Abstract
Background
The triglyceride-glucose (TyG) index is a reliable indicator of insulin resistance. We aimed to investigate the TyG index in relation to cardio-cerebrovascular diseases (CCVDs and mortality.
Methods
This retrospective study included 114,603 subjects. The TyG index was categorized into four quartiles by sex: Q1, <8.249 and <8.063; Q2, 8.249‒<8.614 and 8.063‒<8.403; Q3, 8.614‒< 8.998 and 8.403‒<8.752; and Q4, ≥8.998 and ≥8.752, in men and women, respectively. To calculate hazard ratios (HRs) and 95% confidence intervals (CIs) for the primary outcomes (CCVDs and all-cause mortality) and secondary outcomes (cardiovascular diseases [CVDs], cerebrovascular diseases [CbVDs], CCVD-related deaths, or all-cause deaths), Cox proportional hazards regression models were adopted.
Results
Compared to Q1, the HRs (95% CIs) for the primary outcomes of Q2, Q3, and Q4 were 1.062 (0.981‒1.150), 1.110 (1.024−1.204), and 1.151 (1.058−1.252) in men and 1.099 (0.986−1.226), 1.046 (0.938−1.166), and 1.063 (0.954−1.184) in women, respectively, after adjusted for age, smoking status, drinking status, physical activity, body mass index, systolic blood pressure, low-density lipoprotein cholesterol, economic status, and anti-hypertensive medications. Fully adjusted HRs (95% CIs) for CVDs of Q2, Q3, and Q4 were 1.114 (0.969−1.282), 1.185 (1.031−1.363), and 1.232 (1.068−1.422) in men and 1.238 (1.017−1.508), 1.183 (0.971−1.440), and 1.238 (1.018−1.505) in women, respectively. The adjusted HRs (95% CIs) for ischemic CbVDs of Q2, Q3, and Q4 were 1.005 (0.850−1.187), 1.225 (1.041−1.441), and 1.232 (1.039−1.460) in men and 1.040 (0.821−1.316), 1.226 (0.981−1.532), and 1.312 (1.054−1.634) in women, respectively, while the TyG index was negatively associated with hemorrhagic CbVDs in women but not in men. The TyG index was not significantly associated with CCVD-related death or all-cause death in either sex.
Conclusions
Elevated TyG index was positively associated with the primary outcomes (CCVDs and all-cause mortality) in men and predicted higher risk of CVDs and ischemic CbVDs in both sexes.
Introduction
Cardiovascular diseases (CVDs) and cerebrovascular diseases (CbVDs), which include coronary heart disease, peripheral arterial disease, and deep vein thrombosis in addition to other diseases of the heart and brain, are the leading cause of death worldwide [1]. CVDs and CbVDs are the second and fourth most common causes of death in Korea, respectively [2]. These cardio-cerebrovascular diseases (CCVDs) are a large burden on public health and a considerable number can be avoided by early detection and active management of their risk factors such as a healthy lifestyle.
Insulin functions as an important hormone to stimulate glucose metabolism and lipogenesis and suppress lipid utilization. Insulin resistance means that insulin does not function properly in the target tissue. Thus, insulin resistance plays an important role in the development of diabetes mellitus and may be closely associated with atherosclerotic CCVDs, malignant neoplasms, and mortality [3–5]. The early detection of insulin resistance is important in reducing the burden of chronic diseases. The hyperinsulinemic-euglycemic glucose clamp method is the gold standard to measure insulin resistance [6]. However, it is difficult to apply this technique to a real-world clinical setting because of its complex measurement procedure. The homeostasis model assessment of insulin resistance (HOMA-IR) is frequently used in the clinical setting but also has limited applicability because insulin levels must be measured [7]. The triglyceride-glucose (TyG) index is a novel reliable indicator to detect the early phase of insulin resistance and type 2 diabetes [8–10]. The TyG index has the advantage of having a normal distribution but the disadvantage of being easily affected by ethnic group, dietary pattern, and alcohol intake [11–13]. Several studies have reported that the TyG index was superior to HOMA-IR in predicting type 2 diabetes in the Korean population [10]. In part, the dietary pattern of the Korean populations who consume high levels of carbohydrates and low levels of fat may contribute to the higher predictability of the TyG index.
This study aimed to examine whether the TyG index was associated with primary outcomes (CCVDs and all-cause mortality) in Korean adults, based on the Korean National Health Insurance Service (NHIS)-National Health Screening (HEALS) cohort. In addition, we further investigated the association between the TyG index and each secondary outcome after stratifying the primary outcomes into CVDs, CbVDs, CCVD-related death, and all-cause death.
Materials and methods
Study population
The NHIS-HEALS cohort included 514,794 subjects who were randomly selected from the 5.1 million examinees of the national health check-up program from January 2002 to December 2003. The age of the subjects was between 40 and 79 years at the end of December 2002. The cohort contains the lifestyle information of subjects and past medical history of diseases based on the self-reported questionnaires, diagnostic codes, and prescription information based on national insurance claim data, death information such as the main cause and date, and laboratory information from biennial national health check-up programs. The NHIS-HEALS cohort did not collect specific lipid profile such as triglycerides (TG) except for total cholesterol levels between 2002 and 2008. Thus, we should set 2009–2010 as the baseline because TG have been available since 2009.
