active cervical rotation or lateral flexion; or the infant is 7 months or older with an sternocleidomastoid mass; or if the side of torticollis changes, or the size or location of an SCM mass increases. (Evidence Quality: II, Recommendation Strength: Moderate) | Revised and updated. | TBA |
| B. Action Statement 7. REQUEST IMAGES AND REPORTS. Physical therapists should request and include in the medical record all images and interpretive reports, completed for the diagnostic workup of an infant with suspected or diagnosed CMT, to inform prognosis. (Evidence Quality: II, Recommendation Strength: Moderate). | Revised and updated. | TBA |
B. Action Statement 8: EXAMINE BODY STRUCTURES. Physical therapists should perform and document the initial examination and evaluation of infants with suspected or diagnosed CMT for the following 7 body structures:
|
Revised and updated. | TBA |
| B. Action Statement 9: CLASSIFY THE LEVEL OF SEVERITY. Physical therapists and other health care providers should classify and document the level of CMT severity, choosing one of eight proposed grades (Figure 2), based on infant’s age at examination, the presence of a SCM mass, and the difference in cervical rotation PROM between the left and right sides. (Evidence Quality: II, Recommendation Strength: Moderate) | Upgraded with new evidence. | TBA |
| B. Action Statement 10: EXAMINE ACTIVITY AND DEVELOPMENTAL STATUS. During the initial and subsequent examinations of infants with suspected or diagnosed CMT, physical therapists should examine and document the types of and tolerance to position changes, and motor development for movement symmetry and milestones, using an age appropriate, valid and reliable standardized test. (Evidence quality: II; Recommendation strength: Moderate) | Revised and updated. | TBA |
B. Action Statement 11. EXAMINE PARTICIPATION STATUS. The physical therapist should obtain and document the parent/caregiver responses regarding:
|
Revised and updated. | TBA |
| B. Action Statement 12: DETERMINE PROGNOSIS. Physical therapists should determine and document the prognosis for resolution of CMT and the episode of care after completion of the evaluation, and communicate it to the parents/caregivers. Prognoses for the extent of symptom resolution, the episode of care, and/or the need to refer for more invasive interventions are related to: the age of initiation of treatment, classification of severity (Figure 2), intensity of intervention, presence of comorbidities, rate of change and adherence with home programming. (Evidence Quality: II, Recommendation Strength: Moderate) | Reaffirmed and updated. | TBA |
| PHYSICAL THERAPY INTERVENTION FOR INFANTS WITH CMT | ||
B. Action Statement 13: PROVIDE THESE FIVE COMPONENTS AS THE FIRST CHOICE INTERVENTION. Physical therapists should provide and document these five components as the first choice intervention for infants with CMT:
|
Revised and updated. | TBA |
| C. Action Statement 14. PROVIDE SUPPLEMENTAL INTERVENTION(S), AFTER APPRAISING APPROPRIATENESS FOR THE INFANT, TO AUGMENT THE FIRST CHOICE INTERVENTION. Physical therapists may provide and document supplemental interventions, after evaluating their appropriateness for treating CMT or postural asymmetries, as adjuncts to the first choice intervention when the first choice intervention has not adequately improved range or postural alignment, and/or when access to services is limited, and/or when the infant is unable to tolerate the intensity of the first choice intervention, and if the physical therapist has the appropriate training to administer the intervention. (Evidence Quality: I-IV, Recommendation Strength: Weak) | Revised and updated. | TBA |
| B. Action Statement 15: INITIATE CONSULTATION WHEN THE INFANT IS NOT PROGRESSING AS ANTICIPATED. Physical therapists who are treating infants with CMT or postural asymmetries should initiate consultation with the infant’s physician and/or specialists about other interventions when the infant is not progressing as anticipated. These conditions might include: when asymmetries of the head, neck and trunk are not starting to resolve after 4–6 weeks of comprehensive intervention, or after 6 months of intervention with a plateau in resolution. (Evidence Quality: II, Recommendation Strength: Moderate) | Revised and updated. | TBA |
| PHYSICAL THERAPY DISCONTINUATION, REASSESSMENT, AND DISCHARGE OF INFANTS WITH CMT | ||
| B. Action Statement 16: DISCONTINUE DIRECT SERVICES WHEN THESE 5 CRITERIA ARE ACHIEVED. Physical therapists should discontinue direct physical therapy services and document outcomes when these 5 criteria are met: PROM within 5 degrees of the non-affected side; symmetrical active movement patterns; age appropriate motor development; no visible head tilt; and the parents/caregivers understand what to monitor as the child grows. (Evidence Quality: II-III, Recommendation Strength: Moderate) | Revised and updated. | TBA |
| B. Action Statement 17: REASSESS INFANTS 3–12 MONTHS AFTER DISCONTINUATION OF DIRECT SERVICES, THEN DISCHARGE IF APPROPRIATE. 3–12 months following discontinuation from direct physical therapy intervention OR when the child initiates walking, physical therapists who treat infants with CMT should examine postural preference, the structural and movement symmetry of the neck, face and head, trunk, hips, upper and lower extremities, and developmental milestones to assess for reoccurrence of CMT and evidence of atypical development. (Evidence Quality: II, Recommendation Strength: Moderate) | Revised and updated. | TBA |
LEVELS OF EVIDENCE AND RECOMMENDATION GRADE CRITERIA
Levels of Evidence (Table 1)
Recommendation Grades for Action Statements (Table 2)
Levels of Evidence and Recommendation Grades
Levels of evidence are assigned based on a combination of a risk of bias assessment and the quality of the outcome measures used in a study. Multiple outcome measures in a single study may have stronger or weaker psychometric properties, and thus individual outcomes receive stronger or weaker levels of evidence, respectively. Recommendation grades A-C are consistent with the levels of evidence in the BRIDGE-Wiz software deontics.2 BRIDGE-Wiz is designed to generate clear and implementable recommendations consistent with the Institute of Medicine (IOM) recommendations for transparency.3 These include: a standardized content outline of a title; a recommendation with an observable action statement; indicators of the evidence quality and the strength of the recommendation; a list of benefits, harms and costs associated with the recommendation; a delineation of the assumptions or judgments made by the guideline development group (GDG) in formatting the recommendation; reasons for intentional vagueness in the recommendation; quality improvement, implementation and audit ideas; and a summary and clinical interpretation of the evidence supporting the recommendation. Theoretical/Foundational (Grade D) and Practice Recommendations (Grade P) are not generated with BRIDGE-Wiz. Grade D is based on basic science or theory, and Grade P is determined by the GDG to represent current best physical therapist (PT) practice or exceptional situations for which studies cannot be performed. Research recommendations identify missing or conflicting evidence, for which studies might improve examination and intervention efficacy, or minimize unwarranted variation.
Status Definitions
These terms are used in the Summary of Action Statements table to indicate changes from the 2013 CMT CPG.1
New – an action statement that was not in the prior version.
Upgraded with new evidence - the action statement has a stronger grade than previously with new references.
Downgraded with new evidence - the action statement has a weaker grade than previously with new references.
Revised and updated - the action statement has been reworded for clarity with new references.
Revised; no new evidence - the action statement has been reworded for clarity with no new references.
Reaffirmed and updated – the action statement is unchanged but has new references.
Reaffirmed; no new evidence - the action statement is unchanged and has no new references.
Retired – an action statement that is withdrawn.
SUMMARY AND STATUS OF ACTION STATEMENTS FOR THE 2018 CONGENITAL MUSCULAR TORTICOLLIS CLINICAL PRACTICE GUIDELINE (Table 3 and SDC 3)
INTRODUCTION
Purpose of the 2018 Congenital Muscular Torticollis Clinical Practice Guideline
The American Physical Therapy Association (APTA) Academy of Pediatric Physical Therapy (APPT) supports the development of clinical practice guidelines (CPG) to assist pediatric physical therapists (PTs) with the identification and management of infants and children with participation restrictions, activity limitations and body function and structure impairments, related to developmental, neuromuscular, cardiorespiratory and musculoskeletal conditions, as defined by the World Health Organization’s (WHO) International Classification of Functioning, Disability and Health (ICF) (www.who.int/classification/icf/en/). In general, the purpose of a CPG is to help PTs know who, what, how and when to treat, and who and when to refer, and to whom.
Congenital muscular torticollis (CMT) is a postural deformity evident shortly after birth, typically characterized by lateral flexion/side bending of the head to one side and cervical rotation/head turning to the opposite side due to unilateral shortening of the sternocleidomastoid muscle.4 This CPG for physical therapy management of infants with CMT is intended as a reference document to guide PTs, families, health care professionals, and educators to improve clinical outcomes and health services for children with CMT, and to inform the need for continued research related to physical therapy management of CMT. Current conventions are to update CPGs every 5–10 years: this document replaces the 2013 Congenital Muscular Torticollis (2013 CMT CPG).1
Specifically, for infants (birth to 12 months) and very young children with CMT, the purposes of the 2018 CMT CPG are to:
Update the evidence and guidance for PTs’ management of CMT, including education, screening, examination, evaluation, diagnosis, reasons to refer, classification, prognosis, interventions, outcome measurements, discontinuation, discharge and reassessment.
Update evidence on common CMT limitations of body functions and structures, activity and participation, and where possible, aligns descriptions with ICF terminology, SDC 1; Appendix 1-ICF/ICD 10 Codes.
Update a CPG for PTs, physicians, families and caregivers, other early childhood or healthcare service providers, academic instructors, clinical instructors, students, policy makers and payers, that describes, using internationally accepted terminology, best current practice of pediatric PT management of CMT across health care settings, including prenatal classes, newborn nurseries, physician offices, outpatient pediatric physical therapy offices, and early intervention programs.
Identify areas of research necessary to strengthen the evidence for CMT management.
Background and Changes in the 2018 Congenital Muscular Torticollis Clinical Practice Guideline
The 2013 CMT CPG1 set standards for the identification, referral and physical therapy management of CMT, allowing practices to: align documentation with the recommended measures,5 develop a clinical decision algorithm,6 and provide guidance for intervention and follow-up.7 Implementing the 2013 CMT CPG recommendations improves outcomes.8 Studies on CMT published since the 2013 CMT CPG, in combination with clinician feedback, warranted a review of the evidence and its impact on the original recommendations.
The following changes to the 2013 CMT CPG were made in this 2018 CMT CPG.
A recommendation was added to educate expectant parents and parents of newborns on the importance of preventing asymmetrical positioning, use of prone playtime (tummy time) and the role of PTs in the comprehensive management of persistent asymmetries.
The recommendation to classify severity was upgraded with a level II study that established good reliability for grading.9
The 7 grades were increased to 8, with a very late category for all infants >12 months of age, and to correct an omitted line to allow classification of early mild in 3–6-month olds; Figure 2 (also SDC2) was also simplified.
For infants born preterm, the GDG recommends documenting both chronological and corrected age, and using the corrected age for developmental testing, assigning the severity classification, and designing the plan of care.
For infants who change service providers to treat CMT, the CMT severity should be classified based on the infant’s current age, corrected as needed for preterm birth, and initial examination findings by the new provider.
The major groupings for classification were revised from Early or Late Identification/Intervention to Early, Later and Very Late PT Evaluation/Intervention, to place the emphasis on classifying severity based on the infant’s age at the PT evaluation.
Thirteen recommendations were revised for clarity and updated with new literature, 2 recommendations were reaffirmed and updated with new literature, 1 recommendation was upgraded from Practice to Moderate strength, and no recommendations were retired.
All action statements now include individualized recommendations for quality improvement, implementation and audit. The 2013 CMT CPG section on Implementation and Audit Recommendations at the end of the document provided general recommendations for implementing the guideline as a whole. The 2018 version has 2 additional headings in each Action Statement Profile. The Quality Improvement section provides a rationale for why that recommendation is important to implement; that is, what aspect of health care services or delivery will improve if the action statement is fully implemented. The Implementation and Audit section provides examples of focused recommendations for implementing and monitoring the action statement to ensure quality improvement.
There are 4 evidence tables in this version. Studies on Measurement Approaches and Studies on the First-choice Intervention are updated with new evidence. Studies on Supplemental Interventions and Studies on Long-Term Follow-Up are new additions.
Sections from the 2013 CMT CPG omitted from this update include the historical background on classic studies that identified the types and incidence of CMT, and the rationale for developing the 2013 version.1
Figure 2.

2018 Classification of Severity and Management of Congenital Muscular Torticollis (CMT)
To use this chart: The vertically aligned ovals on the left, list the factors that are most relevant to the classification process (age asymmetry noted, age of referral and PT evaluation, type of CMT); the diamonds below describe the cycle of PT examination, intervention and reassessment. Begin in the larger rectangle with age at evaluation and type of CMT to choose a grade in the ovals below. Abbreviations: PT, physical therapy; TX, treatment; SCM, sternocleidomastoid; L/R, left/right.
The Scope of the Guideline
The 2013 CMT CPG included a systematic review of literature through May 2013.1 The 2018 CMT CPG is based on a systematic review of literature from January 2012 through September 2017,10 supplemented by critical appraisals of the literature published from September 2017- May 2018. It is assumed throughout the document that the PT has newborn and early childhood experience.
The CPG addresses these aspects of CMT management in infants and very young children:
Parent education to prevent or identify postural preference and the role of pediatric physical therapy in its management.
Diagnostic and referral processes.
Importance of early assessment and referral of infants with asymmetries/CMT to physician and physical therapists.
Reliable, valid, and clinically useful screening, examination and evaluation procedures that should be documented.
Determination of a severity classification and a prognosis for intensity of PT intervention and duration of care.
First-choice physical therapy intervention, including dosage guidance, and supplemental interventions.
Conditions under which a child should be referred to the infant’s physician and/or specialist for consideration of additional tests and interventions.
Prognosis if CMT is treated with conservative interventions, or treated with other interventions, and the consequences of CMT left untreated.
Criteria for discontinuation of direct PT intervention, the importance of a reassessment, and criteria for discharge.
Important outcomes of intervention and patient characteristics affecting outcomes.
Statement of Intent
This guideline is intended to inform clinicians, family members, educators, researchers, policy makers and payers. It is not intended to be construed or to serve as a legal standard of care. As rehabilitation knowledge expands, clinical guidelines are promoted as syntheses of current research and provisional proposals of recommended actions under specific conditions. Standards of care are determined on the basis of all clinical data available for an individual patient/client and are subject to change as knowledge and technology advance, patterns of care evolve, and patient/family values are integrated. This CPG is a summary of practice recommendations that are supported with current published literature that has been reviewed by expert practitioners and other stakeholders. These parameters of practice should be considered guidelines only, not mandates. Adherence to them will not ensure a successful outcome in every patient, nor should they be construed as including all proper methods of care or excluding other acceptable methods of care aimed at the same results. The ultimate decision regarding a particular clinical procedure or treatment plan must be made using the clinical data presented by the patient/client/family, the diagnostic and treatment options available, the patient’s values, expectations and preferences, and the clinician’s scope of practice and expertise. The guideline development group (GDG) suggests that significant departures from accepted guidelines should be documented in patient records at the time the relevant clinical decisions are made.
METHODS
The GDG was approved by the APPT to update the 2013 CMT CPG in accordance with Academy procedures.11 The purpose, scope and content outline builds on the 2013 CMT CPG survey; its content validity is further supported by evidence of the integration of recommendations into practice.7
Search Strategy
This CPG update is based on a systematic review (January 2012 – September 2017) on the physical therapy evidence for diagnosis, prognosis and intervention of CMT to inform the 2013 CMT CPG.10 Refer to Heidenreich et al.10 for details of the search strategy, study selection, study appraisal, data extraction, and results for the 20 studies that informed the 2018 CMT CPG: 14 studies informed prognosis and 6 studies informed intervention.
To assure that the updated CMT CPG utilized the most current evidence, a comprehensive search of 5 databases (CINAHL, Cochrane Library, PsycInfo, PubMed, Web of Science) was performed from September 2017 to May 2018 by the GDG with the single search term “torticollis” resulting in 199 studies. No filters were applied for study type or language.
Selection Criteria
Studies meeting the following 2 criteria were added to those from the 2013 CMT CPG and the 2018 systematic review:10 participants included infants and children diagnosed with CMT and studies informed the PT management of CMT. All study designs were included. Studies were excluded based on the following 4 criteria: they focused only on plagiocephaly; dissertations and abstracts; not published in English; and no statistical analysis of results.
Study Appraisal and Data Extraction
Of the 199 studies, 2 newer studies informed the management of CMT as related to PT that were not available for either the 2013 CMT CPG or the systematic review by Heidenreich, Johnson, Sargent 10 One was a study on the measurement properties of the Classification of CMT Severity grades9 that was appraised using the COSMIN checklist. One study was an intervention study12 that was appraised using the APTA’s Critical Appraisal Tool for Experimental Intervention Studies (CAT-EI) and the Cochrane Risk of Bias13 for intervention studies. Two reviewers completed appraisals of 3 articles to establish inter-rater reliability with at least 90% agreement on each appraisal tool. The 2 reviewers then appraised each study independently, scores were compared for agreement, and discrepancies were resolved via discussion. In addition, the intervention study was assigned a level of design rigor (level I, most rigorous, to level V, least rigorous) according to criteria from the American Academy of Cerebral Palsy and Developmental Medicine Systematic Review Methodology.14
Data were extracted to maintain consistency with the 2013 CMT CPG1 and the 2018 CMT systematic review.10 The 4 evidence tables (SDC 4–7) are Table 4 - Studies on Measurement Approaches, Table 5: Studies on the First-choice Intervention, Table 6: Studies in Supplemental Interventions and Table 7: Studies of Long Term Follow-Up. Strengths and limitations of the evidence are included in the aggregate evidence quality and supporting evidence and clinical interpretation section of each action statement.
Recommendation Formulation
Each 2013 recommendation was evaluated for its currency and consistency with the updated literature. This CPG (2018) was informed by the clinical and professional experience of the GDG, trends in practice changes, and the reported impact of the 2013 CMT CPG informed the decision to reaffirm, revise or upgrade an existing recommendation. The new recommendation on Education is consistent with professional roles to prevent conditions as well as treat them.
External Review Process
External review is consistent with the IOM recommendations for trustworthy guidelines.3 The purposes are to ensure clarity, quality and comprehensiveness of the CPG, and to identify potential bias, lapses in logic or alternative perspectives. A first draft of the 2018 CMT CPG was reviewed by 16 stakeholders representing medicine, pediatric nursing, midwifery, parents of infants with CMT, methods experts, PT practice, research, and knowledge translation. Both a rating scale to assess clarity and implementation feasibility and an open-ended invitation for comments and edits were used to gather feedback. Of the 17 statements, 15 were rated as clear and 12 as feasible by at least 75% of the reviewers. After addressing the 1st round of suggested edits, the document was reviewed by selected American Academy of Pediatrics (AAP) members and posted for public review on the APPT website; invitations to review were distributed to APPT members via its electronic newsletters, through a social media posting, and direct email notices to volunteers. Non-members could review if notified by APPT members. Suggested edits were addressed, and the final draft was submitted to the Pediatric Physical Therapy journal for editorial review. Modifications based on comments from the AAP, APPT members and the general public included clarification or expansions of the facilitators and barriers to implementation of individual action statements and use of consistent terminology throughout the document. Many reviewers reinforced APPT plans for knowledge translation, through the production of parent and medical support documents and downloadable selected figures and tables.
AGREE II Review
This CPG was evaluated by two external reviewers using AGREE II.15 AGREE II is an established instrument designed to assess the quality of clinical practice guidelines using 23 items in 6 domains (www.agreetrust.org). Each item is rated using a 7-point scale, with 7 representing the highest score. Each item includes specific criteria, although reviewer judgment is necessary in applying the criteria. The AGREE II appraisal process supported an iterative process to improve the quality of the guideline. Domain scores for the CMT CPG ranged from 86% to 100%. The 2 reviewers unanimously agreed to recommend the Guideline for use. Scores were discussed by the GDG; where possible, items were addressed in the CPG following the AGREE II reviews. Thus, the percentages are likely higher in the final version of the CPG.
Language
The 2013 CMT CPG is referenced the first time it appears and is used without reference hereafter. In contrast, this document is referred to as the 2018 CMT CPG. Additionally, we use the generic phrase ‘infant’s physician’ to reference pediatricians, referring physicians, family physicians or other primary health care provider.
CONGENITAL MUSCULAR TORTICOLLIS
Incidence and Progression of Congenital Muscular Torticollis
Congenital muscular torticollis (CMT) is a common pediatric musculoskeletal condition, described as a postural deformity of the neck evident at birth or shortly thereafter. Synonyms include fibromatosis colli for the mass type,16, 17 wry neck,18 or twisted neck.19 It is typically characterized by a head tilt to one side or lateral neck flexion, with the neck rotated to the opposite side due to unilateral shortening or fibrosis of the sternocleidomastoid (SCM) muscle. It may be accompanied by cranial deformation (CD),20 developmental dysplasia of the hip (DDH),21 brachial plexus injury,22–24 foot or lower extremity anomalies,25–27 and less frequently, presents as a head tilt and neck rotating to the same side, or as a bilateral condition.28 The incidence of CMT ranges from 3.9%29, 30 to 16%20 of newborns, may occur slightly more frequently in males31, 32 and in infants who are exposed in utero to opioids.33 Congenital muscular torticollis may be present at birth when selected morphologic and birth history variables converge, such as in longer babies, breech presentation and/or the use of forceps during delivery,29 or it may evidence itself during the first few months,20, 26 particularly for those with milder forms.
Congenital muscular torticollis is typically categorized as 3 types: postural, muscular, and SCM mass CMT. Postural CMT presents as the infant’s postural preference27, 34 but without muscle or passive range of motion (PROM) restrictions and is the mildest presentation. Muscular CMT presents with SCM tightness and PROM limitations. Infants with a SCM mass, the most severe form of CMT, present with a fibrotic thickening of the SCM and PROM limitations.35 Since 2013, CMT has also been graded using 7 levels of severity distinguished by age at evaluation, type of CMT, and the presence or absence of a SCM mass.1 In general, infants identified early with postural CMT have shorter treatment episodes.36 Those identified later, after 3–6 months of age and who have a SCM mass, typically have the longest episodes of conservative treatment, and may ultimately undergo more invasive interventions.35, 37
Physicians or parents may be the first to notice an asymmetry, and physicians may provide the initial instructions about positioning and stretching to the parents.38 The American Academy of Pediatrics (AAP), in its Bright Futures Guidelines for Health Supervision of Infants, Children, and Adolescents publication, recommends checking the newborn for head dysmorphia or abnormal shape at 1 week and skull deformities at 1 month, but does not specify checking the neck for symmetry until 2 months, when the term torticollis is first mentioned.39 In the past, if the asymmetry did not resolve after initial exercise instructions by the physicians, infants were typically then referred to physical therapy (PT).38 While this pattern of identification and eventual referral to PT is described in prior literature, the GDG is in strong agreement with the AAP Policy on surveillance; that physicians should be providing developmental surveillance for all infants at every well-child preventative care visit from birth and throughout the first 6 months,40 so that infants with any identified postural asymmetries are referred immediately for PT intervention.
Physical therapy management of CMT is comprehensive, going beyond just stretching tight neck muscles. A comprehensive plan of care addresses the following 5 components as the first-choice intervention: neck PROM, neck and trunk active range of motion (AROM), development of symmetrical movement, environmental adaptations, and parent/caregiver education. Earlier PT intervention is more quickly effective than intervention started later.41 If started before 1 month of age, 98% of infants with CMT achieve near normal range within 1.5 months but waiting until after 1 month of age prolongs the PT episode of care to approximately 6 months and waiting until after 6 months can require 9–10 months of PT intervention, with progressively fewer infants achieving near normal range.36
Reports of untreated CMT are rare,42, 43 but there are descriptions of unresolved or reoccurring CMT in older children or adults, who later undergo botulinum neurotoxin therapy injections,4, 44, 45 or surgery for correction of movement limitations, consequent facial asymmetries,43, 46–49 or pain.50 The incidence of spontaneous resolution is unknown, and there are no documented methods for predicting who will resolve and who will progress to more severe or persistent forms.
Finally, CMT has been associated with CD,51 DDH,52 brachial plexus injury,22–24 foot deformities,26 early motor delays,53, 54 compromised cosmesis,55 and temporomandibular joint dysfunction.56 Thus, early identification and treatment is critical for early correction, early identification of secondary or associated impairments, and prevention of future complications.
Importance of Early Referral
The evidence is strong that earlier intervention results in the best outcomes and decreased episodes of care,36, 41, 57 so early referral is the ideal. A referral flow diagram is provided (Figure 1, SDC 1) that outlines the possible referral and communication pathways based on time of observation, identification of non-muscular causes of asymmetry, prior models and current literature.4, 22, 58–60
Figure 1.
