Abstract
Background
Despite a remarkable progress in the reduction of global rate of maternal mortality, cervical cancer has been identified as the leading cause of maternal morbidity and mortality, particularly in sub-Saharan African countries. The uptake of cervical cancer screening service has been consistently shown to be effective in reducing the incidence rate and mortality from cervical cancer. Despite this, there are limited studies in Ethiopia that were conducted to assess the uptake of cervical cancer screening and its predictors, and these studies showed inconsistent and inconclusive findings. Therefore, this systematic review and meta-analysis was conducted to estimate the pooled cervical cancer screening utilization and its predictors among eligible women in Ethiopia.
Methods and findings
Databases like PubMed, Web of Science, SCOPUS, CINAHL, Psychinfo, Google Scholar, Science Direct, and the Cochrane Library were systematically searched. All observational studies reporting cervical cancer screening utilization and/ or its predictors in Ethiopia were included. Two authors independently extracted all necessary data using a standardized data extraction format. Quality assessment criteria for prevalence studies were adapted from the Newcastle Ottawa quality assessment scale. The Cochrane Q test statistics and I2 test were used to assess the heterogeneity of studies. A random effects model of analysis was used to estimate the pooled prevalence of cervical cancer screening utilization and factors associated with it with the 95% confidence intervals (CIs). From 850 potentially relevant articles, twenty-five studies with a total of 18,067 eligible women were included in this study. The pooled national cervical cancer screening utilization was 14.79% (95% CI: 11.75, 17.83). The highest utilization of cervical cancer screening (18.59%) was observed in Southern Nations Nationalities and Peoples’ region (SNNPR), and lowest was in Amhara region (13.62%). The sub-group analysis showed that the pooled cervical cancer screening was highest among HIV positive women (20.71%). This meta-analysis also showed that absence of women’s formal education reduces cervical cancer screening utilization by 67% [POR = 0.33, 95% CI: 0.23, 0.46]. Women who had good knowledge towards cervical screening [POR = 3.01, 95%CI: 2.2.6, 4.00], perceived susceptibility to cervical cancer [POR = 4.9, 95% CI: 3.67, 6.54], severity to cervical cancer [POR = 6.57, 95% CI: 3.99, 10.8] and those with a history of sexually transmitted infections (STIs) [POR = 5.39, 95% CI: 1.41, 20.58] were more likely to utilize cervical cancer screening. Additionally, the major barriers of cervical cancer screening utilization were considering oneself as healthy (48.97%) and lack of information on cervical cancer screening (34.34%).
Conclusions
This meta-analysis found that the percentage of cervical cancer screening among eligible women was much lower than the WHO recommendations. Only one in every seven women utilized cervical cancer screening in Ethiopia. There were significant variations in the cervical cancer screening based on geographical regions and characteristics of women. Educational status, knowledge towards cervical cancer screening, perceived susceptibility and severity to cervical cancer and history of STIs significantly increased the uptake of screening practice. Therefore, women empowerment, improving knowledge towards cervical cancer screening, enhancing perceived susceptibility and severity to cancer and identifying previous history of women are essential strategies to improve cervical cancer screening practice.
Background
Despite a remarkable progress in the reduction of maternal mortality, cervical cancer is the second most commonly diagnosed cancer and the leading cause of cancer related death among African women [1]. There were approximately 236,000 deaths from cervical cancer worldwide and it was the most common cancer in east and middle Africa [2, 3]. About 90% of cases and 85% of these deaths have occurred in Low and Middle-Income Countries (LMICs); the highest has occurred in Sub-Saharan Africa (SSA) and approximately 311,000 women died from cervical cancer [2]. The incidence, the death rate and morbidities associated with cervical cancer significantly varies across the world; higher in the developing nations compared to the developed countries [4]. The high burden of cervical cancer is mainly due to the early onset of sexual intercourse, multiple sexual partners, human immunodeficiency virus (HIV) infection, history of sexually transmitted infections (STIs), human papilloma virus (HPV) infection, cigarette smoking, limited resources for early detection and poor HPV vaccination coverage [5, 6].
Almost all of the maternal deaths associated with cervical cancer could be prevented if early and effective interventions mechanisms to cervical cancer control were available to all women. In particular, a comprehensive approach such as prevention, early diagnosis, effective screening and treatment programmes of pre-cervical lesions are essential for prevention of cervical cancer [7]. Visual inspection with Acetic Acid (VIA) and Visual Inspection with Lugol’s Iodine (VILI) are commonly used in low-resource settings [6]. VIA combined with the immediate treatment of women who tested positive at the first visit was cost saving and was the next most effective strategy, with a 26% decrease in the incidence of CC, further reduce mortality due to CC. A large-cluster randomized trial from rural India showed that a single round of HPV screening could reduce the incidence and mortality from CC of approximately 50% [8].
The guidelines of the World Health Organization (WHO), the United States Preventive Services Task Force (USPSTF) and the American Cancer Society (ACS) recommends that all eligible women should have cervical cancer screening at least once every three years [9]. Ethiopia adopted WHO’s recommendation that woman aged 30 and above should begin screening for cervical cancer at least one to three years of age with a see- and -treat approach. However, sexually active and HIV-positive women (start screening at HIV diagnosis) are suggested to be screened every 3 years regardless of their age [10]. The prevalence of cervical cancer screening is much higher at the Western countries than SSA [11, 12]; 85.0% in the United States, 78.6% in the United Kingdom [13], and ranges from 2% in Ethiopia, 6% in Kenya [14], to 8% in Nigeria [15]. The lower rate of cervical cancer screening programme at LMICs may be related to the complexity of the screening process and the common inherent barriers in the setting such as poverty, limited access to information, lack of knowledge of cervical cancer, lack of healthcare infrastructure required, lack of trained practitioners and the absence of sustained prevention programmes [16].
The government of Ethiopia launched a cervical cancer screening service and has given more emphasis on programs focusing on the early detection of cervical cancer using advocacy efforts by different stakeholders such as academia, professionals, media and partners. However, the prevalence of cervical cancer remains a major problem, and it is one of the leading causes of morbidity and mortality among women in the country [17, 18]. Evidence show success of cervical screening initiatives depend on high participation of the target population, which in turn is determined by the women’s knowledge, perceptions, health orientations and other socio-cultural issues. It is also affected by factors including early marriage, early sexual practice, delivery of the first baby before the age of 20, multiple sexual partners and low socio economic status. Therefore, addressing the different barriers for poor utilization of cervical cancer screening is essential component of intervention. Although, there were previous pocket studies conducted on these issues in Ethiopia, the studies showed fragmented, inconsistent and inconclusive findings. Even the studies were fragmented in different specific population characteristics like among HIV positive women and reproductive age women. Therefore, this systematic review and meta-analysis aimed to estimate the pooled cervical cancer screening utilization and its predictors among all eligible women in Ethiopia. It also aimed to address the common barriers of cervical cancer screening.
Methods
Registration of systematic review, data sources and search strategies
The purpose of this systematic review and meta-analysis was to estimate the pooled utilization level of cervical cancer screening and its predictors among women of reproductive-aged in Ethiopia. The protocol has been registered with the International Prospective Register of Systematic Review (PROSPERO), the University of York Center for Reviews and Dissemination (https://www.crd.york.ac.uk/), registration number CRD42019119626. The findings of this review have been reported as recommended by the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA-P) 2009 statement checklist [19] (S1 Table). All published articles were searched from major international databases like PubMed, Cochrane Library, Psych Info, Scopus, CINAHL, Web of Science, Science Direct, Google Scholar and African Journals Online. Additionally, Google hand searches were used mainly for unpublished studies. A search was also made for the reference list of studies already identified in order to retrieve additional articles. The Population, Exposure, Comparison and Outcomes (PECO) search formula was used to retrieve articles.
All eligible women for cervical cancer screening Ethiopia were the population of interest for this study. The outcome of interest was the utilization of cervical cancer screening among women. The predictor variables of cervical cancer screening utilization included in this study were age of women, educational status, and occupational status, knowledge of cervical cancer screening, perceived susceptibility and severity to cervical cancer and history of sexually transmitted infections. Comparisons were defined for each predictor based on the reported reference group for each predictor in each respective variable.
