Abstract
Background
Stent edge dissection (SED) is a well-known predictor of worse clinical outcomes. However, impact of SED after current-generation drug-eluting stent (DES) implantation remains unknown since there was no study using only current-generation DES to assess impact of SED. This study aimed to investigate a relationship between SED detected by optical coherence tomography (OCT) and clinical outcomes after current-generation DES implantation.
Methods
This study enrolled 175 patients receiving OCT after current-generation DES implantation. The SED group was compared with the non-SED group in terms of the primary study endpoints which was the cumulative incidence of major adverse cardiac event (MACE) composed of cardiac death, target vessel myocardial infarction (TV-MI), and clinically-driven target lesion revascularization (CD-TLR).
Results
Of 175 patients, SED detected by OCT was observed in 32 patients, while 143 patients did not show SED. In the crude population, the SED group showed a significantly higher incidence of CD-TLR, definite stent thrombosis, TV-MI and cardiac death relative to the non-SED group. After adjustment by an inverse probability weighted methods, the SED group showed a significantly higher incidence of MACE compared with the non-SED group (hazard ratio 3.43, 95% confidence interval 1.09–10.81, p = 0.035). Fibrocalcific or lipidic plaques, greater lumen eccentricity, and stent-oversizing were the predictors of SED.
Conclusions
SED detected by OCT after the current-generation DES implantation led to unfavorable outcomes. Aggressive post-dilatation around the stent edge might worse clinical outcomes due to SED, although achievement of optimal stent expansion is strongly encouraged to improve clinical outcomes.
Introduction
Stent implantation can result in vessel wall injury between the edge of a stent and the adjacent vessel wall [1, 2]. It has been previously reported that residual stent edge dissection (SED) has been associated with a high rate of stent thrombosis and major adverse cardiac events (MACE) [3–5]. SED without flow limitation can be treated according to the operator’s discretion, since there are no clear criteria to judge whether SED should be covered by an additional stent. Angiography and intravascular ultrasound (IVUS) have been used to diagnose SED during procedures of percutaneous coronary intervention (PCI) [3–5]. Previous data showing a relationship between SED and worse clinical outcomes has been demonstrated mainly by angiography or IVUS [3–5]. Optical coherence tomography (OCT) is designed to detect intracoronary structures such as luminal surface and intimal components in detail with a high resolution of 10–20 μm [6]. OCT has enabled the detection of SED that IVUS fails to reveal [7, 8]. Recently, several studies showed that SED detected by OCT was associated with worse clinical outcomes, although there is still a controversy as to whether SED detected by OCT affects clinical outcomes [9–14].
Regardless of imaging modalities such as IVUS or OCT, there was no study that evaluated clinical outcomes of SED after only current-generation DES implantation. The current-generation DES has provided a different performance from bare metal stent (BMS) and first-generation DES since it improved safety and feasibility compared with BMS or first-generation DES [15, 16]. It remains unknown how SED detected by OCT affects clinical outcomes in the current-generation DES era. The purpose of this study was to investigate whether SED detected by OCT affected clinical outcomes after current-generation DES implantation.
Materials and methods
Study population
This study was a single-center, retrospective observational study at Saitama Medical Center, Jichi Medical University. Between April 2010 and March 2020, the consecutive patients undergoing PCI were reviewed. The inclusion criteria were as follows: 1) OCT or optical frequency domain imaging (OFDI) were performed, and 2) current-generation DESs were implanted. The exclusion criteria were as follows: 1) stent was not required during the procedure, 2) BMS or first-generation DES were implanted, 3) final OCT or OFDI images after the procedures were not available, and 4) quality of images was poor to analyze. When a patient received OCT procedures more than once during the study period, only the initial procedure was included. Those patients were divided into the SED and non-SED groups according to the presence of SED detected by OCT. SED detected by OCT was defined as a disruption of the vessel luminal surface with flap at an adjacent site to the stent edge (< 5mm). The strategy of procedures was dependent on operators using OCT assessment. If necessary, the lesion preparation such as pre-dilatation, aspiration and rotational atherectomy was performed before stenting. Pre-dilatation was considered when stent underexpansion and the difficulty of device derivability were expected, or when pre-dilatation makes it easy to perform the next procedure for any reasons. Aspiration was performed when an obvious thrombus by angiography was observed, or when the effectiveness of aspiration was expected. Rotational atherectomy was required when heavily calcified lesions by angiography or intra-coronary imaging such as IVUS or OCT was observed, or unsuccessful balloon dilatation or unsuccessful balloon delivery occurred due to calcification. This study was approved by the institutional review board of Saitama Medical Center, Jichi Medical University (S20-124), and written informed consent was waived because of the retrospective study design. Follow-up data until August 2020 were obtained from a review of hospital records based on clinic visits. Saitama Medical Center, Jichi Medical University is a local core hospital. The annual average number of PCI was approximately 400 to 800 cases a year during this study-period. There are two catheter rooms and at least several interventional cardiologists performed the PCI-procedures in this hospital, although they were not consistent for this study-period from 2010 to 2020.
The primary study endpoints were the cumulative incidence of MACE which were composed of cardiac death, target vessel myocardial infarction (TV-MI), and clinically-driven target lesion revascularization (CD-TLR). All-cause of death, stent thrombosis, and target vessel revascularization (TVR) were also evaluated in this study. Definitions of clinical endpoints were based on the Academic Research Consortium (ARC) [17]. Cardiac death was defined as any death due to a proximate cardiac cause, unwitnessed death or death of unknown cause, and all procedure-related death. TV-MI was defined as MI in the treated vessel [17]. TLR was defined as any revascularization (either repeated PCI or coronary artery bypass graft [CABG]) within the stent and 5mm proximally and distally to the stent [17]. TVR was defined as any revascularization (either repeated PCI or repeated CABG) of the target vessel. Revascularization was considered clinically driven if associated with any of the following: (1) positive functional ischemia study, (2) ischemic symptoms and angiographic diameter stenosis ≥50%, and (3) angiographic diameter stenosis ≥70% without angina or positive functional study [17, 18]. The diagnostic certainty of stent thrombosis, i.e., definite or probable, was evaluated according to the ARC definition [17].