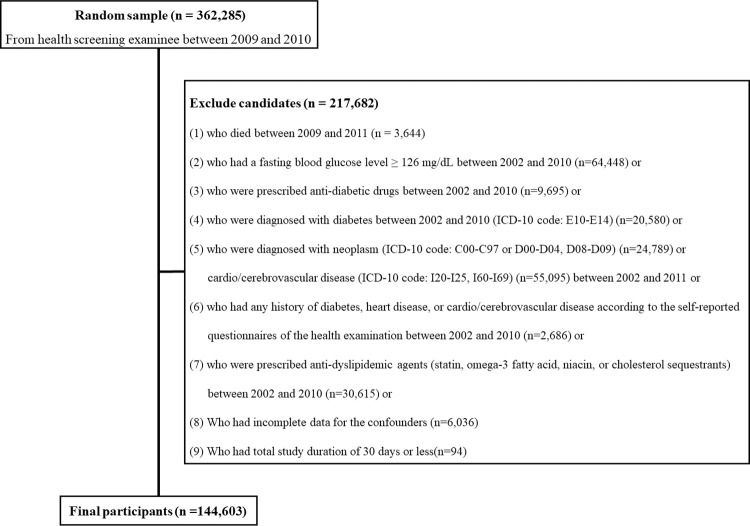
The flowchart describes the inclusions and exclusions of this study (Fig 1). Initially, 362,285 subjects who underwent the national health check-up programs between 2009 and 2010 were included. Among the initial subjects, individuals who met one or more of the following criteria were excluded: 1) subjects who died between 2009 and 2011 (n = 3644); 2) subjects who had a fasting blood glucose levels ≥ 126 mg/dL between 2002 and 2010 (n = 64,448); 3) subjects who were prescribed anti-diabetic drugs between 2002 and 2010 (n = 9695); 4) subjects who were diagnosed with diabetes between 2002 and 2010 (ICD-10 code: E10-E14) (n = 20,580); 5) subjects who were diagnosed with any neoplasm (ICD-10 code: C00-C97 or D00-D09) (n = 24,789) or CCVDs (the International Classification of Diseases [ICD]-10 code: I20-I25 for CVDs or I60-I69 for CbVDs) (n = 55,095) between 2002 and 2011; 6) subjects who had any history of diabetes, heart disease, CVDs, or CbVDs according to the self-reported questionnaires of the national health check-up program between 2002 and 2010 (n = 2,686); 7) subjects who were prescribed lipid-lowering agents (such as statin, omega-3 fatty acids, niacin, or cholesterol sequestrants) between 2002 and 2010 (n = 30,615); 8) subjects who had incomplete data for the confounders (n = 6036); or 9) subjects whose study duration was 30 days or less (n = 94). After these exclusions, 144,603 subjects (78,021 men and 66,582 women) were included in this study.
Fig 1. Flowchart of inclusion and exclusion criteria.
This research followed the 1964 Helsinki Declaration and was approved by the Institutional Review Board of Chungbuk National University Hospital (CBNUH 2021-03-003)
Definition of TyG index, cardio-cerebrovascular disease, death, and study duration
The TyG index was calculated using the following equation: TyG = Ln (TG [mg/dL] × glucose [mg/dL]/2) [14, 15]. Calculated TyG indices were divided into four groups by sex: Q1, <8.249 in men and <8.063 in women; Q2, 8.249 ‒ <8.614 in men and 8.063 ‒ <8.403 in women; Q3, 8.614 ‒ < 8.998 in men and 8.403 ‒ <8.752 in women; and Q4, ≥8.998 in men and ≥8.752 in women.
The primary end point of this study is to compare the occurrence rates of CCVDs and all-cause mortality by TyG quartile groups after enrollment (2009‒2010). CCVDs included CVDs (I20-I25) and CbVDs (I60-I69) based on the ICD-10 code. Furthermore, CbVDs were further stratified in ischemic, hemorrhagic, and other CbVDs according to diagnostic code as follows: ischemic CbVDs were coded as I63 (cerebral infarction), I65 (occlusion and stenosis of precerebral arteries, not resulting in cerebral infarction), and I66 (occlusion and stenosis of cerebral arteries, not resulting in cerebral infarction); hemorrhagic CbVDs as I60 (subarachnoid hemorrhage), I61 (intracranial hemorrhage), and I62 (other nontraumatic intracranial hemorrhage); and other CbVDs as I64 (stroke, not specified as hemorrhage or infarction), I67 (other cerebrovascular diseases), I68 (cerebrovascular disorders in diseases classified elsewhere), and I69 (sequelae of cerebrovascular disease). CCVDs were defined when the main diagnosis (I20-I25 or I60-I69) was recorded at least twice in outpatients or once in hospitalized patients. As secondary outcomes, we considered the subgroup analyses for each CVD, CbVD, CCVD-related deaths, and all-cause deaths. In the case of deceased patients, it was classified by the cause of death recorded in the death certificate. If subjects died from fatal CCVD and the direct cause of death was recorded as CVD or CbVD on his/her death certificate, they were defined as CCVD-related deaths.
The research start date was defined as the day of the first health check-up at baseline (2009–2010). If a subject experienced CCVDs or death, the end date is the earlier date of the events. Otherwise, the end date is the last of the two following cases: the date of the last outpatient clinic visit or the last health check-up.
Definition of covariates
Risk factors that can attribute to CCVDs or deaths were controlled such as age, body mass index (BMI), systolic blood pressure (SBP), low-density lipoprotein cholesterol (LDL-C), lifestyle (cigarette smoking, alcohol consumption, and physical activity), and economic status (monthly household income). These confounding factors were surveyed at baseline (2009‒2010).
BMI (unit, kg/m2) was calculated as body mass (kg) divided by squared height (m). Lifestyle and household income levels were collected from self-reported questionnaires. Smoking status was classified as ever smokers (who had smoked cigarettes in the past) and never smokers (who had never smoked cigarettes). Alcohol consumption was categorized into rare (less than once a week), sometimes (once to twice a week), and often (three or more times a week). Physical activity was divided into rare (less than once a week of any kind of exercise), sometimes (individuals who did not meet the definition of rare or regular physical activity), and regular (five or more times of walking or moderate-intensity exercise a week; three or more times of vigorous-intensity a week; four or more times of walking, moderate-intensity physical activity or vigorous-intensity exercise a week). Economic status was classified into three groups, based on the self-reported monthly household income: low, 0 to 30th percentile; middle, 31st to 70th percentile; and high, 71st to 100th percentile.
Statistical analysis
Continuous and categorical variables were presented as mean ± standard deviations (SDs) and the number of subjects (%), respectively. To check the group difference, analysis of variance (ANOVA) tests for continuous variables and chi-square tests and log-linear models for categorical variables were used. To investigate the association between the TyG index and outcomes (CVD, CbVD, and deaths), outcome-free survival rates were estimated and compared using the Kaplan-Meier method and the log-rank test. The hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated to examine the association between the TyG index and the primary outcomes (CCVDs and all-cause mortality) based on Cox proportional hazards regression models after adjusting for confounders. Three Cox proportional hazards regression models were built after adjusting for age, smoking status, drinking status, physical activity, body mass index, systolic blood pressure, LDL-C, economic status, and anti-hypertensive medications. All p-values were two-sided and statistical significance was set as <0.05. The statistical analyses were performed using the statistical package SAS enterprise version 7.1 (SAS Inc., Cary, NC) and R studio version 3.3.3.