For each of the selected components of PECO, electronic databases were searched using the keyword search and the medical subject heading [MeSH] words. The keywords include “utilization, uptake, cervical cancer, screening, and women of reproductive age as well as Ethiopia”. The search terms were combined by the Boolean operators "OR" and "AND. The specific searching detail in PubMed was putted in S1 Appendix.
Eligibility criteria and study selection
This review included studies that reported either the use of cervical cancer screening or the cervical cancer screening predictors in Ethiopia. All published and unpublished studies through April 7, 2020 and reported in English language were retrieved to assess eligibility for inclusion in this review. However, this review excluded studies that were case reports of populations, surveillance data (demographic health survey), and abstracts of conferences, articles without full access and the outcome of interest not reported. The article selection underwent several steps. Two reviewers (MD and TE) evaluated the retrieved articles for inclusion using their title, abstract and full text review. Any disagreement during the selection process between the reviewers was resolved by consensus. Full texts of selected articles were then evaluated using the prior eligibility. During the encounter of duplication; only the full-text article was retained.
Quality assessment and data collection
The Newcastle-Ottawa Scale (NOS) quality assessment tool was used to assess the quality of the included studies. The tool contains three components- selection of the study groups, comparability of the study groups, and ascertainment of exposure or outcome [20]. The main component of the tool was graded from five stars and mainly emphasized on the methodological quality of each primary study. The other component of the tool graded from two stars and mainly concerned with the comparability of each study. The last component of the tool was graded from three stars and was used to evaluate the results and statistical analysis of each original study. The NOS included three categorical criteria with a maximum score of 9 points. The quality of each study was assessed using the following score algorithms: ≥7 points were considered as “good”, 4 to 6 points were considered as “moderate”, and ≤ 3 point was considered as “poor” quality studies. In order to improve the validity of this systematic review result, only primary studies of fair to good quality have been included. The two reviewers (MD and TE) independently assessed articles for overall study quality and extracted data using a standardized data extraction format. The data extraction format included primary author, year of publication, region of the study, sample size, prevalence, and the selected predictors of cervical cancer screening utilization.
Publication bias and statistical analysis
The publication bias was assessed using the Egger’s [21] and Begg’s [22] tests with a p-value of less than 0.05. The I2 statistic was used to assess heterogeneity between studies and a p-value of less than 0.05 was used to detect heterogeneity. As a result of the presence of heterogeneity, a random-effects model was used as a method of analysis [23]. Data were extracted in Microsoft Excel and exported to Stata version 11 for analysis. Subgroup analysis was conducted by geographic region, population’s characteristics and design or type of study. Moreover, a meta-regression model based on sample size and year of publication was used to identify the sources of random variations in the included studies. The effect of selected determinant variables was analyzed using separate categories of meta-analysis [24]. The findings of the meta-analysis were presented using forest plots and Odds Ratio (OR) with its 95% Confidence intervals (CI). In addition, we conducted a sensitivity analysis to assess whether the pooled prevalence estimates were influenced by individual studies.
Results
Study identification and characteristics of included studies
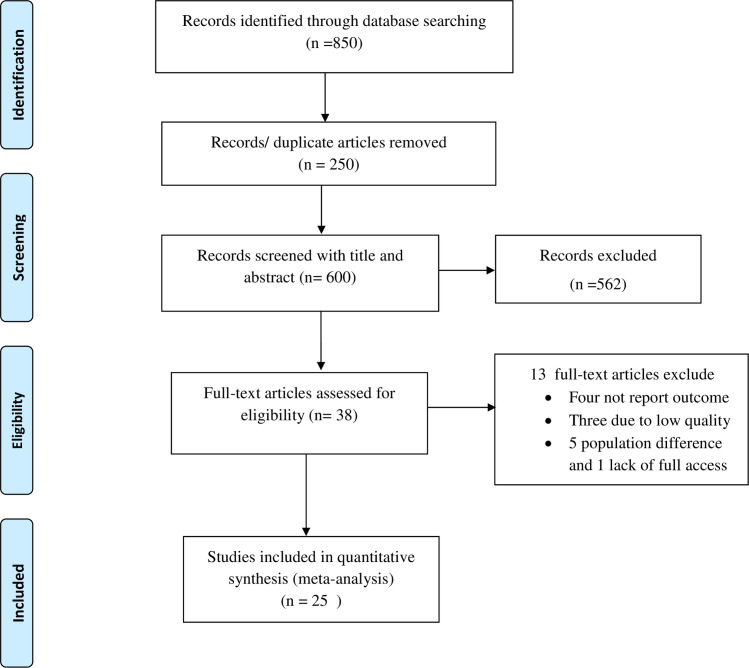
This systematic review and meta-analysis included both published and unpublished studies on the use of cervical cancer screening in Ethiopia. A total of 850 articles were found from the review. Of these, 250 duplicated records were removed and 581 articles were excluded by screening using their titles and abstracts. Subsequently, a total of 38 full-text papers were assessed for eligibility on the basis of the inclusion and exclusion criteria. Thus, four studies were excluded due to lack of the outcome of interest [25–30], three due to low quality [31–33], five due to difference in the study population [34–39] and only one study was excluded due to lack of access to the full text [40]. Finally, 25 studies were included in the final quantitative meta-analysis (Fig 1).
Fig 1. PRISMA flow diagram of cervical cancer screening utilization in Ethiopia.
All of the included studies were cross-sectional. From this, twelve studies were facility- based cross sectional studies (FBCS) and thirteen were community- based cross-sectional studies (CBCS). The review was conducted among 18,067 women to estimate the pooled prevalence of cervical cancer screening. Publication of articles was between 2016 and 2020. The largest sample size was 5,823 women in a national level study [41] and the smallest sample was 250 women from a study conducted in Oromia region [42]. All studies were conducted in five geographic regions of Ethiopia. Four studies (16%) were from Addis Ababa [43–46], nine (36%) were from Amhara [47–55], four (16%) were from Southern Nations, Nationalities and Peoples Representative (SNNPR) [56–60], four (16%) were from Oromia [42, 61–63], two (8%) were from Tigray [64, 65], and the remaining one study [41] was a national- level study. Twelve studies were conducted among eligible women with no specific characteristics of their HIV status [44, 47], five studies on HIV-positive women [43, 48, 53, 61, 63], four studies among healthcare workers [59, 63, 65, 66] and the remaining one study [51] was conducted among women who were commercial sex workers (Table 1).
Table 1. Characteristics of the included studies in the meta-analysis, Ethiopia.
| Author | Year | Region | Prevalence | Design | Sample | Population |
|---|---|---|---|---|---|---|
| Shiferaw H et al. [43] | 2018 | AA | 10.8 | FBCS | 598 | HIV+ |
| Getachew S et al. [44] | 2018 | AA | 25 | FBCS | 520 | All |
| Bante SA et al. [47] | 2019 | Amhara | 20.9 | CBCS | 577 | All |
| Aweke YH et al. [56] | 2017 | SNNPR | 9.9 | CBCS | 583 | all |
| Nega AD et al. [48] | 2018 | Amhara | 10 | FBCS | 496 | HIV+ |
| Nigussie T et al. [49] | 2019 | Amhara | 15.5 | CBCS | 737 | all |
| Bayu H et al. [64] | 2016 | Tigray | 19.8 | CBCS | 1186 | all |
| Assefa AA et al. [57] | 2019 | SNNPR | 40.1 | FBCS | 342 | all |
| Gebreegziabher M et al. [65] | 2016 | Tigray | 10.7 | FBCS | 225 | all |
| Solomon K et al. [61] | 2019 | Oromia | 25 | FBCS | 475 | HIV+ |
| Tefera and Mitiku [50] | 2017 | Amhara | 11 | CBCS | 620 | All |
| Muluneh BA et al. [51] | 2019 | Amhara | 13.28 | CBCS | 467 | CSWs |
| Seyoum T et al. [58] | 2017 | SNNPR | 9.6 | FBCS | 281 | all |
| Geremew AB et al. [26] | 2018 | Amhara | no data | 1152 | 98.7 | |
| Michael E et al. [42] | Unpub | Oromia | 17.6 | CBCS | 250 | all |
| Galibo T et al. [41] | 2017 | National | 2.9 | CBCS | 5823 | all |
| Kassa AS et al. [52] | 2018 | Amhara | 7.3 | CBCS | 735 | all |
| Erku DA et al. [53] | 2017 | Amhara | 23.5 | FBCS | 302 | HIV+ |
| Woldetsadik AB [45] | 2020 | AA | 12.2 | FBCS | 425 | All |
| Aynalem BY et al. [54] | 2020 | Amhara | 5.4 | CBCS | 822 | All |
| Asres T [55] | Unpub | Amhara | 18 | FBCS | 322 | Healthcare |
| Dulla D et al. [59] | 2017 | SNNPR | 11.4 | FBCS | 367 | Healthcare |
| Heyi WD et al. [62] | 2018 | Oromia | 5.8 | CBCS | 845 | All |
| Berhanu T et al. [66] | 2019 | AA | 9.3 | CBCS | 291 | Healthcare |
| Tekle T et al. [60] | 2020 | SNNPR | 22.9 | CBCS | 520 | All |
| Ashagrie A [63] | Unpub | Oromia | 16 | FBCS | 318 | HIV+ |
AA: Addis Ababa; CSWs: Commercial sex workers.