OCT image acquisition
We performed OCT using one of the following systems: M2 OCT system (Light Lab Imaging, Westford, MA, USA; C7XR Fourier-Domain System (St Jude Medical, St Paul, MN, USA), ILUMIEN (St Jude Medical, St Paul, MN, USA), OPTIS (Abbott Vascular, Santa Clara, CA, USA), and LUNAWAVE (Terumo, Tokyo, Japan). Motorized pullback OCT imaging was performed at a rate of 1.0 mm/s through the stent. Images were acquired at 15.6 frames/s and digitally archived. C7XR, ILUMIEN, and OPTIS system were acquired automatically at a pullback rate of 20 mm/s (100 frames/s) or 36 mm/s (180 frames/s) and that of OFDI at a pullback rate of 20 mm/s (160 frames/s). All images were stored digitally and analyzed offline by the LightLab OCT imaging proprietary software (LightLab Imaging), ILUMIEN/OPTIS software (Abbott Vascular, Santa Clara, CA, USA), or Terumo software. Procedure details using each modality have been previously described [19].
OCT image analysis
All OCT images were analyzed based on conventional definitions reported in expert consensus OCT documents [20, 21]. Using automated contour-detection software (OCT system, St Jude Medical or Abbott Vascular, OFDI system, Terumo), stent and lumen cross-sectional areas were measured within the stent and 5mm proximally and distally to the stent. Proximal and distal reference lumen was defined as the largest outside of the stents. In-stent lumen expansion was defined as the percentage of in-stent lumen area/ the average reference lumen area.
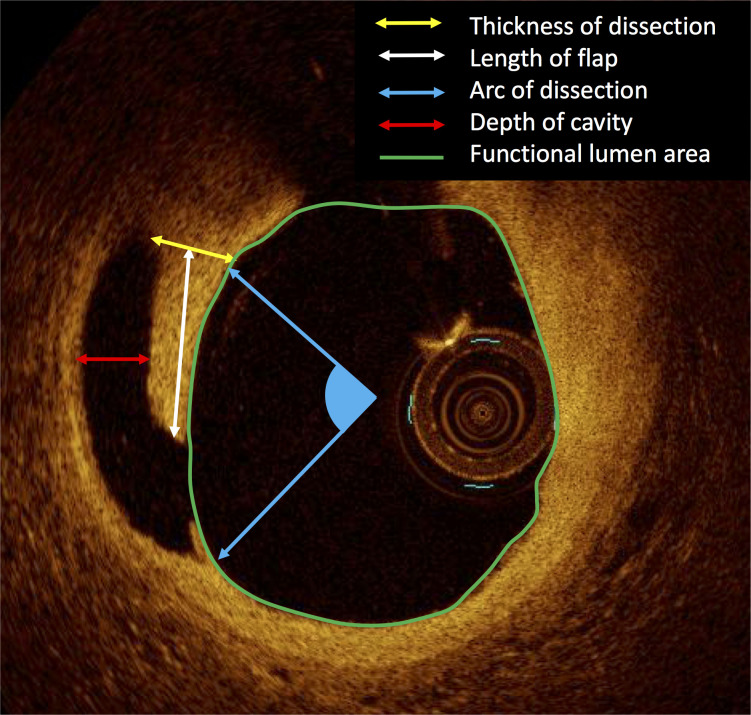
Stent border was defined as the first and last cross-sections of the stented segment where struts could be seen in all 4 quadrants [13]. Stent border area is stent area at the stent border. Peri-stent lumen was defined as the first frame following a stent where any struts could not be seen [13]. At peri-stent lumen, maximum and minimum lumen diameter were measured. Stent-oversizing index was defined as stent border area / reference area. Lumen eccentricity was defined as (maximum lumen diameter–minimum lumen diameter)/ maximum lumen diameter [13]. Type of plaque was categorized as the following 4: 1) normal intima which is characterized by intima with a thickness of < 250μm; 2) lipidic plaque; 3) fibrocalcific plaque, and 4) fibrous plaque [21, 22]. Lipidic plaque was defined as a plaque with a signal poor region with diffuse borders. Fibrocalcific plaque was defined as a plaque with a signal-poor or heterogeneous region and a sharply delineated border. Fibrous plaque was defined as a plaque showing homogeneous and rich OCT signal. If lipidic and fibrocalcific plaque were observed in the same cross-sectional images, either the dominant type was selected. The progressive atherosclerotic lesion was defined as lipidic or fibrocalcific lesions. Most affected cross-sections having the largest dissection were selected to be assessed. The length of the flap was defined as the distance between the tip and bottom of the flap (Fig 1). The thickness of the flap was measured at the bottom of the flap. Arc of dissection was defined as the angle of dissection. The depth of the cavity was defined as a maximal distance in an empty space underneath a flap. The functional lumen area was defined as a lumen area without a flap and space of the cavity. Depth of vessel injury was assessed as the following 3 categories; 1) intima, 2) media, and 3) adventitia.
Fig 1. Measurement of stent edge dissection.
Statistical analysis
Data are presented as values and percentages, mean±SD, or median (interquartile range). Categorical variables were compared between the 2 groups with Fisher’s exact test. Continuous variables were compared between groups using the unpaired t-test or the Mann-Whitney U test, based on the data distribution. For edge-level analysis, the generalized estimated equation (GEE) methods was used. Continuous variables were tested by the GEE method with gamma with the log-link based on the data distribution. Categorical data were tested by the GEE method with an ordinal logistic model. For each endpoint, the cumulative incidence probability through the study-period was estimated using the Kaplan-Meier method. The statistical analysis to adjust the background is necessary since this is a retrospective study. In this study including the small number, the inverse probability treatment weighting (IPTW) using the propensity score is chosen to retain all the patient data since propensity score matching reduces the population after matching patients [23]. Hazard ratio (HR) of SED versus non-SED for MACE was estimated through IPTW Cox model with covariate set. Weights for the IPW methods were estimated through a logistic model for probabilities of SED conditional on covariate set (age, sex, diabetes mellitus, hemodialysis, ejection fraction ≤40%, AHA/ACC type B2 or C). Weights did not highly vary among patients (range: 1.1 to 8.0). Multivariate logistic regression analysis was performed to identify independent predictors for SED. In the multivariate model, variables with P<0.10 in Table 2 were used. If clinically similar variables remained, we selected the variable that we considered to be more clinically relevant. Statistical analysis was performed using JMP version 10, SPSS version 24 and STATA/SE version 15.1. Two-sided P<0.05 was considered to indicate statistical significance.