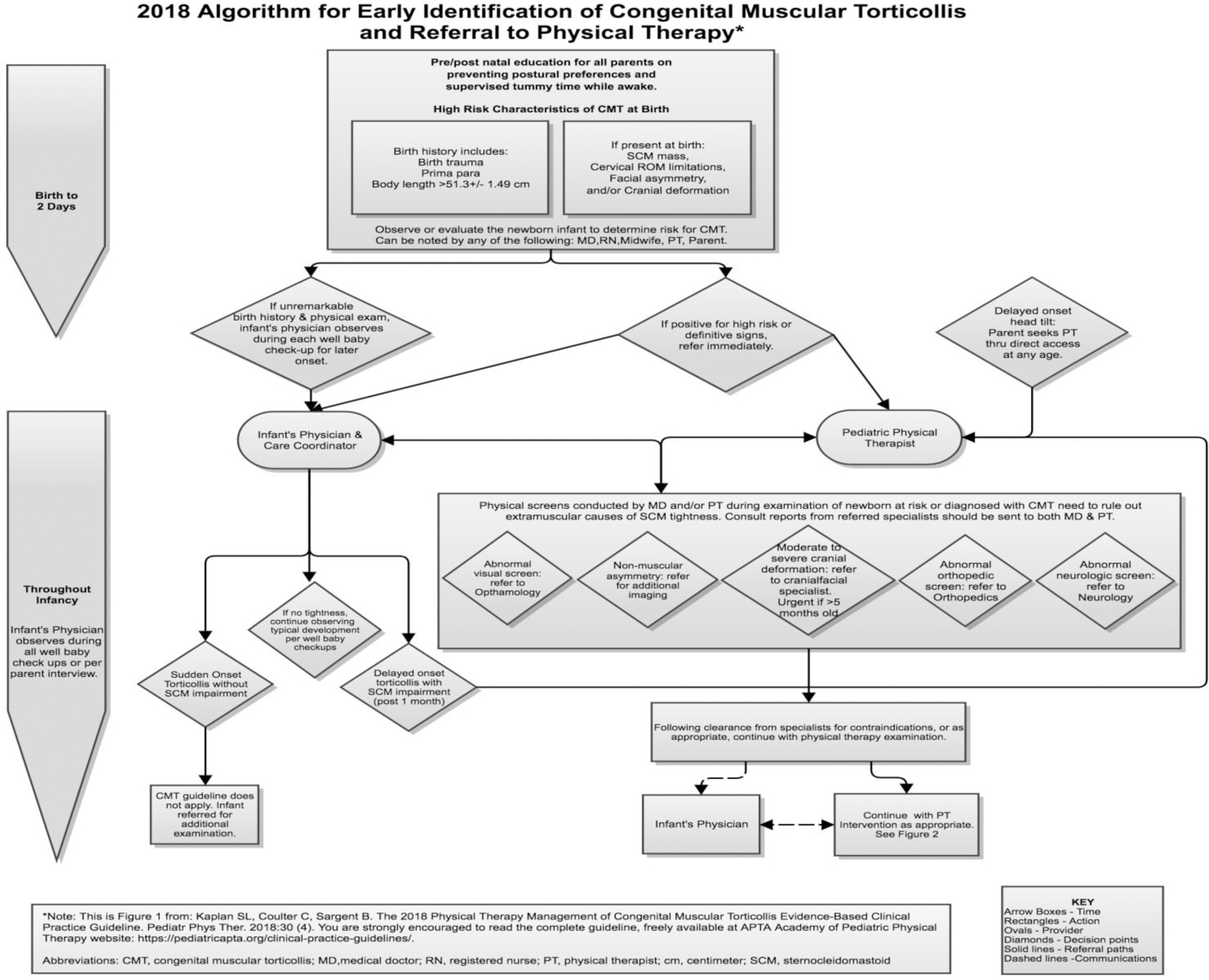
Referral Flow Diagram
The referral flow diagram is divided into 2 distinct time frames: Birth to 2 days, representing the newborn period, and throughout infancy, representing the typical time after discharge to home. During the newborn period, many different healthcare providers may observe the infant because they are involved in the birth and/or postnatal care. These healthcare providers are in the ideal position to observe the symmetry of the head on the shoulders and screen for passive and active movement limitations. After the infant is at home, the most likely observers will be the primary physician and the parents or other caregivers. Regardless of who performs the initial screen, infants with asymmetry should undergo an evaluation to rule out non-muscular causes of CMT. If CMT or a persistent postural preference is diagnosed, the infant should be immediately referred to a pediatric PT.
Early referral to a pediatric PT translates to earlier intervention and prevention of secondary sequelae.26, 61–63 Additionally, reducing the episode of care and avoiding additional or more invasive interventions is cost effective. Preliminary evidence suggests that treatment by a PT may be more efficient in achieving symmetrical movements than when parents are the sole providers of home exercise programs,64 further supporting early referral to PT.
ACTION STATEMENTS
I. EDUCATION, IDENTIFICATION AND REFERRAL OF INFANTS WITH ASYMMETRIES/CONGENITAL MUSCULAR TORTICOLLIS (CMT)
P. Action Statement 1: New. EDUCATE EXPECTANT PARENTS AND PARENTS OF NEWBORNS TO PREVENT ASYMMETRIES/CMT.
Physicians, nurse midwives, prenatal educators, obstetrical nurses, lactation specialists, nurse practitioners or physical therapists should educate and document instruction to all expectant parents and parents of newborns, within the first 2 days of birth, on the importance of supervised prone/tummy play when awake 3 or more times daily, full active movement throughout the body, prevention of postural preferences, and the role of pediatric physical therapists in the comprehensive management of postural preference and optimizing motor development. (Evidence quality: V; Recommendation strength: Best Practice)
Aggregate Evidence Quality:
Clinical experience of the GDG.
Benefits:
Increases parent/caregiver self-efficacy in caring for their newborn.
Informs parents on the importance of supervised tummy time to optimize motor development within the first 6 months.
Teaches parents/caregivers to initiate early surveillance for postural preference and to bring concerns to the infant’s physician, or, in states with direct access, to a pediatric PT.
Informs parents about the role of pediatric PTs in providing a comprehensive and supportive plan of care to manage postural preference associated with CMT and cranial deformation (CD).
May reduce the episode of care and improve outcomes if postural preference is identified and comprehensively managed early.
Risk, Harm, Cost:
May increase parent/caregiver anxiety about the potential for CMT and CD.
May marginally increase the cost of care if prenatal educators, labor and delivery personnel, or postnatal care providers do not incorporate education into usual care.
May increase time needed to spend with a newborn and parents during appointments.
Benefit-Harm Assessment:
Preponderance of Benefit
Value Judgments:
A preponderance of evidence supports that early identification of postural preference and CMT results in shorter episodes of care and full resolution of asymmetries. The GDG feels that if parents know how to monitor their newborn during the first months of life, how to encourage tummy time during awake periods, and are empowered to report their concerns to their physician, that asymmetries could be reduced more quickly or even prevented.
Intentional Vagueness:
Prone positioning for supervised play up to 3 times a day is the recommendation for newborns because the amount of time awake is limited, though the need to start prone positioning right away for short periods should be reinforced. As time awake increases, infants should be placed for supervised prone play as often as tolerated and practical.
Role of Patient/Parent Preferences:
Due to the amount of information that parents of newborns receive during the first days of parenthood, they may benefit from multiple educational opportunities before and after the baby’s arrival. Parents may prefer receiving instruction using different modes of delivery (by video, brochure) or by different healthcare providers (with those they already have a relationship with or as part of prenatal care), or at different phases in their pre to post-natal experience.
Exclusions:
None
Quality Improvement:
Pre-Post-natal education for parents on postural preference and the benefits of early intervention may shorten the episode of care or improve outcomes if an infant is diagnosed and referred to PT early. This is especially true for parents of multiples, whose infants may be at greater risk than singletons for CD that may lead to postural preference.65
Implementation and Audit:
PTs need to do outreach to ensure that health professionals, including but not limited to physicians, nurse midwives, prenatal educators, obstetrical nurses, lactation specialists, nurse practitioners, doulas, and early intervention providers have an accurate understanding of the role of pediatric PTs in the comprehensive management of postural preference and optimizing motor development, and resources for how and to whom to refer parents.
Pediatric PTs can provide community education on the prevention and management of postural preference, including CMT and CD.
Pediatric PTs should distribute the APPT summary brochures on CMT to health care providers or parents as appropriate and educate them about how to access them online (https://pediatricapta.org/clinical-practice-guidelines/).
Pediatric PTs should collaborate with the relevant health care providers in their clinical settings to develop a pathway for parent education to ensure that it is provided both before and within the first 2 days of birth.
Audits of the provision of education to expectant parents and parents of newborns can be completed by quality assurance officers.
Supporting Evidence and Clinical Interpretation:
The first step in the American Academy of Pediatrics’ policy on surveillance for developmental disorders is to “elicit and attend to parents’ concerns about their child’s development”.40 Porter, Qureshi, Caldwell, Echevarria, Dubbs, Sullivan 66 conclude that surveillance does not happen universally, such that others who care for the infant, including parents, should be educated on early surveillance. A mixed methods study determined that 90% of mothers are educated about infant supine sleeping positions, but instruction on awake prone play or rotating prone and supine was only received by 27% of mothers postpartum, and 2 months later, only 8% of mothers used prone positioning during awake time, with 70% positioning only 1–2 times per day.67 The success of the Back to Sleep campaign68 has demonstrably reduced cases of sudden infant death syndrome; however, many ascribe parental adherence to supine positioning, and concomitant avoidance of prone positioning for infant play, as a contributing factor to an increase in CMT. Early and frequent parent education to monitor for asymmetry and about the importance of ‘prone for play’ or ‘tummy time’, in addition to ‘supine or back to sleep’, may help to reduce or prevent asymmetries from developing, particularly when postural preferences are apparent.
R. Research Recommendation:
Studies are needed on the impact of education on:
Health care providers and their knowledge of pediatric PTs’ roles in managing postural preference.
Parents/caregivers about the parental experience of receiving this education.
A. Action Statement 2: Revised and updated. ASSESS NEWBORN INFANTS FOR ASYMMETRIES/CMT.
Physicians, nurse midwives, obstetrical nurses, nurse practitioners, lactation specialists, physical therapists or any clinician or family member must assess and document the presence of neck and/or facial or cranial asymmetry within the first 2 days of birth, using passive cervical rotation and/or visual observation as their respective training supports, when in the newborn nursery or at site of delivery. (Evidence Quality: I, Recommendation Strength: Strong)
Action Statement Profile
Aggregate Evidence Quality:
Level I based on the odds ratios for prediction of CMT from facial asymmetry (OR 21.75, CI 6.60–71.70) and plagiocephaly (OR 23.30, CI 7.01–70.95),69 and level II evidence that starting treatment before 6 weeks of age yields greater reductions in SCM thickness than starting after 6 weeks.41
Benefits:
Early identification of infants at risk for CMT or other conditions that may cause asymmetries.
Early onset of intervention for infants with CMT if referred.
Reduced episode of care to resolve CMT, with consequent reduction in costs.
Reduced risk of needing more invasive interventions (botulinum neurotoxin therapy or surgery) in the future.
Risk, Harm, Cost:
Potential of over-identification of infants may increase costs.
Potential of increasing parent anxiety.
Benefit-Harm Assessment:
Preponderance of Benefit
Value Judgments:
None
Intentional Vagueness:
None
Role of Patient/Parent Preferences:
While parents may not be skilled in formal infant assessment, they are keen observers of their own child. Mothers who are breastfeeding may notice that the infant has greater difficulty feeding on one side, or parents may notice asymmetry in photographs, and these observations should trigger range of motion (ROM) screening by an attending clinician.
Exclusions:
None
Quality Improvement:
Documentation of an assessment for cervical ROM and postural symmetry provides uniform data for more effective communication among clinicians and settings, and for uniform data entry in patient registries.
Early examination can detect asymmetries and support earlier referral to PTs who can provide a comprehensive plan of intervention and follow-up.
Implementation and Audit:
PTs should share the 2018 CMT CPG or the summary brochures (https://pediatricapta.org/clinical-practice-guidelines/) with physicians and other referral sources in their geographic area, highlighting this recommendation and the importance of early cervical ROM screening.
Training of or the development of clinical pathways for health professionals who see the infant at birth may be needed to ensure that a cervical ROM assessment occurs within the first 2 days of delivery.
Documentation forms or electronic records may need revision to reflect the cervical ROM and postural symmetry screen.
Audits of newborn charts may indicate if patterns of examination are changing.
Supporting Evidence and Clinical Interpretation
The intent of this action statement is to increase early identification of infants with CMT for early referral to PT. Newborns (up to the first 3 days of life) can be easily screened by checking for full neck rotation (chin turns past shoulder to 100 degrees)20 and lateral cervical flexion (ear approximates shoulder)20 while stabilized in supine25 during the first postnatal exam. Newborns are at higher risk for CMT if their birth history includes a combination of longer birth body length, primiparity, and birth trauma (including use of instruments for delivery), facial asymmetry and plagiocephaly. Odds ratios from multiple logistic regression for these 5 factors are, from highest to lowest: plagiocephaly 23.30, CI 7.01–70.95; facial asymmetry 21.75, CI 6.60–71.70; primiparity 6.32, CI 2.34–17.04; birth trauma 4.26, CI 1.25–14.52; birth body length 1.88, CI 1.49–2.38. This indicates that infants with asymmetrical heads or faces have as much a 22-fold increase in abnormal sonogram for CMT; primiparity a 6-fold increase; birth trauma a 4-fold increase; and birth body length an almost 2-fold increase.29 Additionally, infants with a history of neonatal abstinence syndrome (NAS) and who require postnatal medication have a higher incidence of CMT than infants without NAS.33 No one item predicts CMT alone but the presence of 2 or more of the above risk factors warrants referral for preventative care and parent education.
The importance of early identification of CMT is well supported. Physicians and PTs in Canada agree that infants identified with CMT should receive formal intervention.38 When intervention is started at earlier ages, it results in shorter episodes of care57 and greater reductions in SCM thickness41 that, anecdotally, may have financial, psychological and quality of life implications for the family.
R. Research Recommendation:
Studies are needed to determine:
Whether routine screening at birth increases the rate of CMT identification and/or increases false positives.
The barriers to early referral of infants with CMT to PT.
B. Action Statement 3: Revised and updated. REFER INFANTS WITH ASYMMETRIES/CMT TO PHYSICIAN AND PHYSICAL THERAPIST.
Physicians, nurse midwives, obstetrical nurses, nurse practitioners, lactation specialists, physical therapists or any clinician or family member should refer infants identified as having postural preference, reduced cervical range of motion, sternocleidomastoid masses, and/or craniofacial asymmetry to their primary physician and a physical therapist with expertise in infants as soon as the asymmetry is noted. (Evidence Quality: II, Recommendation Strength: Moderate)
Action Statement Profile
Aggregate Evidence Quality:
Level II evidence supports that when intervention is started earlier, it takes less time to resolve the ROM limitation,35, 36 there are greater reductions in SCM thickness41 and there is less need for subsequent surgical intervention.36, 61 Importantly, stretching interventions are easier for parents to administer when infants are younger, before the neck musculature strengthens and cooperation declines.36, 63
Benefits:
Early differential diagnosis to determine that the postural asymmetry is due to CMT versus another medical condition, such as a visual impairment or reflux.
Earlier intervention to resolve limited ROM and asymmetries more quickly.
Early parental education to facilitate symmetrical development and self-efficacy with home programs.
Greater infant tolerance with intervention in the first few months of life.
Risk, Harm, Cost:
Increased cost for treatment of asymmetries that some suggest may spontaneously resolve.
Benefit-Harm Assessment:
Preponderance of Benefit
Value Judgments:
Early referral to PT ensures early onset of intervention, which strongly correlates with shorter episodes of care, greater success of conservative measures, and thus can lower overall costs of care. A pediatric PT will also screen and follow the infant for developmental delays, feeding challenges, and environmental factors that may be associated with or contribute to postural preference or CMT.
Intentional Vagueness:
For infants suspected of other causes of asymmetries, i.e. bony anomalies, fractures, neurological conditions, or extra-muscular masses, PTs should collaborate with the infant’s physician to make a definitive diagnosis of CMT prior to onset of PT interventions. The focus and prioritization of interventions may change depending on the type of limitations the infant presents with (e.g. neurological, musculoskeletal, cardiopulmonary, integumentary and/or gastrointestinal).
Role of Patient/Parent Preferences:
Infant tolerance with stretching is easier in the first 2 months than when started after the infant develops greater head control,63, 70 thus infant cooperation is greater and parent adherence to home programs may be optimized. Later referrals put additional stress on parents to adhere to stretching recommendations.
Exclusions:
Infants suspected of having non-muscular conditions that might cause asymmetrical or torticollis posturing should be fully examined by the appropriate specialists to rule out confounding diagnoses prior to initiating PT.
Quality Improvement:
This recommendation will reduce delays in referrals to PTs who can provide a comprehensive plan of intervention and follow-up to ensure that the primary caregivers can adhere to the recommended interventions.
Implementation and Audit:
Training for health professionals and early intervention providers who see infants in early infancy may be needed to ensure that infants are appropriately and quickly referred to PT. Health professionals may be reluctant to refer right away if they perceive parents as being overwhelmed during those early weeks; however, earlier referral translates to better outcomes.
Audits of the age at which parents first noticed the CMT, the date of referral and the age of first PT examination will provide objective measures of delays between identification and referral to PT, and delays between referrals and the 1st scheduled PT examination.
PTs should share the 2018 CMT CPG and/or the APPT summary brochures (https://pediatricapta.org/clinical-practice-guidelines/) with physicians, early intervention providers and other referral sources in their geographic area, highlighting this recommendation and the supporting evidence for early referral.
Clinical pathways for examination and referral processes may reduce delays in the onset of PT services by prioritizing infants with asymmetry/CMT for PT examinations. PTs may need to collaborate with administrators and non-medical professionals to ensure that these infants receive immediate referrals in the pathway, either internally or through external referrals.
Supporting Evidence and Clinical Interpretation:
Clinicians involved with the delivery and care of infants are in the ideal position to assess the presence of CMT. If screening for CMT occurs routinely at birth, infants who are at high risk for CMT, or who have identified SCM tightness or masses, can have PT initiated when the infant is most tolerant of interventions. CMT may not appear until several weeks post-delivery, thus, the 1 month well baby check-up by the physician may be the first point of identification.
Early intervention for infants with CMT, initiated before 3–4 months of age, results in excellent outcomes with 92–100% achieving full passive neck rotation and 0–1% requiring surgical intervention.36, 37 The earlier intervention is started, the shorter the duration of intervention36 and the need for later surgical intervention is significantly reduced.57, 61 Petronic et al. found that when treatment was initiated before 1 month of age, 99% of infants with CMT achieved excellent clinical outcomes (no head tilt, full passive cervical rotation) with an average treatment duration of 1.5 months, but if initiated between 1–3 months of age only 89% of infants achieved excellent outcomes with an average treatment duration of 5.9 months.36 When initiated between 3–6 months of age, 62% of infants achieved excellent outcomes with an average treatment duration of 7.2 months.36 When initiated between 6–12 months of age, 19% of infants achieved excellent outcomes with an average treatment duration of 8.9 months.36 In contrast to recommendations to provide stretching instruction to the parents when CMT is identified at birth, and only refer to PT at 2 months of age if the condition does not resolve,37 recent studies suggest that early PT reduces the time to resolution compared to parent-only stretching,64 that infants become more difficult to stretch as they age and develop neck control,63 and that earlier intervention can negate the need for later surgery.57, 61
Physical therapists address a broad range of developmental and environmental factors that influence outcomes, such as parental ability to perform the home exercise programs, transportation distance from the clinical setting,38 feeding positions, and the infant’s motor and developmental progression.38, 71 Since developmental delays are detectable at 2 months in infants with CMT,54 and the delays may be inversely related to time spent in the prone position,54 instruction to parents and early modeling of prone play time may help to negate potential developmental lags that can occur with CMT.
R. Research Recommendations:
Studies are needed to clarify the predictive baseline measures and characteristics of infants who benefit from immediate follow-up, and to compare the cost benefit of early PT intervention and education as compared to parental instruction and monitoring by physicians.
Longitudinal studies of infants with CMT are needed to clarify how the timing of referral and initiation of intervention impact body structure and functional outcomes, and overall costs of care.
II. PHYSICAL THERAPY EXAMINATION AND EVALUATION OF INFANTS WITH ASYMMETRIES/CMT
B. Action Statement 4: Revised and updated. DOCUMENT INFANT HISTORY.
Physical therapists should obtain a general medical and developmental history of the infant, including 9 specific health history factors, prior to an initial screening. (Evidence Quality: II, Recommendation Strength: Moderate)
Action Statement Profile
Aggregate Evidence Quality:
Level II cohort and outcome studies
Benefits:
A complete history of the pregnancy, delivery, known medical conditions, developmental milestones and daily management of the infant can provide information important to the PT diagnosis, prognosis and intervention.
Risk, Harm, Cost:
None
Benefit-Harm Assessment:
Preponderance of Benefit
Value Judgments:
None
Intentional Vagueness:
None
Role of Patient/Parent Preferences:
Parents/caregivers can provide much of the history through interview and preadmission information packets; however, obtaining medical records may provide specifics that oral histories may not.
Exclusions:
None
Quality Improvement:
Documentation of the 9 specific health history factors provides uniform data for more effective communication among clinicians and settings, and for uniform data entry in patient registries.
Implementation and Audit:
Create parent/caregiver report forms that are completed prior to the initial examination to assist with collecting the 9 items.
Documentation forms or electronic records may need revision to reflect the 9 specific health history factors.
Audit the completeness of history documentation.
Supporting Evidence and Clinical Interpretation:
In addition to documenting the standard intake information (e.g. date of birth, date of examination, gender, birth rank, and reason for referral or parental concerns, general health of the infant, and other health care providers that are seeing the infant) the PT should specifically document the following 9 birth and health history factors.
Chronological age (and corrected age if the infant was born preterm) at initial visit.41, 61, 71
Age of onset of symptoms,26, 71 which may be aided by early photographs.
Pregnancy history including maternal sense of whether the baby was ‘stuck’ in one position during the final 6 weeks of pregnancy.25
Delivery history including birth presentation (cephalic or breech presentation)26, 72, 73 and low birth weight.72
Use of assistance during delivery such as forceps or vacuum suction.29
Head posture/preference20, 27, 74, 75 and asymmetries of the head/face.20, 26, 32, 51, 76
Family history of torticollis or any other congenital or developmental conditions.77, 78
R. Research Recommendation:
Studies are needed to clarify how the health history factors influence PT diagnosis, prognosis, and intervention.
B. Action Statement 5: Revised and updated. SCREEN INFANTS FOR NON-MUSCULAR CAUSES OF ASYMMETRY AND CONDITIONS ASSOCIATED WITH CMT.
When infants present with or without physician referral, and a professional, or the parent or caregiver indicates concern about head or neck posture and/or developmental progression, physical therapists with infant experience should perform and document screens of the neurological, musculoskeletal, integumentary and cardiopulmonary systems, including screens of vision, gastrointestinal history, postural preference and the structural and movement symmetry of the neck, face and head, trunk, hips, upper and lower extremities, consistent with state practice acts. (Evidence Quality: II-IV, Recommendation Strength: Moderate)
Action Statement Profile
Aggregate Evidence Quality:
Level II-IV from cohort and outcome studies, and expert clinical consensus.
Benefits:
Thorough screening can identify asymmetries and determine their consistency with CMT.
Screening for other causes of asymmetry (e.g. DDH, clavicle fracture, brachial plexus injury, congenital and/or genetic conditions) facilitates referral to specialists.
For infants treated for other conditions (i.e., brachial plexus injuries, reflux, and DDH) associated with higher risks for developing CMT, parents can receive preventative instruction for CMT.
In states where PTs may screen and/or treat without physician referral, infants may receive services more quickly.
Risk, Harm, Cost:
The cost of a PT screening if the infant is not already being treated for other conditions.
The risk that PTs without infant experience may miss or misidentify non-muscular causes of asymmetry.
Benefit-Harm Assessment:
Preponderance of Benefit
Value Judgments:
In some geographic locations or practice settings, particularly where direct access to PT is permitted, PTs may be the first to screen an infant for postural asymmetries. Infants may present for reasons other than head or neck postures, but observing overall symmetry is an element of a thorough PT screen.
Intentional Vagueness:
None
Role of Patient/Parent Preferences:
None
Exclusions:
None
Note:
This Action Statement includes conditions for referral after examination, which were in the 2013 CMT CPG Action Statement 14, but are more appropriate in this statement.
Quality Improvement:
Documentation of screens of the neurological, musculoskeletal, integumentary and cardiopulmonary systems provides uniform data for more effective communication among clinicians and settings, and for uniform data entry in patient registries.
Systematic screening ensures that non-muscular causes of asymmetry or associated conditions are ruled out or that timely referral for additional testing occurs.
Implementation and Audit:
Documentation forms or electronic records may need revision to reflect the data collected from the screens.
Clinicians may require training to enhance consistency and reliability of the system screens.
Audit the incidences in which system screens are positive for potential non-muscular causes of CMT or potential associated conditions.
Supporting Evidence and Clinical Interpretation:
It is within the scope of PT practice to screen for non-muscular causes of CMT in the neuromuscular and musculoskeletal systems, including testing for ocular cranial nerve integrity and coordination, abnormal tone, orthopedic alignment and developmental delay.80 The screen is performed to rule out non-muscular causes of observed asymmetrical posturing22, 74, 75, 80 and to determine whether the PT should refer to or consult with the infant’s physician immediately or continue with a detailed examination for CMT. The screen is conducted through parent report and observation of the infant in different positions. Elements of the screen to document include:
History:
per parent report as described in Action Statement 4.
Systems Screen:
Per the APTA Guide to Physical Therapist Practice,80 a systems screen traditionally examines the following 4 domains. For infants with CMT, a gastrointestinal history should be added.
Musculoskeletal Screen:
Screen for symmetrical shape of the face, skull, and spine;19, 56 symmetrical alignment of the shoulder and hip girdles with particular attention to cervical vertebral anomalies, rib cage symmetry,58 and DDH;51 symmetrical PROM of the neck; and palpation for SCM masses or restricted movement.81
Neurological Screen:
Screen for abnormal or asymmetrical tone, retention of primitive reflexes, resistance to movement, cranial nerve integrity, brachial plexus injury; temperament (irritability, alertness); achievement of age appropriate developmental milestones4, 22, 58, 75, 79, 81 inclusive of cognitive and social integration within the family setting.82 Perform a visual screen comprised of symmetrical eye tracking in all directions, noting visual field defects and nystagmus as potential ocular causes of asymmetrical postures.4, 81,83
Integumentary Screen:
Screen for skin fold symmetry of the hips25, 75 and cervical regions;84, 85 color and condition of the skin, with special attention to signs of pressure and trauma that might cause asymmetrical posturing.75
Cardiorespiratory Screen:
Screen for symmetrical coloration, rib cage expansion and clavicle movement to rule out conditions that might cause asymmetrical posturing (e.g. brachial plexus injuries, Grisel’s syndrome);75, 78 check for acute upper respiratory tract distress.24, 86 The infant should be alert and appropriately vocal, without wheezing.
Gastrointestinal History:
Interview the parents for an infant history of reflux or constipation,24 or preferential feeding from one side,27 both of which can contribute to asymmetrical posturing.
Reasons for Consultation or Referral:
The following are the basis for consultation with or referral to the infant’s physician or other specialists.
Cranial deformation and/or facial asymmetry, including plagiocephaly and brachycephaly.19, 20, 29
Atypical presentations, such as tilt and turn to the same side, or plagiocephaly and tilt to the same side.
Late onset torticollis at 6 months or older, which can be associated with neurological conditions, tissue mass, inflammation or acquired asymmetry.24, 75
Visual abnormalities including nystagmus, strabismus, limited or inconsistent visual tracking, and gaze aversion.75, 81
History of acute onset, which is usually associated with trauma or acute illness.22, 87
Changes in the infant’s color during screening of neck PROM.