CBCS: community based cross-sectional study; FBCS: facility based cross-sectional study.
Meta-analysis of cervical cancer screening utilization in Ethiopia
The highest cervical cancer screening utilization was observed in SNNPR, a study conducted at ART health facilities of Hawassa, 40% [57] and Wolayita hospitals, 22.9% [60]. Whereas, the lowest was 2.9% in a national level study [41] and 5.4% from a study conducted in Amhara region [54].
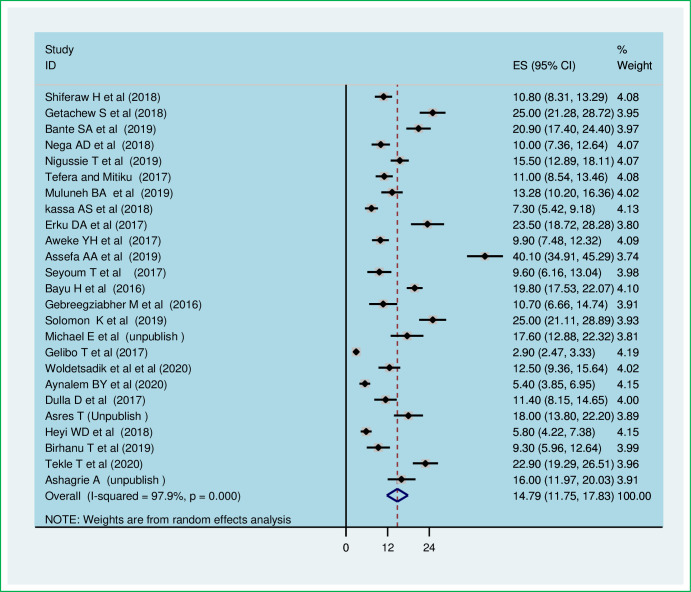
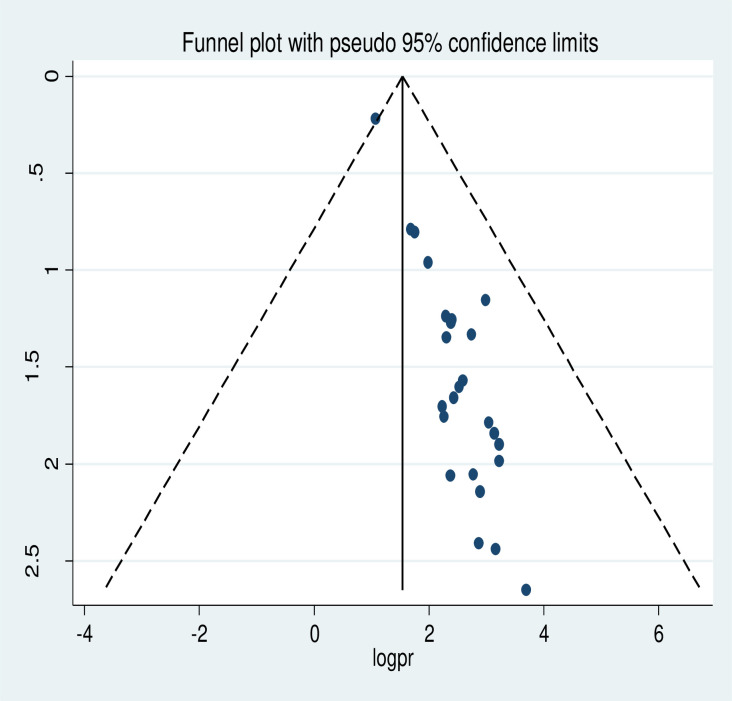
The meta-analysis of twenty-five studies showed that the pooled national level of cervical cancer screening utilization was 14.79% (95% CI: 11.75, 17.83). A random-effect model of analysis was used due to significant heterogeneity (I2 = 97.9%, p-value<0.05) (Fig 2). Publication bias was assessed using Eggers test and it was statistically significant, p-value less than 0.0001. To account for publication bias, the duval and trimmed full analysis was performed. The univariate meta-regression model was also used to identify possible sources of heterogeneity using different covariates like year of publication and sample size. However, none of these variables were found to be statistically significant, p-value > 0.05. Moreover, the sensitivity analysis using a random-effects model showed that no single study had unduly influenced the overall estimate of the use of cervical cancer screening among Ethiopian women (S1 Fig). The funnel plot also showed that there was symmetrical distribution (Fig 3).
Fig 2. The pooled utilization of cervical cancer screening among women in Ethiopia.
Fig 3. Funnel plot of the prevalence of cervical cancer screening utilization in Ethiopia.
Subgroup analysis
The subgroup analysis was conducted based on region of studies, the study design and women’s characteristics. Therefore, this random effect meta-analysis based on the geographic region revealed that the highest cervical cancer screening utilization was observed in the SNNPR, 18.59 (95% CI: 9.65, 27.53) followed by Oromia region, 16.00% (95% CI: 16.00% (95% CI: 6.31, 25.7) and lowest occurred in Amhara region, 13.62% (95% CI: 9.92, 17.32) (Table 2). In addition, the pooled subgroup analysis showed that cervical cancer screening was highest in studies that were institution- based cross-sectional studies, 17.54% (95% CI: 13.16, 21.93). The highest cervical cancer screening was among HIV- positive women, 20.71% (95% CI: 12.8, 28.63) and the lowest was among reproductive age women, 11.54% (95% CI: 8.00, 15.05) (Table 2).
Table 2. Sub-group analysis of cervical cancer screening utilization in Ethiopia: A meta-analysis.
| Subgroup type | Category | No of studies | Prevalence(95%CI) | I2 | P-value |
|---|---|---|---|---|---|
| Study design | FBCS | 12 | 17.54 (13.16,21.93) | 94.6% | <0.0001 |
| CBCS | 13 | 12.29 (8.70,15.88) | 98.0% | <0.0001 | |
| Region | Addis Ababa | 4 | 14.32 (8.09,20.56) | 93.7% | <0.0001 |
| Amhara | 9 | 13.62 (9.92,17.32) | 94.5% | <0.0001 | |
| SNNPR | 5 | 18.59 (9.65,27.53) | 97.3% | <0.001 | |
| Oromia | 4 | 16.00 (6.31, 25.7) | 97.1% | <0.001 | |
| Tigray | 2 | 15.41 (6.5, 24.32) | 93.3% | <0.001 | |
| National level | 1 | 2.9 (2.47,3.33) | - | ||
| Women characteristics | HIV positive | 5 | 20.71 (12.8,28.63) | 96.6% | <0.0001 |
| All women | 12 | 11.54 (8.00, 15.05) | 97.9% | <0.0001 | |
| Healthcare workers | 4 | 12.21 (8.71,15.71) | 72.4% | 0.012 | |
| Commercial sex worker | 1 | 13.28 (10.2,16.36) | - | - |
Predictors of cervical cancer screening utilization
Association of educational status and utilization of cervical cancer screening
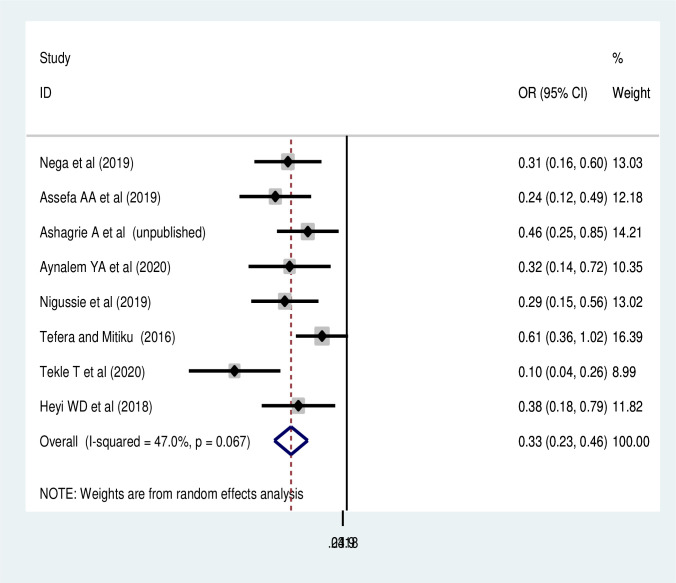
In regard to the social inequities, the effects of three predictors on cervical cancer screening utilization were estimated. Thus, age of women and occupational status were not significantly associated with cervical cancer screening utilization (S2 and S3 Figs). While, women’s educational status was significantly associated with utilization of cervical cancer screening. Accordingly, the pooled random effect of eight studies [48–50, 54, 57, 62, 63, 60] found that women who have no formal education were 66% (POR:0.33, 95% CI: 0.23,0.46) times less likely to utilize cervical cancer screening than those who attended any formal education (Fig 4).