Table 2. Lesion and procedural characteristics and OCT analysis.
| Overall | SED | Non-SED | p-value | |
|---|---|---|---|---|
| (N = 175) | (N = 32) | (N = 143) | ||
| Culprit lesions | 0.96 | |||
| RCA | 42 (24.0) | 8 (25.0) | 34 (23.8) | |
| LAD | 98 (56.0) | 19 (59.4) | 79 (55.2) | |
| LCX | 32 (18.3) | 5 (15.6) | 27 (18.9) | |
| LMT | 2 (1.1) | 0 (0.0) | 2 (1.4) | |
| SVG | 1 (0.6) | 0 (0.0) | 1 (0.7) | |
| AHA/ACC lesion classification | 0.03 | |||
| A | 50 (28.6) | 3 (9.4) | 47 (32.9) | |
| B1 | 48 (27.4) | 12 (37.5) | 36 (25.2) | |
| B2 | 20 (11.4) | 5 (15.6) | 15 (10.5) | |
| C | 57 (32.6) | 12 (37.5) | 45 (31.5) | |
| Type of stent | 0.57 | |||
| DP-EES | 106 (60.6) | 21 (65.6) | 85 (59.4) | |
| R-ZES | 31 (17.7) | 7 (21.9) | 24 (16.8) | |
| BP-EES | 23 (13.1) | 3 (9.4) | 20 (14.0) | |
| BP-SES | 15 (8.6) | 1 (3.1) | 14 (9.8) | |
| Number of stents | ||||
| 1 stent | 162 (92.6) | 27 (84.4) | 135 (94.4) | 0.0504 |
| 2 stents | 12 (6.9) | 4 (12.5) | 8 (5.6) | |
| 3 stents | 1 (0.6) | 1 (3.1) | 0 (0.0) | |
| Stent diameter, mm | 3.0 (2.5–3.0) | 3.0 (2.7–3.0) | 3.0 (2.5–3.0) | 0.57 |
| Total stent length, mm | 20 (16–28) | 22 (18–28) | 20 (16–28) | 0.37 |
| Lesion preparation | ||||
| Pre-dilatation | 116 (66.3) | 20 (62.5) | 96 (67.1) | 0.68 |
| Aspiration | 19 (10.9) | 8 (25.0) | 11 (7.7) | 0.009 |
| Rotational atherectomy | 7 (4.0) | 1 (3.1) | 6 (4.2) | 1.00 |
| Post-balloon dilatation | 77 (44.0) | 18 (56.3) | 59 (41.3) | 0.17 |
| Direct stenting | 63 (36.0) | 14 (43.8) | 49 (34.3) | 0.32 |
| Overlapping stent | 13 (7.4) | 5 (15.6) | 8 (5.6) | 0.06 |
| OCT/OFDI analysis | ||||
| Proximal reference, mm | 6.4 (4.8–8.3) | 6.3 (4.3–8.4) | 6.4 (4.8–8.3) | 0.49 |
| Distal reference, mm | 4.7 (3.5–5.9) | 4.2 (3.3–5.8) | 4.8 (3.6–6.1) | 0.32 |
| Mean reference, mm | 5.7 (4.3–7.2) | 5.9 (3.8–6.6) | 5.7 (4.4–7.4) | 0.60 |
| Minimal stent area, mm2 | 5.0 (3.9–6.1) | 5.3 (3.6–6.4) | 5.0 (3.9–5.9) | 0.64 |
| Percent of stent expansion, % | 89.8 (78.7–103.4) | 95.4 (87.3–100.7) | 87.0 (77.0–104.8) | 0.051 |
Values are presented as median (interquartile range), or n (%) for categorical variables. ACC, American College of Cardiology; AHA, American Heart Association; BP, biodegradable polymer; DP, durable polymer; EES, everolimus-eluting stent; LAD left anterior descending artery; LCX, left circumflex artery; LMT, left main trunk; OCT, optical coherence tomography; OFDI, optical frequency domain imaging; RCA, right coronary artery; SES, sirolimus-eluting stent; SVG, saphenous vein graft.
Results
Patient and lesion characteristics
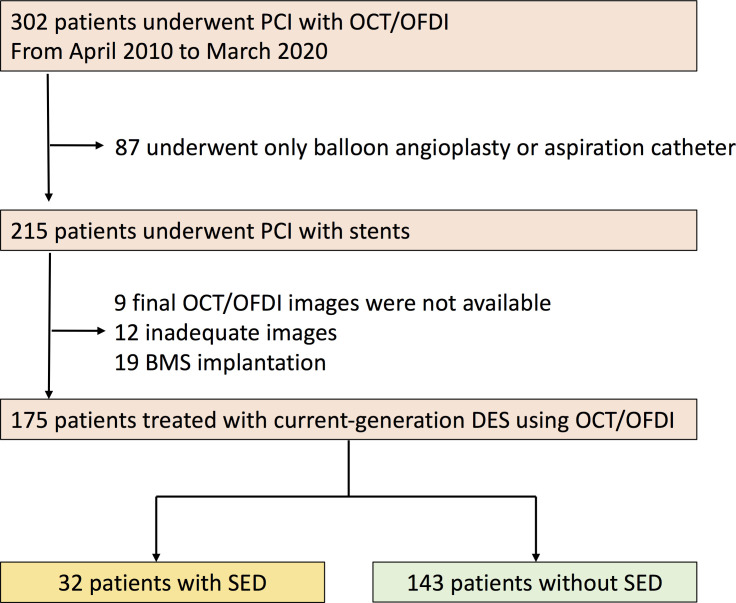
During this study period, 302 patients underwent PCI with OCT. Finally, of these patients, 175 patients who were treated with current-generation DESs and underwent post-procedure imaging by OCT were enrolled in this study (Fig 2). Those patients were divided into 2 groups: 1) the SED group (32 patients with 32 lesions) and 2) the non-SED group (143 patients with 143 lesions). The patient characteristics were comparable except for acute coronary syndrome, which was higher in the SED group relative to the non-SED group (Table 1). The median follow-up period did not significantly differ between the SES and non-SED groups [856 days (234–2098) vs. 870 days (203–1534), respectively, p = 0.37)]. There were no significant differences between the 2 groups in lesion characteristics except for the usage of aspiration for patients with ACS, which was higher in the SED group relative to the non-SED group (Table 2).