If the infant is older than 12 months on initial screening and either facial asymmetry and/or 10–15 degrees of difference exist in active or passive cervical rotation or lateral flexion ROM; or the infant is older than 7 months on initial screening and a SCM mass is present.
R. Research Recommendation:
Studies are needed to identify the precision of screening procedures specific to CMT.
B. Action Statement 6: Revised and updated. REFER INFANTS FROM PHYSICAL THERAPIST TO PHYSICIAN IF INDICATED BY SCREEN.
Physical therapists should document referral of infants to their physicians for additional diagnostic testing when a screen identifies: non-muscular causes of asymmetry (e.g. poor visual tracking, abnormal muscle tone, extra-muscular masses); associated conditions (e.g. cranial deformation); asymmetries inconsistent with CMT; or if the infant is older than 12 months and either facial asymmetry and/or 10–15 degrees of difference exists in passive or active cervical rotation or lateral flexion; or the infant is 7 months or older with a SCM mass; if the side of torticollis changes, or the size or location of an SCM mass increases. (Evidence Quality: II, Recommendation Strength: Moderate)
Action Statement Profile
Aggregate Evidence Quality:
Level II based on cohort follow-up studies of moderate sizes.
Benefits:
Infants with positive screen results are identified and can be co-managed with the infant’s physician and other specialists, e.g. orthotists or surgeons.
Early coordination of care may resolve CMT more quickly and with less cost, as well as initiate appropriate intervention for conditions other than CMT.
Parent support starts earlier for effective home programming, parent education, and the balance of intervention with parental needs to enjoy and bond with their infant.
Risk, Harm, Cost:
Cost of care is increased in the cases when there is a false positive from screening results.
Additional family stress due to concerns about the infant having more serious health conditions.
Benefit-Harm Assessment:
Preponderance of Benefit
Value Judgments:
Level II evidence demonstrates that earlier diagnosis of CMT is better, but there is no literature that documents the risks and consequences of a lack of immediate follow-up for the 18% of infants who have conditions other than CMT.22 While the recommendation strength is categorized as Moderate based on Level II evidence, the GDG believes that referral to the infant’s physician should be categorized as a MUST, when any non-muscular causes of asymmetry are identified to collaborate in the co-management of care of the infant who may have both CMT and other medical conditions.
Intentional Vagueness:
In settings with direct access to PT services, parents may seek evaluation services for an infant with postural asymmetry without referral from the infant’s physician. In either case, a PT should consult with the infant’s physician when any of the aforementioned conditions are present.
Role of Patient/Parent Preferences:
None
Exclusions:
None
Quality Improvement:
Documentation of referral to the infant’s physician when the PT suspects a non-muscular cause of the asymmetry or associated medical conditions provides uniform data for communication across clinicians and settings and and ensures an accurate record of care.
Implementation and Audit:
Consultations or referrals to the physician should include the results of the examination and a rationale for concerns underlying the consult or referral.
Documentation forms or electronic records may need revision with indicators for referrals and rationales for referral.
Audit the incidences in which referrals helped to identify non-muscular causes of CMT and associated conditions.
Supporting Evidence and Clinical Interpretation:
Up to 18% of cases with asymmetrical head posturing may be due to non-muscular causes,22 including Klippel-Feil,22 neurologic disorders,22, 34 ocular disorders,22, 83, 90, 91 brachial plexus injuries including clavicle fractures,22 paroxysmal torticollis that alternates sides,24 spinal abnormalities87, 92 and SCM neoplastic masses34, 85 such as rhabdomyosarcoma.93 Identification of presentations atypical of CMT, including masses that change shape, location, or size, warrant immediate referral to or consultation with the infant’s physician.
R. Research Recommendations:
Studies are needed to clarify the incidence of non-muscular causes of CMT and associated conditions, and how early referral impacts ultimate outcome.
B. Action Statement 7. Revised and updated. REQUEST IMAGES AND REPORTS.
Physical therapists should request, review and include in the medical record all images and interpretive reports, completed for the diagnostic workup of an infant with suspected or diagnosed CMT, to inform prognosis. (Evidence Quality: II, Recommendation Strength: Moderate).
Action Statement Profile
Aggregate Evidence Quality:
Level II based on cohort and outcome studies.
Benefits:
Images and imaging reports, when available, provide a comprehensive picture of the infant’s medical status, including comorbidities.
Images provide visualization of the SCM muscle fiber organization, and the location and size of fibrotic tissue.
Parents appreciate care that is coordinated and shared across disciplines.
Risk, Harm, Cost:
Requesting reports may require additional time for the parents and/or the PTs.
Benefit-Harm Assessment:
Preponderance of Benefit
Value Judgments:
Per the APTA Guide to Physical Therapist Practice,80 requesting relevant clinical reports on an infant’s suspected or diagnosed condition is considered appropriate gathering of medical history.
Intentional Vagueness:
None
Role of Patient/Parent Preferences:
Parents need to formally release information for reports to be forwarded to the PT; parents may arrive with reports and images in their possession.
Exclusions:
None
Quality Improvement:
Document the request for and receipt of reports and images.
Implementation and Audit:
Documentation forms or electronic records may need revision with indicators of requests for and receipt of images and reports.
Audits the incidences in which a report or image helped to inform the prognosis or intervention choices.
Supporting Evidence and Clinical Interpretation:
The current standard of care does not include routine imaging of infants with suspected or diagnosed CMT <1 year of age.94 Rather, infants are typically referred for imaging when there is a specific sign or symptom that raises concern, or there is a lack of progress despite close adherence to the intervention program. Reports and images from specialized exams or laboratory tests can rule out ocular, neurological, skeletal and oncological reasons for asymmetrical posturing.22, 87 In particular, there is a growing body of research using sonoelastography95 or ultrasound imaging to quantify the size, shape, organization and location of fibrous bands or masses,51, 96–98 and to assist with determining an appropriate plan of care and treatment duration.26, 99–102 Ultrasound imaging can also indicate the amount of muscle fiber realignment that occurs over time.96, 98, 103 Emerging evidence suggests that infants with masses or abnormal fiber organization of the SCM are typically identified earlier but require longer episodes of care.73, 102
R. Research Recommendations:
Studies are needed to determine who would benefit from imaging, at what time in the management of CMT images are useful, and how images affect the plan of care.
B. Action Statement 8: Revised and updated. EXAMINE BODY STRUCTURES.
Physical therapists should perform and document the initial examination and evaluation of infants with suspected or diagnosed CMT for the following 7 body structures:
Infant posture and tolerance to positioning in supine, prone, sitting and standing for body symmetry, with or without support, as appropriate for age. (Evidence quality: II; Recommendation strength: Moderate)
Bilateral passive range of motion (PROM) into cervical rotation and lateral flexion. (Evidence quality: II; Recommendation strength: Moderate)
Bilateral active range of motion (AROM) into cervical rotation and lateral flexion. (Evidence quality: II; Recommendation strength: Moderate)
PROM and AROM of the trunk and upper and lower extremities, inclusive of screening for possible developmental dysplasia of the hip (DDH). (Evidence quality: II; Recommendation strength: Moderate)
Pain or discomfort at rest, and during passive and active movement. (Evidence quality: IV; Recommendation strength: Weak)
Skin integrity, symmetry of neck and hip skin folds, presence and location of a SCM mass, and size, shape & elasticity of the SCM muscle and secondary muscles. (Evidence quality: II; Recommendation strength: Moderate)
Craniofacial asymmetries and head/skull shape. (Evidence quality: II; Recommendation strength: Moderate)
Action Statement Profile
Aggregate Evidence Quality:
Preponderance of Level II studies based on well-conducted prospective and retrospective cohort follow-up studies of small to moderate sample sizes.
Benefits:
Confirms the diagnosis of CMT and identifies other problems such as craniosynostosis, DDH, plagiocephaly, brachycephaly, scoliosis, or other orthopedic and medical conditions.
Determines the extent of primary and secondary muscle involvement, to estimate prognosis.
Establishes baselines to measure progress of ROM, strength and alignment, and infant’s ability to incorporate movement through available ranges.
Facilitates systematic linking of interventions to identified impairments.
Standardizes measurement and documentation of body structure limitations from CMT to evaluate group outcomes across clinical settings.
Risk, Harm, Cost:
Examination of passive cervical rotation may result in SCM snapping or a sense of ‘giving way’ in approximately 8% of infants.35
The infant may feel some discomfort or pain, and/or may cry86, 104 due to restricted movement, discomfort with ROM tests, or intolerance of general handling.
In infants with undiagnosed orthopedic conditions, (e.g. osteogenesis imperfecta, hemivertebrae, or cervical instability), there is a risk that overly aggressive testing of PROM could cause secondary injury, though this has not been reported.
Value Judgments:
The evidence for selected measurement approaches varies in strength; however, measures of passive and active ROM, strength, and posture must be documented as part of any PT exam and are consistent with current standards of practice.80 For ROM measurement, the GDG recognizes that clinical practicality has to be weighed against the desire for the most reliable measures. Use of photography, head markers and other devices to increase measurement reliability may create undue burdens for the infant, the family and the PT in daily clinical practice. While there is only moderate to weak evidence to justify the measurement of cervical spine AROM, AROM of the upper and lower extremities, pain or discomfort, condition of the skin folds, condition of the SCM and cervical muscles, and head shape, a lack of evidence is not equated with a lack of clinical relevance. Further, documentation of these initial examination findings sets the baseline for regularly scheduled objective reassessment and outcome measurement.
Intentional Vagueness:
There is no vagueness as to what should be documented. There is variability as to how selected body structures should be measured, due to the limited number of valid tools or methods.
Role of Patient/Parent Preferences:
During testing, parents may perceive that the baby experiences discomfort or that testing positions could potentially harm the baby, resulting in requests to stop testing if the baby is crying. The clinician must be aware and responsive to the parents’ perceptions; it is incumbent on the clinician to fully explain the importance of the measures and the safety precautions used, so that parents and infants can comfortably and accurately complete the testing procedures. Clinicians may need to provide the infant breaks during testing to obtain the baby’s best performance and most reliable measures. Including the parent in the test procedures may help elicit the infant’s best performance, calm the infant if under stress, and generally assist with building trust between the PT and the infant.
Exclusions:
None
Quality Improvement:
Documentation of the 7 elements provides uniform data for more effective communication among clinicians and settings, and uniform data entry in patient registries.
Implementation and Audit:
Documentation forms or electronic records may need revision to reflect the 7 body structure elements.
Additional equipment may need to be procured, such as an arthrodial protractor.
Clinicians may require training to enhance consistency and reliability of the examination elements, specifically cervical PROM using an arthrodial protractor, cervical AROM using the Muscle Function Scale and the stool test, pain assessment using the Face, Legs, Activity, Crying and Consolability (FLACC) scale, and craniofacial asymmetries using the Argenta classification scales.
Use of pictures may require consent and storage procedures for HIPPA compliance.
Audit the incidences in which body structure elements informed intervention.
Supporting Evidence and Clinical Interpretation:
Following a thorough history and screening to rule out asymmetries inconsistent with CMT, the PT conducts a more detailed examination of the infant. The following items appear as a checklist, but in practice, the PT simultaneously observes for asymmetries throughout all exam positions to reduce infant repositioning and increase infant cooperation.
General Posture: Document infant’s posture and tolerance to positioning in supine, prone, sitting and standing for body symmetry, with or without support, as appropriate for age. (Evidence Quality: II, Recommendation Strength: Moderate)
Observe the infant in all positions, documenting symmetrical alignment and preferred positioning or posturing.20, 27, 53, 71, 105 In supine, document the side of torticollis,20, 25, 27, 53 asymmetrical hip positions,25, 27, 76, 106 facial and skull asymmetries, restricted AROM, and asymmetrical use of the trunk and extremities,20, 25, 27, 53, 107 as these are all typical of CMT.
In prone, document asymmetry of the head relative to the trunk, the spine and/or the presence of scoliosis,43 asymmetrical use of the extremities, and the infant’s tolerance to the position. In typically developing infants, greater time spent in prone while awake is positively correlated with higher Alberta Infant Motor Scale (AIMS) scores and fewer delays in achieving prone extension, rolling, unsupported sitting and fine motor control.108, 109 In infants with CMT, positioning in prone at least 3 times per day is correlated with higher AIMS scores.54
In sitting, supported sitting, and supported upright positions (e.g. holding the infant vertically in the air or supported standing as age appropriate), document asymmetrical preferential postures and compensations in the shoulders, trunk and hip.54, 58, 71, 76
If feasible, digital photography may be a fast, reliable method of measuring preferred positioning in supine.110 A baseline is drawn through the acromial processes and another is drawn through the midpoints of both eyes. The intersection angle of the eye line with the shoulder baseline provides an objective measure of preferred head tilt. Care needs to be taken not to record artifacts of the placement of the baby on the surface; photos should represent the typical posture that the baby repeatedly reverts to during the examination session.
PROM: Document the infant’s bilateral passive range of motion (PROM) into cervical rotation and lateral flexion. (Evidence Quality: II, Recommendation Strength: Moderate)
Both passive cervical rotation and lateral flexion / side bending should be measured bilaterally with an arthrodial protractor as described by Öhman.111 The CMT severity grade is determined by the difference between the left and right PROM measures of cervical rotation. Cervical neutral112 needs to be maintained for all measures but is easily compromised when the infant compensates with cervical rotation or extension movements at the end ranges. The PT visually checks the cervical neutral position, assuring that the infant’s nose, chin and visual gaze are directed forwards (neutral rotation), with the nose, mouth and chin vertically aligned (neutral lateral flexion) and the ear lobes and base of the nares are horizontally level (neutral flexion-extension).112
Passive cervical rotation should be measured with the infant in supine, head in cervical neutral, and the nose aligned with the 90 degree vertical reference.32, 111 This approach with an arthrodial protractor is the most commonly referenced standard for measuring passive cervical rotation,20, 25, 26, 31, 32, 70, 111, 113 with a reported inter-rater ICC =.71.114 The benefit of an arthrodial protractor is that the infant’s head is supported beyond the edge of the supporting table, allowing full neck rotation and removing the table surface as a possible barrier to full range. Cervical rotation can be measured reliably by the same rater (ICC = .87–.97) using a standard goniometer aligned along the support surface with the infant lying supine, or in the horizontal plane with children >2 years old if they can independently sit and cooperate115; however, the values from the method used by Klackenberg, Elfving, Haglund-Åkerlind, Carlberg 115 of 49–67 ± 4–9 degrees are distinctly lower than the 110 ± 6 degrees found by others.26, 111
The clinical challenge of using either a goniometer or an arthrodial protractor is that they minimally require 2 adults; one to stabilize the infant’s trunk on the support surface (and this can be the parent/caregiver) and one to rotate the head/neck while measuring range. A 3rd person may be needed to hold the arthrodial protractor in place unless it can be attached to the support surface or stabilized in a stand and calibrated to be level. The GDG strongly values the objective measurement of cervical rotation as a means of establishing a baseline for future comparison. Practice surveys in New Zealand and Canada suggest that PTs often visually estimate, rather than measure rotation range with an instrument; the greatest barrier being the absence of a time efficient and reliable tool.38, 71
Cervical lateral flexion should be measured in supine with the infant’s shoulders stabilized, using an arthrodial protractor for measurement. The PT can either place their hands on the side of the head if the parent stabilizes the trunk and shoulders, or place one hand under the occiput and one hand diagonally across the baby’s chest to palpate for trunk movement and to stabilize the shoulder on the side of the stretch. The head should start in cervical neutral, avoiding neck extension or flexion. The head is laterally flexed until the ear approaches or contacts the stabilized shoulder115 while the opposite shoulder is stabilized; lateral flexion PROM typically reaches 70 ± 2.4 degrees with the limiting factor being cheek size.111 This method is reliable (ICC=.94–.98) when the measures are taken by the same person, using the same set up and procedure, and may be more accurate by 2–3 degrees than photographs taken of the same end range positions.115
When testing cervical PROM, known orthopedic conditions may require modification or avoidance of tests (e.g. osteogenesis imperfecta, congenital hemivertebrae, or children with Down syndrome who have not been cleared for cervical instability). In these cases, the GDG recommends that testing for passive range use only very gentle guidance through the range, ending at the first palpable sign of resistance.
R. Research Recommendation:
Reliable, valid and time efficient methods of measuring infant cervical PROM need to be developed, including lateral flexion, and large-scale normative data of PROM should be established by age in months.
AROM: Document the infant’s bilateral active range of motion (AROM) into cervical rotation and lateral flexion. (Evidence Quality: II, Recommendation Strength: Moderate).
Cervical AROM is considered an important indicator of symmetrical development and neck strength70, 76, 111, 116 and the infant’s integration of PROM for functional activities. Treatment to improve AROM is consistent with the goals of early intervention.82 Asymmetrical movements and movement compensations can indicate muscle tightness, restrictions or weakness.63, 117
Active range is challenging to measure in infants due to behavior and movement variability, difficulty with isolating cervical movements, and a paucity of practical measurement tools that capture infant movements in the clinical setting in a timely manner.38, 71 Studies may list “active movement” as an outcome but do not describe how it is measured and many PTs rely on visual estimation.71
PTs should measure active cervical movement by using one of the following techniques, looking for active and full range in all planes, including diagonals, while the baby is enticed to follow toys, sounds or other forms of stimulation to elicit full range.
For the infant who is <3 months old, head rotation is tested in supine.118
For the infant who is ≥3 months old, test neck rotation while the infant sits in the clinician’s lap who is on a rotating stool, named the stool test. The parent entices the infant to maintain eye contact while the PT rotates the baby away from the parent. The PT observes neck rotation from above using the baby’s nose as a midline indicator as it approaches the shoulder.118 Additionally, neck flexion and extension can be screened in this sitting position.
For infants 2 months and older, the Muscle Function Scale provides an objective categorization of active lateral flexion in developmentally appropriate positions.111, 119 By holding the infant vertically in front of a mirror and tipping the baby horizontally, the PT classifies the head righting position according to a 6-point scale.119 Typically developing infants rarely have a difference between sides, and infants with CMT frequently have a difference of 2–3 points.119 Clinicians should refer to Öhman, Nilsson, Beckung 119 for specific reference values and procedures.
R. Research recommendations:
Determine the sensitivity and specificity of the Muscle Function Scale to differentiate infants with clinically significant limitations from typically developing infants.
Establish a clinically practical, objective method of measuring cervical rotation AROM in infants 0–3 months and infants ≥3 months to assess baselines and changes over time.
Determine what, if any, correlation between active and passive ROM should be used for discontinuation and/or discharge criteria.
Trunk and Extremity ROM: Document the infant’s PROM and AROM of the trunk and upper and lower extremities, inclusive of screening for possible developmental dysplasia of the hip (DDH). (Evidence quality: II; Recommendation strength: Moderate)
The PT should examine passive and active ROM of the spine, shoulder and hip girdle, and arms and legs by observing the natural movements of the infant and by passively moving the arms and legs through all available range at each joint to rule out brachial plexus injuries, clavicle fractures, neurological impairments, hypermobility or CNS lesions.4, 22, 51, 58, 60, 113
To rule out DDH, PTs should observe for symmetry and stability of the hip, and symmetry of the leg lengths and gluteal skin folds.106 The incidence of DDH with CMT ranges from 2.5%53 - 17%21 depending on inclusion criteria, and it increases with the severity of neck rotation restriction.32 While routine screening of all infants for DDH is controversial,120, 121 infants at risk for or those diagnosed with CMT may have a slightly higher incidence.21, 89 Factors such as a history of breech position (OR 4.68 [1.66,13.03]) or cesarean delivery (OR 5.19 [2.06,12.04]),88 family history, maternal age < 20 years, or completion of the evaluation Apgar scores < 8 at 1 minute,122 and being female120 have been associated with greater risk of DDH. No single test or observation is sufficient to diagnose the presence of DDH, nor does the presence of DDH in young infants necessitate immediate treatment, as >90% of newborns with DDH confirmed by ultrasound may resolve on their own.123 Conversely, a missed diagnosis of DDH may cause the infant more suffering if treated later with bracing or surgery, thus the Ortolani and Barlow maneuvers and skin fold assessment are traditionally included in the evaluation of the infant under 3 months of age with CMT.106, 124 Though the sensitivity of the tests vary among studies,120, 125 the specificity for ruling out DDH is stronger.120, 126 After 3 months of age, the Ortolani and Barlow maneuvers may not be sensitive enough to pick up DDH as the joint capsules tighten.126 For infants >3 months old, the Galeazzi sign (asymmetrical shortening of the affected leg), asymmetrical posture of the legs and skin folds, and restrictions in hip abductor PROM may be stronger indicators for DDH, especially since it would be expected to resolve by that time.126
Pain: Document the infant’s pain or discomfort at rest, and during passive and active movement (Evidence Quality: IV, Recommendation Strength: Weak)
PTs should observe for behaviors reflective of body structure discomfort or pain in infants and children during examinations.85, 105, 127 Pain is not typically associated with the initial presentation of CMT59 but may be associated with passive stretching.43, 128 The infant may cry in response to stretching,128 or in response to handling from the therapist; children >2 years old may be able to provide self-reports of pain.127 PTs should differentiate actual pain responses from discomfort or behavioral reactions to stretching, anxiety, or the stress of an unusual environment. Despite acknowledging the possibility of pain, no assessment tools for identifying or rating pain are reported in the CMT literature.
There are 3 clinician-rated pediatric pain scales that quantify infant pain related behaviors and that do not rely on physiological monitoring (e.g. heart rate, blood pressure, oxygen saturation, body temperature). The Children’s and Infants’ Postoperative Pain Scale (CHIPPS)129 has been validated for newborns through 5 years of age for post-surgical pain, and is available in English and Portuguese.130 The Face, Legs, Activity, Crying and Consolability (FLACC) is valid for children from 2 months to 7 years of age131, 132 and in children < 3 years old before and after anesthesia.133 The revised rFLACC134 is valid for children 4–19 years old including those with cognitive impairments. Parent descriptions of their children’s specific pain reactions are part of the rFLACC, and the clinician can observe for those specifically.
Since the FLACC is valid for the typical age range of infants and children treated for CMT, the GDG continues to recommend its use over the CHIPS or rFLACC. The FLACC is administered by having the clinician rate facial expressions, movement and behavior state with a 3 point scale of “0”= no expression or a quiet state, “1”= occasional expression or movements and “2”= inconsolable and large, frequent movements for a maximum of 10 points; lower scores indicate fewer pain related behaviors and higher scores indicate more behaviors. Training in the use of the FLACC is required to achieve adequate reliability.133 One method to differentiate pain from behavioral distress is to hand the inconsolable baby back to its parent/caregiver, observing how quickly the infant quiets. Another option is to have the caregiver do the handling with PT instruction and observe the infant’s reactions to differentiate true pain from discomfort or behavioral reactions.
R. Research Recommendation:
Studies are needed to
Describe and differentiate signs of discomfort from the types of pain reactions typically observed in infants with CMT during specific testing or interventions
Determine the validity of the FLACC in rating true pain reactions during CMT examinations or interventions.
Skin and Muscle: Document the infant’s skin integrity, symmetry of neck and hip skin folds, presence and location of a SCM mass, and size, shape & elasticity of the SCM muscle and secondary muscles (Evidence Quality: II, Recommendation Strength: Moderate)
Skin:
PTs should observe the symmetry and condition of the skin folds around the neck and hips. Typically, the neck skin folds on the anterior affected side are deeper and reddened.81 Infants with brachycephaly and limited cervical ROM in all directions may have deeper posterior folds.85 Observe for symmetry of the hip skin folds in the inguinal and upper thigh area as an indicator of DDH.75, 106
Muscle:
PTs should visually inspect and palpate both SCM muscles and document the side of tightness, the presence or absence of a fibrous band and/or mass, and if a mass is present, note its size and location along the SCM muscle (inferior, middle, superior or entire length).96 The presence of a fibrous band and/or mass, particularly a mass that involves more than the distal 1/3 of the muscle, is correlated with greater severity of the condition.96, 114 These qualities are useful for determining the CMT severity and estimating the episode of care. 26, 32, 35, 51, 63, 96, 102, 114
PTs should document the presence of secondary asymmetries, compensations or atypical tone in the shoulders, trunk, hips and distal extremities while the infant moves through positions during the examination. Typical compensations include tightness of the upper trapezius muscle,135 imbalance of neck muscle strength,111 hiking of the shoulder on the same side of the involved muscle,136 asymmetrical preference for limb use,76, 137 asymmetrical and delayed protective and righting reactions of the head, neck, and trunk,79 Trendelenburg’s sign in children who are walking,106 and scoliosis.76 Secondary compensations and asymmetries of movement need to be continually monitored across the episode of care as they can develop and/or worsen over time.18, 56, 76, 136
Craniofacial: Document the infant’s craniofacial asymmetries and head/skull shape. (Evidence Quality: II, Recommendation Strength: Moderate)
Facial asymmetries involve the relative alignment of each side of the jaw, the cheekbones, eye orbits and ear positions.19, 20, 25, 29, 37, 56, 138 Cranial asymmetries or cranial deformation (CD) refers to asymmetries of the skull, including the frontal, temporal, parietal, and occipital bones, presenting with posterior unilateral flatness (plagiocephaly), bilateral posterior flattening (brachycephaly), asymmetrical brachycephaly, or flattening on both sides of the skull (scaphocephaly).58, 65, 139
Peitsch et al. reported the incidence of localized cranial flattening as 13% in typical singleton infants,65 and 55.6% in twins.65 Cheng et al reported a 90.1% prevalence of craniofacial asymmetry in children with CMT at initial evaluation.26 Untreated CMT can cause craniofacial asymmetries on the side of the torticollis, including: reduced jaw or ramal height, a smaller and elevated eye with changes in the orbit, (recession of the ipsilateral zygoma), recession of the ear on the affected side, a flat appearance of the jaw, malocclusion and possible gum line asymmetry.19, 20, 56, 138
Cranial deformation can either cause or be a result of CMT. Limited AROM from CMT may cause CD as asymmetrical muscle tensions lead to an asymmetrical postural head preference and subsequent skull deformation. 19, 27, 59, 65, 74, 118, 140 Conversely, for infants with CD and no initial CMT, an asymmetrical resting position of the skull may cause persistent neck rotation that can lead to SCM tightness.25,59, 74, 141,118, 140
PTs should document asymmetries of the skull and face. One of the most clinically feasible tools are the classification scales by Argenta.139 The method is clinically practical, does not require equipment other than a copy of the scale, includes pictures to assist with rating, and has moderate inter-rater (mean weighted κ score =.54) and substantial intra-rater reliability (weighted κ scores ranged from =.6–.85).142 Other methods to quantify head shape asymmetries exist, and when more reliable or accurate methods for quantifying head shape are available and feasible, PTs should use them. Examples include: plagiocephalometry,143, 144 the modified Severity Scale for Assessment of Plagiocephaly,145 a craniometer with a headband,146 molding a flexible ruler to the infant’s head shape and tracing the shape,147 3-Dimensional computerized scanning,148 plaster of Paris molds of the infant’s head,149 and the Children’s Healthcare of Atlanta Plagiocephaly Severity Scale.150 These alternative methods are sometimes not tolerated well by the infant, are not clinically practical, or are less intuitive than the Argenta classification scales.