Results
In total, 144,603 subjects (78,021 men and 66,582 women) were included and the median study duration was 5.97 years.
S1 Table displays the p-values for the simultaneous tests of effects by sex and TyG quartile on each variable. For the continuous variables, two-way ANOVA was employed. For the categorical variables, a log-linear model was used. For every variable, we found statistically significant effects of sex and TyG quartile as well as interactions. Based on this significant result, we stratified the data by sex.
Table 1 presents the baseline characteristics of the subjects according to the TyG quartile by sex. Mean age increased in men but decreased in women with the higher quartile group. In both sexes, BMI, SBP, glucose, TG, and percentage of ever smokers increased with increased quartile while the percentage of regular physical activity decreased (all p-values <0.001). Mean TyG index from Q1 to Q4 was 7.945, 8.438, 8.798, and 9.359, respectively, in men and 7.775, 8.240, 8.570, and 9.086, respectively, in women.
Table 1. Baseline characteristics according to the triglyceride-glucose index quartile.
| Male | Q1 (<8.249) | Q2 (8.249‒<8.614) | Q3 (8.614‒<8.998) | Q4 (≥8.998) | p-valueX |
| Number (N) | 19,534 | 19,481 | 19,523 | 19,483 | N.A |
| Age, years | 56.6 ± 8.1 | 56.2 ± 7.9 | 55.7 ± 7.6 | 54.7 ± 6.9 | <0.001 |
| BMI, kg/m2 | 22.7 ± 2.6 | 23.5 ± 2.6 | 24.1 ± 2.6 | 24.8 ± 2.6 | <0.001 |
| SBP, mmHg | 121.8 ± 14.2 | 124.0 ± 14.2 | 125.6 ± 14.2 | 127.4 ± 14.2 | <0.001 |
| Glucose, mg/dL | 89.9 ± 10.4 | 93.5 ± 10.6 | 96.0 ± 10.9 | 99.4 ± 11.2 | <0.001 |
| TG, mg/dL | 65.1 ± 15.0 | 100.6 ± 15.2 | 140.4 ± 21.8 | 248.7 ± 98.3 | <0.001 |
| LDL-C, mg/dL | 112.7 ± 31.6 | 118.8 ± 31.7 | 120.3 ± 32.6 | 109.8 ± 38.2 | <0.001 |
| TyG | 7.945 ± 0.247 | 8.438 ± 0.105 | 8.798 ± 0.109 | 9.359 ± 0.310 | <0.001 |
| Ever smokers, N (%) | 11,437 (58.5) | 12,221 (62.7) | 12,962 (66.4) | 13,800 (70.8) | <0.001 |
| Drinking status, N (%) | <0.001 | ||||
| Rare | 7,623 (39.0) | 7,262 (37.3) | 6,681 (34.2) | 5,708 (29.3) | |
| Sometimes | 7,964 (40.8) | 8,062 (41.4) | 8,281 (42.4) | 8,382 (43.0) | |
| Often | 3,947 (20.2) | 4,157 (21.3) | 4,561 (23.4) | 5,393 (27.7) | |
| Physical activity, N (%) | <0.001 | ||||
| Rare | 3,954 (20.2) | 4,069 (20.9) | 4,068 (20.8) | 4,208 (21.6) | |
| Sometimes | 8,607 (44.1) | 9,077 (46.6) | 9,267 (47.5) | 9,452 (48.5) | |
| Regular | 6,973 (35.7) | 6,335 (32.5) | 6,188 (31.7) | 5,823 (29.9) | |
| Economic status, N (%) | <0.001 | ||||
| Low | 3,172 (16.2) | 3,025 (15.5) | 2,909 (14.9) | 2,871 (14.7) | |
| Middle | 5,890 (30.2) | 5,994 (30.8) | 5,742 (29.4) | 5,924 (30.4) | |
| High | 10,472 (53.6) | 10,462 (53.7) | 10,872 (55.7) | 10,688 (54.9) | |
| Female | Q1 (<8.063) | Q2 (8.063‒<8.403) | Q3 (8.403‒<8.752) | Q4 (≥8.752) | p-value |
| Number (N) | 16,666 | 16,645 | 16,625 | 16,646 | N.A |
| Age, years | 54.1 ± 6.7 | 55.6 ± 7.5 | 56.9 ± 8.2 | 58.3 ± 8.6 | <0.001 |
| BMI, kg/m2 | 22.6 ± 2.6 | 23.2 ± 2.7 | 23.6 ± 2.9 | 24.3 ± 2.9 | <0.001 |
| SBP, mmHg | 117.5 ± 14.3 | 119.8 ± 14.7 | 122.3 ± 15.2 | 124.4 ± 15.3 | <0.001 |
| Glucose, mg/dL | 87.6 ± 9.4 | 91.1 ± 9.5 | 93.1 ± 9.9 | 96.1 ± 10.6 | <0.001 |
| TG, mg/dL | 56.1 ± 12.2 | 84.5 ± 11.8 | 115.0 ± 16.2 | 194.4 ± 73.8 | <0.001 |
| LDL-C, mg/dL | 116.7 ± 30.9 | 122.6 ± 30.3 | 125.5 ± 33.7 | 122.5 ± 36.1 | <0.001 |
| TyG | 7.775 ± 0.237 | 8.240 ± 0.097 | 8.570 ± 0.100 | 9.086 ± 0.290 | <0.001 |
| Ever smokers, N (%) | 282 (1.7) | 308 (1.9) | 351 (2.1) | 423 (2.5) | <0.001 |
| Drinking status, N (%) | <0.001 | ||||
| Rare | 13,545 (81.3) | 13,871 (83.3) | 14,087 (84.7) | 14,233 (85.5) | |
| Sometimes | 2,671 (16.0) | 2,336 (14.0) | 2,133 (12.8) | 1,988 (11.9) | |
| Often | 450 (2.7) | 438 (2.6) | 405 (2.4) | 425 (2.6) | |
| Physical activity, N (%) | <0.001 | ||||
| Rare | 4,334 (26.0) | 4,715 (28.3) | 4,941 (29.7) | 5,181 (31.1) | |
| Sometimes | 7,259 (43.6) | 7,130 (42.8) | 7,078 (42.6) | 6,999 (42.0) | |
| Regular | 5,073 (30.4) | 4,800 (28.8) | 4,606 (27.7) | 4,466 (26.8) | |
| Economic status, N (%) | 0.013 | ||||
| Low | 4,373 (26.2) | 4,291 (25.8) | 4,236 (25.5) | 4,262 (25.6) | |
| Middle | 5,603 (33.6) | 5,694 (34.2) | 5,818 (35.0) | 5,910 (35.5) | |
| High | 6,690 (40.1) | 6,660 (40.0) | 6,571 (39.5) | 6,474 (38.9) |
Abbreviations: BMI, body mass index; SBP, systolic blood pressure; TG, triglycerides; LDL-C, low-density lipoprotein cholesterol; TyG, triglycerides-glucose index.