Fig 2. Study flow chart.
DES, drug-eluting stent; OCT, optical coherence tomography; OFDI, optical frequency domain imaging; PCI, percutaneous coronary intervention; SED, stent edge dissection.
Table 1. Patient characteristics.
| Overall | SED | Non-SED | p-value | |
|---|---|---|---|---|
| (N = 175) | (N = 32) | (N = 143) | ||
| Age, years | 69.3±9.2 | 68.1± 9.2 | 69.6± 9.2 | 0.40 |
| Male | 139 (79.4) | 24 (75.0) | 115 (80.4) | 0.48 |
| Risk Factor | ||||
| Hypertension | 118 (67.4) | 19 (59.4) | 99 (69.2) | 0.30 |
| Hyperlipidemia | 102 (58.3) | 20 (62.5) | 82 (57.3) | 0.69 |
| Diabetes | 72 (41.1) | 13 (40.6) | 59 (41.3) | 1.00 |
| Current smoker | 33 (18.9) | 8 (25.0) | 25 (17.5) | 0.33 |
| Family History of CAD | 32 (18.3) | 5 (15.6) | 27 (18.9) | 0.80 |
| Past medical history | ||||
| Previous MI | 34 (19.4) | 6 (18.8) | 28 (19.6) | 1.00 |
| Previous PCI | 55 (31.4) | 8 (25.0) | 47 (32.9) | 0.53 |
| Previous CABG | 3 (1.7) | 0 (0.0) | 3 (2.1) | 1.00 |
| Hemodialysis | 10 (5.7) | 4 (12.5) | 6 (4.2) | 0.09 |
| Presentation | 0.007 | |||
| Acute coronary syndrome | 46 (26.3) | 15 (46.9) | 31 (21.7) | |
| Stable angina | 129 (73.7) | 17 (53.1) | 112 (78.3) | |
| eGFR, ml/min/1.73m2 | 70.2 (60.2–83.7) | 72.6 (61.4–94.4) | 70.2 (59.9–80.6) | 0.31 |
| Ejection fraction, % | 60.8± 11.9 | 57.5± 13.2 | 61.5± 11.5 | 0.09 |
| Body mass index, kg/m2 | 24.3± 3.5 | 23.8± 4.0 | 24.4± 3.4 | 0.35 |
| Number of disease | 0.85 | |||
| 1 vessel | 110 (62.9) | 20 (62.5) | 90 (62.9) | |
| 2 vessels | 55 (31.4) | 11 (34.4) | 44 (30.8) | |
| 3 vessels | 10 (5.7) | 1 (3.1) | 9 (6.3) | |
| Multi-vessel disease | 65 (37.1) | 12 (37.5) | 53 (37.1) | 1.00 |
| Follow-up period, days | 870 (203–1588) | 856 (234–2098) | 870 (203–1534) | 0.37 |
Values are presented as median (interquartile range), or n (%) for categorical variables. CABG, coronary artery bypass graft; CAD, coronary artery disease; eGFR, estimated glomerular filtration rate; MI, myocardial infarction; PCI, percutaneous coronary intervention; SED, stent edge dissection.
OCT assessment
Proximal, distal and mean reference areas, minimal stent area, and percent expansion were not significantly different between the 2 groups (Table 2). Table 3 listed OCT data at stent edges with and without dissection. Edges with dissection showed significantly smaller reference lumen area relative to edges without dissection. Stent-oversizing index, lumen long diameter to short diameter ratio and lumen eccentricity were significantly greater in edges with dissection than without dissection. Prevalence of plaque-type at stent edge was different between edges with and without dissection. Progressive atherosclerotic plaques (i.e., lipidic and fibrocalcific plaques) were more frequently found in edges with dissection.
Table 3. OCT/OFDI analysis at stent edges.
| Overall | Edge with dissection | Edge without dissection | p-value | |
|---|---|---|---|---|
| (N = 345) | (N = 35) | (N = 310) | ||
| Reference lumen area, mm2 | 5.3 (4.0–7.3) | 4.3 (3.8–5.7) | 5.4 (4.1–7.4) | 0.0009 |
| Stent area at stent border, mm2 | 6.1 (4.7–7.8) | 6.0 (4.7–7.6) | 6.1 (4.6–7.8) | 0.82 |
| Lumen area at adjacent site of stent edge, mm2 | 5.7 (4.3–7.7) | 5.0 (4.0–6.6) | 5.7 (4.3–7.7) | 0.07 |
| Stent-oversizing index | 1.1 (1.0–1.3) | 1.4 (1.1–1.7) | 1.1 (1.0–1.3) | <0.0001 |
| Lumen long diameter / lumen short diameter | 1.1 (1.1–1.2) | 1.2 (1.1–1.3) | 1.1 (1.1–1.2) | 0.009 |
| Lumen eccentricity | 0.12 (0.09–0.17) | 0.16 (0.12–0.24) | 0.12 (0.09–0.16) | 0.002 |
| Type of plaque at stent edge | 0.87 | |||
| Normal | 30 (8.7) | 0 (0.0) | 30 (9.7) | |
| Fibrous | 208 (60.3) | 8 (22.9) | 200 (64.5) | |
| Lipidic | 64 (18.6) | 16 (45.7) | 48 (15.5) | |
| Fibrocalcific | 43 (12.5) | 11 (31.4) | 32 (10.3) | |
| Progressive atherosclerotic plaque | 107 (31.0) | 27 (77.1) | 80 (25.8) | <0.0001 |
| Location of dissection | ||||
| Proximal | - | 12 (34.3) | - | |
| Distal | - | 23 (65.7) | - | |
| Length of flap, mm | - | 0.9 (0.4–1.4) | - | |
| Thickness of flap, mm | - | 0.3 (0.2–0.4) | - | |
| Length of dissection, mm | - | 2.4 (1.5–3.4) | - | |
| Arc of dissection, ° | - | 45 (32–82) | - | |
| Depth of cavity, mm | - | 0.3 (0.2–0.4) | - | |
| Functional lumen area, mm2 | - | 3.7 (2.6–5.6) | - | |
| Depth of dissection | ||||
| Intima | - | 22 (62.9) | - | |
| Media | - | 13 (37.1) | - | |
| Adventitia | - | 0 (0.0) | - | |
| Hematoma | - | 2 (5.7) | - |
Values are presented as median (interquartile range), or n (%) for categorical variables. Generalized estimating equation (GEE) method with gamma with log-link model and was used for continuous valuables and GEE with ordinal logistic model was used for categorical data. OCT, optical coherence tomography; OFDI, optical frequency domain imaging.