PTs should document when CD or facial asymmetry are inconsistent with deformational plagiocephaly or brachycephaly and refer back to the infant’s physician to assess for craniosynostosis.151
B. Action Statement 9: Upgraded with new evidence. CLASSIFY THE LEVEL OF SEVERITY.
Physical therapists and other health care providers should classify and document the level of CMT severity, choosing 1 of 8 proposed grades (Figure 2), based on infant’s age at examination, the presence of a SCM mass, and the difference in cervical rotation PROM between the left and right sides. (Evidence Quality: II, Recommendation Strength: Moderate)
Action Statement Profile
Aggregate Evidence Quality:
A level II cohort reliability study.
Benefits:
Classifying levels of severity may assist with prognosis and parent education.
The 8 grades integrate 2 of the strongest factors related to outcome: the infant’s age at which treatment is initiated and the type of CMT the infant presents with.
More precise classification grades are needed to compare outcomes across research samples.
Risk, Harm, Cost:
Minimal costs to update electronic health records to add Grade 8 and to retrain staff on its use.
Benefit-Harm Assessment:
Preponderance of Benefit
Value Judgments:
The GDG recommends the use of its updated CMT Classification System. Clinician feedback and its uptake into practice7 suggest that the grades assist with educating families about the estimated episode of care.
Intentional Vagueness:
There is no evidence as to whether the chronological or corrected age should be used for infants born preterm to determine the severity grade. Clinicians should document both ages in their practice setting. The GDG recommends using corrected age when determining the severity grade.
Role of Patient/Parent Preferences:
None
Exclusions:
None
Quality Improvement:
Documentation of a severity grade provides a common taxonomy for clinical and research communication, and for uniform data entry in patient registries.
The severity grades are a tool for communicating with parents about the estimated episode of care.
Implementation and Audit:
Documentation forms or electronic records may need revision to reflect the CMT Severity Classification grades, including the addition of Grade 8.
Clinicians may require training to enhance consistency and reliability of the CMT Severity Classification System.
Audit the frequency of documentation of the CMT Classification Severity grades and the accuracy of prognoses with respect to episode of care and functional outcomes.
While there are no studies that correlate the severity of cervical lateral flexion to the severity of CMT or the episode of care, PTs should document objective measures of lateral flexion as a type of asymmetry.
For infants who change service providers to treat CMT, CMT severity should be classified based on the infant’s current age and initial examination findings by the new provider.
Supporting Evidence and Clinical Interpretation:
Multiple taxonomies of CMT classification recur in the literature: age of treatment initiation,36, 57 type of CMT (postural, muscular or SCM mass),26, 57, 113, 114 severity of ROM limitations,26, 32 presence of plagiocephaly,58, 60 and muscle fiber appearance by ultrasound.23, 96, 152 In most studies, these taxonomies are detailed enough to answer the research questions about incidence of various types, incidence of surgical outcomes, and usefulness of ultrasound as a diagnostic tool or classification process. The use of ultrasound to determine a CMT classification is beyond the scope of typical pediatric PT practice.
When looking for guidance on intervention effectiveness for CMT, study samples typically analyze outcomes according to the type of CMT (postural, muscular or SCM mass), the age of presentation,41 or cervical rotation PROM.62, 114, 153 These 3 factors are considered strongly correlated with outcomes, such that the earlier one is treated and the milder the form of CMT, the shorter the episode of care and the higher the probability of complete resolution.36 No studies were found using passive lateral flexion as a factor for categorizing CMT outcomes.
The 2013 CMT CPG proposed a 7-grade CMT Severity Classification System that combined the 3 factors (i.e., age, PROM, mass) to add clarity to research and aid communication among clinicians. The original 7 grades have good inter-rater reliability (ICC (2,1) = 0.83 [95% CI 0.74–0.91]) and good intra-rater reliability (ICC (3,1) = 0.81 [95% CI 0.66–0.91]).9 In a survey of 282 PTs who treat children with CMT, only 3% classified severity with any scale prior to the 2013 CMT CPG; following its publication, the 7-grade CMT Severity Classification System was implemented by 57%.7 This 2018 CMT CPG updates the original 7 grades to 8, based on clinician confusion as to how to grade toddlers >12 months old,9 and because the majority of the evidence estimating episodes of care is based on infants younger than 12 months of age.
Figure 2 presents the updated diagram to include Grade 8 for children who are referred for PT at age 12 months or older, regardless of the type of CMT (postural, muscle tightness or SCM mass). The diagram is best viewed in the color version available at (https://pediatricapta.org/clinical-practice-guidelines/), however to aid clarity with non-color copies, the lines from conditions to grades are patterned. An additional line was added to classify 4–6 month olds with only postural preferences as Grade 1. The vertically aligned ovals, at the left most edge of the diagram, list the factors that are most relevant to the classification process (age asymmetry noted, age of referral and PT evaluation, type of CMT), followed by diamonds that describe the cycle of PT examination, intervention and reassessment. To the right are the range of conditions and actions that link the classification with PT management.
SEVERITY GRADE DEFINITIONS
Grade 1 – Early Mild: Infants between 0–6 months of age with only postural preference or a difference between sides in passive cervical rotation of <15 degrees.
Grade 2 – Early Moderate: Infants between 0–6 months of age, with a difference between sides in passive cervical rotation of 15–30 degrees.
Grade 3 – Early Severe: Infants between 0–6 months of age, with a difference between sides in passive cervical rotation of >30 degrees or a SCM mass.
Grade 4 – Late Mild: Infants between 7–9 months of age with only postural preference or a difference between sides in passive cervical rotation of <15 degrees.
Grade 5 – Late Moderate: Infants between 10–12 months of age with only postural preference or a difference between sides in passive cervical rotation of <15 degrees.
Grade 6 – Late Severe: Infants between 7–9 months of age with a difference between sides in passive cervical rotation of >15 degrees, or between 10–12 months with a difference of 15–30 degrees.
Grade 7 – Late Extreme: Infants between 7– 12 months with a SCM mass, or 10–12 months of age with a difference between sides in passive cervical rotation of >30 degrees.
Grade 8 - Very Late: Infants and children older than 12 months of age with any asymmetry, including postural preference, any difference between sides in passive cervical rotation, or a SCM mass.
The classification process begins at the top of the diagram. Document the age that asymmetry is first noted by a parent or health professional; this may be informed by early infant photos. This age provides history of the condition and may impact the prognosis for the episode of care; however, it does not directly factor into the choice of severity grades. The age of referral for PT evaluation is documented to understand the timeliness between referral and the initial PT evaluation. The age of initial PT evaluation is documented and used in combination with the difference in cervical rotation PROM and/or presence of a SCM mass to determine a severity grade. Classifications are first grouped as “early”, “later” or “very late.” “Early” and “later” have a range of severity within the categories. For example, CMT Classification Grade 2, Early Moderate, is assigned to an infant evaluated by PT either between 0–3 months, or between 4–6 months, with a difference between sides in cervical rotation PROM of 15–30 degrees. The estimated episode of care is based on a constellation of factors including environmental and family resources and would be estimated at a shorter period for the younger infant. A CMT Classification Grade 7, Late Extreme, is assigned to an infant evaluated by a PT between 7–9 months of age with a SCM mass or between 10–12 months of age with difference between sides in cervical rotation PROM of >30 degrees or a SCM mass. Although it would be convenient to assume that there is a linear relationship between the severity grades and the episode of care, there are many factors that may influence an overlap in time frames, with unexpected reductions or extensions in the episode of care. A study by van Vlimmeren, Engelbert, Pelsma, Groenewoud, Boere-Boonekamp, Nijhuis-van der Sanden 154 illustrates how the grades can describe study samples more accurately.
Decisions regarding intervention intensity, frequency and duration take into consideration each of the factors within the large central oval: Severity Classification Grade, Access to Services & Clinician Knowledge and Skill, Patient/Caregiver CMT Knowledge and Program Adherence, Muscle Tissue Characteristics, Infant’s Developmental Stage, and Comorbidities. Action Statement 12 regarding prognosis supports the idea that the earlier and more intense the intervention, the shorter the episode of care and the more complete the resolution of symptoms. No specific recommendation of intensity of intervention is appropriate for all cases. Regardless of severity, when a PT intervention is initiated, the first-choice interventions should be performed frequently throughout each day with responses to intervention regularly reassessed for effectiveness. While a minimum of 1.5 months36 and a maximum of 36 months63 of conservative intervention is reported, the majority of studies cite a range of 4–6 months duration for intervention.
R. Research Recommendation:
Studies are needed to determine a reliable, valid and clinically practical method of measuring cervical lateral flexion, and then to determine how the severity of lateral flexion may relate to the CMT Severity Classification grades.
B. Action Statement 10: Revised and updated. EXAMINE ACTIVITY AND DEVELOPMENTAL STATUS.
During the initial and subsequent examinations of infants with suspected or diagnosed CMT, physical therapists should examine and document the types of and tolerance to position changes, and motor development for movement symmetry and milestones, using an age appropriate, valid and reliable standardized test. (Evidence quality: II; Recommendation strength: Moderate)
Action Statement Profile
Aggregate Evidence Quality:
Level II from cohort and outcome studies.
Benefits:
Early detection of developmental delays, neurological impairments, movement capabilities, muscle function in developmental positions, and infant preferences help to direct the plan of care.
Provides opportunities for parent education on typical development, importance of prone playtime, alternative positioning, and reinforcement of parent adherence to home programs.
Standardizes measurement and documentation of motor activity to evaluate group outcomes across clinical settings for infants with CMT.
Risk, Harm, Cost:
No risks or harms
Norm-referenced developmental standardized tests are proprietary and thus have associated costs for the forms, test manuals and test items. Proficiency in administering the tests may require training.
Benefit-Harm Assessment:
Preponderance of Benefit
Value Judgments:
Measures of the infant’s activity, symmetry of movements, and developmental progression must be documented as part of any PT exam. These are consistent with professional standards of practice,80 and clinical practice specific to CMT.38, 71
Intentional Vagueness:
None
Role of Patient/Parent Preferences:
Parents may perceive that the baby experiences discomfort from the testing positions or that the prone position is harmful and may request that testing not continue if the baby is crying. The clinician should fully explain the importance of varying the infant’s positions, including use of prone positioning, which may be avoided by parents due to misinterpretation of Back to Sleep instructions.54
Exclusions:
None
Quality Improvement:
Routine assessment of development ensures that infants with CMT are achieving age appropriate milestones, and if not, that delays are addressed as they are identified.
Implementation and Audit:
Documentation forms and electronic records may need revision to include the recommended standardized developmental tests and documentation of asymmetries during developmental activities.
Clinicians may require training to enhance consistency and reliability to administer standardized developmental tests.
Audit the incidences in which the standardized developmental tests are completed and inform intervention.
Supporting Evidence and Clinical Interpretation
Infants with CMT have a higher prevalence of gross motor delay at 2 and 6 months of age.53, 54 The motor delay of most infants undergoing PT for CMT resolves by 8–15 months,53, 54 but similar to the general population, some will continue to demonstrate a gross motor delay.53 PTs should use a standardized test with established predictive validity to monitor infants with CMT for potential developmental delays, and if identified, should address remediation of those delays in their plans of care. The GDG recommends using age appropriate, reliable and valid standardized tests, such as the Test of Infant Motor Performance (TIMP) through 4 months of corrected age (http://thetimp.com/), the Alberta Infant Motor Scale (AIMS) from 1– 18 months of corrected age or until walking,155 or the gross motor subtests of the Peabody Developmental Motor Scales, 2nd edition (PDMS-2) from 1 – 72 months of age,156 during the initial evaluation and reassessments. While certification is not required to administer these tests, the validity of the scores and test-retest reliability may be improved following formal training. Additionally, the PT should observe and document asymmetries of age appropriate developmental activity, movement and upper and lower limb use throughout all exam positions.76
R. Research Recommendation:
Studies are needed to identify the best developmental tests to use for infants with suspected or diagnosed CMT, from birth through 12 months, so that the same measures can be documented on all infants, enabling comparison of outcomes across studies.
B. Action Statement 11. Revised and updated. EXAMINE PARTICIPATION STATUS.
The physical therapist should obtain and document the parent/caregiver responses regarding:
Positioning when awake and asleep. (Evidence quality: II; Recommendation strength: Moderate)
Infant time spent in prone. (Evidence quality: II; Recommendation strength: Moderate)
Whether the parent is alternating sides when breast or bottle feeding the infant. (Evidence quality: II; Recommendation strength: Moderate)
Infant time spent in equipment/positioning devices, such as strollers, car seats or swings. (Evidence quality: II; Recommendation strength: Moderate)
Action Statement Profile
Aggregate Evidence Quality:
A predominance of level II prospective cohort follow-up studies with small sample sizes.
Benefits:
Identifies routine passive positioning that facilitates asymmetrical positions of the head, neck and trunk.
Provides information about the general developmental activities and position preferences of the infant.
Provides opportunities for parent/caregiver education and counseling about positioning and activities that facilitate symmetrical development, including successful breastfeeding.
Risk, Harm, Cost:
None
Benefit-Harm Assessment:
Preponderance of Benefit
Value Judgments:
None
Intentional Vagueness:
None
Role of Parent or Patient Preferences:
Parents and caregivers must accurately describe the infant’s daily care routines so positioning and home exercise programs can be tailored to maximize implementation opportunities and enhance the success of early parent roles. Fear of blame for the infant’s condition may lead parents/caregivers to provide inaccurate descriptions. Clinicians should be sensitive to this and may need to build a level of trust with the parents/caregivers before an accurate description can be obtained.
Exclusions:
None
Quality Improvement:
Routine examination of participation ensures that parent–infant dyads are appropriately and successfully interacting during daily routines in ways that optimize motor development.
Implementation and Audit:
Documentation forms and electronic records may need revision to reflect the 4 participation elements listed above.
Clinicians may require training to enhance consistency and reliability for assessing participation.
Audit the incidences in which the participation elements are documented and inform intervention.
Supporting Evidence and Clinical Interpretation:
There is consensus about the need to assess across all the domains of the ICF, including infant participation in daily routines, to develop a comprehensive plan of care.38, 71,79 Moderately strong evidence suggests that specific activities are either preludes for possible asymmetrical development or are the consequences of existing asymmetries.
Positioning When Awake and Asleep, Including Time Spent in Prone:
Documentation should address positioning when awake and asleep, while feeding, and while using positioning devices (e.g. car seats, changing tables, cribs). The purpose of asking parents/caregivers about positioning is to prevent deformational plagiocephaly that may be associated with CMT,60 to correct postural preference that can lead to CMT and plagiocephaly,20, 58, 74, 157 and to treat CMT if present. Three aspects of positioning support an interaction effect with CMT resolution: use of prone positioning, asymmetrical handling to activate weak neck musculature and AROM toward the limited side and feeding from alternate sides.
Prone positioning while awake for greater than 1 cumulative hour per day, with no minimum amounts of time per opportunity, appears to offset the transient effects of supine sleep positions on motor skill acquisition.158, 159 Supine positioning is associated with postural preference and consequently may facilitate asymmetrical neck ROM and secondary development of plagiocephaly.27, 141 Infants who spend more time in prone and side lying positions reduce the impact of preferred positioning27 and achieve motor milestones sooner.54, 160 Though prone sleeping is counter to the Back to Sleep recommendations,161 and is not recommended by the GDG, it has been associated with faster achievement of developmental milestones.162
The conscientious use of positioning during wakeful activities, (e.g. play, feeding, and dressing) facilitates symmetrical development of head shape,60, 163 active and passive neck motion,60, 104 tolerance of prone positioning,159 and achievement of motor milestones.70, 164 Conscientious positioning means that the parent actively places the infant in positions during play, on changing tables, in cribs or carries the infant in ways that require head righting, rotation toward the restricted side, neck and upper body extension,70 or visual attraction toward the affected side. Active movement toward the affected side37 and alternation of trunk and limb movements165 help to counteract asymmetries and prevent potential ones. For the infant with postural preference, these activities may reduce the preference and avoid consequential tightness.
Parents are reported to avoid prone positioning with typically developing infants because the infant does not tolerate the position or because the infant has already achieved independent sitting.159 Education about the importance of prone playtime is critical for infants with suspected or diagnosed CMT, as they have multiple risks of asymmetrical development and delayed motor milestones. PTs should assess each parent’s ability to carry out exercises and home program positioning.
Feeding:
PTs should document the infant’s feeding positions and difficulties as reported by the parent/caregiver during the initial and periodic evaluations. Feeding problems have been identified in infants with CMT and/or plagiocephaly as asymmetrical jaw positioning,166 preference for side of nursing,74, 141 and /or side of bottle feeding.62, 141 As many as 44% of infants with CMT may demonstrate a feeding preference to one side,62 and as many as 2.4% are described as having additional feeding problems.51 In conjunction with infant preference, the parent’s preferred side or hand dominance may also bias positioning to bottle feed from the same side.27 Conversely, infants who breastfeed from both sides have a lower incidence of CD and CMT, possibly due to frequency position changes as compared to infants who are bottle fed on the same side at each feeding.167 Intervention that addresses alternating sides and alternative positions168 for feeding can effectively increase symmetrical positioning, reduce preferred positioning by the infant and improve parent self-efficacy with feeding. Interviewing parents/caregivers about their comfort with alternating feeding positions is common practice,38, 71 is consistent with family centered care,82 and provides an opportunity to suggest positioning strategies.
Equipment/Positioning Devices:
PTs should document the amount of time the infant spends in positioning equipment as reported by the parents (e.g. positioning/seating devices, strollers, car seats, cribs, or swings).118 Persistent use of supportive equipment, in lieu of time spent playing in prone or side lying, may facilitate the deformation of the developing skull due to gravitational forces, which increases the risk of CMT and other asymmetrical developmental movement patterns. The PT should discuss practical strategies with the parents/caregivers regarding positioning and movement facilitation, including alternating positioning of toys and placement in cribs,76, 167 and ensuring frequent opportunities to play in prone from an early age.54, 85, 164 Avoidance of prone placement by parents can occur if the infant does not tolerate it well; the discussion offers an opportunity to assess parent/caregiver comfort and provide graded strategies for prone positioning that build on the infant’s tolerance.
R. Research Recommendations:
Studies are needed to quantify changes in participation and clarify how the participation elements inform the plan of care.
B. Action Statement 12: Reaffirmed and updated. DETERMINE PROGNOSIS.
Physical therapists should determine and document the prognosis for resolution of CMT and the episode of care after completion of the evaluation and communicate it to the parents/caregivers. Prognoses for the extent of symptom resolution, the episode of care, and/or the need to refer for more invasive interventions are related to: the age of initiation of treatment, classification of severity (Figure 2, SDC 2), intensity of intervention, presence of comorbidities, rate of change and adherence with home programming. (Evidence Quality: II, Recommendation Strength: Moderate)
Action Statement Profile
Aggregate Evidence Quality:
Level II-IV cohort studies and case reports with long term follow-up.
Benefits:
Links the exam results and CMT Classification Severity grade to interventions and/or referrals.
Provides guidance on the frequency and dosage of intervention(s) across episodes of care.
Allows parents/caregivers to psychologically prepare for what to expect from PT and the range of possible outcomes for their infant.
Assists parents with understanding and implementing the plan of care.
Articulates the relationship of exam results to expected outcomes for documentation, including letters of medical necessity.
Risk, Harm, Cost:
Lack of a prognosis by either the referring physician or the PT may lead to under-estimation of the CMT severity, resulting in inadequate or untimely delivery of care and/or parent/caregiver confusion about what to expect.
Benefit-Harm Assessment:
Preponderance of Benefit
Value Judgments:
The GDG supports the need to document the potential for improvement of CMT prior to initiating intervention. The PT prognosis is the bridge between the evaluation of initial examination results and classification of severity with the associated interventions within an expected timeframe; thus it should include both objective outcomes to achieve, and time frames in which to achieve them. Articulating the prognosis for PT management ensures clear communication of expectations for the parents/caregivers, and sets objective milestones as a basis for referral back to the primary physician if outcomes are not met. Prognosis is a continual process that occurs throughout the episode of care.
Intentional Vagueness:
None
Role of Patient/Parent Preferences:
The prognosis for improvement, or the time to achieve change, may need to be corrected based on the parent/caregiver ability to perform the exercises and adhere to a home program designed by the PT. Parents should participate in shared decision making with the PT to design a home program that addresses both the infant’s limitations and other parental responsibilities.
Exclusions:
None
Quality Improvement:
Determining a prognosis provides the family and caregivers, healthcare providers, and payors an estimate of the episode of care.
Implementation and Audit:
Educate parents and caregivers about the estimated episode of care and the importance of consistently implementing the home program to maximize outcomes.
Update documentation forms or electronic records to include prognosis based on uniform collection of age of initiation of treatment, CMT Classification Severity grade, intensity of interventions, presence of comorbidities, rate of change, and adherence to home program.
Include the prognosis and estimate of the episode of care on the initial evaluation document and in all professional communications.
Audit the frequency of documentation of prognoses and the accuracy of prognoses with respect to episode of care and functional outcomes.
Supporting Evidence and Clinical Interpretation:
A PT is responsible for determining a prognosis following the patient evaluation.80 A prognostic statement should include the expected outcome in objective measurable terms, the time frame for intervention to achieve the outcomes, and a description of the potential courses of the condition if treated or not. For CMT, the earlier and more intense the intervention, the shorter the episode of care and the more complete the resolution of symptoms.36, 37, 103,32, 43, 169
Demirbilek, Atayurt 57 found the prognosis for full resolution of CMT, treated conservatively prior to 3 months of age, was 100% and lower (75%) when treated after 3 months of age. Five factors have been associated with full or more complete symptom resolution including the infant’s: (1) participation in PT intervention,170 (2) younger age at initiation of treatment,36, 43,41, 62–64 (3) decreased difference in cervical rotation PROM between sides,153 (4) decreased difference in SCM muscle thickness between sides,171 and (5) the caregiver’s ability to frequently implement a home program of active positioning and passive stretching.64
The episode of care has been associated with the severity of the CMT, with mildest forms requiring an average of 2–3 months of treatment, and more severe forms requiring up to 5–6 months of treatment.32 Infants who receive surgical interventions may require an additional 426 to 1118, 172 months of treatment. Seven factors have been associated with a longer episode of care including: (1) older age at initiation of treatment,72 (2) increased restriction of neck rotation PROM,63 (3) increased severity of head tilt,173 (4) motor asymmetry,107 (5) increased thickness72 or stiffness174 of the involved SCM or higher thickness ratio between the involved and uninvolved SCM,72, 173 (6) the presence of a SCM mass or lesion,102,63, 73, 175 and (7) delivery history including infants with lower birth weight72 and breech, compared to cephalic, presentation.72
There is no consensus on the intensity, frequency or delivery of intervention that is appropriate for all cases, except that more frequent stretching and strengthening throughout the day is more effective than less.176 Öhman, Nilsson, Beckung 64 provide preliminary evidence of better outcomes when infants are treated by a PT versus parents, but the combination of PT and home program is the more frequent intervention plan.31, 63, 103, 114 Individual intervention is the most commonly provided delivery model, but a single observational study of group CMT intervention (each group consisted of 6 infant-parent dyads and 2 PTs) suggests that this model may be an alternative to individual intervention.177 Additional research is needed to determine the equivalency of outcomes and the cost-effectiveness of group compared to individual intervention.
R. Research Recommendations:
Studies are needed to:
Clarify the interaction between the factors associated with full symptom resolution and episode of care.
Clarify the accuracy of prognosis with respect to full symptom resolution and episode of care.
Describe and clarify the efficacy of different delivery models, e.g. individual versus group or clinic versus home.
III. PHYSICAL THERAPY INTERVENTION FOR INFANTS WITH CMT
The literature continues to support the following 5 components as the first-choice intervention for CMT: neck PROM; neck and trunk AROM; development of symmetrical movement; environmental adaptations; and parent/caregiver education. The provision of interventions allows for continuous evaluation of progress along all ICF domains, including body structure and function, activities and participation. Moreover, repeated objective measurement of progress can focus intervention choices to achieve goals more quickly.8 It is incumbent on the PT to educate the parents on the importance of the home program178 and to partner with them to incorporate a reasonable and effective program into the home and family schedule. Care should be taken to balance the full scope of the family demands and resources on a case-by-case basis.
It is important to look beyond the infant’s body structure limitations to include perceptual–motor experiences within the context of the infant’s social environment, and gross and fine motor exploration as contributing to the development of cognition.82 Infants with limited or asymmetrical exploration, as seen in CMT and CD,53, 79, 164 have demonstrated delays in early motor development that may affect the development of early perceptual-motor skills and, by inference, cognition.82 Thus, pediatric PTs should treat beyond the body structure level to design and provide interventions that incorporate the infant’s available functional range into activities that promote age appropriate participation, and that promote current and future development and learning across domains.82
B. Action Statement 13: Revised and updated. PROVIDE THESE FIVE COMPONENTS AS THE FIRST-CHOICE INTERVENTION.
Physical therapists should provide and document these 5 components as the first-choice intervention for infants with CMT:
Neck PROM. (Evidence quality: II; Recommendation strength: Moderate)
Neck and trunk AROM. (Evidence quality: II; Recommendation strength: Moderate)
Development of symmetrical movement. (Evidence quality: II; Recommendation strength: Moderate)
Environmental adaptations. (Evidence quality: II; Recommendation strength: Moderate)
Parent/caregiver education. (Evidence quality: II; Recommendation strength: Moderate)
Action Statement Profile
Aggregate Evidence Quality:
Level II randomized controlled trials, cohort and outcome studies.