p-values are calculated from one-way ANOVA in case of continuous variables and from chi square test in case of categorical variables.
Fig 2 shows the estimated cumulative event incidence, based on the Kaplan-Meier’s survival curve: 2A) the primary outcomes (CCVDs and all-cause mortality), 2B) CVDs, and 2C) CbVDs. Cumulative incidence of the primary outcomes was not different in men but significantly increased with the increased quartile groups in women (p-value = 0.247 in men and <0.001 in women) (Fig 2A). Further statistical analyses were conducted after dividing CCVDs into CVDs and CbVDs. The cumulative incidence of CVDs significantly increased with the increasing TyG index (all p-values <0.05) (Fig 2B). Cumulative incidence of CbVDs was not different in men (p-value = .0.673) but the highest in the Q4 of women (p-value <0.010) (Fig 2C). In addition, the Kaplan-Meier estimates for ischemic, hemorrhagic, and other CbVDs were plotted in S1 Fig.
Fig 2. The estimated cumulative incidence of each outcome.
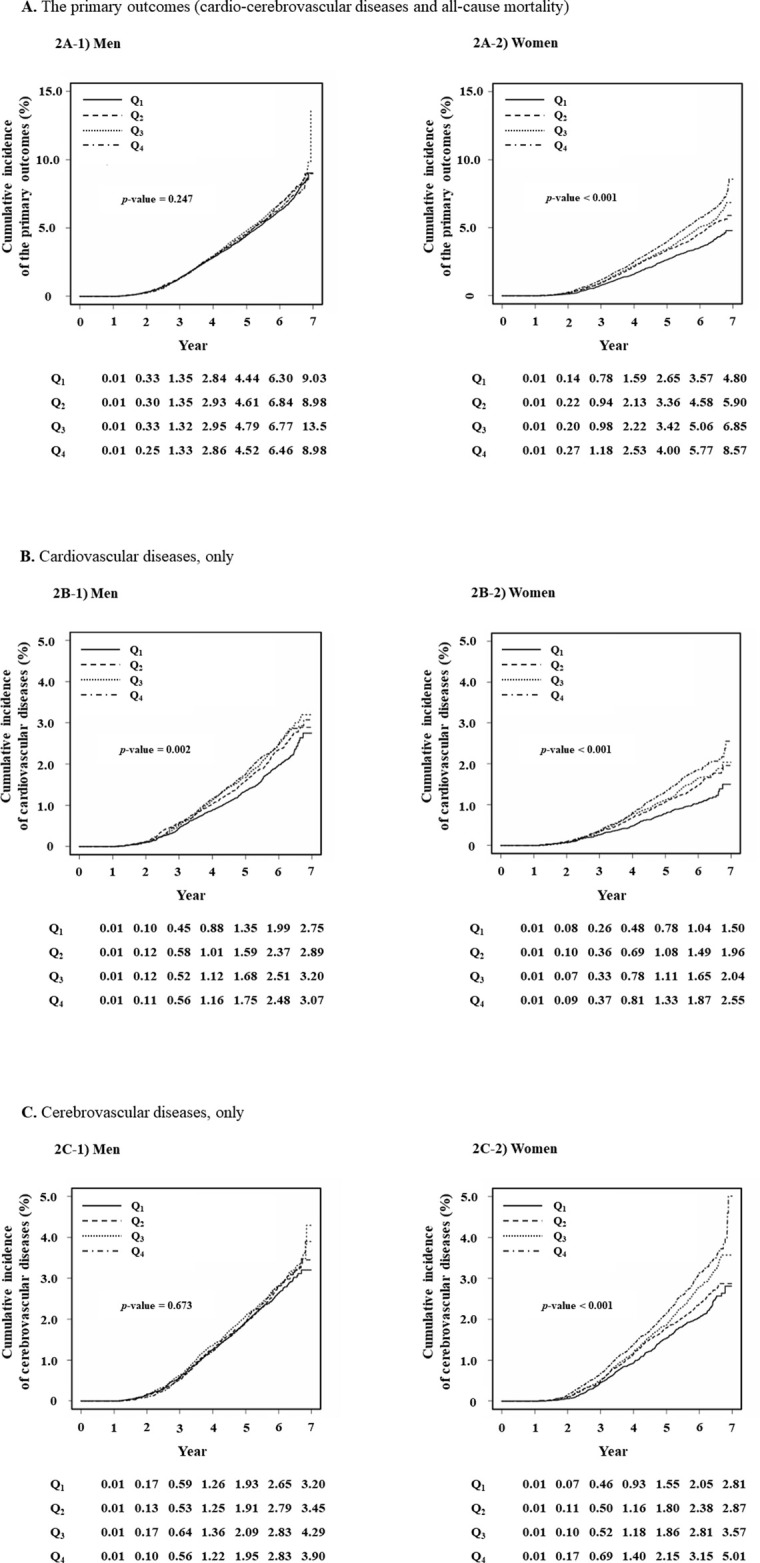
A) The primary outcomes (cardio-cerebrovascular diseases and all-cause mortality). B) Cardiovascular diseases, only. C) Cerebrovascular diseases, only. All p-values are from log-rank tests.