Morphological and quantitative data of SED was shown in Table 3. SED was more frequently observed in distal locations relative to proximal locations (65.7% vs. 34.3%, respectively). SED reached intima in 62.9% and media in 37.1% of cases.
Clinical outcomes between SED and non-SED groups
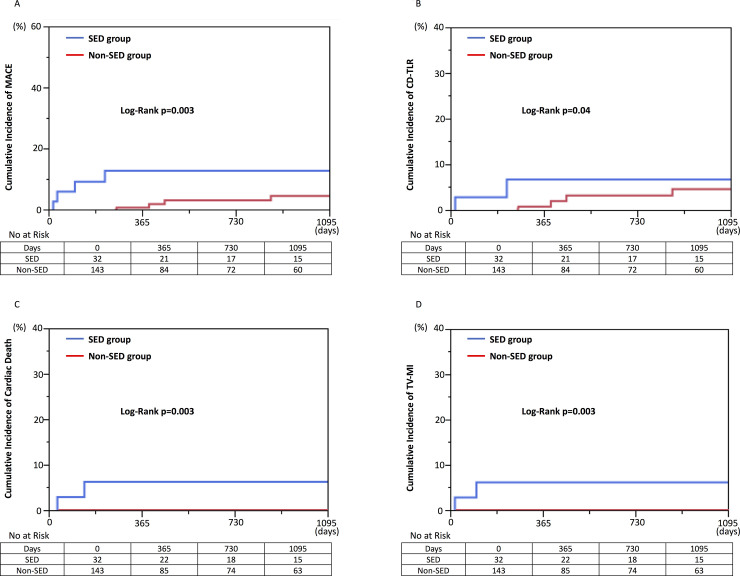
Table 4 summarized clinical outcomes between the SED and non-SED groups. In the crude population, the incidence of MACE was significantly higher in the SED group compared with the non-SED group (21.9% vs. 4.2%, respectively, p = 0.003) (Fig 3A). After adjustment for baseline characteristics by IPW, SED was significantly associated with increased risks for MACE. There were no significant differences of all cause death, cardiac death and non-cardiac death between the 2 groups. The SED group showed significantly higher incidence of CD-TLR, definite stent thrombosis, TV-MI and cardiac death (15.6% vs. 4.2%, p = 0.04; 3.1% vs. 0.0%, p = 0.04; 6.3% vs. 0.0%, p = 0.003; 6.3% vs. 0.0%, p = 0.003) (Fig 3B–3D).
Table 4. Clinical outcomes between SED and non-SED groups.
| Event rate (%) | Crude | IPW Adjusted | ||||
|---|---|---|---|---|---|---|
| Overall (n = 175) | SED (n = 32) | Non-SED (n = 143) | p-value | HR (95% CI) | p-value | |
| All cause death | 8 (4.6) | 3 (9.4) | 5 (3.5) | 0.27 | - | - |
| Cardiac death | 2 (1.1) | 2 (6.3) | 0 (0.0) | 0.003 | - | - |
| Non-cardiac death | 6 (3.4) | 1 (3.1) | 5 (3.5) | 0.71 | - | - |
| Target-vessel MI | 2 (1.1) | 2 (6.3) | 0 (0.0) | 0.003 | - | - |
| Definite stent thrombosis | 1 (0.6) | 1 (3.1) | 0 (0.0) | 0.04 | - | - |
| Probable stent thrombosis | 0 (0.0) | 0 (0.0) | 0 (0.0) | NE | - | - |
| Any TLR | 11 (6.3) | 5 (15.6) | 6 (4.2) | 0.04 | - | - |
| CD-TLR | 11 (6.3) | 5 (15.6) | 6 (4.2) | 0.04 | - | - |
| Any TVR | 12 (6.9) | 5 (15.6) | 7 (4.9) | 0.07 | - | - |
| CD-TVR | 11 (6.3) | 5 (15.6) | 6 (4.2) | 0.04 | - | - |
| MACE | 13 (7.4) | 7 (21.9) | 6 (4.2) | 0.003 | 3.43 (1.09–10.81) | 0.035 |
P-value in the crude-population was analyzed by the log-rank method. CD, clinically-driven; CI, confidence interval; HR, hazard ratio; IPW, inverse probability weighted; MACE, major adverse cardiovascular events; MI, myocardial infarction; NE, not estimable; SED, stent edge dissection; TLR, target lesion revascularization; TVR, target vessel revascularization.
Fig 3. Comparison of clinical outcomes between SED and non-SED groups in the crude population.
Kaplan-Meier curve was expressed up to 3 years. P-value was analyzed based on overall periods. (A) major adverse cardiac event (MACE), (B) clinically-driven target lesion revascularization (CD-TLR), (C) cardiac death, and (D) target-vessel myocardial infarction (TV-MI). SED, stent edge dissection.
The predictors for SED
In the analysis of stent edges, 5 edges were excluded since proximal edges were not observed by OCT due to ostium stenting. The independent risk factor for SED was lumen eccentricity (Odds ratio [OR], 1.59; 95% CI: 1.02–2.43, p = 0.03), stent-oversizing index (OR, 1.24; 95% CI: 1.11–1.40, p = 0.0003) and progressive atherosclerotic lesion (OR, 9.58; 95% CI: 4.16–24.66, p<0.0001) (Table 5).