Fig 4. Association of educational status with cervical cancer screening in Ethiopia.
Association of knowledge and perception of cancer and screening utilization
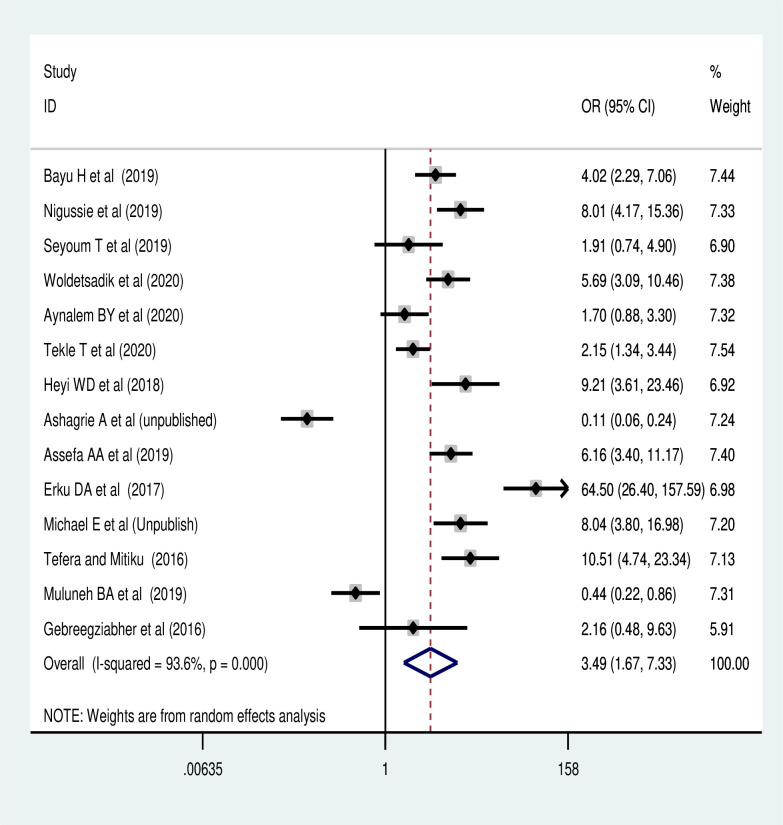
The meta-analysis of 14 studies revealed [42, 45, 49–51, 53, 54, 57, 58, 60, 62–65] that women’s knowledge of cervical cancer screening uptake was the commonest predictor of screening utilization. Women who had good knowledge of cervical cancer screening reuptake were 3.97 times (POR: 3.49, 95% CI: 1.67, 7.33) more likely to have cervical cancer screening than women who had poor knowledge (Fig 5).
Fig 5. Association of knowledge of the screening with cervical cancer screening utilization.
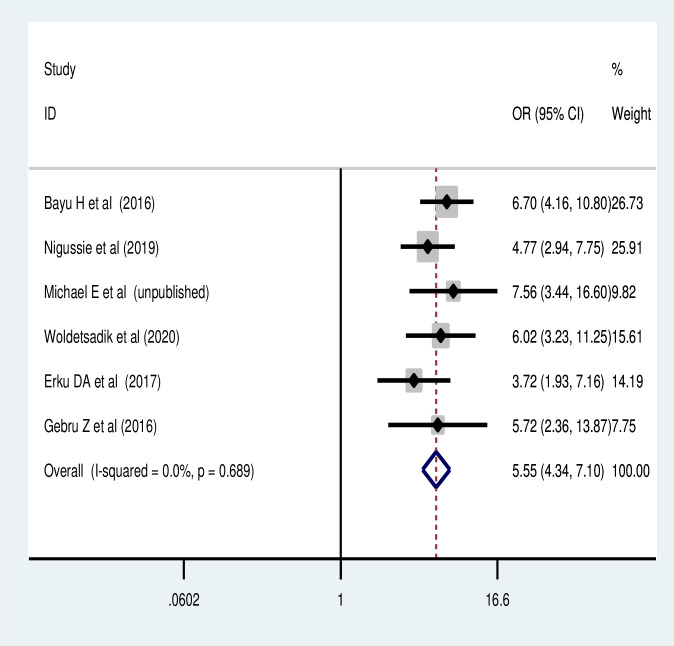
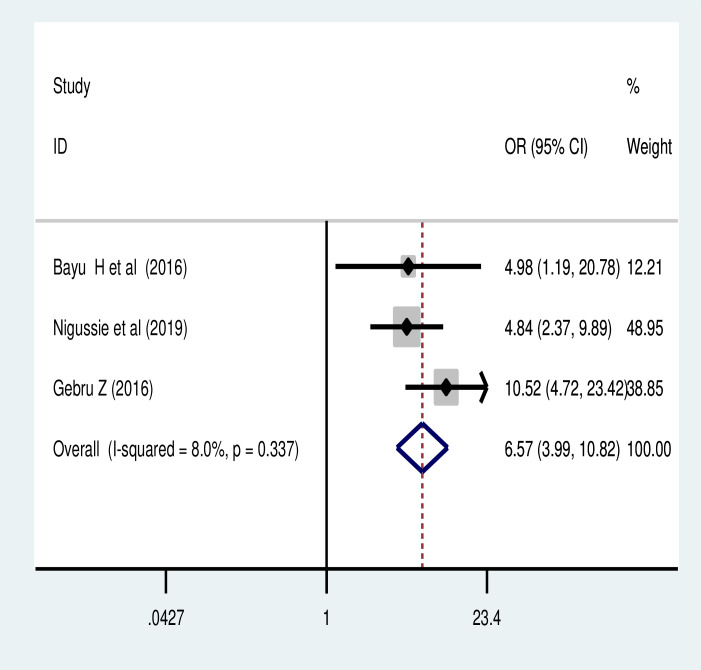
The pooled effect of six studies [33, 42, 45, 49, 53, 64] also revealed that the perceived susceptibility to cervical cancer was another major predictor of cervical cancer screening utilization in Ethiopia. Women who had perceived susceptibility to cervical cancer were 5.5 times more likely to reuptake cervical cancer screening than their counterparts (POR = 5.54, 95% CI: 4.28, 7.16) (Fig 6). Similarly, women who had perceived severity of cervical cancer were more likely to utilize cervical cancer screening (POR = 6.57, 95% CI: 3.99, 10.82) (Fig 7).
Fig 6. Association of perceived susceptibility to cervical cancer with cervical cancer screening.
Fig 7. Association of perceived severity of cancer and cervical cancer screening utilization.