Table 2 demonstrates the results of Cox proportional hazards regression models to examine the association between the TyG index and individual outcomes. Compared to Q1, HRs (95% CIs) for the primary outcomes of Q2, Q3, and Q4 were 1.062 (0.981‒1.150), 1.110 (1.024−1.204), and 1.151 (1.058−1.252), respectively, in men and 1.099 (0.986−1.226), 1.046 (0.938−1.166), and 1.063 (0.954−1.184), respectively, in women after adjusting for age, smoking status, alcohol consumption, physical activity, BMI, SBP, LDL-C, economic status, and anti-hypertensive medications. Subgroup analyses were performed to examine the association between the TyG index and individual outcomes (CVDs, CbVDs, CCVD-death, and all-cause death). Compared to Q1, HRs (95% CIs) for CVDs of Q2, Q3, and Q4 were 1.114 (0.969−1.282), 1.185 (1.031−1.363), and 1.232 (1.068−1.422), respectively, in men and 1.238 (1.017−1.508), 1.183 (0.971−1.440), and 1.238 (1.018−1.505), respectively, in women after fully adjusting. CbVDs were further classified into ischemic, hemorrhagic, and other CbVDs. The adjusted HRs (95% CIs) for ischemic CbVDs of Q2, Q3, and Q4 were 1.005 (0.850−1.187), 1.225 (1.041−1.441), and 1.232 (1.039−1.460) in men and 1.040 (0.821−1.316), 1.226 (0.981−1.532), and 1.312 (1.054−1.634) in women, respectively, while TyG index was negatively associated with hemorrhagic CbVDs in women but not in men. The adjusted HRs (95% CIs) for hemorrhagic CbVDs of Q2, Q3, and Q4 were 0.972 (0.642−1.472), 0.554 (0.347−0.884), and 0.452 (0.278−0.734) in women, compared to Q1. The TyG index was not significantly associated with CCVD-related death and all-cause death in both sexes.
Table 2. Cox-proportional hazards regression model for cardiovascular diseases, cerebrovascular diseases, cardio-cerebrovascular diseases, cardio-cerebrovascular diseases-related death, or all-cause death according to the TyG index quartile.
| Total | Men | Women | |
| The primary outcomes (Cardio-cerebrovascular diseases and all-cause mortality) | Q1 | Reference | Reference |
| Q2 | 1.062 (0.981−1.150) | 1.099 (0.986−1.226) | |
| Q3 | 1.110 (1.024−1.204) | 1.046 (0.938−1.166) | |
| Q4 | 1.151 (1.058−1.252) | 1.063 (0.954−1.184) | |
| Subgroups | Men | Women | |
| Cardiovascular diseases | Q1 | Reference | Reference |
| Q2 | 1.114 (0.969−1.282) | 1.238 (1.017−1.508) | |
| Q3 | 1.185 (1.031−1.363) | 1.183 (0.971−1.440) | |
| Q4 | 1.232 (1.068−1.422) | 1.238 (1.018−1.505) | |
| Ischemic cerebrovascular diseases | Q1 | Reference | Reference |
| Q2 | 1.005 (0.850−1.187) | 1.040 (0.821−1.316) | |
| Q3 | 1.225 (1.041−1.441) | 1.226 (0.981−1.532) | |
| Q4 | 1.232 (1.039−1.460) | 1.312 (1.054−1.634) | |
| Hemorrhagic cerebrovascular diseases | Q1 | Reference | Reference |
| Q2 | 1.126 (0.781−1.625) | 0.972 (0.642−1.472) | |
| Q3 | 0.937 (0.634−1.384) | 0.554 (0.347−0.884) | |
| Q4 | 0.905 (0.602−1.360) | 0.452 (0.278−0.734) | |
| Other cerebrovascular diseases | Q1 | Reference | Reference |
| Q2 | 1.007 (0.833−1.218) | 0.956 (0.789−1.160) | |
| Q3 | 0.949 (0.780−1.155) | 0.943 (0.778−1.143) | |
| Q4 | 0.971 (0.793−1.189) | 0.883 (0.727−1.073) | |
| Cardio-cerebrovascular diseases- | Q1 | Reference | Reference |
| related deaths | Q2 | 1.231 (0.778−1.947) | 0.908 (0.405−2.035) |
| Q3 | 1.157 (0.713−1.876) | 1.452 (0.708−2.976) | |
| Q4 | 1.167 (0.698−1.954) | 1.242 (0.601−2.566) | |
| All-cause deaths | Q1 | Reference | Reference |
| Q2 | 1.048 (0.911−1.205) | 1.216 (0.936−1.580) | |
| Q3 | 1.105 (0.955−1.278) | 1.009 (0.776−1.313) | |
| Q4 | 1.063 (0.907−1.245) | 0.990 (0.763−1.284) |
Adjusted for age, smoking status (ever and never smokers), drinking status (rare, sometimes, and often) and physical activity (rare, sometimes, and regular), body mass index, systolic blood pressure, LDL-C, economic status (low, middle, and high), and anti-hypertensive medications.
Cerebrovascular diseases are as follows: ischemic cerebrovascular diseases, I63 (cerebral infarction), I65 (occlusion and stenosis of precerebral arteries, not resulting in cerebral infarction), and I66 (occlusion and stenosis of cerebral arteries, not resulting in cerebral infarction); hemorrhagic cerebrovascular diseases, I60 (subarachnoid hemorrhage), I61 (intracranial hemorrhage), and I62 (other nontraumatic intracranial hemorrhage); and other cerebrovascular diseases, I64 (stroke, not specified as hemorrhage or infarction), I67 (other cerebrovascular diseases), I68 (cerebrovascular disorders in diseases classified elsewhere), and I69 (sequelae of cerebrovascular disease).
Discussion
This study demonstrated that there was a positive association between the TyG index primary outcomes (CCVDs or all-cause mortality) in men, but not in women. After stratifying the primary outcomes into CVDs, CbVDs, CCVD-related deaths, and all-cause deaths, a higher TyG index predicted a higher risk of CVDs and ischemic CbVDs in both sexes while it was inversely associated with hemorrhagic CbVDs in women. However, TyG index was not significantly associated with CCVD-related deaths and all-cause deaths in either sex.