Table 5. Predictors for SED.
| Univariable Model | Multivariable Model | |||
|---|---|---|---|---|
| Odds ratio (95% CI) | p-value | Odds ratio (95% CI) | p-value | |
| Oversizing index (increase 0.1) | 1.22 (1.11–1.35) | <0.0001 | 1.24 (1.11–1.40) | 0.0003 |
| Lumen eccentricity (increase 0.1) | 1.85 (1.26–2.72) | 0.002 | 1.59 (1.02–2.43) | 0.03 |
| Progressive atherosclerotic lesion | 9.70 (4.42–23.67) | <0.0001 | 9.58 (4.16–24.66) | <0.0001 |
CI, confidence interval; SED, stent edge dissection.
Discussion
The main findings
The main findings in this study were as follows: 1) SED detected by OCT was observed in 18.3% of cases, 2) SED detected by OCT after current-generation DES implantation was significantly associated with MACE, and 3) Risk factors for SED detected by OCT were lumen eccentricity, stent-oversizing, and progressive atherosclerotic lesion (lipidic and fibrocalcific plaques).
The incidence of SED detected by OCT in the current-generation DES era
Each imaging modality such as angiography, IVUS and OCT leads to different incidences of SED due to its resolution. Previous studies showed that SED was observed more often with IVUS (7.8–19.0%) than with angiography (0–4.8%) [1, 2, 24]. OCT can detect SED that IVUS may miss, since OCT has approximately 10 times better resolution than IVUS. Chamie et al. reported that only 16.0% of SEDs by OCT were detected by angiography alone [13]. Moreover, Bouma et al. showed that the prevalence of SED was higher in OCT (19.0%) versus IVUS (4.8%) in the same SEDs [25]. SED detected by OCT has been observed in 19.0% to 39.1% after stent implantation [9, 13, 25]. In the present study, 18.3% of patients underwent OCT-guide PCI had SED, which is concordant with previous OCT studies. Additionally, our study confirmed that SED more frequently occurred almost twice in the distal edges relative to the proximal edges (65.7% vs. 34.3%) [4, 13, 26].
Relationship between SED and clinical outcomes after current-generation DES implantation
Previous studies using IVUS and angiography showed SED resulted in a higher rate of stent thrombosis and TLR, although SED had a high possibility of natural healing [2–5, 14, 27]. The OCT studies are more likely to include not only obvious but also small SED that IVUS or angiography cannot detect. There were several reports about whether SED detected by OCT including small dissection leads to worse clinical outcomes. Recently, in line with previous studies using IVUS and angiography, only a few studies reported that SED detected by OCT after stent implantation was associated with worse clinical outcomes, although earlier studies failed to show this significant association probably due to small number of patients [10, 11, 13, 14]. The previous OCT study reported by Prati et al. showed that distal dissection (>200μm) but not proximal dissection was one of predictors of MACE [10]. Moreover, only a few studies showed the predictors of MACE in patients with SED detected by OCT [9, 12]. The study reported by van Zandvoort et al. including 295 patients with SED showed that a predictor of MACE was only length of dissection [12]. All these studies included BMS and first-generation DES or did not show the prevalence of stents. Therefore, there was no study which focused on only current-generation DES.
Our question was whether these results can be simply applied to the current-generation DES. The current-generation DES equipped with biocompatibility improved safety and efficacy as compared to first-generation DES and BMS [15, 16]. A benefit of the current-generation DES might have generated an expectation for tolerable clinical outcomes of SED cases compared with non-SED cases, which was not observed in cases after first-generation DES or BMS implantation. However, in accordance with previous OCT studies, the present study showed significantly worse clinical outcomes in the SED group than in the non-SED group after current-generation DES implantation. Therefore, our results suggest that avoiding SED would be an important strategy even in the current-generation DES era.
Risk factors for SED after current-generation DES implantation
In this study, progressive atherosclerotic plaques, i.e, lipidic and fibrocalcific plaques, and lumen eccentricity were the risk for SED after current-generation DES implantation, which was concordant with previous reports. Several previous studies showed the factors of SED after stent implantation such as excessive stent expansion, calcified or lipidic plaques, residual plaque eccentricity, stent length, and ST-elevation MI presentation [5, 13, 24]. Stenting on significant plaque such as fibrocalcific or lipidic plaque has been a well-known determinant of SED [5, 13, 24]. In these plaques, high tensile stress is generated at the junction between tissue types with differing elastic properties [28]. In the present study, lumen eccentricity was another predictor of SED. In a lesion with large lumen eccentricity, stenting causes unequal tensile stress in the same cross-section. Higher tensile stress occurs in the direction with a shorter lumen diameter than a longer one when the stent is expanded.
Clinical implications
The previous studies have consistently demonstrated that small minimal stent area (MSA) significantly showed worse TLR and MACE [29]. Therefore, more aggressive post-dilatation especially in vessels with small MSA might be encouraged to achieve better stent expansion [8]. However, aggressive post-dilatation around stent edges to achieve optimal stent expansion might rather have the risk of SED resulting in worse clinical outcomes. The appropriate location of post-dilatation should be carefully chosen to avoid SED. Furthermore, even if the appropriate location was selected, there would be the possibility that post-dilatation affects the unplanned location due to insufficient visibility of stent or heart beat. Therefore, it might be acceptable to choose a downsized balloon when a balloon after stent implantation is in contact with a plaque which is not protected by a stent cage.
This study showed that progressive atherosclerotic plaques (lipidic and fibrocalcific plaques) by OCT and lumen eccentricity were associated with SED. Therefore, operators have to avoid locations with these features as stent landing zones. However, even though using OCT, SED related to stent-oversizing could not be completely avoided. One of the potential reasons for SED related to stent-oversizing is that the actual stent landing zones were different from those initially planned. Those unplanned stent landing zones can be led by heart-beat, longer or shorter length of the stents than planned, or any technical mistakes. Therefore, the possibility of stent landing at unplanned zones should be considered when stent length is selected. A choice of a downsized stent is also an option to avoid SED. When it is difficult to expect the landing zone, a downsized stent can be a choice for safety. The actual landing zone would be checked by an intra-coronary imaging device after an implantation of a downsized stent, and then, the appropriate size of post-balloon should be decided to correct stent malapposition if present.