Association of history of sexual transmitted infection and cervical cancer screening uptake
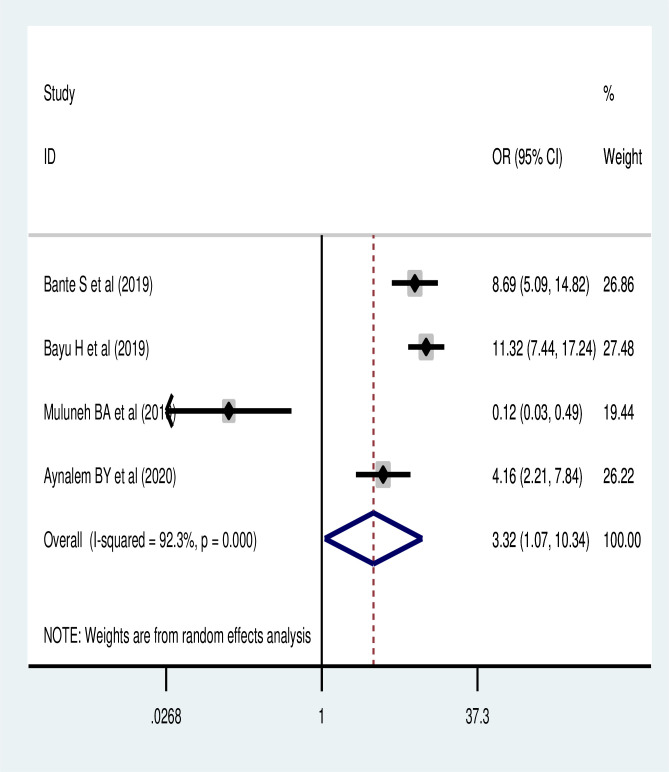
Based on the pooled analysis of four studies [47, 51, 54, 64], women who had history of sexual transmitted infection were more likely to utilize cervical cancer screening (POR: 3.32, 95% CI: 1.07, 10.34) (Fig 8).
Fig 8. Association of history of sexual transmitted infection with cervical cancer screening utilization.
Barriers of cervical cancer screening uptake
The pooled analysis also revealed that the most common reasons that hinder the use of cervical cancer screening were associated with women considered to be healthy, 48.97% (95% CI: 38.3, 59.59) and lack of information on screening, 34.34% (95% CI: (17.93, 50.75) (Table 3).
Table 3. Barriers of the cervical cancer screening utilization in Ethiopia: A meta-analysis.
| Barriers | Studies | Prevalence [95% CI] | I2 | P-value |
|---|---|---|---|---|
| Consider as healthy | 11 | 48.97% [38.3, 59.59] | 98.7% | <0.0001 |
| Fear of screening | 11 | 15.25% [6.77,23.73] | 99.4% | <0.0001 |
| Lack of information | 7 | 34.34% [17.93, 50.75] | 99.4% | <0.0001 |
| Embarrassment | 8 | 11.16% [5.76,16.56] | 99.3 | <0.0001 |
| Long waiting time | 7 | 21.58% [6.87,36.28] | 99.6 | <0.0001 |
| Don’t know place | 5 | 10.06% [3.53,16.59] | 97.0 | <0.0001 |
Discussions
The uptake of cervical cancer screening services in Ethiopia is not well established. Despite, WHO recommends cervical cancer screening tests to be included as part of well-planned and implemented programs in every country’s health care policy. This systematic review and meta-analysis was conducted to estimate the pooled level of cervical cancer screening and its associated factors in Ethiopia. Accordingly, the pooled national level of cervical cancer screening utilization was 14.79 (95% CI: 11.75, 17.83). This was lower than 85% from a study conducted in United States [13], 21.4% in China national population based survey [67], 19.4% in Kenya [68], 19% - 63% from studies conducted in 54 countries [69], 48.9% in Malaysia [70], and also lower than 67% from a national-based study conducted among Vietnamese women [71]. The difference could be explained by the variation in the population characteristics, study settings and quality of health care services and screening programs. Besides, this could be explained by socio-economic inequalities, higher birth order and poor access to reproductive health care service utilization in Ethiopia could lower the cervical screening utilization. Previous report also showed that women with high birth order and poor women are less likely to receive cervical screening service [69]. In Ethiopia, a small proportion of women are in contact with obstetric or gynecological health services and that the health system may not have the capacity to provide effective screening to a larger number of women. Therefore, intervention programs to improve the quality of cervical cancer screening clinics are essential.
The findings of this meta-analysis also showed that the highest prevalence of cervical cancer screening occurred in the SNNPR followed by Oromia region and the lowest was in Amhara region. Regional variation in the burden of cervical cancer screening in Ethiopia might be explained by the difference in maternal health care service utilization that could be explained by in the difference in spousal support, cultural and linguistic diversity across the regions and societal stigmatization. Additionally, health service-related reason like cost of access to services, proximity to facilities, navigation of the facilities, waiting time and attitude of the health care staff may be the reasons for the regional difference and lower use of cervical cancer screening in the country.
The highest screening utilization in SNNPR and Oromia may be due to the nature of included studies in the respective regions. For example, 60% of the studies from SNNP region were institutional based cross-sectional studies and 50% of the included studies from Oromia region were conducted among HIV-positive women. Such differences may have contributed to the higher prevalence of cervical cancer screening in SNNPR and Oromia regions. Furthermore, socio-demographic characteristics and lifestyle activities could also be mentioned as reasons for the variation in screening across the different regions in the country. The pooled cervical cancer screening was also highest among HIV- positive women (20.71%). This may be due to the fact that these women may be given information about the disease during their follow-up visit to antiretroviral therapy [57], which may improve their knowledge about cervical cancer, and therefore, increase service utilization.
This systematic review and meta-analysis found that educational status of women was one of the significant predictors of cervical cancer screening utilization. No formal education reduces the cervical cancer screening uptake by 67%, and this finding was supported by a study done in China [67] and a meta-analysis conducted in developed countries [72]. The possible justification for this might be due to the fact that women who have no formal education are less likely to have gynecological examinations and maternal health service utilization. As the result, they are likely to have limited exposure to visit health institution for antenatal care, health facility delivery and post-natal care.
Uneducated women also have lower possibilities to read and fully understand the information and instructions provided by healthcare providers, and therefore, reduce the rate of cervical cancer screening. Cervical cancer educational interventions and provider recommendation for screening increases the rates of cervical cancer screening [73]. Therefore, more integrated interventions to improve women’s empowerment should be done at national level to improve the rate of cervical cancer screening utilization, and therefore, reduce cervical cancer related morbidity and mortality.
This study also found that women’s knowledge of screening for cervical cancer was a significant predictor of cervical cancer screening service uptake. The finding was supported by studies done in Uganda [74], Malaysia [70], a review done in LMICs [75] and among Arab women [76]. This could be explained by the fact that those women who had good knowledge for cervical cancer screening are more likely to give priority to the issue and improves their decisions on health- seeking screening behavior. Accordingly, findings in Ethiopia, Malawi, Tanzania and Thailand [64, 77–79] have shown that a good flow of information and awareness creation campaigns about cervical cancer increase the uptake of cervical cancer screening.
This meta-analysis also showed that women with a history of STI were more likely to use screening for cervical cancer compared to those with no history of STI. This result was supported by the findings of other studies [64, 80]. This may be explained by the fact that women who have STIs and history of STI will have an increased chance of visiting health institutions for treatment and medical check-ups, and therefore, more likely to get the screening information from the healthcare provider.
This systematic review and meta-analysis also found that perceived susceptibility and severity were also predictors of the use of cervical cancer screening as supported by Wanyenze et al. [74]. This may be those who perceive their susceptibility or severity of cancer may be aware of the severity of the cancer and higher level of education about the disease as a result of the increased screening rate. As a result, those women who have an increased perception of susceptibility or severity of the disease may have higher education that has increased adherence to the cervical cancer screening [68]. These may include those women who are perceived to be more acutely aware of their risk, more interested and knowledgeable about health and behavioral issues, and better access to health information and resources [81]. This finding was also supported by recent studies done in Ghana [82] and Kenya [83] which found that women who perceived the severity of disease were more likely to accept screening due to increased perception of the benefits and barriers to cervical cancer, which increases their cancer screening.