Insulin stimulates glucose metabolism and suppresses fatty acid utilization and lipolysis as an energy source. Insulin resistance is defined as an impaired tissue response to insulin stimulation, resulting in the dysfunction of glucose and lipid metabolism [3]. These metabolic alterations lead to chronic hyperglycemia, dyslipidemia (in particular, increased free fatty acid release in adipose tissue and hepatic TG production), oxidative stress, and inflammation that induce endothelial dysfunction and atherosclerotic change. Eventually, insulin resistance increases CCVDs and premature death through these pathophysiologic mechanisms [3–5]. Thus, early detection and a more intensive management of insulin resistance are very important in reducing the likelihood of these events. Even though several methods to measure insulin resistance have been proposed, the hyperinsulinemic-euglycemic glucose clamp is considered the standard [6]. However, the hyperinsulinemic-euglycemic glucose clamp is not practical for use in real-world clinics because of complex and invasive measurements. Another reliable surrogate marker of insulin resistance is HOMA-IR. However, the disadvantage is that blood insulin levels are not routinely checked for patients without diabetes in the primary clinical setting [7].
The TyG index is newly proposed as a marker to measure insulin resistance. TG and glucose levels, which are used as variables to calculate the TyG index, are widely and commonly measured in clinics. The Korean Society of Lipid and Atherosclerosis recommends that the lipid profile (total cholesterol, TG, HDL-C, and LDL-C) be checked every 4‒6 years for adults to screen for dyslipidemia and CCVD risk [16]. Many guidelines recommend the annual measurement of fasting glucose levels to screen for diabetes or prediabetes [17, 18], while there are few recommendations for the measurement of fasting insulin levels to screen for them. Furthermore, Asian populations may consume more foods with a higher carbohydrate content than Western populations, which increases their blood TG and glucose levels [19]. Carbohydrate-rich diets raise the possibility of developing hypertriglyceridemia and impaired fasting glucose, which are related to the risk of CCVDs [20, 21]. Epidemiological studies support the proposal that the TyG index is a better indicator of insulin resistance in Asian populations than HOMA-IR [10, 22]. In addition, a lot of studies report that the TyG index was more closely associated with atherosclerosis, arterial stiffness, subclinical cerebral vessel diseases, and coronary arterial diseases than HOMA-IR [23–26].
The primary outcomes of this study are CCVDs and all-cause mortality. CCVDs are composed of CVDs (such as angina pectoris and myocardial infarctions) and CbVDs (such as non-traumatic cerebral hemorrhage, cerebral infarction, and cerebral arterial diseases). The statistical significance between the TyG index and primary outcomes differ by statistical method (significant only in women from the log-rank test and only in men from Cox proportional hazards model). The association was not significant from the log-rank test in men (Fig 2). However, it was significant after adjusting for age in the Cox proportional hazards regression model (S2 Table). This phenomenon is the opposite in women. That is, the log-rank test showed a significant association, but the fully adjusted Cox proportional hazards regression model showed non-significance. This might be explained by the different age distribution over TyG index by sex. As TyG quartile increases, the mean age decreases in men but increases in women. Because the log-rank test does not consider any confounding factors, the significance in women might include the compounded effect of age and TyG index. However, the elevated TyG index increased the occurrence of CVDs in both sexes, while it was not independently associated with individual events such as CbVDs, CCVD-related deaths, and all-cause deaths. These findings signify that the TyG index might be a good indicator in predicting CVDs such as ischemic heart diseases. Results from this study are consistent with previous studies by Park and Zhao et. al., who reported that an elevated TyG index forecast risk of ischemic heart disease and cardiovascular events [27, 28]. However, these studies had a smaller population size and did not stratify the study population according to sex. Our study supports the previous studies but clarifies the association between TyG index and individual events. There is lack of evidence to show the inverse association between TyG index and hemorrhagic stroke. Several studies have reported that higher TyG index was not significantly associated with intracranial hemorrhage but increased ischemic stroke [29, 30]. The inverse relationship of the TyG index to hemorrhagic CbVDs in this study is little different from this null association of previous studies. The protective mechanism through which the higher TyG index decreased the risk of hemorrhagic CbVDs should be further investigated.
This study has several strengths to distinguish it from previous studies. First, the NHIS-HEALS cohort represents the entire Korean adult population. The NHIS provides reliable information on individual medical history (diagnostic code, laboratory results from national health screening program, lifestyle factors, anthropometric data, and death information based on a death certificate) and socioeconomic data (based on insurance premiums and self-reported questionnaires). The data from the NHIS-HEALS cohort were based on real-world measurements in a clinical setting. The real-world data showed the true relationship reflecting many variables and environments that we did not expect. Second, an apparently healthy population aged 40 years or older who received a national health screening examination was enrolled in this study, while patients who were diagnosed with diabetes, cancers, or CCVDs were excluded from the analysis. In addition, to control the effects of medications, such as glucose-lowering and lipid-lowering drugs, individuals who had taken these drugs between 2002 and 2010 were excluded. However, individuals treated with these drugs after 2010 were not excluded in the final analyses because the aim of this study was to investigate the association between the initial TyG index and CCVDs or all-cause mortality regardless of medication during the follow-up period. In other words, we just tried to examine whether the elevated TyG index at baseline increases the CCVDs and all-cause mortality. These strict exclusion criteria may minimize the effects of underlying conditions on the primary outcome. Despite strict exclusion, many subjects (144,000 or more) were included in the final analyses. Third, the median follow-up duration was 5.97 years. In the case of apparently healthy individuals, large population size and longer study duration allowed a more accurate relationship to be explored than those from previous studies [27, 28]. Fourth, we widely examined the outcomes, which were related to the TyG index. The primary outcomes were stratified to several individual outcomes and sequentially investigated the relationships between them and the TyG index. In addition, many confounding factors including health behaviors, SBP, LDL-C, economic status, and anti-hypertensive medications for CCVDs were adjusted in the Cox proportional hazards regression models. This conservative approach provides a reliable association between the TyG index and outcomes to minimize the function of these conventional risk factors as confounders.