Study limitation
This study has the following limitations. First, this study was a retrospective and observational study at a single center. Second, the patient background might not be completely adjusted. The prevalence of ACS, which significantly differed between the SED and non-SED groups, was not included as the covariant set for IPTW, because the definition of AHA/ACC lesion classification includes the information of thrombus which is more frequently found in ACS than in stable angina. In the process of variables selection, if clinically similar variables remained, we selected the variable that we considered to be more clinically relevant in order to avoid multicollinearity. Therefore, AHA/ACC type classification was used as the covariant set rather than ACS or not. Third, the population of this study is small. Finally, this study covered the long study-period from 2010 to 2020, which was long enough to change the strategy of the procedure in a single center. In fact, changes in the PCI-strategy and -procedure might affect the occurrence of SED. However, those changes would not affect so much on the main conclusions of this study, since the aim of this study was to investigate the factors of SED and compare clinical outcomes between the SED and non-SED groups. However, in order to exclude possible confounding factors, further prospective and large-scale trials which include enough population during a short period are needed to assess the significance of SEDs.
Conclusions
SED detected by OCT after current-generation DES implantation was associated with worse clinical outcomes. Atherosclerotic lesions, lumen eccentricity, and stent-oversizing were predictors of SED. The operators need to plan strategies to avoid SED, although it is important to achieve optimal stent expansion in order to improve clinical outcomes.
Supporting information
(XLSX)
Acknowledgments
The authors acknowledge all staff in the catheter laboratory in Jichi Medical University, Saitama Medical Center for their technical support in this study.
Data Availability
Data was submitted as Supporting Information.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Hong MK, Park SW, Lee NH, Nah DY, Lee CW, Kang DH, et al. Long-term outcomes of minor dissection at the edge of stents detected with intravascular ultrasound. Am J Cardiol. 2000;86(7):791–95. doi: 10.1016/s0002-9149(00)01085-7 [DOI] [PubMed] [Google Scholar]
- 2.Sheris SJ, Canos MR, Weissman NJ. Natural history of intravascular ultrasound–detected edge dissections from coronary stent deployment. American Heart Journal. 2000;139(1):59–63. doi: 10.1016/s0002-8703(00)90309-0 [DOI] [PubMed] [Google Scholar]
- 3.Cutlip DE, Baim DS, Ho KK, Popma JJ, Lansky AJ, Cohen DJ, et al. Stent thrombosis in the modern era: a pooled analysis of multicenter coronary stent clinical trials. Circulation. 2001;103(15):1967–71. doi: 10.1161/01.cir.103.15.1967 [DOI] [PubMed] [Google Scholar]
- 4.Biondi-Zoccai GG, Agostoni P, Sangiorgi GM, Airoldi F, Cosgrave J, Chieffo A, et al. Incidence, predictors, and outcomes of coronary dissections left untreated after drug-eluting stent implantation. Eur Heart J. 2006;27(5):540–6. doi: 10.1093/eurheartj/ehi618 [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi N, Mintz GS, Witzenbichler B, Metzger DC, Rinaldi MJ, Duffy PL, et al. Prevalence, Features, and Prognostic Importance of Edge Dissection After Drug-Eluting Stent Implantation: An ADAPT-DES Intravascular Ultrasound Substudy. Circ Cardiovasc Interv. 2016;9(7):e003553. doi: 10.1161/CIRCINTERVENTIONS.115.003553 [DOI] [PubMed] [Google Scholar]
- 6.Bezerra HG, Costa MA, Guagliumi G, Rollins AM, Simon DI. Intracoronary optical coherence tomography: a comprehensive review clinical and research applications. JACC Cardiovasc Interv. 2009;2(11):1035–46. doi: 10.1016/j.jcin.2009.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ali ZA, Maehara A, Généreux P, Shlofmitz RA, Fabbiocchi F, Nazif TM, et al. Optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation (ILUMIEN III: OPTIMIZE PCI): a randomised controlled trial. The Lancet. 2016;388(10060):2618–28. doi: 10.1016/S0140-6736(16)31922-5 [DOI] [PubMed] [Google Scholar]
- 8.Otake H, Kubo T, Takahashi H, Shinke T, Okamura T, Hibi K, et al. Optical Frequency Domain Imaging Versus Intravascular Ultrasound in Percutaneous Coronary Intervention (OPINION Trial). JACC: Cardiovascular Imaging. 2018;11(1):111–23. doi: 10.1016/j.jcmg.2017.06.021 [DOI] [PubMed] [Google Scholar]
- 9.Bouki KP, Sakkali E, Toutouzas K, Vlad D, Barmperis D, Phychari S, et al. Impact of coronary artery stent edge dissections on long-term clinical outcome in patients with acute coronary syndrome: an optical coherence tomography study. Catheter Cardiovasc Interv. 2015;86(2):237–46. doi: 10.1002/ccd.25855 [DOI] [PubMed] [Google Scholar]
- 10.Prati F, Romagnoli E, Burzotta F, Limbruno U, Gatto L, La Manna A, et al. Clinical Impact of OCT Findings During PCI: The CLI-OPCI II Study. JACC Cardiovasc Imaging. 2015;8(11):1297–305. doi: 10.1016/j.jcmg.2015.08.013 [DOI] [PubMed] [Google Scholar]
- 11.Prati F, Romagnoli E, Gatto L, La Manna A, Burzotta F, Limbruno U, et al. Clinical Impact of Suboptimal Stenting and Residual Intrastent Plaque/Thrombus Protrusion in Patients With Acute Coronary Syndrome: The CLI-OPCI ACS Substudy (Centro per la Lotta Contro L’Infarto-Optimization of Percutaneous Coronary Intervention in Acute Coronary Syndrome). Circ Cardiovasc Interv. 2016;9(12). [DOI] [PubMed] [Google Scholar]