Furthermore, the results of this systematic review and meta-analysis found that the common barriers to the utilization of cervical cancer screening were considered healthy and lack of information by women. This is supported by additional studies [47, 49, 51, 56, 64]. This may be due to the fact that those who consider their status to be healthy and who have poor knowledge are less likely to perceive the benefits of screening and the severity of cervical cancer., Therefore, multi-disciplinary interventions across the life course, community education and social mobilization on cervical cancer risk and its screening should be improved and emphasized to increase the cervical cancer screening utilization.
This review’s strengths include the very extensive systematic search conducted and the inclusion of articles identified without specifying the population characteristics and period of publications. Our review adopted the international standard definitions to measure the quality of studies. This meta-analysis has its strengths because it has used a pre-specified protocol for search strategy and data abstraction and used internationally accepted tools for a critical appraisal system for the quality assessment of individual studies.
However, the results of this review should be interpreted with some limitation. The high heterogeneity in the characteristics of the studies might lead to insufficient statistical power to detect significant association. However, a meta-regression analysis revealed that there was no variation due to sample size and publication year. This meta-analysis was also unable to assess the type of screening, and therefore, an area of research for future studies. Additionally, the studies included in this review were from only five regions out of the nine regional states and the two administrative cities that might reduce its representativeness for the country. Some studies have small sample size, affect the estimation.
Conclusions
This meta-analysis found that cervical cancer screening rate was lower than the WHO recommendations. Only one in every seven eligible women were screened in Ethiopia, and there was a significant variation in the screening level based on geographical regions and characteristics of women. Women’s educational status, knowledge towards cervical cancer screening, perceived susceptibility and severity to cervical cancer and history of sexual transmitted infections significantly increased uptake of the screening practice. Therefore, women empowerment, improving knowledge towards cervical cancer screening, enhancing perceived susceptibility and severity to cervical cancer and identifying previous history of women are an essential strategy to increase utilization of cancer screening. Moreover, adoption of the better strategies and addressing the barriers of cervical cancer screening uptake mainly improving of the provision of adequate information on cervical cancer screening has a paramount importance to improve cervical cancer screening among reproductive age women.
Supporting information
(TIF)
(TIF)
(TIF)
(DOC)
(DOCX)
(DOCX)
(DOCX)
Abbreviations
- CBCS
Community Based Cross-sectional Studies
- FBCS
Facility- Based Cross-sectional Studies
- HIV
Human Immunodeficiency Virus
- HPV
Human Papilloma Virus
- LMICs
Low and Middle-Income Countries
- POR
Pooled Odds Ratio
- SNNPR
Southern Nations, Nationalities and Peoples Representative
- SSA
Sub Saharan Africa
- STI
Sexually Transmitted Infection, WHO: World Health Organization
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.WHO U, PATH. cryosurgical equipment for the treatment of precancerous cervical lesions and prevention of cervical cancer. 2012.
- 2.WHO/NMH/NMA. UN Joint Global Programme on Cervical Cancer Prevention and Control. The United Nations Global Cervical Cancer Programme 2016.
- 3.Bruni L, Albero G, Serrano B, Mena M, Gómez D, Muñoz J, et al. Human Papillomavirus and Related Diseases in Ethiopia. 2017. [Google Scholar]
- 4.Fitzmaurice C, Dicker D, Pain A, Hamavid H, Moradi-Lakeh M, MacIntyre MF, et al. The Global Burden of Cancer 2013. JAMA oncology. 2015;1(4):505–27. Epub 2015/07/17. doi: 10.1001/jamaoncol.2015.0735 ; PubMed Central PMCID: PMC4500822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hendry M, Lewis R, Clements A, Damery S, Clare W. "HPV? Never heard of it!": A systematic review of girls’ and parents’ information needs, views and preferences about human papillomavirus vaccination 2013. doi: 10.1016/j.vaccine.2013.08.091 [DOI] [PubMed] [Google Scholar]
- 6.Health WHOR, Diseases WHOC, Promotion H. Comprehensive cervical cancer control: a guide to essential practice: World Health Organization; 2006. [PubMed]
- 7.Denny L, Quinn M, Sankaranarayanan R. Screening for cervical cancer in developing countries. Vaccine. 2006;24:S71–S7. doi: 10.1016/j.vaccine.2006.05.121 [DOI] [PubMed] [Google Scholar]
- 8.Sankaranarayanan R et al. HPV screening for cervical cancer in rural India. N Engl J Med. 2009;360: 1385–1394. doi: 10.1056/NEJMoa0808516 [DOI] [PubMed] [Google Scholar]
- 9.Moyer VA. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Annals of internal medicine. 2012;156(12):880–91. doi: 10.7326/0003-4819-156-12-201206190-00424 [DOI] [PubMed] [Google Scholar]
- 10.FMOH. National cancer control plan of Ethiopia. 2015.
- 11.Campos NG, Castle PE, Wright TC Jr, Kim JJ. Cervical cancer screening in low‐resource settings: A cost‐effectiveness framework for valuing tradeoffs between test performance and program coverage. International journal of cancer. 2015;137(9):2208–19. doi: 10.1002/ijc.29594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pimple S, Mishra G, Shastri S. Global strategies for cervical cancer prevention. Current Opinion in Obstetrics and Gynecology. 2016;28(1):4–10. doi: 10.1097/GCO.0000000000000241 [DOI] [PubMed] [Google Scholar]
- 13.(OECD. StatExtracts; 2013. http://stats.oecd.org/Index.aspx?DataSetCode=HEALTH_PROC. Cited May 6 2020.).
- 14.Sudenga SL, Rositch AF, Otieno WA, Smith JS. Knowledge, attitudes, practices, and perceived risk of cervical cancer among Kenyan women: brief report. International Journal of Gynecologic Cancer. 2013;23(5):895–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Idowu A, Olowookere SA, Fagbemi AT, Ogunlaja OA. Determinants of cervical cancer screening uptake among women in Ilorin, North Central Nigeria: a community-based study. Journal of cancer epidemiology. 2016;2016. doi: 10.1155/2016/6469240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ngugi CW BH, Muigai AW, Wanzala P, Mbithi JN. Factors affecting uptake of cervical cancer early detection measures among women in Thika, Kenya. Health Care Women Int. 2012;33(595–613 doi: 10.1080/07399332.2011.646367 ]. [DOI] [PubMed] [Google Scholar]
- 17.De Sanjosé S, Serrano B, Castellsagué X, Brotons M, Muñoz J, Bruni L, et al. Human papillomavirus (HPV) and related cancers in the Global Alliance for Vaccines and Immunization (GAVI) countries. A WHO/ICO HPV Information Centre Report. Vaccine. 2012;30(Suppl 4):D1–83. [DOI] [PubMed] [Google Scholar]
- 18.Chidyaonga-Maseko F, Chirwa ML, Muula AS. Underutilization of cervical cancer prevention services in low and middle income countries: a review of contributing factors. Pan African medical journal. 2015;21(1). doi: 10.11604/pamj.2015.21.231.6350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moher D et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic reviews. 2015;4(1). doi: 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). Jama. 2016;315(8):801–10. doi: 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Egger M SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Begg CB MM. Operating characteristics of a rank correlation testfor publication bias. Biometrics. 1994;50(4):1088–101. [PubMed] [Google Scholar]
- 23.Higgins JP TS, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deeks JJ, Higgins JP, Altman DG. Analysing data and undertaking meta‐analyses. Cochrane handbook for systematic reviews of interventions. 2008:241–84. [Google Scholar]
- 25.Teame H, Gebremariam L, Kahsay T, Berhe K, Gebreheat G. Factors affecting utilization of cervical cancer screening services among women attending public hospitals in Tigray region, Ethiopia, 2018; Case control study. 2019;14(3):e0213546. doi: 10.1371/journal.pone.0213546 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geremew AB, Gelagay AA, Azale T. Comprehensive knowledge on cervical cancer, attitude towards its screening and associated factors among women aged 30–49 years in Finote Selam town, northwest Ethiopia. Reproductive health. 2018;15(1):29. Epub 2018/02/16. doi: 10.1186/s12978-018-0471-1 ; PubMed Central PMCID: PMC5813403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petros H, Ayele AA. Cervical Cancer Screening and Treatment Services in South West Shoa Zone of Oromia Region. Ethiopian Journal of Reproductive Health. 2018;10(1):7–. [Google Scholar]