There are several limitations that should be interpreted cautiously. We could not validate whether the TyG index directly correlated with insulin resistance because the NHIS-HEALS cohort did not contain information on serum insulin levels. However, the TyG index was already validated to indicate insulin resistance in previous studies [14, 31]. The outcomes such as CVDs, CbVDs, and CCVD-related deaths were not directly collected by our research team. There is the possibility of misclassification or a different definition of outcome events. The Korean health insurance system is uniquely different from other countries because all Koreans should subscribe to the NHIS and the Korean government collects and controls health information, insurance claim information, and reimbursement. For example, the NHIS assesses the coverage of insurance and determines the cost of the individual medical service, while Health Insurance Review and Assessment Service (HIRAS), another national agency, reviews the claim data and reimburses the hospital fees. Special diseases such as CCVDs, cancers, and rare diseases are more strictly monitored by these two national agencies because patients with special diseases pay 5% of their hospital fee and the NHIS pays the remaining medical cost. These strict monitoring systems minimize inaccurate diagnosis. In addition, to minimize the misclassification, we more conservatively defined the CCVDs. CCVDs were defined when the relevant diagnostic codes (I20-I25 or I60-I69) as the main diagnostic code were recorded more than once at hospitalization or at least twice at the outpatient visit. Even if there are misclassifications, we believe that there would be very few. Repeatedly measured TyG index was not used in this study. The longitudinal study using repeated measurements of the TyG index was warranted to investigate the more accurate association between the TyG index and the outcomes such as CCVD and all-cause death than one measurement. The TyG index seems to be significantly affected by diet and ethnic group [11, 12]. Thus, the findings from the current study are difficult to generalize to all countries and ethnic groups. Menopausal status was not available in the NHIS-HEALS cohort. Menopause is a well-known risk factor of metabolic abnormality and cardiovascular diseases [32]. If we stratified women according to menopausal status, a more precise relationship could have been shown between TyG index and CCVD-related outcomes in women. Finally, newly prescribed glucose- and lipid-lowering drugs prescribed during the follow-up period was not adjusted in the Cox proportional hazards regression model. These drugs might affect the association between the TyG index and the outcomes.
In conclusion, men with an elevated TyG index were at higher risk for CCVDs or all-cause mortality. Individuals with a higher TyG index were more likely to develop CVDs and ischemic CbVDs in both sexes while the TyG index was not significantly associated with CCVD-related death and all-cause death.
Supporting information
A. Ischemic cerebrovascular diseases. B. Hemorrhagic cerebrovascular diseases. C. Other cerebrovascular diseases. All p-values are from log-rank tests.
(TIF)
(DOCX)
(DOCX)
List of abbreviations
- ANOVA
analysis of variance
- BMI
body mass index
- CbVD
cerebrovascular disease
- CCVD
cardio-cerebrovascular disease
- CI
confidence interval
- CVD
cardiovascular disease
- HEALS
health screening
- HOMA-IR
homeostasis model assessment of insulin resistance
- HR
hazard ratio
- ICD
international classification of diseases
- LDL-C
low-density lipoprotein cholesterol
- NHIS
national health insurance service
- SBP
systolic blood pressure
- SD
standard deviation
- TG
triglycerides
- TyG
triglycerides-glucose
Data Availability
The data underlying the results presented in the study are available from the Korean National Health Insurance Service homepage (URL: https://nhiss.nhis.or.kr/) E-mail: nhiss@nhis.or.kr Phone: +82-1577-1000 Address: 32 Gungang-ro, Wonju-si, Gangwon-do 26464.
Funding Statement
Recipients: Hee-Taik Kang Grant Number: 2021R1G1A1006485 Funder: National Research Foundation of Korea The funder did not play any role in the study design, data collection and analysis, decision to publish or preparation of the manuscript. This research was designed, analyzed, and written by the author.
References
- 1.World Health Organization. Cardiovascular diseases (CVDs) fact sheets. 2021. URL: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds). [Google Scholar]
- 2.Statistics Korea: Causes of Death Statistics in 2018. URL: http://kostat.go.kr/portal/eng/index.action.
- 3.Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. 2018;17(1):122. doi: 10.1186/s12933-018-0762-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ausk KJ, Boyko EJ, Ioannou GN. Insulin resistance predicts mortality in nondiabetic individuals in the U.S. Diabetes Care. 2010;33(6):1179–85. doi: 10.2337/dc09-2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pan K, Nelson RA, Wactawski-Wende J, Lee DJ, Manson JE, Aragaki AK, et al. Insulin Resistance and Cancer-Specific and All-Cause Mortality in Postmenopausal Women: The Women’s Health Initiative. J Natl Cancer Inst. 2020;112(2):170–8. doi: 10.1093/jnci/djz069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214–23. doi: 10.1152/ajpendo.1979.237.3.E214 [DOI] [PubMed] [Google Scholar]
- 7.Fukushima M, Taniguchi A, Sakai M, Doi K, Nagasaka S, Tanaka H, et al. Homeostasis model assessment as a clinical index of insulin resistance. Comparison with the minimal model analysis. Diabetes Care. 1999;22(11):1911–2. doi: 10.2337/diacare.22.11.1911 [DOI] [PubMed] [Google Scholar]
- 8.Du T, Yuan G, Zhang M, Zhou X, Sun X, Yu X. Clinical usefulness of lipid ratios, visceral adiposity indicators, and the triglycerides and glucose index as risk markers of insulin resistance. Cardiovasc Diabetol. 2014;13:146. doi: 10.1186/s12933-014-0146-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park B, Lee HS, Lee YJ. Triglyceride glucose (TyG) index as a predictor of incident type 2 diabetes among nonobese adults: a 12-year longitudinal study of the Korean Genome and Epidemiology Study cohort. Transl Res. 2021;228:42–51. doi: 10.1016/j.trsl.2020.08.003 [DOI] [PubMed] [Google Scholar]
- 10.Lee SH, Kwon HS, Park YM, Ha HS, Jeong SH, Yang HK, et al. Predicting the development of diabetes using the product of triglycerides and glucose: the Chungju Metabolic Disease Cohort (CMC) study. PLoS one. 2014;9(2):e90430. doi: 10.1371/journal.pone.0090430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kouvari M, Boutari C, Chrysohoou C, Fragkopoulou E, Antonopoulou S, Tousoulis D, et al. Mediterranean diet is inversely associated with steatosis and fibrosis and decreases ten-year diabetes and cardiovascular risk in NAFLD subjects: Results from the ATTICA prospective cohort study. Clin Nutr. 2021;40(5):3314–24. doi: 10.1016/j.clnu.2020.10.058 [DOI] [PubMed] [Google Scholar]
- 12.Moon S, Park JS, Ahn Y. The Cut-off Values of Triglycerides and Glucose Index for Metabolic Syndrome in American and Korean Adolescents. J Korean Med Sci. 2017;32(3):427–33. doi: 10.3346/jkms.2017.32.3.427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee YC, Park BJ, Lee JH. Sex Differences in the Relationship Between High-Risk Drinking and the Triglyceride-Glucose (TyG) Index: An Analysis Using 2013 and 2015 Korean National Health and Nutrition Examination Survey Data. Alcohol Alcohol. 2021;56(4):393–400. doi: 10.1093/alcalc/agaa122 [DOI] [PubMed] [Google Scholar]
- 14.Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, Martínez-Abundis E, Ramos-Zavala MG, Hernández-González SO, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95(7):3347–51. doi: 10.1210/jc.2010-0288 [DOI] [PubMed] [Google Scholar]
- 15.Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6(4):299–304. doi: 10.1089/met.2008.0034 [DOI] [PubMed] [Google Scholar]
- 16.Committee of Clinical Practice Guideline of the Korean Society of Lipid and Atherosclerosis (KSoLA). Korean Guidelines for the Management of Dyslipidemia 4th ed. 2018.