Benefits to the Infant
Increases infant’s active and passive ROM.
Facilitates normal and prevents, reduces or eliminates asymmetrical postural, gross motor, skeletal, cognitive, sensory and visual development.
Reduces use of environmental supports/equipment that may increase asymmetry.
Avoids or minimizes need for future, more invasive procedures.
Benefits to the Parent
Enables parents to be active and effective caregivers.
Education and early intervention provide assurances that they did not cause the CMT.
Education empowers parents to implement interventions between PT appointments.
Education provides parents with information about typical developmental milestones and the factors that contribute to asymmetry.
Balances the use of supine as a frequent infant position with activities in prone, side-lying, and sitting during supervised, wakeful activities.
Reduces potential overall cost of care for CMT with early, intense treatment.
Risk, Harm, Cost:
Stretching of the SCM can result in muscle snapping, which may or may not cause momentary infant discomfort, however the documented long-term outcomes are positive.35
Cost of care may be a burden for families.
Parents/caregivers may apply interventions incorrectly.
Parents may decrease the intensity of home exercises if they perceive that the PT is implementing the treatment.70
Value Judgments:
None
Intentional Vagueness:
The GDG supports that stretching should be frequent through the day, every day; however, there is no dosage standard linking technique and duration of stretches, repetitions within each treatment session, frequency of treatment sessions per day, overall duration of care, and frequency of clinic visits, including tapering schedules, to specific CMT severity classifications.
Role of Parent/Caregiver or Patient Preferences:
Parental perceptions of the impact of CMT on their infant’s function and the importance of the intervention program on their infant’s future function are strong factors related to adherence to appointments and home exercises.178 Parent/caregiver adherence to the plan of care under a PT’s guidance64, 176 is optimal for achieving early intense treatment dosages.
Exclusions:
None
Quality Improvement:
This recommendation may reduce unwarranted variation in practice and provides consumers with guidance for evidence-based interventions.
Implementation and Audit:
Develop home exercise program materials, including online demonstrations of the 5 components of the first-choice intervention.
Update documentation forms and electronic records to include the education provided to parents, their understanding and adherence to the exercises.
While there are no studies that correlate the severity of lateral cervical flexion to the severity of CMT or the episode of care, PTs should document objective measures of lateral flexion and treat until resolved.
PTs should consider the corrected age of infants born preterm when designing a plan of care.
Audit PT adherence to providing the 5 components of the first-choice intervention or reasons for deviating from the recommendation.
Supporting Evidence and Clinical Interpretation:
Neck PROM:
Manual stretching remains the most commonly reported form of intervention for CMT,37, 57, 103, 105 with one new randomized controlled study comparing 2 stretching frequencies with < 3-month-old infants with CMT and PROM limitations.176 One group received 10 sessions per day of 10 stretches each (100 stretches) and the other received 5 sessions per day of 10 stretches each (50 stretches) with all other stretching parameters held constant. Both groups had significant improvements in head tilt and cervical rotation at 4 and 8 weeks, but the group receiving 100 stretches/day resulted in greater improvement than 50 stretches/day group. While this one study provides support for an increased stretching frequency, there is no consensus on the techniques to perform the stretches, the number of repetitions, the duration of stretches and rest periods, and the number of individuals required for the stretches.
Stretching as an intervention should not be painful; stretches should be stopped if the infant resists,59, 104 or the parent perceives changes in breathing or circulation.176 Low intensity, sustained, pain free stretches are recommended to avoid micro trauma of the muscle tissue.59
The two-person technique for stretching has one person stabilizing the infant in supine with the head held beyond the support surface and the second person holding the head to guide it through the available range of cervical rotation and side bending.37, 63, 179 Alternatively, the single person technique has the infant positioned in supine on the caregiver’s lap with one hand stabilizing the chest and shoulders and the other guiding the head through the range.25 Hand placement is important when using either the 1 or 2 person stretch to properly stabilize the infant, to minimize compensatory movements and to guide the infant’s head through the available range.25, 63, 179 The choice of technique may depend on the size and age of the infant when stretching is initiated, with younger, smaller infants more easily managed by a single person technique, while larger or more active infants may require two people to provide adequate positioning support.
Cervical PROM can also be achieved through positioning and handling,70, 76, 104 including carrying or placing the infant in side lying to gently stretch the shortened SCM37, 59, 76, 104 and while lying prone with the face turned to the shortened SCM.31, 37, 104, 180 Passive cervical stretching can also be achieved during feeding141, 167 by encouraging turning toward the shortened side to pursue a bottle or breast, through the use of alternative feeding positions168 and when necessary, through positioning in car seats and infant carriers.25, 104, 118, 140
Neck and Trunk AROM:
Active ROM continues to be the standard of care in combination with other interventions.180, 181 Strengthening cervical and trunk muscles can be achieved through AROM during positioning,104 handling,63, 70, 86 carrying the infant,70, 76, 86, 141 while feeding,141, 167, 168 and through exercises isolating the weaker muscles.63, 70, 76, 104 Incorporating righting reactions in upright postures, rolling, side lying or sitting has been used effectively during treatment and daily care routines to strengthen muscles opposite of the affected muscles.31, 104, 182 The affected side of CMT is placed downward, elongating the tighter muscles and encouraging activity of the weaker, non-affected side.70, 76, 104 Positioning the infant in prone encourages bilateral neck flexor elongation and strengthens neck and spine extensors.63, 81 Using visual and auditory tracking to elicit head turning in supported sitting towards the affected muscle37, 76 can strengthen cervical rotation.
Development of Symmetrical Movement.
Observational data (n=173) suggest that up to 25% (n=44) of infants with postural CMT may have transient motor asymmetry; 2/3 of the 33 infants with follow-up data had no asymmetries by age 2 years.107 Developmental exercises should be incorporated into PT interventions and home programs to promote symmetrical movement in weight bearing postures and to prevent the development of asymmetrical movement patterns in prone, sitting, crawling, and walking.86,76, 107, 141
Environmental Adaptations.
Adaptations to the infant’s environment can be incorporated into the home exercise program. Alternating the infant’s position in the crib and changing table encourages head turning in the desired direction.25, 65, 141 Adapting the car seat position to promote desired AROM,104, 160, 167 minimizing the amount of time in a car seat and infant carrier,118, 140 and placing toys on the affected side for the infant to turn the head towards the tighter side104 have been recommended as part of home programming, but not studied.
Parent/Caregiver Education.
Parents and caregivers should be educated about the importance of ‘tummy time’ or prone play,59, 159, 162,54, 67, 164 positioning and handling to encourage symmetry,59, 141,25,70, 81 minimizing the time spent in car seats and carriers to avoid CD as a precursor to CMT,25, 27, 118 and alternating feedings to each side.167, 168 These strategies should be integrated into the daily routines and home programs to enhance adherence.
Parents and caregivers may be inclined to seek advice from Internet sites and support groups. These sources can provide an array of information, but the veracity of information can vary, and the sites cannot tailor interventions to an individual child’s body structures and activity limitations. Information on the use of prone positioning for play varies widely on when to start, how often and for how long a session.67 Parents should be encouraged to review information with their infant’s physician and/or PT regarding exercises or interventions they are considering. Identification of evidence-based, reputable Internet resources would assist both clinicians and families in keeping up with current and valid management approaches.
R. Research Recommendation:
Studies are needed to:
Identify intervention techniques and dosages, including accurate descriptions of active exercises, with links to the CMT Classification Severity grades.
Identify the components of optimal home programs.
Evaluate the benefits of individual versus group therapy conditions.
C. Action Statement 14. Revised and updated. PROVIDE SUPPLEMENTAL INTERVENTION(S), AFTER APPRAISING APPROPRIATENESS FOR THE INFANT, TO AUGMENT THE FIRST-CHOICE INTERVENTION.
Physical therapists may provide and document supplemental interventions, after evaluating their appropriateness for treating CMT or postural asymmetries, as adjuncts to the first-choice intervention when the first-choice intervention has not adequately improved range or postural alignment, and/or when access to services is limited, and/or when the infant is unable to tolerate the intensity of the first-choice intervention, and if the physical therapist has the appropriate training to administer the intervention. (Evidence Quality: I-IV, Recommendation Strength: Weak)
Action Statement Profile
Aggregate Evidence Quality:
Level I-IV studies; 2 new level 1studies on microcurrent (MC) and kinesiology tape (KT).180, 181
Benefits:
On an individual basis, combining supplemental interventions supported by limited evidence with the first-choice intervention:
May be effective in improving outcomes or shortening treatment duration.
May accommodate an infant’s temperament or tolerance to treatment.
May avoid or minimize the need for future, more invasive procedures.
May increase parent/caregiver ability to implement home program.
Risk, Harm, Cost:
Selected supplemental interventions should only be applied by clinicians skilled in that specific technique or modality, and who understand the potential risks or side effects.
There may be an added burden to the parent(s)/caregivers to learn additional intervention techniques.
Some interventions may not be covered by insurance.
Some approaches may increase the cost of care.
Benefit-Harm Assessment:
Preponderance of Benefit for microcurrent (MC) and equal for other supplemental interventions.
Value Judgments:
Clinicians who are seeking to augment their first-choice interventions should choose those supplemental interventions with the strongest evidence first. Thus, if trained, clinicians should choose to use MC before choosing among the others of lesser strength.
Intentional Vagueness:
While evidence supporting the use of MC is increasing, it is not known when it is best to add it to a plan of care.
Role of Parent/Caregiver or Patient Preferences:
Parents may inquire about different interventions for the treatment of CMT.
Exclusions:
None
Quality Improvement:
Providing supplementary interventions may accelerate the resolution of CMT in infants whose progress has slowed.
Implementation and Audit:
Document the application and dosage of supplemental interventions to accurately measure their impact on infants with CMT.
Audit the types and documentation of supplemental interventions to determine their overall benefit to patients.
Supporting Evidence and Clinical Interpretation:
The following interventions are recommended as supplements to the first-choice intervention described in Action Statement 13 and are presented in descending order of evidence strength. In addition to experimental intervention studies, several studies have used a combination of the first-choice intervention with soft tissue mobilization,173 massage41, 153, 173, 174 and therapeutic US.41, 153, 173, 180 While these studies are designed to look at prediction of outcomes or efficacy of other interventions, they provide preliminary evidence that a multimodal approach is effective; additional research is needed to study their individual effects. Finally, there are some interventions described in the common press for which there are no peer reviewed publications to explain their effect on CMT and/or support their effectiveness. Departures from the guideline should be documented in the patient’s record at the time the relevant clinical decisions are made; clinicians are strongly encouraged to publish the clinical reasoning and results of these alternative approaches.
Interventions with New Evidence
Level I Evidence.
Microcurrent (MC) is a low intensity single channel alternating current applied superficially at a level that is not perceived by the patient. Two studies demonstrate reduced treatment duration and improved ROM with the addition of MC to PT intervention. In a 2013 level 1 randomized controlled trial (RCT),180 all 20 infants received a home program, 20 minutes of exercises, 5 minutes of ultrasound, and 30 minutes with the MC unit set up, but only 10 infants received active MC. Treatment sessions were 3 times per week until PROM resolved or there were no improvements after 6 months of ongoing care. Those receiving the active MC had significantly shorter treatment durations (2.6 months) than those who did not (6.3 months). The results are consistent with a prior RCT128 when 30 minutes of MC was applied to the involved SCM of infants with CMT, 3 times per week for 2 weeks, resulting in improved head tilt angle, neck rotation toward the affected side, and less crying during therapy when compared to a control group of infants with CMT who received traditional stretching and exercises. The sample groups were small (n = 7 experimental vs. 8 control) and there was no long-term follow-up, but the average infant age was 7 months, and many had already been treated with stretching programs.
Kinesiological taping (KT) refers to the use of stretchable tape to support muscles and to provide sensory feedback. In contrast to the 2013 CMT CPG recommendation that KT could be a supplemental treatment, a 2016 level 1 study suggests that there is no added value to KT when provided for 3 weeks in conjunction with other conservative methods.181 This was a small prospective single blinded RCT with 3 infant groups who had KT applied 6 days/week for 3 weeks; all groups also received an exercise program and PT intervention. Group 1 had exercise only, group 2 had KT applied to the involved SCM for inhibition and the uninvolved SCM for facilitation, and group 3 had KT applied only to the involved SCM for inhibition. While there were within group changes in neck PROM, Muscle Function Scale (MFS) scores and head shape symmetry from their baselines, there were no significant differences between treatment groups immediately after treatment, at 1 month or at 3 months post treatment. This suggests that there is no added value of KT beyond exercise even over a 3-week treatment period. Öhman reported an immediate effect of KT on MFS scores while the tape is on; however, it is not clear if the change lasts beyond the immediate effect when KT is removed.183 Additional studies of alternative methods of applying KT may further clarify when and if this approach is supported for use with CMT.
Soft Tissue Mobilization (STM) as described by Keklicek, Uygur 12 was applied in three phases: a passive mobilization phase, mobilization with stretching, and mobilization with active cervical rotation. For infants with CMT, a home program with STM 3 days a week for 12 weeks, compared to only a home program, resulted in improved cervical rotation PROM and head tilt after 6 weeks of intervention, but not after 12 weeks of intervention or 18 weeks after the start of the study. Between groups, there was no difference in lateral flexion PROM or AROM throughout the study. It is not clear if the improvements at 6 weeks are due to the treatment technique or intensity of treatment since the intervention for the control group was not dose equivalent and parents performed an unspecified home program of stretching and handling. PTs may choose this approach if an infant is not progressing or is resisting passive stretching.
Interventions with No New Evidence
Level 1 Evidence
Myokinetic stretching as described by Chon, Yoon, You 103 consists of sustained 2-finger overpressure on the taut SCM muscle; 60 repetitions were delivered over 30 minutes, 5 times per week for an average of 1.7 months. Pre and post treatment measures of the SCM thickness in infants with either the muscular torticollis or SCM mass types were made by ultrasound. Results describe significant reductions in SCM thickness and improved cervical rotation and head symmetry with retention at the 1-year reassessment by parent reports. The study had no control group and the average age of sample was 50 days (range of 30–70). Additionally, the parents performed an unspecified home program of stretching and handling, so it is not clear if the improvements are due to the treatment technique, intensity of treatment, and/or age of the infants. Most studies demonstrate that infants under 2 months will resolve with traditional stretching approaches delivered at frequencies of less than 5 days/week. PTs may choose this approach if an infant is not progressing or is resisting passive stretching.
Level IV Evidence
The Tscharnuter Akademie for Motor Organization approach (TAMO) promotes problem solving and movement exploration during treatment, emphasizing light touch and the infant’s responses to gravity and support surfaces. A single case study of TAMO describes the treatment plan for an infant with CMT.86 The subject is a twin born prematurely, hospitalized in the NICU for 5.5 weeks and for other medical conditions during which he appeared to develop asymmetrical posturing. Despite home programming of position changes, encouragement of AROM, and use of prone positioning, SCM tightness developed and the infant was referred for treatment at 6.5 months of age (4.5 months corrected age). The application of TAMO is mixed with AROM activities, soft tissue mobilization, parent instruction for use of home positioning to facilitate muscle lengthening and carrying techniques that facilitate head righting opposite of the tightness. While the changes across time are well documented, it is not clear what contribution the TAMO approach provides separate from the positioning and handling approaches that others have shown to be effective except for the noticeable absence of passive stretching. This approach may be a useful addition for PTs who have received postgraduate training in the TAMO approach, particularly for infants who are resistant to stretching; however, without any other studies to demonstrate its application, generalizability is limited.
Level V Evidence
The Tubular Orthosis for Torticollis (TOT) collar has been described in the literature 63, 84 and online (www.symmetric-designs.com) as a neck orthotic designed to prevent movement toward and stimulate active movement away from the tilted head position. The collars are used as an adjunct to conservative treatment of infants with CMT who demonstrate adequate head control in supported sitting and > 5–6 degrees of head tilt.84, 179 Although noted as part of routine intervention in the treatment of infants with CMT who meet criteria for their use,63, 81, 105, 182 there are no studies that isolate the outcomes of the TOT collar compared to other interventions. Pilot data reported in Karmel-Ross 84 suggest that infants treated with the TOT collar achieve 89.5/90 degrees vertical head position as compared to 84.8/90 for those who did not.
Soft foam collars have been described by Jacques84 and have been used post-surgery,78 post-surgery in conjunction with PT,184–188 and post botulinum toxin189 without specific rationales provided. They may be useful as passive support for the lengthened muscle, to protect incisions from curious hands, or to facilitate active movement away from the previously shortened side. Binder, Eng, Gaiser, Koch 76 describe the use of a soft felt and stockinette collar for infants presenting with < 45 degrees passive cervical rotation and a constant tilt. In all cases, no studies have been found that isolate the impact of foam or soft collars on the outcomes of conservative care.
Custom fabricated cervical orthoses have been described for post-surgical management of CMT in children186, 190 or young adults.191 They reportedly provide greater stabilization of the spine and less mobility than the softer foam collars or semi rigid cervical orthoses,192, 193 but their use with infants has not been reported in the literature.
Interventions without Published Evidence of Efficacy
The following approaches have either not been studied systematically or shown not to provide any additional benefit. Additional approaches have been found on the Internet and in the common press for which no peer reviewed literature was found.
Cervical manipulation of the infant in supine has been compared to standard stretching alone in a small double-blind randomized trial (n=32). Results indicated no differences between the groups, with many confounding variables. The study sample was underpowered, both groups received stretching and home programs, the infants were young, (3–6 months of age when stretching alone is known to be effective), and selected measures are reported as unreliable due to infant cooperation. The actual technique used for cervical manipulation is not well described in the study. Others have concluded that the use of cervical manipulation in infants has no sufficient evidence of benefits and may be associated with higher risks of apnea and possible death.194, 195 In weighing the potential risks against the benefits of other approaches, the GDG does not recommend cervical manipulation as an intervention for infants with CMT.
The following interventions appear in print, online, in continuing education brochures and parent support groups for infants with torticollis and deformational plagiocephaly, but no peer reviewed studies have been found that describe the specific approaches or their effectiveness for resolving CMT: soft tissue massage as a single modality,84,81, 86, 103 craniosacral therapy,84 Total Motion Release (TMR), and Feldenkrais.84 Physicians, therapists, and parents should be aware that these approaches have no peer reviewed publications that describe or study their effect on CMT, and their clinical application, risks and anticipated outcomes may only be anecdotally reported. Due to a lack of studies, the GDG cannot recommend these approaches for management of CMT at this time. Clinicians who choose to use these approaches should document departures from the guideline in patient records at the time the relevant clinical decisions are made, obtain consent to treat from parents that acknowledges the lack of published evidence, carefully document objective measures of change, and consider publication of their outcomes.
R. Research Recommendation:
Studies are needed to describe and clarify the efficacy of all supplementary interventions, including determinants for their choice, principles of application, dosage and outcomes measures.
B. Action Statement 15: Revised and updated. INITIATE CONSULTATION WHEN THE INFANT IS NOT PROGRESSING AS ANTICIPATED.
Physical therapists who are treating infants with CMT or postural asymmetries should initiate consultation with the infant’s physician and/or specialists about other interventions when the infant is not progressing as anticipated. These conditions might include: when asymmetries of the head, neck and trunk are not starting to resolve after 4–6 weeks of comprehensive intervention, or after 6 months of intervention with a plateau in resolution. (Evidence Quality: II, Recommendation Strength: Moderate)
Action Statement Profile
Aggregate Evidence Quality:
Level II based on cohort follow-up studies.
Benefits:
Other interventions (e.g. botulinum neurotoxin therapy or surgery) can be considered to resolve the current asymmetries and prevent further progression of deformities and compensations.
Provides the family/caregivers with alternative management strategies to help resolve asymmetries.
Risk, Harm, Cost:
The consultations and possible subsequent interventions may add to the cost of care.
Benefit-Harm Assessment:
Preponderance of Benefit
Value Judgments:
Collaborative and coordinated care is in the best interest of the infant and family centered care.
Intentional Vagueness:
The GDG is intentionally vague about the range of 4–6 weeks as the amount of time that a PT should treat an infant who is not responding to intervention. Since younger infants typically change more quickly than older infants, the GDG recommends that infants younger than 2 months who are not responding to intervention should be referred to their physician sooner than infants older than 2 months, who may require more time to respond to intervention.
Role of Patient/Parent Preferences:
The age of the infant, severity of the CMT, rate of changes, needs of the family, cooperation and developmental needs of the infant, and available resources of the family/caregivers should help to determine the episode of care before an infant is referred back to the infant’s physician for consideration of alternative interventions.
Exclusions:
None
Note:
The 2013 CMT CPG conditions of referral when an infant presents at older ages with ROM limitations and/or facial asymmetry were incorporated into Action Statement 5 on Screening.
Quality Improvement:
Referral back to the physician when the infant is not progressing as anticipated enhances coordinated communication about the infant, enables the infant to receive additional or specialized interventions, and promotes stronger professional relationships.
Implementation and Audit:
Documentation should include information supporting the reason for referral, the PT’s hypotheses about other factors that might need attention, and the treatment types and intensities that were used.
Survey referral sources for how they would like to receive communication about their patients (e.g. digital versus hard copy reports or letters).
Audit the number of infants that are fully resolved as compared to those who require referral for interventions other than PT.
Supporting Evidence and Clinical Interpretation:
The literature supports a wide range of treatment durations for conservative care, so the question of when to refer an infant who is not progressing as anticipated has no clear answer. The duration of care will vary depending on the age of diagnosis and referral of the infant for services and the severity grade. Infants who are referred within the first 3 months with a severity grade of 1–3 (Figure 2) will most likely NOT require 6 months of conservative intervention if the interventions appropriately address the impairments and there is adherence with home programming. Infants who present with severity grades of 4–7 will more likely require the full 6 months of care, or more, depending on the number of comorbidities. Factors that might extend treatment duration include the presence of motor asymmetries,107 an older age at initiation of treatment,36, 41 the presence or absence of a SCM mass,63, 73, 102, 175 the amount of head tilt,26, 36, 63, 114, 173 the quality of the SCM fibers,72, 102, 173, 174 the presence of facial asymmetry or CD,36 parental preference for conservative care, inconsistent home program adherence by parents/caregivers, and infant health conditions that may interfere with CMT interventions. Throughout the episode of care, the PT should collaborate with the infant’s physician and the family to make a judgment about when to increase the intensity of direct PT treatment or consider alternative approaches. This decision should be based on the rate of change, the persisting impairments, the age of the infant and the needs and values of the family. The literature supports that if infants have treatment initiated before 3 months of age, 98–100% will respond to conservative treatment within a 6-month period of time,37, 57, 61, 63 though full resolution may require longer durations. The determining factors should be documented measures of progressive improvement, with referral triggered by plateaus at or after 6 months of consistent and intensive intervention.
Invasive Interventions:
There are two conditions for which a child may be referred for consideration of more invasive interventions: (1) if after 6 months of conservative intervention there is a lack of progress, or (2) if the child first begins intervention after 1 year of age and presents with significant restrictions and/or a SCM mass. Under these conditions, the PT should consult with the infant’s physician or referring physician about other approaches; the two most reported are botulinum toxin injections and surgical lengthening of the SCM. The following brief descriptions are provided for information but are not exhaustive reviews of these approaches. Clinicians and families should discuss these options with their infants’ physicians when conservative care has not been successful.
Botulinum toxin is a neurotoxin that is postulated to act on the tight SCM in 2 ways: as a neuromuscular block that inhibits acetylcholine release, thus reducing stimulation of an already tight muscle, and as a neurotoxin causing muscle atrophy and weakening that allows for easier stretching.135, 196 While it is not formally approved for use with infants, it is approved for adults with cervical dystonia.196 Three retrospective studies 135, 136, 189 describe the effectiveness of botulinum toxin in increasing ROM in infants with CMT as varying from 25%136 to 74%135 to 93%.189 Adverse effects include pain and bruising,189 temporary dysphagia,135 and neck weakness,135 all of which are reported to resolve.
Surgical release of the SCM is the more traditional alternative for treating recalcitrant CMT.184, 188, 197 It is beyond the scope of this CPG to describe the variety of surgical approaches, which generally fall into 3 categories: tendon lengthening, unipolar release of the distal SCM attachment or bipolar release of both SCM muscle attachments.198, 199 There is emerging evidence that use of acellular dermal matrix may yield better post-surgical cervical ROM for corrections after age 8.200 Criteria that have been used to determine the timing for surgery include: persisting limitations in cervical ROM >15 degrees,114, 169 progressing limitations,59 having a SCM mass and being >12 months of age combined with late age diagnosis,114 persistent visible head tilt,26, 114, 169 not responding to intervention after 6 months,26, 114 and reaching the age of 1 year without resolution;169 surgery before 8 years of age appears to yield better outcomes than after age 8.201 The post op management of CMT is similar to pre-op, and can range from 4–6 weeks202 up to 11 months172, 203 to work on scar management, muscle strength and ROM.
R. Research Recommendations:
Studies are needed to describe the incidence of infants that require invasive care, their history of interventions, the best time for referral, and any associated physical therapy outcomes.
IV. PHYSICAL THERAPY DISCONTINUATION, REASSESSMENT, AND DISCHARGE OF INFANTS WITH CMT
B. Action Statement 16: Revised and updated. DISCONTINUE DIRECT SERVICES WHEN THESE 5 CRITERIA ARE ACHIEVED.
Physical therapists should discontinue direct physical therapy services and document outcomes when these 5 criteria are met: PROM within 5 degrees of the non-affected side; symmetrical active movement patterns; age appropriate motor development; no visible head tilt; and the parents/caregivers understand what to monitor as the child grows. (Evidence Quality: II-III, Recommendation Strength: Moderate)
Action Statement Profile
Aggregate Evidence Quality:
Benefits:
Use of these criteria for discontinuation from direct PT reasonably ensures that:
The CMT has resolved within accepted ranges of measurement error.
There are no lingering secondary compensations or developmental delays.
The parents/caregivers know how to assess for regression as the infant grows and when to contact their infant’s physician and/or the PT for reassessment.
Discontinuation documentation reflects the expected outcomes for the episode of care, relative to the baseline measures taken at the initial examination.