- 12.van Zandvoort LJC, Tomaniak M, Tovar Forero MN, Masdjedi K, Visseren L, Witberg K, et al. Predictors for Clinical Outcome of Untreated Stent Edge Dissections as Detected by Optical Coherence Tomography. Circ Cardiovasc Interv. 2020;13(3):e008685. doi: 10.1161/CIRCINTERVENTIONS.119.008685 [DOI] [PubMed] [Google Scholar]
- 13.Chamie D, Bezerra HG, Attizzani GF, Yamamoto H, Kanaya T, Stefano GT, et al. Incidence, predictors, morphological characteristics, and clinical outcomes of stent edge dissections detected by optical coherence tomography. JACC Cardiovasc Interv. 2013;6(8):800–13. doi: 10.1016/j.jcin.2013.03.019 [DOI] [PubMed] [Google Scholar]
- 14.Radu MD, Raber L, Heo J, Gogas BD, Jorgensen E, Kelbaek H, et al. Natural history of optical coherence tomography-detected non-flow-limiting edge dissections following drug-eluting stent implantation. EuroIntervention. 2014;9(9):1085–94. doi: 10.4244/EIJV9I9A183 [DOI] [PubMed] [Google Scholar]
- 15.Palmerini T, Benedetto U, Biondi-Zoccai G, Della Riva D, Bacchi-Reggiani L, Smits PC, et al. Long-Term Safety of Drug-Eluting and Bare-Metal Stents: Evidence From a Comprehensive Network Meta-Analysis. J Am Coll Cardiol. 2015;65(23):2496–507. doi: 10.1016/j.jacc.2015.04.017 [DOI] [PubMed] [Google Scholar]
- 16.Stone GW, Rizvi A, Newman W, Mastali K, Wang JC, Caputo R, et al. Everolimus-eluting versus paclitaxel-eluting stents in coronary artery disease. N Engl J Med. 2010;362(18):1663–74. doi: 10.1056/NEJMoa0910496 [DOI] [PubMed] [Google Scholar]
- 17.Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115(17):2344–51. doi: 10.1161/CIRCULATIONAHA.106.685313 [DOI] [PubMed] [Google Scholar]
- 18.Jinnouchi H, Kuramitsu S, Shinozaki T, Hiromasa T, Kobayashi Y, Takeji Y, et al. Five-Year Clinical Outcomes After Drug-Eluting Stent Implantation Following Rotational Atherectomy for Heavily Calcified Lesions. Circ J. 2018;82(4):983–91. doi: 10.1253/circj.CJ-17-0564 [DOI] [PubMed] [Google Scholar]
- 19.Jinnouchi H, Kuramitsu S, Shinozaki T, Tomoi Y, Hiromasa T, Kobayashi Y, et al. Difference of Tissue Characteristics Between Early and Late Restenosis After Second-Generation Drug-Eluting Stents Implantation- An Optical Coherence Tomography Study. Circ J. 2017;81(4):450–7. doi: 10.1253/circj.CJ-16-1069 [DOI] [PubMed] [Google Scholar]
- 20.Prati F, Guagliumi G, Mintz GS, Costa M, Regar E, Akasaka T, et al. Expert review document part 2: methodology, terminology and clinical applications of optical coherence tomography for the assessment of interventional procedures. Eur Heart J. 2012;33(20):2513–20. doi: 10.1093/eurheartj/ehs095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tearney GJ, Regar E, Akasaka T, Adriaenssens T, Barlis P, Bezerra HG, et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol. 2012;59(12):1058–72. doi: 10.1016/j.jacc.2011.09.079 [DOI] [PubMed] [Google Scholar]
- 22.Donnelly P, Maurovich-Horvat P, Vorpahl M, Nakano M, Kaple RK, Warger W, et al. Multimodality imaging atlas of coronary atherosclerosis. JACC Cardiovasc Imaging. 2010;3(8):876–80. doi: 10.1016/j.jcmg.2010.06.006 [DOI] [PubMed] [Google Scholar]
- 23.Benedetto U, Head SJ, Angelini GD, Blackstone EH. Statistical primer: propensity score matching and its alternatives. Eur J Cardiothorac Surg. 2018;53(6):1112–7. doi: 10.1093/ejcts/ezy167 [DOI] [PubMed] [Google Scholar]
- 24.Liu X, Tsujita K, Maehara A, Mintz GS, Weisz G, Dangas GD, et al. Intravascular ultrasound assessment of the incidence and predictors of edge dissections after drug-eluting stent implantation. JACC Cardiovasc Interv. 2009;2(10):997–1004. doi: 10.1016/j.jcin.2009.07.012 [DOI] [PubMed] [Google Scholar]
- 25.Bouma BE, Tearney GJ, Yabushita H, Shishkov M, Kauffman CR, Gauthier DD, et al. Evaluation of intracoronary stenting by intravascular optical coherence tomography. Heart. 2003;89(3):317–20. doi: 10.1136/heart.89.3.317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonzalo N, Serruys PW, Okamura T, Shen ZJ, Garcia-Garcia HM, Onuma Y, et al. Relation between plaque type and dissections at the edges after stent implantation: an optical coherence tomography study. Int J Cardiol. 2011;150(2):151–5. doi: 10.1016/j.ijcard.2010.03.006 [DOI] [PubMed] [Google Scholar]
- 27.Kume T, Okura H, Miyamoto Y, Yamada R, Saito K, Tamada T, et al. Natural history of stent edge dissection, tissue protrusion and incomplete stent apposition detectable only on optical coherence tomography after stent implantation—preliminary observation. Circ J. 2012;76(3):698–703. doi: 10.1253/circj.cj-11-0845 [DOI] [PubMed] [Google Scholar]
- 28.Richardson PD, Davies MJ, Born VV. Influence of plaque configuration and stress distribution on fissuring of coronary atherosclerotic plaques. The Lancet. 1989;2:941–4. doi: 10.1016/s0140-6736(89)90953-7 [DOI] [PubMed] [Google Scholar]
- 29.Maehara A, Matsumura M, Ali ZA, Mintz GS, Stone GW. IVUS-Guided Versus OCT-Guided Coronary Stent Implantation: A Critical Appraisal. JACC Cardiovasc Imaging. 2017;10(12):1487–503. doi: 10.1016/j.jcmg.2017.09.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
Data was submitted as Supporting Information.