- 17.Korean Diabetes Association. Treatment guideline for diabetes, 6th edition. 2019. [Google Scholar]
- 18.American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S14–s31. doi: 10.2337/dc20-S002 [DOI] [PubMed] [Google Scholar]
- 19.Jung CH, Choi KM. Impact of High-Carbohydrate Diet on Metabolic Parameters in Patients with Type 2 Diabetes. Nutrients. 2017;9(4):322. doi: 10.3390/nu9040322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jung CH, Son JW, Kang S, Kim WJ, Kim HS, Kim HS, et al. Diabetes Fact Sheets in Korea, 2020: An Appraisal of Current Status. Diabetes Metab J. 2021;45(1):1–10. doi: 10.4093/dmj.2020.0254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwon YJ, Lee JW, Kang HT. Secular Trends in Lipid Profiles in Korean Adults Based on the 2005–2015 KNHANES. Int J Environ Res Public Health. 2019;16(14):2555. doi: 10.3390/ijerph16142555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee SB, Kim MK, Kang S, Park K, Kim JH, Baik SJ, et al. Triglyceride Glucose Index Is Superior to the Homeostasis Model Assessment of Insulin Resistance for Predicting Nonalcoholic Fatty Liver Disease in Korean Adults. Endocrinol Metab (Seoul, Korea). 2019;34(2):179–86. doi: 10.3803/EnM.2019.34.2.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nam KW, Kwon HM, Jeong HY, Park JH, Kwon H, Jeong SM. High triglyceride-glucose index is associated with subclinical cerebral small vessel disease in a healthy population: a cross-sectional study. Cardiovasc Diabetol. 2020;19(1):53. doi: 10.1186/s12933-020-01031-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho YR, Ann SH, Won KB, Park GM, Kim YG, Yang DH, et al. Association between insulin resistance, hyperglycemia, and coronary artery disease according to the presence of diabetes. Sci Rep. 2019;9(1):6129. doi: 10.1038/s41598-019-42700-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Irace C, Carallo C, Scavelli FB, De Franceschi MS, Esposito T, Tripolino C, et al. Markers of insulin resistance and carotid atherosclerosis. A comparison of the homeostasis model assessment and triglyceride glucose index. Int J Clin Pract. 2013;67(7):665–72. doi: 10.1111/ijcp.12124 [DOI] [PubMed] [Google Scholar]
- 26.Lee SB, Ahn CW, Lee BK, Kang S, Nam JS, You JH, et al. Association between triglyceride glucose index and arterial stiffness in Korean adults. Cardiovasc Diabetol. 2018;17(1):41. doi: 10.1186/s12933-018-0692-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park B, Lee YJ, Lee HS, Jung DH. The triglyceride-glucose index predicts ischemic heart disease risk in Koreans: a prospective study using National Health Insurance Service data. Cardiovasc Diabetol. 2020;19(1):210. doi: 10.1186/s12933-020-01186-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao Q, Zhang TY, Cheng YJ, Ma Y, Xu YK, Yang JQ, et al. Triglyceride-Glucose Index as a Surrogate Marker of Insulin Resistance for Predicting Cardiovascular Outcomes in Nondiabetic Patients with Non-ST-Segment Elevation Acute Coronary Syndrome Undergoing Percutaneous Coronary Intervention. J Atheroscler Thromb. 2020. Epub doi: 10.5551/jat.59840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang A, Wang G, Liu Q, Zuo Y, Chen S, Tao B, et al. Triglyceride-glucose index and the risk of stroke and its subtypes in the general population: an 11-year follow-up. Cardiovasc Diabetol. 2021;20(1):46. doi: 10.1186/s12933-021-01238-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Si S, Li J, Li Y, Li W, Chen X, Yuan T, et al. Causal Effect of the Triglyceride-Glucose Index and the Joint Exposure of Higher Glucose and Triglyceride With Extensive Cardio-Cerebrovascular Metabolic Outcomes in the UK Biobank: A Mendelian Randomization Study. Front Cardiovasc Med. 2020;7:583473. doi: 10.3389/fcvm.2020.583473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu X, Wang L, Zhang W, Ming J, Jia A, Xu S, et al. Fasting triglycerides and glucose index is more suitable for the identification of metabolically unhealthy individuals in the Chinese adult population: A nationwide study. J Diabetes Investig. 2019;10(4):1050–8. doi: 10.1111/jdi.12975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pu D, Tan R, Yu Q, Wu J. Metabolic syndrome in menopause and associated factors: a meta-analysis. Climacteric. 2017;20(6):583–91. doi: 10.1080/13697137.2017.1386649 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A. Ischemic cerebrovascular diseases. B. Hemorrhagic cerebrovascular diseases. C. Other cerebrovascular diseases. All p-values are from log-rank tests.
(TIF)
(DOCX)
(DOCX)
Data Availability Statement
The data underlying the results presented in the study are available from the Korean National Health Insurance Service homepage (URL: https://nhiss.nhis.or.kr/) E-mail: nhiss@nhis.or.kr Phone: +82-1577-1000 Address: 32 Gungang-ro, Wonju-si, Gangwon-do 26464.