Risk, Harm, Cost:
There is an unknown amount of risk that discontinuation from PT services with 5 degrees residual asymmetry will progress to other anatomical areas (cervical scoliosis, craniofacial) or return as the infant grows.
Benefit-Harm Assessment:
Preponderance of Benefit
Value Judgments:
The GDG defines cervical rotation and cervical lateral flexion motions as included in PROM. Further, it includes full active cervical rotation and lateral flexion in the phrase symmetrical active movement.
Intentional Vagueness:
None
Role of Patient/Parent Preferences:
Parents/caregivers need to be educated about the importance of screening for asymmetries as the child grows and becomes more active against gravity. They should be advised that preferential positioning is often observed during times of fatigue or illness, and that re-evaluation is only warranted if it persists.
Exclusions:
None
Quality Improvement:
Complete documentation of baseline and discontinuation measures will support more accurate PT outcomes.
Measurements taken at each treatment session provides feedback to parents about the child’s progress and supports fine tuning of the interventions which can shorten the duration of care.8
Implementation and Audit:
PTs should follow up with families that discontinue direct PT services prior to achieving resolution of asymmetries or formal discharge, to determine the reason for discontinuation.
PTs should educate parents/caregivers on signs of recurring CMT when changing from direct PT to monitoring with a reassessment at 3–12 months of age or when the infant starts walking.
Supporting Evidence and Clinical Interpretation:
The 2018 CMT CPG uses the phrase discontinuation of direct services to mean when the infant has achieved the 5 criteria and direct intervention is no longer warranted. Discharge is defined as occurring 3–12 months after the discontinuation of direct services when PT reassessment for potential residual CMT or other developmental concerns are negative.
While the duration of intervention for the individual infant will vary depending on the constellation of factors identified in Figure 2, the criteria for discontinuing direct PT services are based on norms for infant growth and development,111 known risk of early delays,53, 54, 204 and the emerging evidence of possible long-term sequelae.43, 113 Functionally, it is critical that the infant who has achieved full PROM can actively use the available range, so PT criteria for discontinuation should address developmental activity rather than focus solely on biomechanical measures of change.79 Persistent functional limitations or developmental delays, after achievement of full PROM, are reasons to extend or initiate a new episode of care. Finally, these criteria are common across the literature and thus, are in keeping with current practice norms.
R. Research Recommendation:
Longitudinal studies are needed to understand the best criteria and/or timing for discontinuing infants from direct PT intervention and the final discharge from the episode of care.
B. Action Statement 17: Revised and updated. REASSESS INFANTS 3–12 MONTHS AFTER DISCONTINUATION OF DIRECT SERVICES, THEN DISCHARGE IF APPROPRIATE.
3–12 months following discontinuation from direct physical therapy intervention OR when the child initiates walking, physical therapists who treat infants with CMT should examine postural preference, the structural and movement symmetry of the neck, face and head, trunk, hips, upper and lower extremities, and developmental milestones to assess for reoccurrence of CMT and evidence of atypical development. (Evidence Quality: II, Recommendation Strength: Moderate)
Action Statement Profile
Aggregate Evidence Quality:
Level II based on longitudinal follow-up studies with moderately large samples, reasonable follow-up periods and reliable outcome measures.
Benefits:
Detection of postures and movement consistent with relapsing CMT, particularly as infants initiate walking and move against gravity.
Detection of developmental delays.
Ability to restart home exercise programs if asymmetry is identified.
Screening identifies other causes of asymmetry, other than CMT, if asymmetries reappear.
Risk, Harm, Cost:
A single follow-up visit will minimally add to the cost of care.
Benefit-Harm Assessment:
Preponderance of Benefit
Value Judgments:
A single follow-up PT visit for infants with a history of CMT is consistent with the APTA Guide to Physical Therapist Practice that describes the roles of a PT as including prevention of recidivism and preservation of optimal function.80
Intentional Vagueness:
The recommended time at which follow-up is scheduled (3–12 months) is wide because the age of the infant at discontinuation from direct PT intervention will vary. Reassessment of younger infants, discontinued from direct intervention between 4–6 months, may need to occur sooner when the infants are initiating standing and walking. It is not known how far out into early childhood that reassessment should occur. Literature suggests that by 8–15 months, the infant with delays at 2–6 months catches up with their peers,53, 205 and they continue to demonstrate age appropriate motor development at preschool age.204 However, a single follow-up study suggests that some infants are at greater risk for persistent neurodevelopmental conditions, such as developmental coordination disorder and attention deficit hyperactivity, which may not become evident until the early school years.113
Role of Patient/Parent Preferences:
Parents/caregivers may choose to forego a PT reassessment if it places undue burden on the family for travel, time or finances. Parents should be advised at discontinuation of direct PT intervention of the small chance that developmental conditions may evidence themselves when the child enters school, and parents should be educated to observe for persistent asymmetry.
Exclusions:
None
Quality Improvement:
Long-term follow-up reassessments will provide data to understand the incidence of residual asymmetries or functional deficits, and parental satisfaction.
Implementation and Audit:
Provide education to clinicians and families about this recommendation to improve adherence to reassessment.
Determine a method, based on location and health care coverage processes, to facilitate a cost-effective PT reassessment. This may require PTs to educate administrators, service coordinators and non-medical professionals about the importance of a comprehensive reassessment for infants with CMT. PTs should collaborate with their administrative and health care providers to develop pathways for parents to obtain this reassessment, either internally or by referral to other services.
Provide clear instructions to parents about the signs of unresolved or returning CMT.
- After reassessment, document:
- That parents were instructed to notify the PT if there is a persistent return of head tilt or asymmetry in active rotation or lateral flexion ROM.
- The PT recommendation to the physician to check the infant’s cervical ROM and presence of head tilt in well child visits.
- The PT recommendation for a PT reassessment to check the condition of the infant’s CMT and general development at 12 months or when walking begins.
Have the parent complete a reminder post card for a PT reassessment that can be mailed to the family at the appropriate time.
Audit the number of reassessments completed versus the reasons for no reassessment, or premature discontinuation of services.
Supporting Evidence and Clinical Interpretation:
The long-term consequences of CMT are implied from studies of older children and adults who require surgeries for correction of unresolved asymmetry43, 47, 198 and from long term follow-up studies.37, 204 While the short-term outcomes of conservative management are well documented, there is little direct evidence of the long-term effectiveness of early PT intervention, nor the rate of recidivism following early intervention. Studies report an ‘excellent’ resolution of CMT as having less than 5 degrees of passive rotation asymmetry with the opposite side,26, 32, 46, 175 and a ‘good’ resolution with as much as 10 degrees32, 46 residual. It is not known whether the last 5–10 degrees spontaneously resolves or in whom a mild limitation remains, whether achieving cervical rotation PROM equates to full active use of the available range, or whether residual asymmetry influences normal development.
Öhman, Beckung 204 found that although infants with a history of CMT did not exhibit motor delays at preschool age, 7% exhibited a head tilt and 26% had some degree of PROM asymmetry. The clinical significance of asymmetric neck PROM is uncertain because only children with CMT were followed. All had ≥ 85° of rotation PROM to each side, and 7 children had a lateral flexion PROM differences between sides of only 5–10°; it’s not clear if age matched children without CMT would present with similar results. In this study, asymmetric cervical PROM at preschool age was associated with the degree of asymmetric cervical rotation PROM as an infant.206
The documented potential for increasing muscle fibrosis,98 developmental delays,113 and hemi-syndrome76 support that a single PT reassessment is prudent to determine if the resolution of CMT achieved at an earlier age is maintained as the infant continues to develop, and to assess for potential developmental delays or biased limb use. Physicians should be cognizant of the risk for asymmetries and/or motor delays during routine physical exams as infants with a history of CMT are followed through to their teen years.
The length of time after discontinuation that a PT reassessment should be conducted is supported by level IV evidence. Wei, Schwartz, Weaver, Orvidas 51 propose following infants until complete resolution, or a minimum of 12 months. Ultrasound images suggest that while clinical indicators of ROM may improve, they are not correlated with SCM fibrous changes, and these fibrous changes can continue until at least age 3.98 Finally, the potential for developmental delays may not become evident until early school age,113 so a re-examination when the child enters elementary school may be warranted if a parent or teacher reports, or the child presents with, residual asymmetries, developmental delays or preferential positioning. Regional differences as to when a child is seen for their final direct service appointment may differ from the criteria for discharge, when the episode of care for CMT is considered closed.
R. Research Recommendations:
Studies are needed to:
Determine the most reasonable PT reassessment times after discontinuation of direct PT intervention based on initial presentations.
Establish the level of risk of developing asymmetries following an episode of intervention.
Describe parent/caregiver experiences and/or satisfaction with PT intervention and infant outcomes. Limited mentions of parent and/or patient satisfaction are available post surgery46, 199 and post botulinum toxin use,189 but none were found specific to PT management.
Determine the validity and reliability of using telemedicine or virtual meetings as compared to in-person PT reassessment for the 3–12-month reassessment.
SUMMARY
A review of the literature, including a focused systematic review, resulted in 17 graded action statements with varying levels of obligation that address education, referral, screening, examination and evaluation, classification, prognosis, first-choice and supplementary physical therapy interventions, inter-professional consultations, discontinuation, reassessment, and discharge, with suggestions for quality improvement, implementation and audits. Flow sheets for referral paths and classification of CMT severity have been updated. Evidence tables are available as supplemental files. Research recommendations are made for 17 practice issues and summarized at the end of the document.
GENERAL GUIDELINE IMPLEMENTATION STRATEGIES
There is a growing body of evidence on implementing research into practice. The following suggestions are provided as general strategies for clinicians to implement the action statements of this CPG but are not an exhaustive review. Many variables impact the successful translation of evidence into practice; clinicians will need to assess their own practice structures, cultures and clinical skills to determine how to best implement the action statements as individuals and how to facilitate implementation by others.
The GDG recommends that:
Education about the 2018 CMT CPG to be included in physical therapy curricula.
Continuing education programs are provided to PTs on the updates in the 2018 CMT CPG.
PTs distribute brochures developed by the APPT (https://pediatricapta.org/clinical-practice-guidelines/) to parents, physicians, midwives, and other health care providers that summarize the applicable key points of the 2018 CMT CPG.
Strategies for Individual Implementation
Seek training in the use of the recommended standardized measures and/or intervention approaches.207
Build relationships with referral sources to encourage early referral of infants.
Measure individual service outcomes of care (e.g. patient impact across the ICF domains, costs, parent/caregiver satisfaction).208, 209
Strategies for Facilitating CPG Implementation in Other Clinicians
Recognize that adoption of the recommendations by others may require time for learning about the 2018 CMT CPG content, developing a positive attitude toward adopting the action statements, comparing what is already done with the recommended actions, trialing selected changes in practice to determine their efficacy, and finally, routine integration of the tested changes.208, 210
Identify early adopting clinicians as opinion leaders to introduce the guideline via journal clubs or staff presentations.208, 210
Identify gaps in knowledge and skills following content presentations to determine staff needs to implement recommendations.210
Use documentation templates to facilitate standardized collection and implementation of the recommended measures and actions.5, 211, 212
Institute quality assurance processes to monitor the routine collection of recommended data and implementation of recommendations, and to identify barriers to complete collection.208, 213
Measure structural outcomes (e.g. dates of referral, equipment availability), process outcomes (e.g. use of tests and measures, breadth of plan of care), and service outcomes (e.g. patient impact across the ICF domains, costs, parent/caregiver satisfaction)208, 209 to describe service delivery patterns and publish results.
SUMMARY OF RESEARCH RECOMMENDATIONS PER ACTION STATEMENT
Action Statement 1:
Educate Expectant Parents and Parents of Newborns to Prevent Asymmetries/CMT. Studies are needed on the impact of education of:
Health care providers and their knowledge of pediatric PTs’ roles in managing postural preference.
Parents/caregivers about the parental experience of receiving this education.
Action Statement 2:
Assess Newborn Infants for Asymmetries/CMT
Studies are needed to determine:
Whether routine screening at birth increases the rate of CMT identification or increases false positives.
The barriers to early referral of infants with CMT to PT.
Action Statement 3:
Refer Infants with Asymmetries/CMT to Physician and Physical Therapist.
Studies are needed to clarify the predictive baseline measures and characteristics of infants who benefit from immediate follow-up, and to compare the cost benefit of early PT intervention and education as compared to parental instruction and monitoring by physicians.
Longitudinal studies of infants with CMT are needed to clarify how the timing of referral and initiation of intervention impact body structure and functional outcomes, and overall costs of care.
Action Statement 4:
Document Infant History
Studies are needed to clarify how the health history factors influence PT diagnosis, prognosis, and intervention.
Action Statement 5:
Screen Infants for Non-Muscular Causes of Asymmetry and Conditions Associated with CMT.
Studies are needed to identify the precision of screening procedures specific to CMT.
Action Statement 6:
Refer Infants from Physical Therapist to Physician if Indicated by Screen.
Studies are needed to clarify the incidence of non-muscular causes of CMT and associated conditions, and how early referral impacts ultimate outcome.
Action Statement 7:
Request Images and Reports
Studies are needed to determine who would benefit from imaging, at what time in the management of CMT images are useful, and how images affect the plan of care.
Action Statement 8:
Examine Body Structures
Reliable, valid and time efficient methods of measuring infant cervical PROM need to be developed, including lateral flexion, and large-scale normative data of PROM should be established by age in months.
Determine the sensitivity and specificity of the Muscle Function Scale to differentiate infants with clinically significant limitations from typically developing infants.
Establish a clinically practical, objective method of measuring cervical rotation AROM in infants 0–3 months and infants >3 months to assess baseline and change over time.
Determine what, if any, correlation between active and passive ROM should be used for discontinuation and/or discharge criteria.
Studies are needed to describe and differentiate signs of discomfort from the types of pain reactions typically observed in infants with CMT during specific testing or interventions.
Determine the validity of the FLACC in rating true pain reactions during CMT examinations or interventions.
Action Statement 9:
Classify the Level of Severity
Studies are needed to determine a reliable, valid and clinically practical method of measuring cervical lateral flexion, and then to determine how the severity of lateral flexion may relate to the CMT Severity Classification grades.
Action Statement 10:
Examine Activity and Developmental Status
Studies are needed to identify the best developmental tests to use for infants with suspected or diagnosed CMT, from birth through 12 months, so that the same measures can be documented on all infants, enabling comparison of outcomes across studies.
Action Statement 11:
Examine Participation Status
Studies are needed to quantify changes in participation and clarify how the participation elements inform the plan of care.
Action Statement 12:
Determine Prognosis. Studies are needed to:
Clarify the interaction between the factors associated with full symptom resolution and episode of care.
Clarify the accuracy of prognosis with respect to full symptom resolution and episode of care.
Describe and clarify the efficacy of different delivery models, e.g. individual versus group or clinic versus home.
Action Statement 13:
Provide These 5 Components as the First-choice Intervention. Studies are needed to:
Identify intervention techniques and dosages, including accurate descriptions of active exercises, with links to the CMT Classification Severity grades.
Identify the components of optimal home programs.
Evaluate the benefits of individual versus group therapy conditions.
Action Statement 14:
Provide Supplemental Intervention(s), After Appraising Appropriateness For the Infant, to Augment the First-choice Intervention
Studies are needed to describe and clarify the efficacy of all supplementary interventions, including determinants for their choice, principles of application, dosage and outcomes measures.
Action Statement 15:
Initiate Consultation when the Infant is not Progressing as Anticipated
Studies are needed to describe the incidence of infants that require invasive care, their history of interventions, the best time for referral, and any associated physical therapy outcomes.
Action Statement 16:
Discontinue Direct Services when these 5 Criteria are Achieved.
Longitudinal studies are needed to understand the best criteria and/or timing for discontinuing infants from direct PT intervention and the final discharge from the episode of care.
Action Statement 17:
Reassess Infants 3–12 Months after Discontinuation of Direct Services, then Discharge.
Determine the most reasonable reassessment times after discontinuation of direct PT intervention based on initial presentations.
Establish the level of risk of developing asymmetries following an episode of intervention.
Describe parent/caregiver experiences and/or satisfaction with PT intervention and infant outcomes. Limited mentions of parent and/or patient satisfaction are available post surgery46, 199 and post botulinum toxin use,189 but none were found specific to PT management.
Determine the validity and reliability of using telemedicine or virtual meetings as compared to in-person PT reassessment for the 3–12 month reassessment.
DEVELOPMENT OF THE GUIDELINE
This clinical practice guideline is the product of many people’s work and support. At each phase of the update, the GDG has benefitted from the work and advice of clinicians, methodologists and the families with whom we work. The following outlines the phases of this update, and formally acknowledges the contributors in each phase. Contributors are listed alphabetically..
Phase 1: Organization and manuscript development, including determination of scope.
Colleen P. Coulter, PT, DPT, PhD, PCS
Team Lead, Limb Deficiency Program
Orthotics and Prosthetics Department,
Children’s Healthcare of Atlanta
Adjunct Assistant Professor of Rehabilitation Medicine
Emory University School of Medicine
Atlanta, Georgia
Sandra L. Kaplan, PT, DPT, PhD
Professor, Dept. of Rehabilitation and Movement Sciences
Rutgers, The State University of New Jersey
Newark, NJ
Barbara Sargent, PT, PhD, PCS
Assistant Professor of Clinical Physical Therapy
Division of Biokinesiology and Physical Therapy
University of Southern California,
Los Angeles, CA
Phase 2: Literature search and abstract review.
Colleen P. Coulter, PT, DPT, PhD, PCS,
Emily Heidenreich, PT, DPT, PCS
Sandra L. Kaplan, PT, DPT, PhD
Barbara Sargent, PT, PhD, PCS
Phase 3: Literature review, appraiser reliability training and critical appraisal ratings for systematic review.
Emily Heidenreich, PT, DPT, PCS
Barbara Sargent, PT, PhD, PCS
Phase 4: Action Statement generation and literature summarization.
Colleen Coulter, PT, DPT, PhD, PCS,
Sandra L. Kaplan, PT, DPT, PhD
Barbara Sargent, PT, PhD, PCS
Phase 5: First round review by content experts
Cynthia Baker, MD (AAP representative)
Department of Pediatrics
Kaiser Permanente Medical Center
Los Angeles, CA
Ginette Lange, PhD, CNM, FNP
Associate Professor
School of Nursing/ Nurse Midwifery Program
Rutgers, The State University of New Jersey
Christine McDonough, PT, PhD (Methodologist)
Assistant Professor of Physical Therapy
School of Health and Rehabilitation Sciences
University of Pittsburgh
Pittsburgh, PA
Victoria Mena, AuD (Parent and public representative)
Hearing and Speech Lead/Pediatric Audiologist
Department of Rehabilitation Services Children’s Hospital Los Angeles
Los Angeles, CA 90027
Anna Öhman, PT, PhD (Pediatric physical therapist and researcher)
PhD Specialist in Pediatrics
Gothenburg, Sweden.
Scott Parrott, PhD (Methodologist)
Professor, Department of Interdisciplinary Studies,
School of Health Professions
Adjunct Professor, Department of Epidemiology,
School of Public Health
Rutgers, The State University of New Jersey
Melanie Percy, RN, PhD, CPNP, FAAN (Pediatric Nurse Practitioner)
Associate Professor
Advanced Practice Nursing Division
School of Nursing
Rutgers, The State University of New Jersey
Amy Pomrantz, PT, DPT, OCS, ATC (Parent and public representative)
Assistant Professor of Clinical Physical Therapy
Division of Biokinesiology and Physical Therapy at the Herman Ostrow School of Dentistry
University of Southern California,
Los Angeles, CA
Philip Spandorfer, MD, MSCE, FAAP (Pediatrician)
North Atlantic Pediatric Associates, PC
Atlanta, GA
Jordan Steinberg, MD, PhD, FAAP (Pediatric Plastic Surgeon)
Assistant Professor, Department of Plastic Surgery
Johns Hopkins University School of Medicine
Baltimore, MD
APPT Knowledge Translation Committee Project Leaders:
Erin Bompiani, PT, DPT, PCS
Assistant Professor, School of Physical Therapy & Athletic Training
Pacific University
Hillsboro, Oregon
Ellen Brennan, PT, DPT, PCS
Children’s Specialized Hospital
94 Steven’s Rd Toms River, NJ
Catie Christensen, PT, DPT, PCS
Physical Therapist and Evidence-Based Practice Coordinator
Nationwide Children’s Hospital
Westerville, Ohio
Barbara Pizzutillo, PT, DPT, MBA
Clinician, Private Practice/ Early Intervention
Wynnewood, PA
Susan Rabinowicz, DPT, MS
Private Practice Consultant, NY
All 1st round reviewers declared an absence of conflicts of interest with the topic, process and/or financial relationships.
Phase 6: External review of the revised CPG by the public & AGREE II ratings.
Following edits based on the 1st round review, a revised CPG draft was posted for public comment on the APTA Academy of Pediatric Physical Therapy web site. Notices were sent through the APPT electronic newsletter, posted on a physical and occupational therapy social media Web site, and sent individually to any clinicians who had inquired about the CPG during its update regarding the opportunity for comments. Comments were and may be submitted to torticolliscpg@gmail.com.
AGREE II Reviewers
This CPG was evaluated by these reviewers using the AGREE II,15 an established instrument designed to assess the quality of clinical practice guidelines.
Lisa Selby-Silverstein, PT, PhD. NCS
Professor, Program in Physical Therapy
Neumann University, Aston PA
Catherine R. Smith, PT, DPT, PhD, PCS, CNT
Associate Professor, Physical Therapy; Vanderbilt Pediatric Professorship
University of Tennessee, Chattanooga, TN
Phase 7: Submission for publication to Pediatric Physical Therapy.
Colleen P. Coulter, PT, DPT, PhD, PCS
Sandra L. Kaplan, PT, DPT, PhD
Barbara Sargent, PT, PhD, PCS
Linda Fetters, PT, PhD, FAPTA, Pediatric Physical Therapy, Editor-in-Chief
Phase 8: Dissemination of guideline
APTA Academy of Pediatric Physical Therapy Web page.
PEDro Submission –Sandra L. Kaplan, PT, DPT, PhD
Presentations scheduled at the APPT Annual Conference (2018) and the APTA Combined Sections Meeting (2019).
Phase 9: Plan for revision. The GDG recommends that the CPG be updated in five years, as the body of evidence expands.11 The guideline revision will be organized by Barbara Sargent, PT, PhD, PCS. Similar to the 2018 CMT CPG, a systematic review to inform the 2023 CMT CPG will be initiated in 2021 and completed in 2023; the 2023 CMT CPG update will begin in 2022 and be completed in 2023.
Phase 10: Plan for monitoring guideline uptake. The GDG recommends a survey of pediatric PTs in 2021, similar to Kaplan et al,7 to assess implementation of the 2018 CMT CPG guideline.
Supplementary Material
SDC 4 Table 4. Studies on Measurement Approaches
SDC 5 Table 5: Studies on the First-choice Intervention
SDC 6 Table 6. Studies on Supplemental Interventions
SDC 8 Table 8. The 2018 CMT CPG Summary of Action Statements
SDC 7 Table 7. Studies of Long Term Follow-Up
Special Acknowledgements
Pam Corley, Reference librarian, USC 2013 CMT CPG
Robert Johnson, MLIS, Reference librarian, USC, 2018 CMT Systematic Review
Richard Shiffman, MD, BridgeWIZ developer.
Funding
Authors are members of the APTA and the Academy of Pediatric Physical Therapy, both of which provided funds for travel to meetings and clerical services in support of the guideline;
Dr. Sargenťs salary was supported by National Institutes of Health grant K12-HD055929 (PI: Ottenbacher), website: www.nih.gov. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Funding sources did not influence the content or process of updating the guideline.
Grant support:
This study was supported by grants from the American Physical Therapy Association, the Academy of Pediatric Physical Therapy, and the National Institutes of Health.
Appendix 1: ICF and ICD 10 Codes
| ICF codes | CMT presentation |
|---|---|
| Impairments of Body Structures and Functions | |
| B7108 Mobility of joint functions, other specified | Cervical Passive & Active ROM |
| B7300 Power of isolated muscles and muscle groups | Strength of lateral neck flexion and cervical rotation; Strength of neck and back extensors in prone; symmetrical strength of SCM in pull to sit. |
| B7350 Tone of isolated muscles and muscle groups | Hyper or hypotonia; spasm |
| B7600 Control of simple voluntary movements | Active visual pursuit toward the shortened side; symmetrical movements of trunk; UE and LEs in developmental positions |
| S7103 Joints of head and neck region | Cervical AROM, PROM |
| S7104 Muscles of head and neck region | Presence of a SCM mass |
| S7108 Structure of head and neck region, other specified | Facial and skull symmetry |
| S7401/ S5001 Hip Joint | Hip Dysplasia |
| Activity Limitations | |
| D110 Watching | TIMP, AIMS, AROM, ocular torticollis |
| D440 Fine hand use | Hands to midline; hemi-syndrome |
| D445 Hand and arm use | Hands to midline; hemi-syndrome; AIMS, AROM |
| Participation Restrictions | |
| D7600 Parent-child relationships | Parent comfort and knowledge with positioning and home programming |
| D7601 Child-parent relationships | Infant engagement with parent during feeding and play |
| D920 Recreation and leisure | AIMS, attention to toys |
AROM, active range of motion; AIMS, Alberta Infant Motor Scales; CMT, congenital muscular torticollis; ICD, International Statistical Classification of Diseases and Related Health Problems; ICF, International Classification of Functioning, Disability and Health; LEs, lower extremities; PROM, passive range of motion; ROM, range of motion; SCM, sternocleidomastoid; TIMP, Test of Infant Motor Performance; UE, upper extremities
ICD 10 Codes
The following codes may be used by a variety of health care professionals and are offered for reference; they are not intended to be directional for billing purposes.
Q67.0 Facial asymmetry
Q67.3 Plagiocephaly
Q68.0 Congenital deformity of sternocleidomastoid muscle
Q79.8 Other congenital malformations of musculoskeletal system
P15.2 Sternomastoid injury due to birth injury
M43.6 Torticollis
Appendix 2: Operational Definitions
Brachycephaly:
Cranial deformation with flattening of the entire posterior surface of the head.211
Cervical rotation:
Movement in the transverse plane, such that the chin turns toward or past the ipsilateral shoulder.