- 28.Gebrie MH, Belete MA, Lemlem SB, Woreta HK. Knowledge, preventive practice and associated factors of female nurses’ towards cervical cancer in the selected government hospitals in Addis Ababa, Ethiopia. J Diabetes Metab. 2015;6(7):569. [Google Scholar]
- 29.Tarekegn AA, Mengistu MY, Mirach TH. Health professionals’ willingness to pay and associated factors for cervical cancer screening program at College of Medicine and Health Sciences, University of Gondar, Northwest Ethiopia. PloS one. 2019;14(4). doi: 10.1371/journal.pone.0215904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bejiga B. Acceptability of cervical cancer screening using See and Treat (SAT) approachand determinant factors among women of reproductive age in health centers in Addis Ababa, Ethiopia 2017. [Google Scholar]
- 31.Boka A, Nigatu D. Cervical cancer screening and associated factors among women attending gynecology out-patient department and maternal and child health atmettu karlreferralhospital, South West, Ethiopia, 2019. International Journal of Current Research in Life Sciences. 8(01):2934–44. [Google Scholar]
- 32.Deribe L. cervical cancer screening service utilization and associated factors among HIV positive and women with unknown hiv status in alamata generalized hospital, Tigray, Ethiopia 2018: comparative cross sectional study: Addis Ababa Universty; 2018. [Google Scholar]
- 33.Gebru Z, Gerbaba M, Dirar A. Utilization of Cervical Carcinoma Screening Service and Associated Factors among Currently Married Women in Arba Minch Town, Southern Ethiopia Journal of Womens Health Care. 2016;5(1). [Google Scholar]
- 34.Tilahun T, Tulu T, Dechasa W. Knowledge, attitude and practice of cervical cancer screening and associated factors amongst female students at Wollega University, western Ethiopia. BMC research notes. 2019;12(1):518. doi: 10.1186/s13104-019-4564-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mulatu K, Motma A, Seid M, Tadesse M. Assessment of knowledge, attitude and practice on cervical cancer screening among female students of Mizan Tepi University, Ethiopia, 2016. Cancer Biol Ther Oncol. 2017;1(1):1–5. [Google Scholar]
- 36.Mekuria R. cervical cancer screening behavior and associated factors among women attending gynecology out-patient department and maternal and child health Atdilla university referral hospital, Ethiopia, 2018: Addis Ababa University; 2018. [Google Scholar]
- 37.Tsegaye S. Knowledge, Attitude, Practice of Cervical Cancer Screening and Its Associated Factors Among Female Students in Hawassa Universitycollege of Medicine and Health Science Hawassa: Addis Ababa University; 2015. [Google Scholar]
- 38.Abdulkadir IR. Level of knowledge toward human papillomavirus/cervical cancer & practice of Papanicolaou test screening among female Addis Ababa university students in Ethiopia: California State University, Northridge; 2013. [Google Scholar]
- 39.Gebregziabher D, Berhanie E, Birhanu T, Tesfamariam K. Correlates of cervical cancer screening uptake among female under graduate students of Aksum University, College of Health Sciences, Tigray, Ethiopia. BMC research notes. 2019;12(1):520. Epub 2019/08/21. doi: 10.1186/s13104-019-4570-z ; PubMed Central PMCID: PMC6701026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mersha A. Comprehensive knowledge and uptake of cervical cancer screening is low among women living with HIV/AIDS in Northwest Ethiopia. Value in Health. 2017;20(9):A493. doi: 10.1186/s40661-017-0057-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gelibo T, Roets L, Getachew T, Bekele A. Coverage and factors associated with cervical Cancer screening: results from a population-based WHO steps Study in Ethiopia. Adv Oncol Res Treat. 2017;1(115):2. [Google Scholar]
- 42.Michael E. Cervical cancer screening utilization and its associated factors among women aged 30 years and above in Woliso town, South West Showa Zone, Oromia region, Ethiopia: Addis Ababa Universty; 2018. [Google Scholar]
- 43.Shiferaw S, Addissie A. Knowledge about cervical cancer and barriers toward cervical cancer screening among HIV-positive women attending public health centers in Addis Ababa city, Ethiopia. 2018;7(3):903–12. doi: 10.1002/cam4.1334 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Getachew S, Getachew E. Cervical cancer screening knowledge and barriers among women in Addis Ababa, Ethiopia. 2019;14(5):e0216522. doi: 10.1371/journal.pone.0216522 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woldetsadik AB, Amhare AF, Bitew ST, Pei L, Lei J, Han J. Socio-demographic characteristics and associated factors influencing cervical cancer screening among women attending in St. Paul’s Teaching and Referral Hospital, Ethiopia. BMC women’s health. 2020;20(1):70. Epub 2020/04/08. doi: 10.1186/s12905-020-00927-5 ; PubMed Central PMCID: PMC7137499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Birhanu Z, Abdissa A, Belachew T, Deribew A, Segni H, Tsu V, et al. Health seeking behavior for cervical cancer in Ethiopia: a qualitative study. International journal for equity in health. 2012;11:83. Epub 2013/01/01. doi: 10.1186/1475-9276-11-83 ; PubMed Central PMCID: PMC3544623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bante SA, Getie SA, Getu AA, Mulatu K, Fenta SL. Uptake of pre-cervical cancer screening and associated factors among reproductive age women in Debre Markos town, Northwest Ethiopia, 2017. BMC public health. 2019;19(1):1102. Epub 2019/08/16. doi: 10.1186/s12889-019-7398-5 ; PubMed Central PMCID: PMC6692942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nega AD, Woldetsadik MA, Gelagay AA. Low uptake of cervical cancer screening among HIV positive women in Gondar University referral hospital, Northwest Ethiopia: cross-sectional study design. 2018;18(1):87. Epub 2018/07/10. doi: 10.1186/s12905-018-0579-z . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nigussie T, Admassu B, Nigussie A. Cervical cancer screening service utilization and associated factors among age-eligible women in Jimma town using health belief model, South West Ethiopia. BMC women’s health. 2019;19(1):127. Epub 2019/10/30. doi: 10.1186/s12905-019-0826-y ; PubMed Central PMCID: PMC6819648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tefera F, Mitiku I. Uptake of Cervical Cancer Screening and Associated Factors Among 15-49-Year-Old Women in Dessie Town, Northeast Ethiopia. Journal of cancer education: the official journal of the American Association for Cancer Education. 2017;32(4):901–7. Epub 2016/04/15. doi: 10.1007/s13187-016-1021-6 . [DOI] [PubMed] [Google Scholar]
- 51.Muluneh BA, Atnafu DD, Wassie B. Predictors of cervical cancer screening service utilization among commercial sex workers in Northwest Ethiopia: a case-control study. BMC women’s health. 2019;19(1):162. doi: 10.1186/s12905-019-0862-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kasa AS, Tesfaye TD, Temesgen WA. Knowledge, attitude and practice towards cervical cancer among women in Finote Selam city administration, West Gojjam Zone, Amhara Region, North West Ethiopia, 2017. African health sciences. 2018;18(3):623–36. doi: 10.4314/ahs.v18i3.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Erku DA, Netere AK, Mersha AG, Abebe SA, Mekuria AB, Belachew SA. Comprehensive knowledge and uptake of cervical cancer screening is low among women living with HIV/AIDS in Northwest Ethiopia. Gynecologic oncology research and practice. 2017;4(1):20. doi: 10.1186/s40661-017-0057-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aynalem BY, Anteneh KT, Enyew MM. Utilization of cervical cancer screening and associated factors among women in Debremarkos town, Amhara region, Northwest Ethiopia: Community based cross-sectional study. PloS one. 2020;15(4):e0231307. Epub 2020/04/08. doi: 10.1371/journal.pone.0231307 ; PubMed Central PMCID: PMC7138328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.ASERES T. KNOWLEDGE, PRACTICE AND ASSOCIATED FACTORS OF CERVICAL CANCER SCREENING AMONG WOMEN HEALTH WORKERS IN GONDAR UNIVERSITY TEACHING AND REFERRAL HOSPITAL, GONDAR, ETHIOPIA, 2016 2017.