Congenital muscular torticollis (CMT):
Congenital muscular torticollis is a common pediatric orthopedic condition, described as a postural deformity of the neck evident at birth or shortly thereafter. It is typically characterized by a head tilt to one side and the neck rotated to the opposite side, due to unilateral shortening or fibrosis of the sternocleidomastoid muscle. It may be accompanied by cranial deformation or DDH, and less frequently, atypically present as a head tilt and neck twisting to the same side. 29, 110, 212. CMT has been associated with DDH,49 brachial plexus injury,20–22 lower extremity deformities,23–25 early developmental delay,20, 50 persistent developmental delays,109 facial asymmetry, which may impact function and cosmesis,52 and temporomandibular joint dysfunction.53
Cranial deformation:
a distortion of the shape of the skull resulting from mechanical forces that occur pre or postnatally.211 This term includes plagiocephaly and brachycephaly.
Lateral cervical flexion, side bending, or head tilt:
Movement in the coronal plane, such that the infant’s ear approaches the ipsilateral shoulder.
Plagiocephaly:
Cranial deformation with flattening of 1 side of the head.139
Postural preference:
(synonymous with positional preference) refers to the preferred head and neck asymmetry that an infant gravitates to in all positions.
Sternocleidomastoid mass
(synonymous with fibromotosis colli, tumor, pseudotumor or node):
A condition in which the sternocleidomastoid muscle is enlarged due to fibrosing of muscle cells with identifiable histological changes.93 It is referred to as a ‘mass’ throughout this document.
Footnotes
Conflict of Interest statement: The authors declare no conflicts of interest.
The American Physical Therapy Association Academy of Pediatric Physical Therapy welcomes comments on this guideline. Comments may be sent to torticolliscpg@gmail.com. This guideline may be reproduced for educational and implementation purposes.
Reviewers: Cynthia Baker, MD (AAP representative), Ginette Lange, PhD, CNM, FNP (nursing midwifery), Christine McDonough, PT, PhD (methodologist), Victoria Mena, AuD (parent representative), Anna Öhman, PT, PhD (pediatric physical therapist and researcher), Scott Parrott, PhD (methodologist), Melanie Percy, RN, PhD, CPNP, FAAN (pediatric nurse practitioner), Amy Pomrantz, PT, DPT, OCS, ATC (parent representative), Philip Spandorfer, MD, MSCE, FAAP (pediatrician), Jordan Steinberg, MD, PhD, FAAP (pediatric plastic surgeon), and members of the APPT Knowledge Translation Committee, Erin Bompiani, PT, DPT, PCS, Ellen Brennan, PT, DPT, PCS, Catie Christensen, PT, DPT, PCS, Barbara Pizzutillo, PT, DPT, MBA and Susan Rabinowicz, PT, DPT, MS.
Supplemental digital content, SDC, is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (https://journals.lww.com/pedpt/pages/default.aspx). Additionally, supplements can be downloaded from the Academy of Pediatric Physical Therapy Web site (https://pediatricapta.org/clinical-practice-guidelines/).
Conflict of Interest Statements
Colleen P. Coulter, PT, DPT, PhD, PCS - nothing to disclose
Barbara Sargent, PT, PhD, PCS – nothing to disclose
Sandra L. Kaplan, PT, DPT, PhD – nothing to disclose
Contributor Information
Sandra L. Kaplan, Department of Rehabilitation and Movement Sciences, Rutgers, The State University of New Jersey, Newark NJ.
Colleen Coulter, Orthotics and Prosthetics Department, Children’s Healthcare of Atlanta, Atlanta, GA.
Barbara Sargent, Division of Biokinesiology and Physical Therapy at the Herman Ostrow School of Dentistry, University of Southern California, Los Angeles, CA.
REFERENCES
- 1.Kaplan SL, Coulter C, Fetters L. Physical therapy management of congenital muscular torticollis: An evidence-based clinical practice guideline from The Section On Pediatrics Of The American Physical Therapy Association. Pediatr Phys Ther 2013;25(4):348–394. [DOI] [PubMed] [Google Scholar]
- 2.Shiffman RN, Michel G, Rosenfeld RM, Davidson C. Building better guidelines with BRIDGE-Wiz: development and evaluation of a software assistant to promote clarity, transparency, and implementability. J Am Med Inform Assoc 2011;19:94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Institute of Medicine. Clinical practice guidelines we can trust Washington, DC: National Academies Press;2011. 9780309164221 (pbk.), 0309164222 (pbk.), 9780309164238 (pdf). [Google Scholar]
- 4.Do TT. Congenital muscular torticollis: current concepts and review of treatment. [Review] [22 refs]. Curr Opin Pediatr 2006;18(1):26–29. [DOI] [PubMed] [Google Scholar]
- 5.Gutierrez D, Kaplan SL. Aligning documentation with congenital muscular torticollis clinical practice guidelines: Administrative case report. Phys Ther 2016;96(1):111–120. [DOI] [PubMed] [Google Scholar]
- 6.Nichter S A clinical algorithm for early identification and intervention of cervical muscular torticollis. Clin Pediatr 2015;Epub:1-5. [DOI] [PubMed]
- 7.Kaplan SL, Dole RL, Schreiber J. Uptake of the congenital muscular torticollis clinical practice guideline into pediatric practice. Pediatr Phys Ther 2017;29:307–313. [DOI] [PubMed] [Google Scholar]
- 8.Strenk ML, Kiger M, Hawke JL, Mischnick A, Quatman-Yates C. Implementation of a quality improvement initiative: Improved congenital muscular torticollis outcomes in a large hospital setting. Phys Ther 2017;97(6):649–658. [DOI] [PubMed] [Google Scholar]
- 9.Oledzka M, Kaplan SL, Sweeney JK, Coulter C, Evans-Rogers DL. Interrater and intrarater reliabiity of the congenital muscular torticollis severity classification system. Pediatr Phys Ther 2018;May 25, 2018 - Publish Ahead of Print - Issue - p [DOI] [PubMed]
- 10.Heidenreich E, Johnson R, Sargent B. Informing the update to the Physical Therapy Management Of Congenital Muscular Torticollis clinical practice guideline. Pediatr Phys Ther 2018;30(3):164–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaplan SL, Coulter C, Fetters L. Developing evidence-based physical therapy clinical practice guidelines. Pediatr Phys Ther 2013;Publish Ahead of Print: 10.1097/PEP.1090b1013e31829491c31829495. [DOI] [PubMed] [Google Scholar]
- 12.Keklicek H, Uygur F. A randomized controlled study on the efficiency of soft tissue mobilization in babies with congenital muscular torticollis. J Back Musculoskelet Rehabil 2018;31(2):315–321. [DOI] [PubMed] [Google Scholar]
- 13.Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Darrah J, Hickman R, O’Donnell M, Vogtle L, Wiart L. AACPDM Methodology To Develop Systematic Reviews Of Treatment Interventions (Revision 1.2) Milwaukee, WI: American Academy for Cerebral Palsy and Developmental Medicine;2008. [Google Scholar]
- 15.AGREE Next Steps Consortium,. The AGREE II Instrument [Electronic version] 2009.
- 16.Blythe WR, Logan TC, Holmes DK, Drake AF. Fibromatosis colli: A common cause of neonatal torticollis. Am Fam Physician 1996;54(6):1965–1967. [PubMed] [Google Scholar]
- 17.Lowry KC, Estroff JA, Rahbar R. The presentation and management of fibromatosis colli. Ear Nose Throat J 2004;89(9):4–9. [DOI] [PubMed] [Google Scholar]
- 18.Stassen LF, Kerawala CJ. New surgical technique for the correction of congenital muscular torticollis (wry neck). Br J Oral Maxillofac Surg 2000;38(2):142–147. [DOI] [PubMed] [Google Scholar]
- 19.Hollier L, Kim J, Grayson BH, McCarthy JG. Congenital muscular torticollis and the associated craniofacial changes. Plast Reconstr Surg 2000;105(3):827–835. [DOI] [PubMed] [Google Scholar]
- 20.Stellwagen LM, Hubbard E, Chambers C, Jones KL. Torticollis, facial asymmetry and plagiocephaly in normal newborns. Arch Dis Child 2008;93(10):827–831. [DOI] [PubMed] [Google Scholar]
- 21.Tien YC, Su JY, Lin GT, Lin SY. Ultrasonographic study of the coexistence of muscular torticollis and dysplasia of the hip. J Pediatr Orthop 2001;21(3):343–347. [PubMed] [Google Scholar]
- 22.Ballock RT, Song KM. The prevalence of nonmuscular causes of torticollis in children. J Pediatr Orthop 1996;16(August):500–504. [DOI] [PubMed] [Google Scholar]
- 23.Tatli B, Aydinli N, Caliskan M, Ozmen M, Bilir F, Acar G. Congenital muscular torticollis: evaluation and classification. Pediatr Neurol 2006;34(1):41–44. [DOI] [PubMed] [Google Scholar]
- 24.Tomczak KK, Rosman NP. Torticollis. J Child Neurol 2012;28(3):365–378. [DOI] [PubMed] [Google Scholar]
- 25.Stellwagen LM, Hubbard E, Vaux K. Look for the “stuck baby” to identify congenital torticollis. Contemp Pediatr 2004;21(5):55–65. [Google Scholar]
- 26.Cheng JC, Tang SP, Chen TM, Wong MW, Wong EM. The clinical presentation and outcome of treatment of congenital muscular torticollis in infants—A study of 1,086 cases. J Pediatr Surg 2000;35(7):1091–1096. [DOI] [PubMed] [Google Scholar]
- 27.Boere-Boonekamp MM, van der Linden-Kuiper LT. Positional preference: Prevalence in infants and follow-up after two years. Pediatr 2001;107:339–343. [DOI] [PubMed] [Google Scholar]
- 28.Matuszewski L, Pietrzyk D, Kandzierski G, Wilczynski M. Bilateral congenital torticollis: A case report with 25 years of follow-up. J Pediatr Orthop B 2017;26(6):585–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen M-M, Chang H-C, Hsieh C-F, Yen M-F, Chen TH-H. Predictive model for congenital muscular torticollis: Analysis of 1021 infants with sonography. Arch Phys Med Rehabil 2005;86(11):2199–2203. [DOI] [PubMed] [Google Scholar]
- 30.Aarnivala HEI, Valkama AM, Pirttiniemi PM. Cranial shape, size and cervical motion in normal newborns. Early Human Development 2014;90(8):425–430. [DOI] [PubMed] [Google Scholar]
- 31.Cheng JC, Au AW. Infantile torticollis: a review of 624 cases. J Pediatr Orthop 1994;14(6):802–808. [PubMed] [Google Scholar]
- 32.Cheng JC, Tang SP, Chen TM. Sternocleidomastoid pseudotumor and congenital muscular torticollis in infants: a prospective study of 510 cases. J Pediatr 1999;134(6):712–716. [DOI] [PubMed] [Google Scholar]
- 33.McAllister JM, Hall ES, Hertenstein GER, Merhar SL, Uebel PL, Wexelblatt SL. Torticollis in Infants with a History of Neonatal Abstinence Syndrome. J Pediatr May 2018;196:305–308. [DOI] [PubMed] [Google Scholar]
- 34.Nucci P, Kushner BJ, Serafino M, Orzalesi N. A multi-disciplinary study of the ocular, orthopedic, and neurologic causes of abnormal head postures in children. Am J Ophthalmol 2005;140(1):65–68. [DOI] [PubMed] [Google Scholar]
- 35.Cheng JC, Chen TM, Tang SP, Shum SL, Wong MW, Metreweli C. Snapping during manual stretching in congenital muscular torticollis. Clin Orthop Relat Res 2001;384:237–244. [DOI] [PubMed] [Google Scholar]
- 36.Petronic I, Brdar R, Cirovic D, et al. Congenital muscular torticollis in children: Distribution, treatment duration and outcome. Eur J Phys Rehabil Med 2010;45(2):153–158. [PubMed] [Google Scholar]
- 37.Celayir AC. Congenital muscular torticollis: early and intensive treatment is critical. A prospective study. Pediatr Int 2000;42(5):504–507. [DOI] [PubMed] [Google Scholar]
- 38.Fradette J, Gagnon I, Kennedy E, Snider L, Majnemer A. Clinical decision making regarding intervention needs of infants with torticollis. Pediatr Phys Ther 2011;23(3):249–256. [DOI] [PubMed] [Google Scholar]
- 39.Hagan JF, Shaw JS, Duncan PM. Bright futures: Guidelines for health supervision of infants, children and adolescents, 3rd Edition. Elk Grove Village, IL: 2008. 9781581102239. [Google Scholar]
- 40.Council on Children With Disabilities, Section on Developmental Behavioral Pediatrics, Bright Futures Steering Committee, Medical Home Initiatives for Children With Special Needs Project Advisory Committee. Identifying infants and young children with developmental disorders in the medical home: An algorithm for developmental surveillance and screening. Pediatr July 2006;118(1):405–420. [DOI] [PubMed] [Google Scholar]
- 41.Lee K, Chung E, Lee B-H. A comparison of outcomes of asymmetry in infants with congenital muscular torticollis according to age upon starting treatment. J Phys Ther Sci 2017;29:543–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coventry MB, Harris LE. Congenital muscular torticollis in infancy some observations regarding treatment. JBJS 1959;41(5):815–822. [PubMed] [Google Scholar]
- 43.Canale ST, Griffin DW, Hubbard CN. Congenital muscular torticollis. A long-term follow-up. JBJS 1982;64(6):810–816. [PubMed] [Google Scholar]
- 44.Joyce MB, de Chalain TMB. Treatment of recalcitrant idiopathic muscular torticollis in infants with botulinum toxin type a. J Craniofac Surg 2005;16(2):321–327. [DOI] [PubMed] [Google Scholar]
- 45.Bouchard M, Chouinard S, Suchowersky O. Adult cases of congenital muscular torticollis successfully treated with botulinum toxin. Mov Disord 2010;25(14):2453–2456. [DOI] [PubMed] [Google Scholar]
- 46.Shim JS, Noh KC, Park SJ. Treatment of congenital muscular torticollis in patients older than 8 years. J Pediatr Orthop 2004;24(6):683–688. [DOI] [PubMed] [Google Scholar]
- 47.Chen CE, Ko JY. Surgical treatment of muscular torticollis for patients above 6 years of age. Arch Orthop Trauma Surg 2000;120(3–4):149–151. [DOI] [PubMed] [Google Scholar]
- 48.Ajay G, Kothari S. Lower pole release in congenital muscular torticollis – Retrospective analysis of outcomes in 15 cases. IJPMR 2016;27(3):46–48. [Google Scholar]
- 49.Seo SJ, Kim JH, Joh YH, et al. Change of facial asymmetry in patients with congenital muscular torticollis after surgical release. J Craniofac Surg January 2016;27(1):64–69. [DOI] [PubMed] [Google Scholar]
- 50.Uluer MC, Bojovic B. A rare cervical dystonia mimic in adults: Congenital muscular torticollis (fibromatosis colli), a follow-up. Front Neurol 2016;7:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wei JL, Schwartz KM, Weaver AL, Orvidas LJ. Pseudotumor of infancy and congenital muscular torticollis: 170 cases. Laryngoscope 2001;111(4 Pt 1):688–695. [DOI] [PubMed] [Google Scholar]
- 52.Hummer CD, MacEwen GD. The coexistence of torticollis and congenital dysplasia of the hip. JBJS 1972;54(6):1255–1256. [PubMed] [Google Scholar]
- 53.Schertz M, Zuk L, Zin S, Nadam L, Schwartz D, Bienkowski RS. Motor and cognitive development at one-year follow-up in infants with torticollis. Early Hum Dev 2008;84(1):9–14. [DOI] [PubMed] [Google Scholar]
- 54.Öhman A, Nilsson S, Lagerkvist A, Beckung ERE. Are infants with torticollis at risk of a delay in early motor milestones compared with a control group of healthy infants? Dev Med Child Neurol 2009;51:545–550. [DOI] [PubMed] [Google Scholar]
- 55.Tse P, Cheng J, Chow Y, Leung PC. Surgery for neglected congenital torticollis. Acta Orthop Scand 1987;58(3):270–272. [DOI] [PubMed] [Google Scholar]
- 56.Yu C-C, Wong F-H, Lo L-J, Chen Y-R. Craniofacial deformity in patients with uncorrected congenital muscular torticollis: an assessment from three-dimensional computed tomography imaging. Plast Reconstr Surg 2004;113:24–33. [DOI] [PubMed] [Google Scholar]
- 57.Demirbilek S, Atayurt HF. Congenital muscular torticollis and sternomastoid tumor: results of nonoperative treatment. J Pediatr Surg 1999;34(4):549–551. [DOI] [PubMed] [Google Scholar]
- 58.van Vlimmeren LA, Helders PJ, van Adrichem LN, Engelbert RH. Diagnostic strategies for the evaluation of asymmetry in infancy-a review. [Review] [74 refs]. Eur J Pediatr 2004;163(4–5):185–191. [DOI] [PubMed] [Google Scholar]
- 59.van Vlimmeren LA, Helders PJM, van Adrichem LNA, Engelbert RHH. Torticollis and plagiocephaly in infancy: therapeutic strategies. Pediatr Rehabil 2006;9:40–46. [DOI] [PubMed] [Google Scholar]
- 60.de Chalain TMB, Park S. Torticollis associated with positional plagiocephaly: A growing epidemic. J Craniofac Surg 2010;16(3):411–418. [DOI] [PubMed] [Google Scholar]
- 61.Cameron BHLJC, Cameron GS. Success of nonoperative treatment for congenital muscular torticollis is dependent on early therapy. J Pediatr Surg 1994;9:391–393. [Google Scholar]
- 62.Lal S, Abbasi AS, Jamro S. Response of primary torticollis to physiotherapy. J Surg Pakistan 2011;16(December):153–156. [Google Scholar]
- 63.Emery C The determinants of treatment duration for congenital muscular torticollis. Phys Ther 1994;74(10):921–929. [DOI] [PubMed] [Google Scholar]
- 64.Öhman AM, Nilsson S, Beckung ERE. Stretching treatment for infants with congenital muscular torticollis: Physiotherapist or parents? A randomized pilot study. PM R 2010;2:1073–1079. [DOI] [PubMed] [Google Scholar]
- 65.Peitsch WK, H. KC, A. LR, B. MJ. Incidence of cranial asymmetry in health newborns. Pediatr 2002;110(6). [DOI] [PubMed] [Google Scholar]
- 66.Porter S, Qureshi R, Caldwell BA, Echevarria M, Dubbs WB, Sullivan MW. Developmental surveillance and screening practices by pediatric primary care providers implications for early intervention professionals. Infants Young Child 2016;29(2):91–101. [Google Scholar]
- 67.Koren A, Reece SM, Kahn-D’angelo L, Medeiros D. Parental information and behaviors and provider practices related to tummy time and back to sleep. J Pediatr Health Care 2003;24(4):222–230. [DOI] [PubMed] [Google Scholar]
- 68.Moon RY, Task Force On Sudden Infant Death S. SIDS and other sleep-related infant deaths: Evidence base for 2016 updated recommendations for a safe infant sleeping environment. Pediatrics November 2016;138(5):e20162940. [DOI] [PubMed] [Google Scholar]
- 69.Chen CC, Bode RK, Granger CV, Heinemann AW. Psychometric properties and developmental differences in children’s ADL item hierarchy: a study of the WeeFIM instrument. Am J Phys Med Rehabil September 2005;84(9):671–679. [DOI] [PubMed] [Google Scholar]
- 70.Öhman AM, Mardbrink E-L, Stensby J, Beckung E. Evaluation of treatment strategies for muscle function. Physiother Theory Pract 2011;27(7):463–470. [DOI] [PubMed] [Google Scholar]
- 71.Luxford BK. The physiotherapy management of infants with congenital muscuiar torticollis: A survey of current practice in New Zealand. NZ Journal of Physiotherapy 2009;37(3):127–135. [Google Scholar]
- 72.Jung AY, Kang EY, Lee SH, Nam DH, Cheon JH, Kim HJ. Factors that affect the rehabilitation duration in patients with congenital muscular torticollis. Ann Rehabil Med February 2015;39(1):18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Han MH, Kang JY, Do HJ, et al. Comparison of clinical findings of congenital muscular torticollis between patients with and without sternocleidomastoid lesions as determined by ultrasonography. J Pediatr Orthop August 2 2017. [DOI] [PubMed] [Google Scholar]
- 74.van Vlimmeren LA, van der Graaf Y, Boere-Boonekamp MM, L’Hoir MP, Helders PJM, Engelbert RHH. Risk factors for deformational plagiocephaly at birth and at 7 weeks of age: A prospective cohort study. Pediatr 2007;119:e408–418. [DOI] [PubMed] [Google Scholar]
- 75.Nuysink J, van Haastert IC, Takken T, Helders PJM. Symptomatic asymmetry in the first six months of life: Differential diagnosis. Eur J Pediatr 2008;167(6):613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Binder H, Eng GD, Gaiser JF, Koch B. Congenital muscular torticollis: results of conservative management with long-term follow-up in 85 cases. Arch Phys Med Rehabil April 1987;68(4):222–225. [PubMed] [Google Scholar]
- 77.Thompson F, McManus S, Colville J. Familial congenital muscular torticollis: Case report and review of the literature. Clin Orthop Relat Res 1986;202. [PubMed] [Google Scholar]
- 78.Sönmez K, Turkyilmaz Z, Demirogullari B, et al. Congenital muscular torticollis in children. [Review] [16 refs]. J Oto-Rhino-Laryngology 2005;67(6):344–347. [DOI] [PubMed] [Google Scholar]
- 79.Tessmer A, Mooney P, Pelland L. A developmental perspective on congenital muscular torticollis: A critical appraisal of the evidence. Pediatr Phys Ther 2010;22(4):378–383. [DOI] [PubMed] [Google Scholar]
- 80.APTA. Guide to physical therapist practice. Phys Ther January 2001;81(1):1–768. [PubMed] [Google Scholar]
- 81.Gray GMTKH. Differential diagnosis of torticollis: A case report. Pediatr Phys Ther 2009;21:369–374. [DOI] [PubMed] [Google Scholar]
- 82.Lobo MA, Harbourne RT, Dusing SC, McCoy SW. Grounding early intervention: Physical therapy cannot just be about motor skills anymore. Phys Ther 2013;93(1):94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Williams CR, O’Flynn E, Clarke NM, Morris RJ. Torticollis secondary to ocular pathology. J Bone Joint Surg Br 1996;78(4):620–624. [PubMed] [Google Scholar]
- 84.Karmel-Ross K Torticollis: Differential Diagnosis, Assessment And Treatment, Surgical Management And Bracing Binghamton,NY: Haworth Press, Inc.; 1997. [Google Scholar]
- 85.Freed SS, Coulter-O’Berry C. Identification and treatment of congenital muscular torticollis in infants. J Prosthet Orthot. 2004;16:S18–S23. [Google Scholar]
- 86.Rahlin M TAMO therapy as a major component of physical therapy intervention for an infant with congenital muscular torticollis: A case report. Pediatr Phys Ther 2005;17:209–218. [DOI] [PubMed] [Google Scholar]
- 87.Haque S, Shafi BBB, Kaleem M. Imaging of torticollis in children. RadioGraphics 2012;32:557–571. [DOI] [PubMed] [Google Scholar]
- 88.Minihane KP, Grayhack JJ, Simmons TD, Seshadri R, Wysocki RW, Sarwark JF. Developmental dysplasia of the hip in infants with congenital muscular torticollis. Am J Orthop 2008;37(9). [PubMed] [Google Scholar]
- 89.von Heideken J, Green DW, Burke SW, et al. The relationship between developmental dysplasia of the hip and congenital muscular torticollis. J Pediatr Orthop 2006;26(6):805–808. [DOI] [PubMed] [Google Scholar]
- 90.Nucci P, Curiel B. Abnormal head posture due to ocular problems: a review. Curr Pediatr Rev 2009;5(2):105–111. [Google Scholar]
- 91.Brodsky MC, Holmes JM. Torsional augmentation for the treatment of lateropulsion and torticollis in partial ocular tilt reaction. J AAPOS 2012;16(2):141–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ahn AR, Rah UW, Woo JE, Park S, Kim S, Yim SY. Craniovertebral junction abnormalities in surgical patients with congenital muscular torticollis. J Craniofac Surg February 26 2018;Feb 26 Publish Ahead of Print. [DOI] [PubMed] [Google Scholar]
- 93.Brown RE, Harave S. Diagnostic imaging of benign and malignant neck masses in children-a pictorial review. Quant Imaging Med Surg October 2016;6(5):591–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Boyko N, Eppinger MA, Straka-DeMarco D, Mazzola CA. Imaging of congenital torticollis in infants: a retrospective study of an institutional protocol. J Neurosurg Pediatr August 2017;20(2):191–195. [DOI] [PubMed] [Google Scholar]
- 95.Lee SY, Park HJ, Choi YJ, et al. Value of adding sonoelastography to conventional ultrasound in patients with congenital muscular torticollis. Pediatr Radiol December 2013;43(12):1566–1572. [DOI] [PubMed] [Google Scholar]
- 96.Cheng JC-Y, Metreweli C, Chen TM-K, Tang S-P. Correlation of ultrasonographic imaging of congenital muscular torticollis with clinical assessment in infants. Ultrasound Med Biol 2000;26(8):1237–1241. [DOI] [PubMed] [Google Scholar]
- 97.Tang S, Liu Z, Quan X, Qin J, Zhang D. Sternocleidomastoid pseudotumor of infants and congenital muscular torticollis: fine-structure research. J Pediatr Orthop 1998;18(2):214–218. [PubMed] [Google Scholar]
- 98.Tang SFT, Hsu K-H, Wong AMK, Hsu C-C, Chang C-H. Longitudinal followup study of ultrasonography in congenital muscular torticollis. Clin Orthop Relat Res 2002(403):179–185. [DOI] [PubMed] [Google Scholar]
- 99.Dudkiewicz I, Ganel A, Blankstein A. Congenital muscular torticollis in infants: Ultrasound-assisted diagnosis and evaluation. J Pediatr Orthop 2005;25(6):812–814. [DOI] [PubMed] [Google Scholar]
- 100.Kwon DR, Park GY. Diagnostic value of real-time sonoelastography in congenital muscular torticollis. J Ultrasound Med 2012;31:721–727. [DOI] [PubMed] [Google Scholar]
- 101.Hu CF, Fu TC, Chen CY, Chen CP, Lin YJ, Hsu CC. Longitudinal follow-up of muscle echotexture in infants with congenital muscular torticollis. Medicine (Baltimore) February 2017;96(6):e6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee Y-T, Park J-W, Lim M, et al. A clinical comparative study of ultrasound-normal vs. Ultrasound-abnormal congenital muscular torticollis. PM&R 2016;8(3):214–220. [DOI] [PubMed] [Google Scholar]
- 103.Chon SC, Yoon SI, You JH. Use of the novel myokinetic stretching technique to ameliorate fibrotic mass in congenital muscular torticollis: An experimenter-blinded study with 1-year follow-up. J Back Musculoskelet Rehabil 2010;23:63–68. [DOI] [PubMed] [Google Scholar]
- 104.Taylor JL, Norton Ellen S. Developmental muscular torticollis: Outcomes in young children treated by physical therapy. Pediatr Phys Ther 1997;9:173–178. [Google Scholar]
- 105.Burch C, Hudson P, Reder R, Ritchey M, Strenk M, Woosley M. Cincinnati Children’s Hospital Medical Center: Evidence-based clinical care guideline for therapy management of congenital muscular torticollis in children age 0 to 36 months: Cincinnati Children’s Hospital Medical Center;2009.