- 56.Aweke YH, Ayanto SY, Ersado TL. Knowledge, attitude and practice for cervical cancer prevention and control among women of childbearing age in Hossana Town, Hadiya zone, Southern Ethiopia: Community-based cross-sectional study. PloS one. 2017;12(7):e0181415. Epub 2017/07/26. doi: 10.1371/journal.pone.0181415 ; PubMed Central PMCID: PMC5526548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Assefa AA, Astawesegn FH, Eshetu B. Cervical cancer screening service utilization and associated factors among HIV positive women attending adult ART clinic in public health facilities, Hawassa town, Ethiopia: a cross-sectional study. BMC health services research. 2019;19(1):847. Epub 2019/11/21. doi: 10.1186/s12913-019-4718-5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seyoum T, Yesuf A, Kejela G, Gebremeskel F. Utilization of cervical cancer screening and associated factors among female health Workers in Governmental Health Institution of Arba Minch town and Zuria District, Gamo Gofa zone, Arba Minch, Ethiopia, 2016. Arch Cancer Res. 2017;5(4):165. [Google Scholar]
- 59.Dulla D, Daka D, Wakgari N. Knowledge about cervical cancer screening and its practice among female health care workers in southern Ethiopia: a cross-sectional study. International journal of women’s health. 2017;9:365. doi: 10.2147/IJWH.S132202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tekle T, Wolka E, Nega B, Kumma WP, Koyira MM. Knowledge, Attitude and Practice Towards Cervical Cancer Screening Among Women and Associated Factors in Hospitals of Wolaita Zone, Southern Ethiopia. Cancer management and research. 2020;12:993. doi: 10.2147/CMAR.S240364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Solomon K, Tamire M, Kaba M. Predictors of cervical cancer screening practice among HIV positive women attending adult anti-retroviral treatment clinics in Bishoftu town, Ethiopia: the application of a health belief model. BMC cancer. 2019;19(1):989. Epub 2019/10/28. doi: 10.1186/s12885-019-6171-6 ; PubMed Central PMCID: PMC6813043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heyi WD, Bekabil TT, Ebo GG. KNOWLEDGE, ATTITUDE AND PRACTICE OF CERVICAL CANCER SCREENING AMONG WOMEN AGED 15–49 YEARS IN BISHOFTU TOWN, EAST SHEWA ZONE, OROMIA REGION, ETHIOPIA, 2016. Ethiopian Journal of Reproductive Health. 2018;10(2):10–. [Google Scholar]
- 63.Ashagrie A. Knowledge and screening practice on cervical cancer and associated factors among HIV positive women in Adama, Ethiopia: Addis Ababa Universty; 2017. doi: 10.1186/s13104-017-2887-z [DOI] [Google Scholar]
- 64.Bayu H, Berhe Y, Mulat A, Alemu A. Cervical Cancer Screening Service Uptake and Associated Factors among Age Eligible Women in Mekelle Zone, Northern Ethiopia, 2015: A Community Based Study Using Health Belief Model. PloS one. 2016;11(3):e0149908. Epub 2016/03/11. doi: 10.1371/journal.pone.0149908 ; PubMed Central PMCID: PMC4786115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gebreegziabher M, Asefa NG, Berhe S. Factors Affecting the Practices of Cervical Cancer Screening among Female Nurses at Public Health Institutions in Mekelle Town, Northern Ethiopia, 2014: A Cross-Sectional Study. Journal of Cancer Research. 2016;2016. [Google Scholar]
- 66.Berhanu T, Mamo E, Tewolde T, Beshir M. Knowledge of Cervical Cancer and Its Screening Practice among Health Extension Workers in Addis Ababa, Ethiopia. Primary Health Care: Open Access. 2019;9(1):1–5. [Google Scholar]
- 67.Bao H, Zhang L, Wang L, Zhang M, Zhao Z, Fang L, et al. Significant variations in the cervical cancer screening rate in China by individual‐level and geographical measures of socioeconomic status: a multilevel model analysis of a nationally representative survey dataset. Cancer medicine. 2018;7(5):2089–100. doi: 10.1002/cam4.1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tiruneh FN, Chuang K-Y, Ntenda PAM, Chuang Y-C. Individual-level and community-level determinants of cervical cancer screening among Kenyan women: a multilevel analysis of a Nationwide survey. BMC women’s health. 2017;17(1):109. doi: 10.1186/s12905-017-0469-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gakidou E, Nordhagen S, Obermeyer Z. Coverage of cervical cancer screening in 57 countries: low average levels and large inequalities. PLoS medicine. 2008;5(6). doi: 10.1371/journal.pmed.0050132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gan DEH, Dahlui M. Cervical screening uptake and its predictors among rural women in Malaysia. Singapore medical journal. 2013;54(3):163–8. doi: 10.11622/smedj.2013047 [DOI] [PubMed] [Google Scholar]
- 71.Ho V, Yamal JM, Atkinson EN, Basen-Engquist K, Tortolero-Luna G, Follen M. Predictors of Breast and Cervical Screening in Vietnamese Women in Harris County, Houston, Texas. Cancer Nursing. 2005;28(2):119–29. 00002820-200503000-00005. doi: 10.1097/00002820-200503000-00005 [DOI] [PubMed] [Google Scholar]
- 72.Damiani G, Basso D, Acampora A, Bianchi CB, Silvestrini G, Frisicale EM, et al. The impact of level of education on adherence to breast and cervical cancer screening: evidence from a systematic review and meta-analysis. Preventive medicine. 2015;81:281–9. doi: 10.1016/j.ypmed.2015.09.011 [DOI] [PubMed] [Google Scholar]
- 73.Musa J, Achenbach CJ, O’Dwyer LC, Evans CT, McHugh M, Hou L, et al. Effect of cervical cancer education and provider recommendation for screening on screening rates: A systematic review and meta-analysis. PloS one. 2017;12(9). doi: 10.1371/journal.pone.0183924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wanyenze RK, Bwanika JB, Beyeza-Kashesya J, Mugerwa S, Arinaitwe J, Matovu JK, et al. Uptake and correlates of cervical cancer screening among HIV-infected women attending HIV care in Uganda. Global health action. 2017;10(1):1380361. doi: 10.1080/16549716.2017.1380361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Devarapalli P, Labani S, Nagarjuna N, Panchal P, Asthana S. Barriers affecting uptake of cervical cancer screening in low and middle income countries: A systematic review. Indian journal of cancer. 2018;55(4):318. doi: 10.4103/ijc.IJC_253_18 [DOI] [PubMed] [Google Scholar]
- 76.Abboud S, De Penning E, Brawner BM, Menon U, Glanz K, Sommers MS, editors. Cervical cancer screening among Arab women in the United States: an integrative review. Oncology nursing forum; 2017: NIH Public Access. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lyimo FS, Beran TN. Demographic, knowledge, attitudinal, and accessibility factors associated with uptake of cervical cancer screening among women in a rural district of Tanzania: three public policy implications. BMC public health. 2012;12(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chosamata MS, Hong SA, Tiraphat S. Determinants of cervical cancer screening utilization among women aged 30–45 years in Blantyre district, Malawi. 2015. [Google Scholar]
- 79.Visanuyothin S, Chompikul J, Mongkolchati A. Determinants of cervical cancer screening adherence in urban areas of Nakhon Ratchasima Province, Thailand. Journal of infection and public health. 2015;8(6):543–52. doi: 10.1016/j.jiph.2015.04.018 . [DOI] [PubMed] [Google Scholar]
- 80.Teame H, Addissie A, Ayele W, Hirpa S, Gebremariam A, Gebreheat G, et al. Factors associated with cervical precancerous lesions among women screened for cervical cancer in Addis Ababa, Ethiopia: A case control study. PLOS ONE. 2018;13(1):e0191506. doi: 10.1371/journal.pone.0191506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ross CE, Wu C-l. The links between education and health. American sociological review. 1995:719–45.
- 82.Ebu NI, Ogah JK. Predictors of cervical cancer screening intention of HIV-positive women in the central region of Ghana. BMC women’s health. 2018;18(1):43. doi: 10.1186/s12905-018-0534-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.KAMBAGA EM. Determinants of provider-initiated HIV testing and counselling uptake in Jaramogi Oginga Odinga teaching and referral hospital, Kisumu, Kenya: Maseno University; 2017. [Google Scholar]