- 106.Storer SK, Dimaggio J, Skaggs DL, Angeles CHL, Angeles L. Developmental dysplasia of the hip. Am Fam Physician 2006;74:1310–1316. [PubMed] [Google Scholar]
- 107.Watemberg N, Ben-Sasson A, Goldfarb R. Transient motor asymmetry among infants with congenital torticollis-description, characterization, and results of follow-up. Pediatr Neurol June 2016;59:36–40. [DOI] [PubMed] [Google Scholar]
- 108.Majnemer A, Barr RG. Association between sleep position and early motor development. J Pediatr 2006;149(5):623–629. [DOI] [PubMed] [Google Scholar]
- 109.Majnemer A, Barr RG. Influence of supine sleep positioning on early motor milestone acquisition. Dev Med Child Neurol 2005;47(6):370–376. [DOI] [PubMed] [Google Scholar]
- 110.Rahlin M, Sarmiento B. Reliability of still photography measuring habitual head deviation from midline in infants with congenital muscular torticollis. Pediatr Phys Ther 2010;22(4):399–406. [DOI] [PubMed] [Google Scholar]
- 111.Öhman AM, Beckung ERE. Reference values for range of motion and muscle function of the neck in infants. Pediatr Phys Ther 2008;20:53–58. [DOI] [PubMed] [Google Scholar]
- 112.Fletcher JP, Bandy WD. Intrarater reliability of CROM measurement of cervical spine active range of motion in persons with and without neck pain. JOSPT 2008;38(10):640–645. [DOI] [PubMed] [Google Scholar]
- 113.Schertz M, Zuk L, Green D. Long-term neurodevelopmental follow-up of children with congenital muscular torticollis. J Child Neurol 2012(September). [DOI] [PubMed] [Google Scholar]
- 114.Cheng JCY, Wong MWN, Tang SP, Chen TM, Shum SL, Wong EM. Clinical determinants of the outcome of manual stretching in the treatment of congenital muscular torticollis in infants: A prospective study of eight hundred and twenty-one cases. JBJS 2001;83(5):679–687. [DOI] [PubMed] [Google Scholar]
- 115.Klackenberg EP, Elfving B, Haglund-Åkerlind Y, Carlberg EB. Intra-rater reliability in measuring range of motion in infants with congenital muscular torticollis. Adv Physiother 2005;7:84–91. [Google Scholar]
- 116.Campbell SK, Kolobe TH, Osten ET, Lenke M, Girolami GL. Construct validity of the test of infant motor performance. Phys Ther 1995;75(7):585–596. [DOI] [PubMed] [Google Scholar]
- 117.Öhman A, Beckung E. Functional and cosmetic status in children treated for congenital muscular torticollis as infants. Adv Physiother 2005;7(3):135–140. [Google Scholar]
- 118.Laughlin J, Luerssen TG, Dias MS. Prevention and management of positional skull deformities in infants. Pediatr 2011;128(6):1236–1241. [DOI] [PubMed] [Google Scholar]
- 119.Öhman AM, Nilsson S, Beckung ER. Validity and reliability of the muscle function scale, aimed to assess the lateral flexors of the neck in infants. Physiother Theory Pract 2009;25(2):129–137. [DOI] [PubMed] [Google Scholar]
- 120.AHRQ. Screening for developmental dysplasia of the hip: Evidence synthesis Number 422006
- 121.Shaw BA, Segal LS, Section On Orthopaedics. Evaluation and referral for developmental dysplasia of the hip in infants. Pediatr December 2016;138(6). [DOI] [PubMed] [Google Scholar]
- 122.Jimenez C, Delgado-Rodriquez M, Lopez-Moratalla M, Sillero M, Galvez-Vargas R. Validity and diagnostic bias in the clinical screening for congenital dysplasia of the hip. Acta Orthop Belg 1994;60(3):315–321. [PubMed] [Google Scholar]
- 123.U. S. Preventative Services Task Force. Screening for developmental dysplasia of the hip: Recommendation statement 2006.
- 124.Guille JT, Pizzutillo PD, MacEwen GD. Developmental dysplasia of the hip from birth to six months. J Am Acad Orthop Surg 1999;8:232–242. [DOI] [PubMed] [Google Scholar]
- 125.Sulaiman AR, Yusof Z, Munajat I, Lee NAA, Rad MM, Zaki N. Developmental dysplasia of hip screening using Ortolani and Barlow testing on breech delivered neonates. Malays Orthop J 2011;5(3):13–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Committee on Quality Improvement-Subcommittee on Developmental Dysplasia of the Hip. Clinical practice guideline: Early detection of developmental dysplasia. Pediatr 2000;105(4 pt 1):896–905. [DOI] [PubMed] [Google Scholar]
- 127.Herr K, Coyne PJ, Key T, et al. Pain assessment in the nonverbal patient: Position statement with clinical practice recommendations. Pain Manag Nurs 2006;7(2):44–52. [DOI] [PubMed] [Google Scholar]
- 128.Kim MY, Kwon DR, Lee HI. Therapeutic effect of microcurrent therapy in infants with congenital muscular torticollis. Phys Med Rehabil 2009;1(8):736–739. [DOI] [PubMed] [Google Scholar]
- 129.Büttner W, Finke W. Analysis of behavioural and physiological parameters for the assessment of postoperative analgesic demand in newborns, infants and young children: a comprehensive report on seven consecutive studies. Paediatr Anaesth 2000;10(3):303–318. [DOI] [PubMed] [Google Scholar]
- 130.Alves MMO, Carvalho PRA, Wagner MB, Castoldi A, Becker MM, Silva CC. Cross-validation of the children’s and infants’ postoperative pain scale in brazilian children. Pain Practice 2008;8(3):171–176. [DOI] [PubMed] [Google Scholar]
- 131.Merkel S, Voepel-Lewis T, Malviya S. Pain assessment in infants and young children: the FLACC scale. Am J Nurs 2002;102(10):55–58. [DOI] [PubMed] [Google Scholar]
- 132.Merkel SI, Voepel-Lewis T, Shayevitz JR, Malviya S. The FLACC: A behavioral scale for scoring postoperative pain in young children. Pediatr Nurs 1997;23(3):293–297. [PubMed] [Google Scholar]
- 133.Manworren RCB, Hynan LS. Clinical validation of FLACC: Preverbal patient pain scale. Pediatr Nurs 2003;29(2):140–146. [PubMed] [Google Scholar]
- 134.Malviya S, Voepel-Lewis T, Burke C, Merkel S, Tait AR. The revised FLACC observational pain tool: Improved reliability and validity for pain assessment in children with cognitive impairment. Paediatr Anaesth 2006;16(3):258–265. [DOI] [PubMed] [Google Scholar]
- 135.Oleszek JL, Chang N, Apkon SD, Wilson PE. Botulinum toxin type a in the treatment of children with congenital muscular torticollis. Am J Phys Med Rehabil 2005;84(10):813–816. [DOI] [PubMed] [Google Scholar]
- 136.Collins A, Jankovic J. Botulinum toxin injection for congenital muscular torticollis presenting in children and adults. Neurology 2006;67:1083–1085. [DOI] [PubMed] [Google Scholar]
- 137.Parikh SN, Crawford AH, Choudhury S. Magnetic resonance imaging in the evaluation of infantile torticollis. Orthop 2004;27(5):509–515. [DOI] [PubMed] [Google Scholar]
- 138.Chate RA. Facial scoliosis from sternocleidomastoid torticollis: Long-term postoperative evaluation. Br J Oral Maxillofac Surg 2005;43(5):428–434. [DOI] [PubMed] [Google Scholar]
- 139.Argenta L Clinical classification of positional plagiocephaly. J Craniofac Surg 2004;15(3):368–372. [DOI] [PubMed] [Google Scholar]
- 140.Persing J, James H, Swanson J, Kattwinkel J, Medicine A. Prevention and management of positional skull deformities in infants. Pediatr 2003;112(1):199–202. [DOI] [PubMed] [Google Scholar]
- 141.van Vlimmeren La, van der Graaf Y, Boere-Boonekamp MM, L’Hoir MP, Helders PJM, Engelbert RHH. Effect of pediatric physical therapy on deformational plagiocephaly in children with positional preference: a randomized controlled trial. Arch Pediatr Adolesc Med 2008;162:712–718. [DOI] [PubMed] [Google Scholar]
- 142.Spermon J, Spermon-Marijnen R, Scholten-Peeters W. Clinical classification of deformational plagiocephaly according to Argenta: a reliability study. J Craniofac Surg 2008;19:664–668. [DOI] [PubMed] [Google Scholar]
- 143.van Vlimmeren LA, Takken T, van Adrichem LN, van der Graaf Y, Helders PJ, Engelbert RH. Plagiocephalometry: a non-invasive method to quantify asymmetry of the skull; a reliability study. Eur J Pediatr March 2006;165(3):149–157. [DOI] [PubMed] [Google Scholar]
- 144.van Adrichem LNA, van Vlimmeren LA, Cadanova D, et al. Validation of a simple method for measuring cranial deformities (plagiocephalometry). J Craniofac Surg 2008;19(1):15–21. [DOI] [PubMed] [Google Scholar]
- 145.Öhman A The inter-rater and intra-rater reliability of a modified “severity scale for assessment of plagiocephaly” among physical therapists. Physiother Theory Pract 2011;28(5):402–406. [DOI] [PubMed] [Google Scholar]
- 146.Öhman A A craniometer with a headband can be a reliable tool to measure plagiocephaly and brachycephaly in clinical practice. Health 2016;08(12):1258–1265. [Google Scholar]
- 147.Loveday BP, de Chalain TB. Active counterpositioning or orthotic device to treat positional plagiocephaly? J Craniofac Surg 2001;12:308–313. [DOI] [PubMed] [Google Scholar]
- 148.Plank LH, Giavedoni B, Lombardo JR, Geil MD, Reisner A. Comparison of infant head shape changes in deformational plagiocephaly following treatment with a cranial remolding orthosis using a noninvasive laser shape digitizer. J Craniofac Surg 2006;17(6):1084–1091. [DOI] [PubMed] [Google Scholar]
- 149.Golden KA, Beals SP, Littlefield TR, Pomatto JK. Sternocleidomastoid imbalance versus congenital muscular torticollis: their relationship to positional plagiocephaly. Cleft Palate Craniofac J 1999;36(3):256–261. [DOI] [PubMed] [Google Scholar]
- 150.Holowka MA, Reisner A, Giavedoni B, Lombardo JR, Coulter C. Plagiocephaly Severity Scale to aid in clinical treatment recommendations. J Craniofac Surg May 2017;28(3):717–722. [DOI] [PubMed] [Google Scholar]
- 151.Cunningham ML, Heike CL. Evaluation of the infant with an abnormal skull shape. Curr Opin Pediatr 2007;19:645–651. [DOI] [PubMed] [Google Scholar]
- 152.Hsu T-C, Wang C-L, Wong M-K, Hsu K-H, Tang F-T, Chen H-T. Correlation of clinical and ultrasonographic in congenital muscular torticollis. Arch Phys Med Rehabil 1999;80:637–641. [DOI] [PubMed] [Google Scholar]
- 153.Lee K, Chung E, Lee B-H. A study on asymmetry in infants with congenital muscular torticollis according to head rotation. J Phys Ther Sci 2017;29:48–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.van Vlimmeren LA, Engelbert RH, Pelsma M, Groenewoud HM, Boere-Boonekamp MM, Nijhuis-van der Sanden MW. The course of skull deformation from birth to 5 years of age: a prospective cohort study. Eur J Pediatr January 2017;176(1):11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Darrah J, Piper M, Watt MJ. Assessment of gross motor skills of at-risk infants: Predictive validity of the Alberta Infant Motor Scale. Dev Med Child Neurol 1998;40(7):485–491. [DOI] [PubMed] [Google Scholar]
- 156.Folio MR, Fewell RR. Peabody Developmental Motor Scales Austin, Tex.: Pro-Ed; 2000. [Google Scholar]
- 157.Flannery AM, Tamber MS, Mazzola C, et al. Congress of Neurological Surgeons systematic review and evidence-based guidelines for the management of patients with positional plagiocephaly: Executive summary. Neurosurgery 2016;79(5):623–624. [DOI] [PubMed] [Google Scholar]
- 158.Dudek-Shriber L, Zelazny S. The effects of prone positioning on the quality and acquisition of developmental milestones in four-month-old infants. Pediatr Phys Ther 2007;19(1):48–55. [DOI] [PubMed] [Google Scholar]
- 159.Monson RM, Deitz J, Kartin D. The relationship between awake positioning and motor performance among infants who slept supine. Pediatr Phys Ther 2003;15(5):196–203. [DOI] [PubMed] [Google Scholar]
- 160.Pin T, Eldridge B, Galea MP. A review of the effects of sleep position, play position, and equipment use on motor development in infants. Dev Med Child Neurol 2007;49(11):858–867. [DOI] [PubMed] [Google Scholar]
- 161.Moon R, Task Force On Sudden Infant Death Syndrome. SIDS and other sleep-related infant deaths: expansion of recommendations for a safe infant sleeping environment. Pediatrics November 2011;128(5):1030–1039. [DOI] [PubMed] [Google Scholar]
- 162.Davis BE, Moon RY, Sachs HC, Ottolini MC. Effects of sleep position on infant motor development. Pediatr 1998;102(5):1135–1140. [DOI] [PubMed] [Google Scholar]
- 163.Fetters L, Huang H-h. Motor development and sleep, play, and feeding positions in very-low-birthweight infants with and without white matter disease. Dev Med Child Neurol 2007;49(11):807–813. [DOI] [PubMed] [Google Scholar]
- 164.Kennedy E, Majnemer A, Farmer JP, Bar RG, Platt RW. Motor development of infants with positional plagiocephaly. Phys Occup Ther Pediatr 2009;29(3):222–235. [DOI] [PubMed] [Google Scholar]
- 165.Philippi H, Faldum A, Jung T, et al. Patterns of postural asymmetry in infants: A standardized video-based analysis. Eur J Pediatr 2006;165(3):158–164. [DOI] [PubMed] [Google Scholar]
- 166.Wall V, Glass R. Mandibular asymmetry and breastfeeding problems: Experience from 11 cases. J Hum Lact 2006;22(3):328–334. [DOI] [PubMed] [Google Scholar]
- 167.Losee JE, Mason AC, Dudas J, Hua LB, Mooney MP. Nonsynostotic occipital plagiocephaly: Factors impacting onset, treatment, and outcomes. Plast Reconstr Surg 2007;119(6):1866–1873. [DOI] [PubMed] [Google Scholar]
- 168.Genna CW. Breastfeeding infants with congenital torticollis. J Hum Lact May 2015;31(2):216–220. [DOI] [PubMed] [Google Scholar]
- 169.Burstein FD. Long-term experience with endoscopic surgical treatment for congenital muscular torticollis in infants and children: A review of 85 cases. Plast Reconstr Surg 2004;114(2):491–493. [DOI] [PubMed] [Google Scholar]
- 170.Ryu JH, Kim DW, Kim SH, et al. Factors correlating outcome in young infants with congenital muscular torticollis. Can Assoc Radiol J 2016;67(1):82–87. [DOI] [PubMed] [Google Scholar]
- 171.Park HJ, Kim SS, Lee SY, et al. Assessment of follow-up sonography and clinical improvement among infants with congenital muscular torticollis. Am J Neuroradiology 2013;34:890–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Oledzka M, Suhr M. Postsurgical physical therapy management of congenital muscular torticollis. Pediatr Phys Ther April 2017;29(2):159–165. [DOI] [PubMed] [Google Scholar]
- 173.Lee K, Chung E, Koh S-E, Lee B-H. Outcomes of asymmetry in infants with congenital muscular torticollis. J Phys Ther Sci 2015;27:461–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hong SK, Song JW, Woo SB, Kim JM, Kim TE, Lee ZI. Clinical usefulness of sonoelastography in infants with congenital muscular torticollis. Ann Rehabil Med 2016;40(1):28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lee Y-T, Yoon K, Kim Y-B, et al. Clinical features and outcome of physiotherapy in early presenting congenital muscular torticollis with severe fibrosis on ultrasonography: a prospective study. J Pediatr Surg 2011;46(8):1526–1531. [DOI] [PubMed] [Google Scholar]
- 176.He L, Yan X, Li J, et al. Comparison of 2 dosages of stretching treatment in infants with congenital muscular torticollis: A randomized trial. Am J Phys Med Rehabil May 2017;96(5):333–340. [DOI] [PubMed] [Google Scholar]
- 177.Surprenant D, Milne S, Moreau K, Robert ND. Adapting to higher demands: Using innovative methods to treat infants presenting with torticollis and plagiocephaly. Pediatr Phys Ther 2014;26:339–345. [DOI] [PubMed] [Google Scholar]
- 178.Rabino SR, Peretz SR, Kastel-Deutch T, Tirosh E. Factors affecting parental adherence to an intervention program for congenital torticollis. Pediatr Phys Ther 2013;25(3):298–303. [DOI] [PubMed] [Google Scholar]
- 179.Karmel-Ross K, Lepp M. Assessment and treatment of children with congenital muscular torticollis. Phys Occup Ther Pediatr 1997;17(2):21–67. [Google Scholar]
- 180.Kwon DR, Park GY. Efficacy of microcurrent therapy in infants with congenital muscular torticollis involving the entire sternocleidomastoid muscle: a randomized placebo-controlled trial. Clin Rehabil 2014;28(10):983–991. [DOI] [PubMed] [Google Scholar]
- 181.Giray E, Karadag-Saygi E, Mansiz-Kaplan B, Tokgoz D, Bayindir O, Kayhan O. A randomized, single-blinded pilot study evaluating the effects of kinesiology taping and the tape application techniques in addition to therapeutic exercises in the treatment of congenital muscular torticollis. Clin Rehabil August 2017;31(8):1098–1106. [DOI] [PubMed] [Google Scholar]
- 182.Emery C Conservative management of congenital muscular torticollis: A literature review. Phys Occup Ther Pediatr 1997;17(2):13–20. [Google Scholar]
- 183.Öhman A The immediate effect of kinesiology taping on muscular imbalance in the lateral flexors of the neck in infants: a randomized masked study. PM R May 2015;7(5):494–498. [DOI] [PubMed] [Google Scholar]
- 184.Lee IJ, Lim SY, Song HS, Park MC. Complete tight fibrous band release and resection in congenital muscular torticollis. J Plast Reconstr Aesthet Surg 2010;63(6):947–953. [DOI] [PubMed] [Google Scholar]
- 185.Lee J, Moon H, Park M, Yoo W, Choi I, Cho T-J. Change of craniofacial deformity after sternocleidomastoid muscle release in pediatric patients with congenital muscular torticollis. JBJS 2012;94(13):e93–97. [DOI] [PubMed] [Google Scholar]
- 186.Amemiya M, Kikkawa I, Watanabe H, Hoshino Y. Outcome of treatment for congenital muscular torticollis: A study on ages for treatment, treatment methods, and postoperative therapy. Eur J Orthop Surg Traumatol 2009;19(5):303–307. [Google Scholar]
- 187.Swain B Transaxillary endoscopic release of restricting bands in congenital muscular torticollis--a novel technique. JPRAS 2007;60(1):95–98. [DOI] [PubMed] [Google Scholar]
- 188.Kozlov Y, Yakovlev A, Novogilov V, et al. SETT--Subcutaneous endoscopic transaxillary tenotomy for congenital muscular torticollis. J Laparoendosc Adv Surg Tech 2009;19(1):S-179–181. [DOI] [PubMed] [Google Scholar]
- 189.Joyce MB, de Chalain TM. Treatment of recalcitrant idiopathic muscular torticollis in infants with botulinum toxin type A. J Craniofac Surg 2005;16(2):321–327. [DOI] [PubMed] [Google Scholar]
- 190.Itoi E, Funayama K, Suzuki T, Kamio K, Sakurai M. Tenotomy and postoperative brace treatment for muscular torticollis. Contemp Orthop 1990;20(5):515–523. [PubMed] [Google Scholar]
- 191.Lee J-Y, Koh S-E, Lee I-S, et al. The cervical range of motion as a factor affecting outcome in patients with congenital muscular torticollis. Ann Rehabil Med 2013;37(2):183–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Prasad KD, Hegde C, Shah N, Shetty M. Congenital muscular torticollis: Rehabilitation with a customized appliance. J Prosthet Orthot 2013;25:89–92. [Google Scholar]
- 193.Skaggs DL, Lerman LD, Albrektson J, Lerman M, Stewart DG, Tolo VT. Use of a noninvasive halo in children. Spine 2008;33(15):1650–1654. [DOI] [PubMed] [Google Scholar]
- 194.Brand PL, Engelbert RH, Helders PJ, Offringa M. Systematic review of the effects of therapy in infants with the KISS-syndrome (kinetic imbalance due to suboccipital strain). Ned Tijdschr Geneeskd 2005;149(13):703–707. [PubMed] [Google Scholar]
- 195.Gotlib A, Rupert R. Chiropractic manipulation in pediatric health conditions--an updated systematic review. Chiropr Osteopat 2008;16:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Allergan. Botox2013.
- 197.Lee TG, Rah DK, Kim YO. Endoscopic-assisted surgical correction for congenital muscular torticollis. J Craniofac Surg 2012;23(6):1832–1834. [DOI] [PubMed] [Google Scholar]
- 198.Patwardhan S, Shyam AK, Sancheti P, Arora P, Nagda T, Naik P. Adult presentation of congenital muscular torticollis: a series of 12 patients treated with a bipolar release of sternocleidomastoid and Z-lengthening. J Bone Joint Surg Br 2011;93(6):828–832. [DOI] [PubMed] [Google Scholar]
- 199.Shim JS, Jang HP. Operative treatment of congenital torticollis. J Bone Joint Surg Br 2008;90(7):934–939. [DOI] [PubMed] [Google Scholar]
- 200.Hahn HM, Cook KH, Lee IJ, Park DH, Park MC. Use of acellular dermal matrix in treatment of congenital muscular torticollis in patients over eight years of age. J Craniofac Surg May 2017;28(3):610–615. [DOI] [PubMed] [Google Scholar]
- 201.Hung NN, Anh LT. A comparison of outcome of age at time surgery between younger and older than 8 years old in children with congenital muscular torticollis. OALibJ 2017;04(11):1–12. [Google Scholar]
- 202.Burstein FD, Cohen SR. Endoscopic surgical treatment for congenital muscular torticollis. Plast Reconstr Surg 1998;101(1):20–24. [DOI] [PubMed] [Google Scholar]
- 203.Cheng JC, Tang SP. Outcome of surgical treatment of congenital muscular torticollis. Clin Orthop Relat Res 1999;362:190–200. [PubMed] [Google Scholar]
- 204.Öhman A, Beckung E. Children who had congenital torticollis as infants are not at higher risk for a delay in motor development at preschool age. PM R October 2013;5(10):850–855. [DOI] [PubMed] [Google Scholar]
- 205.Öhman A, Nilsson S, Lagerkvist AL, Beckung E. Are infants with torticollis at risk of a delay in early motor milestones compared with a control group of healthy infants? Dev Med Child Neurol 2009;51(7):545–550. [DOI] [PubMed] [Google Scholar]
- 206.Öhman AM. The status of the cervical spine in preschool children with a history of congenital muscular torticollis. Open Journal of Therapy and Rehabilitation 2013;01(02):31–35. [Google Scholar]
- 207.Brusamento S, Legido-Quigley H, Panteli D, et al. Assessing the effectiveness of strategies to implement clinical guidelines for the management of chronic diseases at primary care level in EU member states: a systematic review. Health Policy 2012;107(2–3):168–183. [DOI] [PubMed] [Google Scholar]
- 208.RNAO. Toolkit : Implementation of Best Practice Guidelines (2nd Ed). Toronto, Ontario: 2012. [Google Scholar]
- 209.Hoenig H, Duncan PW, Horner RD, et al. Structure, process, and outcomes in stroke rehabilitation. Med Care 2002;40(11):1036–1047. [DOI] [PubMed] [Google Scholar]
- 210.Moulding NT, Silagy Ca, Weller DP. A framework for effective management of change in clinical practice: dissemination and implementation of clinical practice guidelines. Qual Health Care 1999;8(3):177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Whited K, Aiyagari V, Calderon-Arnulphi M, et al. Standardized admission and discharge templates to improve documentation of The Joint Commission on Accreditation of Healthcare Organization performance markers. J Neurosci Nurs 2010;42(4):225–228. [DOI] [PubMed] [Google Scholar]
- 212.Davies BL. Evidence into clinical practice. JOGNN 2002;31:558–562. [DOI] [PubMed] [Google Scholar]
- 213.Kinsman L, James EL. Evidence-based practice needs evidence-based implementation. Lippincotts Case Manag Sep-Oct 2001;6(5):208–216; quiz 217–209. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SDC 4 Table 4. Studies on Measurement Approaches
SDC 5 Table 5: Studies on the First-choice Intervention
SDC 6 Table 6. Studies on Supplemental Interventions
SDC 8 Table 8. The 2018 CMT CPG Summary of Action Statements
SDC 7 Table 7. Studies of Long Term Follow-Up


